Abstract
Background
Host-plant resistance is the most desirable and economic way to overcome BPH damage to rice. As single-gene resistance is easily lost due to the evolution of new BPH biotypes, it is urgent to explore and identify new BPH resistance genes.
Results
In this study, using F2:3 populations and near-isogenic lines (NILs) derived from crosses between two BPH-resistant Sri Lankan rice cultivars (KOLAYAL and POLIYAL) and a BPH-susceptible cultivar 9311, a new resistance gene Bph33 was fine mapped to a 60-kb region ranging 0.91–0.97 Mb on the short arm of chromosome 4 (4S), which was at least 4 Mb distant from those genes/QTLs (Bph12, Bph15, Bph3, Bph20, QBph4 and QBph4.2) reported before. Seven genes were predicted in this region. Based on sequence and expression analyses, a Leucine Rich Repeat (LRR) family gene (LOC_Os04g02520) was identified as the most possible candidate of Bph33. The gene exhibited continuous and stable resistance from seedling stage to tillering stage, showing both antixenosis and antibiosis effects on BPH.
Conclusion
The results of this study will facilitate map-based cloning and marker-assisted selection of the gene.
Similar content being viewed by others
Background
Brown planthopper (BPH; Nilaparvata lugens Stål) is one of the most destructive insect pests of rice in Asia-Pacific region, often causing hopper burn and severe yield loss due to its monophagy and migration ability (Normile 2008). Presently, spraying insecticides (e.g. imidacloprid) is the chief way to control BPH, which is costly and hazardous to health and environment. In addition, it often makes BPH develop resistance to insecticides and therefore leads to resurrection of BPH population (Tanaka et al. 2000). Hence, using rice resistance to BPH should be the most economic and effective approach for the management of BPH (Jena et al. 2006).
Rice resistance to BPH has been studied in the last half century. To date, more than 30 BPH-resistance genes or quantitative trait loci (QTLs) have been identified from ssp. indica and wild relatives of rice (Prahalada et al. 2017; Guo et al. 2018). Of them, 22 genes or QTLs (Bph1, Bph2/Bph26, Bph3, Bph6, bph7, Bph9, Bph12, Bph14, Bph15, Bph17, Bph18, Bph19, Bph20, Bph21, Bph27, Bph27(t), Bph28(t), bph29, QBph3, QBph4, QBph4.2, Bph31 and Bph32) have been fine-mapped (Cha et al. 2008; Murai et al. 2001; Jairin et al. 2007; Qiu et al. 2010, 2012, 2014; Zhao et al. 2016; Du et al. 2009; Lv et al. 2014; Liu et al. 2015; Jena et al. 2006; Chen et al. 2006; Rahman et al. 2009; Huang et al. 2013; He et al. 2013; Wu et al. 2014; Wang et al. 2015; Hu et al. 2015a,b; Prahalada et al. 2017; Ren et al. 2016). However, only 8 genes (Bph14, Bph26, Bph3, bph29, Bph32, Bph18, Bph9 and Bph6) have been isolated by map-based cloning (Du et al. 2009; Tamura et al. 2014; Liu et al. 2015; Wang et al. 2015; Ren et al. 2016; Ji et al. 2016; Zhao et al. 2016; Guo et al. 2018). Bph14, Bph26 (or Bph2), Bph18 and Bph9 are found to encode coiled-coil, nucleotide-binding, and leucine-rich repeat (CC-NB-LRR) proteins, and the latter three (Bph26, Bph18 and Bph9) are allelic. Four haplotypes in Bph9 locus exhibit different resistance to different BPH biotypes, indicating that allelic variation is an important strategy for rice to resist BPH (Zhao et al. 2016).
Since 1970s, many BPH resistance genes have been utilized in rice breeding and a series of cultivars with improved resistance (e.g. IR26, IR36 and IR50) have been developed and released. However, because of the evolution of new BPH biotypes, the improved cultivars are easy to lose their BPH resistance controlled by a single resistant gene (Jena and Kim 2010). Moreover, only a few resistance genes (Bph3, Bph6, Bph9 and Bph31) show high resistance to more than one BPH biotypes/populations. Therefore, it is urgent to discover new broad-spectrum BPH resistance genes and integrate them into rice cultivars.
Two Sri Lankan indica rice cultivars, KOLAYAL and POLIYAL, were previously found to be highly resistant to the most dominated BPH strain in Wuhan (seedling resistance score RS = 1.3 and 2.2 in two tests) and that in Fuzhou (RS = 1.5 and 1.6 in two tests), China, suggesting that they could be useful donors of BPH resistance genes in rice breeding. The present study aimed to understand the genetic basis and possible mechanism of the BPH resistance in KOLAYAL and POLIYAL, so as to facilitate the utilization of these two varieties as BPH resistance donors in rice breeding.
Results
Segregation of BPH resistance in F2:3 populations
To understand the genetic basis of BPH resistance in KOLAYAL and POLIYAL, we investigated the RS segregation at seedling stage in the two F2:3 populations derived from crosses KOLAYAL × 9311 and POLIYAL × 9311. In both F2:3 populations, the RS at 12 DAI showed a bimodal distribution, with the valley bottom approximately at the point between 6.00 and 6.99 (Fig. 1). According to the valley bottom, the F2:3 lines could be divided into two classes, the resistant (RS ≤ 6.99) and the susceptible (RS ≥ 7.00), which was consistent with the scoring criterion of BPH resistance (Qiu et al. 2010). Thus, the segregation ratios of resistant lines to susceptible lines in the two populations were found to be 71:22 and 69:20, respectively, both of which were in agreement with the theoretical 3:1 ratio (chi-squares = 0.76 and 0.58, P = 0.313 and 0.446). These results indicated that the BPH resistance of KOLAYAL and POLIYAL were both controlled by a major gene.
Mapping of resistance gene
To map the genes in the two resistant parents, we performed BSA-seq based on the two F2:3 populations. A total of 161.5 K SNP and short InDel markers were obtained for gene mapping analysis. The results showed that there was only a common peak of allele frequency difference in the two populations ranging 0–1.5 Mb on the short arm of chromosome 4 (4S) (Fig. 2), suggesting that the resistance genes of KOLAYAL and POLIYAL were possibly the same. We named it Bph33, following the two (Bph31 and Bph32) reported by Prahalada et al. (2017) and Ren et al. (2016), respectively.
Verification of Bph33
To verify the location of Bph33, we examined its effect in both BC1F1:2 and BC2F1 populations of the two crosses. Based on the BSA-seq results, two flanking InDel markers, H25 and D17 (Additional file 1: Table S1), which delimited an interval of ~ 1.5 Mb, were developed to survey Bph33 genotype of the BC1F1:2 lines and BC2F1 plants. At the same time, the resistance levels of BC1F1:2 lines and BC2F1 plants were evaluated. T-test showed that the difference of resistance level was very significant between the homozygous resistant genotype and the homozygous susceptible genotype in the two BC1F1:2 populations (Fig. 3a, b) and between the heterozygous resistant genotype and the homozygous susceptible genotype in the two BC2F1 populations (Fig. 3c, d). These results strongly confirmed the existence of Bph33 on the region between markers H25 and D17.
Comparison of resistance levels among different Bph33 genotypes in four populations. a and b In the BC1F2:3 populations from 9311 × KOLAYLA (a) and 9311 × POLIYAL (b); c and d In the BC2F1 populations from 9311 × KOLAYLA (c) and 9311 × POLIYAL (d). The Bph33 genotype was identified according to its flanking markers H25 and D17. A, KOLAYLA or POLIYAL genotype; B, 9311 genotype; H, heterozygote genotype. P-values are shown for the comparison between A and B (in a and b) or between B and H (in c and d)
Fine mapping of Bph33
Since Bph33 was present in both KOLAYLA and POLIYAL, we only used the BC3F2 plants from KOLAYLA × 9311 to fine map the gene. A total of 3200 BC3F2 plants segregating at the Bph33 locus were genotyped with H25 and D17, from which 184 recombinants between the two markers were identified. Based on these recombinants, a local map of the H25-D17 region containing additional nine markers inside was constructed (Fig. 4b). The 184 recombinants were classified into different groups according to their marker genotypes in this region and 3–4 recombinants was randomly selected from each group for subsequent analysis. Each recombinant selected was selfed and the homozygous recombinants in its progeny line were identified using markers and tested for BPH resistance. According to the genotypes and resistance phenotypes of the homozygous recombinants, it could be inferred that lines 80, 140 and 124, 117 delimited the left and right margins of the Bph33 position, respectively (Fig. 4c). Thus, Bph33 was located between InDel markers H14 and H84 (Additional file 1: Table S1), within an interval of 470 kb in the Nipponbare genome sequence.
Fine mapping of Bph33. a Physical map of part of chromosome 4S containing Bph33. b, d Physical map of marker interval H25-D17 (b) or H14-H84 harboring Bph33. The numbers in brackets show the times of recombination occurred between the marker loci and Bph33 among 3200 (b) or 6600 (d) individuals. c, e Graphical genotypes and resistance phenotypes of the recombinants. Black, white and grey bars denote KOLAYLA, 9311 and recombined segments, respectively. R, resistant; S, susceptible. f Annotated genes within the final 60-kb interval. The nucleotide sequence differences and resultant amino acid changes (in brackets) among the parents and Nipponbare in the CDS of candidate gene LOC Os04g02520 are shown
To narrow down the Bph33 interval, we further screened the genotypes of 6600 BC3F3 seedlings and obtained 128 recombinants between H14 and H84, from which the progeny homozygous recombinants were genotyped using 10 new indel markers evenly distributed between H14 and H84. Thus, a high resolution map of Bph33 locus was constructed (Fig. 4d). By testing the resistance of the homozygous recombinants obtained through the same procedure as described above, we found that the Bph33 position could be confined between markers H99 and H101 ((Additional file 1: Table S1)), which spanned a distance of 60 kb (Fig. 4d, e), covering part of the two BAC clones OSJNBa0073L13 and OSJNBa0050O03 of Nipponbare each.
Candidates of Bph33
According the reference genome of Nipponbare, seven genes are predicted in the 60 kb region harboring Bph33 (Fig. 4f). Among them, two genes encode a zinc finger protein (LOC_Os04g02510) and a Leucine Rich Repeat (LRR) family protein (LOC_Os04g02520), respectively, while the other five genes (LOC_Os04g02530, LOC_Os04g02550, LOC_Os04g02560, LOC_Os04g02570 and LOC_Os04g02580) all encode expressed proteins. To identify the possible candidate gene(s), using the BSA-seq data, we compared the allele sequences of these genes from the resistant parents KOLAYAL and POLIYAL with those from the susceptible parent 9311 and Nipponbare (Additional file 2: Table S3). LOC_Os04g02510 and LOC_Os04g02520 both contained many variations between the resistant and the susceptible varieties, and their alleles from the resistant varieties appeared to be functional. So, they both were potential to be Bph33. Among the five expressed-protein genes, LOC_Os04g02530 and LOC_Os04g02570 showed loss-of-function (start codon lost and frameshift) mutations in KOLAYAL and POLIYAL; LOC_Os04g02580 had no exon variations; LOC_Os04g02550 only contained a SNP in 3′-UTR; and LOC_Os04g02560 had a SNP causing non-synonymous change of a codon. Obviously, the first two genes were impossible to be Bph33 because resistant was dominant to susceptible. The middle two were also unlikely to be Bph33. The possibility for the last one to be Bph33 could not be eliminated, but it should be very small compared with those of LOC_Os04g02510 and LOC_Os04g02520. Hence, we had good reasons to exclude the five expressed-protein genes as the candidates of Bph33.
To further examine the possibility of candidate genes to be Bph33, we analyzed their expression before and after infestation in NIL(+) (near-isogenic lines carrying Bph33 allele from the resistant parent) and NIL(−) (near-isogenic lines carrying Bph33 allele from the susceptible parent), respectively. The results (Additional file 3: Figure S1) indicated that at the time of 0 h (before infestation), five genes, LOC_Os04g02510, LOC_Os04g02520, LOC_Os04g02530, LOC_Os04g02550 and LOC_Os04g02570 showed significantly lower expression level in NIL (+) than those of NIL (−). At the time of 6 h and 48 h (after infestation), four and two genes had lower expression level in NIL (+) that those of NIL (−). However, the expression levels of none of the genes in NIL (+) were significantly up-regulated and significantly higher than those in NIL (−) almost at both of time points after BPH infestation. These results suggested that the structure, not expression variation might be related to the function of Bph33.
The expression level of LOC_Os04g02510 was dramatically reduced at 48 h after infestation in both NIL (+) and NIL (−), but LOC_Os04g02520 did not show significant expression changes. In addition, both genes had a lower expression level in NIL (+) than in NIL(−), but the difference was much greater for LOC_Os04g02510. Considering that resistant was dominant to susceptible as mentioned above, these results suggested that LOC_Os04g02510 should be less likely to be Bph33. So, we took LOC_Os04g02520 as the most possible candidate of Bph33. There were six SNPs and two InDels in the second and the third exons of LOC_Os04g02520 between the two resistant and the two susceptible varieties, causing six amino acid substitutions and one and two amino acid insertions in POLIYAL and KOLAYAL, respectively (Fig. 4f). The possibility for LOC_Os04g02520 to be Bph33 was also indirectly supported by the findings that LRR domain exists in some BPH-resistance genes (Du et al. 2009; Tamura et al. 2014; Ji et al. 2016; Zhao et al. 2016).
Resistance effect of Bph33
To better understand the resistance effect of Bph33, we examined the resistance of NIL(+) and NIL(−) together with the three parents. The NIL(+) and NIL(−) showed similar resistance levels to those of the resistant and the susceptible parents, respectively, exhibiting significant difference between them at both seedling stage (Fig. 5a, c) and tillering stage (Fig. 5b). These results suggested that Bph33 had continuous resistance effect during the plant growth.
Test of resistance effect of Bph33 at seedling stage and tillering stage. a Seedling resistance of NILs and parents. Asterisks indicate significant difference between the resistant and the susceptible genotypes at 0.01 level. Error bar indicates standard error. b NIL(+) and NIL(−) plants at 15 DAI. c Seedlings of NILs and parents at 12 DAI
Antixenosis effect of Bph33
In the host selection experiment, BPHs dispersed inside a bucket soon after release, with most landing on soil and the edge of the bucket. As shown in Fig. 6a, 3h after release, about half of the BPHs were found on plants, with ~ 15 nymphs on NIL (+), KOLAYAL or POLIYAL, and ~ 35 nymphs on NIL (−) or 9311. Afterwards, the number of BPHs on the resistant plants decreased slightly, while that on the susceptible plants increased rapidly, reaching the maximum value of ~ 65 at 24 h and then maintaining approximately stable with only slight decrease at 48 h. The results suggested that after one day the BPHs had basically settled down on the plants for food. The difference in the number of settled BPHs on plants was very great and significant between the resistant and the susceptible genotypes throughout the period of experiment, indicating that antixenosis was a factor in the BPH resistance conferred by Bph33.
Tests of antixenosis and antibiosis conferred by Bph33. a BPH settlement preference on plants of different Bph33 genotypes. b Weights of honeydew secreted by BPH on NILs and parents. c Areas of honeydew secreted by BPH feeding on NIL(+) and NIL(−) plants. d Survival rates of BPH on NILs and parents. The column colors have the same meanings as in a. KOL, KOLAYLA; POL, POLIYAL. Asterisks indicate significant difference between the resistant and the susceptible genotypes at 0.01 level. Error bar indicates standard error
Antibiosis effect of Bph33
The antibiosis experiment showed that the weight of honeydew secreted by BPH on NIL (+) (1.14 mg/female) was significantly lower than that on NIL (−) (5.84 mg/female; Fig. 6b). Likewise, the honeydew weight on the resistant parents KOLAYLA (1.28 mg/female) and POLIYAL (0.95 mg/female) were significantly lower than that on the susceptible parent 9311 (6.84 mg/female; Fig. 6b). Consistently, the area of honeydew stains generated by BPH on NIL (+) was much smaller than that on NIL (−) (Fig. 6c). Moreover, BPH survival rates on NIL (+) and the two resistant parents were significantly lower than those on NIL (−) and 9311 from 3 to 7 DAI, and the difference between the resistant genotype and the susceptible genotype increased rapidly with the time (Fig. 6d). At 7 DAI, only 25.75% BPH survived on NIL (+), while 87.78% were still alive on NIL (−). These results indicated that antibiosis was also a factor in the BPH resistance conferred by Bph33.
Discussion
Up to now, at least 30 BPH resistance genes/QTLs have been found in rice, distributed on chromosomes 3, 4, 6, 11 and 12. Interestingly, multiple BPH resistance genes cluster together on chromosomes 4, 6 and 12. Six genes/QTLs (Bph12, Bph3, Bph15, Bph20, QBph4 and QBph4.2) are located in the region 5–9 Mb on chromosome 4S (Qiu et al. 2012; Lv et al. 2014; Liu et al. 2015; Rahman et al. 2009; Hu et al. 2015a,b). In the present study, Bph33 was fine mapped in a region of ~ 60 kb (0.91–0.97 Mb) on chromosome 4S, which was at least 4 Mb distant from those genes/QTLs reported before (Fig. 7). Recently, a new major QTL, Qbph4.3, was mapped between markers RM551 (0.177 Mb) and RM335 (0.688 Mb) on chromosome 4S (Mohanty et al. 2017). The position of Bph33 was outside this interval (Fig. 7). Therefore, Bph33 should be different from Qbph4.3. There is another QTL, Qbph4.4, claimed beside Qbph4.3, of which the interval covers Bph33 (Mohanty et al. 2017). However, Qbph4.4 is a minor QTL with small effect and its interval is very large, ranging from 0.688 Mb to 13.07 Mb, which covers a total of seven BPH resistance genes/QTLs reported (including Bph33; Fig. 7). This means that Qbph4.4 is indeterminate. In addition, as Qbph4.4 is mapped in the same population with Qbph4.3, it is likely to be a false QTL because two QTLs located in adjacent marker intervals are indistinguishable according to the statistical theory of QTL mapping (Zeng 1994). So, it is hard to say that Qbph4.4 is a new QTL. Hence, we have reason to believe that Bph33 is a novel major gene for BPH resistance.
At the individual level, three general resistance mechanisms have been recognized to defend plants against BPH, including antixenosis, antibiosis and tolerance (Cohen et al. 1997). Among them, antibiosis is the major one conferred by almost all BPH resistance genes (Qiu et al. 2010). The results of this study indicated that Bph33 also exhibited significant antibiosis and antixenosis effects, with its resistant allele acting to strengthen the antibiosis and antixenosis (Fig. 6).
It has been found that a large proportion of rice cultivars from Sri Lanka are probably resistant to different biotypes of BPH (Hu et al. 2016). Rathu Heenati, a famous Sri Lankan cultivar, is found to be resistant to all the four biotypes of BPH (Sun et al. 2005; Jairin et al. 2007). In this study, the two resistant cultivars, KOLAYLA and POLIYAL, were also originated from Sri Lanka. This implies that they (or Bph33 gene) have the potential to resist to multiple biotypes of BPH. In fact, as mentioned above, these two cultivars showed high resistance to BPH from Wuhan (~ 30 N, 114E) and Fuzhou (~ 26 N, 119E). The two cities are far apart to each other (the straight line distance between them is ~ 900 km) and have different climates. So, it is possible that the BPH populations from these two places are divergent in genetic structure with different constitution of biotypes or different predominant biotypes. In addition, the resistance mechanisms of Bph33 discussed above and its feature of continuous resistance during plant growth (Fig. 5) are all similar to those found in broad-spectrum BPH resistance genes such as Bph6 and Bph9 (Zhao et al. 2016; Guo et al. 2018). All these imply that Bph33 could be a potential resistance gene of wide spectrum.
Advances in sequencing technology and functional genomics have significantly facilitated the mapping and cloning of BPH resistance genes. The first BPH resistance gene Bph14 was cloned in 2009, while seven other genes, Bph26, Bph3, bph29, Bph32, Bph18, Bph9 and Bph6, were cloned only within recent two years (Du et al. 2009; Tamura et al. 2014; Liu et al. 2015; Wang et al. 2015; Ren et al. 2016; Ji et al. 2016; Zhao et al. 2016; Guo et al. 2018). BSA-seq is very efficient method for gene/QTL mapping (Tang et al. 2018). In this study, we used BSA-seq to rapidly map Bph33 in a 1.5 Mb region and further fine mapped the gene using closely linked markers developed according to the deep sequencing data, demonstrating the efficiency of BSA-seq mapping. Our results will facilitate map-based cloning of Bph33 and adopting marker-assisted backcrossing (MABC) strategy in BPH resistance breeding.
Conclusion
A new major BPH resistance gene Bph33 was fine mapped within an interval of 60 kb on rice chromosome 4S. Antixenosis and antibiosis were two factors in the BPH resistance conferred by Bph33. Our results will facilitate map-based cloning and marker-assisted selection of the gene.
Methods
Plant materials and mapping populations
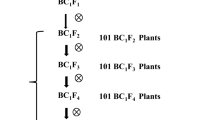
A BPH-susceptible Chinese indica rice cultivar 9311 was crossed with two BPH-resistant Sri Lankan indica rice cultivars, KOLAYAL (IRGC 36295) and POLIYAL (IRGC 36352), which were kindly provided by the International Rice Research Institute (IRRI), to develop two F2:3 populations for genetic analysis and gene mapping. A few highly-resistant F2:3 lines were selected and backcrossed to 9311 to develop several backcross populations for validating the resistance gene. Secondary segregating populations, in which genetic segregation only existed in a few genomic segments including the target region, and isogenic lines (NILs) with 9311 background were further derived from the backcross populations for fine mapping the resistance gene and analyzing its resistance mechanism. The detailed procedure of creating the experimental populations or lines is illustrated in Fig. 8.
Procedure of creating experimental populations or lines used in this study. NIL(+)/NIL(−), near-isogenic lines with 9311 background carrying the BPH resistant/susceptible allele. Both target gene and genetic background were selected. Target gene was selected according to resistance phenotype or flanking markers. Background was selected according to phenotypes of morphological traits
BPH insects and evaluation of BPH resistance
The BPH insects used for infestation in this study were collected from rice fields in Fuzhou, China, and maintained on a susceptible indica rice variety (TN1) under natural conditions at Fujian Agriculture and Forestry University.
Evaluation of BPH resistance was performed by seedling bulk test as described (Huang et al. 2001), with KOLAYAL and POLIYAL as resistant controls and 9311 as susceptible control. Rice seeds were sown in plastic trays of 50 × 30 × 10 cm3 in size, with 20 seeds per line (including control) in a row and 12 lines per tray. Seedlings were grown in a greenhouse at 24–28 °C under natural light. At the three-leaf stage (~ 12 days after sowing), seedlings were thinned out to the density of 10 plants per line, and then infestation was performed with second-instar nymphs in a number of 10 insects per seedling on average. At ~ 12 days after infestation (DAI) when all seedlings of 9311 died, all other lines were examined. The resistance level of a seedling was indicated with a resistance score (RS = 1, 3, 5, 7 or 9). A smaller RS value represented a higher resistance level. The resistance level of a line was indicated by the average RS of all the seedlings it contained.
For evaluation of resistance in tillering stage, two plants (45–50 days old) of NIL (+) or NIL (−), or 9311 (as control) were transplanted into a plastic cup of 17 cm in height and 8 cm in diameter. Then, 400 nymphs of 2–3 instar were released in two plastic cups (one was NIL (+) or NIL (−), the other was 9311) covered by a transparent gauze. At 15 DAI, the plants were evaluated relative to the degree of susceptibility of the control and rated as resistant and susceptible. The experiments were repeated for six times.
Antixenosis test
To test antixenosis, ten 30-day-old rice plants, with five of NIL (+) and NIL (−) each, or of KOLAYAL and 9311 each, or of POLIYAL and 9311 each, were randomly planted in a circle within a plastic bucket of 20 cm in diameter. Plants of the same genotype were marked with small labels. Then, 100 nymphs of 2–3 instar were released in each bucket covered by a transparent gauze. The number of BPHs settling on each plant and the total number of nymphs on 5 plants of the same genotype were recorded at 3 h, 6 h, 24 h and 48 h after release. Six replicates were set, each had 3 buckets in a treatment.
Antibiosis test
To test antibiosis, the amount (weight and area) of honeydew excreted by BPH and the survival rate of BPH were measured. Honeydew collection was performed using the method of Pathak et al. (1982) with slight modification. Newly-emerged fifth-instar female nymphs that had starved for 2 h were enclosed in Parafilm sachets (two nymphs per sachet) and attached to the main stems of rice plants (35 days old) for 24 h. The honeydew in each sachet was then weighed using an electronic balance of 0.1-mg precision. In total, 30 sachets were tested on NIL (+) and NIL (−) each, with three sachets on one plant.
Honeydew area measurement was performed following the method of Du et al. (2009) with modification. A 35-day-old rice plant, of which the leaves and tillers were removed and only the main stem remained, was transplanted into a transparent plastic cup of 17 cm in height and 8 cm in diameter. The cup was covered mouth to mouth by another cup of the same type. The two cups were separated by a piece of filter paper. There was a small hole at the center of the filter paper, through which the main stem stretched into the upper cup. Several small holes were made at the bottom of the upper cup for air exchange. Five fifth-instar BPH nymphs that had starved for 2 h were put into the upper cup. After 2 days, the filter paper was dried under 60 °C for 30 min and then treated with 0.1% solution of ninhydrin in acetone to visualize honeydew stains, which appeared as violet or purple areas on the filter paper. Ten replicates were tested.
BPH survival rate experiment was performed in a similar way to the honeydew area test. Each 35-day-old plant was infested with 15 first-instar nymphs, and the number of survival nymphs in the cup was recorded every day for a period of 7 days. Again, all experiments were conducted in ten replicates.
Marker sources and genotyping
Simple sequence repeats (SSR) markers were obtained from the database Gramene (http://www.gramene.org), and insertion-deletion (InDel) markers (Additional file 1: Table S1) were developed based on the genome sequences of 1,479 rice cultivars (http://ricevarmap.ncpgr.cn) as well as the genome sequencing data of the three parents (see below). Genomic DNA was extracted from fresh leaves using the CTAB method with modification. The PCR system for SSR/InDel assay contained 10.0 μL of 2 × buffer, 2.0 μL of dNTP (2 mM), 0.3 μL of each primer (50 ng/μL), 5.3 μL of ddH2O, 0.1 μL of rTaq E (5 U/μL), and 2.0 μL of DNA template (50–100 ng/μL). The PCR program used was: 94 °C for 4 min; 32 cycles of 94 °C for 30 s, 55 °C for 30 s, and 72 °C for 30 s; and 72 °C for 5 min. PCR products were separated on 4% denaturing polyacrylamide gels and visualized by silver staining.
Gene mapping
The next-generation sequencing-based bulked segregant analysis method (BSA-seq) was used to map the BPH resistance gene. A pair of bulks of individuals with opposite extreme phenotypes (extremely-resistant bulk consisting of 15 or 17 lines vs. extremely-susceptible bulk consisting of 16 or 18 lines) was selected from each of the two F2:3 populations, and genomic DNA pools of the four bulks were prepared. The DNA pools as well as the DNA of the three parents were deeply (~ 20×) sequenced on the platform of Hiseq X Ten. Single nucleotide polymorphism (SNP) and short-InDel markers were identified based on the sequencing data, and gene mapping was conducted using the markers.
Analysis of gene expression response to BPH infestation
Quantitative real-time PCR (qRT-PCR) was conducted to analyze the expression of genes in Bph33 region in NIL (+) and NIL (−) before and after BPH infestation, using the primers described by Du et al. (2009) and Zhao et al. (2016) (Additional file 4: Table S2). A thirty-day-old plant with all leaves removed was transplanted into a transparent plastic cup and covered by another transparent plastic cup as described above. Total RNA was isolated from the stems before BPH infestation and 6 h and 48 h after infestation, respectively, using TRIzol reagent (Invitrogen) following the manufacturer’s instructions, and then converted into first-strand cDNA. Total RNA was also extracted from non-infested NIL (−) plants at every time point. qRT-PCR was performed using SYBR Green PCR Master Mix (Applied Bio systems) and a CFX96 Real-Time System (Bio-Rad) following the manufacturer’s instructions. Three biological replicates were set for each treatment. Each replicate was a mixture of 10 independent NILs of the same genotype in BPH resistance (either resistant or susceptible), and each NIL consisted of 5 plants.
Statistical analysis
Student’s t-test was used to examine the difference between treatment and control. One-way ANOVA and Duncan tests were used to compare multiple samples. Statistical tests were conducted using the software SPSS.
References
Cha YS, Ji H, Yun DW et al (2008) Fine mapping of the rice Bph1 gene, which confers resistance to the brown planthopper (Nilaparvata lugens) and development of STS markers for marker assisted selection. Mol Cells 26:146–151
Chen JW, Wang L, Pang F (2006) Genetic analysis and fine mapping of a rice brown planthopper (Nilaparvata lugens Stål) resistance gene bph19(t). Mol Gen Genomics 275:321–329
Cohen MB, Alam SN, Medina EB, Bernal CC (1997) Brown planthopper, Nilaparvata lugens, resistance in rice cultivar IR64: mechanism and role in successful N. lugens management in Central Luzon, Philippines. Entomol Exp Appl 85:221–229
Du B, Zhang WL, Liu BF et al (2009) Identification and characterization of Bph14, a gene conferring resistance to brown planthopper in rice. P Natl Acad Sci USA 106:22163–22168
Guo JP, Xu CX, Wu D et al (2018) Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat Genet. https://doi.org/10.1038/s41588-018-0039-6
He J, Liu YQ, Liu YL et al (2013) High-resolution mapping of brown planthopper (BPH) resistance gene Bph27(t) in rice (Oryza sativa L.). Mol Breeding 31:549–557
Hu J, Xiao C, Cheng MX et al (2015a) Fine mapping and pyramiding of brown planthopper resistance genes QBph3 and QBph4 in an introgression line from wild rice O. officinalis. Mol Breeding 35:3. https://doi.org/10.1007/s11032-015-0228-2
Hu J, Xiao C, Cheng MX et al (2015b) A new finely mapped Oryza australiensis-derived QTL in rice confers resistance to brown planthopper. Gene 561:132–137
Hu J, Xiao C, He YQ (2016) Recent progress on the genetics and molecular breeding of brown planthopper resistance in rice. Rice 9:30. https://doi.org/10.1186/s12284-016-0099-0
Huang D, Qiu Y, Zhang Y et al (2013) Fine mapping and characterization of BPH27, a brown planthopper resistance gene from wild rice (Oryza rufipogon Griff.). Theor Appl Genet 126:219–229
Huang Z, He GC, Shu LH, Li XH, Zhang QF (2001) Identification and mapping of two brown planthopper resistance genes in rice. Theor Appl Genet 102:929–934
Jairin J, Phengrat K, Teangdeerith S et al (2007) Mapping of a broad-spectrum brown planthopper resistance gene, Bph3, on rice chromosome 6. Mol Breeding 19:35–44
Jena KK, Jeung JU, Lee JH et al (2006) High-resolution mapping of a new brown planthopper (BPH) resistance gene, Bph18(t), and marker-assisted selection for BPH resistance in rice (Oryza sativa L.). Theor Appl Genet 112:288–297
Jena KK, Kim SM (2010) Current status of Brown Planthopper (BPH) resistance and genetics. Rice 3:161–171
Ji H, Kim SR, Kim YH et al (2016) Map-based cloning and characterization of the Bph18 gene from wild rice conferring resistance to brown planthopper (BPH) insect pest. Sci Rep 6:34376
Liu YQ, Wu H, Chen H et al (2015) A gene cluster encoding lectin receptor kinases confers broadspectrum and durable insect resistance in rice. Nat Biotechnol 33:301–305
Lv WT, Du B, Shangguan XX et al (2014) BAC and RNA sequencing reveal the brown planthopper resistance gene BPH15 in a recombination cold spot that mediates a unique defense mechanism. BMC Genomics 15:674–589
Mohanty SK, Panda RS, Mohapatra SL et al (2017) Identification of novel quantitative trait loci associated with brown planthopper resistance in the rice landrace Salkathi. Euphytica 213:38. https://doi.org/10.1007/s10681-017-1835-2
Murai H, Hashimoto Z, Sharma P et al (2001) Construction of a high resolution linkage map of a rice brown planthopper (Nilaparvata lugens Stål) resistance gene bph2. Theor Appl Genet 103:526–532
Normile D (2008) Reinventing rice to feed the world. Science 321:330–333
Pathak PK, Saxena RC, Heinrichs EA (1982) Parafilm sachet for measuring honeydew excretion by Nilaparvata lugens on rice. J Econ Entomol 75:194–195
Prahalada GD, Shivakumar N, Lohithaswa HC et al (2017) Identification and fine mapping of a new gene, BPH31 conferring resistance to brown planthopper biotype 4 of India to improve rice, Oryza sativa L. Rice 10:41. https://doi.org/10.1186/s12284-017-0178-x
Qiu Y, Guo J, Jing S et al (2010) High-resolution mapping of the brown planthopper resistance gene Bph6 in rice and characterizing its resistance in the 93-11 and Nipponbare near isogenic backgrounds. Theor Appl Genet 121:1601–1611
Qiu Y, Guo J, Jing S et al (2012) Development and characterization of japonica rice lines carrying the brown planthopper-resistance genes BPH12 and BPH6. Theor Appl Genet 124:485–494
Qiu YF, Guo JP, Jing SL et al (2014) Fine mapping of the rice brown planthopper resistance gene BPH7 and characterization of its resistance in the 93-11background. Euphytica 198:369–379
Rahman ML, Jiang W, Chu SH et al (2009) High-resolution mapping of two rice brown planthopper resistance genes, Bph20(t) and Bph21(t), originating from Oryza minuta. Theor Appl Genet 119:1237–1246
Ren J, Gao F, Wu X, Zeng L, Lv J, Su X, Luo H, Ren G (2016) BPH32, a novel gene encoding an unknown SCR domain-containing protein, confers resistance against the brown planthopper in rice. Sci Rep 6:37645. https://doi.org/10.1038/srep37645.8
Sun L, Su C, Wang C et al (2005) Mapping of a major resistance gene to brown planthopper in the rice cultivar Rathu Heenati. Breed Sci 55:391–396
Tamura Y, Hattori M, Yoshioka H et al (2014) Map-based cloning and characterization of a brown planthopper resistance gene BPH26 from Oryza sativa L. ssp. indica cultivar ADR52. Sci Rep 4:5872
Tanaka K, Endo S, Kazana H (2000) Toxicity of insecticides to predators of rice planthoppers: spiders, the mirid bug and the dryinid wasp. Appl Entomol Zool 35:177–187
Tang WQ, Huang LK, Bu SH, Zhang XZ, Wu WR (2018) Estimation of QTL heritability based on pooled sequencing data. Bioinformatics 34:978–984
Wang Y, Cao LM, Zhang YX et al (2015) Map-based cloning and characterization of BPH29, a B3 domain-containing recessive gene conferring brown planthopper resistance in rice. J Exp Bot 66:6035–6045
Wu H, Liu YQ, He J et al (2014) Fine mapping of brown planthopper (Nilaparvata lugens Stål) resistance gene Bph28(t) in rice (Oryza sativa L.). Mol Breeding 33:909–918
Zeng ZB (1994) Precision mapping of quantitative trait loci. Genetics 136:1457–1468
Zhao Y, Huang J, Wang ZZ et al (2016) Allelic diversity in an NLR gene BPH9 enables rice to combat planthopper variation. P Natl Acad Sci USA 113:12850–12855
Acknowledgments
We are very grateful to IRRI for providing seeds of resistant parents.
Funding
This work was supported in part by National Key R&D Program of China (2017YFD0100103), Fujian Provincial Natural Science Foundation (2017 J01437), National Natural Science Foundation for the Youth of China (31401355).
Availability of data and materials
All relevant data are provide as Tables within the paper in the Additional files.
Author information
Authors and Affiliations
Contributions
WW and JH conceived the idea and wrote the manuscript. JH, XC and LZ conducted the research. WT analyzed the data in BSA-seq experiment. All authors read and approved of the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study complied with the ethical standards of China, where this research work was carried out.
Consent for publication
All authors are consent for publication.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional files
Additional file 1:
Table S1. Indel markers for fine mapping of Bph33. (DOCX 15 kb)
Additional file 2:
Table S3. Structural variations in candidate genes of Bph33. (XLSX 9 kb)
Additional file 3:
Figure S1. Expression analysis of Bph33 candidate genes in the two NILs with contrary Bph33 genotypes. Letter ‘a’ or ‘b’ indicates significant difference between NIL(+) and NIL(−) at the same time point after BPH infestation at 0.01 or 0.05 level. Asterisks indicate significant difference between the none-infested (0 h) and the infested plants at 0.01 (**) or 0.05 (*) level. Error bar indicates standard error (PDF 523 kb)
Additional file 4:
Table S2. List of primers used for analyzing the expression of genes in Bph33 region. (DOCX 14 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hu, J., Chang, X., Zou, L. et al. Identification and fine mapping of Bph33, a new brown planthopper resistance gene in rice (Oryza sativa L.). Rice 11, 55 (2018). https://doi.org/10.1186/s12284-018-0249-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12284-018-0249-7