Abstract
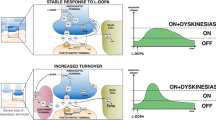
Symptoms of Parkinson’s disease have been controlled with levodopa for many years; however, motor complications consisting of wearing off of medication effect and dyskinesias tend to occur within a few years of starting levodopa. Motor complications can begin a few months after taking levodopa, with the average time to onset estimated to be 6.5 years. Dyskinesias can be troublesome and require intervention. Levodopa-induced dyskinesia can be composed of a variety of movement disorders including chorea, dystonia, ballism, myoclonus, and akathisia. Based on the clinical pattern, the most common dyskinesia is chorea and choreoathetosis. The clinical manifestations can be divided into three main categories based on their clinical movement patterns and the temporal correlation between the occurrence of dyskinesia and the levodopa dosing: on or peak-dose dyskinesias, biphasic dyskinesias, and Off dyskinesias. Severe cases of dyskinesia have been reported, with the extreme being dyskinesia–hyperpyrexia syndrome. The prevalence of LID has been reported in many studies, but the reported incidence varies. The rate of LID development is from 3 to 94%. The prevalence of LID mainly depends on age at onset, disease duration, and severity, and duration of levodopa therapy. Some of the risk factors for the development of dyskinesia are modifiable. Modifiable risk factors include levodopa dose and body weight. Non-modifiable risk factors include age, gender, duration of disease, clinical subtype, disease progression, disease severity, and genetic factors.
Similar content being viewed by others
References
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16:448–458
Ahmed I, Bose SK, Powesc N, Ramlackhansingh A, Turkheimer F, Hotton G, Hammers A, Brooks KJ (2011) Glutamate NMDA receptor dysregulation in Parkinson's disease with dyskinesias. Brain 134(PT 4):979–986
Alegre M, Lopez-Azcarate J, Alonso-Frech F, Rodriguez-Oroz MC, Valencia M, Guridi J, Artieda J, Obeso JA (2012) Subthalamic activity during diphasic dyskinesias in Parkinson’s disease. Mov Disord 27:1178–1181
Antonini A, Fung VS, Boyd JT, Slevin JT, Hall C, Chatamra K, Eaton S, Benesh JA (2016) Effect of levodopa-carbidopa intestinal gel on dyskinesia in advanced Parkinson’s disease patients. Mov Disord 31:530–537
Aquino CC, Fox SH (2015) Clinical spectrum of levodopa-induced complications. Mov Disord 30:80–89
Arabia G, Zappia M, Bosco D, Crescibene L, Bagala A, Bastone L, Caracciolo M, Scornaienghi M, Quattrone A (2002) Body weight, levodopa pharmacokinetics and dyskinesia in Parkinson’s disease. Neurol Sci 23(Suppl 2):S53–S54
Armand S, Landis T, Sztajzel R, Burkhard PR (2009) Dyskinesia-induced postural instability in Parkinson’s disease. Parkinsonism Relat Disord 15:359–364
Ballard PA, Tetrud JW, Langston JW (1985) Permanent human parkinsonism due to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP): seven cases. Neurology 35(7):949–956
Bektas H, Deniz O, Temel S, Keklikoglu HD, Akyol S (2014) Rhabdomyolysis related to dyskinesia in Parkinson’s disease. J Mov Disord 7:25–27
Bjornestad A, Forsaa EB, Pedersen KF, Tysnes OB, Larsen JP, Alves G (2016) Risk and course of major complications in a population-based incident Parkinson's disease cohort. Parkinsonism Relat Disord 22:48–53
Bracco F, Battaglia A, Chouza C, Dupont E, Gershanik O, Marti Masso JF, Montastruc JL (2004) The long-acting dopamine receptor agonist cabergoline in early Parkinson’s disease: final results of a 5-year, double-blind, levodopa-controlled study. CNS Drugs 18:733–746
Bravi D, Mouradian MM, Roberts JW, Davis TL, Chase TN (1993) End-of-dose dystonia in Parkinson’s disease. Neurology 43:2130–2131
Calabresi P, Di Filippo M, Ghiglieri V, Tambasco N, Picconi B (2010) Levodopa-induced dyskinesias in patients with Parkinson’s disease: filling the bench-to-bedside gap. Lancet Neurol 9:1106–1117
Carecchio M, Collini A, Comi C, Cantello R, Bhatia KP, Monaco F (2010) Levodopa-induced belly dancer’s dyskinesias in Parkinson’s disease: report of one case. Mov Disord 25:1760–1762
Catalan MJ, Escribano PM, Alonso-Frech F (2017) Dyskinesias in levodopa-carbidopa intestinal gel infusion era: new challenges, new features. Mov Disord 32:624–625
Chondrogiorgi M, Tatsioni A, Reichmann H, Konitsiotis S (2014) Dopamine agonist monotherapy in Parkinson’s disease and potential risk factors for dyskinesia: a meta-analysis of levodopa-controlled trials. Eur J Neurol 21:433–440
Cilia R, Akpalu A, Sarfo FS, Cham M, Amboni M, Cereda E, Fabbri M, Adjei P, Akassi J, Bonetti A, Pezzoli G (2014) The modern pre-levodopa era of Parkinson’s disease: insights into motor complications from sub-Saharan Africa. Brain 137:2731–2742
Comi C, Ferrari M, Marino F, Magistrelli L, Cantello R, Riboldazzi G, Bianchi ML, Bono G, Cosentino M (2017) Polymorphisms of dopamine receptor genes and risk of l-dopa-induced dyskinesia in Parkinson’s disease. Int J Mol Sci 18:242
Cotzias GC, Van Woert MH, Schiffer LM (1967) Aromatic amino acids and modification of parkinsonism. N Engl J Med 276:374–379
Cotzias GC, Papavasiliou PS, Gellene R (1969) Modification of Parkinsonism—chronic treatment with l-dopa. N Engl J Med 280:337–345
De Keyser J, Vincken W (1985) l-Dopa-induced respiratory disturbance in Parkinson’s disease suppressed by tiapride. Neurology 35:235–237
de Rijk MC, Launer LJ, Berger K, Breteler MM, Dartigues JF, Baldereschi M, Fratiglioni L, Lobo A, Martinez-Lage J, Trenkwalder C, Hofman A (2000) Prevalence of Parkinson’s disease in Europe: a collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 54:S21–S3
de Lau LM, Verbaan D, Marinus J, Heutink P, van Hilten JJ (2012) Catechol-O-methyltransferase Val158Met and the risk of dyskinesias in Parkinson’s disease. Mov Disord 27:132–135
Encarnacion EV, Hauser RA (2008) Levodopa-induced dyskinesias in Parkinson’s disease: etiology, impact on quality of life, and treatments. Eur Neurol 60:57–66
Fabbrini G, Brotchie JM, Grandas F, Nomoto M, Goetz CG (2007) Levodopa-induced dyskinesias. Mov Disord 22:1379–1389 (quiz 523)
Fahn S (1999) Parkinson disease, the effect of levodopa, and the ELLDOPA trial. Earlier vs later L-DOPA. Arch Neurol 56:529–535
Fahn S (2000) The spectrum of levodopa-induced dyskinesias. Ann Neurol 47:S2–S9 (discussion S9–S11)
Fahn S, Oakes D, Shoulson I, Kieburtz K, Rudolph A, Lang A, Olanow CW, Tanner C, Marek K, Group Parkinson Study (2004) Levodopa and the progression of Parkinson’s disease. N Engl J Med 351:2498–2508
Foltynie T, Cheeran B, Williams-Gray CH, Edwards MJ, Schneider SA, Weinberger D, Rothwell JC, Barker RA, Bhatia KP (2009) BDNF val66met influences time to onset of levodopa induced dyskinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 80:141–144
Friedman A (1985) Levodopa-induced dyskinesia: clinical observations. J Neurol 232:29–31
Gil-Navarro S, Grandas F (2010) Dyskinesia–hyperpyrexia syndrome: another Parkinson’s disease emergency. Mov Disord 25:2691–2692
Grotzsch H, Sztajzel R, Burkhard PR (2007) Levodopa-induced ocular dyskinesia in Parkinson’s disease. Eur J Neurol 14:1124–1128
Group PS (1996) Impact of deprenyl and tocopherol treatment on Parkinson’s disease in DATATOP patients requiring levodopa. Ann Neurol 39:37–45
Hametner E, Seppi K, Poewe W (2010) The clinical spectrum of levodopa-induced motor complications. J Neurol 257:S268–S275
Hashim HZ, Norlinah MI, Nafisah WY, Tan HJ, Raymond AA, Tamil AM (2014) Risk factors and predictors of levodopa-induced dyskinesia among multiethnic Malaysians with Parkinson’s disease. Int J Neurosci 124:187–191
Hassin-Baer S, Molchadski I, Cohen OS, Nitzan Z, Efrati L, Tunkel O, Kozlova E, Korczyn AD (2011) Gender effect on time to levodopa-induced dyskinesias. J Neurol 258:2048–2053
Hauser RA, Friedlander J, Zesiewicz TA, Adler CH, Seeberger LC, O’Brien CF, Molho ES, Factor SA (2000) A home diary to assess functional status in patients with Parkinson’s disease with motor fluctuations and dyskinesia. Clin Neuropharmacol 23:75–81
Hauser RA, Hubble JP, Truong DD, U.S. Study Group Istradefylline (2003) Randomized trial of the adenosine A(2A) receptor antagonist istradefylline in advanced PD. Neurology 61:297–303
Hauser RA, Deckers F, Lehert P (2004) Parkinson’s disease home diary: further validation and implications for clinical trials. Mov Disord 19:1409–1413
Hauser RA, Hsu A, Kell S, Espay AJ, Sethi K, Stacy M, Ondo W, O’Connell M, Gupta S (2013) Extended-release carbidopa-levodopa (IPX066) compared with immediate-release carbidopa-levodopa in patients with Parkinson’s disease and motor fluctuations: a phase 3 randomised, double-blind trial. Lancet Neurol 12:346–356
Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney Multicenter Study of Parkinson’s disease: non-l-dopa-responsive problems dominate at 15 years. Mov Disord 20:190–199
Hodgson RA, Bertorelli R, Varty GB, Lachowicz JE, Forlani A, Fredduzzi S, Cohen-Williams ME, Higgins GA, Impagnatiello F, Nicolussi E, Parra LE, Foster C, Zhai Y, Neustadt BR, Stamford AW, Parker EM, Reggiani A, Hunter JC (2009) Characterization of the potent and highly selective A2A receptor antagonists preladenant and SCH 412348 [7-[2-[4-2,4-difluorophenyl]-1-piperazinyl]ethyl]-2-(2-furanyl)-7H-pyrazolo[4,3-e][1,2,4]triazolo[1,5-c]pyrimidin-5-amine] in rodent models of movement disorders and depression. J Pharmacol Exp Ther 330:294–303
Hodgson RA, Bedard PJ, Varty GB, Kazdoba TM, Di Paolo T, Grzelak ME, Pond AJ, Hadjtahar A, Belanger N, Gregoire L, Dare A, Neustadt BR, Stamford AW, Hunter JC (2010) Preladenant, a selective A(2A) receptor antagonist, is active in primate models of movement disorders. Exp Neurol 225:384–90
Holloway RG, Shoulson I, Fahn S, Kieburtz K, Lang A, Marek K, McDermott M, Seibyl J, Weiner W, Musch B, Kamp C, Welsh M, Shinaman A, Pahwa R, Barclay L, Hubble J, LeWitt P, Miyasaki J, Suchowersky O, Stacy M, Russell DS, Ford B, Hammerstad J, Riley D, Standaert D, Wooten F, Factor S, Jankovic J, Atassi F, Kurlan R, Panisset M, Rajput A, Rodnitzky R, Shults C, Petsinger G, Waters C, Pfeiffer R, Biglan K, Borchert L, Montgomery A, Sutherland L, Weeks C, DeAngelis M, Sime E, Wood S, Pantella C, Harrigan M, Fussell B, Dillon S, Alexander-Brown B, Rainey P, Tennis M, Rost-Ruffner E, Brown D, Evans S, Berry D, Hall J, Shirley T, Dobson J, Fontaine D, Pfeiffer B, Brocht A, Bennett S, Daigneault S, Hodgeman K, C O’Connell, T Ross, Richard K, Watts A, Group Parkinson Study (2004) Pramipexole vs levodopa as initial treatment for Parkinson disease: a 4-year randomized controlled trial. Arch Neurol 61:1044–1053
Hong JY, Oh JS, Lee I, Sunwoo MK, Ham JH, Lee JE, Sohn YH, Kim JS, Lee PH (2014) Presynaptic dopamine depletion predicts levodopa-induced dyskinesia in de novo Parkinson disease. Neurology 82:1597–604
Jankovic J (2005) Motor fluctuations and dyskinesias in Parkinson's disease: clinical manifestations. Mov Disord 20(Suppl 11):S11–S16
Jankovic J, Nour F (1986) Respiratory dyskinesia in Parkinson’s disease. Neurology 36:303–304
Kaiser R, Hofer A, Grapengiesser A, Gasser T, Kupsch A, Roots I, Brockmoller J (2003) l-Dopa-induced adverse effects in PD and dopamine transporter gene polymorphism. Neurology 60:1750–1755
Kipfer S, Stephan MA, Schupbach WM, Ballinari P, Kaelin-Lang A (2011) Resting tremor in Parkinson disease: a negative predictor of levodopa-induced dyskinesia. Arch Neurol 68:1037–1039
Klawans HL, Goetz C, Bergen D (1975) Levodopa-induced myoclonus. Arch Neurol 32:330–334
Kostic V, Przedborski S, Flaster E, Sternic N (1991) Early development of levodopa-induced dyskinesias and response fluctuations in young-onset Parkinson’s disease. Neurology 41:202–205
Kostić VS, Marinković J, Svetel M, Stefanova E, Przedborski S (2002) The effect of stage of Parkinson’s disease at the onset of levodopa therapy on development of motor complications. Eur J Neurol 9:9–14
Ku S, Glass GA (2010) Age of Parkinson’s disease onset as a predictor for the development of dyskinesia. Mov Disord 25:1177–1182
Kumar N, Van Gerpen JA, Bower JH, Ahlskog JE (2005) Levodopa-dyskinesia incidence by age of Parkinson’s disease onset. Mov Disord 20:342–344
Lee JY, Cho J, Lee EK, Park SS, Jeon BS (2011) Differential genetic susceptibility in diphasic and peak-dose dyskinesias in Parkinson’s disease. Mov Disord 26:73–79
LeWitt PA (1998) Conjugate eye deviations as dyskinesias induced by levodopa in Parkinson’s disease. Mov Disord 13:731–734
LeWitt PA (2015) Levodopa therapy for Parkinson’s disease: pharmacokinetics and pharmacodynamics. Mov Disord 30:64–72
LeWitt PA, Guttman M, Tetrud JW, Tuite PJ, Mori A, Chaikin P, Sussman NM, U.S. Study Group (2008) Adenosine A2A receptor antagonist istradefylline (KW-6002) reduces “off” time in Parkinson’s disease: a double-blind, randomized, multicenter clinical trial (6002-US-005). Ann Neurol 63:295–302
Luquin MR, Scipioni O, Vaamonde J, Gershanik O, Obeso JA (1992) Levodopa-induced dyskinesias in Parkinson’s disease: clinical and pharmacological classification. Mov Disord 7:117–124
Lyoo CH, Lee MS (2011) Rhabdomyolysis induced by severe levodopa induced dyskinesia in a patient with Parkinson’s disease. J Neurol 258:1893–1894
Manson A, Stirpe P, Schrag A (2012) Levodopa-induced-dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J Parkinsons Dis 2:189–198
Marconi R, Lefebvre-Caparros D, Bonnet AM, Vidailhet M, Dubois B, Agid Y (1994) Levodopa-induced dyskinesias in Parkinson’s disease phenomenology and pathophysiology. Mov Disord 9:2–12
Markham CH (1971) The choreoathetoid movement disorder induced by levodopa. Clin Pharmacol Ther 12:340–343
Melamed E (1979) Early-morning dystonia. A late side effect of long-term levodopa therapy in Parkinson’s disease. Arch Neurol 36:308–310
Meloni M, Solla P, Mascia MM, Marrosu F, Cannas A (2017) Diphasic dyskinesias during levodopa-carbidopa intestinal gel (LCIG) infusion in Parkinson’s disease’. Parkinsonism Relat Disord 37:92–96
Monte FS, da Silva-Junior FP, Braga-Neto P, Nobre e Souza MA, de Bruin VM (2005) Swallowing abnormalities and dyskinesia in Parkinson’s disease. Mov Disord 20:457–462
Muenter MD, Sharpless NS, Tyce GM, Darley FL (1977) Patterns of dystonia (“I-D-I” and “D-I-D-”) in response to l-dopa therapy for Parkinson’s disease. Mayo Clin Proc 52:163–174
Nicoletti A, Zappia M (2015) Coffee consumption and risk of levodopa-induced dyskinesia in Parkinson’s disease: the FRAGAMP study. Mov Disord 30:1854–1856
Nutt JG (1990) Levodopa-induced dyskinesia: review, observations, and speculations. Neurology 40:340–345
Odekerken VJ, van Laar T, Staal MJ, Mosch A, Hoffmann CF, Nijssen PC, Beute GN, van Vugt JP, Lenders MW, Contarino MF, Mink MS, Bour LJ, van den Munckhof P, Schmand BA, de Haan RJ, Schuurman PR, de Bie RM (2013) Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol 12:37–44
Oertel W, Eggert K, Pahwa R, Tanner CM, Hauser RA, Trenkwalder C, Ehret R, Azulay JP, Isaacson S, Felt L, Stempien MJ (2017) Randomized, placebo-controlled trial of ADS-5102 (amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson’s disease (EASE LID 3). Mov Disord 32:1701–1709
Olanow CW, Obeso JA, Stocchi F (2006) Drug insight: continuous dopaminergic stimulation in the treatment of Parkinson’s disease. Nat Clin Pract Neurol 2:382–392
Olanow CW, Stern MB, Sethi K (2009) The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology 72:S1–S136
Olanow CW, Kieburtz K, Odin P, Espay AJ, Standaert DG, Fernandez HH, Vanagunas A, Othman AA, Widnell KL, Robieson WZ, Pritchett Y, Chatamra K, Benesh J, Lenz RA, Antonini A (2014) Continuous intrajejunal infusion of levodopa-carbidopa intestinal gel for patients with advanced Parkinson’s disease: a randomised, controlled, double-blind, double-dummy study. Lancet Neurol 13:141–149
Oliveri RL, Annesi G, Zappia M, Civitelli D, Montesanti R, Branca D, Nicoletti G, Spadafora P, Pasqua AA, Cittadella R, Andreoli V, Gambardella A, Aguglia U, Quattrone A (1999) Dopamine D2 receptor gene polymorphism and the risk of levodopa-induced dyskinesias in PD. Neurology 53:1425–1430
Pahwa R, Tanner CM, Hauser RA, Sethi K, Isaacson S, Truong D, Struck L, Ruby AE, McClure NL, Went GT, Stempien MJ (2015) Amantadine extended release for levodopa-induced dyskinesia in Parkinson’s disease (EASED Study). Mov Disord 30:788–795
Pahwa R, Tanner CM, Hauser RA, Isaacson SH, Nausieda PA, Truong DD, Agarwal P, Hull KL, Lyons KE, Johnson R, Stempien MJ (2017) ADS-5102 (Amantadine) extended-release capsules for levodopa-induced dyskinesia in Parkinson disease (EASE LID Study): a randomized clinical trial. JAMA Neurol 74:941–49
Pechevis M, Clarke CE, Vieregge P, Khoshnood B, Deschaseaux-Voinet C, Berdeaux G, Ziegler M (2005) Effects of dyskinesias in Parkinson’s disease on quality of life and health-related costs: a prospective European study. Eur J Neurol 12:956–963
Pfeiffer RF, LeDoux MS (2015) Levodopa-induced lateral jaw deviation dystonia. Parkinsonism Relat Disord 21:808
Pietracupa S, Fasano A, Fabbrini G, Sarchioto M, Bloise M, Latorre A, Altieri M, Bologna M, Berardelli A (2013) Poor self-awareness of levodopa-induced dyskinesias in Parkinson’s disease: clinical features and mechanisms. Parkinsonism Relat Disord 19:1004–1008
Poewe WH, Lees AJ, Stern GM (1988) Dystonia in Parkinson’s disease: clinical and pharmacological features. Ann Neurol 23:73–78
Quinn NP (1998) Classification of fluctuations in patients with Parkinson’s disease. Neurology 51:S25–S29
Rajput AH (1992) Frequency and cause of Parkinson’s disease. Can J Neurol Sci 19:103–107
Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. N Engl J Med 342:1484–1491
Rice JE, Antic R, Thompson PD (2002) Disordered respiration as a levodopa-induced dyskinesia in Parkinson’s disease. Mov Disord 17:524–527
Rieck M, Schumacher-Schuh AF, Altmann V, Francisconi CL, Fagundes PT, Monte TL, Callegari-Jacques SM, Rieder CR, Hutz MH (2012) DRD2 haplotype is associated with dyskinesia induced by levodopa therapy in Parkinson’s disease patients. Pharmacogenomics 13:1701–1710
Rieck M, Schumacher-Schuh AF, Callegari-Jacques SM, Altmann V, Schneider Medeiros M, Rieder CR, Hutz MH (2015) Is there a role for ADORA2A polymorphisms in levodopa-induced dyskinesia in Parkinson’s disease patients?. Pharmacogenomics 16:573–82
Růžička E, Zárubová K, Nutt JG, Bloem BR (2011) “Silly Walks” in Parkinson’s disease: unusual presentation of dopaminergic-induced dyskinesias. Mov Disord 26:1783–1784
Schrag A, Quinn N (2000) Dyskinesias and motor fluctuations in Parkinson’s disease. A community-based study. Brain 123(Pt 11):2297–305
Schrag A, Ben-Shlomo Y, Brown R, Marsden CD, Quinn N (1998) Young-onset Parkinson’s disease revisited—clinical features, natural history, and mortality. Mov Disord 13:885–94
Schumacher-Schuh AF, Altmann V, Rieck M, Tovo-Rodrigues L, Monte TL, Callegari-Jacques SM, Medeiros MS, Rieder CR, Hutz MH (2014) Association of common genetic variants of HOMER1 gene with levodopa adverse effects in Parkinson’s disease patients. Pharmacogenomics J 14:289–294
Scott NW, Macleod AD, Counsell CE (2016) Motor complications in an incident Parkinson's disease cohort. Eur J Neurol 232(2):304–312
Sharma JC, Macnamara L, Hasoon M, Vassallo M, Ross I (2006) Cascade of levodopa dose and weight-related dyskinesia in Parkinson’s disease (LD-WD-PD cascade). Parkinsonism Relat Disord 12:499–505
Sharma JC, Ross IN, Rascol O, Brooks D (2008) Relationship between weight, levodopa and dyskinesia: the significance of levodopa dose per kilogram body weight. Eur J Neurol 15:493–496
Sharma JC, Bachmann CG, Linazasoro G (2010) Classifying risk factors for dyskinesia in Parkinson’s disease. Parkinsonism Relat Disord 16:490–497
Stacy M, Silver D, Mendis T, Sutton J, Mori A, Chaikin P, Sussman NM (2008) A 12-week, placebo-controlled study (6002-US-006) of istradefylline in Parkinson disease. Neurology 70:2233–2240
Strong JA, Dalvi A, Revilla FJ, Sahay A, Samaha FJ, Welge JA, Gong J, Gartner M, Yue X, Yu L (2006) Genotype and smoking history affect risk of levodopa-induced dyskinesias in Parkinson’s disease. Mov Disord 21:654–659
Tambasco N, Romoli M, Calabresi P (2017) Levodopa in Parkinson’s disease: current status and future developments. Curr Neuropharmacol. https://doi.org/10.2174/1570159X15666170510143821
Tanner CM, Goldman SM (1996) Epidemiology of Parkinson’s disease. Neurol Clin 14:317–335
Thanvi B, Lo N, Robinson T (2007) Levodopa-induced dyskinesia in Parkinson’s disease: clinical features, pathogenesis, prevention and treatment. Postgrad Med J 83:384–388
Tolosa E, Compta Y (2006) Dystonia in Parkinson’s disease. J Neurol 253(Suppl 7):VII7–V13
Van Gerpen JA, Kumar N, Bower JH, Weigand S, Ahlskog JE (2006) Levodopa-associated dyskinesia risk among Parkinson disease patients in Olmsted County, Minnesota, 1976–1990. Arch Neurol 63:205–209
van den Munckhof P, Lenz FA, Chase TN (1998) Square-wave action dystonia in Parkinson’s disease. Mov Disord 13:354–356
Verhagen Metman L, Del Dotto P, Blanchet PJ, van den Munckhof P, Chase TN (1998a) Blockade of glutamatergic transmission as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Amino Acids 14:75–82
Verhagen Metman L, Del Dotto P, van den Munckhof P, Fang J, Mouradian MM, Chase TN (1998b) Amantadine as treatment for dyskinesias and motor fluctuations in Parkinson’s disease. Neurology 50:1323–1326
Warren Olanow C, Kieburtz K, Rascol O, Poewe W, Schapira AH, Emre M, Nissinen H, Leinonen M, Stocchi F (2013) Factors predictive of the development of levodopa-induced dyskinesia and wearing-off in Parkinson’s disease. Mov Disord 28:1064–1071
Wickremaratchi MM, Ben-Shlomo Y, Morris HR (2009) The effect of onset age on the clinical features of Parkinson’s disease. Eur J Neurol 16:450–456
Wills AM, Eberly S, Tennis M, Lang AE, Messing S, Togasaki D, Tanner CM, Kamp C, Chen JF, Oakes D, McDermott MP, Schwarzschild MA, Group Parkinson Study (2013) Caffeine consumption and risk of dyskinesia in CALM-PD. Mov Disord 28:380–383
Zappia M, Annesi G, Nicoletti G, Arabia G, Annesi F, Messina D, Pugliese P, Spadafora P, Tarantino P, Carrideo S, Civitelli D, De Marco EV, Ciro-Candiano IC, Gambardella A, Quattrone A (2005) Sex differences in clinical and genetic determinants of levodopa peak-dose dyskinesias in Parkinson disease: an exploratory study. Arch Neurol 62:601–605
Zhang YH, Tang BS, Song CY, Xu Q, Lou MX, Liu ZH, Yu RH, Yan XX, Guo JF (2013) The relationship between the phenotype of Parkinson’s disease and levodopa-induced dyskinesia. Neurosci Lett 556:109–112
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Drs. Frei, Tran, and Vo have no conflicts of interest to declare. Daniel Truong reports research Grants from Ipsen, Merz, Auspex, Daiichi Sankyo Pharma, AbbVie, National Institute of Neurological Disorders, and Stroke, Kyowa, Neurocrine, Sunovion Pharmaceuticals, Acadia, Accorda, Cynapsus, Neuroderm, Prexton Therapeutics, and Intec. He reports receiving honoraria from Teva Pharmaceutical Industries, Alexza Pharmaceuticals, Inc., Adamas Pharmaceuticals, Revance Therapeutics, and US WorldMeds.
Rights and permissions
About this article
Cite this article
Tran, T.N., Vo, T.N.N., Frei, K. et al. Levodopa-induced dyskinesia: clinical features, incidence, and risk factors. J Neural Transm 125, 1109–1117 (2018). https://doi.org/10.1007/s00702-018-1900-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00702-018-1900-6