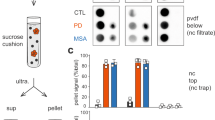
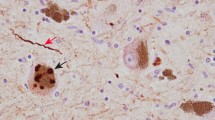
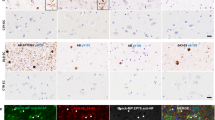
Abstract
Proteinaceous α-synuclein-containing inclusions are found in affected brain regions in patients with Parkinson’s disease (PD), Dementia with Lewy bodies (DLB) and multiple system atrophy (MSA). These appear in neurons as Lewy bodies in both PD and DLB and as glial cytoplasmic inclusions (GCIs) in oligodendrocytes in MSA. The role they play in the pathology of the diseases is unknown, and relatively little is still known about their composition. By purifying the inclusions from the surrounding tissue and comprehensively analysing their protein composition, vital clues to the formation mechanism and role in the disease process may be found. In this study, Lewy bodies were purified from postmortem brain tissue from DLB cases (n = 2) and GCIs were purified from MSA cases (n = 5) using a recently improved purification method, and the purified inclusions were analysed by mass spectrometry. Twenty-one percent of the proteins found consistently in the GCIs and LBs were synaptic-vesicle related. Identified proteins included those associated with exosomes (CD9), clathrin-mediated endocytosis (clathrin, AP-2 complex, dynamin), retrograde transport (dynein, dynactin, spectrin) and synaptic vesicle fusion (synaptosomal-associated protein 25, vesicle-associated membrane protein 2, syntaxin-1). This suggests that the misfolded or excess α-synuclein may be targeted to inclusions via vesicle-mediated transport, which also explains the presence of the neuronal protein α-synuclein within GCIs.



Similar content being viewed by others
References
Alves G, Forsaa EB, Pedersen KF, Dreetz Gjerstad M, Larsen JP (2008) Epidemiology of Parkinson's disease. J Neurol 255(Suppl 5):18–32
Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM (2006) High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet 38:515–517
Borghi R, Marchese R, Negro A, Marinelli L, Forloni G, Zaccheo D, Abbruzzese G, Tabaton M (2000) Full length alpha-synuclein is present in cerebrospinal fluid from Parkinson's disease and normal subjects. Neurosci Lett 287:65–67
Burre J, Volknandt W (2007) The synaptic vesicle proteome. J Neurochem 101:1448–1462
Chen H, Chan DC (2009) Mitochondrial dynamics—fusion, fission, movement, and mitophagy—in neurodegenerative diseases. Hum Mol Genet 18:R169–R176
Cordato DJ, Chan DK (2004) Genetics and Parkinson’s disease. J Clin Neurosci 11:119–123
Culvenor JG, Rietze RL, Bartlett PF, Masters CL, Li QX (2002) Oligodendrocytes from neural stem cells express alpha-synuclein: increased numbers from presenilin 1 deficient mice. Neuroreport 13:1305–1308
Fahn S (2003) Description of Parkinson's disease as a clinical syndrome. Ann N Y Acad Sci 991:1–14
Gai WP, Power JH, Blumbergs PC, Culvenor JG, Jensen PH (1999) Alpha-synuclein immunoisolation of glial inclusions from multiple system atrophy brain tissue reveals multiprotein components. J Neurochem 73:2093–2100
Inoue M, Yagishita S, Ryo M, Hasegawa K, Amano N, Matsushita M (1997) The distribution and dynamic density of oligodendroglial cytoplasmic inclusions (GCIs) in multiple system atrophy: a correlation between the density of GCIs and the degree of involvement of striatonigral and olivopontocerebellar systems. Acta Neuropathol 93:585–591
Iwatsubo T, Yamaguchi H, Fujimuro M, Yokosawa H, Ihara Y, Trojanowski JQ, Lee VM (1996) Purification and characterization of Lewy bodies from the brains of patients with diffuse Lewy body disease. Am J Pathol 148:1517–1529
Jensen PH, Islam K, Kenney J, Nielsen MS, Power J, Gai WP (2000) Microtubule-associated protein 1B is a component of cortical Lewy bodies and binds alpha-synuclein filaments. J Biol Chem 275:21500–21507
Jin LT, Hwang SY, Yoo GS, Choi JK (2006) A mass spectrometry compatible silver staining method for protein incorporating a new silver sensitizer in sodium dodecyl sulfate-polyacrylamide electrophoresis gels. Proteomics 6:2334–2337
Keating DJ (2008) Mitochondrial dysfunction, oxidative stress, regulation of exocytosis and their relevance to neurodegenerative diseases. J Neurochem 104:298–305
Klein C, Schlossmacher MG (2007) Parkinson disease, 10 years after its genetic revolution: multiple clues to a complex disorder. Neurology 69:2093–2104
Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K (2006) Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet 38:518–520
Leverenz JB, Umar I, Wang Q, Montine TJ, McMillan PJ, Tsuang DW, Jin J, Pan C, Shin J, Zhu D, Zhang J (2007) Proteomic identification of novel proteins in cortical lewy bodies. Brain Pathol 17:139–145
Licker V, Kovari E, Hochstrasser DF, Burkhard PR (2009) Proteomics in human Parkinson's disease research. J Proteome 73:10–29
Lindersson E, Lundvig D, Petersen C, Madsen P, Nyengaard JR, Højrup P, Moos T, Otzen D, Gai WP, Blumbergs PC, Jensen PH (2005) p25alpha Stimulates alpha-synuclein aggregation and is co-localized with aggregated alpha-synuclein in alpha-synucleinopathies. J Biol Chem 280:5703–5715
Link AJ, LaBaer J (2009) Proteomics: a Cold Spring Harbor laboratory course manual. Cold Spring Harbor laboratory press, Cold Spring Harbor
McCormack A, Chegeni N, Chegini F, Colella A, Power J, Keating D, Chataway T (2016) Purification of alpha-synuclein containing inclusions from human post mortem brain tissue. J Neurosci Methods 266:141–150
McKeith I, Mintzer J, Aarsland D, Burn D, Chiu H, Cohen-Mansfield J, Dickson D, Dubois B, Duda JE, Feldman H, Gauthier S, Halliday G, Lawlor B, Lippa C, Lopez OL, Carlos Machado J, O'Brien J, Playfer J, Reid W, International Psychogeriatric Association Expert Meeting on DLB (2004) Dementia with Lewy bodies. Lancet Neurol 3:19–28
Miller DW, Johnson JM, Solano SM, Hollingsworth ZR, Standaert DG, Young AB (2005) Absence of alpha-synuclein mRNA expression in normal and multiple system atrophy oligodendroglia. J Neural Transm 112:1613–1624
Onyango IG (2008) Mitochondrial dysfunction and oxidative stress in Parkinson's disease. Neurochem Res 33:589–597
Ozawa T (2006) Pathology and genetics of multiple system atrophy: an approach to determining genetic susceptibility spectrum. Acta Neuropathol 112:531–538
Peiris H, Dubach D, Jessup CF, Unterweger P, Raghupathi R, Muyderman H, Zanin MP, Mackenzie K, Pritchard MA, Keating DJ (2014) RCAN1 regulates mitochondrial function and increases susceptibility to oxidative stress in mammalian cells. Oxidative Med Cell Longev 2014:520316
Pollanen MS, Bergeron C, Weyer L (1993) Deposition of detergent-resistant neurofilaments into Lewy body fibrils. Brain Res 603:121–124
Richter-Landsberg C, Gorath M, Trojanowski JQ, Lee VM (2000) Alpha-synuclein is developmentally expressed in cultured rat brain oligodendrocytes. J Neurosci Res 62:9–14
Shahmoradian SH, Genoud C, Graff-Meyer A, Hench J, Moors T, Schweighauser G, Wang J, Goldie KN, Suetterlin R, Castano-Diez D, Perez-Navarro P, Huisman E, Ipsen S, Ingrassia A, De Gier Y, Rozemuller AJ, Da Paepe A, Erny J, Staempfli A, Hoernschemeyer J, Grosserueschkamp F, Niedieker D, El-Mashtoly S, Quadri M, Van WF, Bonifati V, Gerwert K, Bohrmann B, Frank A, Britschgi M, Stahlberg H, Berg VD, Lauer ME (2017) Lewy pathology in Parkinson’s disease consists of a crowded organellar membranous medley. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Sian J, Hensiek R, Senitz D, Muench G, Jellinger K, Riederer P, Gerlach M (1998) A novel technique for the isolation of Lewy bodies in brain. Acta Neuropathol 96:111–115
Smeyne RJ, Jackson-Lewis V (2005) The MPTP model of Parkinson’s disease. Brain Res Mol Brain Res 134:57–66
Tan EK (2007) The role of common genetic risk variants in Parkinson disease. Clin Genet 72:387–393
Uversky VN, Eliezer D (2009) Biophysics of Parkinson’s disease: structure and aggregation of alpha-synuclein. Curr Protein Pept Sci 10:483–499
Vanacore N (2005) Epidemiological evidence on multiple system atrophy. J Neural Transm 112:1605–1612
Vanacore N, Bonifati V, Fabbrini G, Colosimo C, de Michele G, Marconi R, Nicholl D, Locuratolo N, Talarico G, Romano S, Stocchi F, Bonuccelli U, de Mari M, Vieregge P, Meco G, European Study Group on Atypical Parkinsonism (ESGAP) (2001) Epidemiology of multiple system atrophy. ESGAP Consortium. European Study Group on Atypical Parkinsonisms. Neurol Sci 22:97–99
Wakabayashi K, Takahashi H (2006) Cellular pathology in multiple system atrophy. Neuropathology 26:338–345
Wakabayashi K, Tanji K, Mori F, Takahashi H (2007) The Lewy body in Parkinson’s disease: molecules implicated in the formation and degradation of alpha-synuclein aggregates. Neuropathology 27:494–506
Wenning GK, Tison F, Ben Shlomo Y, Daniel SE, Quinn NP (1997) Multiple system atrophy: a review of 203 pathologically proven cases. Mov Disord 12:133–147
Wenning GK, Stefanova N, Jellinger KA, Poewe W, Schlossmacher MG (2008) Multiple system atrophy: a primary oligodendrogliopathy. Ann Neurol 64:239–246
Wilson CH, Zeile S, Chataway T, Nieuwenhuijs VB, Padbury RT, Barritt GJ (2011) Increased expression of peroxiredoxin 1 and identification of a novel lipid-metabolizing enzyme in the early phase of liver ischemia reperfusion injury. Proteomics 11:4385–4396
Wood-Kaczmar A, Gandhi S, Wood NW (2006) Understanding the molecular causes of Parkinson’s disease. Trends Mol Med 12:521–528
Yoshida M (2007) Multiple system atrophy: alpha-synuclein and neuronal degeneration. Neuropathology 27:484–493
Acknowledgements
We would like to acknowledge Dr. Weiping Gai for the anti-α-synuclein antibody, Ms. Fariba Chegini for her assistance with immunofluoresence and the South Australian Brain Bank for their support.
Funding
This study is financially supported by the Flinders Medical Centre Research Foundation, South Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOC 1040 kb)
Rights and permissions
About this article
Cite this article
McCormack, A., Keating, D.J., Chegeni, N. et al. Abundance of Synaptic Vesicle-Related Proteins in Alpha-Synuclein-Containing Protein Inclusions Suggests a Targeted Formation Mechanism. Neurotox Res 35, 883–897 (2019). https://doi.org/10.1007/s12640-019-00014-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12640-019-00014-0