Abstract
The aquaculture industry is suffering from significant financial setbacks due to an increasing frequency of disease outbreaks, posing a threat to the sector’s sustainability. Various bacterial, viral, parasitic, and fungal pathogens have led to massive mortalities in farmed fish worldwide. Throughout the years, the management of fish diseases has predominantly centered around the utilization of conventional antibiotics and chemicals. Nevertheless, their indiscriminate use has given rise to serious implications, including an increase in resistant pathogens, disruptions in the metabolic processes of fish, degradation of the aquatic environment, the presence of drug residues in aquatic products, and a potential threat to human health. Various effective bio-based and immunoprophylaxis alternative therapies have been developed to overcome these impediments. Recent alternative therapeutic approaches to fish diseases encompass a range of strategies, including phytotherapeutics, nanotherapeutics, probiotics, prebiotics, synbiotics, phage therapy, vaccination, quorum quenching, antimicrobial peptides, biosurfactants, bacteriocins, stem cells, and diagnostic-based therapy. Advancements in biotechnology have significantly enhanced the efficacy of these therapies. However, additional research is essential to refine the utilization of these therapeutic approaches. Critical concerns, such as efficacy, cost, risks, availability, and adverse effects on fish and the ecosystem, need to be addressed to establish guidelines for their sustainable application in aquaculture. This review will increase aquaculturists’ awareness of recent therapies used in fish farming, their mechanisms, challenges, and impacts while promoting the sustainability of commercial aquaculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquaculture stands out as one of the fastest-growing food-producing sectors globally. Its significant contributions to food security, income generation, poverty alleviation, and cultural identity cannot be overstated (Elgendy et al. 2023a). The key to achieving higher fish production, in order to meet the growing demand for fish and address the stagnation of capture fisheries, lies in the intensification of fish farming practices (Araujo et al. 2022). The overstocking of fish farming operations is often accompanied by the adoption of unfavorable management measures, neglecting environmental care and compromising the health of farmed fish (Elgendy et al. 2022a, b, c). Fish farmed under such conditions experience stress, making them more susceptible to attacks from disease-causing agents (Ali et al. 2020; 2022). Currently, infectious disease outbreaks pose significant constraints on the productivity and sustainability of the aquaculture industry (Elgendy et al. 2023b). The intensification of aquaculture practices has resulted in the frequent occurrence of dangerous pathogens, leading to massive fish mortality disease epizootics (Elgendy et al. 2015a, b; Moustafa et al. 2015). Numerous bacterial, viral, parasitic, and fungal diseases break out frequently in intensive and semi-intensive fish culture systems, leading to partial or complete loss in production and colossal economic losses (Moustafa et al. 2014; Radwan et al. 2022a; Elgendy et al. 2023a, b). The potential economic impact of tilapia summer mortality in Egyptian farms was estimated at around 100 million USD (Fathi et al. 2017). Furthermore, in a comprehensive 7-year study conducted by Abdelrahman et al. (2023) in Alabama, USA, the annual financial losses attributed to diseases in commercial catfish farms in West Alabama amounted to a total of 11.1 million USD. Bacterial diseases were identified as the predominant cause of such epizootics, accounting for 83% of disease-related losses.
Aquaculturists have to use substantial quantities of chemotherapeutics, such as antibiotics, disinfectants, and pesticides, to tackle invading pathogens (Salma et al. 2022). The indiscriminate use of these chemotherapeutics has led to the emergence of resistant pathogens and detrimental effects on the ecosystem, posing a threat to human health (Elgendy et al. 2023a, b). The accumulation of antibiotic residues, whether in the environment or within fish tissues, and the emergence of resistant microbial strains serve as discouraging factors for their continued use (Bhat et al. 2022). Moreover, the horizontal transfer of genetic elements, which enables the transmission of drug-resistant genes to bacteria infecting humans, further intensifies antibiotics-associated risks (Jeon et al. 2023). Numerous antiparasitic and antifungal chemotherapeutics are extensively used in aquaculture, including drugs like formalin, formaldehyde, and malachite green. However, their irresponsible application is accompanied by a well-known issue of high toxicity to aquatic animals (UEMS 2016). These substances not only contribute to significant environmental problems but also pose substantial public health hazards (Sudova et al. 2007). The Food and Drug Administration (FDA) recently imposed a ban on formalin and malachite green in food fish production, citing concerns about their lack of consumer-friendliness and, in the case of malachite green, its carcinogenic, mutagenic, and teratogenic properties (Niska et al. 2009; Elgendy et al. 2023b). Therefore, developing effective alternative therapies to conventional chemotherapeutics for controlling pathogenic organisms affecting farmed fish is a global critical concern (Wang et al. 2022; Yilmaz et al. 2022; Bondad-Reantaso et al. 2023). This review, which focused on the alternative therapies recently applied in controlling/mitigating farmed fish diseases, including phytotherapeutics, nanotherapeutics, probiotics, prebiotics, synbiotics, phage therapy, vaccination, quorum quenching, antimicrobial peptides, biosurfactants, bacteriocins, stem cells, and diagnostic-based therapies, is discussed. The therapeutic and health benefits of these therapies against different pathogens affecting farmed fish are highlighted. Moreover, challenges confronting the application of these therapies in aquaculture and their impacts are briefly discussed.
Recent alternative therapies for controlling fish diseases in aquaculture
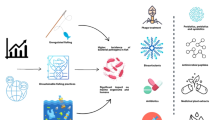
Numerous alternative disease management strategies have been developed to address the limitations of conventional chemotherapeutics in aquaculture (Fig. 1). These measures include the use of various bio-based chemotherapeutics and immunoprophylaxis approaches (Soliman et al. 2019; Wang et al. 2022). The bio-based alternative therapies include probiotics, bacteriophages, vaccination, quorum quenching, antimicrobial peptides, biosurfactants, and bacteriocins (Soliman et al. 2019; Yilmaz et al. 2022; Bondad-Reantaso et al. 2023). Furthermore, active immunoprophylaxis measures, such as using vaccines, and passive approaches, including phytotherapeutics, probiotics, and prebiotics, have been established to counteract attacking pathogens (Hussein et al. 2023; Subasinghe et al. 2023). Developing effective disease diagnostic technologies is essential as it enables accurate pathogen detection, helps prevent disease spread, and facilitates the implementation of targeted therapies (Abdelsalam et al. 2023; Dong et al. 2023).
Phytotherapeutics(medicinal plants and their derivatives)
The application of phytotherapeutics to enhance aquatic animal health involves utilizing medicinal products derived from plants or their derivatives for preventive or therapeutic purposes (Elgendy et al. 2023a; Silva et al. 2024). The use of phytotherapeutics aligns with the growing demand for sustainable and environmentally friendly practices in aquaculture (Ali et al. 2021). Unlike some conventional treatments that may pose adverse impacts on fish, humans, and the ecosystem, plant-based therapeutics offer a natural and eco-friendly approach for controlling diseases affecting farmed fish (Elgendy et al. 2021a).
Medicinal plants and their derivatives possess a diverse range of biologically active components that exhibit growth-promoting, immunostimulatory, antioxidant, and antimicrobial properties (Bondad-Reantaso et al. 2023). The plant bioactive compounds include essential oils, alkaloids, steroids, phenolics, tannins, organosulfur, terpenoids, saponins, glycosides, and flavonoids (Citarasu 2010; Cabello-Gómez et al. 2022; Elgendy et al. 2023a). These compounds often exhibit a broad spectrum of activities (Fig. 2), targeting multiple pathogens simultaneously and providing a holistic approach to disease management (Ergena et al. 2023).
Immunostimulatory outcomes of phytotherapeutics
The administration of medicinal plants or their derivatives to farmed fish has been shown to enhance their immunological responses and disease resistance (Fig. 2). These improvements include increased phagocytic activity, elevated levels of immunoglobulins, enhanced respiratory burst activity, heightened nitrogen oxide production, elevated myeloperoxidase content, boosted complement activity, higher levels of total proteins, as well as enhanced antiprotease activity (Elgendy et al. 2016; 2021a; 2023b; Abbas et al. 2019). Medicinal plants and their derivatives can be employed in various forms, including crude preparations, extracts, or distinct active compounds derived from the plants (Elgendy et al. 2021a). Phytotherapeutics can be used either independently or in combination with other therapeutic approaches (Zhang et al. 2009). Many herbs, spices, and their extracts have proven to be effective against a variety of dangerous fish infections (bacterial, parasitic, viral, and fungal pathogens), as shown in Tables 1, 2, and 3 and Fig. 2.
Antimicrobial efficacy of phytotherapeutics in aquaculture
The potential use of phytotherapeutics exhibiting antibacterial activity in aquaculture has gained considerable attention as an alternative to conventional antibiotics (Elgendy et al. 2021a). Phytotherapeutics, derived from various plant sources, possess diverse bioactive compounds that can efficiently combat bacterial infections in aquaculture (Ali et al. 2021). Several plant extracts and essential oils have demonstrated promising antibacterial properties, making them potential candidates for sustainable disease management in aquaculture, as seen in Fig. 2 and Table 1. Turmeric (Curcuma longa) (Elgendy et al. 2016), lemongrass (Cymbopogon citratus) (Al-Sagheer et al. 2018), moringa (Moringa oleifera) (Elgendy et al. 2021a), rosemary (Rosmarinus officinalis) (Naiel et al. 2019), garlic (Allium sativum) (Guo et al. 2012), and leek extract (Allium ampeloprasum L.) (Ali et al. 2021) are among the promising medicinal plants with significant antibacterial activities.
Moreover, there has been a growing trend in the use of medicinal plants for managing parasitic diseases affecting farmed fish worldwide (Radwan et al. 2022b). Numerous medicinal herbs and their extracts demonstrate strong antagonistic potentials against several detrimental fish parasitic infestations (Table 2). Biological active compounds in medicinal herbs, such as essential oils and diallyl tetrasulfide (allicin), have demonstrated a broad spectrum of antiparasitic activity in aquaculture (Yao et al. 2010; Pérez et al. 2016; Nizio et al. 2018; Adeshina et al. 2021). Bathing the fish in water containing phytotherapeutic agents is the primary method for treating parasitized fish (Radwan et al. 2022b). However, these chemotherapeutics can also be administered to fish through dietary supplementation (Aboud 2010).
Plant-based compounds also have demonstrated significant anti-fungal properties against numerous fish pathogens (Table 3). Essential oils extracted from plants such as Thymus vulgaris and Mentha spicata have shown inhibitory effects against a variety of fungal pathogens (Soković et al. 2009). Furthermore, both crude and extracted forms of Allium cepa dietary supplementation demonstrate antagonistic activity against the highly fatal Saprolegnia parasitica infections in tilapia (Elgendy et al. 2023a). The active components in these medicinal herbs cross the fungal cell membranes, blocking vital enzymes, destroying cell membrane, and suppressing their biochemical process, ultimately causing fungal cell death (Madhuri et al. 2012). Therefore, these compounds can be employed to prevent and mitigate fungal infections of farmed fish. In addition to their anti-fungal properties, certain phytotherapeutics exhibit anti-viral activity (Table 3), making them valuable therapies in controlling viral infections in aquaculture settings. Green tea (Camellia sinensis) extracts and its active compounds such as tea polyphenols, epicatechin gallate, and epigallocatechin displayed excellent antiviral activities against Iridovirus infection in grouper fish, Epinephelus spp. (Li et al. 2022b). Furthermore, a myriad of plant species, including neem (Azadirachta indica), Passiflora spp., Piper longum, and Zanthoxylum bungeanum harbor a diverse array of biologically active compounds, notably alkaloids, renowned for their robust antiviral outcomes (Pal 2023). These compounds hold significant promise in effectively combating viral infections affecting farmed fish in aquaculture operations, as demonstrated in Table 3.
Challenges of phytotherapeutic application in aquaculture
There are some shortcomings facing the widespread application of phytotherapy in aquaculture, as discussed by Barbosa et al. (2023), including the absence of standardized measures for herbal medications and their reliance on practical effectiveness rather than quantified ingredient content. Moreover, the concentrations of bioactive compounds in medicinal herbs are variable and influenced by factors like plant species and extraction methods. Additionally, identifying the specific active compounds responsible for therapeutic effects in plant extracts is a complex process. The bioavailability and absorption of phytochemicals in aquatic environments are influenced by water quality parameters. Lastly, the unavailability of certain plant extracts in adequate quantities also poses an obstacle.
Nanotherapeutics
The application of nanotechnology in aquaculture holds immense potential to bring transformative changes in this industry, including the detection and control of pathogens, water treatment, sterilization of fish ponds, and the efficient delivery of nutrients and drugs (Elgendy et al. 2021b; El-Adawy et al. 2023a, b). The distinctive features of nanotherapeutics, which encompass size-dependent reactivity and precisely targeted delivery mechanisms, make it a potent tool for tackling health issues in farmed fish (Doszpoly et al. 2023). The sizes of nanoparticles range between 1 and 100 nm and are sensitized by numerous physical, chemical, and biological methods (Doszpoly et al. 2023).
Biogenic synthesis of metal nanoparticles, known as green nanotechnology, has paved the way for discovering a broad spectrum of innovative therapies in aquaculture (Khursheed et al. 2023; Salem 2023a, b). This method of nanoparticle synthesis involves the use of various natural biological agents, ranging from simple unicellular organisms to complex multicellular ones, such as bacteria, fungi, actinomycetes, yeast, algae, and plant materials (Moitra et al. 2020; Salem and Fouda 2021; Salem 2023a, b). Extracellular and intracellular filtrates from various microbial cultures can serve as effective reducing agents for nanoparticle production (Li et al. 2011). The biomolecules released by organisms, including enzymes, proteins, polysaccharides, and carbohydrates, oxidize or reduce metallic ions to produce nanoparticles (Salem 2023a, b). Various phytocompounds, such as those from Azadirachta indica (neem) (Rather et al. 2017), Cinnamomum zeylanicum bark (Sathishkumar et al. 2009), and black tea leaf extract (Ara et al. 2009), have been involved in the biosynthesis of numerous nanoparticles (Table 4).
Incorporating selenium nanoparticles (Se NPs) synthesized using the green microalga Pediastrum boryanum into the diets of Nile tilapia, Oreochromis niloticus, improved their immune defense and intestinal integrity compared to control basal diets containing an inorganic Se source (Zahran et al. 2024). Moreover, Gum Arabic-based silver nanoparticles (Ag NPs-GA) displayed significant antibacterial and antibiofilm activities in vitro against Aeromonas hydrophila and Pseudomonas aeruginosa, with minimum inhibitory concentrations of 1.625 µg/ml and 3.25 µg/ml, respectively. These findings make AgNPs-GA suitable for use in commercial antibacterial products for the aquaculture industry (El-Adawy et al. 2021). Biogenic synthesis of metal nanoparticles has garnered significant attention worldwide due to its eco-friendly nature, low cost, and straightforward synthesis process (Singh et al. 2018). Additionally, microbes can be genetically engineered to synthesize nanoparticles with greater control over shape and size (Mabey et al. 2019).
Polymeric nanoparticles also play a prominent role in recent therapeutic applications in aquaculture and are recognized as efficient drug delivery systems (Ahmed et al. 2019a, b; 2020). They are organic-based nanoparticles composed of natural polymers such as chitosan, alginate, and gelatin, as well as synthetic polymers like polyalkylcyanoacrylate (PACA) (Begines et al. 2020). Various methods are used to synthesize polymeric nanoparticles, including solvent evaporation, dialysis, nanoprecipitation, and salting out (Krishnamoorthy and Mahalingam 2015). Nevertheless, biodegradable biopolymer nanoparticles prepared using simple methods without organic solvents are preferred for biological applications (Agarwal et al. 2018). Their unique physical and chemical properties, including large surface areas, enable them to serve a broad spectrum of therapeutic applications (Du et al. 2009).
Polymeric nanoparticles, such as those made from chitosan, can encapsulate active ingredients within their core or adsorb them onto their surfaces (Mohammed et al. 2017; Ahmed et al. 2019a, b). These nanoparticles serve as efficient drug delivery systems by encapsulating drugs, protecting them from degradation, and releasing them in a controlled manner (Ahmed et al. 2019a, b). Polymeric nanoparticles, such as chitosan, are characterized by low toxicity, good biocompatibility, and biodegradation at specific sites, making them suitable for numerous valuable therapeutic applications (Agarwal et al. 2018; Ahmed et al. 2019a, b). Polymeric nanoparticles can be utilized in gene therapy, imaging, diagnostics, and vaccine development (Elsabahy et al. 2015).
Earlier studies have demonstrated the health benefits of chitosan nanoparticles (CSNPs) and their potential therapeutic applications in aquaculture, including immunomodulation against infections (Nikapitiya et al. 2018), as well as antibacterial and antifungal activities (Abdel-Razek 2019a, b). Ahmed et al. (2021) suggested that feeding rainbow trout (Oncorhynchus mykiss) with marine bio-sourced chitosan nanoparticles (CSNPs) enhanced their intestinal immunity and antimicrobial defense mechanisms against Yersinia ruckeri infection. Moreover, the authors observed that CSNPs seemed to be more effective in therapeutic applications rather than for short-term prophylactic use. Additionally, in vitro studies by Ahmed et al. (2020) suggested that CSNPs could be incorporated into disease management strategies in aquaculture. These studies demonstrated that CSNP suspensions (105 nm) possess antimicrobial properties against common bacterial and oomycete pathogens affecting fish. CSNPs demonstrated significant antibacterial efficacy against Pseudomonas fluorescens, Aeromonas hydrophila, and Yersinia ruckeri. Higher doses of CSNPs were effective against Pseudomonas aeruginosa, Pseudomonas putida, Edwardsiella tarda, and Aeromonas salmonicida. Furthermore, CSNPs were capable of reducing the viability of Edwardsiella ictaluri, Francisella noatunensis subsp. orientalis, Aeromonas caviae, Aeromonas veronii, Aphanomyces invadans, and Saprolegnia parasitica.
Several nanomaterials have garnered significant attention and shown potential applications in aquaculture including silver nanoparticles (Ag NPs), zinc oxide nanoparticles (ZnO NPs), copper-based nanoparticles (Cu NPs), gold nanoparticles (Au NPs), and titanium dioxide nanoparticles (TiO2 NPs), as seen in Table 4.
Health benefits and antimicrobial activity of nanotherapeutics against fish pathogens
Nanotherapeutics derived from various metals exhibit powerful antimicrobial properties, positioning them as compelling alternatives to conventional therapies in aquaculture (Doszpoly et al. 2023). These innovative therapies effectively target a wide range of detrimental fish pathogens, including bacteria, parasites, fungi, and viruses (Saleh et al. 2017; Elgendy et al. 2021b; Doszpoly et al. 2023). Nanotherapeutics can be applied in aquaculture through various pathways including injection, bath treatment, or dietary supplementation (Das et al. 2020; Elgendy et al. 2021b). The mechanisms underlying the antimicrobial activities of nanotherapeutics are demonstrated in Fig. 3. These mechanisms include damaging the microbial cell membrane/cell wall, disrupting protein transport, inhibiting vital enzymes, and increasing the generation of reactive oxygen species (Lee et al. 2019). The attachment of nanoparticles to the microbial cell membrane induces damaging changes in membrane potential, disrupts permeability, and leads to a reduction in ATP levels, ultimately culminating in cell death (Saleh et al. 2017). Nanoparticles also attach to nucleic acids, inhibit tRNA binding to the ribosome, and prevent cell division (Huang et al. 2012). Various nanoparticles have been reported to have bactericidal effects against a range of devastating fish bacterial infections, including Aeromonas spp. (Elgendy et al. 2021b), Vibrio spp. (Swain et al. 2014), Streptococcus spp. (Ghetas et al. 2022), Pseudomonas spp. (El-Adawy et al. 2021), Flavobacterium spp. (Shaalan et al. 2020), Edwardsiella spp. (Luis et al. 2020), Ph. damselae subsp. damselae (Cheng et al. 2011), and Yersinia spp. (Demirbas 2021), as seen in Table 4. Moreover, some serious fish parasitic infestations like Ichthyophthirius multifiliis (Saleh et al. 2017), Monogenean parasitic spp. (Malheiros et al. 2020), and Cichlidogyrus spp. (Pimentel-Acosta et al. 2019) have been successfully inhibited by nanotherapeutics, as shown in Table 4. Moreover, certain nanotherapeutics have exhibited inhibitory effects against some fungal fish diseases, like saprolegniasis (AL-Shammari et al. 2022), as well as specific viral diseases, like spring viraemia of carp virus and white spot syndrome virus (WSSV), as seen in Table 4.
The aquatic animal health sector can also leverage the immunostimulatory effects of nanotherapeutics for the prophylaxis and prevention of diseases in farmed fish (Abd Elshafy et al. 2023). Several nanoparticles have shown immunostimulatory effects, enhancing the resistance of fish against detrimental microbial pathogens (Elgendy et al. 2021b). Earlier studies have shown that dietary supplementation of nano-selenium and selenium nanoparticles synthesized using microalgae can significantly enhance the immunological responses and resistance of fish against invading bacterial pathogens (Saffari et al. 2018; Neamat-Allah et al. 2019; Zahran et al. 2024).
Nano-based drugs and vaccines are part of the recent therapeutic approaches employed to address disease challenges in aquaculture. The small size of nanoparticles and their capability to traverse biological barriers have sparked interest in using them as drug and vaccine delivery vehicles (Fenaroli et al. 2014). Moreover, nanomaterials can function as vaccine adjuvants (Myhr and Myskja 2011). Incorporating nanomaterials, such as chitosan nanoparticles, into vaccine formulations enhances the stability of antigens, shields them from enzymatic degradation, preserves immunogenicity, and facilitates the sustained release of the vaccine over the long term (Wang et al. 2011; Zhao et al. 2014). Nano-based vaccines demonstrated promising results in countering certain harmful fish pathogens like Vibrio anguillarum (Vimal et al. 2014) and lymphocystis disease virus (LCDV) (Tian and Yu 2011). Moreover, the combination of nano-probiotics and prebiotics also is an effective approach to deliver these components efficiently to living organisms, enhancing the immune competence of farmed fish against attacking pathogens (Abd Elshafy et al. 2023). The integration of nanoparticles into aquatic nano-feed formulations, as well as the use of nano-derived natural products (green-synthesized nanoparticles), offers powerful health benefits in aquaculture (Ghetas et al. 2022). These nanoparticles enhance the immune responses of farmed fish, thereby serving as a disease-preventive strategy (Elgendy et al. 2021b).
Nanotechnology holds numerous significant applications in aquaculture, spanning from disease diagnosis to the mitigation of antibiotic pollution in the aquatic environment. Numerous nanoscale materials are employed in immune and molecular-based disease diagnostic approaches, with nano-based assays exhibiting superior specificity, sensitivity, and accuracy compared to traditional techniques in detecting fish pathogens (El-Adawy et al. 2023a, b). The distinctive characteristics of nanoparticles, notably their expansive surface area, enable interactions with a greater number of conjugates and compounds (Doszpoly et al. 2023). Consequently, this enhances their sensitivity and specificity in identifying and quantifying disease markers (Wang et al. 2011). Nanobiosensors can detect extremely low levels of pathogens, as well as pollutants in the aquatic ecosystem (Chen et al. 2015). Moreover, the application of nano-based remediation technologies, such as nanofiltration (Ren et al. 2022), nanoadsorbents (Vu et al. 2020), nanocatalytic oxidation processes (Chen et al. 2021), and nanosensors monitoring, proves effective in tackling antibiotic pollution in the aquatic environment (Kumar and Guleria 2020).
Challenges of nanotherapeutics application in aquaculture
The potential toxicity of nanoparticles to aquatic organisms and humans is a significant concern (Naguib et al. 2020). These nanoparticles can pose harm to non-target species or the ecosystem. Therefore, ensuring that the nanoparticles reach the target organisms in a controlled and sustained manner without damaging the surrounding environment is crucial (Desai 2012). The high cost of some nanotherapeutics as well as the persistence of certain nanoparticles in aquatic environments are also critical challenges (Bello and Leong 2017). There is a need for effective standardized testing guidelines to assess the safety of nanomaterials in aquatic environments (Desai 2012).
Probiotics, prebiotics, synbiotics, parabiotics, and postbiotics
Probiotics, prebiotics, and synbiotics represent powerful bio-based alternative approaches to traditional chemotherapeutic methods in fish farming (Yilmaz et al. 2022). These innovative therapies possess significant potential for successfully managing diseases in aquaculture (Hardi et al. 2022; Bondad-Reantaso et al. 2023). Probiotics comprise live microorganisms that exert positive influences on the health and overall well-being of their host organisms when administered in the appropriate amounts (Dawood et al. 2020). Probiotics include bacterial microorganisms, such as Lactobacillus spp., Bacillus spp., and Phaeobacter spp. as well as yeasts like Saccharomyces cerevisiae (Dawood et al. 2021). On the other hand, prebiotics are non-digestible food ingredients usually oligosaccharides, such as inulin, fructooligosaccharides, and galactooligosaccharides, that foster the growth of intestinal beneficial microorganisms of aquatic animals (Dawood et al. 2020; Yilmaz et al. 2022). The modification of the microbiome by these beneficial microorganisms stimulates local and systemic fish immune responses, thereby increasing fish resistance to pathogenic bacteria (Manning et al. 2004). Synbiotic is a combination of prebiotics and probiotics (Hardi et al. 2022). Parabiotics, comprising the deceased cells of probiotics and postbiotics such as supernatants from probiotic cultures (metabolic by-products secreted by bacteria), can also play a crucial role in enhancing fish resistance to serious diseases (Goh et al. 2022).
Live, inactivated, or dead microbial cultures are being used in these bio-based therapeutic formulations; however, the administration of live cells induces more health benefits in fish farming (Abbass et al. 2010). Probiotics can be administered in aquaculture, either as a single-strain (mono) probiotic or as a combination of multiple strains (multi-strains). The delivery methods of probiotics encompass bathing the host in a bacterial suspension, supplementing through artificial inert diets, or employing bioencapsulation (Dawood et al. 2020). These bio-based therapeutics offer numerous advantageous applications in aquaculture, such as disease control in fish (Chen et al. 2016), improving feed utilization, and mitigating the adverse effects of antibiotics and other antimicrobials on fish (El-Saadony et al. 2021). Furthermore, probiotics play a role in modulating the transcription of immune-related genes, providing robust immunity and protection against challenging pathogens (Aly et al. 2008). They also improve water quality via increasing the abundance of beneficial bacteria in water (Mohammadi et al. 2021). Probiotics can also be employed to enhance antioxidant capacity (Abdel-Tawwab et al. 2020) and mitigate the toxicity of other chemotherapeutics (El Euony et al. 2020).
Health benefits and antimicrobial mechanisms
The mechanisms of these bio-based therapeutics are intricate (Fig. 4). Key pathways include competitive exclusion of pathogens and competition for binding sites and nutrients (Brunt et al. 2007; Yilmaz et al. 2022). These mechanisms also involve the production of bactericidal and bacteriostatic antimicrobial compounds. Bacteriocins, proteases, hydrogen peroxide, organic acids, and volatile fatty acids are examples of these antimicrobial substances (Vine et al. 2006). Immunostimulation is another significant mode of action of probiotics and prebiotics (Newaj-Fyzul and Austin 2015). Numerous probiotics exhibited immunostimulatory outcomes in aquaculture such as Spirulina platensis green algae (Ibrahem et al. 2013), Bacillus subtilis, Lactobacillus acidophilus (Aly et al. 2008), and Brewer’s yeast (Gokulakrishnan et al. 2022). Probiotics also may serve as a live vaccine carrier. Recombinant live vector vaccines made with Lactobacillus lactis and B. subtilis stimulate potent protective immune responses in fish (Jiang et al. 2019; Sun et al. 2020). These bio-based therapies have demonstrated antagonistic activities against diverse fish pathogens including bacterial, viral, fungal, and parasitic infections as shown in Table 5.
Challenges of probiotic application in aquaculture
Wang et al. (2008) and Jankovic et al. (2010) discussed the challenges facing the applications of these bio-based therapies. The occurrence of genetic exchange between the viable strains in the probiotics and other microbiota makes the therapy less effective. Furthermore, some probiotic strains could act as potential opportunistic fish pathogens. Therefore, the safety profile of a potential probiotic strain is of critical importance. Ensuring the stability of probiotic strains in the aquatic environment is a significant hurdle. Factors such as water quality, temperature fluctuations, and feed interactions can impact the viability of these therapies. The indigenous microbial community in aquaculture systems may also compete with introduced strains. Regular application of probiotic products, which is required to allow probiotic strains to build up in the system, increases their cost.
Phage therapy as a biocontrol approach for fish bacterial diseases
Therapeutic bacteriophages are viruses that infect bacterial cells. Bacteriophages represent a promising potential alternative to antibiotics for effectively controlling bacterial infections that impact farmed fish (Bondad-Reantaso et al. 2023; Ngoc et al. 2023). These phages are abundant in aquatic environments and persist for extended periods (Cao et al. 2020). Phages replicate through the lytic, lysogenic, pseudo-lysogenic, or chronic cycles, relying entirely on the metabolism of their host for reproduction (Li et al. 2020). Most phages exhaust the resources of their bacterial host, disrupting their metabolism and subsequently destroying them upon releasing their progeny (Rørbo et al. 2018). Phage therapy commonly employs lytic phages, which lyse bacteria, as opposed to lysogenic phages that integrate their genome into the bacterial chromosome (Li et al. 2020).
Health benefits and antimicrobial mechanisms of bacteriophages
Phages possess numerous lytic enzymes with a tremendous structural diversity (Fig. 5). The bacteriophage-encoded enzymes are categorized into three types: endolysins, exolysins, and depolymerases (Latka et al. 2017). These enzymatic proteins hold significant potentials for medicinal applications in aquaculture as novel antimicrobial agents (Katharios et al. 2017; Matamp and Bhat 2019). Phage-derived lysins effectively break down the cell walls and destroy biofilms produced by bacterial fish pathogens (Latka et al. 2017). Bacteriophage also stimulates the fish’s immune responses (Van Belleghem et al. 2017). Bacteriophage therapies possess distinct advantages compared to other therapeutic agents such as (1) they exhibit specific and narrow host ranges, ensuring they do not impact the normal intestinal microbiota of fish; (2) they can self-replicate within the bacterial target, eliminating the requirement for repeated applications; (3) they are safe and have no reported adverse effects on the fish or the environment; (4) phage survival is not affected by aquatic environmental conditions such as temperature, pH, salinity, or organic matter concentration (Mathur et al. 2003; Madsen et al. 2013).
The effectiveness of bacteriophage therapy in aquaculture depends critically on the ability of bacteriophages to reach their target bacterial host (Matamp and Bhat 2019). Bacteriophages can be administered through various routes, including oral administration with feed, injection, topical application on the skin, or via water (Onarinde and Dixon 2018; Akmal et al. 2020). Bath administration of phages is more effective for fish bacterial infection initiated on the skin and gills. On the other hand, phage administration through feed will be more valuable for infections established through the oral route (Nakai and Park 2002).
Phage therapy comes in two forms: active and passive. In active therapy, bacteriophages are administered at a dosage capable of reducing the target host population through multiple replication cycles. In passive therapy, the extensive quantity of administered bacteriophages is sufficient to lyse the entire host population without the necessity for reproduction cycles (Li et al. 2020; Kunttu et al. 2021). Monophage therapy “one single type of bacteriophage” and polyphage therapy “combination of several phages” protocols have been tested in aquaculture (Kunttu et al. 2021). Bacteriophage cocktails, comprising multiple bacteriophages, enable the treatment of a broad spectrum of pathogenic bacterial strains, resulting in more rapid and effective outcomes (Chen et al. 2019). Moreover, the utilization of bacteriophage cocktails targeting diverse receptors of a single bacterium may assist in mitigating the development of resistance to these therapies (Culot et al. 2019). Phage therapy has been effectively used to control numerous destructive bacterial infections affecting fish including, Vibrio spp., Flavobacterium spp., Aeromonas spp., Edwardsiella spp., Streptococcus spp., and Lactococcus garvieae (Table 6).
Challenges of bacteriophage therapy in aquaculture
Earlier studies by Mathur et al. (2003), Laanto et al. (2012), and Middelboe et al. (2009) discussed the limitations of bacteriophage therapy in aquaculture. The occurrence of genetic exchange and mutation resulting from the interaction between phages and their hosts makes phage therapy less effective unless new phage/bacteria combinations are identified. Furthermore, broad-spectrum phages and phage cocktail are required to ensure effective therapy against coinfections with multiple pathogens. Moreover, pathogenic bacteria can develop resistance mechanisms to evade phage infection, potentially reducing the therapy’s efficacy over time. Furthermore, the production of antibodies against phages can result in a decline in their effectiveness. Phage therapy also can distribute antibiotic resistance between strains. Some phages also can enhance the virulence and pathogenicity of bacterial pathogens. This alternative therapeutic approach also encounters limitations against intracellular bacteria. The cost and propagation of phage-based products also may act as an additional challenge in certain aquaculture facilities.
Vaccines as alternative preventive therapies to conventional treatments
Aquatic vaccines are therapeutic formulations composed of intentionally modified pathogenic microorganisms and their by-products, designed to prevent the occurrence of fish infectious diseases (Bondad-Reantaso et al. 2023; Yang et al. 2023). Vaccination stands out as one of the most effective methods for combating disease outbreaks in aquaculture (Abotaleb et al. 2023). The global aquaculture sector is expected to embrace vaccines as a routine preventive measure against a wide spectrum of diseases in the future, as seen in Table 7. This shift is propelled by advancements in aquatic vaccine technology and the decreasing costs associated with their development (Wan et al. 2023).
Types of vaccines and their health benefits in aquaculture
Monovalent and multivalent vaccines are existing. Moreover, live, inactivated, or genetically engineered vaccines are also available (Youssef et al. 2023), as seen in (Fig. 6). Injection, immersion, and oral immunization are the three widely used vaccination methods in aquaculture (Abotaleb et al. 2023). Administering vaccines before pathogen exposure is crucial to allow sufficient time for the development of immunity in fish (Thorarinsson et al. 2021).
Live-attenuated vaccine
Live attenuated aquatic vaccines are composed of pathogenic microbial strains that have been weakened or mutated. The intended microorganism can replicate within the host, generating antigenic stimuli that mimic those found in natural infections (Zhao et al. 2022). These vaccines usually exhibit robust prolonged immunogenicity, often eliminating the necessity for booster shots or the incorporation of adjuvants (Liu et al. 2016a, b; Ahangarzadeh et al. 2023). Various methods exist for generating live attenuated vaccines, such as chemical/physical mutagenesis, genetic engineering, attenuated culture, and antibiotic-induced attenuation (Nguyen et al. 2018a, b; Laith et al. 2019). The advancements in genetic engineering technologies enabled the production of safe and effective live attenuated vaccine strains by knocking out virulence-related genes in virulent strains (Zhao et al. 2022). Attenuated vaccines effectively protected fish against major pathogens like A. hydrophila (Abdel-Hadi et al. 2009), Ed. tarda (Yamasaki et al. 2015b), S. agalactiae (Liu et al. 2019), and Vibrio alginolyticus (Zhou et al. 2020), as seen in Table 7.
Inactivated vaccines
These vaccines employ physical (ultraviolet, high-temperature heating, ultrasound, and gamma irradiation) or chemical techniques (formalin, formaldehyde, hydrogen peroxide, and polyoxylene ethers) to deactivate highly virulent pathogenic microorganisms (Ramos-Espinoza et al. 2020). Despite their deactivation, they retain their immunogenicity and induce specific resistance in vaccinated aquatic animals (Gu et al. 2021). Chemical inactivation is the most frequently employed method for the preparation of these vaccines. Inactivated vaccines have certain drawbacks, notably the vulnerability of the vaccine’s protective efficacy to the preparation process (Mohamad et al. 2021). Additionally, these vaccines necessitate higher doses and result in a shorter duration of immunity. Incorporating suitable adjuvants and creating multivalent or combination vaccines are essential to address these issues (Cimica et al. 2017). Nevertheless, inactivated vaccines offer advantages such as safety, the absence of virulence issues, stable storage, and low cost. They currently stand as the most widely reported commercial vaccines in aquaculture (Thorarinsson et al. 2021). Various deactivated vaccines have been developed to combat major bacterial fish diseases such as motile Aeromonas septicemia (Osman et al. 2009), vibriosis (Abotaleb et al. 2023), enteric red mouth disease, lactococcosis (Abu-Elala et al. 2019), and streptococcosis (Abotaleb et al. 2023). Inactivated vaccines demonstrate notable efficacy against viral fish diseases such as tilapia lake virus (TiLV) (Mai et al. 2022) a us (IHNV) (Anderson et al. 2008), as shown in Table 7.
Genetically engineered vaccines
Genetically engineered vaccines primarily employ genetic engineering technology to extract immune antigen genes from the targeted pathogen and introduce them into recipient organisms (Zhao et al. 2022). This technology enables the organism to generate a substantial quantity of protective antigens, enhancing disease resistance (Thorarinsson et al. 2021). Recombinant subunit vaccines, nucleic acid vaccines, recombinant live vector vaccines, gene deletion/mutation-based vaccines, and plant-based vaccines are the main representatives of these vaccines.
Subunit vaccine
These vaccines target immune responses to specific microbial determinants. They are generated by incorporating a pathogen antigen into a recombinant expression vector. This involves expressing a gene product, such as a recombinant peptide or protein, utilizing either a prokaryotic or eukaryotic expression system (Wan et al. 2023). The antigen is purified and employed in the vaccine products. Subunit vaccines are useful for microorganisms that are challenging to culture. They pose no risk of inducing disease in the host, as they can not replicate within the host (Lin et al. 2023).
The outer bacterial membrane proteins are considered a promising subunit vaccine candidate against numerous dangerous fish pathogens such as Ed. tarda (Liu et al. 2017b), A. hydrophila (Sharma and Dixit 2016), Vibrio mimicus (Fu et al. 2021b), Vibrio harveyi (Nguyen et al. 2018a), and V. anguillarum (Xing et al. 2020). Flagellin is also regarded as a favorable candidate for a subunit vaccine (Gonzalez-Stegmaier et al. 2021). Viral capsid proteins are also considered noteworthy potential antigens for subunit viral vaccine formulations (Rout et al. 2022). Yeast, bacteria, transgenic plants, cyanobacteria, and microalgae have been reported as potential systems to produce recombinant subunit vaccines (Kis et al. 2019). The subunit vaccine, prepared from heat shock protein and outer membrane proteins of F. orientalis and expressed in diatoms, significantly enhanced Nile tilapia resistance against F. orientalis infection (Shahin et al. 2020).
These vaccines can offer protection against multiple infections via combining multiple antigenic genes of different pathogens. However, the expression of recombinant viral and protozoan membrane antigens in their natural structural state poses a challenge for subunit vaccines (Rigano et al. 2009; Cueva et al. 2021). The production of microbial misfolded proteins is a concern. Moreover, subunit vaccines are predominantly administered through injection, and the requirement for adjuvants contributes to an increase in their overall cost (Dhar et al. 2014).
Nucleic acid vaccine
Nucleic acid vaccine technology refers to the introduction of plasmids carrying a specific antigen of pathogens into the host to achieve immune protective effects. These vaccines have gained wide attention for promoting protective immunity against various fish pathogens (Chang 2022). DNA vaccines show high efficacy in combating numerous bacterial infections in fish, such as Ed. tarda (Liu et al. 2016a, b), V. anguillarum (Xing et al. 2021), and V. harveyi (Sun et al. 2020). Nucleic acid vaccines are also effective against various viral pathogens in aquaculture, such as cyprinid herpesviruses (Huo et al. 2020); viral hemorrhagic septicemia (VHSV), and infectious hematopoietic necrosis virus infections (IHNV) (Marsella et al. 2022). Currently, some commercial DNA vaccines are authorized in aquaculture against numerous viral fish diseases (Chang 2022). Studies also reported the efficacy of DNA vaccines against parasitic fish diseases like I. multifiliis (Xu et al. 2020).
DNA vaccines also show promise in protecting fish against some dangerous fungal fish diseases like saprolegniasis, caused by S. parasitica oomycete. Ongoing research in this area indicates that the serine protease from S. parasitica (SpSsp1) holds the potential to protect against this disease, suggesting its possible application as a vaccine candidate against saprolegniasis in aquaculture (Minor et al. 2014).
DNA vaccines are largely free of toxicity. However, it is not possible to determine whether the host genome will successfully integrate with exogenous nucleic acid. Furthermore, immune tolerance of the organism to the expressed antigen is a concern of DNA vaccines (Xu et al. 2020). There is no potential risk of mutagenesis with RNA vaccines as it can be degraded by normal cellular processes (Parenrengi et al. 2022).
Recombinant live vector-based vaccine
These vaccines use a non-pathogenic virus or bacteria as a vector or carrier to deliver genetic material from a pathogen and express immune-related antigens that confer protection in the vaccinated fish (Travieso et al. 2022; Swain et al. 2023). This vaccine can confer protection against multiple infections via inserting the antigen genes of different pathogens into the vector (Parenrengi et al. 2022). Lactobacillus spp. and B. subtilis are suitable as live bacterial vector carriers, while baculovirus and IHNV can serve as potential live viral vectors (Zhao et al. 2019). Zhu et al. (2023) successfully developed a recombinant baculovirus vector vaccine to counter the highly lethal infectious spleen and kidney necrosis virus. The vaccine demonstrated an outstanding level of protection, reaching nearly 100% efficacy in small bass and an impressive 85.7% in larger bass (Micropterus salmoides). Despite the fact that live vector-based vaccines offer numerous advantages, they also come with drawbacks, as certain bacteria with reduced virulence may still pose risks to aquatic animal health. Hence, additional optimization of both vector selection and immunization strategy is essential to enhance the efficacy of live vector vaccines (Travieso et al. 2022).
Revers vaccination
This vaccination approach employs bioinformatics and genomics to predict immunogenic sequences to be used as potential vaccine candidates (Zhang et al. 2023). The predicted antigens are then produced in the laboratory as recombinant proteins and screened in vitro for immunogenicity. Reverse vaccination has been implemented against certain fish pathogens, including Ph. damsella piscicida (Andreoni et al. 2016), Ed. tarda, and Flavobacterium columnare (Mahendran et al. 2016).
Mucosal vaccines
Mucosal vaccines represent innovative vaccination technology in aquaculture. These vaccines stimulate protective responses at mucosal surfaces, thus preventing pathogens from replicating at their initial site of invasion (Muñoz-Atienza et al. 2021). Mucosal vaccines confer a longer period of immunity in vaccinated fishes and can be administered through a variety of routes, such as oral, immersion, and nasal vaccination. Determining the necessary dose of the protective antigen to confer immunity is among the notorious challenges in designing fish mucosal vaccines (Dadar et al. 2016).
Nano-based vaccines
Nano-based vaccines incorporate various nanomaterials, such as alginate, and chitosan in vaccine formulations. Nanoparticles enhance both vaccine delivery and the intensity of immune responses. In the study conducted by Kitiyodom et al. (2019), a nanoparticle-based vaccine targeting the mucosal surface of F. columnare exhibited promising results with a protective efficacy of 60% in Oreochromis spp.
Challenges of aquatic vaccines application in aquaculture
The safety concerns of fish vaccines related to their limited immunogenicity are among their critical limitations (Thorarinsson et al. 2021). This drawback could potentially lead to the development of severe diseases and reduced production in vaccinated fish. Furthermore, live attenuated microorganisms are unstable and may revert to a virulent state. Additionally, the insufficient availability of authorized vaccines and their high cost limit their field application (Mondal and Thomas 2022).
Quorum quenching as a biocontrol method for managing bacterial fish pathogens
Quorum quenching (QQ) serves as a strategic innovative biocontrol approach for fish diseases (Bondad-Reantaso et al. 2023). QQ refers to the disruption or interference with the quorum-sensing systems (QS) of bacterial pathogens (Shaheer et al. 2021). QS is a process through which bacteria communicate with each other by detecting and producing signaling molecules known as autoinducers. These molecules allow bacteria to synchronize their behavior including microbe-microbe and host-microbe interactions (Shaheer et al. 2021).
QQ mode of action
Pathogenic bacteria employ QS to regulate the expression of genes controlling several pathogenic mechanisms such as invasion, biofilm formation, defense pathways, and virulence factors in a manner dependent on cell density (Chen et al. 2020) (Table 8) (Fig. 7). Certain compounds impede QS by inhibiting the synthesis of autoinducers, degrading QS signaling, or disrupting the interaction between autoinducers and their receptors (Santhakumari et al. 2018; Paopradit et al. 2022).
Interfering with QS not only halts the expression of the genes that regulate bacterial virulence and disease mechanisms but also effectively reduces the pathogenicity of bacteria (Shaheer et al. 2021). QQ agents may take the form of microbial strains, enzymes, or quorum-sensing activity inhibitors (QSIs) (Sun et al. 2022b).
Bacillus spp. has emerged as a prominent producer of QQ enzymes (Chen et al. 2020). Moreover, numerous QSIs like sodium houttuyfonate, curcumin, thiazolidine, coumarin, and indole derivatives, are promising in fighting infectious agents (Chaverra Daza et al. 2021). Chen et al. (2020) concluded that Bacillus licheniformis T-1 has promising QQ capabilities against A. hydrophila virulent fish pathogen. The QSI activity of B. licheniformis improved the survival rate of fish infected with A. hydrophila to up to 70%. Moreover, Sun et al. (2022b) investigated the antimicrobial and QQ effects of Bacillus velezensis DH82 against V. parahaemolyticus 17SZ. In vitro studies demonstrated a reduction in the growth and biofilm formation of V. parahaemolyticus. Moreover, the DH82 bacteria not only inhibited QS regulation of V. parahaemolyticus but also mitigated its pathogenicity by downregulating the expression of its virulence genes. Dietary supplementation of B. velezensis to shrimp increased the survival rate of shrimp challenged with V. parahaemolyticus to up to (95%). Furthermore, earlier studies conducted by Shaheer et al. (2021) underscored the robust QQ capabilities of Bacillus spp. strains against V. harveyi, B. subtilis, B. lentus, and B. firmus strains disrupted QS signaling, inhibited biofilm formation, and downregulated the expression of V. harveyi virulence genes. Bacillus spp. strains demonstrated protective effects against V. harveyi challenge in P. monodon larvae, offering a notable 90.66% survival rate.
Challenges of quorum quenching application in aquaculture
There are some challenges associated with QS application in aquaculture as discussed by García-Contreras et al. (2013) and Krzyzek (2019) including the complexity of targeting bacterial QS mechanisms. Ensuring that QQ agents disrupt only the harmful bacteria’s communication without affecting beneficial ones is a multifaceted task. Furthermore, inhibition of the genes responsible for regulating quorum sensing QS can lead to an augmentation of pathogenic traits. Bacteria also could develop resistance to QSI over time. This could reduce the efficacy of these therapies and potentially lead to the evolution of more resilient bacterial strains (García-Contreras et al. 2013).
Antimicrobial peptides
Antimicrobial peptides (AMPs) are one of the best alternative therapies for tackling fish pathogens (Bhat et al. 2022). AMPs are small proteins (< 40 amino acids) encoded in the genomes of both vertebrates and invertebrates, displaying potent antibacterial, antifungal, antiviral, and antiparasitic properties (Colorni et al. 2008; Falco et al. 2008). There are five major classes of AMPs including histone-derived, piscidins, hepcidines, cathelicidins, and defensins (Simora et al. 2021). AMPs’ mode of action is either via direct killing of pathogens or modulation of the fish immune system (Ruangsri et al. 2013; Haney and Hancock 2013) (as seen in Fig. 8). AMPs are effective at low concentrations, and pathogens encounter difficulty in developing resistance against them (Bhat et al. 2022).
In vitro and in vivo studies on natural and synthetic AMPs have demonstrated their antagonistic activity against a variety of fish pathogens such as Y. ruckeri (Chettri et al. 2017); Vibrio spp., Aeromonas spp., Ps. aeruginosa (Bhat et al. 2022); Ed. tarda, and Streptococcus spp. (Peng et al. 2012). The dietary supplementation of O. niloticus with tilapia piscidin 4 (TP4) enhanced fish immune responses and their resistance against Streptococcus iniae challenge. Fish treated with piscidin 4 (TP4) exhibited an enhanced survival rate of up to 70% compared to 25% in non-treated fish (Zahran et al. 2019). AMPs also have potent antifungal, antiviral, and antiparasitic properties. The sensitized KK16 peptide completely inhibited the highly fatal S. parasitica infection in embryonated fish eggs (Bhat et al. 2022). β-defensin 1 derived from Oncorhynchus mykiss (O. mykiss) showed in vitro antiviral activity against (VHSV) infection (Falco et al. 2008). Moreover, Pisicidin 2, isolated from hybrid striped bass, Morone saxatilis L., showed potent anti-parasitic effects against Cryptocaryon irritans and Trichodina spp., A. ocellatum, and I. multifiliis ectoparasites (Colorni et al. 2008). AMP therapy has some drawbacks such as elevated cost, limited stability, and heightened host toxicity (Anunthawan et al. 2015). Additionally, AMPs are susceptible to degradation by both endogenous and microbial proteases and some exhibiting cytotoxic properties (Yang et al. 2018).
Biosurfactants
Biosurfactants (BS) are surface-active amphipathic biomolecules produced by microorganisms, with multifunctional properties. They include acids, peptides, polysaccharides, and fatty acids (Rodriguez-Lopez et al. 2019). BS have valuable antimicrobial (antibacterial, antifungal, and antiviral) activities (Fig. 9). BS disrupt bacterial cell membrane and inhibit biofilm formation (Hamza et al. 2017). BS can also be employed as immunostimulants strengthening farmed fish defense mechanisms against different infections (Rajeswari et al. 2016). Hamza et al. (2017) extracted a glycolipid biosurfactant from Staphylococcus lentus obtained from a marine aquatic environment. This biosurfactant inhibited V. harveyi and Ps. aeruginosa biofilms. The glycolipid biosurfactant also protected Artemia salina nauplii against V. harveyi and Ps. Aeruginosa experimental infections, exhibiting post-challenge survival rates of 85 and 67.5%, respectively.
Moreover, Giri et al. (2017) investigated the therapeutic effects of Bacillus licheniformis VS16-derived biosurfactant against A. hydrophila challenge in Labeo rohita fingerlings. This biosurfactant improved fish defense mechanisms. Fish treated (I/P) with the VS16-derived biosurfactant showed enhanced immune responses such as lysozyme, phagocytic activities, and immunoglobulin levels. Fish I/P injected with BS exhibited the highest post-challenge survival rate (72.7%). Application of biosurfactants in aquaculture faces some challenges including the high cost of substrates, purifying and screening process (Gaur et al. 2022).
Bacteriocins
Bacteriocins are bioactive compounds synthesized by ribosomes and produced by numerous bacteria like Bacillus spp. and Lactococcus spp. (Kumariya et al. 2019; Bondad-Reantaso et al. 2023). Bacteriocins are considered promising eco-friendly substitutes for antibiotics in aquaculture (Fig. 10). Bacteriocins can function in various capacities, acting as colonizing peptides, killing peptides, or signaling peptides activating the fish immune system (Pereira et al. 2022). Bacteriocins kill pathogens through various mechanisms, including the disruption of the bacterial cell wall, inhibition of peptidoglycan synthesis, and the formation of pores in the cell wall, ultimately resulting in rapid cell death (Cotter et al. 2013). Bacteriocins also inhibit Gram-negative bacterial pathogens by interfering with DNA, RNA, and protein metabolism (Prabhakar et al. 2013). Class II peptides, bacteriocins, insert themselves into the bacterial cell membrane, inducing depolarization and ultimately resulting in the lysis of the target cell (Cotter et al. 2005). Moreover, bacteriocins inhibit pathogens through their RNase and phospholipase toxic enzymatic activities (Prabhakar et al. 2013).
Numerous bacteriocins with broad bacteriostatic and bactericidal effects have been identified. Bacteriocins derived from L. lactis effectively inhibit the highly pathogenic L. garvieae (Sequeiros et al. 2015). Additionally, bacteriocins extracted from B. subtilis, isolated from roho fish (Labeo rohita), exhibit antagonistic activity against A. hydrophila, A. salmonicida, and Ps. fluorescens (Banerjee et al. 2017). Moreover, Nisin, produced by L. lactis and isolated from the tilapia gut, inhibits Ps. aeruginosa and Vibrio spp. fish pathogens (Kaktcham et al. 2019). Application of bacteriocins in aquaculture faces constraints.
Toxicity of some bacteriocins crude extracts to the natural fish gut flora, potentially causing alterations in fish bioactive compounds and cytokines, is among the challenges facing their application (Malaczewska al. 2021). It can disrupt the bioremediation process via inhibiting the microbiota. Moreover, the direct use of bacteriocin-producing bacteria poses a risk of disease, as certain strains can revert to pathogens (Feliatra et al. 2018).
Stem cell and aquatic animal health: possibilities and prospective
Stem cell technology has opened new avenues for enhancing all sectors of animal production, including aquaculture, and holds the potential to revolutionize therapies for numerous diseases. Stem cell-assisted therapy affords a new expectation for the treatment of incurable diseases, whereas the conventional therapeutic approaches did not afford complete recovery or treatment of the primary cause (Abdal Dayem et al. 2019). The ultimate goal of stem cell-based therapy, an essential component of regenerative medicine, is to strengthen the body’s ability to recover from the injury itself by stimulating, regulating, and changing the population of endogenous stem cells and/or replenishing the cell pool that is in favor of tissue homeostasis and regeneration (O’Brien and Barry 2009; Hoang et al. 2022).
The unique stem cell properties, such as self-renewal and multi-lineage capacities are key factors in stem cell-mediated damaged tissue repair or replacing damaged cells with new functional cells (Biehl and Russell 2009). Apart from their capacity to regulate inflammation, MSCs exhibit antibacterial properties by impeding microbial growth and mitigating antibiotic resistance in Pseudomonas aeruginosa infections (Ren et al. 2020). Previous reports displayed the potent antimicrobial activity of MSCs, which is attributed to their direct and indirect activity (Alcayaga-Miranda et al. 2017). It has been demonstrated that MSC releases antimicrobial peptides including cathelicidins, beta-defensins, and lipocalin (Sung et al. 2016). Interesting study evidenced the enhanced secretion of the antimicrobial cathelicidin LL-37 by MSC upon the secretion of the bacterial products (Krasnodembskaya et al. 2010).
Another mode of action for the antimicrobial activity of MSCs is their modulatory activity. Studies have shown that exposure to MSC-secreted factors boosts the phagocytosis process and the killing capacity of monocytes and neutrophils, and MSC possesses potent anti-inflammatory activity in sepsis (Brandau et al. 2014; Németh et al. 2009). In addition, the synergistic action of MSCs with antibiotics is reported to promote the survival rate after the systemic infection in the mice sepsis model (Alcayaga-Miranda et al. 2015). The wound-healing capacity of MSC is also one of the key mechanisms of MSC-related regeneration. MSC-assisted wound healing capacity is ascribed to angiogenesis activation, activation of the endogenous stem cell population, alleviation of inflammation, and immune cell recruitment (Motegi and Ishikawa 2017). Therefore, stem cells’ potent anti-microbial activity and wound-healing capacity should be investigated and applied to fish diseases. Then, for the application of stem cells in fish diseases, stem cells could be injected into fish muscles, and topically applied using cream base or biomaterials. Similar to the common routes of drug administration in fish, stem cells can be administered to the fish via topical application using scaffolds or cream base, intramuscular injection, intravenous injection, and supplementation in food pellets. Of note, the suitable cell number that exerts an efficient therapeutic activity should be taken into consideration. In summary, stem cell application in fish diseases is still in its infancy and needs further in-depth research. The application of stem cells in the control of fish diseases offers innovative approaches for managing health in aquaculture. This burgeoning field explores three main applications: developing fish cell lines, disease modeling, and drug screening, as well as germ cell transplantation for treating fish diseases. Stem cells are utilized to create cell lines that model fish diseases, providing a crucial platform for understanding pathogenesis and conducting preliminary drug tests. Germ cell transplantation emerges as a promising method for directly treating diseases or restoring fertility in fish affected by various diseases. By replacing or supplementing diseased cells with healthy germ cells derived from stem cells, researchers aim to produce fish breeds that are resistant to diseases and carry a robust trait. Overall, the integration of stem cell technology in these areas represents a significant advancement in aquatic veterinary medicine, opening up new pathways for disease management and therapeutic innovation.
Diagnostic therapy
The advancement of immunological and molecular diagnostic tools for fish health management is dynamic and constantly evolving, offering substantial potential for the aquatic animal health sector (Abdelsalam et al. 2023). Effective vaccinations and disease management strategies in aquaculture have greatly benefited from early identification and precise diagnosis of fish diseases, facilitated by advancements in disease diagnostic approaches (Jaies et al. 2024). Accurate and early diagnosis of invading pathogens is crucial for the effectiveness of therapy and allows for timely intervention and treatment (Dong et al. 2023). Effective diagnostic technologies (Fig. 11) provide fish farmers with precise information on the health of farmed fish, enabling them to optimize overall health, prevent disease spread, and implement targeted management plans (Li et al. 2022a, b, c).
Collecting case history and conducting clinical and postmortem examinations constitute the initial critical steps in the pathway toward correct diagnosis (Abdelsalam et al. 2023). While wet mounts, microscopic examinations, and isolation and culturing on agar may be helpful, they are not sufficient for accurately identifying the species of pathogens (Abdelsalam et al. 2023). Various diagnostic techniques based on phenotypic, morphological, and serological characteristics of pathogens are available, but some of them have shortcomings that can lead to misdiagnosis (Dong et al. 2023; El-Adawy et al. 2023a, b). Molecular diagnostic methods, relying on PCR-based assays and genome sequencing, offer high accuracy (Austin and Austin 2012). Combining immunological and molecular methodologies (Fig. 11) can lead to a more comprehensive understanding of fish health, immune responses, and the factors influencing farmed fish (Jaies et al. 2024).
Immunological diagnostic techniques
Immunological techniques encompass a broad array of laboratory methods used to diagnose various fish diseases by detecting specific antigens, antibodies, or immune cells in biological specimens (Im et al. 2019). Advancements in immunological techniques are essential for developing, optimizing, and evaluating effective aquatic vaccines in aquaculture. These techniques contribute significantly to vaccination by enabling vaccine efficacy testing, ensuring safety, and conducting quality control measures, thereby enhancing disease prevention and management in aquaculture (Jaies et al. 2024).
The enzyme-linked immunosorbent assay (ELISA)
The ELISA assay is designed to detect and measure specific antigens (such as proteins, peptides, or other compounds) or antibodies with precision and accuracy. The technique is based on enzymatic reactions and the binding of antigens and antibodies (Jaies et al. 2024). The most commonly utilized types of ELISA in immunological assays include Direct ELISA, Indirect ELISA, Sandwich/Capture ELISA, Competitive ELISA, and Multiplex ELISA (El-Adawy et al. 2023a, b). ELISA plays a crucial role in the aquatic health sector, with applications ranging from disease diagnosis and vaccine development to monitoring fish immune responses and environmental conditions (Munangandu et al. 2019; El-Adawy et al. 2023a, b; Jaies et al. 2024). The main limitations of the ELISA assay include cross-reactivity, challenges with specificity, and restricted multiplexing capabilities, which hinder its ability to simultaneously investigate multiple aspects of fish health (Jaies et al. 2024).
Immunofluorescence
Immunofluorescence is an immunohistochemistry technique that utilizes fluorophores to visualize various cellular antigens. The assay is based on the visualization of antigen–antibody interactions using fluorescent labeling and fluorescence microscopy (Jaies et al. 2024). Immunofluorescence has diverse applications, such as diagnosing infections affecting fish, examining the distribution of antigens in fish tissue, studying fish immune responses, and evaluating the effects of environmental pollutants on fish health (Brehm-Stecher and Johnson 2004; Austin 2019). One of the primary limitations of immunofluorescence is its dependence on the permeability of fish tissues, such as scales or skin, to antibody penetration, which significantly influences labeling efficiency (Jaies et al. 2024). Moreover, cross-reactivity and non-specific binding of antibodies to similar antigens can lead to false-positive results, while non-specific background fluorescence can interfere with signal detection (Pina et al. 2022).
Immunohistochemistry
Immunohistochemistry is a powerful immunological diagnostic approach based on antigen–antibody interactions (Jaies et al. 2024). This assay is commonly used to detect and localize specific antigens within tissues (Magaki et al. 2019). Immunohistochemistry has diverse applications across various fields, including disease diagnosis, analysis of histopathological tissue changes associated with diseases, and the study of tissue-specific protein expression. It also plays a crucial role in assessing the effectiveness of medications and vaccines in fish (Ezyaguirre et al. 2011; Zhang et al. 2017; Su and Chen 2021). Primary hurdles in immunohistochemistry methods include cross-reactivity with fish tissues, challenges in fixing fish-specific tissues, and tissue autofluorescence (Jaies et al. 2024).
Molecular diagnostic techniques
Polymerase chain reaction (PCR)
PCR is one of the most widely used techniques in molecular biology and is applied across various sectors. The assay works by amplifying specific DNA sequences from complex mixtures using specific primers and enzymes within a thermal cycler (Abdelsalam et al. 2023). PCR encompasses several types, each serving distinct purposes: Conventional PCR, Reverse transcription PCR (RT-PCR), Quantitative PCR (qPCR), nested PCR, multiplex PCR, multiplex ligation-dependent probe amplification (MLPA), digital PCR, and overlap extension PCR (OE PCR) (Abdelsalam et al. 2023; Jaies et al. 2024). PCR assays have several important applications, including disease diagnosis, drug development, cloning, and recombinant DNA technology. Challenges facing PCR diagnostic techniques include the occurrence of false-positive or false-negative results and interference from various PCR inhibitors (Jaies et al. 2024)
Loop-mediated isothermal amplification (LAMP)
Loop-mediated isothermal amplification (LAMP) is an extremely precise molecular method of nucleic acid amplification (Notomi et al. 2000). The target DNA is recognized by six different regions using a series of four (or six) distinct primers that bind to specific sites on the target gene. The LAMP reaction involves both strand displacement and amplification phases. It amplifies targeted DNA at a constant temperature without the need for a thermal cycler (Abdelsalam et al. 2023). LAMP has numerous applications due to its specificity and simplicity, including genotyping, pathogen detection, and environmental monitoring. However, primer design complexity and cross-reactivity are important constraints facing this diagnostic approach (Jaies et al. 2024).
Next-generation sequencing (NGS)
Next-generation sequencing technology represents a significant advancement in molecular biology, enabling detailed and large-scale genetic analyses that were previously impossible (Satam et al. 2023). NGS can process millions of fragments simultaneously, providing a high-throughput approach to DNA and RNA sequencing, thereby enabling the generation of enormous volumes of valuable data (Qin 2019). This method combines numerous sequencing matrices and bioinformatics technologies. NGS has various applications, including precise pathogen diagnosis, targeted treatment plans, whole genome sequencing, identifying genetic mutations, analyzing epigenetic modifications, and sequencing DNA from environmental samples to study microbial communities (Satam et al. 2023). Challenges facing next-generation sequencing technology include high costs, the need for advanced bioinformatics techniques and knowledge, and the difficulty and time-consuming nature of analyzing raw sequencing data (Jaies et al. 2024).
Recent advancements in fish disease diagnostic approaches
Metagenomics and multi-omics Metagenomics studies genetic material obtained from microbial communities in environmental samples, without the need for culturing individual species (Zhang et al. 2021). Metagenomics enables comprehensive exploration of microbial populations associated with fish ecosystems, including bacteria, viruses, and fungi, using high-throughput DNA sequencing methods (Petrosino et al. 2009; Pérez-Cobas et al. 2020). This approach provides significant new insights into the complex interactions between the microbiome and the fish host, revealing the role of microbial communities in regulating immune responses, preventing disease, and maintaining health (Jaies et al. 2024). Metagenomics approaches have emphasized the role of beneficial microbes in promoting fish health and improving the identification of potential fish pathogens (Yukgehnaish et al. 2020).
A comprehensive understanding of data from proteomics, metabolomics, transcriptomics, and genomics enables the identification of significant biomarkers for diagnostics (Natnan et al. 2021). Proteomic analyses play a crucial role in elucidating pathogen survival strategies and host defense responses, thereby enhancing our understanding of interactions between fish and pathogens (Ahmed et al. 2019a, b). The integration of multi-omics data supports the development of predictive models that reveal how genetic and environmental factors impact fish health and performance (Jaies et al. 2024).
Biosensors and nanotechnology
The aquatic animal health sector benefits from biosensors and nanotechnology in disease diagnosis through enhanced sensitivity, rapid detection, and improved accuracy in identifying pathogens (Bohara et al. 2023). This allows for prompt intervention techniques to mitigate their effects. Biosensors utilize biological molecules to detect specific compounds or biomarkers associated with diseases in fish, offering real-time monitoring and early pathogen detection capabilities (Jaies et al. 2024). Nanotechnology enhances diagnostic tools by miniaturizing sensors, thereby improving their efficiency and enabling on-site testing (El-Adawy et al. 2023a, b). These advancements contribute to proactive disease management, reducing economic losses, and promoting sustainable aquaculture practices. Fernandes et al. (2015) developed a highly sensitive genosensor incorporating multiwalled carbon nanotubes, chitosan, bismuth, and lead sulfide nanoparticles for detecting pathogenic Aeromonas. This biosensor has a high detection limit of 1.0 × 10−14 M and can identify Aeromonas concentrations as low as 102 CFU/mL in spiked tap water.
Artificial intelligence (AI), image processing, and computer vision technology
Integrating AI into disease diagnostic methods can enhance the efficiency and accuracy of pathogen detection in several ways, such as through image analysis, analysis of fish behavior, genetic data analysis, real-time monitoring, and monitoring of water quality (Mandal and Ghosh 2023). Machine learning algorithms can detect anomalies indicating health issues in farmed fish populations, predict disease outbreaks, and optimize feeding schedules (Ditria et al. 2022). Additionally, AI can help determine the most effective treatments and dosages for sick fish, minimizing the use of antibiotics and other medications. AI-driven platforms can assist farmers in making informed decisions using real-time data and predictive models (Jaies et al. 2024).
Automated fish disease detection can also be established through the integration of image-processing technology with computer vision (Torres and Arroyo 2018; Li et al. 2022a, b, c). Developing AR 3D images of fish diseases, utilizing standardized and shared datasets, along with deep learning and data fusion techniques, can enhance the accuracy and efficiency of diagnosing fish diseases (Li et al. 2022a, b, c). This diagnostic approach offers a real-time, non-invasive, and cost-effective method for disease diagnosis (Ohnuma et al. 2017; Li et al. 2022a, b, c).
The complexity of data interpretation, host–pathogen interactions, and high costs are among the most significant challenges faced by these innovative diagnostic approaches (Bahassi and Stambrook 2014; Jaies et al. 2024.
Conclusion
Disease outbreaks pose a substantial obstacle to the sustainability of aquaculture, resulting in massive financial losses. Traditionally, the prevention and control of fish diseases have predominantly leaned on the application of conventional chemotherapeutics and antibiotics. Nevertheless, the prolonged and uncontrolled utilization of these compounds contributes to the rise of microbial resistance, harms fish health, degrades aquaculture environments, leads to the presence of drug residues in aquatic products, induces financial losses, and impedes the trade of fishery products.
These challenges serve as strong motivation to explore innovative and effective therapeutic approaches that can overcome these impediments. Advancements in biotechnology have greatly expanded the development of effective therapies to combat various pathogens in aquaculture. A variety of bio-based and immunoprophylaxis therapies have emerged, including phytotherapeutics, nanotherapeutics, probiotics, prebiotics, synbiotics, phage therapy, vaccination, quorum quenching, antimicrobial peptides, biosurfactants, and bacteriocins. These promising therapies demonstrate significant potential for application in aquaculture. Additionally, germline stem cells and germ cell transplantation offer new prospects for the genetic breeding of disease-resistant fish strains. Regularly screening and monitoring the health status of cultured species are essential for the early detection of diseases. Therefore, developing effective disease diagnostic technologies enables accurate pathogen detection, provides fish farmers with precise information about the health of their farmed fish, and facilitates the application of efficient therapies. Further research is essential to refine the utilization of these alternative therapeutic approaches. There is a need for a deeper understanding of their successes and failures, cost implications, efficacy, risks, practical applications (especially for small fish producers), adverse effects on the aquatic environment, and how these alternatives contribute to improving health and enhancing host immunity. The objective is to devise the safest, economically viable, and most effective methods to prevent aquatic animal diseases. A multidisciplinary approach involving scientists, researchers, industry stakeholders, and regulatory bodies is essential to navigate these challenges for the responsible and sustainable application of these disease-alternative therapeutics in aquaculture.
Recommendations
The primary critical measure in averting disease outbreaks in fish farms revolves around adhering to good farm management and implementing robust biosecurity practices before the event of any disease outbreaks. The second crucial aspect involves the swift and precise diagnosis of invading pathogens. The third focal point is the implementation of cost-effective and safe therapeutic interventions. The fourth critical issue is conducting extensive environmental risk assessment studies before applying any therapy in aquaculture facilities. Application of therapeutic combinations can strongly enhance the efficacy of these therapies. Immunoprophylaxis should be carried out before the occurrence of a disease outbreak to reduce the losses of fish production. While the aforementioned therapeutics show considerable promise in protecting fish from various harmful pathogens, their application carries inherent risks. Therefore, a deeper understanding through research is imperative to address these limitations. A multidisciplinary approach, engaging scientists, industry stakeholders, and regulatory bodies, is essential to navigate these challenges and establish guidelines for responsible and sustainable application of these therapies in aquaculture.
Non-therapeutic preventive measurements for controlling fish diseases
(1) Application of biosecurity and good farm management practices in aquaculture. (2) Development and breading of disease-resistant fish strains. (3) Feeding farmed fish on well-balanced diets. (4) Conducting antibiotic sensitivity testing before administering any antibiotic treatment in fish farms. (5) Strengthen oversight of antibiotic and chemotherapeutic use in fish farms by regulating their sales and ensuring supervision by adequately trained aquatic health professionals. (6) Developing a national strategy on aquatic animal health. (7) Promote ongoing education and training for aquaculturists and fish farmers. (8) Mechanical (filtration) and ozone treatment of water in recirculating aquaculture systems. (9) Employing biological methods to control parasites by using cleaner fish. (10) Enforcing legislation and policies that regulate the use of chemicals in aquaculture.
Phytotherapeutics
(1) Developing standardized phytotherapeutic products with well-defined active ingredients and improving extraction methods. (2) Developing phytotherapeutics with multiple antimicrobial properties through synergistic combinations of different phytotherapeutic compounds or their combination with other bio-enhancers to enhance overall efficacy. (3) Improving the bioavailability and absorption of phytochemicals via adopting encapsulation techniques or nanoformulations. (4) Adjusting dosages and avoiding aquatic environmental conditions that can influence the efficacy and absorption of phytotherapeutics.
Nanotherapeutics
(1) Ensure the safety and biocompatibility of nanotherapeutics for aquatic organisms by conducting comprehensive toxicity studies. (2) Optimize the size of nanoparticles to enhance their uptake and absorption by farmed fish. (3) Conduct thorough environmental impact assessments to understand the potential consequences of nanotherapeutics use on aquatic ecosystem. (4) Improve effective and safe delivery systems for nanotherapeutics, ensuring that nanoparticles reach the target organisms in a controlled and sustained manner without causing harm to the surrounding environment. (5) Adopting green synthesis methods to produce nanoparticles to enhance their therapeutic efficacy. (6) Providing economically viable nanotherapeutics for fish farmers. (7) Enforce legislations controlling the application of nanotherapeutics in aquaculture.
Probiotics, prebiotics, and synbiotics
(1) Selecting probiotic strains that are well-adapted to the target fish species and have proven beneficial effects on growth, disease resistance, and overall health. (2) Conduct microbial analyses to ensure the dominance of beneficial probiotic strains. (3) Select probiotic strains that do not adversely interact with other treatments. (3) Develop new probiotic strain combinations to overcome mutations in microbial strains. (4) Use probiotics with optimal dosage based on the species, size, and environmental condition. (5) Maintain a stable beneficial microbial community through repeated application of probiotics.
Phage therapy
(1) Selecting an appropriate phage that has antagonistic effects against the targeted pathogen. (2) Using phage mixtures, phage cocktails, and new combinations of phages to ensure effective therapy against mixed infections and to avoid bacterial resistance. (3) Phages should be tested for survival against a range of environmental parameters to ensure long-term survival. (4) Phages should be purely lytic to prevent recombination and mutation processes in bacterial strains. (5) Phage therapy should be cost-effective and affordable to farmers. (6) Enforcing legislations regulating the application of bacteriophages in aquaculture.
Vaccines
(1) Confirm the safety requirements of fish vaccines, especially modified live vaccines that need to pass certain safety guidelines. (2) Increasing the availability of commercially authorized vaccines against a wide range of fish pathogens. (3) Developing safe, cost-effective polyvalent vaccines that confer protection against a wide range of pathogens. (4) Fish vaccines should be environmentally friendly, cost-effective, and affordable for small fish farmers. (5) Vaccine technology should be suitable for large-scale production. (6) Developing a new generation of vaccines using recent genetic engineering technology and gene editing to create safer and highly effective vaccines. (7) Improve vaccine delivery methods for fish.
Quorum quenching
(1) Achieving high specificity in targeting the quorum sensing (QS) systems of pathogens without adversely affecting beneficial microorganisms or the host. (2) Using naturally-sourced QSIs and developing newer QSIs agents for resistant bacteria.
Antimicrobial peptides, biosurfactants, and bacteriocins
(1) Improve the purifying and screening process for novel agents. (2) Reduce the cost of applying these therapies. (3) Improve the stability of these therapeutic agents and mitigate potential toxicity, thereby enhancing their overall safety and effectiveness.
Stem cell-based therapy
(1) Adhering to ethical guidelines for the use of stem cells. (2) Optimize the technical aspects of culturing techniques and delivery methods. (3) Conduct continuous research and development to understand the potential of this therapy and identify sources of stem cells. (4) Assess the safety and efficacy of stem cell therapy in aquaculture species. (5) Evaluate the cost-effectiveness of stem cell therapy in aquaculture.
Diagnostic therapy
(1) Selecting appropriate diagnostic tests. (2) Developing reliable antibodies, primers, and probes specific to the pathogens of interest. (3) Standardizing and validating diagnostics to confirm the sensitivity, specificity, and reproducibility of diagnostic tests. (4) Integrating molecular and immunological diagnostics with artificial intelligence based-technologies to significantly enhance the accuracy of pathogen detection. (5) Regular screening and monitoring of the health status of cultured species to detect early signs of disease.
Data availability
No datasets were generated or analysed during the current study.
Code availability
Not applicable.
References
Abbas WT, Abumourad IMK, Mohamed LA, Soliman WSE, Elgendy MY (2019) The role of the dietary supplementation of fenugreek seeds in growth and immunity in Nile tilapia. J Journal of Biological Sciences 12:650–656
Abbass A, Sharifuzzaman SM, Austin B (2010) Cellular components of probiotics control Yersinia ruckeri infection in rainbow trout, Oncorhynchus mykiss (Walbaum). J FishDis 33:31–37
Abd El-Galil MA, Aboelhadid SM (2012) Trials for the control of trichodinosis and gyrodactylosis in hatchery reared Oreochromis niloticus fries by using garlic. Vet Parasitol 185:57–63
Abd El-latif AM, Abd El-Gawad EA, Soror EI, Shourbela RM, Zahran E (2021) Dietary supplementation with miswak (Salvadora persica) improves the health status of Nile tilapia and protects against Aeromonas hydrophila infection. Aquaculture Reports 19
Abd Elshafy MB, Abd EL-Monem AIM, Khattab IM, Fadl SE, Khadiga GA (2023) Nutritional impact of nano zeolite, probiotic, and fatty acids as feed additives on health status of Nile tilapia (Oreochromis niloticus). Sci Rep 13:22740
Abdal Dayem A, Lee SB, Kim K, Lim KM, Jeon T-I, Seok J, Cho S-G (2019) Production of mesenchymal stem cells through stem cell reprogramming. Int J Mol Sci 20:1922–1930
Abdel-Hadi YM, Shamsudin MN, Yusoff K, Zakaria S (2009) Indoor study on the immunization of red tilapia: Oreochromis niloticus × O. mossambicus against aeromonad and pseudomonad septicemias. J Fisheries International 4:45–51
Ahmed F, Soliman FM, Adly MA, Soliman HAM, El-Matbouli M, Saleh M (2020) In vitro assessment of the antimicrobial efficacy of chitosan nanoparticles against major fish pathogens and their cytotoxicity to fish cell lines. J Fish Dis 43:1049–1063
Abdelhamed H, Lawrence ML, Karsi A (2018) Development and characterization of a novel live attenuated vaccine against enteric septicemia of catfish. Front Microbiol 9:1819
Abdelrahman HA, Hemstreet WG, Roy LA, Hanson TR, Beck BH, Kelly AM (2023) Epidemiology and economic impact of disease-related losses on commercial catfish farms: a seven-year case study from Alabama, USA. Aquaculture 566
Abdel-Razek N (2019a) Antimicrobial activities of chitosan nanoparticles against pathogenic microorganisms in Nile tilapia,Oreochromis niloticus. Aquac Int 7:1315–1330
Abdel-Razek N (2019b) Antimicrobial activities of chitosan nanoparticles against pathogenic microorganisms in Nile tilapia, Oreochromis niloticus. Aquac Int 27:1315–1330
Abdelsalam M, Elgendy MY, Elfadadny MR, Sherif AH, Abolghait SK (2023) A review of molecular diagnoses of bacterial fish diseases. Aquacult Int 31:417–434
Abdel-Tawwab M, Khalil RH, Nour AM, Elkhayat BK, Khalifa E, Abdel-Latif HMR (2020) Effects of Bacillus subtilis-fermented rice bran on water quality, performance, antioxidants/oxidants, and immunity biomarkers of white leg shrimp (Litopenaeus vannamei) reared at different salinities with zero water exchange. J Appl Aquac 34:1–26
Abotaleb MM, Soliman HM, Tawfik RG, Mourad A, Khalil RH, Abdel-Latif HM (2023) Efficacy of combined inactivated vaccines against Vibrio alginolyticus and Streptococcus agalactiae infections in Nile tilapia in Egypt. Aquacult Int 12:332–338
Aboud OAE (2010) Application of some Egyptian medicinal plants to eliminate Trichodina sp. and Aeromonas hydrophila in tilapia (Oreochromis niloticus). Researcher 2:12–16
Abu-Elala NM, Samir A, Wasfy M, Elsayed M (2019) Efficacy of injectable and immersion polyvalent vaccine against streptococcal infections in broodstock and offspring of Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 88:293–300
Adel M, Dadar M, Zorriehzahra MJ, Elahi R, Stadtlander T (2020) Antifungal activity and chemical composition of Iranian medicinal herbs against fish pathogenic fungus, Saprolegnia parasitica. Iran J Fish Sci 19:3239–3254
Adeshina I, Abdel-Tawwab M, Tijjani ZA, Tiamiyu LO, Jahanbakhshi A (2021) Dietary Tridax procumbens leaves extract stimulated growth, antioxidants, immunity, and resistance of Nile tilapia, Oreochromis niloticus, to monogenean parasitic infection. Aquaculture 532
Agarwal M, Agarwal MK, Shrivastav N, Pandey S, Das R, Gaur P (2018) Preparation of chitosan nanoparticles and their in-vitro characterization. International Journal of Life-Sciences Scientific Research 4:1713–1720
Ahangarzadeh M, Houshmand H, Torfi Mozanzadeh M, Kakoolaki S, Nazemroaya S, Sepahdari A, Peyghan R, Ajdari A, Sadr AS (2023) Effect of killed autogenous polyvalent vaccines against Vibrio harveyi, V. alginolyticus and Streptococcus iniae on survival and immunogenicity of Asian seabass (Lates calcarifer). Fish Shellfish Immunol 143:109226
Ahmed F, Kumar G, Soliman FM, Adly MA, Soliman HA, El-Matbouli M, Saleh M (2019a) Proteomics for understanding pathogenesis, immune modulation and host pathogen interactions in aquaculture. Comparative Biochemistry and Physiology-Part D 32
Ahmed F, Solimanb FM, Adly MA, Soliman HAM, El-Matbouli M, Saleh M (2019b) Recent progress in biomedical applications of chitosan and its nanocomposites in aquaculture: a review. Res Vet Sci 126:68–82
Ahmed F, Soliman FM, Adly MA, Soliman HAM, El-Matbouli M, Saleh M (2020) In vitro assessment of the antimicrobial efficacy of chitosan nanoparticles against major fish pathogens and their cytotoxicity to fish cell lines. J Fish Dis 43:1049–1063
Ahmed F, Soliman FM, Adly MA, Soliman HAM, El-Matbouli M, Saleh M (2021) Dietary chitosan nanoparticles: potential role in modulation of rainbow trout (Oncorhynchus mykiss) antibacterial defense and intestinal immunity against enteric redmouth disease. Mar Drugs 19:72
Akmal M, Rahimi-Midani A, HafeezurRehman M, Hussain A, Choi TJ (2020) Isolation, characterization, and application of a bacteriophage infecting the fish pathogen Aeromonas hydrophila. Pathogens 9:215
Alcayaga-Miranda F, Cuenca J, Martin A, Contreras L, Figueroa FE, Khoury M (2015) Combination therapy of menstrual derived mesenchymal stem cells and antibiotics ameliorates survival in sepsis. Stem Cell Res Ther 6:1–13
Alcayaga-Miranda F, Cuenca J, Khoury M (2017) Antimicrobial activity of mesenchymal stem cells: current status and new perspectives of antimicrobial peptide-based therapies. Front Immunol 8:248157
Ali SE, Jansen MD, Mohan CV, Delamare-Deboutteville J, Charo-Karisa H (2020) Key risk factors, farming practices and economic losses associated with tilapia mortality in Egypt. Aquaculture 527:735438
Ali SE, Soliman W, Abumourad IMK, Elgendy MY, Songe M (2021) Protective effect of leek extract (Allium ampeloprasum L.) on catfish (Clarias gariepinus) experimentally challenged with Aeromonas hydrophila. Pakistan J Biol Sci 24:199–206
Ali SE, Mahana O, Mohan CV, Delamare-Deboutteville J, Elgendy MY (2022) Genetic characterization and antimicrobial profiling of bacterial isolates collected from Nile tilapia (Oreochromis niloticus) affected by summer mortality syndrome. J Fish Dis 45:1857–1871
Al-Sagheer AA, Mahmoud HK, Reda FM, Mahgoub SA, Ayyat MS (2018) Supplementation of diets for Oreochromis niloticus with essential oil extracts from lemongrass (Cymbopogon citratus) and geranium (Pelargonium graveolens) and effects on growth, intestinal microbiota, antioxidant and immune activities. Aquac Nutr 24:1006–1014
AL-Shammari RH, Alsaady MH, AliSS, (2022) The efficiency of biosynthesized zinc oxide nanoparticles by Fusarium sp. against Saprolegnia parasitica isolated from common carp eggs in fish hatchery. Int J Aquatic Biology 10:378–383
Aly SM, Ahmed YA, Ghareeb AA, Mohamed MF (2008) Studies on Bacillus subtilis and Lactobacillus acidophilus, as potential probiotics, on the immune response and resistance of Tilapia nilotica (Oreochromis niloticus) to challenge infections. Fish Shellfish Immunol 25:128–136
Aly SM, Albutti AS, Rahmani AH, Atti NM (2015) The response of New-season Nile tilapia to Aeromonas hydrophila vaccine. Int J Clin Exp Med 8:4508–4514
Aly SM, Abou El-Gheit SN, Essam El-Din HM (2022) Comparative studies on the efficiency of neem leaves Azadirachta indica and flubendazole treatment against Diplectanum in sea bass Dicentrarchus labrax. Acta Parasitol 67:970–975
Anderson E, Clouthier S, Shewmaker W, Weighall A, LaPatra S (2008) Inactivated infectious haematopoietic necrosis virus (IHNV) vaccines. J Fish Dis 31:729–745
Andreoni F, Amagliani G, Magnani M (2016) Selection of vaccine candidates for fish pasteurellosis using reverse vaccinology and an in vitro screening approach. Methods Mol Biol 1404:181–192
Anunthawan T, De La Fuente-Nú˜nez C, Hancock RE, Klaynongsruang S (2015) Cationic amphipathic peptides KT2 and RT2 are taken up into bacterial cells and kill planktonic and biofilm bacteria. Biochim. Et. Biophys. Acta (BBA)-Biomembr 1848:1352–1358
Aonullah AA, Nuryati S, Alimuddin MS (2017) Efficacy of koi herpesvirus DNA vaccine administration by immersion method on Cyprinus carpio field scale culture. Aquac Res 48:2655–2662
Ara N, Mondal S, Basu S, Laskar RA, Mandal D (2009) Biogenic synthesis of au and Ag nanoparticles using aqueous solutions of black tea leaf extracts. Colloids Surf, B 71:13–118
Araujo GS, Silva JWAd, Cotas J, Pereira L (2022) Fish farming techniques: current situation and trends. J Marine Sci Eng 10:1598
Arijo S, Brunt J, Chabrillón M, Diaz-Rosales P, Austin B (2008) Subcellular components of Vibrio harveyi and probiotics induce immune responses in rainbow trout, Oncorhynchus mykiss (Walbaum), against V. harveyi. J Fish Dis 31:579–590
Austin B (2019) Methods for the diagnosis of bacterial fish diseases. Mar Life Sci Technol 1:41–49
Austin B, Austin DA (2012) Bacterial fish pathogens, disease of farmed and wild fish, 5th edn. Springer, Media Dordrecht
Bahassi EM, Stambrook PJ (2014) Next-generation sequencing technologies: breaking the sound barrier of human genetics. Mutagenesis 29:303–310
Banerjee G, Nandi A, Ray AK (2017) Assessment of hemolytic activity, enzyme production and bacteriocin characterization of Bacillus subtilis LR1 isolated from the gastrointestinal tract of fish. Arch Microbiol 199:115–124
Barbosa FC, Tadine RM, Rezende GD, Lopes GC, Neto EA (2023) Current challenges for the use of phytotherapy. Contribuciones a Las Ciencias Sociales, São José Dos Pinhais 16:2066–2078
Barriga IB, Gonzales AP, Brasiliense AR, Castro KN, Tavares-Dias M (2020) Essential oil of Lippia grata (Verbenaceae) is effective in the control of monogenean infections in Colossoma macropomum gills, a large Serrasalmidae fish from Amazon. Aquac Res 51:3804–3812
Begines B, Ortiz T, Pérez-Aranda M, Martínez G, Merinero M, Argüelles-Arias F, Alcudia A (2020) Polymeric nanoparticles for drug delivery: recent developments and future prospects. Nanomaterials (basel) 10:1403
Bello D, Leong DT (2017) A decade of nanotoxicology: assessing the impact on human health and the environment. NanoImpact 7:15–16
Bhat RH, Khangembam VC, Thakuria D, Pant V, Tandel RS, Tripathi G, Sarma D (2022) Antimicrobial activity of an artificially designed peptide against fish pathogens. Microbiol Res 260
Biehl JK, Russell B (2009) Introduction to stem cell therapy. J Cardiovasc Nurs 24:98–103
Bohara K, Joshi P, Acharya KP, Ramena G (2023) Emerging technologies revolutionising disease diagnosis and monitoring in aquatic animal health. Rev Aquac 16:836–854
Bondad-Reantaso MG, MacKinnon B, Karunasagar I, Fridman S, Alday-Sanz V, Brun E, Groumellec ML, Li A, Surachetpong W, Karunasagar I, Hao B, Dall’Occo A, Urbani R, Caputo, (2023) A review of alternatives to antibiotic use in aquaculture. Rev Aquac 15:1421–1451
Borisutpeth BP, Kanbutra P, Hanjavanit C, Hukanhom KC, Funaki D, Hatai K (2009) Effects of Thai herbs on the control of fungal infection in tilapia eggs and the toxicity to the eggs. Aquaculture Sci 57:475–482
Brandau S, Jakob M, Bruderek K, Bootz F, Giebel B, Radtke S, Mauel K, Jäger M, Flohe SB, Lang S (2014) Mesenchymal stem cells augment the anti-bacterial activity of neutrophil granulocytes. PLoS ONE 9:e106903
Brehm-Stecher BF, Johnson EA (2004) Single-cell microbiology: tools, technologies, and applications. Microbiol Mol Biol Rev 68:538–559
Brunt J, Austin B (2005) Use of a probiotic to control lactococcosis and streptococcosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 28:693–701
Brunt J, Newaj-Fyzul A, Austin B (2007) The development of probiotics for the control of multiple bacterial diseases of rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 30:573–579
Buchmann K (2017) Rainbow trout (Oncorhynchus mykiss) immune response towards a recombinant vaccine targeting the parasitic ciliate Ichthyophthirius multifiliis. J Fish Dis 40:1815–1821
Burbank DR, Shah DH, LaPatra SE, Fornshell G, Cain KD (2011) Enhanced resistance to coldwater disease following feeding of probiotic bacterial strains to rainbow trout (Oncorhynchus mykiss). Aquaculture 321:185–190
Cabello-Gómez JF, Aguinaga-Casañas MA, Falcón-Piñeiro A, González-Gragera E, Márquez-Martín R, Agraso MDM, Bermúdez L, Baños A, Martínez-Bueno M (2022) Antibacterial and antiparasitic activity of propyl-propane-thiosulfinate (PTS) and propyl-propane-thiosulfonate (PTSO) from Allium cepa against gilthead sea bream pathogens in in vitro and in vivo studies. Molecules 14(27):6900
Cao H, Hou S, He S, Lu L, Yang X (2014) Identification of a Bacteriovorax sp. isolate as a potential biocontrol bacterium against snakehead fish-pathogenic Aeromonas veronii. J Fish Dis 37:283–289
Cao Y, Li S, Han S, Wang D, Zhao J, Xu L, Liu H, Lu T (2020) Characterization and application of a novel Aeromonas bacteriophage as treatment for pathogenic Aeromonas hydrophila infection in rainbow trout. Aquaculture 523
Casuso A, Valenzuela-Muñoz V, Benavente BP, Valenzuela-Miranda D, Gallardo-Escárate C (2022) Exploring sea lice vaccines against early stages of infestation in Atlantic salmon (Salmo salar). Vaccines (basel) 1(10):1063
Chang CJ (2022) Methods in molecular biology. In: Thomas S (ed) Development and evaluation of DNA vaccine against salmonid alphavirus. Humana, New York, p 2411
Chang CI, Liu WY (2002) An evaluation of two probiotic bacterial strains, Enterococcus faecium SF68 and Bacillus toyoi, for reducing Edwardsiellosis in cultured European eel, Anguilla anguilla, L. J Fish Dis 25:311–315
Chaverra Daza KE, Silva G´omez E, Moreno Murillo BD, Mayorga Wandurraga H, (2021) Natural and enantiopure alkylglycerols as antibiofilms against clinical bacterial isolates and quorum sensing inhibitors of Chromobacterium violaceum ATCC 12472. Antibiotics 10:430–436
Chen B, Zou L, Wu Z, Sun M (2015) The application of quantum dots in aquaculture pollution detection. Toxicol Environ Chem 98:1–10
Chen Y, Li J, Xiao P, Li GY, Yue S, Huang J, Mo ZL (2016) Isolation and characterization of Bacillus spp. M001 for potential application in turbot (Scophthalmus maximus L.) against Vibrio anguillarum. Aquac Nutr 22:374–381
Chen L, Fan J, Yan T, Liu Q, Yuan S, Zhang H, Yang J, Deng D, Huang S, Ma Y (2019) Isolation and characterization of specific phages to prepare a cocktail preventing Vibrio Sp. Va-F3 infections in shrimp (Litopenaeus vannamei). Front Microbiol 10:2337
Chen B, Peng M, Tong W, Zhang Q, Song Z (2020) The quorum quenching bacterium Bacillus licheniformis T-1 protects zebrafish against Aeromonas hydrophila infection. Probiot Antimicro Prot 12:160–171
Chen Z, Chu X, Huang X et al (2021) Fabrication of visible-light driven CoP/ZnSnO3 composite photocatalyst for high-efficient photodegradation of antibiotic pollutant. Sep Purif Technol 257:117900
Cheng TC, Yao KS, Yeh N et al (2011) Bactericidal effect of blue LED light irradiated TiO2/Fe 3O4 particles on fish pathogen in seawater. Thin Solid Films 519:5002–5006
Cheng Y, Gao D, Xia Y, Wang Z, Bai M, Luo K, Cui X, Wang Y, Zhang S, Xiao W (2021) Characterization of novel bacteriophage AhyVDH1 and its lytic activity against Aeromonas hydrophila. Curr Microbiol 78:329–337
Chettri JK, Mehrdana F, Hansen EB, Ebbensgaard A, Overgaard MT, Lauritsen AH, Dalsgaard I, Buchmann K (2017) Antimicrobial peptide CAP18 and its effect on Yersinia ruckeri infections in rainbow trout Oncorhynchus mykiss (Walbaum): comparing administration by injection and oral routes. J Fish Dis 40:97–104
Chiu C, Cheng CH, Gua WR, Guu YK, Cheng W (2010) Dietary administration of the probiotic Saccharomyces cerevisiae P13, enhanced the growth, innate immune responses, and diseases resistance of the grouper (Epinephelus coioides). Fish Shellfish Immunol 29:1053–1059
Cimica V, Galarza JM (2017) Adjuvant formulations for virus-like particle (VLP) based vaccines. Clin Immunol 183:99–108
Citarasu T (2010) Herbal biomedicines: a new opportunity for aquaculture industry. Aquacult Int 18:403–414
Citarasu T, Sivaram V, Immanuel G, Rout N, Murugan V (2006) Influence of selected Indian immunostimulant herbs against white spot syndrome virus (WSSV) infection in black tiger shrimp, Penaeus monodon with reference to haematological, biochemical and immunological changes. Fish Shellfish Immunol 21:372–384
Colorni A, Ullal A, Heinisch G, Noga EJ (2008) Activity of the antimicrobial polypeptide piscidin 2 against fish ectoparasites. J Fish Dis 31:423–432
Cotter PD, Hill C, Ross RP (2005) Bacteriocins: developing innate immunity for food. Nat Rev Microbiol 3:777–788
Cotter PD, Ross RP, Hill C (2013) Bacteriocins-a viable alternative to antibiotics? Nat Rev Microbiol 11:95–105
Cueva MD, Villena GK, Kitazono AA (2021) Efficient cloning of tilapia lake virus complementary DNAs using an in vivo strategy in baker’s yeast. J World Aquac Soc 52:1209–1220
Cui H, Zhang J, Cong C, Wang L, Li X, Murtaza B, Xu Y (2020) Complete genome analysis of the novel Edwardsiella tarda phage VB_EtaM_ET-ABTNL-9. Arch Virol 165:1241–1244
Culot A, Grosset N, Gautier M (2019) Overcoming the challenges of phage therapy for industrial aquaculture: a review. Aquaculture 513
Dadar M, Dhama K, Vakharia VN, Hoseinifar SH, Karthik K, Tiwari R, Khandia R, Munjal A, Salgado-Miranda C, Joshi SK (2016) Advances in aquaculture vaccines against fish pathogens: global status and current trends. Rev Fish Sci Aquac 25:184–217
Dalgaard M, Buchmann K, Li A (2002) Immunization of rainbow trout fry with Ichthyophthirius multifiliis sonicate: protection of host and immunological changes. Bull Eur Assoc Fish Patho l22 288297
Das S, Aswani R, Midhun SJ, Radhakrishnan EK, Mathew J (2020) Advantage of zinc oxide nanoparticles over silver nanoparticles for the management of Aeromonas veronii infection in Xiphophorus hellerii. Microb Pathog 147
Dawood MAO, Abo-Al-Ela HG, Hasan MT (2020) Modulation of transcriptomic profile in aquatic animals: probiotics, prebiotics and synbiotics scenarios. Fish Shellfish Immunol 97:268–282
Dawood MAO, Abd El-Kader MF, Farid MA, Abd-Elghany M, Alkafafy M, Van Doan H (2021) Saccharomyces cerevisiae enhanced the growth, immune and antioxidative responses of European seabass (Dicentrarchus labrax). Annals of Animal Science 2021:1–12
De la Banda IG, Lobo C, Chabrillón M, Chabrillón M, León-Rubio J, Arijo S, Pazos G, Lucas L, Moriñigo M (2012) Influence of dietary administration of a probiotic strain Shewanella putrefaciens on Senegalese sole (Solea senegalensis, Kaup 1858) growth, body composition and resistance to Photobacterium damselae subsp piscicida. Aquac Res 43:662–669
Demirbas A (2021) Comparison study of synthesized red (or blood) orange peels and juice extract-nanoflowers and their antimicrobial properties on fish pathogen (Yersinia ruckeri). Indian J Microbiol 61:324–330
Desai N (2012) Challenges in development of nanoparticle-based therapeutics. AAPS J 14:282–295
Dhar AK, Manna SK, Thomas Allnutt FC (2014) Viral vaccines for farmed finfish. Virus Dis 25:1–17
Ditria EM, Buelow CA, Gonzalez-Rivero M, Connolly RM (2022) Artificial intelligence and automated monitoring for assisting conservation of marine ecosystems: a perspective. Front Mar Sci 9:918104
Dong HT, Chaijarasphong T, Barnes AC, Delamare-Deboutteville J, Lee PA, Senapin S, Mohan CV, Tang KFJ, McGladdery SE, Bondad-Reantaso MG (2023) From the basics to emerging diagnostic technologies: what is on the horizon for tilapia disease diagnostics? Rev Aquac 15:186–212
Doszpoly A, Shaalan M, El-Matbouli M (2023) Silver nanoparticles proved to be efficient antivirals in vitro against three highly pathogenic fish viruses. Viruses 15:1689
Du WL, Niu SS, Xu YL, Xu ZR, Fan CL (2009) Antibacterial activity of chitosan tripolyphosphate nanoparticles loaded with various metal ions. Carbohyd Polym 75:385–389
Easwaran M, Dananjaya S, Park SC, Lee J, Shin H, De Zoysa M (2017) Characterization of bacteriophage PAh-1 and its protective effects on experimental infection of Aeromonas hydrophila in zebrafish (Danio Rerio). J Fish Dis 40:841–846
El Euony OI, Elblehi SS, Abdel-Latif HM, Abdel-Daim MM, El-Sayed YS (2020) Modulatory role of dietary Thymus vulgaris essential oil and Bacillus subtilis against thiamethoxam-induced hepatorenal damage, oxidative stress, and immunotoxicity in African catfish (Clarias garipenus). Environ Sci Pollut Res 27:23108–23128
El-Adawy MM, Eissa AE, Shaalan M et al (2021) Green synthesis and physical properties of gum Arabic-silver nanoparticles and its antibacterial efficacy against fish bacterial pathogens. Aquac Res 52:1247–1254
El-Adawy MM, Attia MM, Elgendy MY, Abdelsalam M, Fadel A (2023a) Development of silver nano-based indirect ELISA and Dot-ELISA methods for serological diagnosis of a bacterial fish pathogen Aeromonas veronii. J Microbiol Methods 211:106782
El-Adawy MM, Attia MM, Elgendy MY, Abdelsalam M, Fadel A (2023b) Development of silver nano-based indirect ELISA and Dot-ELISA methods for serological diagnosis of a bacterial fish pathogen Aeromonas veronii. J Microbiological Methods 211
Elgendy MY, M. Moustafa AY, Gaafar A, Borhan T, (2015a) Impacts of extreme cold water conditions and some bacterial infections on earthen-pond cultured Nile tilapia, Oreochromis niloticus. RJPBCS 6:136–145
Elgendy MY, Soliman WS, Hassan HA, Kenawy AM, Liala AM (2015b) Effect of abrupt environmental deterioration on the eruption of vibriosis in mari-cultured shrimp, Penaeus indicus, in Egypt. J Fish Aquat Sci 10:146–158
Elgendy MY, Hakim AS, Ibrahim TB, Soliman WS, Ali SE (2016) Immunomodulatory effects of curcumin on Nile tilapia, Oreochromis niloticus and its antimicrobial properties against Vibrio alginolyticus. J Fish Aquat Sci 11:206–215
Elgendy MY, Awad ES, Darwish DA, Abbas HH, Abbas WT (2021a) Investigations on the influence of Moringa oleifera on the growth, haematology, immunity and disease resistance in Oreochromis niloticus with special reference to the analysis of antioxidant activities by PAGE electrophoresis. Aquacult Res 52:4983–4995
Elgendy MY, Shaalan M, Abdelsalam M, Eissa AE, El-Adawy MM, Seida AA (2021b) Antibacterial activity of silver nanoparticles against antibiotic-resistant Aeromonas veronii infections in Nile tilapia, Oreochromis niloticus (L.), in vitro and in vivo assay. Aquacult Res 35:901–920
Elgendy MY, Abdelsalam M, Kenawy AM, Ali SE (2022a) Vibriosis outbreaks in farmed Nile tilapia (Oreochromis niloticus) caused by Vibrio mimicus and V. cholera. Aquacult Int 30:2661–2677
Elgendy MY, Abdelsalam M, Mohamed SA, Ali SE (2022b) Molecular characterization, virulence profiling, antibiotic susceptibility, and scanning electron microscopy of Flavobacterium columnare isolates retrieved from Nile tilapia (Oreochromis niloticus). Aquacult Int 30:845–862
Elgendy MY, Sherif AH, Kenawy AM, Abdelsalam M (2022c) Phenotypic and molecular characterization of the causative agents of Edwardsiellosis causing Nile tilapia (Oreochromis niloticus) summer mortalities. Microb Pathog 169:105620
Elgendy MY, Ali SE, Abbas WT, Algammal AM, Abdelsalam M (2023a) The role of marine pollution on the emergence of fish bacterial diseases. Chemosphere 344
Elgendy MY, Ali SE, Abdelsalam M, Abd El-Aziz TH, Abo-Aziza F, Osman HA, Authman MN, Abbas WT (2023b) Onion (Allium cepa) improves Nile tilapia (Oreochromis niloticus) resistance to saprolegniasis (Saprolegnia parasitica) and reduces immunosuppressive effects of cadmium. Aquacult Int 31:1457–1481
El-Rhman AM, Khattab YA, Shalaby AM (2009) Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 27:175–180
El-Saadony MT, Alagawany M, Patra AK, Kar I, Tiwari R, Dawood MAO, Abdel-Latif HMR (2021) The functionality of probiotics in aquaculture: an overview. Fish Shellfish Immunol 117:36–52
Elsabahy M, Heo GS, Lim SM, Sun G, Wooley KL (2015) Polymeric nanostructures for imaging and therapy. Chem Rev 115:10967–11011
Eraclio G, Tremblay DM, Lacelle-Côté A, Labrie SJ, Fortina MG, Moineau SA (2015) Virulent phage infecting Lactococcus garvieae, with homology to Lactococcus lactis phages. Appl Environ Microbiol 81:8358–8365
Ergena A, NatarajanP BZ (2023) Determination of some haematological parameters and disease resistance capacity to Aeromonas hydrophila infection in the Nile tilapia, Oreochromis niloticus L. fed dietary supplementation of ginger (Zingiber officinale). Egyptian Journal of Aquatic Biology & Fisheries 27:551–568
Essa MA, Mabrouk HA, Mohamed RA, Michael FR (2011) Evaluating different additive levels of yeast, Saccharomyces cerevisiae, on the growth and production performances of a hybrid of two populations of Egyptian African catfish, Clarias gariepinus. Aquaculture 320:137–141
Ezyaguirre EJ, Walker DH, Zaki S (2011) Immunohistology of Infectious Diseases Diagnostic Immunohistochemistry 2011:58–82
Falco A, Chico V, Marroqui L, Perez L, Coll JM, Estepa A (2008) Expression and antiviral activity of a beta-defensin-like peptide identified in the rainbow trout (Oncorhynchus mykiss) EST sequences. Mol Immunol 45:757–765
FAO (2022) The state of world fisheries and aquaculture 2022. Towards Blue Transformation. Rome, FAO. https://doi.org/10.4060/cc0461en
Farsani MN, Hoseinifar SH, Rashidian G, Farsani HG, Ashouri G, Doan HV (2019) Dietary effects of Coriandrum sativum extract on growth performance, physiological and innate immune responses and resistance of rainbow trout (Oncorhynchus mykiss) against Yersinia ruckeri. Fish Shellfish Immunol 91:233–240
Fathi F, Dickson C, Dickson M, Leschen W, Baily J, Muir F, Ulrich K, Weidmann M (2017) Identification of tilapia lake virus in Egypt in Nile tilapia affected by ‘summer mortality’ syndrome. Aquaculture 473:430–432
Fatima R, Priya M, Indurthi L, Radhakrishnan V, Sudhakaran R (2020) Biosynthesis of silver nanoparticles using red algae Portieria hornemannii and its antibacterial activity against fish pathogens. Microb Pathog 138(2020):103780
Feliatra F, Muchlisin ZA, Teruna HY, Utamy WR, Nursyirwani N, Dahliaty A (2018) Potential of bacteriocins produced by probiotic bacteria isolated from tiger shrimp and prawns as antibacterial to Vibrio, Pseudomonas, and Aeromonas species on fish. F1000Research 7:415
Fenaroli F, Westmoreland D, Benjaminsen J et al (2014) Nanoparticles as drug delivery system against tuberculosis in zebrafish embryos: direct visualization and treatment. ACS Nano 8:7014–7026
Fernandes AM, Abdalhai MH, Ji J, Xi B-W, Xie J, Sun J, Noeline R, Lee B, Sun X (2015) Development of highly sensitive electrochemical genosensor based on multiwalled carbon nanotubes–chitosan–bismuth and lead sulfide nanoparticles for the detection of pathogenic Aeromonas. Biosens Bioelectron 63:399–406
Fouad AM, Elkamel AA, Ibrahim S, El-Matbouli M, Soliman H, Abdallah ES (2022) Control of spring viremia of carp in common carp using RNA interference. Aquaculture 559(2022):738417
Fregeneda-Grandes J-M, González-Palacios C, Pérez-Sánchez T, Padilla D, Real F, Aller-Gancedo J-M (2023) Limited probiotic effect of Enterococcus gallinarum L1, Vagococcus fluvialis L21 and Lactobacillus plantarum CLFP3 to protect rainbow trout against saprolegniosis. Animals 13:954
Fridman S, Sinai T, Zilberg D (2014) Efficacy of garlic based treatments against monogenean parasites infecting the guppy (Poecilia reticulata Peters). Vet Parasitol 203:51–58
Fu Y, Guo SQ, Luo JJ et al (2021a) Effectiveness assessment of plant mixtures against Ichthyophthirius multifiliis in grass carp Ctenopharyngodon idella. Aquaculture 530:735742
Fu Y, Zhang YA, Shen J, Tu J (2021b) Immunogenicity study of OmpU subunit vaccine against Vibrio mimicus in yellow catfish, Pelteobagrus fulvidraco. Fish Shellfish Immunol 108:80–85
García-Contreras R, Maeda T, Wood TK (2013) Resistance to quorum-quenching compounds. Appl Environ Microbiol 79:6840–6846
Gaur VK, Sharma P, Gupta S, Varjani S, Srivastava JK, Wong JW, Ngo HH (2022) Opportunities and challenges in omics approaches for biosurfactant production and feasibility of site remediation: strategies and advancements. Environ Technol Innov 25
Gelderen RV (2007) Vaccination of Atlantic salmon (Salmo salar) against marine flexibacteriosis. University of Tasmania. Thesis. https://doi.org/10.25959/23242430.v1
Ghasemi SM, Bouzari M, Yoon BH, Chang HI (2014) Comparative genomic analysis of Lactococcus garvieae phage WP-2, a new member of Picovirinae subfamily of Podoviridae. Gene 551:222–229
Ghetas HA, Abdel-Razek N, Shakweer MS, Abotaleb M, Paray BA, Ali S, Eldessouki EA, Dawood MA, Khalil RH (2022) Antimicrobial activity of chemically and biologically synthesized silver nanoparticles against some fish pathogen. Saudi Journal of Biological Sciences 29:1298–2130
Gholipourkanani H, Buller N, Lymbery A (2019) In vitro antibacterial activity of four nano-encapsulated herbal essential oils against three bacterial fish pathogens. Aquac Res 50:871–875
Ghosh B, Nguyen TD, Crosbie PBB, Nowak BF, Bridle AR (2016) Oral vaccination of first-feeding Atlantic salmon, Salmo salar L., confers greater protection against yersiniosis than immersion vaccination. Vaccine 34:599–608
Ghuglot R, Titus W, Agnihotri A, Krishnakumar V, Krishnamoorthy G, Marimuthu N (2021) Stable copper nanoparticles as potential antibacterial agent against aquaculture pathogens and human fibroblast cell viability. Biocatal Agric Biotechnol 32
Giri SS, Sen SS, Jun JW, Sukumaran V, Park SC (2017) Role of Bacillus licheniformis VS16-derived biosurfactant in mediating immune responses in carp rohu and its application to the food industry. Front Microbiol 28:514–522
Gobeli S, Goldschmidt-Clermont E, Frey J, Burr SE (2009) Pseudomonas chlororaphis strain JF3835 reduces mortality of juvenile perch, Perca fluviatilis L., caused by Aeromonas sobria. J Fish Dis 32:597–602
Goh JX, Tan LT, Law JW, Ser HL, Khaw KY (2022) Harnessing the potentialities of probiotics, prebiotics, synbiotics, paraprobiotics, and postbiotics for shrimp farming. Rev Aquac 14:1–80
Gokulakrishnan M, Kumar R, Ferosekhan S, Siddaiah GM, Nanda S, Pillai B, Swain SK (2023) Bio-utilization of brewery waste (Brewer’s spent yeast) in global aquafeed production and its efficiency in replacing fishmeal: from a sustainability viewpoint. Aquaculture 565
Gonzalez-Stegmaier R, Pena A, Villarroel-Espindola F, Aguila P, Oliver C, MacLeod-Carey D (2021) Full recombinant flagellin b from Vibrio anguillarum (rFLA) and its recombinant D1 domain (rND1) promote a pro-inflammatory state and improve vaccination against P. salmonis in Atlantic salmon (S. salar). Dev Comp Immunol 117:1349–1358
Gopalakannan A, Arul V (2011) Inhibitory activity of probiotic Enterococcus faecium MC13 against Aeromonas hydrophila confers protection against hemorrhagic septicemia in common carp Cyprinus carpio. Aquacult Int 19:973–985.
Goto T, Hirazawa N, Takaishi Y, Kashiwada Y (2015) Antiparasitic effects of Sophora flavescens root extracts on the ciliate, Cryptocaryon irritans. Aquaculture 435:173–177
Gravningen K, Sakai M, Mishiba T, Fujimoto T (2008) The efficacy and safety of an oil-based vaccine against Photobacterium damsela subsp. piscicida in yellowtail (Seriola quinqueradiata): a field study. Fish Shellfish Immunol 24:523–529
Gu Q, Wang G, Li N, Hao D, Liu H, Wang C, Hu Y, Zhang M (2021) Evaluation of the efficacy of a novel Vibrio vulnificus vaccine based on antibacterial peptide inactivation in turbot. Scophthalmus Maximus Fish & Shellfish Immunology 118(2021):197–204
Guo JJ, Kuo CM, Chuang YC, Hong JW, Chou RL, Chen TI (2012) The effects of garlic-supplemented diets on antibacterial activity against Streptococcus iniae and on growth in orange-spotted grouper. Epinephelus Coioides, Aquaculture 365:33–38
Habimana O, Zanoni M, Vitale S, O’Neill T, Scholz D, Xu B, Casey E (2018) One particle, two targets: a combined action of functionalized gold nanoparticles, against Pseudomonas fluorescens biofilms. J Colloid and Interface Science 526:419–428
Halimi M, Alishahi M, Abbaspour M, Ghorbanpoor M, Tabandeh MR (2019) Valuable method for production of oral vaccine by using alginate and chitosan against Lactococcus garvieae/Streptococcus iniae in rainbow trout (Oncorhynchus mykiss). Fish Shellfish Immunol 90:431–439
Hamza F, Satpute S, Banpurkar A, Kumar AR, Zinjarde S (2017) Biosurfactant from a marine bacterium disrupts biofilms of pathogenic bacteria in a tropical aquaculture system. FEMS Microbiol Ecol 1(93):11
Haney EF, Hancock REW (2013) Peptide design for antimicrobial and immunomodulatory applications. Pept Sci 100:572–583
Haq IU, Chaudhry WN, Andleeb S, Qadri I (2012) Isolation and partial characterization of a virulent bacteriophage IHQ1 specific for Aeromonas punctata from stream water. Microb Ecol 63:954–963
Hardi EH, Nugroho RA, Rostika R, Mardliyaha CM, Sukarti K, Rahayu W, Supriansyah A, Saptiani G (2022) Synbiotic application to enhance growth, immune system, and disease resistance toward bacterial infection in catfish (Clarias gariepinus). Aquaculture 549
Harikrishnan R, Balasundaram C, Heo MS (2010) Effect of probiotics enriched diet on Paralichthys olivaceus infected with lymphocystis disease virus (LCDV). Fish Shellfish Immunol 29:868–874
Hashimoto GS, Neto FM, Ruiz ML, Acchile M, Chagas EC, Chaves FC, Martins ML (2016) Essential oils of Lippia sidoides and Mentha piperita against monogenean parasites and their influence on the hematology of Nile tilapia. Aquaculture 450:182–186
Hoang DM, Pham PT, Bach TQ, Ngo AT, Nguyen QT, Phan TT, Nguyen GH, Le PT, Hoang VT, Forsyth NR (2022) Signal Transduct Target Ther 7:1–41
Huang K, Ma H, Liu J et al (2012) Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 6:4483–4493
Huo X, Fan C, Ai T, Su J (2020) The combination of molecular adjuvant CCL35. 2 and DNA vaccine significantly enhances the immune protection of Carassius auratus gibelio against CyHV-2 infection. Vaccines 8:567
Hussein MA, Hassan WA, Yassen HA, Osman AM (2023) Vaccination with bacterial ghosts of Streptococcus iniae and Lactococcus garvieae originated from outbreak of marine fish streptococcosis, induce potential protection against the disease in Nile tilapia, Oreochromis niloticus (Linnaeus, 1758). Fish Shellfish Immunol 141(2023):109008
Ibrahem MD, Mohamed MF, Ibrahim MA (2013) The role of Spirulina platensis (Arthrospira platensis) in growth and immunity of Nile tilapia (Oreochromis niloticus) and its resistance to bacterial infection. J Agric Sci 5:109–117
Im K, Mareninov S, Diaz MFP, Yong WH (2019) An introduction to performing immunofluorescence staining. Methods Protocols 2019:299–311
Ingelbrecht J, Millera T, Lymberya AJ, Maitac M, Torikaic S, Partridge G (2020) Anthelmintic herbal extracts as potential prophylactics or treatments for monogenean infections in cultured yellowtail kingfish (Seriola lalandi). Aquaculture 520
Jaafar RM, Al-Jubury A, Dalsgaard I, Karami AM, Kania PW, Buchmann K (2019) Effect of oral booster vaccination of rainbow trout against Yersinia ruckeri depends on type of primary immunization. Fish Shellfish Immunol 85:61–65
Jaies I, Shah FA, Qadiri SN, Qayoom I, Bhat BA, Dar SA, Bhat FA (2024) Immunological and molecular diagnostic techniques in fish health: present and future prospectus. Mol Biol Rep 2024(51):551
Jankovic I, Sybesma W, Phothirath P, Ananta E, Mercenier A (2010) Application of probiotics in food products—challenges and new approaches. Curr Opin Biotechnol 21:175–181
Jeon JH, Jang K, Lee J, Kang L, Lee S (2023) Transmission of antibiotic resistance genes through mobile genetic elements in Acinetobacter baumannii and gene-transfer prevention. Sci Total Environ 857
Jian J, Wu Z (2003) Effects of traditional Chinese medicine on nonspecific immunity and disease resistance of large yellow croaker, Pseudosciaena crocea (Richardson). Aquaculture 218:1–9
Jiang H, Bian Q, Zeng W, Ren P, Sun H, Lin Z (2019) Oral delivery of bacillus subtilis spores expressing grass carp reovirus VP4 protein produces protection against grass carp reovirus infection. Fish Shellfish Immunol 84:768–780
Kaktcham PM, Tchamani Piame L, Sandjong Sileu GM et al (2019) Bacteriocinogenic Lactococcus lactis subsp. lactis 3MT isolated from freshwater Nile tilapia: isolation, safety traits, bacteriocin characterization, and application for biopreservation in fish pate. Arch Microbiol 201:1249–1258
Kalatzis PG, Bastías R, Kokkari C, Katharios P (2016) Isolation and characterization of two lytic bacteriophages, FSt2 and FGrn1; phage therapy application for biological control of Vibrio alginolyticus in aquaculture live feeds. PLoS ONE 11
Kamei Y, Yoshimizu M, Ezura Y, Kimura T (2008) Screening of bacteria with antiviral activity from fresh water salmonid hatcheries. Microbiol Immunol 32:67–73
Katharios P, Kalatzis PG, Kokkari C, Sarropoulou E, Middelboe M (2017) Isolation and characterization of a N4-like lytic bacteriophage infecting Vibrio splendidus, a pathogen of fish and bivalves. PLoS ONE 12
Kawato Y, Yasuike M, Nakamura Y, Shigenobu Y, Fujiwara A, Sano M, Nakai T (2015) Complete genome sequence analysis of two Pseudomonas plecoglossicida phages, potential therapeutic agents. Appl Environ Microbiol 81:874–881
Kawato Y, Istiqomah I, Gaafar AY, Hanaoka M, Ishimaru K, Yasuike M, Nishiki I, Nakamura Y, Fujiwara A, Nakai T (2020) A novel jumbo Tenacibaculum maritimum lytic phage with head-fiber-like appendages. Arch Virol 165:303–311
Khursheed S, Dutta J, Ahmad I, Rather MA, Badroo IA, Bhat TA, Ahmad I, Amin A, Shah A, Qadri T, Habib H (2023) Biogenic silver nanoparticles: synthesis, applications and challenges in food sector with special emphasis on aquaculture. Food Chem X 20:101051
Kim D, Beck BR, Heo SB et al (2013) Lactococcus lactis BFE920 activates the innate immune system of olive flounder (Paralichthys olivaceus), resulting in protection against Streptococcus iniae infection and enhancing feed efficiency and weight gain in large-scale field studies. Fish Shellfish Immunol 35:1585–1590
Kim SG, Giri SS, Yun S, Kim HJ, Kim SW, Kang JW, Han SJ, Kwon J, Jun JW, Oh WT (2020) Genomic characterization of bacteriophage PEt-SU, a novel PhiKZ-related virus infecting Edwardsiella tarda. Arch Virol 165:219–222
Kim HJ, Kim YT, Kim HB, Choi SH, Lee JH (2021) Characterization of bacteriophage VVP001 and its application for the inhibition of Vibrio vulnificus causing seafood-borne diseases. Food Microbiol 94
Kis Z, Shattock R, Shah N, Kontoravdi C (2019) Emerging technologies for low-cost, rapid vaccine manufacture. Biotechnol 14:1800376
Kitiyodom S, Yata T, Yostawornkul J, Kaewmalun S, Nittayasut N, Suktham K, Surassmo S, Namdee K, Rodkhum C, Pirarat N (2019) Enhanced efficacy of immersion vaccination in tilapia against columnaris disease by chitosan-coated pathogen-like mucoadhesive nanovaccines. Fish Shellfish Immunol 95:213–219
Korkea-Aho TL, Papadopoulou A, Heikkinen J et al (2012) Pseudomonas M162 confers protection against rainbow trout fry syndrome by stimulating immunity. J Applied Microbiology 113:24–35
Krasnodembskaya A, Song Y, Fang X, Gupta N, Serikov V, Lee JW, Matthay MA (2010) Antibacterial effect of human mesenchymal stem cells is mediated in part from secretion of the antimicrobial peptide LL-37. Stem Cells 28:2229–2238
Krishnamoorthy K, Mahalingam M (2015) Selection of a suitable method for the preparation of polymeric nanoparticles: multi-criteria decision making approach. Adv Pharm Bull 5:57–67
Krzyzek P (2019) Challenges and limitations of anti-quorum sensing therapies. Front Microbiol 10:2473
Kumar V, Guleria P (2020) Application of DNA-nanosensor for environmental monitoring: recent advances and perspectives. Curr Pollution Rep 2020:1–21
Kumar A, Raman RP, Kumar K, Pandey PK, Kumar V, Mohanty S, Kumar S (2012) Antiparasitic efficacy of piperine against Argulus spp. on Carassius auratus (Linn. 1758): in vitro and in vivo study. Parasitol Res 111:2071–2076
Kumariya R, Garsa AK, Rajput YS, Sood SK, Akhtar N, Patel S (2019) Bacteriocins: classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb Pathog 128:171–177
Kunttu HM, Runtuvuori-Salmela A, Middelboe M, Clark J, Sundberg LR (2021) Comparison of delivery methods in phage therapy against Flavobacterium columnare Infections in rainbow trout. Antibiotics 10:914
Kwon S, Kang B, Jun S, Yoon S, Lee J, Kang S (2017) Evaluating the effectiveness of Streptococcus parauberis bacteriophage Str-PAP-1 as an environmentally friendly alternative to antibiotics for aquaculture. Aquaculture 468:464–470
Laanto E, Bamford JK, Laakso J, Sundberg LR (2012) Phage-driven loss of virulence in a fish pathogenic bacterium. PLoS ONE 7
Laith AA, Abdullah MA, Nurhafizah WI, Hussein HA, Aya J, Effendy AW, Najiah M (2019) Efficacy of live attenuated vaccine derived from the Streptococcus agalactiae on the immune responses of Oreochromis niloticus. Fish Shellfish Immunol 90:235–243
Lal TM, Sano M, Hatai K, Ransangan J (2016) Complete genome sequence of a giant Vibrio phage ValKK3 infecting Vibrio alginolyticus. Genom Data 8:37–38
Lal TM, Sano M, Ransangan J (2017) Isolation and characterization of large marine bacteriophage (Myoviridae), VhKM4 infecting Vibrio harveyi. J Aquat Anim Health 29:26–30
Lategan MJ, Torpy FR, Gibson LF (2004) Control of saprolegniosis in the eel Anguilla australis Richardson, by Aeromonas media strain A199. Aquaculture 240:19–27
Latka A, Maciejewska B, Majkowska-Skrobek G, Briers Y, Drulis-Kawa Z (2017) Bacteriophage-encoded virion-associated enzymes to overcome the carbohydrate barriers during the infection process. Appl Microbiol Biotechnol 101:3103–3311
Lee NY, Ko WC, Hsueh PR (2019) Nanoparticles in the treatment of infections caused by multidrug-resistant organisms. Front Pharmacol 4(10):1153
Leskinen K, Pajunen MI, Vilanova MV, Kiljunen S, Nelson A, Smith D, Skurnik M (2020) YerA41, a Yersinia ruckeri bacteriophage: determination of a non-sequencable DNA bacteriophage genome via RNA-sequencing. Viruses 12:620
Levy G, Zilberg D, Paladini G, Fridman S (2015) Efficacy of ginger-based treatments against infection with Gyrodactylus turnbulli in the guppy (Poecilia reticulata (Peters). Vet Parasitol 209:235–241
Li X, Xu H, Chen ZS, Chen G (2011) Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater 2011:270974
Li BY, Hu Y, Li J, Shi K, Shen YF, Zhu B, Wang GX (2019) Ursolic acid from Prunella vulgaris L. efficiently inhibits IHNV infection in vitro and in vivo. Virus Res 273:197741
Li GL, Cortez MH, Dushoff J, Weitz JS (2020) When to be temperate: on the fitness benefits of lysis vs. lysogeny. Virus 6:42–49
Li D, Li X, Wang Q, Hao Y (2022a) Advanced techniques for the intelligent diagnosis of fish diseases: a review. Animals 12:2938
Li M, Wei D, Huang S, Huang L, Xu F, Yu Q, Liu M, Li P (2022b) Medicinal herbs and phytochemicals to combat pathogens in aquaculture. Aquacult Int 30:1239–1259
Li P, Huang S, Xiao S, Xu Y, Wei X, Xiao J, Guo Z, Yu Q, Liu M (2022c) Antiviral activities of green tea components against grouper Iridovirus infection in vitro and in vivo. Viruses 5(14):1227
Lim J, Jang Y, Han HJ, Hong S (2023) Molecular mechanisms of the virulence and efficacy of a highly virulent Vibrio anguillarum strain and its formalin-inactivated vaccine in rainbow trout. J Fish Dis 46:563–574
Lin P, Xu M, Yang Q, Chen M, Guo S (2023) Inoculation of Freund’s adjuvant in European eel (Anguilla anguilla) revealed key KEGG pathways and DEGs of host anti-Edwardsiella anguillarum infection. Fish Shellfish Immunol 136
Liu F, Tang X, Sheng X, Xing J, Zhan W (2016a) DNA vaccine encoding molecular chaperone GroEL of Edwardsiella tarda confers protective efficacy against Edwardsiellosis. Mol Immunol 79:55–65
Liu G, Zhu J, Chen K, Gao T, Yao H, Liu Y (2016b) Development of Streptococcus agalactiae vaccines for tilapia. Dis Aquat Organ 122:163–170
Liu F, Tang X, Sheng X, Xing J, Zhan W (2017a) Comparative study of the vaccine potential of six outer membrane proteins of Edwardsiella tarda and the immune responses of flounder (Paralichthys olivaceus) after vaccination. Vet Immunol Immunopathol 185:38–47
Liu Y, Zhang Q, Xu D, Fu Y, Lin D, Zhou S (2017b) Antiparasitic efficacy of commercial curcumin against Ichthyophthirius multifiliis in grass carp (Ctenopharyngodon idellus). Aquaculture 480:65–70
Liu X, Jiao C, Ma Y, Wang Q, Zhang Y (2018) A live attenuated Vibrio anguillarum vaccine induces efficient immunoprotection in tiger puffer (Takifugu rubripes). Vaccine 36:1460–1466
Liu L, Lu DQ, Xu J, Luo HL, Li AX (2019) Development of attenuated erythromycin-resistant streptococcus agalactiae vaccine for tilapia (Oreochromis niloticus) culture. J Fish Dis 42:693–701
Liu X, Xiao H, Chao J, Jian S, Wu X, Lu J, Wang J, Chen C, Liu Y (2023) Polyvalent passive vaccine candidates from egg yolk antibodies (IgY) of important outer membrane proteins (PF1380 and ExbB) of Pseudomonas fluorescens in fish. Fish Shellfish Immunol 143
Luis AIS, Campos EVR, De Oliveira JL, Guilger-Casagrande M, de Lima R, Castanha R, de Castro V, Fraceto L (2020) Zein nanoparticles impregnated with eugenol and garlic essential oils for treating fish pathogens. ACS Omega 5:15557–15566
Lund M, Rosag MV, Krasnov A, Timmerhaus G, Nyman IB, Aspehaug V (2016) Experimental Piscine orthoreovirus infection mediates protection against pancreas disease in Atlantic salmon (Salmo salar). Vet Res 47:107–117
Luo X, Liao G, Liu C, Jiang X, Lin M, Zhao C, Tao J, Huang Z (2018) Characterization of bacteriophage HN 48 and its protective effects in Nile tilapia Oreochromis niloticus against Streptococcus agalactiae infections. J Fish Dis 41:1477–1484
Ma J, Bruce TJ, Jones EM (2019) Cain KD (2019) A review of fish vaccine development strategies: conventional methods and modern biotechnological approaches. Microorganisms 7:569
Mabey T, Andrea Cristaldi D, Oyston P, Lymer KP, Stulz E, Wilks S, William Keevil C, Zhang X (2019) Bacteria and nanosilver: the quest for optimal production. Crit Rev Biotechnol 39:272–287
Madhuri S, Mandloi A, Govind P, Sahni Y (2012) Antimicrobial activity of some medicinal plants against fish pathogens. International Research Journal of Pharmacy 3(4):28–33
Madsen L, Bertelsen SK, Dalsgaard I, Middelboe M (2013) Dispersal and survival of Flavobacterium psychrophilum phages in vivo in rainbow trout and in vitro under laboratory conditions: implications for their use in phage therapy. Appl Environ Microbiol 79:4853–4861
Magaki S, Hojat SA, Wei B, So A, Yong WH (2019) An introduction to the performance of immunohistochemistry. Methods Mol Biol 1897:289–298
Mahendran R, Jeyabaskar S, Sitharaman G, Michael RD, Paul AV (2016) Computer-aided vaccine designing approach against fish pathogens Edwardsiella tarda and Flavobacterium columnare using bioinformatics softwares. Drug Des Dev Ther 10:1703–1714
Mai T, Toullec J, Van Wynsberge S, Besson M, Soulet S, Petek S, Aliotti E, Ekins M, Hall K, Erpenbeck D, Lecchini D, Beniddir MA, Saulnier D, Debitus C (2019) Potential of fascaplysin and palauolide from Fascaplysinopsis cf reticulata to reduce the risk of bacterial infection in fish farming. Fish Aquatic Sci 22:30–37
Mai TT, Kayansamruaj P, Soontara C, Kerddee P, Nguyen D-H, Senapin S, Costa JZ, del-Pozo J, Thompson KD, Rodkhum C (2022) Immunization of Nile tilapia (Oreochromis niloticus) broodstock with tilapia lake virus (TiLV) inactivated vaccines elicits protective antibody and passive maternal antibody transfer. Vaccines 10:167
Malaczewska J, Kaczorek-Lukowska E (2021) Nisin-alantibiotic with immunomodulatory properties: a review. Peptides 2021:137
Malheiros DF, Maciel PO, Videira MN, Tavares-Dias M (2016) Toxicity of the essential oil of Mentha piperita in Arapaima gigas (pirarucu) and antiparasitic effects on Dawestrema spp. (Monogenea). Aquaculture 455:81–86
Malheiros DF, Sarquis IR, Ferreira IM, Delgado Mathews P, Mertins O, Tavares-Dias M (2020) Nanoemulsions with oleoresin of Copaifera reticulata Leguminosae) improve anthelmintic efficacy in the control of monogenean parasites when compared to oleoresin without nanoformulation. J Fish Dis 43:687–695
Mandal A, Ghosh AR (2023) Role of artificial intelligence (AI) in fish growth and health status monitoring: a review on sustainable aquaculture. Aquacult Int 32:1–30
Manning TS, Gibson GR (2004) Prebiotics. Best Prac Res. Clin Gastroenterol 18:287–298
Mansouri-Tehrani HA, Keyhanfar M, Behbahani M, Dini G (2021) Synthesis and characterization of algae-coated selenium nanoparticles as a novel antibacterial agent against Vibrio harveyi, a Penaeus vannamei pathogen. Aquaculture 534:736260
Marsella A, Pascoli F, Pretto T, Buratin A, Biasini L, Abbadi M, Cortinovis L, Berto P, Manfrin A, Vanelli M, Perulli S, Rasmussen JS, Sepúlveda D, Vendramin N, Lorenzen N (2022) Toffan A (2022) Efficacy of DNA vaccines in protecting rainbow trout against VHS and IHN under intensive farming conditions. Vaccines (basel) 10:2062
Matamp N, Bhat S (2019) Phage endolysins as potential antimicrobials against multidrug resistant Vibrio alginolyticus and Vibrio parahaemolyticus: current status of research and challenges ahead. Microorganisms 7:84
Mathur MD, Vidhani S, Mehndiratta PL (2003) Bacteriophage therapy: an alternative to conventional antibiotics. J the Association of Physicians of India 51:593–596
Micol V, Caturla N, Pérez-Fons L, Más V, Pérez L, Estepa A (2005) The olive leaf extract exhibits antiviral activity against viral haemorrhagic septicaemia rhabdovirus (VHSV). Antivir Res 66:129–136
Middelboe M, Holmfeldt K, Riemann L, Nybroe O, Haaber J (2009) Bacteriophages drive strain diversification in a marine Flavobacterium: implications for phage resistance and physiological properties. Environ Microbiol 11:1971–1982
Minor KL, Anderson VL, Davis KS, Van Den Berg AH, Christie JS, Löbach L, Faruk AR, Wawra S, Secombes C, West PV (2014) A putative serine protease, SpSsp1, from Saprolegnia parasitica is recognised by sera of rainbow trout, Oncorhynchus mykiss. Fungal Biol 118:630–639
Modak B, Sandino AM, Arata L, Cárdenas-Jirón G, Torres R (2010) Inhibitory effect of aromatic geranyl derivatives isolated from Heliotropium filifolium on infectious pancreatic necrosis virus replication. Vet Microbiol 141:53–58
Mohamad A, Zamri-Saad M, Amal MNA, Al-saari N, Monir MS, Chin YK, Yasin Md (2021) Vaccine efficacy of a newly developed feed-based whole-cell polyvalent vaccine against vibriosis, streptococcosis and motile aeromonad septicemia in Asian Seabass. Lates Calcarifer Vaccines 9:368
Mohammadi G, Rafiee G, Tavabe KR, Abdel-Latif HMR, Dawood MAO (2021) The enrichment of diet with beneficial bacteria (single- or multi-strain) in biofloc system enhanced the water quality, growth performance, immune responses, and disease resistance of Nile tilapia (Oreochromis niloticus). Aquaculture 539:736640
Mohammed H, Olivares-Fuster O, La Frentz S, Arias CR (2013) New attenuated vaccine against columnaris disease in fish: choosing the right parental strain is critical for vaccine efficacy. Vaccine 31:5276–5280
Mohammed MA, Syeda JTM, Wasan KM, Wasan EK (2017) An overview of chitosan nanoparticles and its application in non-parenteral drug delivery. Pharmaceutics 9:53–60
Moitra P, Alafeef M, Dighe K, Frieman M, Pan D (2020) Selective naked-eye detection of SARS-CoV-2 mediated by N gene targeted antisense oligonucleotide capped plasmonic nanoparticles. ACS Nano 14:7617–7627
Mondal H, Thomas J (2022) A review on the recent advances and application of vaccines against fish pathogens in aquaculture. Aquacult Int 30:1971–2000
Monir MS, Yusoff MSM, Zamri-Saad M, Amal MNA, Mohamad A, Azzam-Sayuti M, Ina-Salwany MY (2022) Effect of an oral bivalent vaccine on immune response and immune gene profiling in vaccinated red tilapia (Oreochromis spp.) during infections with Streptococcus iniae and Aeromonas hydrophila. Biology 11:1268
Mostafa AA, Al-Askar AA, Taha Yassin M (2020) Anti-saprolegnia potency of some plant extracts against Saprolegnia diclina, the causative agent of saprolengiasis. Saudi J Biol Sci 27:1482–1487
Motegi SI, Ishikawa O (2017) Mesenchymal stem cells: the roles and functions in cutaneous wound healing and tumor growth. J Dermatol Sci 86:83–89
Moustafa M, Eissa AE, Laila AM, Gaafar AY, Abumourad IMK, Elgendy MY (2014) Mass mortalities in mari-cultured European sea bass (Dicentrarchus labrax) at Northern Egypt Res J Pharmaceut Biol Chem Sci 5, 95–109
Moustafa M, Eissa AE, Laila AM, Gaafar AY, Abumourad IM, Elgendy MY (2015) Investigations into the potential causes of mass kills in mari-cultured gilthead sea bream, (Sparus aurata) at Northern Egypt. Res J Pharmaceut Biol Chem Sci 6:466–477
Munangandu HM, Evensen Ø (2019) Correlates of protective immunity for fish vaccines. Fish Shellfish Immunol 85:132–140
Muniruzzaman M, Chowdhury MB (2008) Evaluation of medicinal plants through fish feed against bacterial fish disease. Progress Agric 19:151–159
Muñoz-Atienza E, Diaz-Rosales P, Tafalla C (2021) Systemic and mucosal B and T cell responses upon mucosal vaccination of teleost fish. Front Immunol 11:622377
Myhr AI, Myskja BK (2011) Precaution or integrated responsibility approach to nanovaccines in fish farming? A critical appraisal of the UNESCO precautionary principle. NanoEthics 51:73–86
Naguib M, Mahmoud UM, Mekkawy IA, Sayed AEDH (2020) Hepatotoxic effects of silver nanoparticles on Clarias gariepinus; biochemical, histopathological, and histochemical studies. Toxicol Reports 7:133–141
Naiel MA, Ismael NE, Shehata SA (2019) Ameliorative effect of diets supplemented with rosemary (Rosmarinus officinalis) on aflatoxin B1 toxicity in terms of the performance, liver histopathology, immunity and antioxidant activity of Nile tilapia (Oreochromis niloticus). Aquaculture 511:734264
Najafi M, Zamini AA (2013) Comparative analysis of antifungal properties of Zataria multiflora Boiss, Eucalyptus spp. essence and malachite green on eggs of kutum (Rutilus frisii Kutum). Advances in Biological Research 7:163–168
Nakai T, Park S (2002) Bacteriophage therapy of infectious diseases in aquaculture. Res Microbiol 153:13–18
Natnan ME, Low CF, Chong CM, Bunawan H, Baharum SN (2021) Integration of omics tools for understanding the fish immune response due to microbial challenge. Front Mar Sci 8:668771
Neamat-Allah ANF, Mahmoud EA, El Hakim YA (2019) Efficacy of dietary nano-selenium on growth, immune response, antioxidant, transcriptomic profile and resistance of Nile tilapia, Oreochromis niloticus against Streptococcus iniae infection. Fish Shellfish Immunol 94:280–287
Németh K, Leelahavanichkul A, Yuen PS, Mayer B, Parmelee A, Doi K, Robey PG, Leelahavanichkul K, Koller BH, Brown JM (2009) Bone marrow stromal cells attenuate sepsis via prostaglandin E2–dependent reprogramming of host macrophages to increase their interleukin-10 production. Nature medicine 15:42–49
Newaj-Fyzul A, Austin B (2015) Probiotics, immunostimulants, plant products and oral vaccines, and their role as feed supplements in the control of bacterial fish diseases. J Fish Dis 38:937–955
Ngoc Th, Oanh DH, Duyen LM, Xuan TT, Hoang HA, Nga LP (2023) Bacteriophage PVN06 protected catfish Pangasianodon hypophthalmus from Edwardsiella ictaluri infection. Journal of Microorganism Control 28:57–64
Nguyen HT, Nguyen TTT, Chen YC, Vu-Khac H, Wang PC, Chen SC (2018a) Enhanced immune responses and effectiveness of refined outer membrane protein vaccines against Vibrio harveyi in orange-spotted grouper (Epinephelus coioides). J Fish Dis 41:1349–1358
Nguyen TD, Crosbie PBB, Nowak BF, Bridle AR (2018b) The effects of inactivation methods of Yersinia ruckeri on the efficacy of single dip vaccination in Atlantic salmon (Salmo salar). J Fish Dis 41:1173–1176
Nikapitiya C, Dananjaya SHS, De Silva BCJ, Heo GJ, Oh C, De Zoysa M, Lee J (2018) Chitosan nanoparticles: a positive immune response modulator as display in zebrafish larvae against Aeromonas hydrophila infection. Fish Shellfish Immunol 76:240–246
Nikapitiya C, Chandrarathna H, Dananjaya S, De Zoysa M, Lee J (2020) Isolation and characterization of phage (ETP-1) specific to multidrug resistant pathogenic Edwardsiella tarda and its in vivo biocontrol efficacy in zebrafish (Danio rerio). Biologicals 63:14–23
Niska K, Korkea-aho T, Lindfors E, Kiuru T, Tuomainen M, Taskinen J, Peltonen K (2009) Disappearance of malachite green residues in fry of rainbow trout (Oncorhynchus mykiss) after treatment of eggs at the hatching stage. Aquaculture 297:25–30
Nizio DAC, Fujimoto RY, Maria AN, Carneiro PCF, França CC, Sousa NC, Brito FA, Sampaio TS, Arrigoni-Blank MF, Blank AF (2018) Essential oils of Varronia curassavica accessions have different activity against white spot disease in freshwater fish. Parasitol Res 117:97–105
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:63–63
Nuidate T, Kuaphiriyakul A, Surachat K, Mittraparp-Arthorn P (2021) Induction and genome analysis of HY01, a newly reported prophage from an emerging shrimp pathogen Vibrio campbellii. Microorganisms 9:400
O’Brien T, Barry FP (2009) In stem cell therapy and regenerative medicine. Mayo Clinic Proceedings, Elsevier 2009:859–861
Ochoa-Meza A, Alvarez-Sanchez A, Romo-Quinonez C, Barraza A, Magallon-Barajas F, Chavez-Sanchez A, Garcia-Ramos J, Toledano-Magana Y, Bogdanchikova N, Pestryakov A (2019) Silver nanoparticles enhance survival of white spot syndrome virus infected Penaeus vannamei shrimps by activation of its immunological system. Fish Shellfish Immunol 84:1083–1089
Ohnuma S, Katagiri T, Maita M, Endo M, Futami K (2017) Application of tissue clearing techniques to 3d study of infectious disease pathology in fish. Fish Pathol 52:96–99
Okeke MI, Iroegbu CU, Jideofor CO, Okoli A, Esimone CO (2001) Anti-microbial activity of ethanol extracts of two indigenous Nigerian spices. J Herbs Spices Med Plants 8:39–48
Onarinde BA, Dixon RA (2018) Prospects for biocontrol of Vibrio parahaemolyticus contamination in blue mussels (Mytilus edulus) a year-long study. Front Microbiol 9:1043
Ooyama T, Hirokawa Y, Minami1 T, Yasuda H, Nakai T, Endo M, Ruangpan L, Yoshida T (2002) Cell-surface properties of Lactococcus garvieae strains and their immunogenicity in the yellowtail Seriola quinqueradiata. Dis Aquat Org 51:169–177
Osman KM, Mohamed LA, Rahman EHA, Soliman WS (2009) Trials for vaccination of tilapia fish against Aeromonas and Pseudomonas infections using monovalent, bivalent and polyvalent vaccines. World J Fish Mar Sci 1:297–304
Pal D (2023) Anti-viral metabolites from medicinal plants. Springer Nature, Switzerland
Pan X, Wu T, Song Z, Tang H, Zhao Z (2008) Immune responses and enhanced disease resistance in Chinese drum, Miichthys miiuy (Basilewsky), after oral administration of live or dead cells of Clostridium butyricum CB2. J Fish Dis 31:679–686
Paopradit P, Aksonkird T, Mittraparp-arthorn P (2022) Indole inhibits quorum sensing-dependent phenotypes and virulence of acute hepatopancreatic necrosis disease-causing Vibrio parahaemolyticus. Aquac Res 53:3586–3597
Parenrengi A, Tenriulo A, Suryati E, Rosmiati R, Lante S, Azis AA (2022) Application of dsRNA VP15-WSSV by oral vaccination to increase survival rate and response immunes of tiger shrimp Penaeus monodon. Indian J Anim Res 56:893–898
Park J, Wendt M, Heo G (2016) Antimicrobial activity of essential oil of Eucalyptus globulus against fish pathogenic bacteria. Laboratory Animal Research 32:87
Park SC, Shimamura I, Fukunaga M, Mori KI, Nakai T (2000) Isolation of bacteriophages specific to a fish pathogen, Pseudomonas plecoglossicida, as a candidate for disease control. Appl Environ Microbiol 66:1416–1422
Patil JR, Desai SN, Roy P, Durgaiah M, Saravanan RS, Vipra A (2014) Simulated hatchery system to assess bacteriophage efficacy against Vibrio harveyi. Dis Aquat Org 112:113–119
Peng K-C, Lee S-H, Hour A-L, Pan C-Y, Lee L-H, Chen J-Y (2012) Five different piscidins from Nile tilapia, Oreochromis niloticus: analysis of their expressions and biological functions. PLoS ONE 7:e50263
Pereira WA, Piazentin AC, de Oliveira RC et al (2022) Bacteriocinogenic probiotic bacteria isolated from an aquatic environment inhibit the growth of food and fish pathogens. Sci Rep 12:5530
Pérez MG, Moll CN, Espinosa GM, López AV (2016) Evaluation of different Mediterranean essential oils as prophylactic agents in anisakidosis. Pharm Biol 55:456–461
Pérez-Cobas AE, Gomez-Valero L, Buchrieser C (2020) Metagenomic approaches in microbial ecology: an update on whole-genome and marker gene sequencing analyses. Microb Genom 6(8):mgen000409
Petrosino JF, Highlander S, Luna RA, Gibbs RA, Versalovic J (2009) Metagenomic pyrosequencing and microbial identification. Clin Chem 55:856–866
Pieters N, Brunt J, Austin B, Lyndon AR (2008) Efficacy of in-feed probiotics against Aeromonas bestiarum and Ichthyophthirius multifiliis skin infections in rainbow trout (Oncorhynchus mykiss, Walbaum). J Appl Microb 105:723–732
Pimentel-Acosta CA, Morales-Serna FN, Chávez-Sánchez MC, Lara HH, Pestryakov A, Bogdanchikova N, Fajer-Ávila EJ (2019) Efficacy of silver nanoparticles against the adults and eggs of monogenean parasites of fish. Parasitol Res 118:1741–1749
Pina R, Santos-Díaz AI, Orta-Salazar E, Aguilar-Vazquez AR, Mantellero CA, Acosta-Galeana I, Estrada-Mondragon A, Prior-Gonzalez M, Martinez-Cruz JI, Rosas-Arellano A (2022) Ten approaches that improve immunostaining: a review of the latest advances for the optimization of immunofluorescence. Int J Mol Sci 23(3):1426
Pirarat N, Kobayashi T, Katagiri T, Maita M, Endo M (2006) Protective effects and mechanisms of a probiotic bacterium Lactobacillus rhamnosus against experimental Edwardsiella tarda infection in tilapia (Oreochromis niloticus). Vet Immunol Immunopathol 113:339–347
Prabhakar KV, Lingala VK, Peelei A, Mikkili I, Venkateswarulu TC, Dulla JB (2013) Biosynthesis and potential application of bacteriocins. Pure and Applied Microbiology 7:2–13
Prasad Y, Kumar D, Sharma A (2011) Lytic bacteriophages specific to Flavobacterium columnare rescue catfish, Clarias batrachus (Linn.) from columnaris disease. J Environ Biol 32:161–168
Puvanendran V, Rud I, Breiland MS, Arnesen JA, Axelsson L (2021) Probiotic Carnobacterium divergens increase growth parameters and disease resistance in farmed Atlantic cod (Gadus morhua) larvae without influencing the microbiota. Aquaculture 532(2021):736072
Qin D (2019) Next-generation sequencing and its clinical application. Cancer Biology Med 16:4
Radwan M, Abbas MMM, Afifi MAM, Mohammadein A, Al Malki JS (2022a) Fish parasites and heavy metals relationship in wild and cultivated fish as potential health risk assessment in Egypt. Front Environ Sci 10:890039
Radwan M, Abbas MMM, Mohammadein A, Al Malki JS, Elraey SMA, Magdy M (2022b) Growth performance, immune response, antioxidative status, and antiparasitic and antibacterial capacity of the Nile tilapia (Oreochromis niloticus) after dietary supplementation with bottle gourd (Lagenaria siceraria, Molina) seed powder. Front Mar Sci 9:901439
Raida MK, Larsen JL, Nielsen ME, Buchmann K (2003) Enhanced resistance of rainbow trout, Oncorhynchus mykiss (Walbaum), against Yersinia ruckeri challenge following oral administration of Bacillus subtilis and B. licheniformis (BioPlus2B). J Fish Dis 26:495–498
Rajeswari V, Priyadarshini SK, Saranya V, Suguna P, Shenbagarathai R (2016) Immunostimulation by phospholipopeptide biosurfactant from Staphylococcus hominis in Oreochromis mossambicus. Fish Shellfish Immunol 48:244–253
Ramos-Espinoza FC, Cueva-Quiroz VA, Yunis-Aguinaga J, de Moraes JR (2020) A comparison of novel inactivation methods for production of a vaccine against Streptococcus agalactiae in Nile tilapia. Oreochromis Niloticus Aquaculture 528
Rather MA, Bhat IA, Sharma N, Gora A, Ganie PA, Sharma R (2017) Synthesis and characterization of Azadirachta indica constructed silver nanoparticles and their immunomodulatory activity in fish. Aquac Res 48:3742–3754
Rattanachaikunsopon P, Phumkhachorn P (2010) Use of Asiatic pennywort Centella asiatica aqueous extract as a bath treatment to control columnaris in Nile tilapia. J Aquatic Animal Health 22:14–20
Reen FJ, Mooij MJ, Holcombe LJ, Mcsweeney CM, Mcglacken GP, Morrissey JP (2011) The Pseudomonas quinolone signal (PQS), and its precursor HHQ, modulate interspecies and interkingdom behaviour. FEMS Microbiol Ecol 77:413–428
Reina JC, P´erez-Victoria I, Martín J, Llamas I, (2019) A quorum-sensing inhibitor strain of Vibrio alginolyticus blocks Qs-controlled phenotypes in Chromobacterium violaceum and Pseudomonas aeruginosa. Mar Drugs 17:494–503
Reina JC, Romero M, Salto R, C´amara M, Llamas I (2021) AhaP, A quorum quenching acylase from Psychrobacter sp. M9–54–1 that attenuates Pseudomonas aeruginosa and Vibrio coralliilyticus virulence. Mar Drugs 19:16
Ren Z, Zheng X, Yang H, Zhang Q, Liu X, Zhang X, Yang S, Xu F, Yang J (2020) Human umbilical-cord mesenchymal stem cells inhibit bacterial growth and alleviate antibiotic resistance in neonatal imipenem-resistant Pseudomonas aeruginosa infection. Innate Immun 26:215–221
Ren L, Zhang S, Ma Z, Qiu Y, Ying D, Jia J, Shao J, He Y (2022) Antibiotics separation from saline wastewater by nanofiltration membrane based on tannic acid-ferric ions coordination complexes. Desalination 541(2022):116034
Reyes-Becerril M, Ruvalcaba F, Sanchez V, López MG, Silva-Jara J, Hernandez-Adame L, CAngulo, (2021) Green synthesis of gold nanoparticles using Turnera diffusa Willd enhanced antimicrobial properties and immune response in longfin yellowtail leukocytes. Aquac Res 52:3391–3402
Rigano MM, Manna C, Giulini A, Vitale A, Cardi T (2009) Plants as biofactories for the production of subunit vaccines against bio-security-related bacteria and viruses. Vaccine 27:3463–3466
Robertsen B, Chang CJ, Bratland L (2016) IFN-adjuvanted DNA vaccine against infectious salmon anemia virus: antibody kinetics and longevity of IFN expression. Fish Shellfish Immunol 54:328–332
Robertson PA, O’Dowd C, Burrells C, Williams P, Austin B (2000) Use of Carnobacterium sp. as a probiotic for Atlantic salmon (Salmo salar L.) and rainbow trout (Oncorhynchus mykiss Walbaum). Aquaculture 185:235–243
Rodriguez-Estrada U, Satoh S, Haga Y, Fushimi H, Sweetman J (2009) Effects of single and combined supplementation of Enterococcus faecalis, mannan oligosaccharide and polyhydrobutyric acid on growth performance and immune response of rainbow trout Oncorhynchus mykiss. Aquaculture Science 57:609–617
Rodriguez-Lopez L, Rincon-Fontan M, Vecino X, Cruz JM, Moldes AB (2019) Preservative and irritant capacity of biosurfactants from different sources: a comparative study. J Pharm Sci 108:2296–2304
Rørbo N, Rønneseth A, Kalatzis PG (2018) Exploring the effect of phage therapy in preventing Vibrio anguillarum infections in cod and turbot larvae. Antibiotics 7:42
Rout SS, de Grahl I, Yu X, Reumann S (2022) Production of a viral surface protein in Nannochloropsis oceanica for fish vaccination against infectious pancreatic necrosis virus. Appl Microbiol Biotechnol 106:6535–6549
Ruangsri J, Kitani Y, Kiron V, Lokesh J, Brinchmann MF, Karlsen BO, Fernandes JMO (2013) A novel beta-defensin antimicrobial peptide in Atlantic cod with stimulatory effect on phagocytic activity. PLoS ONE 8(4):e62302
Saeidi MR, Adel M, Caipang CM, Dawood MA (2017) Immunological responses and disease resistance of rainbow trout (Oncorhynchus mykiss) juveniles following dietary administration of stinging nettle (Urtica dioica). Fish Shellfish Immunol 71:230–238
Saffari S, Keyvanshokooh S, Zakeri M, Johari SA, Pasha-Zanoosi H, Mozanzadeh MT (2018) Effects of dietary organic, inorganic, and nanoparticulate selenium sources on growth, hemato-immunological, and serum biochemical parameters of common carp (Cyprinus carpio) Fish Physiol. Biochem 44, 1087-1097
Saha M, Bandyopadhyay PK (2019) Green biosynthesis of silver nanoparticle using garlic, Allium sativum with reference to its antimicrobial activity against the pathogenic strain of Bacillus sp. and Pseudomonas sp. infecting goldfish. Carassius Auratus Proc Zool Soc 72:180–186
Saleh M, Abdel-Baki AA, Dkhil MA, El-Matbouli M, Al-Quraishy S (2017) Antiprotozoal effects of metal nanoparticles against Ichthyophthirius multifiliis. Parasitology 144:1802–1810
Salem SS (2023a) A mini review on green nanotechnology and its development in biological effects. Archives of Microbiol 205:128
Salem SS (2023b) A comprehensive review of nanomaterials: types, synthesis, characterization, and applications. Biointerface Res Appl Chem 13(1):41
Salem SS, Fouda A (2021) Green synthesis of metallic nanoparticles and their prospective biotechnological applications: an overview. Biol Trace Elem Res 199:344–370
Salini R, Santhakumari S, Veera Ravi A, Karutha PS (2019) Synergistic antibiofilm efficacy of undecanoic acid and auxins against quorum sensing mediated biofilm formation of luminescent Vibrio harveyi. Aquaculture 498:162–170
Salma U, Shafiujjaman M, Al Zahid M, Faruque MH, Habibullah-Al-Mamun M, Hossain A (2022) Widespread use of antibiotics, pesticides, and other aqua-chemicals in finfish aquaculture in Rajshahi district of Bangladesh. Sustainability 14:17038
Santhakumari S, Jayakumar R, Logalakshmi R, Prabhu NM, Abdul Nazar AK, Karutha Pandian S, Veera Ravi A (2018) In vitro and in vivo effect of 2,6-Di-tert-butyl-4-methylphenol as an antibiofilm agent against quorum sensing mediated biofilm formation of Vibrio spp. Int J Food Microbiol 281:60–71
Satam H, Joshi K, Mangrolia U, Waghoo S, Zaidi G, Rawool S, Thakare RP, Banday S, Mishra AK, Das G, Malonia SK (2023) Next-generation sequencing technology: current trends and advancements. Biology (basel) 12:997
Sathishkumar M, Forbes E, Sneha K, Yun Y (2009) Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Int J Mater Sci 73:332–338
Sequeiros C, Garcés ME, Vallejo M, Marguet ER, Olivera NL (2015) Potential aquaculture probiont Lactococcus lactis TW34 produces nisin Z and inhibits the fish pathogen Lactococcus garvieae. Arch Microbiol 197:449–458
Shaalan M, Sellyei B, El-Matbouli M, Székely C (2020) Efficacy of silver nanoparticles to control flavobacteriosis caused by Flavobacterium johnsoniae in common carp, Cyprinus carpio. Dis Aquat Organ 137:175–183
Shaheer P, Sreejith VN, Joseph TC, Murugadas V, Lalitha KV (2021) Quorum quenching Bacillus spp.: an alternative biocontrol agent for Vibrio harveyi infection in aquaculture. Dis Aquat Organ 146:117–128
Shahin K, Shinn AP, Metselaar M, Ramirez-Paredes JG, Monaghan SJ, Thompson KD (2019) Efficacy of an inactivated whole-cell injection vaccine for Nile tilapia, Oreochromis niloticus (L), against multiple isolates of Francisella noatunensis subsp. orientalis from diverse geographical regions. Fish Shellfish Immunol 89:486–553
Shahin K, Pirezan F, Rogge M, LaFrentz BR, Shrestha RP, Hildebrand M (2020) Development of IglC and GroEL recombinant vaccines for Francisellosis in Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 105:341–349
Sharma M, Dixit A (2016) Immune response characterization and vaccine potential of a recombinant chimera comprising B-cell epitope of Aeromonas hydrophila outer membrane protein c and LTB. Vaccine 34:6259–6266
Silva RO, Copatti CE, Pereira GA, Macedo JS, de Souza AM, Dutra LM, Almeida JR, Reste G, JB Melo (2024) Promotion of growth and resistance against Aeromonas hydrophila in Nile tilapia juveniles supplemented with Citrus limon extract. Aquaculture 578
Simora RMC, Wang W, Coogan M, El Husseini N, Terhune JS, Dunham RA (2021) Effectiveness of cathelicidin antimicrobial peptide against ictalurid catfish bacterial pathogens. Aquat Anim Health 33:178–189
Singh J, Dutta T, Kim KH (2018) Green synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol 16:1–24
Singh T, Choudhary P and Singh S (2022) Antimicrobial peptides: mechanism of action. Insights on antimicrobial peptides. IntechOpen. https://doi.org/10.5772/intechopen.99190
Sivaram V, Babu M, Immanuel G, Murugadass S, Citarasu T, Marian M (2004) Growth and immune response of juvenile greasy groupers (Epinephelus tauvina) fed with herbal antibacterial active principle supplemented diets against Vibrio harveyi infections. Aquaculture 237:9–20
Sohn H, Kwon H, Lee S, Wan Q, Lee J (2023) Development of a trivalent vaccine for prevention of co-infection by Miamiensis avidus and Tenacibaculum maritimum in farmed olive flounder. Fish Aquat Sci 26:605–616
Soković MD, Vukojević J, Marin PD, Brkić DD, Vajs V, van Griensven LJ (2009) Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 7(14):238–249
Soliman WS, Shaapan RM, Mohamed LA, Gayed SSR (2019) Recent biocontrol measures for fish bacterial diseases, in particular to probiotics, bio-encapsulated vaccines, and phage therapy. Open Vet J 9:190–195
Son VM, Chang CC, Wu MC, Guu YK, Chiu CH, Cheng W (2009) Dietary administration of the probiotic, Lactobacillus plantarum, enhanced the growth, innate immune responses, and disease resistance of the grouper Epinephelus coioides. Fish Shellfish Immun 26:691–698
Sorroza L, Padilla D, Acosta F et al (2012) Characterization of the probiotic strain Vagococcus fluvialis in the protection of European sea bass (Dicentrarchus labrax) against vibriosis by Vibrio anguillarum. Vet Microbiol 155:369–373
Soto E, Wiles J, Elzer P, Macaluso K, Hawke JP (2011) Attenuated Francisella asiatica iglC mutant induces protective immunity to Francisellosis in tilapia. Vaccine 29:593–598
Stalin N, Srinivasan P (2017) Efficacy of potential phage cocktails against Vibrio harveyi and closely related Vibrio species isolated from shrimp aquaculture environment in the south east coast of India. Vet Microbiol 207:83–96
Su FJ, Chen MM (2021) Protective efficacy of novel oral biofilm vaccines against Lactococcus garvieae infection in mullet. Mugil Cephalus Vaccines 9:844
Su FJ, Chen MM (2021) Protective efficacy of novel oral biofilm vaccines against Lactococcus garvieae infection in mullet. Mugil Cephalus Vaccines 9(8):844
Su FJ, Chen MM (2022) Protective efficacy of novel oral biofilm vaccines against Photobacterium damselae subsp. damselae infection in giant grouper, Epinephelus lanceolatus. Vaccines (Basel) 10(2):207
Subasinghe R, Alday-Sanz V, Bondad-Reantaso MG, Jie H, Shinn AP, Sorgeloos P (2023) Biosecurity: reducing the burden of disease. World Aquaculture Society 54:397–426
Sudova E, Machova J, Svobodova Z, Vesely T (2007) Negative effects of malachite green and possibilities of its replacement in the treatment of fish eggs and fish: a review. Vet Med 52:527–539
Sun H, Shang M, Tang Z, Jiang H, Dong H, Zhou X (2020) Oral delivery of bacillus subtilis spores expressing Clonorchis sinensis paramyosin protects grass carp from cercaria infection. Appl Microbiol Biotechnol 104:1633–1646
Sun J, Zhang M, Zhao D, Yang J, Shi Y, Xu B, Liu X, Guan X, Shi W, Liu M (2022a) Immunological effects of recombinant Lactobacillus casei expressing IHNV G protein and rainbow trout (Oncorhynchus mykiss) chemokine CK6 as an oral vaccine. Front Immunol 13:927443
Sun X, Liu J, Deng S, Li R, Lv W, Zhou S, Tang X, Sun Y-z, Ke M, Wang K (2022b) Quorum quenching bacteria Bacillus velezensis DH82 on biological control of Vibrio parahaemolyticus for sustainable aquaculture of Litopenaeus vannamei. Front Mar Sci 9:780055
Sung PH, Chiang HJ, Chen CH, Chen YL, Huang TH, Zhen YY, Chang MW, Liu CF, Chung SY, Chen YL (2016) Combined therapy with adipose-derived mesenchymal stem cells and ciprofloxacin against acute urogenital organ damage in rat sepsis syndrome induced by intrapelvic injection of cecal bacteria. Stem Cells Transl Med 5:782–792
Swain B, Campodonico VA, Curtiss R III (2023) Recombinant attenuated Edwardsiella piscicida vaccine displaying regulated lysis to confer biological containment and protect catfish against Edwardsiellosis. Vaccines 11:1470
Swain P, Nayak SK, Sasmal A et al (2014) Antimicrobial activity of metal based nanoparticles against microbes associated with diseases in aquaculture. World J Microbiol Biotechnol 30:2491–2502
Tan D, Gram L, Middelboe M (2014) Vibriophages and their interactions with the fish pathogen Vibrio anguillarum. Appl Environ Microbiol 80:3128–3140
Thanigaivel S, Vickram S, Saranya V, Ali H, Alarifi S, Kumar J, Modigunta R, Anbarasu K, Lakshmipathy R, Rohini K (2022) Seaweed polysaccharide mediated synthesis of silver nanoparticles and its enhanced disease resistance in Oreochromis mossambicus. J King Saud Univ Sci 34:101771
Thorarinsson R, Wolf JC, Inami M, Phillips L, Jones G, Macdonald AM (2021) Effect of a novel DNA vaccine against pancreas disease caused by salmonid alphavirus subtype 3 in Atlantic salmon (Salmo salar). Fish Shellfish Immunol 108:116–126
Tian J, Yu J (2011) Poly (lactic-co-glycolic acid) nanoparticles as candidate DNA vaccine carrier for oral immunization of Japanese flounder (Paralichthys olivaceus) against lymphocystis disease virus. Fish Shellfish Immunol 30:109–117
Torres S, Arroyo C (2018) Automatic measurement of fish weight and size by processing underwater hatchery images. Eng Lett 26:461–472
Torres-Corral Y, Girons A, González-Barreiro O, Seoane R, Riaza A, Santos Y (2021) Effect of bivalent vaccines against Vibrio anguillarum and Aeromonas salmonicida subspecie achromogenes on health and survival of turbot. Vaccines 9:906
Travieso T, Li J, Mahesh S (2022) The use of viral vectors in vaccine development. Vaccines 7:9
Triet TH, Tinh BTT, Hau LV, Huong TV, Binh NQ (2019) Development and potential use of an Edwardsiella ictaluri wzz mutant as a live attenuated vaccine against enteric septicemia in Pangasius hypophthalmus (Tra catfish). Fish Shellfish Immunol 87:87–95
Udomkusonsri P, TrongvanichnamK LM, Klangkaew N, Kusucharit N (2007) In vitro efficacy of the antifungal activity of some Thai medicinal-plants on the pathogenic fungus, Saprolegnia parasitica H2, from Fish. Kasetsart J 41:56–61
Union Européenne Des Médecins Spécialistes (UEMS) (2016) Formalin banning in Europe in 2016. https://www.uems.eu/data/assets/pdf.file/0005/39641/Draft-statement-Formalin-Banning-UEMS-Council.pdf
Valentim DSS, Duarte JL, Oliveira AEMFM, Cruz RAS, Carvalho JCT, Solans C, Fernandes CP, Tavares-Dias M (2018) Effects of a nanoemulsion with Copaifera officinalis oleoresin against monogenean parasites of Colossoma macropomum: a neotropical Serrasalmidae. J Fish Dis 41:1041–1048
Valladão GM, Gallani SU, Ikefuti CV, da Cruz C, Levy-Pereira N, Rodrigues MV, Pilarski F (2016) Essential oils to control ichthyophthiriasis in pacu, Piaractus mesopotamicus (Holmberg): special emphasis on treatment with Melaleuca alternifolia. J Fish Dis J Fish Dis 39:1–10
Van Belleghem JD, Clement F, Merabishvili M, Lavigne R, Vaneechoutte M (2017) Pro-and anti-inflammatory responses of peripheral blood mononuclear cells induced by Staphylococcus aureus and Pseudomonas aeruginosa phages. Sci Rep 7:1–13
Vendrell D, Balcázar JL, Ruiz-Zarzuela I, de Blas I, Gironés O, Múzquiz JL (2007) Safety and efficacy of an inactivated vaccine against Lactococcus garvieae in rainbow trout (Oncorhynchus mykiss). Prev Vet Med 80:222–229
Vendrell D, Balcazar JL, de Blas I, Ruiz-Zarzuela I, Girones O, Muzquiz JL (2008) Protection of rainbow trout (Oncorhynchus mykiss) from lactococcosis by probiotic bacteria. Comp Immunol Microbiol Infect Dis 31:337–345
Veyrand-Quirós B, Gómez-Gil B, Lomeli-Ortega CO, Escobedo-Fregoso C, Millard AD, Tovar-Ramírez D, Balcázar JL, Quiroz-Guzmán E (2020) Use of bacteriophage VB_Pd_PDCC-1 as biological control agent of Photobacterium damselae subsp. damselae during hatching of long fin yellowtail (Seriola rivoliana) eggs. J Appl Microbiol 129:1497–1510
Vijayakumar S, Vaseeharan B, Malaikozhundan B et al (2017) A novel antimicrobial therapy for the control of Aeromonas hydrophila infection in aquaculture using marine polysaccharide coated gold nanoparticle. Microb Pathog 110:140–151
Vijayakumar S, Chen J, Kalaiselvi V, Divya M, González-Sánchez Z, Durán-Lara EF, Vaseeharan B (2021) Antibacterial and antibiofilm activities of marine polysaccharide laminarin formulated gold nanoparticles: an ecotoxicity and cytotoxicity assessment. J Environ Chem Eng 9
Vimal S, Abdul Majeed S, Nambi KSN, Madan N, Farook MA, Venkatesan C, Taju G, Venu S, Subburaj R, Thirunavukkarasu AR, Sahul Hameed AS (2014) Delivery of DNA vaccine using chitosan–tripolyphosphate (CS/TPP) nanoparticles in Asian sea bass, Lates calcarifer (Bloch, 1790) for protection against nodavirus infection. Aquaculture 420:240–246
Vine NG, Leukes WD, Kaiser H (2006) Probiotics in marine larviculture. FEMS Microbiol Rev 30:404–427
Vu TH, Ngo TMV, Duong TTA et al (2020) Removal of tetracycline from aqueous solution using nanocomposite based on polyanion modified laterite material. Anal Methods Chem 243:1172
Wan M, Ding Y (2023) Study on immunogenicity of Lrp subunit vaccine against Vibrio alginolyticus in pearl gentian grouper (♀Epinephelus fuscoguttatus ×♂Epinephelus lanceolatus). Front Mar Sci 10:1098816
Wang Y-B, Li J-R, Lin J (2008) Probiotics in aquaculture: challenges and outlook. Aquaculture 281:1–4
Wang JJ, Zeng ZW, Xiao RZ et al (2011) Recent advances of chitosan nanoparticles as drug carriers. Int J Nanomedicine 6:765
Wang JL, Meng XL, Lu RH, Wu C, Luo YT, Yan X (2015) Effects of Rehmannia glutinosa on growth performance, immunological parameters and disease resistance to Aeromonas hydrophila in common carp (Cyprinus carpio L.). Aquaculture 435:293–300
Wang JB, Lin NT, Tseng YH, Weng SF (2016) Genomic characterization of the novel Aeromonas hydrophila phage Ahp1 suggests the derivation of a new subgroup from PhiKMV-like family. PLoS ONE 11:e0162060
Wang H, Chen Yq Ru, Yq GX, Lq Lu (2018) EGCG: potential application as a protective agent against grass carp reovirus in aquaculture. J Fish Dis 41:1–9
Wang B, Thompson KD, Wangkahart E, Yamkasem J, Bondad-Reantaso MG, Tattiyapong P, Jian J, Surachetpong W (2022) Strategies to enhance tilapia immunity to improve their health in aquaculture. Rev Aquac 15:41–56
Wright E, Elliman J, Owens L (2013) Induction and characterization of lysogenic bacteriophages from Streptococcus iniae. J Appl Microbiol 114:1616–1624
Xiaoyan Wu, Xing J, Tang X, Sheng X, Chi H, Zhan W (2022) Protective cellular and humoral immune responses to Edwardsiella tarda in flounder (Paralichthys olivaceus) immunized by an inactivated vaccine. Mol Immunol 149(2022):77–86
Xing CF, Hu HH, Huang JB, Fang H, Kai Y, Wu Y, Chi S (2013) Diet supplementation of Pediococcus pentosaceus in cobia (Rachycentron canadum) enhances growth rate, respiratory burst and resistance against photobacteriosis. Fish Shellfish Immunol 35:1122–1128
Xing J, Zhang Z, Luo K, Tang X, Sheng X, Zhan WT (2020) T and B lymphocytes immune responses in flounder (Paralichthys olivaceus) induced by two forms of outer membrane protein K from Vibrio anguillarum: subunit vaccine and DNA vaccine. Mol Immunol 118:40–51
Xing J, Jiang X, Xu H, Sheng X, Tang X, Chi H (2021) Local immune responses to VAA DNA vaccine against Listonella anguillarum in flounder (Paralichthys olivaceus). Mol Immunol 134:141–149
Xu DH, Klesius PH, Shoemaker CA (2008) Protective immunity of Nile tilapia against Ichthyophthirius multifiliis postimmunization with live theronts and sonicated trophonts. Fish Shellfish Immunol 25:124–127
Xu DH, Zhang D, Shoemaker C, Beck B (2020) Dose effects of a DNA vaccine encoding immobilization antigen on immune response of channel catfish against Ichthyophthirius multifiliis. Fish Shellfish Immunol 106:1031–1041
Yamaki S, Kawai Y, Yamazaki K (2015) Characterization of a novel bacteriophage, Phda1, infecting the histamine-producing Photobacterium damselae subsp. damselae. J Appl Microbiol 118:1541–1550
Yamasaki M, Araki K, Maruyoshi K, Matsumoto M, Nakayasu C, Moritomo T (2015) Comparative analysis of adaptive immune response after vaccine trials using live attenuated and formalin-killed cells of Edwardsiella tarda in Ginbuna crucian carp (Carassius auratus langsdorfii). Fish Shellfish Immunol 45:437–442
Yambot AV, SongYL, (2006) Immunization of grouper, Epinephelus coioides, confers protection against a protozoan parasite, Cryptocaryon irritans. Aquaculture 260:1–9
Yang Q, Pande GSJ, Wang Z, Lin B, Rubin RA, Vora GJ, Defoirdt T (2017) Indole signalling and (micro)algal auxins decrease the virulence of Vibrio campbellii, a major pathogen of aquatic organisms. Environ Microbiol 19:1987–2004
Yang M, Zhang C, Zhang MZ, Zhang S (2018) Beta-defensin derived cationic antimicrobial peptides with potent killing activity against gram negative and gram positive bacteria. BMC Microbiol 18:1–14
Yang S, Mkingule I, Liu L, Chen W, Yuan X, Ma Z, Liang L, Qian S, Huang M, Fei H (2023) Protective efficacy evaluation of immunogenic protein AHA_3793 of Aeromonas hydrophila as vaccine candidate for largemouth bass Micropterus salmoides. J Ocean Limnol 41:392–400
Yao JY, Shen JY, Li XL, Xu Y, Hao GJ, Pan XY, Wang GX, Yin WL (2010) Effect of sanguinarine from the leaves of Macleaya cordata against Ichthyophthirius multifiliis in grass carp (Ctenopharyngodon idella). Parasitol Res 107:1035–1042
Yasuike M, Nishiki I, Iwasaki Y, Nakamura Y, Fujiwara A, Sugaya E, Kawato Y, Nagai S, Kobayashi T, Ototake M (2015) Full-genome sequence of a novel myovirus, GF-2, infecting Edwardsiella tarda: comparison with other Edwardsiella myoviral genomes. Arch Virol 160:2129–2133
Yilmaz S, Yilmaz E, Dawood MAO, Ringø E, Ahmadifar E, Abdel-Latif HMR (2022) Probiotics, prebiotics, and synbiotics used to control vibriosis in fish: a review. Aquaculture 547
Youssef HA, Ayoub HF, Soror EI, Matter AF (2023) Virulence genes contributing to Aeromonas veronii pathogenicity in Nile tilapia (Oreochromis niloticus): approaching the development of live and inactivated vaccines. Aquac Int 31:1253–1267
Yu N, Zeng W, Xiong Z, Liu Z (2022) A high efficacy DNA vaccine against tilapia lake virus in Nile tilapia (Oreochromis niloticus). Aquaculture Reports 24
Yukgehnaish K, Kumar P, Sivachandran P, Marimuthu K, Arshad A, Paray BA, Arockiaraj J (2020) Gut microbiota metagenomics in aquaculture: factors influencing gut microbiome and its physiological role in fish. Reviews Aquaculture 12:1903–1927
Zahran E, Risha E, Elbahnaswy S, Mahgoub H, Abd El-Moaty A (2019) Tilapia piscidin 4 (TP4) enhances immune response, antioxidant activity, intestinal health and protection against Streptococcus iniae infection in Nile tilapia. Aquaculture 513(2019):734451
Zahran E, Elbahnaswy S, Ahmed F, Risha E, Mansour AT, Alqahtani AS, Awadin W, El Sebaei MJ (2024) Dietary microalgal-fabricated selenium nanoparticles improve Nile tilapia biochemical indices, immune-related gene expression, and intestinal immunity. BMC Vet Res 20:107
Zhang G, Gong S, Yu D, Yuan H (2009) Propolis and Herba Epimedii extracts enhance the non-specific immune response and disease resistance of Chinese sucker, Myxocyprinus asiaticus. Fish Shellfish Immunol 26:467–472
Zhang XP, Li WX, Ai TS, Zou H, Wu SG, Wang GT (2014) The efficacy of four common anthelmintic drugs and traditional Chinese medicinal plant extracts to control Dactylogyrus vastator (Monogenea). Aquaculture 420:302–307
Zhang H, Wen W, Yan J (2017) Application of immunohistochemistry technique in hydrobiological studies. Aquaculture and Fisheries 2:140–144
Zhang Y, Tan X, Tu X, Ling F, Wang G (2020) Efficacy and antiparasitic mechanism of curdione from Curcuma zedoaria against Gyrodactylus kobayashii in goldfish. Aquaculture 523
Zhang L, Chen F, Zeng Z, Xu M, Sun F, Yang L, Bi X, Lin Y, Gao Y, Hao H, Yi W, Li M, Xie Y (2021) Advances in metagenomics and its application in environmental microorganisms. Front Microbiol 12
Zhang T, Zhang M, Xu Z, He Y, Zhao X, Cheng H, Chen X, Xu J, Ding Z (2023) The screening of the protective antigens of Aeromonas hydrophila using the reverse vaccinology approach: potential candidates for subunit vaccine development. Vaccines 11:1266
Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg AP (2014) Nanoparticle vaccines. Vaccine 32:327–337
Zhao Z, Zhang C, Jia Y-J, Qiu D-K, Lin Q, Li N-Q, Huang Z-B, Fu X-Z, Wang G-X, Zhu B (2019) Immersion vaccination of fish Siniperca chuatsi against infectious spleen and kidney necrosis virus with a SWCNTs-based subunit vaccine. Fish Shellfish Immunol 92:133–140
Zhao Z, Wang H, Zhang H, Guan Y, Siddiqui SA, Feng-Shan X, Cong B (2022) Oral vaccination with recombinant Lactobacillus casei expressing Aeromonas hydrophila Aha1 against A. hydrophila infections in common carps. Virulence 13:794–807
Zhou S, Song D, Zhou X, Mao X, Zhou X, Wang S, Wei J, Huang Y, Wang W, Xiao SM, Qin Q (2019) Characterization of Bacillus subtilis from gastrointestinal tract of hybrid Hulong grouper (Epinephelus fuscoguttatus × E. lanceolatus) and its effects as probiotic additives. Fish Shellfish Immunol 84:1115–1124
Zhou S, Tu X, Pang H, Hoare R, Monaghan SJ, Luo J (2020) A T3SS regulator mutant of Vibrio alginolyticus affects antibiotic susceptibilities and provides significant protection to Danio rerio as a live attenuated vaccine. Front Cell Infection Microbiol 10:1–15
Zhu M, Shen Z, Gu Y, Tong X, Zhang Y, Pan J, Feng Y, Hu X, Wang Y, Cao G, Xue R, Gong C (2023) A recombinant baculovirus vector vaccine (BacMCP) against the infectious spleen and kidney necrosis virus (ISKNV). J Fish Dis 46:165–176
Zilberg D, Tal A, Froyman N, Abutbul S, Dudai N, Golan-Goldhirsh A (2010) Dried leaves of Rosmarinus officinalis as a treatment for streptococcosis in tilapia. J Fish Dis 33:361–369
Acknowledgements
We would like to acknowledge the assistance provided by the staff members of the Hydrobiology Department, Veterinary Research Institute, National Research Centre.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). Open access (OA) in Springer Nature’s portfolio of journals is covered by the transformative agreement between Springer Nature and Science, Technology & Innovation Funding Authority (STDF) in cooperation with the Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
This study was conducted in cooperation with all authors. M Y. E: Conceptualization; writing-original draft; writing–review and editing. A. A, A.S. E, and M. A: writing–review and editing.; R H. K, M. M. M: review and editing.
Corresponding author
Ethics declarations
Ethics approval
The study followed the guidelines and instructions of the Institutional Animal Care and Use Committee, National Research Centre, Cairo, Egypt.
Conflict of interests
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elgendy, M.Y., Ali, S.E., Dayem, A.A. et al. Alternative therapies recently applied in controlling farmed fish diseases: mechanisms, challenges, and prospects. Aquacult Int (2024). https://doi.org/10.1007/s10499-024-01603-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10499-024-01603-3