Abstract
Objective: to evaluate the efficacy of US, both qualitatively and semi-quantitatively, in the selection of treatment for the Covid-19 patient, using patient triage as the gold standard. Methods: Patients admitted to the Covid-19 clinic to be treated with monoclonal antibodies (mAb) or retroviral treatment and undergoing lung ultrasound (US) were selected from the radiological data set between December 2021 and May 2022 according to the following inclusion criteria: patients with proven Omicron variant and Delta Covid-19 infection; patients with known Covid-19 vaccination with at least two doses. Lung US (LUS) was performed by experienced radiologists. The presence, location, and distribution of abnormalities, such as B-lines, thickening or ruptures of the pleural line, consolidations, and air bronchograms, were evaluated. The anomalous findings in each scan were classified according to the LUS scoring system. Nonparametric statistical tests were performed. Results: The LUS score median value in the patients with Omicron variant was 1.5 (1–20) while the LUS score median value in the patients with Delta variant was 7 (3–24). A difference statistically significant was observed for LUS score values among the patients with Delta variant between the two US examinations (p value = 0.045 at Kruskal Wallis test). There was a difference in median LUS score values between hospitalized and non-hospitalized patients for both the Omicron and Delta groups (p value = 0.02 on the Kruskal Wallis test). For Delta patients groups the sensitivity, specificity, positive and negative predictive values, considering a value of 14 for LUS score for the hospitalization, were of 85.29%, 44.44%, 85.29% and 76.74% respectively. Conclusions: LUS is an interesting diagnostic tool in the context of Covid-19, it could allow to identify the typical pattern of diffuse interstitial pulmonary syndrome and could guide the correct management of patients.
Similar content being viewed by others
Introduction
Although almost three years have passed since the first documented case of Covid-19 infection, and despite the introduction of vaccines that are effective in preventing severe forms of the disease, the management of the infected patient remains a health emergency, especially about hospital admission [1-34]. Globally, at May 2023, there have been 766,404.339 confirmed cases of COVID-19, including 6,935.958 deaths, reported to WHO [35]. As of 26 October 2022, a total of 12,830.378.906 vaccine doses have been administered [35].
While the imaging methods have improved the diagnosis, also thanks to the contribution of artificial intelligence techniques, thanks to which it is also possible to define a stratification of the patient's risk, both in terms of disease progression and death, and in terms of post-infection sequelae [36-54], the progress in the field of treatment still remains to be understood. To date, a defined treatment for Covid-19 infection has not been introduced. There may be different therapeutic options, many of them based on symptom control, and the possibility of using one class or another depends on the disease severity and timing of onset [55-66]. Monoclonal antibodies (mAbs) have transformed the treatment of several diseases, including cancer and inflammatory or autoimmune conditions, and are a new first line for the treatment of infectious diseases. An unprecedented number of mAbs have been developed over the past year to combat coronavirus disease 2019 (COVID-19) [67-73]. However, considering that RNA viruses, as SARS-CoV-2, are evolving biological entities, it is known that these entities can escape the immunity elicited by infection, vaccination, or mAb administration [74]. Antiviral agents represent a major advance in the COVID-19 patient therapeutic management, leading to a substantial reduction in SARS-CoV-2-related complications and mortality [73]. Currently available agents are expensive and the efficacy has not been demonstrated for several subtypes [73]. In this scenario, a correct patient selection should be carried out according to a rigorous study protocol that considers the time of drug administration so as the imaging tools that could objectively select the patient [75-91]. Although ultrasounds (US) have been suggested by various scientific societies as a tool to be used in selected cases [92-94], the possibility of a wide using make them a suitable tool in the patient risk assessment [95-104]. The introduction of different evaluation scores also make the assessment objectively and non-operator dependent [105-115].
Aim is this study is to assess the US role and efficacy, as qualitatively as semi-quantitatively diagnostic tool, in the Covid-19 patient treatment selection, using patient triage as gold standard.
Methods
Patient characteristics
This retrospective study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Ethics Committee IRCCS L. Spallanzani. Data acquisition and analysis were performed in compliance with the protocols approved by the National Institute for Infectious Diseases IRCCS L. Spallanzani Ethics Committee (Ethical Approval Number 164, 26 June 2020). The Local Ethical Committee board, considering the ongoing epidemic emergency, renounced patient informed consent.
Out-patients admitted to the Covid-19 clinic to be treated with mAb or retroviral therapies, within five days of Covid-19 symptoms onset, and undergoing lung ultrasound were selected between December 2021 and May 2022, according to the following inclusion criteria: (1) patients with proven variant COVID-19 infection Omicron and Delta; (2) patients with known COVID-19 vaccination with at least two doses; (3) patients with ultrasound images at baseline. Exclusion criteria were: (1) patients without ultrasound images at baseline, (2) patients with no clinical follow-up.
Ultrasound procedures and images assessment
Lung US (LUS) was performed by expert radiologists, with > 5 years of experience in lung evaluation, and an adequate personal protective equipment. An US system (Acuson Juniper, Siemens-Healthineers, Germany) with convex 3.5–5 MHz and linear 4–8 MHz probes, dedicated to COVID-19 patients, was used. Covers for the probes and the US machine console were used during the procedure.
Examinations were performed with patients in a sitting position, systematically scanning the anterior and posterior portions of each hemithorax. A convex probe was initially used to obtain a panoramic view of the pleural line and ultrasound artifacts associated with the status of the lung parenchyma (A-lines, B-lines, and consolidations). Then, to obtain a more detailed study of the appearance of the pleural line and subpleural abnormalities, the radiologist used a linear probe.
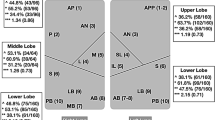
According to Mongodi et al. [116], LUS score was computed in six regions per hemithorax: regions 1 and 2, anterior regions; regions 3 and 4, lateral regions; and regions 5 and 6, posterior regions. Complete examinations taken 10–15 min for each patient.
Images were saved using a dedicated software and reviewed by radiologist after the assessment, to avoid prolonged exposure with the infected patient.
The presence, location, and distribution of abnormalities, such as B-lines, thickening or ruptures of the pleural line, consolidations, and air bronchograms, were evaluated.
The anomalous findings in each scan were also classified according to the scoring system (LUS score) proposed by Soldati et al. [117] (0 = regular pleural line, “A” lines present; 1 = notched pleural line, focal B lines; 2 = broken pleural line, subpleural consolidations; and 3 = blank lung with or without consolidations).
Patient clinical data
Patient Clinical data, including vital signs and oxygen saturation in room air at the moment of admission, SARS-CoV-2 testing, timing of symptom onset, and main comorbidities, were also retrieved from each clinical record.
Statistical analysis
Continuous data were expressed in terms of median values and range. Chi square test and Kruskal Wallis test were used to verify differences among groups. Kolmogorov–Smirnov was used methods to test the normality of the data.
P value < 0.05 was considered significant for all tests.
The statistical analyses were performed using the Statistics and Machine Toolbox of MATLAB R2021b (MathWorks, Natick, MA, USA).
Results
Our study group included thirty-six patients (median age 58 years, range 28–82 years) with Omicron variant subjected to US evaluation and forty-three patients (median age 68 years, range 31–84 years) with Delta variant with US evaluation. No Omicron patients were re-assessed by US, since this evaluation it is not required according to symptoms. Among Delta patients, 16 patients were subjected to US examination in the follow-up (8 days after the baseline US as median value; range 6–23 days after the baseline US).
Kolmogorov–Smirnov verified the normality of the data (p value < 0.05).
With regard to qualitative assessment, among Omicron patients: 5 had single B-lines (Figs. 1, 2), 4 confluent B-lines, 7 low grade pleural line thickening, 2 medium grade pleural line thickening, 1 high grade pleural line thickening and one consolidative pattern (Fig. 3).
Among Delta patients: 3 had single B-lines, 12 confluent B-lines, 6 low grade pleural line thickening, 6 medium grade pleural line thickening (Fig. 4), 5 high grade pleural line thickening and 3 consolidative pattern (Fig. 5).
Among Omicron patients, 25 patients had fever (range 37.5°–40°), 22 cough, 22 pharyngitis, 1 dyspnea, 2 fibromyalgia and 2 had headache.
Among Delta patients: 4 dyspnea, 22 fever (range 37.5–40), 22 cough, 11 fibromyalgia, 10 headache and 2 dyspepsia.
Table 1 reports LUS and US patterns score median value and range for single pattern. There was a statistically significant difference in median LUS score values between different patient subgroups grouped by US pattern for both the Omicron and Delta groups (p value ≪ 0.01 at Kruskal Wallis test).
There was no statistically significant difference in the US pattern between the Omicron and Delta patient groups (p value = 0.43 at Chi square test).
No statistical differences were found between Lung score and symptoms (p value = 0.25 at Kruskal Wallis test) and between US pattern and symptoms (p value = 0.37 at Kruskal Wallis test).
A difference statistically significant was observed for LUS score median values among the patients with Omicron variant compared to the patients with Delta variant (p value < 0.001 at Kruskal Wallis test, Fig. 6). The LUS score median value in the patients with Omicron variant was 1.5 (1–20) while the LUS score median value in the patients with Delta variant was 7 (3–24).
A difference statistically significant was observed for LUS score values among the patients with Delta variant between the two US examinations (p value = 0.045 at Kruskal Wallis test, Fig. 7).
The LUS score median value in the patients with Delta variant in the follow-up was 3.
Among the Delta patients, 9 patients were hospitalized while among the Omicron patients 2 patients were hospitalized. There was a statistically significant difference in median LUS score values between hospitalized and non-hospitalized patients for both the Omicron and Delta groups (p value = 0.02 on the Kruskal Wallis test). For both Delta and Omicron patients, the cut-off value of LUS score for the hospitalized patients was 14 (Fig. 8).
For Delta patients groups the sensitivity, specificity, positive and negative predictive values, considering a value of 14 for LUS score for the hospitalization, were of 85.29%, 44.44%, 85.29% and 76.74% respectively. Considering that only 2 patients were hospitalized in Omicron patient groups the performance of LUS score was not calculated.
Discussion
Covid-19 infection remains a health emergency considering the high transmission rate of the virus, the variability of the clinical symptoms and the need for careful monitoring of patients, since, although 80% of patients have mild symptoms, approximately 14% of patients have moderate to severe disease with 5% becoming critically ill [35]. Also, considering that a proper coronavirus treatment has not yet been established and several therapies are aimed at resolving the symptoms, an assessment of the risk of an infected patient, both in terms of disease progression and the need for admission to intensive care unit, is crucial [118, 119]. In this context, the need for an objective evaluation of pulmonary involvement appears evident.
Today, several Computed Tomography (CT) severity scores have been proposed [120-125] and, although CT is thought to play a key role COVID-19 patient diagnostic work-up [4-13, 108, 126-143], however the transport of infected patients for a CT scan followed requires necessary decontamination procedures that makes this procedure risky and time consuming [118]. According to the WHO [35], COVID‐19 patient, that require hospitalization for moderate–severe disease, will need supplementary oxygen and consistent observing to facilitate early recognition and escalation of the deteriorating patient. In this scenario, LUS is an effective diagnostic tool, since this procedure do not require patient transport since it can be performed in patient room [118].
In COVID-19 pneumonia, LUS helps to identify the typical pattern of diffuse interstitial pulmonary syndrome, characterized by multiple or confluent B-lines, pleural line thickening, pleural line irregularity, and consolidations [127]. LUS findings correlate with CT findings and have several advantages such as lack of radiation exposure, repeatability during follow-up, low cost, and easier application [127, 128].
We evaluated 36 Omicron patients and 43 Delta patients, which have been assessed with US, showing that no statistical differences were found between Lung score and symptoms and between US pattern and symptoms. Considering this result, it is clear as the symptoms should not be the only data to be evaluated in order to hospitalize a patient, while the possibility of objectifying the disease allows an optimization of patient management, avoiding overtreatment and under treatment.
With regard to severity, although, there was no statistically significant difference in the US pattern between the Omicron and Delta patient groups, a difference statistically significant was observed for LUS score values among the patients with Omicron variant compared to the patients with Delta variant. In fact, the LUS score median value in the patients with Omicron was 1.5, while in Delta patients was 7. These results are in line with previous studies that demonstrated that in-hospital mortality was 7.6% for alpha, 12.2% for delta, and 7.1% for omicron [129]. Among unvaccinated patients with hospitalized Covid-19, severity on the WHO Clinical Progression Scale was higher for delta than alpha and lower for omicron than delta [129]. Compared with unvaccinated patients, severity was lower for vaccinated patients for each variant, including alpha and omicron [129].
Among Delta patients, 9 patients were hospitalized while among Omicron patients 2 patients were hospitalized. There was a statistically significant difference in median LUS score values between hospitalized and non-hospitalized patients for both the Omicron and Delta groups. For both Delta and Omicron patients, the cut-off value of LUS score for the hospitalized patients was 14. For Delta patient groups the sensitivity, specificity, positive and negative predictive values, considering a value of 14 for LUS score for the hospitalization, were of 85.29%, 44.44%, 85.29% and 76.74% respectively. However, due to the small number of patients and the extreme overlapping box plots, the evidence for the difference between the two groups should be further validated.
Our results are in line with the data reported by previous study [130]. A recent systematic review showed as LUS can be used at home assistance to prevent unnecessary hospital admission, since LUS, integrated with clinic and physical evaluation, results in more accurate diagnosis of COVID-19. Additionally, LUS can be used in the prognostic stratification of patients with pneumonia through the extension of specific patterns and their evolution to the consolidation phase in emergency setting [130]. These data were, also, confirmed by multicenter prospective cohort study [129]. The authors evaluated 398 patients and reported that LUS predicts mechanical ventilation, ICU admission, and high-flow oxygen treatment but not survival [108].
During follow-up, a difference statistically significant was observed for LUS score values among the patients with Delta variant between the two US examinations. The LUS median score value in the patients with Delta variant in the follow-up was 3. These results are similar to Lichter et al. results [131], which showed that clinical deterioration was associated with increased follow-up LUS scores, mostly due to loss of aeration in anterior lung segments. A multicenter study evaluated patients with COVID-19-related acute respiratory distress syndrome (ARDS) with at least one LUS study within 5 days of initiating invasive mechanical ventilation [132] evaluating 137 patients and demonstrating that the global LUS score was associated with successful exit from mechanical ventilation regardless of ARDS severity, but not with 28-day mortality [132].
LUS is interesting diagnostic tool for assessing the severity of COVID-19 related pneumonia since this examination could potentially decrease or eliminate the need for hospitalization. In addition, decreasing global LUS score was associated with a better treatment response and clinical outcome.
Our study has several limitations: (a) patient selection, considering only patients admitted to the Covid-19 clinic to be treated with mAbs or retroviral treatment, this could cause biased selection; (b) retrospective study; (c) The prognostic value of changes in LUS score over time could not be assessed for all patients. Post-treatment changes in LUS scores should be further evaluated as a monitoring tool for lung parenchymal re-aeration; (d) heart failure or extensive emphysema are not considered in our study, however, they are not comorbidities that may affect the ultrasound image and the presence, location, and distribution of abnormalities, such as B-lines, thickening, or ruptures of the pleural line, consolidations and air bronchograms that were evaluated on the images.
Conclusion
LUS is attractive diagnostic tool in Covid-19 setting, it could allowing to identify the typical pattern of diffuse interstitial lung syndrome, and could guide the proper patient management. We found a difference in median LUS score values between hospitalized and non-hospitalized patients for both the Omicron and Delta groups. A possible cut-off value of LUS score for the hospitalized patients could be 14.
Availability of data and materials
All data are reported in the manuscript.
References
Ochani R, Asad A, Yasmin F, Shaikh S, Khalid H, Batra S, Sohail MR, Mahmood SF, Ochani R, Hussham Arshad M, Kumar A, Surani S. COVID-19 pandemic: from origins to outcomes. A comprehensive review of viral pathogenesis, clinical manifestations, diagnostic evaluation, and management. Infez Med. 2021;29(1):20–36.
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, Kang L, Guo L, Liu M, Zhou X, Luo J, Huang Z, Tu S, Zhao Y, Chen L, Xu D, Li Y, Li C, Peng L, Li Y, Xie W, Cui D, Shang L, Fan G, Xu J, Wang G, Wang Y, Zhong J, Wang C, Wang J, Zhang D, Cao B. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–32. https://doi.org/10.1016/S0140-6736(20)32656-8.
Safiabadi Tali SH, LeBlanc JJ, Sadiq Z, Oyewunmi OD, Camargo C, Nikpour B, Armanfard N, Sagan SM, Jahanshahi-Anbuhi S. Tools and techniques for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)/COVID-19 detection. Clin Microbiol Rev. 2021;34(3):e00228-e320. https://doi.org/10.1128/CMR.00228-20.
Grassi R, Belfiore MP, Montanelli A, Patelli G, Urraro F, Giacobbe G, Fusco R, Granata V, Petrillo A, Sacco P, Mazzei MA, Feragalli B, Reginelli A, Cappabianca S. COVID-19 pneumonia: computer-aided quantification of healthy lung parenchyma, emphysema, ground glass and consolidation on chest computed tomography (CT). Radiol Med. 2021;126(4):553–60. https://doi.org/10.1007/s11547-020-01305-9.
Fusco R, Grassi R, Granata V, Setola SV, Grassi F, Cozzi D, Pecori B, Izzo F, Petrillo A. Artificial intelligence and COVID-19 using chest CT scan and chest X-ray images: machine learning and deep learning approaches for diagnosis and treatment. J Pers Med. 2021;11(10):993. https://doi.org/10.3390/jpm11100993.
Davenport MS, Bruno MA, Iyer RS, Johnson AM, Herrera R, Nicola GN, Ortiz D, Pedrosa I, Policeni B, Recht MP, Willis M, Zuley ML, Weinstein S. ACR statement on safe resumption of routine radiology care during the coronavirus disease 2019 (COVID-19) pandemic. J Am Coll Radiol. 2020;17(7):839–44. https://doi.org/10.1016/j.jacr.2020.05.001.
Dalpiaz G, Gamberini L, Carnevale A, Spadaro S, Mazzoli CA, Piciucchi S, Allegri D, Capozzi C, Neziri E, Bartolucci M, Muratore F, Coppola F, Poerio A, Giampalma E, Baldini L, Tonetti T, Cappellini I, Colombo D, Zani G, Mellini L, Agnoletti V, Damiani F, Gordini G, Laici C, Gola G, Potalivo A, Montomoli J, Ranieri VM, Russo E, Taddei S, Volta CA, Scaramuzzo G. Clinical implications of microvascular CT scan signs in COVID-19 patients requiring invasive mechanical ventilation. Radiol Med. 2022;127(2):162–73. https://doi.org/10.1007/s11547-021-01444-7.
Granata V, Ianniello S, Fusco R, Urraro F, Pupo D, Magliocchetti S, Albarello F, Campioni P, Cristofaro M, Di Stefano F, Fusco N, Petrone A, Schininà V, Villanacci A, Grassi F, Grassi R, Grassi R. Quantitative analysis of residual COVID-19 lung CT features: consistency among two commercial software. J Pers Med. 2021;11(11):1103. https://doi.org/10.3390/jpm11111103.
Fernandes Q, Inchakalody VP, Merhi M, Mestiri S, Taib N, Moustafa Abo El-Ella D, Bedhiafi T, Raza A, Al-Zaidan L, Mohsen MO, Yousuf Al-Nesf MA, Hssain AA, Yassine HM, Bachmann MF, Uddin S, Dermime S. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. 2022;54(1):524–40. https://doi.org/10.1080/07853890.2022.2031274.
Reginelli A, Grassi R, Feragalli B, Belfiore MP, Montanelli A, Patelli G, La Porta M, Urraro F, Fusco R, Granata V, Petrillo A, Giacobbe G, Russo GM, Sacco P, Grassi R, Cappabianca S. Coronavirus disease 2019 (COVID-19) in Italy: double reading of chest CT examination. Biology (Basel). 2021;10(2):89. https://doi.org/10.3390/biology10020089.
Granata V, Fusco R, Setola SV, Galdiero R, Picone C, Izzo F, D’Aniello R, Miele V, Grassi R, Grassi R, Petrillo A. Lymphadenopathy after BNT162b2 Covid-19 vaccine: preliminary ultrasound findings. Biology (Basel). 2021;10(3):214. https://doi.org/10.3390/biology10030214.
Grassi R, Fusco R, Belfiore MP, Montanelli A, Patelli G, Urraro F, Petrillo A, Granata V, Sacco P, Mazzei MA, Feragalli B, Reginelli A, Cappabianca S. Coronavirus disease 2019 (COVID-19) in Italy: features on chest computed tomography using a structured report system. Sci Rep. 2020;10(1):17236. https://doi.org/10.1038/s41598-020-73788-5.
Granata V, Fusco R, Villanacci A, Magliocchetti S, Urraro F, Tetaj N, Marchioni L, Albarello F, Campioni P, Cristofaro M, Di Stefano F, Fusco N, Petrone A, Schininà V, Grassi F, Girardi E, Ianniello S. Imaging severity COVID-19 assessment in vaccinated and unvaccinated patients: comparison of the different variants in a high volume Italian reference center. J Pers Med. 2022;12(6):955. https://doi.org/10.3390/jpm12060955.
Granata V, Fusco R, Vallone P, Setola SV, Picone C, Grassi F, Patrone R, Belli A, Izzo F, Petrillo A. Not only lymphadenopathy: case of chest lymphangitis assessed with MRI after COVID 19 vaccine. Infect Agent Cancer. 2022;17(1):8. https://doi.org/10.1186/s13027-022-00419-1.
Palmisano A, Scotti GM, Ippolito D, Morelli MJ, Vignale D, Gandola D, Sironi S, De Cobelli F, Ferrante L, Spessot M, Tonon G, Tacchetti C, Esposito A. Chest CT in the emergency department for suspected COVID-19 pneumonia. Radiol Med. 2021;126(3):498–502. https://doi.org/10.1007/s11547-020-01302-y.
Özel M, Aslan A, Araç S. Use of the COVID-19 reporting and data system (CO-RADS) classification and chest computed tomography involvement score (CT-IS) in COVID-19 pneumonia. Radiol Med. 2021;126(5):679–87. https://doi.org/10.1007/s11547-021-01335-x.
Borghesi A, Sverzellati N, Polverosi R, Balbi M, Baratella E, Busso M, Calandriello L, Cortese G, Farchione A, Iezzi R, Palmucci S, Pulzato I, Rampinelli C, Romei C, Valentini A, Grassi R, Larici AR. Impact of the COVID-19 pandemic on the selection of chest imaging modalities and reporting systems: a survey of Italian radiologists. Radiol Med. 2021;126(10):1258–72. https://doi.org/10.1007/s11547-021-01385-1.
Ierardi AM, Gaibazzi N, Tuttolomondo D, Fusco S, La Mura V, Peyvandi F, Aliberti S, Blasi F, Cozzi D, Carrafiello G, De Filippo M. Deep vein thrombosis in COVID-19 patients in general wards: prevalence and association with clinical and laboratory variables. Radiol Med. 2021;126(5):722–8. https://doi.org/10.1007/s11547-020-01312-w.
Cardobi N, Benetti G, Cardano G, Arena C, Micheletto C, Cavedon C, Montemezzi S. CT radiomic models to distinguish COVID-19 pneumonia from other interstitial pneumonias. Radiol Med. 2021;126(8):1037–43. https://doi.org/10.1007/s11547-021-01370-8.
Alsharif W, Qurashi A. Effectiveness of COVID-19 diagnosis and management tools: a review. Radiography (Lond). 2021;27(2):682–7. https://doi.org/10.1016/j.radi.2020.09.010.
Moroni C, Cozzi D, Albanesi M, Cavigli E, Bindi A, Luvarà S, Busoni S, Mazzoni LN, Grifoni S, Nazerian P, Miele V. Chest X-ray in the emergency department during COVID-19 pandemic descending phase in Italy: correlation with patients’ outcome. Radiol Med. 2021;126(5):661–8. https://doi.org/10.1007/s11547-020-01327-3.
Masselli G, Almberger M, Tortora A, Capoccia L, Dolciami M, D’Aprile MR, Valentini C, Avventurieri G, Bracci S, Ricci P. Role of CT angiography in detecting acute pulmonary embolism associated with COVID-19 pneumonia. Radiol Med. 2021;126(12):1553–60. https://doi.org/10.1007/s11547-021-01415-y.
Kao YS, Lin KT. A meta-analysis of the diagnostic test accuracy of CT-based radiomics for the prediction of COVID-19 severity. Radiol Med. 2022;127(7):754–62. https://doi.org/10.1007/s11547-022-01510-8.
Cereser L, Girometti R, Da Re J, Marchesini F, Como G, Zuiani C. Inter-reader agreement of high-resolution computed tomography findings in patients with COVID-19 pneumonia: a multi-reader study. Radiol Med. 2021;126(4):577–84. https://doi.org/10.1007/s11547-020-01320-w.
Thompson MG, Burgess JL, Naleway AL, Tyner H, Yoon SK, Meece J, Olsho LEW, Caban-Martinez AJ, Fowlkes AL, Lutrick K, Groom HC, Dunnigan K, Odean MJ, Hegmann K, Stefanski E, Edwards LJ, Schaefer-Solle N, Grant L, Ellingson K, Kuntz JL, Zunie T, Thiese MS, Ivacic L, Wesley MG, Mayo Lamberte J, Sun X, Smith ME, Phillips AL, Groover KD, Yoo YM, Gerald J, Brown RT, Herring MK, Joseph G, Beitel S, Morrill TC, Mak J, Rivers P, Poe BP, Lynch B, Zhou Y, Zhang J, Kelleher A, Li Y, Dickerson M, Hanson E, Guenther K, Tong S, Bateman A, Reisdorf E, Barnes J, Azziz-Baumgartner E, Hunt DR, Arvay ML, Kutty P, Fry AM, Gaglani M. Prevention and attenuation of Covid-19 with the BNT162b2 and mRNA-1273 vaccines. N Engl J Med. 2021;385(4):320–9. https://doi.org/10.1056/NEJMoa2107058.
Shaw B, Daskareh M, Gholamrezanezhad A. The lingering manifestations of COVID-19 during and after convalescence: update on long-term pulmonary consequences of coronavirus disease 2019 (COVID-19). Radiol Med. 2021;126(1):40–6. https://doi.org/10.1007/s11547-020-01295-8.
Caruso D, Polici M, Zerunian M, Pucciarelli F, Polidori T, Guido G, Rucci C, Bracci B, Muscogiuri E, De Dominicis C, Laghi A. Quantitative Chest CT analysis in discriminating COVID-19 from non-COVID-19 patients. Radiol Med. 2021;126(2):243–9. https://doi.org/10.1007/s11547-020-01291-y.
Somma F, Negro A, D’Agostino V, Piscitelli V, Pace G, Tortora M, Tortora F, Gatta G, Caranci F. COVID-19 and low back pain: previous infections lengthen recovery time after intradiscal ozone therapy in patients with herniated lumbar disc. Radiol Med. 2022;127(6):673–80. https://doi.org/10.1007/s11547-022-01500-w.
Francolini G, Desideri I, Stocchi G, Ciccone LP, Salvestrini V, Garlatti P, Aquilano M, Greto D, Bonomo P, Meattini I, Scotti V, Scoccianti S, Simontacchi G, Livi L. Impact of COVID-19 on workload burden of a complex radiotherapy facility. Radiol Med. 2021;126(5):717–21. https://doi.org/10.1007/s11547-021-01338-8.
Fusco R, Simonetti I, Ianniello S, Villanacci A, Grassi F, Dell’Aversana F, Grassi R, Cozzi D, Bicci E, Palumbo P, Borgheresi A, Giovagnoni A, Miele V, Barile A, Granata V. Pulmonary lymphangitis poses a major challenge for radiologists in an oncological setting during the COVID-19 pandemic. J Pers Med. 2022;12(4):624. https://doi.org/10.3390/jpm12040624.
Mungmunpuntipantip R, Wiwanitkit V. COVID-19, intradiscal ozone therapy and back pain: a correspondence. Radiol Med. 2022;127(10):1179. https://doi.org/10.1007/s11547-022-01544-y.
Cellini F, Di Franco R, Manfrida S, Borzillo V, Maranzano E, Pergolizzi S, Morganti AG, Fusco V, Deodato F, Santarelli M, Arcidiacono F, Rossi R, Reina S, Merlotti A, Jereczek-Fossa BA, Tozzi A, Siepe G, Cacciola A, Russi E, Gambacorta MA, Scorsetti M, Ricardi U, Corvò R, Donato V, Muto P, Valentini V. Palliative radiotherapy indications during the COVID-19 pandemic and in future complex logistic settings: the NORMALITY model. Radiol Med. 2021;126(12):1619–56. https://doi.org/10.1007/s11547-021-01414-z.
Lombardi AF, Afsahi AM, Gupta A, Gholamrezanezhad A. Severe acute respiratory syndrome (SARS), Middle East respiratory syndrome (MERS), influenza, and COVID-19, beyond the lungs: a review article. Radiol Med. 2021;126(4):561–9. https://doi.org/10.1007/s11547-020-01311-x.
Gerasia R, Mamone G, Amato S, Cucchiara A, Gallo GS, Tafaro C, Fiorello G, Caruso C, Miraglia R. COVID-19 safety measures at the Radiology Unit of a Transplant Institute: the non-COVID-19 patient’s confidence with safety procedures. Radiol Med. 2022;127(4):426–32. https://doi.org/10.1007/s11547-022-01454-z.
Gabelloni M, Faggioni L, Cioni D, Mendola V, Falaschi Z, Coppola S, Corradi F, Isirdi A, Brandi N, Coppola F, Granata V, Golfieri R, Grassi R, Neri E. Extracorporeal membrane oxygenation (ECMO) in COVID-19 patients: a pocket guide for radiologists. Radiol Med. 2022;127(4):369–82. https://doi.org/10.1007/s11547-022-01473-w.
Masci GM, Iafrate F, Ciccarelli F, Pambianchi G, Panebianco V, Pasculli P, Ciardi MR, Mastroianni CM, Ricci P, Catalano C, Francone M. Tocilizumab effects in COVID-19 pneumonia: role of CT texture analysis in quantitative assessment of response to therapy. Radiol Med. 2021;126(9):1170–80. https://doi.org/10.1007/s11547-021-01371-7.
Bianchi A, Mazzoni LN, Busoni S, Pinna N, Albanesi M, Cavigli E, Cozzi D, Poggesi A, Miele V, Fainardi E, Gadda D. Assessment of cerebrovascular disease with computed tomography in COVID-19 patients: correlation of a novel specific visual score with increased mortality risk. Radiol Med. 2021;126(4):570–6. https://doi.org/10.1007/s11547-020-01313-9.
De Felice F, D’Angelo E, Ingargiola R, Iacovelli NA, Alterio D, Franco P, Bonomo P, Merlotti A, Bacigalupo A, Maddalo M, Di Rito A, Fanetti G, D’Onofrio I, Dionisi F, Miccichè F, Trignani M, Musio D, Paiar F, Romanello DA, Donato V, Orlandi E. A snapshot on radiotherapy for head and neck cancer patients during the COVID-19 pandemic: a survey of the Italian Association of Radiotherapy and Clinical Oncology (AIRO) head and neck working group. Radiol Med. 2021;126(2):343–7. https://doi.org/10.1007/s11547-020-01296-7.
Pecoraro M, Cipollari S, Marchitelli L, Messina E, Del Monte M, Galea N, Ciardi MR, Francone M, Catalano C, Panebianco V. Cross-sectional analysis of follow-up chest MRI and chest CT scans in patients previously affected by COVID-19. Radiol Med. 2021;126(10):1273–81. https://doi.org/10.1007/s11547-021-01390-4.
Agostini A, Borgheresi A, Carotti M, Ottaviani L, Badaloni M, Floridi C, Giovagnoni A. Third-generation iterative reconstruction on a dual-source, high-pitch, low-dose chest CT protocol with tin filter for spectral shaping at 100 kV: a study on a small series of COVID-19 patients. Radiol Med. 2021;126(3):388–98. https://doi.org/10.1007/s11547-020-01298-5.
Caruso D, Zerunian M, Polici M, Pucciarelli F, Guido G, Polidori T, Rucci C, Bracci B, Tremamunno G, Laghi A. Diagnostic performance of CT lung severity score and quantitative chest CT for stratification of COVID-19 patients. Radiol Med. 2022;127(3):309–17. https://doi.org/10.1007/s11547-022-01458-9.
Novelli F, Pinelli V, Chiaffi L, Carletti AM, Sivori M, Giannoni U, Chiesa F, Celi A. Prognostic significance of peripheral consolidations at chest x-ray in severe COVID-19 pneumonia. Radiol Med. 2022;127(6):602–8. https://doi.org/10.1007/s11547-022-01487-4.
Borghesi A, Golemi S, Scrimieri A, Nicosia CMC, Zigliani A, Farina D, Maroldi R. Chest X-ray versus chest computed tomography for outcome prediction in hospitalized patients with COVID-19. Radiol Med. 2022;127(3):305–8. https://doi.org/10.1007/s11547-022-01456-x.
Cartocci G, Colaiacomo MC, Lanciotti S, Andreoli C, De Cicco ML, Brachetti G, Pugliese S, Capoccia L, Tortora A, Scala A, Valentini C, Almberger M, D’Aprile MR, Avventurieri G, Giura R, Kharrub Z, Leonardi A, Boccia M, Catalano C, Ricci P. Correction to: Chest CT for early detection and management of coronavirus disease (COVID-19): a report of 314 patients admitted to Emergency Department with suspected pneumonia. Radiol Med. 2021;126(4):642. https://doi.org/10.1007/s11547-020-01292-x.
Cappabianca S, Fusco R, de Lisio A, Paura C, Clemente A, Gagliardi G, Lombardi G, Giacobbe G, Russo GM, Belfiore MP, Urraro F, Grassi R, Feragalli B, Miele V. Correction to: Clinical and laboratory data, radiological structured report findings and quantitative evaluation of lung involvement on baseline chest CT in COVID-19 patients to predict prognosis. Radiol Med. 2021;126(4):643. https://doi.org/10.1007/s11547-020-01322-8.
Salman R, Sammer MB, Serrallach BL, Sangi-Haghpeykar H, Annapragada AV, Paul GR. Lower skeletal muscle mass on CT body composition analysis is associated with adverse clinical course and outcome in children with COVID-19. Radiol Med. 2022;127(4):440–8. https://doi.org/10.1007/s11547-022-01462-z.
Barra S, Guarnieri A, di Monale E Bastia MB, Marcenaro M, Tornari E, Belgioia L, Magrini SM, Ricardi U, Corvò R. Short fractionation radiotherapy for early prostate cancer in the time of COVID-19: long-term excellent outcomes from a multicenter Italian trial suggest a larger adoption in clinical practice. Radiol Med. 2021;126(1):142–6. https://doi.org/10.1007/s11547-020-01216-9.
Palmisano A, Vignale D, Boccia E, Nonis A, Gnasso C, Leone R, Montagna M, Nicoletti V, Bianchi AG, Brusamolino S, Dorizza A, Moraschini M, Veettil R, Cereda A, Toselli M, Giannini F, Loffi M, Patelli G, Monello A, Iannopollo G, Ippolito D, Mancini EM, Pontone G, Vignali L, Scarnecchia E, Iannacone M, Baffoni L, Sperandio M, de Carlini CC, Sironi S, Rapezzi C, Antiga L, Jagher V, Di Serio C, Furlanello C, Tacchetti C, Esposito A. AI-SCoRE (artificial intelligence-SARS CoV2 risk evaluation): a fast, objective and fully automated platform to predict the outcome in COVID-19 patients. Radiol Med. 2022;127(9):960–72. https://doi.org/10.1007/s11547-022-01518-0.
Filograna L, Manenti G, Ampanozi G, Calcagni A, Ryan CP, Floris R, Thali MJ. Potentials of post-mortem CT investigations during SARS-COV-2 pandemic: a narrative review. Radiol Med. 2022;127(4):383–90. https://doi.org/10.1007/s11547-022-01457-w.
Rawashdeh MA, Saade C. Radiation dose reduction considerations and imaging patterns of ground glass opacities in coronavirus: risk of over exposure in computed tomography. Radiol Med. 2021;126(3):380–7. https://doi.org/10.1007/s11547-020-01271-2.
Anastasi E, Manganaro L, Guiducci E, Ciaglia S, Dolciami M, Spagnoli A, Alessandri F, Angeloni A, Vestri A, Catalano C, Ricci P. Association of serum Krebs von den Lungen-6 and chest CT as potential prognostic factors in severe acute respiratory syndrome SARS-CoV-2: a preliminary experience. Radiol Med. 2022;127(7):725–32. https://doi.org/10.1007/s11547-022-01504-6.
Rizzo S, Catanese C, Puligheddu C, Epistolio S, Ramelli G, Frattini M, Pereira Mestre R, Nadarajah N, Rezzonico E, Magoga F, Milan L, Del Grande F, Giovanella L, Ceriani L. CT evaluation of lung infiltrates in the two months preceding the Coronavirus disease 19 pandemic in Canton Ticino (Switzerland): were there suspicious cases before the official first case? Radiol Med. 2022;127(4):360–8. https://doi.org/10.1007/s11547-022-01466-9.
Ippolito D, Giandola T, Maino C, Pecorelli A, Capodaglio C, Ragusi M, Porta M, Gandola D, Masetto A, Drago S, Allegranza P, Corso R, Talei Franzesi C, Sironi S. Acute pulmonary embolism in hospitalized patients with SARS-CoV-2-related pneumonia: multicentric experience from Italian endemic area. Radiol Med. 2021;126(5):669–78. https://doi.org/10.1007/s11547-020-01328-2.
Omer SB, Benjamin RM, Brewer NT, Buttenheim AM, Callaghan T, Caplan A, Carpiano RM, Clinton C, DiResta R, Elharake JA, Flowers LC, Galvani AP, Lakshmanan R, Maldonado YA, McFadden SM, Mello MM, Opel DJ, Reiss DR, Salmon DA, Schwartz JL, Sharfstein JM, Hotez PJ. Promoting COVID-19 vaccine acceptance: recommendations from the lancet commission on vaccine refusal, acceptance, and demand in the USA. Lancet. 2021;398(10317):2186–92. https://doi.org/10.1016/S0140-6736(21)02507-1.
Hodgson SH, Mansatta K, Mallett G, Harris V, Emary KRW, Pollard AJ. What defines an efficacious COVID-19 vaccine? A review of the challenges assessing the clinical efficacy of vaccines against SARS-CoV-2. Lancet Infect Dis. 2021;21(2):e26–35. https://doi.org/10.1016/S1473-3099(20)30773-8.
Karim SSA, Karim QA. Omicron SARS-CoV-2 variant: a new chapter in the COVID-19 pandemic. Lancet. 2021;398(10317):2126–8. https://doi.org/10.1016/S0140-6736(21)02758-6.
Young B, Tan TT, Leo YS. The place for remdesivir in COVID-19 treatment. Lancet Infect Dis. 2021;21(1):20–1. https://doi.org/10.1016/S1473-3099(20)30911-7.
Song Y, Zhang M, Yin L, Wang K, Zhou Y, Zhou M, Lu Y. COVID-19 treatment: close to a cure? A rapid review of pharmacotherapies for the novel coronavirus (SARS-CoV-2). Int J Antimicrob Agents. 2020;56(2):106080. https://doi.org/10.1016/j.ijantimicag.2020.106080.
Meo SA, Klonoff DC, Akram J. Efficacy of chloroquine and hydroxychloroquine in the treatment of COVID-19. Eur Rev Med Pharmacol Sci. 2020;24(8):4539–47. https://doi.org/10.26355/eurrev_202004_21038.
Pascarella G, Strumia A, Piliego C, Bruno F, Del Buono R, Costa F, Scarlata S, Agrò FE. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. https://doi.org/10.1111/joim.13091.
Mouffak S, Shubbar Q, Saleh E, El-Awady R. Recent advances in management of COVID-19: a review. Biomed Pharmacother. 2021;143:112107. https://doi.org/10.1016/j.biopha.2021.112107.
Bhavana V, Thakor P, Singh SB, Mehra NK. COVID-19: pathophysiology, treatment options, nanotechnology approaches, and research agenda to combating the SARS-CoV2 pandemic. Life Sci. 2020;261:118336. https://doi.org/10.1016/j.lfs.2020.118336.
Babaei F, Mirzababaei M, Nassiri-Asl M, Hosseinzadeh H. Review of registered clinical trials for the treatment of COVID-19. Drug Dev Res. 2021;82(4):474–93. https://doi.org/10.1002/ddr.21762.
Shrestha DB, Budhathoki P, Khadka S, Shah PB, Pokharel N, Rashmi P. Favipiravir versus other antiviral or standard of care for COVID-19 treatment: a rapid systematic review and meta-analysis. Virol J. 2020;17(1):141. https://doi.org/10.1186/s12985-020-01412-z.
Montesarchio V, Parrela R, Iommelli C, Bianco A, Manzillo E, Fraganza F, Palumbo C, Rea G, Murino P, De Rosa R, Atripaldi L, D’Abbraccio M, Curvietto M, Mallardo D, Celentano E, Grimaldi AM, Palla M, Trojaniello C, Vitale MG, Million-Weaver SL, Ascierto PA. Outcomes and biomarker analyses among patients with COVID-19 treated with interleukin 6 (IL-6) receptor antagonist sarilumab at a single institution in Italy. J Immunother Cancer. 2020;8(2):e001089. https://doi.org/10.1136/jitc-2020-001089.
Cruz-Teran C, Tiruthani K, McSweeney M, Ma A, Pickles R, Lai SK. Challenges and opportunities for antiviral monoclonal antibodies as COVID-19 therapy. Adv Drug Deliv Rev. 2021;169:100–17. https://doi.org/10.1016/j.addr.2020.12.004.
Taylor PC, Adams AC, Hufford MM, de la Torre I, Winthrop K, Gottlieb RL. Neutralizing monoclonal antibodies for treatment of COVID-19. Nat Rev Immunol. 2021;21(6):382–93. https://doi.org/10.1038/s41577-021-00542-x.
Hwang YC, Lu RM, Su SC, Chiang PY, Ko SH, Ke FY, Liang KH, Hsieh TY, Wu HC. Monoclonal antibodies for COVID-19 therapy and SARS-CoV-2 detection. J Biomed Sci. 2022;29(1):1. https://doi.org/10.1186/s12929-021-00784-w.
Jaworski JP. Neutralizing monoclonal antibodies for COVID-19 treatment and prevention. Biomed J. 2021;44(1):7–17. https://doi.org/10.1016/j.bj.2020.11.011.
Li D, Sempowski GD, Saunders KO, Acharya P, Haynes BF. SARS-CoV-2 neutralizing antibodies for COVID-19 prevention and treatment. Annu Rev Med. 2022;27(73):1–16. https://doi.org/10.1146/annurev-med-042420-113838.
Tabll AA, Shahein YE, Omran MM, Elnakib MM, Ragheb AA, Amer KE. A review of monoclonal antibodies in COVID-19: role in immunotherapy, vaccine development and viral detection. Hum Antibodies. 2021;29(3):179–91. https://doi.org/10.3233/HAB-200441.
Aiello TF, García-Vidal C, Soriano A. Antiviral drugs against SARS-CoV-2. Rev Esp Quimioter. 2022;35(Suppl 3):10–5. https://doi.org/10.37201/req/s03.03.2022.
Hoffmann M, Krüger N, Schulz S, Cossmann A, Rocha C, Kempf A, Nehlmeier I, Graichen L, Moldenhauer AS, Winkler MS, Lier M, Dopfer-Jablonka A, Jäck HM, Behrens GMN, Pöhlmann S. The Omicron variant is highly resistant against antibody-mediated neutralization: implications for control of the COVID-19 pandemic. Cell. 2022;185(3):447-456.e11. https://doi.org/10.1016/j.cell.2021.12.032.
Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, Seymour L. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer. 2016;62:132–7. https://doi.org/10.1016/j.ejca.2016.03.081.
Schwartz LH, Seymour L, Litière S, Ford R, Gwyther S, Mandrekar S, Shankar L, Bogaerts J, Chen A, Dancey J, Hayes W, Hodi FS, Hoekstra OS, Huang EP, Lin N, Liu Y, Therasse P, Wolchok JD, de Vries E. RECIST 1.1: standardisation and disease-specific adaptations: perspectives from the RECIST working group. Eur J Cancer. 2016;62:138–45. https://doi.org/10.1016/j.ejca.2016.03.082.
Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol. 2020;72(2):288–306. https://doi.org/10.1016/j.jhep.2019.09.026.
Granata V, Grassi R, Fusco R, Belli A, Palaia R, Carrafiello G, Miele V, Grassi R, Petrillo A, Izzo F. Local ablation of pancreatic tumors: state of the art and future perspectives. World J Gastroenterol. 2021;27(23):3413–28. https://doi.org/10.3748/wjg.v27.i23.3413.
Cholangiocarcinoma Working Group. Italian clinical practice guidelines on cholangiocarcinoma: part II—treatment. Dig Liver Dis. 2020;52(12):1430–42. https://doi.org/10.1016/j.dld.2020.08.030.
Seymour L, Bogaerts J, Perrone A, Ford R, Schwartz LH, Mandrekar S, Lin NU, Litière S, Dancey J, Chen A, Hodi FS, Therasse P, Hoekstra OS, Shankar LK, Wolchok JD, Ballinger M, Caramella C, de Vries EGE, RECIST working group. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol. 2017;18(3):e143–52. https://doi.org/10.1016/S1470-2045(17)30074-8.
Fushimi Y, Yoshida K, Okawa M, Maki T, Nakajima S, Sakata A, Okuchi S, Hinoda T, Kanagaki M, Nakamoto Y. Vessel wall MR imaging in neuroradiology. Radiol Med. 2022;30:1–14. https://doi.org/10.1007/s11547-022-01528-y.
Granata V, Simonetti I, Fusco R, Setola SV, Izzo F, Scarpato L, Vanella V, Festino L, Simeone E, Ascierto PA, Petrillo A. Management of cutaneous melanoma: radiologists challenging and risk assessment. Radiol Med. 2022;127(8):899–911. https://doi.org/10.1007/s11547-022-01522-4.
Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U, ESMO Guidelines Committee. Cutaneous melanoma: ESMO clinical practice guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(12):1884–901. https://doi.org/10.1093/annonc/mdz411.
Granata V, Fusco R, De Muzio F, Cutolo C, Setola SV, Dell’Aversana F, Grassi F, Belli A, Silvestro L, Ottaiano A, Nasti G, Avallone A, Flammia F, Miele V, Tatangelo F, Izzo F, Petrillo A. Radiomics and machine learning analysis based on magnetic resonance imaging in the assessment of liver mucinous colorectal metastases. Radiol Med. 2022;127(7):763–72. https://doi.org/10.1007/s11547-022-01501-9.
Tagliafico AS, Campi C, Bianca B, Bortolotto C, Buccicardi D, Francesca C, Prost R, Rengo M, Faggioni L. Blockchain in radiology research and clinical practice: current trends and future directions. Radiol Med. 2022;127(4):391–7. https://doi.org/10.1007/s11547-022-01460-1.
Granata V, Fusco R, Salati S, Petrillo A, Di Bernardo E, Grassi R, Palaia R, Danti G, La Porta M, Cadossi M, Gašljević G, Sersa G, Izzo F. A systematic review about imaging and histopathological findings for detecting and evaluating electroporation based treatments response. Int J Environ Res Public Health. 2021;18(11):5592. https://doi.org/10.3390/ijerph18115592.
Chiti G, Grazzini G, Flammia F, Matteuzzi B, Tortoli P, Bettarini S, Pasqualini E, Granata V, Busoni S, Messserini L, Pradella S, Massi D, Miele V. Gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): a radiomic model to predict tumor grade. Radiol Med. 2022. https://doi.org/10.1007/s11547-022-01529-x.
Granata V, Grassi R, Fusco R, Setola SV, Belli A, Ottaiano A, Nasti G, La Porta M, Danti G, Cappabianca S, Cutolo C, Petrillo A, Izzo F. Intrahepatic cholangiocarcinoma and its differential diagnosis at MRI: how radiologist should assess MR features. Radiol Med. 2021;126(12):1584–600. https://doi.org/10.1007/s11547-021-01428-7.
Cholangiocarcinoma Working Group. Italian clinical practice guidelines on cholangiocarcinoma: part I—classification, diagnosis and staging. Dig Liver Dis. 2020;52(11):1282–93. https://doi.org/10.1016/j.dld.2020.06.045.
Granata V, Fusco R, De Muzio F, Cutolo C, Setola SV, Grassi R, Grassi F, Ottaiano A, Nasti G, Tatangelo F, Pilone V, Miele V, Brunese MC, Izzo F, Petrillo A. Radiomics textural features by MR imaging to assess clinical outcomes following liver resection in colorectal liver metastases. Radiol Med. 2022;127(5):461–70. https://doi.org/10.1007/s11547-022-01477-6.
Fusco R, Setola SV, Raiano N, Granata V, Cerciello V, Pecori B, Petrillo A. Analysis of a monocentric computed tomography dosimetric database using a radiation dose index monitoring software: dose levels and alerts before and after the implementation of the adaptive statistical iterative reconstruction on CT images. Radiol Med. 2022;127(7):733–42. https://doi.org/10.1007/s11547-022-01481-w.
Tan BS, Dunnick NR, Gangi A, Goergen S, Jin ZY, Neri E, Nomura CH, Pitcher RD, Yee J, Mahmood U. RSNA international trends: a global perspective on the COVID-19 pandemic and radiology in late 2020. Radiology. 2021;299(1):E193–203. https://doi.org/10.1148/radiol.2020204267.
Simpson S, Kay FU, Abbara S, Bhalla S, Chung JH, Chung M, Henry TS, Kanne JP, Kligerman S, Ko JP, Litt H. Radiological Society of North America expert consensus statement on reporting chest CT findings related to COVID-19. Endorsed by the society of thoracic radiology, the American College of Radiology, and RSNA: secondary publication. J Thorac Imaging. 2020;35(4):219–27. https://doi.org/10.1097/RTI.0000000000000524.
Ruscitti P, Esposito M, Gianneramo C, Di Cola I, De Berardinis A, Martinese A, Nkamtse Tochap G, Conforti A, Masciocchi C, Cipriani P, Barile A, Fargnoli MC. Nail and enthesis assessment in patients with psoriatic disease by high frequency ultrasonography: findings from a single-centre cross-sectional study. Radiol Med. 2022. https://doi.org/10.1007/s11547-022-01568-4.
Salaffi F, Carotti M, Di Matteo A, Ceccarelli L, Farah S, Villota-Eraso C, Di Carlo M, Giovagnoni A. Ultrasound and magnetic resonance imaging as diagnostic tools for sarcopenia in immune-mediated rheumatic diseases (IMRDs). Radiol Med. 2022;127(11):1277–91. https://doi.org/10.1007/s11547-022-01560-y.
Ventura C, Baldassarre S, Cerimele F, Pepi L, Marconi E, Ercolani P, Floridi C, Argalia G, Goteri G, Giovagnoni A. 2D shear wave elastography in evaluation of prognostic factors in breast cancer. Radiol Med. 2022;127(11):1221–7. https://doi.org/10.1007/s11547-022-01559-5.
Bartolotta TV, Orlando AAM, Dimarco M, Zarcaro C, Ferraro F, Cirino A, Matranga D, Vieni S, Cabibi D. Diagnostic performance of 2D-shear wave elastography in the diagnosis of breast cancer: a clinical appraisal of cutoff values. Radiol Med. 2022;127(11):1209–20. https://doi.org/10.1007/s11547-022-01546-w.
Fresilli D, Di Leo N, Martinelli O, Di Marzo L, Pacini P, Dolcetti V, Del Gaudio G, Canni F, Ricci LI, De Vito C, Caiazzo C, Carletti R, Di Gioia C, Carbone I, Feinstein SB, Catalano C, Cantisani V. 3D-Arterial analysis software and CEUS in the assessment of severity and vulnerability of carotid atherosclerotic plaque: a comparison with CTA and histopathology. Radiol Med. 2022;127(11):1254–69. https://doi.org/10.1007/s11547-022-01551-z.
Bruno F, Marrelli A, Tommasino E, Martinese G, Gagliardi A, Pertici L, Pagliei V, Palumbo P, Arrigoni F, Di Cesare E, Barile A, Masciocchi C, Splendiani A. Advanced MRI imaging of nerve roots in lumbar radiculopathy due to discoradicular conflict: DWI, DTI, and T2 mapping with clinical and neurophysiological correlations. Radiol Med. 2022;127(11):1270–6. https://doi.org/10.1007/s11547-022-01550-0.
Pizzini FB, Conti E, Bianchetti A, Splendiani A, Fusco D, Caranci F, Bozzao A, Landi F, Gandolfo N, Farina L, Miele V, Trabucchi M, Frisoni GB, Bastianello S. Radiological assessment of dementia: the Italian inter-society consensus for a practical and clinically oriented guide to image acquisition, evaluation, and reporting. Radiol Med. 2022;127(9):998–1022. https://doi.org/10.1007/s11547-022-01534-0.
Spinelli MS, Balbaa MF, Gallazzi MB, Eid ME, Kotb HT, Shafei ME, Ierardi AM, Daolio PA, Barile A, Carrafiello G. Role of percutaneous CT-guided radiofrequency ablation in treatment of intra-articular, in close contact with cartilage and extra-articular osteoid osteomas: comparative analysis and new classification system. Radiol Med. 2022;127(10):1142–50. https://doi.org/10.1007/s11547-022-01542-0.
Song W, Chen Q, Guo D, Jiang C. Preoperative estimation of the survival of patients with unresectable hepatocellular carcinoma achieving complete response after conventional transcatheter arterial chemoembolization: assessments of clinical and LI-RADS MR features. Radiol Med. 2022;127(9):939–49. https://doi.org/10.1007/s11547-022-01517-1.
Kang YJ, Cho JH, Hwang SH. Diagnostic value of various criteria for deep lobe involvement in radiologic studies with parotid mass: a systematic review and meta-analysis. Radiol Med. 2022;127(10):1124–33. https://doi.org/10.1007/s11547-022-01540-2.
Bardakci O, Daş M, Akdur G, Akman C, Siddikoğlu D, Şimşek G, Kaya F, Atalay Ü, Topal MT, Beyazit F, Ünal Çetin E, Akdur O, Beyazit Y. Point-of-care lung ultrasound, lung CT and NEWS to predict adverse outcomes and mortality in COVID-19 associated pneumonia. J Intensive Care Med. 2022;1:8850666221111731. https://doi.org/10.1177/08850666221111731.
Cantinotti M, Marchese P, Assanta N, Pizzuto A, Corana G, Santoro G, Franchi E, Viacava C, Van den Eynde J, Kutty S, Gargani L, Giordano R. Lung ultrasound findings in healthy children and in those who had recent, not severe COVID-19 infection. J Clin Med. 2022;11(20):5999. https://doi.org/10.3390/jcm11205999.
Banai A, Lupu L, Shetrit A, Hochstadt A, Lichter Y, Levi E, Szekely Y, Schellekes N, Jacoby T, Zahler D, Itach T, Taieb P, Gefen S, Viskin D, Shidlansik L, Adler A, Levitsky E, Havakuk O, Banai S, Ghantous E, Topilsky Y. Systematic lung ultrasound in Omicron-type vs. wild-type COVID-19. Eur Heart J Cardiovasc Imaging. 2022. https://doi.org/10.1093/ehjci/jeac212.
Skaarup SH, Aagaard R, Ovesen SH, Weile J, Kirkegaard H, Espersen C, Lassen MCH, Skaarup KG, Posth S, Laursen CB, Bock A, Dan Arvig M, Biering-Sørensen T. Focused lung ultrasound to predict respiratory failure in patients with symptoms of COVID-19: a multicentre prospective cohort study. ERJ Open Res. 2022;8(4):00128–2022. https://doi.org/10.1183/23120541.00128-2022.
Durrani N, Vukovic D, van der Burgt J, Antico M, van Sloun RJG, Canty D, Steffens M, Wang A, Royse A, Royse C, Haji K, Dowling J, Chetty G, Fontanarosa D. Automatic deep learning-based consolidation/collapse classification in lung ultrasound images for COVID-19 induced pneumonia. Sci Rep. 2022;12(1):17581. https://doi.org/10.1038/s41598-022-22196-y.
Heldeweg MLA, Mousa A, van Ekeren J, Lieveld AWE, Walburgh-Schmidt RS, Smit JM, Haaksma ME, de Grooth HJ, Heunks LMA, Tuinman PR. Lung ultrasound to predict gas-exchange response to prone positioning in COVID-19 patients: a prospective study in pilot and confirmation cohorts. J Crit Care. 2022;73:154173. https://doi.org/10.1016/j.jcrc.2022.154173.
Orosz G, Gyombolai P, Tóth JT, Szabó M. Reliability and clinical correlations of semi-quantitative lung ultrasound on BLUE points in COVID-19 mechanically ventilated patients: the ’BLUE-LUSS’-A feasibility clinical study. PLoS ONE. 2022;17(10):e0276213. https://doi.org/10.1371/journal.pone.0276213.
García AF, Ángel-Isaza AM, Chica J, Estrada DE, Vargas-Morales CA, Revelo-Noguera J, Morell T, Gómez JA, Rodríguez Holguín F, Umaña M, Serna JJ, Carvajal S. Lung ultrasound as a screening tool for SARS-CoV-2 infection in surgical patients. J Clin Ultrasound. 2022. https://doi.org/10.1002/jcu.23358.
Han J, Yang X, Xu W, Jin R, Meng S, Ding L, Zhang Y, Hu X, Liu W, Li H, Meng F. Lung ultrasonography findings of coronavirus disease 2019 patients: comparison between primary and secondary regions of China. Immun Inflamm Dis. 2022;10(10):e713. https://doi.org/10.1002/iid3.713.
Russo G, Flor N, Casella F, Ippolito S, Leidi F, Casazza G, Radovanovic D, Vezzulli F, Santus P, Cogliati C. Lung ultrasound in the follow-up of severe COVID-19 pneumonia: six months evaluation and comparison with CT. Intern Emerg Med. 2022;14:1–8. https://doi.org/10.1007/s11739-022-03084-9.
Lombardi FA, Franchini R, Morello R, Casciaro E, Ianniello S, Serra M, Satriano F, Mojoli F, Mongodi S, Pignatelli D, Di Paola M, Casciaro S. A new standard scoring for interstitial pneumonia based on quantitative analysis of ultrasonographic data: a study on COVID-19 patients. Respir Med. 2021;189:106644. https://doi.org/10.1016/j.rmed.2021.106644.
Mongodi S, De Luca D, Colombo A, Stella A, Santangelo E, Corradi F, Gargani L, Rovida S, Volpicelli G, Bouhemad B, Mojoli F. Quantitative lung ultrasound: technical aspects and clinical applications. Anesthesiology. 2021;134(6):949–65. https://doi.org/10.1097/ALN.0000000000003757.
Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF, Perlini S, Torri E, Mariani A, Mossolani EE, Tursi F, Mento F, Demi L. Proposal for international standardization of the use of lung ultrasound for patients With COVID-19: a simple, quantitative, reproducible method. J Ultrasound Med. 2020;39(7):1413–9. https://doi.org/10.1002/jum.15285.
Smith MJ, Hayward SA, Innes SM, Miller ASC. Point-of-care lung ultrasound in patients with COVID-19: a narrative review. Anaesthesia. 2020;75(8):1096–104. https://doi.org/10.1111/anae.15082.
Feldstein LR, Tenforde MW, Friedman KG, Newhams M, Rose EB, Dapul H, Soma VL, Maddux AB, Mourani PM, Bowens C, Maamari M, Hall MW, Riggs BJ, Giuliano JS Jr, Singh AR, Li S, Kong M, Schuster JE, McLaughlin GE, Schwartz SP, Walker TC, Loftis LL, Hobbs CV, Halasa NB, Doymaz S, Babbitt CJ, Hume JR, Gertz SJ, Irby K, Clouser KN, Cvijanovich NZ, Bradford TT, Smith LS, Heidemann SM, Zackai SP, Wellnitz K, Nofziger RA, Horwitz SM, Carroll RW, Rowan CM, Tarquinio KM, Mack EH, Fitzgerald JC, Coates BM, Jackson AM, Young CC, Son MBF, Patel MM, Newburger JW, Randolph AG, Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with multisystem inflammatory syndrome in children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325(11):1074–87. https://doi.org/10.1001/jama.2021.2091.
Francone M, Iafrate F, Masci GM, Coco S, Cilia F, Manganaro L, Panebianco V, Andreoli C, Colaiacomo MC, Zingaropoli MA, Ciardi MR, Mastroianni CM, Pugliese F, Alessandri F, Turriziani O, Ricci P, Catalano C. Chest CT score in COVID-19 patients: correlation with disease severity and short-term prognosis. Eur Radiol. 2020;30(12):6808–17. https://doi.org/10.1007/s00330-020-07033-y.
Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time course of lung changes at chest CT during recovery from Coronavirus disease 2019 (COVID-19). Radiology. 2020;295(3):715–21. https://doi.org/10.1148/radiol.2020200370.
Akdur G, Daş M, Bardakci O, Akman C, Sıddıkoğlu D, Akdur O, Akçalı A, Erbaş M, Reşorlu M, Beyazit Y. Prediction of mortality in COVID-19 through combing CT severity score with NEWS, qSOFA, or peripheral perfusion index. Am J Emerg Med. 2021;50:546–52. https://doi.org/10.1016/j.ajem.2021.08.079.
Li K, Wu J, Wu F, Guo D, Chen L, Fang Z, Li C. The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol. 2020;55(6):327–31. https://doi.org/10.1097/RLI.0000000000000672.
Sayeed S, Faiz BY, Aslam S, Masood L, Saeed R. CT chest severity score for COVID 19 pneumonia: a quantitative imaging tool for severity assessment of disease. J Coll Physicians Surg Pak. 2021;30(4):388–92. https://doi.org/10.29271/jcpsp.2021.04.388.
Lieveld AWE, Azijli K, Teunissen BP, van Haaften RM, Kootte RS, van den Berk IAH, van der Horst SFB, de Gans C, van de Ven PM, Nanayakkara PWB. Chest CT in COVID-19 at the ED: validation of the COVID-19 reporting and data system (CO-RADS) and CT severity score: a prospective, multicentre, observational study. Chest. 2021;159(3):1126–35. https://doi.org/10.1016/j.chest.2020.11.026.
Volpicelli G, Gargani L, Perlini S, Spinelli S, Barbieri G, Lanotte A, Casasola GG, Nogué-Bou R, Lamorte A, Agricola E, Villén T, Deol PS, Nazerian P, Corradi F, Stefanone V, Fraga DN, Navalesi P, Ferre R, Boero E, Martinelli G, Cristoni L, Perani C, Vetrugno L, McDermott C, Miralles-Aguiar F, Secco G, Zattera C, Salinaro F, Grignaschi A, Boccatonda A, Giostra F, Infante MN, Covella M, Ingallina G, Burkert J, Frumento P, Forfori F, Ghiadoni L, on behalf of the International Multicenter Study Group on LUS in COVID-19. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: an international multicenter study. Intensive Care Med. 2021;47(4):444–54. https://doi.org/10.1007/s00134-021-06373-7.
Allinovi M, Parise A, Giacalone M, Amerio A, Delsante M, Odone A, Franci A, Gigliotti F, Amadasi S, Delmonte D, Parri N, Mangia A. Lung ultrasound may support diagnosis and monitoring of COVID-19 pneumonia. Ultrasound Med Biol. 2020;46(11):2908–17. https://doi.org/10.1016/j.ultrasmedbio.2020.07.018.
Volpicelli G, Fraccalini T, Cardinale L, Stranieri G, Senkeev R, Maggiani G, Pacielli A, Basile D. Feasibility of a new lung ultrasound protocol to determine the extent of lung injury in COVID-19 pneumonia. Chest. 2023;163(1):176–84. https://doi.org/10.1016/j.chest.2022.07.014.
Lauring AS, Tenforde MW, Chappell JD, Gaglani M, Ginde AA, McNeal T, Ghamande S, Douin DJ, Talbot HK, Casey JD, Mohr NM, Zepeski A, Shapiro NI, Gibbs KW, Files DC, Hager DN, Shehu A, Prekker ME, Erickson HL, Exline MC, Gong MN, Mohamed A, Johnson NJ, Srinivasan V, Steingrub JS, Peltan ID, Brown SM, Martin ET, Monto AS, Khan A, Hough CL, Busse LW, Ten Lohuis CC, Duggal A, Wilson JG, Gordon AJ, Qadir N, Chang SY, Mallow C, Rivas C, Babcock HM, Kwon JH, Halasa N, Grijalva CG, Rice TW, Stubblefield WB, Baughman A, Womack KN, Rhoads JP, Lindsell CJ, Hart KW, Zhu Y, Adams K, Schrag SJ, Olson SM, Kobayashi M, Verani JR, Patel MM, Self WH. Influenza and other viruses in the acutely Ill (IVY) network. Clinical severity of, and effectiveness of mRNA vaccines against, covid-19 from omicron, delta, and alpha SARS-CoV-2 variants in the United States: prospective observational study. BMJ. 2022;376:e069761. https://doi.org/10.1136/bmj-2021-069761.
Peixoto AO, Costa RM, Uzun R, Fraga AMA, Ribeiro JD, Marson FAL. Applicability of lung ultrasound in COVID-19 diagnosis and evaluation of the disease progression: a systematic review. Pulmonology. 2021;27(6):529–62. https://doi.org/10.1016/j.pulmoe.2021.02.004.
Lichter Y, Topilsky Y, Taieb P, Banai A, Hochstadt A, Merdler I, Gal Oz A, Vine J, Goren O, Cohen B, Sapir O, Granot Y, Mann T, Friedman S, Angel Y, Adi N, Laufer-Perl M, Ingbir M, Arbel Y, Matot I, Szekely Y. Lung ultrasound predicts clinical course and outcomes in COVID-19 patients. Intensive Care Med. 2020;46(10):1873–83. https://doi.org/10.1007/s00134-020-06212-1. Erratum in: Intensive Care Med. 2020.
Pierrakos C, Lieveld A, Pisani L, Smit MR, Heldeweg M, Hagens LA, Smit J, Haaksma M, Veldhuis L, Schmidt RW, Errico G, Marinelli V, Attou R, David CE, Zimatore C, Murgolo F, Grasso S, Mirabella L, Cinnella G, De Bels D, Schultz MJ, Tuinman PR, Bos LD. A lower global lung ultrasound score is associated with higher likelihood of successful extubation in invasively ventilated COVID-19 patients. Am J Trop Med Hyg. 2021;105(6):1490–7. https://doi.org/10.4269/ajtmh.21-0545.PMID:34662857;PMCID:PMC8641363.
Granata V, de Lutio di Castelguidone E, Fusco R, Catalano O, Piccirillo M, Palaia R, Izzo F, Gallipoli AD, Petrillo A. Irreversible electroporation of hepatocellular carcinoma: preliminary report on the diagnostic accuracy of magnetic resonance, computer tomography, and contrast-enhanced ultrasound in evaluation of the ablated area. Radiol Med. 2016;121(2):122–31. https://doi.org/10.1007/s11547-015-0582-5.
Granata V, Fusco R, Catalano O, Avallone A, Palaia R, Botti G, Tatangelo F, Granata F, Cascella M, Izzo F, Petrillo A. Diagnostic accuracy of magnetic resonance, computed tomography and contrast enhanced ultrasound in radiological multimodality assessment of peribiliary liver metastases. PLoS ONE. 2017;12(6):e0179951. https://doi.org/10.1371/journal.pone.0179951.
Granata V, Fusco R, Costa M, Picone C, Cozzi D, Moroni C, La Casella GV, Montanino A, Monti R, Mazzoni F, Grassi R, Malagnino VG, Cappabianca S, Grassi R, Miele V, Petrillo A. Preliminary report on computed tomography radiomics features as biomarkers to immunotherapy selection in lung adenocarcinoma patients. Cancers (Basel). 2021;13(16):3992. https://doi.org/10.3390/cancers13163992.
Fusco R, Granata V, Mazzei MA, Meglio ND, Roscio DD, Moroni C, Monti R, Cappabianca C, Picone C, Neri E, Coppola F, Montanino A, Grassi R, Petrillo A, Miele V. Quantitative imaging decision support (QIDSTM) tool consistency evaluation and radiomic analysis by means of 594 metrics in lung carcinoma on chest CT scan. Cancer Control. 2021. https://doi.org/10.1177/1073274820985786.
Granata V, Fusco R, Filice S, Catalano O, Piccirillo M, Palaia R, Izzo F, Petrillo A. The current role and future prospectives of functional parameters by diffusion weighted imaging in the assessment of histologic grade of HCC. Infect Agent Cancer. 2018;3(13):23. https://doi.org/10.1186/s13027-018-0194-5.
Bimonte S, Leongito M, Barbieri A, Del Vecchio V, Barbieri M, Albino V, Piccirillo M, Amore A, Di Giacomo R, Nasto A, Granata V, Petrillo A, Arra C, Izzo F. Inhibitory effect of (-)-epigallocatechin-3-gallate and bleomycin on human pancreatic cancer MiaPaca-2 cell growth. Infect Agent Cancer. 2015;29(10):22. https://doi.org/10.1186/s13027-015-0016-y.
Fusco R, Sansone M, Granata V, Setola SV, Petrillo A. A systematic review on multiparametric MR imaging in prostate cancer detection. Infect Agent Cancer. 2017;30(12):57. https://doi.org/10.1186/s13027-017-0168-z.
Cascella M, Bimonte S, Barbieri A, Del Vecchio V, Caliendo D, Schiavone V, Fusco R, Granata V, Arra C, Cuomo A. Dissecting the mechanisms and molecules underlying the potential carcinogenicity of red and processed meat in colorectal cancer (CRC): an overview on the current state of knowledge. Infect Agent Cancer. 2018;15(13):3. https://doi.org/10.1186/s13027-018-0174-9.
Grassi R, Cappabianca S, Urraro F, Feragalli B, Montanelli A, Patelli G, Granata V, Giacobbe G, Russo GM, Grillo A, De Lisio A, Paura C, Clemente A, Gagliardi G, Magliocchetti S, Cozzi D, Fusco R, Belfiore MP, Grassi R, Miele V. Chest CT computerized aided quantification of PNEUMONIA lesions in COVID-19 infection: a comparison among three commercial software. Int J Environ Res Public Health. 2020;17(18):6914. https://doi.org/10.3390/ijerph17186914.
Granata V, Fusco R, Barretta ML, Picone C, Avallone A, Belli A, Patrone R, Ferrante M, Cozzi D, Grassi R, Grassi R, Izzo F, Petrillo A. Radiomics in hepatic metastasis by colorectal cancer. Infect Agent Cancer. 2021;16(1):39. https://doi.org/10.1186/s13027-021-00379-y.
Granata V, Fusco R, Avallone A, Catalano O, Filice F, Leongito M, Palaia R, Izzo F, Petrillo A. Major and ancillary magnetic resonance features of LI-RADS to assess HCC: an overview and update. Infect Agent Cancer. 2017;28(12):23. https://doi.org/10.1186/s13027-017-0132-y.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Each author contributed equally at manuscript methodology definition and implementation, writing and revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors have no conflicts of interest to be disclosed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Granata, V., Fusco, R., Villanacci, A. et al. Qualitative and semi-quantitative ultrasound assessment in delta and Omicron Covid-19 patients: data from high volume reference center. Infect Agents Cancer 18, 34 (2023). https://doi.org/10.1186/s13027-023-00515-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13027-023-00515-w