Abstract
In the past century, there have been great achievements in identifying resistance (R) genes and quantitative trait loci (QTLs) as well as revealing the corresponding molecular mechanisms for resistance in rice to major diseases and insect pests. The introgression of R genes to develop resistant rice cultivars has become the most effective and eco-friendly method to control pathogens/insects at present. However, little attention has been paid to durable and broad-spectrum resistance, which determines the real applicability of R genes. Here, we summarize all the R genes and QTLs conferring durable and broad-spectrum resistance in rice to fungal blast, bacterial leaf blight (BLB), and the brown planthopper (BPH) in molecular breeding. We discuss the molecular mechanisms and feasible methods of improving durable and broad-spectrum resistance to blast, BLB, and BPH. We will particularly focus on pyramiding multiple R genes or QTLs as the most useful method to improve durability and broaden the disease/insect spectrum in practical breeding regardless of its uncertainty. We believe that this review provides useful information for scientists and breeders in rice breeding for multiple stress resistance in the future.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rice has become one of the most important staple crops, feeding over half of the world’s population (Cheng et al. 2013a). Annually, up to 37% of rice yield is lost due to diseases and arthropod pests on average (Sparks et al. 2012). Among them, blast, BLB, and BPH are the three main biotic stresses (Ji et al. 2016). Over approximately 4 million tons of pesticides are used annually (FAO 2021) to control detrimental insects, fungal, and bacterial diseases. As a result, they have caused adverse effects on water, soil, air, and various taxa, which finally severely affect global ecosystem, biodiversity, and human health (Sharma et al. 2019).
The development of resistant cultivars by introducing resistance (R) genes into rice has become a sustainable and environmental-friendly approach for disease and insect management (Cheng et al. 2013a; Yin and Qiu 2019). A major limitation of host plant resistance is that targeted pest species can overcome resistance mediated by specific resistance genes by producing new virulent strains of the pathogens and insects (Palloix et al. 2009). For example, the resistance effects of the blast disease gene were overcome within 3 years after planting (Kiyosawa 1982). The pathogen and insect populations have such extremely rapid evolutionary speed, but the process of discovering a new R gene and incorporating it into a rice cultivar is quite slow and inefficient (Li et al. 2021b). Thus, durable resistance in cultivars has increasingly attracted attention since durable resistance can remain effective over a longer time (Johnson 1979, 1984). This descriptive term provides no implications for genetic background or molecular mechanism but only two empirical elements—time and area—which are difficult to define clearly for different farmers (Brown 2015). For a subsistence farmer, durable resistance is desired to maintain stable, predictable, and adequate crop production other than an above-average or maximizing yield, while a farmer in industrial agriculture prefers new crop cultivars with the most commercial value in the market to those that are out of date, which requires durable resistance to maintain crops with the highest yield and quality (Summers and Brown 2013; Brown 2015; Anuradha et al. 2022).Unfortunately, little is known about the molecular mechanisms for durable resistance in rice (Johnson 1993; Brown 2015).
Rice varieties with broad-spectrum resistance are highly desirable since this type of resistance is effective to more than one pathogen species or multiple races/strains of the same pathogen (Kou and Wang 2010; Hu et al. 2023). Many organizations and governments have accepted that the current norm for the public release of new rice varieties since these varieties have been evaluated for resistance to major diseases and insects based on standard protocols (Bank; Benedict and Linscombe 2009; IRRI, 2022). Recently, the term broad-spectrum resistance has expanded to include resistance to at least two different insect species or multiple biotypes/populations of the same insect pest (Jairin et al. 2007a; Liu et al. 2015; Kloth et al. 2021). The genetic basis for broad-spectrum resistance may include membrane-associated pattern recognition receptors (PRRs) and R proteins in the plant innate immunity system, defense-signaling proteins, pathogenesis-related (PR) proteins, susceptibility (S) proteins, quantitative trait loci (QTLs), and non-host resistance (NHR) (Liu et al. 2015; Fonseca and Mysore 2019; Li et al. 2020a). Both broad-spectrum and durable resistance have become increasingly prominent for practical applications since their combination provides an effective strategy for better pest managements to avoid heavy economic losses.
Another constraint on breeding for disease and insect resistance in rice for commercial crop varieties is the pleiotropic effects of resistance, leading to a growth–defense trade-off (Huot et al. 2014). In most cases, plant defense responses to pathogens and insects can evoke fitness costs associated with crop growth and development, the valuable agronomic property of the cultivar, and resistance to other environmental stresses, which lead to economic losses in yield and/or cooking and taste quality and unpredictable ecological consequences (Brown and Rant 2013; Huot et al. 2014; Wiesner-Hanks and Nelson 2016; Nelson et al. 2018). Therefore, farmers prefer to growing rice varieties with higher yields and better quality instead of growing cultivars with pest resistance but lower commercial values even though those varieties with higher economic values need to use chemical pesticides for pest control. During the post-Green Revolution era, many elite rice cultivars were developed with high yield and superior quality. Hence, those R genes that would not alter yield-associated traits and superior grain quality of the elite rice cultivars have been used in breeding programs with the aim of obtaining the most benefits of resistance against diseases and pests.
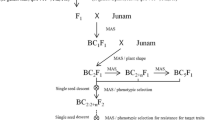
Marker-assisted selection (MAS) refers to the indirect selection process of target traits according to a DNA marker linked to a desired trait. Since it was first used in tomato breeding four decades ago (Tanksley and Rick 1980; Soller and Beckmann 1983), it has made a profound impact on crop genetic improvement by manipulating non-observable traits in conventional breeding. It has accelerated resistance breeding for diseases and pests, tolerance breeding for drought and hypersalinity, and the introgression of important agronomic traits, such as plant architecture and seed morphology (Ben-Ari and Lavi 2012). The extensive use of MAS has even remodeled the general procedures of conventional breeding, such as backcrossing, pyramiding, and recurrent selection, to marker-assisted backcrossing, marker-assisted gene pyramiding (MAGP), and marker-assisted recurrent selection. Thus far, it has become a powerful new methodology in crop breeding (Jiang 2015). In particular, MAGP of multiple R genes and/or QTLs in crop resistance breeding has empirical applications in improving durability and broad-spectrum resistance against disease and insects (Mekonnen et al. 2017; Fukuoka 2018).
For the past thirty years, there has been an increasing number of reports about R genes and/or QTLs identified from germplasm resistant lines with good resistance against diseases and insects, and in some cases, molecular mechanisms underlying rice defense against biotic stress have been studied and their potential applications in practical breeding have been explored. However, little attention has been paid to durable and broad-spectrum resistance to diseases and insects, which are really two most important parameters to determine the practical values in breeding programs and rice production. Here, we summarize recent significant progress on durable and broad-spectrum resistance to blast, leaf blight, and brown planthopper in rice. We focus on the genetic basis and feasible methods for improving durable and broad-spectrum resistance to blast, BLB, and BPH in practical breeding. Additionally, our work provides the primary basis of molecular design for breeding for multiple stress resistance in the future.
Durable and broad-spectrum resistance to rice blast disease
Rice blast disease, caused by the phytopathogenic fungus Magnaporthe oryzae (anamorph: Pyricularia grisea), is one of the most devastating rice diseases in the world. Because this fungal disease leads to annual losses of 10–30% of the global rice yield with an estimated cost of about $ 66 billion (Pennisi 2010; Boddy 2016). It uses an appressorium to penetrate the plant cell wall and then manipulates host cells for parasitism (Galhano and Talbot 2011). The pathogen attacks all aboveground parts of a rice plant, including the leaf, collar, node, neck, panicle, and leaf sheath in the field and even roots in the laboratory (Sesma and Osbourn 2004; Marcel et al. 2010).
The rice blast pathosystem follows a typical gene-for-gene model of plant–microbe interactions, showing that the avirulence (AVR) gene from M. oryzae triggers resistance responses in the host rice harboring the corresponding R gene (Wang et al. 2017a). More than 100 blast resistance genes/alleles and 500 related QTLs have been identified (Li et al. 2019a; Dixit et al. 2020), most of which have been identified by molecular and QTL mapping (Tanweer et al. 2015). Primarily, these approaches are built on rice germplasm identified as donors of blast resistance in laboratory or field work. Considering the potentially applicable values of the improvement of rice varieties, the genes/loci conferring broad-spectrum resistance have attracted a great deal of attention. Thus far, approximately 44 genes/loci with broad-spectrum resistance have been identified (Table 1 and Supplemental Table 1), and 38 have been used for MAS in rice breeding programs (Supplemental Table 2) (Rao et al. 2014; Junjie et al. 2019; Mehta et al. 2019). Among the identified genes, Pi1, Pi2/Piz-5, Pi9, pi21, Pi54/Pi-kh, Pb1, and Pigm have been widely used in breeding programs. The genes Pi1, Pi9, pi21, Pb1, and Pigm confer broad-spectrum and durable resistance to rice blast (Fukuoka et al. 2009; Hayashi et al. 2010; Hua et al. 2012; Rathour et al. 2016; Deng et al. 2017). Pb1 has been widely used in Japan for over 40 years and still remains resistance to blast in fields (Fujii et al. 2023). As shown in Supplemental Table 2, only one or two Pi genes have been more successfully introduced into rice varieties/lines to improve blast resistance. Pyramiding multiple genes (three or more Pi) remains to be developed.
Defense regulating genes, genes involved in NHR, and QTLs related to blast resistance are important keys to engineering durable resistance in crops (Fonseca and Mysore 2019; Li et al. 2019a). Seventy-seven defense regulating genes have been extensively studied regarding the mechanisms underlying rice responses to blast pathogens (Li et al. 2019a). bsr-d1, spl11, spl33, OsBBI1, OsMYB30, OsNAC60, PIBP1, and LHCB5 confer broad-spectrum resistance to the rice blast disease (Zeng et al. 2004; Li et al. 2011, 2017, 2020b; Wang et al. 2017b, 2018; Liu et al. 2019; Zhai et al. 2019). In particular, bsr-d1 shows broad-spectrum blast resistance without a significant yield penalty (Li et al. 2017). However, there are rare examples of their practical utilization in breeding programs.
Diverse genes are involved in NHR, such as physical or chemical barriers, cell death, reactive oxygen species (ROS) accumulation, lignification, and callose deposition. Thus far, many attempts have been made to introduce or overexpress NHR genes from other plant species into crops (Fonseca and Mysore 2019). In particular, overexpression of NPR1 from Arabidopsis and RXO1 from maize in rice has provided valuable information to engineer ideal broad-spectrum disease resistance in the laboratory (Zhao et al. 2005; Xu et al. 2017). However, to date, the effective utilization of NHR genes in practical applications is still very limited.
QTLs usually improve plant resistance partially, which shows smaller additive effects than major R genes. However, QTL-mediated resistance is usually non-race-specific. Thus, weak but additive resistance mediated by QTLs is considered a potential alternative to engineer durable resistance against a broad range of pathogens (Fukuoka et al. 2015; Srivastava et al. 2017). Although less studied than R genes, four QTLs, pi21, Pi35, Pi63, and Pb1, have been cloned (Fukuoka et al. 2009, 2014; Hayashi et al. 2010; Xu et al. 2014a). QTLs can confer durable resistance in practical utilization (Fujii et al. 2023), but the allele alone is probably not able to control the disease under high disease pressure or in a complex environment. Therefore, pyramiding appropriate R genes together with QTLs may provide an applicable means to achieve durable and broad-spectrum resistance in the field.
Pyramided Pi genes are considered an artificially effective way to increase the blast resistance spectrum and durability (Liu et al. 2008; Luo et al. 2017; Guan et al. 2019). However, this method always leads to unexpected resistant effects with positive or negative deviations (Ning et al. 2020). Therefore, it seems greatly challenging for rice breeders to deploy and utilize such a high number of Pi genes to improve durable and broad-spectrum resistance. There are three major concerns in current resistance breeding programs. First, the genetic background usually alters the resistance spectrum and durability conferred by R genes (Cao et al. 2007; Gallois et al. 2018), which makes R genes with optimistic molecular demonstrations in laboratories often fall short in the field tests after being used in rice breeding (Brutus and Yang He 2010). Second, durable and broad-spectrum resistance to blast disease conferred by R genes in rice is inevitably at the expense of yield, grain quality, cooking and eating quality, or resistance to other abiotic stresses. This conflict propels breeders and farmers to find a balance between disease resistance and fitness costs according to their own experience and economic benefits. This is subjective and debatable, as it is not determined by an index but rather personal preference. Thus, a certain balance is difficult to apply extensively in rice breeding and production. Third, R gene frequency is diversified in different rice-planting regions, and the core effective R genes in rice varieties show obvious differences in different rice-growing zones. For example, the gene frequencies of Pi9 were recorded as 50%, 21.4%, and 15.22% in Thai, Korea, and India, respectively. Thus, Pi9 is the core effective R gene in Thai, but not in Korea or India. The Pit gene is the core effective R gene with a 60.24% gene frequency in India but cannot be detected in Korea (Cho et al. 2007; Yadav et al. 2019; Sooklim et al. 2022). However, different ecological zones can share similar key effective R genes. For example, Guangdong province (20°13′–25°31′N, 109°39′–117°19′E) in China is characterized by high ambient temperatures, and Hunan province (24°37′–30° 08′N, 108°47′–114°16′E) has a high summer and low fall temperature. However, both provinces share the same key effective R genes, Pi1, Pik, Pik-m, and Piz (Zhang et al. 2017). In addition, similar ecological zones have different key effective genes, such as Jiangsu province in China with Pish, Pit, and Pia (Qi et al. 2023) and Jiangxi province with Piz and Pid3 (Lan et al. 2019). These factors may constrain the improvement of durable and broad-spectrum resistance to rice blast disease in a limited region and a short time.
There are other approaches to improving durable and broad-spectrum resistance to blast disease. Due to the rapid development of CRISPR/Cas9‐mediated genome editing, it is feasible to knock out disease-susceptibility (S) genes (sometime recessive R genes) or related disease-susceptibility factors to generate new varieties with broad-spectrum resistance to blast disease, e.g., pi21 and RESISTANCE TO BLAST1 (RBL1) (Nawaz et al. 2020; Tao et al. 2021; Sha et al. 2023). However, the authority’s approval is required before the commercialization of crops and foods created using genome editing technology, which is always debatable and takes a lot of time. Another strategy for the long-term control of blast pathogens is rice multilines, which are made of several near-isogenic lines (NILs) with different R genes showing uniform phenotypic agricultural traits to reduce blast pathogen development and prevent the breakdown of blast R genes (Koizumi et al. 2004). Rice multilines have shown stable blast disease control in Japan for more than 10 years (Koizumi and Tani 2000; Ishikawa et al. 2022). However, the breeding of multiple NILs and strict maintenance increases the cost of developing and growing rice multilines. In addition, breeders need to pay continuous attention to the race distribution of the blast fungus (Fujii et al. 2023). Therefore, rice multilines controlling blast disease have not been accepted by farmers outside Japan.
Durable and broad-spectrum resistance to rice blight disease
Rice blight disease, the most destructive bacterial disease among rice and rice-related species (Jiang et al. 2023; Savary et al. 2019; Sanya et al. 2022), leading to yield losses varying from 2 to 74% (Reddy 1979; Naqvi et al. 2018), is caused by the gram-negative and non-spore-forming bacteria Xanthomonas oryzae pv. oryzae (Xoo) (Ming et al. 1991; Ishiyama 1992). The disease is also called bacterial leaf blight (BLB) since it induces yellowing and drying of leaves, as well as wilting of seedlings (also called kresek). The pathogen enters rice tissues through wounds on leaves and roots and natural openings, such as hydathodes, stomata, and growth cracks caused by the emergence of new roots at the base of the leaf sheath; it multiplies in epithem cells, moves to the xylem vessels, and blocks the phloem (Gnanamanickam et al. 1999; Shekhar et al. 2020). The pathogen infects rice at all growth stages, resulting in severe losses. Infection at early growth stages can lead to yield losses of up to 90% (Shekhar et al. 2020). Infection at the later booting stage does not decrease the yield too much but leads to poor-quality grains and a high proportion of broken kernels (Niones et al. 2022). This disease was first observed in 1884–1885 in Japan, and the pathogen Xoo was identified in 1911. BLB is prevalent throughout rice-cropping regions, including Asia, Africa, Australia, Europe, Latin America, the Caribbean, and North America. This disease spreads easily through water, rain, typhoons, plant-to-plant contact, farm implements, and seeds (Niones et al. 2022).
The rice–Xoo pathosystem has been regarded as a genetic model for host–pathogen interactions and co-evolution (Niño-Liu et al. 2006; Chen et al. 2020). R gene-mediated BLB resistance usually follows gene-for-gene theory and is race-specific (Dai et al. 2007; Chen et al. 2020). To date, over 47 BLB resistance genes/loci (Supplemental Table 3) and 100 s of QTLs have been identified from cultivated and wild rice and artificial and natural mutants (Yang et al. 2021; Lu et al. 2022). Some genes confer resistance to a wide spectrum of Xoo races from different geographical locations, including Xa2, Xa3/Xa26, xa5, Xa6/Xa3, Xa7, xa8, xa13, xa19, xa20, Xa21, Xa22(t), Xa23, xa24, Xa25, Xa27(t), Xa31(t), Xa32(t), Xa36(t), Xa39, xa41(t), Xa45(t), and Xa47, whereas others are effective against only one or a few strains that may be limited to a particular geographical location, such as Xa1 and Xa10 (Table 1 and Supplemental Table 3). Among these R genes, Xa3/Xa26, Xa4, Xa7, and Xa47 have been reported to confer durable resistance against Xoo (Hu et al. 2017; Deng et al. 2018; Chen et al. 2021; Lu et al. 2022).
Thus far, 14 Xa genes (Xa3/Xa26, Xa4, xa5, Xa7, Xa10, xa13, Xa21, Xa22, Xa23, Xa27, Xa33, xa33(t), Xa38, and Xa40) have been used in breeding practices (Fiyaz et al. 2022; Yang et al. 2022). Xa4 is the most widely deployed BLB R gene in modern commercial rice varieties, including the famous ‘Green Revolution’ rice variety IR64, and has been deployed in 55 countries and over 80% of the total rice-farming area since the late 1960s (Khush et al. 1989; Khush 2005; Arif et al. 2010; Mackill and Khush 2018; Quibod et al. 2020). As a result, the regional Xoo race frequency has changed, especially the major population of Xoo races, and new strains virulent to Xa4 have emerged and prevailed in different geographical locations, causing new BLB epidemics (Mew et al. 1992; Zhang 2009; Quibod et al. 2020). Therefore, seeking new genes with broad-spectrum and durable resistance and deploying these genes into rice varieties are important tasks for scientists and breeders. After the frequency of Xa4 virulent outbreaks increased during the 1990s, xa5, Xa7, xa13, and Xa21 were used in BLB resistance breeding throughout Asia (Furuya et al. 2012; Yugander et al. 2017; Chukwu et al. 2019; Quibod et al. 2020; Nugroho et al. 2022).
Many attempts have been made to pyramid multiple R genes and delay the breakdown of BLB resistance conferred by a single R gene. Thus far, two, three, four, and even five BLB R genes have been successfully pyramided in rice cultivars (Huang et al. 1997; Chukwu et al. 2019; Hsu et al. 2020). As the Xoo populations and Xoo pathotype distribution are continuously evolving with both local environments and modern agricultural practices, different R genes and pyramided R genes have been deployed in different counties in recent decades. For example, Xa23 has been widely used in Chinese breeding programs over the past decade (Zhou et al. 2011; Zhang et al. 2015). While japonica rice cultivars carrying Xa3/Xa26 have been widely grown in Korea (Shin et al. 2011). The gene combination of xa5 and xa13 confers resistance to all isolates throughout Vietnam (NODA et al. 1999), while the pyramided xa5, xa13, and Xa21 are the most suitable gene combination applied in Indian BLB breeding programs (Mishra et al. 2013).
In addition to R genes and relative MAS breeding, there are other genes and up-to-date technologies that can potentially broaden the resistance spectrum of rice cultivars. For example, S genes to BLB have been engineered by CRISPR/Cas9-mediated gene editing to generate new rice lines with broad-spectrum resistance to Xoo races (Oliva et al. 2019; Xu et al. 2019). The genetic engineering of Xa10 can enhance broad-spectrum and durable resistance to Xoo (Zeng et al. 2015). They provide novel strategies for creating new rice germplasm with broad-spectrum resistance, although further investigations to evaluate the performance of those edited lines in the field are still underway. Among the 44 defense regulator genes known to be involved in BLB resistance, 21 genes contribute to resistance to at least two Xoo isolates (Liu et al. 2021b). Compared with R genes and S genes, these genes have attracted much less attention in current molecular breeding. However, they enrich the gene pool for broad-spectrum resistance to disease, which might provide a new method for resistance breeding in the future.
At present, a new problem of BLB resistance breeding brings out as the new virulent pathogens causing bacterial leaf blight-like symptoms occur in different rice-growing regions. Pantoea ananatis was first reported as a new BLB pathogen, showing the same symptomatic rice leaves as Xoo in India (Mondal et al. 2011), Malaysia (Azizi et al. 2019), and the USA (Luna et al. 2023). In Korea, only P. agglomerans has been found (Lee et al. 2010). Both P. ananatis and P. stewartii have been discovered in Africa (Kini et al. 2017b, 2017a) and Thailand (Arayaskul et al. 2020). In Malaysia, both P. ananatis and P. dispersa have been discovered (Toh et al. 2019), and in China, both P. ananatis and E. asburiae have been reported (Xue et al. 2021). Specially, in southeast China, the BLB incidence caused by P. ananatis is 45–60%, which alarms the new present pathogen is replacing the traditional Xoo to cause disease and even a new BLB pandemic (Yu et al. 2022). Therefore, these new pathogens pose a great challenge to the rice cultivars incorporated with Xa genes, since we have not determined the occurrence, spread, and control of new emerging pathogens yet.
Durable and broad-spectrum resistance to the brown planthopper
The brown planthopper, Nilaparvata lugens (Stål) (Hemiptera: Delphacidae, abbreviated as BPH), is the most devastating insect pest of rice in major rice producing countries. It can cause up to over 20% of yield loss and more than $ 300 million of economic loss every year, due to its high intrinsic fecundity, rapid development of insecticide resistance, and long-distance migration (Dyck and Thomas 1979; Stout 2014; Liu and Sun 2016; Muduli et al. 2021). It causes direct damage by feeding exclusively on rice sap from phloem tissues. The high population of BPHs in the field causes rice leaves to wilt and become brown, which is a phenomenon known as ‘hopperburn’. BPH can cause indirect damage by transmitting pathogens, such as grassy stunt virus and ragged stunt virus (Cheng et al. 2013a; Stout 2014).
Today, the deployment of the BPH resistance gene in rice cultivars has inevitably been considered the most effective and long-lasting method of BPH management, although it was seriously questioned at the beginning of the application of BPH-resistant varieties (Sogawa 2015). The first BPH-resistant variety carrying Bph1, IR26, defended against the prevalent BPH population biotype 1 and was released to the public in 1973 (Peng et al. 2000; Jena and Kim 2010). In the subsequent 3–4 years, BPH resistance was easily broken down as biotype 2 emerged and became predominant (Jena and Kim 2010). Variety IR36 harboring bph2, which confers resistance to biotype 2, was deployed widely for over 10 years after its release in 1976 (Peng et al. 2000; Guan et al. 2022). However, in 1981, biotype 3, which could overcome bph2-mediated resistance, was detected in the field (Zhao et al. 2016; Baehaki and Iswanto 2017). Bph3 and bph4 were discovered later and incorporated into rice cultivars with durable and broad-spectrum resistance to all BPH biotypes (Kawaguchi et al. 2001; Jairin et al. 2007a, 2010; Liu et al. 2015). This typical boom-and-bust cycle (Priestley 1978) forced scientists and breeders to explore new germplasm resources and new technologies that could confer durable and broad-spectrum resistance to rice cultivars.
Thus far, over 45 BPH resistance genes and genetic loci have been identified in wild and cultivated rice (Du et al. 2020; Sani Haliru et al. 2020). Among these, 21 genes/loci have been confirmed to confer broad-spectrum resistance (Table 1 and Supplemental Table 4). BPH3, BPH6, bph25, BPH30, and qBPH6(t) also exhibit resistance to another notorious pest, the white-backed planthopper (WBPH, Sogatella furcifera) (Khush and Brar 1991; Jairin et al. 2007a, 2007b; Jena and Kim 2010; Myint et al. 2012; Liu et al. 2015; Guo et al. 2018; Shi et al. 2021). The allelic polymorphism of BPH9 confers diverse levels of resistance to multiple BPH biotypes (Zhao et al. 2016). However, 38 genes/loci could not be determined because only 1 biotype or population of BPH or new laboratory-selected colonies were used during the period of BPH resistance identification. As new biotypes of BPH are continuously increasing (Thanysiriwat et al. 2009; Listihani et al. 2022), more efforts are needed to identify the virulence shift in wild populations and to keep updating new biotype nomenclature over time. In rice breeding programs, 34 genes/loci have been introduced into rice cultivars to develop new varieties or breeding lines. A total of 7 genes/loci have been introgressed into rice cultivars and released to the public, while the other 27 genes/loci have just been introduced into advanced breeding lines (Supplemental Table 5).
Pyramided R genes have been discovered with additive resistance effects. For example, BPH14 and BPH15 have shown more durable resistance to BPH, while qBPH3 and qBPH4 display more resistance than qBPH3 and qBPH4 alone (Huang et al. 2001; Hu et al. 2015). BPH1-introgressed cultivars have lost control of BPH in Southeast Asia, where biotype 2 is widely distributed, but IR64 has shown durable and broad-spectrum resistance to different biotypes over two decades due to the major genes BPH1 and BPH37 and associated minor QTLs (Alam and Cohen 1998; Mackill and Khush 2018; Yang et al. 2019). However, gene combinations do not always lead to positive effects compared to a single R gene (Jena et al. 2017). Gene stacking has become the most effective way to develop a new variety harboring durable and broad-spectrum resistance in practical modern rice breeding programs (Mundt 2014; Muduli et al. 2021). To improve stable and durable resistance to BPH in rice and develop promising genetic sources for emerging BPH biotypes in advance, 2–4 BPH genes are stacked to develop new breeding materials (Supplemental Table 5) (Rahman et al. 2009; Zhu et al. 2013; Jena et al. 2017; Li et al. 2019b).
As a new method of improving disease resistance, the CRISPR/Cas9-mediated gene knockout of the cytochrome P450 gene CYP71A1 (S-related factors) improves rice insect resistance, not only to BPH but also to WBPH and the striped stem borer (Chilo suppressalis) (Lu et al. 2018).
Multiple disease or stress resistance in rice
Multiple disease resistance (MDR) refers to host plant resistance to a minimum of two diseases, excluding non-host resistance (Wiesner-Hanks and Nelson 2016). Although it was regarded as a valued trait for plant pathologists and breeders for over a century, genetic evidence and molecular mechanisms were not well studied until Wiesner-Hanks and Nelson’s review. MDR is considered a peculiar form of broad-spectrum resistance and is particularly difficult to overcome due to multiple unlinked genes/loci, clusters of tightly linked genes, and individual genes with pleiotropic effects (Wiesner-Hanks and Nelson 2016). MDR has been described as potentially important in barley and wheat (Pooja et al. 2014; Pal et al. 2022), while increasing genetic evidence in other plants, including rice, maize, coffee, and cotton, has been reported (Ali et al. 2013; de Almeida et al. 2021; Jamaloddin et al. 2021; Huo et al. 2023). In regard to sessile plants and ongoing climate change, the idea of MDR might extend to the resistance or tolerance to two or more multiple stresses, both abiotic and biotic, such as drought, heat, chilling, flood, fungi, bacteria, viruses, parasitic plants, and insects. This concept has been accepted and cited in the second-generation ‘new plant type’ (NPT-2) initiative and the Green Super Rice (GSR) project (Bin Rahman and Zhang 2023).
In rice, 40 defense regulator genes coding various proteins in different biological activities, as summarized by Liu et al. (2021b), have been found to be involved in MDR, 29 of which contribute to resistance to both blast and BLB (Liu et al. 2021b). Even a series of developmental genes, protein networks, signaling pathways, pleiotropic genes, and a set of response factors in plants can confer multiple stress resistance (Chun et al. 2012; Jacob et al. 2017; Cohen and Leach 2019; Zhang et al. 2021b; Husaini 2022). However, there have been few practical applications of these genes in crop breeding. Most plant R genes are race-specific, conforming to the typical gene-for-gene relationship, but some exhibit non-race-specific resistance to at least two pathogens, insects, or stresses. For example, wheat Lr34 and Lr67 confer resistance to both rust disease and powdery mildew (Krattinger et al. 2009; Moore et al. 2015), even Lr34 confers resistance to rust disease and northern corn leaf blight in maize and barley and blast disease in rice (Risk et al. 2013; Krattinger et al. 2016; Sucher et al. 2017). As stated previously, BPH3, BPH6, bph25, BPH30, and qBPH6(t) confer resistance to both BPH and WBPH in rice (Khush and Brar 1991; Jairin et al. 2007a, 2007b; Jena and Kim 2010; Myint et al. 2012; Liu et al. 2015; Guo et al. 2018; Shi et al. 2021). Rice OslecRK can induce an immune response to fungal and bacterial diseases, as well as BPH (Cheng et al. 2013b).
Thus far, an effective strategy for producing MDR or multiple stress resistance has been to combine multiple R genes into a single crop genotype, which can simultaneously increase the durability of resistance (Wiesner-Hanks and Nelson 2016; Mundt 2018). Due to MAS, it is possible to pyramid multiple stress resistance genes using molecular markers rather than phenotyping in different stress environments. Marker-assisted forward breeding and marker-assisted backcross breeding have been used to develop rice cultivars with multiple R genes or QTLs (Dixit et al. 2020; Ramalingam et al. 2020). For example, Pi9 for blast, Xa21 for BLB, Gm8 for gall midge (a serious rice pest), and three major QTLs, qDTY1.1, qDTY2.2, and qDTY4.1, were introduced into an elite Indian rice variety, ‘Naveen,’ in six years (Janaki Ramayya et al. 2021). The new approaches could not reduce the time of the breeding project, as the selected crop plants had to be tested and evaluated in the field (Ben-Ari and Lavi 2012). In addition, the development of multiple R gene pyramids usually takes a great deal of time because a series of backcrosses and subsequent MAS are needed to avoid the loss of gene effectiveness and durability before the cultivars introgressed with multiple R genes are released to the public (Stam and McDonald 2018). As Supplemental Table 6 shows, among 133 super rice cultivars in China, only 40 cultivars show resistance to one stress, 2 cultivars exhibit resistance to two stresses, and none show resistance to blast, BLB, and BPH. Similarly, among 154 varieties released by the Central Varietal Release Committee in India (Supplemental Table 7), a total of 64 cultivars show resistance to one stress, 20 cultivars exhibit resistance to two stresses, and 4 varieties show resistance to three stresses (Table 2). Rice breeders now know of many resistance genes; however, most breeding programs emphasize monogenic resistance. It seems that pyramiding of R genes to produce multiple stress or disease resistance has attracted less attention and effort than stress or disease resistance to a single stress.
Another strategy for stacking resistance is multigene transformation. At first, scientists combined a single gene transformation with hybrid polymerization (Datta et al. 2002). However, this method is time-consuming and requires hybridization and screening work to confirm pyramided transgenic plants. Another method, multi-vector co-transformation, has been used to transfer multiple genes in separate vectors into the same recipient plant at the same time, but this method is uncertain and unstable. Multigene single-vector transformation utilizes multiple genes on one plasmid, which can then be transferred to the same recipient plant at one time. This method has received much more attention due to its easy manipulation and time and labor efficiency (Shehryar et al. 2020; Li et al. 2021a). The only hurdle is placing multiple genes on a single T-DNA, which is being resolved by the development of a high-efficiency transgene stacking system, such as the TransGene Stacking II (TGSII) system (Zhu et al. 2017) and GAANTRY (Gene Assembly in Agrobacterium by Nucleic acid Transfer using Recombinase Technology) system (Collier et al. 2018). A new method, named HACKing (Highly Efficient and Accessible System by CracKing Genes into the Genome) system based on CRISPR/Cas9 genome editing, has been developed to assemble multigene pathways in fungi. This system provides high and stable expression of multiple genes and more choice for different conditions based on the researcher’s demands (Yue et al. 2023). Therefore, it can be quickly modified for use in other organisms, especially plants. Recently, a new approach to obtaining MDR in rice has been reported, in which multiple S genes are modified by CRISPR/Cas9-mediated gene knockout. A rice mutant inhibiting pi21, bsr-d1, and xa5 shows enhanced resistance to blast disease and BLB (Tao et al. 2021). Both transgenic stacking genes and modifying genes are logically time- and labor-saving methods compared to multiple gene pyramiding. However, it will still be a long time before they are widely used, as transgenic and cisgenic gene cassettes are strictly required for government approval before field deployment (Stam and McDonald 2018). For example, since the first transgenic tobacco plants were developed in 1983 (Herrera-Estrella et al. 1983), only 463 transgenic events from the 32 main crops have been approved according to the genetically modified crops database of the International Service for the Acquisition of Agri-Biotech Applications (ISAAA, http://www.isaaa.org/gmapprovaldatabase/default.asp). Among them, only 6 events from rice and only 2 events for 2 genes conferring resistance to 2 Lepidopteran insects have been reported.
Conclusion and perspectives
Rice breeding is an ancient activity that dates back to the dawn of agriculture (Wallace et al. 2018; Xu and Sun 2021). The multi-origin domestications of Oryza sativa from wild to cultivated rice began more than 10,000 years ago, when the morphological traits, physiological characteristics, and ecological adaptability were greatly improved by local and independent famers’ conscious and unconscious selection in the fields (Sweeney and McCouch 2007; Xue et al. 2021; Zhang et al. 2021a). After Mendelian genetics was found and introduced into breeding programs between the late nineteenth and early twentieth century, plant breeding entered scientific conventional breeding (Wallace et al. 2018). With the fast development of molecular genetics and biotechnology, especially MAS applied extensively in plant breeding programs, marker-assisted breeding has developed a great number of crop cultivars with important agronomic traits to alleviate global hunger in the past half century. For example, the semidwarf-1 (sd1) gene, a ‘Green Revolution’ gene in rice, has been widely deployed in modern rice varieties, increasing rice yields to a new peak in the world (Cheng et al. 2022). Plant breeding has now developed into an applied, multidisciplinary science utilizing the maximum benefits from genomic, phenomic, and enviromic data (Gepts and Hancock 2006; Crossa et al. 2021).
Thus far, pyramiding multiple R genes/QTLs is the most effective method for increasing the durability of resistance and broadening the pathogen spectrum in rice breeding. Although this approach has many empirical applications, scientists and breeders are not certain about the final effects of multiple R gene combinations. The main reason is that multiple genes do not mean one gene function added to another gene function, as there is a potentially complex relationship that could result in an unexpected phenotype. This complex relationship cannot be understood clearly only from the R gene/QTLs studied from a single perspective or several related perspectives, such as gene function, signal pathway, or regulatory mechanism, but also from systemic and multi-omics studies, such as genomics, transcriptomics, metabolomics, and phenomics. Such great systemic and multi-omics work cannot be accomplished in only a wet laboratory but a dry laboratory.
Machine learning (ML) refers to computational algorithms that turn datasets from large-scale observations into usable models (Edgar and Manz 2017). It has shown rapid development and has been deployed in multi-dimensional phenotyping, plant–pathogen interactions, and plant breeding, including QTL mapping, genetic architecture assessment, and genomic prediction (Sperschneider 2020; Wang et al. 2020; van Dijk et al. 2021). Multiple stress resistance genes have been identified in rice using ML (Shaik and Ramakrishna 2013). Artificial intelligence (AI) refers to systems imitated by a computer to display intelligent behavior similar to humans but with some degree of autonomy (Sheikh et al. 2023). It has acted as a revolution in our society, impacting industry, business, agriculture, and science. It can turn traditional experience-based selection into precision molecular design in breeding (Liu et al. 2021a) and reshape modern crop breeding into smart breeding (Khan et al. 2022; Xu et al. 2022). In addition, AI integrated with speed breeding can accelerate crop breeding due to resistance to multiple stresses from unfavorable environmental conditions and infectious living organisms (Watson et al. 2018; Rai 2022). Therefore, ML and AI can help us not only discover the genetic basis of durable and broad-spectrum resistance but also quickly design and develop new rice cultivars with durable and broad-spectrum resistance to major diseases and insects for specific geographic locations in the near future.
References
Alam SN, Cohen MB (1998) Detection and analysis of QTLs for resistance to the brown planthopper, Nilaparvata lugens, in a doubled-haploid rice population. Theor Appl Genet 97:1370–1379
Ali F, Pan Q, Chen G, Zahid KR, Yan J (2013) Evidence of multiple disease resistance (MDR) and implication of meta-analysis in marker assisted selection. PLoS ONE 8:e68150
Anuradha N, Patro TSSK, Singamsetti A, Sandhya Rani Y, Triveni U, Nirmala Kumari A, Govanakoppa N, Lakshmi Pathy T, Tonapi VA (2022) Comparative study of AMMI- and BLUP-based simultaneous selection for grain yield and stability of finger millet [Eleusine coracana (L.) Gaertn.] genotypes. Front Plant Sci 12:786839
Arayaskul N, Poompouang S, Lithanatudom P, Lithanatudom SK (2020) First report of a leaf blight in rice (Oryza sativa) caused by pantoea ananatis and pantoea stewartii in Thailand. Plant Dis 104:562
Arif M, Jaffar M, Baber M, Sheikh M, Kousar S, Arif A, Zafar Y (2010) Identification of bacterial blight resistance genes Xa4 in Pakistani rice germplasm using PCR. Afr J Biotechnol 7:541–545
Ashikawa I, Hayashi N, Yamane H, Kanamori H, Wu J, Matsumoto T, Ono K, Yano M (2008) Two adjacent nucleotide-binding site–leucine-rich repeat class genes are required to confer pikm-specific rice blast resistance. Genetics 180:2267–2276
Azizi MMF, Zulperi D, Rahman MAA, Abdul-Basir B, Othman NA, Ismail SI, Hata EM, Ina-Salwany MY, Abdullah MAF (2019) First report of pantoea ananatis causing leaf blight disease of rice in peninsular Malaysia. Plant Dis 103:2122
Baehaki SE, Iswanto EH (2017) The filtering of rice resistance and population buildup to determine antibiosis and tolerance as characteristics of rice resistance to brown planthopper biotype 3. Am J Eng Res (AJER) 6:188–196
Ballini E, Berruyer R, Morel J-B, Lebrun M-H, Nottéghem J-L, Tharreau D (2007) Modern elite rice varieties of the ‘Green Revolution’ have retained a large introgression from wild rice around the Pi33 rice blast resistance locus. New Phytol 175:340–350
Ben-Ari G, Lavi U (2012) Marker-assisted selection in plant breeding. In: Altman A, Hasegawa PM (eds) Plant biotechnology and agriculture. Academic Press, San Diego, pp 163–184
Benedict LF, Linscombe SD (2009) Developing New Varieties of Rice. In Louisiana Agriculture (LSU AgCenter, Crowley, La.)
Berruyer R, Adreit H, Milazzo J, Gaillard S, Berger A, Dioh W, Lebrun MH, Tharreau D (2003) Identification and fine mapping of Pi33, the rice resistance gene corresponding to the Magnaporthe grisea avirulence gene ACE1. Theor Appl Genet 107:1139–1147
Bin Rahman ANMR, Zhang J (2023) Trends in rice research: 2030 and beyond. Food Energy Secur. 12:e390
Boddy L (2016) Chapter 8 - pathogens of autotrophs. In: Watkinson SC, Boddy L, Money NP (eds) The Fungi (Third Edition). Academic Press, Boston, pp 245–292
Brown JKM (2015) Durable resistance of crops to disease: a darwinian perspective. Annu Rev Phytopathol 53:513–539
Brown JKM, Rant JC (2013) Fitness costs and trade-offs of disease resistance and their consequences for breeding arable crops. Plant Pathol 62:83–95
Brutus A, Yang He S (2010) Broad-spectrum defense against plant pathogens. Nat Biotechnol 28:330–331
Bryan GT, Wu K-S, Farrall L, Jia Y, Hershey HP, McAdams SA, Faulk KN, Donaldson GK, Tarchini R, Valent B (2000) A Single amino acid difference distinguishes resistant and susceptible alleles of the rice blast resistance gene Pi-ta. Plant Cell 12:2033–2045
Busungu C, Taura S, Sakagami J-I, Ichitani K (2016) Identification and linkage analysis of a new rice bacterial blight resistance gene from XM14, a mutant line from IR24. Breed Sci 66:636–645
Busungu C, Taura S, Sakagami J-I, Anai T, Ichitani K (2018) High-resolution mapping and characterization of xa42, a resistance gene against multiple Xanthomonas oryzae pv. oryzae races in rice (Oryza sativa L.). Breed Sci 68:188–199
Cao Y, Ding X, Cai M, Zhao J, Lin Y, Li X, Xu C, Wang S (2007) The expression pattern of a rice disease resistance gene Xa3/Xa26 is differentially regulated by the genetic backgrounds and developmental stages that influence its function. Genetics 177:523–533
Cheema KK, Grewal NK, Vikal Y, Sharma R, Lore JS, Das A, Bhatia D, Mahajan R, Gupta V, Bharaj TS, Singh K (2008) A novel bacterial blight resistance gene from Oryza nivara mapped to 38 kb region on chromosome 4L and transferred to Oryza sativa L. Genet Res 90:397–407
Chen XW, Li SG, Xu JC, Zhai WX, Ling ZZ, Ma BT, Wang YP, Wang WM, Cao G, Ma YQ, Shang JJ, Zhao XF, Zhou KD, Zhu LH (2004) identification of two blast resistance genes in a rice variety, digu. J Phytopathol 152:77–85
Chen J, Shi Y, Liu W, Chai R, Fu Y, Zhuang J, Wu J (2011a) A Pid3 allele from rice cultivar Gumei2 confers resistance to Magnaporthe oryzae. J Genet Genom 38:209–216
Chen S, Liu X, Zeng L, Ouyang D, Yang J, Zhu X (2011b) Genetic analysis and molecular mapping of a novel recessive gene xa34(t) for resistance against Xanthomonas oryzae pv. oryzae. Theor Appl Genet 122:1331–1338
Chen S, Wang C, Yang J, Chen B, Wang W, Su J, Feng A, Zeng L, Zhu X (2020) Identification of the novel bacterial blight resistance gene Xa46(t) by mapping and expression analysis of the rice mutant H120. Sci Rep 10:12642
Chen X, Liu P, Mei L, He X, Chen L, Liu H, Shen S, Ji Z, Zheng X, Zhang Y, Gao Z, Zeng D, Qian Q, Ma B (2021) Xa7, a new executor R gene that confers durable and broad-spectrum resistance to bacterial blight disease in rice. Plant Commun 2:100143
Cheng X, Zhu L, He G (2013a) Towards understanding of molecular interactions between rice and the brown planthopper. Mol Plant 6:621–634
Cheng X, Wu Y, Guo J, Du B, Chen R, Zhu L, He G (2013b) A rice lectin receptor-like kinase that is involved in innate immune responses also contributes to seed germination. Plant J 76:687–698
Cheng X, Huang Y, Tan Y, Tan L, Yin J, Zou G (2022) Potentially useful dwarfing or semi-dwarfing genes in rice breeding in addition to the sd1 gene. Rice 15:66
Cho Y-C, Kwon S-W, Choi I-S, Lee S-K, Jeon J-S, Oh M-K, Roh J-H, Suh J-P, Yang S-J, Kim Y-G (2007) Identification of major blast resistance genes in Korean rice varieties (Oryza sativa L.) using molecular markers. J Crop Sci Biotech 10:265–276
Chu Z, Fu B, Yang H, Xu C, Li Z, Sanchez A, Park YJ, Bennetzen JL, Zhang Q, Wang S (2006) Targeting xa13, a recessive gene for bacterial blight resistance in rice. Theor Appl Genet 112:455–461
Chukwu SC, Rafii MY, Ramlee SI, Ismail SI, Hasan MM, Oladosu YA, Magaji UG, Akos I, Olalekan KK (2019) Bacterial leaf blight resistance in rice: a review of conventional breeding to molecular approach. Mol Biol Rep 46:1519–1532
Chun HJ, Park HC, Koo SC, Lee JH, Park CY, Choi MS, Kang CH, Baek D, Cheong YH, Yun D-J, Chung WS, Cho MJ, Kim MC (2012) Constitutive expression of mammalian nitric oxide synthase in tobacco plants triggers disease resistance to pathogens. Mol Cells 34:463–471
Cohen SP, Leach JE (2019) Abiotic and biotic stresses induce a core transcriptome response in rice. Sci Rep 9:6273
Collier R, Thomson JG, Thilmony R (2018) A versatile and robust Agrobacterium-based gene stacking system generates high-quality transgenic Arabidopsis plants. Plant J 95:573–583
Crossa J, Fritsche-Neto R, Montesinos-Lopez OA, Costa-Neto G, Dreisigacker S, Montesinos-Lopez A, Bentley AR (2021) The modern plant breeding triangle: optimizing the use of genomics, phenomics, and enviromics data. Front Plant Sci 12:651480
Dai L-Y, Liu X-L, Xiao Y-H, Wang G-L (2007) Recent advances in cloning and characterization of disease resistance genes in rice. J Integr Plant Biol 49:112–119
Datta K, Baisakh N, Maung Thet K, Tu J, Datta S (2002) Pyramiding transgenes for multiple resistance in rice against bacterial blight, yellow stem borer and sheath blight. Theor Appl Genet 106:1–8
de Almeida DP, Caixeta ET, Moreira KF, de Oliveira ACB, de Freitas KNP, Pereira AA, Rosado RDS, Zambolim L, Cruz CD (2021) Marker-assisted pyramiding of multiple disease resistance genes in coffee genotypes (Coffea arabica). Agronomy 11:1763
Deng Y, Zhai K, Xie Z, Yang D, Zhu X, Liu J, Wang X, Qin P, Yang Y, Zhang G, Li Q, Zhang J, Wu S, Milazzo J, Mao B, Wang E, Xie H, Tharreau D, He Z (2017) Epigenetic regulation of antagonistic receptors confers rice blast resistance with yield balance. Science 355:962–965
Deng Y, Liu H, Zhou Y, Zhang Q, Li X, Wang S (2018) Exploring the mechanism and efficient use of a durable gene-mediated resistance to bacterial blight disease in rice. Mol Breed 38:18
Dixit S, Singh UM, Singh AK, Alam S, Venkateshwarlu C, Nachimuthu VV, Yadav S, Abbai R, Selvaraj R, Devi MN, Ramayya PJ, Badri J, Ram T, Lakshmi J, Lakshmidevi G, Lrk JV, Padmakumari AP, Laha GS, Prasad MS, Seetalam M, Singh VK, Kumar A (2020) Marker assisted forward breeding to combine multiple biotic-abiotic stress resistance/tolerance in rice. Rice 13:29
Dong L, Liu S, Xu P, Deng W, Li X, Tharreau D, Li J, Zhou J, Wang Q, Tao D, Yang Q (2017) Fine mapping of Pi57(t) conferring broad spectrum resistance against Magnaporthe oryzae in introgression line IL-E1454 derived from Oryza longistaminata. PLoS ONE 12:e0186201
Du B, Chen R, Guo J, He G (2020) Current understanding of the genomic, genetic, and molecular control of insect resistance in rice. Mol Breed 40:24
Dyck VA, Thomas B (1979) The brown planthopper problem, pp 3–16
Edgar TW, Manz DO (2017) Machine learning. In: Edgar TW, Manz DO (eds) Research methods for cyber security. Syngress, Rockland, pp 153–173
FAO. (2021). World Food and Agriculture - Statistical Yearbook 2021. (Rome).
Fiyaz RA, Shivani D, Chaithanya K, Mounika K, Chiranjeevi M, Laha GS, Viraktamath BC, Rao LVS, Sundaram RM (2022) Genetic improvement of rice for bacterial blight resistance: present status and future prospects. Rice Sci 29:118–132
Fonseca JP, Mysore KS (2019) Genes involved in nonhost disease resistance as a key to engineer durable resistance in crops. Plant Sci 279:108–116
Fujii K, Suzuki T, Nakamura M, Yoshida T, Uchikawa Y, Suwazono H, Hayashi N, Kanda Y, Inoue H (2023) Investigating the mechanisms underlying the durability and sustainable use of Pb1 gene-mediated high field resistance to rice panicle blast. Agronomy 13:1751
Fukuoka S (2018) Marker-assisted gene pyramiding for durable resistance to blast. In: Sasaki T, Ashikari M (eds) Rice genomics, genetics and breeding. Springer, Singapore, pp 393–415
Fukuoka S, Okuno K (2001) QTL analysis and mapping of pi21, a recessive gene for field resistance to rice blast in Japanese upland rice. Theor Appl Genet 103:185–190
Fukuoka S, Saka N, Koga H, Ono K, Shimizu T, Ebana K, Hayashi N, Takahashi A, Hirochika H, Okuno K, Yano M (2009) Loss of function of a proline-containing protein confers durable disease resistance in rice. Science 325:998–1001
Fukuoka S, Yamamoto S-I, Mizobuchi R, Yamanouchi U, Ono K, Kitazawa N, Yasuda N, Fujita Y, Nguyen T, Koizumi S, Sugimoto K, Matsumoto T, Yano M (2014) Multiple functional polymorphisms in a single disease resistance gene in rice enhance durable resistance to blast. Sci Rep 4:4550
Fukuoka S, Saka N, Mizukami Y, Koga H, Yamanouchi U, Yoshioka Y, Hayashi N, Ebana K, Mizobuchi R, Yano M (2015) Gene pyramiding enhances durable blast disease resistance in rice. Sci Rep 5:7773
Furuya N, Taura S, Goto T, Thuy BT, Ton PH, Tsuchiya K, Yoshimura A (2012) Diversity in Virulence of Xanthomonas oryzae pv. oryzae from Northern Vietnam. Japan Agric Res q: JARQ 46:329–338
Galhano R, Talbot NJ (2011) The biology of blast: understanding how Magnaporthe oryzae invades rice plants. Fungal Biol Rev 25:61–67
Gallois J-L, Moury B, German-Retana S (2018) Role of the genetic background in resistance to plant viruses. Int J Mol Sci 19:2856
Gao DY, Xu ZG, Chen ZY, Sun LH, Sun QM, Lu F, Hu BS, Liu YF (2002) Identification of a resistance gene to bacterial blight (Xanthomonas oryzae pv. oryzae) in a somaclonal mutant HX-3 of indica rice. Yi Chuan Xue Bao 29:138–143
Gao D-Y, Liu A-M, Zhou Y-H, Cheng Y-J, Xiang Y-H, Sun L-H, Zhai W-X (2005) Molecular mapping of a bacterial blight resistance gene Xa-25 in rice. Yi Chuan Xue Bao=acta Genetica Sinica 32:183–188
Gepts P, Hancock J (2006) The future of plant breeding. Crop Sci 46:1630–1634
Gnanamanickam SS, Priyadarisini VB, Narayanan NN, Vasudevan P, Kavitha S (1999) An overview of bacterial blight disease of rice and strategies for its management. Curr Sci 77:1435–1444
Goto T, Matsumoto T, Furuya N, Tsuchiya K, Yoshimura A (2009) Mapping of bacterial blight resistance gene Xa11 on rice chromosome 3. Japan Agric Res q: JARQ 43:221–225
Gu K, Tian D, Yang F, Wu L, Sreekala C, Wang D, Wang GL, Yin Z (2004) High-resolution genetic mapping of Xa27(t), a new bacterial blight resistance gene in rice, Oryza sativa L. Theor Appl Genet 108:800–807
Gu K, Sangha JS, Li Y, Yin Z (2008) High-resolution genetic mapping of bacterial blight resistance gene Xa10. Theor Appl Genet 116:155–163
Guan H, Hou X, Jiang Y, Srivastava V, Mao D, Pan R, Chen M, Zhou Y, Wang Z, Chen Z (2019) Feature of blast resistant near-isogenic lines using an elite maintainer line II-32B by marker-assisted selection. J Plant Pathol 101:491–501
Guan W, Shan J, Gao M, Guo J, Wu D, Zhang Q, Wang J, Chen R, Du B, Zhu L, He G (2022) Bulked segregant RNA sequencing revealed difference between virulent and avirulent brown planthoppers. Front Plant Sci 13:8432275
Guo S, Zhang D, Lin X (2010) Identification and mapping of a novel bacterial blight resistance gene Xa35(t) originated from Oryza minuta. Sci Agric Sin 43:2611–2618
Guo J, Xu C, Wu D, Zhao Y, Qiu Y, Wang X, Ouyang Y, Cai B, Liu X, Jing S, Shangguan X, Wang H, Ma Y, Hu L, Wu Y, Shi S, Wang W, Zhu L, Xu X, Chen R, Feng Y, Du B, He G (2018) Bph6 encodes an exocyst-localized protein and confers broad resistance to planthoppers in rice. Nat Genet 50:297–306
Hayashi K, Yoshida H (2009) Refunctionalization of the ancient rice blast disease resistance gene Pit by the recruitment of a retrotransposon as a promoter. Plant J 57:413–425
Hayashi N, Inoue H, Kato T, Funao T, Shirota M, Shimizu T, Kanamori H, Yamane H, Hayano-Saito Y, Matsumoto T, Yano M, Takatsuji H (2010) Durable panicle blast-resistance gene Pb1 encodes an atypical CC-NBS-LRR protein and was generated by acquiring a promoter through local genome duplication. Plant J 64:498–510
He Q, Li D, Zhu Y, Tan M, Zhang D, Lin X (2006) Fine mapping of Xa2, a bacterial blight resistance gene in rice. Mol Breed 17:1–6
Herrera-Estrella L, Depicker A, Van Montagu M, Schell J (1983) Expression of chimaeric genes transferred into plant cells using a Ti-plasmid-derived vector. Nature 303:209–213
Hisatoshi K (1993) infection types in rice-xanthomonas campestris pv. oryzae interaction. Japan Agric Res Q 27:81–87
Hou L-Y, Yu P, Xu Q, Yuan X-P, Yu H-Y, Wang Y-P, Wang C-H, Wan G, Tang S-X, Peng S-T, Wei X-H (2011) Genetic analysis and preliminary mapping of two recessive resistance genes to brown planthopper, Nilaparvata lugens Stål in Rice. Rice Sci 18:238–242
Hsu Y-C, Chiu C-H, Yap R, Tseng Y-C, Wu Y-P (2020) Pyramiding bacterial blight resistance genes in Tainung82 for broad-spectrum resistance using marker-assisted selection. Int J Mol Sci 21:1281
Hu J, Xiao C, Cheng M, Gao G, Zhang Q, He Y (2015) Fine mapping and pyramiding of brown planthopper resistance genes QBph3 and QBph4 in an introgression line from wild rice O. officinalis. Mol Breed 35:3
Hu K, Cao J, Zhang J, Xia F, Ke Y, Zhang H, Xie W, Liu H, Cui Y, Cao Y, Sun X, Xiao J, Li X, Zhang Q, Wang S (2017) Improvement of multiple agronomic traits by a disease resistance gene via cell wall reinforcement. Nat Plants 3:17009
Hu X-H, Shen S, Wu J-L, Liu J, Wang H, He J-X, Yao Z-L, Bai Y-F, Zhang X, Zhu Y, Li G-B, Zhao J-H, You X, Xu J, Ji Y-P, Li D-Q, Pu M, Zhao Z-X, Zhou S-X, Zhang J-W, Huang Y-Y, Li Y, Ning Y, Lu Y, Huang F, Wang W-M, Fan J (2023) A natural allele of proteasome maturation factor improves rice resistance to multiple pathogens. Nat Plants 9:228–237
Hua L, Wu J, Chen C, Wu W, He X, Lin F, Wang L, Ashikawa I, Matsumoto T, Wang L, Pan Q (2012) The isolation of Pi1, an allele at the Pik locus which confers broad spectrum resistance to rice blast. Theor Appl Genet 125:1047–1055
Hua L-X, Liang L-Q, He X-Y, Wang L, Zhang W-S, Liu W, Liu X-Q, Lin F (2015) Development of a marker specific for the rice blast resistance gene Pi39 in the Chinese cultivar Q15 and its use in genetic improvement. Biotechnol Biotechnol Equip 29:448–456
Huang N, Angeles ER, Domingo J, Magpantay G, Singh S, Zhang G, Kumaravadivel N, Bennett J, Khush GS (1997) Pyramiding of bacterial blight resistance genes in rice: marker-assisted selection using RFLP and PCR. Theor Appl Genet 95:313–320
Huang Z, He G, Shu L, Li X, Zhang Q (2001) Identification and mapping of two brown planthopper resistance genes in rice. Theor Appl Genet 102:929–934
Huang H, Huang L, Feng G, Wang S, Wang Y, Liu J, Jiang N, Yan W, Xu L, Sun P, Li Z, Pan S, Liu X, Xiao Y, Liu E, Dai L, Wang G-L (2011) Molecular mapping of the new blast resistance genes Pi47 and Pi48 in the durably resistant local rice cultivar Xiangzi 3150. Phytopathology 101:620–626
Huo W-Q, Zhang Z-Q, Ren Z-Y, Zhao J-J, Song C-X, Wang X-X, Pei X-Y, Liu Y-G, He K-L, Zhang F, Li X-Y, Li W, Yang D-G, Ma X-F (2023) Unraveling genomic regions and candidate genes for multiple disease resistance in upland cotton using meta-QTL analysis. Heliyon 9:e18731
Huot B, Yao J, Montgomery BL, He SY (2014) Growth-defense tradeoffs in plants: a balancing act to optimize fitness. Mol Plant 7:1267–1287
Husaini AM (2022) High-value pleiotropic genes for developing multiple stress-tolerant biofortified crops for 21st-century challenges. Heredity 128:460–472
Hutin M, Sabot F, Ghesquière A, Koebnik R, Szurek B (2015) A knowledge-based molecular screen uncovers a broad-spectrum OsSWEET14 resistance allele to bacterial blight from wild rice. Plant J 84:694–703
(IRRI), I.R.R.I. (2022) Nepal releases seven new and improved rice varieties to increase domestic rice production
Ishii T, Brar DS, Multani DS, Khush GS (1994) Molecular tagging of genes for brown planthopper resistance and earliness introgressed from Oryza australiensis into cultivated rice. O Sativa Genome 37:217–221
Ishikawa K, Kuroda T, Hori T, Iwata D, Matsuzawa S, Nakabayashi J, Sasaki A, Ashizawa T (2022) Long-term blast control in high eating quality rice using multilines. Sci Rep 12:14880–14880
Ishiyama S (1992) Studies of bacterial leaf blight of rice. Report of the imperial agricultural station. Nishigahara (Konosu) 45:233–261
Iyer AS, McCouch SR (2004) The rice bacterial blight resistance gene xa5 encodes a novel form of disease resistance. Mol Plant-Microbe Interact 17:1348–1354
Jacob P, Hirt H, Bendahmane A (2017) The heat-shock protein/chaperone network and multiple stress resistance. Plant Biotechnol J 15:405–414
Jairin J, Phengrat K, Teangdeerith S, Vanavichit A, Toojinda T (2007a) Mapping of a broad-spectrum brown planthopper resistance gene, Bph3, on rice chromosome 6. Mol Breed 19:35–44
Jairin J, Teangdeerith S, Leelagud P, Phengrat K, Vanavichit A, Toojinda T (2007b) Detection of brown planthopper resistance genes from different rice mapping populations in the same genomic location. ScienceAsia 33:347–352
Jairin J, Sansen K, Wongboon W, Kothcharerk JJBS (2010) Detection of a brown planthopper resistance gene bph4 at the same chromosomal position of Bph3 using two different genetic backgrounds of rice. Breed Sci 60:71–75
Jamaloddin M, Mahender A, Gokulan CG, Balachiranjeevi C, Maliha A, Patel HK, Ali J (2021) Molecular approaches for disease resistance in Rice. In: Ali J, Wani SH (eds) Rice improvement: physiological, molecular breeding and genetic perspectives. Springer International Publishing, Cham, pp 315–378
Janaki Ramayya P, Vinukonda VP, Singh UM, Alam S, Venkateshwarlu C, Vipparla AK, Dixit S, Yadav S, Abbai R, Badri J, Phani Padmakumari A (2021) Marker-assisted forward and backcross breeding for improvement of elite Indian rice variety Naveen for multiple biotic and abiotic stress tolerance. PLoS ONE 16:e0256721
Jena KK, Kim S-M (2010) Current status of brown planthopper (BPH) resistance and genetics. Rice 3:161–171
Jena KK, Hechanova SL, Verdeprado H, Prahalada GD, Kim S-R (2017) Development of 25 near-isogenic lines (NILs) with ten BPH resistance genes in rice (Oryza sativa L.): production, resistance spectrum, and molecular analysis. Theor Appl Genet 130:2345–2360
Jena K, Suh J, Jeung J, Cho Y, Roh J, Han S, Kim Y, Brar D (2007) Molecular breeding for durable blast disease resistance in rice
Jeon JS, Chen D, Yi GH, Wang GL, Ronald PC (2003) Genetic and physical mapping of Pi5(t), a locus associated with broad-spectrum resistance to rice blast. Mol Genet Genom 269:280–289
Jeung J-U, Kim B, Cho YC, Han SS, Moon HP, Lee YT, Jena KK (2007) A novel gene, Pi40(t), linked to the DNA markers derived from NBS-LRR motifs confers broad spectrum of blast resistance in rice. Theor Appl Genet 115:1163–1177
Ji Z-J, Yang S-D, Zeng Y-X, Liang Y, Yang C-D, Qian Q (2016) Pyramiding blast, bacterial blight and brown planthopper resistance genes in rice restorer lines. J Integr Agric 15:1432–1440
Ji C, Ji Z, Liu B, Cheng H, Liu H, Liu S, Yang B, Chen G (2020) Xa1 allelic R genes activate rice blight resistance suppressed by interfering TAL effectors. Plant Commun 1:100087–100087
Jiang G-L (2015) Molecular marker-assisted breeding: a plant breeder’s review. In: Al-Khayri JM, Jain SM, Johnson DV (eds) Advances in plant breeding strategies: breeding, biotechnology and molecular tools. Springer International Publishing, Cham, pp 431–472
Jiang H, Luo J, Liu Q, Ogunyemi SO, Ahmed T, Li B, Yu S, Wang X, Yan C, Chen J, Li B (2023) Rice bacterial leaf blight drives rhizosphere microbial assembly and function adaptation. Microbiol Spectr 11:e01059-e11023
Jin XW, Wang C, Yang Q, Jiang QX, Fan YL, Liu GC, Zhao K (2007) Breeding of near-isogenic line CBB30 and molecular mapping of Xa30(t), a new resistance gene to bacterial blight in rice. Sci Agric Sin 40:1094–1100
Johnson R (1979) Letter to the editor: the concept of durable resistance. Phytopathology 69:198–199
Johnson R (1984) A critical analysis of durable resistance. Annu Rev Phytopathol 22:309–330
Johnson R (1993) Durability of disease resistance in crops: some closing remarks about the topic and the symposium. In: Jacobs T, Parlevliet JE (eds) Durability of disease resistance. Springer, The Netherlands, pp 283–300
Junjie X, Huafeng D, Longping Y (2019) The utilization of rice blast resistance genes in hybrid rice breeding in China. In: Yulin J (ed) Protecting rice grains in the post-genomic era. IntechOpen, Rijeka
Kaku H, Ogawa T (2000) The relationship between browning reaction and bacterial blight resistance gene Xa3 in rice
Kaku H, Ogawa T (2001) Genetic analysis of the relationship between the browning reaction and bacterial blight resistance gene Xa3 in rice. J Gen Plant Pathol 67:228–230
Kawaguchi M, Murata K, Ishii T, Takumi S, Mori N, Nakamura C (2001) Assignment of a brown planthopper(Nilaparvata lugens Stal) resistance gene bph4 to the rice chromosome 6. Breed Sci 51:13–18
Khan MHU, Wang S, Wang J, Ahmar S, Saeed S, Khan SU, Xu X, Chen H, Bhat JA, Feng X (2022) Applications of artificial intelligence in climate-resilient smart-crop breeding. Int J Mol Sci 23:11156
Khush GS, Brar DS (1991) Genetics of resistance to insects in crop plants. In: Brady NC (ed) Advances in agronomy. Academic Press, Cambridge, pp 223–274
Khush GS, Bacalangco E, Ogawa T (1990) 18. A new gene for resistance to bacterial blight from O. longistaminata. Rice Genet News Lett 7:121–122
Khush G, Mackill D, Sidhu G (1989) Breeding rice for resistance to bacterial blight. Bacterial blight of rice, 207–217
Khush GS (2005) IR varieties and their impact. (Int. Rice Res. Inst.).
Kim S-M (2018) Identification of novel recessive gene xa44(t) conferring resistance to bacterial blight races in rice by QTL linkage analysis using an SNP chip. Theor Appl Genet 131:2733–2743
Kim S-M, Reinke RF (2019) A novel resistance gene for bacterial blight in rice, Xa43(t) identified by GWAS, confirmed by QTL mapping using a bi-parental population. PLoS ONE 14:e0211775–e0211775
Kim S-M, Suh J-P, Qin Y, Noh T-H, Reinke RF, Jena KK (2015) Identification and fine-mapping of a new resistance gene, Xa40, conferring resistance to bacterial blight races in rice (Oryza sativa L.). Theor Appl Genet 128:1933–1943
Kim J, An X, Yang K, Miao S, Qin Y, Hu Y, Du B, Zhu L, He G, Chen R (2022) Molecular mapping of a new brown planthopper resistance gene Bph43 in rice (Oryza sativa L.). Agronomy 12:808
Kini K, Agnimonhan R, Afolabi O, Soglonou B, Silué D, Koebnik R (2017a) First report of a new bacterial leaf blight of rice caused by pantoea ananatis and pantoea stewartii in togo. Plant Dis 101:241
Kini K, Agnimonhan R, Afolabi O, Milan B, Soglonou B, Gbogbo V, Koebnik R, Silué D (2017b) First report of a new bacterial leaf blight of rice caused by pantoea ananatis and pantoea stewartii in benin. Plant Dis 101:242–242
Kiyosawa S (1982) Genetics and epidemiological modeling of breakdown of plant disease resistance. Annu Rev Phytopathol 20:93–117
Kloth KJ, Shah P, Broekgaarden C, Ström C, Albrectsen BR, Dicke M (2021) SLI1 confers broad-spectrum resistance to phloem-feeding insects. Plant Cell Environ 44:2765–2776
Koide Y, Kawasaki A, Telebanco-Yanoria MJ, Hairmansis A, Nguyet NTM, Bigirimana J, Fujita D, Kobayashi N, Fukuta Y (2010) Development of pyramided lines with two resistance genes, Pish and Pib, for blast disease (Magnaporthe oryzae B. Couch) in rice (Oryza sativa L.). Plant Breed 129:670–675
Koizumi S, Tani T (2000) Effective Control of rice blast disease with ‘sasanishiki’ multiline. Springer, The Netherlands, pp 137–145
Koizumi S, Ashizawa T, Zenbayashi KS (2004) Durable control of rice blast disease with multilines. Springer, The Netherlands, pp 191–199
Korinsak S, Sriprakhon S, Sirithanya P, Jairin J, Korinsak S, Vanavichit A, Toojinda T (2009) Identification of microsatellite markers (SSR) linked to a new bacterial blight resistance gene xa33(t) in rice cultivar ‘Ba7.’ Maejo Int J Sci Technol 3:235–247
Kou Y, Wang S (2010) Broad-spectrum and durability: understanding of quantitative disease resistance. Curr Opin Plant Biol 13:181–185
Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H, Bossolini E, Selter LL, Keller B (2009) A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science 323:1360–1363
Krattinger SG, Sucher J, Selter LL, Chauhan H, Zhou B, Tang M, Upadhyaya NM, Mieulet D, Guiderdoni E, Weidenbach D, Schaffrath U, Lagudah ES, Keller B (2016) The wheat durable, multipathogen resistance gene Lr34 confers partial blast resistance in rice. Plant Biotechnol J 14:1261–1268
Kumar PN, Sujatha K, Laha GS, Rao KS, Mishra B, Viraktamath BC, Hari Y, Reddy CS, Balachandran SM, Ram T, Madhav MS, Rani NS, Neeraja CN, Reddy GA, Shaik H, Sundaram RM (2012) Identification and fine-mapping of Xa33, a novel gene for resistance to Xanthomonas oryzae pv. oryzae. Phytopathology 102:222–228
Lan B, Yang Y-Q, Chen J, Chen H, Li X (2019) Molecular marker detection and analysis of blast resistance genes in main rice cultivars in Jiangxi Province. Mol Plant Breed 17:2559–2567
Lee K, Rasabandith S, Angeles ER, Khush G (2003) Inheritance of resistance to bacterial blight in 21 cultivars of rice. Phytopathology 93:147–152
Lee HB, Hong JP, Kim SB (2010) First report of leaf blight caused by pantoea agglomerans on rice in Korea. Plant Dis 94:1372–1372
Li ZK, Sanchez A, Angeles E, Singh S, Domingo J, Huang N, Khush GS (2001) Are the dominant and recessive plant disease resistance genes similar? A case study of rice R genes and Xanthomonas oryzae pv. oryzae races. Genetics 159:757–765
Li R, Li L, Wei S, Wei Y, Chen Y, Bai D, Yang L, Huang F, Lu W, Zhang X, Li X, Yang X, Wei Y (2006) The evaluation and utilization of new genes for brown planthopper resistance in common wild rice (Oryza rufipogon Griff.). Mol Plant Breed 14:365–371
Li W, Zhong S, Li G, Li Q, Mao B, Deng Y, Zhang H, Zeng L, Song F, He Z (2011) Rice RING protein OsBBI1 with E3 ligase activity confers broad-spectrum resistance against Magnaporthe oryzae by modifying the cell wall defence. Cell Res 21:835–848
Li W, Zhu Z, Chern M, Yin J, Yang C, Ran L, Cheng M, He M, Wang K, Wang J, Zhou X, Zhu X, Chen Z, Wang J, Zhao W, Ma B, Qin P, Chen W, Wang Y, Liu J, Wang W, Wu X, Li P, Wang J, Zhu L, Li S, Chen X (2017) A natural allele of a transcription factor in rice confers broad-spectrum blast resistance. Cell 170:114-126.e115
Li W, Chern M, Yin J, Wang J, Chen X (2019a) Recent advances in broad-spectrum resistance to the rice blast disease. Curr Opin Plant Biol 50:114–120
Li Z, Xue Y, Zhou H, Li Y, Usman B, Jiao X, Wang X, Liu F, Qin B, Li R, Qiu Y (2019b) High-resolution mapping and breeding application of a novel brown planthopper resistance gene derived from wild rice (Oryza. rufipogon Griff). Rice 12:41
Li W, Deng Y, Ning Y, He Z, Wang G-L (2020a) Exploiting broad-spectrum disease resistance in crops: from molecular dissection to breeding. Annu Rev Plant Biol 71:575–603
Li W, Wang K, Chern M, Liu Y, Zhu Z, Liu J, Zhu X, Yin J, Ran L, Xiong J, He K, Xu L, He M, Wang J, Liu J, Bi Y, Qing H, Li M, Hu K, Song L, Wang L, Qi T, Hou Q, Chen W, Li Y, Wang W, Chen X (2020b) Sclerenchyma cell thickening through enhanced lignification induced by OsMYB30 prevents fungal penetration of rice leaves. New Phytol 226:1850–1863
Li C, Zhang J, Ren Z, Xie R, Yin C, Ma W, Zhou F, Chen H, Lin Y (2021a) Development of ‘multiresistance rice’ by an assembly of herbicide, insect and disease resistance genes with a transgene stacking system. Pest Manag Sci 77:1536–1547
Li Q, Wang B, Yu J, Dou D (2021b) Pathogen-informed breeding for crop disease resistance. J Integr Plant Biol 63:305–311
Liang LQ, Wang CY, Zeng LX, Wang WJ, Feng JQ, Chen B, Su J, Chen S, Shang FD, Zhu XY, Lin F (2017) The rice cultivar Baixiangzhan harbours a recessive gene xa42(t) determining resistance against Xanthomonas oryzae pv. oryzae. Plant Breed 136:603–609
Lin X, Zhang D, Xie Y, Gao H, Zhang Q (1996) Identifying and mapping a new gene for bacterial blight resistance in rice based on RFLP markers. Phytopathology 86:1156–1159
Listihani L, Ariati PEP, Yuniti IGAD, Selangga DGW (2022) The brown planthopper (Nilaparvata lugens) attack and its genetic diversity on rice in Bali, Indonesia. Biodiversitas 23:4696–4704
Liu X-D, Sun Q-H (2016) Early assessment of the yield loss in rice due to the brown planthopper using a hyperspectral remote sensing method. Int J Pest Manag 62:205–213
Liu G, Lu G, Zeng L, Wang GL (2002) Two broad-spectrum blast resistance genes, Pi9(t) and Pi2(t), are physically linked on rice chromosome 6. Mol Genet Genom 267:472–480
Liu W, Wang F, Jin S, Zhu X, Li J, Liu Z, Liao Y, Zhu M, Huang H, Fu F, Liu Y (2008) Improvement of rice blast resistance in TGMS line by pyramiding of Pi-1 and Pi-2 through molecular marker-assisted selection. Acta Agron Sin 34:1128–1136
Liu Y, Liu B, Zhu X, Yang J, Bordeos A, Wang G, Leach JE, Leung H (2013) Fine-mapping and molecular marker development for Pi56(t), a NBS-LRR gene conferring broad-spectrum resistance to Magnaporthe oryzae in rice. Theor Appl Genet 126:985–998
Liu Y, Wu H, Chen H, Liu Y, He J, Kang H, Sun Z, Pan G, Wang Q, Hu J, Zhou F, Zhou K, Zheng X, Ren Y, Chen L, Wang Y, Zhao Z, Lin Q, Wu F, Zhang X, Guo X, Cheng X, Jiang L, Wu C, Wang H, Wan J (2015) A gene cluster encoding lectin receptor kinases confers broad-spectrum and durable insect resistance in rice. Nat Biotechnol 33:301–305
Liu M, Zhang S, Hu J, Sun W, Padilla J, He Y, Li Y, Yin Z, Liu X, Wang W, Shen D, Li D, Zhang H, Zheng X, Cui Z, Wang G-L, Wang P, Zhou B, Zhang Z (2019) Phosphorylation-guarded light-harvesting complex II contributes to broad-spectrum blast resistance in rice. Proc Natl Acad Sci 116:17572–17577
Liu J, Fernie AR, Yan J (2021a) Crop breeding – From experience-based selection to precision design. J Plant Physiol 256:153313
Liu Z, Zhu Y, Shi H, Qiu J, Ding X, Kou Y (2021b) Recent progress in rice broad-spectrum disease resistance. Int J Mol Sci 22:11658
Lu H-P, Luo T, Fu H-W, Wang L, Tan Y-Y, Huang J-Z, Wang Q, Ye G-Y, Gatehouse AMR, Lou Y-G, Shu Q-Y (2018) Resistance of rice to insect pests mediated by suppression of serotonin biosynthesis. Nat Plants 4:338–344
Lu Y, Zhong Q, Xiao S, Wang B, Ke X, Zhang Y, Yin F, Zhang D, Jiang C, Liu L, Li J, Yu T, Wang L, Cheng Z, Chen L (2022) A new NLR disease resistance gene Xa47 confers durable and broad-spectrum resistance to bacterial blight in rice. Front Plant Sci 13:1037901–1037901
Luna E, Lang J, McClung A, Wamishe Y, Jia Y, Leach JE (2023) First report of rice bacterial leaf blight disease caused by pantoea ananatis in the United States. Plant Dis 107:2214
Luo W, Huang M, Guo T, Xiao W, Wang J, Yang G, Liu Y, Wang H, Chen Z, Zhuang C (2017) Marker-assisted selection for rice blast resistance genes Pi2 and Pi9 through high-resolution melting of a gene-targeted amplicon. Plant Breed 136:67–73
Ma J, Lei C, Xu X, Hao K, Wang J, Cheng Z, Ma X, Ma J, Zhou K, Zhang X, Guo X, Wu F, Lin Q, Wang C, Zhai H, Wang H, Wan J (2015) Pi64, encoding a novel CC-NBS-LRR protein, confers resistance to leaf and neck blast in rice. Mol Plant-Microbe Interact 28:558–568
Mackill DJ, Khush GS (2018) IR64: a high-quality and high-yielding mega variety. Rice 11:18
Marcel S, Sawers R, Oakeley E, Angliker H, Paszkowski U (2010) Tissue-adapted invasion strategies of the rice blast fungus Magnaporthe oryzae. Plant Cell 22:3177–3187
Mehta S, Singh B, Dhakate P, Rahman M, Islam MA (2019) Rice, marker-assisted breeding, and disease resistance. In: Wani SH (ed) Disease resistance in crop plants: molecular, genetic and genomic perspectives. Springer International Publishing, Cham, pp 83–111
Mekonnen T, Haileselassie T, Kassahun T (2017) Identification, mapping and pyramiding of genes/quantitative trait loci (QTLs) for durable resistance of crops to biotic stresses. J Plant Pathol Microbiol 8:1000412
Meng X, Xiao G, Telebanco-Yanoria MJ, Siazon PM, Padilla J, Opulencia R, Bigirimana J, Habarugira G, Wu J, Li M, Wang B, Lu G-D, Zhou B (2020) The broad-spectrum rice blast resistance (R) gene Pita2 encodes a novel R protein unique from Pita. Rice 13:19
Mew T, Vera Cruz C, Medalla E (1992) Changes in race frequency of Xanthomonas oryzae pv. oryzae in response to rice cultivars planted in the Philippines. Plant Dis 76:1029–1032
Miao L, Wang C, Zheng C, Che J, Gao Y, Wen Y, Li G, Zhao K (2010) Molecular mapping of a new gene for resistance to rice bacterial blight. Sci Agric Sin 43:3051–3058
Ming D, Ye H, Schaad NW, Roth DA (1991) Selective recovery of Xanthomonas spp. from rice seed. Phytopathology 81:1358–1363
Mishra D, Vishnupriya MR, Anil MG, Konda K, Raj Y, Sonti RV (2013) Pathotype and genetic diversity amongst indian isolates of xanthomonas oryzae pv. oryzae. PLoS ONE 8:e81996
Mondal KK, Mani C, Singh J, Kim J-G, Mudgett MB (2011) A new leaf blight of rice caused by pantoea ananatis in India. Plant Dis 95:1582–1582
Moore JW, Herrera-Foessel S, Lan C, Schnippenkoetter W, Ayliffe M, Huerta-Espino J, Lillemo M, Viccars L, Milne R, Periyannan S, Kong X, Spielmeyer W, Talbot M, Bariana H, Patrick JW, Dodds P, Singh R, Lagudah E (2015) A recently evolved hexose transporter variant confers resistance to multiple pathogens in wheat. Nat Genet 47:1494–1498
Msami JA, Kawaguchi Y, Ichitani K, Taura S (2021) Linkage analysis of rice bacterial blight resistance gene xa20 in XM6, a mutant line from IR24. Breed Sci 71:144–154
Muduli L, Pradhan SK, Mishra A, Bastia DN, Samal KC, Agrawal PK, Dash M (2021) Understanding brown planthopper resistance in rice: genetics, biochemical and molecular breeding approaches. Rice Sci 28:532–546
Mundt CC (2014) Durable resistance: a key to sustainable management of pathogens and pests. Infect Genet Evol 27:446–455
Mundt CC (2018) Pyramiding for resistance durability: theory and practice. Phytopathology® 108:792–802
Murata K, Fujiwara M, Murai H, Takumi S, Mori N, Nakamura C (2001) Mapping of a brown planthopper (Nilaparvata lugens Stål) resistance gene Bph9 on the long arm of rice chromosome 12. Cereal Res Commun 29:245–250
Myint KKM, Fujita D, Matsumura M, Sonoda T, Yoshimura A, Yasui H (2012) Mapping and pyramiding of two major genes for resistance to the brown planthopper (Nilaparvata lugens [Stål]) in the rice cultivar ADR52. Theor Appl Genet 124:495–504
Nakai H, Nakamura K, Kuwahara S, Saito M (1988) Genetic studies of an induced rice mutant resistant to multiple races of bacterial leaf blight. Rice Genet Newsl 5:101
Naqvi SAH, Umar U, Hasnain A, Rehman A, Perveen R (2018) Effect of botanical extracts: a potential biocontrol agent for xanthomonas Oryzae Pv. Oryzae, causing bacterial leaf blight disease of rice. Pak J Agric Res 32:59–72
Nawaz G, Usman B, Peng H, Zhao N, Yuan R, Liu Y, Li R (2020) Knockout of Pi21 by CRISPR/Cas9 and iTRAQ-based proteomic analysis of mutants revealed new insights into M. oryzae resistance in elite rice line. Genes 11:735
Neelam K, Mahajan R, Gupta V, Bhatia D, Gill BK, Komal R, Lore JS, Mangat GS, Singh K (2020) High-resolution genetic mapping of a novel bacterial blight resistance gene xa-45(t) identified from Oryza glaberrima and transferred to Oryza sativa. Theor Appl Genet 133:689–705
Nelson R, Wiesner-Hanks T, Wisser R, Balint-Kurti P (2018) Navigating complexity to breed disease-resistant crops. Nat Rev Genet 19:21–33
Ning X, Yunyu W, Aihong L (2020) Strategy for use of rice blast resistance genes in rice molecular breeding. Rice Sci 27:263–277
NiÑo-Liu DO, Ronald PC, Bogdanove AJ (2006) Xanthomonas oryzae pathovars: model pathogens of a model crop. Mol Plant Pathol 7:303–324
Niones J, Sharp R, Donayre DK, Oreiro E, Milne A, Oliva R (2022) Dynamics of bacterial blight disease in resistant and susceptible rice varieties. Eur J Plant Pathol 163:1–17
Noda T, van Du P, Van EL, Dinh HD, Kaku H (1999) Pathogenicity of Xanthomonas oryzae pv. oryzae strains in Vietnam. Jpn J Phytopathol 65:293–296
Nugroho C, Cumagun CJR, Oliva R (2022) Diversity of Xanthomonas oryzae pv. Oryzae on susceptible and resistant rice lines in bacterial blight hot spot areas of the Philippines. Eur J Plant Pathol 163:951–960
Oliva R, Ji C, Atienza-Grande G, Huguet-Tapia JC, Perez-Quintero A, Li T, Eom J-S, Li C, Nguyen H, Liu B, Auguy F, Sciallano C, Luu VT, Dossa GS, Cunnac S, Schmidt SM, Slamet-Loedin IH, Vera Cruz C, Szurek B, Frommer WB, White FF, Yang B (2019) Broad-spectrum resistance to bacterial blight in rice using genome editing. Nat Biotechnol 37:1344–1350
Pal N, Jan I, Saini DK, Kumar K, Kumar A, Sharma PK, Kumar S, Balyan HS, Gupta PK (2022) Meta-QTLs for multiple disease resistance involving three rusts in common wheat (Triticum aestivum L.). Theor Appl Genet 135:2385–2405
Palloix A, Ayme V, Moury B (2009) Durability of plant major resistance genes to pathogens depends on the genetic background, experimental evidence and consequences for breeding strategies. New Phytol 183:190–199
Peng S, Laza RC, Visperas RM, Sanico AL, Cassman KG, Khush GS (2000) Grain yield of rice cultivars and lines developed in the Philippines since 1966. Crop Sci 40:307–314
Pennisi E (2010) Armed and dangerous. In Science, pp 804–805
Pooja S, Sharma R, Singh A, Rakesh S, Sundeep K (2014) Multiple disease resistance in wheat: need of today. Wheat Inf Serv 118:7–16
Porter BW, Chittoor JM, Yano M, Sasaki T, White FF (2003) Development and mapping of markers linked to the rice bacterial blight resistance gene Xa7. Crop Sci 43:1484–1492
Prahalada GD, Shivakumar N, Lohithaswa HC, Sidde Gowda DK, Ramkumar G, Kim S-R, Ramachandra C, Hittalmani S, Mohapatra T, Jena KK (2017) Identification and fine mapping of a new gene, BPH31 conferring resistance to brown planthopper biotype 4 of India to improve rice. Oryza Sativa l Rice 10:41
Priestley R (1978) Detection of increased cirulence in populations of yellow rust. In: Scott P, Bainbridge A (eds) Plant disease epidemiology. Blackwell Scientific, Oxford, pp 63–70
Qi Z, Du Y, Yu J, Zhang R, Yu M, Cao H, Song T, Pan X, Liang D, Liu Y (2023) Molecular detection and analysis of blast resistance genes in rice main varieties in Jiangsu Province. China Agron 13:157
Qiu Y, Guo J, Jing S, Zhu L, He G (2014) Fine mapping of the rice brown planthopper resistance gene BPH7 and characterization of its resistance in the 93–11 background. Euphytica 198:369–379
Qu S, Liu G, Zhou B, Bellizzi M, Zeng L, Dai L, Han B, Wang G-L (2006) The broad-spectrum blast resistance gene Pi9 encodes a nucleotide-binding site–leucine-rich repeat protein and is a member of a multigene family in rice. Genetics 172:1901–1914
Quibod IL, Atieza-Grande G, Oreiro EG, Palmos D, Nguyen MH, Coronejo ST, Aung EE, Nugroho C, Roman-Reyna V, Burgos MR, Capistrano P, Dossa SG, Onaga G, Saloma C, Cruz CV, Oliva R (2020) The Green Revolution shaped the population structure of the rice pathogen Xanthomonas oryzae pv. oryzae. ISME J 14:492–505
Rahman ML, Jiang W, Chu SH, Qiao Y, Ham T-H, Woo M-O, Lee J, Khanam MS, Chin J-H, Jeung J-U, Brar DS, Jena KK, Koh H-J (2009) High-resolution mapping of two rice brown planthopper resistance genes, Bph20(t) and Bph21(t), originating from Oryza minuta. Theor Appl Genet 119:1237–1246
Rai KK (2022) Integrating speed breeding with artificial intelligence for developing climate-smart crops. Mol Biol Rep 49:11385–11402
Ram T, Laha GS, Gautam SK, Deen R, Madhav MS, Brar DS, Viraktamath BC (2010) Identification of a new gene from Oryza brachyantha with broad-spectrum resistance to bacterial blight of rice in India. Rice Genet Newsl 25:57
Ramalingam J, Raveendra C, Savitha P, Vidya V, Chaithra TL, Velprabakaran S, Saraswathi R, Ramanathan A, Arumugam Pillai MP, Arumugachamy S, Vanniarajan C (2020) Gene pyramiding for achieving enhanced resistance to bacterial blight, blast, and sheath blight diseases in rice. Front Plant Sci 11:591457
Rao Y, Li Y, Qian Q (2014) Recent progress on molecular breeding of rice in China. Plant Cell Rep 33:551–564
Rathour R, Gopala Krishnan S, Khanna A, Dhatwalia S, Kaachra A, Sharma TR, Singh AK (2016) Development and validation of co-dominant gene based markers for Pi9, a gene governing broad-spectrum resistance against blast disease in rice. Mol Breed 36:168
Reddy APK (1979) Relationship of bacterial leaf blight severity to grain yield of rice. Phytopathology 69:967
Risk JM, Selter LL, Chauhan H, Krattinger SG, Kumlehn J, Hensel G, Viccars LA, Richardson TM, Buesing G, Troller A, Lagudah ES, Keller B (2013) The wheat Lr34 gene provides resistance against multiple fungal pathogens in barley. Plant Biotechnol J 11:847–854
Ronald PC, Albano B, Tabien R, Abenes L, Wu K-S, McCouch S, Tanksley SD (1992) Genetic and physical analysis of the rice bacterial blight disease resistance locus, Xa21. Mol Gen Genet MGG 236:113–120
Sani Haliru B, Rafii MY, Mazlan N, Ramlee SI, Muhammad II, Silas Akos I, Halidu J, Swaray S, Rini Bashir Y (2020) Recent strategies for detection and improvement of brown planthopper resistance genes in rice: a review. Plants 9:1202
Sanya DRA, Syed-Ab-Rahman SF, Jia A, Onésime D, Kim K-M, Ahohuendo BC, Rohr JR (2022) A review of approaches to control bacterial leaf blight in rice. World J Microbiol Biotechnol 38:113
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A (2019) The global burden of pathogens and pests on major food crops. Nat Ecol Evol 3:430–439
Sesma A, Osbourn AE (2004) The rice leaf blast pathogen undergoes developmental processes typical of root-infecting fungi. Nature 431:582–586
Sha G, Sun P, Kong X, Han X, Sun Q, Fouillen L, Zhao J, Li Y, Yang L, Wang Y, Gong Q, Zhou Y, Zhou W, Jain R, Gao J, Huang R, Chen X, Zheng L, Zhang W, Qin Z, Zhou Q, Zeng Q, Xie K, Xu J, Chiu T-Y, Guo L, Mortimer JC, Boutté Y, Li Q, Kang Z, Ronald PC, Li G (2023) Genome editing of a rice CDP-DAG synthase confers multipathogen resistance. Nature 618:1017–1023
Shaik R, Ramakrishna W (2013) Machine learning approaches distinguish multiple stress conditions using stress-responsive genes and identify candidate genes for broad resistance in rice. Plant Physiol 164:481–495
Shang J, Tao Y, Chen X, Zou Y, Lei C, Wang J, Li X, Zhao X, Zhang M, Lu Z, Xu J, Cheng Z, Wan J, Zhu L (2009) Identification of a new rice blast resistance gene, Pid3, by genomewide comparison of paired nucleotide-binding site–leucine-rich repeat genes and their pseudogene alleles between the two sequenced rice genomes. Genetics 182:1303–1311
Sharma TR, Madhav MS, Singh BK, Shanker P, Jana TK, Dalal V, Pandit A, Singh A, Gaikwad K, Upreti HC, Singh NK (2005) High-resolution mapping, cloning and molecular characterization of the Pi-khgene of rice, which confers resistance to Magnaporthe grisea. Mol Genet Genom 274:569–578
Sharma A, Kumar V, Shahzad B, Tanveer M, Sidhu GPS, Handa N, Kohli SK, Yadav P, Bali AS, Parihar RD, Dar OI, Singh K, Jasrotia S, Bakshi P, Ramakrishnan M, Kumar S, Bhardwaj R, Thukral AK (2019) Worldwide pesticide usage and its impacts on ecosystem. SN Appl Sci 1:1446
Shehryar K, Khan RS, Iqbal A, Hussain SA, Imdad S, Bibi A, Hamayun L, Nakamura I (2020) Transgene stacking as effective tool for enhanced disease resistance in plants. Mol Biotechnol 62:1–7
Sheikh H, Prins C, Schrijvers E (2023) Artificial intelligence: definition and background. In Mission AI: the new system technology, Springer, pp 15–41
Shekhar S, Sinha D, Kumari A (2020) An overview of bacterial leaf blight disease of rice and different strategies for its management. Int J Curr Microbiol App Sci 9:2250–2265
Shi S, Wang H, Nie L, Tan D, Zhou C, Zhang Q, Li Y, Du B, Guo J, Huang J, Wu D, Zheng X, Guan W, Shan J, Zhu L, Chen R, Xue L, Walling LL, He G (2021) Bph30 confers resistance to brown planthopper by fortifying sclerenchyma in rice leaf sheaths. Mol Plant 14:1714–1732
Shin M-S, Kim K-Y, Park H-SP, Ko J-K (2011) Breeding for resistance to bacterial blight in rice. Korean J Breed Sci 43:251–261
Sidhu GS, Khush GS (1978) Dominance reversal of a bacterial blight resistance gene in some rice cultivars. Phytopathology 68:461
Singh RJ, Khush GS, Mew TW (1983) A new gene for resistance to bacterial blight in rice. Crop Sci 23:558–560
Sogawa K (2015) Planthopper outbreaks in different paddy ecosystems in Asia: man-made hopper plagues that threatened the green revolution in rice. In: Heong KL, Cheng J, Escalada MM (eds) Rice planthoppers: ecology, management, socio economics and policy. Springer, The Netherlands, pp 33–63
Soller M, Beckmann JS (1983) Genetic polymorphism in varietal identification and genetic improvement. Theor Appl Genet 67:25–33
Song W-Y, Wang G-L, Chen L-L, Kim H-S, Pi L-Y, Holsten T, Gardner J, Wang B, Zhai W-X, Zhu L-H, Fauquet C, Ronald P (1995) A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 270:1804–1806
Sooklim C, Chomnunti P, Jantasuriyarat C, Chukeatirote E, Nilthong R, Nilthong S (2022) Genetic diversity and population structure of blast resistance genes in Thai upland rice germplasm. Eur J Plant Pathol 163:587–599
Sparks A, Nelson A, Castilla N (2012) Where rice pests and diseases do the most damage. In Rice Today (the International Rice Research Institute (IRRI))
Sperschneider J (2020) Machine learning in plant–pathogen interactions: empowering biological predictions from field scale to genome scale. New Phytol 228:35–41
Sreewongchai T, Toojinda T, Thanintorn N, Kosawang C, Vanavichit A, Tharreau D, Sirithunya P (2010) Development of elite indica rice lines with wide spectrum of resistance to Thai blast isolates by pyramiding multiple resistance QTLs. Plant Breed 129:176–180
Srivastava D, Shamim M, Kumar M, Mishra A, Pandey P, Kumar D, Yadav P, Siddiqui MH, Singh KN (2017) Current status of conventional and molecular interventions for blast resistance in rice. Rice Sci 24:299–321
Stam R, McDonald BA (2018) When resistance gene pyramids are not durable-the role of pathogen diversity. Mol Plant Pathol 19:521–524
Stout MJ (2014) Host-plant resistance in pest management. In: Abrol DP (ed) Integrated pest management. Academic Press, San Diego, pp 1–21
Su J, Wang W, Han J, Chen S, Wang C, Zeng L, Feng A, Yang J, Zhou B, Zhu X (2015) Functional divergence of duplicated genes results in a novel blast resistance gene Pi50 at the Pi2/9 locus. Theor Appl Genet 128:2213–2225
Sucher J, Boni R, Yang P, Rogowsky P, Büchner H, Kastner C, Kumlehn J, Krattinger SG, Keller B (2017) The durable wheat disease resistance gene Lr34 confers common rust and northern corn leaf blight resistance in maize. Plant Biotechnol J 15:489–496
Suh JP, Roh JH, Cho YC, Han SS, Kim YG, Jena KK (2009) The Pi40 gene for durable resistance to rice blast and molecular analysis of Pi40-advanced backcross breeding lines. Phytopathology 99:243–250
Summers RW, Brown JKM (2013) Constraints on breeding for disease resistance in commercially competitive wheat cultivars. Plant Pathol 62:115–121
Sun X, Cao Y, Yang Z, Xu C, Li X, Wang S, Zhang Q (2004) Xa26, a gene conferring resistance to Xanthomonas oryzae pv. oryzae in rice, encodes an LRR receptor kinase-like protein. Plant J 37:517–527
Sun L, Su C, Wang C, Zhai H-Q, Wan JJBS (2005) Mapping of a major resistance gene to the brown planthopper in the rice cultivar rathu heenati. Breed Sci 55:391–396
Sun P, Liu J, Wang Y, Jiang N, Wang S, Dai Y, Gao J, Li Z, Pan S, Wang D, Li W, Liu X, Xiao Y, Liu E, Wang G-L, Dai L (2013) Molecular mapping of the blast resistance gene Pi49 in the durably resistant rice cultivar Mowanggu. Euphytica 192:45–54
Sweeney M, McCouch S (2007) The complex history of the domestication of rice. Ann Bot 100:951–957
Takagi H, Uemura A, Yaegashi H, Tamiru M, Abe A, Mitsuoka C, Utsushi H, Natsume S, Kanzaki H, Matsumura H, Saitoh H, Yoshida K, Cano LM, Kamoun S, Terauchi R (2013) MutMap-Gap: whole-genome resequencing of mutant F2 progeny bulk combined with de novo assembly of gap regions identifies the rice blast resistance gene Pii. New Phytol 200:276–283
Tanksley SD, Rick CM (1980) Isozymic gene linkage map of the tomato: applications in genetics and breeding. Theor Appl Genet 58:161–170
Tanweer FA, Rafii MY, Sijam K, Rahim HA, Ahmed F, Latif MA (2015) Current advance methods for the identification of blast resistance genes in rice. CR Biol 338:321–334
Tao H, Shi X, He F, Wang D, Xiao N, Fang H, Wang R, Zhang F, Wang M, Li A, Liu X, Wang G-L, Ning Y (2021) Engineering broad-spectrum disease-resistant rice by editing multiple susceptibility genes. J Integr Plant Biol 63:1639–1648
Taura S, Ichitani K (2023) Chromosomal location of xa19, a broad-spectrum rice bacterial blight resistant gene from XM5, a mutant line from IR24. Plants (Basel) 12:602
Taura S, Ogawa T, Yoshimura A, Ikeda R, Omura T (1991) Identification of a recessive resistance gene in induced mutant line XM5 of rice to rice bacterial blight. Jpn J Breed 41:427–432
Taura S, Ogawa T, Yoshimura A, Ikeda R, IWata N (1992) ldentification of a recessive resistance gene to rice bacterial blight of mutant line XM6, Oryza sativa L. Jpn J Breed 42:7–13
Telebanco-Yanoria MJ, Koide Y, Fukuta Y, Imbe T, Kato H, Tsunematsu H, Kobayashi N (2010) Development of near-isogenic lines of Japonica-type rice variety Lijiangxintuanheigu as differentials for blast resistance. Breed Sci 60:629–638
Thanysiriwat T, Pattwatang P, Angeles ER (2009) New biotypes of brown planthopper in Thailand. In Proceedings of the rice and temperate cereal crops annual conference 2009 (Bangkok, Thailand), pp 386–389
Toh WK, Loh PC, Wong HL (2019) First report of leaf blight of rice caused by pantoea ananatis and pantoea dispersa in Malaysia. Plant Dis 103:1764–1764
van Dijk ADJ, Kootstra G, Kruijer W, de Ridder D (2021) Machine learning in plant science and plant breeding. iScience 24:101890
Vera Cruz CM, Bai J, Oña I, Leung H, Nelson RJ, Mew T-W, Leach JE (2000) Predicting durability of a disease resistance gene based on an assessment of the fitness loss and epidemiological consequences of avirulence gene mutation. Proc Natl Acad Sci 97:13500–13505
Vikal Y, Das A, Patra B, Goel RK, Sidhu JS, Singh K (2007) Identification of new sources of bacterial blight (Xanthomonas oryzae pv. oryzae) resistance in wild Oryza species and O. glaberrima. Plant Genet Resour 5:108–112
Vikal Y, Chawla H, Sharma R, Lore JS, Singh K (2014) Mapping of bacterial blight resistance gene xa8 in rice (Oryza sativa L.). Indian J Genet Plant Breed 74:589–595
Wallace JG, Rodgers-Melnick E, Buckler ES (2018) On the road to breeding 4.0: unraveling the good, the bad, and the boring of crop quantitative genomics. Annu Rev Genet 52:421–444
Wang GL, Mackill DJ, Bonman JM, McCouch SR, Champoux MC, Nelson RJ (1994) RFLP mapping of genes conferring complete and partial resistance to blast in a durably resistant rice cultivar. Genetics 136:1421–1434
Wang Z-X, Yano M, Yamanouchi U, Iwamoto M, Monna L, Hayasaka H, Katayose Y, Sasaki T (1999) The Pib gene for rice blast resistance belongs to the nucleotide binding and leucine-rich repeat class of plant disease resistance genes. Plant J 19:55–64
Wang W, Zhai W, Luo M, Jiang G, Chen X, Li X, Wing RA, Zhu L (2001) Chromosome landing at the bacterial blight resistance gene Xa4 locus using a deep coverage rice BAC library. Mol Genet Genom 265:118–125
Wang C, Tan M, Xu X, Wen G, Zhang D, Lin X (2003) Localizing the bacterial blight resistance gene, Xa22(t), to a 100-kilobase bacterial artificial chromosome. Phytopathology ® 93:1258–1262
Wang C-L, Zhao B, Zhang Q, Zhao K-J, Xing Q-D (2004) Identification of a new rice germplasm with resistance to bacterial blight and the breeding of a near-isogenic line. J Plant Genet Resour 5:26–30
Wang C, Wen G, Lin X, Liu X, Zhang D (2009a) Identification and fine mapping of the new bacterial blight resistance gene, Xa31(t), in rice. Eur J Plant Pathol 123:235–240
Wang L, Xu X, Lin F, Pan Q (2009b) Characterization of rice blast resistance genes in the pik cluster and fine mapping of the Pik-p locus. Phytopathology 99:900–905
Wang C, Qin T, Yu H, Zhang X, Che J, Gao Y, Zheng C, Yang B, Zhao K (2014) The broad bacterial blight resistance of rice line CBB23 is triggered by a novel transcription activator-like (TAL) effector of Xanthomonas oryzae pv. oryzae. Mol Plant Pathol 15:333–341
Wang C, Zhang X, Fan Y, Gao Y, Zhu Q, Zheng C, Qin T, Li Y, Che J, Zhang M, Yang B, Liu Y, Zhao K (2015) XA23 is an executor R protein and confers broad-spectrum disease resistance in rice. Mol Plant 8:290–302
Wang B-H, Ebbole DJ, Wang Z-H (2017a) The arms race between Magnaporthe oryzae and rice: Diversity and interaction of Avr and R genes. J Integr Agric 16:2746–2760
Wang S, Lei C, Wang J, Ma J, Tang S, Wang C, Zhao K, Tian P, Zhang H, Qi C, Cheng Z, Zhang X, Guo X, Liu L, Wu C, Wan J (2017b) SPL33, encoding an eEF1A-like protein, negatively regulates cell death and defense responses in rice. J Exp Bot 68:899–913
Wang Z, Xia Y, Lin S, Wang Y, Guo B, Song X, Ding S, Zheng L, Feng R, Chen S, Bao Y, Sheng C, Zhang X, Wu J, Niu D, Jin H, Zhao H (2018) Osa-miR164a targets OsNAC60 and negatively regulates rice immunity against the blast fungus Magnaporthe oryzae. Plant J 95:584–597
Wang H, Cimen E, Singh N, Buckler E (2020) Deep learning for plant genomics and crop improvement. Curr Opin Plant Biol 54:34–41
Watson A, Ghosh S, Williams MJ, Cuddy WS, Simmonds J, Rey MD, Asyraf Md Hatta M, Hinchliffe A, Steed A, Reynolds D, Adamski NM (2018) Speed breeding is a powerful tool to accelerate crop research and breeding. Nat Plants 4:23–29
Wiesner-Hanks T, Nelson R (2016) Multiple disease resistance in plants. Annu Rev Phytopathol 54:229–252
Wongsaprom C, Sirithunya P, Vanavichit A, Pantuwan G, Jongdee B, Sidhiwong N, Lanceras-Siangliw J, Toojinda T (2010) Two introgressed quantitative trait loci confer a broad-spectrum resistance to blast disease in the genetic background of the cultivar RD6 a Thai glutinous jasmine rice. Field Crop Res 119:245–251
Wu JL, Fan YY, Li DB, Zheng KL, Leung H, Zhuang JY (2005) Genetic control of rice blast resistance in the durably resistant cultivar Gumei 2 against multiple isolates. Theor Appl Genet 111:50–56
Wu X, Li X, Xu C, Wang S (2008) Fine genetic mapping of xa24, a recessive gene for resistance against Xanthomonas oryzae pv. oryzae in rice. Theor Appl Genet 118:185–191
Xiang Y, Cao Y, Xu C, Li X, Wang S (2006) Xa3, conferring resistance for rice bacterial blight and encoding a receptor kinase-like protein, is the same as Xa26. Theor Appl Genet 113:1347–1355
Xiao W, Yang Q, Wang H, Guo T, Liu Y, Zhu X, Chen Z (2011) Identification and fine mapping of a resistance gene to Magnaporthe oryzae in a space-induced rice mutant. Mol Breed 28:303–312
Xie Z, Yan B, Shou J, Tang J, Wang X, Zhai K, Liu J, Li Q, Luo M, Deng Y, He Z (2019) A nucleotide-binding site-leucine-rich repeat receptor pair confers broad-spectrum disease resistance through physical association in rice. Philos Trans R Soc B: Biol Sci 374:20180308
Xing J, Zhang D, Yin F, Zhong Q, Wang B, Xiao S, Ke X, Wang L, Zhang Y, Zhao C, Lu Y, Chen L, Cheng Z, Chen L (2021) Identification and fine-mapping of a new bacterial blight resistance gene, Xa47(t), in G252, an introgression line of yuanjiang common wild rice (Oryza rufipogon). Plant Dis 105:4106–4112
Xu R, Sun C (2021) What happened during domestication of wild to cultivated rice. Crop J 9:564–576
Xu X, Hayashi N, Wang C-T, Fukuoka S, Kawasaki S, Takatsuji H, Jiang C-J (2014a) Rice blast resistance gene Pikahei-1(t), a member of a resistance gene cluster on chromosome 4, encodes a nucleotide-binding site and leucine-rich repeat protein. Mol Breed 34:691–700
Xu X, Lv Q, Shang J, Pang Z, Zhou Z, Wang J, Jiang G, Tao Y, Xu Q, Li X, Zhao X, Li S, Xu J, Zhu L (2014b) Excavation of Pid3 orthologs with differential resistance spectra to magnaporthe oryzae in rice resource. PLoS ONE 9:e93275
Xu G, Yuan M, Ai C, Liu L, Zhuang E, Karapetyan S, Wang S, Dong X (2017) uORF-mediated translation allows engineered plant disease resistance without fitness costs. Nature 545:491–494
Xu Z, Xu X, Gong Q, Li Z, Li Y, Wang S, Yang Y, Ma W, Liu L, Zhu B, Zou L, Chen G (2019) Engineering broad-spectrum bacterial blight resistance by simultaneously disrupting variable TALE-binding elements of multiple susceptibility genes in rice. Mol Plant 12:1434–1446
Xu Y, Zhang X, Li H, Zheng H, Zhang J, Olsen MS, Varshney RK, Prasanna BM, Qian Q (2022) Smart breeding driven by big data, artificial intelligence, and integrated genomic-enviromic prediction. Mol Plant 15:1664–1695
Xue Y, Hu M, Chen S, Hu A, Li S, Han H, Lu G, Zeng L, Zhou J (2021) Enterobacter asburiae and pantoea ananatis causing rice bacterial blight in China. Plant Dis 105:2078–2088
Yadav MK, Aravindan S, Ngangkham U, Raghu S, Prabhukarthikeyan SR, Keerthana U, Marndi BC, Adak T, Munda S, Deshmukh R, Pramesh D, Samantaray S, Rath PC (2019) Blast resistance in Indian rice landraces: genetic dissection by gene specific markers. PLoS ONE 14:e0211061
Yamamoto T, Ogawa T (1990) Inheritance of resistance in rice cultivars, Toyonishiki, Milyang 23 and IR24 to Myanmar isolates of bacterial leaf blight pathogen. JARQ 24:74–77
Yang Q, Lin F, Wang L, Pan Q (2009) Identification and mapping of Pi41, a major gene conferring resistance to rice blast in the Oryza sativa subsp. indica reference cultivar, 93–11. Theor Appl Genet 118:1027–1034
Yang M, Cheng L, Yan L, Shu W, Wang X, Qiu Y (2019) Mapping and characterization of a quantitative trait locus resistance to the brown planthopper in the rice variety IR64. Hereditas 156:22
Yang W, Zhao J, Zhang S, Chen L, Yang T, Dong J, Fu H, Ma Y, Zhou L, Wang J, Liu W, Liu Q, Liu B (2021) Genome-wide association mapping and gene expression analysis reveal the negative role of OsMYB21 in regulating bacterial blight resistance in rice. Rice 14:58
Yang Y, Zhou Y, Sun J, Liang W, Chen X, Wang X, Zhou J, Yu C, Wang J, Wu S, Yao X, Zhou Y, Zhu J, Yan C, Zheng B, Chen J (2022) Research progress on cloning and function of Xa genes against rice bacterial blight. Front Plant Sci 13:847199
Yin K, Qiu J-L (2019) Genome editing for plant disease resistance: applications and perspectives. Philos Trans R Soc B: Biol Sci 374:20180322
Yogesh V, Dharminder B (2017) Genetics and genomics of bacterial blight resistance in rice. In: Jinquan L (ed) Advances in international rice research. IntechOpen, Rijeka
Young-Soon C, Hyeonso J, Doh-Won Y, Byoung-Ohg A, Myung Chul L, Seok-Cheol S, Chun Seok L, Eok Keun A, Yong-Hee J, Il-Doo J, Jae-Keun S, Hee-Jong K, Moo-Young E (2008) Fine mapping of the rice Bph1 gene, which confers resistance to the brown planthopper (Nilaparvata lugens Stal), and development of STS markers for marker-assisted selection. Mol Cells 26:146–151
Yu L, Yang C, Ji Z, Zeng Y, Liang Y, Hou Y (2022) First report of new bacterial leaf blight of rice caused by pantoea ananatis in Southeast China. Plant Dis 106:310
Yuan B, Zhai C, Wang W, Zeng X, Xu X, Hu H, Lin F, Wang L, Pan Q (2011) The Pik-p resistance to Magnaporthe oryzae in rice is mediated by a pair of closely linked CC-NBS-LRR genes. Theor Appl Genet 122:1017–1028
Yue Q, Meng J, Qiu Y, Yin M, Zhang L, Zhou W, An Z, Liu Z, Yuan Q, Sun W, Li C, Zhao H, Molnár I, Xu Y, Shi S (2023) A polycistronic system for multiplexed and precalibrated expression of multigene pathways in fungi. Nat Commun 14:4267
Yugander A, Sundaram RM, Ladhalakshmi D, Hajira SK, Prakasam V, Prasad MS, Sheshu Madhav M, Ravindra Babu V, Laha GS (2017) Virulence profiling of Xanthomonas oryzae pv. oryzae isolates, causing bacterial blight of rice in India. Eur J Plant Pathol 149:171–191
Zeng L-R, Qu S, Bordeos A, Yang C, Baraoidan M, Yan H, Xie Q, Nahm BH, Leung H, Wang G-L (2004) Spotted leaf11, a negative regulator of plant cell death and defense, encodes a U-box/armadillo repeat protein endowed with E3 ubiquitin ligase activity. Plant Cell 16:2795–2808
Zeng L, Cheng T, Zhu X (2009) Breeding of rice inbred cultivar Baixiangzhan first reported resistance to rice bacterial blight pathogen type V severe virulent strain in Guangdong. Guangdong Agric Sci 5(19):28
Zeng X, Yang X, Zhao Z, Lin F, Wang L, Pan Q (2011) Characterization and fine mapping of the rice blast resistance gene Pia. Sci China Life Sci 54:372–378
Zeng X, Tian D, Gu K, Zhou Z, Yang X, Luo Y, White FF, Yin Z (2015) Genetic engineering of the Xa10 promoter for broad-spectrum and durable resistance to Xanthomonas oryzae pv. oryzae. Plant Biotechnol J 13:993–1001
Zhai C, Lin F, Dong Z, He X, Yuan B, Zeng X, Wang L, Pan Q (2011) The isolation and characterization of Pik, a rice blast resistance gene which emerged after rice domestication. New Phytol 189:321–334
Zhai K, Deng Y, Liang D, Tang J, Liu J, Yan B, Yin X, Lin H, Chen F, Yang D, Xie Z, Liu J-Y, Li Q, Zhang L, He Z (2019) RRM transcription factors interact with NLRs and regulate broad-spectrum blast resistance in Rice. Mol Cell 74:996-1009.e1007
Zhang Q (2009) Genetics and improvement of bacterial blight resistance of hybrid rice in China. Rice Sci 16:83–92
Zhang Q, Wang C, Zhao K, Zhao Y, Caslana V, Zhu X, Li D, Jiang Q (2001) The effectiveness of advanced rice lines with new resistance gene Xa23 to rice bacterial blight. Rice Genet Newsl 18:71–72
Zhang F, Zhuo D-L, Zhang F, Huang L-Y, Wang W-S, Xu J-L, Vera Cruz C, Li Z-K, Zhou Y-L (2015) Xa39, a novel dominant gene conferring broad-spectrum resistance to Xanthomonas oryzae pv. oryzae in rice. Plant Pathol 64:568–575
Zhang Y, Zhu Q, Yao Y, Zhao Z, Correll JC, Wang L, Pan Q (2017) The race structure of the rice blast pathogen across southern and northeastern China. Rice 10:46
Zhang F, Wang C, Li M, Cui Y, Shi Y, Wu Z, Hu Z, Wang W, Xu J, Li Z (2021a) The landscape of gene–CDS–haplotype diversity in rice: Properties, population organization, footprints of domestication and breeding, and implications for genetic improvement. Mol Plant 14:787–804
Zhang H, Liu P, Wang B, Yuan F (2021b) The roles of trichome development genes in stress resistance. Plant Growth Regul 95:137–148
Zhao B, Lin X, Poland J, Trick H, Leach J, Hulbert S (2005) A maize resistance gene functions against bacterial streak disease in rice. Proc Natl Acad Sci 102:15383–15388
Zhao Y, Huang J, Wang Z, Jing S, Wang Y, Ouyang Y, Cai B, Xin X-F, Liu X, Zhang C, Pan Y, Ma R, Li Q, Jiang W, Zeng Y, Shangguan X, Wang H, Du B, Zhu L, Xu X, Feng Y-Q, He SY, Chen R, Zhang Q, He G (2016) Allelic diversity in an NLR gene BPH9 enables rice to combat planthopper variation. Proc Natl Acad Sci 113:12850–12855
Zheng C-K, Wang C-L, Yu Y-J, Liang Y-T, Zhao K-J (2009) Identification and molecular mapping of Xa32(t), a novel resistance gene for bacterial blight (Xanthomonas oryzae pv. oryzae) in rice. Acta Agron Sin 35:1173–1180
Zhou B, Qu S, Liu G, Dolan M, Sakai H, Lu G, Bellizzi M, Wang G-L (2006) The eight amino-acid differences within three leucine-rich repeats between Pi2 and Piz-t resistance proteins determine the resistance specificity to magnaporthe grisea. Mol Plant-Microbe Interact ® 19:1216–1228
Zhou Y-L, Uzokwe VNE, Zhang C-H, Cheng L-R, Wang L, Chen K, Gao X-Q, Sun Y, Chen J-J, Zhu L-H, Zhang Q, Ali J, Xu J-L, Li Z-K (2011) Improvement of bacterial blight resistance of hybrid rice in China using the Xa23 gene derived from wild rice (Oryza rufipogon). Crop Prot 30:637–644
Zhu X, Chen S, Yang J, Zhou S, Zeng L, Han J, Su J, Wang L, Pan Q (2012) The identification of Pi50(t), a new member of the rice blast resistance Pi2/Pi9 multigene family. Theor Appl Genet 124:1295–1304
Zhu R, Huang W, Hu J, Liu W, Zhu Y (2013) Breeding of new sterile line Luohong 4A of Honglian type hybrid rice. J Wuhan Univ (Nat Sci Ed) 59:33–36
Zhu Q, Yu S, Zeng D, Liu H, Wang H, Yang Z, Xie X, Shen R, Tan J, Li H, Zhao X, Zhang Q, Chen Y, Guo J, Chen L, Liu Y-G (2017) Development of “purple endosperm rice” by engineering anthocyanin biosynthesis in the endosperm with a high-efficiency transgene stacking system. Mol Plant 10:918–929
Acknowledgements
We apologize for the omission of any pertinent original references owing to space limitations. We thank Dr. Ming-Shun Chen (USDA-ARS) for proof reading and reviewing.
Funding
This work was supported by Jiangxi ‘Double Thousand Plan’ (2F2522100), Modern Agricultural Research Collaborative Innovation Special Project (JXXTCXBSJJ202205), Key Technological Research in Agriculture of Jiangxi Province, China (JXNK202301-03), Joint research on improved rice varieties of Jiangxi (JXNZWZY01), and Breeding of new rice variety with high adaptability and superior quality (20192BBF60004).
Author information
Authors and Affiliations
Contributions
XYC, GXZ, and YT proposed the concept. XYC, GHZ, WC, Lin Tan, QSL, FSC, and Lei Tan searched the studies and prepared the supplemental materials. XYC wrote the manuscript. GXZ and YT revised and finalized the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval and consent to participate
Not applicable.
Consent for publication
All authors agree to publication in the journal.
Additional information
Communicated by Rajeev K. Varshney.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, X., Zhou, G., Chen, W. et al. Current status of molecular rice breeding for durable and broad-spectrum resistance to major diseases and insect pests. Theor Appl Genet 137, 219 (2024). https://doi.org/10.1007/s00122-024-04729-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00122-024-04729-3