Abstract
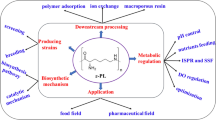
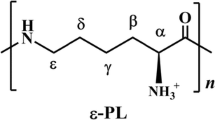
The naturally occurring homo-polyamide biopolymer, ε-poly-L-lysine (ε-PL) consists of 25–35 L-lysine residues with amide linkages between α-carboxyl groups and ε-amino groups. ɛ-PL exhibits several useful properties because of its unusual structure, such as biodegradability, water solubility, no human toxicity, and broad-spectrum antibacterial activities; it is widely applied in the fields of food, medicine, clinical chemistry and electronics. However, current industrial production of ε-PL is only performed in a few countries. Based on an analysis of the physiological characteristics of ε-PL fermentation, current advances that enhance ε-PL fermentation, from strain improvement to product isolation are systematically reviewed, focusing on: (1) elucidating the metabolic pathway and regulatory mechanism of ε-PL synthesis; (2) enhancing biosynthetic performance through mutagenesis, fermentation optimization and metabolic engineering; and (3) understanding and improving the biological activity and functional properties of ε-PL. Finally, perspectives on engineering and exploiting ε-PL as a source material for the production of various advanced materials are also discussed, providing scientific guidelines for researchers to further improve the ε-PL fermentation process.
Similar content being viewed by others
Introduction
As an important polycationic peptide, ε-Poly-L-lysine (ε-PL) is a homopolymer consisting of 25–35 L-lysine residues (molecular weight (Mw) range of 3,200–4,500 Da) with linkages between α-carboxyl and ε-amino groups [1, 2]. Because of the presence of many free amino groups in the main chain and many cationic amino groups on the side chains, ε-PL displays multication characteristics in acidic to slightly alkaline environments. In general, ε-PL is a slightly bitter tasting, hygroscopic, light-yellow natural cationic polymer, and can be completely digested into lysine by the body. Because of its novel structure, ɛ-PL exhibits several excellent physicochemical and biological properties, including water solubility, selective removal of endotoxins, anti-obesity properties, biodegradability, thermostability and nontoxicity toward humans and the environment [3,4,5,6,7]. More importantly, ε-PL exhibits broad-spectrum antimicrobial ability against Gram-negative and Gram-positive bacteria, such as Staphylococcus aureus, fungi, yeasts and some viruses [8], in which ε-PL strongly inhibits several food-borne pathogens, including Escherichia coli O157:H7 [9], Listeria monocytogenes, Staphylococcus aureus, Serratia marcescens [10]. Therefore, ε-PL and its derivatives have been of interest in preservatives, biodegradable fibers, hydrogels, drug carriers, biochip coatings in food and in the cosmetics and pharmaceutical fields [11, 12], making ɛ-PL has received considerable research and development attention [13, 14]. For example, based on its strong antibacterial activity, biodegradability and low toxicity, ε-PL has been generally recognized as a safe (GRAS No.000135) food preservative for routinely using in Japan, Korea, USA, China and other countries[15, 16].
Compared with chemical method, microorganisms are more practical, efficient and environment friendly for ɛ-PL production [17], and even microbial ε-PL exhibits more activity than chemically synthesized ε-PL [8]. As a secondary metabolite, ε-PL is mainly produced from sustainable resources such as sugars and glycerol through microbial fermentation by various Streptomycetaceae, a few filamentous fungi and some Bacilli [18, 19], but the production of ε-PL is unstable and dependent on cell density [20]. Recently, with the development of genetic engineering, bioinformatics, and advanced precision instruments and testing equipment, microbial ɛ-PL fermentation has been systematically investigated, focusing on screening of higher ɛ-PL-producing strains [21], development of new fermentation mode, regulation of the polymerization degree of ɛ-PL and understanding the biosynthetic mechanism of ɛ-PL [22], optimization of culture conditions [23], and illuminating the inhibitory effect of ε-PL on microorganisms [24]. Therefore, the intention of this review is to summarize recent developments in ɛ-PL fermentation, from strain-breeding to process optimization, focusing particularly on the update progress on the regulation mechanism of microbial ɛ-PL. In addition, perspectives on exploiting and modifying ε-PL to produce various advanced materials are also discussed, providing scientific guidelines and valuable insights for further enhancing the overall performance of ɛ-PL fermentation.
Occurrence of ε-PL in microorganisms
Assay methods for ε-PL
The most convenient and rapid method for determining ε-PL is a colorimetric procedure with the dye-methyl orange, which interacts with ε-PL to produce a water-insoluble compound, allowing the concentration of ε-PL to be calculated from the absorbance at 465 nm [25]. Recently, a series of qualitative, or quantitative methods have been developed to measure ε-PL concentration, including: (1) colorimetric methods with the acidic dye [26], e.g., molybdosilicate anion [27], poly R-478.24, or dipicrylamine [28]; (2) an improved high sensitivity colorimetric assay with glucose oxidase, in which ε-PL is determined without any pretreatment [29]; (3) integrating high-throughput screening strategy using ribosome engineering technology, permitting ε-PL to be determined in < 5 min per plate without compromising accuracy [30]; (4) high-performance liquid chromatography (HPLC) methods, which can determine the concentration and degree of polymerization (DP) of ε-PL. This wide choice of assays has permitted a large number of ε-PL-producing microorganisms to be screened, including various Streptomycetes, some Bacilli and a few filamentous fungi [31] (Table 1). Notably, Streptomycetes are considered as the best producers of ε-PL and commercial production of ε-PL is mainly depended on the fermentation with Streptomyces albulus [32, 33].
Separation and purification of ε-PL
Few studies have focused on exploring methods for separating and purifying ε-PL from the fermentation medium [34, 35, 36]. In general, ε-PL exists in a fully protonated form (εPLHn+) in solution at pH < 4, but the εPLHn+ cation can interact with tetraphenylborate ion (TPB–) anion to form a precipitate, εPLH(TPB)n, at around pH 3.5. Addition of NaTPB precipitated both polycationic εPLHn+, and ammonium and potassium ions; the latter were removed from the mixture through washing with acetone. After dissolving εPLH(TPB)n in HCl solution, ε-PL was precipitated with acetone, as the hydrochloride salt, providing a rapid and simple method to purify basic peptides or polyamines [37]. Recently, an alternative strategy, including flocculation, filtration, ultrafiltration, ion-exchange chromatography, and decolorization, has been developed and applied to purify ε-PL from fermentation medium. Under the optimal conditions for adsorption and desorption on Amberlite IRC-50 resin, a final ε-PL with a purity of 92.4% was obtained with a 75% recovery [38]. Furthermore, an alcohol/salt aqueous two-phase system (ATPS) was also developed, in which an ATPS, consisting of 20% (w/w) ethanol and 20% (w/w) ammonium sulfate at pH 9.5, was applied to purify ε-PL from fermentation medium with triplicate extractions, and a final ε-PL product with 92.4% purity and 87.7% recovery was obtained with the combination of desalting by ultrafiltration [39].
Metabolic pathway and regulation mechanism for microbial ε-PL
As a secondary metabolite, ε-PL was found in the culture filtrate of S. albulus No. 346 (currently designated S. albulus NBRC14147) [40], and mainly produced from sustainable resources such as sugars and glycerol by microbial fermentation by various Streptomycetaceae, such as Streptomyces MZ18 [18], Streptomyces griseus [19], Streptomyces aureofaciens [41], a few filamentous fungi and some Bacilli [18, 19] (Table 1).
Genome sequence for ɛ-PL-producing strains
In general, the synthesis of biopolymer is a sophisticated process that involves cell growth, precursor synthesis, energy provision, redox equilibrium, cofactor regulation, transportation of substrates and products, and complex global regulatory system [42]. Fortunately, the draft genome sequences of several ɛ-PL-producing strains (including S. albulus CCRC 11814 [43], S. albulus PD-1 [44], S. albulus NK660 [45], Kitasatospora sp. MY5-36 and S. albulus NBRC14147 [46] have been published. The genomes of the Streptomycetes exhibit high similarity, having genomes within the scope of 8.7 to 11.9 Mb and GC contents between 69 and 76% [42, 44]. However, the ɛ-PL-producing strains also have special characteristics; S. albulus ZPM has 44 gene clusters related to secondary metabolites, almost twice those of Streptomyces coelicolor A3 (25 gene clusters) [47]. In addition, S. albulus CCRC 11814 contains 69 tRNA genes and 4 rRNA genes with 1 rRNA operon located on contig 198, resulting in 9,177 protein-coding sequences being identified [48]. More importantly, S. albulus CCRC 11814 can produce enough L-lysine to support a high production level of ε-PL due to the lack of feedback regulation of aspartate kinase. Based on the genomic data, most genes encoding proteins for synthetic pathway, energy metabolic, transportation mechanism and regulation information in the ɛ-PL-producing strains have been successfully annotated, indicating that ɛ-PL is closely related to the biosynthesis and assembly of L-lysine, as well as being associated with multiple cellular processes [49]. Therefore, the genomic information was considered as a powerful platform for analyzing metabolic pathways and identifying candidate genes, enabling more complicated projects on metabolic analysis and pathway engineering, to further enhance the fermentation performance of ɛ-PL-producing strain.
Biosynthetic pathway for microbial ε-PL
As the direct monomeric precursor of ε-PL, L-lysine can be synthesized through the aspartate pathway from L-aspartate. As shown in Fig. 1, aspartokinase (Ask, catalyzing the phosphorylation of L-aspartic acid to form L-4-phosphoaspartic acid) and aspartate semialdehyde dehydrogenase (Asd, reducing L-4-phosphoaspartic acid into L-aspartate 4-semialdehyde) are the two key enzymes that regulate the synthesis of amino acids [50]. However, due to the complicacy of aspartate pathway, bacterial species have evolved diverse modes for Ask regulation, in which the Ask enzyme(s) of S. albulus appear to be resistant to feedback inhibition by L-lysine and/or L-threonine, thereby providing sufficient L-lysine for high-yield ε-PL biosynthesis [51].
In microorganisms, two mechanisms for the activation of amino acids are involved in peptide biosynthesis, i.e., adenylation by AMP, catalyzed by non-ribosomal peptide synthetases (NRPSs) and phosphorylation by ADP, catalyzed by amide ligases [52]. The mechanism of the assembly of L-lysine monomers into an ε-PL polymer is not completely understood, but it appears that ε-PL is biosynthesized in a manner similar to the action of AMP and NRPSs [53]. L-lysine monomers are adenylated on the carboxyl group, then transferred onto the ε-PL synthetase active site sulfhydryl group, to produce activated aminoacyl thioester intermediates [54]. The ε-PL synthetases (Pls) can catalyze repeated peptide bond formation reactions between the thio-esterified lysine and the ε-amino group of a free lysine, or of an ε-PL peptide, thereby progressively elongating the ε-PL [50, 55].
ε-PL synthetase (Pls) and ε-PL-degrading enzyme (Pld)
ε-PL synthetase is the essential enzyme for ε-PL synthesis (Fig. 1), but its catalytic mechanism was poorly understood until relatively recently. To probe the mechanism of Pls, some native enzymes were purified from the membrane fraction of S. albulus, and found that the affinity of L-lysine for Pls is relatively high. Pls is a single-module NRPS with the classic A- and T-domains of NRPSs, with six transmembrane (TM) domains surrounding three tandem soluble domains, without any thioesterase, or condensation domain, and acts as a ligase for peptide bond formation [56, 57]. During the biosynthesis of ε-PL, L-lysine monomers are polymerized via a three-step enzymatic reaction, involving adenylation, thiolation and peptide bond formation; and the catalytic mechanism is initiated at the N-terminus by the A- and T-domains with the adenylation and transfer of an incoming L-lysine monomer as an extending unit (described in detail by Hamano [58] and Kazuya [59]). The C-terminal tandem domains (C1-, C2- and C3-domains) catalyze peptide bond formation between the extending unit and a freely diffusible L-lysine molecule (priming unit) to produce an L-lysine dimer, then the dimer is used as a freely diffusible substrate (acceptor substrate) for the next polymerization reaction. The growing chain of ε-PL is never covalently bound to the enzyme, indicating that the enzyme holds the growing chain at the active site by non-covalent interactions, until the polymerization reactions are complete [58]. In summary, this catalytic cycle has no defined endpoint and Pls acts iteratively during ε-PL chain growth to produce a variety of chain lengths, normally between 25 and 35 residues. The linker regions of Pls are important in binding the growing chain of ε-PL to the active site and providing insights into their function, i.e., connecting the TM domains and regulating the chain length of ε-PL [56]. Although a detailed description of the process that the growing polymer interacts with the enzyme remains unclear, the improved understanding of the physiological function of Pls has contributed to the creation of new classes of biopolymers, by biosynthetic engineering.
Furthermore, Pls exhibited a typical hyperbolic saturation curve with respect to the L-lysine concentration at a constant ATP concentration during the catalytic reaction, indicating that Pls is allosterically regulated by ATP [60]. However, the affinity of Pls for ATP is lower than that of L-lysine (approximately 2 mM) and at low concentrations (0.25–2 mM) and high concentrations (3–5 mM) of ATP, the enzyme exhibits negative and positive allosteric interactions, respectively, indicating that Pls requires a high concentration (> 3 mM) of ATP for full activity [60]. However, no Pls polymerization catalytic activity was tested with other nucleotides, such as GTP, CTP, and TTP. Therefore, ATP, generated by the glycolytic pathway and electron-transport chain, is accumulated as an important cofactor for ε-PL biosynthesis, in which allosteric regulation of Pls avoids excessive consumption of ATP for primary metabolism.
Since ε-PL has antibiotic activity, ε-PL producers generally produce a membrane-bound ε-PL-degrading enzyme (Pld), which degrades ɛ-PL to protect the producing organism. During the ε-PL fermentation, ε-PL can be accumulated when the pH decreases to around 3.2, but Pld activity is detectable at neutral pH; Pld acts via an exopeptidase-type mode, releasing N-terminal L-lysine residues one by one [60]. However, although S. virginiae IFO12827 and S. norsei IFO15452 have high ε-PL degrading activity, both strains can still produce a high yield of ε-PL, indicating an inverse correlation between the distributions of membrane-bound Pls and Pld [61]. More importantly, a high level of ε-PL-degrading activity was also found in a pld (encoding ε-PL-degrading enzyme) knockout mutant, suggesting that ε-PL degradation into shorter chains is also catalyzed by endo-peptidases, without the release of L-lysine monomers [60]. Recently, the gene encoding an unidentified ε-PL-degrading enzyme (Pld II), with endo-type peptidase activity, was identified in S. albulus NBRC14147 [60], and the Pld II activity was found on the cell surface, indicating that Pld II is bound to the outside of the cytoplasmic membrane. However, a knockout mutant of both pldI and pldII still could not produce ε-PL under neutral pH conditions [60]. Therefore, an acidic pH is an important factor, both to activate Pls and inhibit ε-PL degradation by Pld, as well as accumulating intracellular ATP, resulting in the production and accumulation of ε-PL [60, 56].
Regulation of the peptide chain length of microbial ɛ-PL
Similar to other biopolymers, the biological functions of ε-PL closely rely on its molecular weight, so the ability to produce it with a specific chain length, or a defined range of chain lengths, would be very beneficial to widen the potential applications of ε-PL [62]. Currently, the commercial ε-PL commonly ranges from 3.0 to 4.5 kDa (corresponding to 25–35-mer) [48] and this chain-length diversity is generated directly by the synthetase, rather than the degrading enzyme [56, 63]. However, despite the improved understanding of the mechanism of ε-PL biosynthesis, the processes that control the polymerization degree of ε-PL are still not fully elucidated, resulting in few commercially available ε-PLs with controlled molecular weight ranges. At present, five strategies have been applied to selectively produce short-chain ε-PL, but all have drawbacks: (1) Screening strains for ability to produce short chain (5–20 residues) ε-PL, but this approach gives low yields (4.5 g/L) [64, 65]; (2) Adding aliphatic short chain polyols, or cyclodextrins (such as sulfated cyclodextrin) to the growth medium, to regulate the chain-length [66] or molecular weight [67] of ε-PL, but such polyols present food safety risks; (3) Shortening the chain length by engineering ε-PL-synthetase through mutagenesis of its linker regions [58], but the yield is low and the synthesis mechanism is not fully understood; (4) Using ε-PL-degrading enzymes to produce short chain ε-PL from naturally occurring ε-PL [68], but consistent control of the chain length between different batches is difficult, the enzymes are costly and the process is inefficient; (5) Modification of fermentation conditions, using glycerol as carbon source, can produce short chain ε-PL (8–32 residues) in high yield (39.8 g/L), high purity (98.8%) and high recovery ratio (72.6%); however, the chain length range is wide and poorly controlled [69].
Strategies for improving the performance of ε-PL fermentation
During the conventional ε-PL fermentation, the viscosity of culture medium increases with the accumulation of ε-PL, requiring slow and difficult processing to separate the ε-PL from the bacterial cells, thereby decreasing the process yield. Furthermore, lysis of the bacterial cells during fermentation makes semi-continuous culture difficult and significantly increases the production costs of ε-PL, restricting industrial production to a few countries. To meet the rapidly increasing demand for ɛ-PL, various approaches have been employed to enhance the synthetic efficiency of ε-PL, achieving higher yields in less time.
Breeding higher yielding ε-PL-producing strains by mutagenesis
In the aspartate pathway, L-lysine can partially repress the synthesis of aspartokinase and glycine inhibits aspartokinase activity. Therefore, some resistant mutants (S-(2-aminoethyl)-L-cysteine and glycine-resistant) were screened for reduced feedback inhibition, increased cell growth and increased ε-PL production. For example, resistant mutant was screened using sulfaguanidine, glycine, L-lysine and DL-3 hydroxynorvaline as selection markers, in which S. diastatochromogenes L9 was selected and produced 0.77 g/L ε-PL, 15% higher than that of the original strain [70]. Meanwhile, genome shuffling has also been applied to simultaneously improve the glucose tolerance, ε-PL tolerance and ε-PL productivity of S. graminearus [71, 72]. With the help of genome shuffling in five species (Streptomyces padanus, Streptomyces griseofuscus, Streptomyces graminearus, Streptomyces hygroscopicus and Streptomyces albulus), the hybrid FEEL-1 was identified through morphology and spore color, and ε-PL production correspondingly increased to 1.12 g/L, about 2.75-fold higher than that of the control [73]. Furthermore, mutagenesis combined with streptomycin resistance (such as streptomycin, gentamicin, and rifampicin) was also developed to improve the fermentation performance of ε-PL-producing strains [74, 75]. For example, after atmospheric and room temperature plasma (ARTP) mutagenesis and genome shuffling, S. albulus AG3-28 was screened with an increased concentration of gentamicin and could produce 3.43 g/L of ε-PL with a lower molecular weight in shake-flask culture [76].
Optimization of fermentation processes to improve ε-PL production
The original ɛ-PL fermentation strategy was the two-phase pH control approach, in which the culture medium pH is maintained above 5.0 for optimal cell growth, then lowered to pH 4.0 for optimal ɛ-PL biosynthesis, giving an ɛ-PL yield of 48.3 g/L [77]. However, because of the toxicity of ε-PL to its own producer organism and the largely unknown self-protection mechanism, further enhancing ɛ-PL production is very difficult. Several fermentation strategies have been developed to increase ɛ-PL production, including: (1) The pH control strategy, (2) the dissolved oxygen control strategy, (3) optimization of the fermentation medium and (4) improvement of the fermentation conditions. These strategies have significantly improved ɛ-PL production and decreased the overall processing costs [31] and are discussed in detail below.
Improving ɛ-PL production by pH-control
In the conventional ε-PL fermentation, ε-PL could be only accumulated when the pH was below 5.0 and the maximum synthesis rate was detected at about pH 4.0 [78]. Therefore, strict pH control strategy is essential to achieve high ε-PL production, in which low pH increases membrane permeability and prevents the binding of ε-PL onto the cells [79]. More importantly, when the ε-PL-producing strain was subjected to acid stress, the enhancement of acid tolerance was contributed to improved fermentation performance [80]. According to the analysis of specific cell growth and the rate of ε-PL production, a novel two-stage pH control method was developed by changing the culture pH from 3.5 to 3.8 after 36 h, which increased the ε-PL yield and productivity from 7.8 g/L and 3.1 g/L/day to 9.1 g/L and 4.8 g/L/day, respectively [81]. Meanwhile, after evaluation of the effects of acidic pH-shock on mycelial metabolic activity, an integrated pH-shock strategy was proposed, involving a pre-acid shock adaptation period at pH 5.0, followed by an acidic pH 3.0 shock for 12 h (including a gradual pH decrease from 4.0 to 3.0), finally increasing the pH to 4.0 to promote ε-PL accumulation. As a result, both cell growth and ɛ-PL yield were greatly improved, increasing the maximum ε-PL yield and productivity to 54.70 g/L and 6.84 g/L/day, respectively [78]. Similarly, another novel two-stage fermentation, including culture and fermentation stages, was also developed based on the analysis of conventional pH shock fermentation, achieving ε-PL yield and productivity increases to 32.22 g/L and 5.86 g/L/day, which were 32.3% and 36.6% higher, respectively, than those of the conventional fermentation [82].
In summary, the fermentation performance of ε-PL can be remarkably improved by pH shock strategy, but the underlying mechanism is poorly understood [83]. Based on the analysis of transcriptomic and physiological data, several physiological mechanisms have been proposed (described in detail by Wang [80]), as follows [84, 85]: (1) Changes in cellular morphology during fed-batch fermentation, providing a predictor for determining the ε-PL production rate; (2) physiological changes (such as the enhancement of mycelial respiration and up-regulation of key genes involved in central metabolic and ε-PL biosynthetic pathways) induced by acidic pH shock have contributed synergistically to the improvement of ε-PL biosynthesis [84]; (3) the synthesis of ε-PL is a cellular response to acid stress, providing new insights into enhancing ε-PL production via adaptive evolution or metabolic engineering [80]; (4) signal transduction systems play a major role in responding to pH shock, enhancing metabolic vitality and intracellular redox homeostasis [85]. (5) pH shock influences a wide range of proteins including global regulators, fatty acid desaturase, respiratory chain components and ATP-binding cassette transporter, thereby improving cellular respiratory activity and enhancing ε-PL productivity [83].
Improving the production of ɛ-PL by dissolved oxygen control strategy
The dissolved oxygen (DO) level is another key factor for ɛ-PL fermentation, in which the high DO level is beneficial for the formation of ATP, and then improves cell growth and ɛ-PL biosynthesis. A DO control strategy, with a fixed DO level at 40% during the cell growth phase, followed by a reduction to 20% in the ɛ-PL biosynthesis phase, increased the biomass and ɛ-PL production to 1.99 and 20.73 g/L, respectively [23]. However, due to the intertwined hyphae, high cell density and high ε-PL yield, the culture medium becomes viscous, resulting in decreased oxygen transfer efficiency [86], making it hard to increase the DO level by simply enhancing agitation and aeration. In addition, stronger agitation and higher shear stress could result in undesired effects on mycelial morphology, ε-PL productivity and the polymerization degree of ε-PL. Therefore, addition of oxygen-vectors may be an effective strategy to improve the oxygen supply during the ε-PL fermentation, in which 0.5% n-dodecane maintained DO levels above 32% saturation, and then increased the ε-PL production from 23.4 to 30.8 g/L [49]. Similarly, with the introduction of Vitreoscilla hemoglobin gene in the chromosome of S. albulus PD-1, the capability of binding oxygen was correspondingly enhanced for S. albulus PD-1, making the ε-PL production increased by 50.7% than that of wild-type strain [87].
Improving the production of ɛ-PL by fermentation medium optimization
In ε-PL fermentation, the most common culture medium is Medium 3G (M3G), which contains a high proportion of glucose, with the most expensive component being yeast extract [88]. To decrease the cost of ε-PL fermentation, alternative carbon sources (such as glycerol, or molasses) were used to produce ɛ-PL [89, 90]. A mixed glucose/glycerol carbon source accelerated cell growth and L-lysine biosynthesis and greatly shortened the fermentation time, resulting in a higher yield of ε-PL and biomass [91]. For example, glycerol as carbon source effectively enhanced ɛ-PL production, reaching 62.36 g/L in a 5-L bioreactor after 192 h [92]. However, the positive effects of mixed carbon sources on ε-PL production were still incompletely understood [93]. Consequently, chemostat culture was applied to investigate the ability of ε-PL biosynthesis with diverse carbon sources at the same dilution rate. Glucose and glycerol were consumed synergistically to accelerate energy metabolism and carbon fluxes, resulting in a higher energy charge and NADH/NAD+ ratio, providing sufficient carbon skeletons and ATP to supply precursors and energy for high ε-PL production [94, 95]. However, the simultaneous consumption of glucose and glycerol generated a high level of reactive oxygen species (ROS) in the batch fermentation stage, damaging cells and decreasing the specific ε-PL formation rate, suggesting that intensive cellular respiration enhanced ε-PL production [96].
As a result of the self-inhibition of cell growth by ε-PL, insufficient biomass can become another key problem during the ε-PL fermentation. To deal with this problem, several nitrogen-rich nutrients were added after 16 h of culture, of which the addition of 0.5% (w/v) of yeast extract increased ε-PL production to 2.24 g/L. Meanwhile, the final ε-PL yield was further increased to 28.2 g/L by coupling a two-stage pH control strategy with continuous supplementation of yeast extract at 0.5 g/h [97]. However, organic nitrogen (yeast extract) is the most expensive component of the medium for ɛ-PL production, but selection of alternative nitrogen sources to replace yeast extract has contributed to decreasing the cost of ɛ-PL fermentation. Therefore, agro-industrial by-products, i.e., fish meal and corn steep liquor, have been applied as alternative nitrogen sources for industrial ɛ-PL fermentation. With the combination of optimized medium and two-stage pH control, a cost-effective and efficient process for ɛ-PL production was developed in the fed-fermentation, in which the yield and productivity of ɛ-PL increased to 35.24 g/L and 4.85 g/L/day, respectively [86].
Improving ε-PL production by the addition of exogenous substances
As shown in Fig. 1, L-lysine is the direct precursor for ε-PL biosynthesis, and addition of 3 mM L-lysine or D-lysine could effectively improve the fermentation performance, increased the ε-PL production to 1.16 g/L and 1.20 g/L, respectively, which were 41.4% and 46.3% increments over control [98]. More importantly, with the combination of adding L-lysine with glucose–glycerol co-fermentation, the production of ε-PL could be further increased to 37.6 g/L under optimal conditions, suggesting that the bottleneck of ε-PL synthesis is the availability of L-lysine [99]. Furthermore, high ATP levels are essential for optimal Pls activity, and the tricarboxylic acid cycle is the main source of ATP [60]. Therefore, addition of some intermediates (such as L-aspartic acid and citric acid) can not only promote the accumulation of intracellular L-lysine, but also provides a substrate to produce sufficient ATP [100]. For example, the addition of citric acid inhibited the metabolic pathway from phosphoenolpyruvate to the TCA cycle, and consequently enhanced the metabolic flux from phosphoenolpyruvate to oxaloacetate and L-aspartate, thereby improving ε-PL production [100]. However, even though the levels of L-lysine and ATP are high enough, ε-PL cannot be accumulated efficiently if there is insufficient Pls activity. With the synergistic effect of 2 g/L sodium citrate, the ε-PL yield of the pls gene overexpressing strain (Q-PL2) were 211% higher than that of the wild strain. During the fed-batch fermentation, recombinant Q-PL2 produced 20.1 g/L of ε-PL in 72 h, which was 3.2-fold than that of wild-type strain. Therefore, ε-PL synthase is one of the rate-limiting enzymes in the ε-PL synthesis pathway [101].
Iron, manganese and cobalt can be added to the growth medium to regulate ε-PL biosynthesis [102, 103]. For example, adding 10 g/L of talc microparticles improved mycelial morphology and ε-PL production by decreasing pellet diameter from 297.63 to 205.65 μm and increasing ε-PL yield from 1.67 g/L to 2.51 g/L. Combination of talc microparticles with acidic pH shock increased the yield of ε-PL to 62.36 g/L from 54.70 g/L after 192 h [92]. However, the toxicity of ε-PL, significantly decreased the growth-vigor of Streptomyces as ε-PL accumulated and sharply increased the level of reactive oxygen species (ROS) after 24 h. Addition of antioxidants (such as glutathione and astaxanthin) further enhanced ε-PL production by limiting the increase in ROS [104]. Addition of 1.0 g/L astaxanthin increased ε-PL production by 36.3% to 36.1 g/L, suggesting that adding an antioxidant maintains cell vigor by reducing the excess ROS, and thus, improving the ε-PL yield [105].
To minimize the self-inhibition of ε-PL on cell growth, the immobilization approach has been developed and applied to ε-PL fermentation by entrapment or adsorption of Kitasatospora sp. on bagasse, synthetic sponge, macro-porous silica gel, or loofah sponge. As a result, the immobilized cells on loofah sponge could be reused five times over a period of 526 h, and lag phase was only detected in the first batch and the fermentation period was significantly shortened, increasing the yield and productivity of ε-PL from 22.53 g/L and 3.30 g/L/day to 34.11 g/L and 9.34 g/L/day, respectively [106]. More importantly, combining cell immobilization with in situ adsorption (ISA) of ɛ-PL was able to overcome the feedback inhibition and toxic effects of the accumulated ɛ-PL, in which loofah sponge-immobilized cells combined with ISA produced 3.64 g/L of ε-PL compared with 2.73 g/L by free cells combined with ISA [107, 108].
Improvement of ε-PL production by metabolic engineering
In the post-genomic era, genetic engineering has become an important method to improve the fermentation performance of industrial microbial strains. However, up to now, few researches employing genetic engineering have been carried out to improve ε-PL production, as follow: (1) enhancing the efficiency of oxygen transfer during the ε-PL fermentation. To alleviate oxygen limitation, the Vitreoscilla hemoglobin gene (vgb) was integrated into the chromosome of S. albulus PD-1, increasing the ε-PL yield from 22.7 to 34.2 g/L with a productivity of 4.9 g/L/day [87]. Similarly, with the overexpression of heterologous vgb and SAM synthetase gene (metK) in S. albulus NK660, strain S. albulus NK660-VHb could produce 26.67% higher ε-PL and 14.57% higher biomass than the wild-type strain, respectively [109]; (2) improving the efficiency of nitrogen translocation and utilization. With the overexpression of ammonium transporter gene amtB, the production of ε-PL could be significantly increased from 22.7 g/L to 35.7 g/L when the optimum carbon-to-nitrogen ratio increased from 3 to 4.71 in the synthesis stage of fermentation [110]; (3) engineering ε-PL synthase to break the rate-limiting polymerization reaction [111, 112], in which ε-PL synthase was over-expressed in S. albulus, and then the recombinant Q-PL2 could produce 88.2% more than that of wild strain [101]. However, only two ɛ-PL-producing strains (S. albulus IFO14147 and S. albulus PD-1) have well-developed gene delivery systems, seriously limiting the potential for genetic modification of ɛ-PL-producing strains [60]. Therefore, a deeper understanding of the regulation, interactions with other metabolic pathways and mechanism of ɛ-PL biosynthesis, could facilitate a rational genetic approach, integrating high-throughput techniques, omics data and in silico models, and then provide extensive information on the regulatory and biosynthetic systems of ε-PL production and promote enhanced metabolic flux towards ε-PL biosynthesis [14].
Antimicrobial mechanism of ε-PL and its functional modification
Compared with synthetic chemical preservatives, ɛ-PL exhibits biodegradability, water solubility and no human toxicity, and has been approved as a commercial food preservative by the U.S. Food and Drug Administration and equivalent organizations in several other countries [33, 113, 114]. ε-PL has been successfully used in the fields of food preservation (reviewed in detail by Tuersuntuoheti et al. [13], anti-microbial treatments [115, 116, 117], food packaging [118], control of environmental pathogens [119], medicine (reviewed in detail by Shukla et al. [120]) and agriculture (Fig. 2). However, the antimicrobial activity of ɛ-PL is closely related to its molecular weight (MW), and the antimicrobial mechanisms of different MW ranges are different and not well-understood. In general, low-MW ε-PL (< 1 kDa) slightly damages the cell membrane/wall and weakly inhibits the glycolytic pathway, but high-MW ε-PL (1–3 kDa and > 3 kDa) causes cell-wall lesions and increases cell membrane permeability, resulting in protoplasm leakage and cell death [121]. More importantly, the antimicrobial activity of ε-PL can be markedly reduced by chemical modification of its α-amino groups, the presence of anionic polymers, or alkaline conditions [116]. However, the antimicrobial activity of ε-PL can be enhanced by the presence of some water-soluble and biocompatible materials, such as chitosan oligomers or food additives [122]. In summary, ε-PL has a complex antimicrobial mode of action, with multiple cellular targets and mechanisms, which are not well-understood.
Various applications of ε-PL and modification strategies for broadening ε-PL application potential [117]
At present, membrane disruption is the main antimicrobial mode of action of ε-PL and appears to operate by a number of potential mechanisms, i.e.: (1) electrostatic adsorption onto the cell surface [123]. The cationic nature of ε-PL allows it to adsorb onto the cell membrane, disrupting membrane integrity, decreasing respiratory activity, and damaging cell walls and membranes [124, 125]; (2) changing the membrane fatty acid and cell wall composition, resulting in leakage of electrolytes and proteins [116]; (3) inducing osmotic stress by disrupting the ionic balance between the inside and outside of the membrane [116]; and (4) stimulating the accumulation of intracellular reactive oxygen species (ROS), decreasing the integrity of the cell wall and membrane [126, 127]. In summary, cell membrane disruption and generation of oxidative stress, are the two general modes of action involved in ε-PL antimicrobial activity (Fig. 3).
Potential antibacterial mechanisms of ε-PL and the self-protection mechanism of ε-PL-producing organisms. Extracellular ε-PL can disrupt the cell membrane, increasing its permeability and causing leakage of cytoplasmic material. Intracellular ε-PL increases the level of reactive oxygen species (ROS), resulting in damage to genomic DNA and enzymes. Both extra- and intra-cellular mechanisms can result in cell death [152, 126, 140]
There are, however, some limitations to the application of ε-PL. Because of the polycationic nature, ε-PL has poor antioxidant properties, which confers strong binding affinity to anionic materials and affects its antibacterial activity. Modification with several monosaccharides, using the Maillard reaction increased its DPPH radical scavenging capacity by 40.5–69.4%, increased its reducing power (from 0.9 to 1.3) with low cytotoxicity, and caused a slight decrease in antimicrobial activity [128]. Tea polyphenols, micro-encapsulated with ε-PL, had long-acting and slow-release antibacterial properties, with potential applications in food preservation [129].
The antimicrobial activity of ε-PL is modulated by interaction with various biopolymers with different charge distributions, both when there is little binding interaction, e.g., with polycationic chitosan or neutral dextran, and when there are strong electrostatic interactions and/or complex formation, e.g., with anionic carrageenan, alginate, or pectin [130, 131]. For example, ε-PL can interact with polysaccharide and/or protein components in food [132], but the polysaccharides protect ε-PL’s antimicrobial activity [129, 133]. Reacting a mixture of ɛ-PL and D-fructose produced ɛ-PL/fructose complexes with antimicrobial activity, low toxicity and biodegradability, as well as enhanced mechanical properties and water resistance [134].
ɛ-PL forms useful antimicrobial polycationic chains (PLn+) in water because of its high water-solubility. This high water-solubility limits the applicability of ɛ-PL in biopolymer-based plastics, but preparation of a complex (PLn+/BEHS−) between ɛ-PL and an anionic surfactant, bis(2-ethylhexyl) sulfosuccinate (BEHS−) made the ɛ-PL soluble in organic solvents, permitting its use as a coating material to produce a water-resistant antimicrobial membrane. In addition, the thermoplastic PLn+/BEHS− complex was able to be uniformly mixed with polypropylene by heating, creating several new materials with antimicrobial activities [135]. Similarly, a series of dialdehyde microcrystalline cellulose (DAMC) particles, crosslinked with ɛ-PL (ɛ-PL–DAMCs) was prepared by reacting DAMC with varying amounts of ɛ-PL. The ɛ-PL–DAMC complexes had broad-spectrum antibacterial activity, suggesting great potential for use in food packaging [118].
Conclusions
As an important biopolymer, ɛ-PL has several excellent properties (such as water solubility, no human toxicity, biodegradability and broad-spectrum antibacterial activity), and has been widely used in the chemical, food, environmental, medical, biotechnology and other industries. Since the discovery of ɛ-PL in 1977, great progress in fermentation improvement has been made through selective-breeding of producing strains, combined with process optimization [11, 136, 137]. The rapidly increasing demand for ɛ-PL has attracted increasing research interest in ε-PL fermentation, resulting in the development of a number of bioprocesses for effectively producing ε-PL [137, 138]. However, ɛ-PL synthesis is a complex process, involving cell growth, precursor biosynthesis, energy metabolic, redox equilibria, and transportation of substrates and products, making the metabolic pathway of ɛ-PL is strictly regulated by complicated global regulatory systems. More importantly, little is known about the catalytic mechanisms of ɛ-PL synthase (Pls) and ε-PL-degrading enzyme (Pld) [58, 139], how the polymerization of L-lysine to ɛ-PL is regulated at the molecular level, or how regulatory interactions control the chain length of ε-PL [63, 140]. Therefore, there are considerable knowledge gaps relating to various aspects of ε-PL production and downstream processing, resulting in current industrial production of ε-PL being carried out in only a few countries, and very few ε-PL products with controlled molecular weight ranges available commercially.
Considering the huge potential of ε-PL in various applications, there is considerable interest in further improving the economic viability of ε-PL production by fermentation. Future research should follow a three-pronged approach, involving upstream (strain improvement), midstream (advanced fermentation strategies) and downstream (cost-effective recovery) processes (Tuersuntuoheti et al., 2019) [141]. Specific research targets include:
-
Considering the intrinsic complexity of ε-PL biosynthesis, targeting global regulatory mechanisms and transcription factors to further enhance microbial production performance (i.e., superior pH tolerance, higher ε-PL yield and production rate, and precise molecular weight control) at the global level by integrating systems biology and synthetic biology.
-
Creating a superior ε-PL bioprocess by integrating fermentation and downstream processes, such as the use of immobilization supports and continuous-flow systems, the design of suitable bioreactors, and coupling with in situ methods for extraction and recovery, achieving a higher yield, in less time, at reduced cost.
-
Modify the structure and composition of ε-PL by biotechnological approaches, to improve its physicochemical properties and broaden its application potential.
Availability of data and materials
Not applicable.
References
Xu Z, Sha YY, Liu C, Li S, Liang JF, Zhou JH, Xu H. L-Ribose isomerase and mannose-6-phosphate isomerase: properties and applications for L-ribose production. Appl Microbiol Biotechnol. 2016;100:9003–11.
Miao JY, Zhou JL, Liu G, Chen FL, Chen YJ, Gao XY, Dixon W, Song MY, Xiao H, Cao Y. Membrane disruption and DNA binding of Staphylococcus aureus cell induced by a novel antimicrobial peptide produced by Lactobacillus paracasei subsp tolerans FX-6. Food Control. 2016;59:609–13.
Xu ZX, Xu Z, Feng XH, Xu DL, Liang JF, Xu H. Recent advances in the biotechnological production of microbial poly(epsilon-L-lysine) and understanding of its biosynthetic mechanism. Appl Microbiol Biotechnol. 2016;100:6619–30.
Bucatariu F, Schwarz D, Zaharia M, Steinbach C, Ghiorghita CA, Schwarz S, Mihai M. Nanostructured polymer composites for selective heavy metal ion sorption. Collo Surf Physicochem Eng Aspects. 2020. https://doi.org/10.1016/j.colsurfa.2020.125211.
Shih IL, Shen MH. Optimization of cell growth and poly(epsilon-lysine) production in batch and fed-batch cultures by Streptomyces albulus IFO 14147. Process Biochem. 2006;41:1644–9.
Lan WQ, Zhang NN, Liu SC, Chen ML, Xie J. Epsilon-polylysine inhibits shewanella putrefaciens with membrane disruption and cell damage. Molecules. 2019. https://doi.org/10.3390/molecules24203727.
Shao ZP, Yang Y, Fang S, Li YH, Chen J, Meng YC. Mechanism of the antimicrobial activity of whey protein-epsilon-polylysine complexes against escherichia coli and its application in sauced duck products. Int J Food Microbiol. 2020. https://doi.org/10.1016/j.ijfoodmicro.2020.108663.
Hyldgaard M, Mygind T, Vad BS, Stenvang M, Otzen DE, Meyer RL. The antimicrobial mechanism of action of epsilon- poly-L-lysine. Appl Environ Microbiol. 2014;80:7758–70.
Ye RS, Xu HY, Wan CX, Peng SS, Wang LJ, Xu H, Aguilar ZP, Xiong YH, Zeng ZL, Wei H. Antibacterial activity and mechanism of action of epsilon-poly-L-lysine. Biochem Biophys Res Commun. 2013;439:148–53.
Li YQ, Han Q, Feng JL, Tian WL, Mo HZ. Antibacterial characteristics and mechanisms of epsilon-poly-lysine against Escherichia coli and Staphylococcus aureus. Food Control. 2014;43:22–7.
Pandey AK, Kumar A. Improved microbial biosynthesis strategies and multifarious applications of the natural biopolymer epsilon-poly-L-lysine. Process Biochem. 2014;49:496–505.
Hamano Y. Occurrence, biosynthesis, biodegradation, and industrial and medical applications of a naturally occurring epsilon-poly-L-lysine. Biosci Biotechnol Biochem. 2011;75:1226–33.
Tuersuntuoheti T, Wang Z, Wang Z, Liang S, Li X, Zhang M. Review of the application of ε-poly-L-lysine in improving food quality and preservation. J Food Process Preservation. 2019. https://doi.org/10.1111/jfpp.14153.
Yamanaka K, Hamano Y, Oikawa T. Enhancement of metabolic flux toward epsilon-poly-l-lysine biosynthesis by targeted inactivation of concomitant polyene macrolide biosynthesis in Streptomyces albulus. J Biosci Bioeng. 2020;129:558–64.
Fadli M, Saad A, Sayadi S, Chevalier J, Mezrioui NE, Pages JM, Hassani L. Antibacterial activity of thymus maroccanus and thymus broussonetii essential oils against nosocomial infection - bacteria and their synergistic potential with antibiotics. Phytomedicine. 2012;19:464–71.
Su RH, Li TF, Fan DM, Huang JL, Zhao JX, Yan BW, Zhou WG, Zhang WH, Zhang H. The inhibition mechanism of epsilon-polylysine against Bacillus cereus emerging in surimi gel during refrigerated storage. J Sci Food Agric. 2019;99:2922–30.
Tao YH, Chen XY, Jia F, Wang SX, Xiao CS, Cui FC, Li YQ, Bian Z, Chen XS, Wang XH. New chemosynthetic route to linear epsilon-poly-lysine. Chem Sci. 2015;6:6385–91.
Chen X-S, Gao Y, Zhen B, Han D, Zhang J-H, Mao Z-G. Separation and purification of epsilon-poly-L-lysine from fermentation broth. Process Biochem. 2016;51:134–41.
Li S, Tang L, Chen X, Liao L, Li F, Mao Z. Isolation and characterization of a novel epsilon-poly-L-lysine producing strain: Streptomyces griseofuscus. J Ind Microbiol Biotechnol. 2011;38:557–63.
Hirohara H, Takehara M, Saimura M, Ikezaki A, Miyamoto M. Biosynthesis of poly(ε-l-lysine)s in two newly isolated strains of Streptomyces sp. Appl Microbiol Biotechnol. 2006;73:1–1.
El-Sersy NA, Abdelwahab AE, Abouelkhiir SS, Abou-Zeid DM, Sabry SA. Antibacterial and anticancer activity of epsilon-poly-L-lysine (epsilon-PL) produced by a marine Bacillus subtilis sp. J Basic Microbiol. 2012;52:513–22.
Geng WT, Yang C, Gu YY, Liu RH, Guo WB, Wang XM, Song CJ, Wang SF. Cloning of e-poly-L-lysine ( e-PL) synthetase gene from a newly isolated e-PL-producing Streptomyces albulus NK660 and its heterologous expression in Streptomyces lividans. Microb Biotechnol. 2014;7:155–64.
Bankar SB, Singhal RS. Improved Poly-epsilon-lysine biosynthesis using Streptomyces noursei NRRL 5126 by controlling dissolved oxygen during fermentation. J Microbiol Biotechnol. 2011;21:652–8.
Chang SS, Lu WYW, Park SH, Kang DH. Control of foodborne pathogens on ready-to-eat roast beef slurry by epsilon-polylysine. Int J Food Microbiol. 2010;141:236–41.
Nishikawa M, Ogawa K. Distribution of Microbes producing antimicrobial ε-poly-L-lysine polymers in soil microflora determined by a novel method. Appl Envir Microbiol. 2002. https://doi.org/10.1128/AEM.68.7.3575-3581.2002.
Katano H, Uematsu K, Maruyama C, Hamano Y. Analytical methods for the detection and purification of ε-poly-L-lysine for studying biopolymer synthetases, and bioelectroanalysis methods for its functional evaluation. Anal Sci Int J Japan Soc Anal Chem. 2014;30:17.
Katano H, Maruyama C, Hamano Y. Detection of biopolymer ε-poly-L-lysine with molybdosilicate anion for screening of synthetic enzymes. Int J Polym Anal Charact. 2011;16:542–50.
Katano H, Kasahara Y, Ushimaru K, Maruyama C, Hamano Y. Separation and purification of ε-Poly-L-lysine with Its colorimetric determination using dipicrylamine. Anal Ences Int J Japan Soc Anal Chem. 2015;31:1273.
Uematsu K, Ueno T, Ushimaru K, Maruyama C, Hamano Y, Katano H. Colorimetric method to detect epsilon-poly-L-lysine using glucose oxidase. J Biosci Bioeng. 2016;122:513–8.
Liu YJ, Chen XS, Zhao JJ, Pan L, Mao ZG. Development of microtiter plate culture method for rapid screening of ε-Poly-L-lysine-producing strains. Appl Biochem Biotechnol. 2017;183:1209–23.
Wang L, Li S, Zhao JJ, Liu YJ, Chen XS, Tang L, Mao ZG. Efficiently activated epsilon-poly-L-lysine production by multiple antibiotic-resistance mutations and acidic pH shock optimization in Streptomyces albulus. Microbiologyopen. 2019. https://doi.org/10.1002/mbo3.728.
Nishikawa M, Kobayashi K. Streptomyces roseoverticillatus produces two different poly(amino acid)s: lariat-shaped gamma-poly(L-glutamic acid) and epsilon-poly(L-lysine). Microbiology. 2009;155:2988–93.
Wang YC, Cao H, Wang XY. Synthesis and characterization of an injectable epsilon-polylysine/carboxymethyl chitosan hydrogel used in medical application. Mater Chem Phy. 2020. https://doi.org/10.1016/j.matchemphys.2020.122902.
Jia S, Fan B, Dai Y, Wang G, Jia Y. Fractionation and characterization of ε-Poly-L-lysine from Streptomyces albulus CGMCC 1986. Food Sci Biotechnol. 2010;19:361–6.
Gao H, Luo SZ. Biosynthesis, isolation, and structural characterization of ε-poly-L-lysine produced by Streptomyces sp. DES20. Rsc Adv. 2016;16:1–18.
Zhen B, Chen X, Han D, Mao Z. An alternative method for the decoloration of ɛ-poly-l-lysine eluate by macroporous resin in the separation and purification of ɛ-poly-l-lysine from fermentation broth. Food Bioprod Process. 2015;3:1–7.
Katano H, Yoneoka T, Kito N, Maruyama C, Hamano Y. Separation and purification of ε-poly-L-lysine from the culture broth based on precipitation with the tetraphenylborate anion. Anal Sci. 2012;28:1153–7.
Xu-Sheng C, Yang G, Bin Z, Dai H, Jian-Hua Z. Separation and purification of ϵ-poly-l-lysine from fermentation broth. Process Biochem. 2016. https://doi.org/10.1016/j.procbio.2015.11.010.
Chen X, Diao W, Ma Y, Mao Z. Extraction and purification of ε-poly-l-lysine from fermentation broth using an ethanol/ammonium sulfate aqueous two-phase system combined with ultrafiltration. RSC Adv. 2020;10:29587–93.
Oppermann-Sanio FB, Steinbuchel A. Occurrence, functions and biosynthesis of polyamides in microorganisms and biotechnological production. Naturwissenschaften. 2002;89:11–22.
Takehara M, Hibino A, Saimura M, Hirohara H. High-yield production of short chain length poly(epsilon-l-lysine) consisting of 5–20 residues by Streptomyces aureofaciens, and its antimicrobial activity. Biotech Lett. 2010;32:1299–303.
Harrison J, Studholme DJ. Recently published Streptomyces genome sequences. Microb Biotechnol. 2014;7:373–80.
Dodd A, Swanevelder D, Featherston J, Rumbold K. Draft genome sequence of streptomyces albulus strain CCRC 11814, an epsilon-poly-L-lysine-producing actinomycete. Genome Announc. 2013. https://doi.org/10.1128/genomeA.00696-13.
Xu Z, Xia J, Feng X, Li S, Xu H, Bo F, Sun Z. Genome sequence of Streptomyces albulus PD-1, a productive strain for epsilon-poly-L-lysine and poly-L-diaminopropionic acid. Genome Announc. 2014. https://doi.org/10.1128/genomeA.00297-14.
Gu Y, Yang C, Wang X, Geng W, Sun Y, Feng J, Wang Y, Quan Y, Che Y, Zhang C, et al. Genome sequence of the epsilon-poly-l-lysine-producing strain Streptomyces albulus NK660 isolated from soil in gutian. Genome Announc. 2014. https://doi.org/10.1128/genomeA.00532-14.
Yamanaka K, Hamano Y. Draft genome sequence of the most traditional ε-poly-l-lysine producer, Streptomyces albulus NBRC14147. Microbiol Resour Announc. 2019. https://doi.org/10.1128/MRA.01515-18.
Wang L, Gao CH, Tang N, Hu SN, Wu QF. Identification of genetic variations associated with epsilon-poly-lysine biosynthesis in Streptomyces albulus ZPM by genome sequencing. Sci Rep. 2015;5(1):1–9.
Hirohara H, Saimura M, Takehara M, Miyamoto M, Ikezaki A. Substantially monodispersed poly(epsilon-L-lysine)s frequently occurred in newly isolated strains of Streptomyces sp. Appl Microbiol Biotechnol. 2007;76:1009–16.
Xu ZX, Bo FF, Xia J, Sun ZZ, Li S, Feng XH, Xu H. Effects of oxygen-vectors on the synthesis of epsilon-poly-lysine and the metabolic characterization of Streptomyces albulus PD-1. Biochem Eng J. 2015;94:58–64.
Bankar SB, Singhal RS. Panorama of poly-ε-lysine. Rsc. Advances. 2013;3:8586–603.
Hamano Y, Nicchu I, Shimizu T, Onji Y, Hiraki J, Takagi H. epsilon-Poly-L: -lysine producer, Streptomyces albulus, has feedback-inhibition resistant aspartokinase. Appl Microbiol Biotechnol. 2007;76:873–82.
Hamano Y, Arai T, Ashiuchi M, Kino K. NRPSs and amide ligases producing homopoly(amino acid)s and homooligo(amino acid)s. Nat Prod Rep. 2013;30:1087–97.
Kawai T, Kubota T, Hiraki J, Izumi Y. Biosynthesis of epsilon-poly-L-lysine in a cell-free system of Streptomyces albulus. Biochem Biophys Res Commun. 2003;311:635–40.
Finking R, Marahiel MA. Biosynthesis of nonribosomal peptides. Annu Rev Microbiol. 2004;58:453–88.
Saimura M, Takehara M, Mizukami S, Kataoka K, Hirohara H. Biosynthesis of nearly monodispersed poly(epsilon-L-lysine) in Streptomyces species. Biotech Lett. 2008;30:377–85.
Yamanaka K, Maruyama C, Takagi H, Hamano Y. Epsilon-poly-L-lysine dispersity is controlled by a highly unusual nonribosomal peptide synthetase. Nat Chem Biol. 2008;4:766–72.
Purev E, Kondo T, Takemoto D, Niones JT, Ojika M. Identification of ε-Poly-L-lysine as an antimicrobial product from an epichlo endophyte and isolation of fungal ε-PL synthetase gene. Molecules. 2020;25(5):1032.
Hamano Y, Kito N, Kita A, Imokawa Y, Yamanaka K, Maruyama C, Katano H. ε-Poly-L-lysine peptide chain length regulated by the linkers connecting the transmembrane domains of ε-Poly-L-lysine synthetase. Appl Environ Microbiol. 2014;80:4993–5000.
Kazuya Y, Naoko K, Akihiro K, Yuuki. Development of a recombinant ε-poly-L-lysine synthetase expression system to perform mutational analysis. J Bio Bioeng. 2011;111:646–9.
Yamanaka K, Kito N, Imokawa Y, Maruyama C, Utagawa T, Hamano Y. Mechanism of epsilon-poly-L-lysine production and accumulation revealed by identification and analysis of an epsilon-poly-L-lysine-degrading enzyme. Appl Environ Microbiol. 2010;76:5669–75.
Kito M, Onji Y, Yoshida T, Nagasawa T. Occurrence of ε-poly-l-lysine-degrading enzyme in ε-poly-l-lysine-tolerant Sphingobacterium multivorum OJ10: purification and characterization. FEMS Microbiol Lett. 2002;207:147–51.
Zhou CC, Li P, Qi XB, Sharif ARM, Poon YF, Cao Y, Chang MW, Leong SSJ, Chan-Park MB. A photopolymerized antimicrobial hydrogel coating derived from epsilon-poly-L-lysine. Biomaterials. 2011;32:2704–12.
Naoko K, Chitose M, Kazuya Y, Yuuki. Mutational analysis of the three tandem domains of ε-poly-l-lysine synthetase catalyzing the l-lysine polymerization reaction. J Biosci Bioeng. 2013;115:523–6.
Takehara M, Hibino A, Saimura M, Hirohara H. High-yield production of short chain length poly(ε-L-lysine) consisting of 5–20 residues by Streptomyces aureofaciens, and its antimicrobial activity. Biotech Lett. 2010;32:1299–303.
Hirohara H, Takehara M, Saimura M, Masayuki A, Miyamoto M. Biosynthesis of poly(epsilon-L-lysine)s in two newly isolated strains of Streptomyces sp. Appl Microbiol Biotechnol. 2006;73:321–31.
Nishikawa M, Ogawa K. Inhibition of epsilon-poly-L-lysine biosynthesis in Streptomycetaceae bacteria by short-chain polyols. Appl Environ Microbiol. 2006;72:2306–12.
Nishikawa M. Molecular mass control using polyanionic cyclodextrin derivatives for the epsilon-poly-L-lysine biosynthesis by Streptomyces. Enzyme Microb Technol. 2009;45:295–8.
Liu Q, Chen X, Zeng X, Han D, Mao Z. Purification, characterization and application of ε-poly-Llysine-degrading enzyme from Streptomyces sp. M-Z18. Acta Microbiol Sinica. 2014;54(9):1022–32.
Chen XS, Wang KF, Zheng GC, Gao Y, Mao ZG. Preparation, characterization and antimicrobial activity of epsilon-poly-L-lysine with short chain length produced from glycerol by Streptomyces albulus. Process Biochem. 2018;68:22–9.
Wang T, Jia S, Tan Z, Dai Y, Song S, Wang G. Mutagenesis and selective breeding of a high producing poly-L-lysine strain. Front Chem Sci Eng. 2012;6:179–83.
Li S, Li F, Chen XS, Wang L, Xu J, Tang L, Mao ZG. Genome shuffling enhanced ε-poly-L-lysine production by improving glucose tolerance of Streptomyces graminearus. Appl Biochem Biotechnol. 2012;166:414–23.
Zhou Y-P, Ren X-D, Wang L, Chen X-S, Mao Z-G. Enhancement of epsilon-poly-lysine production in epsilon-poly-lysine-tolerant Streptomyces sp by genome shuffling. Bioprocess Biosyst Eng. 2015;38:1705–13.
Li S, Chen XS, Dong CL, Zhao FL, Tang L, Mao ZG. Combining genome shuffling and interspecific hybridization among Streptomyces improved epsilon-poly-l-Lysine production. Appl Biochem Biotechnol. 2013;169:338–50.
Xiang JH, Yang Y, Dabbour M, Mintah BK, Zhang ZL, Dai CH, He RH, Huang GP, Ma HL. Metabolomic and genomic profiles of Streptomyces albulus with a higher epsilon-polylysine production through ARTP mutagenesis. Biochem Eng J. 2020. https://doi.org/10.1016/j.bej.2020.107720.
Wang L, Chen XS, Wu GY, Li S, Zeng X, Ren XD, Tang L, Mao ZG. Improved epsilon-poly-L-lysine production of Streptomyces sp FEEL-1 by atmospheric and room temperature plasma mutagenesis and streptomycin resistance screening. Ann Microbiol. 2015;65:2009–17.
Wang L, Chen XS, Wu GY, Zeng X, Ren XD, Li S, Tang L, Mao ZG. Genome shuffling and gentamicin-resistance to improve epsilon-poly-l-lysine productivity of Streptomyces albulus W-156. Appl Biochem Biotechnol. 2016;180:1601–17.
Kahar P, Iwata T, Hiraki J, Park EY, Okabe M. Enhancement of epsilon-polylysine production by Streptomyces albulus strain 410 using pH control. J Biosci Bioeng. 2001;91:190–4.
Ren XD, Chen XS, Zeng X, Wang L, Tang L, Mao ZG. Acidic pH shock induced overproduction of epsilon-poly-l-lysine in fed-batch fermentation by Streptomyces sp M-Z18 from agro-industrial by-products. Bioprocess Biosyst Eng. 2015;38:1113–25.
Liu SR, Wu QP, Zhang JM, Mo SP, Xiao C, Yang XJ. Investigation on the effects of epsilon-Poly-L-Lysine on a producing strain Streptomyces ahygroscopicus GIM8, for better understanding its biosynthesis. J Basic Microbiol. 2015;55:172–9.
Wang C, Ren X, Yu C, et al. Physiological and transcriptional responses of Streptomyces albulus to acid stress in the biosynthesis of ε-poly-L-lysine. Front Microbiol 2020;11:1379
Xu-Sheng C, Shu Li, Li-Juan L, Xi-Dong R, Feng Li. Production of ε-poly-l-lysine using a novel two-stage pH control strategy by Streptomyces sp. M-Z18 from glycerol. Bioprocess Biosys Eng. 2011;34:561–7.
Pan L, Chen XS, Liu MM, Liu YJ, Mao ZG. Efficient production of epsilon-poly-L-lysine from glucose by two-stage fermentation using pH shock strategy. Process Biochem. 2017;63:8–15.
Pan L, Chen X, Wang K, Mao Z. Understanding high ε-poly-l-lysine production by Streptomyces albulus using pH shock strategy in the level of transcriptomics. J Ind Microbiol Biotechnol. 2019;46:1781–92.
Ren XD, Chen XS, Tang L, Zeng X, Wang L, Mao ZG. Physiological mechanism of the overproduction of epsilon-poly-L-lysine by acidic pH shock in fed-batch fermentation. Bioprocess Biosyst Eng. 2015;38:2085–94.
Pan L, Chen XS, Wang KF, Mao ZG. A temporal transcriptomic dynamics study reveals the reason of enhanced epsilon-poly-L-lysine production in Streptomyces albulus M-Z18 by pH shock. Process Biochem. 2019;85:1–11.
Ren XD, Chen XS, Tang L, Sun QX, Zeng X, Mao ZG. Efficient production of epsilon-poly-L-lysine from agro-industrial by-products by Streptomyces sp M-Z18. Ann Microbiol. 2015;65:733–43.
Xu ZX, Cao CH, Sun ZZ, Li S, Xu Z, Feng XH, Xu H. Construction of a genetic system for Streptomyces albulus PD-1 and improving poly(epsilon-L-lysine) production through expression of Vitreoscilla Hemoglobin. J Microbiol Biotechnol. 2015;25:1819–26.
Posada JA, Rincon LE, Cardona CA. Design and analysis of biorefineries based on raw glycerol: addressing the glycerol problem. Biores Technol. 2012;111:282–93.
Bankar SB, Singhal RS. Optimization of poly-epsilon-lysine production by Streptomyces noursei NRRL 5126. Biores Technol. 2010;101:8370–5.
Xia J, Xu ZX, Xu H, Liang JF, Li S, Feng XH. Economical production of poly(epsilon-L-lysine) and poly(L-diaminopropionic acid) using cane molasses and hydrolysate of streptomyces cells by Streptomyces albulus PD-1. Biores Technol. 2014;164:241–7.
Zeng X, Chen XS, Ren XD, Liu QR, Wang L, Sun QX, Tang L, Mao ZG. Insights into the role of glucose and glycerol as a mixed carbon source in the improvement of epsilon-poly-L-Lysine productivity. Appl Biochem Biotechnol. 2014;173:2211–24.
Ren X-D, Xu Y-J, Zeng X, Chen X-S, Tang L, Mao Z-G. Microparticle-enhanced production of ε-poly-l-lysine in fed-batch fermentation. RSC Adv. 2015;5:82138–43.
Zeng X, Miao W, Wen B, Mao Z, Zhu M, Chen X. Transcriptional study of the enhanced ε-poly- l -lysine productivity in culture using glucose and glycerol as a mixed carbon source. Bioprocess Biosyst Eng. 2019;42:555–66.
Zhang JH, Zeng X, Chen XS, Mao ZG. Metabolic analyses of the improved ε-poly- l -lysine productivity using a glucose–glycerol mixed carbon source in chemostat cultures. Bioprocess Biosyst Eng. 2018;41:1143–51.
Xin Z, Junjie Z, Xusheng C, Zhonggui M, Wenyun M. Insights into the simultaneous utilization of glucose and glycerol by Streptomyces albulus M-Z18 for high ε-poly-l-lysine productivity. Bioprocess Biosyst Eng. 2017;40:1775–85.
Zeng X, Chen XS, Gao Y, Ren XD, Wang L, Mao ZG. Continuously high reactive oxygen species generation decreased the specific epsilon-poly-L-lysine formation rate in fed-batch fermentation using glucose and glycerol as a mixed carbon source. Process Biochem. 2015;50:1993–2003.
Liu SR, Wu QP, Zhang JM, Mo SP. Efficient production of epsilon-poly-L-lysine by Streptomyces ahygroscopicus using one-stage pH control fed-batch fermentation coupled with nutrient feeding. J Microbiol Biotechnol. 2015;25:358–65.
Liu SR, Wu QP, Zhang JM, Mo SP. Effects of L-lysine and D-lysine on epsilon-poly-L-lysine biosynthesis and their metabolites by Streptomyces ahygroscopicus GIM8. Biotechnol Bioprocess Eng. 2012;17:1205–12.
Xu-Sheng C, Xi-Dong R, Xin Z, Fu-Lin Z, Lei T. Enhancement of ε-poly-l-lysine production coupled with precursor l-lysine feeding in glucose–glycerol co-fermentation by Streptomyces sp. M-Z18. Bioprocess Biosy Eng. 2013;36:1843–9.
Xia J, Xu ZX, Xu H, Feng XH, Bo FF. The regulatory effect of citric acid on the co-production of poly(epsilon-lysine) and poly(L-diaminopropionic acid) in Streptomyces albulus PD-1. Bioprocess Biosyst Eng. 2014;37:2095–103.
Wang AX, Tian WZ, Cheng L, Xu YQ, Wang XW, Qin JY, Yu B. Enhanced epsilon-Poly-L-Lysine production by the synergistic effect of epsilon-poly-L-Lysine synthetase overexpression and citrate in Streptomyces albulus. Frontiers Bioeng Biotechnol. 2020. https://doi.org/10.3389/fbioe.2020.00288.
Kobayashi K, Nishikawa M. Promotion of epsilon-poly-L-lysine production by iron in Kitasatospora kifunense. World J Microbiol Biotechnol. 2007;23:1033–6.
Wang GL, Jia SR, Wang TA, Chen LY, Song QC, Li WN. Effect of Ferrous Ion on epsilon-Poly-l-Lysine biosynthesis by Streptomyces diastatochromogenes CGMCC3145. Curr Microbiol. 2011;62:1062–7.
Yan P, Sun HB, Lu PQ, Liu HL, Tang L. Enhancement of epsilon-poly-L-lysine synthesis in Streptomyces by exogenous glutathione. Bioprocess Biosyst Eng. 2018;41:129–34.
Li S, Ji J, Hu S, Chen G. Enhancement of ε-poly-L-lysine production in Streptomyces griseofuscus by addition of exogenous astaxanthin. Bioprocess Biosys Eng. 2020;43(10):1813–21.
Zhang Y, Feng XH, Xu H, Yao Z, Ouyang PK. Epsilon-Poly-L-lysine production by immobilized cells of Kitasatospora sp MY 5–36 in repeated fed-batch cultures. Biores Technol. 2010;101:5523–7.
Liu SR, Wu QP, Zhang JM, Mo SP. Production of epsilon-poly-L-lysine by Streptomyces sp using resin-based, in situ product removal. Biotech Lett. 2011;33:1581–5.
Xiao Q, Yang C, X., S., Liu, Zhang, J. Enhanced ε-poly-l-lysine production from Streptomyces ahygroscopicus by a combination of cell immobilization and in situ adsorption. J Microbiol Biotechnol. 2012;22:1218–23.
Gu Y, Wang X, Yang C, Geng W, Feng J, Wang Y, Wang S, Song C. Effects of chromosomal integration of the Vitreoscilla Hemoglobin Gene (vgb) and S-Adenosylmethionine synthetase gene (metK) on ε-Poly-l-Lysine synthesis in Streptomyces albulus NK660. Appl Biochem Biotechnol. 2016;178:1445–57.
Xu D, Yao H, Cao C, Xu Z, Li S, Xu Z, Zhou J, Feng X, Xu H. Enhancement of ε-poly-L-lysine production by overexpressing the ammonium transporter gene in Streptomyces albulus PD-1. Bioprocess Biosyst Eng. 2018;41:1–9.
Xu DL, Wang R, Xu ZX, Xu Z, Li S, Wang MX, Feng XH, Xu H. Discovery of a short-chain epsilon-poly-L-lysine and its highly efficient production via synthetase swap strategy. J Agric Food Chem. 2019;67:1453–62.
Geng WT, Yang C, Gu YY, Liu RH, Guo WB, Wang XM, Song CJ, Wang SF. Cloning of epsilon-poly-L-lysine ( epsilon-PL) synthetase gene from a newly isolated epsilon-PL-producing Streptomyces albulus NK660 and its heterologous expression in Streptomyces lividans. Microb Biotechnol. 2014;7:155–64.
Liu H, Zhao XX, Yu M, Meng LX, Zhou T, Shan YH, Liu XY, Xia ZH, An MN, Wu YH. Transcriptomic and functional analyses indicate novel anti-viral mode of actions on tobacco mosaic virus of a microbial natural product epsilon-poly-L-lysine. J Agric Food Chem. 2021;69:2076–86.
Chen JG, Liu H, Xia ZH, Zhao XX, Wu YH, An MN. Purification and structural analysis of the effective anti-TMV compound epsilon-Poly-l-lysine produced by Streptomyces ahygroscopicus. Molecules. 2019;24(6):1156.
Xi YW, Guo Y, Wang M, Ge J, Liu YL, Niu W, Chen M, Xue YM, Winston DD, Dai WT, et al. Biomimetic bioactive multifunctional poly(citrate-siloxane)-based nanofibrous scaffolds enable efficient multidrug-resistant bacterial treatment/non-invasive tracking in vitro/in vivo. Chem Eng J. 2020;383:123078.
Bo T, Han PP, Su QZ, Fu P, Guo FZ, Zheng ZX, Tan ZL, Zhong C, Jia SR. Antimicrobial epsilon-poly-L-lysine induced changes in cell membrane compositions and properties of Saccharomyces cerevisiae. Food Control. 2016;61:123–34.
Patil NA. Kandasubramanian B: functionalized polylysine biomaterials for advanced medical applications: a review. Eur Poly J. 2021. https://doi.org/10.1016/j.eurpolymj.2020.110248.
He XH, Li Y, Zhang LM, Du R, Dai YJ, Tan ZL. Preparation of 2,3-dialdehyde microcrystalline cellulose particles crosslinked with epsilon-poly-L-lysine and their antibacterial activity. Cellulose. 2021;28:2833–47.
Wu H, Hu SQ, Nie CL, Zhang JH, Tian HF, Hu WC, Shen T, Wang JG. Fabrication and characterization of antibacterial epsilon-poly-L-lysine anchored dicarboxyl cellulose beads. Carbohydr Polym. 2021. https://doi.org/10.1016/j.carbpol.2020.117337.
Shukla SC, Singh A, Pandey AK, Mishra A. Review on production and medical applications of epsilon-polylysine. Biochem Eng J. 2012;65:70–81.
Hou Y, Wang FP, Tan ZL, Cui JD, Jia SR. Antifungal mechanisms of epsilon-poly-L-Lysine with different molecular weights on Saccharomyces cerevisiae. Korean J Chem Eng. 2020;37:482–92.
Buzon-Duran L, Martin-Gil J, Perez-Lebena E, Ruano-Rosa D, Revuelta JL, Casanova-Gascon J, Ramos-Sanchez MC, Martin-Ramos P. Antifungal agents based on chitosan oligomers epsilon-polylysine and Streptomyces sp secondary metabolites against three botryosphaeriaceae species. Antibiotics Basel. 2019. https://doi.org/10.3390/antibiotics8030099.
Lin L, Gu YL, Li CZ, Vittayapadung S, Cui HY. Antibacterial mechanism of epsilon-Poly-lysine against Listeria monocytogenes and its application on cheese. Food Control. 2018;91:76–84.
Wei ML, Ge YH, Li CY, Chen YR, Wang WH, Duan B, Li X. Antifungal activity of epsilon-poly-L-lysine on Trichothecium roseum in vitro and its mechanisms. Physiol Mol Plant Pathol. 2018;103:23–7.
Jiao W, Liu X, Chen Q, Du Y, Fu M. Epsilon-poly-l-lysine (ε-PL) exhibits antifungal activity in vivo and in vitro against Botrytis cinerea and mechanism involved. Postharvest Biol Technol. 2020;168: 111270.
Zhilei T, Tao B, Fengzhu G, Jiandong C, Shiru J. Effects of ε-Poly- l -lysine on the cell wall of Saccharomyces cerevisiae and its involved antimicrobial mechanism. Int J Biol Macromol. 2018. https://doi.org/10.1016/j.ijbiomac.2018.07.094.
Dou Y, Routledge MN, Gong YY, Godana EA, Dhanasekaran S, Yang QY, Zhang XY, Zhang HY. Efficacy of epsilon-poly-L-lysine inhibition of postharvest blue mold in apples and potential mechanisms. Postharvest Biol Technol. 2021;171:1–10.
Zhang ZH, Zeng XA, Brennan CS, Ma HL, Aadil RM. Preparation and characterisation of novelty food preservatives by maillard reaction between epsilon-polylysine and reducing sugars. Int J Food Sci Technol. 2019;54:1824–35.
Yang H, Li QY, Yang LL, Sun T, Li XP, Zhou B, Li JR. The competitive release kinetics and synergistic antibacterial characteristics of tea polyphenols/epsilon-poly-l-lysine hydrochloride core-shell microcapsules against Shewanella putrefaciens. Int J Food Sci Technol. 2020;55:3542–52.
Chang Y, Mclandsborough L, Mcclements DJ. Interaction of cationic antimicrobial (ɛ-polylysine) with food-grade biopolymers: dextran, chitosan, carrageenan, alginate, and pectin. Food Res Int. 2014;64:396–401.
Li S, Wang N, Du ZJ, Chen GJ. Intergeneric hybridization between Streptomyces albulus and Bacillus subtilis facilitates production of epsilon-poly-L-lysine from corn starch residues. Biotechnol Bioprocess Eng. 2018;23:580–7.
Chang YH, McLandsborough L, McClements DJ. Interactions of a cationic antimicrobial (epsilon-Polylysine) with an anionic biopolymer (Pectin): an isothermal titration calorimetry, microelectrophoresis, and turbidity study. J Agric Food Chem. 2011;59:5579–88.
Li T, Wen C, Dong Y, Li D, Liu M, Wang Z, Janaswamy S, Zhu B, Song S. Effect of ε-polylysine addition on K-carrageenan gel properties: rheology, water mobility, thermal stability and microstructure. Food Hydrocolloids. 2019;95:212–8.
Ushimaru K, Morita T, Fukuoka T. Bio-based, flexible, and tough material derived from ε-Poly- l -lysine and fructose via the maillard reaction. ACS Omega. 2020;5:22793–9.
Ushimar K, Haman Y, Katano H. Antimicrobial activity of ε-poly-l-lysine after forming a water-insoluble complex with an anionic surfactant. Biomacromol. 2017;18:1387–92.
Chang SL, Li H, Liu JN, Zhao MX, Tan MH, Xu PW, Wang XD, Wang LW, Yuan XF, Zhao QS, Zhao B. Effect of hydrogen peroxide treatment on the quality of epsilon-poly-L-lysine products. Biochem Eng J. 2021. https://doi.org/10.1016/j.bej.2021.108017.
Li WC, Lv JG, Dong TY, Li XY, Li XN, Tan ZL, Jia SR. Effects of amino acids and overexpression of dapa gene on the production of epsilon-poly-L-lysine by Streptomyces diastatochromogenes strains. Current Microbiol. 2021. https://doi.org/10.1007/s00284-021-02510-z.
Liu SR, Yang XJ, Sun DF. Enhanced production of epsilon-poly-l-lysine by immobilized Streptomyces ahygroscopicus through repeated-batch or fed-batch fermentation with in situ product removal. Bioprocess Biosys Eng. 2021. https://doi.org/10.1007/s00449-021-02587-7.
Jiang XL, Radko Y, Gren T, Palazzotto E, Jorgensen TS, Cheng T, Xian M, Weber T, Lee SY. Distribution of epsilon-poly-L-lysine synthetases in Coryneform bacteria isolated from cheese and human skin. Appl Environ Microbiol. 2021. https://doi.org/10.1128/AEM.01841-20.
Zhou YP, Yan P, Tang L. Self-protection of Streptomyces to epsilon-poly-L-lysine improves fermentation efficacy. Biochem Eng J. 2021. https://doi.org/10.1016/j.bej.2021.107935.
Nie CL, Shen T, Hu WC, Ma Q, Zhang JH, Hu SQ, Tian HF, Wu H, Luo XG, Wang JG. Characterization and antibacterial properties of epsilon-poly- L-lysine grafted multi-functional cellulose beads. Carbohydr Polym. 2021;1:262.
Zhou YP, Ren XD, Wang L, Chen XS, Mao ZG, Tang L. Enhancement of epsilon-poly-lysine production in epsilon-poly-lysine-tolerant Streptomyces sp by genome shuffling. Bioprocess Biosyst Eng. 2015;38:1705–13.
Sun QX, Chen XS, Ren XD, Mao ZG. Improvement of epsilon-poly-L-lysine production through seed stage development based on in situ pH monitoring. Appl Biochem Biotechnol. 2015;175:802–12.
Bankar S, Dhumal V, Bhotmange D, Bhagwat S, Singhal R. Empirical predictive modelling of poly-lysine biosynthesis in resting cells of Streptomyces noursei. Food Sci Biotechnol. 2014;23:201–7.
Wang L, Chen XS, Wu GY, Li S, Zeng X, Ren XD, Tang L, Mao ZG. Enhanced e-poly-L-lysine production by inducing double antibiotic-resistant mutations in Streptomyces albulus. Bioprocess Biosyst Eng. 2017;40:271–83.
Xu DL, Yao HQ, Cao CH, Xu ZX, Li S, Xu Z, Zhou JH, Feng XH, Xu H. Enhancement of epsilon-poly-L-lysine production by overexpressing the ammonium transporter gene in Streptomyces albulus PD-1. Bioprocess Biosyst Eng. 2018;41:1337–45.
Chheda AH, Vernekar MR. Enhancement of epsilon-poly-L-lysine (epsilon-PL) production by a novel producer Bacillus cereus using metabolic precursors and glucose feeding. 3 Biotech. 2015;5:839–46.
Singh P. Distribution of antimicrobial ε-polylysine producing marine microbe in sea water along west coast of India. Biomater Med. 2018;7:2577–268.
Li S, Ji JY, Hu SJ, Chen GJ. Enhancement of epsilon-poly-l-lysine production in Streptomyces griseofuscus by addition of exogenous astaxanthin. Bioprocess Biosyst Eng. 2020;43:1813–21.
Zeng X, Zhao JJ, Chen XS, Mao ZG, Miao WY. Insights into the simultaneous utilization of glucose and glycerol by Streptomyces albulus M-Z18 for high epsilon-poly-L-lysine productivity. Bioprocess Biosyst Eng. 2017;40:1775–85.
Xu ZX, Sun ZZ, Li S, Xu Z, Cao CH, Xu ZQ, Feng XH, Xu H. Systematic unravelling of the biosynthesis of poly (L-diaminopropionic acid) in Streptomyces albulus PD1. Sci Rep. 2015. https://doi.org/10.1038/srep17400.
Ry A, Hx A, Cw B, Sp A, Lw A, Hx C, Zpa C, Yx B, Zz D, Hw A. Antibacterial activity and mechanism of action of ε-poly- l -lysine. Biochem Biophys Res Commun. 2013;439:148–53.
Acknowledgements
Not applicable.
Funding
This work was funded by Key projects in Guangxi (grant number: 2019GXNSFDA245008) and the “Bagui Young Scholars” Special Project.
Author information
Authors and Affiliations
Contributions
SBL: literature searches and review, manuscript writing, figure design; YRM and LZ: literature searches and review, drawing; MW and JHM: literature searches and review; XLL: supervision, writing—review and editing; YXB: project administration; YG: literature searches and review, manuscript writing and revision. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors have no conflict of interest to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Li, S., Mao, Y., Zhang, L. et al. Recent advances in microbial ε-poly-L-lysine fermentation and its diverse applications. Biotechnol Biofuels 15, 65 (2022). https://doi.org/10.1186/s13068-022-02166-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13068-022-02166-2