Abstract
This study aimed to develop and evaluate live and inactivated vaccines to Aeromonas veronii pathogenicity in Nile tilapia. Therefore, five well-identified Aeromonas veronii isolates, including A (HY1), A (HY2), A (HY3), A (HY4), and A (HY6) isolated from diseased Nile tilapia (Oreochromis niloticus), were used for vaccine preparation. Virulence genes detected by a polymerase chain reaction (PCR) and lethal dose determination were conducted. Nile tilapia, each with a body weight of 25 ± 0.5 g were divided into six experimental groups (each of 20): T1 group (control), fish were injected with saline as a negative control, T2 group (formalin-killed vaccine) for the A (HY2) strain, T3 group ( formalized killed vaccine) for the A (HY4), T4 group (autoclaved vaccine) for the A (HY2), T5 group (autoclaved vaccine) for A (HY4), and T6 (live vaccine) for A (HY1), triplicate. At the end of the immunization period, all groups were challenged by A. veronii, A (HY2). Blood samples were drawn 21 days post-immunization and 3 days after the challenge test for antibody titer assay. The results showed that the pathogenicity of strains A (HY2) and A (HY4) was the strongest, as the lethality rates (LR) were 100% and 90%, respectively, whereas the pathogenicity was moderate for strains A (HY3) and A (HY6) (LR 60% for each). A (AY1) was the weakest strain as no dead fish was found for this strain. The presence of alt, act, aerolysin, lipase, and fla genes as the main cause of the pathogenesis. The best protective efficacy was obtained from the live vaccine, A (HY1) with a protective rate of about 94.12% (relative percentage of survival, RPS), compared to autoclaved killed vaccines and formalin-killed vaccines. Based on immunoglobulin estimation (IgM) and RPS%, our data concluded that A (HY1) live vaccine had the best vaccine prophylactic effect against the highly pathogenic strain A(HY2).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Bacterial diseases caused by motile aeromonads represent the most common problems in aquaculture freshwater fish. They are considered the body of knowledge that generally links Aeromonas hydrophila and A. veronii infection (Austin and Austin 2016; Dong et al. 2017a, 2017b). Aeromonas hydrophila and other motile Aeromonas species, including A. sobria, A. veronii, and A. cavieae are pathogens that cause motile Aeromonas septicemia and are also linked to public health risks in humans (Janda and Abbott 2010). Aeromonas veronii belongs to the Aeromonadaceae family and it is a Gram-negative, rod-shaped, mesophilic, motile bacterium. Furthermore, it can be found in a variety of aquatic settings (Parte 2014).
Previous research shows that many number of interconnected factors cause the pathogenicity of Aeromonas. There are confirmed virulence factors, such as outer membrane proteins, toxins, proteases, motility-related factors, secretion systems, quorum, and iron ion acquisition systems, as well as hemolytic and cytotoxic activities (Qin et al. 2022). Pathogenicity was related to the presence of virulence genes in Aeromonas strains, which play an essential role in disease development (Hossain et al. 2018; Sun et al. 2016; Zhang et al. 2019). Antibiotics are used to control bacterial diseases in aquaculture. However, this can lead to the development of antibiotic resistance and antimicrobial residues in aquaculture (Vivekanandhan et al. 2002). A recent study by Salah et al. (2021) demonstrated a multi-drug resistance of Aeromonas hydophila chloramphenicol (67.4%), followed by amikacin (51.9%) and gentamicin (47.1%).
Many researchers have developed vaccines against A. hydrophila, primarily in warm water fishes. The experimental vaccine can protect many fish species from A. hydrophila infection following vaccination (Aoki 1999). The commercial vaccine efficacy is complicated by many phenotypic and serological specificities found in Aeromonas spp. (Nielsen et al. 2001; Toranzo et al. 2009). Vaccines are formulated using either antigens (pathogenic bacterial), or whole bacterial killed cells to activate the innate and the cellular and humeral immune (Adams 2019) The effectiveness of a good vaccine is related to the appropriate immunization routes for stimulating where intraperitoneal (i.p.) injection has mostly provided good results, although it is stressful to the animals, impractical for farmer level, labor intensive, and expensive (Assefa and Abunna 2018). Another route of immunization is recently used by feed-based oral immunization that proved to be less laborious, more applicable for mass vaccination at farmer level (Monir et al. 2020). There are different types of vaccines used in tilapia including inactivated monovalent vaccine, inactivated polyvalent vaccine, live bacterial vaccine, and recombinant vaccines ( Shirajum Monir et al. 2020).
Nile tilapia (Oreochromis niloticus) comprise a well-known freshwater fish genus that includes one of the world’s most popular cultivated fish kinds. They are cultivated in many countries ranging from extensive to intensive/commercial ponds. It is the world’s second-largest group of cultured freshwater fish (Mapenzi and Mmochi 2016). Although tilapia aquaculture has improved rapidly, it still faces enormous problems from the number of devastating bacterial and viral diseases (Austin and Austin 2016).
Six virulence genes related to pathogenicity including aerolysin, cytotonic enterotoxins, elastase, glycerophospholipid: cholesterol acyltransferase, lipase, and serine protease were identified A. veronii isolate from diseased crucian carp (Carassius auratus gibelio) (chen et al 2019). Moreover, The prevalence of the virulence genes detected in the isolated motile aeromonads from tilapia was aerolysin (aer), 52.2%; elastase (ahp), 26.25%; hemolysin (hyl), 35%; and lipase (lip), 3.75% (El-Gohary et al. 2013).
Vaccines for aquaculture have been successful in reducing the use of antibiotics, especially in developed countries (Sommerset et al. 2005; Gudding and Van Muiswinkel 2013). The use of antibiotics has obvious drawbacks, more predominantly, the risk to public health because of the development of antimicrobial resistance. In order to reduce the use of chemicals and antibiotics in aquaculture and marine environment, the use of vaccination is suggested (Adams 2019).
In this study, different kinds of Aeromonas veronii vaccines have been developed and their efficacy was tested in O. niloticus Nile tilapia. The virulence genes were detected using PCR to develop live and inactivated vaccines. Their efficacy was determined by measuring antibody titers in fish sera.
Materials and methods
Bacterial strains
Five well-identified Aeromonas veronii isolates (A (HY1), A (HY2), A (HY3), A (HY4), and A (HY6) were isolated from diseased fish as previously (Youssuf et al. 2020). The accession number of A. veronii isolates is represented in Table 1. PCR products were purified using QIA quick PCR Purification kit (QIAGEN, USA) following the manufacturer’s protocol. Then the purified product was sequenced in the forward and/or reverse directions on an Applied Biosystems 3130 automated DNA Sequencer (ABI3130, USA) using a ready reaction Bigdye Terminator V3.1 cycle sequencing kit (Cat. No. 4336817, Perkin- Elmer/Applied Biosystems, Foster City, CA). The resultant sequenced Aeromonas strains were analyzed using the National Center for Biotechnology Information (NCBI), Basic Local Alignment Search Tool (BLAST) program, and the neighbor-joining blast tree against the database of strain types (the most prevalent and pathogenic Aeromonas isolates) and published valid prokaryotic nomenclature (Youssuf et al. 2020).
PCR detection of virulence genes
DNA extraction
A loopful from gills, liver, kidneys, and spleen of the clinically diseased fish were inoculated into Tryptic soy broth (TSB, Oxoid, UK) then streaked over Tryptic soy agar (TSA, Oxoid, UK), incubated for 24 h at 28 °C (Austin and Austin 2016). Pure colonies were stored at − 80 °C in TSB containing 20% glycerol for further studies following Youssuf et al. (2020).
The QIAamp DNA Mini kit (Qiagen, Germany, GmbH) was used to extract bacterial DNA following the manufacturer’s recommendations, with some modifications. First, 200-µl bacterial sample suspension was treated at 56 °C for 10 min in a 10-µl proteinase K and 200-µl lysis buffer mixture. After the incubation, 200-µl ethanol (100%) was added to the lysate. Then, nucleic acid was eluted using the 100-µl elution buffer that was provided in the kit. Finally, the sample was washed and centrifuged following the manufacturer’s recommendations.
PCR detection of virulence genes
The Primers used were supplied by Metabion (Germany) and represented in Table 2. They were used in a 25-µl reaction that included 12.5-µl Emerald Amp Max PCR Master Mix (Takara, Japan), 1 µl each primer of 20-pmol concentration, 5.5-µl water, and 5-µl DNA template. The reaction was performed in an Applied Biosystem 2720 thermal cycle conditions and was adjusted for each primer according to the reference ( Table 2).
PCR product analysis
PCR products were separated using electrophoresis on 1.5% agarose gel (Applichem, Germany, GmbH) in 1 × TBE buffer at room temperature using gradients of 5 V/cm. For gel analysis, 20-µl PCR products were loaded in each gel slot. The fragment sizes were determined by Generuler 100 bp ladder (Qiagen, Gmbh, Germany). A gel documentation system (Alpha Innotech, Biometra) was used to photograph the gel, and the data was processed using Bio Doc Analyze Analysis Software (BDA).
Lethal dose determination
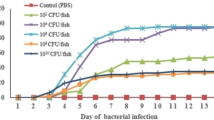
One hundred eighty healthy Nile tilapia with an average weight of 25 ± 0.5 g were acclimated for 14 days at a temperature of 25 ± 1 °C. Fish were randomly divided into six groups, ten fish in triplicate. A. veronii isolates were inoculated into (BHI) broth and grown overnight at 28 °C. Bacterial cells were obtained by centrifugation then adjusted by spectrophotometer apparatus at OD600 of 0.6 using sterile 0.85% NaCl (saline solution). In these conditions, the number of bacteria was about 5 × 108 CFU/ml. To resolve the pathogenicity of isolates, O. niloticus were injected with 0.1-ml bacteria ranging from 5 × 104 to 5 × 107 CFU/fish. These bacterial suspensions were prepared through a series of fivefold serial dilution steps. For each A. veronii isolate, five groups (150 fish) were injected, except the control group that was injected with a sterile saline solution. All groups were monitored for 7 days and the dead fish were recorded every 12 h. The LD50 was determined following Hodson et al. (1984). 1 × 107 CFU/fish bacterial dose in the challenge test was used to confirm the pathogenicity of the isolates. O. niloticus were monitored every 12 h for 7 days, and the lethality rate (LR) was calculated as previously described (Bailonea et al. 2010). Random samples of fish were sacrificed and examined for parasites, mycotic, and bacterial pathogens as described by Austin and Austin (2007) to ensure their normal health status.
Experimental design and vaccine preparation
A. veronii vaccines (inactivated and live) were fabricated as described by Li et al. (2011). Briefly, formalin-kill vaccine (FKV) from A (HY2) and A (HY4) strains were cultivated until they reached an OD600 of 1.0, then the bacteria were harvested to produce an inactivated vaccine in 0.5% formalin. Cells were centrifuged, washed three times, and resuspended in sterile saline solution after 24-h incubation at 37 °C. Autoclaved-kill vaccine (AKV) from the same previous strains was prepared by autoclaving the bacterial culture in the nutrient broth in the autoclave at 121 °C for 15 min. The treated culture was centrifuged at 7000 × g for 30 min. Cell pellets were washed twice using PBS and resuspended again in PBS following Bactol et al. (2018). Strain A (HY1) that was chosen to produce the live vaccine was diluted using sterile saline solution until an OD600 of 0.2 to adjust 1 × 108 CFU/ml of bacterial concentration. We used this strain A (HY1) because it is a naturally attenuated strain where no mortalities were recorded in the control group and A. veronii HY1 group throughout the experimental period in the previous study (Youssuf et al. 2020). All the procedures were performed under sterile conditions. Vaccines were kept at 4 °C until further use. These vaccines were subjected to sterility and safety tests as previously conducted (Anderson et al. 1970).
Experimental design and vaccination
The experiment included six groups as shown in the following table:
Group | Treatment | Strains |
|---|---|---|
T1 group | Control (not vaccinated) | |
T2 group | Formalized killed vaccine | A (HY2) |
T3 group | Formalized killed vaccine | A (HY4) |
T4 group | Autoclave killed vaccine | A (HY2) |
T5 group | Autoclave killed vaccine | A (HY4) |
T6 group | Live vaccine | A (HY1) |
To prevent cross-infection, each group was kept in a separate tank for the trial duration. For 3 weeks, fish were fed at a rate of 5% body weight twice daily (at 9:00 and 17:00). Feed amounts were adjusted bi-weekly to fit the new fish biomass. Every day, fish waste was drained and 50% of the water was replaced with aerated and dechlorinated water from the storage tank. Groups T2, T3, T4, T5, and T6 received 0.1 ml from the prepared vaccines as described previously (Surgha et al. 2021). The T1 control group received a sterile saline post-intraperitoneal injection (I/P) and stay as a negative control. The immunization period was extended for 21 days. The water temperature and dissolved oxygen (DO) were monitored twice a day at 8:00 and 16:00, while the other water quality parameters were measured once a week. Water temperature and DO were 26 ± 1 °C and 6 ± 0.2 mg/L, respectively. The pH value was 7.5 ± 0.5. All these parameters were within the optimum fish growth requirement.
Challenge test
After 21 days post-vaccination, each fish (10 fish/duplicates), vaccinated and positive control was challenged by 0.1-ml intraperitoneal injection (I/P) of strain A (HY2), (1.0 × 108 CFU/ml). Another 20 fish were randomly selected and injected with (1.0 × 108 CFU/ml) of PBS and used as a negative control (NC) following Zhang et al. (2019), the typical signs of diseased fish were external hemorrhages and inflammation or ulcers in gills, fin base, and other tissues. Mortalities were renowned every 12 h for 7 days, and the cumulative mortality rate was determined. The relative percentage of survival (RPS) was calculated according to Amend (1981) as follows:
RPS = [1 − (mortality in vaccinated group/mortality in control group)] × 100%.
Serum collection
Blood was obtained from three fish/replicates 21 days post-vaccination and 3 days post-challenge test. Three samples from each group post-vaccination and post-challenge test were obtained from each fish using a 1-mL syringe with a 25-G needle attached. The needle was inserted into caudal blood vessels. Collected blood was stored in Eppendorf tubes and was left overnight to clot. Clot bordering was done and centrifuged. The collected serum was preserved at − 20 °C until use.
Immunoglobulin assay
Antibody titers were measured spectrophotometrically in fish sera following the protocol of ELISA kits (Cusabio Biotech Co. Ltd., USA). Briefly, 50 μl of standard or sample per well was added per well. Then 50 μl of HRP-conjugate mixed solution to each well (not to Blank. well) was added and mixed well and then incubated for 1 h at 37 °C. Each well was aspirated and washed, repeating the process two times for a total of three washes. The wash occurred by filling each well with wash buffer (200 μl) let it stand for 10 s. Complete removal of the liquid at each step is essential to good performance. After the last wash, any remaining wash buffer was removed by aspirating or decanting. The plate was then inverted and blotted against clean paper towels. Fifty μl of substrate A and 50 μl of substrate B were added to each well and mixed well. The plate was incubated for 15 min at 37 °C. Then 50 μl of stop solution was added to each well, and the plate was thoroughly mixed. The optical density was determined of each well within 10 min, using a microplate reader set to 450 nm.
Statistical analysis
Statistical significance across groups was analyzed using one-way analysis of variance with post hoc Tukey’s tests, using SPSS 20.0 software to determine the significant changes in immunoglobulin levels 21 days post-immunization and 3 days post-challenge with A (HY1). A p-value < 0.05 was considered significant.
Results
Virulence genes of Aeromonas veronii
Among the Aeromonas isolates, DNA analysis showed five A. vernoii strains. PCR was used to detect virulence factors in previous isolates. The results indicated that they have many genotypes.
Fla genes were found in all strains. Act and alt genes were also found in all strains except A (HY1) for act and A(HY6) for alt. lipase gene were found in three strains, while aerolysin gene was detected in all strains except A (HY3) and A (HY6). The ast gene was absent in all strains (Table 3).
Virulence genotype and pathogenicity
Previous research has shown that virulence factor genes are responsible for the pathogenicity of A. veronii. To investigate the relationship between virulence genotype and pathogenicity, the lethal dose (LD50) was determined using different bacterial cell concentrations for the five isolates. Virulence genes, pathogenicity test, and lethality rate (LR) results of A. veronii are represented in Table 3. The pathogenicity of strains A (HY2) and A (HY4) was the strongest, whereas the LRs were 100% and 90%, respectively and these strains explained expression for all genes except for ast gene only. The strains of A (HY3) and A (HY6) pathogenicity were moderate, whereas the LR was 60%, and these strains explained the expression for three virulent genes. A (HY1) was the weakest strain as the expression was only for two genes, and no dead fish was found for this strain. Because of the high pathogenicity of A (HY2) and A (HY4) strains, they were used for vaccine formulation.
Immunoglobulin response for live and inactivated vaccines
After 21 days post-vaccination, the IgM level for live A (HY1) vaccine was the highest (59.29a ± 2.97) followed by AKV for A (HY4) and A (HY2) (50.24b ± 0.70), (45.65bc ± 1.28) respectively. While the lowest levels of IgM were (44.29c ± 0.26), (37.08d ± 2.14) for FKV A (HY4), FKV A (HY2), respectively, they were still relatively high compared to the control group (Table 4). However, the result for IgM levels began to reduce 3 days post-challenge. Although it remained significantly higher (p < 0.05) than the control group. Also, IgM levels were high for live (HY1) vaccine groups (57.29a ± 3.55) followed by AKV groups for A (HY4) and A (HY2) (46.80b ± 3.04), (44.15b ± 1.57), respectively. Also, the FKV for A (HY4), A (HY2) explained the low IgM level (36.65c ± 0.66), (37.08c ± 1.56) compared with other groups (Table 4).
Relative percentage of survival (RPS)
The RPS% of the live vaccine A (HY1) group was higher than the AAKV A (HY2), A(HY4) and higher than FKV (HY2) A (HY4). The mortality rate was 0% in the negative control group (Table 5, Fig. 1).
Discussion
Aeromonads have been identified as the causative agent of fish disease by researchers. The distribution of Aeromonas species in aquatic environments proposes that their interactions with fish are continual and inescapable, which most likely explains their opportunistic pathogenicity (Ottaviani et al. 2011). Over the past decade, Aeromonads, including A. hydrophila and A. veronii have been linked to fish mortality worldwide, and are considered the primary cause of fish outbreaks and causing massive economic losses (Janda and Abbott 2010; Noga 2010). Motile aeromonads are extremely diverse, and not only A. hydrophila can cause the disease but A. veronii and other Aeromonas species have also been recorded as a major threat to freshwater fish aquaculture (Dong et al. 2015; Zhu et al. 2016). Several virulence genes have been discovered in A. veronii, which help to explain its pathogenicity. Additionally, the detection of aerolysin and alt genes has recently been suggested as the main cause of virulence (Wang et al. 2003). Some virulence genes were discovered here in A. veronii isolates that were isolated from diseased fish. Reports of A. veronii in the USA and A. hydrophila in China demonstrated that aerolysin is the major contributor to the virulence of the pathogenic Aeromonas strains (Nawaz et al. 2010; Zheng et al. 2012a, b). Consistent with this, our results show A. veronii virulence does not arise from one gene, but it is probably the result of the collaborative effects of different virulence genes. Our investigation is accordant with the alt + , act + , and fla + genotypes are playing an essential role in A. veronii pathogenicity since A (HY2) and A (HY4) produced a tremendous mortality rate, with an average LD50 value of 5 × 106 and 5 × 107 CFU/fish.
Many studies have been targeted on the immunoprotective characteristics of secreted proteins, just as these consistently affect bacterial virulence (Zhang et al. 2014). For instance, in A. hydrophila, hemolysin, and aerolysin are extracellular proteins that are well-known virulence factors (wang et al. 2019). However, it was discovered that aerolysin is the most important gene for A. veronii virulence. In all the types of infections with A. veronii, signs such as hemorrhagic and ulcerative lesions on the skin and other organs are produced by aerolysin (aer)‐mediated aerotaxis (Bücker et al. 2011). The gene aer is a cytotoxic pore‐forming enterotoxin which was reported to be one of the major virulence factors in the pathogenesis of A. veronii‐associated fish diseases (Baumgartner et al. 2017; Zheng et al. 2012a, b). This study describes the protective capacity of a live vaccine for A. veronii in O. niloticus delivered through injection vaccination. Additionally, the immunization was tested by infecting fish with A(HY1) through intraperitoneal injection. The vaccines are administered to stimulate humoral immune responses (IgM) of immunized fish. The agglutination test is a simple and easy assay to perform that measures antibody amount in the serum of vaccinated hosts or organisms (Tizard 1996). The assessment of agglutinating antibody titer is an easy approach to measure circulating antibodies in serum samples collected from fish previously immunized with particulate antigen preparations (Sugahara and Eguchi 2012). In addition, Morrison and Nowak (2002) reported that the agglutination assay is considered quick, simple, and inexpensive among the serological tests. Blood extraction was drawn 21 days post-vaccination and 3 days post-challenge test from O. niloticus; the results of the study showed that vaccinated O. niloticus with live vaccine produced greater antibody levels in serum than non-vaccinated fish. Alternatively, immunoglobulin was elevated post-immunization and even after the challenge test. Similar to the results obtained from Zhang et al. (2017), who reported that immunized catfish (Ictalurus punctatus) with recombinant proteins significantly increased the relative percentage of survival (RPS), compared with the unimmunized catfish. Interestingly, the formalin-killed vaccine (FKV) results are consistent with Wang et al. (2014), who reported a remarkable increase in antibody (IgM) levels to post-intraperitoneal vaccination with an FKV against S. iniae infection in tilapia. An I/P infection route was used to infect the fish to ensure a systemic immune response from the oral vaccination. The vaccination routes have a significant effect on vaccine efficacy. Systemic immune responses are readily elicited by intramuscular intraperitoneal injections and provide better protection levels than oral routes. However, the oral route is easy and simple in its application (Grabowski et al. 2004). Although, this study demonstrated superior antigenicity of formalized and autoclaved vaccines.
These vaccines were prepared by attenuating live bacterial cells with physical methods, like formalin and autoclaving, which is alike to that of Dehghani et al. (2012), who documented that formalin-killed and heat-killed A. hydrophila vaccines are evenly antigenic in red trout. Nevertheless, this study revealed that formalin-killed whole-cell vaccine has lower antigenicity.
Additionally, the genotype alt − /act − /fla − /lipase − /aerolysin + exhibits loss of virulence and unharmed immunogenicity that could become potential candidates that are used as live vaccines for fish immunization. Anyhow, further studies are crucial to determine the efficacy of these alternatives across different A. veronii strains.
The overall results of the study paved the way for the future development of different types of vaccines to control the bacterial diseases hindering the aquaculture industry, particularly Aeromonas, as alternatives for the intensive use of antimicrobials that have side effects exceeding their therapeutic interventions. In addition, this study could represent the initial step to control the pseudomonas septicemia in the intensive tilapia culture in Egypt, where dependance on this species as a source of protein is a priority. However, the vaccine development should be carefully evaluated to properly assess its efficacy and monitor any inadvertent health effects on tilapia aquaculture.
Conclusion
In conclusion, our study reports the link among virulence genotype, pathogenicity, and immunogenicity of A. veronii isolated from the diseased fish. Different genotypes were applied to identify novel antigens for new vaccine development. The live vaccine was the best against A. veronii infection for O. niloticus protection.
Data availability
The data that support the findings of this study are available from the corresponding author upon request.
References
Adams A (2019) Progress, challenges and opportunities in fish vaccine development. Fish Shellfish Immunol 90:210–214. https://doi.org/10.1016/j.fsi.2019.04.066
Aly, T (1981) Studies on the effect of different adjuvant on the efficiency of FMD vaccine in farm animals. Ph.D. thesis, Faculty of Vet. Med. Zagazig University.
Amend, DF (1981) Potency testing of fish vaccines. In: Anderson DP, Hennessen H, editors. Fish biologics: sero diagnostics and vaccines. Developments in biological standardization. Basel: Karger; pp. 447–54.
Anderson E, Capstick P, Mowat G, Leech F (1970) In vitro method for safety testing of foot-and-mouth disease vaccines. Epidemiol Infect 68:159–172
Aoki T (1999) Motile aeromonads (Aeromonas hydrophila). Fish Diseases and Disorders 3:427–454
Assefa A, Abunna F (2018) Maintenance of fish health in aquaculture: review of epidemiological approaches for prevention and control of infectious disease of fish. Vet Med Int 2018:5432497
Austin B, Austin DA (2016) Aeromonadaceae representative (Aeromonas salmonicida), Bacterial fish pathogens. Springer, pp 215–321
Bactol IDC, Padilla LV, Hilario AL (2018) The immune response of tilapia (Oreochromis niloticus) after vaccination with autoclavekilled, heat-killed, and formalin-killed whole-cell Aeromonas hydrophila vaccines as possible serotype-independent vaccines. Int J Agric Biol 20:846–850
Bailonea RL, Martinsa ML, Mouriñoa JL, Vieiraa FN, Pedrottia FS, Nunesa GC, Silvaa BC (2010) Hematology and agglutination titer after polyvalent immunization and subsequent challenge with Aeromonas hydrophila in Nile tilapia (Oreochromis niloticus). Arch Med Vet 42:221–227
Baumgartner WA, Ford L, Hanson L (2017) Lesions caused by virulent Aeromonas hydrophila in farmed catfish ( Ictalurus punctatus and I. punctatus × I. furcatus) in Mississippi. J Veter Diagn Investig 29(5):747–751
Bücker R, Krug SM, Rosenthal R, Günzel D, Fromm A, Zeitz M, Schulzke JD (2011) Aerolysin from Aeromonas hydrophila perturbs tight junction integrity and cell lesion repair in intestinal epithelial HT-29/B6 cells. J Infect Dis 204(8):1283–1292
Chen F, Sun J, Han Z, Yang X, Xian J-a, Lv A, Hu X, Shi H (2019) Isolation, identification and characteristics of Aeromonas veronii from diseased crucian carp (Carassius auratus gibelio). Front Microbiol 10:2742
Dehghani S, Akhlaghi M, Dehghani M (2012) Efficacy of formalin-killed, heat-killed, and lipopolysaccharide vaccines against motile aeromonads infection in rainbow trout (Oncorhynchus mykiss). Global Veterinaria 9:409–415
Dong HT, Nguyen VV, Le HD, Sangsuriya P, Jitrakorn S, Saksmerprome V, Senapin S, Rodkhum C (2015) Naturally concurrent infections of bacterial and viral pathogens in disease outbreaks in cultured Nile tilapia (Oreochromis niloticus) farms. Aquaculture 448:427–435
Dong H, Siriroob S, Meemetta W, Santimanawong W, Gangnonngiw W, Pirarat N, Khunrae P, Rattanarojpong T, Vanichviriyakit R, Senapin S (2017a) The emergence of tilapia lake virus in Thailand and an alternative semi-nested RT-PCR for detection. Aquaculture 476:111–118
Dong H, Techatanakitarnan C, Jindakittikul P, Thaiprayoon A, Taengphu S, Charoensapsri W, Khunrae P, Rattanarojpong T, Senapin S (2017b) Aeromonas jandaei and Aeromonas veronii caused disease and mortality in Nile tilapia, Oreochromis niloticus (L.). J Fish Dis 40:1395–1403
El-Gohary FA, Zahran E, Abd El-Gawad EA, El-Gohary AH, Abdelhamid MF, El-Mleeh A, Elmahallawy EK, Elsayed MM (2020) Investigation of the prevalence, virulence genes, and antibiogram of motile Aeromonads isolated from Nile tilapia fish farms in Egypt and assessment of their water quality. Animals: an open access journal from MDPI 10(8):1432
Grabowski L, LaPatra S, Cain K (2004) Systemic and mucosal antibody response in tilapia, Oreochromis niloticus (L.), following immunization with Flavobacterium columnare. J Fish Dis 27:573–581
Gudding R, Van-Muiswinkel WB (2013) A history of fish vaccination: science-based disease prevention in aquaculture. Fish Shellfish Immunol 35(6):1683–1688
Hodson PV, Dixon DG, Kaiser KLE (1984) Lethal dose versus lethal concentration as indicator of contaminant toxicity to fish. In: Kaiser KLE (ed) QSAR in Environmental Toxicology. Springer, Dordrecht
Hossain S, De Silva B, Dahanayake P, Heo GJ (2018) Characterization of virulence properties and multi-drug resistance profiles in motile Aeromonas spp. isolated from zebrafish (Danio rerio). Lett Appl Microbiol 67:598–605
Janda JM, Abbott SL (2010) The genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev 23:35–73
Li J, Ni X, Liu Y, Lu C (2011) Detection of three virulence genes alt, ahp, and aerA in Aeromonas hydrophila and their relationship with actual virulence to zebrafish. J Appl Microbiol 110:823–830
Mapenzi LL, Mmochi AJ (2016) Role of salinity on growth performance of Oreochromis niloticus and Oreochromis urolepis urolepis hybrids. J Aquac Res Development 7:431
Monir M, Yusoff SBM, Zulperi ZBM et al (2020) Haemato-immunological responses and effectiveness of feed-based bivalent vaccine against Streptococcus iniae and Aeromonas hydrophila infections in hybrid red tilapia (Oreochromis mossambicus × O. niloticus). BMC Vet Res 16:226
Morrison RN, Nowak BF (2002) The antibody response of teleost fish. Semin Avian Exotic Pet Med 11(1):46–54
Nawaz M, Khan SA, Khan AA, Sung K, Tran Q, Kerdahi K, Steele R (2010) Detection and characterization of virulence genes and integrons in Aeromonas veronii isolated from catfish. Food Microbiol 27:327–331
Nielsen ME, Høi L, Schmidt A, Qian D, Shimada T, Shen J, Larsen J (2001) Is Aeromonas hydrophila the dominant motile Aeromonas species that cause disease outbreaks in aquaculture production in the Zhejiang Province of China? Dis Aquat Org 46:23–29
Noga EJ (2010) Fish disease: diagnosis and treatment. John Wiley & Sons
Ottaviani D, Parlani C, Citterio B, Masini L, Leoni F, Canonico C, Sabatini L, Bruscolini F, Pianetti A (2011) Putative virulence properties of Aeromonas strains isolated from food, environmental and clinical sources in Italy: A comparative study. Int J Food Microbiol 144:538–545
Parte AC (2014) LPSN—list of prokaryotic names with standing in nomenclature. Nucleic Acids Res 42:D613–D616
Qin S, Xiao W, Zhou C, Pu Q, Deng X, Lan L, Liang H, Song X, Wu M (2022) Pseudomonas aeruginosa: pathogenesis, virulence factors, antibiotic resistance, interaction with host, technology advances and emerging therapeutics. Signal Transduct Target Ther 7(1):199
Saleh A, Elkenany R, Younis G (2021) Virulent and Multiple Antimicrobial Resistance Aeromonas hydrophila Isolated from Diseased Nile Tilapia Fish (Oreochromis niloticus) in Egypt with Sequencing of Some Virulence-Associated Genes. Biocontrol science 26(3):167–176
Sen K, Rodgers M (2004) Distribution of six virulence factors in Aeromonas species isolated from US drinking water utilities: a PCR identification. J Appl Microbiol 97:1077–1086
ShirajumMonir M, Yusoff SM, Mohamad A, Ina-Salwany MY (2020) Vaccination of tilapia against motile Aeromonas septicemia: a review. J Aquat Anim Health 32(2):65–76
Singh V, Rathore G, Kapoor D, Mishra BN, Lakra WS (2008) Detection of the aerolysin gene in Aeromonas hydrophila isolated from fish and pond water. Indian J Microbiol 48:453–458
Sommerset I, Krossoy B, Biering E, Frost P (2005) Vaccines for fish in aquaculture. Expert Rev Vaccines 4(1):89–101
Sugahara K, Eguchi M (2012) The use of warmed water treatment to induce protective immunity against the bacterial cold-water disease pathogen Flavobacterium psychrophilum in ayu (Plecoglossus altivelis). Fish Shellfish Immunol 32(3):489–493
Sughra F., Rahman M. Hafeez-ur, Abbas F., Altaf I. (2023) Evaluation of three alum-precipitated Aeromonas hydrophila vaccines administered to Labeo rohita, Cirrhinus mrigala and Ctenopharyngodon idella: immunokinetics, immersion challenge and histopathology. Brazilian Journal of Biology 83. https://doi.org/10.1590/1519-6984.249913
Sun J, Zhang X, Gao X, Jiang Q, Wen Y, Lin L (2016) Characterization of virulence properties of Aeromonas veronii isolated from diseased Gibel Carp (Carassius gibelio). Int J Mol Sci 17:496
Tizard I (1996) Inflammation and veterinary immunology: an introduction. WB Saunders, Philadelphia, Pennsylvania
Toranzo A, Romalde J, Magariños B, Barja J (2009) Present and future of aquaculture vaccines against fish bacterial diseases. Options Mediterraneennes 86:155–176
Vivekanandhan G, Savithamani K, Hatha A, Lakshmanaperumalsamy P (2002) Antibiotic resistance of Aeromonas hydrophila isolated from marketed fish and prawn of South India. Int J Food Microbiol 76:165–168
Wang G, Clark CG, Liu C, Pucknell C, Munro CK, Kruk TM, Caldeira R, Woodward DL, Rodgers FG (2003) Detection and characterization of the hemolysin genes in Aeromonas hydrophila and Aeromonas sobria by multiplex PCR. J Clin Microbiol 41:1048–1054
Wang J, Zou L, Li A (2014) Construction of a Streptococcus iniae sortase A mutant and evaluation of its potential as an attenuated modified live vaccine in Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol 40:392–398
Wang Y, Wang X, Ali F, Li Z, Fu Y, Yang X, Lin W, Lin X (2019) Comparative extracellular proteomics of Aeromonas hydrophila reveals iron-regulated secreted proteins as potential vaccine candidates. Front Immunol 10:256
Youssuf H, El Asely AM, Abdel Gawad E, Elabd H, Matter A, Shaheen A, Abbass A (2020) Insight into summer mortality syndrome in farmed Nile tilapia (Oreochromis niloticus) associated with a bacterial infection. Benha Vet Med J 39:111–118
Zhang D, Pridgeon JW, Klesius PH (2014) Vaccination of channel catfish with extracellular products of Aeromonas hydrophilprotectson against infection by the pathogen. Fish Shellfish Immunol 36:270–275
Zhang D, Xu DH, Shoemaker CA (2017) Immunization with recombinant aerolysin and hemolysin protected channel catfish against virulent Aeromonas hydrophila. Aquac Res 48:875–882
Zhang DX, Kang YH, Song MF, Shu HP, Guo SN, Jia JP, Tao LT, Zhao ZL, Zhang L, Wang CF (2019) Identity and virulence properties of Aeromonas isolates from healthy Northern snakehead (Channa argus) in China. Lett Appl Microbiol 69:100–109
Zheng W, Cao H, Yang X (2012a) Aeromonas veronii infection in the cultured snakehead fish, Ophiocephalus argus (Cantor). Afr J Microbiol Res 6(44):7218–7223
Zheng W, Cao H, Yang X (2012b) Grass carp (Ctenopharyngodon idellus) infected with multiple strains of Aeromonas hydrophila. Afr J Microbiol Res 6:4512–4520
Zhu M, Wang X, Li J, Li G, Liu Z, Mo Z (2016) Identification and virulence properties of Aeromonas veronii bv. sobria isolates causing an ulcerative syndrome of loach Misgurnus anguillicaudatus. J Fish Dis 39:777–781
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB). The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
HY and AM designed the study, HA and AM performed the experimental part, and AM, HA and ES drafted the manuscript. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
This study has followed the guidelines of the Committee of Animal Welfare and Research Ethics, Faculty of Veterinary Medicine, Benha University, Egypt.
Conflict of interest
The authors declare no competing interests.
Additional information
Handling Editor: Brian Austin
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Youssef, H.A., Ayoub, H.F., Soror, E.I. et al. Virulence genes contributing to Aeromonas veronii pathogenicity in Nile tilapia (Oreochromis niloticus): approaching the development of live and inactivated vaccines. Aquacult Int 31, 1253–1267 (2023). https://doi.org/10.1007/s10499-022-01023-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-022-01023-1