Abstract
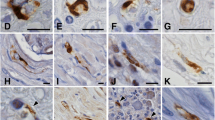
In this autopsy-based study, α-synuclein immunohistochemistry and lipofuscin pigment-Nissl architectonics in serial sections of 100 μm thickness were used to investigate the spinal cords and brains of 46 individuals: 28 patients with clinically and neuropathologically confirmed Parkinson’s disease, 6 cases with incidental Lewy body disease, and 12 age-matched controls. α-Synuclein inclusions (particulate aggregations, Lewy neurites/bodies) in the spinal cord were present between neuropathological stages 2–6 in all cases whose brains were staged for Parkinson’s disease-related synucleinopathy. The only individuals who did not have Lewy pathology in the spinal cord were a single stage 1 case (incidental Lewy body disease) and all controls. Because the Parkinson’s disease-related lesions were observable in the spinal cord only after Lewy pathology was seen in the brain, it could be concluded that, within the central nervous system, sporadic Parkinson’s disease does not begin in the spinal cord. In addition: (1) α-Synuclein-immunoreactive axons clearly predominated over Lewy bodies throughout the spinal cord and were visible in medial and anterior portions of the anterolateral funiculus. Their terminal axons formed dense α-synuclein-immunoreactive networks in the gray matter and were most conspicuous in the lateral portions of layers 1, 7, and in the cellular islands of layer 9. (2) Notably, this axonopathy increased remarkably in density from cervicothoracic segments to lumbosacral segments of the cord. (3) Topographically, it is likely that the spinal cord α-synuclein immunoreactive axonal networks represent descending projections from the supraspinal level setting nuclei (locus coeruleus, lower raphe nuclei, magnocellular portions of the reticular formation). (4) Following the appearance of the spinal cord axonal networks, select types of projection neurons in the spinal cord gray matter displayed α-synuclein-immunoreactive inclusions: chiefly, nociceptive neurons of the dorsal horn in layer 1, sympathetic and parasympathetic preganglionic neurons in layer 7, the cellular pools of α-motoneurons in layer 9, and the smaller motoneurons in Onuf’s nucleus in layer 9 (ventral horn). The spinal cord lesions may contribute to clinical symptoms (e.g., pain, constipation, poor balance, lower urinary tract complaints, and sexual dysfunction) that occur during the premotor and motor phases of sporadic Parkinson’s disease.







Similar content being viewed by others
References
Abbott RD, Petrovitch H, White LR et al (2001) Frequency of bowel movements and the risk of Parkinson’s disease. Neurology 57:456–462
Abbott RD, Ross GW, Petrovitch H et al (2007) Bowel movement frequency in late-life and incidental Lewy bodies. Mov Disord 22:1581–1586
Ashraf W, Pfeiffer RF, Quigley EMM (1994) Anorectal manometry in the assessment of anorectal function in Parkinson’s disease: a comparison with chronic idiopathic constipation. Mov Disord 9:655–663
Aston-Jones G, Cohen JD (2005) Adaptive gain and the role of the locus coeruleus-norepinephrine system in optimal performance. J Comp Neurol 493:99–110
Awerbuch GI, Sandyk R (1994) Autonomic functions in the early stages of Parkinson’s disease. Int J Neurosci 74:9–16
Barson AJ, Sands J (1977) Regional and segmental characteristics of the human spinal cord. J Anat 123:797–803
Basbaum AI, Fields HL (1984) Endogeneous pain control systems: brainstem spinal pathways and endorphin circuitry. Ann Rev Neurosci 7:309–338
Beach TG, Adler CH, Sue LI et al (2010) Multi-organ distribution of phospohorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol 119:689–702
Benarroch EE (1993) The central autonomic network: functional organization, dysfunction, and perspective. Mayo Clin Proc 68:988–1001
Benarroch EE (2001) Pain-autonomic interactions: a selective review. Clin Auton Res 11:343–349
Benarroch EE (2007) Enteric nervous system: functional organization and neurologic implications. Neurology 69:1953–1957
Benarroch EE (2010) Neural control of the bladder: recent advances and neurologic implications. Neurology 75:1839–1846
Benarroch EE, Schmeichel AM, Low PA et al (2005) Involvement of medullary regions controlling sympathetic output in Lewy body disease. Brain 128:338–344
Blessing WW (2004) Lower brain stem regulation of visceral, cardiovascular, and respiratory function. In: Paxinos G, Mai JK (eds) The human nervous system, 2nd edn. Academic Press, San Diego, pp 464–478
Bloch A, Probst A, Bissig H, Adams H, Tolnay M (2006) α-Synuclein pathology of the spinal and peripheral autonomic nervous system in neurologically unimpaired elderly subjects. Neuropathol Appl Neurobiol 12:284–295
Bohus B, Koolhaas JM, Luiten PGM et al (1996) The neurobiology of the central nucleus of the amygdala in relation to neuroendocrine and autonomic outflow. Progr Brain Res 107:447–460
Bowsher D, Abdel-Maguid TE (1984) Superficial dorsal horn of the adult human spinal cord. Neurosurgery 15:893–899
Braak E, Sandmann-Keil D, Rüb U et al (2001) Alpha-synuclein immunopositive Parkinson’s disease-related inclusion bodies in lower brain stem nuclei. Acta Neuropathol 101:195–201
Braak H (1984) Architectonics as seen by lipofuscin stains. In: Jones EG, Peters A (eds) Cerebral cortex. Cellular components of the cerebral cortex, vol I. Plenum Press, New York, pp 59–104
Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82:239–259
Braak H, Braak E, Yilmazer D et al (1994) Amygdala pathology in Parkinson’s disease. Acta Neuropathol 88:493–500
Braak H, Sandmann-Keil D, Gai WP, Braak E (1999) Extensive axonal Lewy neurites in Parkinson’s disease: a novel pathological feature revealed by α-synuclein. Neurosci Lett 265:67–69
Braak H, Rüb U, Sandmann-Keil D et al (2000) Parkinson’s disease: affection of brain stem nuclei controlling premotor and motor neurons of the somatomotor system. Acta Neuropathol 99:489–495
Braak H, Del Tredici K, Rüb U et al (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–210
Braak H, Rüb U, Gai WP, Del Tredici K (2003) Idiopathic Parkinson’s disease: possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J Neural Transm 110:517–536
Braak H, de Vos RAI, Bohl J, Del Tredici K (2006) Gastric α-synuclein immunoreactive inclusions in Meissner’s and Auerbach’s plexuses in cases staged for Parkinson’s disease-related brain pathology. Neurosci Lett 396:67–72
Braak H, Sastre M, Bohl JR, de Vos RA, Del Tredici K (2007) Parkinson’s disease: lesions in dorsal horn layer I, involvement of parasympathetic and sympathetic pre- and postganglionic neurons. Acta Neuropathol 113:421–429
Brown RG, Jahanshahi M, Quinn N, Marsden CD (1990) Sexual function in patients with Parkinson’s disease and their partners. J Neurol Neurosurg Psychiatry 53:480–486
Buzas B, Max MB (2004) Pain in Parkinson disease. Neurology 62:2156–2157
Cersósimo MG, Benarroch EE (2008) Neural control of the gastrointestinal tract: implications for Parkinson disease. Mov Disord 23:1065–1075
Cersósimo MG, Perandonas C, Micheli FE et al (2011) Alpha-synuclein immunoreactivity in minor salivary gland biopsies of Parkinson’s disease patients. Mov Disord 26:188–190
Chalmers D, Swash M (1987) Selective vulnerability of urinary Onuf motoneurons in Shy–Drager syndrome. J Neurol 234:259–260
Clark FM, Proudfit HK (1991) The projection of locus coeruleus neurons to the spinal cord in the rat determined by anterograde tracing combined with immunocytochemistry. Brain Res 538:231–245
Craig AD (1993) Propriospinal input to thoracolumbar sympathetic nuclei from cervical and lumbar lamina I neurons in the cat and monkey. J Comp Neurol 331:517–530
Craig AD (2003) Pain mechanisms: labeled lines versus convergence in central processing. Ann Rev Neurosci 26:1–30
Dale GE, Probst A, Luthert P et al (1992) Relationships between Lewy bodies and pale bodies in Parkinson’s disease. Acta Neuropathol 83:525–529
Danzer KM, Krebs SK, Wolff M, Birk G, Hengerer B (2009) Seeding induced by alpha-synuclein oligomers provides evidence for spreading of alpha-synuclein pathology. J Neurochem 111:192–203
Defazio G, Beradelli A, Fabrini G et al (2008) Pain as a nonmotor symptom of Parkinson disease: evidence from a case-control study. Arch Neurol 65:1191–1194
de Jong TR, Veening JG, Waldinger MD, Cools AR, Olivier B (2006) Serotonin and the neurobiology of the ejaculatory threshold. Neurosci Biobehav Rev 30:893–907
Delledonne A, Klos KJ, Fujishiro H et al (2008) Incidental Lewy body disease and preclinical Parkinson disease. Arch Neurol 65:1074–1080
Del Tredici K, Braak H (2012) Lewy pathology and neurodegeneration in premotor Parkinson’s disease. Mov Disord 27:597–607
Del Tredici K, Rüb U, de Vos RAI, Bohl JRE, Braak H (2002) Where does Parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol 61:413–426
Del Tredici K, Hawkes CH, Ghebremedhin E, Braak H (2010) Lewy pathology in the submandibular gland of individuals with incidental Lewy body disease and sporadic Parkinson’s disease. Acta Neuropathol 119:703–713
den Hartog Jager WA, Bethlem J (1960) The distribution of Lewy bodies in the central and autonomic nervous systems in idiopathic paralysis agitans. J Neurol Neurosurg Psychiatry 23:283–290
Desplats P, Lee HJ, Bae EJ et al (2009) Inclusion formation and neuronal cell death through neuron-to-neuron transmission of α-synuclein. Proc Natl Acad Sci USA 106:13010–13015
Dickson DW (2001) α-Synuclein and the Lewy body disorders. Curr Opin Neurol 14:423–432
Dickson DW, Fujishiro H, DelleDonne A et al (2008) Evidence that incidental Lewy body disease is presymptomatic Parkinson’s disease. Acta Neuropathol 115:437–444
Dickson DW, Braak H, Duda JE et al (2009) Neuropathological assessment of Parkinson disease: refining the diagnostic criteria. Lancet Neurol 8:1150–1157
Dickson DW, Uchikado H, Fujishiro H, Tsuboi Y (2010) Evidence in favor of Braak staging of Parkinson’s disease. Mov Disord 25(Suppl 1):S78–S82
Djaldetti R, Shifrin A, Rogowski Z et al (2004) Quantitative measurement of pain sensation in patients with Parkinson disease. Neurology 62:2171–2175
Duda JE, Lee VMY, Trojanowski JQ (2000) Neuropathology of synuclein aggregates: new insights into mechanism of neurodegenerative diseases. J Neurosi Res 61:121–127
Dugger BN, Murray ME, Boeve BR et al (2012) Neuropathological analysis of brainstem cholinergic and catecholaminergic nuclei in relation to rapid eye movement (REM) sleep behaviour disorder. Neuropathol Appl Neurol 38:142–152
Dunning CJ, Reyes JF, Steiner JA, Brundin P (2012) Can Parkinson’s disease pathology be propagated from one neuron to another? Progr Neurobiol 97:205–219
Eadie MJ, Tyrer JH (1965) Alimentary disorder in parkinsonism. Austral Ann Med 14:13–22
Edwards LL, Pfeiffer RF, Quigley EMM, Hofman R, Balluff M (1991) Gastrointestinal symptoms in Parkinson’s disease. Mov Disord 6:151–156
Edwards LL, Quigley EMM, Hofman R, Pfeiffer RF (1993) Gastrointestinal symptoms in Parkinson Disease: 18-month follow-up study. Mov Disord 8:83–86
Edwards LL, Quigley EMM, Harned RK et al (1994) Characterization of swallowing and defecation in Parkinson’s disease. Am J Gastroenterol 89:15–25
Ford B (1998) Pain in Parkinson’s disease. Clin Neurosci 5:63–72
Foreman RD, Blair RW (1988) Central organization of sympathetic cardiovascular response to pain. Ann Rev Physiol 50:607–622
Forger N, Breedlove SM (1986) Sexual dimorphism in human and canine spinal cord: role of early androgen. Proc Natl Acad of Sci USA 83:7527–7531
Forno LS (1969) Concentric hyalin intraneuronal inclusions of Lewy type in the brain of elderly persons (50 incidental cases): relationship to parkinsonism. J Am Geriatr Soc 17:557–575
Forno LS (1987) The Lewy body in Parkinson’s disease. Adv Neurol 45:35–43
Fowler CJ (2006) Integrated control of lower urinary tract—clinical perspective. Br J Pharmacol 147:S14–S24
Frigerio R, Fujishiro H, Maraganore DM et al (2009) Comparison of risk factor profiles in incidental Lewy body disease and Parkinson disease. Arch Neurol 66:1114–1119
Fürst S (1999) Transmitters involved in antinociception in the spinal cord. Brain Res Bull 48:129–141
Gallagher DA, Lees AJ, Schrag A (2010) What are the most important nonmotor symptoms in patients with Parkinson’s disease and are we missing them? Mov Disord 25:2493–2500
Gelb DJ, Oliver E, Gilman S (1999) Diagnostic criteria for Parkinson’s disease. Arch Neurol 56:33–39
Gibb WR, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Goetz CG, Tanner CM, Levy M, Wilson RS, Garron DC (1986) Pain in Parkinson’s disease. Mov Disord 1:45–49
Grillner S, Wallén S, Saitoh K, Kozlov A, Robertson B (2008) Neural basis of goal-directed locomotion in vertebrates—an overview. Brain Res Rev 57:2–12
Guyenet PG, Koshiya N, Huangfu D et al (1996) Role of medulla oblongata in generation of sympathetic and vagal outflows. Prog Brain Res 107:127–144
Hansen C, Li J-Y (2012) Beyond alpha-synuclein transfer: pathology propagation in Parkinson’s disease. Trends Mol Med 18:248–255
Hansen C, Angot E, Bergström A-L et al (2011) α-Synuclein propagates from mouse brain to grafted dopaminergic neurons and seeds aggregation in cultured human cells. J Clin Invest 121:715–725
Hilz MJ, Axelrod FB, Braeske K, Stemper B (2002) Cold pressor test demonstrates residual sympathetic cardiovascular activation in familial dysautonomia. J Neurol Sci 196:81–89
Holstege G (1992) The emotional motor system. Europ J Morphol 30:67–79
Holstege G (1996) The somatic motor system. Progr Brain Res 107:9–26
Holstege G (2005) Micturition and the soul. J Comp Neurol 493:15–20
Holstege G (2005) Central nervous system control of ejaculation. World J Urol 23:109–114
Holstege G, Mouton LJ, Gerrits NM (2004) Emotional motor system. In: Paxinos G, Mai JK (eds) The human nervous system, 2nd edn. Academic Press, San Diego, pp 1306–1325
Hopkins DA, Bieger D, de Vente J, Steinbusch HWM (1996) Vagal efferent projections: viscerotopy, neurochemistry and effects of vagotomy. Progr Brain Res 107:79–96
Hornung J-P (2003) The human raphe nuclei and the serotonergic system. J Chem Neuroanat 26:331–343
Iwanaga K, Wakabayashi K, Yoshimoto M et al (1999) Lewy body-type degeneration in cardiac plexus in Parkinson’s and incidental Lewy body diseases. Neurology 52:1269–1271
Jacobs BL, Fornal CA (1993) 5-HT and motor control: a hypothesis. Trends Neurosci 16:346–352
Jänig W (1996) Spinal cord reflex organization of sympathetic systems. Progr Brain Res 107:43–77
Jankovic J (2008) Parkinson’s disease: clinical features and diagnosis. J Neurol Neurosurg Psychiatry 79:368–376
Jellinger KA (1991) Pathology of Parkinson’s disease. Changes other than the nigrostriatal pathway. Mol Chem Neuropathol 14:153–197
Jost WH, Eckardt VF (2003) Constipation in idiopathic Parkinson’s disease. Scand J Gastroenterol 38:681–686
Kinder MV, Bastiaanssen EH, Janknegt RA, Marani E (1995) Neuronal circuitry of the lower urinary tract; central and peripheral neuronal control of the micturition cycle. Anat Embryol 192:195–209
Klos KJ, Ahlskog JE, Josephs KA et al (2006) α-Synuclein pathology in the spinal cord of neurologically asymptomatic aged individuals. Neurology 66:1100–1102
Kojima M, Sano Y (1983) The organization of serotonin fibers in the anterior column of the mammalian spinal cord. Anat Embryol 167:1–11
Kojima H, Furuta Y, Fujita M, Fujioka Y, Nagashima K (1989) Onuf’s motoneuron is resistant to poliovirus. J Neurol Sci 93:85–92
Koller WC (1984) Sensory symptoms in Parkinson’s disease. Neurology 34:957–959
Korczyn AD (1990) Autonomic nervous system disturbances in Parkinson’s disease. Adv Neurol 53:463–468
Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW (2008) Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat Med 14:504–506
Kuusisto E, Parkkinen L, Alafuzoff I (2003) Morphogenesis of Lewy bodies: dissimilar incorporation of α-synuclein, ubiquitin, and p62. J Neuropathol Exp Neurol 62:1241–1253
Lee SJ (2008) Origins and effects of extracellular alpha synuclein: implications in Parkinson’s disease. J Mol Neurosci 34:17–22
Lee HJ, Patel S, Lee SJ (2005) Intravesicular localization and exocytosis of α-synuclein and its aggregates. J Neurosci 25:6016–6024
Lee SJ, Desplats P, Sigurdson C, Tsigelny I, Masliah E (2010) Cell-to-cell transmission of non-prion protein aggregates. Nat Rev Neurol 26:702–706
Light AR (1988) Normal anatomy and physiology of the spinal cord dorsal horn. Appl Neurophysiol 51:78–88
Loewy AD (1990) Central autonomic pathways. In: Loewy AD, Spyer KM (eds) Central regulation of autonomic functions. Oxford University Press, New York, pp 88–103
Luk KC, Song C, O’Brien P et al (2009) Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc Natl Acad Sci USA 106:20051–20056
Magerkurth C, Schnitzer R, Braune S (2005) Symptoms of autonomic failure in Parkinson’s disease: prevalence and impact on daily life. Clin Auton Res 15:76–82
Makaroff L, Gunn A, Gervasoni C, Richy F (2011) Gastrointestinal disorders in Parkinson’s disease: prevalence and health outcomes in a US claims database. J Parkinsons Dis 1:65–74
Mannen T (2000) Neuropathological findings of Onuf’s nucleus and its significance. Neuropathology 20(Suppl):S30–S33
Mannen T, Iwata M, Toyokura Y, Nagashima K (1977) Preservation of a certain motoneurone group of the sacral cord in amyotrophic lateral sclerosis: its clinical significance. J Neurol Neurosurg Psychiatry 40:464–469
Mannen T, Iwata M, Toyokura Y, Nagashima K (1982) The Onuf’s nucleus and the external anal sphincter muscles in amyotrophic lateral sclerosis and Shy–Drager syndrome. Acta Neuropathol 58:255–260
Markesbery WR, Jicha GA, Liu H, Schmitt FA (2009) Lewy body pathology in normal elderly subjects. J Neuropathol Exp Neurol 68:816–822
Martignoni E, Pacchetti C, Godi L, Miceli G, Nappi G (1995) Autonomic disorders in Parkinson’s disease. J Neural Transm 45(Suppl):11–19
Martin GF, Holstege G, Mehler WM (1990) Reticular formation of the pons and medulla. In: Paxinos G (ed) The human nervous system, 1st edn. Academic Press, New York, pp 203–220
Mathers SE, Kempster PA, Swash M, Lees AJ (1988) Constipation and paradoxical puborectalis contraction in anismus and Parkinson’s disease: a dystonic phenomenon? J Neurol Neu-rosurg Psy-chia-try 51:1503–1507
Mathers SE, Kempster PA, Law PJ et al (1989) Anal sphincter dysfunction in Parkinson’s disease. Arch Neurol 46:1061–1064
McHugh JM, McHugh WB (2000) Pain: neuroanatomy, chemical mediators, and clinical implications. AACN Clin Issues 2:168–178
McKeith IG, Dickson DW, Lowe J et al (2005) Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65:1863–1862
Millan MJ (2002) Descending control of pain. Prog Neurbiol 66:355–474
Minguez-Castellanos A, Chamorro CE, Escamilla-Sevilla F et al (2007) Do α-synuclein aggregates in autonomic plexuses predate Lewy body disorders? A cohort study. Neurology 68:2012–2018
Molony V, Steedman WM, Cervero F, Iggo A (1981) Intracellular marking of identified neurons in the superficial dorsal horn of the cat spinal cord. Quart J Exp Physiol 66:211–223
Mylius V, Brebbemann J, Dohmann H et al (2011) Pain sensitivity and clinical progression in Parkinson’s disease. Mov Disord 26:2220–2225
The National Institute on Aging (1997) Consensus recommendations for the post-mortem diagnosis of Alzheimer’s disease. The National Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer’s Disease. Neurobiol Aging 18:S1–S2
Nieuwenhuys R (1996) The greater limbic system, the emotional motor system and the brain. Progr Brain Res 107:551–580
Norris EH, Giasson BI, Lee VM (2004) α-Synuclein: normal function and role in neurodegenerative diseases. Curr Top Dev Biol 60:17–54
Nyberg LG, Olson L (1977) A new major projection from locus coeruleus: the main source of noradrenergic nerve terminals in the ventral and dorsal columns of the spinal cord. Brain Res 132:85–93
Oinas M, Paetau A, Myillykangas L et al (2010) alpha-Synuclein pathology in the spinal cord autonomic nuclei associates with alpha-synuclein pathology in the brain: a population-based Vantaa 85+ study. Acta Neuropathol 119:715–722
Onufronwicz B (1899) Notes on the arrangement and function of the cell groups of the sacral regions of the spinal cord. J Nerv Med Dis 26:498–504
O’Sullivan SS, Holton JL, Massey LA et al (2008) Parkinson’s disease with Onuf’s nucleus involvement mimicking multiple system atrophy. J Neurol Neurosurg Psychiatry 79:232–234
O’Sullivan SS, Williams DR, Gallagher DW et al (2008) Non-motor symptoms as presenting complaints in Parkinson’s disease: a clinicopathological study. Mov Disord 23:101–106
Oyanagi K, Wakabayashi K, Ohama E et al (1990) Lewy bodies in lower sacral parasympathetic neurons of a patient with Parkinson’s disease. Acta Neuropathol 80:558–559
Pan-Montojo F, Anichtchik O, Dening Y et al (2010) Progression of Parkinson’s disease pathology is reproduced by intragastric administration of rotenone in mice. PLoS ONE 5:38762
Parkinson JD (1817) An essay on the shaking palsy. Sherwood, Neely and Jones, London
Paulus W, Jellinger KA (1991) The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol 50:743–755
Paxinos G, Huang X-F, Sengul G, Watson C (2012) Organization of brainstem nuclei: In: Mai JK, Paxinos G (eds) The human nervous system, 3rd edn. Academic Press, San Diego, pp 260–327
Perrin FE, Gerber YN, Teigell M et al (2011) Anatomical study of serotonergic innervation and 5-HT1A receptor in the human spinal cord. Cell Death Dis 2:3218
Perrotta A, Sandrini G, Serrao M et al (2011) Facilitated temporal summation of pain at spinal level in Parkinson’s disease. Mov Disord 26:442–448
Pfeiffer RF (2011) Gastrointestinal dysfunction in Parkinson’s disease. Parkinsonism Rel Disord 17:10–15
Pouclet H, Lebouvier T, Coron E et al (2012) A comparison between rectal and colonic biopsies to detect Lewy pathology in Parkinson’s disease. Neurobiol Dis 45:305–309
Prasad K, Beach TG, Hedreen J, Richfield EK (2012) Critical role for truncated α-synuclein and aggregates in Parkinson’s disease and incidental Lewy body disease. Brain Pathol March 27 [Epub ahead of print] PMID: 22452578
Pullen AH, Tucker D, Martin JE (1997) Morphological and morphometric characterization of Onuf’s nucleus in the spinal cord in man. J Anat 191:201–213
Puskár Z, Polgár E, Todd AJ (2001) A population of large lamina I projections neurons with selective inhibitory input in rat spinal cord. Neuroscience 102:167–176
Quinn NP, Koller WC, Lang AE, Marsden CD (1996) Painful Parkinson’s disease. Lancet 1:1366–1369
Rajaofetra N, Passagia JG, Marlier L et al (1992) Serotoninergic, noradrenergic, and peptidergic innervation of Onuf’s nucleus of normal and transacted spinal cords of baboons (Papio papio). J Comp Neurol 318:1–17
Ransmayr GN, Holliger S, Schletterer K et al (2008) Lower urinary tract symptoms in dementia with Lewy bodies, Parkinson disease, and Alzheimer disease. Neurology 70:299–303
Rexed BA (1954) A cytoarchitectonic atlas of the spinal cord in the cat. J Comp Neurol 100:297–379
Ross GW, Abbott RD, Petrovitch H et al (2006) Association of olfactory dysfunction with incidental Lewy bodies. Mov Disord 21:2062–2067
Sage JI (2004) Pain in Parkinson’s disease. Curr Treat Options Neurol 6:191–200
Sakakibara R, Shinotoh H, Uchiyama T et al (2001) Questionnaire-based assessment of pelvic organ dysfunction in Parkinson’s disease. Auton Neurosci 92:76–85
Sakakibara R, Uchiyama T, Yamanishi T, Kishi M (2010) Genitourinary dysfunction in Parkinson’s disease. Mov Disord 25:2–12
Sakakibara R, Tateno F, Kishi M et al (2012) Pathophysiology of bladder dysfunction in Parkinson’s disease. Neurobiol Dis 46:565–571
Samuels ER, Szabadi E (2009) Functional neuroanatomy of the noradrenergic locus coeruleus: its roles in the regulation of arousal and autonomic function part I: principles of functional organization. Curr Neuropharmacol 6:235–253
Sandrini G, Serrae M, Rossi P et al (2005) The lower limb flexion reflex in humans. Prog Neurobiol 77:353–395
Saper CB, Sorrentino DM, German DC, de Lacalle S (1991) Medullary catecholaminergic neurons in the normal human brain and in Parkinson’s disease. Ann Neurol 29:577–584
Sasa M, Yoshimura N (1994) Locus coeruleus noradrenergic neurons as a micturition center. Microsc Res Tech 29:226–230
Savica R, Cartin JM, Grossardt BR et al (2009) Medical records documentation of constipation preceding Parkinson disease: a case–control study. Neurology 73:1752–1758
Scatton B, Dennis T, L’Heureux R et al (1986) Degeneration of noradrenergic and serotonergic but not dopaminergic neurones in the lumbar spinal cord of Parkinson patients. Brain Res 380:181–185
Scherder E, Wolters E, Polman C, Serfeant J, Swaab D (2005) Pain in Parkinson’s disease and multiple sclerosis: its relation to the medial and lateral pain systems. Neurosci Biobehav Rev 29:1047–1056
Schrøder HD (1981) Onuf’s nucleus X: a morphological study of a human spinal nucleus. Anat Embryol 162:443–453
Sengul G, Watson C (2012) Spinal cord: regional anatomy, cytoarchitecture, and chemoarchitecture. In: Mai JK, Paxinos G (eds) The human nervous system, 3rd edn. Academic Press, Boston, pp 186–232
Serrao M, Ranavolo A, Anderson OK et al (2012) Adaptive behaviour of the spinal cord in the transition from quiet stance to walking. BMC Neurosci 16:80
Sethi K (2008) Levodopa unresponsive symptoms in Parkinson’s disease. Mov Disord 23(Suppl 3):S521–S533
Shannon KM, Keshavarzian A, Dodiya HB, Jakate S, Kordower JH (2012) Is alpha-synuclein in the colon a biomarker for premotor Parkinson’s disease? Evidence from 3 cases. Mov Disord 27:716–719
Siddiqui MF, Rast S, Lynn MJ, Auchus AP, Pfeiffer RF (2002) Autonomic dysfunction in Parkinson’s disease: a comprehensive symptom survey. Parkinsonism Rel Disord 8:277–284
Sims KS, Williams RS (1990) The human amygdaloid complex: a cytologic and histochemical atlas using Nissl, myelin, acetylcholinesterase and nicotinamide adenine dinucleotide phosphate diaphorase staining. Neuroscience 36:449–472
Singer C, Weiner WJ, Sanchez-Ramos JR (1992) Autonomic dysfunction in men with Parkinson’s disease. Eur Neurol 32:134–140
Snider SR, Fahn S, Isgreen WP, Cote LJ (1976) Primary sensory symptoms in Parkinsonism. Neurology 76:423–429
Sofic E, Riederer P, Gsell W et al (1991) Biogenic amines and metabolites in spinal cord of patients with Parkinson’s disease and amyotrophic lateral sclerosis. J Neural Transm Park Dis Dement Sect 3:133–142
Spillantini MG, Schmidt ML, Lee VMY et al (1997) α-Synuclein in Lewy bodies. Nature 388:839–840
Steinbusch HWM (1981) Distribution of serotonin-immunoreactivity in the central nervous system of the rat. Cell bodies and terminals. Neuroscience 6:557–618
Strack AM, Sawyer WB, Hughes JH, Platt KB, Loewy AD (1989) A general pattern of CNS innervation of the sympathetic outflow demonstrated by transneuronal pseudorabies viral infections. Brain Res 491:156–162
Sun MK (1995) Central neural organization and control of sympathetic nervous system in mammals. Prog Neurobiol 47:157–233
Tamura T, Yoshida M, Hashizume Y, Sobue G (2012) Lewy body-related α-synucleinopathy in the spinal cord of cases with incidental Lewy body disease. Neuropathology 32:13–22
Thal DR, Rüb U, Orantes M, Braak H (2002) Phases of Aβ-deposition in the human brain and its relevance for the development of AD. Neurology 58:1791–1800
Thor KB (2003) Serotonin and norepinephrine involvement in efferent pathways to the urethral rhabdosphincter: implications for treating stress urinary incontinence. Urology 62(Suppl 1):3–9
Thor KB (2004) Targeting serotonin and norepinephrin receptors in stress urinary incontinence. Int J Gynaecol Obstet 86(Suppl 1):38–52
Todd AJ (2010) Neuronal circuitry for pain processing in the dorsal horn. Nature Rev Neurosci 11:823–836
Tofaris GK, Spillantini MG (2007) Physiological and pathological properties of α-synuclein. Cell Mol Life Sci 64:2194–2201
Tracey I (2005) Nociceptive processing in the human brain. Curr Opin Neurobiol 15:478–487
Trojanowski JQ, Lee VM (2007) Parkinson’s disease and related neurodegenerative synucleinopathies linked to progressive accumulations of synuclein aggregates in the brain. Parkinsonism Relat Disord 7:247–251
van de Berg WD, Hepp DH, Dijkstra AA et al (2012) Patterns of alpha-synuclein pathology in incidental cases and clinical subtypes of Parkinson’s disease. Parkinsonsim Relat Disord 18(Suppl 1):S28–S30
Verbaan D, Marinus J, Visser M et al (2007) Patient-reported autonomic symptoms in Parkinson disease. Neurology 69:333–341
Vivacqua G, Casini A, Vaccaro R et al (2011) Spinal cord and parkinsonism: neuromorphological evidences in humans and experimental studies. J Chem Neuroanat 42:327–340
Vogt BA, Sikes RW (2000) The medial pain system, cingulate cortex, and parallel processing of nociceptive information. Progr Brain Res 122:223–235
Wakabayashi K, Takahashi H (1997) Neuropathology of autonomic nervous system in Parkinson’s disease. Eur Neurol 38(Suppl 2):2–7
Wakabayashi K, Takahashi H (1997) The intermediolateral nucleus and Clarke’s column in Parkinson’s disease. Acta Neuropathol 94:287–289
Wakabayashi K, Takahashi H, Takeda S, Ohama E, Ikuta F (1988) Parkinson’s disease: the presence of Lewy bodies in Auerbach’s and Meissner’s plexuses. Acta Neuropathol 76:217–221
Wakabayashi K, Takahashi H, Ohama E, Ikuta F (1990) Parkinson’s disease: an immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol 79:581–583
Wakabayashi K, Takahashi H, Ohama E, Takeda S, Ikuta F (1993) Lewy bodies in the visceral autonomic nervous system in Parkinson’s disease. Adv Neurol 60:609–612
Waseem S, Gwinn-Hardy K (2001) Pain in Parkinson’s disease. Postgrad Med 110:1–5
Watson C, Paxinos G, Kayalioglu G (eds) (2009) The spinal cord: a Christopher and Dana Reeve Foundation Text and Atlas. Academic Press, Boston
Waxman EA, Giasson BI (2009) Molecular mechanisms of alpha-synuclein neurodegeneration. Biochim Biophys Acta 1792:616–624
Westlund KN, Coulter JD (1980) Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in the monkey: axonal transport studies and dopamine-β-hydroxylase immunocytochemistry. Brain Res Rev 2:235–264
Westlund KN, Craig (1996) LC receives ascending input from nociceptive fibers located in lamina I of the spinal cord
Willis WD, Westlund KN (1997) Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol 14:2–31
Winge K, Fowler CJ (2006) Bladder dysfunction in parkinsonism: mechanisms, prevalence, symptoms, and management. Mov Disord 21:737–745
Yamamoto T, Satomi H, Ise H, Takatama H, Takahashi K (1978) Sacral spinal innervations of the rectal and vesical smooth muscles and the sphincteric striated muscles as demonstrated by the horseradish peroxidase method. Neurosci Lett 7:41–47
Yeo L, Singh R, Gundeti M, Barua JM, Masood J (2012) Urinary tract dysfunction in Parkinson’s disease: a review. Int Urol Nephrol 44:415–424
Zarow C, Lyness SA, Mortimer JA, Chui HC (2003) Neuronal loss is greater in the locus coeruleus than nucleus basalis and substantia nigra in Alzheimer and Parkinson diseases. Arch Neurol 60:337–341
Zhang ET, Craig AD (1997) Morphology and distribution of spinothalamic lamina I neurons in the monkey. J Neurosci 17:3274–3284
Zesiewicz TA, Sullivan KL, Arnulf I et al (2010) Practice parameter: treatment of nonmotor symptoms of Parkinson disease: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 74:924–931
Acknowledgments
This study was made possible by funding from the German Research Council (Deutsche Forschungsgemeinschaft, DFG, Grant number TR 1000/1-1). The authors wish to thank Albert C. Ludolph, M.D. (Director, Department of Neurology, University of Ulm) for his ongoing support. They also gratefully acknowledge Ms. Siegrid Baumann, Ms. Simone Feldengut, Ms. Gabriele Ehmke (immunohistochemistry), and Mr. David Ewert (graphics) for their technical expertise. The Braak Collection (Goethe University Frankfurt) provided autopsy material.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Del Tredici, K., Braak, H. Spinal cord lesions in sporadic Parkinson’s disease. Acta Neuropathol 124, 643–664 (2012). https://doi.org/10.1007/s00401-012-1028-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-012-1028-y
Keywords
- α-Synuclein
- Autonomic nervous system
- Central nervous system
- Incidental Lewy body disease
- Intermediolateral nucleus
- Lewy body disease
- Lewy bodies/neurites
- Locus coeruleus
- Micturition
- Neurodegeneration
- Nucleus of Onuf
- Pain system
- Lower raphe nuclei
- Noradrenalin
- Renshaw cells
- Serotonin
- Sporadic Parkinson’s disease
- Premotor Parkinson’s disease
- Sacral parasympathetic nucleus
- Spinal cord
- Synucleinopathy