Abstract
Extrapyramidal movement disorders include hypokinetic rigid and hyperkinetic or mixed forms, most of them originating from dysfunction of the basal ganglia (BG) and their information circuits. The functional anatomy of the BG, the cortico-BG–thalamocortical, and BG–cerebellar circuit connections are briefly reviewed. Pathophysiologic classification of extrapyramidal movement disorder mechanisms distinguish (1) parkinsonian syndromes, (2) chorea and related syndromes, (3) dystonias, (4) myoclonic syndromes, (5) ballism, (6) tics, and (7) tremor syndromes. Recent genetic and molecular–biologic classifications distinguish (1) synucleinopathies (Parkinson’s disease, dementia with Lewy bodies, Parkinson’s disease–dementia, and multiple system atrophy); (2) tauopathies (progressive supranuclear palsy, corticobasal degeneration, FTLD-17; Guamian Parkinson–dementia; Pick’s disease, and others); (3) polyglutamine disorders (Huntington’s disease and related disorders); (4) pantothenate kinase-associated neurodegeneration; (5) Wilson’s disease; and (6) other hereditary neurodegenerations without hitherto detected genetic or specific markers. The diversity of phenotypes is related to the deposition of pathologic proteins in distinct cell populations, causing neurodegeneration due to genetic and environmental factors, but there is frequent overlap between various disorders. Their etiopathogenesis is still poorly understood, but is suggested to result from an interaction between genetic and environmental factors. Multiple etiologies and noxious factors (protein mishandling, mitochondrial dysfunction, oxidative stress, excitotoxicity, energy failure, and chronic neuroinflammation) are more likely than a single factor. Current clinical consensus criteria have increased the diagnostic accuracy of most neurodegenerative movement disorders, but for their definite diagnosis, histopathological confirmation is required. We present a timely overview of the neuropathology and pathogenesis of the major extrapyramidal movement disorders in two parts, the first one dedicated to hypokinetic-rigid forms and the second to hyperkinetic disorders.

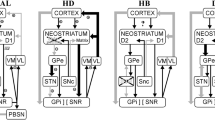
Modified from (Haber 2016) with permission from Association La Conférence Hippocrate-Servier. © AICH-Servier

From (Jellinger 2016)

Modified from (Helmich et al. 2011)

From (Jellinger 2012a)

From (Jellinger 2012a)

Modified from (Tsang and Chung 2009)

From (Jellinger and Wenning 2016)
Similar content being viewed by others
Abbreviations
- AGs:
-
Argyrophilic grains
- ALS:
-
Amyptrophic lateral sclerosis
- ALS/PDC:
-
Guamanian ALS–Parkinson’s disease complex
- AP:
-
Astroglial plaque
- APP:
-
Amyloid precursor protein
- AR-PD:
-
Akinesia-and-rigidity type PD
- AS:
-
α-Synuclein
- AutD:
-
Autosomal dominant
- AutR:
-
Autosomal recessive
- βSyn:
-
β-Synuclein
- BG:
-
Basal ganglia
- BHC:
-
Benign hereditary chorea
- BIBD:
-
Basophilic inclusion body disease
- CAA:
-
Cerebral amyloid angiopathy
- CAG:
-
Polyglutamine
- CBD:
-
Corticobasal degeneration
- CBGTC:
-
Cortico-BG-thalamocortical
- CBS:
-
Corticobasal syndrome
- ChAc:
-
Chorea-acanthocytosis
- ChAT:
-
Choline-acetyl transferase
- CI:
-
Cognitive impairment
- CN:
-
Caudate nucleus
- CNS:
-
Central nervous system
- CS–TD:
-
Cortico-striatal–temporal difference
- DA:
-
Dopamine
- DLB:
-
Dementia with Lewy bodies
- DLB-AD:
-
Dementia with Lewy bodies and Alzheimer’s disease
- DRD:
-
Dopa-responsive dystonia
- DRPLA:
-
Dentatorubral-pallidoluysian atrophy
- DS:
-
Dystonia syndrome
- ENK:
-
Enkephalin
- ET:
-
Essential tremor
- FTDP-17:
-
Frontotemporal degeneration and parkinsonism linked to chromosome 17
- FTLD:
-
Frontotemporal lobar degeneration
- GABA:
-
γ-Aminobutyric acid
- GBA:
-
Glucocerebrosidase gene
- GCase:
-
Glucocerebrosidase
- GCIs:
-
Glial cytoplasmic inclusions
- GDNF:
-
Glia-derived neurotrophic factor
- GNIs:
-
Glial nuclear inclusions
- GPe:
-
External segment of globus pallidus
- GPi:
-
Internal segment of globus pallidus
- GTP:
-
Guanosine triphosphate
- HD:
-
Huntington’s disease
- HTT:
-
Huntingtin
- iLBD:
-
Incidental Lewy body disease
- IT:
-
Intratelencephalic
- LB:
-
Lewy body
- LC:
-
Locus ceruleus
- LID:
-
l-Dopa-induced dyskinesia
- LP:
-
Lewy body pathology
- MCI:
-
Mild cognitive impairment
- MD:
-
Menkes’ disease
- MJD:
-
Machado-Joseph disease
- MSA:
-
Multiple system atrophy
- MSA-C:
-
Multiple system atrophy with predominant cerebellar features
- MSA-P:
-
Multiple system atrophy with predominant parkinsonism
- MSN:
-
Medium spiny projection neuron
- NA:
-
Neuroacanthocytosis
- NBIA:
-
Neurodegeneration with brain iron accumulation
- NBM:
-
Nucleus basalis of Meynert
- NCIs:
-
Neuronal cytoplasmic inclusions
- NFTs:
-
Neurofibrillary tangles
- NIID:
-
Neuronal intranuclear inclusion disease
- NM:
-
Neuromelanin
- NNIs:
-
Neuronal nuclear inclusions
- NT:
-
Neuropil threads
- OCD:
-
Obsessive-compulsive disorder
- OPC:
-
Olivopontocerebellar
- OPCA:
-
Olivopontocerebellar atrophy
- OS:
-
Oxidative stress
- pAS:
-
Phosphorylated α-synuclein
- PC:
-
Purkinje cell
- PD:
-
Parkinson’s disease
- PDC:
-
Parkinson’s disease complex
- PDD:
-
Parkinson’s disease dementia
- PEP:
-
Postencephalitic parkinsonism
- PGF:
-
PSP with progressive gait freezing
- PHFs:
-
Paired helical filaments
- PiD:
-
Pick’s disease
- PKAN:
-
Pantothenate-kinase associated neurodegeneration
- PPN:
-
Pedunculopontine nucleus
- PPT:
-
Pedunculo-pontine tegmental
- PSP:
-
Progressive supranuclear palsy
- PSP-CBS:
-
PSP presenting with corticobasal syndrom
- PSP-P:
-
Progressive supranuclear palsy-parkinsonism
- PSP-RS:
-
Richardson’s syndrome
- Put:
-
Putamen
- SCA3:
-
Spinocerebellar ataxia type 3
- SN:
-
Substantia nigra
- SNc:
-
Substantia nigra pars compacta
- SNr:
-
Substantia nigra pars reticulata
- SP:
-
Substance P
- STN:
-
Subthalamic nucleus
- TA:
-
Tufted astrocyte
- TDPD:
-
Tremor-dominant type of PD
- TH:
-
Tyrosine hydoxylase
- TS:
-
Tourette’s syndrome
- VaP:
-
Vascular parkinsonism
- VM:
-
Ventromedial
- VTA:
-
Ventral tegmental area
- WD:
-
Wilson’s disease
- XDP:
-
X-linked dystonia-parkinsonism
References
Aarsland D, Kurz MW (2010) The epidemiology of dementia associated with Parkinson’s disease. Brain Pathol 20:633–639
Abbott RD, Nelson JS, Ross GW, Uyehara-Lock JH, Tanner CM, Masaki KH, Launer LJ, White LR, Petrovitch H (2017) Marinesco bodies and substantia nigra neuron density in Parkinson’s disease. Neuropathol Appl Neurobiol 43:621–630
Abeliovich A, Gitler AD (2016) Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature 539:207–216
Adamowicz DH, Roy S, Salmon DP, Galasko DR, Hansen LA, Masliah E, Gage FH (2017) Hippocampal alpha-synuclein in dementia with Lewy bodies contributes to memory impairment and is consistent with spread of pathology. J Neurosci 37:1675–1684
Adler CH, Caviness JN, Sabbagh MN, Shill HA, Connor DJ, Sue L, Evidente VG, Driver-Dunckley E, Beach TG (2010a) Heterogeneous neuropathological findings in Parkinson’s disease with mild cognitive impairment. Acta Neuropathol 120:827–828
Adler CH, Connor DJ, Hentz JG, Sabbagh MN, Caviness JN, Shill HA, Noble B, Beach TG (2010b) Incidental Lewy body disease: clinical comparison to a control cohort. Mov Disord 25:642–646
Adler CH, Dugger BN, Hentz JG, Hinni ML, Lott DG, Driver-Dunckley E, Mehta S, Serrano G, Sue LI, Duffy A, Intorcia A, Filon J, Pullen J, Walker DG, Beach TG (2016) Peripheral synucleinopathy in early Parkinson’s disease: submandibular gland needle biopsy findings. Mov Disord 31:250–256
Agarwal S, Gilbert R (2019) Progressive supranuclear palsy, 2018/09/26 edn. StatPearls Publishing, Treasure Island
Ahmed Z, Josephs KA, Gonzalez J, DelleDonne A, Dickson DW (2008) Clinical and neuropathologic features of progressive supranuclear palsy with severe pallido-nigro-luysial degeneration and axonal dystrophy. Brain 131:460–472
Ahmed Z, Asi YT, Sailer A, Lees AJ, Houlden H, Revesz T, Holton JL (2012) The neuropathology, pathophysiology and genetics of multiple system atrophy. Neuropathol Appl Neurobiol 38:4–24
Alafuzoff I, Hartikainen P (2017) Alpha-synucleinopathies. In: Kovacs GG, Alafuzoff I (eds) Handbook of clinical neurology. Neuropathology, vol 145. Elsevier B.V., New York, pp 339–353
Alafuzoff I, Ince PG, Arzberger T, Al-Sarraj S, Bell J, Bodi I, Bogdanovic N, Bugiani O, Ferrer I, Gelpi E, Gentleman S, Giaccone G, Ironside JW, Kavantzas N, King A, Korkolopoulou P, Kovacs GG, Meyronet D, Monoranu C, Parchi P, Parkkinen L, Patsouris E, Roggendorf W, Rozemuller A, Stadelmann-Nessler C, Streichenberger N, Thal DR, Kretzschmar H (2009) Staging/typing of Lewy body related alpha-synuclein pathology: a study of the BrainNet Europe Consortium. Acta Neuropathol 117:635–652
Alberico SL, Cassell MD, Narayanan NS (2015) The vulnerable ventral tegmental area in Parkinson’s disease. Basal Ganglia 5:51–55
Albrecht F, Bisenius S, Morales Schaack R, Neumann J, Schroeter ML (2017) Disentangling the neural correlates of corticobasal syndrome and corticobasal degeneration with systematic and quantitative ALE meta-analyses. NPJ Parkinsons Dis 3:12
Al-Chalabi A, Durr A, Wood NW, Parkinson MH, Camuzat A, Hulot JS, Morrison KE, Renton A, Sussmuth SD, Landwehrmeyer BG, Ludolph A, Agid Y, Brice A, Leigh PN, Bensimon G (2009) Genetic variants of the alpha-synuclein gene SNCA are associated with multiple system atrophy. PLoS One 4:e7114. https://doi.org/10.1371/journal.pone.0007114
Alecu I, Bennett SAL (2019) Dysregulated lipid metabolism and its role in alpha-synucleinopathy in Parkinson’s disease. Front Neurosci 13:328
Alegre-Abarrategui J, Brimblecombe KR, Roberts RF, Velentza-Almpani E, Tilley BS, Bengoa-Vergniory N, Proukakis C (2019) Selective vulnerability in alpha-synucleinopathies. Acta Neuropathol. https://doi.org/10.1007/s00401-00019-02010-00402
Alexander SK, Rittman T, Xuereb JH, Bak TH, Hodges JR, Rowe JB (2014) Validation of the new consensus criteria for the diagnosis of corticobasal degeneration. J Neurol Neurosurg Psychiatry 85:925–929
Alexandris A, Walker L, Liu AKL, McAleese KE, Johnson M, Pearce RK, Gentleman SM, Attems J (2019) Cholinergic deficits and galaninergic hyperinnervation of the nucleus basalis of Meynert in Alzheimer’s disease and Lewy body disorders. Neuropathol Appl Neurobiol (accepted article)
Ali F, Josephs K (2018a) The diagnosis of progressive supranuclear palsy: current opinions and challenges. Expert Rev Neurother 18:603–616
Ali F, Josephs KA (2018b) Corticobasal degeneration: key emerging issues. J Neurol 265:439–445
Ali F, Martin PR, Botha H, Ahlskog JE, Bower JH, Masumoto JY, Maraganore D, Hassan A, Eggers S, Boeve BF, Knopman DS, Drubach D, Petersen RC, Dunkley ED, van Gerpen J, Uitti R, Whitwell JL, Dickson DW, Josephs KA (2019) Sensitivity and specificity of diagnostic criteria for progressive supranuclear palsy. Mov Disord. https://doi.org/10.1002/mds.27619
Allen M, Burgess JD, Ballard T, Serie D, Wang X, Younkin CS, Sun Z, Kouri N, Baheti S, Wang C, Carrasquillo MM, Nguyen T, Lincoln S, Malphrus K, Murray M, Golde TE, Price ND, Younkin SG, Schellenberg GD, Asmann Y, Ordog T, Crook J, Dickson D, Ertekin-Taner N (2016) Gene expression, methylation and neuropathology correlations at progressive supranuclear palsy risk loci. Acta Neuropathol 132:197–211
Allen M, Wang X, Serie DJ, Strickland SL, Burgess JD, Koga S, Younkin CS, Nguyen TT, Malphrus KG, Lincoln SJ, Alamprese M, Zhu K, Chang R, Carrasquillo MM, Kouri N, Murray ME, Reddy JS, Funk C, Price ND, Golde TE, Younkin SG, Asmann YW, Crook JE, Dickson DW, Ertekin-Taner N (2018) Divergent brain gene expression patterns associate with distinct cell-specific tau neuropathology traits in progressive supranuclear palsy. Acta Neuropathol 136:709–727
American P, Association, Force D-T (2013) Diagnostic and statistical manual of mental disorders: DSM–5, 5th edn. American Psychiatric Publishing Inc., Arlington
Ammal Kaidery N, Thomas B (2018) Current perspective of mitochondrial biology in Parkinson’s disease. Neurochem Int 117:91–113
Angot E, Steiner JA, Lema Tome CM, Ekstrom P, Mattsson B, Bjorklund A, Brundin P (2012) Alpha-synuclein cell-to-cell transfer and seeding in grafted dopaminergic neurons In vivo. PLoS One 7:e39465
Ansari M, Adib Moradi S, Ghazi Sherbaf F, Hedayatnia A, Aarabi MH (2019) Comparison of structural connectivity in Parkinson’s disease with depressive symptoms versus non-depressed: a diffusion MRI connectometry study. Int Psychogeriatr 31:5–12
Aoki N, Boyer PJ, Lund C, Lin WL, Koga S, Ross OA, Weiner M, Lipton A, Powers JM, White CL 3rd, Dickson DW (2015) Atypical multiple system atrophy is a new subtype of frontotemporal lobar degeneration: frontotemporal lobar degeneration associated with alpha-synuclein. Acta Neuropathol 130:93–105
Arawaka S, Sato H, Sasaki A, Koyama S, Kato T (2017) Mechanisms underlying extensive Ser129-phosphorylation in alpha-synuclein aggregates. Acta Neuropathol Commun 5:48
Arima K (2006) Ultrastructural characteristics of tau filaments in tauopathies: immuno-electron microscopic demonstration of tau filaments in tauopathies. Neuropathology 26:475–483
Armstrong RA (2013) White matter pathology in progressive supranuclear palsy (PSP): a quantitative study of 8 cases. Clin Neuropathol 32:399–405
Armstrong MJ (2018) Progressive supranuclear palsy: an update. Curr Neurol Neurosci Rep 18:12
Armstrong MJ (2019) Lewy body dementias. Continuum (Minneap Minn) 25:128–146
Armstrong MJ, Litvan I, Lang AE, Bak TH, Bhatia KP, Borroni B, Boxer AL, Dickson DW, Grossman M, Hallett M, Josephs KA, Kertesz A, Lee SE, Miller BL, Reich SG, Riley DE, Tolosa E, Troster AI, Vidailhet M, Weiner WJ (2013) Criteria for the diagnosis of corticobasal degeneration. Neurology 80:496–503
Armstrong RA, Kotzbauer PT, Perlmutter JS, Campbell MC, Hurth KM, Schmidt RE, Cairns NJ (2014) A quantitative study of alpha-synuclein pathology in fifteen cases of dementia associated with Parkinson disease. J Neural Transm 121:171–181
Arnaoutoglou NA, O’Brien JT, Underwood BR (2019) Dementia with Lewy bodies—from scientific knowledge to clinical insights. Nat Rev Neurol 15:103–112
Aroso M, Ferreira R, Freitas A, Vitorino R, Gomez-Lazaro M (2016) New insights on the mitochondrial proteome plasticity in Parkinson’s disease. Proteom Clin Appl 10:416–429
Asi YT, Ling H, Ahmed Z, Lees AJ, Revesz T, Holton JL (2014) Neuropathological features of multiple system atrophy with cognitive impairment. Mov Disord 29:884–888
Atias M, Tevet Y, Sun J, Stavsky A, Tal S, Kahn J, Roy S, Gitler D (2019) Synapsins regulate alpha-synuclein functions. Proc Natl Acad Sci USA. https://doi.org/10.1073/pnas.1903054116
Attems J, Jellinger KA (2008) The dorsal motor nucleus of the vagus is not an obligatory trigger site of Parkinson’s disease. Neuropathol Appl Neurobiol 34:466–467
Attems J, Walker L, Jellinger KA (2014) Olfactory bulb involvement in neurodegenerative diseases. Acta Neuropathol 127:459–475
Ayers JI, Giasson BI, Borchelt DR (2018) Prion-like spreading in tauopathies. Biol Psychiatry 83:337–346
Bachhuber T, Katzmarski N, McCarter JF, Loreth D, Tahirovic S, Kamp F, Abou-Ajram C, Nuscher B, Serrano-Pozo A, Muller A, Prinz M, Steiner H, Hyman BT, Haass C, Meyer-Luehmann M (2015) Inhibition of amyloid-beta plaque formation by alpha-synuclein. Nat Med 21:802–807
Bae EJ, Yang NY, Song M, Lee CS, Lee JS, Jung BC, Lee HJ, Kim S, Masliah E, Sardi SP, Lee SJ (2014) Glucocerebrosidase depletion enhances cell-to-cell transmission of alpha-synuclein. Nat Commun 5:4755
Bagetta V, Ghiglieri V, Sgobio C, Calabresi P, Picconi B (2010) Synaptic dysfunction in Parkinson’s disease. Biochem Soc Trans 38:493–497
Bailey RM, Covy JP, Melrose HL, Rousseau L, Watkinson R, Knight J, Miles S, Farrer MJ, Dickson DW, Giasson BI, Lewis J (2013) LRRK2 phosphorylates novel tau epitopes and promotes tauopathy. Acta Neuropathol 126:809–827
Balestrino R, Schapira AHV (2018) Glucocerebrosidase and Parkinson disease: molecular, clinical, and therapeutic implications. Neuroscientist 24:540–559
Ballanger B, Klinger H, Eche J, Lerond J, Vallet AE, Le Bars D, Tremblay L, Sgambato-Faure V, Broussolle E, Thobois S (2012) Role of serotonergic 1A receptor dysfunction in depression associated with Parkinson’s disease. Mov Disord 27:84–89
Bandres-Ciga S, Saez-Atienzar S, Bonet-Ponce L, Billingsley K, Vitale D, Blauwendraat C, Gibbs JR, Pihlstrom L, Gan-Or Z, Cookson MR, Nalls MA, Singleton AB (2019) The endocytic membrane trafficking pathway plays a major role in the risk of Parkinson’s disease. Mov Disord 34:460–468
Barca E, Kleiner G, Tang G, Ziosi M, Tadesse S, Masliah E, Louis ED, Faust P, Kang UJ, Torres J, Cortes EP, Vonsattel JP, Kuo SH, Quinzii CM (2016) Decreased coenzyme q10 levels in multiple system atrophy cerebellum. J Neuropathol Exp Neurol 75:663–672
Bassil F, Guerin PA, Dutheil N, Li Q, Klugmann M, Meissner WG, Bezard E, Fernagut PO (2017) Viral-mediated oligodendroglial alpha-synuclein expression models multiple system atrophy. Mov Disord 32:1230–1239
Batla A, De Pablo-Fernandez E, Erro R, Reich M, Calandra-Buonaura G, Barbosa P, Balint B, Ling H, Islam S, Cortelli P, Volkmann J, Quinn N, Holton JL, Warner TT, Bhatia KP (2018) Young-onset multiple system atrophy: clinical and pathological features. Mov Disord 33:1099–1107
Beach TG, Adler CH, Sue LI, Peirce JB, Bachalakuri J, Dalsing-Hernandez JE, Lue LF, Caviness JN, Connor DJ, Sabbagh MN, Walker DG (2008) Reduced striatal tyrosine hydroxylase in incidental Lewy body disease. Acta Neuropathol 115:445–451
Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, Sasse J, Boyer S, Shirohi S, Brooks R, Eschbacher J, White CL 3rd, Akiyama H, Caviness J, Shill HA, Connor DJ, Sabbagh MN, Walker DG (2009) Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta Neuropathol 117:613–634
Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, Akiyama H, Caviness JN, Shill HA, Sabbagh MN, Walker DG (2010) Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119:689–702
Beck G, Singh A, Papa SM (2018) Dysregulation of striatal projection neurons in Parkinson’s disease. J Neural Transm (Vienna) 125:449–460
Bengoa-Vergniory N, Roberts RF, Wade-Martins R, Alegre-Abarrategui J (2017) Alpha-Synuclein oligomers: a new hope. Acta Neuropathol 134:819–838
Benskey MJ, Perez RG, Manfredsson FP (2016) The contribution of alpha synuclein to neuronal survival and function—implications for Parkinson’s disease. J Neurochem 137:331–359
Berciano J, Valldeoriola F, Ferrer I, Rumia J, Pascual J, Marin C, Rey MJ, Tolosa E (2002) Presynaptic parkinsonism in multiple system atrophy mimicking Parkinson’s disease: a clinicopathological case study. Mov Disord 17:812–816
Bereczki E, Francis PT, Howlett D, Pereira JB, Hoglund K, Bogstedt A, Cedazo-Minguez A, Baek JH, Hortobagyi T, Attems J, Ballard C, Aarsland D (2016) Synaptic proteins predict cognitive decline in Alzheimer’s disease and Lewy body dementia. Alzheimers Dement 12:1149–1158
Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T, Goetz CG, Halliday GM, Hardy J, Lang AE, Litvan I, Marek K, Obeso J, Oertel W, Olanow CW, Poewe W, Stern M, Deuschl G (2014) Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease. Mov Disord 29:454–462
Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F (1973) Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci 20:415–455
Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, Raethjen J, Stamelou M, Testa CM, Deuschl G (2018) Consensus Statement on the classification of tremors from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord 33:75–87
Bhattacharjee P, Ohrfelt A, Lashley T, Blennow K, Brinkmalm A, Zetterberg H (2019) Mass spectrometric analysis of Lewy body-enriched alpha-synuclein in Prkinson’s disease. J Proteome Res 18:2109–2120
Bieri G, Brahic M, Bousset L, Couthouis J, Kramer NJ, Ma R, Nakayama L, Monbureau M, Defensor E, Schule B, Shamloo M, Melki R, Gitler AD (2019) LRRK2 modifies alpha-syn pathology and spread in mouse models and human neurons. Acta Neuropathol 137:961–980
Birdi S, Rajput AH, Fenton M, Donat JR, Rozdilsky B, Robinson C, Macaulay R, George D (2002) Progressive supranuclear palsy diagnosis and confounding features: report on 16 autopsied cases. Mov Disord 17:1255–1264
Bitan G, Dutta S, Del Rosario I, Paul K, Palma JA, Perlman SL, Poon WW, Kaufmann H, Fogel BL, Bronstein JM, Ritz B (2019) Alpha-synuclein in brain-derived exosomes distinguishes Parkinson’s disease from multiple system atrophy (abstr.). AD/PD Conference 2019, Lisbon 14th Int. Conf. on Alzheimer’s & Parkinson’s Disease, March 26–31, 2019, Lisbon. https://cmoffice.kenes.com/cmsearchableprogrammeV15/conferencemanager/programme/personid/anonymous/abstractdetails/0000253720
Bizzarri JV, Giupponi G, Maniscalco I, Schroffenegger P, Conca A, Kapfhammer HP (2015) Parkinson’s disease and psychoses. Neuropsychiatry 29:1–13
Blandini F, Cilia R, Cerri S, Pezzoli G, Schapira AHV, Mullin S, Lanciego JL (2018) Glucocerebrosidase mutations and synucleinopathies: toward a model of precision medicine. Mov Disord 34:9–21
Blauwendraat C, Heilbron K, Vallerga CL, Bandres-Ciga S, von Coelln R, Pihlstrom L, Simon-Sanchez J, Schulte C, Sharma M, Krohn L, Siitonen A, Iwaki H, Leonard H, Noyce AJ, Tan M, Gibbs JR, Hernandez DG, Scholz SW, Jankovic J, Shulman LM, Lesage S, Corvol JC, Brice A, van Hilten JJ, Marinus J, Eerola-Rautio J, Tienari P, Majamaa K, Toft M, Grosset DG, Gasser T, Heutink P, Shulman JM, Wood N, Hardy J, Morris HR, Hinds DA, Gratten J, Visscher PM, Gan-Or Z, Nalls MA, Singleton AB (2019) Parkinson’s disease age at onset genome-wide association study: defining heritability, genetic loci, and alpha-synuclein mechanisms. Mov Disord. https://doi.org/10.1002/mds.27659
Bleasel JM, Wong JH, Halliday GM, Kim WS (2014) Lipid dysfunction and pathogenesis of multiple system atrophy. Acta Neuropathol Commun 2:15
Bleasel JM, Halliday GM, Kim WS (2016) Animal modeling an oligodendrogliopathy–multiple system atrophy. Acta Neuropathol Commun 4:12
Bodea LG, Eckert A, Ittner LM, Piguet O, Gotz J (2016) Tau physiology and pathomechanisms in frontotemporal lobar degeneration. J Neurochem 138(Suppl 1):71–94
Bohnen NI, Albin RL, Muller ML, Petrou M, Kotagal V, Koeppe RA, Scott PJ, Frey KA (2015) Frequency of cholinergic and caudate nucleus dopaminergic deficits across the predemented cognitive spectrum of Parkinson disease and evidence of interaction effects. JAMA Neurol 72:194–200
Bohnen NI, Muller M, Frey KA (2017) Molecular imaging and updated diagnostic criteria in Lewy body dementias. Curr Neurol Neurosci Rep 17:73
Bohnen NI, Kanel P, Zhou Z, Koeppe RA, Frey KA, Dauer WT, Albin RL, Müller MLTM (2019) Cholinergic system changes of falls and freezing of gait in Parkinson disease. Ann Neurol 85:538–549
Book A, Guella I, Candido T, Brice A, Hattori N, Jeon B, Farrer MJ (2018) A meta-analysis of alpha-synuclein multiplication in familial parkinsonism. Front Neurol 9:1021
Bordia T, Perez XA (2019) Cholinergic control of striatal neurons to modulate l-dopa-induced dyskinesias. Eur J Neurosci 49:859–868
Borroto-Escuela DO, Perez De La Mora M, Manger P, Narvaez M, Beggiato S, Crespo-Ramirez M, Navarro G, Wydra K, Diaz-Cabiale Z, Rivera A, Ferraro L, Tanganelli S, Filip M, Franco R, Fuxe K (2018) Brain dopamine transmission in health and Parkinson’s disease: modulation of synaptic transmission and plasticity through volume transmission and dopamine heteroreceptors. Front Synaptic Neurosci 10:20
Bostan AC, Strick PL (2018) The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 19:338–350
Botsford E, George J, Buckley EE (2018) Parkinson’s disease and metal storage disorders: a systematic review. Brain Sci 8:194. https://doi.org/10.3390/brainsci8110194
Bourdenx M, Koulakiotis NS, Sanoudou D, Bezard E, Dehay B, Tsarbopoulos A (2017) Protein aggregation and neurodegeneration in prototypical neurodegenerative diseases: examples of amyloidopathies, tauopathies and synucleinopathies. Prog Neurobiol 155:171–193
Bove C, Travagli RA (2019) Neurophysiology of the brain stem in Parkinson’s disease. J Neurophysiol 121:1856–1864
Braak H, Del Tredici K (2008) Nervous system pathology in sporadic Parkinson disease. Neurology 70:1916–1925
Braak H, Del Tredici K (2009) Neuroanatomy and pathology of sporadic Parkinson’s disease. Adv Anat Embryol Cell Biol 201:1–119
Braak H, Del Tredici K (2017) Neuropathological staging of brain pathology in sporadic Parkinson’s disease: separating the wheat from the chaff. J Parkinsons Dis 7:S71–S85
Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K (2006) Stanley Fahn Lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson’s disease reconsidered. Mov Disord 21:2042–2051
Breen DP, Halliday GM, Lang AE (2019) Gut-brain axis and the spread of alpha-synuclein pathology: Vagal highway or dead end? Mov Disord 34:307–316
Brettschneider J, Irwin DJ, Boluda S, Byrne MD, Fang L, Lee EB, Robinson JL, Suh E, Van Deerlin VM, Toledo JB, Grossman M, Hurtig H, Dengler R, Petri S, Lee VM, Trojanowski JQ (2017) Progression of alpha-synuclein pathology in multiple system atrophy of the cerebellar type. Neuropathol Appl Neurobiol 43:315–329
Brettschneider J, Suh E, Robinson JL, Fang L, Lee EB, Irwin DJ, Grossman M, Van Deerlin VM, Lee VM, Trojanowski JQ (2018) Converging patterns of alpha-synuclein pathology in multiple system atrophy. J Neuropathol Exp Neurol 77:1005–1016
Bridi JC, Hirth F (2018) Mechanisms of alpha-synuclein induced synaptopathy in Parkinson’s disease. Front Neurosci 12:80
Bringmann G, God R, Feineis D, Wesemann W, Riederer P, Rausch WD, Reichmann H, Sontag KH (1995) The TaClo concept: 1-trichloromethyl-1,2,3,4-tetrahydro-beta-carboline (TaClo), a new toxin for dopaminergic neurons. J Neural Transm Suppl 46:235–244
Brockmann K, Srulijes K, Pflederer S, Hauser AK, Schulte C, Maetzler W, Gasser T, Berg D (2015) GBA-associated Parkinson’s disease: reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord 30:407–411
Brooks JA, Houlden H, Melchers A, Islam AJ, Ding J, Li A, Paudel R, Revesz T, Holton JL, Wood N, Lees A, Singleton AB, Scholz SW (2011) Mutational analysis of parkin and PINK1 in multiple system atrophy. Neurobiol Aging 32(548):e545–e547
Brück D, Wenning GK, Stefanova N, Fellner L (2016) Glia and alpha-synuclein in neurodegeneration: A complex interaction. Neurobiol Dis 85:262–274
Brudek T, Winge K, Rasmussen NB, Bahl JM, Tanassi J, Agander TK, Hyde TM, Pakkenberg B (2016) Altered alpha-synuclein, parkin, and synphilin isoform levels in multiple system atrophy brains. J Neurochem 136:172–185
Brundin P, Melki R, Kopito R (2010) Prion-like transmission of protein aggregates in neurodegenerative diseases. Nat Rev Mol Cell Biol 11:301–307
Bu J, Liu J, Liu K, Wang Z (2019) Diagnostic utility of gut alpha-synuclein in Parkinson’s disease: a systematic review and meta-analysis. Behav Brain Res 364:340–347
Buchman AS, Nag S, Leurgans SE, Miller J, VanderHorst V, Bennett DA, Schneider JA (2018) Spinal Lewy body pathology in older adults without an antemortem diagnosis of Parkinson’s disease. Brain Pathol 28:560–568
Burke RE, Dauer WT, Vonsattel JP (2008) A critical evaluation of the Braak staging scheme for Parkinson’s disease. Ann Neurol 64:485–491
Burre J, Sharma M, Sudhof TC (2018) Cell biology and pathophysiology of alpha-synuclein. Cold Spring Harb Perspect Med. https://doi.org/10.1101/cshperspect.a024091
Cairns NJ, Perrin RJ, Franklin EE, Carter D, Vincent B, Xie M, Bateman RJ, Benzinger T, Friedrichsen K, Brooks WS, Halliday GM, McLean C, Ghetti B, Morris JC (2015) Neuropathologic assessment of participants in two multi-center longitudinal observational studies: the Alzheimer Disease Neuroimaging Initiative (ADNI) and the Dominantly Inherited Alzheimer Network (DIAN). Neuropathology 35:390–400
Calabresi P, Mercuri NB, Di Filippo M (2009) Synaptic plasticity, dopamine and Parkinson’s disease: one step ahead. Brain 132:285–287
Calafate S, Buist A, Miskiewicz K, Vijayan V, Daneels G, de Strooper B, de Wit J, Verstreken P, Moechars D (2015) Synaptic contacts enhance cell-to-cell tau pathology propagation. Cell Rep 11:1176–1183
Callizot N, Combes M, Henriques A, Poindron P (2019) Necrosis, apoptosis, necroptosis, three modes of action of dopaminergic neuron neurotoxins. PLoS One 14:e0215277
Calo L, Wegrzynowicz M, Santivanez-Perez J, Grazia Spillantini M (2016) Synaptic failure and alpha-synuclein. Mov Disord 31:169–177
Caminiti SP, Sala A, Iaccarino L, Beretta L, Pilotto A, Gianolli L, Iannaccone S, Magnani G, Padovani A, Ferini-Strambi L, Perani D (2019) Brain glucose metabolism in Lewy body dementia: implications for diagnostic criteria. Alzheimers Res Ther 11:20
Campbell BC, McLean CA, Culvenor JG, Gai WP, Blumbergs PC, Jakala P, Beyreuther K, Masters CL, Li QX (2001) The solubility of alpha-synuclein in multiple system atrophy differs from that of dementia with Lewy bodies and Parkinson’s disease. J Neurochem 76:87–96
Candelise N, Schmitz M, Llorens F, Villar-Pique A, Cramm M, Thom T, da Silva Correia SM, Gomes Eriton, da Cunha J, Mobius W, Outeiro TF, Alvarez VG, Banchelli M, D’Andrea C, de Angelis M, Zafar S, Rabano A, Matteini P, Zerr I (2019) Seeding variability of different alpha synuclein strains in synucleinopathies. Ann Neurol 85:691–703
Canerina-Amaro A, Pereda D, Diaz M, Rodriguez-Barreto D, Casanas-Sanchez V, Heffer M, Garcia-Esparcia P, Ferrer I, Puertas-Avendano R, Marin R (2019) Differential aggregation and phosphorylation of alpha synuclein in membrane compartments associated with Parkinson disease. Front Neurosci 13:382
Cazorla M, Kang UJ, Kellendonk C (2015) Balancing the basal ganglia circuitry: a possible new role for dopamine D2 receptors in health and disease. Mov Disord 30:895–903
Cenci MA, Crossman AR (2018) Animal models of l-dopa-induced dyskinesia in Parkinson’s disease. Mov Disord 33:889–899
Cenci MA, Jorntell H, Petersson P (2018) On the neuronal circuitry mediating l-DOPA-induced dyskinesia. J Neural Transm (Vienna) 125:1157–1169
Cersosimo MG (2018) Propagation of alpha-synuclein pathology from the olfactory bulb: possible role in the pathogenesis of dementia with Lewy bodies. Cell Tissue Res 373:233–243
Cersosimo MG, Benarroch EE (2012a) Autonomic involvement in Parkinson’s disease: pathology, pathophysiology, clinical features and possible peripheral biomarkers. J Neurol Sci 313:57–63
Cersosimo MG, Benarroch EE (2012b) Pathological correlates of gastrointestinal dysfunction in Parkinson’s disease. Neurobiol Dis 46:559–564
Chang A, Fox SH (2016) Psychosis in parkinson’s disease: epidemiology, pathophysiology, and management. Drugs 76:1093–1118
Chang D, Nalls MA, Hallgrimsdottir IB, Hunkapiller J, van der Brug M, Cai F, Kerchner GA, Ayalon G, Bingol B, Sheng M, Hinds D, Behrens TW, Singleton AB, Bhangale TR, Graham RR (2017) A meta-analysis of genome-wide association studies identifies 17 new Parkinson’s disease risk loci. Nat Genet 49:1511–1516
Chartier S, Duyckaerts C (2018) Is Lewy pathology in the human nervous system chiefly an indicator of neuronal protection or of toxicity? Cell Tissue Res 373:149–160
Chavarria C, Rodriguez-Bottero S, Quijano C, Cassina P, Souza JM (2018) Impact of monomeric, oligomeric and fibrillar alpha-synuclein on astrocyte reactivity and toxicity to neurons. Biochem J 475:3153–3169
Chelban V, Manole A, Pihlstrom L, Schottlaender L, Efthymiou S, Oconnor E, Meissner WG, Holton JL, Houlden H (2017) Analysis of the prion protein gene in multiple system atrophy. Neurobiol Aging 49:216 e15–216 e18
Chen YF, Tseng YL, Lan MY, Lai SL, Su CS, Liu JS, Chang YY (2014) The relationship of leukoaraiosis and the clinical severity of vascular parkinsonism. J Neurol Sci 346:255–259
Chen ZC, Zhang W, Chua LL, Chai C, Li R, Lin L, Cao Z, Angeles DC, Stanton LW, Peng JH, Zhou ZD, Lim KL, Zeng L, Tan EK (2017) Phosphorylation of amyloid precursor protein by mutant LRRK2 promotes AICD activity and neurotoxicity in Parkinson’s disease. Sci Signal. https://doi.org/10.1126/scisignal.aam6790
Chen C, Turnbull DM, Reeve AK (2019a) Mitochondrial dysfunction in Parkinson’s disease-cause or consequence? Biology (Basel) 8:38
Chen Z, Chen JA, Shatunov A, Jones AR, Kravitz SN, Huang AY, Lawrence L, Lowe JK, Lewis CM, Payan CAM, Lieb W, Franke A, Deloukas P, Amouyel P, Tzourio C, Dartigues JF, Ludolph A, Bensimon G, Leigh PN, Bronstein JM, Coppola G, Geschwind DH, Al-Chalabi A (2019b) Genome-wide survey of copy number variants finds MAPT duplications in progressive supranuclear palsy. Mov Disord
Cheng HC, Ulane CM, Burke RE (2010) Clinical progression in Parkinson disease and the neurobiology of axons. Ann Neurol 67:715–725
Chiu CC, Lu CS, Weng YH, Chen YL, Huang YZ, Chen RS, Cheng YC, Huang YC, Liu YC, Lai SC, Lin KJ, Lin YW, Chen YJ, Chen CL, Yeh TH, Wang HL (2019) PARK14 (D331Y) PLA2G6 causes early-onset degeneration of substantia nigra dopaminergic neurons by inducing mitochondrial dysfunction, er stress, mitophagy impairment and transcriptional dysregulation in a knockin mouse model. Mol Neurobiol 56:3835–3853
Christopher L, Duff-Canning S, Koshimori Y, Segura B, Boileau I, Chen R, Lang AE, Houle S, Rusjan P, Strafella AP (2015) Salience network and parahippocampal dopamine dysfunction in memory-impaired Parkinson disease. Ann Neurol 77:269–280
Chu Y, Morfini GA, Langhamer LB, He Y, Brady ST, Kordower JH (2012) Alterations in axonal transport motor proteins in sporadic and experimental Parkinson’s disease. Brain 135:2058–2073
Chu HY, Atherton JF, Wokosin D, Surmeier DJ, Bevan MD (2015) Heterosynaptic regulation of external globus pallidus inputs to the subthalamic nucleus by the motor cortex. Neuron 85:364–376
Clavaguera F, Akatsu H, Fraser G, Crowther RA, Frank S, Hench J, Probst A, Winkler DT, Reichwald J, Staufenbiel M, Ghetti B, Goedert M, Tolnay M (2013) Brain homogenates from human tauopathies induce tau inclusions in mouse brain. Proc Natl Acad Sci USA 110:9535–9540
Colloby SJ, McParland S, O’Brien JT, Attems J (2012) Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain 135:2798–2808
Colom-Cadena M, Gelpi E, Charif S, Belbin O, Blesa R, Marti MJ, Clarimon J, Lleo A (2013a) Confluence of alpha-synuclein, tau, and beta-amyloid pathologies in dementia with Lewy bodies. J Neuropathol Exp Neurol 72:1203–1212
Colom-Cadena M, Gelpi E, Marti MJ, Charif S, Dols-Icardo O, Blesa R, Clarimon J, Lleo A (2013b) MAPT H1 haplotype is associated with enhanced alpha-synuclein deposition in dementia with Lewy bodies. Neurobiol Aging 34:936–942
Colom-Cadena M, Grau-Rivera O, Planellas L, Cerquera C, Morenas E, Helgueta S, Munoz L, Kulisevsky J, Marti MJ, Tolosa E, Clarimon J, Lleo A, Gelpi E (2017a) Regional overlap of pathologies in Lewy body disorders. J Neuropathol Exp Neurol 76:216–224
Colom-Cadena M, Pegueroles J, Herrmann AG, Henstridge CM, Munoz L, Querol-Vilaseca M, Martin-Paniello CS, Luque-Cabecerans J, Clarimon J, Belbin O, Nunez-Llaves R, Blesa R, Smith C, McKenzie CA, Frosch MP, Roe A, Fortea J, Andilla J, Loza-Alvarez P, Gelpi E, Hyman BT, Spires-Jones TL, Lleo A (2017b) Synaptic phosphorylated alpha-synuclein in dementia with Lewy bodies. Brain 140:3204–3214
Coon EA, Cutsforth-Gregory JK, Benarroch EE (2018) Neuropathology of autonomic dysfunction in synucleinopathies. Mov Disord 33:349–358
Coughlin D, Xie SX, Liang M, Williams A, Peterson C, Weintraub D, McMillan CT, Wolk DA, Akhtar RS, Hurtig HI, Branch Coslett H, Hamilton RH, Siderowf AD, Duda JE, Rascovsky K, Lee EB, Lee VM, Grossman M, Trojanowski JQ, Irwin DJ (2018) Cognitive and pathological influences of tau pathology in Lewy body disorders. Ann Neurol 85:259–271
Coughlin DG, Petrovitch H, White LR, Noorigian JV, Masaki K, Webster RG, Duda JE (2019) Most cases with Lewy pathology in a population-based cohort adhere to the Braak progression pattern but ‘failure to fit’ is highly dependent on staging system applied. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2019.1003.1023
Cousins O, Yousaf T, Wilson H, Pagano G, Politis M (2019) Molecular imaging of dementia with Lewy bodies. Int Rev Neurobiol 144:59–93
Covell DJ, Robinson JL, Akhtar RS, Grossman M, Weintraub D, Bucklin HM, Pitkin RM, Riddle D, Yousef A, Trojanowski JQ, Lee VM (2017) Novel conformation-selective alpha-synuclein antibodies raised against different in vitro fibril forms show distinct patterns of Lewy pathology in Parkinson’s disease. Neuropathol Appl Neurobiol 43:604–620
Coyle-Gilchrist IT, Dick KM, Patterson K, Vazquez Rodriquez P, Wehmann E, Wilcox A, Lansdall CJ, Dawson KE, Wiggins J, Mead S, Brayne C, Rowe JB (2016) Prevalence, characteristics, and survival of frontotemporal lobar degeneration syndromes. Neurology 86:1736–1743
Critchley M (1981) Arteriosclerotic pseudoparkinsonism. In: Rose FC, Capildeo R (eds) Research Progress in Parkinson’s disease. Pitman, London
Cykowski MD, Coon EA, Powell SZ, Jenkins SM, Benarroch EE, Low PA, Schmeichel AM, Parisi JE (2015) Expanding the spectrum of neuronal pathology in multiple system atrophy. Brain 138:2293–2309
Daher JP (2017) Interaction of LRRK2 and alpha-synuclein in Parkinson’s disease. Adv Neurobiol 14:209–226
Dale GE, Probst A, Luthert P, Martin J, Anderton BH, Leigh PN (1992) Relationships between Lewy bodies and pale bodies in Parkinson’s disease. Acta Neuropathol (Berl) 83:525–529
Das T, Eliezer D (2019) Membrane interactions of intrinsically disordered proteins: the example of alpha-synuclein. Biochim Biophys Acta Proteins Proteomics. https://doi.org/10.1016/j.bbapap.2019.1005.1001
Dash D, Pandey S (2019) Movement disorders associated with neuronal antibodies. Acta Neurol Scand 139:106–117
Davis MY, Johnson CO, Leverenz JB, Weintraub D, Trojanowski JQ, Chen-Plotkin A, Van Deerlin VM, Quinn JF, Chung KA, Peterson-Hiller AL, Rosenthal LS, Dawson TM, Albert MS, Goldman JG, Stebbins GT, Bernard B, Wszolek ZK, Ross OA, Dickson DW, Eidelberg D, Mattis PJ, Niethammer M, Yearout D, Hu SC, Cholerton BA, Smith M, Mata IF, Montine TJ, Edwards KL, Zabetian CP (2016) Association of GBA mutations and the E326K polymorphism with motor and cognitive progression in Parkinson disease. JAMA Neurol 73:1217–1224
Davis AA, Leyns CEG, Holtzman DM (2018) Intercellular spread of protein aggregates in neurodegenerative disease. Annu Rev Cell Dev Biol 34:545–568
De Cecco E, Legname G (2018) The role of the prion protein in the internalization of alpha-synuclein amyloids. Prion 12:23–27
de Iure A, Napolitano F, Beck G, Quiroga Varela A, Durante V, Sciaccaluga M, Mazzocchetti P, Megaro A, Tantucci M, Cardinale A, Punzo D, Mancini A, Costa C, Ghiglieri V, Tozzi A, Picconi B, Papa SM, Usiello A, Calabresi P (2019) Striatal spreading depolarization: Possible implication in levodopa-induced dyskinetic-like behavior. Mov Disord. https://doi.org/10.1002/mds.27632
de la Fuente-Fernandez R, Schulzer M, Kuramoto L, Cragg J, Ramachandiran N, Au WL, Mak E, McKenzie J, McCormick S, Sossi V, Ruth TJ, Lee CS, Calne DB, Stoessl AJ (2011) Age-specific progression of nigrostriatal dysfunction in Parkinson’s disease. Ann Neurol 69:803–810
De Pablo-Fernandez E, Breen DP, Bouloux PM, Barker RA, Foltynie T, Warner TT (2017) Neuroendocrine abnormalities in Parkinson’s disease. J Neurol Neurosurg Psychiatry 88:176–185
De Pablo-Fernandez E, Cerdan Santacruz D, Warner TT, Holton JL (2018) No evidence of iatrogenic human transmission in autopsy confirmed multiple system atrophy. Mov Disord 33:1183–1184
De Reuck J, Auger F, Durieux N, Cordonnier C, Maurage CA, Deramecourt V, Pasquier F, Leys D, Bordet R (2018) The impact of cerebral amyloid angiopathy in Lewy body dementia: a neuropathological study with magnetic resonance imaging correlations. J Neurodegener Dis Res 1:101
Dehay B (2014) Evidence piles up for prion-like propagation mechanisms in synucleinopathies. Mov Disord 29:187
Dehay B, Fernagut PO (2016) Alpha-synuclein-based models of Parkinson’s disease. Rev Neurol (Paris) 172:371–378
Del Rey NL, Quiroga-Varela A, Garbayo E, Carballo-Carbajal I, Fernandez-Santiago R, Monje MHG, Trigo-Damas I, Blanco-Prieto MJ, Blesa J (2018) Advances in Parkinson’s disease: 200 years later. Front Neuroanat 12:113
Del Tredici K, Braak H (2012) Spinal cord lesions in sporadic Parkinson’s disease. Acta Neuropathol 124:643–664
Del Tredici K, Braak H (2013) Dysfunction of the locus coeruleus-norepinephrine system and related circuitry in Parkinson’s disease-related dementia. J Neurol Neurosurg Psychiatry 84:774–783
Delacourte A, Robitaille Y, Sergeant N, Buee L, Hof PR, Wattez A, Laroche-Cholette A, Mathieu J, Chagnon P, Gauvreau D (1996) Specific pathological Tau protein variants characterize Pick’s disease. J Neuropathol Exp Neurol 55:159–168
DelleDonne A, Klos KJ, Fujishiro H, Ahmed Z, Parisi JE, Josephs KA, Frigerio R, Burnett M, Wszolek ZK, Uitti RJ, Ahlskog JE, Dickson DW (2008) Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol 65:1074–1080
Deramecourt V, Bombois S, Maurage CA, Ghestem A, Drobecq H, Vanmechelen E, Lebert F, Pasquier F, Delacourte A (2006) Biochemical staging of synucleinopathy and amyloid deposition in dementia with Lewy bodies. J Neuropathol Exp Neurol 65:278–288
Dettmer U, Selkoe D, Bartels T (2016) New insights into cellular alpha-synuclein homeostasis in health and disease. Curr Opin Neurobiol 36:15–22
Dhillon JS, Trejo-Lopez JA, Riffe C, Levites Y, Sacino AN, Borchelt DR, Yachnis AY, Giasson BI (2019a) Comparative analyses of the in vivo induction and transmission of alpha-synuclein pathology in transgenic mice by MSA brain lysate and recombinant alpha-synuclein fibrils. Acta Neuropathol Commun 7:80
Dhillon JS, Trejo-Lopez JA, Riffe C, McFarland NR, Hiser WM, Giasson BI, Yachnis AT (2019b) Dissecting alpha-synuclein inclusion pathology diversity in multiple system atrophy: implications for the prion-like transmission hypothesis. Lab Invest. https://doi.org/10.1038/s41374-41019-40198-41379
di Domenico A, Carola G, Calatayud C, Pons-Espinal M, Munoz JP, Richaud-Patin Y, Fernandez-Carasa I, Gut M, Faella A, Parameswaran J, Soriano J, Ferrer I, Tolosa E, Zorzano A, Cuervo AM, Raya A, Consiglio A (2019) Patient-specific IPSC-derived astrocytes contribute to non-cell-autonomous neurodegeneration in Parkinson’s disease. Stem Cell Rep 12:213–229
Dickson DW (2012) Parkinson’s disease and parkinsonism: neuropathology. Cold Spring Harb Perspect Med 2:a009258
Dickson DW (2018) Neuropathology of Parkinson disease. Parkinsonism Relat Disord 46(Suppl 1):S30–S33
Dickson DW, Fujishiro H, Delledonne A, Menke J, Ahmed Z, Klos KJ, Josephs KA, Frigerio R, Burnett M, Parisi JE, Ahlskog JE (2008) Evidence that incidental Lewy body disease is pre-symptomatic Parkinson’s disease. Acta Neuropathol 115:437–444
Dickson DW, Braak H, Duda JE, Duyckaerts C, Gasser T, Halliday GM, Hardy J, Leverenz JB, Del Tredici K, Wszolek ZK, Litvan I (2009) Neuropathological assessment of Parkinson’s disease: refining the diagnostic criteria. Lancet Neurol 8:1150–1157
Dickson DW, Ahmed Z, Algom AA, Tsuboi Y, Josephs KA (2010a) Neuropathology of variants of progressive supranuclear palsy. Curr Opin Neurol 23:394–400
Dickson DW, Uchikado H, Fujishiro H, Tsuboi Y (2010b) Evidence in favor of Braak staging of Parkinson’s disease. Mov Disord 25(Suppl 1):S78–S82
Dickson DW, Hauw JJ, Agid Y, Litvan I (2011a) Progressive supranuclear palsy and corticobasal degeneration. In: Dickson DW, Weller RO (eds) Neurodegeneration: the molecular pathology of dementia and movement disorders, 2nd edn. Blackwell Publishing Ltd., Oxford, pp 135–155
Dickson DW, Kouri N, Murray ME, Josephs KA (2011b) Neuropathology of frontotemporal lobar degeneration-tau (FTLD-tau). J Mol Neurosci 45:384–389
Dickson DW, Heckman MG, Murray ME, Soto AI, Walton RL, Diehl NN, van Gerpen JA, Uitti RJ, Wszolek ZK, Ertekin-Taner N, Knopman DS, Petersen RC, Graff-Radford NR, Boeve BF, Bu G, Ferman TJ, Ross OA (2018) APOE epsilon4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology 91:e1182–e1195
Dijkstra AA, Voorn P, Berendse HW, Groenewegen HJ, Rozemuller AJ, van de Berg WD (2014) Stage-dependent nigral neuronal loss in incidental Lewy body and Parkinson’s disease. Mov Disord 29:1244–1251
Dirkx MF, den Ouden HE, Aarts E, Timmer MH, Bloem BR, Toni I, Helmich RC (2017) Dopamine controls Parkinson’s tremor by inhibiting the cerebellar thalamus. Brain 140:721–734
Djaldetti R, Lorberboym M, Karmon Y, Treves TA, Ziv I, Melamed E (2011) Residual striatal dopaminergic nerve terminals in very long-standing Parkinson’s disease: a single photon emission computed tomography imaging study. Mov Disord 26:327–330
Dolgacheva LP, Berezhnov AV, Fedotova EI, Zinchenko VP, Abramov AY (2019) Role of DJ-1 in the mechanism of pathogenesis of Parkinson’s disease. J Bioenerg Biomembr 51:175–188
Don AS, Hsiao JH, Bleasel JM, Couttas TA, Halliday GM, Kim W (2014) Altered lipid levels provide evidence for myelin dysfunction in multiple system atrophy. Acta Neuropathol Commun 2:150
Donadio V (2018) Skin nerve alpha-synuclein deposits in Parkinson’s disease and other synucleinopathies: a review. Clin Auton Res. https://doi.org/10.1007/s10286-10018-10581-10284
Donadio V, Doppler K, Incensi A, Kuzkina A, Janzen A, Mayer G, Volkmann J, Rizzo G, Antelmi E, Plazzi G, Sommer C, Liguori R, Oertel WH (2019) Abnormal alpha-synuclein deposits in skin nerves: intra- and inter-laboratory reproducibility. Eur J Neurol. https://doi.org/10.1111/ene.13939
Donaghy PC, McKeith I (2014) The clinical characteristics of dementia with Lewy bodies and a consideration of prodromal diagnosis. Alzheimers Res Ther 6:46
Donker Kaat L, Boon AJ, Azmani A, Kamphorst W, Breteler MM, Anar B, Heutink P, van Swieten JC (2009) Familial aggregation of parkinsonism in progressive supranuclear palsy. Neurology 73:98–105
Doppler K, Weis J, Karl K, Ebert S, Ebentheuer J, Trenkwalder C, Klebe S, Volkmann J, Sommer C (2015) Distinctive distribution of phospho-alpha-synuclein in dermal nerves in multiple system atrophy. Mov Disord 30:1688–1692
Dorsey ER, Sherer T, Okun MS, Bloem BR (2018) The emerging evidence of the Parkinson pandemic. J Parkinsons Dis 8:S3–S8
Double KL, Reyes S, Werry EL, Halliday GM (2010) Selective cell death in neurodegeneration: why are some neurons spared in vulnerable regions? Prog Neurobiol 92:316–329
Driver-Dunckley ED, Zhang N, Adler CH, Serrano GE, Sue LI, Shill H, Mehta SH, Belden CM, Zamrini E, Davis K, Beach T (2019) Brain Lewy-type synucleinopathy density is associated with a lower prevalence of atherosclerotic cardiovascular disease risk factors in patients with Parkinson’s disease. J Parkinsons Dis (in print)
Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, Dickson D, Duyckaerts C, Cummings J, Gauthier S, Korczyn A, Lees A, Levy R, Litvan I, Mizuno Y, McKeith IG, Olanow CW, Poewe W, Sampaio C, Tolosa E, Emre M (2007) Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord 22:2314–2324
Duffy MF, Collier TJ, Patterson JR, Kemp CJ, Luk KC, Tansey MG, Paumier KL, Kanaan NM, Fischer DL, Polinski NK, Barth OL, Howe JW, Vaikath NN, Majbour NK, El-Agnaf OMA, Sortwell CE (2018) Lewy body-like alpha-synuclein inclusions trigger reactive microgliosis prior to nigral degeneration. J Neuroinflamm 15:129
Dugger BN, Dickson DW (2010) Cell type specific sequestration of choline acetyltransferase and tyrosine hydroxylase within Lewy bodies. Acta Neuropathol 120:633–639
Dugger BN, Hoffman BR, Scroggins A, Serrano GE, Adler CH, Shill HA, Belden CM, Sabbagh MN, Caviness JN, Driver Dunckley E, Beach TG (2018) Tau immunoreactivity in peripheral tissues of human aging and select tauopathies. Neurosci Lett 696:132–139
Durcan R, Donaghy P, Osborne C, Taylor JP, Thomas AJ (2019) Imaging in prodromal dementia with Lewy bodies: where do we stand? Int J Geriatr Psychiatry 34:635–646
Duyckaerts C, Clavaguera F, Potier MC (2019) The prion-like propagation hypothesis in Alzheimer’s and Parkinson’s disease. Curr Opin Neurol 32:266–271
Dzamko N, Gysbers AM, Bandopadhyay R, Bolliger MF, Uchino A, Zhao Y, Takao M, Wauters S, van de Berg WD, Takahashi-Fujigasaki J, Nichols RJ, Holton JL, Murayama S, Halliday GM (2017) LRRK2 levels and phosphorylation in Parkinson’s disease brain and cases with restricted Lewy bodies. Mov Disord 32:423–432
Ebashi M, Ito Y, Uematsu M, Nakamura A, Hirokawa K, Kamei S, Uchihara T (2019) How to demix Alzheimer-type and PSP-type tau lesions out of their mixture-hybrid approach to dissect comorbidity. Acta Neuropathol Commun 7:71
Ekman U, Eriksson J, Forsgren L, Mo SJ, Riklund K, Nyberg L (2012) Functional brain activity and presynaptic dopamine uptake in patients with Parkinson’s disease and mild cognitive impairment: a cross-sectional study. Lancet Neurol 11:679–687
Elder GJ, Mactier K, Colloby SJ, Watson R, Blamire AM, O’Brien JT, Taylor JP (2017) The influence of hippocampal atrophy on the cognitive phenotype of dementia with Lewy bodies. Int J Geriatr Psychiatry 32:1182–1189
Elias S, Israel Z, Bergman H (2008) Physiology of Parkinson’s disease. In: Hallett M, Poewe W (eds) Therapeutics of Parkinson’s disease and other movement disorders. Wiley-Blackwell, New York, pp 25–36
Emmanouilidou E, Vekrellis K (2016) Exocytosis and spreading of normal and aberrant alpha-synuclein. Brain Pathol 26:398–403
Emre M, Aarsland D, Brown R, Burn DJ, Duyckaerts C, Mizuno Y, Broe GA, Cummings J, Dickson DW, Gauthier S, Goldman J, Goetz C, Korczyn A, Lees A, Levy R, Litvan I, McKeith I, Olanow W, Poewe W, Quinn N, Sampaio C, Tolosa E, Dubois B (2007) Clinical diagnostic criteria for dementia associated with Parkinson’s disease. Mov Disord 22:1689–1707
Engeln M, De Deurwaerdere P, Li Q, Bezard E, Fernagut PO (2015) Widespread monoaminergic dysregulation of both motor and non-motor circuits in parkinsonism and dyskinesia. Cereb Cortex 25:2783–2792
Erro R, Picillo M, Amboni M, Savastano R, Scannapieco S, Cuoco S, Santangelo G, Vitale C, Pellecchia MT, Barone P (2019) Comparing postural instability and gait disorder and akinetic-rigid subtyping of Parkinson disease and their stability over time. Eur J Neurol 1:5. https://doi.org/10.1111/ene.13968
Erskine D, Thomas AJ, Taylor JP, Savage MA, Attems J, McKeith IG, Morris CM, Khundakar AA (2017) Neuronal loss and alpha-synuclein pathology in the superior colliculus and its relationship to visual hallucinations in dementia with Lewy bodies. Am J Geriatr Psychiatry 25:595–604
Erskine D, Ding J, Thomas AJ, Kaganovich A, Khundakar AA, Hanson PS, Taylor JP, McKeith IG, Attems J, Cookson MR, Morris CM (2018) Molecular changes in the absence of severe pathology in the pulvinar in dementia with Lewy bodies. Mov Disord 33:982–991
Espay AJ, LeWitt PA, Kaufmann H (2014) Norepinephrine deficiency in Parkinson’s disease: the case for noradrenergic enhancement. Mov Disord 29:1710–1719
Espay AJ, Vizcarra JA, Marsili L, Lang AE, Simon DK, Merola A, Josephs KA, Fasano A, Morgante F, Savica R, Greenamyre JT, Cambi F, Yamasaki TR, Tanner CM, Gan-Or Z, Litvan I, Mata IF, Zabetian CP, Brundin P, Fernandez HH, Standaert DG, Kauffman MA, Schwarzschild MA, Sardi SP, Sherer T, Perry G, Leverenz JB (2019) Revisiting protein aggregation as pathogenic in sporadic Parkinson and Alzheimer diseases. Neurology 92:329–337
Evans T, Kok WL, Cowan K, Hefford M, Anichtchik O (2018) Accumulation of beta-synuclein in cortical neurons is associated with autophagy attenuation in the brains of dementia with Lewy body patients. Brain Res 1681:1–13
Falcon B, Zhang W, Murzin AG, Murshudov G, Garringer HJ, Vidal R, Crowther RA, Ghetti B, Scheres SHW, Goedert M (2018) Structures of filaments from Pick’s disease reveal a novel tau protein fold. Nature 561:137–140
Fanciulli A, Wenning GK (2015) Multiple-system atrophy. N Engl J Med 372:249–263
Fathy YY, Jonker AJ, Oudejans E, de Jong FJJ, van Dam AW, Rozemuller AJM, van de Berg WDJ (2019) Differential insular cortex subregional vulnerability to alpha-synuclein pathology in Parkinson’s disease and dementia with Lewy bodies. Neuropathol Appl Neurobiol 45:262–277
Feany MB, Bender WW (2000) A Drosophila model of Parkinson’s disease. Nature 404:394–398
Federoff M, Price TR, Sailer A, Scholz S, Hernandez D, Nicolas A, Singleton AB, Nalls M, Houlden H (2016) Genome-wide estimate of the heritability of multiple system atrophy. Parkinsonism Relat Disord 22:35–41
Felice VD, Quigley EM, Sullivan AM, O’Keeffe GW, O’Mahony SM (2016) Microbiota-gut-brain signalling in Parkinson’s disease: Implications for non-motor symptoms. Parkinsonism Relat Disord 27:1–8
Fellner L, Jellinger KA, Wenning GK, Stefanova N (2011) Glial dysfunction in the pathogenesis of alpha-synucleinopathies: emerging concepts. Acta Neuropathol 121:675–693
Fellner L, Wenning GK, Stefanova N (2015) Models of multiple system atrophy. Curr Top Behav Neurosci 22:369–393
Fellner L, Buchinger E, Brueck D, Irschick R, Wenning GK, Stefanova N (2018) Limited effects of dysfunctional macroautophagy on the accumulation of extracellularly derived alpha-synuclein in oligodendroglia: implications for MSA pathogenesis. BMC Neurosci 19:32
Fereshtehnejad SM, Zeighami Y, Dagher A, Postuma RB (2017) Clinical criteria for subtyping Parkinson’s disease: biomarkers and longitudinal progression. Brain 140:1959–1976
Ferguson MC, Garland EM, Hedges L, Womack-Nunley B, Hamid R, Phillips JA 3rd, Shibao CA, Raj SR, Biaggioni I, Robertson D (2014) SHC2 gene copy number in multiple system atrophy (MSA). Clin Auton Res 24:25–30
Ferman TJ, Aoki N, Crook JE, Murray ME, Graff-Radford NR, van Gerpen JA, Uitti RJ, Wszolek ZK, Graff-Radford J, Pedraza O, Kantarci K, Boeve BF, Dickson DW (2018) The limbic and neocortical contribution of alpha-synuclein, tau, and amyloid beta to disease duration in dementia with Lewy bodies. Alzheimers Dement 14:330–339
Ferrari R, Kia DA, Tomkins JE, Hardy J, Wood NW, Lovering RC, Lewis PA, Manzoni C (2018) Stratification of candidate genes for Parkinson’s disease using weighted protein-protein interaction network analysis. BMC Genom 19:452
Ferreira SA, Romero-Ramos M (2018) Microglia response during Parkinson’s disease: alpha-synuclein intervention. Front Cell Neurosci 12:247
Ferrer I (2018) Oligodendrogliopathy in neurodegenerative diseases with abnormal protein aggregates: the forgotten partner. Prog Neurobiol 169:24–54
Ferrer I, Lopez-Gonzalez I, Carmona M, Arregui L, Dalfo E, Torrejon-Escribano B, Diehl R, Kovacs GG (2014) Glial and neuronal tau pathology in tauopathies: characterization of disease-specific phenotypes and tau pathology progression. J Neuropathol Exp Neurol 73:81–97
Ferrer I, Aguiló García M, Carmona M, Andrés-Benito P, Torrejón-Escribano B, Garcia-Esparcia P, del Rio JA (2019) Involvement of oligodendrocytes in tau seeding and spreading in tauopathies. Front Aging Neurosci 11:112
Ffytche DH, Creese B, Politis M, Chaudhuri KR, Weintraub D, Ballard C, Aarsland D (2017) The psychosis spectrum in Parkinson disease. Nat Rev Neurol 13:81–95
Filippini A, Gennarelli M, Russo I (2019) Alpha-synuclein and glia in Parkinson’s disease: a beneficial or a detrimental duet for the endo-lysosomal system? Cell Mol Neurobiol 39:161–168
Fitzgerald E, Murphy S, Martinson HA (2019) Alpha-synuclein pathology and the role of the microbiota in Parkinson’s disease. Front Neurosci 13:369
Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB (2013) Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain 136:2405–2418
Foguem C, Manckoundia P (2018) Lewy body disease: clinical and pathological “overlap syndrome” between synucleinopathies (Parkinson disease) and tauopathies (Alzheimer disease). Curr Neurol Neurosci Rep 18:24
Formisano R, Zasler ND (2014) Posttraumatic parkinsonism. J Head Trauma Rehabil 29:387–390
Forno LS (1996) Neuropathology of Parkinson’s disease. J Neuropathol Exp Neurol 55:259–272
Forno LS, DeLanney LE, Irwin I, Langston JW (1996) Electron microscopy of Lewy bodies in the amygdala-parahippocampal region. Comparison with inclusion bodies in the MPTP-treated squirrel monkey. Adv Neurol 69:217–228
Forrest SL, Kril JJ, Stevens CH, Kwok JB, Hallupp M, Kim WS, Huang Y, McGinley CV, Werka H, Kiernan MC, Gotz J, Spillantini MG, Hodges JR, Ittner LM, Halliday GM (2018) Retiring the term FTDP-17 as MAPT mutations are genetic forms of sporadic frontotemporal tauopathies. Brain 141:521–534
Forrest SL, Crockford DR, Sizemova A, McCann H, Shepherd CE, McGeachie AB, Affleck AJ, Carew-Jones F, Bartley L, Kwok JB, Kim WS, Jary E, Tan RH, McGinley CV, Piguet O, Hodges JR, Kril JJ, Halliday GM (2019a) Coexisting Lewy body disease and clinical parkinsonism in frontotemporal lobar degeneration. Neurology 92:e2472–e2482
Forrest SL, Halliday GM, McCann H, McGeachie AB, McGinley CV, Hodges JR, Piguet O, Kwok JB, Spillantini MG, Kril JJ (2019b) Heritability in frontotemporal tauopathies. Alzheimers Dement (Amst) 11:115–124
Foti SC, Hargreaves I, Carrington S, Kiely AP, Houlden H, Holton JL (2019) Cerebral mitochondrial electron transport chain dysfunction in multiple system atrophy and Parkinson’s disease. Sci Rep 9:6559
Francis PT, Perry EK (2007) Cholinergic and other neurotransmitter mechanisms in Parkinson’s disease, Parkinson’s disease dementia, and dementia with Lewy bodies. Mov Disord 22(Suppl 17):S351–S357
Franquet E, Salvado-Figueres M, Lorenzo-Bosquet C, Cuberas-Borros G, Rovira A, Castell-Conesa J, Hernandez-Vara J (2012) Nigrostriatal pathway dysfunction in a methanol-induced delayed dystonia-parkinsonism. Mov Disord 27:1220–1221
Freestone PS, Chung KK, Guatteo E, Mercuri NB, Nicholson LF, Lipski J (2009) Acute action of rotenone on nigral dopaminergic neurons—involvement of reactive oxygen species and disruption of Ca homeostasis. Eur J Neurosci 30:1849–1859
French IT, Muthusamy KA (2018) A review of the pedunculopontine nucleus in Parkinson’s disease. Front Aging Neurosci 10:99
Freundt EC, Maynard N, Clancy EK, Roy S, Bousset L, Sourigues Y, Covert M, Melki R, Kirkegaard K, Brahic M (2012) Neuron-to-neuron transmission of alpha-synuclein fibrils through axonal transport. Ann Neurol 72:517–524
Friedman JH (2018) Dementia with Lewy bodies and Parkinson’s disease dementia become the same disease. Parkinsonism Relat Disord 46(Suppl 1):S6–S9
Frigerio R, Fujishiro H, Ahn TB, Josephs KA, Maraganore DM, Delledonne A, Parisi JE, Klos KJ, Boeve BF, Dickson DW, Ahlskog JE (2011) Incidental Lewy body disease: Do some cases represent a preclinical stage of dementia with Lewy bodies? Neurobiol Aging 32:857–863
Frisina PG, Haroutunian V, Libow LS (2009) The neuropathological basis for depression in Parkinson’s disease. Parkinsonism Relat Disord 15:144–148
Fujioka S, Ogaki K, Tacik PM, Uitti RJ, Ross OA, Wszolek ZK (2014a) Update on novel familial forms of Parkinson’s disease and multiple system atrophy. Parkinsonism Relat Disord 20(Suppl 1):S29–S34
Fujioka S, Van Gerpen JA, Uitti RJ, Dickson DW, Wszolek ZK (2014b) Familial progressive supranuclear palsy: a literature review. Neurodegener Dis 13:180–182
Fujishiro H, Ferman TJ, Boeve BF, Smith GE, Graff-Radford NR, Uitti RJ, Wszolek ZK, Knopman DS, Petersen RC, Parisi JE, Dickson DW (2008a) Validation of the neuropathologic criteria of the third consortium for dementia with Lewy bodies for prospectively diagnosed cases. J Neuropathol Exp Neurol 67:649–656
Fujishiro H, Tsuboi Y, Lin WL, Uchikado H, Dickson DW (2008b) Co-localization of tau and alpha-synuclein in the olfactory bulb in Alzheimer’s disease with amygdala Lewy bodies. Acta Neuropathol 116:17–24
Fujishiro H, Iseki E, Higashi S, Kasanuki K, Murayama N, Togo T, Katsuse O, Uchikado H, Aoki N, Kosaka K, Arai H, Sato K (2010) Distribution of cerebral amyloid deposition and its relevance to clinical phenotype in Lewy body dementia. Neurosci Lett 486:19–23
Fujiyama F, Unzai T, Karube F (2019) Thalamostriatal projections and striosome-matrix compartments. Neurochem Int 125:67–73
Gadad BS, Britton GB, Rao KS (2011) Targeting oligomers in neurodegenerative disorders: lessons from alpha-synuclein, tau, and amyloid-beta peptide. J Alzheimers Dis 24(Suppl 2):223–232
Gai WP, Pountney DL, Power JH, Li QX, Culvenor JG, McLean CA, Jensen PH, Blumbergs PC (2003) Alpha-synuclein fibrils constitute the central core of oligodendroglial inclusion filaments in multiple system atrophy. Exp Neurol 181:68–78
Galts CPC, Bettio LEB, Jewett DC, Yang CC, Brocardo PS, Rodrigues ALS, Thacker JS, Gil-Mohapel J (2019) Depression in neurodegenerative diseases: common mechanisms and current treatment options. Neurosci Biobehav Rev 102:56–84
Gallucci M, Dell’Acqua C, Boccaletto F, Fenoglio C, Galimberti D, Di Battista ME (2019) Overlap between frontotemporal dementia and dementia with Lewy bodies: a Treviso Dementia (TREDEM) registry case report. J Alzheimers Dis 69:839–847
Gao LL, Zhang JR, Chan P, Wu T (2017) Levodopa effect on basal ganglia motor circuit in Parkinson’s disease. CNS Neurosci Ther 23:76–86
Gash DM, Rutland K, Hudson NL, Sullivan PG, Bing G, Cass WA, Pandya JD, Liu M, Choi DY, Hunter RL, Gerhardt GA, Smith CD, Slevin JT, Prince TS (2008) Trichloroethylene: parkinsonism and complex 1 mitochondrial neurotoxicity. Ann Neurol 63:184–192
Gasser T, Hardy J, Mizuno Y (2011) Milestones in PD genetics. Mov Disord 26:1042–1048
Gegg ME, Schapira AHV (2018) The role of glucocerebrosidase in Parkinson disease pathogenesis. FEBS J 285:3591–3603
Geibl FF, Henrich MT, Oertel WH (2019) Mesencephalic and extramesencephalic dopaminergic systems in Parkinson’s disease. J Neural Transm (Vienna) 126:377–396
Geiger JT, Ding J, Crain B, Pletnikova O, Letson C, Dawson TM, Rosenthal LS, Pantelyat A, Gibbs JR, Albert MS, Hernandez DG, Hillis AE, Stone DJ, Singleton AB, Hardy JA, Troncoso JC, Scholz SW (2016) Next-generation sequencing reveals substantial genetic contribution to dementia with Lewy bodies. Neurobiol Dis 94:55–62
Gelpi E, Colom-Cadena M (2019) Oligomers: a hot topic for neurodegeneration and a note of caution for experimental models. Brain 142:228–230
Gelpi E, Navarro-Otano J, Tolosa E, Gaig C, Compta Y, Rey MJ, Marti MJ, Hernandez I, Valldeoriola F, Rene R, Ribalta T (2014) Multiple organ involvement by alpha-synuclein pathology in Lewy body disorders. Mov Disord 29:1010–1018
George G, Valiya Parambath S, Lokappa SB, Varkey J (2019) Construction of Parkinson’s disease marker-based weighted protein-protein interaction network for prioritization of co-expressed genes. Gene 697:67–77
Gerson JE, Farmer KM, Henson N, Castillo-Carranza DL, Carretero Murillo M, Sengupta U, Barrett A, Kayed R (2018) Tau oligomers mediate alpha-synuclein toxicity and can be targeted by immunotherapy. Mol Neurodegener 13:13
Geut H, Vergouw LJM, Galis Y, Ingrassia A, de Jong FJ, Quadri M, Bonifati V, Lemstra AW, Rozemuller AJM, van de Berg WDJ (2019) Neuropathological and genetic characteristics of a post-mortem series of cases with dementia with Lewy bodies clinically suspected of Creutzfeldt-Jakob’s disease. Parkinsonism Relat Disord. https://doi.org/10.1016/j.parkreldis.2019.1002.1011
Ghazi Sherbaf F, Same K, Aarabi MH (2018) High angular resolution diffusion imaging correlates of depression in Parkinson’s disease: a connectometry study. Acta Neurol Belg 118:573–579
Ghetti B, Wszolek ZK, Boeve BF, Spina S, Goedert M (2011) Frontotemporal dementia and parkinsonism linked to chromosome 17. In: Dickson DW, Weller RO (eds) Neurodegeneration: the molecular pathology of dementia and movement disorders, 2nd edn. Blackwell Publishing Ltd., Oxford, pp 110–134
Ghiglieri V, Calabrese V, Calabresi P (2018) Alpha-synuclein: from early synaptic dysfunction to neurodegeneration. Front Neurol 9:295
Gibbons GS, Lee VMY, Trojanowski JQ (2019) Mechanisms of cell-to-cell transmission of pathological tau: a review. JAMA Neurol 76:101–108
Giguere N, Burke Nanni S, Trudeau LE (2018) On cell loss and selective vulnerability of neuronal populations in Parkinson’s disease. Front Neurol 9:455
Gil MJ, Manzano MS, Cuadrado ML, Fernandez C, Gomez E, Matesanz C, Calero M, Rabano A (2018a) Frontotemporal lobar degeneration: Study of a clinicopathological cohort. J Clin Neurosci 58:172–180
Gil MJ, Manzano MS, Cuadrado ML, Fernandez C, Gomez E, Matesanz C, Calero M, Rabano A (2018b) Argyrophilic grain pathology in frontotemporal lobar degeneration: demographic, clinical, neuropathological, and genetic features. J Alzheimers Dis 63:1109–1117
Gilman S, Wenning GK, Low PA, Brooks DJ, Mathias CJ, Trojanowski JQ, Wood NW, Colosimo C, Durr A, Fowler CJ, Kaufmann H, Klockgether T, Lees A, Poewe W, Quinn N, Revesz T, Robertson D, Sandroni P, Seppi K, Vidailhet M (2008) Second consensus statement on the diagnosis of multiple system atrophy. Neurology 71:670–676
Ginsberg MD (1985) Carbon monoxide intoxication: clinical features, neuropathology and mechanisms of injury. J Toxicol Clin Toxicol 23:281–288
Godini R, Fallahi H, Ebrahimie E (2019) A comparative system-level analysis of the neurodegenerative diseases. J Cell Physiol 234:5215–5229
Goedert M, Eisenberg DS, Crowther RA (2017a) Propagation of tau aggregates and neurodegeneration. Annu Rev Neurosci 40:189–210
Goedert M, Jakes R, Spillantini MG (2017b) The synucleinopathies: twenty years on. J Parkinsons Dis 7:S51–S69
Goedert M, Masuda-Suzukake M, Falcon B (2017c) Like prions: the propagation of aggregated tau and alpha-synuclein in neurodegeneration. Brain 140:266–278
Goedert M, Falcon B, Zhang W, Ghetti B, Scheres SHW (2019) Distinct conformers of assembled tau in Alzheimer’s and Pick’s diseases. Cold Spring Harb Symp Quant Biol. https://doi.org/10.1101/sqb.2018.1183.037580
Goetz CG, Emre M, Dubois B (2008) Parkinson’s disease dementia: definitions, guidelines, and research perspectives in diagnosis. Ann Neurol 64(Suppl 2):S81–S92
Gomez G, Escande MV, Suarez LM, Rela L, Belforte JE, Moratalla R, Murer MG, Gershanik OS, Taravini IRE (2019) Changes in dendritic spine density and inhibitory perisomatic connectivity onto medium spiny neurons in l-dopa-induced dyskinesia. Mol Neurobiol. https://doi.org/10.1007/s12035-12019-11515-12034
Gomez-Suaga P, Fdez E, Blanca Ramirez M, Hilfiker S (2012) A link between autophagy and the pathophysiology of LRRK2 in Parkinson’s disease. Parkinsons Dis 2012:324521
Gomperts SN (2016) Lewy body dementias: dementia with Lewy bodies and Parkinson disease dementia. Continuum (Minneap Minn) 22:435–463
Gonzalez N, Arcos-Lopez T, Konig A, Quintanar L, Menacho Marquez M, Outeiro TF, Fernandez CO (2019) Effects of alpha-synuclein posttranslational modifications on metal binding. J Neurochem. https://doi.org/10.1111/jnc.14721
Gotz J, Halliday G, Nisbet RM (2019) Molecular pathogenesis of the tauopathies. Annu Rev Pathol 14:239–261
Grabli D, Auré K, Vidailhet M, Roze E (2011) Movement disorders in neurometabolic diseases. In: Gálvez-Jiménez N, Tuite P (eds) Uncommon causes of movement disorders. Cambridge University Press, Cambridge, pp 245–257
Grant R, Graus F (2009) Paraneoplastic movement disorders. Mov Disord 24:1715–1724
Greffard S, Verny M, Bonnet AM, Seilhean D, Hauw JJ, Duyckaerts C (2010) A stable proportion of Lewy body bearing neurons in the substantia nigra suggests a model in which the Lewy body causes neuronal death. Neurobiol Aging 31:99–103
Grigoletto J, Pukass K, Gamliel A, Davidi D, Katz-Brull R, Richter-Landsberg C, Sharon R (2017) Higher levels of myelin phospholipids in brains of neuronal alpha-synuclein transgenic mice precede myelin loss. Acta Neuropathol Commun 5:37
Grimm MJ, Respondek G, Stamelou M, Arzberger T, Ferguson L, Gelpi E, Giese A, Grossman M, Irwin DJ, Pantelyat A, Rajput A, Roeber S, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Colosimo C, van Eimeren T, Kassubek J, Levin J, Meissner WG, Nilsson C, Oertel WH, Piot I, Poewe W, Wenning GK, Boxer A, Golbe LI, Josephs KA, Litvan I, Morris HR, Whitwell JL, Compta Y, Corvol JC, Lang AE, Rowe JB, Hoglinger GU (2019) How to apply the Movement Disorder Society criteria for diagnosis of progressive supranuclear palsy. Mov Disord. https://doi.org/10.1002/mds.27666
Grozdanov V, Danzer KM (2018) Release and uptake of pathologic alpha-synuclein. Cell Tissue Res 373:175–182
Grunblatt E, Ruder J, Monoranu CM, Riederer P, Youdim MB, Mandel SA (2018) Differential alterations in metabolism and proteolysis-related proteins in human Parkinson’s disease substantia nigra. Neurotox Res 33:560–568
Guan X, Zhang Y, Wei H, Guo T, Zeng Q, Zhou C, Wang J, Gao T, Xuan M, Gu Q, Xu X, Huang P, Pu J, Zhang B, Liu C, Zhang M (2019) Iron-related nigral degeneration influences functional topology mediated by striatal dysfunction in Parkinson’s disease. Neurobiol Aging 75:83–97
Guerreiro R, Ross OA, Kun-Rodrigues C, Hernandez DG, Orme T, Eicher JD, Shepherd CE, Parkkinen L, Darwent L, Heckman MG, Scholz SW, Troncoso JC, Pletnikova O, Ansorge O, Clarimon J, Lleo A, Morenas-Rodriguez E, Clark L, Honig LS, Marder K, Lemstra A, Rogaeva E, St George-Hyslop P, Londos E, Zetterberg H, Barber I, Braae A, Brown K, Morgan K, Troakes C, Al-Sarraj S, Lashley T, Holton J, Compta Y, Van Deerlin V, Serrano GE, Beach TG, Lesage S, Galasko D, Masliah E, Santana I, Pastor P, Diez-Fairen M, Aguilar M, Tienari PJ, Myllykangas L, Oinas M, Revesz T, Lees A, Boeve BF, Petersen RC, Ferman TJ, Escott-Price V, Graff-Radford N, Cairns NJ, Morris JC, Pickering-Brown S, Mann D, Halliday GM, Hardy J, Trojanowski JQ, Dickson DW, Singleton A, Stone DJ, Bras J (2018) Investigating the genetic architecture of dementia with Lewy bodies: a two-stage genome-wide association study. Lancet Neurol 17:64–74
Guerrero E, Vasudevaraju P, Hegde ML, Britton GB, Rao KS (2013) Recent advances in alpha-synuclein functions, advanced glycation, and toxicity: implications for Parkinson’s disease. Mol Neurobiol 47:525–536
Guiney SJ, Adlard PA, Bush AI, Finkelstein DI, Ayton S (2017) Ferroptosis and cell death mechanisms in Parkinson’s disease. Neurochem Int 104:34–48
Gundner AL, Duran-Pacheco G, Zimmermann S, Ruf I, Moors T, Baumann K, Jagasia R, van de Berg WDJ, Kremer T (2019) Path mediation analysis reveals GBA impacts Lewy body disease status by increasing alpha-synuclein levels. Neurobiol Dis 121:205–213
Guo JL, Lee VM (2014) Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat Med 20:130–138
Habas C, Manto M, Cabaraux P (2019) The cerebellar thalamus. Cerebellum 18:635–648
Haber SN (2016) Corticostriatal circuitry. Dialogues Clin Neurosci 18:7–21
Hall JM, Lewis SJG (2019) Neural correlates of cognitive impairment in Parkinson’s disease: a review of structural MRI findings. Int Rev Neurobiol 144:1–28
Hall JM, Shine JM, Walton CC, Gilat M, Kamsma YP, Naismith SL, Lewis SJ (2014) Early phenotypic differences between Parkinson’s disease patients with and without freezing of gait. Parkinsonism Relat Disord 20:604–607
Halliday GM, McCann H (2010) The progression of pathology in Parkinson’s disease. Ann N Y Acad Sci 1184:188–195
Halliday G, Hely M, Reid W, Morris J (2008) The progression of pathology in longitudinally followed patients with Parkinson’s disease. Acta Neuropathol 115:409–415
Halliday G, Herrero MT, Murphy K, McCann H, Ros-Bernal F, Barcia C, Mori H, Blesa FJ, Obeso JA (2009) No Lewy pathology in monkeys with over 10 years of severe MPTP parkinsonism. Mov Disord 24:1519–1523
Halliday GM, Song YJ, Harding AJ (2011) Striatal beta-amyloid in dementia with Lewy bodies but not Parkinson’s disease. J Neural Transm 118:713–719
Halliday G, McCann H, Shepherd C (2012) Evaluation of the Braak hypothesis: how far can it explain the pathogenesis of Parkinson’s disease? Expert Rev Neurother 12:673–686
Halliday GM, Leverenz JB, Schneider JS, Adler CH (2014) The neurobiological basis of cognitive impairment in Parkinson’s disease. Mov Disord 29:634–650
Hamed M, Schraml F, Wilson J, Galvin J, Sabbagh MN (2018) Occipital and cingulate hypometabolism are significantly under-reported on 18-fluorodeoxyglucose positron emission tomography scans of patients with Lewy body dementia. J Alzheimers Dis Parkinsonism 8:428
Hansen LA, Daniel SE, Wilcock GK, Love S (1998) Frontal cortical synaptophysin in Lewy body diseases: relation to Alzheimer’s disease and dementia. J Neurol Neurosurg Psychiatry 64:653–656
Hansen D, Ling H, Lashley T, Holton JL, Warner TT (2019) Clinical, neuropathological and genetic features of Lewy body dementias. Neuropathol Appl Neurobiol 11:78. https://doi.org/10.1111/nan.12554
Hara K, Watanabe H, Bagarinao E, Kawabata K, Yoneyama N, Ohdake R, Imai K, Masuda M, Yokoi T, Ogura A, Tsuboi T, Ito M, Atsuta N, Niwa H, Taoka T, Maesawa S, Naganawa S, Katsuno M, Sobue G (2018) Corpus callosal involvement is correlated with cognitive impairment in multiple system atrophy. J Neurol 265:2079–2087
Harding AJ, Stimson E, Henderson JM, Halliday GM (2002) Clinical correlates of selective pathology in the amygdala of patients with Parkinson’s disease. Brain 125:2431–2445
Hasegawa M, Arai T, Nonaka T, Kametani F, Yoshida M, Hashizume Y, Beach TG, Buratti E, Baralle F, Morita M, Nakano I, Oda T, Tsuchiya K, Akiyama H (2008) Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Ann Neurol 64:60–70
Hata Y, Ma N, Yoneda M, Morimoto S, Okano H, Murayama S, Kawanishi S, Kuzuhara S, Kokubo Y (2018) Nitrative stress and tau accumulation in amyotrophic lateral sclerosis/parkinsonism-dementia complex (ALS/PDC) in the Kii Peninsula, Japan. Front Neurosci 11:751
Hayakawa H, Nagai M, Kawanami A, Nakata Y, Nihira T, Ogino M, Takada M, Saido T, Takano J, Saegusa M, Mikami T, Hamada J, Nishiyama K, Mochizuki H, Mizuno Y (2013) Loss of DARPP-32 and calbindin in multiple system atrophy. J Neural Transm 120:1689–1698
Heckman MG, Schottlaender L, Soto-Ortolaza AI, Diehl NN, Rayaprolu S, Ogaki K, Fujioka S, Murray ME, Cheshire WP, Uitti RJ, Wszolek ZK, Farrer MJ, Sailer A, Singleton AB, Chinnery PF, Keogh MJ, Gentleman SM, Holton JL, Aoife K, Mann DM, Al-Sarraj S, Troakes C, Dickson DW, Houlden H, Ross OA (2014) LRRK2 exonic variants and risk of multiple system atrophy. Neurology 83:2256–2261
Heckman MG, Brennan RR, Labbe C, Soto AI, Koga S, DeTure MA, Murray ME, Petersen RC, Boeve BF, van Gerpen JA, Uitti RJ, Wszolek ZK, Rademakers R, Dickson DW, Ross OA (2019) Association of MAPT subhaplotypes with risk of progressive supranuclear palsy and severity of tau pathology. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2019.0250
Hegeman DJ, Hong ES, Hernandez VM, Chan CS (2016) The external globus pallidus: progress and perspectives. Eur J Neurosci 43:1239–1265
Heitz C, Noblet V, Cretin B, Philippi N, Kremer L, Stackfleth M, Hubele F, Armspach JP, Namer I, Blanc F (2015) Neural correlates of visual hallucinations in dementia with Lewy bodies. Alzheimers Res Ther 7:6
Helmich RC (2018) The cerebral basis of Parkinsonian tremor: a network perspective. Mov Disord 33:219–231
Helmich RC, Janssen MJ, Oyen WJ, Bloem BR, Toni I (2011) Pallidal dysfunction drives a cerebellothalamic circuit into Parkinson tremor. Ann Neurol 69:269–281
Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG (2008) The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord 23:837–844
Henderson MX, Trojanowski JQ, Lee VM (2019) α-Synuclein pathology in Parkinson's disease and related alpha-synucleinopathies. Neurosci Lett. https://doi.org/10.1016/j.neulet.2019.134316
Hepp DH, Vergoossen DL, Huisman E, Lemstra AW, Berendse HW, Rozemuller AJ, Foncke EM, van de Berg WD (2016) Distribution and load of amyloid-beta pathology in Parkinson disease and dementia with Lewy bodies. J Neuropathol Exp Neurol 75:936–945
Heras-Garvin A, Weckbecker D, Ryazanov S, Leonov A, Griesinger C, Giese A, Wenning GK, Stefanova N (2019) Anle138b modulates alpha-synuclein oligomerization and prevents motor decline and neurodegeneration in a mouse model of multiple system atrophy. Mov Disord 34:255–263
Hernandez D, Paisan Ruiz C, Crawley A, Malkani R, Werner J, Gwinn-Hardy K, Dickson D, Wavrant Devrieze F, Hardy J, Singleton A (2005) The dardarin G 2019 S mutation is a common cause of Parkinson’s disease but not other neurodegenerative diseases. Neurosci Lett 389:137–139
Herz DM, Haagensen BN, Christensen MS, Madsen KH, Rowe JB, Lokkegaard A, Siebner HR (2015) Abnormal dopaminergic modulation of striato-cortical networks underlies levodopa-induced dyskinesias in humans. Brain 138:1658–1666
Hintzen A, Pelzer EA, Tittgemeyer M (2018) Thalamic interactions of cerebellum and basal ganglia. Brain Struct Funct 223:569–587
Hirsch EC, Jenner P, Przedborski S (2013) Pathogenesis of Parkinson’s disease. Mov Disord 28:24–30
Hoffmann A, Ettle B, Battis K, Reiprich S, Schlachetzki JCM, Masliah E, Wegner M, Kuhlmann T, Riemenschneider MJ, Winkler J (2019) Oligodendroglial a-synucleinopathy-driven neuroinflammation in multiple system atrophy. Brain Pathol 29:380–396
Hoglinger GU (2018) Is it useful to classify progressive supranuclear palsy and corticobasal degeneration as different disorders? No. Mov Disord Clin Pract 5:141–144
Hoglinger GU, Oertel WH, Hirsch EC (2006) The rotenone model of parkinsonism—the 5 years inspection. J Neural Transm Suppl (70):269–272
Hoglinger GU, Melhem NM, Dickson DW, Sleiman PM, Wang LS, Klei L, Rademakers R, de Silva R, Litvan I, Riley DE, van Swieten JC, Heutink P, Wszolek ZK, Uitti RJ, Vandrovcova J, Hurtig HI, Gross RG, Maetzler W, Goldwurm S, Tolosa E, Borroni B, Pastor P, Cantwell LB, Han MR, Dillman A, van der Brug MP, Gibbs JR, Cookson MR, Hernandez DG, Singleton AB, Farrer MJ, Yu CE, Golbe LI, Revesz T, Hardy J, Lees AJ, Devlin B, Hakonarson H, Muller U, Schellenberg GD (2011) Identification of common variants influencing risk of the tauopathy progressive supranuclear palsy. Nat Genet 43:699–705
Hoglinger GU, Respondek G, Stamelou M, Kurz C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Nilsson C, Whitwell JL, Arzberger T, Englund E, Gelpi E, Giese A, Irwin DJ, Meissner WG, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Antonini A, Bhatia KP, Bordelon Y, Compta Y, Corvol JC, Colosimo C, Dickson DW, Dodel R, Ferguson L, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris HR, Nestor P, Oertel WH, Poewe W, Rabinovici G, Rowe JB, Schellenberg GD, Seppi K, van Eimeren T, Wenning GK, Boxer AL, Golbe LI, Litvan I (2018) Clinical diagnosis of progressive supranuclear palsy: the movement disorder society criteria. Mov Disord 32:853–864
Holmqvist S, Chutna O, Bousset L, Aldrin-Kirk P, Li W, Bjorklund T, Wang ZY, Roybon L, Melki R, Li JY (2014) Direct evidence of Parkinson pathology spread from the gastrointestinal tract to the brain in rats. Acta Neuropathol 128:805–820
Holton JL, Lees AL, Revesz T (2011) Multiple system atrophy. In: Dickson DW, Weller RO (eds) Neurodegeneration: the molecular pathology of dementia and movement disorders, 2nd edn. Blackwell Publishing Ltd., Oxford, pp 242–252
Homma T, Mochizuki Y, Komori T, Isozaki E (2016) Frequent globular neuronal cytoplasmic inclusions in the medial temporal region as a possible characteristic feature in multiple system atrophy with dementia. Neuropathology 36:421–431
Horowitz MP, Greenamyre JT (2010) Mitochondrial iron metabolism and its role in neurodegeneration. J Alzheimers Dis 20(Suppl 2):S551–S568
Horvath J, Burkhard PR, Bouras C, Kovari E (2013a) Etiologies of parkinsonism in a century-long autopsy-based cohort. Brain Pathol 23:28–33
Horvath J, Herrmann FR, Burkhard PR, Bouras C, Kovari E (2013b) Neuropathology of dementia in a large cohort of patients with Parkinson’s disease. Parkinsonism Relat Disord 19:864–868 (discussion 864)
Houlden H, Singleton AB (2012) The genetics and neuropathology of Parkinson’s disease. Acta Neuropathol 124:325–338
Houlden H, Baker M, Morris HR, MacDonald N, Pickering-Brown S, Adamson J, Lees AJ, Rossor MN, Quinn NP, Kertesz A, Khan MN, Hardy J, Lantos PL, St George-Hyslop P, Munoz DG, Mann D, Lang AE, Bergeron C, Bigio EH, Litvan I, Bhatia KP, Dickson D, Wood NW, Hutton M (2001) Corticobasal degeneration and progressive supranuclear palsy share a common tau haplotype. Neurology 56:1702–1706
Hu D, Sun X, Liao X, Zhang X, Zarabi S, Schimmer A, Hong Y, Ford C, Luo Y, Qi X (2019) Alpha-synuclein suppresses mitochondrial protease ClpP to trigger mitochondrial oxidative damage and neurotoxicity. Acta Neuropathol 137:939–960
Huang M, Wang B, Li X, Fu C, Wang C, Kang X (2019) Alpha-synuclein: a multifunctional player in exocytosis, endocytosis, and vesicle recycling. Front Neurosci 13:28
Hwang J, Bank AM, Mortazavi F, Oakley DH, Frosch MP, Schmahmann JD (2019) Spinal cord a-synuclein deposition associated with myoclonus in patients with MSA-C. Neurology (accepted paper)
Iljina M, Garcia GA, Horrocks MH, Tosatto L, Choi ML, Ganzinger KA, Abramov AY, Gandhi S, Wood NW, Cremades N, Dobson CM, Knowles TP, Klenerman D (2016) Kinetic model of the aggregation of alpha-synuclein provides insights into prion-like spreading. Proc Natl Acad Sci USA 113:E1206–E1215
Imbriani P, Schirinzi T, Meringolo M, Mercuri NB, Pisani A (2018) Centrality of early synaptopathy in Parkinson’s disease. Front Neurol 9:103
Inzelberg R, Estrada-Cuzcano A, Laitman Y, De Vriendt E, Friedman E, Jordanova A (2018) Kufor–Rakeb syndrome/PARK9: one novel and one possible recurring Ashkenazi ATP13A2 mutation. J Parkinsons Dis 8:399–403
Iodice V, Lipp A, Ahlskog JE, Sandroni P, Fealey RD, Parisi JE, Matsumoto JY, Benarroch EE, Kimpinski K, Singer W, Gehrking TL, Gehrking JA, Sletten DM, Schmeichel AM, Bower JH, Gilman S, Figueroa J, Low PA (2012) Autopsy confirmed multiple system atrophy cases: Mayo experience and role of autonomic function tests. J Neurol Neurosurg Psychiatry 83:453–459
Irwin DJ, Hurtig HI (2018) The contribution of tau, amyloid-beta and alpha-synuclein pathology to dementia in Lewy body disorders. J Alzheimers Dis Parkinsonism. https://doi.org/10.4172/2161-0460.1000444
Irwin DJ, Abrams JY, Schonberger LB, Leschek EW, Mills JL, Lee VM, Trojanowski JQ (2013a) Evaluation of potential infectivity of Alzheimer and Parkinson disease proteins in recipients of cadaver-derived human growth hormone. JAMA Neurol 70:462–468
Irwin DJ, Cohen TJ, Grossman M, Arnold SE, McCarty-Wood E, Van Deerlin VM, Lee VM, Trojanowski JQ (2013b) Acetylated tau neuropathology in sporadic and hereditary tauopathies. Am J Pathol 183:344–351
Irwin DJ, Lee VM, Trojanowski JQ (2013c) Parkinson’s disease dementia: convergence of alpha-synuclein, tau and amyloid-beta pathologies. Nat Rev Neurosci 14:626–636
Irwin DJ, Cairns NJ, Grossman M, McMillan CT, Lee EB, Van Deerlin VM, Lee VM, Trojanowski JQ (2015) Frontotemporal lobar degeneration: defining phenotypic diversity through personalized medicine. Acta Neuropathol 129:469–491
Irwin DJ, Brettschneider J, McMillan CT, Cooper F, Olm C, Arnold SE, Van Deerlin VM, Seeley WW, Miller BL, Lee EB, Lee VM, Grossman M, Trojanowski JQ (2016) Deep clinical and neuropathological phenotyping of Pick disease. Ann Neurol 79:272–287
Ishiyama M, Yagishita S, Hasegawa K, Yokoyama T (2006) Ultrastructural study (tEM and sEM) of cortical Lewy bodies (abstract). Neuropathol 26(2):A58
Ito K, Arai K, Yoshiyama Y, Kashiwado K, Sakakibara Y, Hattori T (2008) Astrocytic tau pathology positively correlates with neurofibrillary tangle density in progressive supranuclear palsy. Acta Neuropathol 115:623–628
Itoh N, Ishiguro K, Arai H, Kokubo Y, Sasaki R, Narita Y, Kuzuhara S (2003) Biochemical and ultrastructural study of neurofibrillary tangles in amyotrophic lateral sclerosis/parkinsonism-dementia complex in the Kii peninsula of Japan. J Neuropathol Exp Neurol 62:791–798
Iyer A, Claessens M (2019) Disruptive membrane interactions of alpha-synuclein aggregates. Biochim Biophys Acta Proteins Proteom 1867:468–482
Jabbari E, Woodside J, Tan MMX, Shoai M, Pittman A, Ferrari R, Mok KY, Zhang D, Reynolds RH, de Silva R, Grimm MJ, Respondek G, Muller U, Al-Sarraj S, Gentleman SM, Lees AJ, Warner TT, Hardy J, Revesz T, Hoglinger GU, Holton JL, Ryten M, Morris HR (2018) Variation at the TRIM11 locus modifies progressive supranuclear palsy phenotype. Ann Neurol 84:485–496
Janda E, Isidoro C, Carresi C, Mollace V (2012) Defective autophagy in Parkinson’s disease: role of oxidative stress. Mol Neurobiol 46:639–661
Jellinger KA (2003) Prevalence of vascular lesions in dementia with Lewy bodies. A postmortem study. J Neural Transm (Vienna) 110:771–778
Jellinger KA (2004) Lewy body-related alpha-synucleinopathy in the aged human brain. J Neural Transm 111:1219–1235
Jellinger KA (2006) Pathological substrate of dementia in Parkinson’s disease–its relation to DLB and DLBD. Parkinsonism Relat Disord 12:119–120
Jellinger KA (2008a) Vascular parkinsonism. Therapy 5:237–255
Jellinger KA (2008b) Different tau pathology pattern in two clinical phenotypes of progressive supranuclear palsy. Neurodegener Dis 5:339–346
Jellinger KA (2009a) A critical evaluation of current staging of alpha-synuclein pathology in Lewy body disorders. Biochim Biophys Acta 1792:730–740
Jellinger KA (2009b) Significance of brain lesions in Parkinson disease dementia and Lewy body dementia. Front Neurol Neurosci 24:114–125
Jellinger KA (2011) Postencephalitic Parkinsonism. In: Dickson DW, Weller RO (eds) Neurodegeneration: the molecular pathology of dementia and movement disorders, 2nd edn. Blackwell Publishing Ltd., Oxford, pp 179–187
Jellinger KA (2012a) The role of alpha-synuclein in neurodegeneration—an update. Transl Neurosci 3:75–122
Jellinger KA (2012b) Neuropathology of sporadic Parkinson’s disease: evaluation and changes of concepts. Mov Disord 27:8–30
Jellinger KA (2013a) Synuclein and Parkinson’s disease: an update. In: Martinez A, Gil C (eds) Emerging drugs and targets for Parkinson’s disease. The Royal Society of Chemistry, London, pp 175–214
Jellinger KA (2013b) Mild cognitive impairment in Parkinson disease: heterogenous mechanisms. J Neural Transm (Vienna) 120:157–167
Jellinger KA (2014) Neuropathology. In: Wenning GK, Fanciulli A (eds) Multiple system atrophy. Springer, Vienna, pp 17–55
Jellinger KA (2015) Neuropathobiology of non-motor symptoms in Parkinson disease. J Neural Transm (Vienna) 122:1429–1440
Jellinger KA (2016) Neuropathology of movement disorders, vol 1. In: Winn HR (ed) Youmans Neurological surgery, 7th edn. Elsevier-Saunders, Philadelphia, pp e840–e880
Jellinger KA (2017a) Neuropathology of movement disorders. In: Falup-Pecurariu C, Ferreira J, Martinez-Martin P, Chaudhuri KR (eds) Movement disorders curricula. Springer, Wien, pp 43–48
Jellinger KA (2017b) Neuropathology of nonmotor symptoms of Parkinson’s disease. Int Rev Neurobiol 133:13–62
Jellinger KA (2018a) Multiple system atrophy: an oligodendroglioneural synucleinopathy. J Alzheimers Dis 62:1141–1179
Jellinger KA (2018b) Dementia with Lewy bodies and Parkinson’s disease-dementia: current concepts and controversies. J Neural Transm (Vienna) 125:615–650
Jellinger KA (2018c) Young-onset multiple system atrophy. Mov Disord 33:1974–1975
Jellinger KA (2019) Is Braak staging valid for all types of Parkinson’s disease? J Neural Transm (Vienna) 126:423–431
Jellinger KA, Attems J (2006) Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta Neuropathol 112:253–260
Jellinger KA, Attems J (2008) Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol 115:127–136
Jellinger KA, Korczyn AD (2018) Are dementia with Lewy bodies and Parkinson’s disease dementia the same disease? BMC Med 16:34
Jellinger KA, Lantos PL (2010) Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta Neuropathol 119:657–667
Jellinger KA, Wenning GK (2016) Multiple system atrophy: pathogenic mechanisms and biomarkers. J Neural Transm (Vienna) 123:555–572
Jellinger KA, Seppi K, Wenning GK (2005) Grading of neuropathology in multiple system atrophy: proposal for a novel scale. Mov Disord 20(Suppl 12):S29–S36
Jiang P, Dickson DW (2018) Parkinson’s disease: experimental models and reality. Acta Neuropathol 135:13–32
Jiang P, Gan M, Yen SH, Moussaud S, McLean PJ, Dickson DW (2016) Proaggregant nuclear factor(s) trigger rapid formation of alpha-synuclein aggregates in apoptotic neurons. Acta Neuropathol 132:77–91
Johnson ME, Stecher B, Labrie V, Brundin L, Brundin P (2019) Triggers, facilitators, and aggravators: redefining Parkinson’s disease pathogenesis. Trends Neurosci 42:4–13
Joki H, Higashiyama Y, Nakae Y, Kugimoto C, Doi H, Kimura K, Kishida H, Ueda N, Nakano T, Takahashi T, Koyano S, Takeuchi H, Tanaka F (2018) White matter hyperintensities on MRI in dementia with Lewy bodies, Parkinson’s disease with dementia, and Alzheimer’s disease. J Neurol Sci 385:99–104
Josephs KA, Ishizawa T, Tsuboi Y, Cookson N, Dickson DW (2002) A clinicopathological study of vascular progressive supranuclear palsy: a multi-infarct disorder presenting as progressive supranuclear palsy. Arch Neurol 59:1597–1601
Jost WH, Lingor P, Tonges L, Schwarz J, Buhmann C, Kassubek J, Schrag A (2019) Dyskinesia in multiple system atrophy and progressive supranuclear palsy. J Neural Transm (Vienna). https://doi.org/10.1007/s00702-00019-02012-00700
Joutsa J, Gardberg M, Roytta M, Kaasinen V (2014) Diagnostic accuracy of parkinsonism syndromes by general neurologists. Parkinsonism Relat Disord 20:840–844
Kaindlstorfer C, Granata R, Wenning GK (2013) Tremor in multiple system atrophy—a review. Tremor Other Hyperkinet Mov (N Y). https://doi.org/10.7916/d7918nv7919gz7919
Kaindlstorfer C, Jellinger KA, Eschlbock S, Stefanova N, Weiss G, Wenning GK (2018) The relevance of iron in the pathogenesis of multiple system atrophy: a viewpoint. J Alzheimers Dis 61:1253–1273
Kalaitzakis ME, Pearce RK (2009) The morbid anatomy of dementia in Parkinson’s disease. Acta Neuropathol 118:587–598
Kalaitzakis ME, Christian LM, Moran LB, Graeber MB, Pearce RK, Gentleman SM (2009a) Dementia and visual hallucinations associated with limbic pathology in Parkinson’s disease. Parkinsonism Relat Disord 15:196–204
Kalaitzakis ME, Pearce RK, Gentleman SM (2009b) Clinical correlates of pathology in the claustrum in Parkinson’s disease and dementia with Lewy bodies. Neurosci Lett 461:12–15
Kalaitzakis ME, Walls AJ, Pearce RK, Gentleman SM (2011) Striatal Abeta peptide deposition mirrors dementia and differentiates DLB and PDD from other Parkinsonian syndromes. Neurobiol Dis 41:377–384
Kalia LV, Kalia SK (2015) Alpha-synuclein and Lewy pathology in Parkinson’s disease. Curr Opin Neurol 28:375–381
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386:896–912
Kam TI, Mao X, Park H, Chou SC, Karuppagounder SS, Umanah GE, Yun SP, Brahmachari S, Panicker N, Chen R, Andrabi SA, Qi C, Poirier GG, Pletnikova O, Troncoso JC, Bekris LM, Leverenz JB, Pantelyat A, Ko HS, Rosenthal LS, Dawson TM, Dawson VL (2018) Poly(ADP-ribose) drives pathologic alpha-synuclein neurodegeneration in Parkinson’s disease. Science. https://doi.org/10.1126/science.aat8407
Kamin J, Manwani S, Hughes D (2000) Emergency psychiatry: extrapyramidal side effects in the psychiatric emergency service. Psychiatr Serv 51:287–289
Kanazawa T, Uchihara T, Takahashi A, Nakamura A, Orimo S, Mizusawa H (2008) Three-layered structure shared between Lewy bodies and Lewy neurites—three-dimensional reconstruction of triple-labeled sections. Brain Pathol 18:415–422
Kanazawa T, Adachi E, Orimo S, Nakamura A, Mizusawa H, Uchihara T (2012) Pale neurites, premature alpha-synuclein aggregates with centripetal extension from axon collaterals. Brain Pathol 22:67–78
Kane JPM, Surendranathan A, Bentley A, Barker SAH, Taylor JP, Thomas AJ, Allan LM, McNally RJ, James PW, McKeith IG, Burn DJ, O’Brien JT (2018) Clinical prevalence of Lewy body dementia. Alzheimers Res Ther 10:19
Kang SW, Jeon S, Yoo HS, Chung SJ, Lee PH, Sohn YH, Yun M, Evans AC, Ye BS (2019) Effects of Lewy body disease and Alzheimer disease on brain atrophy and cognitive dysfunction. Neurology 92:e2015–e2026
Karpowicz RJ Jr, Trojanowski JQ, Lee VM (2019) Transmission of alpha-synuclein seeds in neurodegenerative disease: recent developments. Lab Investig. https://doi.org/10.1038/s41374-41019-40195-z
Kempster PA, O’Sullivan SS, Holton JL, Revesz T, Lees AJ (2010) Relationships between age and late progression of Parkinson’s disease: a clinico-pathological study. Brain 133:1755–1762
Kett LR, Dauer WT (2016) Endolysosomal dysfunction in Parkinson’s disease: recent developments and future challenges. Mov Disord 31:1433–1443
Kiely AP, Asi YT, Kara E, Limousin P, Ling H, Lewis P, Proukakis C, Quinn N, Lees AJ, Hardy J, Revesz T, Houlden H, Holton JL (2013) Alpha-synucleinopathy associated with G51D SNCA mutation: a link between Parkinson’s disease and multiple system atrophy? Acta Neuropathol 125:753–769
Kiely AP, Ling H, Asi YT, Kara E, Proukakis C, Schapira AH, Morris HR, Roberts HC, Lubbe S, Limousin P, Lewis PA, Lees AJ, Quinn N, Hardy J, Love S, Revesz T, Houlden H, Holton JL (2015) Distinct clinical and neuropathological features of G51D SNCA mutation cases compared with SNCA duplication and H50Q mutation. Mol Neurodegener 10:41
Kiely AP, Murray CE, Foti SC, Benson BC, Courtney R, Strand C, Lashley T, Holton JL (2018) Immunohistochemical and molecular investigations show alteration in the inflammatory profile of multiple system atrophy brain. J Neuropathol Exp Neurol 77:598–607
Kiely AP, Miners JS, Courtney R, Strand C, Love S, Holton JL (2019) Exploring the putative role of kallikrein-6, calpain-1 and cathepsin-D in the proteolytic degradation of alpha-synuclein in multiple system atrophy. Neuropathol Appl Neurobiol 45:347–360
Kim HJ, Jeon BS (2012) Multiple system atrophy with prolonged survival. Mov Disord 27:1834
Ryu H-S, Oh M, Oh JS, Moon H, Park KW, Lee CS, You S, Kim M-J, Kim Y, J., Kim J, Kim KM, Kim JS, Chung SJ (2019) Distinct clinical features of predominant pre-synaptic and transsynaptic nigrostriatal dysfunction in multiple system atrophy. J Neurol Sci (accepted article)
Keber U, Klietz M, Carlsson T, Oertel WH, Weihe E, Schafer MK, Hoglinger GU, Depboylu C (2015) Striatal tyrosine hydroxylase-positive neurons are associated with L-DOPA-induced dyskinesia in hemiparkinsonian mice. Neuroscience 298:302–317
Kim YD, Kim JS, Lee ES, Yang DW, Lee KS, Kim YI (2009) Progressive “vascular” corticobasal syndrome due to bilateral ischemic hemispheric lesions. Intern Med 48:1699–1702
Kim JS, Yang JJ, Lee DK, Lee JM, Youn J, Cho JW (2015) Cognitive impairment and its structural correlates in the parkinsonian subtype of multiple system atrophy. Neurodegener Dis 15:294–300
Kim EJ, Brown JA, Deng J, Hwang JL, Spina S, Miller ZA, DeMay MG, Valcour V, Karydas A, Ramos EM, Coppola G, Miller BL, Rosen HJ, Seeley WW, Grinberg LT (2018) Mixed TDP-43 proteinopathy and tauopathy in frontotemporal lobar degeneration: nine case series. J Neurol 265:2960–2971
Kingsbury AE, Bandopadhyay R, Silveira-Moriyama L, Ayling H, Kallis C, Sterlacci W, Maeir H, Poewe W, Lees AJ (2010) Brain stem pathology in Parkinson’s disease: an evaluation of the Braak staging model. Mov Disord 25:2508–2515
Kishore A, Popa T (2014) Cerebellum in levodopa-induced dyskinesias: the unusual suspect in the motor network. Front Neurol 5:157
Klaus A, da Silva JA, Costa RM (2019) What, if, and when to move: basal ganglia circuits and self-paced action initiation. Annu Rev Neurosci. https://doi.org/10.1146/annurev-neuro-072116-031033
Klein AD, Mazzulli JR (2018) Is Parkinson’s disease a lysosomal disorder? Brain 141:2255–2262
Klein C, Lochte T, Delamonte SM, Braenne I, Hicks AA, Zschiedrich-Jansen K, Simon DK, Friedman JH, Lohmann K (2016) PLA2G6 mutations and parkinsonism: long-term follow-up of clinical features and neuropathology. Mov Disord 31:1927–1929
Klietz M, Keber U, Carlsson T, Chiu WH, Hoglinger GU, Weihe E, Schafer MK, Depboylu C (2016) l-DOPA-induced dyskinesia is associated with a deficient numerical downregulation of striatal tyrosine hydroxylase mRNA-expressing neurons. Neuroscience 331:120–133
Klingelhoefer L, Reichmann H (2017) Parkinson’s disease as a multisystem disorder. J Neural Transm (Vienna) 124:709–713
Ko WKD, Bezard E (2017) Experimental animal models of Parkinson’s disease: a transition from assessing symptomatology to alpha-synuclein targeted disease modification. Exp Neurol 298:172–179
Koga S, Dickson DW (2018) Recent advances in neuropathology, biomarkers and therapeutic approach of multiple system atrophy. J Neurol Neurosurg Psychiatry 89:175–184
Koga S, Dickson DW (2019) “Minimal change” multiple system atrophy with limbic-predominant alpha-synuclein pathology. Acta Neuropathol 137:167–169
Koga S, Aoki N, Uitti RJ, van Gerpen JA, Cheshire WP, Josephs KA, Wszolek ZK, Langston JW, Dickson DW (2015) When DLB, PD, and PSP masquerade as MSA: an autopsy study of 134 patients. Neurology 85:404–412
Koga S, Parks A, Uitti RJ, van Gerpen JA, Cheshire WP, Wszolek ZK, Dickson DW (2017a) Profile of cognitive impairment and underlying pathology in multiple system atrophy. Mov Disord 32:405–413
Koga S, Roemer SF, Kasanuki K, Dickson DW (2019) Cerebrovascular pathology presenting as corticobasal syndrome: an autopsy case series of “vascular CBS”. Parkinsonism Relat Disord (in press)
Koga S, Sanchez-Contreras M, Josephs KA, Uitti RJ, Graff-Radford N, van Gerpen JA, Cheshire WP, Wszolek ZK, Rademakers R, Dickson DW (2017b) Distribution and characteristics of transactive response DNA binding protein 43 kDa pathology in progressive supranuclear palsy. Mov Disord 32:246–255
Koga S, Kouri N, Walton RL, Ebbert MTW, Josephs KA, Litvan I, Graff-Radford N, Ahlskog JE, Uitti RJ, van Gerpen JA, Boeve BF, Parks A, Ross OA, Dickson DW (2018a) Corticobasal degeneration with TDP-43 pathology presenting with progressive supranuclear palsy syndrome: a distinct clinicopathologic subtype. Acta Neuropathol 136:389–404
Koga S, Lin WL, Walton RL, Ross OA, Dickson DW (2018b) TDP-43 pathology in multiple system atrophy: colocalization of TDP-43 and alpha-synuclein in glial cytoplasmic inclusions. Neuropathol Appl Neurobiol 44:707–721
Kokubo Y, Taniguchi A, Hasegawa M, Hayakawa Y, Morimoto S, Yoneda M, Hirokawa Y, Shiraishi T, Saito Y, Murayama S, Kuzuhara S (2012) Alpha-synuclein pathology in the amyotrophic lateral sclerosis/parkinsonism dementia complex in the Kii Peninsula, Japan. J Neuropathol Exp Neurol 71:625–630
Köllensperger M, Geser F, Seppi K, Stampfer-Kountchev M, Sawires M, Scherfler C, Boesch S, Mueller J, Koukouni V, Quinn N, Pellecchia MT, Barone P, Schimke N, Dodel R, Oertel W, Dupont E, Ostergaard K, Daniels C, Deuschl G, Gurevich T, Giladi N, Coelho M, Sampaio C, Nilsson C, Widner H, Sorbo FD, Albanese A, Cardozo A, Tolosa E, Abele M, Klockgether T, Kamm C, Gasser T, Djaldetti R, Colosimo C, Meco G, Schrag A, Poewe W, Wenning GK (2008) Red flags for multiple system atrophy. Mov Disord 23:1093–1099
Konno T, Ross OA, Teive HAG, Slawek J, Dickson DW, Wszolek ZK (2017) DCTN1-related neurodegeneration: Perry syndrome and beyond. Parkinsonism Relat Disord 41:14–24
Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, Halliday GM, Bartus RT (2013) Disease duration and the integrity of the nigrostriatal system in Parkinson’s disease. Brain 136:2419–2431
Kosaka K, Tsuchiya K, Yoshimura M (1988) Lewy body disease with and without dementia: a clinicopathological study of 35 cases. Clin Neuropathol 7:299–305
Kotzbauer PT, Giasson BI, Kravitz AV, Golbe LI, Mark MH, Trojanowski JQ, Lee VM (2004) Fibrillization of alpha-synuclein and tau in familial Parkinson’s disease caused by the A53T alpha-synuclein mutation. Exp Neurol 187:279–288
Kouli A, Torsney KM, Kuan WL (2018) Parkinson’s disease: etiology, neuropathology, and pathogenesis. In: Stoker TB, Greenland JC (eds) Parkinson’s disease: pathogenesis and clinical aspects, 2019/02/01 edn. Codon Publications, Brisbane, pp 3–26. https://doi.org/10.15586/codonpublications.parkinsonsdisease.12018
Kouri N, Oshima K, Takahashi M, Murray ME, Ahmed Z, Parisi JE, Yen SH, Dickson DW (2013) Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: an unusual clinicopathologic variant of CBD. Acta Neuropathol 125:741–752
Kouri N, Carlomagno Y, Baker M, Liesinger AM, Caselli RJ, Wszolek ZK, Petrucelli L, Boeve BF, Parisi JE, Josephs KA, Uitti RJ, Ross OA, Graff-Radford NR, DeTure MA, Dickson DW, Rademakers R (2014) Novel mutation in MAPT exon 13 (p. N410H) causes corticobasal degeneration. Acta Neuropathol 127:271–282
Kouri N, Ross OA, Dombroski B, Younkin CS, Serie DJ, Soto-Ortolaza A, Baker M, Finch NC, Yoon H, Kim J, Fujioka S, McLean CA, Ghetti B, Spina S, Cantwell LB, Farlow MR, Grafman J, Huey ED, Ryung Han M, Beecher S, Geller ET, Kretzschmar HA, Roeber S, Gearing M, Juncos JL, Vonsattel JP, Van Deerlin VM, Grossman M, Hurtig HI, Gross RG, Arnold SE, Trojanowski JQ, Lee VM, Wenning GK, White CL, Hoglinger GU, Muller U, Devlin B, Golbe LI, Crook J, Parisi JE, Boeve BF, Josephs KA, Wszolek ZK, Uitti RJ, Graff-Radford NR, Litvan I, Younkin SG, Wang LS, Ertekin-Taner N, Rademakers R, Hakonarsen H, Schellenberg GD, Dickson DW (2015) Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun 6:7247
Kovacs GG, Rozemuller AJ, van Swieten JC, Gelpi E, Majtenyi K, Al-Sarraj S, Troakes C, Bodi I, King A, Hortobagyi T, Esiri MM, Ansorge O, Giaccone G, Ferrer I, Arzberger T, Bogdanovic N, Nilsson T, Leisser I, Alafuzoff I, Ironside JW, Kretzschmar H, Budka H (2013) Neuropathology of the hippocampus in FTLD-Tau with Pick bodies: a study of the BrainNet Europe Consortium. Neuropathol Appl Neurobiol 39:166–178
Kovacs GG, Robinson JL, Xie SX, Lee EB, Grossman M, Wolk DA, Irwin DJ, Weintraub D, Kim CF, Schuck T, Yousef A, Wagner ST, Suh E, Van Deerlin VM, Lee VM, Trojanowski JQ (2017) Evaluating the patterns of aging-related tau astrogliopathy unravels novel insights into brain aging and neurodegenerative diseases. J Neuropathol Exp Neurol 76:270–288
Kreisler A, Mastain B, Tison F, Fenelon G, Destee A (2007) Multi-infarct disorder presenting as corticobasal degeneration (DCB): vascular pseudo-corticobasal degeneration? Rev Neurol (Paris) 163:1191–1199
Krejciova Z, Carlson GA, Giles K, Prusiner SB (2019) Replication of multiple system atrophy prions in primary astrocyte cultures from transgenic mice expressing human alpha-synuclein. Acta Neuropathol Commun 7:81
Krismer F, Wenning GK (2017) Multiple system atrophy: insights into a rare and debilitating movement disorder. Nat Rev Neurol 13:232–243
Kruer MC (2013) The neuropathology of neurodegeneration with brain iron accumulation. Int Rev Neurobiol 110:165–194
Kübler D, Wachter T, Cabanel N, Su Z, Turkheimer FE, Dodel R, Brooks DJ, Oertel WH, Gerhard A (2019) Widespread microglial activation in multiple system atrophy. Mov Disord 34:564–568
Kujawska M, Jodynis-Liebert J (2018) What is the evidence that Parkinson’s disease is a prion disorder, which originates in the gut? Int J Mol Sci 19:3573
Kun-Rodrigues C, Orme T, Carmona S, Hernandez DG, Ross OA, Eicher JD, Shepherd C, Parkkinen L, Darwent L, Heckman MG, Scholz SW, Troncoso JC, Pletnikova O, Dawson T, Rosenthal L, Ansorge O, Clarimon J, Lleo A, Morenas-Rodriguez E, Clark L, Honig LS, Marder K, Lemstra A, Rogaeva E, St George-Hyslop P, Londos E, Zetterberg H, Barber I, Braae A, Brown K, Morgan K, Troakes C, Al-Sarraj S, Lashley T, Holton J, Compta Y, Van Deerlin V, Serrano GE, Beach TG, Lesage S, Galasko D, Masliah E, Santana I, Pastor P, Diez-Fairen M, Aguilar M, Tienari PJ, Myllykangas L, Oinas M, Revesz T, Lees A, Boeve BF, Petersen RC, Ferman TJ, Escott-Price V, Graff-Radford N, Cairns NJ, Morris JC, Pickering-Brown S, Mann D, Halliday GM, Hardy J, Trojanowski JQ, Dickson DW, Singleton A, Stone DJ, Guerreiro R, Bras J (2019) A comprehensive screening of copy number variability in dementia with Lewy bodies. Neurobiol Aging 75:223e1–223e10
Kuusisto E, Parkkinen L, Alafuzoff I (2003) Morphogenesis of Lewy bodies: dissimilar incorporation of alpha-synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol 62:1241–1253
Kuzumaki N, Suda Y, Iwasawa C, Narita M, Sone T, Watanabe M, Maekawa A, Matsumoto T, Akamatsu W, Igarashi K, Tamura H, Takeshima H, Tawfik VL, Ushijima T, Hattori N, Okano H (2019) Cell-specific overexpression of COMT in dopaminergic neurons of Parkinson’s disease. Brain 142:1675–1689
La Vitola P, Beeg M, Balducci C, Santamaria G, Restelli E, Colombo L, Caldinelli L, Pollegioni L, Gobbi M, Chiesa R, Forloni G (2019) Cellular prion protein neither binds to alpha-synuclein oligomers nor mediates their detrimental effects. Brain 142:249–254
Lam B, Khan A, Keith J, Rogaeva E, Bilbao J, St George-Hyslop P, Ghani M, Freedman M, Stuss DT, Chow T, Black SE, Masellis M (2018) Characterizing familial corticobasal syndrome due to Alzheimer’s disease pathology and PSEN1 mutations. Alzheimers Dement 13:520–530
Lanciego JL, Luquin N, Obeso JA (2012) Functional neuroanatomy of the basal ganglia. Cold Spring Harb Perspect Med 2:a009621
Lane EL (2019) l-DOPA for Parkinson’s disease-a bittersweet pill. Eur J Neurosci 49:384–398
Lang AE (2011) A critical appraisal of the premotor symptoms of Parkinson’s disease: potential usefulness in early diagnosis and design of neuroprotective trials. Mov Disord 26:775–783
Langston JW, Forno LS, Tetrud J, Reeves AG, Kaplan JA, Karluk D (1999) Evidence of active nerve cell degeneration in the substantia nigra of humans years after 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine exposure. Ann Neurol 46:598–605
Laurens B, Vergnet S, Lopez MC, Foubert-Samier A, Tison F, Fernagut PO, Meissner WG (2017) Multiple system atrophy—state of the art. Curr Neurol Neurosci Rep 17:41
Lautenschlager J, Stephens AD, Fusco G, Strohl F, Curry N, Zacharopoulou M, Michel CH, Laine R, Nespovitaya N, Fantham M, Pinotsi D, Zago W, Fraser P, Tandon A, St George-Hyslop P, Rees E, Phillips JJ, De Simone A, Kaminski CF, Schierle GSK (2018) C-terminal calcium binding of alpha-synuclein modulates synaptic vesicle interaction. Nat Commun 9:712
Lawton M, Baig F, Rolinski M, Ruffman C, Nithi K, May MT, Ben-Shlomo Y, Hu MT (2015) Parkinson’s disease subtypes in the Oxford Parkinson Disease Centre (OPDC) discovery cohort. J Parkinsons Dis 5:269–279
Lawton M, Ben-Shlomo Y, May MT, Baig F, Barber TR, Klein JC, Swallow DMA, Malek N, Grosset KA, Bajaj N, Barker RA, Williams N, Burn DJ, Foltynie T, Morris HR, Wood NW, Grosset DG, Hu MTM (2018) Developing and validating Parkinson’s disease subtypes and their motor and cognitive progression. J Neurol Neurosurg Psychiatry 89:1279–1287
Lee J-H, Lee M-S (2019) Iron accumulation in atypical parkinsonian syndromes: in vivo MRI evidences for distinctive patterns. Front Neurol 10:74. https://doi.org/10.3389/fneur.2019.00074
Lee MJ, Shin JH, Seoung JK, Lee JH, Yoon U, Oh JH, Jung DS, Kim EJ (2015) Cognitive impairments associated with morphological changes in cortical and subcortical structures in multiple system atrophy of the cerebellar type. Eur J Neurol 23:92–100
Lees AJ, Hardy J, Revesz T (2009) Parkinson’s disease. Lancet 373:2055–2066
Lehtonen S, Sonninen TM, Wojciechowski S, Goldsteins G, Koistinaho J (2019) Dysfunction of cellular proteostasis in Parkinson’s disease. Front Neurosci 13:457
Lema Tomé CM, Tyson T, Rey NL, Grathwohl S, Britschgi M, Brundin P (2013) Inflammation and alpha-synuclein’s prion-like behavior in Parkinson’s disease—is there a link? Mol Neurobiol 47:561–574
Lenka A, Ingalhalikar M, Shah A, Saini J, Arumugham SS, Hegde S, George L, Reddy V, Reddy YCJ, Yadav R, Pal PK (2018) Hippocampal subfield atrophy in patients with Parkinson’s disease and psychosis. J Neural Transm (Vienna) 125:1361–1372
Leverenz JB, Umar I, Wang Q, Montine TJ, McMillan PJ, Tsuang DW, Jin J, Pan C, Shin J, Zhu D, Zhang J (2007) Proteomic identification of novel proteins in cortical lewy bodies. Brain Pathol 17:139–145
Leverenz JB, Hamilton R, Tsuang DW, Schantz A, Vavrek D, Larson EB, Kukull WA, Lopez O, Galasko D, Masliah E, Kaye J, Woltjer R, Clark C, Trojanowski JQ, Montine TJ (2008) Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain Pathol 18:220–224
Li A, Paudel R, Johnson R, Courtney R, Lees AJ, Holton JL, Hardy J, Revesz T, Houlden H (2013) Pantothenate kinase-associated neurodegeneration is not a synucleinopathy. Neuropathol Appl Neurobiol 39:121–131
Li M, Ma Q, Zhao X, Wang C, Wu H, Li J, Yang W (2018) Dilemma of multiple system atrophy and spinocerebellar ataxias. J Neurol 265:2764–2772
Li X, Koudstaal W, Fletcher L, Costa M, van Winsen M, Siregar B, Inganas H, Kim J, Keogh E, Macedo J, Holland T, Perry S, Bard F, Hoozemans JJ, Goudsmit J, Apetri A, Pascual G (2019) Naturally occurring antibodies isolated from PD patients inhibit synuclein seeding in vitro and recognize Lewy pathology. Acta Neuropathol 137:825–836
Licker V, Turck N, Kovari E, Burkhardt K, Cote M, Surini-Demiri M, Lobrinus JA, Sanchez JC, Burkhard PR (2014) Proteomic analysis of human substantia nigra identifies novel candidates involved in Parkinson’s disease pathogenesis. Proteomics 14:784–794
Lill CM (2016) Genetics of Parkinson’s disease. Mol Cell Probes 30:386–396
Lim KL, Zhang CW (2013) Molecular events underlying Parkinson’s disease—an interwoven tapestry. Front Neurol 4:33
Lim EW, Aarsland D, Ffytche D, Taddei RN, van Wamelen DJ, Wan YM, Tan EK, Ray Chaudhuri K (2018) Amyloid-beta and Parkinson’s disease. J Neurol. https://doi.org/10.1007/s00415-00018-09100-00418
Lima VA, do Nascimento LA, Eliezer D, Follmer C (2019) Role of Parkinson’s disease-linked mutations and n-terminal acetylation on the oligomerization of alpha-synuclein induced by 3,4-dihydroxyphenylacetaldehyde. ACS Chem Neurosci 10:690–703
Limphaibool N, Iwanowski P, Holstad MJV, Perkowska K (2018) Parkinsonism in inherited metabolic disorders: key considerations and major features. Front Neurol 9:857
Lin YW, Truong D (2019) Diffuse Lewy body disease. J Neurol Sci 399:144–150
Lin CH, Tan EK, Yang CC, Yi Z, Wu RM (2015) COQ2 gene variants associate with cerebellar subtype of multiple system atrophy in Chinese. Mov Disord 30:436–437
Lin G, Wang L, Marcogliese PC, Bellen HJ (2019) Sphingolipids in the pathogenesis of Parkinson’s disease and parkinsonism. Trends Endocrinol Metab 30:106–117
Ling H, Holton JL, Lees AJ, Revesz T (2013) TDP-43 pathology is present in most post-encephalitic parkinsonism brains (Scientific correspondence). Neuropathol Appl Neurobiol 40:654–657
Ling H, de Silva R, Massey LA, Courtney R, Hondhamuni G, Bajaj N, Lowe J, Holton JL, Lees A, Revesz T (2014) Characteristics of progressive supranuclear palsy presenting with corticobasal syndrome: a cortical variant. Neuropathol Appl Neurobiol 40:149–163
Ling H, Asi YT, Petrovic IN, Ahmed Z, Prashanth LK, Hazrati LN, Nishizawa M, Ozawa T, Lang A, Lees AJ, Revesz T, Holton JL (2015) Minimal change multiple system atrophy: an aggressive variant? Mov Disord 30:960–967
Ling H, Kovacs GG, Vonsattel JP, Davey K, Mok KY, Hardy J, Morris HR, Warner TT, Holton JL, Revesz T (2016) Astrogliopathy predominates the earliest stage of corticobasal degeneration pathology. Brain 139:3237–3252
Lionnet A, Leclair-Visonneau L, Neunlist M, Murayama S, Takao M, Adler CH, Derkinderen P, Beach TG (2018) Does Parkinson’s disease start in the gut? Acta Neuropathol 135:1–12
Liu M, Choi DY, Hunter RL, Pandya JD, Cass WA, Sullivan PG, Kim HC, Gash DM, Bing G (2010) Trichloroethylene induces dopaminergic neurodegeneration in Fisher 344 rats. J Neurochem 112:773–783
Liu AKL, Lim EJ, Ahmed I, Chang RC, Pearce RKB, Gentleman SM (2018) Review: revisiting the human cholinergic nucleus of the diagonal band of Broca. Neuropathol Appl Neurobiol 44:647–662
Liu AKL, Chau TW, Lim EJ, Ahmed I, Chang RC, Kalaitzakis ME, Graeber MB, Gentleman SM, Pearce RKB (2019a) Hippocampal CA2 Lewy pathology is associated with cholinergic degeneration in Parkinson’s disease with cognitive decline. Acta Neuropathol Commun 7:61
Liu X, Hebron M, Shi W, Lonskaya I, Moussa CE (2019b) Ubiquitin specific protease-13 independently regulates parkin ubiquitination and alpha-synuclein clearance in alpha-synucleinopathies. Hum Mol Genet 28:548–560
Liu JQ, Chu SF, Zhou X, Zhang DY, Chen NH (2019c) Role of chemokines in Parkinson’s disease. Brain Res Bull. https://doi.org/10.1016/j.brainresbull.2019.1005.1020
Longhena F, Faustini G, Missale C, Pizzi M, Spano P, Bellucci A (2017) The contribution of alpha-synuclein spreading to Parkinson’s disease synaptopathy. Neural Plast 2017:5012129
Longhena F, Faustini G, Varanita T, Zaltieri M, Porrini V, Tessari I, Poliani PL, Missale C, Borroni B, Padovani A, Bubacco L, Pizzi M, Spano P, Bellucci A (2018) Synapsin III is a key component of alpha-synuclein fibrils in Lewy bodies of PD brains. Brain Pathol 28:875–888
Lopez KLR, Simpson JE, Watson LC, Mortiboys H, Hautbergue GM, Bandmann O, Highley JR (2019) TIGAR inclusion pathology is specific for Lewy body diseases. Brain Res 1706:218–223
Lu X, Kim-Han JS, Harmon S, Sakiyama-Elbert SE, O’Malley KL (2014) The Parkinsonian mimetic, 6-OHDA, impairs axonal transport in dopaminergic axons. Mol Neurodegener 9:17
Lubbe S, Morris HR (2014) Recent advances in Parkinson’s disease genetics. J Neurol 261:259–266
Ludtmann MHR, Angelova PR, Horrocks MH, Choi ML, Rodrigues M, Baev AY, Berezhnov AV, Yao Z, Little D, Banushi B, Al-Menhali AS, Ranasinghe RT, Whiten DR, Yapom R, Dolt KS, Devine MJ, Gissen P, Kunath T, Jaganjac M, Pavlov EV, Klenerman D, Abramov AY, Gandhi S (2018) Alpha-synuclein oligomers interact with ATP synthase and open the permeability transition pore in Parkinson’s disease. Nat Commun 9:2293
Lue L-F, Walker DG, Adler CH, Shill H, Tran H, Akiyama H, Sue LI, Caviness J, Sabbagh MN, Beach TG (2012) Biochemical increase in phosphorylated a-synuclein precedes histopathology of Lewy-type synucleinopathies. Brain Pathol 22:745–756
Lunati A, Lesage S, Brice A (2018) The genetic landscape of Parkinson’s disease. Rev Neurol (Paris) 174:628–643
Ma LY, Liu GL, Wang DX, Zhang MM, Kou WY, Feng T (2019) Alpha-synuclein in peripheral tissues in Parkinson’s disease. ACS Chem Neurosci 10:812–823
Maeda S, Sato Y, Takashima A (2018) Frontotemporal dementia with parkinsonism linked to chromosome-17 mutations enhance tau oligomer formation. Neurobiol Aging 69:26–32
Maffeo E, Montuschi A, Stura G, Giordana MT (2014) Chronic acquired hepatocerebral degeneration, pallidal T1 MRI hyperintensity and manganese in a series of cirrhotic patients. Neurol Sci 35:523–530
Maillet A, Krack P, Lhommee E, Metereau E, Klinger H, Favre E, Le Bars D, Schmitt E, Bichon A, Pelissier P, Fraix V, Castrioto A, Sgambato-Faure V, Broussolle E, Tremblay L, Thobois S (2016) The prominent role of serotonergic degeneration in apathy, anxiety and depression in de novo Parkinson’s disease. Brain 139:2486–2502
Maiti P, Manna J, Dunbar GL (2017) Current understanding of the molecular mechanisms in Parkinson’s disease: targets for potential treatments. Transl Neurodegener 6:28
Mak E, Donaghy PC, McKiernan E, Firbank MJ, Lloyd J, Petrides GS, Thomas AJ, O’Brien JT (2019) Beta amyloid deposition maps onto hippocampal and subiculum atrophy in dementia with Lewy bodies. Neurobiol Aging 73:74–81
Malandrini A, Rubegni A, Battisti C, Berti G, Federico A (2013) Electron-dense lamellated inclusions in 2 siblings with Kufor-Rakeb syndrome. Mov Disord 28:1751–1752
Mandel RJ, Marmion DJ, Kirik D, Chu Y, Heindel C, McCown T, Gray SJ, Kordower JH (2017) Novel oligodendroglial alpha synuclein viral vector models of multiple system atrophy: studies in rodents and nonhuman primates. Acta Neuropathol Commun 5:47
Maor G, Rapaport D, Horowitz M (2019) The effect of mutant GBA1 on accumulation and aggregation of alpha-synuclein. Hum Mol Genet 28:1768–1781
Marder K (2010) Cognitive impairment and dementia in Parkinson’s disease. Mov Disord 25(Suppl 1):S110–S116
Markesbery WR (2010) Neuropathologic alterations in mild cognitive impairment: a review. J Alzheimers Dis 19:221–228
Markesbery WR, Jicha GA, Liu H, Schmitt FA (2009) Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol 68:816–822
Markopoulou K, Dickson DW, McComb RD, Wszolek ZK, Katechalidou L, Avery L, Stansbury MS, Chase BA (2008) Clinical, neuropathological and genotypic variability in SNCA A53T familial Parkinson’s disease. Variability in familial Parkinson’s disease. Acta Neuropathol 116:25–35
Marras C, Alcalay RN, Caspell-Garcia C, Coffey C, Chan P, Duda JE, Facheris MF, Fernandez-Santiago R, Ruiz-Martinez J, Mestre T, Saunders-Pullman R, Pont-Sunyer C, Tolosa E, Waro B (2016) Motor and nonmotor heterogeneity of LRRK2-related and idiopathic Parkinson’s disease. Mov Disord 31:1192–1202
Marras C, Lang A, van de Warrenburg BP, Sue CM, Tabrizi SJ, Bertram L, Mercimek-Mahmutoglu S, Ebrahimi-Fakhari D, Warner TT, Durr A, Assmann B, Lohmann K, Kostic V, Klein C (2017) Nomenclature of genetic movement disorders: recommendations of the International Parkinson and Movement Disorder Society task force. Mov Disord 32:724–725
Marras C, Beck JC, Bower JH, Roberts E, Ritz B, Ross GW, Abbott RD, Savica R, Van Den Eeden SK, Willis AW, Tanner CM (2018) Prevalence of Parkinson’s disease across North America. NPJ Parkinsons Dis 4:21
Martin-Bastida A, Lao-Kaim NP, Roussakis AA, Searle GE, Xing Y, Gunn RN, Schwarz ST, Barker RA, Auer DP, Piccini P (2019) Relationship between neuromelanin and dopamine terminals within the Parkinson's nigrostriatal system. Brain. https://doi.org/10.1093/brain/awz1120
Masaracchia C, Hnida M, Gerhardt E, Lopes da Fonseca T, Villar-Pique A, Branco T, Stahlberg MA, Dean C, Fernandez CO, Milosevic I, Outeiro TF (2018) Membrane binding, internalization, and sorting of alpha-synuclein in the cell. Acta Neuropathol Commun 6:79
Masui K, Nakata Y, Fujii N, Iwaki T (2012) Extensive distribution of glial cytoplasmic inclusions in an autopsied case of multiple system atrophy with a prolonged 18-year clinical course. Neuropathology 32:69–76
Matsuo A, Akiguchi I, Lee GC, McGeer EG, McGeer PL, Kimura J (1998) Myelin degeneration in multiple system atrophy detected by unique antibodies. Am J Pathol 153:735–744
Matsusue E, Fujii S, Kanasaki Y, Sugihara S, Miyata H, Ohama E, Ogawa T (2008) Putaminal lesion in multiple system atrophy: postmortem MR-pathological correlations. Neuroradiology 50:559–567
Mavroeidi P, Arvanitaki F, Karakitsou AK, Vetsi M, Kloukina I, Zweckstetter M, Giller K, Becker S, Sorrentino ZA, Giasson BI, Jensen PH, Stefanis L, Xilouri M (2019) Endogenous oligodendroglial alpha-synuclein and TPPP/p25alpha orchestrate alpha-synuclein pathology in experimental multiple system atrophy models. Acta Neuropathol. https://doi.org/10.1007/s00401-00019-02014-y
McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J (2017) TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol 27:472–479
McCormack A, Chegeni N, Chegini F, Colella A, Power J, Keating D, Chataway T (2016) Purification of alpha-synuclein containing inclusions from human post mortem brain tissue. J Neurosci Methods 266:141–150
McCormack A, Keating DJ, Chegeni N, Colella A, Wang JJ, Chataway T (2019) Abundance of synaptic vesicle-related proteins in alpha-synuclein-containing protein inclusions suggests a targeted formation mechanism. Neurotox Res 35:883–897
McGregor MM, Nelson AB (2019) Circuit mechanisms of Parkinson’s disease. Neuron 101:1042–1056
McKee AC, Stein TD, Kiernan PT, Alvarez VE (2015) The neuropathology of chronic traumatic encephalopathy. Brain Pathol 25:350–364
McKee AC, Abdolmohammadi B, Stein TD (2018) The neuropathology of chronic traumatic encephalopathy. Handb Clin Neurol 158:297–307
McKeith I (2007) Dementia with Lewy bodies and Parkinson’s disease with dementia: where two worlds collide. Pract Neurol 7:374–382
McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1872
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K (2017) Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89:88–100
Mehanna R, Jankovic J (2013) Movement disorders in cerebrovascular disease. Lancet Neurol 12:597–608
Mehra S, Sahay S, Maji SK (2019) Alpha-synuclein misfolding and aggregation: implications in Parkinson’s disease pathogenesis. Biochim Biophys Acta Proteins Proteomics. https://doi.org/10.1016/j.bbapap.2019.1003.1001
Melzer TR, Stark MR, Keenan RJ, Myall DJ, MacAskill MR, Pitcher TL, Livingston L, Grenfell S, Horne KL, Young BN, Pascoe MJ, Almuqbel MM, Wang J, Marsh SH, Miller DH, Dalrymple-Alford JC, Anderson TJ (2019) Beta amyloid deposition is not associated with cognitive impairment in Parkinson’s disease. Front Neurol 10:391
Mendoza-Santiesteban CE, Palma JA, Martinez J, Norcliffe-Kaufmann L, Hedges TR 3rd, Kaufmann H (2015) Progressive retinal structure abnormalities in multiple system atrophy. Mov Disord 30:1944–1953
Mensikova K, Tuckova L, Kolarikova K, Bartonikova T, Vodicka R, Ehrmann J, Vrtel R, Prochazka M, Kanovsky P, Kovacs GG (2019) Atypical parkinsonism of progressive supranuclear palsy-parkinsonism (PSP-P) phenotype with rare variants in FBXO7 and VPS35 genes associated with Lewy body pathology. Acta Neuropathol 137:171–173
Meredith GE, Rademacher DJ (2011) MPTP mouse models of Parkinson’s disease: an update. J Parkinsons Dis 1:19–33
Michel PP, Hirsch EC, Hunot S (2016) Understanding dopaminergic cell death pathways in Parkinson disease. Neuron 90:675–691
Mietelska-Porowska A, Wasik U, Goras M, Filipek A, Niewiadomska G (2014) Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci 15:4671–4713
Mihaescu AS, Masellis M, Graff-Guerrero A, Kim J, Criaud M, Cho SS, Ghadery C, Valli M, Strafella AP (2018) Brain degeneration in Parkinson’s disease patients with cognitive decline: a coordinate-based meta-analysis. Brain Imaging Behav. https://doi.org/10.1007/s11682-11018-19922-11680
Mikasa M, Kanai K, Li Y, Yoshino H, Mogushi K, Hayashida A, Ikeda A, Kawajiri S, Okuma Y, Kashihara K, Sato T, Kondo H, Funayama M, Nishioka K, Hattori N (2018) COQ2 variants in Parkinson’s disease and multiple system atrophy. J Neural Transm (Vienna) 125:937–944
Miklossy J, Steele JC, Yu S, McCall S, Sandberg G, McGeer EG, McGeer PL (2008) Enduring involvement of tau, beta-amyloid, alpha-synuclein, ubiquitin and TDP-43 pathology in the amyotrophic lateral sclerosis/parkinsonism-dementia complex of Guam (ALS/PDC). Acta Neuropathol 116:625–637
Milosevic L, Gramer R, Kim TH, Algarni M, Fasano A, Kalia SK, Hodaie M, Lozano AM, Popovic MR, Hutchison WD (2019) Modulation of inhibitory plasticity in basal ganglia output nuclei of patients with Parkinson’s disease. Neurobiol Dis 124:46–56
Mimuro M, Yoshida M, Kuzuhara S, Kokubo Y (2018) Amyotrophic lateral sclerosis and parkinsonism-dementia complex of the Hohara focus of the Kii Peninsula: a multiple proteinopathy? Neuropathology 38:98–107
Mishima T, Koga S, Lin WL, Kasanuki K, Castanedes-Casey M, Wszolek ZK, Oh SJ, Tsuboi Y, Dickson DW (2017) Perry syndrome: a distinctive type of TDP-43 proteinopathy. J Neuropathol Exp Neurol 76:676–682
Mishima T, Fujioka S, Tomiyama H, Yabe I, Kurisaki R, Fujii N, Neshige R, Ross OA, Farrer MJ, Dickson DW, Wszolek ZK, Hattori N, Tsuboi Y (2018) Establishing diagnostic criteria for Perry syndrome. J Neurol Neurosurg Psychiatry 89:482–487
Mitsui J, Matsukawa T, Sasaki H, Yabe I, Matsushima M, Durr A, Brice A, Takashima H, Kikuchi A, Aoki M, Ishiura H, Yasuda T, Date H, Ahsan B, Iwata A, Goto J, Ichikawa Y, Nakahara Y, Momose Y, Takahashi Y, Hara K, Kakita A, Yamada M, Takahashi H, Onodera O, Nishizawa M, Watanabe H, Ito M, Sobue G, Ishikawa K, Mizusawa H, Kanai K, Hattori T, Kuwabara S, Arai K, Koyano S, Kuroiwa Y, Hasegawa K, Yuasa T, Yasui K, Nakashima K, Ito H, Izumi Y, Kaji R, Kato T, Kusunoki S, Osaki Y, Horiuchi M, Kondo T, Murayama S, Hattori N, Yamamoto M, Murata M, Satake W, Toda T, Filla A, Klockgether T, Wullner U, Nicholson G, Gilman S, Tanner CM, Kukull WA, Stern MB, Lee VM, Trojanowski JQ, Masliah E, Low PA, Sandroni P, Ozelius LJ, Foroud T, Tsuji S (2015) Variants associated with Gaucher disease in multiple system atrophy. Ann Clin Transl Neurol 2:417–426
Miyaji Y, Koyama K, Kurokawa T, Mitomi M, Suzuki Y, Kuroiwa Y (2013) Vascular corticobasal syndrome caused by unilateral internal carotid artery occlusion. J Stroke Cerebrovasc Dis 22:1193–1195
Molano J, Boeve B, Ferman T, Smith G, Parisi J, Dickson D, Knopman D, Graff-Radford N, Geda Y, Lucas J, Kantarci K, Shiung M, Jack C, Silber M, Pankratz VS, Petersen R (2010) Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study. Brain 133:540–556
Monzio Compagnoni G, Kleiner G, Samarani M, Aureli M, Faustini G, Bellucci A, Ronchi D, Bordoni A, Garbellini M, Salani S, Fortunato F, Frattini E, Abati E, Bergamini C, Fato R, Tabano S, Miozzo M, Serratto G, Passafaro M, Deleidi M, Silipigni R, Nizzardo M, Bresolin N, Comi GP, Corti S, Quinzii CM, Di Fonzo A (2018) Mitochondrial dysregulation and impaired autophagy in iPSC-derived dopaminergic neurons of multiple system atrophy. Stem Cell Rep 11:1185–1198
Mor DE, Tsika E, Mazzulli JR, Gould NS, Kim H, Daniels MJ, Doshi S, Gupta P, Grossman JL, Tan VX, Kalb RG, Caldwell KA, Caldwell GA, Wolfe JH, Ischiropoulos H (2017) Dopamine induces soluble alpha-synuclein oligomers and nigrostriatal degeneration. Nat Neurosci 20:1560–1568
Mor DE, Daniels MJ, Ischiropoulos H (2019) The usual suspects, dopamine and alpha-synuclein, conspire to cause neurodegeneration. Mov Disord 34:167–179
Morbelli S, Chincarini A, Brendel M, Rominger A, Bruffaerts R, Vandenberghe R, Kramberger MG, Trost M, Garibotto V, Nicastro N, Frisoni GB, Lemstra AW, van der Zande J, Pilotto A, Padovani A, Garcia-Ptacek S, Savitcheva I, Ochoa-Figueroa MA, Davidsson A, Camacho V, Peira E, Arnaldi D, Bauckneht M, Pardini M, Sambuceti G, Aarsland D, Nobili F (2019) Metabolic patterns across core features in dementia with Lewy bodies. Ann Neurol 85:715–725
Mori F, Inenaga C, Yoshimoto M, Umezu H, Tanaka R, Takahashi H, Wakabayashi K (2002) Alpha-synuclein immunoreactivity in normal and neoplastic Schwann cells. Acta Neuropathol 103:145–151
Mori F, Nishie M, Kakita A, Yoshimoto M, Takahashi H, Wakabayashi K (2006) Relationship among alpha-synuclein accumulation, dopamine synthesis, and neurodegeneration in Parkinson disease substantia nigra. J Neuropathol Exp Neurol 65:808–815
Mori F, Okada KI, Nomura T, Kobayashi Y (2016) The pedunculopontine tegmental nucleus as a motor and cognitive interface between the cerebellum and basal ganglia. Front Neuroanat 10:109
Morimoto S, Hatsuta H, Kokubo Y, Nakano Y, Hasegawa M, Yoneda M, Hirokawa Y, Kuzuhara S, Shiraishi T, Murayama S (2018) Unusual tau pathology of the cerebellum in patients with amyotrophic lateral sclerosis/parkinsonism-dementia complex from the Kii Peninsula, Japan. Brain Pathol 28:287–291
Morita K, Kawaguchi Y (2019) A dual role hypothesis of the cortico-basal-ganglia pathways: opponency and temporal difference through dopamine and adenosine. Front Neural Circuits 12:111
Mosharov EV, Borgkvist A, Sulzer D (2015) Presynaptic effects of levodopa and their possible role in dyskinesia. Mov Disord 30:45–53
Moskvina V, Harold D, Russo G, Vedernikov A, Sharma M, Saad M, Holmans P, Bras JM, Bettella F, Keller MF, Nicolaou N, Simon-Sanchez J, Gibbs JR, Schulte C, Durr A, Guerreiro R, Hernandez D, Brice A, Stefansson H, Majamaa K, Gasser T, Heutink P, Wood N, Martinez M, Singleton AB, Nalls MA, Hardy J, Owen MJ, O’Donovan MC, Williams J, Morris HR, Williams NM (2013) Analysis of genome-wide association studies of Alzheimer disease and of Parkinson disease to determine if these 2 diseases share a common genetic risk. JAMA Neurol 70:1268–1276
MSA-Research-Collaboration, Collaboration M-SAR (2013) Mutations in COQ2 in familial and sporadic multiple-system atrophy. N Engl J Med 369:233–244
Mudher A, Colin M, Dujardin S, Medina M, Dewachter I, Alavi Naini SM, Mandelkow EM, Mandelkow E, Buee L, Goedert M, Brion JP (2017) What is the evidence that tau pathology spreads through prion-like propagation? Acta Neuropathol Commun 5:99
Mulak A, Bonaz B (2015) Brain-gut-microbiota axis in Parkinson’s disease. World J Gastroenterol 21:10609–10620
Muller SK, Bender A, Laub C, Hogen T, Schlaudraff F, Liss B, Klopstock T, Elstner M (2013) Lewy body pathology is associated with mitochondrial DNA damage in Parkinson’s disease. Neurobiol Aging 34:2231–2233
Mullin S, Schapira A (2013) Alpha-synuclein and mitochondrial dysfunction in Parkinson’s disease. Mol Neurobiol 47:587–597
Mullin S, Hughes D, Mehta A, Schapira AHV (2019) Neurological effects of glucocerebrosidase gene mutations. Eur J Neurol 26:e329–e388
Munch G, Luth HJ, Wong A, Arendt T, Hirsch E, Ravid R, Riederer P (2000) Crosslinking of alpha-synuclein by advanced glycation endproducts—an early pathophysiological step in Lewy body formation? J Chem Neuroanat 20:253–257
Munoz DG, Morris HR, Rossor M (2011) Pick’s disease. In: Dickson DW, Weller RO (eds) Neurodegeneration: the molecular pathology of dementia and movement disorders, 2nd edn. Blackwell Publishing Ltd., Oxford, pp 156–164
Murray ME, Kouri N, Lin WL, Jack CR Jr, Dickson DW, Vemuri P (2014) Clinicopathologic assessment and imaging of tauopathies in neurodegenerative dementias. Alzheimers Res Ther 6:1
Naasan G, Shany-Ur T, Sidhu M, Barton C, Ketelle R, Shdo SM, Kramer JH, Miller BL, Seeley WW (2019) Corticobasal syndrome with visual hallucinations and probable REM-sleep behavior disorder: an autopsied case report of a patient with CBD and LBD pathology. Neurocase 25:26–33
Nakamoto FK, Okamoto S, Mitsui J, Sone T, Ishikawa M, Yamamoto Y, Kanegae Y, Nakatake Y, Imaizumi K, Ishiura H, Tsuji S, Okano H (2018) The pathogenesis linked to coenzyme Q10 insufficiency in iPSC-derived neurons from patients with multiple-system atrophy. Sci Rep 8:14215
Nakamura K, Mori F, Kon T, Tanji K, Miki Y, Tomiyama M, Kurotaki H, Toyoshima Y, Kakita A, Takahashi H, Yamada M, Wakabayashi K (2015) Filamentous aggregations of phosphorylated alpha-synuclein in Schwann cells (Schwann cell cytoplasmic inclusions) in multiple system atrophy. Acta Neuropathol Commun 3:29
Nakata Y, Yasuda T, Fukaya M, Yamamori S, Itakura M, Nihira T, Hayakawa H, Kawanami A, Kataoka M, Nagai M, Sakagami H, Takahashi M, Mizuno Y, Mochizuki H (2012) Accumulation of alpha-synuclein triggered by presynaptic dysfunction. J Neurosci 32:17186–17196
Narasimhan S, Guo JL, Changolkar L, Stieber A, McBride JD, Silva LV, He Z, Zhang B, Gathagan RJ, Trojanowski JQ, Lee VMY (2017) Pathological tau strains from human brains recapitulate the diversity of tauopathies in nontransgenic mouse brain. J Neurosci 37:11406–11423
Nardone R, Holler Y, Brigo F, Versace V, Sebastianelli L, Florea C, Schwenker K, Golaszewski S, Saltuari L, Trinka E (2019) Spinal cord involvement in Lewy body-related alpha-synucleinopathies. J Spinal Cord Med. https://doi.org/10.1080/10790268.10792018.11557863
Ndayisaba A, Jellinger K, Berger T, Wenning GK (2019) TNFalpha inhibitors as targets for protective therapies in MSA: a viewpoint. J Neuroinflamm 16:80
Nejad-Davarani S, Koeppe RA, Albin RL, Frey KA, Muller M, Bohnen NI (2019) Quantification of brain cholinergic denervation in dementia with Lewy bodies using PET imaging with [(18)F]-FEOBV. Mol Psychiatry 24:322–327
Nervi A, Reitz C, Tang MX, Santana V, Piriz A, Reyes D, Lantigua R, Medrano M, Jimenez-Velazquez IZ, Lee JH, Mayeux R (2011) Familial aggregation of dementia with Lewy bodies. Arch Neurol 68:90–93
Neumann M, Mackenzie IRA (2019) Review: neuropathology of non-tau frontotemporal lobar degeneration. Neuropathol Appl Neurobiol 45:19–40
Neumann WJ, Schroll H, de Almeida Marcelino AL, Horn A, Ewert S, Irmen F, Krause P, Schneider GH, Hamker F, Kuhn AA (2018) Functional segregation of basal ganglia pathways in Parkinson’s disease. Brain 141:2655–2669
Nguyen M, Wong YC, Ysselstein D, Severino A, Krainc D (2019) Synaptic, mitochondrial, and lysosomal dysfunction in Parkinson’s disease. Trends Neurosci 42:140–149
Ni Z, Pinto AD, Lang AE, Chen R (2010) Involvement of the cerebellothalamocortical pathway in Parkinson disease. Ann Neurol 68:816–824
Nishida N, Yoshida K, Hata Y, Arai Y, Kinoshita K (2015) Pathological features of preclinical or early clinical stages of corticobasal degeneration: a comparison with advanced cases. Neuropathol Appl Neurobiol 41:893–905
Niu H, Shen L, Li T, Ren C, Ding S, Wang L, Zhang Z, Liu X, Zhang Q, Geng D, Wu X, Li H (2018) Alpha-synuclein overexpression in the olfactory bulb initiates prodromal symptoms and pathology of Parkinson’s disease. Transl Neurodegener 7:25
Nouraei N, Mason DM, Miner KM, Carcella MA, Bhatia TN, Dumm BK, Soni D, Johnson DA, Luk KC, Leak RK (2018) Critical appraisal of pathology transmission in the alpha-synuclein fibril model of Lewy body disorders. Exp Neurol 299:172–196
Nussbaum RL (2018) Genetics of synucleinopathies. Cold Spring Harb Perspect Med 8:78. https://doi.org/10.1101/cshperspect.a024109
Nykjaer C, Brudek T, Salvesen L, Pakkenberg B (2017) Changes in the cell population in brain white matter in multiple system atrophy. Mov Disord 32:1074–1082
Obeso JA, Stamelou M, Goetz CG, Poewe W, Lang AE, Weintraub D, Burn D, Halliday GM, Bezard E, Przedborski S, Lehericy S, Brooks DJ, Rothwell JC, Hallett M, DeLong MR, Marras C, Tanner CM, Ross GW, Langston JW, Klein C, Bonifati V, Jankovic J, Lozano AM, Deuschl G, Bergman H, Tolosa E, Rodriguez-Violante M, Fahn S, Postuma RB, Berg D, Marek K, Standaert DG, Surmeier DJ, Olanow CW, Kordower JH, Calabresi P, Schapira AHV, Stoessl AJ (2017) Past, present, and future of Parkinson’s disease: a special essay on the 200th Anniversary of the Shaking Palsy. Mov Disord 32:1264–1310
Odagiri S, Tanji K, Mori F, Kakita A, Takahashi H, Wakabayashi K (2012) Autophagic adapter protein NBR1 is localized in Lewy bodies and glial cytoplasmic inclusions and is involved in aggregate formation in alpha-synucleinopathy. Acta Neuropathol 124:173–186
Ogaki K, Martens YA, Heckman MG, Koga S, Labbe C, Lorenzo-Betancor O, Wernick AI, Walton RL, Soto AI, Vargas ER, Nielsen HM, Fujioka S, Kanekiyo T, Uitti RJ, van Gerpen JA, Cheshire WP, Wszolek ZK, Low PA, Singer W, Dickson DW, Bu G, Ross OA (2018) Multiple system atrophy and apolipoprotein E. Mov Disord 33:647–650
Oinas M, Paetau A, Myllykangas L, Notkola IL, Kalimo H, Polvikoski T (2010) Alpha-synuclein pathology in the spinal cord autonomic nuclei associates with alpha-synuclein pathology in the brain: a population-based Vantaa 85+ study. Acta Neuropathol 119:715–722
O’Keeffe GW, Sullivan AM (2018) Evidence for dopaminergic axonal degeneration as an early pathological process in Parkinson’s disease. Parkinsonism Relat Disord 56:9–15
Olah J, Ovadi J (2019) Pharmacological targeting of alpha-synuclein and TPPP/p25 in Parkinson’s disease: challenges and opportunities in a Nutshell. FEBS Lett. https://doi.org/10.1002/1873-3468.13464
Olah J, Bertrand P, Ovadi J (2017) Role of the microtubule-associated TPPP/p25 in Parkinson’s and related diseases and its therapeutic potential. Expert Rev Proteom 14:301–309
Olanow CW, Savolainen M, Chu Y, Halliday GM, Kordower JH (2019) Temporal evolution of microglia and alpha-synuclein accumulation following foetal grafting in Parkinson’s disease. Brain 142:1690–1700
Oppedal K, Ferreira D, Cavallin L, Lemstra AW, Ten Kate M, Padovani A, Rektorova I, Bonanni L, Wahlund LO, Engedal K, Nobili F, Kramberger M, Taylor JP, Hort J, Snaedal J, Blanc F, Walker Z, Antonini A, Westman E, Aarsland D (2019) A signature pattern of cortical atrophy in dementia with Lewy bodies: a study on 333 patients from the European DLB consortium. Alzheimers Dement 15:400–409
O’Regan G, deSouza RM, Balestrino R, Schapira AH (2017) Glucocerebrosidase mutations in Parkinson disease. J Parkinsons Dis 7:411–422
Orimo S, Uchihara T, Nakamura A, Mori F, Kakita A, Wakabayashi K, Takahashi H (2008) Axonal alpha-synuclein aggregates herald centripetal degeneration of cardiac sympathetic nerve in Parkinson’s disease. Brain 131:642–650
Orme T, Guerreiro R, Bras J (2018) The genetics of dementia with Lewy bodies: current understanding and future directions. Curr Neurol Neurosci Rep 18:67
Ortega-de San Luis C, Sanchez-Garcia MA, Nieto-Gonzalez JL, Garcia-Junco-Clemente P, Montero-Sanchez A, Fernandez-Chacon R, Pascual A (2018) Substantia nigra dopaminergic neurons and striatal interneurons are engaged in three parallel but interdependent postnatal neurotrophic circuits. Aging Cell 17:e12821
Ortuno-Lizaran I, Esquiva G, Beach TG, Serrano GE, Adler CH, Lax P, Cuenca N (2018) Degeneration of human photosensitive retinal ganglion cells may explain sleep and circadian rhythms disorders in Parkinson’s disease. Acta Neuropathol Commun 6:90
Osaki Y, Ben-Shlomo Y, Lees AJ, Wenning GK, Quinn NP (2009) A validation exercise on the new consensus criteria for multiple system atrophy. Mov Disord 24:2272–2276
Ou R, Wei Q, Hou Y, Yuan X, Song W, Cao B, Liu H, Zhang L, Chen Y, Shang H (2018) Vascular risk factors and depression in Parkinson’s disease. Eur J Neurol 25:637–643
Ouchi H, Toyoshima Y, Tada M, Oyake M, Aida I, Tomita I, Satoh A, Tsujihata M, Takahashi H, Nishizawa M, Shimohata T (2014) Pathology and sensitivity of current clinical criteria in corticobasal syndrome. Mov Disord 29:238–244
Outeiro TF, Harvey K, Dominguez-Meijide A, Gerhardt E (2019) LRRK2, alpha-synuclein, and tau: partners in crime or unfortunate bystanders? Biochem Soc Trans. https://doi.org/10.1042/BST20180466
Outeiro TF, Koss DJ, Erskine D, Walker L, Kurzawa-Akanbi M, Burn D, Donaghy P, Morris C, Taylor JP, Thomas A, Attems J, McKeith I (2019b) Dementia with Lewy bodies: an update and outlook. Mol Neurodegener 14:5
Overk C, Rockenstein E, Valera E, Stefanova N, Wenning G, Masliah E (2018) Multiple system atrophy: experimental models and reality. Acta Neuropathol 135:33–47
Oyanagi K, Hashimoto T, Yamazaki M (2011) Parkinsonism-dementia complex of Guam. In: Dickson DW, Weller RO (eds) Neurodegeneration: the molecular pathology of dementia and movement disorders, 2nd edn. Blackwell Publishing Ltd., Oxford, pp 171–178
Ozawa T (2007) Morphological substrate of autonomic failure and neurohormonal dysfunction in multiple system atrophy: impact on determining phenotype spectrum. Acta Neuropathol (Berl) 114:201–211
Ozawa T, Onodera O (2017) Multiple system atrophy: clinicopathological characteristics in Japanese patients. Proc Jpn Acad Ser B Phys Biol Sci 93:251–258
Pagano G, Niccolini F, Politis M (2018) The serotonergic system in Parkinson’s patients with dyskinesia: evidence from imaging studies. J Neural Transm (Vienna) 125:1217–1223
Paisan-Ruiz C, Guevara R, Federoff M, Hanagasi H, Sina F, Elahi E, Schneider SA, Schwingenschuh P, Bajaj N, Emre M, Singleton AB, Hardy J, Bhatia KP, Brandner S, Lees AJ, Houlden H (2010) Early-onset l-dopa-responsive parkinsonism with pyramidal signs due to ATP13A2, PLA2G6, FBXO7 and spatacsin mutations. Mov Disord 25:1791–1800
Pan T, Kondo S, Le W, Jankovic J (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131:1969–1978
Pan PY, Zhu Y, Shen Y, Yue Z (2019) Crosstalk between presynaptic trafficking and autophagy in Parkinson’s disease. Neurobiol Dis 122:64–71
Pan-Montojo F, Anichtchik O, Dening Y, Knels L, Pursche S, Jung R, Jackson S, Gille G, Spillantini MG, Reichmann H, Funk RH (2010) Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS One 5:e8762
Paolicelli RC, Bergamini G, Rajendran L (2019) Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience 405:148–157
Papa SM, Wichmann T (2015) Interaction between hyperdirect and indirect basal ganglia pathways. Mov Disord 30:909
Park JS, Blair NF, Sue CM (2015) The role of ATP13A2 in Parkinson’s disease: clinical phenotypes and molecular mechanisms. Mov Disord 30:770–779
Parkkinen L, Hartikainen P, Alafuzoff I (2007) Abundant glial alpha-synuclein pathology in a case without overt clinical symptoms. Clin Neuropathol 26:276–283
Parkkinen L, Pirttila T, Alafuzoff I (2008) Applicability of current staging/categorization of alpha-synuclein pathology and their clinical relevance. Acta Neuropathol 115:399–407
Parkkinen L, O’Sullivan SS, Collins C, Petrie A, Holton JL, Revesz T, Lees AJ (2011) Disentangling the relationship between Lewy bodies and nigral neuronal loss in Parkinson’s disease. J Parkinsons Dis 1:277–286
Pasquini J, Ceravolo R, Qamhawi Z, Lee JY, Deuschl G, Brooks DJ, Bonuccelli U, Pavese N (2018) Progression of tremor in early stages of Parkinson’s disease: a clinical and neuroimaging study. Brain 141:811–821
Paulus W, Jellinger K (1991) The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol 50:743–755
Pedersen KM, Marner L, Pakkenberg H, Pakkenberg B (2005) No global loss of neocortical neurons in parkinson’s disease: a quantitative stereological study. Mov Disord 20:164–171
Peelaerts W, Bousset L, Baekelandt V, Melki R (2018) a-Synuclein strains and seeding in Parkinson’s disease, incidental Lewy body disease, dementia with Lewy bodies and multiple system atrophy: similarities and differences. Cell Tissue Res 373:195–212
Pelzer EA, Melzer C, Timmermann L, von Cramon DY, Tittgemeyer M (2017) Basal ganglia and cerebellar interconnectivity within the human thalamus. Brain Struct Funct 222:381–392
Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL, Zhang B, Pitkin RM, Olufemi MF, Luk KC, Trojanowski JQ, Lee VM (2018a) Cellular milieu imparts distinct pathological alpha-synuclein strains in alpha-synucleinopathies. Nature 557:558–563
Peng C, Gathagan RJ, Lee VM (2018b) Distinct alpha-synuclein strains and implications for heterogeneity among alpha-synucleinopathies. Neurobiol Dis 109:209–218
Perez XA, Zhang D, Bordia T, Quik M (2017) Striatal D1 medium spiny neuron activation induces dyskinesias in parkinsonian mice. Mov Disord 32:538–548
Perez XA, Bordia T, Quik M (2018) The striatal cholinergic system in l-dopa-induced dyskinesias. J Neural Transm (Vienna) 125:1251–1262
Perez-Pardo P, Kliest T, Dodiya HB, Broersen LM, Garssen J, Keshavarzian A, Kraneveld AD (2017) The gut-brain axis in Parkinson’s disease: possibilities for food-based therapies. Eur J Pharmacol 817:86–95
Perl DP, Olanow CW (2007) The neuropathology of manganese-induced parkinsonism. J Neuropathol Exp Neurol 66:675–682
Pessoa Rocha N, Reis HJ, Vanden Berghe P, Cirillo C (2014) Depression and cognitive impairment in Parkinson’s disease: a role for inflammation and immunomodulation? Neuroimmunomodulation 21:88–94
Petersen RC, Parisi JE, Dickson DW, Johnson KA, Knopman DS, Boeve BF, Jicha GA, Ivnik RJ, Smith GE, Tangalos EG, Braak H, Kokmen E (2006) Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol 63:665–672
Petrovic IN, Ling H, Asi Y, Ahmed Z, Kukkle PL, Hazrati LN, Lang AE, Revesz T, Holton JL, Lees AJ (2012) Multiple system atrophy-parkinsonism with slow progression and prolonged survival: a diagnostic catch. Mov Disord 27:1186–1190
Picconi B, Paille V, Ghiglieri V, Bagetta V, Barone I, Lindgren HS, Bernardi G, Angela Cenci M, Calabresi P (2008) l-DOPA dosage is critically involved in dyskinesia via loss of synaptic depotentiation. Neurobiol Dis 29:327–335
Picillo M, Lizarraga KJ, Friesen EL, Chau H, Zhang M, Sato C, Rooke G, Munhoz RP, Rogaeva E, Fraser PE, Kalia SK, Kalia LV (2018) Parkinsonism due to A53E alpha-synuclein gene mutation: clinical, genetic, epigenetic, and biochemical features. Mov Disord 33:1950–1955
Pietracupa S, Martin-Bastida A, Piccini P (2017) Iron metabolism and its detection through MRI in parkinsonian disorders: a systematic review. Neurol Sci 38:2095–2101
Pihlstrom L, Blauwendraat C, Cappelletti C, Berge-Seidl V, Langmyhr M, Henriksen SP, van de Berg WDJ, Gibbs JR, Cookson MR, Singleton AB, Nalls MA, Toft M (2018) A comprehensive analysis of SNCA-related genetic risk in sporadic Parkinson disease. Ann Neurol 84:117–129
Pinho R, Paiva I, Jercic KG, Fonseca-Ornelas L, Gerhardt E, Fahlbusch C, Garcia-Esparcia P, Kerimoglu C, Pavlou MAS, Villar-Pique A, Szego E, Lopes da Fonseca T, Odoardi F, Soeroes S, Rego AC, Fischle W, Schwamborn JC, Meyer T, Kugler S, Ferrer I, Attems J, Fischer A, Becker S, Zweckstetter M, Borovecki F, Outeiro TF (2019) Nuclear localization and phosphorylation modulate pathological effects of alpha-synuclein. Hum Mol Genet 28:31–50
Poletti M, Frosini D, Pagni C, Baldacci F, Nicoletti V, Tognoni G, Lucetti C, Del Dotto P, Ceravolo R, Bonuccelli U (2012) Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 83:601–606
Polinski NK, Volpicelli-Daley LA, Sortwell CE, Luk KC, Cremades N, Gottler LM, Froula J, Duffy MF, Lee VMY, Martinez TN, Dave KD (2018) Best practices for generating and using alpha-synuclein pre-formed fibrils to model Parkinson’s disease in rodents. J Parkinsons Dis 8:303–322
Politis M, Wu K, Loane C, Kiferle L, Molloy S, Brooks DJ, Piccini P (2010) Staging of serotonergic dysfunction in Parkinson’s disease: an in vivo 11C-DASB PET study. Neurobiol Dis 40:216–221
Politis M, Wu K, Loane C, Brooks DJ, Kiferle L, Turkheimer FE, Bain P, Molloy S, Piccini P (2014) Serotonergic mechanisms responsible for levodopa-induced dyskinesias in Parkinson’s disease patients. J Clin Investig 124:1340–1349
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL (1997) Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease. Science 276:2045–2047
Pont-Sunyer C, Tolosa E, Caspell-Garcia C, Coffey C, Alcalay RN, Chan P, Duda JE, Facheris M, Fernandez-Santiago R, Marek K, Lomena F, Marras C, Mondragon E, Saunders-Pullman R, Waro B (2017) The prodromal phase of leucine-rich repeat kinase 2-associated Parkinson disease: clinical and imaging Studies. Mov Disord 32:726–738
Poplawska-Domaszewicz K, Florczak-Wyspianska J, Kozubski W, Michalak S (2018) Paraneoplastic movement disorders. Rev Neurosci 29:745–755
Post MR, Lieberman OJ, Mosharov EV (2018) Can interactions between alpha-synuclein, dopamine and calcium explain selective neurodegeneration in Parkinson’s disease? Front Neurosci 12:161
Power JH, Barnes OL, Chegini F (2017) Lewy bodies and the mechanisms of neuronal cell death in Parkinson’s disease and dementia with Lewy bodies. Brain Pathol 27:3–12
Prasad V, Wasser Y, Hans F, Goswami A, Katona I, Outeiro TF, Kahle PJ, Schulz JB, Voigt A (2019) Monitoring alpha-synuclein multimerization in vivo. FASEB J 33:2116–2131
Prots I, Grosch J, Brazdis RM, Simmnacher K, Veber V, Havlicek S, Hannappel C, Krach F, Krumbiegel M, Schutz O, Reis A, Wrasidlo W, Galasko DR, Groemer TW, Masliah E, Schlotzer-Schrehardt U, Xiang W, Winkler J, Winner B (2018) Alpha-synuclein oligomers induce early axonal dysfunction in human iPSC-based models of synucleinopathies. Proc Natl Acad Sci USA 115:7813–7818
Puska G, Lutz MI, Molnar K, Regelsberger G, Ricken G, Pirker W, Laszlo L, Kovacs GG (2018) Lysosomal response in relation to alpha-synuclein pathology differs between Parkinson’s disease and multiple system atrophy. Neurobiol Dis 114:140–152
Qamhawi Z, Towey D, Shah B, Pagano G, Seibyl J, Marek K, Borghammer P, Brooks DJ, Pavese N (2015) Clinical correlates of raphe serotonergic dysfunction in early Parkinson’s disease. Brain 138:2964–2973
Quadri M, Mandemakers W, Grochowska MM, Masius R, Geut H, Fabrizio E, Breedveld GJ, Kuipers D, Minneboo M, Vergouw LJM, Carreras Mascaro A, Yonova-Doing E, Simons E, Zhao T, Di Fonzo AB, Chang HC, Parchi P, Melis M, Correia Guedes L, Criscuolo C, Thomas A, Brouwer RWW, Heijsman D, Ingrassia AMT, Calandra Buonaura G, Rood JP, Capellari S, Rozemuller AJ, Sarchioto M, Fen Chien H, Vanacore N, Olgiati S, Wu-Chou YH, Yeh TH, Boon AJW, Hoogers SE, Ghazvini M, Ijpma AS, van Ijcken WFJ, Onofrj M, Barone P, Nicholl DJ, Puschmann A, De Mari M, Kievit AJ, Barbosa E, De Michele G, Majoor-Krakauer D, van Swieten JC, de Jong FJ, Ferreira JJ, Cossu G, Lu CS, Meco G, Cortelli P, van de Berg WDJ, Bonifati V (2018) LRP10 genetic variants in familial Parkinson’s disease and dementia with Lewy bodies: a genome-wide linkage and sequencing study. Lancet Neurol 17:597–608
Quinn JG, Coulson DT, Brockbank S, Beyer N, Ravid R, Hellemans J, Irvine GB, Johnston JA (2012) Alpha-synuclein mRNA and soluble alpha-synuclein protein levels in post-mortem brain from patients with Parkinson’s disease, dementia with Lewy bodies, and Alzheimer’s disease. Brain Res 1459:71–80
Quinzii CM, Hirano M, DiMauro S (2014) Mutant COQ2 in multiple-system atrophy. N Engl J Med 371:81–82
Racette BA, Criswell SR, Lundin JI, Hobson A, Seixas N, Kotzbauer PT, Evanoff BA, Perlmutter JS, Zhang J, Sheppard L, Checkoway H (2012) Increased risk of parkinsonism associated with welding exposure. Neurotoxicology 33:1356–1361
Rajput AH, Sitte HH, Rajput A, Fenton ME, Pifl C, Hornykiewicz O (2008) Globus pallidus dopamine and Parkinson motor subtypes: clinical and brain biochemical correlation. Neurology 70:1403–1410
Ray NJ, Bradburn S, Murgatroyd C, Toseeb U, Mir P, Kountouriotis GK, Teipel SJ, Grothe MJ (2018) In vivo cholinergic basal forebrain atrophy predicts cognitive decline in de novo Parkinson’s disease. Brain 141:165–176
Rebeiz JJ, Kolodny EH, Richardson EP Jr (1968) Corticodentatonigral degeneration with neuronal achromasia. Arch Neurol 18:20–33
Reed X, Bandres-Ciga S, Blauwendraat C, Cookson MR (2019) The role of monogenic genes in idiopathic Parkinson’s disease. Neurobiol Dis 124:230–239
Reetz K, Gaser C, Klein C, Hagenah J, Buchel C, Gottschalk S, Pramstaller PP, Siebner HR, Binkofski F (2009) Structural findings in the basal ganglia in genetically determined and idiopathic Parkinson’s disease. Mov Disord 24:99–103
Refolo V, Bez F, Polissidis A, Kuzdas-Wood D, Sturm E, Kamaratou M, Poewe W, Stefanis L, Angela Cenci M, Romero-Ramos M, Wenning GK, Stefanova N (2018) Progressive striatonigral degeneration in a transgenic mouse model of multiple system atrophy: translational implications for interventional therapies. Acta Neuropathol Commun 6:2
Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF (2008) A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord 23:183–189 (quiz 313)
Rektor I, Bohnen NI, Korczyn AD, Gryb V, Kumar H, Kramberger MG, de Leeuw FE, Pirtosek Z, Rektorova I, Schlesinger I, Slawek J, Valkovic P, Vesely B (2018) An updated diagnostic approach to subtype definition of vascular parkinsonism—recommendations from an expert working group. Parkinsonism Relat Disord 49:9–16
Ren S, Zhang H, Zheng W, Liu M, Gao F, Wang Z, Chen Z (2019) Altered functional connectivity of cerebello-cortical circuit in multiple system atrophy (cerebellar-type). Front Neurosci 12:996
Respondek G, Kurz C, Arzberger T, Compta Y, Englund E, Ferguson LW, Gelpi E, Giese A, Irwin DJ, Meissner WG, Nilsson C, Pantelyat A, Rajput A, van Swieten JC, Troakes C, Josephs KA, Lang AE, Mollenhauer B, Muller U, Whitwell JL, Antonini A, Bhatia KP, Bordelon Y, Corvol JC, Colosimo C, Dodel R, Grossman M, Kassubek J, Krismer F, Levin J, Lorenzl S, Morris H, Nestor P, Oertel WH, Rabinovici GD, Rowe JB, van Eimeren T, Wenning GK, Boxer A, Golbe LI, Litvan I, Stamelou M, Hoglinger GU (2017) Which ante mortem clinical features predict progressive supranuclear palsy pathology? Mov Disord 32:995–1005
Rey NL, Wesson DW, Brundin P (2018) The olfactory bulb as the entry site for prion-like propagation in neurodegenerative diseases. Neurobiol Dis 109:226–248
Reyes JF, Rey NL, Bousset L, Melki R, Brundin P, Angot E (2014) Alpha-synuclein transfers from neurons to oligodendrocytes. Glia 62:387–398
Reyes JF, Sackmann C, Hoffmann A, Svenningsson P, Winkler J, Ingelsson M, Hallbeck M (2019) Binding of alpha-synuclein oligomers to Cx32 facilitates protein uptake and transfer in neurons and oligodendrocytes. Acta Neuropathol. https://doi.org/10.1007/s00401-00019-02007-x
Reynolds RH, Botia J, Nalls MA, Hardy J, Gagliano Taliun SA, Ryten M (2019) Moving beyond neurons: the role of cell type-specific gene regulation in Parkinson’s disease heritability. NPJ Parkinsons Dis 5:6
Rietdijk CD, Perez-Pardo P, Garssen J, van Wezel RJ, Kraneveld AD (2017) Exploring Braak’s hypothesis of Parkinson’s disease. Front Neurol 8:37
Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G (2016) Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology 86:566–576
Rizzo G, Arcuti S, Copetti M, Alessandria M, Savica R, Fontana A, Liguori R, Logroscino G (2018) Accuracy of clinical diagnosis of dementia with Lewy bodies: a systematic review and meta-analysis. J Neurol Neurosurg Psychiatry 89:358–366
Robakis D, Cortes E, Clark LN, Vonsattel JP, Virmani T, Alcalay RN, Crary JF, Levy OA (2016) The effect of MAPT haplotype on neocortical Lewy body pathology in Parkinson disease. J Neural Transm (Vienna) 123:583–588
Roberts HL, Brown DR (2015) Seeking a mechanism for the toxicity of oligomeric alpha-synuclein. Biomolecules 5:282–305
Robinson JL, Lee EB, Xie SX, Rennert L, Suh E, Bredenberg C, Caswell C, Van Deerlin VM, Yan N, Yousef A, Hurtig HI, Siderowf A, Grossman M, McMillan CT, Miller B, Duda JE, Irwin DJ, Wolk D, Elman L, McCluskey L, Chen-Plotkin A, Weintraub D, Arnold SE, Brettschneider J, Lee VM, Trojanowski JQ (2018) Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 141:2181–2193
Rocha Cabrero F, Morrison EH (2019) Lewy Bodies, 2019/02/07 edn. StatPearls Publishing, Treasure Island
Rocha EM, De Miranda B, Sanders LH (2018) Alpha-synuclein: pathology, mitochondrial dysfunction and neuroinflammation in Parkinson’s disease. Neurobiol Dis 109:249–257
Rockenstein E, Nuber S, Overk CR, Ubhi K, Mante M, Patrick C, Adame A, Trejo-Morales M, Gerez J, Picotti P, Jensen PH, Campioni S, Riek R, Winkler J, Gage HH, Winner B, Masliah E (2014) Accumulation of oligomer-prone a-synuclein exacerbates synaptic and neuronal degeneration in vivo. Brain 137:1496–1513
Rohan Z, Rahimi J, Weis S, Kapas I, Auff E, Mitrovic N, Liberski PP, Sikorska B, Matej R, Kovacs GG (2015) Screening for alpha-synuclein immunoreactive neuronal inclusions in the hippocampus allows identification of atypical MSA (FTLD-synuclein). Acta Neuropathol 130:299–301
Rohan Z, Milenkovic I, Lutz MI, Matej R, Kovacs GG (2016) Shared and distinct patterns of oligodendroglial response in alpha-synucleinopathies and tauopathies. J Neuropathol Exp Neurol 75:1100–1109
Rohani M, Lang AE, Sina F, Elahi E, Fasano A, Hardy J, Bras J, Alavi A (2017) Action myoclonus and seizure in Kufor–Rakeb syndrome. Mov Disord Clin Pract 5:195–199
Ronchi D, Di Biase E, Franco G, Melzi V, Del Sorbo F, Elia A, Barzaghi C, Garavaglia B, Bergamini C, Fato R, Mora G, Del Bo R, Fortunato F, Borellini L, Trezzi I, Compagnoni GM, Monfrini E, Frattini E, Bonato S, Cogiamanian F, Ardolino G, Priori A, Bresolin N, Corti S, Comi GP, Di Fonzo A (2016) Mutational analysis of COQ2 in patients with MSA in Italy. Neurobiol Aging 45:213e1–213e2
Rongve A, Aarsland D (2013) Dementia in Parkinson’s disease and dementia with Lewy bodies. In: Dening T, Thomas A (eds) Oxford textbook of old age psychiatry 2e. Oxford University Press, Oxford, pp 469–478
Rosborough K, Patel N, Kalia LV (2017) Alpha-synuclein and parkinsonism: updates and future perspectives. Curr Neurol Neurosci Rep 17:31
Rosqvist K, Horne M, Hagell P, Iwarsson S, Nilsson MH, Odin P (2018) Levodopa effect and motor function in late stage Parkinson’s disease. J Parkinsons Dis 8:59–70
Rudakou U, Ouled Amar Bencheikh B, Ruskey JA, Krohn L, Laurent SB, Spiegelman D, Liong C, Fahn S, Waters C, Monchi O, Fon EA, Dauvilliers Y, Alcalay RN, Dupre N, Gan-Or Z (2019) Common and rare GCH1 variants are associated with Parkinson’s disease. Neurobiol Aging 73:231
Rudow G, O’Brien R, Savonenko AV, Resnick SM, Zonderman AB, Pletnikova O, Marsh L, Dawson TM, Crain BJ, West MJ, Troncoso JC (2008) Morphometry of the human substantia nigra in ageing and Parkinson’s disease. Acta Neuropathol 115:461–470
Ruf VC, Nubling GS, Willikens S, Shi S, Schmidt F, Levin J, Botzel K, Kamp F, Giese A (2019) Different effects of alpha-synuclein mutants on lipid binding and aggregation detected by single molecule fluorescence spectroscopy and tht fluorescence-based measurements. ACS Chem Neurosci 10:1649–1659
Ruffmann C, Zini M, Goldwurm S, Bramerio M, Spinello S, Rusconi D, Gambacorta M, Tagliavini F, Pezzoli G, Giaccone G (2012) Lewy body pathology and typical Parkinson disease in a patient with a heterozygous (R275 W) mutation in the Parkin gene (PARK2). Acta Neuropathol 123:901–903
Ruffmann C, Calboli FC, Bravi I, Gveric D, Curry LK, de Smith A, Pavlou S, Buxton JL, Blakemore AI, Takousis P, Molloy S, Piccini P, Dexter DT, Roncaroli F, Gentleman SM, Middleton LT (2016) Cortical Lewy bodies and Abeta burden are associated with prevalence and timing of dementia in Lewy body diseases. Neuropathol Appl Neurobiol 42:436–450
Rui Q, Ni H, Li D, Gao R, Chen G (2018) The role of LRRK2 in neurodegeneration of Parkinson disease. Curr Neuropharmacol 16:1348–1357
Rumpf JJ, Albers J, Fricke C, Mueller W, Classen J (2018) Structural abnormality of substantia nigra induced by methamphetamine abuse. Mov Disord 32:1784–1788
Russ C, Lovestone S, Baker M, Pickering-Brown SM, Andersen PM, Furlong R, Mann D, Powell JF (2001) The extended haplotype of the microtubule associated protein tau gene is not associated with Pick’s disease. Neurosci Lett 299:156–158
Sailer A, Scholz SW, Nalls MA, Schulte C, Federoff M, Price TR, Lees A, Ross OA, Dickson DW, Mok K, Mencacci NE, Schottlaender L, Chelban V, Ling H, O’Sullivan SS, Wood NW, Traynor BJ, Ferrucci L, Federoff HJ, Mhyre TR, Morris HR, Deuschl G, Quinn N, Widner H, Albanese A, Infante J, Bhatia KP, Poewe W, Oertel W, Hoglinger GU, Wullner U, Goldwurm S, Pellecchia MT, Ferreira J, Tolosa E, Bloem BR, Rascol O, Meissner WG, Hardy JA, Revesz T, Holton JL, Gasser T, Wenning GK, Singleton AB, Houlden H (2016) A genome-wide association study in multiple system atrophy. Neurology 87:1591–1598
Sakamoto R, Tsuchiya K, Yoshida R, Itoh Y, Furuta N, Kosuga A, Sugai Y, Mimura M (2009) Progressive supranuclear palsy combined with Alzheimer’s disease: a clinicopathological study of two autopsy cases. Neuropathology 29:219–229
Salvesen L, Ullerup BH, Sunay FB, Brudek T, Lokkegaard A, Agander TK, Winge K, Pakkenberg B (2015) Changes in total cell numbers of the basal ganglia in patients with multiple system atrophy—a stereological study. Neurobiol Dis 74:104–113
Sampedro F, Marin-Lahoz J, Martinez-Horta S, Pagonabarraga J, Kulisevsky J (2019) Dopaminergic degeneration induces early posterior cortical thinning in Parkinson’s disease. Neurobiol Dis 124:29–35
Samudra N, Patel N, Womack KB, Khemani P, Chitnis S (2016) Psychosis in Parkinson disease: a review of etiology, phenomenology, and management. Drugs Aging 33:855–863
Sanford AM (2018) Lewy body dementia. Clin Geriatr Med 34:603–615
Santiago RM, Vital MABF, Sato MDO, Adam GP (2016) Depression in Parkinson’s disease is associated with a serotoninergic system change secondary to neuroinflammation. Int J Neurol Neurother 3:061
Sassone J, Valtorta F, Ciammola A (2019) Early dyskinesias in Parkinson’s disease patients with parkin mutation: a primary corticostriatal synaptopathy? Front Neurosci 13:273
Savica R, Grossardt BR, Bower JH, Ahlskog JE, Rocca WA (2013a) Incidence and pathology of synucleinopathies and tauopathies related to parkinsonism. JAMA Neurol 70:859–866
Savica R, Grossardt BR, Bower JH, Boeve BF, Ahlskog JE, Rocca WA (2013b) Incidence of dementia with Lewy bodies and Parkinson disease dementia. JAMA Neurol 70:1396–1402
Savica R, Beach TG, Hentz JG, Sabbagh MN, Serrano GE, Sue LI, Dugger BN, Shill HA, Driver-Dunckley E, Caviness JN, Mehta SH, Jacobson SA, Belden CM, Davis KJ, Zamrini E, Shprecher DR, Adler CH (2019) Lewy body pathology in Alzheimer’s disease: a clinicopathological prospective study. Acta Neurol Scand 139:76–81
Schapira AHV, Chaudhuri KR, Jenner P (2017) Non-motor features of Parkinson disease. Nat Rev Neurosci 18:435–450
Schmidt R, Berke JD (2017) A pause-then-cancel model of stopping: evidence from basal ganglia neurophysiology. Philos Trans R Soc Lond B Biol Sci. https://doi.org/10.1098/rstb.2016.0202
Schneider SA, Alcalay RN (2017) Neuropathology of genetic synucleinopathies with parkinsonism: review of the literature. Mov Disord 32:1504–1523
Schofield EC, Hodges JR, Macdonald V, Cordato NJ, Kril JJ, Halliday GM (2011) Cortical atrophy differentiates Richardson’s syndrome from the parkinsonian form of progressive supranuclear palsy. Mov Disord 26:256–263
Scholz SW, Houlden H, Schulte C, Sharma M, Li A, Berg D, Melchers A, Paudel R, Gibbs JR, Simon-Sanchez J, Paisan-Ruiz C, Bras J, Ding J, Chen H, Traynor BJ, Arepalli S, Zonozi RR, Revesz T, Holton J, Wood N, Lees A, Oertel W, Wullner U, Goldwurm S, Pellecchia MT, Illig T, Riess O, Fernandez HH, Rodriguez RL, Okun MS, Poewe W, Wenning GK, Hardy JA, Singleton AB, Del Sorbo F, Schneider S, Bhatia KP, Gasser T (2009) SNCA variants are associated with increased risk for multiple system atrophy. Ann Neurol 65:610–614
Schottlaender LV, Bettencourt C, Kiely AP, Chalasani A, Neergheen V, Holton JL, Hargreaves I, Houlden H (2016) Coenzyme Q10 levels are decreased in the cerebellum of multiple-system atrophy patients. PLoS One 11:e0149557
Schulte C, Gasser T (2011) Genetic basis of Parkinson’s disease: inheritance, penetrance, and expression. Appl Clin Genet 4:67–80
Schulz J, Pagano G, Fernandez Bonfante JA, Wilson H, Politis M (2018) Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson’s disease. Brain 141:1501–1516
Schulz J, Takousis P, Wohlers I, Itua IOG, Dobricic V, Rucker G, Binder H, Middleton L, Ioannidis JPA, Perneczky R, Bertram L, Lill CM (2019) Meta-analyses identify differentially expressed microRNAs in Parkinson’s disease. Ann Neurol 85:835–851
Schulz-Schaeffer WJ (2010) The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol 120:131–143
Schulz-Schaeffer WJ (2015) Is cell death primary or secondary in the pathophysiology of idiopathic Parkinson’s disease? Biomolecules 5:1467–1479
Schumacher J, Peraza LR, Firbank M, Thomas AJ, Kaiser M, Gallagher P, O’Brien JT, Blamire AM, Taylor JP (2019) Dysfunctional brain dynamics and their origin in Lewy body dementia. Brain 142:1767–1782
Scudamore O, Ciossek T (2018) Increased oxidative stress exacerbates alpha-synuclein aggregation in vivo. J Neuropathol Exp Neurol 77:443–453
Seidel K, Schols L, Nuber S, Petrasch-Parwez E, Gierga K, Wszolek Z, Dickson D, Gai WP, Bornemann A, Riess O, Rami A, Den Dunnen WF, Deller T, Rub U, Kruger R (2010) First appraisal of brain pathology owing to A30P mutant alpha-synuclein. Ann Neurol 67:684–689
Seidel K, Mahlke J, Sonny S, Krüger R, Heinsen H, Auburger G, Bouzrou M, Grinberg LT, Wicht H, Korf H-W, den Dunnen W, Rüb U (2015) The brainstem pathologies of Parkinson’s disease and dementia with Lewy bodies. Brain Pathol 25:121–135
Sekiya H, Kowa H, Koga H, Takata M, Satake W, Futamura N, Funakawa I, Jinnai K, Takahashi M, Kondo T, Ueno Y, Kanagawa M, Kobayashi K, Toda T (2019) Wide distribution of alpha-synuclein oligomers in multiple system atrophy brain detected by proximity ligation. Acta Neuropathol 137:455–466
Selikhova M, Williams DR, Kempster PA, Holton JL, Revesz T, Lees AJ (2009) A clinico-pathological study of subtypes in Parkinson’s disease. Brain 132:2947–2957
Sestini S, Alongi P, Berti V, Calcagni ML, Cecchin D, Chiaravalloti A, Chincarini A, Cistaro A, Guerra UP, Pappatà S, Tiraboschi P, Nobili F (2019) The role of molecular imaging in the frame of the revised dementia with Lewy body criteria. Clin Transl Imaging 7:83–98
Seto-Salvia N, Pagonabarraga J, Houlden H, Pascual-Sedano B, Dols-Icardo O, Tucci A, Paisan-Ruiz C, Campolongo A, Anton-Aguirre S, Martin I, Munoz L, Bufill E, Vilageliu L, Grinberg D, Cozar M, Blesa R, Lleo A, Hardy J, Kulisevsky J, Clarimon J (2012) Glucocerebrosidase mutations confer a greater risk of dementia during Parkinson’s disease course. Mov Disord 27:393–399
Shah A, Prasad S, Rastogi B, Dash S, Saini J, Pal PK, Ingalhalikar M (2019) Altered structural connectivity of the motor subnetwork in multiple system atrophy with cerebellar features. Eur Radiol 29:2783–2791
Shahmoradian SH, Lewis AJ, Genoud C, Graff-Meyer A, Hench J, Moors T, Schweighauser G, Wang J, Goldie KN, Suetterlin R, Castano-Diez D, Perez-Navarro P, Huisman E, Ipsen S, Ingrassia A, de Gier Y, Rozemuller AJM, Da Paepe A, Erny J, Staempfli A, Hoernschemeyer J, Grosserueschkamp F, Niedieker D, El-Mashtoly SF, Quadri M, van Ijcken WFJ, Bonifati V, Gerwert K, Bohrmann B, Frank S, Britschgi M, Stahlberg H, van de Berg W, Lauer ME (2018) Lewy pathology in Parkinson’s disease consists of a crowded organellar, membranous medley (This article is a preprint and has not been peer-reviewed). bioRxiv. https://doi.org/10.1101/137976
Sharma M, Wenning G, Krüger R (2014) Mutant COQ2 in multiple-system atrophy. N Engl J Med 371:80–83
Sharp ME, Marder KS, Cote L, Clark LN, Nichols WC, Vonsattel JP, Alcalay RN (2014) Parkinson’s disease with Lewy bodies associated with a heterozygous PARKIN dosage mutation. Mov Disord 29:566–568
Shimohata T, Aiba I, Nishizawa M (2015) Criteria for the diagnosis of corticobasal degeneration. Brain Nerve 67:513–523
Shimozawa A, Ono M, Takahara D, Tarutani A, Imura S, Masuda-Suzukake M, Higuchi M, Yanai K, Hisanaga SI, Hasegawa M (2017) Propagation of pathological alpha-synuclein in marmoset brain. Acta Neuropathol Commun 5:12
Shine JM, Matar E, Ward PB, Frank MJ, Moustafa AA, Pearson M, Naismith SL, Lewis SJ (2013) Freezing of gait in Parkinson’s disease is associated with functional decoupling between the cognitive control network and the basal ganglia. Brain 136:3671–3681
Shinotoh H, Shimada H, Kokubo Y, Tagai K, Niwa F, Kitamura S, Endo H, Ono M, Kimura Y, Hirano S, Mimuro M, Ichise M, Sahara N, Zhang MR, Suhara T, Higuchi M (2019) Tau imaging detects distinctive distribution of tau pathology in ALS/PDC on the Kii Peninsula. Neurology 92:e136–e147
Shuaib UA, Rajput AH, Robinson CA, Rajput A (2016) Neuroleptic-induced parkinsonism: clinicopathological study. Mov Disord 31:360–365
Sian-Hulsmann J, Mandel S, Youdim MB, Riederer P (2011) The relevance of iron in the pathogenesis of Parkinson’s disease. J Neurochem 118:939–957
Sian-Hulsmann J, Monoranu C, Strobel S, Riederer P (2015) Lewy bodies: a spectator or salient killer? CNS Neurol Disord Drug Targets 14:947–955
Sibon I, Tison F (2004) Vascular Parkinsonism. Curr Opin Neurol 17:49–54
Sidransky E, Lopez G (2012) The link between the GBA gene and parkinsonism. Lancet Neurol 11:986–998
Siepel FJ, Bronnick KS, Booij J, Ravina BM, Lebedev AV, Pereira JB, Gruner R, Aarsland D (2014) Cognitive executive impairment and dopaminergic deficits in de novo Parkinson’s disease. Mov Disord 29:1802–1808
Simonyan K (2019) Recent advances in understanding the role of the basal ganglia [version 1; peer review: 2 approved]. F1000 Res 8:122. https://doi.org/10.12688/f11000research.16524.12681
Simonyan K, Cho H, Hamzehei Sichani A, Rubien-Thomas E, Hallett M (2017) The direct basal ganglia pathway is hyperfunctional in focal dystonia. Brain 140:3179–3190
Singleton AB, Farrer MJ, Bonifati V (2013) The genetics of Parkinson’s disease: progress and therapeutic implications. Mov Disord 28:14–23
Sklerov M, Kang UJ, Liong C, Clark L, Marder K, Pauciulo M, Nichols WC, Chung WK, Honig LS, Cortes E, Vonsattel JP, Alcalay RN (2017) Frequency of GBA variants in autopsy-proven multiple system atrophy. Mov Disord Clin Pract 4:574–581
Skogseth RE, Hortobagyi T, Soennesyn H, Chwiszczuk L, Ffytche D, Rongve A, Ballard C, Aarsland D (2017) Accuracy of clinical diagnosis of dementia with Lewy bodies versus neuropathology. J Alzheimers Dis 59:1139–1152
Smith C, Malek N, Grosset KA, Cullen B, Gentleman SM, Grosset DG (2019) The neuropathology of dementia in patients with Parkinson’s disease: a systematic review of autopsy studies. J Neurol Neurosurg Psychiatry (in press)
Snead D, Eliezer D (2014) Alpha-synuclein function and dysfunction on cellular membranes. Exp Neurobiol 23:292–313
Snijders AH, Takakusaki K, Debu B, Lozano AM, Krishna V, Fasano A, Aziz TZ, Papa SM, Factor SA, Hallett M (2016) Physiology of freezing of gait. Ann Neurol 80:644–659
Sorrentino ZA, Giasson BI, Chakrabarty P (2019) Alpha-synuclein and astrocytes: tracing the pathways from homeostasis to neurodegeneration in Lewy body disease. Acta Neuropathol. https://doi.org/10.1007/s00401-00019-01977-00402
Spay C, Meyer G, Welter ML, Lau B, Boulinguez P, Ballanger B (2018) Functional imaging correlates of akinesia in Parkinson’s disease: still open issues. Neuroimage Clin 21:101644
Spillantini MG, Goedert M (2013) Tau pathology and neurodegeneration. Lancet Neurol 12:609–622
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M (1998) Alpha-synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with Lewy bodies. Proc Natl Acad Sci USA 95:6469–6473
Spina S, Brown JA, Deng J, Gardner RC, Nana AL, Hwang JL, Gaus SE, Huang EJ, Kramer JH, Rosen HJ, Kornak J, Neuhaus J, Miller BL, Grinberg LT, Boxer AL, Seeley WW (2019) Neuropathological correlates of structural and functional imaging biomarkers in 4-repeat tauopathies. Brain. https://doi.org/10.1093/brain/awz1122
Spires-Jones TL, Attems J, Thal DR (2017) Interactions of pathological proteins in neurodegenerative diseases. Acta Neuropathol 134:187–205
Srulijes K, Hauser AK, Guella I, Asselta R, Brockmann K, Schulte C, Solda G, Cilia R, Maetzler W, Schols L, Wenning GK, Poewe W, Barone P, Wullner U, Oertel W, Berg D, Goldwurm S, Gasser T (2013) No association of GBA mutations and multiple system atrophy. Eur J Neurol 20:e61–e62
Stankovic I, Quinn N, Vignatelli L, Antonini A, Berg D, Coon E, Cortelli P, Fanciulli A, Ferreira JJ, Freeman R, Halliday G, Hoglinger GU, Iodice V, Kaufmann H, Klockgether T, Kostic V, Krismer F, Lang A, Levin J, Low P, Mathias C, Meissner WG, Kaufmann LN, Palma JA, Panicker JN, Pellecchia MT, Sakakibara R, Schmahmann J, Scholz SW, Singer W, Stamelou M, Tolosa E, Tsuji S, Seppi K, Poewe W, Wenning GK (2019) A critique of the second consensus criteria for multiple system atrophy. Mov Disord. https://doi.org/10.1002/mds.27701
Steele JC, McGeer PL (2008) The ALS/PDC syndrome of Guam and the cycad hypothesis. Neurology 70:1984–1990
Stefanis L (2012) Alpha-synuclein in Parkinson’s disease. Cold Spring Harb Perspect Med 2:a009399
Stefanis L, Emmanouilidou E, Pantazopoulou M, Kirik D, Vekrellis K, Tofaris GK (2019) How is alpha-synuclein cleared from the cell? J Neurochem. https://doi.org/10.1111/jnc.14704
Stefanova N (2014) Animal models. In: Wenning GK, Fanciulli A (eds) Multiple system atrophy. Springer, Vienna, pp 83–96
Stejskalova Z, Rohan Z, Rusina R, Tesar A, Kukal J, Kovacs GG, Bartos A, Matej R (2019) Pyramidal system involvement in progressive supranuclear palsy—a clinicopathological correlation. BMC Neurol 19:42
Strang KH, Croft CL, Sorrentino ZA, Chakrabarty P, Golde TE, Giasson BI (2018) Distinct differences in prion-like seeding and aggregation between tau protein variants provide mechanistic insights into tauopathies. J Biol Chem 293:2408–2421
Strang KH, Golde TE, Giasson BI (2019) MAPT mutations, tauopathy, and mechanisms of neurodegeneration. Lab Invest. https://doi.org/10.1038/s41374-41019-40197-x
Su Y, Deng MF, Xiong W, Xie AJ, Guo J, Liang ZH, Hu B, Chen JG, Zhu X, Man HY, Lu Y, Liu D, Tang B, Zhu LQ (2019) MicroRNA-26a/death-associated protein kinase 1 signaling induces synucleinopathy and dopaminergic neuron degeneration in Parkinson’s disease. Biol Psychiatry 85:769–781
Sulzer D, Surmeier DJ (2013) Neuronal vulnerability, pathogenesis, and Parkinson’s disease. Mov Disord 28:41–50
Sumikura H, Takao M, Hatsuta H, Ito S, Nakano Y, Uchino A, Nogami A, Saito Y, Mochizuki H, Murayama S (2015) Distribution of alpha-synuclein in the spinal cord and dorsal root ganglia in an autopsy cohort of elderly persons. Acta Neuropathol Commun 3:57
Sun QY, Guo JF, Han WW, Zuo X, Wang L, Yao LY, Pan Q, Xia K, Yan XX, Tang BS (2013) Genetic association study of glucocerebrosidase gene L444P mutation in essential tremor and multiple system atrophy in mainland China. J Clin Neurosci 20:217–219
Sun Z, Xiang X, Tang B, Chen Z, Peng H, Xia K, Jiang H (2015) SNP rs11931074 of the SNCA gene may not be associated with multiple system atrophy in Chinese population. Int J Neurosci 125:612–615
Sun Z, Ohta Y, Yamashita T, Sato K, Takemoto M, Hishikawa N, Abe K (2016) New susceptible variant of COQ2 gene in Japanese patients with sporadic multiple system atrophy. Neurol Genet 2:e54
Sun Y, Pham AN, Hare DJ, Waite TD (2018) Kinetic modeling of pH-dependent oxidation of dopamine by iron and its relevance to Parkinson’s disease. Front Neurosci 12:859
Sun R, Yang S, Zheng B, Liu J, Ma X (2019) Apolipoprotein E polymorphisms and Parkinson disease with or without dementia: a meta-analysis including 6453 participants. J Geriatr Psychiatry Neurol 32:3–15
Surmeier DJ (2018) Determinants of dopaminergic neuron loss in Parkinson’s disease. FEBS J 285:3657–3668
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18:101–113
Suryanarayana SM, Hellgren Kotaleski J, Grillner S, Gurney KN (2019) Roles for globus pallidus externa revealed in a computational model of action selection in the basal ganglia. Neural Netw 109:113–136
Svensson E, Horvath-Puho E, Thomsen RW, Djurhuus JC, Pedersen L, Borghammer P, Sorensen HT (2015) Vagotomy and subsequent risk of Parkinson’s disease. Ann Neurol 78:522–529
Swirski M, Miners JS, de Silva R, Lashley T, Ling H, Holton J, Revesz T, Love S (2014) Evaluating the relationship between amyloid-ß and a-synuclein phosphorylated at Ser129 in dementia with Lewy bodies and Parkinson’s disease. Alzheimers Res Ther 6:77
Taba P (2017) Toxic-induced parkinsonism. In: Falup-Pecurariu C, Ferreira J, Martinez-Martin P, Chaudhuri KR (eds) Movement disorders curricula. Springer, Wien, pp 225–232
Tacik P, Fiesel FC, Fujioka S, Ross OA, Pretelt F, Castaneda Cardona C, Kidd A, Hlavac M, Raizis A, Okun MS, Traynor S, Strongosky AJ, Springer W, Wszolek ZK (2014) Three families with Perry syndrome from distinct parts of the world. Parkinsonism Relat Disord 20:884–888
Taghdiri F, Sato C, Ghani M, Moreno D, Rogaeva E, Tartaglia MC (2016) Novel GRN mutations in patients with corticobasal syndrome. Sci Rep 6:22913
Tagliaferro P, Burke RE (2016) Retrograde axonal degeneration in Parkinson disease. J Parkinsons Dis 6:1–15
Taguchi K, Watanabe Y, Tsujimura A, Tanaka M (2019) Expression of alpha-synuclein is regulated in a neuronal cell type-dependent manner. Anat Sci Int 94:11–22
Taipa R, Pereira C, Reis I, Alonso I, Bastos-Lima A, Melo-Pires M, Magalhaes M (2016) DJ-1 linked parkinsonism (PARK7) is associated with Lewy body pathology. Brain 139:1680–1687
Takanashi M, Funayama M, Matsuura E, Yoshino H, Li Y, Tsuyama S, Takashima H, Nishioka K, Hattori N (2018) Isolated nigral degeneration without pathological protein aggregation in autopsied brains with LRRK2 p.R1441H homozygous and heterozygous mutations. Acta Neuropathol Commun 6:105
Tamguney G, Korczyn AD (2018) A critical review of the prion hypothesis of human synucleinopathies. Cell Tissue Res 373:213–220
Tamtaji OR, Behnam M, Pourattar MA, Jafarpour H, Asemi Z (2019) Aquaporin 4: a key player in Parkinson’s disease. J Cell Physiol. https://doi.org/10.1002/jcp.28871
Tanaka G, Yamanaka T, Furukawa Y, Kajimura N, Mitsuoka K, Nukina N (2019) Sequence- and seed-structure-dependent polymorphic fibrils of alpha-synuclein. Biochim Biophys Acta Mol Basis Dis 1865:1410–1420
Taniguchi-Watanabe S, Arai T, Kametani F, Nonaka T, Masuda-Suzukake M, Tarutani A, Murayama S, Saito Y, Arima K, Yoshida M, Akiyama H, Robinson A, Mann DMA, Iwatsubo T, Hasegawa M (2016) Biochemical classification of tauopathies by immunoblot, protein sequence and mass spectrometric analyses of sarkosyl-insoluble and trypsin-resistant tau. Acta Neuropathol 131:267–280
Tanji K, Miki Y, Mori F, Kon T, Kakita A, Takahashi H, Wakabayashi K (2019) Phosphorylated NUB1 distinguishes alpha-synuclein in Lewy bodies from that in glial cytoplasmic inclusions in multiple system atrophy. Brain Pathol. https://doi.org/10.1111/bpa.12728
Tardivel M, Begard S, Bousset L, Dujardin S, Coens A, Melki R, Buee L, Colin M (2016) Tunneling nanotube (TNT)-mediated neuron-to neuron transfer of pathological tau protein assemblies. Acta Neuropathol Commun 4:117
Tatsumi S, Mimuro M, Iwasaki Y, Takahashi R, Kakita A, Takahashi H, Yoshida M (2014a) Argyrophilic grains are reliable disease-specific features of corticobasal degeneration. J Neuropathol Exp Neurol 73:30–38
Tatsumi S, Uchihara T, Aiba I, Iwasaki Y, Mimuro M, Takahashi R, Yoshida M (2014b) Ultrastructural differences in pretangles between Alzheimer disease and corticobasal degeneration revealed by comparative light and electron microscopy. Acta Neuropathol Commun 2:161
Tecuapetla F, Jin X, Lima SQ, Costa RM (2016) Complementary contributions of striatal projection pathways to action initiation and execution. Cell 166:703–715
Tercjak A, Bergareche A, Caballero C, Tunon T, Linazasoro G (2014) Lewy bodies under atomic force microscope. Ultrastruct Pathol 38:1–5
Thakur P, Breger LS, Lundblad M, Wan OW, Mattsson B, Luk KC, Lee VMY, Trojanowski JQ, Bjorklund A (2017) Modeling Parkinson’s disease pathology by combination of fibril seeds and alpha-synuclein overexpression in the rat brain. Proc Natl Acad Sci USA 114:E8284–E8293
Thal DR, von Arnim CA, Griffin WS, Mrak RE, Walker L, Attems J, Arzberger T (2015) Frontotemporal lobar degeneration FTLD-tau: preclinical lesions, vascular, and Alzheimer-related co-pathologies. J Neural Transm (Vienna) 122:1007–1018
Thenganatt MA, Jankovic J (2014) Parkinson disease subtypes. JAMA Neurol 71:499–504
Thobois S, Prange S, Sgambato-Faure V, Tremblay L, Broussolle E (2017) Imaging the etiology of apathy, anxiety, and depression in Parkinson’s disease: implication for treatment. Curr Neurol Neurosci Rep 17:76
Thomas AJ, Mahin-Babaei F, Saidi M, Lett D, Taylor JP, Walker L, Attems J (2018) Improving the identification of dementia with Lewy bodies in the context of an Alzheimer’s-type dementia. Alzheimers Res Ther 10:27
Thomas AJ, Donaghy P, Roberts G, Colloby SJ, Barnett NA, Petrides G, Lloyd J, Olsen K, Taylor JP, McKeith I, O’Brien JT (2019) Diagnostic accuracy of dopaminergic imaging in prodromal dementia with Lewy bodies. Psychol Med 49:396–402
Tiraboschi P, Attems J, Thomas A, Brown A, Jaros E, Lett DJ, Ossola M, Perry RH, Ramsay L, Walker L, McKeith IG (2015) Clinicians’ ability to diagnose dementia with Lewy bodies is not affected by beta-amyloid load. Neurology 84:496–499
Titova N, Qamar MA, Chaudhuri KR (2017) The nonmotor features of Parkinson’s disease. Int Rev Neurobiol 132:33–54
Togo T, Cookson N, Dickson DW (2002) Argyrophilic grain disease: neuropathology, frequency in a dementia brain bank and lack of relationship with apolipoprotein E. Brain Pathol 12:45–52
Tomiyama M (2017) Symptoms and pathophysiology of dyskinesias. Brain Nerve 69:1409–1416
Tomiyama H, Hatano T, Hattori N (2007) Clinical molecular genetics for PARK8 (LRRK2). Brain Nerve 59:839–850
Tong J, Wong H, Guttman M, Ang LC, Forno LS, Shimadzu M, Rajput AH, Muenter MD, Kish SJ, Hornykiewicz O, Furukawa Y (2010) Brain alpha-synuclein accumulation in multiple system atrophy, Parkinson’s disease and progressive supranuclear palsy: a comparative investigation. Brain 133:172–188
Tran TN, Vo TNN, Frei K, Truong DD (2018) Levodopa-induced dyskinesia: clinical features, incidence, and risk factors. J Neural Transm (Vienna) 125:1109–1117
Tremblay ME, Cookson MR, Civiero L (2019) Glial phagocytic clearance in Parkinson’s disease. Mol Neurodegener 14:16
Trinh J, Zeldenrust FMJ, Huang J, Kasten M, Schaake S, Petkovic S, Madoev H, Grunewald A, Almuammar S, Konig IR, Lill CM, Lohmann K, Klein C, Marras C (2018) Genotype-phenotype relations for the Parkinson’s disease genes SNCA, LRRK2, VPS35: MDSGene systematic review. Mov Disord 33:1857–1870
Trojanowski JQ, Revesz T (2007) Proposed neuropathological criteria for the post mortem diagnosis of multiple system atrophy. Neuropathol Appl Neurobiol 33:615–620
Tsang AH, Chung KK (2009) Oxidative and nitrosative stress in Parkinson’s disease. Biochim Biophys Acta 1792:643–650
Tzoulis C, Schwarzlmuller T, Biermann M, Haugarvoll K, Bindoff LA (2016) Mitochondrial DNA homeostasis is essential for nigrostriatal integrity. Mitochondrion 28:33–37
Ubhi K, Rockenstein E, Mante M, Inglis C, Adame A, Patrick C, Whitney K, Masliah E (2010) Neurodegeneration in a transgenic mouse model of multiple system atrophy is associated with altered expression of oligodendroglial-derived neurotrophic factors. J Neurosci 30:6236–6246
Ubhi K, Low P, Masliah E (2011) Multiple system atrophy: a clinical and neuropathological perspective. Trends Neurosci 34:581–590
Uchihara T (2014) Pretangles and neurofibrillary changes: Similarities and differences between AD and CBD based on molecular and morphological evolution. Neuropathology 34:571–577
Uchikado H, DelleDonne A, Ahmed Z, Dickson DW (2006a) Lewy bodies in progressive supranuclear palsy represent an independent disease process. J Neuropathol Exp Neurol 65:387–395
Uchikado H, Lin WL, DeLucia MW, Dickson DW (2006b) Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol 65:685–697
Uemura N, Yagi H, Uemura MT, Hatanaka Y, Yamakado H, Takahashi R (2018) Inoculation of alpha-synuclein preformed fibrils into the mouse gastrointestinal tract induces Lewy body-like aggregates in the brainstem via the vagus nerve. Mol Neurodegener 13:21
Uitti RJ, Rajput AH, Ashenhurst EM, Rozdilsky B (1985) Cyanide-induced parkinsonism: a clinicopathologic report. Neurology 35:921–925
Vaikath NN, Erskine D, Morris CM, Majbour NK, Vekrellis K, Li JY, El-Agnaf OMA (2018) Heterogeneity in alpha-synuclein subtypes and their expression in cortical brain tissue lysates from Lewy body diseases and Alzheimer’s disease. Neuropathol Appl Neurobiol. https://doi.org/10.1111/nan.12531
Valdinocci D, Radford RAW, Goulding M, Hayashi J, Chung RS, Pountney DL (2018) Extracellular Interactions of alpha-synuclein in multiple system atrophy. Int J Mol Sci 19:4129
Valera E, Spencer B, Mott J, Trejo M, Adame A, Mante M, Rockenstein E, Troncoso JC, Beach TG, Masliah E, Desplats P (2017) MicroRNA-101 modulates autophagy and oligodendroglial alpha-synuclein accumulation in multiple system atrophy. Front Mol Neurosci 10:329
van de Berg WD, Hepp DH, Dijkstra AA, Rozemuller JA, Berendse HW, Foncke E (2012) Patterns of alpha-synuclein pathology in incidental cases and clinical subtypes of Parkinson’s disease. Parkinsonism Relat Disord 18(Suppl 1):S28–S30
Van Do B, Gouel F, Jonneaux A, Timmerman K, Gele P, Petrault M, Bastide M, Laloux C, Moreau C, Bordet R, Devos D, Devedjian JC (2016) Ferroptosis, a newly characterized form of cell death in Parkinson’s disease that is regulated by PKC. Neurobiol Dis 94:169–178
van Swieten J, Spillantini MG (2007) Hereditary frontotemporal dementia caused by Tau gene mutations. Brain Pathol 17:63–73
Vasili E, Dominguez-Meijide A, Outeiro TF (2019) Spreading of alpha-synuclein and tau: a systematic comparison of the mechanisms involved. Front Mol Neurosci 12:107
Vergouw LJM, van Steenoven I, van de Berg WDJ, Teunissen CE, van Swieten JC, Bonifati V, Lemstra AW, de Jong FJ (2017) An update on the genetics of dementia with Lewy bodies. Parkinsonism Relat Disord 43:1–8
Verheijen BM, Oyanagi K, van Leeuwen FW (2018) Dysfunction of protein quality control in parkinsonism–dementia complex of Guam. Front Neurol 9:173
Vermeiren Y, De Deyn PP (2017) Targeting the norepinephrinergic system in Parkinson’s disease and related disorders: the locus coeruleus story. Neurochem Int 102:22–32
Verstraeten A, Theuns J, Van Broeckhoven C (2015) Progress in unraveling the genetic etiology of Parkinson disease in a genomic era. Trends Genet 31:140–149
Veys L, Vandenabeele M, Ortuno-Lizaran I, Baekelandt V, Cuenca N, Moons L, De Groef L (2019) Retinal alpha-synuclein deposits in Parkinson’s disease patients and animal models. Acta Neuropathol 137:379–395
Vilensky JA (ed) (2011) Encephalitis lethargica: during and after the epidemic. Oxford University Press, Oxford
Virmani T, Moskowitz CB, Vonsattel JP, Fahn S (2015) Clinicopathological characteristics of freezing of gait in autopsy-confirmed Parkinson’s disease. Mov Disord 30:1874–1884
Vitale F, Capozzo A, Mazzone P, Scarnati E (2018) Neurophysiology of the pedunculopontine tegmental nucleus. Neurobiol Dis. https://doi.org/10.1016/j.nbd.2018.1003.1004
Volpicelli-Daley LA (2017) Effects of alpha-synuclein on axonal transport. Neurobiol Dis 105:321–327
Volta M, Milnerwood AJ, Farrer MJ (2015) Insights from late-onset familial parkinsonism on the pathogenesis of idiopathic Parkinson’s disease. Lancet Neurol 14:1054–1064
Voon V, Napier TC, Frank MJ, Sgambato-Faure V, Grace AA, Rodriguez-Oroz M, Obeso J, Bezard E, Fernagut PO (2017) Impulse control disorders and levodopa-induced dyskinesias in Parkinson’s disease: an update. Lancet Neurol 16:238–250
Voronkov DN, Salkov VN, Anufriev PL, Khudoerkov RM (2018) Lewy bodies in Parkinson’s disease: histological, immunohistochemical, and interferometric examinations. Arkhiv Patologii 80:9–13
Voutsinas GE, Stavrou EF, Karousos G, Dasoula A, Papachatzopoulou A, Syrrou M, Verkerk AJ, van der Spek P, Patrinos GP, Stoger R, Athanassiadou A (2010) Allelic imbalance of expression and epigenetic regulation within the alpha-synuclein wild-type and p.Ala53Thr alleles in Parkinson disease. Hum Mutat 31:685–691
Vriend C, Raijmakers P, Veltman DJ, van Dijk KD, van der Werf YD, Foncke EM, Smit JH, Berendse HW, van den Heuvel OA (2014) Depressive symptoms in Parkinson’s disease are related to reduced [123I]FP-CIT binding in the caudate nucleus. J Neurol Neurosurg Psychiatry 85:159–164
Wakabayashi K, Miki Y (2018) Multi-organ distribution of alpha-synuclein pathology in dementia with Lewy bodies. Brain Nerve 70:489–500
Wakabayashi K, Mori F, Nishie M, Oyama Y, Kurihara A, Yoshimoto M, Kuroda N (2005) An autopsy case of early (“minimal change”) olivopontocerebellar atrophy (multiple system atrophy-cerebellar). Acta Neuropathol 110:185–190
Wakabayashi K, Mori F, Tanji K, Orimo S, Takahashi H (2010) Involvement of the peripheral nervous system in synucleinopathies, tauopathies and other neurodegenerative proteinopathies of the brain. Acta Neuropathol 120:1–12
Wakabayashi K, Tanji K, Odagiri S, Miki Y, Mori F, Takahashi H (2013) The Lewy body in Parkinson’s disease and related neurodegenerative disorders. Mol Neurobiol 47:495–508
Walker DG, Lue LF, Adler CH, Shill HA, Caviness JN, Sabbagh MN, Akiyama H, Serrano GE, Sue LI, Beach TG (2013) Changes in properties of serine 129 phosphorylated alpha-synuclein with progression of Lewy-type histopathology in human brains. Exp Neurol 240:190–204
Walker L, McAleese KE, Thomas AJ, Johnson M, Martin-Ruiz C, Parker C, Colloby SJ, Jellinger K, Attems J (2015) Neuropathologically mixed Alzheimer’s and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta Neuropathol 129:729–748
Wang Y, Balaji V, Kaniyappan S, Kruger L, Irsen S, Tepper K, Chandupatla R, Maetzler W, Schneider A, Mandelkow E, Mandelkow EM (2017) The release and trans-synaptic transmission of Tau via exosomes. Mol Neurodegener 12:5
Wang B, Underwood R, Kamath A, Britain C, McFerrin MB, McLean PJ, Volpicelli-Daley LA, Whitaker RH, Placzek WJ, Becker K, Ma J, Yacoubian TA (2018) 14-3-3 proteins reduce cell-to-cell transfer and propagation of pathogenic alpha-synuclein. J Neurosci 38:8211–8232
Wang X, Becker K, Levine N, Zhang M, Lieberman AP, Moore DJ, Ma J (2019) Pathogenic alpha-synuclein aggregates preferentially bind to mitochondria and affect cellular respiration. Acta Neuropathol Commun 7:41
Waring SC, Esteban-Santillan C, Reed DM, Craig UK, Labarthe DR, Petersen RC, Kurland LT (2004) Incidence of amyotrophic lateral sclerosis and of the parkinsonism-dementia complex of Guam, 1950-1989. Neuroepidemiology 23:192–200
Watanabe H, Riku Y, Nakamura T, Hara K, Ito M, Hirayama M, Yoshida M, Katsuno M, Sobue G (2016) Expanding concept of clinical conditions and symptoms in multiple system atrophy. Rinsho Shinkeigaku 56:457–464
Wei L, Hu X, Yuan Y, Liu W, Chen H (2018) Abnormal ventral tegmental area-anterior cingulate cortex connectivity in Parkinson’s disease with depression. Behav Brain Res 347:132–139
Weismiller HA, Murphy R, Wei G, Ma B, Nussinov R, Margittai M (2018) Structural disorder in four-repeat Tau fibrils reveals a new mechanism for barriers to cross-seeding of Tau isoforms. J Biol Chem 293:17336–17348
Wenning GK, Quinn N, Magalhaes M, Mathias C, Daniel SE (1994) “Minimal change” multiple system atrophy. Mov Disord 9:161–166
Wenning GK, Jellinger K, Litvan I (1997) Supranuclear gaze palsy and eyelid apraxia in postencephalitic parkinsonism. J Neural Transm 104:845–865
Wenning GK, Litvan I, Tolosa E (2011) Milestones in atypical and secondary parkinsonisms. Mov Disord 26:1083–1095
Wenning GK, Geser F, Krismer F, Seppi K, Duerr S, Boesch S, Kollensperger M, Goebel G, Pfeiffer KP, Barone P, Pellecchia MT, Quinn NP, Koukouni V, Fowler CJ, Schrag A, Mathias CJ, Giladi N, Gurevich T, Dupont E, Ostergaard K, Nilsson CF, Widner H, Oertel W, Eggert KM, Albanese A, del Sorbo F, Tolosa E, Cardozo A, Deuschl G, Hellriegel H, Klockgether T, Dodel R, Sampaio C, Coelho M, Djaldetti R, Melamed E, Gasser T, Kamm C, Meco G, Colosimo C, Rascol O, Meissner WG, Tison F, Poewe W (2013) The natural history of multiple system atrophy: a prospective European cohort study. Lancet Neurol 12:264–274
Wenning G, Trojanowski JQ, Kaufmann H, Wisniewski T, Rocca WA, Low PA (2018) Is multiple system atrophy an infectious disease? Ann Neurol 83:10–12
Whitwell JL (2018) Tau imaging in parkinsonism: what have we learned so far? Mov Disord Clin Pract 5:118–130
Wichmann T, Bergman H, DeLong MR (2018) Basal ganglia, movement disorders and deep brain stimulation: advances made through non-human primate research. J Neural Transm (Vienna) 125:419–430
Wider C, Dickson DW, Stoessl AJ, Tsuboi Y, Chapon F, Gutmann L, Lechevalier B, Calne DB, Personett DA, Hulihan M, Kachergus J, Rademakers R, Baker MC, Grantier LL, Sujith OK, Brown L, Calne S, Farrer MJ, Wszolek ZK (2009) Pallidonigral TDP-43 pathology in Perry syndrome. Parkinsonism Relat Disord 15:281–286
Wilkaniec A, Lenkiewicz AM, Czapski GA, Jesko HM, Hilgier W, Brodzik R, Gassowska-Dobrowolska M, Culmsee C, Adamczyk A (2019) Extracellular alpha-synuclein oligomers induce parkin s-nitrosylation: relevance to sporadic Parkinson’s disease etiopathology. Mol Neurobiol 56:125–140
Williams DR, Holton JL, Strand K, de Silva R, Lees AJ, Revesz T (2007) Differences in tau load are associated with different clinical phenotypes in progressive supranuclear palsy observations (abstr.). Neuropathol Appl Neurobiol 33:248–275
Williams-Gray CH, Foltynie T, Lewis SJ, Barker RA (2006) Cognitive deficits and psychosis in Parkinson’s disease: a review of pathophysiology and therapeutic options. CNS Drugs 20:477–505
Wilson H, Niccolini F, Pellicano C, Politis M (2019) Cortical thinning across Parkinson’s disease stages and clinical correlates. J Neurol Sci 398:31–38
Woerman AL, Kazmi SA, Patel S, Freyman Y, Oehler A, Aoyagi A, Mordes DA, Halliday GM, Middleton LT, Gentleman SM, Olson SH, Prusiner SB (2018) MSA prions exhibit remarkable stability and resistance to inactivation. Acta Neuropathol 135:49–63
Woerman AL, Oehler A, Kazmi SA, Lee J, Halliday GM, Middleton LT, Gentleman SM, Mordes DA, Spina S, Grinberg LT, Olson SH, Prusiner SB (2019) Multiple system atrophy prions retain strain specificity after serial propagation in two different Tg(SNCA*A53T) mouse lines. Acta Neuropathol 137:437–454
Wong YC, Krainc D (2017) Alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 23:1–13
Wszolek ZK, Slowinski J, Golan M, Dickson DW (2005) Frontotemporal dementia and parkinsonism linked to chromosome 17. Folia Neuropathol 43:258–270
Xia Y, Zhang G, Han C, Ma K, Guo X, Wan F, Kou L, Yin S, Liu L, Huang J, Xiong N, Wang T (2019) Microglia as modulators of exosomal alpha-synuclein transmission. Cell Death Dis 10:174
Yabe I, Soma H, Takei A, Fujiki N, Yanagihara T, Sasaki H (2006) MSA-C is the predominant clinical phenotype of MSA in Japan: analysis of 142 patients with probable MSA. J Neurol Sci 249:115–121
Yamasaki TR, Holmes BB, Furman JL, Dhavale DD, Su BW, Song ES, Cairns NJ, Kotzbauer PT, Diamond MI (2019) Parkinson’s disease and multiple system atrophy have distinct alpha-synuclein seed characteristics. J Biol Chem 294:1045–1058
Yamashita S, Sakashita N, Yamashita T, Tawara N, Tasaki M, Kawakami K, Komohara Y, Fujiwara Y, Kamikawa M, Nakagawa T, Hirano T, Maeda Y, Hasegawa M, Takeya M, Ando Y (2014) Concomitant accumulation of alpha-synuclein and TDP-43 in a patient with corticobasal degeneration. J Neurol 261:2209–2217
Yamazaki M, Hasegawa M, Mori O, Murayama S, Tsuchiya K, Ikeda K, Chen KM, Katayama Y, Oyanagi K (2005) Tau-positive fine granules in the cerebral white matter: a novel finding among the tauopathies exclusive to parkinsonism-dementia complex of Guam. J Neuropathol Exp Neurol 64:839–846
Yan X, Uronen RL, Huttunen HJ (2018) The interaction of alpha-synuclein and Tau: a molecular conspiracy in neurodegeneration? Semin Cell Dev Biol. https://doi.org/10.1016/j.semcdb.2018.1005.1005
Yang HS, Yu L, White CC, Chibnik LB, Chhatwal JP, Sperling RA, Bennett DA, Schneider JA, De Jager PL (2018) Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE epsilon4 haplotype status: a community-based cohort study. Lancet Neurol 17:773–781
Yap SM, Lynch T, MacMahon P, Murray B (2017) Paraneoplastic atypical parkinsonism with anti-crmp5 antibodies and severe caudate and putaminal hypometabolism on 18-fluorodeoxyglucose positron emission tomography of the brain. Mov Disord Clin Pract 4:263–265. https://doi.org/10.1002/mdc1003.12370
Yasuda T, Nakata Y, Mochizuki H (2013) Alpha-synuclein and neuronal cell death. Mol Neurobiol 47:466–483
Yokota O, Tsuchiya K, Terada S, Ishizu H, Uchikado H, Ikeda M, Oyanagi K, Nakano I, Murayama S, Kuroda S, Akiyama H (2008) Basophilic inclusion body disease and neuronal intermediate filament inclusion disease: a comparative clinicopathological study. Acta Neuropathol 115:561–575
Yokoyama Y, Toyoshima Y, Shiga A, Tada M, Kitamura H, Hasegawa K, Onodera O, Ikeuchi T, Someya T, Nishizawa M, Kakita A, Takahashi H (2016) Pathological and clinical spectrum of progressive supranuclear palsy: with special reference to astrocytic tau pathology. Brain Pathol 26:155–166
Yokoyama JS, Karch CM, Fan CC, Bonham LW, Kouri N, Ross OA, Rademakers R, Kim J, Wang Y, Hoglinger GU, Muller U, Ferrari R, Hardy J, Momeni P, Sugrue LP, Hess CP, James Barkovich A, Boxer AL, Seeley WW, Rabinovici GD, Rosen HJ, Miller BL, Schmansky NJ, Fischl B, Hyman BT, Dickson DW, Schellenberg GD, Andreassen OA, Dale AM, Desikan RS (2017) Shared genetic risk between corticobasal degeneration, progressive supranuclear palsy, and frontotemporal dementia. Acta Neuropathol 133:825–837
Yoshida M (2014) Astrocytic inclusions in progressive supranuclear palsy and corticobasal degeneration. Neuropathology 34:555–570
Yoshida K, Hata Y, Kinoshita K, Takashima S, Tanaka K, Nishida N (2017) Incipient progressive supranuclear palsy is more common than expected and may comprise clinicopathological subtypes: a forensic autopsy series. Acta Neuropathol 133:809–823
Yoshino H, Nishioka K, Li Y, Oji Y, Oyama G, Hatano T, Machida Y, Shimo Y, Hayashida A, Ikeda A, Mogushi K, Shibagaki Y, Hosaka A, Iwanaga H, Fujitake J, Ohi T, Miyazaki D, Sekijima Y, Oki M, Kusaka H, Fujimoto KI, Ugawa Y, Funayama M, Hattori N (2018) GCH1 mutations in dopa-responsive dystonia and Parkinson’s disease. J Neurol 265:1860–1870
Young CB, Sonne J (2018) Neuroanatomy, Basal Ganglia. StatPearls Publishing, Treasure Island
Yousaf T, Dervenoulas G, Valkimadi PE, Politis M (2019) Neuroimaging in Lewy body dementia. J Neurol 266:1–26
Yuan X, Chen Y, Cao B, Zhao B, Wei Q, Guo X, Yang Y, Yuan L, Shang H (2015) An association analysis of the R1628P and G2385R polymorphisms of the LRRK2 gene in multiple system atrophy in a Chinese population. Parkinsonism Relat Disord 21:147–149
Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG (2008) Patterns and stages of alpha-synucleinopathy: relevance in a population-based cohort. Neurology 70:1042–1048
Zahodne LB, Fernandez HH (2008) Pathophysiology and treatment of psychosis in Parkinson’s disease: a review. Drugs Aging 25:665–682
Zaltieri M, Grigoletto J, Longhena F, Navarria L, Favero G, Castrezzati S, Colivicchi MA, Della Corte L, Rezzani R, Pizzi M, Benfenati F, Spillantini MG, Missale C, Spano P, Bellucci A (2015a) Alpha-synuclein and synapsin III cooperatively regulate synaptic function in dopamine neurons. J Cell Sci 128:2231–2243
Zaltieri M, Longhena F, Pizzi M, Missale C, Spano P, Bellucci A (2015b) Mitochondrial dysfunction and alpha-synuclein synaptic pathology in Parkinson’s disease: who’s on first? Parkinsons Dis 2015:108029
Zange L, Noack C, Hahn K, Stenzel W, Lipp A (2015) Phosphorylated alpha-synuclein in skin nerve fibres differentiates Parkinson’s disease from multiple system atrophy. Brain 138:2310–2321
Zeng XS, Geng WS, Jia JJ, Chen L, Zhang PP (2018) Cellular and molecular basis of neurodegeneration in Parkinson disease. Front Aging Neurosci 10:109
Zhai S, Shen W, Graves SM, Surmeier DJ (2019) Dopaminergic modulation of striatal function and Parkinson’s disease. J Neural Transm (Vienna) 126:411–422
Zhang Y, Larcher KM, Misic B, Dagher A (2017) Anatomical and functional organization of the human substantia nigra and its connections. Elife 6:e26653
Zhang G, Xia Y, Wan F, Ma K, Guo X, Kou L, Yin S, Han C, Liu L, Huang J, Xiong N, Wang T (2018a) New perspectives on roles of alpha-synuclein in Parkinson’s disease. Front Aging Neurosci 10:370
Zhang X, Gao F, Wang D, Li C, Fu Y, He W, Zhang J (2018b) Tau pathology in Parkinson’s disease. Front Neurol 9:809
Zhao Q, Yang X, Tian S, An R, Zheng J, Xu Y (2016) Association of the COQ2 V393A variant with risk of multiple system atrophy in East Asians: a case-control study and meta-analysis of the literature. Neurol Sci 37:423–430
Zhou ZD, Selvaratnam T, Lee JCT, Chao YX, Tan EK (2019) Molecular targets for modulating the protein translation vital to proteostasis and neuron degeneration in Parkinson’s disease. Transl Neurodegener 8:6
Zhu Y, Song X, Xu M, Hu X, Li E, Liu J, Yuan Y, Gao JH, Liu W (2016) Impaired interhemispheric synchrony in Parkinson’s disease with depression. Sci Rep 6:27477
Zhukareva V, Shah K, Uryu K, Braak H, Del Tredici K, Sundarraj S, Clark C, Trojanowski JQ, Lee VM (2002) Biochemical analysis of tau proteins in argyrophilic grain disease, Alzheimer’s disease, and Pick’s disease : a comparative study. Am J Pathol 161:1135–1141
Zijlmans JC, Daniel SE, Hughes AJ, Revesz T, Lees AJ (2004) Clinicopathological investigation of vascular parkinsonism, including clinical criteria for diagnosis. Mov Disord 19:630–640
Zondler L, Kostka M, Garidel P, Heinzelmann U, Hengerer B, Mayer B, Weishaupt JH, Gillardon F, Danzer KM (2017) Proteasome impairment by alpha-synuclein. PLoS One 12:e0184040
Ztaou S, Amalric M (2019) Contribution of cholinergic interneurons to striatal pathophysiology in Parkinson’s disease. Neurochem Int 126:1–10
Zucca FA, Segura-Aguilar J, Ferrari E, Munoz P, Paris I, Sulzer D, Sarna T, Casella L, Zecca L (2017) Interactions of iron, dopamine and neuromelanin pathways in brain aging and Parkinson’s disease. Prog Neurobiol 155:96–119
Zunke F, Moise AC, Belur NR, Gelyana E, Stojkovska I, Dzaferbegovic H, Toker NJ, Jeon S, Fredriksen K, Mazzulli JR (2018) Reversible conformational conversion of alpha-synuclein into toxic assemblies by glucosylceramide. Neuron 97(92–107):e110
Acknowledgements
The author thanks Mr. E. Mitter-Ferstl, PhD, for secretarial and graphical work. The study was partially funded by the Society for the Promotion of Research in Experimental Neurology, Vienna, Austria.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that he has no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jellinger, K.A. Neuropathology and pathogenesis of extrapyramidal movement disorders: a critical update—I. Hypokinetic-rigid movement disorders. J Neural Transm 126, 933–995 (2019). https://doi.org/10.1007/s00702-019-02028-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-019-02028-6