Abstract
Background
Radiological evaluation of dementia is expected to increase more and more in routine practice due to both the primary role of neuroimaging in the diagnostic pathway and the increasing incidence of the disease. Despite this, radiologists often do not follow a disease-oriented approach to image interpretation, for several reasons, leading to reports of limited value to clinicians. In our work, through an intersocietal consensus on the main mandatory knowledge about dementia, we proposed a disease-oriented protocol to optimize and standardize the acquisition/evaluation/interpretation and reporting of radiological images. Our main purpose is to provide a practical guideline for the radiologist to help increase the effectiveness of interdisciplinary dialogue and diagnostic accuracy in daily practice.
Results
We defined key clinical and imaging features of the dementias (A), recommended MRI protocol (B), proposed a disease-oriented imaging evaluation and interpretation (C) and report (D) with a glimpse to future avenues (E). The proposed radiological practice is to systematically evaluate and score atrophy, white matter changes, microbleeds, small vessel disease, consider the use of quantitative measures using commercial software tools critically, and adopt a structured disease-oriented report.
Summary statement
In the expanding field of cognitive disorders, the only effective assessment approach is the standardized disease-oriented one, which includes a multidisciplinary integration of the clinical picture, MRI, CSF and blood biomarkers and nuclear medicine.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Key Results
-
1.
The assessment of dementia is based on clinical, radiological, nuclear and lab evaluation.
-
2.
Brain atrophy, white matter lesions, microhemorrhages, and vascular diseases should be radiologically evaluated.
-
3.
Radiological assessment should be based on visual assessment, scoring, and volumetry.
-
4.
The disease-oriented structured radiology report increases its clinical value.
-
5.
Multidisciplinary teamwork increases diagnostic accuracy.
Background
Dementias are taking up more space in everyday clinical and radiological scenarios, considering that the worldwide population is aging [78] and that brain imaging plays a key role in the assessment of cognitive impairment [8]. In addition, the scientific community is increasingly focused on the debate over the use of AI [51], FDA- and EC-approved automated segmentation software [73], and the optimal use of neuroimaging in new drug trials [68].” A recent survey, carried out in Europe among academic and non-academic institutions [74], disclosed that the current practice in dementia imaging presents some homogeneity (mainly in imaging acquisition and image interpretation) but also differences in training and reporting, in using advanced imaging techniques and volumetric measures, as well as in communication between clinicians and radiologists. This work stems from the need of different Italian scientific societies to standardize and optimize the radiological approach for the assessment and follow-up of the aging brain and cognitive disorders.
We aim to fill this gap of variability and uncertainty, providing a practical approach in evaluating, interpreting, and monitoring the aging brain and main cognitive disorders.
Methods
The promoters of the initiative (FBP and SB) representatives of the Neuroradiological Section of the Italian Society of Radiology (SIRM), with the clinical support of EC and GBF, created a core panel with experts in dementia and cognitive disorders representatives of the Italian Association of Diagnostic and Interventional Neuroradiology (AINR). The purpose of their work is to submit a preliminary consensus draft to the representatives of SIGG (Italian Society of Geriatrics and Gerontology, Società Italiana di Geriatria e Gerontologia) and AIP (Italian Association of Psychogeriatrics, Associazione Italiana di Psicogeriatria) for their revision and final approval.
The main research questions were:
-
Define the key concepts (A) of what the radiologist needs to know: main clinical features (definition of brain aging, cognitive syndromes, and primary and secondary dementias) and imaging findings.
-
Frame the MRI radiological approach for the assessment and follow-up of the aging brain and cognitive disorders—MRI protocol (B), imaging evaluation and interpretation (C), and reporting (D) for clinical use
-
Identify the main factors that will influence clinical and radiological practice in this population in the near future (E)
-
The consensus between experts was reached using a similar approach to the previously published paper (Pizzini FB et. Insights Imaging) and consisted in:
-
A critical review of previous literature by European/American task forces and scientific societies related to A–D
-
Circulation and discussion of the draft based on this review among the core panel and then between the experts in more rounds
-
Changes of the original draft till the group converged towards an agreement on all the points A–E
-
Literature review
Literature review was performed through the PubMed database and on the web through Google and Google Scholar platforms, as well as specialized websites (Radiopaedia.org and radiologyassistant.nl/neuroradiology) and textbooks. The standardized strings used to search the database for literature were structured by combining the keywords (1) disease of interest, (2) biomarker (3) guidelines/recommendations/evaluation. As shown in the flowchart (Fig. 1), only original texts (abstract and full text) published in English were considered, without filtering for article type and publication date. Articles were selected after a review of the titles and abstracts of the first fifteen “best matches” to determine relevance and affinity to the research purpose. When it was useful, consultation was extended to the bibliographic references of the selected articles. Finally, in our bibliography, articles range from 1988 to 2022, with a predominance of the last decade.
Results
(A) Introduction to aging, cognitive impairment, and dementia
The following boxes represent consensus findings related to the key concepts (Box 1) and clinical features and imaging findings (Boxes 2, 3, 4, 5, 6, 7, 8, 9, 10 and 11) of the major primary and secondary dementias.
(B) MRI protocol (acquisition)
-
1.
Field of the MRI suite (1.5; 3T). The choice of field strength does not affect the evaluation of atrophy or white matter load (WML), but it can affect the detection rate of microbleeds [46].
-
2.
Standard protocol (core sequences and parameters). They have been found to have a major impact on image resolution [15] thus on the detection rate of atrophy, white matter changes, and microbleeds (e.g. in GRE detection of 33% of the microbleeds identified by thin-section SW [46, 65]) (Table 1).
-
3.
Optional additional sequences (i.e. functional MRI, microstructural DWI, spectroscopy, Magnetization Transfer Imaging) are useful at the group level [25] when comparing a specific disease with healthy subjects or other clinically overlapping diseases, Level 1 of a five-level scale of Imaging biomarkers [18, 77]. While ASL (Arterial Spin Labeling) is useful at the individual level because it reaches a sensitivity and specificity > 80% for the clinical diagnosis of a given patient (Level 2 of the same scale). None of these sequences can be considered effective for early clinical diagnosis (Level 3) or could be used as surrogate criteria for pathological diagnosis (Level 4) or provide a direct measure of the underlying neuropathological changes (Level 5).
-
4.
Contrast-enhanced MR: Contrast Media injection not indicated in aging and cognitive impairment (except for CAA-ri, see Box 8. CAA).
(C) Evaluation and interpretation
-
1.
Visual (qualitative) assessment
Table 2.
-
2.
Visual rating scales
-
(a)
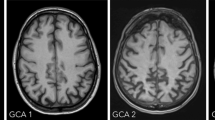
ATROPHY (see Tables 3 and 4; Figs. 2 and 3, and 4) should be evaluated in multiple planes on T1 (or FLAIR, but not T2), comparing the most preserved sulci/gyri (usually the occipital ones) to the most affected ones, symmetrically, using these scales:
Global cerebral Atrophy (GCA) [49], Koedam for posterior lobes—which are most affected in atypical AD [34], MTA-Scheltens for medial temporal lobe [60].
-
(b)
WHITE MATTER CHANGES (WMC) (see Tables 5 and 6; Fig. 5) should be evaluated in FLAIR/T2. The most used scales are Fazekas [17] and age-related white matter changes (ARWMC) [76]. The WMC have variable size, but minimal diameter of the lesions at imaging > 1 mm (in any plane).
-
(c)
MICROBLEEDS should be evaluated in GRE T2*/SWI and the minimal diameter of the lesions at imaging is < 10 mm in any plane (Fig. 6). They could be a feature of small vessel disease (hypertension or cerebral amyloid angiopathy, CAA) and could be related to antithrombotic bleeding risk. All possible microbleeds mimics should be excluded (i.e., vessels, small cavernomas, mineralization foci, artifacts at the air-bone interface and due to partial volume, small hemorrhagic areas due to infarcts or other bleeds).
Axial GRE T2*. On the left image and middle images, infratentorial [pons (n. 1), left middle cerebellar peduncle (n. 1) and hemisphere (n. 1)] and deep [right thalamus (n. 5), posterior putamen (n. 1)] microbleeds, correlated to systemic hypertension. On the right image 3 lobar microbleeds associated with Fazekas 3, in keeping with amyloid angiopathy
An example of scale with high intrarater and interrater reliability is MARS (Microbleed Anatomical Rating Scale) which describes their number and location in lobar and/or infratentorial and/or deep regions [23].
-
(d)
SMALL VESSEL DISEASE (SVD) (see Figs. 5 and 6; Figs. 7 and 8) should be evaluated in FLAIR/T2, GRE T2*/SWI, and T1 acquisitions. Parenchymal changes such as (1) microbleeds; (2) lacunae; (3) perivascular spaces; and (4) white matter changes indicate the presence of small vessel disease, but each type of imaging finding has a different risk weight. In fact, the presence of a single lacuna or microbleed adds one point of the SVD score (Total 4 points) [69], which is equivalent to that of a severe enlargement of the perivascular spaces and/or Fazekas 3, thus indicating a higher risk of clinical consequence (ischemic and hemorrhagic brain events, dementia) [47].
-
3.
Volumetric measures
Visual differentiation between brain changes due to aging or to an early stage of the disease can be difficult, so the quantification of brain structures from a single patient and its comparison to age and sex-specific reference MRI data of healthy population can improve the diagnosis (Fig. 7). Several volumetric brain assessment methods and commercial Regulatory Authority approved (e.g., FDA, CE, CFDA marking) software are clinically available and implemented in radiology reporting, but without a clear strategy in the assessment. One way to improve the diagnostic accuracy of the use of the software in clinical practice is the double assessment—visual and quantitative—which combines the visual rating and the atrophy measurements [73].
-
4.
Follow up
If there are any vascular findings at MRI, an annual follow-up is recommended.
In case of trials or other pathologies, the control MRI should be scheduled according to clinical indications.
(D) Reporting
-
1.
A structured reporting is often not considered useful in clinical practice and could present other critical issues [74], so a guided report is preferable (Fig. 8). We recommend using the following template, modified from a previous ESNR dementia working group 2019 proposal.
Please consider that it is advisable to mention in the "Conclusions/Impressions" of the report:
-
Any individual differences from a control population (cross-sectional assessment) by applying the visual qualitative and rating scale or/and the volumetric assessment
-
Any stability or longitudinal worsening of the radiological findings (longitudinal evaluation) from previous radiological examinations, if appropriate for comparison
To do this, when evaluating brain atrophy, it is useful to take into account existing reference standards for assessing differences between a subject and the control population and individual rates of change over the life course even with respect to the trajectories of volumetric brain imaging markers [7, 75].
According to these large and inclusive datasets currently available (BrainChart open source and Rotterdam study), the trajectories of volumetric changes in gray matter, white matter, and third ventricles show nonlinear curves, with accelerated change with advancing age and some differences between men and women.
Regarding the "mixed pathology" reported in "Conclusions/Impressions," it should be emphasized that the diagnosis of "mixed dementia" is clinical, not neuroradiological. The neuroradiological description of, for example, hippocampal atrophy and Fazekas 3, does not mean that the patient's cognitive impairment is equally attributable to neurodegeneration and microangiopathy. It is up to the clinician to determine how much of the clinical picture is attributable to one or the other component.
Sample Case Report (in Supplemental material)
(E) Future avenues
In the future, the use of volumetric information in routine radiology may be increasingly widespread, and we recommend dual assessment (combining visual scoring with volumetry, see C 3). These measurements are reproducible and automatic, but are depending on scan protocol, software, and the reference population. Other critical issues include the limited access to volumetric tools in the clinical setting (data must be transferred to the workstation and results to the PACS), and the training required to properly read the results.
Differences between men and women in neuroimaging biomarkers of neurodegeneration are reported [7, 75] and these should be considered in the near future when normative reference values will be applied in a clinical setting to assess pathology in individual patients.
The new Alzheimer drugs (i.e., Aducanumab) are rapidly changing the clinical scenario and the role of MRI, leading to the need for specific MRI protocols and precise reporting of the side effects of ARIA (amyloid-related imaging abnormalities, referring to cerebral edema or microhemorrhages).
One of the next frontiers is the clinical application of artificial intelligence, as it can offer solutions and interpretation of complex, multimodal medical information, such as that provided by imaging (radiology and nuclear medicine), biology, and neurocognitive testing, thus improving the diagnostic and prognostic process. But the process of identifying international medico-legal rules is still at an early stage [51].
Discussion
To complement what was presented in the results, the main practical recommendation that emerged is to try to fit all the radiological steps presented—MRI protocol (B), image evaluation and interpretation (C), and reporting (D)—to the clinical diagnosis. Unfortunately, in radiological practice, there are still several general obstacles to this [74], such as:
(1) reduced confidence about the most correct approach to reading images (especially in the use of scales and volumetry), (2) report variability (with no use of structured or guided reports), and (3) generic requisition forms that do not allow radiologists to conclude whether imaging results are in line with clinical suspicion. The latter problem could be solved by better communication among specialists (e.g., at interdisciplinary meetings), which is essential in challenging clinical settings.
The use of all proposed scores is highly recommended, possibly accompanied by a visual description, except for MARS—which in routine is best replaced by a description of the number and distribution of microbleeds, according to the major patterns (superficial distribution in cerebral amyloid angiopathy versus deep infratentorial localization in hypertension)—and for the SVD score—which can be replaced by a description of the findings of small vessel disease according to the priority of their clinical relevance.
Although MRI findings are diagnostic only for a few conditions (e.g., late-onset AD, vascular dementia, CAA, iNPH, etc.), they support the clinical diagnosis of all forms of dementia (see Boxes, above) and provide important information on differential diagnosis, overlapping/coexisting forms (e.g., AD and VaD; FTLD and VaD; DLB and VaD), and possible side effects of new drugs.
More generally, neuroimaging is crucial for the diagnosis of dementia and is recommended in every patient with cognitive decline. In older adults, especially in the oldest old or in patients with multiple comorbidities, severe disability or behavioral disorders, completion of an MRI or nuclear imaging protocol can be troublesome, due to limited collaboration. The indications for the examination should be discussed with the treating physicians, ideally in a multidisciplinary team. Limited to these cases, volumetric CT is acceptable [2], at least to rule out some secondary and potentially reversible causes of cognitive impairment, such as subdural hematoma or brain masses.
Conclusions
The diagnostic process of cognitive disorders requires a combined assessment of the clinical picture and imaging, including CT, MRI, and nuclear medicine, and can only be achieved through the dialogue between disciplines and the ongoing review of shared knowledge, information, and reports.
Availability of data and materials
Data sharing is not applicable to this article as no datasets were generated.
Abbreviations
- AD:
-
Alzheimer disease
- ADC:
-
Apparent diffusion coefficient
- AIDS:
-
Acquired immunodeficiency syndrome
- AINR:
-
Italian Association of diagnostic and interventional NeuroRadiology (Associazione Italiana di Neuroradiologia Diagnostica ed Interventistica)
- AIP:
-
Italian Association of Psychogeriatrics (Associazione Italiana di Psicogeriatria)
- aka:
-
Also known as
- ALS:
-
Amyotrophic lateral sclerosis
- APOE:
-
Apolipoprotein E
- APP:
-
Amyloid precursor protein
- ARIA:
-
Amyloid related imaging abnormalities
- ARWMC:
-
Age-related white matter changes
- ASL:
-
Arterial spin labeling
- AVIM:
-
Asymptomatic ventriculomegaly with features of idiopathic normal pressure hydrocephalus on MRI
- AVM:
-
Arterio-venous malformations
- bvFTD:
-
Behavioral variant frontotemporal dementia
- CA:
-
Callosal angle
- CAA:
-
Cerebral amyloid angiopathy
- CAA-ri:
-
Cerebral amyloid angiopathy-related inflammation
- CADASIL:
-
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy
- CAPPAH:
-
Convexity apparent hyperperfusion
- CARASIL:
-
Cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy
- CBD:
-
Corticobasal degeneration
- CBF:
-
Cerebral blood flow
- CBS:
-
Corticobasal syndrome
- CE:
-
European community (comunità europea)
- CFDA:
-
China food and drugs administration
- CJD:
-
Creutzfeldt–Jakob disease
- cMBS:
-
Cortical microbleeds
- CNS:
-
Central nervous system
- cSAH:
-
Convexity subarachnoid hemorrhage
- CSF:
-
Cerebrospinal fluid
- cSS:
-
Cortical superficial siderosis
- CT:
-
Computed tomography
- DESH:
-
Disproportionately enlarged subarachnoid space hydrocephalus
- DLB:
-
Dementia with Lewy bodies
- DWI:
-
Diffusion weighted imaging
- EMT:
-
Endovascular mechanical thrombectomy
- Eo-AD:
-
Early-onset Alzheimer disease
- ESNR:
-
European society of neuroradiology—diagnostic and Interventional
- FDA:
-
Food and drug administration
- FDG:
-
Fluorodeoxyglucose
- FLAIR:
-
Fluid attenuated inversion recovery
- FSE:
-
Fast spin echo
- FTD:
-
Frontotemporal dementias
- FTLD:
-
Frontotemporal lobar degeneration
- FU:
-
Follow up
- GCA:
-
Global cerebral atrophy
- gCJD:
-
Genetic Creutzfeldt-Jakob disease
- GRE:
-
Gradient-recalled-echo
- ICH:
-
Intracerebral hemorrhage
- iCJD:
-
Iatrogenic Creutzfeldt-Jakob disease
- iNPH:
-
Idiopathic normal pressure hydrocephalus
- IVT:
-
Intravenous thrombolysis
- KS:
-
Korsakoff syndrome
- LBD:
-
Lewy body dementia
- Lo-AD:
-
Late-onset Alzheimer Disease
- LOVA:
-
Long-standing overt ventriculomegaly in adults
- lvPPA:
-
Logopenic variant primary progressive aphasia
- MARS:
-
Microbleed anatomical rating scale
- MCA:
-
Middle cerebral artery
- MCI:
-
Mild cognitive impairment
- MND:
-
Motor neuron disease
- MRA:
-
Magnetic resonance angiography
- MRI:
-
Magnetic resonance imaging
- MTA:
-
Medial temporal lobe atrophy
- MTR:
-
Magnetization transfer ratio
- nfvPPA:
-
Nonfluent/agrammatic variant primary progressive aphasia
- NI:
-
Neuroimaging
- NPH:
-
Normal pressure hydrocephalus
- PACS:
-
Picture archiving and communication system
- PCA:
-
Posterior cortical atrophy
- PD:
-
Parkinson's disease
- PDD:
-
Parkinson disease dementia
- PET:
-
Positron emission tomography
- PiB:
-
Pittsburgh compound B
- PPA:
-
Primary progressive aphasia
- prnp:
-
Prion protein
- PRNP:
-
Prion protein
- PSEN1:
-
Presenilin 1
- PSEN2:
-
Presenilin 2
- PSP:
-
Progressive supranuclear palsy
- PVS:
-
Perivascular spaces
- REM:
-
Rapid eye movement
- sCJD:
-
Sporadic Creutzfeldt–Jakob disease
- SIGG:
-
Italian Society of Geriatrics and Gerontology
- SIRM:
-
Italian Society of Medical and Interventional Radiology (Società Italiana di Radiologia Medica e Interventistica)
- SPECT:
-
Single-photon emission computerized tomography
- SVD:
-
Small vessel disease
- svPPA:
-
Semantic variant primary progressive aphasia
- SW:
-
Susceptibility weighted
- SWI:
-
Susceptibility weighted imaging
- T:
-
Tesla
- TDP-43:
-
Transactive response DNA-binding protein 43
- TFNE:
-
Transient focal neurologic episodes
- TSE:
-
Turbo spin echo
- VaD:
-
Vascular dementia
- VCI:
-
Vascular cognitive impairment
- vCJD:
-
Variant Creutzfeldt–Jakob disease
- WE:
-
Wernicke encephalopathy
- WK:
-
Wernicke–Korsakoff syndrome
- WM:
-
White matter
- WMC:
-
White matter changes
- WMH:
-
White matter hyperintensities
- WML:
-
White matter load
- Yo:
-
Years old
References
Alzheimer’s Association, (2018) 2018 Alzheimer’s disease facts and figures. Alzheimer’s Dementia 14:367–429. https://doi.org/10.1016/j.jalz.2018.02.001
Auriel E, Charidimou A, Gurol ME et al (2016) Validation of clinicoradiological criteria for the diagnosis of cerebral amyloid angiopathy-related inflammation. JAMA Neurol 73:197–202. https://doi.org/10.1001/jamaneurol.2015.4078
Banerjee G, Carare R, Cordonnier C et al (2017) The increasing impact of cerebral amyloid angiopathy: essential new insights for clinical practice. J Neurol Neurosurg Psychiatry 88:982–994. https://doi.org/10.1136/jnnp-2016-314697
Bartsch AJ, Homola G, Biller A et al (2007) Manifestations of early brain recovery associated with abstinence from alcoholism. Brain 130:36–47. https://doi.org/10.1093/brain/awl303
Bavis J, Reynolds P, Tegeler C, Clark P (2003) Asymmetric neuroimaging in Creutzfeldt-Jakob disease: a ruse. J Neuroimaging 13:376–379
Bejanin A, Schonhaut DR, La Joie R et al (2017) Tau pathology and neurodegeneration contribute to cognitive impairment in Alzheimer’s disease. Brain 140:3286–3300. https://doi.org/10.1093/brain/awx243
Bethlehem RAI, Seidlitz J, White SR et al (2022) Brain charts for the human lifespan. Nature. https://doi.org/10.1038/s41586-022-04554-y
Boccardi M, Nicolosi V, Festari C et al (2020) Italian consensus recommendations for a biomarker-based aetiological diagnosis in mild cognitive impairment patients. Eur J Neurol 27:475–483. https://doi.org/10.1111/ene.14117
Boukobza M, Ilic-Habensus E, Duval X, Laissy J-P (2020) Acute convexity subarachnoid hemorrhage (cSAH) in infectious endocarditis (IE): imaging features and follow-up. J Neurol 267:2971–2982. https://doi.org/10.1007/s00415-020-09953-7
García C, de León S, Cabello JP, Ortiz R, Vaamonde J (2018) Parkinsonism associated with pathological 123I-FP-CIT SPECT (DaTSCAN) Results as the initial manifestation of sporadic Creutzfeldt–Jakob disease. Case Rep Neurol Med 2018:1–3. https://doi.org/10.1155/2018/5157275
Charidimou A, Linn J, Vernooij MW et al (2015) Cortical superficial siderosis: detection and clinical significance in cerebral amyloid angiopathy and related conditions. Brain 138:2126–2139. https://doi.org/10.1093/brain/awv162
Cohen OS, Hoffmann C, Lee H et al (2009) MRI detection of the cerebellar syndrome in Creutzfeldt–Jakob disease. Cerebellum 8:373–381. https://doi.org/10.1007/s12311-009-0106-8
Day GS, Gordon BA, Perrin RJ et al (2018) In vivo [18F]-AV-1451 tau-PET imaging in sporadic Creutzfeldt–Jakob disease. Neurology 90:e896–e906. https://doi.org/10.1212/WNL.0000000000005064
Deng F, Sharma R (2016) Modified Boston criteria for cerebral amyloid angiopathy. Radiopaedia.org. https://doi.org/10.53347/rID-48897
Di Giuliano F, Minosse S, Picchi E et al (2021) Qualitative and quantitative analysis of 3D T1 silent imaging. Radiol med 126:1207–1215. https://doi.org/10.1007/s11547-021-01380-6
Eckert T, Barnes A, Dhawan V et al (2005) FDG PET in the differential diagnosis of parkinsonian disorders. Neuroimage 26:912–921. https://doi.org/10.1016/j.neuroimage.2005.03.012
Fazekas F, Chawluk J, Alavi A et al (1987) MR signal abnormalities at 1.5 T in Alzheimer’s dementia and normal aging. Am J Roentgenol 149:351–356. https://doi.org/10.2214/ajr.149.2.351
Frisoni GB, Boccardi M, Barkhof F et al (2017) Strategic roadmap for an early diagnosis of Alzheimer’s disease based on biomarkers. The Lancet Neurology 16:661–676. https://doi.org/10.1016/S1474-4422(17)30159-X
Gazdzinski S, Durazzo TC, Mon A et al (2010) Cerebral white matter recovery in abstinent alcoholics—a multimodality magnetic resonance study. Brain 133:1043–1053. https://doi.org/10.1093/brain/awp343
Gorno-Tempini ML, Hillis AE, Weintraub S et al (2011) Classification of primary progressive aphasia and its variants. Neurology 76:1006–1014. https://doi.org/10.1212/WNL.0b013e31821103e6
Graff-Radford J, Murray ME, Lowe VJ et al (2014) Dementia with Lewy bodies: basis of cingulate island sign. Neurology 83:801–809. https://doi.org/10.1212/WNL.0000000000000734
Greenberg SM, Charidimou A (2018) Diagnosis of cerebral amyloid angiopathy: evolution of the Boston Criteria. Stroke 49:491–497. https://doi.org/10.1161/STROKEAHA.117.016990
Gregoire SM, Chaudhary UJ, Brown MM et al (2009) The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology 73:1759–1766. https://doi.org/10.1212/WNL.0b013e3181c34a7d
Gregoire SM, Jäger HR, Yousry TA et al (2010) Brain microbleeds as a potential risk factor for antiplatelet-related intracerebral haemorrhage: hospital-based, case-control study. J Neurol Neurosurg Psychiatry 81:679–684. https://doi.org/10.1136/jnnp.2009.198994
Gunbey HP, Has AC, Aslan K et al (2021) Microstructural white matter abnormalities in hypothyroidism evaluation with diffusion tensor imaging tract-based spatial statistical analysis. Radiol med 126:283–290. https://doi.org/10.1007/s11547-020-01234-7
Gurol ME, Dierksen G, Betensky R et al (2012) Predicting sites of new hemorrhage with amyloid imaging in cerebral amyloid angiopathy. Neurology 79:320–326. https://doi.org/10.1212/WNL.0b013e31826043a9
Hamaguchi T, Kitamoto T, Sato T et al (2005) Clinical diagnosis of MM2-type sporadic Creutzfeldt–Jakob disease. Neurology 64:643–648. https://doi.org/10.1212/01.WNL.0000151847.57956.FA
Harding A, Halliday G, Caine D, Kril J (2000) Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain 123(Pt 1):141–154. https://doi.org/10.1093/brain/123.1.141
Hassan A, Whitwell JL, Boeve BF et al (2010) Symmetric corticobasal degeneration (S-CBD). Parkinsonism Relat Disord 16:208–214. https://doi.org/10.1016/j.parkreldis.2009.11.013
Hogan DB, Jetté N, Fiest KM et al (2016) The prevalence and incidence of frontotemporal dementia: a systematic review. Can J Neurol Sci 43(Suppl 1):S96–S109. https://doi.org/10.1017/cjn.2016.25
Ishii K, Kanda T, Harada A et al (2008) Clinical impact of the callosal angle in the diagnosis of idiopathic normal pressure hydrocephalus. Eur Radiol 18:2678–2683. https://doi.org/10.1007/s00330-008-1044-4
Johnson JK, Diehl J, Mendez MF et al (2005) Frontotemporal lobar degeneration: demographic characteristics of 353 patients. Arch Neurol 62:925–930. https://doi.org/10.1001/archneur.62.6.925
Khurram A, Kleinig T, Leyden J (2014) Clinical associations and causes of convexity subarachnoid hemorrhage. Stroke 45:1151–1153. https://doi.org/10.1161/STROKEAHA.113.004298
Koedam ELGE, Lehmann M, van der Flier WM et al (2011) Visual assessment of posterior atrophy development of a MRI rating scale. Eur Radiol 21:2618–2625. https://doi.org/10.1007/s00330-011-2205-4
Lee SE, Rabinovici GD, Mayo MC et al (2011) Clinicopathological correlations in corticobasal degeneration. Ann Neurol 70:327–340. https://doi.org/10.1002/ana.22424
Linn J, Halpin A, Demaerel P et al (2010) Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology 74:1346–1350. https://doi.org/10.1212/WNL.0b013e3181dad605
Lummel N, Wollenweber FA, Demaerel P et al (2015) Clinical spectrum, underlying etiologies and radiological characteristics of cortical superficial siderosis. J Neurol 262:1455–1462. https://doi.org/10.1007/s00415-015-7736-1
Manners DN, Parchi P, Tonon C et al (2009) Pathologic correlates of diffusion MRI changes in Creutzfeldt–Jakob disease. Neurology 72:1425–1431. https://doi.org/10.1212/WNL.0b013e3181a18846
Matías-Guiu JA, Guerrero-Márquez C, Cabrera-Martín MN et al (2017) Amyloid- and FDG-PET in sporadic Creutzfeldt–Jakob disease: correlation with pathological prion protein in neuropathology. Prion 11:205–213. https://doi.org/10.1080/19336896.2017.1314427
Meissner B, Kallenberg K, Sanchez-Juan P et al (2009) MRI lesion profiles in sporadic Creutzfeldt–Jakob disease. Neurology 72:1994–2001. https://doi.org/10.1212/WNL.0b013e3181a96e5d
Mendez MF (2019) Early-onset Alzheimer disease and its variants. Contin Lifelong Learn Neurol 25:34–51. https://doi.org/10.1212/CON.0000000000000687
Mendez MF, Lee AS, Joshi A, Shapira JS (2012) Nonamnestic Presentations of early-onset Alzheimer’s disease. Am J Alzheimers Dis Other Demen 27:413–420. https://doi.org/10.1177/1533317512454711
Murray R, Neumann M, Forman MS et al (2007) Cognitive and motor assessment in autopsy-proven corticobasal degeneration. Neurology 68:1274–1283. https://doi.org/10.1212/01.wnl.0000259519.78480.c3
Nagahama Y, Fukuyama H, Turjanski N et al (1997) Cerebral glucose metabolism in corticobasal degeneration: comparison with progressive supranuclear palsy and normal controls. Mov Disord 12:691–696. https://doi.org/10.1002/mds.870120510
Nakajima M, Yamada S, Miyajima M et al (2021) Guidelines for management of idiopathic normal pressure hydrocephalus (third edition): endorsed by the Japanese Society of Normal Pressure Hydrocephalus. Neurol Med Chir (Tokyo) 61:63–97. https://doi.org/10.2176/nmc.st.2020-0292
Nandigam RNK, Viswanathan A, Delgado P et al (2009) MR imaging detection of cerebral microbleeds: effect of susceptibility-weighted imaging, section thickness, and field strength. AJNR Am J Neuroradiol 30:338–343. https://doi.org/10.3174/ajnr.A1355
Pasi M, Cordonnier C (2020) Clinical relevance of cerebral small vessel diseases. Stroke 51:47–53. https://doi.org/10.1161/STROKEAHA.119.024148
Pasi M, Pongpitakmetha T, Charidimou A et al (2019) Cerebellar microbleed distribution patterns and cerebral amyloid angiopathy. Stroke 50:1727–1733. https://doi.org/10.1161/STROKEAHA.119.024843
Pasquier F, Leys D, Weerts JGE et al (1996) Inter-and Intraobserver reproducibility of cerebral atrophy assessment on MRI scans with hemispheric infarcts. Eur Neurol 36:268–272. https://doi.org/10.1159/000117270
Pitel A-L, Chételat G, Le Berre AP et al (2012) Macrostructural abnormalities in Korsakoff syndrome compared with uncomplicated alcoholism. Neurology 78:1330–1333. https://doi.org/10.1212/WNL.0b013e318251834e
Pizzini FB, Pesapane F, Niessen W et al (2020) ESMRMB round table report on “can europe lead in machine learning of MRI-data?” Magn Reson Mater Phy 33:217–219. https://doi.org/10.1007/s10334-019-00821-8
Rabinovici GD (2019) Late-onset Alzheimer Disease. CONTINUUM: Lifelong Learning in Neurology 25:14–33. https://doi.org/10.1212/CON.0000000000000700
Rademakers R, Neumann M, Mackenzie IR (2012) Advances in understanding the molecular basis of frontotemporal dementia. Nat Rev Neurol 8:423–434. https://doi.org/10.1038/nrneurol.2012.117
Relkin N, Marmarou A, Klinge P et al (2005) Diagnosing idiopathic normal-pressure hydrocephalus. Neurosurgery 57:S24–S216. https://doi.org/10.1227/01.NEU.0000168185.29659.C5
Rodrigues MA, Samarasekera N, Lerpiniere C et al (2018) The Edinburgh CT and genetic diagnostic criteria for lobar intracerebral haemorrhage associated with cerebral amyloid angiopathy: model development and diagnostic test accuracy study. Lancet Neurol 17:232–240. https://doi.org/10.1016/S1474-4422(18)30006-1
Román GC, Tatemichi TK, Erkinjuntti T et al (1993) Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN international workshop. Neurology 43:250–260. https://doi.org/10.1212/wnl.43.2.250
Rotman J, Zimmerman R (2016) Patterns of ischemic stroke from Lacunar to territorial to multiple embolic to watershed hypotensive. In: Saba L, Raz E (eds) Neurovascular imaging. Springer, New York, pp S329–S362
Sachdev P, Kalaria R, O’Brien J et al (2014) Diagnostic criteria for vascular cognitive disorders: A VASCOG statement. Alzheimer Dis Assoc Disord 28:206–218. https://doi.org/10.1097/WAD.0000000000000034
Savoiardo M, Grisoli M, Girotti F (2000) Magnetic resonance imaging in CBD, related atypical parkinsonian disorders, and dementias. Adv Neurol 82:197–208
Scheltens P, Launer LJ, Barkhof F et al (1995) Visual assessment of medial temporal lobe atrophy on magnetic resonance imaging: interobserver reliability. J Neurol 242:557–560. https://doi.org/10.1007/BF00868807
Schroeter ML, Raczka K, Neumann J, Yves von Cramon D (2007) Towards a nosology for frontotemporal lobar degenerations—a meta-analysis involving 267 subjects. Neuroimage 36:497–510. https://doi.org/10.1016/j.neuroimage.2007.03.024
Schroth G, Naegele T, Klose U et al (1988) Reversible brain shrinkage in abstinent alcoholics, measured by MRI. Neuroradiology 30:385–389. https://doi.org/10.1007/BF00404102
Seeley WW (2019) Behavioral variant frontotemporal dementia. Contin Lifelong Learn Neurol 25:76–100. https://doi.org/10.1212/CON.0000000000000698
Shams S, Fällmar D, Schwarz S et al (2017) MRI of the swallow tail sign: a useful marker in the diagnosis of Lewy body dementia? AJNR Am J Neuroradiol 38:1737–1741. https://doi.org/10.3174/ajnr.A5274
Shams S, Martola J, Cavallin L et al (2015) SWI or T2*: which MRI sequence to use in the detection of cerebral microbleeds? The Karolinska imaging dementia study. AJNR Am J Neuroradiol 36:1089–1095. https://doi.org/10.3174/ajnr.A4248
Sibon I, Foubert A, Menegon P et al (2005) Creutzfeldt–Jakob disease mimicking radiologic posterior reversible leukoencephalopathy. Neurology 65:329. https://doi.org/10.1212/01.wnl.0000175231.07913.e2
Soliveri P, Monza D, Paridi D et al (1999) Cognitive and magnetic resonance imaging aspects of corticobasal degeneration and progressive supranuclear palsy. Neurology 53:502–507. https://doi.org/10.1212/wnl.53.3.502
Sperling R, Salloway S, Brooks DJ et al (2012) Amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with bapineuzumab: a retrospective analysis. Lancet Neurol 11:241–249. https://doi.org/10.1016/S1474-4422(12)70015-7
Staals J, Makin SDJ, Doubal FN et al (2014) Stroke subtype, vascular risk factors, and total MRI brain small-vessel disease burden. Neurology 83:1228–1234. https://doi.org/10.1212/WNL.0000000000000837
Svenningsson P (2019) Corticobasal degeneration: advances in clinicopathology and biomarkers. Curr Opin Neurol 32:597–603. https://doi.org/10.1097/WCO.0000000000000707
Ter Telgte A, Scherlek AA, Reijmer YD et al (2020) Histopathology of diffusion-weighted imaging-positive lesions in cerebral amyloid angiopathy. Acta Neuropathol 139:799–812. https://doi.org/10.1007/s00401-020-02140-y
Tsai H-H, Pasi M, Tsai L-K et al (2021) Centrum semiovale perivascular space and amyloid deposition in spontaneous intracerebral hemorrhage. Stroke 52:2356–2362. https://doi.org/10.1161/STROKEAHA.120.032139
Vernooij MW, Jasperse B, Steketee R et al (2018) Automatic normative quantification of brain tissue volume to support the diagnosis of dementia: a clinical evaluation of diagnostic accuracy. NeuroImage Clin 20:374–379. https://doi.org/10.1016/j.nicl.2018.08.004
Vernooij MW, Pizzini FB, Schmidt R et al (2019) Dementia imaging in clinical practice: a European-wide survey of 193 centres and conclusions by the ESNR working group. Neuroradiology 61:633–642. https://doi.org/10.1007/s00234-019-02188-y
Vinke EJ, de Groot M, Venkatraghavan V et al (2018) Trajectories of imaging markers in brain aging: the Rotterdam study. Neurobiol Aging 71:32–40. https://doi.org/10.1016/j.neurobiolaging.2018.07.001
Wahlund LO, Barkhof F, Fazekas F et al (2001) A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke 32:1318–1322. https://doi.org/10.1161/01.STR.32.6.1318
Whitwell JL, Höglinger GU, Antonini A et al (2017) Radiological biomarkers for diagnosis in PSP: where are we and where do we need to be? Neuroimaging biomarkers for diagnosis in PSP. Mov Disord 32:955–971. https://doi.org/10.1002/mds.27038
Winblad B, Amouyel P, Andrieu S et al (2016) Defeating Alzheimer’s disease and other dementias: a priority for European science and society. Lancet Neurol 15:455–532. https://doi.org/10.1016/S1474-4422(16)00062-4
Xia C, Makaretz SJ, Caso C et al (2017) Association of in vivo [18F]AV-1451 Tau PET imaging results with cortical atrophy and symptoms in typical and atypical Alzheimer disease. JAMA Neurol 74:427. https://doi.org/10.1001/jamaneurol.2016.5755
Yamauchi H, Fukuyama H, Nagahama Y et al (1998) Atrophy of the corpus callosum, cortical hypometabolism, and cognitive impairment in corticobasal degeneration. Arch Neurol 55:609–614. https://doi.org/10.1001/archneur.55.5.609
Zhou Y, Li J, Nordberg A, Ågren H (2021) Dissecting the binding profile of PET tracers to corticobasal degeneration tau fibrils. ACS Chem Neurosci 12:3487–3496. https://doi.org/10.1021/acschemneuro.1c00536
Zipursky RB, Lim KC, Pfefferbaum A (1989) MRI study of brain changes with short-term abstinence from alcohol. Alcohol Clin Exp Res 13:664–666. https://doi.org/10.1111/j.1530-0277.1989.tb00401.x
Acknowledgements
AINR (Associazione Italiana di NeuroRadiologia diagnostica ed interventistica), AIP (Associazione Italiana di Psicogeriatria), SIGG (Società Italiana di Geriatria e Gerontologia), SIRM (Società Italiana di Radiologia Medica e interventistica).
Funding
Open access funding provided by Università degli Studi di Verona within the CRUI-CARE Agreement. None.
Author information
Authors and Affiliations
Contributions
The conception of the study was done by Francesca B. Pizzini (FBP), Giovanni B. Frisoni (GBF) and Stefano Bastianello (SB). The study design was done by FBP, GBF and SB. Images collection was done by FBP and GBF. First drafting was done by FBP and EC and revised by all the authors/experts. Editing and final approval was done by all the authors/experts.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pizzini, F.B., Conti, E., Bianchetti, A. et al. Radiological assessment of dementia: the Italian inter-society consensus for a practical and clinically oriented guide to image acquisition, evaluation, and reporting. Radiol med 127, 998–1022 (2022). https://doi.org/10.1007/s11547-022-01534-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11547-022-01534-0