Abstract
Dementia with Lewy bodies (DLB) is an age-associated neurodegenerative disorder producing progressive cognitive decline that interferes with normal life and daily activities. Neuropathologically, DLB is characterised by the accumulation of aggregated α-synuclein protein in Lewy bodies and Lewy neurites, similar to Parkinson’s disease (PD). Extrapyramidal motor features characteristic of PD, are common in DLB patients, but are not essential for the clinical diagnosis of DLB. Since many PD patients develop dementia as disease progresses, there has been controversy about the separation of DLB from PD dementia (PDD) and consensus reports have put forward guidelines to assist clinicians in the identification and management of both syndromes. Here, we present basic concepts and definitions, based on our current understanding, that should guide the community to address open questions that will, hopefully, lead us towards improved diagnosis and novel therapeutic strategies for DLB and other synucleinopathies.
Similar content being viewed by others
Synucleinopathies: a general overview
The synucleinopathies comprise several neurodegenerative disorders characterised by the accumulation of aggregated forms of the protein α-synuclein (α-syn) in both neuronal and non-neuronal cells in the brain. Most idiopathic synucleinopathies are age-associated and, therefore, their prevalence is increasing in parallel with the world wide increase in life expectancy [1]. Synucleinopathies are second to Alzheimer’s disease (AD) amongst the most common neurodegenerative disorders known to cause dementia [2]. As with most neurodegenerative disorders, there are still no disease-modifying drugs, limiting treatment options to symptomatic relief and palliative measures. Therefore, synucleinopathies pose a growing socio-economic burden to modern societies, and demand urgent attention.
Most synucleinopathies are Lewy body diseases (LBD), as they are characterised by the accumulation of aggregated a α-syn into Lewy bodies (LBs) within vulnerable neurons and Lewy neurites (LN) in neuronal processes [3]. The LBD comprise Parkinson’s disease (PD), Parkinson’s disease dementia (PDD), and dementia with Lewy bodies (DLB), among other less common disorders [4]. The central role of α-syn in LBD originated from almost simultaneous findings of mutations in the gene encoding for α-syn (SNCA) in familial forms of PD [5], and of α-syn comprising the major protein component of Lewy bodies [3].
Multiple system atrophy (MSA) is neuropathologically characterised by accumulation of aggregated α-syn in oligodendrocytes, inclusions known as glial cytoplasmic inclusions (GCIs) [4, 6], while LB pathology is absent and, therefore, MSA is not an LBD.
The initial clinical and neuropathological studies which established the distinct clinical and neuropathological phenotype of the disorder now known as DLB, preceded immunohistochemical methods to detect α-syn in human brain tissue, but later revisions of international consensus for diagnostic guidelines now recommend the use of immunohistochemistry [7,8,9,10,11].
Clinical under-diagnosis of DLB [12], and over-diagnosis of PD [13, 14], have led to most studies of LBD focusing on PD and PDD, leaving DLB historically under-researched relative to its population prevalence. Increasing recognition of DLB as a distinct and prevalent age-associated neurodegenerative dementia has stimulated increasing numbers of high-quality studies on its aetiology and pathogenesis. Here, we summarise contemporary findings from this rapidly expanding field, focusing on genetics, diagnostic biomarkers and molecular mechanisms.
The clinical definition of DLB
DLB is now the preferred term [8, 10, 11] for a variety of previously used clinical diagnoses including diffuse LB disease (DLBD) [15,16,17], LB dementia [18], dementia associated with cortical Lewy bodies (DCLB) [19], the LB variant of Alzheimer’s disease (LBVAD) [20, 21], and senile dementia of LB type (SDLT) [22].
Recognition and definition of the DLB syndrome originally occurred through post-mortem neuropathological observations, of a particular distribution of LB and LN in the brains of elderly subjects with dementia, followed by a retrospective review of their clinical histories [23]. This revealed two major findings – the first was that a significant number of LB pathology cases had a clinical presentation that was discernibly different from other dementia subtypes, even at an early stage in the disease. Fluctuating levels of cognitive impairment, recurrent visual hallucinations, spontaneous extrapyramidal motor features and a history of rapid eye movement (REM) sleep behavior disorder (RBD) were the most prominent symptoms, and the presence of two or more of these symptoms in an individual with dementia is now considered sufficient for a clinical diagnosis of probable DLB.
The other major observation was that approximately 50% of subjects showing full blown DLB pathology at neuropathological post-mortem examination did not show the characteristic clinical picture of DLB during life but typically presented with global cognitive decline reminiscent of AD. Unsurprisingly, such cases usually show additional high levels of AD neuropathological change [24, 25]. The true prevalence of such mixed pathology cases is unknown but autopsy studies indicate that between a third and a half of carefully clinically diagnosed AD show at least some degree of LB pathology at autopsy [20, 26]. Complex visual hallucinations are the only clinical feature indicating the likely presence of LB pathology in an otherwise typical AD case [27], but robust data on progression, prognosis and response to treatments of “mixed AD+DLB” (i.e., cases showing both full blown AD and DLB pathology) are lacking.
While a recent UK estimate found that only 4.6% of specialist dementia service referrals were clinically diagnosed with DLB [28], substantial LB pathology was present in about 20% of post-mortem brains, further underpinning the general under-diagnosis of DLB during life. Moreover, there was substantial variability in DLB clinical diagnosis rates (2.4% - 5.9%) between individual clinicians working in geographically proximal services suggesting that performance could be improved simply by better application of clinical methods and by increased use of biomarkers (see section "Biomarkers in LBD").
The current clinical diagnostic criteria for DLB are shown in Table 1. Dementia, defined as a progressive cognitive decline of sufficient magnitude to interfere with normal social or occupational functions, or with usual daily activities, is an essential requirement. Disproportionate attentional, executive function and visual processing deficits relative to memory and naming are typical features. Diagnostic toolkits have been published to assist clinicians to identify the core clinical features [29,30,31] but no DLB-specific cognitive batteries have yet been developed.
The item generally causing the most difficulty in assessment is the identification of cognitive fluctuation. It is recommended to use one of several published methods which typically use a series of structured questions asking: (i) about changes in the patient’s level of functioning during the day; (ii) about excessive daytime drowsiness; or (iii) about difficulty in arousing the patient so they maintain attention throughout the day. RBD can be difficult to differentiate from the numerous other sleep disturbances that can occur in dementia unless the care-taker is specifically asked whether they have ever seen the patient appear to "act out his/her dreams" while sleeping (punching or flailing arms in the air, shouting or screaming). Assessment of parkinsonism can be problematic, especially when the clinician is not an expert movement disorder neurologist, since motor features may be absent in up to 25% of autopsy confirmed DLB cases and, even when present, may be very mild. Documentation of only one of the cardinal features, bradykinesia, resting tremor, or rigidity, is required for DLB, while at least two are required to diagnose PD. Co-morbidities, e.g. arthritis, or inability to comply with neurological examination because of cognitive impairment may lead to false positive diagnoses.
Recurrent, complex visual hallucinations, which occur in the majority of DLB patients, pose less problems of recognition, provided that the clinician asks directly about them and quantifies their severity with an appropriate scale. They are typically well formed, featuring people or animals, and may be accompanied by related phenomena including passage hallucinations, sense of presence and visual illusions. Patients are typically able to report these experiences, as are observant caregivers [23].
A case of probable DLB established using consensus criteria has been estimated as having a diagnostic specificity at autopsy of ~85%, possibly the highest of the common neurodegenerative dementia subtypes. The extent to which the addition of indicative biomarkers in the revised DLB criteria will increase this specificity, remains to be determined [32].
Additional clinical features are known to be supportive of a DLB diagnosis. These are symptoms that are commonly present, sometimes early [33] and which may indicate DLB in a patient with dementia, particularly when they persist over time or if several occur in combination (Table 1).
Another important issue to consider is the relationship between the diagnosis of DLB and that of dementia occurring in a patient with a pre-existing clinical diagnosis of PD, usually referred to as PDD. This has been a source of controversy and, therefore, needs clarification and continued research efforts. Although the end stage neuropathological findings in such cases may be similar, there can be little doubt that the clinical experience of the patients and their families will have been very different. DLB is typically a disorder associated with cognitive impairment in which extrapyramidal motor features are often mild or absent, at least until the late stages. In contrast, PDD is characterised by early and prominent extrapyramidal motor features required for PD diagnosis, with neuropsychiatric and cognitive symptoms occurring later. Undoubtedly, the two distinct clinical syndromes of DLB and PD/PDD share underlying pathomechanisms and, while the reasons for the clinical heterogeneity may be due to different propagation patterns of α-syn pathology across different neuronal pathways, the additive effects of concomitant AD pathology which is more common and severe in DLB as compared to PD/PDD should be taken into consideration. Hence, it is inappropriate to simply use PD as an umbrella term for all LBD, and this is reflected in the original formulation of the “one-year rule” (bottom of Table 1) by which DLB should be diagnosed when dementia occurs before, or concurrently with parkinsonism, while the term PDD should be used to describe dementia that occurs in the context of well-established PD ([34] for further discussion). This approach, adopted by DSM5 [35] and the final draft of ICD-11 [36], both of which recommend the distinction of DLB and PDD, suggests that this convention will remain in use until new scientific insight allows to distinguish between DLB and PD/PDD based on specific and well characterized differences in their respective pathomechanisms.
The mean age of onset of PDD and DLB is similar at >70 years whereas PD onset is typically earlier with a mean of 60 years. Data regarding the comparative age related prevalence of PDD and DLB are limited with some suggesting that DLB patients are younger at symptom onset than those with PDD and with more hallucinations and cognitive fluctuations; and others reporting younger age at disease onset in PDD or no essential differences between disorders [37].
Biomarkers in DLB
The diagnostic criteria of DLB identify ‘indicative’ and ‘supportive’ biomarkers based upon their diagnostic specificity and the volume of good quality evidence available (Table 1) [11]. The presence of an indicative biomarker in combination with a single core clinical feature is sufficient for a diagnosis of probable DLB. Supportive biomarkers are consistent with DLB but lack the specificity of the indicative biomarkers.
Indicative biomarkers
Striatal dopamine transporter imaging
Like PD, DLB is associated with nigrostriatal dopaminergic neuron loss. This can be detected using SPECT or PET imaging using a ligand that binds to presynaptic dopamine transporters (e.g. N-ω-fluoropropyl-2β-carbomethoxy-3β-(4-iodophenyl) nortropane (FP-CIT)). Visually rated FP-CIT SPECT has a sensitivity of 78% and specificity of 90% to differentiate probable DLB from other dementias when compared with clinical diagnosis [38]. This has been confirmed with post-mortem diagnosis [39]. The upper limit of sensitivity of FP-CIT SPECT reflects the absence of substantia nigra pathology sufficient to cause an abnormal scan in some cases of DLB [40, 41].
FP-CIT SPECT images can be rated visually using a scale developed for PD [42], though many cases of DLB are difficult to classify using this scale (Fig. 1a) [43]. Clinical reports often use a combination of visual interpretation and semi-quantitative analysis, which has been shown to increase reader confidence [44, 45].
Indicative biomarkers for dementia with Lewy bodies. A. N-ωfluoropropyl-2β-carbomethoxy- 3β-(4-iodophenyl) nortropane (123I-FP-CIT SPECT) single photon emission tomography (SPECT). Axial images from FP-CIT SPECT at the level of the striatum. Grade 0 – normal uptake in left and right striatum. Grade 1 – unilateral decreased uptake in putamen [42]. Grade 2: bilateral uptake in putamen. Grade 3: virtually absent uptake bilaterally in the caudate and putamen. Balanced bilateral loss in the caudate and putamen is often seen in DLB, which does not fit easily into any Benamer scale category. B. Cardiac Meta-iodobenzylguanidine (MIBG SPECT) Imaging. The top image is normal, with a clear cardiac outline visible (arrow, HMR=3.14). The bottom image is abnormal with no visible cardiac outline (HMR=1.03). C. Polysomnography (PSG) recording demonstrating episodes of REM sleep without atonia on electro-oculogram (EOG) measuring eye movements, electroencephalogram (EEG) and electromyogram (EMG) measuring chin movement. With thanks to Dr Sean Colloby (a), Ms Gemma Roberts (b) and Dr Kirstie Anderson (c)
Dopamine transporter imaging should not be used to differentiate DLB from frontotemporal dementia, progressive supranuclear palsy, corticobasal syndrome or multiple system atrophy as these conditions can also be associated with reduced striatal dopamine transporters [46].
MIBG Myocardial scintigraphy
Cardiac autonomic denervation is found in Lewy body diseases such as PD, DLB and pure autonomic failure [47]. Meta-iodobenzylguanidine (MIBG) is a noradrenaline analogue that binds to presynaptic cardiac autonomic nerve terminals. MIBG binding in the heart is compared to non-specific binding in the mediastinum (H:M ratio, Fig. 1b). Single centre studies have demonstrated high sensitivity and specificity of MIBG scintigraphy [48,49,50]. The only multicentre study to date found a sensitivity of 69% and specificity of 89% [51]. The diagnostic accuracy of MIBG in this study improved when compared with clinical diagnosis 3 years after the scan (sensitivity 77%, specificity 97%) [52].
A limitation of MIBG scintigraphy is that comorbid conditions and medications can reduce cardiac MIBG uptake [53]. As a result, studies have excluded participants with common conditions such as heart failure, ischaemic heart disease and poorly-controlled diabetes [51]. Thresholds for abnormality in H:M ratio differ between centres, due in part to differences in collimators (from 1.60 to 2.20 in the above studies) [48, 52]. Individual centres should therefore develop local thresholds prior to clinical application.
Polysomnography
Polysomnography (Fig. 1c) allows for the objective identification of RBD by measuring EEG, eye movements and muscle movement during sleep. Polysomnography-confirmed RBD has a very high diagnostic specificity of 98% for synucleinopathies (PD, DLB or MSA) [54]. It had an 84% sensitivity in post-mortem confirmed DLB cases [55]. Sensitivity may be limited by the absence of REM sleep in some polysomnography sessions.
Supportive biomarkers
Imaging
DLB is associated with less severe medial temporal lobe atrophy on structural imaging when compared to AD [56]. However, the sensitivity of this biomarker to detect DLB is limited by the presence of AD pathology and associated medial temporal lobe atrophy in a significant proportion of DLB cases [57]. Occipital hypoperfusion and hypometabolism can also be seen on functional imaging, though FDG-PET is more effective in identifying DLB than HMPAO-SPECT [58].
EEG
DLB is characterised by the presence of prominent posterior slow wave activity [59,60,61] and temporal slow wave activity [62]. The dominant EEG rhythm, normally within the alpha range, is slowed toward pre-alpha/fast theta and the variability of dominant frequency over time is increased [60, 61, 63, 64]. Single centre studies have reported good to excellent discrimination of DLB from AD using quantification of EEG by a variety of methods [59]; multicentre studies have been more equivocal [60]. However, EEG may be an important biomarker for DLB in the future as changes can be detected early in the disease course [65, 66].
Other biomarkers
Fluid biomarkers
CSF α-syn levels have variously been found to be increased, decreased or unchanged in DLB [67]. The reasons for these conflicting results may include contamination (e.g. with blood) and differences in CSF acquisition, processing and analysis [68]. The differentiation of DLB from AD using CSF markers is further complicated by the presence of AD pathology in a significant proportion of DLB cases as discussed above. At present, CSF measures cannot discriminate between DLB and AD, but markers of AD pathology may be useful in stratifying DLB patients for future clinical trials [69].
Novel Biomarkers
The development of biomarkers for the diagnosis of LBD such as DLB is an active area of research. Much of this effort is focussed on the development of an α-syn biomarker to complement the β-amyloid (Aβ) and tau biomarkers that have been developed for AD. α-syn imaging ligands are currently in the pre-clinical stage [70]. α-syn biomarkers in other tissues such as skin [71], and gut [72] are currently being investigated.
Genetics of DLB
Our present understanding of the genetic aetiology of DLB is limited. Nevertheless, the available studies suggest that genetic factors are as important in DLB as in AD or PD. Positive family history of dementia and DLB is a strong risk factor for DLB and siblings of affected individuals are at 2.3 fold risk of developing the disease themselves [73, 74]. Nonetheless, DLB pedigrees with highly penetrant alleles are rare and frequencies of genetic variants in genes linked with DLB are poorly understood.
Whilst families with DLB are rare, such families are informative in providing genetic insight to the aetiology of DLB. Most cases of suggested familial DLB show a predominant PD phenotype where many family members have motor impairment as a presentation long before onset of cognitive symptoms. Very few families with suggested DLB show cognitive problems at presentation. Consequently many families, while they do show cognitive changes and dementia as part of the disease process, do not have typical DLB meeting consensus criteria in all family members. For example, individuals in families with rare point mutation in the SNCA gene such as the Contursi kindred [75, 76] often have profound dementia as part of the disease process, although this is variable and often a later symptom. Typically, cases with point mutations in SNCA present as early onset PD [77,78,79]. Similarly, in the Waters-Miller-Muenter kindred with triplication of SNCA [80], onset is typically motor impairment with very few cases showing cognitive impairment at presentation and which can be described as having DLB [81]. Families with SNCA duplication do present clinically with certain features of DLB and show typical pathology of neocortical α-syn deposition, but again, dementia is often a later feature or not prominent [82,83,84,85,86,87]. Therefore, SNCA mutations are not a common finding in DLB [88].
Often families and individuals that have AD and causal mutations in APP or PSEN1 along with concurrent presence of LBs, typically in the amygdala, have been described as having DLB or LBD. While these cases fit with a wider view of LBD, most do not meet consensus clinical criteria for DLB [89].
There are however families which do meet clinical criteria for DLB and where familial inheritance is shown. In a description of two families with typical late onset dementia showing typical DLB, analysis showed widespread neocortical α-syn pathology with typically only mild AD pathology, although a genetic defect was not identified [90]. One family with dementia at onset and later development of parkinsonism was reported where age at onset of dementia was variable [91]. Neuropathology of the proband showed widespread neocortical type α-syn pathology and Braak stage V neurofibrillary tangles fulfilling neuropathologic criteria for both DLB and AD. Sequence analysis of this family has shown the presence of a P123H SNCB mutation near the C-terminus of the protein, although no deposition of β-synuclein protein in brain tissue was observed [92].
Two unrelated families with suggested DLB have been reported as carrying a mutation in the EIF4G gene [93] known to be associated with increased risk of PD [94]. In these affected families, presentation was typically a dementia syndrome with variable parkinsonian features and pathology indicative of diffuse neocortical α-syn deposition with only age related AD pathology. Siblings with clinically and neuropathologically confirmed DLB have been reported [95, 96]. However, a shared genetic mutation has not yet been identified [97]. Individuals with DLB do show potentially causative mutations in certain autosomal dominant or recessive genes associated with other neurodegenerative disorders and individuals with mutations in PARK2, CHMP2B, PSEN2, SQSTM1, EIF4G1, and GIGYF2 have been identified [97].
Although families with SNCA mutations do not show clinical characteristics of DLB, association with the SNCA locus is also strongly apparent in large studies of sporadic DLB [98, 99]. Association with the SNCA gene is not surprising due to the protein product α-syn being present in LB and believed to be central in the pathophysiology of DLB, PD and PDD. However, there seems to be an interesting correlation, with the 3’ of the SNCA gene being associated with the PD phenotype and the 5’ region linking with DLB. This may impact on the gene expression and distribution of LB pathology in the brain.
Multiple studies dissecting the genetic component of DLB have been published to date (for a comprehensive review see [100, 101]), and the genetic landscape of DLB mirrors that of the clinical and neuropathological overlap between DLB, PD and AD. To date, no high penetrance pathogenic mutations have been identified. However, a number of common (>1% in population) and rare genetic risk variants have been established. Genes reported to be associated with DLB are SNCA, LRRK2, PSEN1, PSEN2, APP, SNCB, MAPT, SCARB2, GBA and APOE (Table 2). The finding of rare variants in AD genes (PSEN1, PSEN2 and APP) in cases of dementia, as previously noted, might be in part due to misdiagnosis, particularly when the neuropathological assessment has not been possible. The co-occurrence of LB pathology in AD is common and may influence the disease phenotype towards DLB [102]. The recent genome wide association study confirmed several of the previously reported associations (APOE, SNCA and GBA) and identified a new probable locus CNTN1 [99], providing an unbiased and the most comprehensive study of DLB genetics to date.
The strongest and most replicated genetic risk factors for DLB are unequivocally APOE ε4 allele and Glucocerebrosidase (GBA). APOE ε4 carriers often develop mixed DLB-AD pathology. However, the ε4 allele is also over-represented in pure DLB and PDD [103]. Multiple studies have found an association of APOE ε4 with an increased risk of DLB and, recently, a greater severity of LB pathology in cases with APOE ε4 and low AD pathology has been reported [97, 104, 105]. These findings imply an involvement of APOE in the mechanism of pure LB pathology spread and not only an increased risk of developing DLB, or Aβ associated DLB. Interestingly, no association of APOE genotype is observed for PD [106].
The association of GBA and DLB is well established [107]. The GBA gene encodes a lysosomal enzyme involved in the metabolism of complex glycosphingolipids (OMIM 606463). DLB patients are 8 times more likely to be carriers of GBA mutations than controls [107]. This risk is higher than that reported for PD [108], and seems to associate with earlier age at onset, severity and disease progression. Similar to APOE, GBA is likely involved in the mechanism of LB pathology formation and/or spread, although the exact cause of this predisposition is unknown. The recently reported association of DLB with PD-linked SCARB2 emphasises the importance of lysosomal pathways in DLB [98].
DLB appears to be genetically heterogeneous, with a rare contribution of pathogenic causative mutations and relatively common risk factors, which may explain why DLB is a relatively common disorder, but with a reduced aggregation in families [97]. Our knowledge of DLB is undoubtedly evolving and interrogation of currently known risk factors will improve our understanding of DLB pathophysiology.
Neuropathology of DLB
The majority of DLB cases show loss of pigmented, dopaminergic neurons in the substantia nigra (SN), similar to that which is seen in PD (Fig. 2a-c). However, as the main pathological changes in DLB affect the neocortex and limbic system, additional macroscopic changes are observed in patients with DLB. Some structural changes are similar to those seen in AD, with widespread cerebral atrophy being a feature of both AD and DLB [109]. Unlike AD, there is a relative preservation of the medial temporal lobe in DLB [110] (Fig. 2d-f).
Macroscopic features of DLB. Dopaminergic cell loss is observed in the substantia nigra of a DLB patient (black arrows) (a) compared to AD (b) and control (c). In the same patients, atrophy of the medial temporal lobe is evident in AD, blue arrows (e) whilst it is relatively spared in DLB (d), and controls (f). Both scale bars represent 1cm
Microscopically, DLB is characterised by the abnormal accumulation of α-syn in neuronal somata and processes (i.e., LB and LN). Under pathological conditions, α-syn undergoes a conformational change from random coil to a cross-β sheet-rich structure [111, 112]. Electron microscopy has revealed that LB and LN are composed of unbranched α-syn filaments with a typical length of 200-600nm and a width of 5-10nm [113]. Two types of LB have been described: i) brainstem LB have an acidophilic and argyrophilic core with a pale stained halo, classically seen using H&E staining (Fig. 3a and b). Typically they are 8-30μm in diameter and predominantly seen in pigmented neurons of the SN (Fig. 3c); ii) cortical LB are eosinophilic, rounded, angular or reniform structures without a halo and can be visualized using α-syn immunohistochemistry, most notably in layers V and VI of the neocortex (Fig. 3d-f).
Histopathological features of DLB. Midbrain section at the level of the superior colliculus stained with H&E where dopaminergic neurons in the substantia nigra are vulnerable in DLB patients (a). Brainstem LBs are classically detected using H&E (b – black arrow) and frequently in the pigmented neurons of the SN (c – white arrows). Cortical LB pathology (e.g. cingulate cortex) affects all layers of the neocortex, most notably layers V and VI (d – red arrows). Cortical LBs and LNs can be visualised by α-syn immunohistochemistry (e - LB blue arrow head, LN blue arrow). α-syn phosphorylated at serine 129 detects a greater abundance of LB pathology compared to staining with phosphorylation independent antibodies (f - green arrows illustrate LBs, LNs, and Lewy dots). Alzheimer’s disease pathology is also a frequent finding in post-mortem tissue from DLB patients including hyperphosphorylated tau tangles (g) and Aβ plaques (h). Of note photomicrographs E-H were taken from sequential sections of the cingulate cortex of the same DLB patient. Abbreviations: SN, substantia nigra; WM, white matter; LB, Lewy body; LN, Lewy neurite; α-syn, α-synuclein. Scale bar represents 0.5cm in A, 20μm in B and C, 500 μm D, and 50μm in E-H
α-syn can undergo extensive posttranslational modifications (PTM), with phosphorylated, nitrated, and SUMOylated forms of α-syn identified in LB [114,115,116]. Immunohistochemistry of α-syn phosphorylated at serine 129 in DLB has revealed far more abundance of α-syn than phosphorylation-independent antibodies and, in addition to LB and LN, more threads and dot-like structures (Lewy dots) are immunopositive for this modified form of α-syn (Fig. 3f) [117, 118]. Therefore, it is tempting to speculate that cell types in individual brain regions could accumulate differently modified forms of α-syn, which may have implications in the design of disease modifying therapeutics, or in defining previously unidentified discrete clinico-pathological subtypes of DLB.
Based on current international neuropathological staging systems it is impossible to distinguish DLB from PDD, which shares similar clinical, neurochemical and morphological characteristics with DLB. However, imaging and post-mortem studies have suggested DLB cases exhibit elevated limbic and striatal AD related pathologies, and a lesser degree of dopaminergic cell loss compared to PDD [119,120,121].
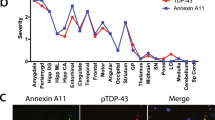
The common occurrence of additional pathologies in DLB (e.g. AD related neurofibrillary tangles and Aβ plaques (Fig. 3g and h), or fronto-temporal lobar degeneration related (FTLD)) is of current interest [122,123,124,125,126,127]. The presence of multiple pathological lesions has implications for disease prognosis, and has been shown to alter the clinical phenotype; an elevated burden of hyperphosphorylated tau has been associated with a shorter survival time from the onset of dementia [128], and a summated score of hyperphosphorylated tau, Aβ, and α-syn is a better predictor of cognitive decline as measured by MMSE compared to individual pathology scores [129]. Intracellular inclusions of TDP-43 (Transactive response DNA-binding protein 43), the hallmark pathology in FTLD, are also often observed in DLB, with prevalence rates reported to be between 0-56% [127, 130, 131]. The distribution of TDP-43 pathology differs in DLB compared to FTLD, with limbic structures affected early in the degenerative process[127, 132]. The presence of TDP-43 pathology has been shown to modify the clinical and radiological findings in neurodegenerative diseases, as patients with additional TDP-43 pathology are more cognitively impaired and display greater hippocampal atrophy as seen on MRI compared to patients lacking TDP-43 pathology[133, 134]. Concomitant cerebrovascular pathology is also commonly observed, appearing in 50% of autopsy-confirmed DLB cases[125]. Reduced cerebral blood flow and microvessel density associated with decreased vascular endothelial growth factor, maybe secondary to α-syn accumulation in the occipital cortex[135], have been suggested. However, there is a still a gap in the knowledge of the exact pathogenesis of CVP in DLB and the cumulative effect on clinical phenotype. Unsurprisingly additional pathologies may impede the clinicians’ ability to provide an accurate diagnosis of DLB [24, 27, 128, 136,137,138].
There are several internationally recognised neuropathological staging systems to assess the topographical distribution of α-syn [11, 41, 139, 140] incorporating a semi-quantitative grading of α-syn to assess the severity in individual brain regions (Fig. 4). The majority of cases can be classified in accordance with the suggested rostral-caudal propagation of α-syn. However, other factors such as concomitant AD type pathology (often observed in DLB and taken into account in the fourth consensus report of the DLB Consortium [11]), or a genetic susceptibility may influence α-syn aggregation, and it is possible that certain brain regions may become more vulnerable to further abnormal protein deposition. α-syn deposits have also been detected in the peripheral nervous system of patients with synucleinopathies[141, 142]. Further investigations highlighted a multi-organ distribution of α-syn including the gastrointestinal, cardiovascular, endocrine, and respiratory systems[143]. A high prevalence of submandibular gland α-syn has been reported in autopsied-confirmed cases, with 89% / 71% of PD / DLB exhibiting α-syn positive lesions and α-syn positivity has been reported in skin nerve fibres of DLB patients [144]. However, the relation between peripheral and central nervous system α-syn pathology is not fully understood and warrants further investigation.
Schematic diagrams illustrating the neuropathological staging systems for LBD. The Newcastle-McKeith criteria distinguishes between brainstem predominant (regions affected including IX/X motor nucleus, locus coeruleus, and substantia nigra), limbic (transitional, regions include amygdala, transentorhinal cortex, and cingulate cortex), and diffuse neocortical (frontal, temporal, parietal, lobes are affected). N.B. the most recent consensus included the addition of olfactory only, and amygdala predominant stages [11] (a). Braak staging of α-syn deposition: Braak stage 1, IX/X motor nucleus of the medulla oblongata, Braak stage 2, addition of lesions to the locus coeruleus, Braak stage 3, α-syn progresses to the substantia nigra of the midbrain, Braak stage 4, α-syn lesions now detected in the transentorhinal region and CA2 of the hippocampus, Braak stage 5, higher association of the neocortex are affected, and Braak stage 6, α-syn is visible in the premotor and motor regions [139] (b). Leverenz and colleagues modified the original Newcastle-McKeith criteria to include cases that lack α-syn pathology in any other regions with the exception of the amygdala, known as amygdala predominant LB disease [140] (c). Beach and colleagues proposed a unified staging system to include cases that have α-syn confined to the olfactory bulb or bypass the brainstem to the limbic predominant pathway [41] (d)
α-syn is assumed to spread throughout the brain in a prion-like manner [145, 146] (see section "Molecular Mechanisms"). The staging system proposed by Braak and colleagues is based on the assumption that cerebral α-syn pathology initially manifests in the medulla from where it propagates, to the SN (at which stage clinical symptoms of parkinsonism are evident), and further to the neocortex (when clinical symptoms associated with dementia emerge) [10]. However, in DLB, which initially manifests with clinical dementia and only rarely with extrapyramidal symptoms, this topographical spreading pattern is not applicable and α-syn pathology may initially manifest in limbic and/or neocortical areas. In cases with additional limbic and neocortical AD pathology, α-syn pathology may be aggravated as it is tempting to speculate that neurons already subjected to insult by concomitant tau and/ or Aβ pathology could act as trigger sites contributing to the aggregation and deposition of α-syn in the neocortex. Evidence in support of this hypothesis is provided in cases that neuropathologically fulfill criteria for DLB and AD, where the concurrent presence of hyperphosphorylated tau, Aβ, and α-syn has been demonstrated to alter the topographical distribution of pathological protein aggregates compared to cases that do not harbor multiple lesions within the same brain region [137]. The notion that hyperphosphorylated tau, Aβ, and α-syn can influence each other, promoting simultaneous aggregation, is also supported by data from in vitro and transgenic animal studies [147,148,149,150,151], however as this is yet to be fully recapitulated in human tissue [152], future work in this area will help to establish the presence of a mechanistic link between multiple pathologies.
The relevance of Lewy pathology to the patho-mechanisms responsible for eliciting the clinical phenotype is still controversial. Numerous clinico-pathological studies have failed to correlate LB density with disease duration, age of onset, presence or absence of cognitive fluctuations, visual hallucinations, delusions, recurrent falls, severity of parkinsonism or cognitive decline [153,154,155,156]. This is not entirely surprising, as two of the core clinical features of DLB (fluctuations in cognition and recurrent visual hallucinations) are transient in nature. Therefore, other dynamic factors (such as perhaps the levels of oligomeric species of α-syn, or specific PTMs of α-syn) may be better predictors of clinical features of DLB rather than overall LB density. Another hypothesis is that formation of LB represents a neuroprotective mechanism in affected neurons [157, 158], which may account for the lack of association in cognitive decline with increasing LB burden.
Molecular mechanisms
Despite the controversy about the causal role of LB pathology in LBD, the aggregation of α-syn is considered a central process in all synucleinopathies. The aggregation of α-syn follows a two-step process, initiated by a rate limiting nucleation phase in which soluble monomers associate into transient intermediate oligomers, which are built upon during the exponential elongation phase, producing primary filaments that are in turn integrated into fibrillary assembles [159]. This process conforms to a generalised scheme of protein fibrillation established not only for α-syn [160] but also for other proteins such as tau [161] or Aβ [162]. The conversion between nucleation and elongation likely requires small disordered oligomeric arrangements to adopt more stable ordered configuration, resistant to degradation and capable of promoting further fibrillation [163]. Each step of fibrillation can be modulated by a number of factors including familial α-syn mutations [164,165,166,167] as well as by a variety of PTMs, such as acetylation [168], glycation [169], nitration [170], oxidation [171], phosphorylation [114, 172, 173], or truncation [174].
The initial lag phase of the primary nucleation can be bypassed by the presence of “seed competent” fibrils [175], resulting in a secondary nucleation event, which likely facilitates the formation of new aggregates on the surface of existing fibrils [176].
The apparent induction of de-novo fibrillation via the uptake of transmitted α-syn arrangements may underlie the prion-like spread of pathology initially observed as the transmission of Lewy pathology to transplanted fetal neurons [145, 177]. Additional studies demonstrated α-syn aggregates may spread between neurons by sequestering native α-syn thereby promoting aggregate growth [178].
The suggestion that α-syn may spread like a prion is an attractive hypothesis, as it may explain the stereotyped topography of Lewy pathology and clinical heterogeneity across LBD. Importantly, it has also considerable translational potential. However, the regional spread of α-syn does not appear to be solely determined by the strength of anatomical connectivity or a ‘nearest neighbor’ rule, indicating cell- or region- autonomous factors may govern the development of LB pathology [179].
The lymphocyte activation gene 3 (LAG-3) binds α-syn with high specificity and induces endocytosis from the extracellular milieu, and its knockdown impedes the cellular uptake of α-syn fibrils [180]. However, data from our group on the distribution of LAG-3 in post-mortem brain tissue indicate it is a pan-neuronal marker, and is expressed by neurons that do not typically manifest LB (unpublished data).
We have also recently shown that, similarly to Aβ, α-syn interacts with the prion protein (PrP), triggering a signaling cascade that culminates with neuronal dysfunction [181].
Low expression of native α-syn has been described in regions that do not develop LB pathology [182] and decreased cellular expression is prohibitive to intracellular aggregation [183]. Therefore, low expression levels of native α-syn within particular neuronal sub-types may inhibit intracellular aggregation by limiting the initiation nucleation phase.
Nevertheless, the consequences for those cells affected depends on the configuration of the prion-like agent. Somewhat surprisingly, the uptake of fibrils in vitro has been associated with a protective outcome despite accelerated aggregation, and is in contrast to the induction of apoptosis triggered upon the uptake of monomeric or oligomeric preparations [184]. Accordingly, as mentioned above, it remains unclear if mature fibrils which comprise LBs are the primary toxic agent of the disease. Indeed, whilst the presence of cortical LBs is associated with cognitive impairments [185], there is little evidence to support a correlative relationship between LB burden and the severity of dysfunction [154, 155, 186, 187]. This disconnect is not only evident symptomatically, but also at the cellular level, as key pathological changes are often reported independent and/or assumed prior to LB formation. These include synaptic dysfunction [188], decreased neurofilament mRNA production [189], the accumulation of axonal trafficked proteins [190], the induction of apoptotic cascades [191] and neuronal loss [192, 193].
Thus, despite the stable prominent nature of α-syn fibrils, it is likely that toxicity is instead driven by a pool of ill-defined heterogeneous oligomers. These oligomers may dynamically shift in equilibrium, altering their properties and substrates, either acting as intermediates of aggregation (on-pathway oligomers) or terminal assemblies (off-pathway oligomers) from which fibrillation is no longer favorable [160]. Owing to their transient nature, the investigation of oligomers has been somewhat problematic. Nevertheless a variety of oligomers have been defined by their structure, as observed in vitro. These include annular [194] and globular [184] and/or by their involvement in fibrillation [195]. A truncated breakdown product from the incomplete lysosomal processing of fibrils, so called “pα-syn*”, has recently been demonstrated as highly toxic [196], highlighting the potential for the retroactive production of toxins. Mechanistically, an array of cellular insults conducive to dysfunction and death have been attributed to α-syn oligomers; including membrane permeabilization [195, 197, 198], altered synaptic transmission and plasticity [36, 169, 181], the breakdown of protein degradation [199], as well as impairment of cellular organelles such as mitochondria and endoplasmic reticulum [196, 200,201,202]. Despite our progress in understanding the molecular basis of α-syn toxicity, it must be conceded that the generalised terms “oligomers” and “fibrils” lacks the fidelity required for the evaluation of physiological aggregates. Multiple conformations of these assemblies exist, which dictates their biological profile, and may account for specific strains of aggregates resulting in differential clinical diseases [203,204,205]. As such, the extrapolation or generalisation of outcomes observed from in vitro systems, synthetic preparations or from differing protocols of biological extractions must be made with extreme caution.
Conclusions and outlook
DLB is a devastating disorder for which we lack effective therapies. This is, at least partly, due to our lack of detailed understanding of the molecular underpinnings of the disease. Importantly, consensus guidelines have improved the diagnosis and management of DLB, and the 1-year rule remains valid for distinguishing DLB from PDD in the clinical setting [37]. However, we still need additional guidelines (including better stratification of patient cohorts) and outcome measures for future clinical trials in DLB. In addition, we need to continue to improve our understanding of genetic factors, of neuropathological hallmarks, and of the underlying molecular mechanisms.
At the molecular level, we need to identify factors that may justify that the same proteins, such as α-syn, tau, or Aβ, may behave differently and lead to distinct disease manifestations. In this context, PTMs emerge as likely suspects, as they could influence the behavior and accumulation of the various proteins in different brain regions. Given that PTMs can be either transient or irreversible, they may operate together or independently, and may influence the formation of prion-like strains that could then spread in different ways depending on the disease.
Progress is challenging due to the considerable heterogeneity observed in DLB. The hope is that the knowledge acquired will enable us to define better biomarkers for early diagnosis and for following disease progression, and to identify novel targets for therapeutic intervention. Ultimately, our collective goal as a community, should be to distinguish DLB from other similar disorders, in order to better assist patients and families not only with disease management but also, and more importantly, modifying, stopping, or altogether prevent this terrible disease.
Abbreviations
- AD:
-
Alzheimer’s disease
- DCLB:
-
Dementia associated with cortical Lewy bodies
- DLB:
-
Dementia with Lewy bodies
- LB:
-
Lewy body
- LBD:
-
Lewy body disease
- LBVAD:
-
LB variant of Alzheimer’s disease
- PD:
-
Parkinson’s disease
- PDD:
-
Parkinson’s disease dementia
- PET:
-
Positron emission tomography
- RBD:
-
REM sleep behavior disorder
- REM:
-
Rapid eye movement
- SDLT:
-
Senile dementia of LB type
- SPECT:
-
Single-photon emission computed tomography
- α-syn:
-
Alpha-synuclein
References
Van Den Eeden SK, Tanner CM, Bernstein AL, Fross RD, Leimpeter A, Bloch DA, et al. Incidence of Parkinson's disease: variation by age, gender, and race/ethnicity. American journal of epidemiology. 2003;157(11):1015–22.
Bostrom F, Jonsson L, Minthon L, Londos E. Patients with Lewy body dementia use more resources than those with Alzheimer's disease. International journal of geriatric psychiatry. 2007;22(8):713–9.
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–40.
Jellinger KA. Neuropathological spectrum of synucleinopathies. Movement disorders : official journal of the Movement Disorder Society. 2003;18(Suppl 6):S2–12.
Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, et al. Mutation in the alpha-synuclein gene identified in families with Parkinson's disease. Science. 1997;276(5321):2045–7.
Jellinger KA, Lantos PL. Papp-Lantos inclusions and the pathogenesis of multiple system atrophy: an update. Acta neuropathologica. 2010;119(6):657–67.
Kosaka K. Lewy bodies in cerebral cortex, report of three cases. Acta neuropathologica. 1978;42(2):127–34.
McKeith IG, Galasko D, Kosaka K, Perry EK, Dickson DW, Hansen LA, et al. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the consortium on DLB international workshop. Neurology. 1996;47(5):1113–24.
McKeith IG, Perry EK, Perry RH. Report of the second dementia with Lewy body international workshop: diagnosis and treatment. Consortium on Dementia with Lewy Bodies. Neurology. 1999;53(5):902–5.
McKeith IG, Dickson DW, Lowe J, Emre M, O'Brien JT, Feldman H, et al. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–72.
McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017;89(1):88–100.
Vann Jones SA, O'Brien JT. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol Med. 2014;44(4):673–83.
Schrag A, Ben-Shlomo Y, Quinn N. How valid is the clinical diagnosis of Parkinson's disease in the community? Journal of neurology, neurosurgery, and psychiatry. 2002;73(5):529–34.
Jellinger KA. How valid is the clinical diagnosis of Parkinson's disease in the community? Journal of neurology, neurosurgery, and psychiatry. 2003;74(7):1005–6.
Kosaka K, Yoshimura M, Ikeda K, Budka H. Diffuse type of Lewy body disease: progressive dementia with abundant cortical Lewy bodies and senile changes of varying degree--a new disease? Clinical neuropathology. 1984;3(5):185–92.
Dickson DW, Davies P, Mayeux R, Crystal H, Horoupian DS, Thompson A, et al. Diffuse Lewy body disease. Neuropathological and biochemical studies of six patients. Acta neuropathologica. 1987;75(1):8–15.
Lennox G, Lowe JS, Godwin-Austen RB, Landon M, Mayer RJ. Diffuse Lewy body disease: an important differential diagnosis in dementia with extrapyramidal features. Progress in clinical and biological research. 1989;317:121–30.
Gibb WR, Esiri MM, Lees AJ. Clinical and pathological features of diffuse cortical Lewy body disease (Lewy body dementia). Brain : a journal of neurology. 1987;110(Pt 5):1131–53.
Byrne EJ, Lennox GG, Godwin-Austen RB, Jefferson D, et al. Dementia associated with cortical Lewy bodies: Proposed clinical diagnostic criteria. Dementia. 1991;2(5):283–4.
Hansen L, Salmon D, Galasko D, Masliah E, Katzman R, DeTeresa R, et al. The Lewy body variant of Alzheimer's disease: a clinical and pathologic entity. Neurology. 1990;40(1):1–8.
Forstl H, Burns A, Luthert P, Cairns N, Levy R. The Lewy-body variant of Alzheimer's disease. Clinical and pathological findings. The British journal of psychiatry : the journal of mental science. 1993;162:385–92.
Perry RH, Irving D, Blessed G, Fairbairn A, Perry EK. Senile dementia of Lewy body type. A clinically and neuropathologically distinct form of Lewy body dementia in the elderly. Journal of the neurological sciences. 1990;95(2):119–39.
Mckeith I. Dementia with Lewy bodies: A Clinical Overview. In: Ames D, O'Brien, Burns, A, editor. Dementia. Boca Raton: CRC Press; 2017.
Tiraboschi P, Attems J, Thomas A, Brown A, Jaros E, Lett DJ, et al. Clinicians' ability to diagnose dementia with Lewy bodies is not affected by beta-amyloid load. Neurology. 2015;84(5):496–9.
Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, et al. National Institute on Aging-Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease: a practical approach. Acta neuropathologica. 2012;123(1):1–11.
Toledo JB, Cairns NJ, Da X, Chen K, Carter D, Fleisher A, et al. Clinical and multimodal biomarker correlates of ADNI neuropathological findings. Acta neuropathologica communications. 2013;1:65.
Thomas AJ, Mahin-Babaei F, Saidi M, Lett D, Taylor JP, Walker L, et al. Improving the identification of dementia with Lewy bodies in the context of an Alzheimer's-type dementia. Alzheimer's research & therapy. 2018;10(1):27.
Kane JPM, Surendranathan A, Bentley A, Barker SAH, Taylor JP, Thomas AJ, et al. Clinical prevalence of Lewy body dementia. Alzheimer's research & therapy. 2018;10(1):19.
Galvin JE. Improving the clinical detection of lewy body dementia with the lewy body composite risk score. Alzheimer's & dementia (Amsterdam, Netherlands). 2015;1(3):316–24.
Thomas AJ, Taylor JP, McKeith I, Bamford C, Burn D, Allan L, et al. Development of assessment toolkits for improving the diagnosis of the Lewy body dementias: feasibility study within the DIAMOND Lewy study. International journal of geriatric psychiatry. 2017;32(12):1280–304.
Thomas AJ, Taylor JP, McKeith I, Bamford C, Burn D, Allan L, et al. Revision of assessment toolkits for improving the diagnosis of Lewy body dementia: The DIAMOND Lewy study. International journal of geriatric psychiatry. 2018;33(10):1293–304.
Rizzo G, Arcuti S, Copetti M, Alessandria M, Savica R, Fontana A, et al. Accuracy of clinical diagnosis of dementia with Lewy bodies: a systematic review and meta-analysis. Journal of neurology, neurosurgery, and psychiatry. 2018;89(4):358–66.
Donaghy PC, Taylor JP, O'Brien JT, Barnett N, Olsen K, Colloby SJ, et al. Neuropsychiatric symptoms and cognitive profile in mild cognitive impairment with Lewy bodies. Psychol Med. 2018;48(14):2384–90.
Boeve BF, Dickson DW, Duda JE, Ferman TJ, Galasko DR, Galvin JE, et al. Arguing against the proposed definition changes of PD. Movement disorders : official journal of the Movement Disorder Society. 2016;31(11):1619–22.
American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders DSM-5. 5th ed. Washington, DC: American Psychiatric Association; 2013.
Emanuele M, Esposito A, Camerini S, Antonucci F, Ferrara S, Seghezza S, et al. Exogenous Alpha-Synuclein Alters Pre- and Post-Synaptic Activity by Fragmenting Lipid Rafts. EBioMedicine. 2016;7:191–204.
Jellinger KA, Korczyn AD. Are dementia with Lewy bodies and Parkinson's disease dementia the same disease? BMC medicine. 2018;16(1):34.
McKeith I, O'Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, et al. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. Lancet neurology. 2007;6(4):305–13.
Thomas AJ, Attems J, Colloby SJ, O'Brien JT, McKeith I, Walker R, et al. Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology. 2017;88(3):276–83.
Zaccai J, Brayne C, McKeith I, Matthews F, Ince PG. Patterns and stages of alpha-synucleinopathy: Relevance in a population-based cohort. Neurology. 2008;70(13):1042–8.
Beach TG, Adler CH, Lue L, Sue LI, Bachalakuri J, Henry-Watson J, et al. Unified staging system for Lewy body disorders: correlation with nigrostriatal degeneration, cognitive impairment and motor dysfunction. Acta neuropathologica. 2009;117(6):613–34.
Benamer TS, Patterson J, Grosset DG, Booij J, de Bruin K, van Royen E, et al. Accurate differentiation of parkinsonism and essential tremor using visual assessment of [123I]-FP-CIT SPECT imaging: the [123I]-FP-CIT study group. Movement disorders : official journal of the Movement Disorder Society. 2000;15(3):503–10.
Lloyd JJ, Petrides G, Donaghy PC, Colloby SJ, Attems J, O'Brien JT, et al. A new visual rating scale for Ioflupane imaging in Lewy body disease. NeuroImage: Clinical. 2018;20:823–9.
Booij J, Dubroff J, Pryma D, Yu J, Agarwal R, Lakhani P, et al. Diagnostic Performance of the Visual Reading of (123)I-Ioflupane SPECT Images With or Without Quantification in Patients With Movement Disorders or Dementia. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017;58(11):1821–6.
Nicastro N, Garibotto V, Allali G, Assal F, Burkhard PR. Added Value of Combined Semi-Quantitative and Visual [123I]FP-CIT SPECT Analyses for the Diagnosis of Dementia With Lewy Bodies. Clinical nuclear medicine. 2017;42(2):e96–e102.
Morgan S, Kemp P, Booij J, Costa DC, Padayachee S, Lee L, et al. Differentiation of frontotemporal dementia from dementia with Lewy bodies using FP-CIT SPECT. Journal of neurology, neurosurgery, and psychiatry. 2012;83(11):1063–70.
Kashihara K, Ohno M, Kawada S, Okumura Y. Reduced cardiac uptake and enhanced washout of 123I-MIBG in pure autonomic failure occurs conjointly with Parkinson's disease and dementia with Lewy bodies. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006;47(7):1099–101.
Tiraboschi P, Corso A, Guerra UP, Nobili F, Piccardo A, Calcagni ML, et al. (123) I-2beta-carbomethoxy-3beta-(4-iodophenyl)-N-(3-fluoropropyl) nortropane single photon emission computed tomography and (123) I-metaiodobenzylguanidine myocardial scintigraphy in differentiating dementia with lewy bodies from other dementias: A comparative study. Annals of neurology. 2016;80(3):368–78.
Yoshita M, Taki J, Yokoyama K, Noguchi-Shinohara M, Matsumoto Y, Nakajima K, et al. Value of 123I-MIBG radioactivity in the differential diagnosis of DLB from AD. Neurology. 2006;66(12):1850–4.
Hanyu H, Shimizu S, Hirao K, Kanetaka H, Iwamoto T, Chikamori T, et al. Comparative value of brain perfusion SPECT and [(123)I]MIBG myocardial scintigraphy in distinguishing between dementia with Lewy bodies and Alzheimer's disease. European journal of nuclear medicine and molecular imaging. 2006;33(3):248–53.
Yoshita M, Arai H, Arai H, Arai T, Asada T, Fujishiro H, et al. Diagnostic accuracy of 123I-meta-iodobenzylguanidine myocardial scintigraphy in dementia with Lewy bodies: a multicenter study. PloS one. 2015;10(3):e0120540.
Komatsu J, Samuraki M, Nakajima K, Arai H, Arai H, Arai T, et al. (123)I-MIBG myocardial scintigraphy for the diagnosis of DLB: a multicentre 3-year follow-up study. Journal of neurology, neurosurgery, and psychiatry. 2018.
Flotats A, Carrio I, Agostini D, Le Guludec D, Marcassa C, Schafers M, et al. Proposal for standardization of 123I-metaiodobenzylguanidine (MIBG) cardiac sympathetic imaging by the EANM Cardiovascular Committee and the European Council of Nuclear Cardiology. European journal of nuclear medicine and molecular imaging. 2010;37(9):1802–12.
Boeve BF, Silber MH, Ferman TJ, Lin SC, Benarroch EE, Schmeichel AM, et al. Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder. Sleep medicine. 2013;14(8):754–62.
Ferman TJ, Boeve BF, Smith GE, Lin SC, Silber MH, Pedraza O, et al. Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies. Neurology. 2011;77(9):875–82.
Harper L, Fumagalli GG, Barkhof F, Scheltens P, O'Brien JT, Bouwman F, et al. MRI visual rating scales in the diagnosis of dementia: evaluation in 184 post-mortem confirmed cases. Brain : a journal of neurology. 2016;139(Pt 4):1211–25.
Nedelska Z, Ferman TJ, Boeve BF, Przybelski SA, Lesnick TG, Murray ME, et al. Pattern of brain atrophy rates in autopsy-confirmed dementia with Lewy bodies. Neurobiology of aging. 2015;36(1):452–61.
O'Brien JT, Firbank MJ, Davison C, Barnett N, Bamford C, Donaldson C, et al. 18F-FDG PET and Perfusion SPECT in the Diagnosis of Alzheimer and Lewy Body Dementias. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55(12):1959–65.
Bonanni L, Thomas A, Tiraboschi P, Perfetti B, Varanese S, Onofrj M. EEG comparisons in early Alzheimer's disease, dementia with Lewy bodies and Parkinson's disease with dementia patients with a 2-year follow-up. Brain : a journal of neurology. 2008;131(Pt 3):690–705.
Bonanni L, Franciotti R, Nobili F, Kramberger MG, Taylor JP, Garcia-Ptacek S, et al. EEG Markers of Dementia with Lewy Bodies: A Multicenter Cohort Study. Journal of Alzheimer's disease : JAD. 2016;54(4):1649–57.
Stylianou M, Murphy N, Peraza LR, Graziadio S, Cromarty R, Killen A, et al. Quantitative electroencephalography as a marker of cognitive fluctuations in dementia with Lewy bodies and an aid to differential diagnosis. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2018;129(6):1209–20.
Briel RC, McKeith IG, Barker WA, Hewitt Y, Perry RH, Ince PG, et al. EEG findings in dementia with Lewy bodies and Alzheimer's disease. Journal of neurology, neurosurgery, and psychiatry. 1999;66(3):401–3.
Babiloni C, Del Percio C, Lizio R, Noce G, Cordone S, Lopez S, et al. Abnormalities of cortical neural synchronization mechanisms in patients with dementia due to Alzheimer's and Lewy body diseases: an EEG study. Neurobiology of aging. 2017;55:143–58.
Peraza LR, Cromarty R, Kobeleva X, Firbank MJ, Killen A, Graziadio S, et al. Electroencephalographic derived network differences in Lewy body dementia compared to Alzheimer's disease patients. Scientific reports. 2018;8(1):4637.
Bonanni L, Perfetti B, Bifolchetti S, Taylor JP, Franciotti R, Parnetti L, et al. Quantitative electroencephalogram utility in predicting conversion of mild cognitive impairment to dementia with Lewy bodies. Neurobiology of aging. 2015;36(1):434–45.
Babiloni C, Del Percio C, Lizio R, Noce G, Lopez S, Soricelli A, et al. Abnormalities of resting-state functional cortical connectivity in patients with dementia due to Alzheimer's and Lewy body diseases: an EEG study. Neurobiology of aging. 2018;65:18–40.
Mukaetova-Ladinska EB, Monteith R, Perry EK. Cerebrospinal fluid biomarkers for dementia with lewy bodies. International journal of Alzheimer's disease. 2010;2010:536538.
Mollenhauer B, Schlossmacher MG. CSF synuclein: adding to the biomarker footprint of dementia with Lewy bodies. Journal of neurology, neurosurgery, and psychiatry. 2010;81(6):590–1.
Irwin DJ, Xie SX, Coughlin D, Nevler N, Akhtar RS, McMillan CT, et al. CSF tau and beta-amyloid predict cerebral synucleinopathy in autopsied Lewy body disorders. Neurology. 2018;90(12):e1038–e46.
Kotzbauer PT, Tu Z, Mach RH. Current status of the development of PET radiotracers for imaging alpha synuclein aggregates in Lewy bodies and Lewy neurites. Clinical and Translational Imaging. 2017;5(1):3–14.
Donadio V, Incensi A, Rizzo G, Capellari S, Pantieri R, Stanzani Maserati M, et al. A new potential biomarker for dementia with Lewy bodies: Skin nerve alpha-synuclein deposits. Neurology. 2017;89(4):318–26.
Stokholm MG, Danielsen EH, Hamilton-Dutoit SJ, Borghammer P. Pathological alpha-synuclein in gastrointestinal tissues from prodromal Parkinson disease patients. Annals of neurology. 2016;79(6):940–9.
Nervi A, Reitz C, Tang MX, Santana V, Piriz A, Reyes D, et al. Familial aggregation of dementia with Lewy bodies. Arch Neurol. 2011;68(1):90–3.
Woodruff BK, Graff-Radford NR, Ferman TJ, Dickson DW, DeLucia MW, Crook JE, et al. Family history of dementia is a risk factor for Lewy body disease. Neurology. 2006;66(12):1949–50.
Nussbaum RL, Polymeropoulos MH. Genetics of Parkinson's disease. Hum Mol Genet. 1997;6(10):1687–91.
Golbe LI, Di Iorio G, Sanges G, Lazzarini AM, La Sala S, Bonavita V, et al. Clinical genetic analysis of Parkinson's disease in the Contursi kindred. Annals of neurology. 1996;40(5):767–75.
Yamaguchi K, Cochran EJ, Murrell JR, Polymeropoulos MH, Shannon KM, Crowther RA, et al. Abundant neuritic inclusions and microvacuolar changes in a case of diffuse Lewy body disease with the A53T mutation in the alpha-synuclein gene. Acta neuropathologica. 2005;110(3):298–305.
Zarranz JJ, Alegre J, Gomez-Esteban JC, Lezcano E, Ros R, Ampuero I, et al. The new mutation, E46K, of alpha-synuclein causes Parkinson and Lewy body dementia. Annals of neurology. 2004;55(2):164–73.
Chen CY, Bonham AC, Schelegle ES, Gershwin LJ, Plopper CG, Joad JP. Extended allergen exposure in asthmatic monkeys induces neuroplasticity in nucleus tractus solitarius. The Journal of allergy and clinical immunology. 2001;108(4):557–62.
Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, et al. alpha-Synuclein locus triplication causes Parkinson's disease. Science. 2003;302(5646):841.
Muenter MD, Forno LS, Hornykiewicz O, Kish SJ, Maraganore DM, Caselli RJ, et al. Hereditary form of parkinsonism--dementia. Annals of neurology. 1998;43(6):768–81.
Uchiyama T, Ikeuchi T, Ouchi Y, Sakamoto M, Kasuga K, Shiga A, et al. Prominent psychiatric symptoms and glucose hypometabolism in a family with a SNCA duplication. Neurology. 2008;71(16):1289–91.
Kara E, Kiely AP, Proukakis C, Giffin N, Love S, Hehir J, et al. A 6.4 Mb duplication of the alpha-synuclein locus causing frontotemporal dementia and Parkinsonism: phenotype-genotype correlations. JAMA Neurol. 2014;71(9):1162–71.
Kojovic M, Sheerin UM, Rubio-Agusti I, Saha A, Bras J, Gibbons V, et al. Young-onset parkinsonism due to homozygous duplication of alpha-synuclein in a consanguineous family. Movement disorders : official journal of the Movement Disorder Society. 2012;27(14):1827–9.
Ikeuchi T, Kakita A, Shiga A, Kasuga K, Kaneko H, Tan CF, et al. Patients homozygous and heterozygous for SNCA duplication in a family with parkinsonism and dementia. Arch Neurol. 2008;65(4):514–9.
Wakabayashi K, Hayashi S, Ishikawa A, Hayashi T, Okuizumi K, Tanaka H, et al. Autosomal dominant diffuse Lewy body disease. Acta neuropathologica. 1998;96(2):207–10.
Ishikawa A, Takahashi H, Tanaka H, Hayashi T, Tsuji S. Clinical features of familial diffuse Lewy body disease. Eur Neurol. 1997;38(Suppl 1):34–8.
Johnson J, Hague SM, Hanson M, Gibson A, Wilson KE, Evans EW, et al. SNCA multiplication is not a common cause of Parkinson disease or dementia with Lewy bodies. Neurology. 2004;63(3):554–6.
Geiger JT, Ding J, Crain B, Pletnikova O, Letson C, Dawson TM, et al. Next-generation sequencing reveals substantial genetic contribution to dementia with Lewy bodies. Neurobiol Dis. 2016;94:55–62.
Tsuang DW, Dalan AM, Eugenio CJ, Poorkaj P, Limprasert P, La Spada AR, et al. Familial dementia with lewy bodies: a clinical and neuropathological study of 2 families. Arch Neurol. 2002;59(10):1622–30.
Bonner LT, Tsuang DW, Cherrier MM, Eugenio CJ, Du Jennifer Q, Steinbart EJ, et al. Familial dementia with Lewy bodies with an atypical clinical presentation. J Geriatr Psychiatry Neurol. 2003;16(1):59–64.
Ohtake H, Limprasert P, Fan Y, Onodera O, Kakita A, Takahashi H, et al. Beta-synuclein gene alterations in dementia with Lewy bodies. Neurology. 2004;63(5):805–11.
Fujioka S, Sundal C, Strongosky AJ, Castanedes MC, Rademakers R, Ross OA, et al. Sequence variants in eukaryotic translation initiation factor 4-gamma (eIF4G1) are associated with Lewy body dementia. Acta neuropathologica. 2013;125(3):425–38.
Chartier-Harlin MC, Dachsel JC, Vilarino-Guell C, Lincoln SJ, Lepretre F, Hulihan MM, et al. Translation initiator EIF4G1 mutations in familial Parkinson disease. Am J Hum Genet. 2011;89(3):398–406.
Brett FM, Henson C, Staunton H. Familial diffuse Lewy body disease, eye movement abnormalities, and distribution of pathology. Arch Neurol. 2002;59(3):464–7.
SantaCruz KS, Walker Z, Swagerty D, Piggott MA, Ryo-Yang M, McKeith IG, et al. Clinical presentations in monozygotic twins with dementia with Lewy bodies. J Am Med Dir Assoc. 2002;3(3):175–9.
Keogh MJ, Kurzawa-Akanbi M, Griffin H, Douroudis K, Ayers KL, Hussein RI, et al. Exome sequencing in dementia with Lewy bodies. Transl Psychiatry. 2016;6:e728.
Bras J, Guerreiro R, Darwent L, Parkkinen L, Ansorge O, Escott-Price V, et al. Genetic analysis implicates APOE, SNCA and suggests lysosomal dysfunction in the etiology of dementia with Lewy bodies. Hum Mol Genet. 2014;23(23):6139–46.
Guerreiro R, Ross OA, Kun-Rodrigues C, Hernandez DG, Orme T, Eicher JD, et al. Investigating the genetic architecture of dementia with Lewy bodies: a two-stage genome-wide association study. Lancet neurology. 2018;17(1):64–74.
Orme T, Guerreiro R, Bras J. The Genetics of Dementia with Lewy Bodies: Current Understanding and Future Directions. Curr Neurol Neurosci Rep. 2018;18(10):67.
Vergouw LJM, van Steenoven I, van de Berg WDJ, Teunissen CE, van Swieten JC, Bonifati V, et al. An update on the genetics of dementia with Lewy bodies. Parkinsonism Relat Disord. 2017;43:1–8.
Leverenz JB, Fishel MA, Peskind ER, Montine TJ, Nochlin D, Steinbart E, et al. Lewy body pathology in familial Alzheimer disease: evidence for disease- and mutation-specific pathologic phenotype. Arch Neurol. 2006;63(3):370–6.
Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, et al. APOE epsilon4 increases risk for dementia in pure synucleinopathies. JAMA Neurol. 2013;70(2):223–8.
Dickson DW, Heckman MG, Murray ME, Soto AI, Walton RL, Diehl NN, et al. APOE epsilon4 is associated with severity of Lewy body pathology independent of Alzheimer pathology. Neurology. 2018;91(12):e1182–e95.
Singleton AB, Wharton A, O'Brien KK, Walker MP, McKeith IG, Ballard CG, et al. Clinical and neuropathological correlates of apolipoprotein E genotype in dementia with Lewy bodies. Dement Geriatr Cogn Disord. 2002;14(4):167–75.
Moskvina V, Harold D, Russo G, Vedernikov A, Sharma M, Saad M, et al. Analysis of genome-wide association studies of Alzheimer disease and of Parkinson disease to determine if these 2 diseases share a common genetic risk. JAMA Neurol. 2013;70(10):1268–76.
Nalls MA, Duran R, Lopez G, Kurzawa-Akanbi M, McKeith IG, Chinnery PF, et al. A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol. 2013;70(6):727–35.
Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, et al. Multicenter analysis of glucocerebrosidase mutations in Parkinson's disease. The New England journal of medicine. 2009;361(17):1651–61.
Burton EJ, Karas G, Paling SM, Barber R, Williams ED, Ballard CG, et al. Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. NeuroImage. 2002;17(2):618–30.
Burton EJ, Barber R, Mukaetova-Ladinska EB, Robson J, Perry RH, Jaros E, et al. Medial temporal lobe atrophy on MRI differentiates Alzheimer's disease from dementia with Lewy bodies and vascular cognitive impairment: a prospective study with pathological verification of diagnosis. Brain : a journal of neurology. 2009;132(Pt 1):195–203.
Serpell LC, Berriman J, Jakes R, Goedert M, Crowther RA. Fiber diffraction of synthetic alpha-synuclein filaments shows amyloid-like cross-beta conformation. Proceedings of the National Academy of Sciences of the United States of America. 2000;97(9):4897–902.
Goedert M, Spillantini MG, Del Tredici K, Braak H. 100 years of Lewy pathology. Nature reviews Neurology. 2013;9(1):13–24.
Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with lewy bodies. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(11):6469–73.
Fujiwara H, Hasegawa M, Dohmae N, Kawashima A, Masliah E, Goldberg MS, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nature cell biology. 2002;4(2):160–4.
Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, et al. Oxidative damage linked to neurodegeneration by selective alpha-synuclein nitration in synucleinopathy lesions. Science. 2000;290(5493):985–9.
Rott R, Szargel R, Shani V, Hamza H, Savyon M, Abd Elghani F, et al. SUMOylation and ubiquitination reciprocally regulate alpha-synuclein degradation and pathological aggregation. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(50):13176–81.
Saito Y, Kawashima A, Ruberu NN, Fujiwara H, Koyama S, Sawabe M, et al. Accumulation of phosphorylated alpha-synuclein in aging human brain. Journal of neuropathology and experimental neurology. 2003;62(6):644–54.
Obi K, Akiyama H, Kondo H, Shimomura Y, Hasegawa M, Iwatsubo T, et al. Relationship of phosphorylated alpha-synuclein and tau accumulation to Abeta deposition in the cerebral cortex of dementia with Lewy bodies. Experimental neurology. 2008;210(2):409–20.
Jellinger KA, Attems J. Does striatal pathology distinguish Parkinson disease with dementia and dementia with Lewy bodies? Acta neuropathologica. 2006;112(3):253–60.
Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, et al. Amyloid load in Parkinson's disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. Journal of neurology, neurosurgery, and psychiatry. 2008;79(12):1331–8.
Tsuboi Y, Dickson DW. Dementia with Lewy bodies and Parkinson's disease with dementia: are they different? Parkinsonism Relat Disord. 2005;11(Suppl 1):S47–51.
Kovacs GG, Alafuzoff I, Al-Sarraj S, Arzberger T, Bogdanovic N, Capellari S, et al. Mixed brain pathologies in dementia: the BrainNet Europe consortium experience. Dement Geriatr Cogn Disord. 2008;26(4):343–50.
Kovacs GG, Milenkovic I, Wohrer A, Hoftberger R, Gelpi E, Haberler C, et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: a community-based autopsy series. Acta neuropathologica. 2013;126(3):365–84.
Jellinger KA, Attems J. Neuropathological evaluation of mixed dementia. Journal of the neurological sciences. 2007;257(1-2):80–7.
Dugger BN, Adler CH, Shill HA, Caviness J, Jacobson S, Driver-Dunckley E, et al. Concomitant pathologies among a spectrum of parkinsonian disorders. Parkinsonism Relat Disord. 2014.
Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204.
McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J. TDP-43 pathology in Alzheimer's disease, dementia with Lewy bodies and ageing. Brain pathology (Zurich, Switzerland). 2017;27(4):472–9.
Irwin DJ, Grossman M, Weintraub D, Hurtig HI, Duda JE, Xie SX, et al. Neuropathological and genetic correlates of survival and dementia onset in synucleinopathies: a retrospective analysis. Lancet neurology. 2017;16(1):55–65.
Howlett DR, Whitfield D, Johnson M, Attems J, O'Brien JT, Aarsland D, et al. Regional Multiple Pathology Scores Are Associated with Cognitive Decline in Lewy Body Dementias. Brain pathology (Zurich, Switzerland). 2015;25(4):401–8.
Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, et al. Phosphorylated TDP-43 in Alzheimer's disease and dementia with Lewy bodies. Acta neuropathologica. 2009;117(2):125–36.
Nakashima-Yasuda H, Uryu K, Robinson J, Xie SX, Hurtig H, Duda JE, et al. Co-morbidity of TDP-43 proteinopathy in Lewy body related diseases. Acta neuropathologica. 2007;114(3):221–9.
Josephs KA, Murray ME, Whitwell JL, Parisi JE, Petrucelli L, Jack CR, et al. Staging TDP-43 pathology in Alzheimer's disease. Acta neuropathologica. 2014;127(3):441–50.
Josephs KA, Whitwell JL, Knopman DS, Hu WT, Stroh DA, Baker M, et al. Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology. 2008;70(19 Pt 2):1850–7.
Josephs KA, Whitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, et al. TDP-43 is a key player in the clinical features associated with Alzheimer's disease. Acta neuropathologica. 2014;127(6):811–24.
Miners S, Moulding H, de Silva R, Love S. Reduced vascular endothelial growth factor and capillary density in the occipital cortex in dementia with Lewy bodies. Brain pathology (Zurich, Switzerland). 2014, 24(4):334–43.
Serby M, Brickman AM, Haroutunian V, Purohit DP, Marin D, Lantz M, et al. Cognitive burden and excess Lewy-body pathology in the Lewy-body variant of Alzheimer disease. The American journal of geriatric psychiatry : official journal of the American Association for Geriatric Psychiatry. 2003;11(3):371–4.
Walker L, McAleese KE, Thomas AJ, Johnson M, Martin-Ruiz C, Parker C, et al. Neuropathologically mixed Alzheimer's and Lewy body disease: burden of pathological protein aggregates differs between clinical phenotypes. Acta neuropathologica. 2015;129(5):729–48.
Merdes AR, Hansen LA, Jeste DV, Galasko D, Hofstetter CR, Ho GJ, et al. Influence of Alzheimer pathology on clinical diagnostic accuracy in dementia with Lewy bodies. Neurology. 2003;60(10):1586–90.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiology of aging. 2003;24(2):197–211.
Leverenz JB, Hamilton R, Tsuang DW, Schantz A, Vavrek D, Larson EB, et al. Empiric refinement of the pathologic assessment of Lewy-related pathology in the dementia patient. Brain pathology (Zurich, Switzerland). 2008;18(2):220–4.
Braak H, de Vos RA, Bohl J, Del Tredici K. Gastric alpha-synuclein immunoreactive inclusions in Meissner's and Auerbach's plexuses in cases staged for Parkinson's disease-related brain pathology. Neuroscience letters. 2006;396(1):67–72.
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F. Parkinson's disease: the presence of Lewy bodies in Auerbach's and Meissner's plexuses. Acta neuropathologica. 1988;76(3):217–21.
Beach TG, Adler CH, Sue LI, Vedders L, Lue L, White Iii CL, et al. Multi-organ distribution of phosphorylated alpha-synuclein histopathology in subjects with Lewy body disorders. Acta neuropathologica. 2010;119(6):689–702.
Beach TG, Adler CH, Serrano G, Sue LI, Walker DG, Dugger BN, et al. Prevalence of Submandibular Gland Synucleinopathy in Parkinson's Disease, Dementia with Lewy Bodies and other Lewy Body Disorders. Journal of Parkinson's disease. 2016;6(1):153–63.
Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson's disease suggest host-to-graft disease propagation. Nature medicine. 2008;14(5):501–3.
Brundin P, Li JY, Holton JL, Lindvall O, Revesz T. Research in motion: the enigma of Parkinson's disease pathology spread. Nature reviews Neuroscience. 2008;9(10):741–5.
Clinton LK, Blurton-Jones M, Myczek K, Trojanowski JQ, LaFerla FM. Synergistic Interactions between Abeta, tau, and alpha-synuclein: acceleration of neuropathology and cognitive decline. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(21):7281–9.
Masliah E, Rockenstein E, Veinbergs I, Sagara Y, Mallory M, Hashimoto M, et al. beta-amyloid peptides enhance alpha-synuclein accumulation and neuronal deficits in a transgenic mouse model linking Alzheimer's disease and Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(21):12245–50.
Swirski M, Miners JS, de Silva R, Lashley T, Ling H, Holton J, et al. Evaluating the relationship between amyloid-beta and alpha-synuclein phosphorylated at Ser129 in dementia with Lewy bodies and Parkinson's disease. Alzheimer's research & therapy. 2014;6(5-8):77.
Giasson BI, Forman MS, Higuchi M, Golbe LI, Graves CL, Kotzbauer PT, et al. Initiation and synergistic fibrillization of tau and alpha-synuclein. Science. 2003;300(5619):636–40.
Lee VM, Giasson BI, Trojanowski JQ. More than just two peas in a pod: common amyloidogenic properties of tau and alpha-synuclein in neurodegenerative diseases. Trends in neurosciences. 2004;27(3):129–34.
Colom-Cadena M, Gelpi E, Charif S, Belbin O, Blesa R, Marti MJ, et al. Confluence of alpha-synuclein, tau, and beta-amyloid pathologies in dementia with Lewy bodies. Journal of neuropathology and experimental neurology. 2013;72(12):1203–12.
Gomez-Isla T, Growdon WB, McNamara M, Newell K, Gomez-Tortosa E, Hedley-Whyte ET, et al. Clinicopathologic correlates in temporal cortex in dementia with Lewy bodies. Neurology. 1999;53(9):2003–9.
Gomez-Tortosa E, Newell K, Irizarry MC, Albert M, Growdon JH, Hyman BT. Clinical and quantitative pathologic correlates of dementia with Lewy bodies. Neurology. 1999;53(6):1284–91.
Mattila PM, Rinne JO, Helenius H, Dickson DW, Roytta M. Alpha-synuclein-immunoreactive cortical Lewy bodies are associated with cognitive impairment in Parkinson's disease. Acta neuropathologica. 2000;100(3):285–90.
Weisman D, Cho M, Taylor C, Adame A, Thal LJ, Hansen LA. In dementia with Lewy bodies, Braak stage determines phenotype, not Lewy body distribution. Neurology. 2007;69(4):356–9.
Bodner RA, Outeiro TF, Altmann S, Maxwell MM, Cho SH, Hyman BT, et al. Pharmacological promotion of inclusion formation: a therapeutic approach for Huntington's and Parkinson's diseases. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(11):4246–51.
Tanaka M, Kim YM, Lee G, Junn E, Iwatsubo T, Mouradian MM. Aggresomes formed by alpha-synuclein and synphilin-1 are cytoprotective. The Journal of biological chemistry. 2004;279(6):4625–31.
Morris AM, Watzky MA, Agar JN, Finke RG. Fitting neurological protein aggregation kinetic data via a 2-step, minimal/"Ockham's razor" model: the Finke-Watzky mechanism of nucleation followed by autocatalytic surface growth. Biochemistry. 2008;47(8):2413–27.
Fink AL. The aggregation and fibrillation of alpha-synuclein. Accounts of chemical research. 2006;39(9):628–34.
Kuret J, Chirita CN, Congdon EE, Kannanayakal T, Li G, Necula M, et al. Pathways of tau fibrillization. Biochimica et biophysica acta. 2005;1739(2-3):167–78.
Serpell LC. Alzheimer's amyloid fibrils: structure and assembly. Biochimica et biophysica acta. 2000;1502(1):16–30.
Iljina M, Garcia GA, Horrocks MH, Tosatto L, Choi ML, Ganzinger KA, et al. Kinetic model of the aggregation of alpha-synuclein provides insights into prion-like spreading. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(9):E1206–15.
Conway KA, Lee SJ, Rochet JC, Ding TT, Harper JD, Williamson RE, et al. Accelerated oligomerization by Parkinson's disease linked alpha-synuclein mutants. Annals of the New York Academy of Sciences. 2000;920:42–5.
Lashuel HA, Petre BM, Wall J, Simon M, Nowak RJ, Walz T, et al. Alpha-synuclein, especially the Parkinson's disease-associated mutants, forms pore-like annular and tubular protofibrils. Journal of molecular biology. 2002;322(5):1089–102.
Fredenburg RA, Rospigliosi C, Meray RK, Kessler JC, Lashuel HA, Eliezer D, et al. The impact of the E46K mutation on the properties of alpha-synuclein in its monomeric and oligomeric states. Biochemistry. 2007;46(24):7107–18.
Lazaro DF, Rodrigues EF, Langohr R, Shahpasandzadeh H, Ribeiro T, Guerreiro P, et al. Systematic comparison of the effects of alpha-synuclein mutations on its oligomerization and aggregation. PLoS genetics. 2014;10(11):e1004741.
de Oliveira RM, Vicente Miranda H, Francelle L, Pinho R, Szego EM, Martinho R, et al. The mechanism of sirtuin 2-mediated exacerbation of alpha-synuclein toxicity in models of Parkinson disease. PLoS biology. 2017;15(3):e2000374.
Vicente Miranda H, Szego EM, Oliveira LMA, Breda C, Darendelioglu E, de Oliveira RM, et al. Glycation potentiates alpha-synuclein-associated neurodegeneration in synucleinopathies. Brain : a journal of neurology. 2017;140(5):1399–419.
Yamin G, Uversky VN, Fink AL. Nitration inhibits fibrillation of human alpha-synuclein in vitro by formation of soluble oligomers. FEBS letters. 2003;542(1-3):147–52.
Uversky VN, Yamin G, Souillac PO, Goers J, Glaser CB, Fink AL. Methionine oxidation inhibits fibrillation of human alpha-synuclein in vitro. FEBS letters. 2002;517(1-3):239–44.
Paleologou KE, Schmid AW, Rospigliosi CC, Kim HY, Lamberto GR, Fredenburg RA, et al. Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein. The Journal of biological chemistry. 2008;283(24):16895–905.
Ma MR, Hu ZW, Zhao YF, Chen YX, Li YM. Phosphorylation induces distinct alpha-synuclein strain formation. Scientific reports. 2016;6:37130.
Li W, West N, Colla E, Pletnikova O, Troncoso JC, Marsh L, et al. Aggregation promoting C-terminal truncation of alpha-synuclein is a normal cellular process and is enhanced by the familial Parkinson's disease-linked mutations. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(6):2162–7.
Buell AK, Galvagnion C, Gaspar R, Sparr E, Vendruscolo M, Knowles TP, et al. Solution conditions determine the relative importance of nucleation and growth processes in alpha-synuclein aggregation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(21):7671–6.
Gaspar R, Meisl G, Buell AK, Young L, Kaminski CF, Knowles TPJ, et al. Secondary nucleation of monomers on fibril surface dominates alpha-synuclein aggregation and provides autocatalytic amyloid amplification. Quarterly reviews of biophysics. 2017;50:e6.
Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson's disease. Nature medicine. 2008;14(5):504–6.
Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, et al. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):13010–5.
Surmeier DJ, Obeso JA, Halliday GM. Selective neuronal vulnerability in Parkinson disease. Nature reviews Neuroscience. 2017;18(2):101–13.
Mao X, Ou MT, Karuppagounder SS, Kam TI, Yin X, Xiong Y, et al. Pathological alpha-synuclein transmission initiated by binding lymphocyte-activation gene 3. Science. 2016;353(6307).
Ferreira DG, Temido-Ferreira M, Vicente Miranda H, Batalha VL, Coelho JE, Szego EM, et al. alpha-synuclein interacts with PrP(C) to induce cognitive impairment through mGluR5 and NMDAR2B. Nature neuroscience. 2017;20(11):1569–79.
Erskine D, Patterson L, Alexandris A, Hanson PS, McKeith IG, Attems J, et al. Regional levels of physiological alpha-synuclein are directly associated with Lewy body pathology. Acta neuropathologica. 2018;135(1):153–4.
Luna E, Decker SC, Riddle DM, Caputo A, Zhang B, Cole T, et al. Differential alpha-synuclein expression contributes to selective vulnerability of hippocampal neuron subpopulations to fibril-induced toxicity. Acta neuropathologica. 2018;135(6):855–75.
Pinotsi D, Michel CH, Buell AK, Laine RF, Mahou P, Dobson CM, et al. Nanoscopic insights into seeding mechanisms and toxicity of alpha-synuclein species in neurons. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(14):3815–9.
Hurtig HI, Trojanowski JQ, Galvin J, Ewbank D, Schmidt ML, Lee VM, et al. Alpha-synuclein cortical Lewy bodies correlate with dementia in Parkinson's disease. Neurology. 2000;54(10):1916–21.
Harding AJ, Halliday GM. Cortical Lewy body pathology in the diagnosis of dementia. Acta neuropathologica. 2001;102(4):355–63.
Ballard CG, Jacoby R, Del Ser T, Khan MN, Munoz DG, Holmes C, et al. Neuropathological substrates of psychiatric symptoms in prospectively studied patients with autopsy-confirmed dementia with lewy bodies. The American journal of psychiatry. 2004;161(5):843–9.
Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(6):1405–10.
Bergeron C, Petrunka C, Weyer L, Pollanen MS. Altered neurofilament expression does not contribute to Lewy body formation. The American journal of pathology. 1996;148(1):267–72.
Katsuse O, Iseki E, Marui W, Kosaka K. Developmental stages of cortical Lewy bodies and their relation to axonal transport blockage in brains of patients with dementia with Lewy bodies. Journal of the neurological sciences. 2003;211(1-2):29–35.
Tompkins MM, Hill WD. Contribution of somal Lewy bodies to neuronal death. Brain research. 1997;775(1-2):24–9.
Milber JM, Noorigian JV, Morley JF, Petrovitch H, White L, Ross GW, et al. Lewy pathology is not the first sign of degeneration in vulnerable neurons in Parkinson disease. Neurology. 2012;79(24):2307–14.
Parkkinen L, O'Sullivan SS, Collins C, Petrie A, Holton JL, Revesz T, et al. Disentangling the relationship between lewy bodies and nigral neuronal loss in Parkinson's disease. Journal of Parkinson's disease. 2011;1(3):277–86.
Pountney DL, Voelcker NH, Gai WP. Annular alpha-synuclein oligomers are potentially toxic agents in alpha-synucleinopathy. Hypothesis. Neurotoxicity research. 2005;7(1-2):59–67.
Giehm L, Svergun DI, Otzen DE, Vestergaard B. Low-resolution structure of a vesicle disrupting α-synuclein oligomer that accumulates during fibrillation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(8):3246–51.
Grassi D, Howard S, Zhou M, Diaz-Perez N, Urban NT, Guerrero-Given D, et al. Identification of a highly neurotoxic alpha-synuclein species inducing mitochondrial damage and mitophagy in Parkinson's disease. Proceedings of the National Academy of Sciences of the United States of America. 2018;115(11):E2634–e43.
Pacheco C, Aguayo LG, Opazo C. An extracellular mechanism that can explain the neurotoxic effects of alpha-synuclein aggregates in the brain. Frontiers in physiology. 2012;3:297.
Pacheco CR, Morales CN, Ramirez AE, Munoz FJ, Gallegos SS, Caviedes PA, et al. Extracellular alpha-synuclein alters synaptic transmission in brain neurons by perforating the neuronal plasma membrane. Journal of neurochemistry. 2015;132(6):731–41.
Emmanouilidou E, Stefanis L, Vekrellis K. Cell-produced alpha-synuclein oligomers are targeted to, and impair, the 26S proteasome. Neurobiology of aging. 2010;31(6):953–68.
Ingelsson M. Alpha-Synuclein Oligomers-Neurotoxic Molecules in Parkinson's Disease and Other Lewy Body Disorders. Frontiers in neuroscience. 2016;10:408.
Bengoa-Vergniory N, Roberts RF, Wade-Martins R, Alegre-Abarrategui J. Alpha-synuclein oligomers: a new hope. Acta neuropathologica. 2017;134(6):819–38.
Paiva I, Jain G, Lazaro DF, Jercic KG, Hentrich T, Kerimoglu C, et al. Alpha-synuclein deregulates the expression of COL4A2 and impairs ER-Golgi function. Neurobiol Dis. 2018;119:121–35.
Li B, Ge P, Murray KA, Sheth P, Zhang M, Nair G, et al. Cryo-EM of full-length alpha-synuclein reveals fibril polymorphs with a common structural kernel. Nature communications. 2018;9(1):3609.
Peng C, Gathagan RJ, Covell DJ, Medellin C, Stieber A, Robinson JL, et al. Cellular milieu imparts distinct pathological alpha-synuclein strains in alpha-synucleinopathies. Nature. 2018;557(7706):558–63.
Jung BC, Lim YJ, Bae EJ, Lee JS, Choi MS, Lee MK, et al. Amplification of distinct alpha-synuclein fibril conformers through protein misfolding cyclic amplification. Experimental & molecular medicine. 2017;49(4):e314.
Labbe C, Heckman MG, Lorenzo-Betancor O, Soto-Ortolaza AI, Walton RL, Murray ME, et al. MAPT haplotype H1G is associated with increased risk of dementia with Lewy bodies. Alzheimers Dement. 2016;12(12):1297–304.
Meeus B, Verstraeten A, Crosiers D, Engelborghs S, Van den Broeck M, Mattheijssens M, et al. DLB and PDD: a role for mutations in dementia and Parkinson disease genes? Neurobiology of aging. 2012;33(3):629 e5–e18.
Labbe C, Ogaki K, Lorenzo-Betancor O, Soto-Ortolaza AI, Walton RL, Rayaprolu S, et al. Role for the microtubule-associated protein tau variant p.A152T in risk of alpha-synucleinopathies. Neurology. 2015;85(19):1680–6.
Heckman MG, Soto-Ortolaza AI, Contreras MYS, Murray ME, Pedraza O, Diehl NN, et al. LRRK2 variation and dementia with Lewy bodies. Parkinsonism Relat Disord. 2016;31:98–103.
Piscopo P, Marcon G, Piras MR, Crestini A, Campeggi LM, Deiana E, et al. A novel PSEN2 mutation associated with a peculiar phenotype. Neurology. 2008;70(17):1549–54.
Keogh MJ, Wei W, Wilson I, Coxhead J, Ryan S, Rollinson S, et al. Genetic compendium of 1511 human brains available through the UK Medical Research Council Brain Banks Network Resource. Genome Res. 2017;27(1):165–73.
Guyant-Marechal I, Berger E, Laquerriere A, Rovelet-Lecrux A, Viennet G, Frebourg T, et al. Intrafamilial diversity of phenotype associated with app duplication. Neurology. 2008;71(23):1925–6.
Acknowledgements
TFO is supported by the DFG Center for Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB). Photomicrographs were taken from tissue provided by the Newcastle Brain Tissue Resource, which is funded in part by a grant from the UK Medical Research Council (G0400074), by Brains for Dementia research, a joint venture between Alzheimer’s Society and Alzheimer’s Research UK and by the NIHR Newcastle Biomedical Research Centre awarded to the Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University.
DLB research is supported by NIHR Newcastle Biomedical Research Centre in Ageing and Long-Term Conditions. CMM is funded by the UK Medical Research Council, National Institutes of Health and the Lewy Body Society. LW is funded by the Alzheimer’s Society.
Funding
TFO is supported by the DFG Center for Nanoscale Microscopy and Molecular Physiology of the Brain (CNMPB). Newcastle Brain Tissue Resource is funded in part by a grant from the UK Medical Research Council (G0400074), by Brains for Dementia research, a joint venture between Alzheimer’s Society and Alzheimer’s Research UK and by the NIHR Newcastle Biomedical Research Centre awarded to the Newcastle upon Tyne Hospitals NHS Foundation Trust and Newcastle University.
DLB research is supported by NIHR Newcastle Biomedical Research Centre in Ageing and Long-Term Conditions. CMM is funded by the UK Medical Research Council, National Institutes of Health and the Lewy Body Society. LW is funded by the Alzheimer’s Society.
Availability of data and materials
This is a review article. All data and materials are available.
Author information
Authors and Affiliations
Contributions
TFO and IM conceived the manuscript and wrote the manuscript. DK, DE, LW, DB, CM, MK-A, J-PT, AT, JA, and PD wrote the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
This is a review article. All ethical approvals have been obtained as well as consent to participate.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Outeiro, T.F., Koss, D.J., Erskine, D. et al. Dementia with Lewy bodies: an update and outlook. Mol Neurodegeneration 14, 5 (2019). https://doi.org/10.1186/s13024-019-0306-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13024-019-0306-8