Abstract
Background
Cancer is a significant global health issue, resulting from uncontrolled cell division leading to abnormal cell or tissue growth. Traditional chemotherapeutic techniques have investigated a wide variety of pharmaceutically active molecules despite their poor bioavailability, quick renal clearance, inconsistent distribution, and unavoidable side effects. Green synthesis, unlike chemical methods, prioritizes eco-friendliness and cost-effectiveness. Using natural sources like plant extracts, it minimizes environmental impact, reduces costs, and aligns with sustainability goals. Operating under milder conditions, it consumes less energy compared to traditional approaches. Green synthesis is a highly promising and efficient method for producing nanoparticles due to its versatility and scalability.
Main body
Nanotechnology is making progress in cancer treatment because of nanoparticles' tiny size, large surface area, adaptability, and functionality, as well as their potential to induce apoptotic pathways and fast penetration or internalization into cancer cells. Biosynthesis of metallic nanoparticles using plant or microbe extracts is attracting attention to replace toxic chemicals with phytochemicals that can act as reducing, capping, or stabilizing agents and improve metallic nanoparticles biocompatibility, antitumor, and antioxidant properties. This review focuses on biosynthesized metallic nanoparticles and their anticancer effects on breast, prostate, skin, cervical, colorectal, lung, and liver cancer.
Conclusion
Biosynthesis of nanoparticles for cancer therapy stands at the forefront of innovative and sustainable approaches. Despite challenges, ongoing research demonstrates the potential of biosynthesis to revolutionize cancer nanomedicine, emphasizing the need for continued exploration and collaboration in this rapidly advancing field. Overall, this review offers a comprehensive understanding of the most recent developments in biosynthesized metallic nanoparticles for the treatment of cancer as well as their potential future applications in medicine.
Similar content being viewed by others
Background
Cancer is caused by the excessive proliferation of normal cells, which causes genetic instability and mutations to accumulate within cells and tissues, transforming them into malignant cells. Radiation, smoking, nicotine, toxins in drinking water, food, air, chemicals, certain metals, and infectious agents are all potential external causes of cancer, in addition to internal ones such genetic mutations, weakened immunity, and hormone imbalances [1]. Despite significant efforts by scientists to overcome cancer, it remains difficult to effectively treat. Hair loss, exhaustion, nausea, and other symptoms are possible side effects of conventional chemotherapy, which uses chemicals to destroy cancer cells. Because of these side effects and drug resistance, it is difficult to take advantage of conventional chemotherapy for the complete treatment of cancer [2]. Nanomedicine has made significant advancements in the treatment of cancer over the past several years and is useful as a drug carrier for chemotherapeutics because of its size, shape, selective binding capability, high permeability and retention impact, surface modification, etc. This allows them to deliver drugs directly to the cancer cells while preserving healthy tissue [3]. Nanoparticles (NPs) have been explored as pharmaceutical carriers for more than three decades to increase the in vivo effectiveness of several existing anticancer molecules. The investigations conducted during 1970s explored anticancer drug-loaded liposomes [4]. NPs are widely used for the delivery of imaging agents, genes, or chemotherapeutics, exploiting their intrinsic toxicity, such as related to the release of hazardous species [5]. Inherent properties, such as antioxidant action, or activities dependent on the application of external stimuli, like hyperthermia in response to the introduction of infrared rays or magnetic fields, may account for the physicochemical characteristics that give NPs their anticancer activity [4]. Metal and metal oxide NPs are being used experimentally to directly kill tumor cells by converting applied magnetic fields into strong hyperthermia or by performing effective photodynamic therapies that can reach even internal tissues by converting in situ penetrating infrared radiation into visible light inside the tumor [6].
NPs are commonly synthesized using top-down (synthesized via size reduction) and bottom-up strategies (NPs are generated from small entities such as atoms and molecules) [7, 8]. Hazardous and poisonous chemicals, expensive laboratory equipment and infrastructure, and the ability to operate under a variety of circumstances, including high temperature and pressure, are all utilized in the various chemical and physical procedures used to create and synthesize NPs. NP synthesis is expensive, and it results in highly toxic and harmful compounds that pose a variety of biological risks. So, it is advised to create an eco-friendly process combining biological and green synthesis techniques [9].
Green synthesis of metal or metal oxide NPs involves reducing metal complexes in diluted solutions to form metal colloidal dispersions. Despite being commonly used reducing agents, sodium borohydride and hydrazine hydrate are not preferred due to their toxicity from nanoparticle contamination. Green resources including plant and microbial extracts contain compounds that can convert metal precursors into NPs. All green methods typically include combining one or more of these biological extracts with metal salt solutions. Biomolecules change the oxidation state of metal salts from positive to zero, stabilizing newly formed NPs or acting as in situ reducing and capping agents [10]. The formation of a monolayer around the NPs prevents them from aggregating together due to chemical and physical interactions. Khan et al. synthesized palladium NPs using a root extract from Salvadora persica. The extract contained polyphenols with bioreduction and stabilizing properties [11]. The quantity of natural compound present in the extract affects the size and size distribution of NPs. Khatami et al. synthesized 15-nm-sized silver NPs (Ag NPs) from dried grass. At 5 μg/mL, Ag NPs suppress cancer cell multiplication and decrease cancer cell survival by 30% [12].
Biological processes are preferred to chemical and physical ones because they are less expensive, safer for the environment, do not require extreme conditions, and do not generate harmful by-product molecules. Biological nanoparticle synthesis employing living organisms is a green technique for synthesizing NPs with desired properties. Biosynthesis allows unicellular and multicellular organisms to react [13]. Biogenic synthesis can produce large numbers of contamination free well-defined NPs. It also has a lower environmental impact than alternative physicochemical manufacturing methods [14]. Cheng et al. used zinc oxide NPs (ZnO NPs) synthesized from Rehmanniae radix (RR) as a target drug delivery vehicle to inhibit bone cancer (MG-63) cell proliferation. Increasing dosage caused apoptosis [15]. Caesalpinia pulcherrima extract-loaded Ag NPs are cytotoxic to HCT116 cell lines [16]. Artemisia turcomanica leaf extract-loaded Ag NPs were cytotoxic against normal fibroblast cells (L-929) and gastric cancer cells (AGS) [17]. Metal oxides are toxic for cancer cells and nontoxic for normal cells (Fig. 1A). Due to the presence of capping agent on metal oxide NPs, these bind to the surface of healthy and cancerous cells through electrostatic attraction. Ionic species, namely metal and oxygen radicals, are formed when the biosynthesized NPs dissociate. The elevated metal ion concentration prevents the growth of cancer cells by changing their metal cation homeostasis. Superoxide dismutase (SOD) eliminates the oxygen radical, resulting in hydrogen peroxide, which peroxisome catalases and phytocompounds then convert into water and oxygen. As a result, the superoxide radical is converted into antioxidants before reactive oxygen species (ROS) develop in normal cells [18].
ROS produced by oxygen radicals generates hydrogen peroxide, which causes oxidative stress and kills cancer cells. However, the lack of experimental evidence for these routes motivates more research into the lethal mechanism of photosynthesized metal oxide NPs in both healthy and malignant cells [19]. This article discusses the probable mechanism of action, the green synthesis of metallic NPs from plant extracts, and their anticancer effectiveness against different cancer cells. It also summarizes characterization methods and the most significant findings from recent studies.
Metallic nanoparticles in cancer treatment
The NPs have been used for many years in biomedical operations such as imaging and drug delivery [20]. Inorganic NPs and their numerous applications, such as cellular absorption, diagnostics, and therapy, have received significant attention in recent years. Most of the inorganic nanoparticle research is focused on materials such as gold, silica, and others. Together with a facilitator, the mesoporous silica bestows a very cutting-edge approach to imaging and drug release [21]. The activity of this facilitator is triggered with the help of external stimuli. Due to their outstanding optical, magnetic, and photothermal characteristics, metallic NPs are widely explored in biological imaging and targeted drug delivery. Gold NPs (Au NPs), Ag NPs, iron-based NPs, and copper NPs are some of the most often utilized metallic NPs. Since their size and surface qualities can be easily adjusted, Au NPs are utilized as drug carriers for intracellular targeting [22]. The visible light extinction behavior of metallic NPs makes it possible to track their trajectories in the cells.
Anti-human epidermal growth factor receptor 2 (HER2)-functionalized gold-on-silica nanoshells have been found to target HER2-positive breast cancer cells [23]. Clinical trials for the detection of nodal metastases using Combidex®, an iron oxide-based nanoformulation, are close to complete [24]. Iron deficiency anemia can be treated with Feraheme®, an iron oxide nanoformulation that contains ferumoxytol. This was approved by the FDA in June 2009 to treat nodal metastases in prostate and testicular cancer [25, 26]. Metallic NPs cause apoptotic, autophagic, and necrotic cancer cell death through ROS generation, caspase-3 activation, mitochondrial outer membrane permeabilization, and specific DNA cleavage [27].
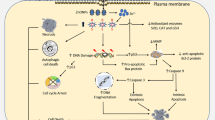
Metal nanoparticle delivery pathway and cell damage can be linked to interaction of NPs with ions in circulation, ingestion by phagocytic cells, opsonization or enzymic degradation, internalization via endocytosis, membrane perforating and damage of its components and function, chromosomal aberrations and changes in cell replication rate, lysosome rupture, mitochondria damage, lower growth rate, structural changes, and shorter lifespan of microtubules of the cytoskeleton, generation of ROS, oxidative stress, and subsequent processes (Fig. 1B). NPs of different sizes enter cells via distinct pathways. Smaller NPs penetrate cells via receptor-mediated uptake by interacting with the caveolin receptor on the cell membrane. Larger NPs are more likely to enter cells via clathrin-mediated endocytosis. When NPs enter a cell, they can proceed one of two ways: either they interact with cytosolic proteins in a direct fashion, or they are transported to the lysosome–endosome complex, where their surfaces are modified before they are released into the cytosol [27]. Inside the cell, NPs trigger up a chain reaction that releases ROS and initiates the release of metal ions. These metal ions tend to connect with proteins' SH groups, breaking their S–S bridges. As a result, the cell physiology is altered, resulting in activation of various signaling pathways that lead to programmed cell death [28]. Apoptosis is frequently induced by either intrinsic or extrinsic pathways. Nanomaterials can induce apoptotic signaling via both intrinsic and extrinsic pathways. In the case of intrinsic apoptosis, ROS production causes mitochondrial membrane depolarization, which results in the release of cytochrome C into the cytosol. This cytochrome C promotes the caspase-9/3 apoptotic cascade by activating pro-apoptotic proteases in apoptosis initiated by the extrinsic pathway (Fig. 1C) [29].
Ag NPs play a significant role in breast cancer treatment as well as skin wound healing therapy. In this review, most of the studies mentioned are about Ag NPs tested on breast cancer cell lines. For example, the IC50 value of paclitaxel is 80 g/dL, while the IC50 value of Ag NPs loaded with Elaeodendron croceum extract against MDA-MB-231 breast cancer cell line is 138.8 µg/mL [30]. Moringa oleifera flower aqueous extract-loaded Au NPs showed anticancer activity against A549 lung cancer cells. Au NPs (50 µg/mL) showed significant anticancer activity against lung cancer cell line [31]. ZnO NPs kill tumor cells through NADPH-dependent oxidative burst and apoptotic signaling. ZnO NPs of various sizes and specific surface areas had a similar effect on cytotoxicity and DNA fragmentation in macrophages of mice in an ap47phox- and Nrf2-independent manner. Because of their critical function in the modulation of immunological responses during inflammation and the clearing of inhaled particles, ZnO NPs trigger necrosis and apoptosis in macrophages. ZnO NPs promote the rapid induction of nuclear condensation, DNA fragmentation, and formation of hypodiploid DNA-containing nuclei and apoptotic bodies [32]. Furthermore, the delivery of cerium oxide NPs (CeO2 NPs) might cause DNA damage, which results in tumor cell death. CeO2 NPs enhance ROS in tumor cells, causing apoptosis without genotoxicity. The antitumor activity of CeO2 NPs is greatly dependent on their size and shape. Both small- and large-sized NPs induce DNA damage in tumor cell lines [33].
Silver nanoparticles
Due to their unique physical and chemical characteristics, such as high electrical conductivity and optical, electrical, thermal, and biological properties, Ag NPs are gaining considerable interest in the healthcare sector [36]. Ag NP aggregates enter mammalian cells via endocytosis and can cross blood–brain barrier due to their small size. After entering an endocytic vesicle, they are intracellularly transported to the cytoplasm and nucleus [37]. The antimicrobial properties of silver have been observed since ancient times. Silver is currently employed in various applications to regulate bacterial proliferation, such as in dental procedures, catheters, and the treatment of burn injuries. Ag ions and Ag-based compounds are generally recognized for their severe toxicity to microorganisms, exhibiting potent biocidal properties [38]. Ag NPs, measuring around 32.2 nm, were manufactured using an extract derived from Teucrium polium. These NPs were incorporated into a film made of polylactic acid and polyethylene glycol (PLA/PEG). The resulting film serves as a biodegradable wound dressing that possesses antioxidant and antibacterial properties. The incorporation of biogenic silver NPs into PLA/PEG nanofibers resulted in the total inhibition of growth in P. aeruginosa and S. aureus, demonstrating substantial antibacterial properties [39]. The Ag NP-loaded amorphous calcium polyphosphate NPs, which were synthesized using wet chemical precipitation, exhibited effective antibacterial activity against E. coli, Staphylococcus aureus (S. aureus), and Enterococcus faecium [40]. A recent study shown that the production of Ag NPs using a crude leaf extract of Lycium shawii exhibited a minimum inhibitory concentration (MIC) ranging from 1 mg/mL to 15 mg/mL against several microorganisms. The measured MIC clearly demonstrates the significant antibacterial properties of the produced NPs [41]. Multiple in vitro and in vivo studies have demonstrated the anticancer effects of Ag NPs, rendering them a highly promising choice for cancer therapy [42]. At a dosage of 1.0 mg/L, enzyme-responsive Ag NPs coated with adenosine triphosphate killed 56.04% of HepG2 cell line [43]. Ag NPs produced by the one-step caffeic acid-mediated reduction are anticipated to enter cells via endocytosis and effectively suppress HepG2 cell growth through apoptosis induction [44]. The aqueous extract of Panax ginseng roots was used to synthesize Ag NPs with the assistance of ultrasound. The resulting NPs exhibited an IC50 value of 157 µg/mL against the PC14 cancer cell line. In PC14 cells, the biosynthesized Ag NPs modulated the PI3K/AKT/mTOR signaling pathway and elevated ROS levels, apoptosis, and LDH release [45]. Chen et al. studied the function and mechanism of Ag NPs in prostate cancer. Ag NPs diminished lysozyme membrane integrity, number, and protease activity. This blocked autophagy. In PC-3 cell lines, sublethal Ag NP doses can produce hypoxia and energy deficiency [46].
Gold nanoparticles
Au NPs are synthesized using chemical, physical, and biological methods. Conversion of metallic gold into nano-particulate gold by chemical reduction is a common menthod for the synthesis of Au NPs. Citrate mediated reduction method has been described by Turkevich in 1951 to synthesize stable and size controlled Au NPs. Brust and Schriffin explored sodium borohydride mediated reduction to synthesize Au NPs. In 1996 Schmid et. al. described seed mediated growth, the most explored chemical method, to synthesize Au NPs [47]. Surface modification of Au NPs can be done using amine and thiol groups. This has the potential to benefit biomedical applications such as targeted delivery, imaging, and sensing for electron microscopy markers [48]. Murawala et al. synthesized Au NPs with a bovine serum albumin cap and methotrexate loading that impede MCF-7 proliferation and cause G1-S phase arrest, DNA breakage, and eventually apoptosis [49]. Gum acacia (GA) was utilized successfully to synthesize gemcitabine hydrochloride (GEM)-loaded colloidal Au NPs. Cell viability was 64.8% and 51.8% for naked GEM-treated cells at doses ranging from 0.25 to 0.5 µg/mL, respectively. GEM-GA-Au NPs decreased cell viability by 51.2% and 42.8%, respectively. GEM-GA-Au NPs exhibited superior anti-proliferation effects on MDA-MB 231 human breast cancer cells compared to naked GEM [50]. In comparison with free TA-peptide, the conjugation of Au NPs and a thioctic acid-DMPGTVLP peptide (TA-peptide) conjugate led to a more substantial release of cytochrome c after the activation of caspase-3/7. However, after intratumoral injection in tumor-bearing mice, TA-peptide Au NPs exhibited superior antitumor effectiveness compared to TA-peptide [51].
Iron oxide
Over the past 20 years, iron-based NPs have gained interdisciplinary scientific interest due to their distinctive properties and nanotechnological possibilities [52]. Iron oxide NPs (Fe3O4/Fe2O3 NPs) exhibit good superparamagnetic characteristics of Fe3O4 and Fe2O3, leading to significant performance in drug delivery applications [53]. Superparamagnetic Fe3O4/Fe2O3 NPs have attracted considerable attention because of their potential use in imaging, drug delivery, and hyperthermia management. They are non-toxic, biodegradable, and biocompatible and effectively eliminated from the human body via iron metabolism pathways [54]. Unique physical and chemical properties of Fe3O4/Fe2O3 NPs (large surface area, superparamagnetic properties, and nanoscale dimensions with a spherical form and an adjustable size of less than 50 nm) make them highly efficient [55]. Fe3O4/Fe2O3 NPs have an intrinsically therapeutic impact on malignancies [56] and resist tumor cell growth in a better way when compared to untreated control cells [57]. Sun et al. synthesized multifunctional methotrexate-loaded iron oxide NPs conjugated with chlorotoxin (a targeting ligand). Due to tumor cell cytotoxicity, these NPs may be employed in cancer diagnosis and treatment [58].
Zinc oxide
Zinc oxide nanoparticles (ZnO NPs) have emerged as a promising contender for use in biomedical research, food packaging, optical, electrical, and food processing applications. ZnO NPs are harmful to cancer cells because, at low pH levels, they decompose into Zn2+ ions. These Zn2+ ions produce ROS, which kill cancer cells. Additionally, ZnO NPs have been successfully employed as a vehicle for the precise delivery of anticancer drugs into tumor cells [59]. Wahab et al. found that ZnO NPs, when utilized at very low concentrations and in a dose-dependent manner, were effective against MCF-7 (breast cancer) and HepG2 (liver cancer) cells. At 25 µg/mL, HepG2 cell viability was below 10% [60].
Copper oxide
CuO, a well-known p-type semiconductor, has long been studied for its monoclinic structure. Cupric oxide (tenorite monoclinic CuO) and cuprous oxide (cuprite cubic Cu2O) are two crystalline forms of copper oxide [61]. Potential metal ion leaching and dissolving, as well as oxidative stress, DNA damage, lipid peroxidation, membrane damage, and mitochondrial damage have all been explored in the literature as toxicity pathways. A small amount of CuO NPs can generate large amounts of ROS such as O2, OH, and H2O2. CuO NPs cause membrane disruption and ROS generation after they enter the mitochondria [62]. Wang et al. found that CuO NPs increased the survival rate of tumor-bearing animals, inhibited the metastasis of B16-F10 cells, and significantly delayed the growth of melanoma. The data revealed that CuO NPs had minimal systemic toxicity and were promptly eliminated from the organs. When CuO NPs penetrated the cells, they preferentially targeted the mitochondria, causing cytochrome C to be released and caspase-3 and caspase-9 to be activated. Thus, CuO NPs can kill cancer cells via mitochondrion-mediated apoptosis to treat melanoma and other cancers [63].
Titanium oxide
Bioengineered titanium oxide nanoparticles (TiO2 NPs) have been shown to have good stability, chemical neutrality, hydrophilicity, oxidizing power, and electrical, optical, physical, and photocatalytic properties. Because of their powerful antibacterial and odor-removing properties, TiO2 NPs are employed in filters and cosmetics. TiO2 photocatalysts have been extensively explored for the killing or suppression of bacterial growth due to their excellent chemical stability and nontoxicity [64]. Plant extracts may exhibit properties of the metals or metal oxides that make up their composition in addition to the presence of phytoconstituents. These features may ultimately result in their many critical activities in the prevention or treatment of cancer. It has been shown that the tiny size of TiO2 NPs gives them potent anticancer activity against cancer cells [65].
Biological synthesis of metallic nanoparticles
Over the past decade, there has been an increase in efforts to discover efficient, low-cost, eco-friendly, and long-lasting strategies for producing green NPs [66]. Researchers across the globe are interested in green synthesis since it is an environmentally safe technique and a fascinating study topic for the synthesis of metallic NPs for biomedical applications [67]. Using biological agents to make NPs of various sizes, shapes, compositions, and physicochemical characteristics is safe, non-toxic, and environmentally sustainable [68]. Biosynthesis of these NPs is done at mild pH, pressure, and temperature without using an external reducing agent, capping agent, or stabilizing agent [69]. Capping agents have a significant role in the synthesis of metallic NPs. The major role of capping agent is to functionalize and stabilize the NPs, along with controlling size and morphology [70]. NPs synthesized by green synthesis methods are exceptionally stable, well dispersed, and have a narrow size distribution [71].
Nucleation and production of stable metallic NPs during biological synthesis are affected by several factors such as temperature, reactant concentrations, pH, and reaction time. For example, when employing biomass from Avena sativa (oats) at pH 2, rod-shaped Au NPs were larger, ranging from 25 to 85 nm. In contrast, at pH 3 and 4, the Au NPs were comparably smaller, with sizes ranging from 5 to 20 nm. The functional groups present in extract were more easily accessible for particle nucleation within the pH range of 3 to 4. In contrast, a reduced number of functional groups were present at pH 2, leading to the aggregation and formation of bigger Au NPs [72]. Ag NPs were synthesized using bark extract derived from Cinnamon zeylanicum. The particle yield exhibited a positive correlation with the concentration of the bark extract, whereas the NPs assumed a mostly spherical morphology at pH values of 5 and higher [73]. Prathna et al. found that the combination of Ag(NO)3 with Azadirachta indica leaf extract led to the formation of progressively bigger particles as the reaction time increased. By adjusting reaction time from 30 to 240 min, the particle size changed from 10 to 35 nm. The concentration of NPs produced at various stages of reaction was determined using inductively coupled plasma optical emission spectroscopy measurements. After 2 h, the yield of the process had significantly increased to 78%. Subsequently, there was a progressive and continuous increase in the yield [74]. The photosynthesized NPs are safer than their chemically synthesized counterparts for usage in healthcare applications since they do not contain any harmful contaminants. When applying metal in healthcare products, safety risks related to nanosize, penetration, and tissue permeability must be considered. In vitro cytocompatibility of phytonanoparticles has been documented by multiple researchers. Extensive research is still required to determine their pharmacodynamics, immunogenicity, absorption, biodistribution, excretion, and acute and chronic toxicity. Although plant-mediated nanoparticle production is often considered an environmentally beneficial approach, there is still a dearth of evidence addressing the direct and indirect ecological impacts of these particles [75].
Green synthesis using plant extract
Plants, which include grasses, ferns, trees, bushes, flowers, and other varieties of green algae and lichens, are among the most essential forms of life. NPs can help plants by acting as fertilizers, pesticides, growth regulators, and antibacterial agents. However, flora can also assist with the development of nanotechnology. Plants can be used to produce NPs in two different ways: directly through extraction or indirectly via plant-mediated biosynthesis. Agriculture, food science, nanotechnology, and pharmaceutical science are just some of the fields that could benefit from a better understanding of the interaction between NPs and plant extracts [76]. Plant-mediated green NP synthesis is one of the most preferred approaches because it normally requires a neutral pH and can occur at room temperature [77]. Plants and plant extracts are sustainable and renewable resources for NP production, unlike prokaryotic bacteria, which require expensive methods for maintaining microbial cultures and downstream processing [7]. Using the various plant parts (Fig. 2A), such as fruits, seeds, calluses, stems, peels, leaves, and flowers, biological processes synthesize metal NPs in a range of sizes and shapes. Metal NPs are synthesized using metal precursors and plant extracts as reducing and capping agents under suitable conditions [78]. A green chemistry approach for the synthesis of metal NPs can be achieved in three stages: (i) the activation phase, in which the phytoconstituents reduce the metal ions, followed by the nucleation of reduced metal atoms; (ii) the growth phase, in which small NPs join to form larger NPs; (iii) the termination phase, in which the NPs take on their final shape [79]. Bioactive alkaloids, phenolic acids, polyphenols, proteins, carbohydrates, and terpenoids in plant extracts reduce and stabilize metallic ions (Fig. 2B) [80].
Green synthesis of nanoparticles: A different plant parts used for nanoparticles formation, B general method for biosynthesized nanoparticles formation, C different microorganisms used for nanoparticles formation, D different shapes of nanoparticles that can be synthesized, and E possible mechanism of nanoparticle synthesis using microbes (Figure E: recreated with permission
Tabebuia berteroi leaf extract is rich in polyphenol, and Withania coagulans plant extract is rich in flavonoids, tannins, and phenolics. These extracts have been used to reduce Fe and Pd ions to form their respective metal NPs, which are then mixed with graphene oxide to make a nanocomposite [81]. Anisomeles indica leaf extract reduced silver ions to spherical Ag NPs (50–100 nm) in 10 min at room temperature [82]. Lee et al. reported Au NPs synthesis utilizing sequential fractional extracts from Ocimum sanctum leaves. For the sequential fraction extraction of O. sanctum leaves, different polarity solvents (hexane, chloroform, n-butanol, and water) were utilized, and it was discovered that unique solvent fractions (extract) are responsible for the creation of morphologically varied Au NPs. Water extract produced anisotropic NPs, hexane extract produced spherical Au NPs, chloroform extract produced a circular disk-shaped structure with rough edges, and n-butanol extract produced Au NP aggregates [83]. Possible mechanisms of the anticancer effect of phytosynthesized metal or metal oxide NPs include the formation of pro-apoptotic caspases, activation of reactive oxygen species, damage to cell membranes and mitochondria, damage to DNA, and DNA fragmentation [84]. Table 1 represents various plant extracts utilized in the synthesis of metallic NPs along with their corresponding applications.
Biosynthesis of nanoparticles using microbes
Actinomycetes, bacteria, fungi, marine algae, viruses, and yeasts have all been effective in the production of NPs employing unicellular and multicellular organisms (Fig. 2C) [113]. These organisms can produce reducing and stabilizing agents in the synthesis of NPs with a wide range of shapes, compositions, and physiochemical properties (Fig. 2D) [114]. Microorganisms can synthesize metallic NPs both intracellularly and extracellularly (Fig. 2E) [115]. To produce NPs extracellularly, microbes are cultivated in the appropriate environments. The microorganism-containing enzyme-rich broth is centrifuged to synthesize NPs [116]. The intracellular synthesis of NPs is carried out via the cellular mechanism of microbial cells [117]. Several studies explored extracellular methods for producing metal NPs [118]. Kalimuthu et al. investigated the role of the nitrate reductase enzyme in the production of Ag NPs by Bacillus licheniformis. The enzyme is responsible for converting Ag+ to Ag°, and they hypothesized that nitrate ions might play a role in its induction. Cofactors like NADH in NADH-dependent nitrate reductase enzymes are required for generating metal NPs. Bioreduction of Ag+ to Ag° may be caused by the release of cofactors NADH and NADH-dependent enzymes by B. licheniformis, in addition to other components, especially nitrate reductase [119]. Pseudomonas stutzeri was treated with a concentrated Ag NO3 solution to produce Ag NPs in the periplasm [120]. Numerous fungi strains have been described for the extracellular biofabrication of NPs using CdS, Au, Zirconia, Si, Ti, and magnetite [121]. The method of NP production varies depending on the microorganisms. However, the basic concept involves microorganisms entrapping metal ions on their surface or within their cells, followed by their reduction to NPs. Electrostatic forces are frequently used as a trapping medium [122].
Bacteria
Bacteria can precipitate metals at nanoscale level as well as regulating interaction pathways for metal ion reduction [123]. E. coli biosynthesized Ag NPs of 50 nm size by a reliable and cost-effective approach [124]. Pseudomonas aeruginosa (P. aeruginosa) and other species have been studied for their ability to synthesize Zn, iron, nickel, Au, and Ag NPs [125]. The NADPH-dependent reductase enzyme may be involved in the reduction of Au3+ to Au° and stabilization via capping molecules in the presence of Stenotrophomonas maltophilia [126]. Different bacterial species, as listed in Table 2, have been employed to produce metal NPs.
Fungi
Fungi are non-phototrophic eukaryotic microorganisms with rigid cell walls [147]. Fungi are among the biological sources that are exploited in bioremediation, can mineralize, and are known as mediators in the synthesis of NPs. This is due to their ability to create huge volumes of biomass [148]. Fungi can reduce the size of metal ions to NPs via two different processes: (i) through the contact of the fungus biomass with the metal inside the fungal cell and (ii) through the interaction of the fungus filtrate with the mineral solution outside the fungal cell [149]. This whole process is accomplished through two distinct mechanisms. First, the fungal cell wall traps metal ions on its surface due to the electrostatic interaction of the positively charged groups in the enzymes, and then, the cell enzymes reduce the metal ions to produce NPs. Second, the method involves the reduction of nitrates using NADPH secreted by fungi, followed by its conversion into NADP to produce extracellular NPs [150]. The myogenic pathway produces better NPs than bacteria and plants because they accumulate metals more efficiently. Triangle-shaped intracellular Au NPs (20–35 nm) synthesized by Aspergillus clavatus isolated from Azadirachta indica have been explored to demonstrate mycosynthesis [151]. Phoma glomerata can be used to synthesize Ag NPs that are antibacterial against resistant strains of E. coli, P. aeruginosa, and S. aureus [152]. Trichoderma viride, Chaetomium globosum, Aspergillus niger, and Pleurotus ostreatus can produce selenium NPs [153]. Cladosporium perangustum aqueous extract-derived Ag NPs decreased MCF-7 cell viability by increasing caspase-3, caspase-7, caspase-8, and caspase-9 expression [154]. Metallic NPs derived from various fungi are presented in Table 3.
Algae
Algae have the capacity to accumulate heavy metal ions. These aquatic microorganisms have been explored to synthesize NPs [176]. Using Tetraselmis kochinensis, spherical Au NPs with sizes ranging from 5–35 nm were produced intracellularly [177]. Sargassum polycystum (a brown algae)-based CuO NPs had excellent anticancer characteristics and great potential against pathogenic bacteria [178]. Priyadharshini et al. used Gracilaria edulis (macroalgae) extract to synthesize Ag and ZnO NPs. The synthesized NPs exhibited excellent antitumor activity against human PC3 cells [179]. Hypnea musciformis (Wulfen), a red macroalga, has been explored to synthesize Au NPs for its anti-fungal activity against Aspergillus niger and Mucor spp [180]. Ecklonia cava extract-loaded Ag NPs have shown significant anti-bacterial activity against E. coli and S. aureus. These NPs had antioxidant properties and anti-cancer activity against human cervical (HeLa) cells [181]. Numerous algal components and precursor salts employed in the synthesis and capping of metallic NPs are shown in Table 4.
Green-synthesized metallic nanoparticles for cancer treatment
Cancer is defined as an abnormal growth of tissue or cells characterized by uncontrolled autonomous division, with the number of cell divisions rising over time [4]. More than 200 distinct cancers have been identified, and they have six common basic characteristics: replicative immortality; the ability to generate new blood vessels; the ability to invade and spread to other organs; resistance to apoptosis; proliferative signaling; and evasion of growth [198]. NPs are used to increase the compatibility and bioavailability of natural bioactives for the treatment of different chronic disorders, including cancer [199]. NPs are often considered as a possible solution for this due to evidence of their ability to induce the apoptotic pathway in vitro, which implies their anticancer effect [200]. NPs have been demonstrated to produce ROS, which can activate pro-apoptotic pathways. Different biogenic metal oxide NPs have shown promising results in the treatment of cancer by producing cytotoxicity in malignant cells while having no effect on normal cells. The specific mechanism by which various metal and metal oxide NPs kill cancer cell types is unclear [201]. It is widely believed that mitochondrial signaling pathways play a crucial role in NP-based activation of apoptosis in cancer cells. Metallic NPs usually produce ROS, which causes oxidative stress and apoptosis [202]. Apoptosis begins with apoptotic protein activation, DNA damage, mitochondrial breakdown, apoptosome formation, and cell shrinkage [203]. Prostate and lung cancer cells are sensitive to cytotoxic activities in Pinus roxburghii bioactive-loaded Ag NPs. The ability of mitochondrial depolarization and DNA damage to trigger apoptosis via the intrinsic route have been reported. ROS, cell cycle arrest, and caspase-3 activation cause cancer cell apoptosis [204]. A class of protease enzymes called caspases is important for the apoptotic process. By activating the executioner caspase-3 through cleavage, the initiators caspase-8 and caspase-9 specifically cause the proteolysis of poly(ADP-ribose) polymerase (PARP) and apoptosis by impairing DNA repair [205].
Breast cancer
Breast cancer has surpassed lung cancer as the most frequent cancer in the world, with 2.26 million recorded cases per year, 11.7% of all cancer cases, and 24.5% of malignancies in women. Furthermore, it is the most frequent disease among women, accounting for 15.5% of all female cancer mortality each year [206]. With an emphasis on more biologically directed medicines and treatment deescalation to lessen side effects, therapeutic approaches have evolved over the past 10–15 years to take this heterogeneity into consideration [207]. Capping ZnO NPs with R. fairholmianus inhibited cellular development while increasing cytotoxicity and ROS. Apoptosis was also accompanied by an increase in pro-apoptotic proteins (p53, Bax), a decrease in anti-apoptotic proteins (Bcl-2), and a marked elevation in cytoplasmic cytochrome c and caspase 3/7 (apoptosis indicators) [208]. Calendula officinalis leaf extract was used as a green reducing and stabilizing agent in the biosynthesis of Sn NPs. These NPs showed remarkable potential in breast cancer treatment. The IC50 values for biosynthesized Sn NPs were 132, 126, and 119 µg/mL for the MCF7, Hs 319.T, and MCF10 cell lines, respectively [209]. Gloriosa superba rhizome extract was used in the synthesis of biomolecule-coated nanotitania catalysts. For the MCF-7 (cancer) and L929 (normal) cell lines, the IC50 of nanotitania catalysts was 46.64 and 61.81 µg/mL, respectively. Figure 3A shows that when nanotitania catalysts (46.64 µg/mL) were added to MCF-7 cells, they made a lot more intracellular ROS than control cells. This demonstrated that metal and metal oxide nanoparticle exposure increased ROS levels and decreased mitochondrial membrane potential. This suggested that the NPs induced apoptotic cell death. ROS generation can stimulate cell death by apoptosis and necrosis [210].
A Effects of Gloriosa superba rhizome and Gloriosa superba rhizome extract mediated titanium dioxide nanoparticles on intracellular ROS generation in treated MCF-7 cells, shown as follows: (a) nanotitania catalyst-treated cells, (b) rhizome extract, (c) control cells, B anticancer activity of CuO NPs synthesized from S. alternifolium stem bark extract, C Relative mRNA expression percentages following treatment of MCF7 cells with Z. mauritiana fruit extract-mediated Ag/AgCl NPs. A dashed line denotes an expression level of 1.0, D Pt NPs containing P. granatum stained by propidium iodide (a) control; (b) IC50 molarity (25 µg/mL); (c) maximum molarity (100 µg/mL). (A: under copyright (CC BY) from Taylor and Francis, B: under copyright (CC BY) from Springer, C: under copyright (CC BY) from ACS publication, and D: under copyright (CC BY) from Elsevier)
Yugandhar et al. reported that Syzygium alternifolium bark extract-loaded CuO NPs reduced treated cell lines by 50% in comparison with untreated cell lines with an IC50 value of 50 µg/mL (Fig. 3B) [211]. Artabotrys hexapetalus leaf extracts loaded CeO2 NPs potent cytotoxicity against MCF-7 cancer cells at an IC50 value of 48 0.05 µg/mL [212]. Al-Nuairi et al. used MTT assay to examine the effects of Ag NPs from Cyperus conglomeratus root extract on MCF-7 breast cancer cells and normal fibroblasts. The selective cytotoxicity was found against MCF-7 with an IC50 of 5 μg/mL [213]. Kabir et al. treated MCF-7 cells for 48 h with Zizyphus mauritiana fruit extract-loaded Ag/AgCl NPs. A real-time polymerase chain reaction (PCR) was used to monitor the expression levels of eight apoptosis-related genes. FAS, caspase-8, and FADD expression levels were increased, and PARP expression levels were decreased (Fig. 3C) [214]. To confirm the antiproliferative activity of P. granatum crust extract-loaded platinum NPs (Pt NPs), Sahin et al. examined nuclear densification and apoptotic alterations using the propidium iodide staining in MCF-7 cell line. Only a few control cells responded positively to propidium iodide. A progressive increase in the proportion of cells that responded favorably to propidium iodide was seen in the cells treated with 25 µg/mL of Pt NPs after 48-h exposure (Fig. 3D) [215]. Table 5 shows various plant extracts and precursor salts explored to synthesize metallic NPs for their breast cancer applications.
Lung cancer
Lung cancer is the sixth-leading cause of mortality worldwide [226]. With estimated yearly occurrences of 2.21 million (11.4% of cancer cases) and a mortality rate of 1.79 million lung cancer patients per year, lung cancer is still prevalent in all nations. Lung cancer is the second-leading cause of death in women after breast cancer [227]. Because of the long-term adaptation of cancer-causing behaviors including smoking, physical inactivity, and westernized diets, the global incidence of lung cancer is quickly rising [228]. Au NPs containing leaf extract of Alternanthera bettzickiana reduced cancer cell growth and triggered apoptosis, DNA breakage, and altered mitochondrial membrane potential in lung cancer cell lines. Au NPs had an impact on cellular M-phase entry. Au+ may activate p53 and other cell cycle genes, delaying the entry of cells into the M-phase and increasing apoptosis [229].
Cleistanthus collinus extract loaded into Ag NPs has been explored as a reducing and capping agent. The scavenging of free radicals was significantly impacted by the in vitro antioxidant activity of Ag NPs. The IC50 for human lung cancer cells (A549) and normal cells (HBL-100) was 30 µg/mL and 60 µg/mL, respectively. Ag NPs do not have any adverse effects on mice organs [230]. A549 cells exposed to 50 µg/mL Magnolia officinalis extract-loaded Au NPs showed substantial cell death. TUNEL and DAPI staining of A549 lung cancer cells Magnolia officinalis extract-loaded Au NPs confirmed ROS-arbitrated apoptosis (Fig. 4A). The TUNEL assay stained with green fluorescence showed live cells. Magnolia officinalis loaded Au NPs confirmed increased apoptotic cells with DAPI staining with blue fluorescent cells [231]. MTT assay to test the anti-lung cancer activity of Ledebouria revoluta bulb extract-loaded TiO2 NPs showed an IC50 value of 53.65 µg/mL and showed improved antitumor activity against A549 cells [232]. At 100 µg/mL, biogenic Au NPs showed that Vero cells remained alive; however, these NPs were cytotoxic (IC50 60 µg/mL) against A549 lung cancer cells (Fig. 4B) [233]. The proliferative activity of A549 cells gradually decreased over time in proportion to the increasing concentration of the test substance during the biological synthesis of Pt NPs using Ononidis radix extract. The mortality of cells cultured with platinum NPs increased [234]. Lonicera japonica extract-loaded Ag NPs at 75 µg/mL concentration showed 52% cell viability (Fig. 4C) [235]. In a xenograft severe combined immunodeficient mouse model, H1299 tumor growth was inhibited by Ag NPs synthesized from longan peel powder. After 36 days of treatment, the lung tumor size was 1.13 ± 0.21 mm2 and 0.49 ± 0.07 mm2 in the control and Ag NP-treated groups, respectively [236]. Valodkar et al. conducted in vitro toxicity research on human lung cancer cells using plant latex-capped Ag NPs. At the higher dose, more dead cells (in red) and very few live cells (in green) were seen, indicating a dose-dependent mortality of the cells ranging from 20 to 80% (Fig. 4D) [237]. Table 6 shows various plant extracts and precursor salts explored to synthesize metallic NPs for their lung cancer applications.
A By using DAPI/TUNEL dual staining, Au NPs synthesized by Magnolia officinalis were determined to increase ROS-arbitrated apoptosis. B The cytotoxicity of biogenic Au NPs using bael fruit juice was studied against A549. C Anti-cancer ability of synthesized silver nanoparticle (Ag NPs) using phytochemical rich medicinal plant Lonicera japonica proved against A549 lung cancer cells by cell viability assay. D AO/EB staining of plant latex-capped silver nanoparticles in A549 (cells more dead cells (in red) and very few live cells (in green) in highest dose of LAgNP) exposed to (a) 0 μg/mL LAg NPs, (b) 1 μg/mL LAg NPs, (c) 10 μg/mL LAg NPs, (d) 20 μg/mL LAg NPs,(e) 50 μg/mL LAg NPs and (f) 100 μg/mL LAg NPs (A: under copyright (CC BY) from Taylor and Francis, B, C and D under copyright (CC BY) from Elsevier)
Cervical cancer
About 604,127 new cases and 341,831 fatalities from cervical cancer are reported in 2020 [253]. Solanum nigrum leaf extract-loaded ZnO NPs inhibited β-catenin, increased the levels of p53, caspase-3, and caspase-9, and showed a dose-dependent cytotoxic effect against HeLa cell lines [254]. After 24 h of treatment, Catharanthus roseus extract (5 µg/mL) loaded in Au NPs induced apoptosis in HeLa cells dual stained with acridine orange (AO)/ethiduim bromide (EtBr). The control cells showed homogeneous bright green nuclei and cytoplasm for AO-positive cells. In Ao/EtBr staining, the cells treated with the synthesized NPs showed characteristics of apoptosis such as nuclear condensation, cell shrinkage, and the formation of apoptosis bodies (Fig. 5A). HeLa cells were incubated with Catharanthus roseus extract-loaded Au NPs at different concentrations (5 and 10 µg/mL) for 24 h to measure the level of ROS production (Fig. 5B) [255]. A. officinalis root extract-loaded Ag NPs are toxic to SiHa cell lines, with an IC50 of 44 µg/mL. The biosynthesized Ag NPs arrested cell division in the G2/M phases and accelerated the cell cycle in the G1 and S phases [256]. Extract of Euphorbia antiquorum L. latex loaded in Ag NPs inhibited the growth of HeLa cell line with an IC50 value of 28 µg/mL [257]. After 24 h of incubation, Au NPs containing an aqueous extract of Alternanthera sessilis (1–15 µg/mL) showed cytotoxicity against HeLa cells (Fig. 5C) [258]. CuO NPs containing dry black beans (0.5 µg/mL and 1 µg/mL) have shown cytotoxic effects against HeLa cells. CuO NPs inhibited cervical carcinoma colonies and influenced the generation of ROS. The number of cervical carcinoma cell colonies was much lower in CuO NPs-treated cells than in the control group (Fig. 5D) [259]. Table 7 shows various plant extracts and precursor salts explored to synthesize metallic NPs for their cervical cancer applications.
A Ao/EtBr staining after 24 h of treatment with various concentrations (5 and 10 µg/mL) of photosynthesized Au NPs from Catharanthus roseus. B Using DCFH-DA staining assay photosynthesized Au NPs from Catharanthus roseus induces ROS production in HeLa cells. C The ability of Au NPs from A. sessilis to cause cytotoxicity in HeLa cervical cancer cell lines. D Clonogenic survival assay on HeLa cells following incubation with CuO NPs synthesized using an aqueous black bean extract NPs (A, B and C under copyright (CC BY) from Taylor and Francis, D. under copyright (CC BY) from Elsevier)
Colorectal cancer
Colorectal cancer (CRC) is the second most lethal and third-most prevalent cancer worldwide. It accounts for 9.2% of all cancer-related deaths and 10.2% of all new cases. Aqueous extract of Allium cepa loaded in Ag NPs promotes apoptosis by suppressing expression of Bcl2 family genes [267]. Albizia lebbeck extract (40 and 60 µg/mL)-loaded CuO NPs showed early apoptosis (orange stained) and late apoptosis (red stained) apoptotic cells (Fig. 6A) for 24 h through the activation of a dual staining method by AO/EtBr in HCT-116 colon cancer cells [268]. Ag NPs containing Pimpinella anisum seed extract showed cytotoxicity against CRC cells. Ag NPs destroyed cancer cells through cell growth inhibition, cell cycle arrest in the G2/M phase, and induction of apoptosis [269]. AO/EtBr staining assay in HCT-116 cells showed that the Trichosanthes kirilowii extract loaded in Au NPs increased ROS production, damaged mitochondrial membrane, induced morphological alterations (Fig. 6B), induced G0/G1 phase cell-cycle arrest (Fig. 6C), activated caspase expression, and downregulated anti-apoptotic expression [270]. The cytotoxic effect of lead oxide and CeO2 NPs synthesized using an aqueous extract of Prosopis fracta fruit was investigated in colon (HT-29) cancer cell lines. These NPs were not harmful at 500 µg/mL and 62.5 µg/mL [271]. Ag NPs containing Curcuma longa and Zingiber officinale rhizomes extract had an IC50 of 150.8 µg/mL. At a 25–500 µg/mL dose, the synthesized NPs were cytotoxic to HT-29 cells (Fig. 6D) [272]. Table 8 shows various plant extracts and precursor salts explored to synthesize metallic NPs for their colorectal cancer applications.
A Dual staining method by AO/EtBr in HCT-116 colon cancer cells. B Induction of apoptosis on HCT-116 cells treated with Au NPs synthesized from Trichosanthes kirilowii at various concentrations upto 24 h studied using AO/EB staining assay. C Cell-cycle analysis of HCT-116 cells treatment with Au NPs (15 and 20 µg/mL) synthesized from Trichosanthes kirilowii. D Anticancer activity of Zingiber officinale and Curcuma longa synthesized Ag NPs at different concentrations (a) control, (b) 500 µg/mL, (c) 250 µg/mL, (d) 100 µg/mL, (e) 50 µg/mL and (f) 25 µg/mL (A, B and C under copyright (CC BY) from Taylor and Francis, D under copyright (CC BY) from Elsevier)
Prostate cancer
Prostate cancer is the second most common cancer in men, with 1.41 million incidences annually (14.1% of all cancer cases in men) [275]. Green-synthesized nanosilver containing Rosmarinus officinalis extract exhibited cytotoxic effects against prostate cancer cells through the activation of caspase 3 and caspase 9 mRNA [276]. Firdhouse et al. examined the cytotoxic effect of nanosilver containing Alternanthera sessilis extract against prostate cancer cells (PC3) at 1.56, 3.12, 6.25, 12.5, and 25 µL/mL doses. The highest concentration (12.5 and 25 µL/mL) of Ag NPs showed a decrease in PC3 cancer cells (Fig. 7A) [277]. Camellia sinensis L extract loaded in Au NPs reduced PC-3 cell growth with an IC50 of 19.71 µg/mL. Surface detachment, cell shrinkage, and body distortion were observed in PC-3 cells. This demonstrated the cytotoxic effect of green tea extract containing Au NPs [278]. The cytotoxic effect of Au NPs containing an extract of desert truffles (Tirmania nivea) against normal human prostate cell lines and prostate cancer cell lines is shown in Fig. 7B [279]. Salvia miltiorrhiza extract has been used as a capping agent to synthesize Ag NPs to explore its cytotoxic property against PCa LNcap cell lines. The proliferation of LNcap cells was dramatically inhibited for 24 h with increasing concentration of Ag NPs (Fig. 7C) [280]. The IC50 for the PC3 cell line treated with green-synthesized ZnO NPs made from Hyssopus officinalis extract for 24 h and 48 h was 8.07 µg/mL and 5 µg/mL, respectively. The percentage of PC3 cells that underwent induced apoptosis was 26.6% ± 0.05, 44% ± 0.12, and 80% ± 0.07 [281]. The Trypan blue exclusion test was used to assess in vitro cytotoxicity in PC-3 cells. Ag NPs of Dimocarpus longan Lour. peel extract showed cytotoxic effect at a dose between 5 to 10 µg/mL with an IC50 value less than 10 µg/mL (about 50% of PC-3 cells died) (Fig. 7D) [282]. Table 9 shows various plant extracts and precursor salts explored to synthesize metallic NPs for their prostate cancer applications.
A Cytomorphological changes such as cancer cell membrane lyses, coiling with the addition of silver (a, b) and nanosilver synthesized using Alternanthera sessilis in (d, e) after 48 h compared to that of control. B The cytotoxicity of Au NPs synthesized using extract of desert truffles (Tirmania nivea) against normal human cell line (a) untreated cells, (b) treated cells with synthesized Au NPs, and against cancer cell line (c) control untreated VCaP cells, (d) Treated VCaP cells with synthesized Au NPs. C Cytotoxic potential of Ag NPs from leaf extract of Salvia miltiorrhiza in prostate cancer LNcap cell. D Dose-dependent cytotoxic effects of Ag NPs biosynthesized using Dimocarpus Longan Lour. Peel Extract on prostate cancer PC-3 cells in vitro (A and D under copyright (CC BY) from Springer, B under copyright (CC BY) from Elsevier, C under copyright (CC BY) from Taylor and Francis)
Skin cancer
In a study by Wu et al., aqueous Siberian ginseng extract was used as an organic reducing agent to biosynthesize Au NPs. These Au NPs were then tested against murine melanoma B16 cells for their anticancer properties. The results demonstrated that the synthesized Au NPs increased ROS levels and decreased mitochondrial membrane potential (Fig. 8A). The BH3 mimics by biosynthesized Au NPs increased the expression of pro-apoptotic proteins while decreasing the expression of anti-apoptotic proteins in melanoma cells [287]. Cassia fistula leaf extract reduced silver ions to Ag NPs. The estimated IC50 values for the leaf extract, Ag NPs, and AgNO3 were 96.36 1.01 µg/mL, 92.207 1.24 µg/mL, and 84.246 2.41 µg/mL, respectively. The percentage cell viability in Fig. 8B shows the dose-dependent effect of synthesized Ag NPs against cancer cell line [288]. The in vivo therapeutic efficacy of Quisqualis indica flower extract-derived Cu NPs was investigated by Mukhopadhyay et al. in mice carrying B16F10 melanoma tumors. A substantial reduction in tumor development was recorded. Quisqualis indica flower extract-derived Cu NPs triggered cytotoxicity and death in melanoma cells due to the gene expression [289]. In a study, polyphenols from Vitis vinifera L. (grape) peels were used as reducing and stabilizing agents for the synthesis of Au NPs. The IC50 value for V. vinifera peel extract was 319.14 µg/mL. The IC50 values of V. vinifera peel loaded Au NPs and fluorouracil (standard drug) were 23.6 µM and 23.43 µM, respectively. Figure 8C displays the percentage of inhibition plotted against the concentration of fluorouracil and peel extract-loaded Au NPs [290]. Ag NPs of Indigofera longeracemosa leaf extract revealed a dose–response relationship with an IC50 value of 48 µg/mL against the human skin cancer cell line SK MEL-28. Biosynthesized NPs upregulated the tumor suppressor gene p53 and significantly downregulated the anti-apoptotic gene Bcl-2 [291]. Elephantopus scaber-loaded Ag NPs were more effective against the A375 skin carcinoma cell line than its pure extract. After 48 h of incubation, morphological changes in treated A375 cells were observed under an inverted phase contrast tissue culture microscope (Fig. 8D) [292]. Table 10 shows various plant extracts and precursor salts explored to synthesize metallic NPs for their skin cancer applications.
A Using a 1-mM Rhodamine 123 staining approach, the apoptotic effect of Siberian ginseng synthesized Au NPs on the mitochondrial membrane permeability in murine melanoma cell line B16 was evaluated. B Ag NPs from the leaf extracts of Cassia fistula have been shown to be toxic to A-431 epidermal cancer cells (IC50 values for the leaf extract, Ag NPs, and AgNO3 are anticipated to be 96.36 ± 1.01, 92.207 ± 1.24, and 84.246 ± 2.41 µg/mL, respectively). C Fluorouracil and Vitis vinifera peel Au NPs had an inhibitory effect on A431 cells 24 h after incubation. D Morphological changes induced on treated A375 cells by Ag NPs using the phytoreducing agent Elephantopus scaber (A and D under copyright (CC BY) from Taylor and Francis, B under copyright (CC BY) from Wiley Online Library, C under copyright (CC BY) from Elsevier)
Liver cancer
Liver cancer is the sixth-most common primary malignancy and the fourth-leading cause of cancer-related deaths in the world. Cholangiocarcinoma (CCA) and hepatocellular carcinoma (HCC) are the two most common histologic types of primary liver cancer, accounting for more than 80% of all cases. Liver fibrosis and inflammation-induced necrosis are the major causes of malignancy [296]. The effect of Cordyceps militaris-loaded Au NPs on the mitochondrial membrane potential of HepG2 cells revealed a strong green fluorescence in untreated cells with high membrane potential. Green fluorescence intensity decreased in HepG2 cells treated to 10 and 12.5 µg/mL Au NPs. The mitochondrial membrane potential remained intact in untreated cells (Fig. 9A). Untreated cells did not exhibit apoptosis, as shown by green fluorescence staining in Fig. 9B. HepG2 cells treated with Au NPs (10 and 12.5 µg/mL) showed a substantial increase in apoptotic cells as visualized by orange fluorescence staining. HepG2 cells were found to undergo apoptosis after being exposed to Au NPs coated with an extract of Cordyceps militaris [297]. Coriandrum sativum leaf aqueous extract-loaded iron NPs were green-synthesized by Zhan et al., and they demonstrated dose-dependent anticancer activity and very poor cell viability against LMH/2A, McA-RH7777, N1-S1 Fudr, and SNU-387 cell lines while having no cytotoxicity on the normal cell line (HUVEC) [298]. The MTT assay was used to test the in vitro cytotoxicity of Ag NPs loaded with extract from the Punica granatum leaf against the HepG2 cell line. This study found that Ag NPs had substantial anti-cancer efficacy at a dosage of 70 µg/mL, causing 50% cell death (Fig. 9C). Ag NPs significantly inhibited cell growth by more than 90% [299]. With an IC50 value of 93.75 µg/mL, Morinda pubescens extract-loaded Ag NPs have been shown to exhibit considerable cytotoxic effect against HEPG2 cell lines [300]. In a time- and dose-dependent manner, Artemisia scoparia extract and its biosynthesized ZnO NPs decreased cell proliferation and induced apoptosis in Huh-7 cancer cells. Artemisia scoparia extract and its biosynthesized ZnO NPs had IC50 values of 10.26 and 310.24 µg/mL, respectively. Figure 9D shows that the anti-apoptotic genes were downregulated while the pro-apoptotic genes were upregulated by the Artemisia scoparia extract-loaded ZnO NPs [301]. With an IC50 value of 62.5 µg/mL, Seripheidium quettense-mediated green synthesis of biogenic Ag NPs inhibited the proliferation of HepG2 cells [302]. Table 11 shows various plant extracts and precursor salts explored to synthesize metallic NPs for their liver cancer applications.
A Represents that the mitochondrial membrane permeability of Cordyceps militaris extract synthesized Au NPs, B effect of Au NPs from C. militaris induces apoptotic morphological changes in HepG2 cells. Green fluorescence labeling revealed that apoptosis had not occurred in the untreated cells. Orange fluorescence staining in HepG2 cells at 10 mg and 12.5 µg/mL shows that the Au NPs treatment dramatically boosted the apoptotic cells. C anticancer activity of various concentrations of synthesized Ag NPs synthesized using Punica granatum leaves against the liver cancer cell line—HepG2, and D cytotoxic effects of biosynthesized ZnO NPs using Artemisia scoparia leaf extract against Huh-7 liver cancer cells (under copyright (CC BY) from Taylor and Francis online)
Theranostic applications of green-synthesized nanoparticles
Theranostics is a multidisciplinary scientific field focused on creating a wide range of complex diagnostic and therapeutic agents. By utilizing nanotechnology, theranostics enhance bioavailability by delivering bioactives to the sites of absorption. Theranostics utilizing metallic NPs could be useful in treating a wide range of conditions, including cancer, malaria, microbial infections, and cardiovascular disorders [308]. There has been a significant increase in the production of metallic NPs from medicinal plants. These metallic NPs play a crucial role in the advancement of theranostics. Anisotropic Au NPs were produced by an aqueous method employing cocoa extract. These NPs exhibited favorable biocompatibility when subjected to in vitro testing utilizing A431, MDA-MB231, L929, and NIH-3T3 cell lines, at doses of up to 200 μg/mL. The use of green-synthesized NIR absorbing anisotropic Au NPs was effective in causing cell death in epidermoid carcinoma A431 cells when irradiated with a femtosecond laser at 800 nm with a low power density of 6 W/cm2. This demonstrates the suitability of NPs for photothermal ablation of cancer cells. These Au NPs exhibited high X-ray contrast during computed tomography testing, thus confirming their suitability as a contrast agent [309].
The synthesis of Au NPs using cinnamon proved to be an effective diagnostic agent for imaging both in laboratory settings and within living organisms. These NPs possess both biocompatibility and purity, making them suitable for use in in vivo applications. Photoacoustic emissions based in vitro study confirmed internalization of NPs in PC-3 and MCF-7 cells. Additionally, biodistribution investigations conducted on healthy mice demonstrated that these Au NPs accumulated in the lungs. This finding further supports the potential of using Au NPs as contrast agents for targeting [310]. In a radiotherapy investigation, thymoquinone-loaded green-synthesized Ag NPs in combination with the MDA-MB-231 mammary adenocarcinoma cells showed improved radiotherapy, significantly increased cancer cell killing, and DNA damage in comparison to the radiation alone. This was carried out via radiotherapy enhancement and the delivery of thymoquinone to the cancer cells. The developed system is proposed to be a promising combined regimen for efficient cancer therapy [311].
Rutin-loaded CoFe2O4 and ZnFe2O4 NPs (29 nm and 25 nm) displayed ferromagnetic and superparamagnetic properties. The saturation magnetization values were measured to be 56.2 emu/g and 6 emu/g, respectively. Thus, these NPs exhibited exceptional and efficient magnetic properties, making them crucial for magnetic hyperthermia therapy. Significant photothermal efficacy of green-synthesized CoFe2O4 and ZnFe2O4 NPs combined with laser radiation against MCF-7 cells was indicated by the results of the inverted stage microscopy and MTT assay [312]. The Fe3O4 NPs produced utilizing the fruit peel of P. granatum, exhibited an excellent relaxivity rate and generated strong magnetic resonance imaging (MRI) signals in the study. NPs containing 2% P. granatum fruit peel extract were loaded with 5-FU, which displayed 62 ± 0.3% entrapment efficiency. Based on in vitro cytotoxicity studies conducted on CCD112 normal and HCT116 colorectal cancer cell lines, it was observed that the 5-Fluorouracil loaded in the P. granatum fruit peel extract-based Fe3O4 NPs at 15.62% µg/mL resulted in a 11% and 29% reduction in cell viability in healthy and colorectal cancer cells, respectively. In the future, green-synthesized Fe3O4 NPs may play an important role as an eco-friendly nanocarrier in thermo-chemotherapy and MRI for the treatment of cancer [313].
Future perspectives
The subject of biosynthesized metallic NPs loaded with plant extracts is a novel and intriguing area of study. These NPs, often called "green nanoparticles," are extensively explored in the fields of drug delivery. Biosynthesized metallic nanoparticles loaded with plant extracts hold significant promise in medicine. Researchers are investigating their potential as drug delivery systems, where these nanoparticles can be loaded with therapeutic compounds and targeted to specific cells or tissues in the body. They might also be utilized for imaging purposes, such as in cancer detection or tracking the progression of diseases. These NPs can be designed to release drugs in a controlled and sustained manner. This characteristic permits for prolonged drug action, reducing the frequency of dosing and enhancing patient compliance with medication regimens. Biosynthesized metallic NPs loaded with plant extracts can be engineered to deliver drugs to specific cells or tissues in the body with high precision. This targeted drug delivery approach minimizes the side effects associated with conventional drug delivery methods and improves the therapeutic efficacy of medications. Drug resistance is a major challenge in many diseases. By using biosynthesized NPs, it might be possible to enhance the effectiveness of existing drugs against resistant strains of pathogens or cancer cells. Some drugs have low bioavailability, meaning that they are poorly absorbed by the body. Biosynthesized metallic NPs loaded with plant extracts can improve the solubility and bioavailability of such drugs, leading to more efficient therapeutic outcomes. Green nanoparticles derived from plant extracts generally exhibit lower toxicity compared to synthetic nanoparticles. By using biocompatible and biodegradable materials, the risk of adverse reactions and long-term side effects can be minimized. Researchers can combine the unique properties of metallic nanoparticles with the medicinal properties of plant extracts. This results in multifunctional nanoparticles that not only deliver drugs but also possess inherent therapeutic effects derived from the plant extracts, such as anti-inflammatory or antioxidant properties. Despite the promising future of biosynthesized metallic NPs loaded with plant extracts, comprehensive safety evaluations and regulation will be required. It will be of the uttermost importance to ensure that these nanoparticles are safe for human health, the environment, and non-target organisms. The commercialization and scalability of the production of these nanoparticles will be one of the most significant future challenges as research in this field advances. To make these technologies accessible and practical for a variety of applications, it will be necessary to develop large-scale, cost-effective production methods.
Conclusions
Cancer remains one of the most prevalent causes of mortality across the world, despite recent advances in diagnosis and treatment. No effective cancer treatment has been identified to date, and all the anticancer medications now on the market have the potential to cause negative effects. Nanotechnology has the potential to significantly improve current methods for diagnosing and treating cancer patients. To find better diagnostics and therapies that are as effective, specific, and low-toxic as feasible, researchers are currently attempting to develop novel approaches. Recent biomedical research has focused extensively on biological, or "green," synthesis of NPs. Green synthesis is less expensive, less toxic, and more ecologically friendly than conventional methods of producing NPs. This article will assist formulation scientists and nanotechnologists working on the green production of metal or metal oxide NPs by utilizing plant extracts. It also explored their therapeutic potential of plant extracts against various cancers. The science of metallic NPs is one of the most intriguing areas of study for cutaneous or transdermal drug administration. Thus, lipid nanocarriers are predicted to open new avenues in biomedical science while also improving an essential area of dermatologic literature.
Availability of data and materials
This work is not an original research paper but a review paper. Availability of data is not applicable.
References
Iqbal J, Abbasi BA, Mahmood T, Kanwal S, Ali B, Shah SA, Khalil AT (2017) Plant-derived anticancer agents: a green anticancer approach. Asian Pac J Trop Biomed 7:1129–1150. https://doi.org/10.1016/j.apjtb.2017.10.016
Biswas AK, Islam MR, Choudhury ZS, Mostafa A, Kadir MF (2014) Nanotechnology based approaches in cancer therapeutics. Adv Nat Sci Nanosci Nanotechnol 5:043001. https://doi.org/10.1088/2043-6262/5/4/043001
Menon S, Ks SD, Santhiya R, Rajeshkumar S, Kumar V (2018) Selenium nanoparticles: a potent chemotherapeutic agent and an elucidation of its mechanism. Colloids Surf B Biointerfaces 170:280–292. https://doi.org/10.1016/j.colsurfb.2018.06.006
Vinardell MP, Mitjans M (2015) Antitumor activities of metal oxide nanoparticles. Nanomaterials 5:1004–1021. https://doi.org/10.3390/nano5021004
Racca L, Cauda V (2021) Remotely activated nanoparticles for anticancer therapy. Nanomicro Lett 13:11. https://doi.org/10.1007/s40820-020-00537-8
Caputo F, De Nicola M, Ghibelli L (2014) Pharmacological potential of bioactive engineered nanomaterials. Biochem Pharmacol 92:112–130. https://doi.org/10.1016/j.bcp.2014.08.015
Narayanan KB, Sakthivel N (2011) Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv Colloid Interface Sci 169:59–79. https://doi.org/10.1016/j.cis.2011.08.004
Pamkhande PG, Ghule NW, Bamer AH, Kalaskar MG (2019) Metal nanoparticles synthesis: an overview on methods of preparation, advantages and disadvantages, and applications. J Drug Deliv Sci Technol 53:101174. https://doi.org/10.1016/j.jddst.2019.101174
Namvar F, Moniri M, Tahir M, Azizi S, Mohamad R (2015) Nanoparticles biosynthesized by fungi and yeast: a review of their preparation, properties, and medical applications. Molecules 20:16540–16565. https://doi.org/10.3390/molecules200916540
Patil SP, Chaudhari RY, Nemade MS (2022) Azadirachta indica leaves mediated green synthesis of metal oxide nanoparticles: a review. Talanta Open 5:100083. https://doi.org/10.1016/j.talo.2022.100083
Khan M, Albalawi GH, Shaik MR, Khan M, Adil SF, Kuniyil M, Alkhathlan HZ, Al-Warthan A, Siddiqui MR (2017) Miswak mediated green synthesized palladium nanoparticles as effective catalysts for the Suzuki coupling reactions in aqueous media. J Saudi Chem Soc 21:450–457. https://doi.org/10.1016/j.jscs.2016.03.008
Khatami M, Sharifi I, Nobre MA, Zafarnia N, Aflatoonian MR (2018) Waste-grass-mediated green synthesis of silver nanoparticles and evaluation of their anticancer, antifungal and antibacterial activity. Green Chem Lett Rev 11:125–134. https://doi.org/10.1080/17518253.2018.1444797
Mohanpuria P, Rana NK, Yadav SK (2008) Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res 10:507–517. https://doi.org/10.1007/s11051-007-9275-x
Hutchison JE (2008) Greener nanoscience: a proactive approach to advancing applications and reducing implications of nanotechnology. ACS Nano 2:395–402. https://doi.org/10.1021/nn800131j
Cheng J, Wang X, Qiu L, Li Y, Marraiki N, Elgorban AM, Xue L (2020) Green synthesized zinc oxide nanoparticles regulates the apoptotic expression in bone cancer cells MG-63 cells. J Photochem Photobiol B. https://doi.org/10.1016/j.jphotobiol.2019.111644
Deepika S, Selvaraj CI, Roopan SM (2020) Screening bioactivities of Caesalpinia pulcherrima L. swartz and cytotoxicity of extract synthesized silver nanoparticles on HCT116 cell line. Mater Sci Eng. https://doi.org/10.1016/j.msec.2019.110279
Mousavi B, Tafvizi F, Zaker Bostanabad S (2018) Green synthesis of silver nanoparticles using Artemisia turcomanica leaf extract and the study of anti-cancer effect and apoptosis induction on gastric cancer cell line (AGS). Artif Cells Nanomed Biotechnol 46:499–510. https://doi.org/10.1080/21691401.2018.1430697
Das B, Dash SK, Mandal D, Ghosh T, Chattopadhyay S, Tripathy S, Das S, Dey SK, Das D, Roy S (2017) Green synthesized silver nanoparticles destroy multidrug resistant bacteria via reactive oxygen species mediated membrane damage. Arab J Chem 10(6):862–876. https://doi.org/10.1016/j.arabjc.2015.08.008
Andra S, Balu SK, Jeevanandham J, Muthalagu M, Vidyavathy M, Chan YS, Danquah MK (2019) Phytosynthesized metal oxide nanoparticles for pharmaceutical applications. Naunyn Schmiedebergs Arch Pharmacol 392:755–771. https://doi.org/10.1007/s00210-019-01666-7
Shreyash N, Sonker M, Bajpai S, Tiwary SK (2021) Review of the mechanism of nanocarriers and technological developments in the field of nanoparticles for applications in cancer theragnostics. ACS Appl Bio Mater 4:2307–2334. https://doi.org/10.1021/acsabm.1c00020
Peng H, Xu Z, Wang Y, Feng N, Yang W, Tang J (2020) Biomimetic mesoporous silica nanoparticles for enhanced blood circulation and cancer therapy. ACS Appl Bio Mater 3:7849–7857. https://doi.org/10.1021/acsabm.0c01014
Bagalkot V, Zhang L, Levy-Nissenbaum E, Jon S, Kantoff PW, Langer R, Farokhzad OC (2007) Quantum dot-aptamer conjugates for synchronous cancer imaging, therapy, and sensing of drug delivery based on Bi-fluorescence resonance energy transfer. Nano Lett 7:3065–3070. https://doi.org/10.1021/nl071546n
Xu ZP, Zeng QH, Lu GQ, Yu AB (2006) Inorganic nanoparticles as carriers for efficient cellular delivery. Chem Eng Sci 61:1027–1040. https://doi.org/10.1016/j.ces.2005.06.019
Zhao X, Hilliard LR, Mechery SJ, Wang Y, Bagwe RP, Jin S, Tan W (2004) A rapid bioassay for single bacterial cell quantitation using bioconjugated nanoparticles. Proc Natl Acad Sci 101(42):15027–15032. https://doi.org/10.1073/pnas.0404806101
Mousa SA, Bharali DJ (2011) Nanotechnology-based detection and targeted therapy in cancer: nano-bio paradigms and applications. Cancers (Basel) 3:2888–2903. https://doi.org/10.3390/cancers3032888
Schroeder A, Heller DA, Winslow MM, Dahlman JE, Pratt GW, Langer R, Jacks T, Anderson DG (2012) Treating metastatic cancer with nanotechnology. Nat Rev Cancer 12:39–50. https://doi.org/10.1038/nrc3180
Singh AK (2017) Comparative therapeutic effects of plant-extract synthesized and traditionally synthesized gold nanoparticles on alcohol-induced inflammatory activity in sh-sy5y cells in vitro. Biomedicines. https://doi.org/10.3390/biomedicines5040070
Slavin YN, Asnis J, Hńfeli UO, Bach H (2017) Metal nanoparticles: understanding the mechanisms behind antibacterial activity. J Nanobiotechnol. https://doi.org/10.1186/s12951-017-0308-z
Ouyang L, Shi Z, Zhao S, Wang FT, Zhou TT, Liu B, Bao JK (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 45:487–498. https://doi.org/10.1111/j.1365-2184.2012.00845.x
Odeyemi SW, De La Mare J, Edkins AL, Afolayan AJ (2019) In vitro and in vivo toxicity assessment of biologically synthesized silver nanoparticles from Elaeodendron croceum. J Complement Integr Med 16(3):20180184. https://doi.org/10.1515/jcim-2018-0184
Anand K, Gengan RM, Phulukdaree A, Chuturgoon A (2015) Agroforestry waste Moringa oleifera petals mediated green synthesis of gold nanoparticles and their anti-cancer and catalytic activity. J Ind Eng Chem 21:1105–1111. https://doi.org/10.1016/j.jiec.2014.05.021
Rao PV, Nallappan D, Madhavi K, Rahman S, Jun Wei L, Gan SH (2016) Phytochemicals and biogenic metallic nanoparticles as anticancer agents. Oxid Med Cell Longev. https://doi.org/10.1155/2016/3685671
Wason MS, Colon J, Das S, Seal S, Turkson J, Zhao J, Baker CH (2013) Sensitization of pancreatic cancer cells to radiation by cerium oxide nanoparticle-induced ROS production. Nanomedicine 9:558–569. https://doi.org/10.1016/j.nano.2012.10.010
Nikolova MP, Chavali MS (2020) Metal oxide nanoparticles as biomedical materials. Biomimetics 5(2):27. https://doi.org/10.3390/BIOMIMETICS5020027
Kim YJ, Perumalsamy H, Castro-Aceituno V, Kim D, Markus J, Lee S, Kim S, Liu Y, Yang DC (2019) Photoluminescent and self-assembled hyaluronic acid-zinc oxide-ginsenoside rh2 nanoparticles and their potential caspase-9 apoptotic mechanism towards cancer cell lines. Int J Nanomed 14:8195–8208. https://doi.org/10.2147/IJN.S221328
Zhang XF, Liu ZG, Shen W, Gurunathan S (2016) Silver nanoparticles: Synthesis, characterization, properties, applications, and therapeutic approaches. Int J Mol Sci 17:1534. https://doi.org/10.3390/ijms17091534
Greulich C, Diendorf J, Simon T, Eggeler G, Epple M, Köller M (2011) Uptake and intracellular distribution of silver nanoparticles in human mesenchymal stem cells. Acta Biomater 7:347–354. https://doi.org/10.1016/j.actbio.2010.08.003
Raj S, Trivedi R, Soni V (2021) Biogenic synthesis of silver nanoparticles, characterization and their applications—a review. Surfaces 5(1):67–90. https://doi.org/10.3390/surfaces5010003
Bardania H, Mahmoudi R, Bagheri H, Salehpour Z, Fouani MH, Darabian B et al (2020) Facile preparation of a novel biogenic silver-loaded Nanofilm with intrinsic anti-bacterial and oxidant scavenging activities for wound healing. Sci Rep. https://doi.org/10.1038/s41598-020-63032-5
Ekram B, Tolba E, El-Sayed AF, Müller WEG, Schröder HC, Wang X et al (2024) Cell migration, DNA fragmentation and antibacterial properties of novel silver doped calcium polyphosphate nanoparticles. Sci Rep. https://doi.org/10.1038/s41598-023-50849-z
Kaur N, Kumar R, Alhan S, Sharma H, Singh N, Yogi R et al (2024) Lycium shawii mediated green synthesis of silver nanoparticles, characterization and assessments of their phytochemical, antioxidant, antimicrobial properties. Inorg Chem Commun 1:159. https://doi.org/10.1016/j.inoche.2023.111735
Miranda RR, Sampaio I, Zucolotto V (2022) Exploring silver nanoparticles for cancer therapy and diagnosis. Colloids Surf B 210:112254. https://doi.org/10.1016/j.colsurfb.2021.112254
Datta LP, Chatterjee A, Acharya K, De P, Das M (2017) Enzyme responsive nucleotide functionalized silver nanoparticles with effective antimicrobial and anticancer activity. New J Chem 41(4):1538–1548. https://doi.org/10.1039/C6NJ02955H
Guo D, Dou D, Ge L, Huang Z, Wang L, Gu N (2015) A caffeic acid mediated facile synthesis of silver nanoparticles with powerful anti-cancer activity. Colloids Surf B 134:229–234. https://doi.org/10.1016/j.colsurfb.2015.06.070
He J, Feizipour S, Veisi H, Amraii SA, Zangeneh MM, Hemmati S (2024) Panax ginseng root aqueous extract mediated biosynthesis of silver nanoparticles under ultrasound condition and investigation of the treatment of human lung adenocarcinoma with following the PI3K/AKT/mTOR signaling pathway. Inorg Chem Commun 160:11. https://doi.org/10.1016/j.inoche.2024.112021
Chen Y, Yang T, Chen S, Qi S, Zhang Z, Xu Y (2020) Silver nanoparticles regulate autophagy through lysosome injury and cell hypoxia in prostate cancer cells. J Biochem Mol Toxicol 34:1–9. https://doi.org/10.1002/jbt.22474
Sharma N, Bhatt G, Kothiyal P (2015) Gold nanoparticles synthesis, properties, and forthcoming applications—a review. Indian J Pharm Biol Res. https://doi.org/10.30750/IJPBR.3.2.3
Zhang Q, Yang M, Zhu Y, Mao C (2017) Metallic nanoclusters for cancer imaging and therapy. Curr Med Chem 25:1379–1396. https://doi.org/10.2174/0929867324666170331122757
Murawala P, Tirmale A, Shiras A, Prasad BL (2014) In situ synthesized BSA capped gold nanoparticles: effective carrier of anticancer drug methotrexate to MCF-7 breast cancer cells. Mater Sci Eng C 34:158–167. https://doi.org/10.1016/j.msec.2013.09.004
Devi L, Gupta R, Jain SK, Singh S, Kesharwani P (2020) Synthesis, characterization and in vitro assessment of colloidal gold nanoparticles of Gemcitabine with natural polysaccharides for treatment of breast cancer. J Drug Deliv Sci Technol. https://doi.org/10.1016/j.jddst.2020.101565
Akrami M, Samimi S, Alipour M, Bardania H, Ramezanpour S, Najafi N, Hosseinkhani S, Kamankesh M, Haririan I, Hassanshahi F (2021) Potential anticancer activity of a new pro-apoptotic peptide–thioctic acid gold nanoparticle platform. Nanotechnology 32(14):145101. https://doi.org/10.1088/1361-6528/abd3cb
Niculescu AG, Chircov C, Grumezescu AM (2022) Magnetite nanoparticles: synthesis methods—a comparative review. Methods 199:16–27. https://doi.org/10.1016/j.ymeth.2021.04.018
Yusefi M, Shameli K, Ali RR, Pang SW, Teow SY (2020) Evaluating anticancer activity of plant-mediated synthesized iron oxide nanoparticles using Punica Granatum fruit peel extract. J Mol Struct. https://doi.org/10.1016/j.molstruc.2019.127539
Murthy S, Effiong P, Fei CC (2020) Metal oxide nanoparticles in biomedical applications. In: Al-Douri Y (ed) Metal oxide powder technologies: fundamentals, processing methods and applications. Elsevier, pp 233–251. https://doi.org/10.1016/b978-0-12-817505-7.00011-7
Attia NF, Abd El-Monaem EM, El-Aqapa HG, Elashery SE, Eltaweil AS, El Kady M, Khalifa SA, Hawash HB, El-Seedi HR (2022) Iron oxide nanoparticles and their pharmaceutical applications. Appl Surf Sci Adv. https://doi.org/10.1016/j.apsadv.2022.100284
Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM (2016) Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol 11:986–994. https://doi.org/10.1038/nnano.2016.168
Das RK, Pachapur VL, Lonappan L, Naghdi M, Pulicharla R, Maiti S, Cledon M, Dalila LM, Sarma SJ, Brar SK (2017) Biological synthesis of metallic nanoparticles: plants, animals and microbial aspects. Nanotechnol Environ Eng 2:18. https://doi.org/10.1007/s41204-017-0029-4
Sun C, Fang C, Stephen Z, Veiseh O, Hansen S, Lee D, Ellenbogen RG, Olson J, Zhang M (2008) Tumor-targeted drug delivery and MRI contrast enhancement by chlorotoxin-conjugated iron oxide nanoparticles. Nanomedicine 3:495–505. https://doi.org/10.2217/17435889.3.4.495
Singh TA, Das J, Sil PC (2020) Zinc oxide nanoparticles: A comprehensive review on its synthesis, anticancer and drug delivery applications as well as health risks. Adv Colloid Interface Sci. https://doi.org/10.1016/j.cis.2020.102317
Wahab R, Siddiqui MA, Saquib Q, Dwivedi S, Ahmad J, Musarrat J, Al-Khedhairy AA, Shin HS (2014) ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity. Colloids Surf B Biointerfaces 117:267–276. https://doi.org/10.1016/j.colsurfb.2014.02.038
Fortunato E, Figueiredo V, Barquinha P, Elamurugu E, Barros R, Gonçalves G, Park SH, Hwang CS, Martins R (2010) Thin-film transistors based on p-type Cu2O thin films produced at room temperature. Appl Phys Lett. https://doi.org/10.1063/1.3428434
Xia T, Kovochich M, Nel AE (2007) Impairment of mitochondrial function by particulate matter (PM) and their toxic components: Implications for PM-induced cardiovascular and lung disease. Front Biosci 12:1238–1246. https://doi.org/10.2741/2142
Wang Y, Yang F, Zhang HX, Zi XY, Pan XH, Chen F, Luo WD, Li JX, Zhu HY, Hu YP (2013) Cuprous oxide nanoparticles inhibit the growth and metastasis of melanoma by targeting mitochondria. Cell Death Dis 4:e783. https://doi.org/10.1038/cddis.2013.314
Anandgaonker P, Kulkarni G, Gaikwad S, Rajbhoj A (2019) Synthesis of TiO2 nanoparticles by electrochemical method and their antibacterial application. Arab J Chem 12:1815–1822. https://doi.org/10.1016/j.arabjc.2014.12.015
Nadeem M, Tungmunnithum D, Hano C, Abbasi BH, Hashmi SS, Ahmad W, Zahir A (2018) The current trends in the green syntheses of titanium oxide nanoparticles and their applications. Green Chem Lett Rev 11(4):492–502. https://doi.org/10.1080/17518253.2018.1538430
Tadele KT, Abire TO, Feyisa TY (2021) Green synthesized silver nanoparticles using plant extracts as promising prospect for cancer therapy: a review of recent findings. J Nanomed 4:1040
Shafey AM (2020) Green synthesis of metal and metal oxide nanoparticles from plant leaf extracts and their applications: a review. Green Process Synth 9:304–339. https://doi.org/10.1515/gps-2020-0031
Singh KR, Nayak V, Sarkar T, Singh RP (2020) Cerium oxide nanoparticles: properties, biosynthesis and biomedical application. RSC Adv 10(45):27194–27214. https://doi.org/10.1039/D0RA04736H
Usman AI, Aziz AA, Khaniabadi PM (2020) Sonochemical synthesis of gold nanoparticles via palm oil fronds extracts for cytotoxicity assay. IOP Conf Ser Mater Sci Eng. https://doi.org/10.1088/1757-899X/839/1/012004
Gittins DI, Bethell D, Schiffrin DJ, Nichols RJ (2000) A nanometre-scale electronic switch consisting of a metal cluster and redox-addressable groups. Nature 408(6808):67–69
Shyam A, Chandran SS, George B, Sreelekha E (2021) Plant mediated synthesis of AgNPs and its applications: an overview. Inorg Nano-Met Chem 51:1646–1662. https://doi.org/10.1080/24701556.2020.1852254
Armendariz V, Herrera I, Peralta-Videa JR, Jose-Yacaman M, Troiani H, Santiago P, Gardea-Torresdey JL (2004) Size controlled gold nanoparticle formation by Avena sativa biomass: use of plants in nanobiotechnology. J Nanopart Res. https://doi.org/10.1007/s11051-004-0741-4
Sathishkumar M, Sneha K, Won SW, Cho CW, Kim S, Yun YS (2009) Cinnamon zeylanicum bark extract and powder mediated green synthesis of nano-crystalline silver particles and its bactericidal activity. Colloids Surf B Biointerfaces 73(2):332–338. https://doi.org/10.1016/j.colsurfb.2009.06.005
Prathna TC, Chandrasekaran N, Raichur AM, Mukherjee A (2011) Kinetic evolution studies of silver nanoparticles in a bio-based green synthesis process. Colloids Surf A Physicochem Eng Asp 377(1–3):212–216. https://doi.org/10.1016/j.colsurfa.2010.12.047
Augustine R, Hasan A (2020) Emerging applications of biocompatible phytosynthesized metal/metal oxide nanoparticles in healthcare. J Drug Deliv Sci Technol 56:101516. https://doi.org/10.1016/j.jddst.2020.101516
Hu J, Xianyu Y (2021) When nano meets plants: a review on the interplay between nanoparticles and plants. Nano Today. https://doi.org/10.1016/j.nantod.2021.101143
Velusamy P, Kumar GV, Jeyanthi V, Das J, Pachaiappan R (2016) Bio-inspired green nanoparticles: synthesis, mechanism, and antibacterial application. Toxicol Res 32:95–102. https://doi.org/10.5487/TR.2016.32.2.095
Kumar V, Yadav SK (2009) Plant-mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol 84:151–157. https://doi.org/10.1002/jctb.2023
Karmous I, Pandey A, Haj KB, Chaoui A (2020) Efficiency of the green synthesized nanoparticles as new tools in cancer therapy: insights on plant-based bioengineered nanoparticles, biophysical properties, and anticancer roles. Biol Trace Elem Res 196:330–342. https://doi.org/10.1007/s12011-019-01895-0
Marshall AT, Haverkamp RG, Davies CE, Parsons JG, Gardea-Torresdey JL, van Agterveld D (2007) Accumulation of gold nanoparticles in Brassic juncea. Int J Phytoremediat 9:197–206. https://doi.org/10.1080/15226510701376026
El-Seedi HR, El-Shabasy RM, Khalifa SA, Saeed A, Shah A, Shah R, Iftikhar FJ, Abdel-Daim MM, Omri A, Hajrahand NH, Sabir JS (2019) Metal nanoparticles fabricated by green chemistry using natural extracts: biosynthesis, mechanisms, and applications. RSC Adv 9:24539–24559. https://doi.org/10.1039/c9ra02225b
Govindarajan M, Rajeswary M, Veerakumar K, Muthukumaran U, Hoti SL, Benelli G (2016) Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp Parasitol 161:40–47. https://doi.org/10.1016/j.exppara.2015.12.011
Lee SY, Krishnamurthy S, Cho CW, Yun YS (2016) Biosynthesis of gold nanoparticles using Ocimum sanctum extracts by solvents with different polarity. ACS Sustain Chem Eng 4:2651–2659. https://doi.org/10.1021/acssuschemeng.6b00161
Patil MP, Kim GD (2017) Eco-friendly approach for nanoparticles synthesis and mechanism behind antibacterial activity of silver and anticancer activity of gold nanoparticles. Appl Microbiol Biotechnol 101:79–92. https://doi.org/10.1007/s00253-016-8012-8
Mohamed HE, Afridi S, Khalil AT, Ali M, Zohra T, Salman M, Ikram A, Shinwari ZK, Maaza M (2020) Bio-redox potential of Hyphaene thebaica in bio-fabrication of ultrafine maghemite phase iron oxide nanoparticles (Fe2O3 NPs) for therapeutic applications. Mater Sci Eng C 112:110890. https://doi.org/10.1016/j.msec.2020.110890
Üstün E, Önbaş SC, Çelik SK, Ayvaz MÇ, Şahin N (2022) Green synthesis of iron oxide nanoparticles by using Ficus carica leaf extract and its antioxidant activity. Biointerface Res Appl Chem 12:2108–2116. https://doi.org/10.33263/BRIAC122.21082116
Velsankar K, Parvathy G, Mohandoss S, Krishna Kumar M, Sudhahar S (2022) Celosia argentea leaf extract-mediated green synthesized iron oxide nanoparticles for bio-applications. J Nanostruct Chem 12:625–640. https://doi.org/10.1007/s40097-021-00434-5
Devi HS, Boda MA, Shah MA, Parveen S, Wani AH (2019) Green synthesis of iron oxide nanoparticles using Platanus orientalis leaf extract for antifungal activity. Green Process Synth 8:38–45. https://doi.org/10.1515/gps-2017-0145
Usha V, Amutha E, Pushpalaksmi E, Samraj JJ, Rajaduraipandian S, Gandhimathi S, Annadurai G (1970) Green synthesis and characterization of antibacterial studies by iron oxide nanoparticles using Carica papaya leaf extract. J Appl Sci Environ Manag 26:421–427. https://doi.org/10.4314/jasem.v26i3.8
Padalia H, Chanda S (2017) Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artif Cells Nanomed Biotechnol 45:1751–1761. https://doi.org/10.1080/21691401.2017.1282868
Naiel B, Fawzy M, Halmy MW, Mahmoud AE (2022) Green synthesis of zinc oxide nanoparticles using Sea Lavender (Limonium pruinosum L. Chaz.) extract: characterization, evaluation of anti-skin cancer, antimicrobial and antioxidant potentials. Sci Rep 12:1–12. https://doi.org/10.1038/s41598-022-24805-2
Gharpure S, Yadwade R, Ankamwar B (2022) Non-antimicrobial and non-anticancer properties of ZnO nanoparticles biosynthesized using different plant parts of Bixa orellana. ACS Omega 7:1914–1933. https://doi.org/10.1021/acsomega.1c05324
Shnawa BH, Hamad SM, Barzinjy AA, Kareem PA, Ahmed MH (2022) Scolicidal activity of biosynthesized zinc oxide nanoparticles by Mentha longifolia L. leaves against Echinococcus granulosus protoscolices. Emergent Mater 5:683–693. https://doi.org/10.1007/s42247-021-00264-9
Faisal S, Jan H, Abdullah AI, Rizwan M, Hussain Z, Sultana K, Ali Z, Uddin MN (2022) In vivo analgesic, anti-inflammatory, and anti-diabetic screening of Bacopa monnieri-synthesized copper oxide nanoparticles. ACS Omega 7:4071–4082. https://doi.org/10.1021/acsomega.1c05410
Qamar H, Rehman S, Chauhan DK, Tiwari AK, Upmanyu V (2020) Green synthesis, characterization and antimicrobial activity of copper oxide nanomaterial derived from Momordica charantia. Int J Nanomed 15:2541–2553. https://doi.org/10.2147/IJN.S240232
Al-Khafaji MA, Al-Refai’a RA, Al-Zamely OM (2021) Green synthesis of copper nanoparticles using Artemisia plant extract. Mater Today Proc 49:2831–2835. https://doi.org/10.1016/j.matpr.2021.10.067
Pillai RR, Sreelekshmi PB, Meera AP (2020) Enhanced biological performance of green sythesized copper oxide nanoparticles using Pimenta dioica leaf extract. Mater Today Proc 50:163–172. https://doi.org/10.1016/j.matpr.2021.11.547
Chandrasekar A, Vasantharaj S, Jagadeesan NL, Shankar SN, Pannerselvam B, Bose VG, Arumugam G, Shanmugavel M (2021) Studies on phytomolecules mediated synthesis of copper oxide nanoparticles for biomedical and environmental applications. Biocatal Agric Biotechnol 33:101994. https://doi.org/10.1016/j.bcab.2021.101994
Rajeshkumar S, Santhoshkumar J, Jule LT, Ramaswamy K (2021) Phytosynthesis of titanium dioxide nanoparticles using king of bitter Andrographis paniculata and its embryonic toxicology evaluation and biomedical potential. Bioinorg Chem Appl. https://doi.org/10.1155/2021/6267634
Santhoshkumar T, Rahuman AA, Jayaseelan C, Rajakumar G, Marimuthu S, Kirthi AV, Velayutham K, Thomas J, Venkatesan J, Kim SK (2014) Green synthesis of titanium dioxide nanoparticles using Psidium guajava extract and its antibacterial and antioxidant properties. Asian Pac J Trop Med 7:968–976. https://doi.org/10.1016/S1995-7645(14)60171-1
Suman TY, Ravindranath RR, Elumalai D, Kaleena PK, Ramkumar R, Perumal P, Aranganathan L, Chitrarasu PS (2015) Larvicidal activity of titanium dioxide nanoparticles synthesized using Morinda citrifolia root extract against Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus and its other effect on non-target fish. Asian Pac J Trop Dis 5:224–230. https://doi.org/10.1016/S2222-1808(14)60658-7
Sivaranjani V, Philominathan PJ (2016) Synthesize of Titanium dioxide nanoparticles using Moringa oleifera leaves and evaluation of wound healing activity. Wound Med 12:1–5. https://doi.org/10.1016/j.wndm.2015.11.002
Gomaa EZ (2017) Antimicrobial, antioxidant and antitumor activities of silver nanoparticles synthesized by Allium cepa extract: a green approach. J Genet Eng Biotechnol 15:49–57. https://doi.org/10.1016/j.jgeb.2016.12.002
Vijayan R, Joseph S, Mathew B (2018) Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif Cells Nanomed Biotechnol 46:861–871. https://doi.org/10.1080/21691401.2017.1345930
Suman TY, Rajasree SR, Kanchana A, Elizabeth SB (2013) Biosynthesis, characterization and cytotoxic effect of plant mediated silver nanoparticles using Morinda citrifolia root extract. Colloids Surf B Biointerfaces 106:74–78. https://doi.org/10.1016/j.colsurfb.2013.01.037
Hawar SN, Al-Shmgani HS, Al-Kubaisi ZA, Sulaiman GM, Dewir YH, Rikisahedew JJ (2022) Green synthesis of silver nanoparticles from Alhagi graecorum leaf extract and evaluation of their cytotoxicity and antifungal activity. J Nanomater. https://doi.org/10.1155/2022/1058119
Hawadak J, Kojom Foko LP, Pande V, Singh V (2022) In vitro antiplasmodial activity, hemocompatibility and temporal stability of Azadirachta indica silver nanoparticles. Artif Cells Nanomed Biotechnol 50:286–300. https://doi.org/10.1080/21691401.2022.2126979
Meléndez-Villanueva MA, Morán-Santibañez K, Martínez-Sanmiguel JJ, Rangel-López R, Garza-Navarro MA, Rodríguez-Padilla C, Zarate-Triviño DG, Trejo-Ávila LM (2019) Virucidal activity of gold nanoparticles synthesized by green chemistry using garlic extract. Viruses 11:1–13. https://doi.org/10.3390/v11121111
Peivandi S, Dehghanzadeh H, Baghizadeh A (2023) Biosynthesis of gold nanoparticles using sansevieria plant extract and its biomedical application. Inorg Nano-Met Chem 53(5):482–489. https://doi.org/10.1080/24701556.2022.2078355
Muniyappan N, Pandeeswaran M, Amalraj A (2021) Green synthesis of gold nanoparticles using Curcuma pseudomontana isolated curcumin: Its characterization, antimicrobial, antioxidant and anti- inflammatory activities. Environ Toxicol Chem 3:117–124. https://doi.org/10.1016/j.enceco.2021.01.002
Patra JK, Kwon Y, Baek KH (2016) Green biosynthesis of gold nanoparticles by onion peel extract: synthesis, characterization and biological activities. Adv Powder Technol 27:2204–2213. https://doi.org/10.1016/j.apt.2016.08.005
Suriyakala G, Sathiyaraj S, Babujanarthanam R, Alarjani KM, Hussein DS, Rasheed RA, Kanimozhi K (2022) Green synthesis of gold nanoparticles using Jatropha integerrima Jacq. flower extract and their antibacterial activity. J King Saud Univ Sci 34:101830. https://doi.org/10.1016/j.jksus.2022.101830
Gu H, Chen X, Chen F, Zhou X, Parsaee Z (2018) Ultrasound-assisted biosynthesis of CuO-NPs using brown alga Cystoseira trinodis: characterization, photocatalytic AOP, DPPH scavenging and antibacterial investigations. Ultrason Sonochem 41:109–119. https://doi.org/10.1016/j.ultsonch.2017.09.006
Gahlawat G, Choudhury AR (2019) A review on the biosynthesis of metal and metal salt nanoparticles by microbes. RSC Adv 9(23):12944–12967. https://doi.org/10.1039/C8RA10483b
Jain N, Bhargava A, Majumdar S, Tarafdar JC, Panwar J (2011) Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: a mechanism perspective. Nanoscale 3:635–641. https://doi.org/10.1039/c0nr00656d
Das VL, Thomas R, Varghese RT, Soniya EV, Mathew J, Radhakrishnan EK (2014) Extracellular synthesis of silver nanoparticles by the Bacillus strain CS 11 isolated from industrialized area. Biotech 4:121–126. https://doi.org/10.1007/s13205-013-0130-8
Prasad R, Pandey R (2016) Barman I (2016) Engineering tailored nanoparticles with microbes: Quo vadis? Wiley Interdiscip Rev Nanomed Nanobiotechnol 8(2):316–330. https://doi.org/10.1002/wnan.1363
Durán N, Marcato PD, Alves OL, De Souza GI, Esposito E (2005) Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol 3:1–7. https://doi.org/10.1186/1477-3155-3-8
Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S (2008) Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces 65:150–153. https://doi.org/10.1016/j.colsurfb.2008.02.018
Klaus T, Joerger R, Olsson E, Granqvist CG (1999) Silver-based crystalline nanoparticles, microbially fabricated. Proc Natl Acad Sci 96(24):13611–13614. https://doi.org/10.1073/pnas.96.24.13611
Khalil AT, Ovais M, Iqbal J, Ali A, Ayaz M, Abbas M, Ahmad I, Devkota HP (2022) Microbes-mediated synthesis strategies of metal nanoparticles and their potential role in cancer therapeutics. Semin Cancer Biol 86:693–705. https://doi.org/10.1016/j.semcancer.2021.06.006
Ali J, Ali N, Wang L, Waseem H, Pan G (2019) Revisiting the mechanistic pathways for bacterial mediated synthesis of noble metal nanoparticles. J Microbiol Methods 159:18–25. https://doi.org/10.1016/j.mimet.2019.02.010
Fariq A, Khan T, Yasmin A (2017) Microbial synthesis of nanoparticles and their potential applications in biomedicine. J Appl Biomed 15:241–248. https://doi.org/10.1016/j.jab.2017.03.004
Gurunathan S, Kalishwaralal K, Vaidyanathan R, Venkataraman D, Pandian SR, Muniyandi J, Hariharan N, Eom SH (2009) Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Colloids Surf B Biointerfaces 74:328–335. https://doi.org/10.1016/j.colsurfb.2009.07.048
Koul B, Poonia AK, Yadav D, Jin JO (2021) Microbe-mediated biosynthesis of nanoparticles: applications and future prospects. Biomolecules. https://doi.org/10.3390/biom11060886
Nangia Y, Wangoo N, Goyal N, Shekhawat G, Suri CR (2009) A novel bacterial isolate Stenotrophomonas maltophilia as living factory for synthesis of gold nanoparticles. Microb Cell Fact. https://doi.org/10.1186/1475-2859-8-39
Zhao H, Maruthupandy M, Al-mekhlafi FA, Chackaravarthi G, Ramachandran G, Chelliah CK (2022) Biological synthesis of copper oxide nanoparticles using marine endophytic actinomycetes and evaluation of biofilm producing bacteria and A549 lung cancer cells. J King Saud Univ Sci 34:101866. https://doi.org/10.1016/j.jksus.2022.101866
Bukhari SI, Hamed MM, Al-Agamy MH, Gazwi HS, Radwan HH, Youssif AM (2021) Biosynthesis of copper oxide nanoparticles using Streptomyces MHM38 and its biological applications. J Nanomater. https://doi.org/10.1155/2021/6693302
John MS, Nagoth JA, Zannotti M, Giovannetti R, Mancini A, Ramasamy KP, Miceli C, Pucciarelli S (2021) Biogenic synthesis of copper nanoparticles using bacterial strains isolated from an antarctic consortium associated to a psychrophilic marine ciliate: characterization and potential application as antimicrobial agents. Mar Drugs. https://doi.org/10.3390/md19050263
Nabila MI, Kannabiran K (2018) Biosynthesis, characterization and antibacterial activity of copper oxide nanoparticles (CuO NPs) from actinomycetes. Biocatal Agric Biotechnol 15:56–62. https://doi.org/10.1016/j.bcab.2018.05.011
Ebadi M, Zolfaghari MR, Aghaei SS, Zargar M, Shafiei M, Zahiri HS, Noghabi KA (2019) A bio-inspired strategy for the synthesis of zinc oxide nanoparticles (ZnO NPs) using the cell extract. RSC Adv 9(41):23508–23525. https://doi.org/10.1039/c9ra03962g
Faisal S, Rizwan M, Ullah R, Alotaibi A, Khattak A, Bibi N, Idrees M (2022) Paraclostridium benzoelyticum bacterium-mediated zinc oxide nanoparticles and their in vivo multiple biological applications. Oxid Med Cell Longev. https://doi.org/10.1155/2022/5994033
Hamk M, Akçay FA, Avcı A (2022) Green synthesis of zinc oxide nanoparticles using Bacillus subtilis ZBP4 and their antibacterial potential against foodborne pathogens. Prep Biochem Biotechnol. https://doi.org/10.1080/10826068.2022.2076243
Motazedi R, Rahaiee S, Zare M (2020) Efficient biogenesis of ZnO nanoparticles using extracellular extract of Saccharomyces cerevisiae: evaluation of photocatalytic, cytotoxic and other biological activities. Bioorg Chem 101:103998. https://doi.org/10.1016/j.bioorg.2020.103998
Mansoor A, Khan MT, Mehmood M, Khurshid Z, Ali MI, Jamal A (2022) Synthesis and characterization of titanium oxide nanoparticles with a novel biogenic process for dental application. Nanomaterials. https://doi.org/10.3390/nano12071078
Meenatchisundaram N, Chellamuthu J, Jeyaraman AR, Arjunan N, Muthuramalingam JB, Karuppuchamy S (2022) Biosynthesized TiO2 nanoparticles an efficient biogenic material for photocatalytic and antibacterial applications. Energy Environ 33:377–398. https://doi.org/10.1177/0958305X211000261
Agceli GK, Hammachi H, Kodal SP, Cihangir N, Aksu Z (2020) A Novel approach to synthesize TiO2 nanoparticles: biosynthesis by using Streptomyces sp. HC1. J Inorg Organomet Polym Mater 30:3221–3229. https://doi.org/10.1007/s10904-020-01486-w
Elsilk SE, Khalil MA, Aboshady TA, Alsalmi FA, Ali SS (2022) Streptomyces rochei MS-37 as a novel marine actinobacterium for green biosynthesis of silver nanoparticles and their biomedical applications. Molecules 27:7296. https://doi.org/10.3390/molecules27217296
Al-Dhabi NA, Ghilan AK, Esmail GA, Arasu MV, Duraipandiyan V, Ponmurugan K (2019) Environmental friendly synthesis of silver nanomaterials from the promising Streptomyces parvus strain Al-Dhabi-91 recovered from the Saudi Arabian marine regions for antimicrobial and antioxidant properties. J Photochem Photobiol B 197:111529. https://doi.org/10.1016/j.jphotobiol.2019.111529
Khalil MA, El-Shanshoury AE, Alghamdi MA, Alsalmi FA, Mohamed SF, Sun J, Ali SS (2022) Biosynthesis of silver nanoparticles by marine Actinobacterium Nocardiopsis dassonvillei and exploring their therapeutic potentials. Front Microbiol. https://doi.org/10.3389/fmicb.2021.705673
Samuel MS, Jose S, Selvarajan E, Mathimani T, Pugazhendhi A (2020) Biosynthesized silver nanoparticles using Bacillus amyloliquefaciens; application for cytotoxicity effect on A549 cell line and photocatalytic degradation of p-nitrophenol. J Photochem Photobiol B 202:111642. https://doi.org/10.1016/j.jphotobiol.2019.111642
Saravanan M, Barik SK, MubarakAli D, Prakash P, Pugazhendhi A (2018) Synthesis of silver nanoparticles from Bacillus brevis (NCIM 2533) and their antibacterial activity against pathogenic bacteria. Microb Pathog 116:221–226. https://doi.org/10.1016/j.micpath.2018.01.038
Składanowski M, Wypij M, Laskowski D, Golińska P, Dahm H, Rai M (2017) Silver and gold nanoparticles synthesized from Streptomyces sp. isolated from acid forest soil with special reference to its antibacterial activity against pathogens. J Clust Sci 28:59–79. https://doi.org/10.1007/s10876-016-1043-6
Shunmugam R, Balusamy SR, Kumar V, Menon S, Lakshmi T, Perumalsamy H (2021) Biosynthesis of gold nanoparticles using marine microbe (Vibrio alginolyticus) and its anticancer and antioxidant analysis. J King Saud Univ Sci. https://doi.org/10.1016/j.jksus.2020.101260
Patil MP, Kang MJ, Niyonizigiye I, Singh A, Kim JO, Seo YB, Kim GD (2019) Extracellular synthesis of gold nanoparticles using the marine bacterium Paracoccus haeundaensis BC74171T and evaluation of their antioxidant activity and antiproliferative effect on normal and cancer cell lines. Colloids Surf B Biointerfaces. https://doi.org/10.1016/j.colsurfb.2019.110455
Vairavel M, Devaraj E, Shanmugam R (2020) An eco-friendly synthesis of Enterococcus sp.—mediated gold nanoparticle induces cytotoxicity in human colorectal cancer cells. Environ Sci Pollut Res 27:8166–8175. https://doi.org/10.1007/s11356-019-07511-x
Bowman SM, Free SJ (2006) The structure and synthesis of the fungal cell wall. BioEssays 28:799–808. https://doi.org/10.1002/bies.20441
Jhansi K, Jayarambabu N, Reddy KP, Reddy NM, Suvarna RP, Rao KV, Kumar VR, Rajendar V (2017) Biosynthesis of MgO nanoparticles using mushroom extract: effect on peanut (Arachis hypogaea L.) seed germination. 3 Biotech. https://doi.org/10.1007/s13205-017-0894-3
Yadav A, Kon K, Kratosova G, Duran N, Ingle AP, Rai M (2015) Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: progress and key aspects of research. Biotechnol Lett 37:2099–2120. https://doi.org/10.1007/s10529-015-1901-6
Kashyap PL, Kumar S, Srivastava AK, Sharma AK (2013) Myconanotechnology in agriculture: a perspective. World J Microbiol Biotechnol 29:191–207. https://doi.org/10.1007/s11274-012-1171-6
Verma VC, Singh SK, Solanki R, Prakash S (2011) Biofabrication of anisotropic gold nanotriangles using extract of endophytic Aspergillus clavatus as a dual functional reductant and stabilizer. Nanoscale Res Lett 6:1–7. https://doi.org/10.1007/s11671-010-9743-6
Birla SS, Tiwari VV, Gade AK, Ingle AP, Yadav AP, Rai MK (2009) Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol 48:173–179. https://doi.org/10.1111/j.1472-765X.2008.02510.x
Sarkar J, Dey P, Saha S, Acharya K (2011) Mycosynthesis of selenium nanoparticles. Micro Nano Lett 6:599–602. https://doi.org/10.1049/mnl.2011.0227
Govindappa M, Lavanya M, Aishwarya P, Pai K, Lunked P, Hemashekhar B, Arpitha BM, Ramachandra YL, Raghavendra VB (2020) Synthesis and characterization of endophytic fungi, Cladosporium perangustum mediated silver nanoparticles and their antioxidant, anticancer and nano-toxicological study. Bionanoscience 10:928–941. https://doi.org/10.1007/s12668-020-00719-z
Zakariya NA, Majeed S, Jusof WH (2022) Investigation of antioxidant and antibacterial activity of iron oxide nanoparticles (IONPS) synthesized from the aqueous extract of Penicillium spp. Sens Int 3:1–9. https://doi.org/10.1016/j.sintl.2022.100164
Sidkey NB (2020) Biosynthesis, characterization and antimicrobial activity of iron oxide nanoparticles synthesized by fungi. Al-Azhar J Pharm Sci 62(2):164–179. https://doi.org/10.21608/AJPS.2020.118382
Baskar G, Chandhuru J, Praveen AS, Sheraz Fahad K (2017) Anticancer activity of iron oxide nanobiocomposite of fungal asparaginase. Int J Mod Sci Technol 2:98–104
Saravanakumar K, Shanmugam S, Varukattu NB, MubarakAli D, Kathiresan K, Wang MH (2019) Biosynthesis and characterization of copper oxide nanoparticles from indigenous fungi and its effect of photothermolysis on human lung carcinoma. J Photochem Photobiol B 190:103–109. https://doi.org/10.1016/j.jphotobiol.2018.11.017
Ghareib M, Abdallah W, Tahon M, Tallima A (2019) Biosynthesis of copper oxide nanoparticles using the preformed biomass of Aspergillus fumigatus and their antibacterial and photocatalytic activities. Dig J Nanomater Biostruct 14(2):291–303
Mani VM, Kalaivani S, Sabarathinam S, Vasuki M, Soundari AJ, Das MA, Elfasakhany A, Pugazhendhi A (2021) Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: bioactivity and anti-cancer evaluations. Environ Res. https://doi.org/10.1016/j.envres.2021.111502
Fatima F, Wahid I (2022) Eco-friendly synthesis of silver and copper nanoparticles by Shizophyllum commune fungus and its biomedical applications. Int J Environ Sci Technol 19:7915–7926. https://doi.org/10.1007/s13762-021-03517-6
Mkhize SS, Pooe OJ, Khoza S, Mongalo IN, Khan R, Simelane MB (2022) Characterization and biological evaluation of zinc oxide nanoparticles synthesized from Pleurotus ostreatus mushroom. Appl Sci (Switzerland). https://doi.org/10.3390/app12178563
Mani VM, Nivetha S, Sabarathinam S, Barath S, Das MA, Basha S, Elfasakhany A, Pugazhendhi A (2022) Multifunctionalities of mycosynthesized zinc oxide nanoparticles (ZnO NPs) from Cladosporium tenuissimum FCBGr: antimicrobial additives for paints coating, functionalized fabrics and biomedical properties. Prog Org Coat. https://doi.org/10.1016/j.porgcoat.2021.106650
Abdelkader DH, Negm WA, Elekhnawy E, Eliwa D, Aldosari BN, Almurshedi AS (2022) Zinc oxide nanoparticles as potential delivery carrier: green synthesis by Aspergillus niger endophytic fungus, characterization, and in vitro/in vivo antibacterial activity. Pharmaceuticals. https://doi.org/10.3390/ph1091057
Arya S, Sonawane H, Math S, Tambade P, Chaskar M, Shinde D (2021) Biogenic titanium nanoparticles (TiO2 NPs) from Tricoderma citrinoviride extract: synthesis, characterization and antibacterial activity against extremely drug-resistant Pseudomonas aeruginosa. Int Nano Lett 11:35–42. https://doi.org/10.1007/s40089-020-00320-y
Rehman S, Jermy R, Asiri SM, Shah MA, Farooq R, Ravinayagam V, Ansari MA, Alsalem Z, Al Jindan R, Reshi Z, Khan FA (2020) Using Fomitopsis pinicola for bioinspired synthesis of titanium dioxide and silver nanoparticles, targeting biomedical applications. RSC Adv 10:32137–32147. https://doi.org/10.1039/d0ra02637a
Gupta P, Rai N, Verma A, Saikia D, Singh SP, Kumar R, Singh SK, Kumar D, Gautam V (2022) Green-based approach to synthesize silver nanoparticles using the fungal endophyte. ACS Omega 7(50):46653–46673. https://doi.org/10.1021/acsomega.2c05605
Vijayan S, Divya K, George TK, Jisha MS (2016) Biogenic synthesis of silver nanoparticles using endophytic fungi Fusarium oxysporum isolated from Withania somnifera (L.), its antibacterial and cytotoxic activity. J Bionanosci 10:369–376. https://doi.org/10.1166/jbns.2016.1390
Omran BA, Nassar HN, Fatthallah NA, Hamdy A, El-Shatoury EH, El-Gendy NS (2018) Characterization and antimicrobial activity of silver nanoparticles mycosynthesized by Aspergillus brasiliensis. J Appl Microbiol 125:370–382. https://doi.org/10.1111/jam.13776
Ramos MM, Morais EDS, Sena IDS, Lima AL, de Oliveira FR, de Freitas CM, Fernandes CP, de Carvalho JC, Ferreira IM (2020) Silver nanoparticle from whole cells of the fungi Trichoderma spp. isolated from Brazilian Amazon. Biotechnol Lett 42:833–843. https://doi.org/10.1007/s10529-020-02819-y
Win TT, Khan S, Fu P (2020) Fungus- (Alternaria sp) mediated silver nanoparticles synthesis, characterization, and screening of antifungal activity against some phytopathogens. J Nanotechnol. https://doi.org/10.1155/2020/8828878
Munawer U, Raghavendra VB, Ningaraju S, Krishna KL, Ghosh AR, Melappa G, Pugazhendhi A (2020) Biofabrication of gold nanoparticles mediated by the endophytic Cladosporium species: photodegradation, in vitro anticancer activity and in vivo antitumor studies. Int J Pharm 588:119729. https://doi.org/10.1016/j.ijpharm.2020.119729
Abdel-Kareem MM, Zohri AA (2018) Extracellular mycosynthesis of gold nanoparticles using Trichoderma hamatum: optimization, characterization and antimicrobial activity. Lett Appl Microbiol 67:465–475. https://doi.org/10.1111/lam.13055
Sarkar J, Ray S, Chattopadhyay D, Laskar A, Acharya K (2012) Mycogenesis of gold nanoparticles using a phytopathogen Alternaria alternata. Bioprocess Biosyst Eng 35:637–643. https://doi.org/10.1007/s00449-011-0646-4
Clarance P, Luvankar B, Sales J, Khusro A, Agastian P, Tack JC, Al Khulaifi MM, Al-Shwaiman HA, Elgorban AM, Syed A, Kim HJ (2020) Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J Biol Sci 27:706–712. https://doi.org/10.1016/j.sjbs.2019.12.026
Shah M, Fawcett D, Sharma S, Tripathy SK, Poinern GE (2015) Green synthesis of metallic nanoparticles via biological entities. Materials 8:7278–7308. https://doi.org/10.3390/ma8115377
Senapati S, Syed A, Moeez S, Kumar A, Ahmad A (2012) Intracellular synthesis of gold nanoparticles using alga Tetraselmis kochinensis. Mater Lett 79:116–118. https://doi.org/10.1016/j.matlet.2012.04.009
Ramaswamy SV, Narendhran S, Sivaraj R (2016) Potentiating effect of ecofriendly synthesis of copper oxide nanoparticles using brown alga: antimicrobial and anticancer activities. Bull Mater Sci 39:361–364
Priyadharshini RI, Prasannaraj G, Geetha N, Venkatachalam P (2014) Microwave-mediated extracellular synthesis of metallic silver and zinc oxide nanoparticles using macro-algae (Gracilaria edulis) extracts and its anticancer activity against human PC3 cell lines. Appl Biochem Biotechnol 174:2777–2790. https://doi.org/10.1007/s12010-014-1225-3
Murugesan S, Bhuvaneswari S, Shanthi N, Murugakoothan P, Sivamurugan V (2015) Red alga Hypnea musciformis (Wulf) lamour mediated environmentally benign synthesis and antifungal activity of gold nano particles. Int J Nanosci Nanotechnol 6:71–83
Venkatesan J, Kim SK, Shim MS (2016) Antimicrobial, antioxidant, and anticancer activities of biosynthesized silver nanoparticles using marine algae Ecklonia cava. Nanomaterials 6(12):235. https://doi.org/10.3390/nano6120235
Bensy AD, Christobel GJ, Muthusamy K, Alfarhan A, Anantharaman P (2022) Green synthesis of iron nanoparticles from Ulva lactuca and bactericidal activity against enteropathogens. J King Saud Univ Sci 34:101888. https://doi.org/10.1016/j.jksus.2022.101888
Salem DM, Ismail MM, Aly-Eldeen MA (2019) Biogenic synthesis and antimicrobial potency of iron oxide (Fe3O4) nanoparticles using algae harvested from the Mediterranean Sea. Egypt Egypt J Aquat Res 45:197–204. https://doi.org/10.1016/j.ejar.2019.07.002
Subhashini G, Ruban P, Daniel T (2018) Biosynthesis and characterization of magnetic (Fe3O4) iron oxide nanoparticles from a red seaweed Gracilaria edulis and its antimicrobial activity. Int J Adv Sci Res Manag 3:184–189
Haris M, Fatima N, Iqbal J, Chalgham W, Mumtaz AS, El-Sheikh MA, Tavafoghi M (2023) Oscillatoria limnetica mediated green synthesis of iron oxide (Fe2O3) nanoparticles and their diverse in vitro bioactivities. Molecules. https://doi.org/10.3390/molecules28052091
Abboud Y, Saffaj T, Chagraoui A, El Bouari A, Brouzi K, Tanane O, Ihssane B (2014) Biosynthesis, characterization and antimicrobial activity of copper oxide nanoparticles (CONPs) produced using brown alga extract (Bifurcaria bifurcata). Appl Nanosci (Switzerland) 4:571–576. https://doi.org/10.1007/s13204-013-0233-x
Saran S, Sharma G, Kumar M, Ali MI (2017) Biosynthesis of copper oxide nanoparticles using cyanobacteria Spirulina platensis and its antibacterial activity. Int J Pharm Sci Res 8:3887–3892. https://doi.org/10.13040/IJPSR.0975-8232.8(9).3887-92
Kothai R, Arul B, Anbazhagan V (2022) Anti-dengue activity of ZnO nanoparticles of crude fucoidan from brown seaweed S. marginatum. Appl Biochem Biotechnol. https://doi.org/10.1007/s12010-022-03966-w
Sanaeimehr Z, Javadi I, Namvar F (2018) Antiangiogenic and antiapoptotic effects of green-synthesized zinc oxide nanoparticles using Sargassum muticum algae extraction. Cancer Nanotechnol. https://doi.org/10.1186/s12645-018-0037-5
Bhattacharya P, Chatterjee K, Swarnakar S, Banerjee S (2020) Green synthesis of zinc oxide nanoparticles via algal route and its action on cancerous cells and pathogenic microbes. Adv Nano Res 3:15–27. https://doi.org/10.21467/anr.3.1.15-27
Kathiraven T, Sundaramanickam A, Shanmugam N, Balasubramanian T (2015) Green synthesis of silver nanoparticles using marine algae Caulerpa racemosa and their antibacterial activity against some human pathogens. Appl Nanosci (Switzerland) 5:499–504. https://doi.org/10.1007/s13204-014-0341-2
El-Sheekh MM, Shabaan MT, Hassan L, Morsi HH (2020) Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herpes Simplex (HSV-1) virus in vitro using cell-line culture technique. Int J Environ Health Res 32:1–12. https://doi.org/10.1080/09603123.2020.1789946
Sinha SN, Paul D, Halder N, Sengupta D, Patra SK (2015) Green synthesis of silver nanoparticles using fresh water green alga Pithophora oedogonia (Mont.) Wittrock and evaluation of their antibacterial activity. Appl Nanosci (Switzerland) 5:703–709. https://doi.org/10.1007/s13204-014-0366-6
Aboelfetoh EF, El-Shenody RA, Ghobara MM (2017) Eco-friendly synthesis of silver nanoparticles using green algae (Caulerpa serrulata): reaction optimization, catalytic and antibacterial activities. Environ Monit Assess. https://doi.org/10.1007/s10661-017-6033-0
Dhanasezhian A, Srivani S, Govindaraju K, Parija P, Sasikala S, Kumar MR (2019) Anti-herpes simplex virus (HSV-1 and HSV-2) activity of biogenic gold and silver nanoparticles using seaweed Sargassum wightii. Indian J Mar Sci 48:1252–1257
Naveena BE, Prakash S (2013) Biological synthesis of gold nanoparticles using marine algae Gracilaria corticata and its application as a potent antimicrobial and antioxidant agent. Asian J Pharm Clin Res 6:179–182
Singh M, Saurav K, Majouga A, Kumari M, Kumar M, Manikandan S, Kumaraguru AK (2015) The cytotoxicity and cellular stress by temperature-fabricated polyshaped gold nanoparticles using marine macroalgae, Padina gymnospora. Biotechnol Appl Biochem 62:424–432. https://doi.org/10.1002/bab.1271
Singh KR, Nayak V, Singh J, Singh AK, Singh RP (2021) Potentialities of bioinspired metal and metal oxide nanoparticles in biomedical sciences. RSC Adv 11:24722–24746. https://doi.org/10.1039/d1ra04273d
Al-kawmani AA, Alanazi KM, Farah MA, Ali MA, Hailan WA, Al-Hemaid FM (2020) Apoptosis-inducing potential of biosynthesized silver nanoparticles in breast cancer cells. J King Saud Univ Sci 32(4):2480–2488. https://doi.org/10.1016/j.jksus.2020.04.002
Eid AM, Fouda A, Niedbała G, Hassan SE, Salem SS, Abdo AM, F. Hetta H, Shaheen TI, (2020) Endophytic Streptomyces laurentii mediated green synthesis of Ag-NPs with antibacterial and anticancer properties for developing functional textile fabric properties. Antibiotics 9:1–18. https://doi.org/10.3390/antibiotics9100641
Biswas AK, Islam MR, Choudhury ZS, Mostafa A, Kadir MF (2014) Nanotechnology based approaches in cancer therapeutics. Adv Nat Sci J Nanosci Nanotechnol 5(4):043001. https://doi.org/10.1088/2043-6262/5/4/043001
Ullah I, Khalil AT, Ali M, Iqbal J, Ali W, Alarifi S, Shinwari ZK (2020) Green-synthesized silver nanoparticles induced apoptotic cell death in MCF-7 breast cancer cells by generating reactive oxygen species and activating caspase 3 and 9 enzyme activities. Oxid Med Cell Longev 1215395:1–14. https://doi.org/10.1155/2020/1215395
Jain N, Jain P, Rajput D, Patil UK (2021) Green synthesized plant-based silver nanoparticles: therapeutic prospective for anticancer and antiviral activity. Micro Nano Syst Lett. https://doi.org/10.1186/s40486-021-00131-6
Kumari R, Saini AK, Kumar A, Saini RV (2020) Apoptosis induction in lung and prostate cancer cells through silver nanoparticles synthesized from Pinus roxburghii bioactive fraction. J Biol Inorg Chem 25:23–37. https://doi.org/10.1007/s00775-019-01729-3
Li Y, Ke Y, Zou H, Wang K, Huang S, Rengarajan T, Wang L (2019) Gold nano particles synthesized from Strychni semen and its anticancer activity in cholangiocarcinoma cell (KMCH-1). Artif Cells Nanomed Biotechnol 47:1610–1616. https://doi.org/10.1080/21691401.2019.1594860
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F (2021) Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F (2019) Breast cancer. Nat Rev Dis Primers. https://doi.org/10.1038/s41572-019-0111-2
George BP, Rajendran NK, Houreld NN, Abrahamse H (2022) Rubus capped zinc oxide nanoparticles induce apoptosis in MCF-7. Breast Cancer Cell Mol. https://doi.org/10.3390/molecules27206862
Zhai C, Shi C, Hu Y, Xu Z, Wang R (2022) Anti-breast carcinoma effects of green synthesized tin nanoparticles from Calendula officinalis leaf aqueous extract inhibits MCF7, Hs 319.T, and MCF10 cells proliferation. J Exp Nanosci 17:351–361. https://doi.org/10.1080/17458080.2022.2076836
Mahendran D, Kavi Kishor PB, Geetha N, Manish T, Sahi SV, Venkatachalam P (2021) Efficient antibacterial/biofilm, anti-cancer and photocatalytic potential of titanium dioxide nanocatalysts green synthesised using Gloriosa superba rhizome extract. J Exp Nanosci 16:11–31. https://doi.org/10.1080/17458080.2021.1872781
Yugandhar P, Vasavi T, Uma Maheswari Devi P, Savithramma N (2017) Bioinspired green synthesis of copper oxide nanoparticles from Syzygium alternifolium (Wt.) Walp: characterization and evaluation of its synergistic antimicrobial and anticancer activity. Appl Nanosci (Switzerland) 7:417–427. https://doi.org/10.1007/s13204-017-0584-9
Parvathy S, Manjula G, Balachandar R, Subbaiya R (2022) Green synthesis and characterization of cerium oxide nanoparticles from Artabotrys hexapetalus leaf extract and its antibacterial and anticancer properties. Mater Lett. https://doi.org/10.1016/j.matlet.2022.131811
Al-Nuairi AG, Mosa KA, Mohammad MG, El-Keblawy A, Soliman S, Alawadhi H (2020) Biosynthesis, characterization, and evaluation of the cytotoxic effects of biologically synthesized silver nanoparticles from Cyperus conglomeratus root extracts on breast cancer cell line MCF-7. Biol Trace Elem Res 194:560–569. https://doi.org/10.1007/s12011-019-01791-7
Kabir SR, Asaduzzaman AK, Amin R, Haque AT, Ghose R, Rahman MM, Islam J, Amin MB, Hasan I, Debnath T, Chun BS (2020) Zizyphus mauritiana fruit extract-mediated synthesized silver/silver chloride nanoparticles retain antimicrobial activity and induce apoptosis in MCF-7 cells through the Fas pathway. ACS Omega 5:20599–20608. https://doi.org/10.1021/acsomega.0c02878
Şahin B, Aygün A, Gündüz H, Şahin K, Demir E, Akocak S, Şen F (2018) Cytotoxic effects of platinum nanoparticles obtained from pomegranate extract by the green synthesis method on the MCF-7 cell line. Colloids Surf B Biointerfaces 163:119–124. https://doi.org/10.1016/j.colsurfb.2017.12.042
Gomathi AC, Rajarathinam SX, Sadiq AM, Rajeshkumar S (2020) Anticancer activity of silver nanoparticles synthesized using aqueous fruit shell extract of Tamarindus indica on MCF-7 human breast cancer cell line. J Drug Deliv Sci Technol 55:101376. https://doi.org/10.1016/j.jddst.2019.101376
Khateef R, Khadri H, Almatroudi A, Alsuhaibani SA, Mobeen SA, Khan RA (2019) Potential in-vitro anti-breast cancer activity of green-synthesized silver nanoparticles preparation against human MCF-7 cell-lines. Adv Nat Sci Nanosci Nanotechnol. https://doi.org/10.1088/2043-6254/ab47ff
Oves M, Aslam M, Rauf MA, Qayyum S, Qari HA, Khan MS, Alam MZ, Tabrez S, Pugazhendhi A, Ismail IM (2018) Antimicrobial and anticancer activities of silver nanoparticles synthesized from the root hair extract of Phoenix dactylifera. Mater Sci Eng C 89:429–443. https://doi.org/10.1016/j.msec.2018.03.035
Oves M, Rauf MA, Aslam M, Qari HA, Sonbol H, Ahmad I, Zaman GS, Saeed M (2022) Green synthesis of silver nanoparticles by Conocarpus Lancifolius plant extract and their antimicrobial and anticancer activities. Saudi J Biol Sci 29:460–471. https://doi.org/10.1016/j.sjbs.2021.09.007
Li S, Al-Misned FA, El-Serehy HA, Yang L (2021) Green synthesis of gold nanoparticles using aqueous extract of Mentha Longifolia leaf and investigation of its anti-human breast carcinoma properties in the in vitro condition. Arab J Chem 14:102931. https://doi.org/10.1016/j.arabjc.2020.102931
Hosny M, Fawzy M, El-Badry YA, Hussein EE, Eltaweil AS (2022) Plant-assisted synthesis of gold nanoparticles for photocatalytic, anticancer, and antioxidant applications. J Saudi Chem Soc. https://doi.org/10.1016/j.jscs.2022.101419
Biresaw SS, Taneja P (2020) Copper nanoparticles green synthesis and characterization as anticancer potential in breast cancer cells (MCF7) derived from Prunus nepalensis phytochemicals. Mater Today Proc 49:3501–3509. https://doi.org/10.1016/j.matpr.2021.07.149
Sivaraj R, Rahman PK, Rajiv P, Narendhran S, Venckatesh R (2014) Biosynthesis and characterization of Acalypha indica mediated copper oxide nanoparticles and evaluation of its antimicrobial and anticancer activity. Spectrochim Acta A Mol Biomol Spectrosc 129:255–258. https://doi.org/10.1016/j.saa.2014.03.027
Rajagopal G, Nivetha A, Sundar M, Panneerselvam T, Murugesan S, Parasuraman P, Kumar S, Ilango S, Kunjiappan S (2021) Mixed phytochemicals mediated synthesis of copper nanoparticles for anticancer and larvicidal applications. Heliyon. https://doi.org/10.1016/j.heliyon.2021.e07360
Iqbal H, Razzaq A, Uzair B, Ul Ain N, Sajjad S, Althobaiti NA et al (2021) Breast cancer inhibition by biosynthesized titanium dioxide nanoparticles is comparable to free doxorubicin but appeared safer in balb/c mice. Materials 14:12. https://doi.org/10.3390/ma14123155
Sargazi S, Laraib U, Er S, Rahdar A, Hassanisaadi M, Zafar MN, Diez-Pascual AM, Bilal M (2022) Application of green gold nanoparticles in cancer therapy and diagnosis. Nanomaterials 12(7):1102. https://doi.org/10.3390/nano12071102
Barta JA, Powell CA, Wisnivesky JP (2019) Global epidemiology of lung cancer. Ann Glob Health. https://doi.org/10.5334/aogh.2419
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90. https://doi.org/10.3322/caac.20107
Nagalingam M, Kalpana VN, Panneerselvam A (2018) Biosynthesis, characterization, and evaluation of bioactivities of leaf extract-mediated biocompatible gold nanoparticles from Alternanthera bettzickiana. Biotechnol Rep. https://doi.org/10.1016/j.btre.2018.e00268
Kanipandian N, Kannan S, Ramesh R, Subramanian P, Thirumurugan R (2014) Characterization, antioxidant and cytotoxicity evaluation of green synthesized silver nanoparticles using Cleistanthus collinus extract as surface modifier. Mater Res Bull 49:494–502. https://doi.org/10.1016/j.materresbull.2013.09.016
Zheng Y, Zhang J, Zhang R, Luo Z, Wang C, Shi S (2019) Gold nano particles synthesized from Magnolia officinalis and anticancer activity in A549 lung cancer cells. Artif Cells Nanomed Biotechnol 47:3101–3109. https://doi.org/10.1080/21691401.2019.1645152
Aswini R, Murugesan S, Kannan K (2021) Bio-engineered TiO2 nanoparticles using Ledebouria revoluta extract: Larvicidal, histopathological, antibacterial and anticancer activity. Int J Environ Anal Chem 101:2926–2936. https://doi.org/10.1080/03067319.2020.1718668
Devendrapandi G, Sahay MI, Padmanaban D, Panneerselvam A, Palraj R, Thanikasalam R, Sadaiyandi V, Balu R, Rajendiran N (2023) Biogenic synthesis of gold nanoparticles using bael fruit juice and its efficacy against human A-549 lung cancer cell line. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2023.110636
Dobrucka R, Romaniuk-Drapała A, Kaczmarek M (2019) Evaluation of biological synthesized platinum nanoparticles using Ononidis radix extract on the cell lung carcinoma A549. Biomed Microdev. https://doi.org/10.1007/s10544-019-0424-7
Rajivgandhi GN, Chackaravarthi G, Ramachandran G, Manoharan N, Ragunathan R, Siddiqi MZ, Alharbi NS, Khaled JM, Li WJ (2022) Synthesis of silver nanoparticle (Ag NPs) using phytochemical rich medicinal plant Lonicera japonica for improve the cytotoxicity effect in cancer cells. J King Saud Univ Sci. https://doi.org/10.1016/j.jksus.2021.101798
He Y, Du Z, Ma S, Liu Y, Li D, Huang H, Jiang S, Cheng S, Wu W, Zhang K, Zheng X (2016) Effects of green-synthesized silver nanoparticles on lung cancer cells in vitro and grown as xenograft tumors in vivo. Int J Nanomed 11:1879–1887. https://doi.org/10.2147/IJN.S103695
Valodkar M, Jadeja RN, Thounaojam MC, Devkar RV, Thakore S (2011) In vitro toxicity study of plant latex capped silver nanoparticles in human lung carcinoma cells. Mater Sci Eng C 31:1723–1728. https://doi.org/10.1016/j.msec.2011.08.001
Chen F, Zheng Q, Li X, Xiong J (2022) Citrus sinensis leaf aqueous extract green-synthesized silver nanoparticles: characterization and cytotoxicity, antioxidant, and anti-human lung carcinoma effects. Arab J Chem 15:103845. https://doi.org/10.1016/j.arabjc.2022.103845
Zhang H, Li T, Luo W, Peng GX, Xiong J (2022) Green synthesis of Ag nanoparticles from Leucus aspera and its application in anticancer activity against alveolar cancer. J Exp Nanosci 17:47–60. https://doi.org/10.1080/17458080.2021.2007886
Tian S, Saravanan K, Mothana RA, Ramachandran G, Rajivgandhi G, Manoharan N (2020) Anti-cancer activity of biosynthesized silver nanoparticles using Avicennia marina against A549 lung cancer cells through ROS/mitochondrial damages. Saudi J Biol Sci 27:3018–3024. https://doi.org/10.1016/j.sjbs.2020.08.029
Lakshmanan G, Sathiyaseelan A, Kalaichelvan PT, Murugesan K (2018) Plant-mediated synthesis of silver nanoparticles using fruit extract of Cleome viscosa L.: assessment of their antibacterial and anticancer activity. Karbala Int J Mod Sci 4:61–68. https://doi.org/10.1016/j.kijoms.2017.10.007
Zhang X, Tan Z, Jia K, Zhang W, Dang M (2019) Rabdosia rubescens Linn: green synthesis of gold nanoparticles and their anticancer effects against human lung cancer cells A549. Artif Cells Nanomed Biotechnol 47:2171–2178. https://doi.org/10.1080/21691401.2019.1620249
Vijayakumar S, Vaseeharan B, Malaikozhundan B, Gopi N, Ekambaram P, Pachaiappan R, Velusamy P, Murugan K, Benelli G, Kumar RS, Suriyanarayanamoorthy M (2017) Therapeutic effects of gold nanoparticles synthesized using Musa paradisiaca peel extract against multiple antibiotic resistant Enterococcus faecalis biofilms and human lung cancer cells (A549). Microb Pathog 102:173–183. https://doi.org/10.1016/j.micpath.2016.11.029
Sun B, Hu N, Han L, Pi Y, Gao Y, Chen K (2019) Anticancer activity of green synthesised gold nanoparticles from Marsdenia tenacissima inhibits A549 cell proliferation through the apoptotic pathway. Artif Cells Nanomed Biotechnol 47:4012–4019. https://doi.org/10.1080/21691401.2019.1575844
Rani N, Rawat K, Shrivastava A, Yadav S, Gupta K, Saini K (2022) In Vitro study of green synthesized ZnO nanoparticles on human lung cancer cell lines. Mater Today Proc 49:1436–1442. https://doi.org/10.1016/j.matpr.2021.07.203
Zhang H, Liang Z, Zhang J, Wang WP, Zhang H, Lu Q (2020) Zinc oxide nanoparticle synthesized from Euphorbia fischeriana root inhibits the cancer cell growth through modulation of apoptotic signaling pathways in lung cancer cells. Arab J Chem 13:6174–6183. https://doi.org/10.1016/j.arabjc.2020.05.020
Rajeshkumar S, Kumar SV, Ramaiah A, Agarwal H, Lakshmi T, Roopan SM (2018) Biosynthesis of zinc oxide nanoparticles using Mangifera indica leaves and evaluation of their antioxidant and cytotoxic properties in lung cancer (A549) cells. Enzyme Microb Technol 117:91–95. https://doi.org/10.1016/j.enzmictec.2018.06.009
Gu J, Aidy A, Goorani S (2022) Anti-human lung adenocarcinoma, cytotoxicity, and antioxidant potentials of copper nanoparticles green-synthesized by Calendula officinalis. J Exp Nanosci 17:285–296. https://doi.org/10.1080/17458080.2022.2066082
Sankar R, Maheswari R, Karthik S, Shivashangari KS, Ravikumar V (2014) Anticancer activity of Ficus religiosa engineered copper oxide nanoparticles. Mater Sci Eng C 44:234–239. https://doi.org/10.1016/j.msec.2014.08.030
Majid A, Faraj HR (2022) Green synthesis of copper nanoparticles using aqueous extract of Yerba Mate (llex Paraguarients St Hill) and its anticancer activity. Int J Nanosci Nanotechnol 18(2):99–108
Chandrasekaran R, Yadav SA, Sivaperumal S (2020) Phytosynthesis and characterization of copper oxide nanoparticles using the aqueous extract of Beta vulgaris L and evaluation of their antibacterial and anticancer activities. J Clust Sci 31:221–230. https://doi.org/10.1007/s10876-019-01640-6
Gu J, Chen F, Zheng Z, Bi L, Morovvati H, Goorani S (2023) Novel green formulation of copper nanoparticles by Foeniculum vulgare: Chemical characterization and determination of cytotoxicity, anti-human lung cancer and antioxidant effects. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2023.110442
Singh D, Vignat J, Lorenzoni V, Eslahi M, Ginsburg O, Lauby-Secretan B, Arbyn M, Basu P, Bray F, Vaccarella S (2020) Articles Global estimates of incidence and mortality of cervical cancer in 2020: a baseline analysis of the WHO Global Cervical Cancer Elimination Initiative. Lancet Glob Health 11:197–206. https://doi.org/10.1016/S2214-109X(22)00501-0
Thomas S, Gunasangkaran G, Arumugam VA, Muthukrishnan S (2022) Synthesis and characterization of zinc oxide nanoparticles of Solanum nigrum and its anticancer activity via the induction of apoptosis in cervical cancer. Biol Trace Elem Res 200:2684–2697. https://doi.org/10.1007/s12011-021-02898-6
Ke Y, Al Aboody MS, Alturaiki W, Alsagaby SA, Alfaiz FA, Veeraraghavan VP, Mickymaray S (2019) Photosynthesized gold nanoparticles from Catharanthus roseus induces caspase-mediated apoptosis in cervical cancer cells (HeLa). Artif Cells Nanomed Biotechnol 47:1938–1946. https://doi.org/10.1080/21691401.2019.1614017
Tripathi D, Modi A, Smita SS, Narayan G, Pandey-Rai S (2022) Biomedical potential of green synthesized silver nanoparticles from root extract of Asparagus officinalis. J Plant Biochem Biotechnol 31:213–218. https://doi.org/10.1007/s13562-021-00684-y
Rajkuberan C, Prabukumar S, Sathishkumar G, Wilson A, Ravindran K, Sivaramakrishnan S (2017) Facile synthesis of silver nanoparticles using Euphorbia antiquorum L. latex extract and evaluation of their biomedical perspectives as anticancer agents. J Saudi Chem Soc 21:911–919. https://doi.org/10.1016/j.jscs.2016.01.002
Qian L, Su W, Wang Y, Dang M, Zhang W, Wang C (2019) Synthesis and characterization of gold nanoparticles from aqueous leaf extract of Alternanthera sessilis and its anticancer activity on cervical cancer cells (HeLa). Artif Cells Nanomed Biotechnol 47:1173–1180. https://doi.org/10.1080/21691401.2018.1549064
Nagajyothi PC, Muthuraman P, Sreekanth TV, Kim DH, Shim J (2017) Green synthesis: In-vitro anticancer activity of copper oxide nanoparticles against human cervical carcinoma cells. Arab J Chem 10:215–225. https://doi.org/10.1016/j.arabjc.2016.01.011
Al-Sheddi ES, Farshori NN, Al-Oqail MM, Al-Massarani SM, Saquib Q, Wahab R, Musarrat J, Al-Khedhairy AA, Siddiqui MA (2018) Anticancer potential of green synthesized silver nanoparticles using extract of Nepeta deflersiana against human cervical cancer cells (HeLA). Bioinorg Chem Appl. https://doi.org/10.1155/2018/9390784
asanth K, Ilango K, MohanKumar R, Agrawal A, Dubey GP, (2014) Anticancer activity of Moringa oleifera mediated silver nanoparticles on human cervical carcinoma cells by apoptosis induction. Colloids Surf B Biointerfaces 117:354–359. https://doi.org/10.1016/j.colsurfb.2014.02.052
Adebayo IA, Arsad H, Gagman HA, Ismail NZ, Samian MR (2020) Inhibitory effect of eco-friendly naturally synthesized silver nanoparticles from the leaf extract of medicinal Detarium microcarpum plant on pancreatic and cervical cancer cells. Asian Pac J Cancer Prev 5:1247–1252. https://doi.org/10.31557/APJCP.2020.21.5.1247
Khatua A, Prasad A, Priyadarshini E, Patel AK, Naik A, Saravanan M, Barabadi H, Ghosh L, Paul B, Paulraj R, Meena R (2020) Emerging antineoplastic plant-based gold nanoparticle synthesis: a mechanistic exploration of their anticancer activity toward cervical cancer cells. J Clust Sci 31:329–1340. https://doi.org/10.1007/s10876-019-01742-1
Sundaram CS, Kumar JS, Kumar SS, Ramesh PL, Zin T, Rao UM (2020) Antibacterial and anticancer potential of Brassica oleracea var acephala using biosynthesised copper nanoparticles. Med J Malays 75(6):677–684
Chen H, Feng X, Gao L, Mickymaray S, Paramasivam A, Abdulaziz Alfaiz F et al (2021) Inhibiting the PI3K/AKT/mTOR signalling pathway with copper oxide nanoparticles from Houttuynia cordata plant: attenuating the proliferation of cervical cancer cells. Artif Cells Nanomed Biotechnol 49(1):240–249. https://doi.org/10.1080/21691401.2021.1890101
Narayanan M, Vigneshwari P, Natarajan D, Kandasamy S, Alsehli M, Elfasakhany A, Pugazhendhi A (2021) Synthesis and characterization of TiO2 NPs by aqueous leaf extract of Coleus aromaticus and assess their antibacterial, larvicidal, and anticancer potential. Environ Res. https://doi.org/10.1016/j.envres.2021.111335
Abdellatif AA, Mahmood A, Alsharidah M, Mohammed HA, Alenize SK, Bouazzaoui A, Al Rugaie O, Alnuqaydan AM, Ahmad R, Vaali-Mohammad MA, Alfayez M (2022) Bioactivities of the green synthesized silver nanoparticles reduced using Allium cepa L aqueous extracts induced apoptosis in colorectal cancer cell lines. J Nanomater. https://doi.org/10.1155/2022/1746817
Malaikolundhan H, Mookkan G, Krishnamoorthi G, Matheswaran N, Alsawalha M, Veeraraghavan VP, Krishna Mohan S, Di A (2020) Anticarcinogenic effect of gold nanoparticles synthesized from Albizia lebbeck on HCT-116 colon cancer cell lines. Artif Cells Nanomed Biotechnol 48:1206–1213. https://doi.org/10.1080/21691401.2020.1814313
Devanesan S, Al Salhi MS, Vishnubalaji R, Alfuraydi AA, Alajez NM, Alfayez M, Murugan K, Sayed SR, Nicoletti M, Benelli G (2017) Rapid biological synthesis of silver nanoparticles using plant seed extracts and their cytotoxicity on colorectal cancer cell lines. J Clust Sci 28:595–605. https://doi.org/10.1007/s10876-016-1134-4
Han X, Jiang X, Guo L, Wang Y, Veeraraghavan VP, Krishna Mohan S, Wang Z, Cao D (2019) Anticarcinogenic potential of gold nanoparticles synthesized from Trichosanthes kirilowii in colon cancer cells through the induction of apoptotic pathway. Artif Cells Nanomed Biotechnol 47:3577–3584. https://doi.org/10.1080/21691401.2019.1626412
Nazaripour E, Mousazadeh F, Moghadam MD, Najafi K, Borhani F, Sarani M, Ghasemi M, Rahdar A, Iravani S, Khatami M (2021) Biosynthesis of lead oxide and cerium oxide nanoparticles and their cytotoxic activities against colon cancer cell line. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2021.108800
Venkatadri B, Shanparvish E, Rameshkumar MR, Arasu MV, Al-Dhabi NA, Ponnusamy VK, Agastian P (2020) Green synthesis of silver nanoparticles using aqueous rhizome extract of Zingiber officinale and Curcuma longa: i3n-vitro anti-cancer potential on human colon carcinoma HT-29 cells. Saudi J Biol Sci 27:2980–2986. https://doi.org/10.1016/j.sjbs.2020.09.021
Dehghanizade S, Arasteh J, Mirzaie A (2018) Green synthesis of silver nanoparticles using Anthemis atropatana extract: characterization and in vitro biological activities. Artif Cells Nanomed Biotechnol 46:160–168. https://doi.org/10.1080/21691401.2017.1304402
Prabhu D, Arulvasu C, Babu G, Manikandan R, Srinivasan P (2013) Biologically synthesized green silver nanoparticles from leaf extract of Vitex negundo L. induce growth-inhibitory effect on human colon cancer cell line HCT15. Process Biochem 48(2):317–324. https://doi.org/10.1016/j.procbio.2012.12.013
Chhikara BS, Parang K (2023) Global Cancer Statistics 2022: the trends projection analysis. Chem Biol Lett 10(1):451–451
Vimal TG (2022) Green synthesis of silver nanoparticles using Rosmarinus officinalis leaf extract and study of anticancer effect and apoptosis induction on prostate cancer cell line (PC-3). J Pharm Negat Results 13:3531–3536. https://doi.org/10.47750/pnr.2022.13.S06.472
Firdhouse MJ, Lalitha P (2013) Biosynthesis of silver nanoparticles using the extract of Alternanthera sessilis-antiproliferative effect against prostate cancer cells. Cancer Nanotechnol 4:137–143. https://doi.org/10.1007/s12645-013-0045-4
Prema P, Boobalan T, Arun A, Rameshkumar K, Babu RS, Veeramanikandan V, Nguyen VH, Balaji P (2022) Green tea extract mediated biogenic synthesis of gold nanoparticles with potent anti-proliferative effect against PC-3 human prostate cancer cells. Mater Lett. https://doi.org/10.1016/j.matlet.2021.130882
Obaid AS, Hassan KT, Hassan OM, Ali HH, Ibraheem IJ, Salih TA, Adil BH, Almoneef MM (2023) In-vitro antibacterial, cytotoxicity, and anti-prostate cancer effects of gold nanoparticles synthesized using extract of desert truffles (Tirmania nivea). Mater Chem Phys. https://doi.org/10.1016/j.matchemphys.2023.127673
Zhang K, Liu X, Samuel Ravi SO, Ramachandran A, Aziz Ibrahim IA, Nassir MA, Yao J (2019) Synthesis of silver nanoparticles (AgNPs) from leaf extract of Salvia miltiorrhiza and its anticancer potential in human prostate cancer LNCaP cell lines. Artif Cells Nanomed Biotechnol 47:2846–2854. https://doi.org/10.1080/21691401.2019.1638792
Rahimi Kalateh Shah Mohammad G, Karimi E, Oskoueian E, Homayouni-Tabrizi M (2020) Anticancer properties of green-synthesised zinc oxide nanoparticles using Hyssopus officinalis extract on prostate carcinoma cells and its effects on testicular damage and spermatogenesis in Balb/C mice. Andrologia. https://doi.org/10.1111/and.13450
He Y, Du Z, Ma S, Cheng S, Jiang S, Liu Y, Li D, Huang H, Zhang K, Zheng X (2016) Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using Dimocarpus Longan Lour. Peel Extract Nanoscale Res Lett. https://doi.org/10.1186/s11671-016-1511-9
Singh SP, Mishra A, Shyanti RK, Singh RP, Acharya A (2021) Silver nanoparticles synthesized using Carica papaya leaf extract (AgNPs-PLE) causes cell cycle arrest and apoptosis in human prostate (DU145) cancer cells. Biol Trace Elem Res 199:1316–1331. https://doi.org/10.1007/s12011-020-02255-z
Adeyemi JO, Onwudiwe DC, Oyedeji AO (2022) Biogenic synthesis of CuO, ZnO, and CuO–ZnO nanoparticles using leaf extracts of Dovyalis caffra and their biological properties. Molecules. https://doi.org/10.3390/molecules27103206
Netala VR, Bukke S, Domdi L, Soneya S, Reddy GS, Bethu MS, Kotakdi VS, Saritha KV, Tartte V (2018) Biogenesis of silver nanoparticles using leaf extract of Indigofera hirsuta L. and their potential biomedical applications (3-in-1 system). Artif Cells Nanomed Biotechnol 46:1138–1148. https://doi.org/10.1080/21691401.2018.1446967
Zhao W, Wang L, Chen H, Qi L, Yang R, Ouyang T, Ning L (2022) Green synthesis, characterization and determination of anti-prostate cancer, cytotoxicity and antioxidant effects of gold nanoparticles synthesized using Alhagi maurorum. Inorg Chem Commun. https://doi.org/10.1016/j.inoche.2022.109525
Wu F, Zhu J, Li G, Wang J, Veeraraghavan VP, Krishna Mohan S, Zhang Q (2019) Biologically synthesized green gold nanoparticles from Siberian ginseng induce growth-inhibitory effect on melanoma cells (B16). Artif Cells Nanomed Biotechnol 47:3297–3305. https://doi.org/10.1080/21691401.2019.1647224
Mohanta YK, Panda SK, Biswas K, Tamang A, Bandyopadhyay J, De D, Mohanta D, Bastia AK (2016) Biogenic synthesis of silver nanoparticles from Cassia fistula (Linn.): in vitro assessment of their antioxidant, antimicrobial and cytotoxic activities. IET Nanobiotechnol 10:438–444. https://doi.org/10.1049/iet-nbt.2015.0104
Mukhopadhyay R, Kazi J, Debnath MC (2018) Synthesis and characterization of copper nanoparticles stabilized with Quisqualis indica extract: evaluation of its cytotoxicity and apoptosis in B16F10 melanoma cells. Biomed Pharmacother 97:1373–1385. https://doi.org/10.1016/j.biopha.2017.10.167
Nirmala JG, Akila S, Narendhirakannan RT, Chatterjee S (2017) Vitis vinifera peel polyphenols stabilized gold nanoparticles induce cytotoxicity and apoptotic cell death in A431 skin cancer cell lines. Adv Powder Technol 28:1170–1184. https://doi.org/10.1016/j.apt.2017.02.003
Suseela V (2015) Cytotoxic effect of green synthesized silver nanoparticles using Indigofera longeracemosa on skin cancer SK MEL-28 cell lines. Int J Preclin Pharm Res 6(3):118–125
Francis S, Joseph S, Koshy EP, Mathew B (2018) Microwave assisted green synthesis of silver nanoparticles using leaf extract of Elephantopus scaber and its environmental and biological applications. Artif Cells Nanomed Biotechnol 46:795–804. https://doi.org/10.1080/21691401.2017.1345921
Nayak D, Pradhan S, Ashe S, Rauta PR, Nayak B (2015) Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J Colloid Interface Sci 457:329–338. https://doi.org/10.1016/j.jcis.2015.07.012
Saber MM, Mirtajani SB, Karimzadeh K (2018) Green synthesis of silver nanoparticles using Trapa natans extract and their anticancer activity against A431 human skin cancer cells. J Drug Deliv Sci Technol 47:375–379. https://doi.org/10.1016/j.jddst.2018.08.004
Vakayil R, Muruganantham S, Kabeerdass N, Rajendran M, MahadeoPalve A, Ramasamy S et al (2021) Acorus calamus-zinc oxide nanoparticle coated cotton fabrics shows antimicrobial and cytotoxic activities against skin cancer cells. Process Biochem 111:1–8. https://doi.org/10.1016/j.procbio.2021.08.024
Wu H, Wang MD, Zhu JQ, Li ZL, Wang WY, Gu LH, Shen F, Yang T (2022) Mesoporous nanoparticles for diagnosis and treatment of liver cancer in the era of precise medicine. Pharmaceutics. https://doi.org/10.3390/pharmaceutics14091760
Ji Y, Cao Y, Song Y (2019) Green synthesis of gold nanoparticles using a Cordyceps militaris extract and their antiproliferative effect in liver cancer cells (HepG2). Artif Cells Nanomed Biotechnol 47:2737–2745. https://doi.org/10.1080/21691401.2019.1629952
Zhan Q, Han J, Sheng L (2021) Iron nanoparticles green-formulated by Coriandrum sativum leaf aqueous extract: investigation of its anti-liver cancer effects. Arch Med Sci. https://doi.org/10.5114/aoms/144627
Saratale RG, Shin HS, Kumar G, Benelli G, Kim DS, Saratale GD (2018) Exploiting antidiabetic activity of silver nanoparticles synthesized using Punica granatum leaves and anticancer potential against human liver cancer cells (HepG2). Artif Cells Nanomed Biotechnol 46:211–222. https://doi.org/10.1080/21691401.2017.1337031
Inbathamizh L, Ponnu TM, Mary EJ (2013) In vitro evaluation of antioxidant and anticancer potential of Morinda pubescens synthesized silver nanoparticles. J Pharm Res 6:32–38. https://doi.org/10.1016/j.jopr.2012.11.010
Mohammadi Shivyari A, Tafvizi F, Noorbazargan H (2022) Anti-cancer effects of biosynthesized zinc oxide nanoparticles using Artemisia scoparia in Huh-7 liver cancer cells. Inorg Nano-Met 52:375–386. https://doi.org/10.1080/24701556.2021.1980018
Qasim Nasar M, Zohra T, Khalil AT, Saqib S, Ayaz M, Ahmad A, Shinwari ZK (2019) Seripheidium quettense mediated green synthesis of biogenic silver nanoparticles and their theranostic applications. Green Chem Lett Rev 12:310–322. https://doi.org/10.1080/17518253.2019.1643929
Taghavizadeh Yazdi ME, Darroudi M, Amiri MS, Hosseini HA, Nourbakhsh F, Mashreghi M, Farjadi M, Mousavi Kouhi SM, Mousavi SH (2020) Anticancer, antimicrobial, and dye degradation activity of biosynthesised silver nanoparticle using Artemisia kopetdaghensis. Micro Nano Lett 15:1065–1070. https://doi.org/10.1049/mnl.2020.0387
Ashokkumar T, Prabhu D, Geetha R, Govindaraju K, Manikandan R, Arulvasu C, Singaravelu G (2014) Apoptosis in liver cancer (HepG2) cells induced by functionalized gold nanoparticles. Colloids Surf B Biointerfaces 123:549–556. https://doi.org/10.1016/j.colsurfb.2014.09.051
Amuthavalli P, Hwang JS, Dahms HU, Wang L, Anitha J, Vasanthakumaran M, Gandhi AD, Murugan K, Subramaniam J, Paulpandi M, Chandramohan B (2021) Zinc oxide nanoparticles using plant Lawsonia inermis and their mosquitocidal, antimicrobial, anticancer applications showing moderate side effects. Sci Rep. https://doi.org/10.1038/s41598-021-88164-0
Chung IM, Abdul Rahuman A, Marimuthu S, Vishnu Kirthi A, Anbarasan K, Rajakumar G (2015) An investigation of the cytotoxicity and caspase-mediated apoptotic effect of green synthesized zinc oxide nanoparticles using Eclipta prostrata on human liver carcinoma cells. Nanomaterials 5:1317–1330. https://doi.org/10.3390/nano5031317
Chung IM, Abdul Rahuman A, Marimuthu S, Vishnu Kirthi A, Anbarasan K, Padmini P, Rajakumar G (2017) Green synthesis of copper nanoparticles using Eclipta prostrata leaves extract and their antioxidant and cytotoxic activities. Exp Ther Med 14:18–24. https://doi.org/10.3892/etm.2017.4466
Liu L, Kshirsagar PG, Gautam SK, Gulati M, Wafa EI, Christiansen JC, White BM, Mallapragada SK, Wannemuehler MJ, Kumar S, Solheim JC (2022) Nanocarriers for pancreatic cancer imaging, treatments, and immunotherapies. Theranostics 12(3):1030. https://doi.org/10.7150/thno.64805
Fazal S, Jayasree A, Sasidharan S, Koyakutty M, Nair SV, Menon D (2014) Green synthesis of anisotropic gold nanoparticles for photothermal therapy of cancer. ACS Appl Mater Interfaces 11:8080–8089. https://doi.org/10.1021/am500302t
Chanda N, Shukla R, Zambre A, Mekapothula S, Kulkarni RR, Katti K et al (2011) An effective strategy for the synthesis of biocompatible gold nanoparticles using cinnamon phytochemicals for phantom CT imaging and photoacoustic detection of cancerous cells. Pharm Res 2:279–291. https://doi.org/10.1007/s11095-010-0276-6
Fathy MM (2020) Biosynthesis of silver nanoparticles using thymoquinone and evaluation of their radio-sensitizing activity. Bionanoscience 10(1):260–266. https://doi.org/10.1007/s12668-019-00702-3
Mosleh-Shirazi S, Kasaee SR, Dehghani F, Kamyab H, Kirpichnikova I, Chelliapan S, Firuzyar T, Akhtari M, Amani AM (2023) Investigation through the anticancer properties of green synthesized spinel ferrite nanoparticles in present and absent of laser photothermal effect. Ceram Int 49(7):11293–11301. https://doi.org/10.1016/j.ceramint.2022.11.329
Yusefi M, Shameli K, Hedayatnasab Z, Teow SY, Ismail UN, Azlan CA et al (2021) Green synthesis of Fe3O4 nanoparticles for hyperthermia, magnetic resonance imaging and 5-fluorouracil carrier in potential colorectal cancer treatment. Res Chem Intermed 47(5):1789–1808. https://doi.org/10.1007/s11164-020-04388-1
Acknowledgements
Authors are thankful to the UPES University for providing all necessary facilities to write this communication.
Funding
This work was not funded or supported by any persons or group.
Author information
Authors and Affiliations
Contributions
MT was involved in the methodology, software, data curation and writing-original draft, DM assisted in conceptualization of work, RA was involved in the visualization, supervision, validation, writing-reviewing and editing.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
No animal or human tissue was used in this work, and ethics approval and consent were not applicable.
Consent for publication
All authors gave their full consent for publication of this work.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Todaria, M., Maity, D. & Awasthi, R. Biogenic metallic nanoparticles as game-changers in targeted cancer therapy: recent innovations and prospects. Futur J Pharm Sci 10, 25 (2024). https://doi.org/10.1186/s43094-024-00601-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43094-024-00601-9