Abstract
In materials science, “green” synthesis has gained extensive attention as a reliable, sustainable, and eco-friendly protocol for synthesizing a wide range of materials/nanomaterials including metal/metal oxides nanomaterials, hybrid materials, and bioinspired materials. As such, green synthesis is regarded as an important tool to reduce the destructive effects associated with the traditional methods of synthesis for nanoparticles commonly utilized in laboratory and industry. In this review, we summarized the fundamental processes and mechanisms of “green” synthesis approaches, especially for metal and metal oxide [e.g., gold (Au), silver (Ag), copper oxide (CuO), and zinc oxide (ZnO)] nanoparticles using natural extracts. Importantly, we explored the role of biological components, essential phytochemicals (e.g., flavonoids, alkaloids, terpenoids, amides, and aldehydes) as reducing agents and solvent systems. The stability/toxicity of nanoparticles and the associated surface engineering techniques for achieving biocompatibility are also discussed. Finally, we covered applications of such synthesized products to environmental remediation in terms of antimicrobial activity, catalytic activity, removal of pollutants dyes, and heavy metal ion sensing.
Similar content being viewed by others
Introduction
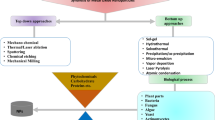
Over the last decade, novel synthesis approaches/methods for nanomaterials (such as metal nanoparticles, quantum dots (QDs), carbon nanotubes (CNTs), graphene, and their composites) have been an interesting area in nanoscience and technology [1,2,3,4,5,6,7,8,9]. To obtain nanomaterials of desired sizes, shape, and functionalities, two different fundamental principles of synthesis (i.e., top down and bottom up methods) have been investigated in the existing literature (Fig. 1). In the former, nanomaterials/nanoparticles are prepared through diverse range of synthesis approaches like lithographic techniques, ball milling, etching, and sputtering [10]. The use of a bottom up approach (in which nanoparticles are grown from simpler molecules) also includes many methods like chemical vapor deposition, sol–gel processes, spray pyrolysis, laser pyrolysis, and atomic/molecular condensation.
Interestingly, the morphological parameters of nanoparticles (e.g., size and shape) can be modulated by varying the concentrations of chemicals and reaction conditions (e.g., temperature and pH). Nevertheless, if these synthesized nanomaterials are subject to the actual/specific applications, then they can suffer from the following limitation or challenges: (i) stability in hostile environment, (ii) lack of understanding in fundamental mechanism and modeling factors, (iii) bioaccumulation/toxicity features, (iv) expansive analysis requirements, (v) need for skilled operators, (vi) problem in devices assembling and structures, and (vii) recycle/reuse/regeneration. In true world, it is desirable that the properties, behavior, and types of nanomaterials should be improved to meet the aforementioned points. On the other hand, these limitations are opening new and great opportunities in this emerging field of research.
To counter those limitations, a new era of ‘green synthesis’ approaches/methods is gaining great attention in current research and development on materials science and technology. Basically, green synthesis of materials/nanomaterials, produced through regulation, control, clean up, and remediation process, will directly help uplift their environmental friendliness. Some basic principles of “green synthesis” can thus be explained by several components like prevention/minimization of waste, reduction of derivatives/pollution, and the use of safer (or non-toxic) solvent/auxiliaries as well as renewable feedstock.
‘Green synthesis’ are required to avoid the production of unwanted or harmful by-products through the build-up of reliable, sustainable, and eco-friendly synthesis procedures. The use of ideal solvent systems and natural resources (such as organic systems) is essential to achieve this goal. Green synthesis of metallic nanoparticles has been adopted to accommodate various biological materials (e.g., bacteria, fungi, algae, and plant extracts). Among the available green methods of synthesis for metal/metal oxide nanoparticles, utilization of plant extracts is a rather simple and easy process to produce nanoparticles at large scale relative to bacteria and/or fungi mediated synthesis. These products are known collectively as biogenic nanoparticles (Fig. 2).
Green synthesis methodologies based on biological precursors depend on various reaction parameters such as solvent, temperature, pressure, and pH conditions (acidic, basic, or neutral). For the synthesis of metal/metal oxide nanoparticles, plant biodiversity has been broadly considered due to the availability of effective phytochemicals in various plant extracts, especially in leaves such as ketones, aldehydes, flavones, amides, terpenoids, carboxylic acids, phenols, and ascorbic acids. These components are capable of reducing metal salts into metal nanoparticles [11]. The basic features of such nanomaterials have been investigated for use in biomedical diagnostics, antimicrobials, catalysis, molecular sensing, optical imaging, and labelling of biological systems [12].
Here, we summarized the current state of research on the green synthesis of metal/metal oxide nanoparticles with their advantages over chemical synthesis methods. In addition, we also discussed the role of solvent systems (synthetic materials), various biological (natural extracts) components (like bacteria, algae, fungi, and plant extracts) with their advantages over other conventional components/solvents. The main aim of this literature study is to provide detailed mechanisms for green synthesis and their real world environmental remediation applications. Overall, our goal is to systematically describe “green” synthesis procedures and their related components that will benefit researchers involved in this emerging field while serving as a useful guide for readers with a general interest in this topic.
Biological components for “green” synthesis
Innumerable physical and chemical synthesis approaches require high radiation, highly toxic reductants, and stabilizing agents, which can cause pernicious effects to both humans and marine life. In contrast, green synthesis of metallic nanoparticles is a one pot or single step eco-friendly bio-reduction method that requires relatively low energy to initiate the reaction. This reduction method is also cost efficient [13,14,15,16,17,18,19].
Bacteria
Bacterial species have been widely utilized for commercial biotechnological applications such as bioremediation, genetic engineering, and bioleaching [20]. Bacteria possess the ability to reduce metal ions and are momentous candidates in nanoparticles preparation [21]. For the preparation of metallic and other novel nanoparticles, a variety of bacterial species are utilized. Prokaryotic bacteria and actinomycetes have been broadly employed for synthesizing metal/metal oxide nanoparticles.
The bacterial synthesis of nanoparticles has been adopted due to the relative ease of manipulating the bacteria [22]. Some examples of bacterial strains that have been extensively exploited for the synthesis of bioreduced silver nanoparticles with distinct size/shape morphologies include: Escherichia coli, Lactobacillus casei, Bacillus cereus, Aeromonas sp. SH10 Phaeocystis antarctica, Pseudomonas proteolytica, Bacillus amyloliquefaciens, Bacillus indicus, Bacillus cecembensis, Enterobacter cloacae, Geobacter spp., Arthrobacter gangotriensis, Corynebacterium sp. SH09, and Shewanella oneidensis. Likewise, for the preparation of gold nanoparticles, several bacterial species (such as Bacillus megaterium D01, Desulfovibrio desulfuricans, E. coli DH5a, Bacillus subtilis 168, Shewanella alga, Rhodopseudomonas capsulate, and Plectonema boryanum UTEX 485) have been extensively used. Information on the size, morphology, and applications of various nanoparticles is summarized in Table 1.
Fungi
Fungi-mediated biosynthesis of metal/metal oxide nanoparticles is also a very efficient process for the generation of monodispersed nanoparticles with well-defined morphologies. They act as better biological agents for the preparation of metal and metal oxide nanoparticles, due to the presence of a variety of intracellular enzyme [23]. Competent fungi can synthesize larger amounts of nanoparticles compared to bacteria [24]. Moreover, fungi have many merits over other organisms due to the presence of enzymes/proteins/reducing components on their cell surfaces [25]. The probable mechanism for the formation of the metallic nanoparticles is enzymatic reduction (reductase) in the cell wall or inside the fungal cell. Many fungal species are used to synthesize metal/metal oxide nanoparticles like silver, gold, titanium dioxide and zinc oxide, as discussed in Table 1.
Yeast
Yeasts are single-celled microorganisms present in eukaryotic cells. A total of 1500 yeast species have been identified [26]. Successful synthesis of nanoparticles/nanomaterials via yeast has been reported by numerous research groups. The biosynthesis of silver and gold nanoparticles by a silver-tolerant yeast strain and Saccharomyces cerevisiae broth has been reported. Many diverse species are employed for the preparation of innumerable metallic nanoparticles, as discussed in Table 1.
Plants
Plants have the potential to accumulate certain amounts of heavy metals in their diverse parts. Consequently, biosynthesis techniques employing plant extracts have gained increased consideration as a simple, efficient, cost effective and feasible methods as well as an excellent alternative means to conventional preparation methods for nanoparticle production. There are various plants that can be utilized to reduce and stabilize the metallic nanoparticles in “one-pot” synthesis process. Many researchers have employed green synthesis process for preparation of metal/metal oxide nanoparticles via plant leaf extracts to further explore their various applications.
Plants have biomolecules (like carbohydrates, proteins, and coenzyme) with exemplary potential to reduce metal salt into nanoparticles. Like other biosynthesis processes, gold and silver metal nanoparticles were first investigated in plant extract-assisted synthesis. Various plants [including aloe vera (Aloe barbadensis Miller), Oat (Avena sativa), alfalfa (Medicago sativa), Tulsi (Osimum sanctum), Lemon (Citrus limon), Neem (Azadirachta indica), Coriander (Coriandrum sativum), Mustard (Brassica juncea) and lemon grass (Cymbopogon flexuosus)] have been utilized to synthesize silver nanoparticles and gold nanoparticles, as listed in Table 2. The major part of this type of research has explored the ex vivo synthesis of nanoparticles, while metallic nanoparticles can be formed in living plants (in vivo) by reducing metal salt ions absorbed as soluble salts. The in vivo synthesis of nanoparticles like zinc, nickel, cobalt, and copper was also observed in mustard (Brassica juncea), alfalfa (Medicago sativa), and sunflower (Helianthus annuus) [27]. Also, ZnO nanoparticles have been prepared with a great variety of plant leaf extracts such as coriander (Coriandrum sativum) [28], crown flower (Calotropis gigantean) [29], copper leaf (Acalypha indica) [30], China rose (Hibiscus rosa-sinensis) [31], Green Tea (Camellia sinensis) [32], and aloe leaf broth extract (Aloe barbadensis Miller) [33]. Readers can refer to the work of Iravani [34] for a comprehensive overview of plant materials utilized for the biosynthesis of nanoparticles.
Solvent system-based “green” synthesis
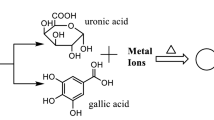
Solvent systems are a fundamental component in the synthesis process, whether it is “green” synthesis or not. Water is always considered an ideal and suitable solvent system for synthesis processes. According to Sheldon, “the best solvent is no solvent, and if a solvent is desirable then water is ideal” [35]. Water is the cheapest and most commonly accessible solvent on earth. Since the advent of nanoscience and nanotechnology, the use of water as a solvent for the synthesis of various nanoparticles has been carried out. For instance, synthesized Au and Ag nanoparticles at room temperature using gallic acid, a bifunctional molecule, in an aqueous medium [36]. Gold nanoparticles were produced via a laser ablation technique in an aqueous solution. The oxygen present in the water leads to partial oxidation of the synthesized gold nanoparticles, which finally enhanced its chemical reactivity and had a great impact on its growth [37].
In the literature, “green” synthesis consists of two major routes:
-
Wherein water is used as a solvent system.
-
Wherein a natural source/extract is utilized as the main component.
Both of these routes have been covered in the coming section according to the present literature. Hopefully, our efforts will help researchers gain a better knowledge of ‘green’ synthesis methods, the role of toxic/non-toxic solvents (or components), and renewable resources derived from natural sources. Ionic and supercritical liquids are one of the best examples in this emerging area. Ionic liquids (ILs) are composed of ions that have melting points below 100 °C. Ionic liquids are also acknowledged as “room temperature ionic liquids.” Several metal nanoparticles (e.g., Au, Ag, Al, Te, Ru, Ir, and Pt) have been synthesized in ionic liquids [38,39,40,41]. The process of nanoparticle synthesis is simplified since the ionic liquid can serve as both a reductant and a protective agent.
ILs can be hydrophilic or hydrophobic depending on the nature of the cations and anions. For example, 1-butyl-3-methyl imidazolium (Bmim) hexafluorophosphate (PF6) is hydrophobic, whereas its tetrafluoroborate (BF4) analogue is hydrophilic. Since both species are ionic in nature, they can act as catalysts [40, 42,43,44,45]. Bussamara et al. have performed a comparative study by controlling the synthesis of manganese oxide (Mn3O4) nanoparticles using imidazolium ionic liquids and oleylamine (a conventional solvent). They found that smaller sized nanoparticles (9.9 ± 1.8 nm) were formed with better dispersity in ionic liquids than in the oleylamine solvent (12.1 ± 3.0 nm) [46]. Lazarus et al. synthesized silver nanoparticles in an ionic liquid (BmimBF4). The synthesized nanoparticles were in both smaller isotropic spherical and large-sized anisotropic hexagonal shaped forms [47]. An electrochemical method was employed for this purpose [48]. Ionic liquid was used in the electrolytic reaction as a substitute for water without mechanical stirring. For the first time, Kim et al. developed a one-phase preparation technique for gold (Au) and platinum (Pt) nanoparticles by means of thiol-functionalized ionic liquids (TFILs). TFILs acted as a stabilizing agent to produce crystalline structures with small sizes [49]. Dupont et al. used 1-n-butyl-3-methylimidazolium hexafluorophosphate (which is room temperature ionic liquid) for synthesizing Ir(0) nanoparticles by Ir(I) reduction. The average size of synthesized nanoparticles was ~ 2 nm. Interestingly, the ionic liquid medium is impeccable for the production of recyclable biphasic catalytic systems for hydrogenation reactions [50].
The benefits of using ionic liquids instead of other solvents include the following. (a) Many metal catalysts, polar organic compounds, and gases are easily dissolved in ILs to support biocatalysts. (b) ILs have constructive thermal stabilities to operate in a broad temperature range. Most of these melt below room temperature and begin to decompose above 300 or 400 °C. As such, they allow a broader synthesis temperature range (e.g., three to four times) than that of water. (c) The solubility properties of IL can be modulated by modifying the cations and anions associated with them. (d) Unlike other polar solvents or alcohols, ILs are non-coordinating. However, they have polarities comparable to alcohol. (e) ILs do not evaporate into the environment like volatile solvents because they have no vapor pressure. (f) ILs have dual functionality because they have both cations and anions. The problems associated with the biodegradability of ionic liquids make them not acceptable for synthesis of metallic nanoparticles. To diminish these non-biodegradability issues, many new potentially benign ionic liquids are being developed with maximum biodegradation efficiency [51,52,53,54]. The innumerable ILs are used to synthesize various metallic nanoparticles as listed in Table 3.
Likewise, ordinary solvents can be converted into super critical fluids at temperatures and pressures above critical point. In the supercritical state, solvent properties such as density, thermal conductivity, and viscosity are significantly altered. Carbon dioxide is the most feasible super critical, non-hazardous, and inert fluid [55, 56]. Also, supercritical water can serve as a good solvent system for several reactions. As, water has critical temperature of 646 K and pressure of 22.1 MPa [57]. Silver and copper NPs can be synthesized in supercritical carbon dioxide [58]. Sue et al. suggested that decreasing the solubility of metal oxides around the critical point can lead to super saturation and the ultimate formation of nanoparticles [59]. Kim et al. synthesized tungsten oxide (WO3) and tungsten blue oxide nanoparticles by using sub- and supercritical water and methanol [60].
Stability and toxicity of the nanoparticles
The environmental distribution and transport of released nanoparticles depend on their ability to make metastable aqueous suspensions or aerosols in environmental fluids. The stability of the nanoparticles in the environment can therefore be evaluated by estimating their propensity to aggregate or interact with the surrounding media. Aggregation is a time-dependent phenomena associated with the rate of particle collision while the stability of the suspension is largely determined by the size of the particles and affinity toward other environmental constituents. The “green” synthesis of AgNPs from tea leaf extraction was found to be stable after entering the aquatic environment [61]. Likewise, the stability of AgNPs (in aqueous medium) manufactured using plant extracts and plant metabolites was confirmed from the resulting material [62]. Surface complexation is also reported to affect the intrinsic stability of nanoparticles by regulating its colloidal stability. The nature and stability of nanoparticles were theoretically predicted through a mechanistic understanding of the surface complexation processes [63]. The colloidal stability (or rate of dissolution) of nanoparticles can be regulated by controlling the particle size and surface capping or through functionalization techniques [64, 65]).
Transformation of nanoparticles is an essential property to consider when assessing their environmental impact or toxicity. For instance, sulfurization of AgNPs greatly reduced their toxicity due to the lower solubility of silver sulfide [66]. For similar reasons, the use of biocompatible stabilizing agents (e.g., biodegradable polymers and copolymers) have opened up a “greener” avenue of nanomaterial surface engineering. Such techniques can impart remarkable stability, e.g., in situ synthesis of AuNPs capped with Korean red ginseng root [67]. Apart from surface chemistry, other key structural features determining the nanomaterial toxicity are the size, shape, and composition of the nanomaterials [68]. Toxicity analysis of AgNP synthesized using plant leaf extracts showed enhanced seed germination rates in the AgNP chemical treatment for activation than the corresponding control treatments [69]. However, the mechanism of such rate enhancement effects was not reported.
Mechanism of “green” synthesis for metals and their oxide nanoparticles
Microorganism-based mechanism
There are different mechanisms for the formation of nanoparticles using different microorganisms. First, metallic ions are captured on the surface or inside the microbial cells, and then these arrested metal ions are reduced into metal nanoparticles by the action of enzymes. Sneha et al. [70] described the mechanism of microorganism-assisted silver and gold nanoparticles formed via Verticillium sp. or algal biomass based on the following hypothesis. (a) First, the silver or gold ions were captured on the surface of fungal cells via electrostatic interactions between ions and negatively charged cell wall enzymes. (b) Then, silver or gold ions were bioreduced into silver or gold nuclei, which subsequently grew. The two key aspects in the biosynthesis of nanoparticles are NADH (nicotinamide adenine dinucleotide) and NADH-dependent nitrate reductase. Kalishwaralal et al. [71] demonstrated that the nitrate reductase was responsible for the production of bioreduced silver nanoparticles by B. licheniformis. Nonetheless, the bioreduction mechanisms associated with the production of metal salt ions and the resulting metallic nanoparticles formed by microorganisms remain unexplored.
Plant leaf extract-based mechanism
For nanoparticle synthesis mediated by plant leaf extract, the extract is mixed with metal precursor solutions at different reaction conditions [72]. The parameters determining the conditions of the plant leaf extract (such as types of phytochemicals, phytochemical concentration, metal salt concentration, pH, and temperature) are admitted to control the rate of nanoparticle formation as well as their yield and stability [73]. The phytochemicals present in plant leaf extracts have uncanny potential to reduce metal ions in a much shorter time as compared to fungi and bacteria, which demands the longer incubation time [74]. Therefore, plant leaf extracts are considered to be an excellent and benign source for metal as well as metal oxide nanoparticle synthesis. Additionally, plant leaf extract play a dual role by acting as both reducing and stabilizing agents in nanoparticles synthesis process to facilitate nanoparticles synthesis [75]. The composition of the plant leaf extract is also an important factor in nanoparticle synthesis, for example different plants comprise varying concentration levels of phytochemicals [76, 77]. The main phytochemicals present in plants are flavones, terpenoids, sugars, ketones, aldehydes, carboxylic acids, and amides, which are responsible for bioreduction of nanoparticles [78].
Flavonoids contain various functional groups, which have an enhanced ability to reduce metal ions. The reactive hydrogen atom is released due to tautomeric transformations in flavonoids by which enol-form is converted into the keto-form. This process is realized by the reduction of metal ions into metal nanoparticles. In sweet basil (Ocimum basilicum) extracts, enol- to keto-transformation is the key factor in the synthesis of biogenic silver nanoparticles [79]. Sugars such as glucose and fructose exist in plant extracts can also be responsible for the formation of metallic nanoparticles. Note that glucose was capable of participating in the formation of metallic nanoparticles with different size and shapes, whereas fructose-mediated gold and silver nanoparticles are monodisperse in nature [80].
An FTIR analysis of green synthesized nanoparticles via plant extracts confirmed that nascent nanoparticles were repeatedly found to be associated with proteins [81]. Also, amino acids have different ways of reducing the metal ions. Gruen et al. [82] observed that amino acids (viz cysteine, arginine, lysine, and methionine are proficient in binding with silver ions. Tan et al. [83] tested all of the 20 natural α-amino acids to establish their efficient potential behavior towards the reduction of Au0 metal ions.
Plant extracts are made up of carbohydrates and proteins biomolecules, which act as a reducing agent to promote the formation of metallic nanoparticles [34]. Also, the proteins with functionalized amino groups (–NH2) available in plant extracts can actively participate in the reduction of metal ions [84]. The functional groups (such as –C–O–C–, –C–O–, –C=C–, and –C=O–) present in phytochemicals such as flavones, alkaloids, phenols, and anthracenes can help to generate metallic nanoparticles. According to Huang et al. [85], the absorption peaks of FTIR spectra at (1) 1042 and 1077, (2) 1606 and 1622, and (3) 1700–1800 cm−1 imply the stretching of (1) –C–O–C– or –C–O–, (2) –C=C– and (3) –C=O, respectively. Based on FTIR analysis, they confirmed that functional groups like –C–O–C–, –C–O–, –C=C–, and –C=O, are the capping ligands of the nanoparticles [86]. The main role of the capping ligands is to stabilize the nanoparticles to prevent further growth and agglomeration. Kesharwani et al. [87] covered photographic films using an emulsion of silver bromide. When light hit the film, the silver bromide was sensitized; this exposed film was placed into a solution of hydroquinone, which was further oxidized to quinone by the action of sensitized silver ion. The silver ion was reduced to silver metal, which remained in the emulsion.
Based on the chemistry of photography, we assumed that hydroquinone or plastohydroquinone or quinol (alcoholic compound) serve as a main reducing agent for the reduction of silver ions to silver nanoparticles through non-cyclic photophosphorylation [87]. Thus, this experiment proves that the biomolecules and heterocyclic compounds exist in plant extract were accountable for the extracellular synthesis of metallic nanoparticles by plants. It has already been well established that numerous plant phytochemicals including alkaloids, terpenoids, phenolic acids, sugars, polyphenols, and proteins play a significant role in the bioreduction of metal salt into metallic nanoparticles. For instance, Shanakr et al. [88] confirmed that the terpenoids present in geranium leaf extract actively take part in the conversion of silver ions into nanoparticles. Eugenol is a main terpenoid component of Cinnamomum zeylanisum (cinnamon) extracts, and it plays a crucial role for the bioreduction of HAuCl4 and AgNO3 metal salts into their respective metal nanoparticles. FTIR data showed that –OH groups originating from eugenol disappear during the formation of Au and Ag nanoparticles. After the formation of Au nanoparticles, carbonyl, alkenes, and chloride functional groups appeared. Several other groups [e.g., R–CH and –OH (aqueous)] were also found both before and after the production of Au nanoparticles [89]. Thus, they proposed the possible chemical mechanism shown in Fig. 3. Nonetheless, the exact fundamental mechanism for metal oxide nanoparticle preparation via plant extracts is still not fully tacit. In general, there are three phases of metallic nanoparticle synthesis from plant extracts: (1) the activation phase (bioreduction of metal ions/salts and nucleation process of the reduced metal ions), (2) the growth phase (spontaneous combination of tiny particles with greater ones) via a process acknowledged as Ostwald ripening, and (3) the last one is termination phase (defining the final shape of the nanoparticles) [90, 91]. The process of nanoparticle formation by plant extract is depicted in Fig. 4 [92].
Schematic for the reduction of Au and Ag ions [89]
Mechanism of nanoparticle formation by plant leaf extract [228]
Environmental remediation applications
Antimicrobial activity
Various studies have been carried out to ameliorate antimicrobial functions because of the growing microbial resistance towards common antiseptic and antibiotics. According to in vitro antimicrobial studies, the metallic nanoparticles effectively obstruct the several microbial species [93]. The antimicrobial effectiveness of the metallic nanoparticles depends upon two important parameters: (a) material employed for the synthesis of the nanoparticles and (b) their particle size. Over the time, microbial resistance to antimicrobial drugs has become gradually raised and is therefore a considerable threat to public health. For instance, antimicrobial drug resistant bacteria contain methicillin-resistant, sulfonamide-resistant, penicillin-resistant, and vancomycin-resistant properties [94]. Antibiotics face many current challenges such as combatting multidrug-resistant mutants and biofilms. The effectiveness of antibiotic is likely to decrease rapidly because of the drug resistance capabilities of microbes. Hence, even when bacteria are treated with large doses of antibiotics, diseases will persist in living beings. Biofilms are also an important way of providing multidrug resistance against heavy doses of antibiotics. Drug resistance occurs mainly in infectious diseases such as lung infection and gingivitis [95]. The most promising approach for abating or avoiding microbial drug resistance is the utilization of nanoparticles. Due to various mechanisms, metallic nanoparticles can preclude or overwhelm the multidrug-resistance and biofilm formation, as described in Figs. 5 and 6.
Schematic for the multiple antimicrobial mechanisms in different metal nanoparticles against microbial cells [96]
Various mechanisms of antimicrobial activity of metal nanoparticles [93]
Various nanoparticles employ multiple mechanisms concurrently to fight microbes [e.g., metal-containing nanoparticles, NO-releasing nanoparticles (NO NPs), and chitosan-containing nanoparticles (chitosan NPs)]. Nanoparticles can fight drug resistance because they operate using multiple mechanisms. Therefore, microbes must simultaneously have multiple gene mutations in their cell to overcome the nanoparticle mechanisms. However, simultaneous multiple biological gene mutations in the same cell are unlikely [96].
Multiple mechanisms observed in nanoparticles are discussed in Table 4. Silver nanoparticles are the most admired inorganic nanoparticles, and they are utilized as efficient antimicrobial, antifungal, antiviral, and anti-inflammatory agents [97]. According to a literature survey, the antimicrobial potential of silver nanoparticles can be described in the following ways: (1) denaturation of the bacterial outer membrane [98], (2) generation of pits/gaps in the bacterial cell membrane leading to fragmentation of the cell membrane [99, 100], and (3) interactions between Ag NPs and disulfide or sulfhydryl groups of enzymes disrupt metabolic processes; this step leads to cell death [101]. The shape-dependent antimicrobial activity was also examined. According to Pal et al. [102], truncated triangular nanoparticles are highly reactive in nature because their high-atom-density surfaces have enhanced antimicrobial activity.
The synthesis of Au nanoparticles is highly useful in the advancement of effective antibacterial agents because of their non-toxic nature, queer ability to be functionalized, polyvalent effects, and photo-thermal activity [103,104,105]. However, the antimicrobial action of gold nanoparticles is not associated with the production of any reactive oxygen species-related process [106]. To investigate the antibacterial potential of the Au nanoparticles, researchers attempted to attach nanoparticles to the bacterial membrane followed by modifying the membrane potential, which lowered the ATP level. This attachment also inhibited tRNA binding with the ribosome [106]. Azam et al. [107] examined the antimicrobial potential of zinc oxide (ZnO), copper oxide (CuO), and iron oxide (Fe2O3) nanoparticles toward gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa) and gram-positive bacteria (Staphylococcus Aureus and Bacillus subtilis). Accordingly, the most intense antibacterial activity was reported for the ZnO nanoparticles. In contrast, Fe2O3 nanoparticles exhibited the weakest antibacterial effects. The order of antibacterial activities of nanoparticles was found to be as ZnO (19.89 ± 1.43 nm), CuO (29.11 ± 1.61 nm), and Fe2O3 (35.16 ± 1.47 nm). These results clearly depicts that the size of the nanoparticles also play a momentous role in the antibacterial potential of each sample [107]. The anticipated mechanism of antimicrobial action of ZnO nanoparticles is: (1) ROS generation, (2) zinc ion release on the surface, (3) membrane dysfunction, and (4) entry into the cell. Also, the antimicrobial potential of ZnO nanoparticles is concentration and surface area dependent [108]. Mahapatra et al. [109] determined the antimicrobial action of copper oxide nanoparticles towards several bacterial species such as Klebsiella pneumoniae, P. aeruginosa, Shigella Salmonella paratyphi s. They found that CuO nanoparticles exhibited suitable antibacterial activity against those bacteria. It was assumed that nanoparticles should cross the bacterial cell membrane to damage the crucial enzymes of bacteria, which further induce cell death. For instance, green synthesized nanoparticles show enhanced antimicrobial activity compared to chemically synthesized or commercial nanoparticles. This is because the plants [such as Ocimum sanctum (Tulsi) and Azadirachta indica (neem)] employed for synthesis of nanoparticles have medicinal properties [110, 111]. For example, green synthesized silver nanoparticles showed an efficient and large zone of clearance against various bacterial strains compared to commercial silver nanoparticles (Fig. 7) [112].
Schematic for the antimicrobial activity for the five bacterial strains: a Staphylococcus aureus, b Klebsiella pneumonia, c Pseudomonas aeruginosa, d Vibrio cholera, and e Proteus vulgaris. Numbers of 1 through 6 inside each strain denote: (1) nickel chloride, (2) control ciprofloxacin, (3) Desmodium gangeticum root extract, (4) negative control, (5) nickel NPs prepared by a green method, and (6) nickel NPs prepared by a chemical method [229]
Catalytic activity
4-Nitrophenol and its derivatives are used to manufacture herbicides, insecticides, and synthetic dyestuffs, and they can significantly damage the ecosystem as common organic pollutants of wastewater. Due to its toxic and inhibitory nature, 4-nitrophenol is a great environmental concern. Therefore, the reduction of these pollutants is crucial. The 4-nitrophenol reduction product, 4-aminophenol, has been applied in diverse fields as an intermediate for paracetamol, sulfur dyes, rubber antioxidants, preparation of black/white film developers, corrosion inhibitors, and precursors in antipyretic and analgesic drugs [113, 114]. The simplest and most effective way to reduce 4-nitrophenol is to introduce NaBH4 as a reductant and a metal catalyst such as Au NPs [115], Ag NPs [116], CuO NPs [117], and Pd NPs [118]. Metal NPs exhibit admirable catalytic potential because of the high rate of surface adsorption ability and high surface area to volume ratio. Nevertheless, the viability of the reaction declines as a consequence of the substantial potential difference between donor (H3BO3/NaBH4) and acceptor molecules (nitrophenolate ion), which accounts for the higher activation energy barrier.
Metallic NPs can promote the rate of reaction by increasing the adsorption of reactants on their surface, thereby diminishing activation energy barriers [119, 120] (Fig. 8). The UV–visible spectrum of 4-nitrophenol was characterized by a sharp band at 400 nm as a nitrophenolate ion was produced in the presence of NaOH. The addition of Ag NPs (synthesized by Chenopodium aristatum L. stem extract) to the reaction medium led to a fast decay in the absorption intensity at 400 nm, which was concurrently accompanied by the appearance of a comparatively wide band at 313 nm, demonstrating the formation of 4-aminophenol [121] (Fig. 9).
Schematic of the metallic NP-mediated catalytic reduction of 4-nitrophenol to 4-aminophenol [120]
UV-visible spectra illustrating Chenopodium aristatum L. stem extract synthesized Ag NP-mediated catalytic reduction of 4-NP to 4-AP at three different temperatures a 30 °C, b 50 °C, and c 70 °C. Reduction in the absorption intensity of the characteristic nitrophenolate band at 400 nm accompanied by concomitant appearance of a wider absorption band at 313 nm indicates the formation of 4-AP [121]
Removal of pollutant dyes
Cationic and anionic dyes are a main class of organic pollutants used in various applications [122]. Organic dyes play a very imperative role due to their gigantic demand in paper mills, textiles, plastic, leather, food, printing, and pharmaceuticals industries. In textile industries, about 60% of dyes are consumed in the manufacturing process of pigmentation for many fabrics [123]. After the fabric process, nearly 15% of dyes are wasted and are discharged into the hydrosphere, and they represent a significant source of pollution due to their recalcitrance nature [124]. The pollutants from these manufacturing units are the most important sources of ecological pollution. They produce undesirable turbidity in the water, which will reduce sunlight penetration, and this leads to the resistance of photochemical synthesis and biological attacks to aquatic and marine life [125,126,127]. Therefore, the management of effluents containing dyes is one of the daunting challenge in the field of environmental chemistry [128].
The need for hygienic and safe drinking water is increasing day by day. Considering this fact, the use of metal and metal oxide semiconductor nanomaterials for oxidizing toxic pollutants has become of great interest in recent material research fields [129,130,131]. In the nano regime, semiconductor nanomaterials have superior photocatalytic activity relative to the bulk materials. Metal oxide semiconductor nanoparticles (like ZnO, TiO2, SnO2, WO3, and CuO) have been applied preferentially for the photocatalytic activity of synthetic dyes [31, 132,133,134]. The merits of these nanophotocatalysts (e.g., ZnO and TiO2 nanoparticles) are ascribable to their high surface area to mass ratio to enhance the adsorption of organic pollutants. The surface energy of the nanoparticles increases due to the large number of surface reactive sites available on the nanoparticle surfaces. This leads to an increase in rate of contaminant removal at low concentrations. Consequently, a lower quantity of nanocatalyst will be required to treat polluted water relative to the bulk material [135,136,137,138]. Like metal oxide nanoparticles, metal nanoparticles also show enhanced photocatalytic degradation of various pollutant dyes; for example, silver nanoparticles synthesized from Z. armatum leaf extract were utilized for the degradation of various pollutant dyes [127] (Fig. 10).
Schematic for the reduction of a safranine O, b methyl red, c methyl orange, and d methylene blue dyes using silver NPs synthesized from Z. armatum leaf extract by metallic nanoparticles [136]
Heavy metal ion sensing
Heavy metals (like Ni, Cu, Fe, Cr, Zn, Co, Cd, Pb, Cr, Hg, and Mn) are well-known for being pollutants in air, soil, and water. There are innumerable sources of heavy metal pollution such as mining waste, vehicle emissions, natural gas, paper, plastic, coal, and dye industries [139]. Some metals (like lead, copper, cadmium, and mercury ions) shows enhanced toxicity potential even at trace ppm levels [140, 141]. Therefore, the identification of toxic metals in the biological and aquatic environment has become a vital need for proper remedial processes [142,143,144]. Conventional techniques based on instrumental systems generally offer excellent sensitivity in multi-element analysis. However, experimental set ups to perform such analysis are highly expensive, time-consuming, skill-dependent, and non-portable.
Due to the tunable size and distance-dependent optical properties of metallic nanoparticles, they have been preferably employed for the detection of heavy metal ions in polluted water systems [145, 146]. The advantages of using metal NPs as colorimetric sensors for heavy metal ions in environmental systems/samples include simplicity, cost effectiveness, and high sensitivity at sub ppm levels. Karthiga et al. [147] synthesized AgNPs using various plant extracts used as colorimetric sensors for heavy metal ions like cadmium, chromium, mercury, calcium, and zinc (Cd2+, Cr3+, Hg2+, Ca2+, and Zn2+) in water. Their as-synthesized Ag nanoparticles showed colorimetric sensing of zinc and mercury ions (Zn2+ and Hg2+). Likewise, AgNPs synthesized using mango fresh leaves and dried leaves (fresh, MF-AgNPs and sun-dried, MD-AgNPs) exhibited selective sensing for mercury and lead ions (Hg2+ and Pb2+). Also, AgNPs prepared from pepper seed extract and green tea extract (GT-AgNPs) showed selective sensing properties for Hg2+, Pb2+, and Zn2+ ions [147] (Fig. 11).
Schematic of metal removal using metal oxides prepared by green synthesis. Left—a digital images and b absorption spectra of neem bark extract-mediated silver NPs (NB-AgNPs) with different metal ions and concentration-dependent studies of c Hg2+ and d Zn2+. Right—a digital images and b absorption spectra of fresh mango leaf extract-mediated silver NPs (MF-AgNPs) with different metal ions and c concentration-dependent studies of Pb2+ removal [147]
Conclusion and future prospects
‘Green’ synthesis of metal and metal oxide nanoparticles has been a highly attractive research area over the last decade. Numerous kinds of natural extracts (i.e., biocomponents like plant, bacteria, fungi, yeast, and plant extract) have been employed as efficient resources for the synthesis and/or fabrication of materials. Among them, plant extract has been proven to possess high efficiency as stabilizing and reducing agents for the synthesis of controlled materials (i.e., controlled shapes, sizes, structures, and other specific features). This review article was organized to encompass the ‘state of the art’ research on the ‘green’ synthesis of metal/metal oxide nanoparticles and their use in environmental remediation applications. Detailed synthesis mechanisms and an updated literature study on the role of solvents in synthesis have been reviewed thoroughly based on the literature available to help encounter the existing problems in ‘green’ synthesis. In summary, future research and development of prospective ‘green’ materials/nanoparticle synthesis should be directed toward extending laboratory-based work to an industrial scale by considering traditional/present issues, especially health and environmental effects. Nevertheless, ‘green’ material/nanoparticle synthesis based on biocomponent-derived materials/nanoparticles is likely to be applied extensively both in the field of environmental remediation and in other important areas like pharmaceutical, food, and cosmetic industries. Biosynthesis of metals and their oxide materials/nanoparticles using marine algae and marine plants is an area that remains largely unexplored. Accordingly, ample possibilities remain for the exploration of new green preparatory strategies based on biogenic synthesis.
References
Hoffmann MR, Martin ST, Choi W, Bahnemann DW. Environmental applications of semiconductor photocatalysis. Chem Rev. 1995;95:69–96. https://doi.org/10.1021/cr00033a004.
Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–20. https://doi.org/10.1021/ja057254a.
Kim JS, Kuk E, Yu KN, et al. Antimicrobial effects of silver nanoparticles. Nanomed Nanotechnol Biol Med. 2007;3:95–101. https://doi.org/10.1016/j.nano.2006.12.001.
Laurent S, Forge D, Port M, et al. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem Rev. 2008;108:2064–110. https://doi.org/10.1021/cr068445e.
Livage J, Henry M, Sanchez C. Sol–gel chemistry of transition metal oxides. Prog Solid State Chem. 1988;18:259–341. https://doi.org/10.1016/0079-6786(88)90005-2.
O’Neal DP, Hirsch LR, Halas NJ, et al. Photo-thermal tumor ablation in mice using near infrared-absorbing nanoparticles. Cancer Lett. 2016;209:171–6. https://doi.org/10.1016/j.canlet.2004.02.004.
Oskam G. Metal oxide nanoparticles: synthesis, characterization and application. J Sol–gel Sci Technol. 2006;37:161–4.
Sastry M, Ahmad A, Khan MI, Kumar R. Biosynthesis of metal nanoparticles using fungi and actinomycete. Curr Sci. 2003;85:162–70. https://doi.org/10.1016/S0927-7765(02)00174-1.
Su X-Y, Liu P-D, Wu H, Gu N. Enhancement of radiosensitization by metal-based nanoparticles in cancer radiation therapy. Cancer Biol Med. 2014;11:86–91. https://doi.org/10.7497/j.issn.2095-3941.2014.02.003.
Cao G. Nanastructures and nanomaterials—synthesis, properties and applications. Singapore: World Scientific; 2004.
Doble M, Kruthiventi AK. Green chemistry and engineering. Cambridge: Academic Press; 2007.
Aguilar Z. Nanomaterials for medical applications. Boston: Elsevier; 2013.
Dahoumane SA, Yéprémian C, Djédiat C, et al. Improvement of kinetics, yield, and colloidal stability of biogenic gold nanoparticles using living cells of Euglena gracilis microalga. J Nanoparticle Res. 2016. https://doi.org/10.1007/s11051-016-3378-1.
El-Rafie HM, El-Rafie MH, Zahran MK. Green synthesis of silver nanoparticles using polysaccharides extracted from marine macro algae. Carbohydr Polym. 2013;96:403–10. https://doi.org/10.1016/j.carbpol.2013.03.071.
Husen A, Siddiqi KS. Plants and microbes assisted selenium nanoparticles: characterization and application. J Nanobiotechnol. 2014;12:28.
Khan M, Al-Marri AH, Khan M, et al. Green approach for the effective reduction of graphene oxide using Salvadora persica L. root (Miswak) extract. Nanoscale Res Lett. 2015;10:1–9. https://doi.org/10.1186/s11671-015-0987-z.
Patel V, Berthold D, Puranik P, Gantar M. Screening of cyanobacteria and microalgae for their ability to synthesize silver nanoparticles with antibacterial activity. Biotechnol Reports. 2015;5:112–9. https://doi.org/10.1016/j.btre.2014.12.001.
Siddiqi KS, Husen A. Fabrication of metal nanoparticles from fungi and metal salts: scope and application. Nanoscale Res Lett. 2016;11:1–15.
Wadhwani SA, Shedbalkar UU, Singh R, Chopade BA. Biogenic selenium nanoparticles: current status and future prospects. Appl Microbiol Biotechnol. 2016;100:2555–66.
Gericke M, Pinches A. Microbial production of gold nanoparticles. Gold Bull. 2006;39:22–8. https://doi.org/10.1007/BF03215529.
Iravani S. Bacteria in nanoparticle synthesis: current status and future prospects. Int Sch Res Not. 2014;2014:1–18. https://doi.org/10.1155/2014/359316.
Thakkar KN, Mhatre SS, Parikh RY. Biological synthesis of metallic nanoparticles. Nanomed Nanotechnol Biol Med. 2010;6:257–62.
Chen Y-L, Tuan H-Y, Tien C-W, et al. Augmented biosynthesis of cadmium sulfide nanoparticles by genetically engineered Escherichia coli. Biotechnol Prog. 2009;25:1260–6. https://doi.org/10.1002/btpr.199.
Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanoparticle Res. 2008;10:507–17.
Narayanan KB, Sakthivel N. Synthesis and characterization of nano-gold composite using Cylindrocladium floridanum and its heterogeneous catalysis in the degradation of 4-nitrophenol. J Hazard Mater. 2011;189:519–25. https://doi.org/10.1016/j.jhazmat.2011.02.069.
Yurkov AM, Kemler M, Begerow D. Species accumulation curves and incidence-based species richness estimators to appraise the diversity of cultivable yeasts from beech forest soils. PLoS ONE. 2011;1:1. https://doi.org/10.1371/journal.pone.0023671.
Marchiol L. Synthesis of metal nanoparticles in living plants. Ital J Agron. 2012;7:274–82.
Anastas PT, Warner JC. 12 principles of green chemistry. Green chemistry: theory and practice. Oxford: Oxford University Press; 1998.
Vidya C, Hiremath S, Chandraprabha MN, et al. Green synthesis of ZnO nanoparticles by Calotropis gigantea. Int J Curr Eng Technol. 2013;1:118–20.
Gnanasangeetha D, Saralathambavani D. Biogenic production of zinc oxide nanoparticles using Acalypha indica. J Chem Biol Phys Sci. 2014;4:238–46.
Devi HS, Singh TD. Synthesis of copper oxide nanoparticles by a novel method and its application in the degradation of methyl orange. Adv Electron Electr Eng. 2014;4:83–8.
Maensiri S, Laokul P, Klinkaewnarong J, et al. Indium oxide (in 2O3) nanoparticles using aloe vera plant extract: synthesis and optical properties. J Optoelectron Adv Mater. 2008;10:161–5.
Gunalan S, Sivaraj R, Rajendran V. Green synthesized ZnO nanoparticles against bacterial and fungal pathogens. Prog Nat Sci Mater Int. 2012;22:693–700. https://doi.org/10.1016/j.pnsc.2012.11.015.
Iravani S. Green synthesis of metal nanoparticles using plants. Green Chem. 2011;13:2638. https://doi.org/10.1039/c1gc15386b.
Shanker U, Jassal V, Rani M, Kaith BS. Towards green synthesis of nanoparticles: from bio-assisted sources to benign solvents. A review. Int J Environ Anal Chem. 2016;96:801–35.
Yoosaf K, Ipe BI, Suresh CH, Thomas KG. In situ synthesis of metal nanoparticles and selective naked-eye detection of lead ions from aqueous media. J Phys Chem C. 2007;111:12839–47. https://doi.org/10.1021/jp073923q.
Sylvestre J, Poulin S, Kabashin AV, et al. Surface chemistry of gold nanoparticles produced by laser ablation in aqueous media. J Phys Chem B. 2004;108:16864–9. https://doi.org/10.1021/jp047134.
Er H, Yasuda H, Harada M, et al. Formation of silver nanoparticles from ionic liquids comprising N-alkylethylenediamine: effects of dissolution modes of the silver(I) ions in the ionic liquids. Colloids Surf A Physicochem Eng Asp. 2017;522:503–13. https://doi.org/10.1016/j.colsurfa.2017.03.046.
Srivastava V. In situ generation of ru nanoparticles to catalyze CO2 hydrogenation to formic acid. Catal Lett. 2014;144:1745–50. https://doi.org/10.1007/s10562-014-1321-6.
Vollmer C, Redel E, Abu-Shandi K, et al. Microwave irradiation for the facile synthesis of transition-metal nanoparticles (NPs) in ionic liquids (ILs) from metal-carbonyl precursors and Ru-, Rh-, and Ir-NP/IL dispersions as biphasic liquid-liquid hydrogenation nanocatalysts for cyclohexene. Chem A Eur J. 2010;16:3849–58. https://doi.org/10.1002/chem.200903214.
Zhang H, Cui H. Synthesis and characterization of functionalized ionic liquid-stabilized metal (gold and platinum) nanoparticles and metal nanoparticle/carbon nanotube hybrids. Langmuir. 2009;25:2604–12. https://doi.org/10.1021/la803347h.
Zhang ZC. Catalysis in ionic liquids. Adv Catal. 2006;49:153–237.
Dupont J, De Souza RF, Suarez PAZ. Ionic liquid (molten salt) phase organometallic catalysis. Chem Rev. 2002;102:3667–92. https://doi.org/10.1021/cr010338r.
van Rantwijk F, Sheldon RA. Biocatalysis in ionic liquids. Chem Rev. 2007;107:2757–85.
Welton T. Ionic liquids in catalysis. Coord Chem Rev. 2004;248:2459–77.
Bussamara R, Melo WWM, Scholten JD, et al. Controlled synthesis of Mn3O4 nanoparticles in ionic liquids. Dalton Trans. 2013;42:14473. https://doi.org/10.1039/c3dt32348j.
Lazarus LL, Riche CT, Malmstadt N, Brutchey RL. Effect of ionic liquid impurities on the synthesis of silver nanoparticles. Langmuir. 2012;28:15987–93. https://doi.org/10.1021/la303617f.
Li N, Bai X, Zhang S, et al. Synthesis of silver nanoparticles in ionic liquid by a simple effective electrochemical method. J Dispers Sci Technol. 2008;29:1059–61. https://doi.org/10.1080/01932690701815606.
Kim K-S, Demberelnyamba D, Lee H. Size-selective synthesis of gold and platinum nanoparticles using novel thiol-functionalized ionic liquids. Langmuir. 2004;20:556–60. https://doi.org/10.1021/la0355848.
Dupont J, Fonseca GS, Umpierre AP, et al. Transition-metal nanoparticles in imidazolium ionic liquids: recyclable catalysts for biphasic hydrogenation reactions. J Am Chem Soc. 2002;124:4228–9. https://doi.org/10.1021/ja025818u.
Bouquillon S, Courant T, Dean D, et al. Biodegradable ionic liquids: selected synthetic applications. Aust J Chem. 2007;60:843–7. https://doi.org/10.1071/CH07257.
Carter EB, Culver SL, Fox PA, et al. Sweet success: ionic liquids derived from non-nutritive sweeteners. Chem Commun (Camb). 2004. https://doi.org/10.1039/b313068a.
Harjani JR, Singer RD, Garcia MT, Scammells PJ. Biodegradable pyridinium ionic liquids: design, synthesis and evaluation. Green Chem. 2009;11:83–90. https://doi.org/10.1039/B811814K.
Imperato G, König B, Chiappe C. Ionic green solvents from renewable resources. Eur J Org Chem. 2007;2007:1049–58.
Fürstner A, Ackermann L, Beck K, et al. Olefin metathesis in supercritical carbon dioxide. J Am Chem Soc. 2001;123:9000–6. https://doi.org/10.1021/ja010952k.
Wittmann K, Wisniewski W, Mynott R, et al. Supercritical carbon dioxide as solvent and temporary protecting group for rhodium-catalyzed hydroaminomethylation. Chem A Eur J. 2001;7:4584–9. https://doi.org/10.1002/1521-3765(20011105)7:21%3c4584:AID-CHEM4584%3e3.0.CO;2-P.
Pollet P, Eckert CA, Liotta CL. Solvents for sustainable chemical processes. WIT Trans Ecol Environ. 2011;154:21–31. https://doi.org/10.2495/CHEM110031.
Ohde H, Hunt F, Wai CM. Synthesis of silver and copper nanoparticles in a water-in-supercritical-carbon dioxide microemulsion. Chem Mater. 2001;13:4130–5. https://doi.org/10.1021/cm010030g.
Sue K, Adschiri T, Arai K. Predictive model for equilibrium constants of aqueous inorganic species at subcritical and supercritical conditions. Ind Eng Chem Res. 2002;41:3298–306. https://doi.org/10.1021/ie010956y.
Kim M, Lee BY, Ham HC, et al. Facile one-pot synthesis of tungsten oxide (WO3− x) nanoparticles using sub and supercritical fluids. J Supercrit Fluids. 2016;111:8–13. https://doi.org/10.1016/j.supflu.2016.01.011.
Sun Q, Cai X, Li J, et al. Green synthesis of silver nanoparticles using tea leaf extract and evaluation of their stability and antibacterial activity. Colloids Surf A Physicochem Eng Asp. 2014;444:226–31. https://doi.org/10.1016/j.colsurfa.2013.12.065.
Sadeghi B, Gholamhoseinpoor F. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;134:310–5. https://doi.org/10.1016/j.saa.2014.06.046.
Fukushi K, Sato T. Using a surface complexation model to predict the nature and stability of nanoparticles. Environ Sci Technol. 2005;39:1250–6. https://doi.org/10.1021/es0491984.
Sharma VK, Siskova KM, Zboril R, Gardea-Torresdey JL. Organic-coated silver nanoparticles in biological and environmental conditions: fate, stability and toxicity. Adv Colloid Interface Sci. 2014;204:15–34. https://doi.org/10.1016/j.cis.2013.12.002.
Tejamaya M, Römer I, Merrifield RC, Lead JR. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ Sci Technol. 2012;46:7011–7. https://doi.org/10.1021/es2038596.
Levard C, Hotze EM, Lowry GV, Brown GE. Environmental transformations of silver nanoparticles: impact on stability and toxicity. Environ Sci Technol. 2012;46:6900–14.
Leonard K, Ahmmad B, Okamura H, Kurawaki J. In situ green synthesis of biocompatible ginseng capped gold nanoparticles with remarkable stability. Colloids Surf B Biointerfaces. 2011;82:391–6. https://doi.org/10.1016/j.colsurfb.2010.09.020.
Virkutyte J, Varma RS. Green synthesis of metal nanoparticles: biodegradable polymers and enzymes in stabilization and surface functionalization. Chem Sci. 2011;2:837. https://doi.org/10.1039/c0sc00338g.
Banerjee P, Satapathy M, Mukhopahayay A, Das P. Leaf extract mediated green synthesis of silver nanoparticles from widely available Indian plants: synthesis, characterization, antimicrobial property and toxicity analysis. Bioresour Bioprocess. 2014;1:1–10. https://doi.org/10.1186/s40643-014-0003-y.
Sneha K, Sathishkumar M, Mao J, et al. Corynebacterium glutamicum-mediated crystallization of silver ions through sorption and reduction processes. Chem Eng J. 2010;162:989–96. https://doi.org/10.1016/j.cej.2010.07.006.
Kalishwaralal K, Deepak V, Ramkumarpandian S, et al. Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater Lett. 2008;62:4411–3. https://doi.org/10.1016/j.matlet.2008.06.051.
Mittal AK, Chisti Y, Banerjee UC. Synthesis of metallic nanoparticles using plant extracts. Biotechnol Adv. 2013;31:346–56.
Dwivedi AD, Gopal K. Biosynthesis of silver and gold nanoparticles using Chenopodium album leaf extract. Colloids Surf A Physicochem Eng Asp. 2010;369:27–33. https://doi.org/10.1016/j.colsurfa.2010.07.020.
Jha AK, Prasad K, Kumar V, Prasad K. Biosynthesis of silver nanoparticles using eclipta leaf. Biotechnol Prog. 2009;25:1476–9. https://doi.org/10.1002/btpr.233.
Malik P, Shankar R, Malik V, et al. Green chemistry based benign routes for nanoparticle synthesis. J Nanoparticles. 2014;2014:1–14. https://doi.org/10.1155/2014/302429.
Li X, Xu H, Chen ZS, Chen G. Biosynthesis of nanoparticles by microorganisms and their applications. J Nanomater. 2011. https://doi.org/10.1155/2011/270974.
Mukunthan KS, Balaji S. Cashew apple juice (Anacardium occidentale L.) speeds up the synthesis of silver nanoparticles. Int J Green Nanotechnol. 2012;4:71–9. https://doi.org/10.1080/19430892.2012.676900.
Prathna TC, Mathew L, Chandrasekaran N, et al. Biomimetic synthesis of nanoparticles: science, technology and applicability. Biomimetics Learn Nat. 2010. https://doi.org/10.5772/8776.
Ahmad N, Sharma S, Alam MK, et al. Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf B Biointerfaces. 2010;81:81–6. https://doi.org/10.1016/j.colsurfb.2010.06.029.
Panigrahi S, Kundu S, Ghosh S, et al. General method of synthesis for metal nanoparticles. J Nanoparticle Res. 2004;6:411–4. https://doi.org/10.1007/s11051-004-6575-2.
Zayed MF, Eisa WH, Shabaka AA. Malva parviflora extract assisted green synthesis of silver nanoparticles. Spectrochim Acta Part A Mol Biomol Spectrosc. 2012;98:423–8. https://doi.org/10.1016/j.saa.2012.08.072.
Gruen LC. Interaction of amino acids with silver(I) ions. BBA Protein Struct. 1975;386:270–4. https://doi.org/10.1016/0005-2795(75)90268-8.
Tan YN, Lee JY, Wang DIC. Uncovering the design rules for peptide synthesis of metal nanoparticles. J Am Chem Soc. 2010;132:5677–86. https://doi.org/10.1021/Ja907454f.
Li S, Shen Y, Xie A, et al. Green synthesis of silver nanoparticles using Capsicum annuum L. extract. Green Chem. 2007;9:852. https://doi.org/10.1039/b615357g.
Huang Q, Li D, Sun Y, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnol. 2007;1:1. https://doi.org/10.1088/0957-4484/18/10/105104.
Mude N, Ingle A, Gade A, Rai M. Synthesis of silver nanoparticles using callus extract of Carica papaya—a first report. J Plant Biochem Biotechnol. 2009;18:83–6. https://doi.org/10.1007/BF03263300.
Kesharwani J, Yoon KY, Hwang J, Rai M. Phytofabrication of silver nanoparticles by leaf extract of Datura metel: hypothetical mechanism involved in synthesis. J Bionanosci. 2009;3:39–44. https://doi.org/10.1166/jbns.2009.1008.
Shankar SS, Ahmad A, Pasricha R, Sastry M. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem. 2003;13:1822. https://doi.org/10.1039/b303808b.
Singh AK, Talat M, Singh DP, Srivastava ON. Biosynthesis of gold and silver nanoparticles by natural precursor clove and their functionalization with amine group. J Nanoparticle Res. 2010;12:1667–75. https://doi.org/10.1007/s11051-009-9835-3.
Glusker JP, Katz AK, Bock CW. Metal ions in biological systems. Rigaku J. 1999;16:8–17.
Si S, Mandal TK. Tryptophan-based peptides to synthesize gold and silver nanoparticles: a mechanistic and kinetic study. Chem A Eur J. 2007;13:3160–8. https://doi.org/10.1002/chem.200601492.
Shah M, Fawcett D, Sharma S, et al. Green synthesis of metallic nanoparticles via biological entities. Materials (Basel). 2015;8:7278–308.
Dizaj SM, Lotfipour F, Barzegar-Jalali M, et al. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C. 2014;44:278–84.
Fair RJ, Tor Y. Antibiotics and bacterial resistance in the 21st century. Perspect Med Chem. 2014. https://doi.org/10.4137/pmc.s14459.
Jayaraman R. Antibiotic resistance: an overview of mechanisms and a paradigm shift. Curr Sci. 2009;96:1475–84.
Pelgrift RY, Friedman AJ. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv Drug Deliv Rev. 2013;65:1803–15.
Zinjarde S. Bio-inspired nanomaterials and their applications as antimicrobial agents. Chron Young Sci. 2012;3:74. https://doi.org/10.4103/2229-5186.94314.
Lok C, Ho C, Chen R, et al. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5:916–24. https://doi.org/10.1021/pr0504079.
Iavicoli I, Fontana L, Leso V, Bergamaschi A. The effects of nanomaterials as endocrine disruptors. Int J Mol Sci. 2013;14:16732–801. https://doi.org/10.3390/ijms140816732.
Yun H, Kim JD, Choi HC, Lee CW. Antibacterial activity of CNT-Ag and GO-Ag nanocomposites against gram-negative and gram-positive bacteria. Bull Korean Chem Soc. 2013;34:3261–4. https://doi.org/10.5012/bkcs.2013.34.11.3261.
Egger S, Lehmann RP, Height MJ, et al. Antimicrobial properties of a novel silver-silica nanocomposite material. Appl Environ Microbiol. 2009;75:2973–6. https://doi.org/10.1128/AEM.01658-08.
Tak YK, Pal S, Naoghare PK, et al. Shape-dependent skin penetration of silver nanoparticles: does it really matter. Sci Rep. 2015. https://doi.org/10.1038/srep16908.
Lima E, Guerra R, Lara V, Guzmán A. Gold nanoparticles as efficient antimicrobial agents for Escherichia coli and Salmonella typhi. Chem Cent J. 2013. https://doi.org/10.1186/1752-153x-7-11.
Tiwari PM, Vig K, Dennis VA, Singh SR. Functionalized gold nanoparticles and their biomedical applications. Nanomaterials. 2011;1:31–63. https://doi.org/10.3390/nano1010031.
Zhou Y, Kong Y, Kundu S, et al. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guérin. J Nanobiotechnol. 2012;1:1. https://doi.org/10.1186/1477-3155-10-19.
Cui Y, Zhao Y, Tian Y, et al. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials. 2012;33:2327–33. https://doi.org/10.1016/j.biomaterials.2011.11.057.
Azam A, Ahmed AS, Oves M, et al. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: a comparative study. Int J Nanomed. 2012;7:6003–9. https://doi.org/10.2147/IJN.S35347.
Buzea C, Pacheco II, Robbie K. Nanomaterials and nanoparticles: sources and toxicity. Biointerphases. 2007;2:MR17–71.
Mahapatra O, Bhagat M, Gopalakrishnan C, Arunachalam KD. Ultrafine dispersed CuO nanoparticles and their antibacterial activity. J Exp Nanosci. 2008;3:185–93. https://doi.org/10.1080/17458080802395460.
Ramteke C, Chakrabarti T, Sarangi BK, Pandey R. Synthesis of silver nanoparticles from the aqueous extract of leaves of Ocimum sanctum for enhanced antibacterial activity. Hindawi Publ Corp J Chem. 2013;2013:1–8. https://doi.org/10.1155/2013/278925.
Verma A, Mehata MS. Controllable synthesis of silver nanoparticles using neem leaves and their antimicrobial activity. J Radiat Res Appl Sci. 2016;9:109–15. https://doi.org/10.1016/j.jrras.2015.11.001.
Velmurugan P, Hong S-C, Aravinthan A, et al. Comparison of the physical characteristics of green-synthesized and commercial silver nanoparticles: evaluation of antimicrobial and cytotoxic effects. Arab J Sci Eng. 2017;42:201–8. https://doi.org/10.1007/s13369-016-2254-8.
Panigrahi S, Basu S, Praharaj S, et al. Synthesis and size-selective catalysis by supported gold nanoparticles: study on heterogeneous and homogeneous catalytic process. J Phys Chem C. 2007;111:4596–605. https://doi.org/10.1021/jp067554u.
Woo Y, Lai DY. Aromatic amino and nitro–amino compounds and their halogenated derivatives. In: Bingham E, Cohrssen B, Powell CH, editors. Patty’s toxicology. Wiley; 2012. https://doi.org/10.1002/0471435139.tox058.pub2.
Lim SH, Ahn E-Y, Park Y. Green synthesis and catalytic activity of gold nanoparticles synthesized by Artemisia capillaris water extract. Nanoscale Res Lett. 2016;11:474. https://doi.org/10.1186/s11671-016-1694-0.
Rostami-Vartooni A, Nasrollahzadeh M, Alizadeh M. Green synthesis of perlite supported silver nanoparticles using Hamamelis virginiana leaf extract and investigation of its catalytic activity for the reduction of 4-nitrophenol and Congo red. J Alloys Compd. 2016;680:309–14. https://doi.org/10.1016/j.jallcom.2016.04.008.
Sharma JK, Akhtar MS, Ameen S, et al. Green synthesis of CuO nanoparticles with leaf extract of Calotropis gigantea and its dye-sensitized solar cells applications. J Alloys Compd. 2015;632:321–5. https://doi.org/10.1016/j.jallcom.2015.01.172.
Gopalakrishnan R, Loganathan B, Dinesh S, Raghu K. Strategic green synthesis, characterization and catalytic application to 4-nitrophenol reduction of palladium nanoparticles. J Clust Sci. 2017;28:2123–31. https://doi.org/10.1007/s10876-017-1207-z.
Gangula A, Podila R, Rao AM, et al. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from Breynia rhamnoides. Langmuir. 2011;27:15268–74. https://doi.org/10.1021/la2034559.
Singh J, Kukkar P, Sammi H, et al. Enhanced catalytic reduction of 4-nitrophenol and congo red dye By silver nanoparticles prepared from Azadirachta indica leaf extract under direct sunlight exposure. Part Sci Technol. 2017. https://doi.org/10.1080/02726351.2017.1390512.
Yuan CG, Huo C, Gui B, et al. Green synthesis of silver nanoparticles using Chenopodium aristatum L. stem extract and their catalytic/antibacterial activities. J Clust Sci. 2017;28:1319–33. https://doi.org/10.1007/s10876-016-1147-z.
Habibi MH, Rezvani Z. Photocatalytic degradation of an azo textile dye (C.I. Reactive Red 195 (3BF)) in aqueous solution over copper cobaltite nanocomposite coated on glass by Doctor Blade method. Spectrochim Acta Part A Mol Biomol Spectrosc. 2015;147:173–7. https://doi.org/10.1016/j.saa.2015.03.077.
Carmen Z, Daniel S. Textile organic dyes—characteristics, polluting effects and separation/elimination procedures from industrial effluents—a critical overview. Organic pollutants ten years after the Stockholm convention—environmental and analytical update. London: InTech; 2012.
Ratna PBS. Pollution due to synthetic dyes toxicity and carcinogenicity studies and remediation. Int J Environ Sci. 2012;3:940–55. https://doi.org/10.6088/ijes.2012030133002.
Dutta AK, Maji SK, Adhikary B. γ-Fe2O3 nanoparticles: an easily recoverable effective photo-catalyst for the degradation of rose bengal and methylene blue dyes in the waste-water treatment plant. Mater Res Bull. 2014;49:28–34. https://doi.org/10.1016/j.materresbull.2013.08.024.
Gonawala KH, Mehta MJ. Removal of color from different dye wastewater by using ferric oxide as an adsorbent. Int J Eng Res Appl. 2014;4:102–9.
Jyoti K, Singh A. Green synthesis of nanostructured silver particles and their catalytic application in dye degradation. J Genet Eng Biotechnol. 2016;14:311–7. https://doi.org/10.1016/j.jgeb.2016.09.005.
Wesenberg D, Kyriakides I, Agathos SN. White-rot fungi and their enzymes for the treatment of industrial dye effluents. Biotechnol Adv. 2003;22:161–87. https://doi.org/10.1016/j.biotechadv.2003.08.011.
Fowsiya J, Madhumitha G, Al-Dhabi NA, Arasu MV. Photocatalytic degradation of Congo red using Carissa edulis extract capped zinc oxide nanoparticles. J Photochem Photobiol B Biol. 2016;162:395–401. https://doi.org/10.1016/j.jphotobiol.2016.07.011.
Nakkala JR, Bhagat E, Suchiang K, Sadras SR. Comparative study of antioxidant and catalytic activity of silver and gold nanoparticles synthesized from Costus pictus leaf extract. J Mater Sci Technol. 2015;31:986–94. https://doi.org/10.1016/j.jmst.2015.07.002.
Varadavenkatesan T, Selvaraj R, Vinayagam R. Phyto-synthesis of silver nanoparticles from Mussaenda erythrophylla leaf extract and their application in catalytic degradation of methyl orange dye. J Mol Liquids. 2016;221:1063–70. https://doi.org/10.1016/j.molliq.2016.06.064.
Bhuyan T, Mishra K, Khanuja M, et al. Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Mater Sci Semicond Process. 2015;32:55–61. https://doi.org/10.1016/j.mssp.2014.12.053.
Stan M, Popa A, Toloman D, et al. Enhanced photocatalytic degradation properties of zinc oxide nanoparticles synthesized by using plant extracts. Mater Sci Semicond Process. 2015;39:23–9. https://doi.org/10.1016/j.mssp.2015.04.038.
Thandapani K, Kathiravan M, Namasivayam E, et al. Enhanced larvicidal, antibacterial, and photocatalytic efficacy of TiO2 nanohybrids green synthesized using the aqueous leaf extract of Parthenium hysterophorus. Environ Sci Pollut Res. 2017;25:1–12. https://doi.org/10.1007/s11356-017-9177-0.
Astruc D. Nanoparticles and catalysis. Weinheim: Wiley; 2008.
Dror I, Baram D, Berkowitz B. Use of nanosized catalysts for transformation of chloro-organic pollutants. Environ Sci Technol. 2005;39:1283–90. https://doi.org/10.1021/es0490222.
Pradeep T, Anshup. Noble metal nanoparticles for water purification: a critical review. Thin Solid Films. 2009;517:6441–78.
Tsuda A, Konduru NV. The role of natural processes and surface energy of inhaled engineered nanoparticles on aggregation and corona formation. NanoImpact. 2016;2:38–44.
Zhang M, Liu Y-Q, Ye B-C. Colorimetric assay for parallel detection of Cd2+, Ni2+ and Co2+ using peptide-modified gold nanoparticles. Analyst. 2012;137:601–7. https://doi.org/10.1039/c1an15909g.
Mehta VN, Kumar MA, Kailasa SK. Colorimetric detection of copper in water samples using dopamine dithiocarbamate-functionalized au nanoparticles. Ind Eng Chem Res. 2013;52:4414–20. https://doi.org/10.1021/ie302651f.
Que EL, Domaille DW, Chang CJ. Metals in neurobiology: probing their chemistry and biology with molecular imaging. Chem Rev. 2008;108:1517–49.
Aragay G, Pons J, Merkoçi A. Recent trends in macro-, micro-, and nanomaterial-based tools and strategies for heavy-metal detection. Chem Rev. 2011;111:3433–58. https://doi.org/10.1021/cr100383r.
Nolan EM, Lippard SJ. Tools and tactics for the optical detection of mercuric ion. Chem Rev. 2008;108:3443–80.
Ray PC. Size and shape dependent second order nonlinear optical properties of nanomaterials and their application in biological and chemical sensing. Chem Rev. 2010;110:5332–65. https://doi.org/10.1021/cr900335q.
Annadhasan M, Muthukumarasamyvel T, Sankar Babu VR, Rajendiran N. Green synthesized silver and gold nanoparticles for colorimetric detection of Hg2+, Pb2+, and Mn2+ in aqueous medium. ACS Sustain Chem Eng. 2014;2:887–96. https://doi.org/10.1021/sc400500z.
Maiti S, Gadadhar B, Laha JK. Detection of heavy metals (Cu+2, Hg+2) by biosynthesized silver nanoparticles. Appl Nanosci. 2016;6:529–38. https://doi.org/10.1007/s13204-015-0452-4.
Karthiga D, Anthony SP. Selective colorimetric sensing of toxic metal cations by green synthesized silver nanoparticles over a wide pH range. RSC Adv. 2013;3:16765–74. https://doi.org/10.1039/C3RA42308E.
Hulkoti NI, Taranath TC. Biosynthesis of nanoparticles using microbes—a review. Colloids Surf B Biointerfaces. 2014;121:474–83.
Setua P, Pramanik R, Sarkar S, et al. Synthesis of silver nanoparticle in imidazolium and pyrolidium based ionic liquid reverse micelles: a step forward in nanostructure inorganic material in room temperature ionic liquid field. J Mol Liq. 2011;162:33–7. https://doi.org/10.1016/j.molliq.2011.05.015.
Ge L, Chen L, Guo R. Microstructure and lubrication properties of lamellar liquid crystal in Brij30/[Bmim]PF6/H2O system. Tribol Lett. 2007;28:123–30. https://doi.org/10.1007/s11249-007-9256-3.
Obliosca JM, Arellano IHJ, Huang MH, Arco SD. Double layer micellar stabilization of gold nanocrystals by greener ionic liquid 1-butyl-3-methylimidazolium lauryl sulfate. Mater Lett. 2010;64:1109–12. https://doi.org/10.1016/j.matlet.2010.02.029.
Itoh H, Naka K, Chujo Y. Synthesis of gold nanoparticles modified with ionic liquid based on the imidazolium cation. J Am Chem Soc. 2004;126:3026–7. https://doi.org/10.1021/ja039895g.
Lazarus LL, Yang AS-J, Chu S, et al. Flow-focused synthesis of monodisperse gold nanoparticles using ionic liquids on a microfluidic platform. Lab Chip. 2010;10:3377. https://doi.org/10.1039/c0lc00297f.
Khare V, Li ZH, Mantion A, et al. Strong anion effects on gold nanoparticle formation in ionic liquids. J Mater Chem. 2010;20:1332–9. https://doi.org/10.1039/B917467b.
Bhatt AI, Mechler Á, Martin LL, Bond AM. Synthesis of Ag and Au nanostructures in an ionic liquid: thermodynamic and kinetic effects underlying nanoparticle, cluster and nanowire formation. J Mater Chem. 2007;17:2241. https://doi.org/10.1039/b618036a.
Raut D, Wankhede K, Vaidya V, et al. Copper nanoparticles in ionic liquids: recyclable and efficient catalytic system for 1,3-dipolar cycloaddition reaction. Catal Commun. 2009;10:1240–3. https://doi.org/10.1016/j.catcom.2009.01.027.
Sunkar S, Nachiyar CV. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac J Trop Biomed. 2012;2:953–9. https://doi.org/10.1016/S2221-1691(13)60006-4.
Shivaji S, Madhu S, Singh S. Extracellular synthesis of antibacterial silver nanoparticles using psychrophilic bacteria. Process Biochem. 2011;46:1800–7. https://doi.org/10.1016/j.procbio.2011.06.008.
Korbekandi H, Iravani S, Abbasi S. Optimization of biological synthesis of silver nanoparticles using Lactobacillus casei subsp. casei. J Chem Technol Biotechnol. 2012;87:932–7. https://doi.org/10.1002/jctb.3702.
Fu M, Li Q, Sun D, et al. Rapid preparation process of silver nanoparticles by bioreduction and their characterizations. Chin J Chem Eng. 2006;14:114–7. https://doi.org/10.1016/S1004-9541(06)60046-3.
Lengke MF, Fleet ME, Southam G. Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold(I)− thiosulfate and gold(III)− chloride complexes. Nano. 2006. https://doi.org/10.1021/es061040r.
Southam G, Beveridge TJ. The in vitro formation of placer gold by bacteria. Geochim Cosmochim Acta. 1994;58:4527–30. https://doi.org/10.1016/0016-7037(94)90355-7.
Wen L, Lin Z, Gu P, et al. Extracellular biosynthesis of monodispersed gold nanoparticles by a SAM capping route. J Nanoparticle Res. 2009;11:279–88. https://doi.org/10.1007/s11051-008-9378-z.
Konishi Y, Tsukiyama T, Tachimi T, et al. Microbial deposition of gold nanoparticles by the metal-reducing bacterium Shewanella algae. Electrochim Acta. 2007;53:186–92. https://doi.org/10.1016/j.electacta.2007.02.073.
Du L, Jiang H, Liu X, Wang E. Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5α and its application on direct electrochemistry of hemoglobin. Electrochem Commun. 2007;9:1165–70. https://doi.org/10.1016/j.elecom.2007.01.007.
Deplanche K, Macaskie LE. Biorecovery of gold by Escherichia coli and Desulfovibrio desulfuricans. Biotechnol Bioeng. 2008;99:1055–64. https://doi.org/10.1002/bit.21688.
He S, Guo Z, Zhang Y, et al. Biosynthesis of gold nanoparticles using the bacteria Rhodopseudomonas capsulata. Mater Lett. 2007;61:3984–7. https://doi.org/10.1016/j.matlet.2007.01.018.
Philipse AP, Maas D. Magnetic colloids from magnetotactic bacteria: chain formation and colloidal stability. Langmuir. 2002;18:9977–84. https://doi.org/10.1021/la0205811.
Mann S, Frankel RB, Blakemore RP. Structure, morphology and crystal growth of bacterial magnetite. Nature. 1984;310:405–7. https://doi.org/10.1038/310405a0.
Marshall MJ, Beliaev AS, Dohnalkova AC, et al. c-Type cytochrome-dependent formation of U(IV) nanoparticles by Shewanella oneidensis. PLoS Biol. 2006;4:1324–33. https://doi.org/10.1371/journal.pbio.0040268.
Holmes JD, Smith PR, Richardson DJ, et al. Energy-dispersive X-ray analysis of the extracellular cadmium sulfide crystallites of Klebsiella aerogenes. Arch Microbiol. 1995;163:143–7.
Ravindra BK, Rajasab AH. A comparative study on biosynthesis of silver nanoparticles using four different fungal species. Int J Pharm Pharm Sci. 2014;6(1):372–6.
Mukherjee P, Ahmad A, Mandal D, et al. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 2001;1:515–9. https://doi.org/10.1021/nl0155274.
Bhainsa KC, D’Souza SF. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B Biointerfaces. 2006;47:160–4. https://doi.org/10.1016/j.colsurfb.2005.11.026.
Vigneshwaran N, Ashtaputre NM, Varadarajan PV, et al. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett. 2007;61:1413–8. https://doi.org/10.1016/j.matlet.2006.07.042.
Vigneshwaran N, Kathe AA, Varadarajan PV, et al. Biomimetics of silver nanoparticles by white rot fungus, Phaenerochaete chrysosporium. Colloids Surf B Biointerfaces. 2006;53:55–9. https://doi.org/10.1016/j.colsurfb.2006.07.014.
Gade AK, Bonde P, Ingle AP, et al. Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J Biobased Mater Bioenergy. 2008;2:243–7. https://doi.org/10.1166/jbmb.2008.401.
Basavaraja S, Balaji SD, Lagashetty A, et al. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater Res Bull. 2008;43:1164–70. https://doi.org/10.1016/j.materresbull.2007.06.020.
Balaji DS, Basavaraja S, Deshpande R, et al. Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Colloids Surf B Biointerfaces. 2009;68:88–92. https://doi.org/10.1016/j.colsurfb.2008.09.022.
Sanghi R, Verma P. Biomimetic synthesis and characterisation of protein capped silver nanoparticles. Bioresour Technol. 2009;100:501–4. https://doi.org/10.1016/j.biortech.2008.05.048.
Ingle A, Rai M, Gade A, Bawaskar M. Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. J Nanoparticle Res. 2009;11:2079–85. https://doi.org/10.1007/s11051-008-9573-y.
Shaligram NS, Bule M, Bhambure R, et al. Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process Biochem. 2009;44:939–43. https://doi.org/10.1016/j.procbio.2009.04.009.
Kathiresan K, Manivannan S, Nabeel MA, Dhivya B. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B Biointerfaces. 2009;71:133–7. https://doi.org/10.1016/j.colsurfb.2009.01.016.
Birla SS, Tiwari VV, Gade AK, et al. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol. 2009;48:173–9. https://doi.org/10.1111/j.1472-765X.2008.02510.x.
Gajbhiye M, Kesharwani J, Ingle A, et al. Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomed Nanotechnol Biol Med. 2009;5:382–6. https://doi.org/10.1016/j.nano.2009.06.005.
Fayaz AM, Balaji K, Girilal M, et al. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomed Nanotechnol Biol Med. 2010. https://doi.org/10.1016/j.nano.2009.04.006.
Binupriya AR, Sathishkumar M, Yun SI. Biocrystallization of silver and gold ions by inactive cell filtrate of Rhizopus stolonifer. Colloids Surf B Biointerfaces. 2010;79:531–4. https://doi.org/10.1016/j.colsurfb.2010.05.021.
Ahmad A, Senapati S, Khan MI, et al. Extra-/intracellular biosynthesis of gold nanoparticles by an alkalotolerant fungus, Trichothecium sp. J Biomed Nanotechnol. 2005;1:47–53. https://doi.org/10.1166/jbn.2005.012.
Senapati S, Ahmad A, Khan MI, et al. Extracellular biosynthesis of bimetallic Au–Ag alloy nanoparticles. Small. 2005;1:517–20. https://doi.org/10.1002/smll.200400053.
Raliya R, Tarafdar JC. Biosynthesis and characterization of zinc, magnesium and titanium nanoparticles: an eco-friendly approach. Int Nano Lett. 2014;4:93. https://doi.org/10.1007/s40089-014-0093-8.
Raliya R, Biswas P, Tarafdar JC. TiO2 nanoparticle biosynthesis and its physiological effect on mung bean (Vigna radiata L.). Biotechnol Rep. 2015;5:22–6. https://doi.org/10.1016/j.btre.2014.10.009.
Kowshik M, Vogel W, Urban J, et al. Microbial synthesis of semiconductor PbS nanocrystallites. Adv Mater. 2002;14:815–8. https://doi.org/10.1002/1521-4095(20020605)14:11%3c815:AID-ADMA815%3e3.0.CO;2-K.
Mourato A, Gadanho M, Lino AR, Tenreiro R. Biosynthesis of crystalline silver and gold nanoparticles by extremophilic yeasts. Bioinorg Chem Appl. 2011;1:1. https://doi.org/10.1155/2011/546074.
Chandran SP, Chaudhary M, Pasricha R, et al. Synthesis of gold nanotriangles and silver nanoparticles using aloe vera plant extract. Biotechnol Prog. 2006. https://doi.org/10.1021/bp0501423.
Krishnaraj C, Jagan EG, Rajasekar S, et al. Synthesis of silver nanoparticles using Acalypha indica leaf extracts and its antibacterial activity against water borne pathogens. Colloids Surf B Biointerfaces. 2010;1:1. https://doi.org/10.1016/j.colsurfb.2009.10.008.
Kasthuri J, Veerapandian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B Biointerfaces. 2009;68:55–60. https://doi.org/10.1016/j.colsurfb.2008.09.021.
Armendariz V, Herrera I, Peralta-Videa JR, et al. Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J Nanoparticle Res. 2004;6:377–82. https://doi.org/10.1007/s11051-004-0741-4.
Shankar SS, Rai A, Ahmad A, Sastry M. Rapid synthesis of Au, Ag, and bimetallic Au core Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 2004;1:1. https://doi.org/10.1016/j.jcis.2004.03.003.
Mondal S, Roy N, Laskar RA, et al. Biogenic synthesis of Ag, Au and bimetallic Au/Ag alloy nanoparticles using aqueous extract of mahogany (Swietenia mahogani JACQ.) leaves. Colloids Surfaces B Biointerfaces. 2011;82:497–504. https://doi.org/10.1016/j.colsurfb.2010.10.007.
Haverkamp RG, Marshall AT. The mechanism of metal nanoparticle formation in plants: limits on accumulation. J Nanoparticle Res. 2009;11:1453–63. https://doi.org/10.1007/s11051-008-9533-6.
Prathna TC, Chandrasekaran N, Raichur AM, Mukherjee A. Biomimetic synthesis of silver nanoparticles by Citrus limon (lemon) aqueous extract and theoretical prediction of particle size. Colloids Surf B Biointerfaces. 2011;82:152–9. https://doi.org/10.1016/j.colsurfb.2010.08.036.
Narayanan KB, Sakthivel N. Coriander leaf mediated biosynthesis of gold nanoparticles. Mater Lett. 2008;62:4588–90. https://doi.org/10.1016/j.matlet.2008.08.044.
Shankar SS, Rai A, Ahmad A, Sastry M. Controlling the optical properties of lemongrass extract synthesized gold nanotriangles and potential application in infrared-absorbing optical coatings. Chem Mater. 2005;17:566–72. https://doi.org/10.1021/cm048292g.
Jha AK, Prasad K. Green synthesis of silver nanoparticles using cycas leaf. Int J Green Nanotechnol Phys Chem. 2010;1:110–7. https://doi.org/10.1080/19430871003684572.
Song JY, Kim BS. Biological synthesis of bimetallic Au/Ag nanoparticles using Persimmon (Diopyros kaki) leaf extract. Korean J Chem Eng. 2008;25:808–11. https://doi.org/10.1007/s11814-008-0133-z.
Ankamwar B, Chaudhary M, Sastry M. Gold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensing. Synth React Inorg Metal Org Nano-Metal Chem. 2005;35:19–26. https://doi.org/10.1081/SIM-200047527.
Ravindra S, Murali Mohan Y, Narayana Reddy N, Mohana Raju K. Fabrication of antibacterial cotton fibres loaded with silver nanoparticles via “green approach”. Colloids Surf A Physicochem Eng Asp. 2010;367:31–40. https://doi.org/10.1016/j.colsurfa.2010.06.013.
Dubey M, Bhadauria S, Kushwah BS. Green synthesis of nanosilver particles from extract of Eucalyptus hybrida (Safeda) leaf. Dig J Nanomater Biostruct. 2009;4:537–43.
Veerasamy R, Xin TZ, Gunasagaran S, et al. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc. 2010. https://doi.org/10.1016/j.jscs.2010.06.004.
Jia L, Zhang Q, Li Q, Song H. The biosynthesis of palladium nanoparticles by antioxidants in Gardenia jasminoides Ellis: long lifetime nanocatalysts for p-nitrotoluene hydrogenation. Nanotechnology. 2009. https://doi.org/10.1088/0957-4484/20/38/385601.
Raghunandan D, Bedre MD, Basavaraja S, et al. Rapid biosynthesis of irregular shaped gold nanoparticles from macerated aqueous extracellular dried clove buds (Syzygium aromaticum) solution. Colloids Surf B Biointerfaces. 2010;79:235–40. https://doi.org/10.1016/j.colsurfb.2010.04.003.
Bar H, Bhui DK, Sahoo GP, et al. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A Physicochem Eng Asp. 2009. https://doi.org/10.1016/j.colsurfa.2009.02.008.
Mochochoko T, Oluwafemi OS, Jumbam DN, Songca SP. Green synthesis of silver nanoparticles using cellulose extracted from an aquatic weed; water hyacinth. Carbohydr Polym. 2013;98:290–4. https://doi.org/10.1016/j.carbpol.2013.05.038.
Gardea-Torresdey JL, Gomez E, Peralta-Videa JR, et al. Alfalfa sprouts: a natural source for the synthesis of silver nanoparticles. Langmuir. 2003. https://doi.org/10.1021/la020835i.
Gardea-Torresdey JL, Parsons JG, Gomez E, et al. Formation and growth of au nanoparticles inside live alfalfa plants. Nano Lett. 2002;2:397–401. https://doi.org/10.1021/nl015673+.
Gardea-Torresdey JL, Tiemann KJ, Gamez G, et al. Gold nanoparticles obtained by bio-precipitation from gold(III) solutions. J Nanoparticle Res. 1999;1:397–404. https://doi.org/10.1023/A:1010008915465.
Parashar UK, Saxena PS. Bioinspired synthesis of silver nanoparticles. J Nanomater. 2009;4:159–66.
Herrera-Becerra R, Zorrilla C, Rius JL, Ascencio JA. Electron microscopy characterization of biosynthesized iron oxide nanoparticles. Appl Phys A Mater Sci Process. 2008;91:241–6.
Singh J, Singh N, Rathi A, et al. Facile approach to synthesize and characterization of silver nanoparticles by using mulberry leaves extract in aqueous medium and its application in antimicrobial activity. J Nanostructures. 2017;7:134–40. https://doi.org/10.22052/jns.2017.02.007.
Santhoshkumar T, Rahuman AA, Rajakumar G, et al. Synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res. 2011;108:693–702. https://doi.org/10.1007/s00436-010-2115-4.
Singh J, Mehta A, Rawat M, Basu S. Green synthesis of silver nanoparticles using sun dried tulsi leaves and its catalytic application for 4-nitrophenol reduction. J Environ Chem Eng. 2018;6:1468–74. https://doi.org/10.1016/j.jece.2018.01.054.
Philip D, Unni C. Extracellular biosynthesis of gold and silver nanoparticles using Krishna tulsi (Ocimum sanctum) leaf. Phys E Low Dimens Syst Nanostructures. 2011;43:1318–22. https://doi.org/10.1016/j.physe.2010.10.006.
Ghodake GS, Deshpande NG, Lee YP, Jin ES. Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf B Biointerfaces. 2010;75:584–9. https://doi.org/10.1016/j.colsurfb.2009.09.040.
Raghunandan D, Basavaraja S, Mahesh B, et al. Biosynthesis of stable polyshaped gold nanoparticles from microwave-exposed aqueous extracellular anti-malignant guava (Psidium guajava) leaf extract. NanoBiotechnology. 2009;5:34–41. https://doi.org/10.1007/s12030-009-9030-8.
Qu J, Luo C, Hou J. Synthesis of ZnO nanoparticles from Zn-hyperaccumulator (Sedum alfredii Hance) plants. IET Micro Nano Lett. 2011;6:174–6.
Dubey SP, Lahtinen M, Sillanpää M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010;45:1065–71. https://doi.org/10.1016/j.procbio.2010.03.024.
Ankamwar B. Biosynthesis of gold nanoparticles (green-gold) using leaf extract of Terminalia catappa. J Chem. 2010;7:1334–9. https://doi.org/10.1155/2010/745120.
Makarov VV, Love AJ, Sinitsyna OV, Makarova SS, Yaminsky IV, Taliansky ME et al. “Green” nanotechnologies: synthesis of metal nanoparticles using plants. Acta Naturae. 2014;6:35–44.
Sudhasree S, Shakila Banu A, Brindha P, Kurian GA. Synthesis of nickel nanoparticles by chemical and green route and their comparison in respect to biological effect and toxicity. Toxicol Environ Chem. 2014;96:743–54.
Authors’ contributions
JS, KHK and PK made substantial contributions to interpretation of literature; drafted the article and revised it critically. All made substantial contributions to draft the article and revised it critically for important intellectual content and gave approval to the submitted manuscript. All authors read and approved the final manuscript.
Acknowledgements
The corresponding author (KHK) acknowledges a supporting Grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (No. 2016R1E1A1A01940995). Dr. Pawan Kumar would like to thank SERB and UGC, New Delhi, for the ‘Empowerment and Equity Opportunities for Excellence in Science’ video file No. EEQ/2016/00484 and the UGC-BSR Start Up-Research Project.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
Without restrictions.
Consent for publication
Not applicable.
Ethics approval and consent to participate
All authors read and approved the final manuscript.
Funding
The corresponding author (KHK and PK) acknowledges a supporting grant from the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, & Future Planning (No. 2016R1E1A1A01940995) and ‘Empowerment and Equity Opportunities for Excellence in Science’ video file No. EEQ/2016/00484 and the UGC-BSR Start Up-Research Project.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Singh, J., Dutta, T., Kim, KH. et al. ‘Green’ synthesis of metals and their oxide nanoparticles: applications for environmental remediation. J Nanobiotechnol 16, 84 (2018). https://doi.org/10.1186/s12951-018-0408-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12951-018-0408-4