Abstract
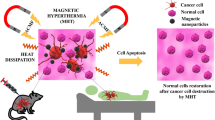
Magnetite nanoparticles (Fe3O4 NPs) have received considerable attention in various biomedical applications due to their fascinating properties and multiple functionalities. In this multidisciplinary study, Fe3O4 NPs were produced by an inexpensive co-precipitation technique and using four different weight percentages of Punica granatum fruit peel extract as a green stabilizer. From the image of transmission electron microscopy, the NPs showed spherical shapes with an average size of 14.38 nm. Results of UV–VIS spectroscopy and bandgap indicated successful preparation of the Fe3O4 NPs stabilized with the extract. Adding the stabilizer concentration improved the particle zeta potential from −29.24 to −35.62 mV. Thermoresponsive performance of the Fe3O4 nanofluids with the green extract could render a remarkable heating capability under the hyperthermia condition. Magnetic resonance imaging (MRI) analysis presented that the samples possessed acceptable MRI signals. An anticancer drug 5-fluorouracil was successfully loaded onto the Fe3O4 NPs containing 2 weight percentage of the extract, which indicated a maximum release of 79% in a media with pH 7.4. In cytotoxicity assays, the drug-loaded Fe3O4 NPs at 15.62 and 31.25 µg.ml−1 concentration eliminated 29% and 35% of HCT116 colorectal cancer cells, respectively. This study, therefore, introduced that the green-synthesized Fe3O4 NPs can be a promising candidate for magnetic hyperthermia therapy, MRI nanoagents and drug delivery in colorectal cancer.








Similar content being viewed by others
References
L.H. Reddy, J.L. Arias, J. Nicolas, P. Couvreur, Chem. Rev. 112, 5818–5878 (2012). https://doi.org/10.1021/cr300068p
Z. Hedayatnasab, A. Dabbagh, F. Abnisa, W.M.A.W. Daud, Eur. Polym. J. 133, 109789 (2020). https://doi.org/10.1016/j.eurpolymj.2020.109789
L.S. Arias, J.P. Pessan, A.P.M. Vieira, T.M.T.d. Lima, A.C.B. Delbem, D.R. Monteiro, Antibiotics 7, 46 (2018) . https://doi.org/10.3390/antibiotics7020046
Y.P. Yew, K. Shameli, M. Miyake, N.B.B.A. Khairudin, S.E.B. Mohamad, T. Naiki, K.X. Lee, Arab. J. Chem. 13, 2287–2308 (2020). https://doi.org/10.1016/j.arabjc.2018.04.013
H. Jahangirian, S. Azizi, R. Rafiee-Moghaddam, B. Baratvand, T.J. Webster, Biomolecules 9, 619 (2019). https://doi.org/10.3390/biom9100619
M. Magro, F. Vianello, Nanomaterials 9, 1608 (2019). https://doi.org/10.3390/nano9111608
Z. Izadiyan, K. Shameli, M. Miyake, S.-Y. Teow, S.-C. Peh, S.E. Mohamad, S.H.M. Taib, Mater. Sci. Eng. C 96, 51–57 (2019). https://doi.org/10.1016/j.msec.2018.11.008
M. Kalhor, Z. Zarnegar, F. Janghorban, S.A. Mirshokraei, Res. Chem. Intermed. 46, 821–836 (2020). https://doi.org/10.1007/s11164-019-03992-0
M. Herlekar, S. Barve, R. Kumar, J. Nanomater. 2014, (2014). https://doi.org/10.1155/2014/140614
M. Kallumadil, M. Tada, T. Nakagawa, M. Abe, P. Southern, Q.A. Pankhurst, J. Magn. Magn. Mater. 321, 1509–1513 (2009). https://doi.org/10.1016/j.jmmm.2009.02.075
A.B. Ogholbeyg, A. Kianvash, A. Hajalilou, E. Abouzari-Lotf, A. Zarebkohan, J. Mater. Sci. Mater. Electron. 29, 12135–12143 (2018). https://doi.org/10.1007/s10854-018-9321-8
D. Tungmunnithum, A. Thongboonyou, A. Pholboon, A. Yangsabai, Medicines. 5, 93 (2018). https://doi.org/10.3390/medicines5030093
P. Pan-In, S. Wanichwecharungruang, J. Hanes, A.J. Kim, Int. J. Nanomedicine 9, 3677 (2014). https://doi.org/10.2147/IJN.S66511
W. Suttirak, S. Manurakchinakorn, J. Food Sci. Technol. 51, 3546–3558 (2014). https://doi.org/10.1007/s13197-012-0887-5
M. Yusefi, K. Shameli, R.R. Ali, S.-W. Pang, S.-Y. Teow, J. Mol. Struct. 1204, 127539 (2020). https://doi.org/10.1016/j.molstruc.2019.127539
S. El-Faham, M. Mohsen, A. Sharaf, A. Zaky, Curr. Sci. Int. 5, 529–542 (2016)
S. Groiss, R. Selvaraj, T. Varadavenkatesan, R. Vinayagam, J. Mol. Struct. 1128, 572–578 (2017). https://doi.org/10.1016/j.molstruc.2016.09.031
P. Boyle, B. Levin, World Cancer Report 2008 (IARC Press, International Agency for Research on Cancer, 2008).
M. Mühlberger, C. Janko, H. Unterweger, E. Schreiber, J. Band, C. Lehmann, D. Dudziak, G. Lee, C. Alexiou, R. Tietze, J. Magn. Magn. Mater. 473, 61–67 (2019). https://doi.org/10.2147/IJN.S218488
A.H. Atta, M.A. El-ghamry, A. Hamzaoui, M.S. Refat, J. Mol. Struct. 1086, 246–254 (2015). https://doi.org/10.1016/j.molstruc.2014.12.085
S. Arsalani, E.J. Guidelli, M.A. Silveira, C.E. Salmon, J.F. Araujo, A.C. Bruno, O. Baffa, J. Magn. Magn. Mater. 475, 458–464 (2019). https://doi.org/10.1016/j.jmmm.2018.11.132
Z. Zahedi-Tabar, S. Bagheri-Khoulenjani, S. Amanpour, H. Mirzadeh, Basic & Clinical Cancer Research (2019). https://doi.org/10.18502/bccr.v11i1.1653
Z. Hedayatnasab, F. Abnisa, W.M.A.W. Daud, Mater. Des. 123, 174–196 (2017). https://doi.org/10.1016/j.matdes.2017.03.036
B.A. Evans, M.D. Bausch, K.D. Sienerth, M.J. Davern, J. Magn. Magn. Mater. 465, 559–565 (2018). https://doi.org/10.1016/j.jmmm.2018.06.051
Y. Javed, K. Akhtar, H. Anwar, Y. Jamil, J. Nanopart. Res. 19, 366 (2017). https://doi.org/10.1007/s11051-017-4045-x
Q. Liu, L. Song, S. Chen, J. Gao, P. Zhao, J. Du, Biomaterials 114, 23–33 (2017). https://doi.org/10.1016/j.biomaterials.2016.10.027
E.J. Bernstein, C. Schmidt-Lauber, J. Kay, Best Pract. Res. CL. RH. 26, 489–503 (2012). https://doi.org/10.1016/j.berh.2012.07.008
J. Qin, Q. Liu, J. Zhang, J. Chen, S. Chen, Y. Zhao, J. Du, A.C.S. Appl, Mater. Interfaces 7, 14043–14052 (2015). https://doi.org/10.1021/acsami.5b03222
M.R. Jahn, T. Nawroth, S.r. Fütterer, U. Wolfrum, U. Kolb, P. Langguth, , Mol. Pharm. 9, 1628–1637 (2012). https://doi.org/10.1021/mp200628u
T. Ren, Q. Liu, H. Lu, H. Liu, X. Zhang, J. Du, J. Mater. Chem. 22, 12329–12338 (2012). https://doi.org/10.1039/C2JM31891A
N. Pothayee, S. Balasubramaniam, N. Pothayee, N. Jain, N. Hu, Y. Lin, R.M. Davis, N. Sriranganathan, A.P. Koretsky, J. Riffle, J. Mater. Chem. B 1, 1142–1149 (2013). https://doi.org/10.1039/C2TB00275B
R. Hao, R. Xing, Z. Xu, Y. Hou, S. Gao, S. Sun, Adv. Mater. 22, 2729–2742 (2010). https://doi.org/10.1002/adma.201000260
G. Biliuta, L. Sacarescu, V. Socoliuc, M. Iacob, L. Gheorghe, D. Negru, S. Coseri, Macromol. Chem. Phys. 218, 1700062 (2017). https://doi.org/10.1002/macp.201700062
S. Srivastava, S. Mohammad, S. Gupta, A.A. Mahdi, R.K. Dixit, V. Singh, F.M. Samadi, Natl. J. Maxillofac 9, 160 (2018). https://doi.org/10.1080/10717544.2019.1582729
M. Yusefi, K. Shameli, H. Jahangirian, S.-Y. Teow, H. Umakoshi, B. Saleh, R. Rafiee-Moghaddam, T.J. Webster, Int. J. Nanomedicine 15, 5417–5432 (2020). https://doi.org/10.2147/IJN.S250047
M. Ebadi, B. Saifullah, K. Buskaran, M.Z. Hussein, S. Fakurazi, Int. J. Nanomed. 14, 6661 (2019). https://doi.org/10.2147/IJN.S214923
Z. Hedayatnasab, A. Dabbagh, F. Abnisa, W.M.A.W. Daud, Mater Res Bull. 132, 110975 (2020). https://doi.org/10.1016/j.materresbull.2020.110975
N.A. Ismail, K. Shameli, M.M.-T. Wong, S.-Y. Teow, J. Chew, S.N.A.M. Sukri, Mater. Sci. Eng. C 104, 109899 (2019). https://doi.org/10.1016/j.msec.2019.109899
S.N.A.M. Sukri, K. Shameli, M.M.-T. Wong, S.-Y. Teow, J. Chew, N.A. Ismail, J. Mol. Struct. 1189, 57–65 (2019). https://doi.org/10.1016/j.molstruc.2019.04.026
Z. Izadiyan, K. Shameli, M. Miyake, H. Hara, S.E.B. Mohamad, K. Kalantari, S.H.M. Taib,E. Rasouli, Arab. J. Chem. 13, (2018) https://doi.org/10.1016/j.arabjc.2018.02.019
J.R. van Ommen, J.M. Valverde, R. Pfeffer, J. Nanopart. Res. 14, 737 (2012). https://doi.org/10.1007/s11051-012-0737-4
S. Sisodiya, L.R. Wallenberg, E. Lewin, O.F. Wendt, Appl. Catal. A 503, 69–76 (2015). https://doi.org/10.1016/j.apcata.2015.07.001
B. Kumar, K. Smita, L. Cumbal, A. Debut, S. Galeas, V.H. Guerrero, Mater. Chem. Phys. 179, 310–315 (2016). https://doi.org/10.1016/j.matchemphys.2016.05.045
G.M. Sulaiman, A.T. Tawfeeq, A.S. Naji, Artif. Cells Nanomed. Biotechnol. 46, 1215–1229 (2018). https://doi.org/10.1080/21691401.2017.1366335
J. Tauc, Amorphous and liquid semiconductors. Springer Science & Business Media: 2012
A.J. Deotale, R. Nandedkar, Today 3, 2069–2076 (2016). https://doi.org/10.1016/j.matpr.2016.04.110
Z. Zhang, C. Boxall, G. Kelsall, Photoelectrophoresis of colloidal iron oxides 1. Hematite (α-Fe 2 O 3), in Colloids in the Aquatic Environment, Elsevier 145–163 (1993)
C. Boxall, G. Kelsall, Z. Zhang, J. Chem. Soc. Faraday Trans. 92, 791–802 (1996). https://doi.org/10.1039/FT9969200791
S. Yu, G.M. Chow, J. Mater. Chem. 14, 2781–2786 (2004). https://doi.org/10.1039/B404964K
N. Zhu, H. Ji, C. Shen, J. Wu, J. Niu, J. Yang, M. Farooq, H. Li, X. Niu, IEEE Trans. Appl. Supercond. 29, 1–5 (2018). https://doi.org/10.1109/TASC.2018.2882416
A. Ramirez-Nuñez, L. Jimenez-Garcia, G. Goya, B. Sanz, J. Santoyo-Salazar, Nanotechnology 29, 074001 (2018). https://doi.org/10.1088/1361-6528/aaa2c1
T. Lam, P. Pouliot, P.K. Avti, F. Lesage, A.K. Kakkar, Adv. Coll. Interf. Sci. 199, 95–113 (2013). https://doi.org/10.1016/j.cis.2013.06.007
U.E. Illangakoon, D.-G. Yu, B.S. Ahmad, N.P. Chatterton, G.R. Williams, Int. J. Pharm. 495, 895–902 (2015). https://doi.org/10.1016/j.ijpharm.2015.09.044
A. Kadam, R.G. Saratale, S. Shinde, J. Yang, K. Hwang, B. Mistry, G.D. Saratale, S. Lone, D.-Y. Kim, J.-S. Sung, Bioresour. Technol. 273, 386–393 (2019). https://doi.org/10.1016/j.biortech.2018.11.041
Y. Li, Y. Feng, J. Jing, F. Yang, BioResources 14, 3615–3629 (2019). https://doi.org/10.15376/biores.14.2.3615-3629
I.C. Radu, A. Hudita, C. Zaharia, B. Galateanu, H. Iovu, E. Tanasa, S. Georgiana Nitu, O. Ginghina, C. Negrei, A. Tsatsakis, Drug Deliv. 26, 318–327 (2019). https://doi.org/10.1080/10717544.2019.1582729
Acknowledgements
This research was funded by Takasago Thermal Engineering Co. Ltd. grant (#4B422) from the research management center (RMC) of Universiti Teknologi Malaysia (UTM) and Malaysia-Japan International Institute of Technology (MJIIT).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yusefi, M., Shameli, K., Hedayatnasab, Z. et al. Green synthesis of Fe3O4 nanoparticles for hyperthermia, magnetic resonance imaging and 5-fluorouracil carrier in potential colorectal cancer treatment. Res Chem Intermed 47, 1789–1808 (2021). https://doi.org/10.1007/s11164-020-04388-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11164-020-04388-1