Abstract
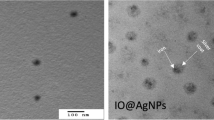
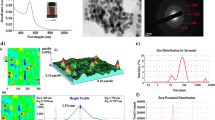
Delivering an optimum radiation dose to the target and preserving the surrounding normal tissues are the main tasks in radiation therapy. Latterly, there is a special interest in formulations of metallic-based nanoparticles to be used as radio-sensitizers. One of these silver nanoparticles (AgNPs) have been used for a wide range of biomedical applications depending on their surface modifications, size, and shape. This study aims to fabricate and characterize green synthesized silver nano-formulations using thymoquinone (TQ), as reducing and capping agent and assessment of their radio-sensitizing effect against the highly aggressive MDA-MB-231 mammary adenocarcinoma. AgNPs were prepared with different sizes by changing the amounts of added TQ. The prepared silver nano-formulations have been characterized using different physical techniques. The radio-sensitization efficacy of prepared AgNPs has been assessed using MTT cytotoxicity assay. Further assessment, neutral comet assay, was carried out to investigate the amount of DNA double-strand breaks which occurred due to AgNPs radio-sensitization. TEM micrograph revealed that as the amount of the added TQ increased from 10, 20, and 40 mg, the average size of prepared silver nanoparticles (AgNPs-20, AgNPs-15, and AgNPs-10) was gradually decreased to be 20 ± 1.5 nm, 15 ± 2 nm, and 10 ± 1.8 nm, respectively. This was confirmed by DLS measurements and UV-Vis spectroscopy. Comet and MTT assay results showed that AgNPs have a size and concentration-dependent radio-sensitization activity, whereas the radiation dose enhancement for small-sized nanoparticles (AgNPs-10) was greater than that of larger sized (AgNPs-20 and AgNPs-15). Therefore, thymoquinone capping silver nanoparticles represent a promising engineered nano-formulation for enhancing cancer radiosensitivity.




Similar content being viewed by others
References
Klębowski, B., Depciuch, J., Parlińska-Wojtan, M., & Baran, J. (2018). Applications of noble metal-based nanoparticles in medicine. International Journal of Molecular Sciences, 19(12), 4031.
Pagáčová, E., Štefančíková, L., Schmidt-Kaler, F., Hildenbrand, G., Vičar, T., Depeš, D., Lee, J. H., Bestvater, F., Lacombe, S., Porcel, E., & Roux, S. (2019). Challenges and contradictions of metal nano-particle applications for radio-sensitivity enhancement in cancer therapy. International Journal of Molecular Sciences, 20(3), 588.
Mathur, P., Jha, S., Ramteke, S., & Jain, N. K. (2018). Pharmaceutical aspects of silver nanoparticles. Artificial cells, Nanomedicine, and Biotechnology, 46(sup1), 115–126.
Huang, P., Yang, D. P., Zhang, C., Lin, J., He, M., Bao, L., & Cui, D. (2011). Protein-directed one-pot synthesis of Ag microspheres with good biocompatibility and enhancement of radiation effects on gastric cancer cells. Nanoscale, 3(9), 3623–3626.
Rai, M., Kon, K., Ingle, A., Duran, N., Galdiero, S., & Galdiero, M. (2014). Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Applied Microbiology and Biotechnology, 98(5), 1951–1961.
Wei, G., Wang, L., Liu, Z., Song, Y., Sun, L., Yang, T., & Li, Z. (2005). DNA-network-templated self-assembly of silver nanoparticles and their application in surface-enhanced Raman scattering. The Journal of Physical Chemistry B, 109(50), 23941–23947.
Franco-Molina, M. A., Mendoza-Gamboa, E., Sierra-Rivera, C. A., Gómez-Flores, R. A., Zapata-Benavides, P., Castillo-Tello, P., Alcocer-González, J. M., Miranda-Hernández, D. F., Tamez-Guerra, R. S., & Rodríguez-Padilla, C. (2010). Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. Journal of Experimental & Clinical Cancer Research, 29(1), 148.
Asharani, P. V., Hande, M. P., & Valiyaveettil, S. (2009). Anti-proliferative activity of silver nanoparticles. BMC Cell Biology, 10(1), 65.
Su, X. Y., Liu, P. D., Wu, H., & Gu, N. (2014). Enhancement of radiosensitization by metal-based nanoparticles in cancer radiation therapy. Cancer Biology & Medicine, 11(2), 86.
Retif, P., Pinel, S., Toussaint, M., Frochot, C., Chouikrat, R., Bastogne, T., & Barberi-Heyob, M. (2015). Nanoparticles for radiation therapy enhancement: the key parametersx. Cancer Biology & Medicine, 11(2), 86.
Liu, P., Huang, Z., Chen, Z., Xu, R., Wu, H., Zang, F., Wang, C., & Gu, N. (2013). Silver nanoparticles: a novel radiation sensitizer for glioma? Nanoscale, 5(23), 11829–11836.
Xu, R., Ma, J., Sun, X., Chen, Z., Jiang, X., Guo, Z., Huang, L., Li, Y., Wang, M., Wang, C., & Liu, J. (2009). Ag nanoparticles sensitize IR-induced killing of cancer cells. Cell Research, 19(8), 1031.
Ma, J., Xu, R., Sun, J., Zhao, D., Tong, J., & Sun, X. (2013). Nanoparticle surface and nanocore properties determine the effect on radiosensitivity of cancer cells upon ionizing radiation treatment. Journal of Nanoscience and Nanotechnology, 13(2), 1472–1475.
Turkevich, J., Stevenson, P. C., & Hillier, J. (1951). A study of the nucleation and growth processes in the synthesis of colloidal gold. Discussions of the Faraday Society, 11, 55–75.
Brust, M., Walker, M., Bethell, D., Schiffrin, D. J., & Whyman, R. (1994). Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. Journal of the Chemical Society, Chemical Communications, 7, 801–802.
Yu, D. G. (2007). Formation of colloidal silver nanoparticles stabilized by Na + –poly (γ-glutamic acid)–silver nitrate complex via chemical reduction process. Colloids and Surfaces B: Biointerfaces, 59(2), 171–178.
Linthorst, J. A. (2010). An overview: origins and development of green chemistry. Foundations of Chemistry, 12(1), 55–68.
Javaid, A., Oloketuyi, S. F., Khan, M. M., & Khan, F. (2018). Diversity of bacterial synthesis of silver nanoparticles. BioNanoScience, 8(1), 43–59.
de Souza, T. A. J., Souza, L. R. R., & Franchi, L. P. (2019). Silver nanoparticles: an integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicology and Environmental Safety, 171, 691–700.
Zhao, X., Zhou, L., Riaz Rajoka, M. S., Yan, L., Jiang, C., Shao, D., Zhu, J., Shi, J., Huang, Q., Yang, H., & Jin, M. (2018). Fungal silver nanoparticles: synthesis, application and challenges. Critical Reviews in Biotechnology, 38(6), 817–835.
Schneider-Stock, R., Fakhoury, I. H., Zaki, A. M., El-Baba, C. O., & Gali-Muhtasib, H. U. (2014). Thymoquinone: fifty years of success in the battle against cancer models. Drug Discovery Today, 19(1), 18–30.
Banerjee, S., Padhye, S., Azmi, A., Wang, Z., Philip, P. A., Kucuk, O., Sarkar, F. H., & Mohammad, R. M. (2010). Review on molecular and therapeutic potential of thymoquinone in cancer. Nutrition and Cancer, 62(7), 938–946.
ElKhoely, A., Hafez, H. F., Ashmawy, A. M., Badary, O., Abdelaziz, A., Mostafa, A., & Shouman, S. A. (2015). Chemopreventive and therapeutic potentials of thymoquinone in HepG2 cells: mechanistic perspectives. Journal of Natural Medicines, 69(3), 313–323.
Talib, W. H., & Mahasneh, A. M. (2012). Combination of Ononis hirta and Bifidobacterium longum decreases syngeneic mouse mammary tumor burden and enhances immune response. Journal of Cancer Research and Therapeutics, 8(3), 417.
Alsammarraie, F. K., Wang, W., Zhou, P., Mustapha, A., & Lin, M. (2018). Green synthesis of silver nanoparticles using turmeric extracts and investigation of their antibacterial activities. Colloids and Surfaces B: Biointerfaces, 171, 398–405.
Fathy, M. M., Mohamed, F. S., Elbialy, N., & Elshemey, W. M. (2018). Multifunctional chitosan-capped gold nanoparticles for enhanced cancer chemo-radiotherapy: an in vitro study. Physica Medica, 48, 76–83.
Buckton, G. (2000). Interfacial phenomena in drug delivery and targeting. CRC press.
Paolino, D., Sinha, P., Fresta, M., & Ferrari, M. (2006). Drug delivery systems. Encyclopedia of medical devices and instrumentation.
Alam, S., Khan, Z. I., Mustafa, G., Kumar, M., Islam, F., Bhatnagar, A., & Ahmad, F. J. (2012). Development and evaluation of thymoquinone-encapsulated chitosan nanoparticles for nose-to-brain targeting: a pharmacoscintigraphic study. International Journal of Nanomedicine, 7, 5705.
Fahmy, H. M., Fathy, M. M., Abd-Elbadia, R. A., & Elshemey, W. M. (2019). Targeting of Thymoquinone-loaded mesoporous silica nanoparticles to different brain areas: In vivo study. Life Sciences, 222, 94–102.
Cui, Y., Dong, H., Cai, X., Wang, D., & Li, Y. (2012). Mesoporous silica nanoparticles capped with disulfide-linked PEG gatekeepers for glutathione-mediated controlled release. ACS Applied Materials & Interfaces, 4(6), 3177–3183.
Gliga, A. R., Skoglund, S., Wallinder, I. O., Fadeel, B., & Karlsson, H. L. (2014). Size-dependent cytotoxicity of silver nanoparticles in human lung cells: the role of cellular uptake, agglomeration and Ag release. Particle and Fibre Toxicology, 11(1), 11.
Carlson, C., Hussain, S. M., Schrand, A. M., Braydich-Stolle, L. K., Hess, K. L., Jones, R. L., & Schlager, J. J. (2008). Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. The Journal of Physical Chemistry B, 112(43), 13608–13619.
Liu, W., Wu, Y., Wang, C., Li, H. C., Wang, T., Liao, C. Y., Cui, L., Zhou, Q. F., Yan, B., & Jiang, G. B. (2010). Impact of silver nanoparticles on human cells: effect of particle size. Nanotoxicology, 4(3), 319–330.
McMahon, S. J., Hyland, W. B., Muir, M. F., Coulter, J. A., Jain, S., Butterworth, K. T., Schettino, G., Dickson, G. R., Hounsell, A. R., O’Sullivan, J. M., & Prise, K. M. (2011). Nanodosimetric effects of gold nanoparticles in megavoltage radiation therapy. Radiotherapy and Oncology, 100(3), 412–416.
Carter, J. D., Cheng, N. N., Qu, Y., Suarez, G. D., & Guo, T. (2007). Nanoscale energy deposition by X-ray absorbing nanostructures. The Journal of Physical Chemistry B, 111(40), 11622–11625.
Lin, Y., McMahon, S. J., Paganetti, H., & Schuemann, J. (2015). Biological modeling of gold nanoparticle enhanced radiotherapy for proton therapy. Physics in Medicine & Biology, 60(10), 4149.
Ahmed, B. S., Rao, A. G., Sankarshan, B. M., Vicas, C. S., Namratha, K., Umesh, T. K., Somashekar, R., & Byrappa, K. (2016). Evaluation of gold, silver and silver–gold (bimetallic) nanoparticles as radiosensitizers for radiation therapy in cancer treatment. Cancer and Oncology Research, 4(3), 42–51.
Khoshgard, K., Kiani, P., Haghparast, A., Hosseinzadeh, L., & Eivazi, M. T. (2017). Radiation dose rate affects the radiosensitization of MCF-7 and HeLa cell lines to X-rays induced by dextran-coated iron oxide nanoparticles. International Journal of Radiation Biology, 93(8), 757–763.
Fathy, M. M., Fahmy, H. M., Saad, O. A., & Elshemey, W. M. (2019). Silica-coated iron oxide nanoparticles as a novel nano-radiosensitizer for electron therapy. Life Sciences, 234, 116756.
Olive, P. L., Wlodek, D., & Banáth, J. P. (1991). DNA double-strand breaks measured in individual cells subjected to gel electrophoresis. Cancer Research, 51(17), 4671–4676.
Havaki, S., Kotsinas, A., Chronopoulos, E., Kletsas, D., Georgakilas, A., & Gorgoulis, V. G. (2015). The role of oxidative DNA damage in radiation induced bystander effect. Cancer Letters, 356(1), 43–51.
Funding
None
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
None
Research Involving Humans and Animals Statement
None
Informed Consent
None
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fathy, M.M. Biosynthesis of Silver Nanoparticles Using Thymoquinone and Evaluation of Their Radio-Sensitizing Activity. BioNanoSci. 10, 260–266 (2020). https://doi.org/10.1007/s12668-019-00702-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12668-019-00702-3