Abstract
Dengue fever is a rapidly spreading infection that affects people all over the tropics and subtropics, posing a significant public health threat. The brown seaweed Stoechospermum marginatum was found all over the world, from South Africa (Indian Ocean) to Australia (Pacific Ocean), among other places. In India, it is only available along the coast of the Bay of Bengal, which is a small region. Various metal oxides were proved to be successful in the formation of nanoparticles and zinc is one among them. In this present study, an attempt was made to study the anti-dengue activity of green synthesized zinc oxide nanoparticles of crude fucoidan isolated from brown seaweed S. marginatum. The fucoidan was isolated from the seaweed by acid extraction method and then characterized by UV, HPLC, and Fourier Transform Infra-Red (FT-IR) Spectroscopy. Then it was biosynthesized into ZnO nanoparticles and characterized by SEM-EDAX analysis. The results showed the formation of fucoidans and SEM studies showed the crystalline nature of the synthesized nanoparticles. The size of nanoparticles was in the range of 80–126 nm. The synthesized nanoparticles were tested with the C6/36 cell line and it was shown 99.09% of anti-dengue activity against the tested cell line. As an antiviral agent, the ZnO nanoparticles of fucoidans have been shown to be an excellent lead molecule for the treatment of dengue fever.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Green nanoparticles are being synthesized using plant extracts, which is an exciting new development in the field of nanotechnology. When compared to chemical and physical methods of synthesis, the biosynthesis of green nanoparticles using plant extracts has both economic and environmental advantages, according to the researchers [1]. Nanoparticles (NPs) are typically less than 100 nm in size, and it has been hypothesized that their biocidal efficacy is due to a combination of their small size and high surface-to-volume ratio, which allows them to form intimate interactions with bacterial membranes [2,3,4,5]. The greater stability of inorganic antibacterial agents such as metals and metal oxides over organic antibacterial agents gives them a distinct advantage over organic antibacterial agents[6, 7]. Zinc oxide (ZnO), one of these metal oxides, has gotten a lot of attention because it acts as an antibacterial agent.
There are at least 72 viruses in the Flaviviridae family, including Japanese encephalitis, Yellow fever, Zika, and West Nile virus. Dengue is the most well-known mosquito-borne viral infection. Dengue virus is an enveloped, single-stranded, positive-sense RNA virus that is spread by the Aedes aegypti and Aedes albopictus mosquitoes. Dengue viral infections are one of the most important mosquito borne diseases in the world [8, 9]. It is found in 4 different serotypes in different regions of the world. Dengue fever has been extremely dangerous from its inception, but its prevalence has increased significantly in recent decades. Despite the fact that there is no particular surveillance, the WHO estimates that the dengue virus is present in more than 100 countries, including the United States [10]. According to WHO, there was a six foldincrease in the number of cases reported (from 0.5 million in 2010 to over 3.34 million in 2016) and also there was a sharp increase in 2019[11]. At this time, there is no appropriate treatment available for dengue fever. The use of medicinal plants has been around since the dawn of civilization, and they have been used to treat a wide range of illnesses, from the common to the life-threatening. When used in conjunction with conventional antiviral medications, herbal medications can be used to treat or increase the therapeutic effect of the drugs [12]. Deliberate use of many herbal remedies against dengue fever infection is becoming more common as an alternative approach to alleviating disease symptoms [13].
Brown seaweeds belonging to the Dictyoceae family are a rich source of a heteropolymer of sulfated L-fucose, known as sulfated polysaccharides. It was called as fucoidans because they were the first sulfated polysaccharides isolated from the Fucales of the Phaeophyceae, which are algae-derived polysaccharides. Fucoidans are found embedded in the intercellular thallus tissues. These, sulfated polysaccharides, also known as fucans, have been shown to be the source of potent bioactive principles that have shown promise in the treatment of viral infections, ulcer, and adhesion problems and also they possess anticoagulant, anti-inflammatory, anti-proliferative, and anti-tumor properties [14].
It is revealed in the report that fucoidans have an inhibitory effect on vascular smooth muscle cells and that it inhibits the proliferation and binding of sperm-ova binding in various species [15]. Different seaweed species produce polysaccharides that are structurally distinct from one another, and the composition of these polysaccharides varies with different parts of the thallus. Fucoidans, which are polysaccharides found in the cell wall surface of the brown algae, are a valuable source of nutrition [16]. It was discovered that fucoidans (sulfated polysaccharides composed of L-fucose and sulfuric acid esters) have numerous and diverse pharmaceutical and biomedical applications [17] and can be used in a variety of therapeutic and diagnostic procedures [18]. This is due to the presence of the sulfate moiety in its chemical structures, which makes it a powerful antimicrobial agent [19].
The brown seaweed S.marginatum was distributed from South Africa (Indian Ocean) to Australia (Pacific Ocean). In India, it is only available along the coast of the Bay of Bengal, which is a small region. S.marginatum has a thick and dichotomously branched thallus with a flat surface, but it does not have a midrib. The margin was complete, the apex was bifid, or flatly truncated. The apex of the branches has a notch in it. It is a species of forking plant that can grow to be 40 cm in length and 8–11 mm breadth. Fertile plants are easily distinguished from nonfertile plants by the dark lines that run along the margins of densely packed sporangia. S. marginatum has a thallus that is flat and isolateral in shape. It contains a sporangium with four tetraspores, which is unique to this species. Each sporangium has many elongated, cylindrical spores, which are arranged in a spiral pattern. The spores have echinate surfaces and stain darkly when exposed to light.
The epidermal layers of S. marginatum are cutinized and distinct. The epidermal cell is tiny, square-shaped, with thick walls and a dark stain on its surface. Between the epidermal layers, there were approximately 8 to 9 layers of relatively homogenous compact parenchyma cells present. Between the epidermal layer and the dermal layer, there seem to be approximately five layers of huge laterally elongated cells with move up and down walls arranged in a layering pattern. The stalk’s marginal part is thin, thick, and rounded, with a slight incline [20].
High sulfate content in the various species demonstrates significant antiviral and antimicrobial properties, indicating that they are beneficial [21]. So the isolation of novel fucoidans from brown seaweed will serve as potent biomolecules with diverse pharmacological activities. Fucoidan extraction, isolation, and characterization from seaweeds are still in the early stages in India, despite the large number of seaweeds that occur in the 7200 km of Indian coastline that can be harvested. When it comes to being a source of fucoidan, Indian brown macroalgae are not subjected to a comprehensive examination. The Gulf of Mannar, located on India’s southeast coast, has been designated as a biosphere reserve, and the seaweeds that grow abundantly in the Gulf region’s 21 islands are being harvested for commercial purposes [20].
To explore the anti-dengue properties of green synthesized ZnO nanoparticles of crude fucoidan isolated from brown seaweed S.marginatum, as well as their characterization, the current study is being conducted.
Materials and Methods
Materials
Cysteine hydrochloride, sorbitol, phenol, HCl, H2SO4, potassium bromide, and barium chloride were of analytical grade and procured from LobaChemi India. HPLC grade methanol and water were purchased from Rankem, India and precoated TLC Plates for chromatographic separation were obtained from Anchrom Enterprises India Private Limited from Mumbai, Maharashtra, India. 3-(4, 5-dimethylthiazol-2-yl) -2, 5-diphenyltetrazolium bromide (MTT), phosphate buffer saline (PBS), minimum essential medium (MEM), and trypsin, were obtained from Hi-Media Laboratories in Mumbai, India. The foetal calf serum (FCS) was obtained from Gibco Laboratories (NV, USA). Geno-Dengue Sen’s S1-S4 PCR kit, contained commercially available chemicals such as buffer, enzymes, dNTPs, a dengue-specific primer and probes was used in this study.
Collection of Seaweed S.marginatum
Thorough washing in seawater followed by thorough washing in tap water was performed to remove epiphytes and other superfluous matters from freshly harvested, matured, free of disease, and robust brown algae S. marginatum (C.Ag.) Kuetz. collected along the coastline of Tuticorin, South India during July 2020. For future reference, the voucher specimens (SMA-1) of S.marginatum were kept in our museum.
Extraction of Crude Fucoidan
100 g of fresh S.marginatum was soaked in 100 mL of 0.1 N HCl at 95oC for 12 h before being used. The extraction procedure was carried out thrice and the extracts were combined and filtered using Whatman No. 1 filter paper to remove any impurities and contaminants. The filtrate was dialyzed against water and then lyophilized to remove any remaining water. The determination of true fucoidans was carried out using the gravimetric method, and the findings have been represented as a percentage of the algae weight [22].
Characterization of Fucoidans
The biochemical composition of crude fucoidan was derived by analyzing the total carbohydrates, L-fucose, and sulfate contents.
Estimation of Total Carbohydrate
Using the methods described by Dubois and Rezzonico, the total carbohydrates present in a sample of water were estimated[23]. To obtain a homogenate solution, 100 mg dried crude fucoidan was ground into a fine powder with 10 mL of distilled water in a mortar and pestle for 30 minwith intermittent grinding. An aliquot of 200µL of the sample was placed in a test tube, 200 µLof 5% phenol, and 1 mL of the con. H2SO4 was added to the sample. The addition of concentrated H2SO4 in a short span of time aided in the mixing and heat development required for the test. In this experiment, the samples were left to stand at room temperature before being measured for absorbance at 485 nm, the results were recorded in comparison to a blank. Different concentrations of D-galactose were used to prepare a standard graph, and the amount of total carbohydrates could be calculated from the graph.
Estimation of L-fucose
Using the method developed by Dishe and Shettles, L-fucose concentrations can be estimated with high accuracy [24]. 1 mL of the sample solution was poured into the test tubes, which were then placed in an ice-water bath to cool until they were completely chilled. Next, the tube was filled with 4.5 mL of H2SO4 reagent, which was then thoroughly mixed together. The tubes were brought to room temperature by placedin a water bath (25oC) for 3–4 min and then placed in hot boiling water for 3 min. Each tube was treated with 5% cysteine hydrochloride solution and mixed after the tubes were allowed to cool under running tap water for a short period. At 396 and 427 nm, the absorbance was measured in comparison to a blank prepared with reagents without a sample[25]. To calculate the absorbance values, the following equation was used:
Whereas A396 = Absorbance at 396 nm.
A427 = Absorbance at 427 nm.
Estimation of Sulfate by the Turbidometric Method
According to Czerwinska and colleagues, the accuracy of the estimated sulfate contents was confirmed through comparison with reference compounds [26]. S.marginatum fucoidan extract (10 mg) was homogenized with 6 mL of 1 N HCl and transferred to a hydrolyzing tube with proper sealing. Boiling the sample in a water bath for 4 h results in hydrolysis of the sample. After that, the sample was allowed to cool down to room temperature. A Whatman filter paper No. 1 was used to filter the solution, and the volume was increased to 25 mL by adding double distilled water to the filter paper after it had been filtered. 1 mL of the filtrate solution was mixed with 1 mL of 6 M HCl and 5 mL of 70% sorbitol. It was stirred with a magnetic stirrer and 1 g of barium chloride crystals was added while stirring andthe absorbance at 470 nm was measured immediately.
Chromatographic Techniques
TLC Analysis
On 10 × 20 cm silica gel-G plates (Merck, Germany), a Thin Layer Chromatography experiment was carried out. It was determined that S. marginatum contains polysaccharides that were qualitatively evaluated and identified. A 9:1 mixture of methanol and water was used to separate the polysaccharides, and when Barfode’s reagent was sprayed on developed plates, the appearance of a red-colored spot confirmed the presence of polysaccharides.
HPLC Analysis
After being centrifuged at 3000 rpm for 10 min, the extracts of S. marginatum were filtered through Whatman filter paper No. 1 with the help of a high-pressure vacuum pump [27]. Using a Shimadzu LC-10AT-VP HPLC system equipped with an LC-10AT pump, SPD-10AT UV-Vis detector, Rheodyne injector with a 20 µL injection loop, and manual injector, the separation of monosaccharides was accomplished. The column used was a Hypersil ODS C-18 column (4.6 × 250 mm, 5 micron size) with a C-18 guard column. In order to achieve the best elution results, gradient solvent systems with a flow rate of 1mL/minwere used at room temperature (25–28 °C). In this experiment, the mobile phase was 0.1% v/v of methanol (solvent A) and water (solvent B). During the experiment, the mobile phase was freshly prepared every day, passed through a 0.45 mm filter, and sonicated prior to use. The entire performance lasted 15 min. The sample injection volume was 20 µL, and the detector wavelength was UV-254 nm, which wasused in this experiment. A Whatman filter paper No.1 was used to filter the sample, and it was then preserved. 2 mol/L of trifluoroacetic acid was used to hydrolyze the fucoidans and convert them into their respective monosaccharides. The monosaccharide was isolated by reverse-phase HPLC using a gradient elution technique, and the concentration was monitored using ultraviolet detection at 254 nm[28]. Table 1 depicts the chromatographic conditions that existed at the time of the experiment.
Fourier Transform Infrared Spectrometry (FTIR) Analysis
The polysaccharide samples (10 mg) were mixed with 90 mg of potassium bromide (KBr) powder of spectroscopic grade and ground before being compressed into 1 mm pellets for FT-IR analysis (Perkin Elmer USA) recorded in the frequency range of 400 to 4000 cm− 1[29].
Synthesis of Zinc Oxide Nanoparticles of Crude Fucoidan
Zinc nitrate hexahydrate (Zn (NO3)2·6H2O) solution 0.1 M was prepared by dissolving 6.58 g zinc nitrate hexahydrate in 300 mL of double-distilled water. To obtain complex formation, 10 mL of the fucoidan solution were added slowly drop by drop while magnetic stirring at 60o C for approximately 2 h. After stirring, the complex was collected and centrifuged at 10,000 rpm for 10 min. The collected pellets were dried for 8 h at 80oC and then stored in airtight glass bottles for further investigation.
Green synthesis of ZnO nanoparticles was carried out using crude fucoidan isolated from S.marginatum and zinc nitrate hexahydrate as a precursor. The addition of a dark brown crude fucoidan solution to a colourless zinc nitrate hexahydrate solution resulted in the formation of a yellowish-white precipitate, which indicated the formation of zinc oxide nanoparticles. When greenish Andrographis paniculata leaf extract was added to the zinc nitrate precursor, it resulted a similar colour change was observed by Rajakumar et al. [30]. Additionally, Bhuyan et al., reported the formation of a whitish precipitate [31]. However, Umar et al. discovered that when Albizia lebbeck leaf extract was added to the precursor zinc acetate, the colour changed from brown to dark brown [32].
Characterization of Zinc Oxide Nanoparticles
UV- Visible Spectrophotometry Analysis
The preliminary investigation includes the use of ultraviolet spectroscopy (Model: Shimadzu UV 1601), which confirms the formation of ZnO nanoparticles in the first place. Standard procedures were used to evaluate the compounds in ultraviolet light (UV) in the range of 300-800 nm.
SEM with EDX Analysis
A sample of ZnO nanoparticles was prepared on carbon-coated tape by placing a small quantity of dried sample on a grid and allowing the film to dry under a mercury lamp for 5 min, followed by a second drying step. The morphology of the biosynthesized ZnO nanoparticles was determined using scanning electron microscopy (SEM). The TESCAN VEGA3 SBH was used to carry out SEM and EDX analysis on the samples under investigation.
Antidengueassays
C6/36 Cells Culture
It was necessary to maintain the Aedes cell line (ATCC® CRL-1660 TM) C6/36 in a minimal essential medium (MEM) containing 10% foetal calf serum (FCS), 2 mL of glutamine, penicillin (100 U/mL), and streptomycin (100 µg/ml). A humidified environment containing 5% CO2 was used to incubate the cells in culture at 28o C [33, 34]. The medium was changed 14 days once.
Preparation of Stock Solution of Fucoidan ZnO Nanoparticles for Cell Viability and Antiviral Assays
Approximately 3000 µg of crude fucoidan ZnO nanoparticles were weighed and dissolved in 1 mL MEM based on their solubility. The pH of the medium was kept at 7.0 throughout the experiment. In the 96 well plates, the solution was diluted to various serial dilution concentrations (1500 µg/mL to 23.44 µg/mL) and then tested. In order to remove impurities from the extract, it was filtered through a 0.22 µmsyringe filter (MILLEX® GV). Extracted stocks were stored at a temperature of -20oC for future use.
In-vitro Anti-Dengue Assay
Antiviral testing was performed in a laboratory setting using monolayers of the C6/36 cell line which were cultured in a 96-well plate. It consists of a dengue-2 virus (TR-1751) control that contained 10, 100, and 1000 viral copies/mLin duplicatecopies as a positive control, and just the cells alone (negative control). The experiment was done by mixing 100 copies of the virus suspension, with the results being recorded. Replicated plates of ZnO nanoparticles were used to pre-treat virus suspension for 55 min with gentle shaking every 10 min. After being pretreated, the virus was redistributed to individual wells of C6/36 cell lines and incubated for 55 min, with gentle shaking every 10 min, until the virus was completely destroyed. After the inoculums had adsorption, the medium was sucked out of each well and flushed off.
In the following step, 100 µL of virus growth medium was added to the cell layer without disturbing the layer. A CO2 incubator was used to maintain a constant temperature of 28o C for 7 days without the cells disturbing the culture plates. Following the completion of the incubation period, the plate was frozen at -70o C, and the lysates were collected and stocked into a 2 mL glass vial for further investigation. Each vial was then subjected to RNA extraction procedure [35, 36]. Furthermore, the antiviral activity of the plant extract was performed by using real-time RT-PCR against the dengue-2 serotype cells with positive control in a subsequent study.
RNA Extraction
Qiagen’s QIAmp Viral RNA kit (Qiagen, Germany) was used to isolate viral RNA from a total of 140 µL of culture supernatant, which was then processed as per the manufacturer’s instructions. The final elution was performed in 50 µL of buffer before being stored at -80 °C until used in the anti-viral testing.
Quantitative Anti-Viral Assay
To determine the inhibitory effect of ZnO nanoparticles of crude fucoidan on the DENV-2 serotype, real-time RT-PCR was used. The experiment was carried out with the aid of a commercially available quantitative kit (Geno-Dengue Sen’s 1–4 PCR kit). Extracted samples were also treated in the same way as quantitation standards for dengue 1–4 serotypes (101–105copies/µl), anda similar volume (15 µL) was used in each case as well. The kit was used to specifically amplify and quantify dengue viruses. Each lysate had previously been subjected to RNA extraction (sample). It was carried out in a real-time PCR instrument, the ABI 7500, for this experiment. All of the PCR reagents were thawed and thoroughly mixed before the experiment began. The master mix was created in accordance with the instructions provided by the kit manufacturer. Following that, the required number of PCR tubes were prepared by adding 10 µL of master mix and 15 µL of extracted RNA to each lysate tube, along with 15 µL of the standards (Dengue 1–4, S 1–5) as a positive control and 15 µL of water (PCR grade) as a negative control. Following that, all of the reagents in the PCR tubes were thoroughly mixed and transferred into the ABI 7500 in near real-time. Conditions of thermocycler amplification include reverse transcription at 50oCfor 15 min, denaturation at 95oCfor 10 min, followed by 45 cycles of denaturation at 95oCfor 15 s, annealing at 55o Cfor 30s and a final extension step at 72oCfor 15s. While the annealing process was taking place, the fluorescence emission data was collected. The standard curve was used for quantitation in subsequent runs, provided that at least one/two standards are used in the current run with back titration.
Data Analysis
Tukey’s test was used to determine the percentages of cell viability of the ZnO nanoparticles of crude fucoidan. The data were analyzed using Microsoft Excel 2007 and a total of three samples were used in the analysis. The results were expressed as the mean of ± SD of all wells, and the cell viability of the plant and the test compound were calculated using the following formula;
Results
The present investigation was carried out on the Zinc oxide nanoparticles of crude fucoidan isolated from the brown seaweed S.marginatum in order to determine its potential in-vitro anti-dengue activity. In this present study, fucoidans were extracted from S.marginatum using an acid extraction process, and the yield was calculated in terms of its weight% (%w/w). The acid extraction of fucoidan at 65 °C for 3 h resulted in a yield of 3.81 ± 0.73% after the extraction process.
Thin Layer Chromatography Analysis
It was determined that S. marginatum contains polysaccharides that were qualitatively evaluated and identified. Upon spraying the TLC plates with Barfode’s reagent, the plates developed the appearance of red-colored spots, which confirmed the presence of polysaccharides in the solution.
RP-HPLC Analysis
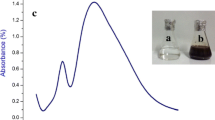
The optimal wavelength for the qualitative HPLC fingerprint profile of S. marginatum was selected at 254 nm due to the sharpness of the peaks and the proper baseline of the profile (Fig. 1). The S. marginatum was subjected to HPLC in order to isolate and identify the constituents present in the alcoholic extract prepared by acid extraction. This is confirmed by the presence of a sharp peak with a retention time (Rt) of 4.430 min, which indicates the presence of sulfated polysaccharides.
FT-IR Spectroscopy Analysis of Fucoidan Fractions
When fucoidans were extracted from S.marginatum, the FT-IR spectra revealed the characteristic absorption bands of fucoidans (Fig. 2). The major functional groups of fucoidan were the hydroxyl group, sulphate group, and methyl group (Fig. 3). The preliminary identification of fucoidan functional groups is always performed by FTIR analysis in the range of 400–4000 cm− 1. The typical IR characteristic of fucoidans can be evaluated by interpreting the O–H group of monomeric monosaccharides at 3400–3450 cm− 1, C–H asymmetric stretching of methyl group at 2900–2950 cm− 1, and S = O asymmetric stretching of sulfate group at 1114 − 1062 cm− 1. The FTIR spectrum showed the peeks at 3460.30, 2933.73 and 850.51 cm− 1, which confirms the hydroxyl, methyl and sulfate groups of fucoidans which suggested a complex pattern of substitution in the sample [28].
SEM and EDX Analysis
The surface morphology of the biosynthesized ZnO nanoparticles of crude fucoidan was shown in Fig. 4 and the Energy-dispersive X-ray spectroscopic analysis was shown in Fig. 5. It was observed that the synthesized nanoparticles were crystal in nature with the size range of 400-700 nm. Elemental analysis of synthesized ZnO nanoparticles characterized by SEM-EDX showed the presence of Zn in the sample ZnO nanoparticles. A strong peak signal was observed in EDX, which confirms the purity of synthesized ZnO nanoparticles.
Maximum Non-toxic Dose of ZnO Nanoparticles
The cell viability and toxicity of the C6/36 cell line were assessed using the MTT assay, and the maximum non-toxic dose of crude fucoidan was calculated to be 46.87 µg/ml (Fig. 6). Similarly, the IC50 was determined using the MTT assay in the Vero cell line using ZnO nanoparticles of crude fucoidan of S. marginatum and was found to be 149.33 µg/ml.
Quantitative Anti-Viral Assay by RT-PCR
According to the findings of this study, the ZnO nanoparticles of crude fucoidan isolated from S. marginatum were 99.09% effective against the dengue 2 viruses when treated with a concentration of 46.88 µg/ml of the virus. As shown in Table 2, the anti-dengue activity of ZnO nanoparticles derived from crude fucoidan isolated from S. marginatum as determined by real-time RT-PCR was superior to that of crude fucoidan isolated from S. marginatum.
Discussion
Dengue and other viruses continue to pose a threat to the world and have emerged as a major public health concern on a global scale. The World Health Organization is extremely concerned about dengue because it affects a large proportion of the world’s population.
Conventional antivirals used for the ailments of viral diseases were subjected to a high incidence of resistance. As a result, the determination of the antiviral effect of seaweeds as potent agents against clinical pathogens becomes extremely important in the treatment of dreadful viral infections, which are becoming increasingly common.
The anti-dengue studies of the seaweed S. marginatum and its isolated fucoidan were not performed. It was attempted in the present work to investigate the anti-dengue activity of ZnO nanoparticles of crude fucoidan isolated from S. marginatum by green biosynthesis in relation to dengue transmission.
The presence of the components of fucoidans was confirmed through chemical analysis. The presence of fucoidans is demonstrated by the appearance of distinct spots with characteristics that are distinct from carbohydrates in the TLC analysis, which is further supported by the appearance of an individual peak in the HPLC studies. FTIR studies provide conclusive evidence of the presence of functional groups in fucoidans, including the presence of prominent hydroxyl and carbonyl groups.
Some antiviral compounds are derived from natural sources, and microalgae are one of the most promising candidates for the production of these compounds. According to the researchers [37, 38], algae are responsible for 9% of biomedical compounds and some of the compounds are unable to replicate through chemical methods [39,40,41]. Furthermore, microalgae are able to produce huge amounts of biomass, and as a result, extremely high levels of compounds, without incurring any excess energy or costs [42,43,44].
Umezawa et al. conducted a study on the antiviral property of microalgae. They discovered that an extract of Chlorella pyrenoidosa, containing acid polysaccharides, had an inhibitory effect in mice against the vesicular stomatitis virus (VSV) [45].
Anti-HSV1 and HSV2 activity of seaweed Arthsospirafusiforme, which produces a sulfate polysaccharide were proved [46]. Porphyridium is a red microalgaethathas received a great deal of attention in recent years because of its potential biotechnological applications in the biomedical field. It is covered by a sulfate polysaccharide envelope that has antitumor, antibacterial, and antiviral activity, among other things [47].
High antiviral activity of sulfate polysaccharides was produced by Porphyridiumcruentum. Both Varicella zoster (HH3), a double-stranded DNA virus belonging to the Herpesvirus family, and vaccinia virus are susceptible to their effects in some cases [48, 49]. This species’ methanol extracts, which contained sulfated polysaccharides, were found to have high in-vitroantiviral activity against Piscine novirhabdovirus (VHSV) and African swine fever virus (ASFV) [50].
The present study discovered that the biosynthesized ZnO nanoparticles of crude fucoidan isolated from the brown seaweed S. marginatum had 99.09% activity against the dengue-2 virus. This high level of activity is likely due to the crude nature of fucoidan, but the formation of ZnO nanoparticles is responsible for the reduction in the percentage of activity seen so far. It has been shown that fucoidan in its purified and sulfated forms may have 100% activity against the dengue2 virus in cell lines that have been studied.
Conclusion
Seaweeds have been discovered to be a rich and viable source of biomolecules with therapeutic potential. A study on biosynthesized ZnO nanoparticles of crude fucoidan from brown seaweed S.marginatum was conducted to determine whether they had anti-dengue activity against C6/36 cell lines in vitro. The crude extract was analyzed by TLC, HPLC, and FTIR studies, and the results showed that fucoidans were present in significant amounts. Isolated fucoidans were synthesized into ZnO nanoparticles and tested for anti-dengue activity against a variety of cell lines. The results showed that the nanoparticles had 99.09% activity against the cell lines tested. As an antiviral agent, the ZnO nanoparticles of fucoidans have been shown to be an excellent lead molecule for the treatment of dengue fever.
References
Azizi, S., Ahmad, M. B., Namvar, F., & Mohamad, R. (2014). Green biosynthesis and characterization of zinc oxide nanoparticles using brown marine macroalga Sargassum muticum aqueous extract. Materials Letters, 116, 275–277
Allaker, R. P. (2010). The use of nanoparticles to control oral biofilm formation. Journal of Dental Research, 89(11), 1175–86
Morones, J. R., Elechiguerra, J. L., Camacho, A., Holt, K., Kouri, J. B., Ramírez, J. T., et al. (2005). The bactericidal effect of silver nanoparticles. Nanotechnology, 16(10), 2346–2353
Kaviyarasu, K., Kanimozhi, K., Matinise, N., Maria Magdalane, C., Mola, G. T., Kennedy, J., et al. (2017). Antiproliferative effects on human lung cell lines A549 activity of cadmium selenide nanoparticles extracted from cytotoxic effects: Investigation of bio-electronic application. Materials Science and Engineering: C [Internet], 76, 1012–25. Available from: https://www.sciencedirect.com/science/article/pii/S0928493117300127
Kaviyarasu, K., Geetha, N., Kanimozhi, K., Maria Magdalane, C., Sivaranjani, S., Ayeshamariam, A., et al. (2017). In vitro cytotoxicity effect and antibacterial performance of human lung epithelial cells A549 activity of Zinc oxide doped TiO2 nanocrystals: Investigation of bio-medical application by chemical method. Materials Science and Engineering: C [Internet], 74, 325–33. Available from: https://www.sciencedirect.com/science/article/pii/S0928493116316940
Sawai, J. (2003). Quantitative evaluation of antibacterial activities of metallic oxide powders (ZnO, MgO and CaO) by conductimetric assay. Journal of Microbiol Methods, 54(2), 177–182
Sondi, I., & Salopek-Sondi, B. (2004). Silver nanoparticles as antimicrobial agent: A case study on E. Coli as a Model for Gram-Negative Bacteria. Journal of Colloid and Interface Science, 275, 177–182
Gurugama, P., Garg, P., Perera, J., Wijewickrama, A., & Seneviratne, S. L. (2010). Dengue viral infections. Indian Journal of Dermatology[Internet], 55(1), 68–78. Available from: https://pubmed.ncbi.nlm.nih.gov/20418983
Malavige, G. N., Fernando, S., Fernando, D. J., & Seneviratne, S. L. (2004). Dengue viral infections. Postgraduate Medical Journal [Internet], 80(948), 588 LP – 601. Available from: http://pmj.bmj.com/content/80/948/588.abstract
Ramalingam, K., Varghese, C. S., Elias, C., Mathew, G. M., & Balasubramanian, A. (2019). A retrospective study on the effect of vitamin C in the management of dengue fever in three different states of India. International Journal of Research in Pharmaceutical Sciences, 10(4), 2670–2673
Brady, O. J., Gething, P. W., Bhatt, S., Messina, J. P., Brownstein, J. S., Hoen, A. G., et al. (2012). Refining the global spatial limits of dengue virus transmission by evidence-based consensus. Plos Neglected Tropical Diseases, 6(8), e1760
Hozumi, T., Matsumoto, T., Ooyama, H., Namba, T., Shiraki, K., Hattori, M., et al. (1995). Antiviral agent containing crude drug. Google Patents
Handel, A. S., Ayala, E. B., Borbor-Cordova, M. J., Fessler, A. G., Finkelstein, J. L., Espinoza, R. X. R., et al. (2016). Knowledge, attitudes, and practices regarding dengue infection among public sector healthcare providers in Machala, Ecuador. Tropical Diseases, Travel Medicine and Vaccines, 2, 8
Eluvakkal, T., Sivakumar, S. R., & Arunkumar, K. (2010). Fucoidan in some Indian brown seaweeds found along the coast gulf of Mannar. International Journal of Botany, Vol. 6, 176–181
Berteau, O., & Mulloy, B. (2003). Sulfated fucans, fresh perspectives: structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiology, 13(6), 29R–40R
Zhang, J., Peng, J., Chen, X., Gong, Y., Wan, L., Gao, F., et al. (2016). Rapid identification of bile acids in snake bile using ultrahigh-performance liquid chromatography with electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences, 1036–1037, 157–169
Bilan, M. I., Grachev, A. A., Shashkov, A. S., Nifantiev, N. E., & Usov, A. I. (2006). Structure of a fucoidan from the brown seaweed Fucus serratus L. Carbohydrate Research, 341(2), 238–245
Guven, K. C. B., Guvener, & Guler, E. (1999). In I. Akat (Ed.), Pharmacological activities of marine algae. Introduction Applied Phycology (pp. 67–92). SPB Academic Publishing by The Hague,
Adhikari, U., Mateu, C. G., Chattopadhyay, K., Pujol, C. A., Damonte, E. B., & Ray, B. (2006). Structure and antiviral activity of sulfated fucans from Stoechospermum marginatum. Phytochemistry, 67(22), 2474–2482
Kumaresan, S., Margret, R. J., & Mohan, V. (2008). Microscopic and preliminary Phytochemical studies on marine algae Padinatetra stromatica and Stoechospermum marginatum from Tuticorin coast, Tamil Nadu. Journal of Medicinal and Aromatic Plant Sciences, 30, 375–380
Saravanan, R., Sadiq, I. S., & Jawahar, P. (2017). Sea urchin diversity and its resources from the Gulf of Mannar, 4th Indian Biodiversity Congress 180–182
Xing, R., Liu, S., Yu, H., Chen, X., Qin, Y., Li, K., et al. (2013). Extraction and separation of fucoidan from Laminaria japonica with chitosan as extractant. Biomed Research International, 2013, 193689
Grignon-Dubois, M., & Rezzonico, B. (2018). Phenolic chemistry of the seagrass Zostera noltei Hornem. Part 1: First evidence of three infraspecific flavonoid chemotypes in three distinctive geographical regions. Phytochemistry, 146, 91–101
Dische, Z., & Shettles, L. B. (1948). A specific color reaction of methylpentoses and a spectrophotometric micromethod for their determination. Journal Of Biological Chemistry, 175(2), 595–603
Chale-Dzul, J., Pérez-Cabeza de Vaca, R., Quintal-Novelo, C., Olivera-Castillo, L., & Moo-Puc, R. (2020). Hepatoprotective effect of a fucoidan extract from Sargassum fluitans Borgesen against CCl(4)-induced toxicity in rats. International Journal of Biological Macromolecules, 145, 500–509
Czerwinska, J., Parkin, M. C., Dargan, P. I., George, C., Kicman, A. T., & Abbate, V. (2019). Stability of mephedrone and five of its phase I metabolites in human whole blood. Drug Testing and Analysis, 11(4), 586–94
Marimuthu, J., @Antonisamy, Essakimuthu, P., Narayanan, J., Anantham, B., Tharmaraj, R. J. J. M., et al. (2012). Phytochemical characterization of brown seaweed Sargassum wightii. Asian Pacific Journal of Tropical Disease [Internet], 2, S109–13. Available from: https://www.sciencedirect.com/science/article/pii/S2222180812601340
Liu, S., Wang, Q., Song, Y., He, Y., Ren, D., Cong, H., et al. (2018). Studies on the hepatoprotective effect of fucoidans from brown algae Kjellmaniella crassifolia. Carbohydrate Polymers [Internet], 193, 298–306. Available from: https://www.sciencedirect.com/science/article/pii/S0144861718303412
Bilan, M. I., Grachev, A. A., Ustuzhanina, N. E., Shashkov, A. S., Nifantiev, N. E., & Usov, A. I. (2002). Structure of a fucoidan from the brown seaweed Fucus evanescens C.Ag. Carbohydrate Research, 337(8), 719–730
Rajakumar, G., Thiruvengadam, M., Mydhili, G., Gomathi, T., & Chung, I. M. (2018). Green approach for synthesis of zinc oxide nanoparticles from Andrographis paniculata leaf extract and evaluation of their antioxidant, anti-diabetic, and anti-inflammatory activities. Bioprocess and Biosystems Engineering, 41(1), 21–30
Bhuyan, T., Mishra, K., Khanuja, M., Prasad, R., & Varma, A. (2015). Biosynthesis of zinc oxide nanoparticles from Azadirachta indica for antibacterial and photocatalytic applications. Materials Science in Semiconductor Processing, 32, 55–61
Umar, H., Kavaz, D., & Rizaner, N. (2019). Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. International Journal of Nanomedicine, 14, 87–100
Medina, F., Medina, J. F., Colón, C., Vergne, E., Santiago, G. A., & Muñoz-Jordán, J. L. (2012). Dengue virus: isolation, propagation, quantification, and storage. Current Protocols in Microbiology, Chap. 15, Unit 15D.2
Acosta, E. G., Castilla, V., & Damonte, E. B. (2011). Infectious dengue-1 virus entry into mosquito C6/36 cells. Virus Research, 160(1–2), 173–179
Rothan, H. A., Zulqarnain, M., Ammar, Y. A., Tan, E. C., Rahman, N. A., & Yusof, R. (2014). Screening of antiviral activities in medicinal plants extracts against dengue virus using dengue NS2B-NS3 protease assay. Tropical Biomedicine, 31(2), 286–296
Ramalingam, S., Karupannan, S., Padmanaban, P., Vijayan, S., Sheriff, K., Palani, G., et al. (2018). Anti-dengue activity of Andrographis paniculata extracts and quantification of dengue viral inhibition by SYBR green reverse transcription polymerase chain reaction. Ayu, 39(2), 87–91
Ahmadi, A., Zorofchian Moghadamtousi, S., Abubakar, S., & Zandi, K. (2015). Antiviral potential of algae polysaccharides isolated from marine sources: A review. Biomed Research International, 2015, 825203
Yasuhara-Bell, J., & Lu, Y. (2010). Marine compounds and their antiviral activities. Antiviral Research, 86(3), 231–240
Carbone, D. A., Olivieri, G., Pollio, A., & Melkonian, M. (2020). Biomass and phycobiliprotein production of Galdieria sulphuraria, immobilized on a twin-layer porous substrate photobioreactor. Applied Microbiology and Biotechnology, 104(7), 3109–3119
Priyadarshani, I., & Rath, B. (2012). Commercial and industrial applications of micro algae-a review. Journal of Algal Biomass Utilization, 3(4), 89–100
Yamaguchi, K. (1996). Recent advances in microalgal bioscience in Japan, with special reference to utilization of biomass and metabolites: a review. Journal of Applied Phycology, 8(6), 487–502
Borowitzka, M. A. (1999). Commercial production of microalgae: ponds, tanks, tubes and fermenters. Journal of Biotechnology, 70(1-3), 313–321
Pulz, O., & Gross, W. (2004). Valuable products from biotechnology of microalgae. Applied Microbiology and Biotechnology, 65(6), 635–648
Carbone, D. A., Olivieri, G., Pollio, A., & Gabriele, Melkonian, M. (2017). Growth and biomass productivity of Scenedesmus vacuolatus on a twin layer system and a comparison with other types of cultivations. Applied Microbiology And Biotechnology, 101(23–24), 8321–8329
Umezawa, I., & Komiyama, K. (1985). Acidic polysaccharide CH-1 isolated from Chlorella pyrenoidosa and the use thereof. US Patent (4533548A)
Sharaf, M., Amara, A., Aboul-Enein, A., Helmi, S., Ballot, A., Astani, A., et al. (2010). Molecular authentication and characterization of the antiherpetic activity of the cyanobacterium Arthrospira fusiformis. Die Pharmazie, 65(2), 132–136
Kavitha, M. D., Kathiresan, S., Bhattacharya, S., & Sarada, R. (2016). Culture media optimization of Porphyridium purpureum: production potential of biomass, total lipids, arachidonic and eicosapentaenoic acid. Journal Of Food Science And Technology, 53(5), 2270–2278
Huleihel, M., Ishanu, V., Tal, J., & Arad, S. M. (2001). Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. Journal of Applied Phycology, 13(2), 127–134
Radonic, L. M., Zimmermann, J. M., Zavallo, D., López, N., & López Bilbao, M. (2006). Rooting in Km selective media as efficient in vitro selection method for sunflower genetic transformation. Electronic Journal of Biotechnology, 9(3), 0
Fabregas, J., García, D., Fernandez-Alonso, M., Rocha, A. I., Gómez-Puertas, P., Escribano, J. M., et al. (1999). In vitro inhibition of the replication of haemorrhagic septicaemia virus (VHSV) and African swine fever virus (ASFV) by extracts from marine microalgae. Antiviral Research, 44(1), 67–73
Acknowledgements
The authors are acknowledged to the Vinayaka Mission’s Research Foundation (Deemed to be University), Salem for providing the necessary facilities to carry out this research.
Funding
The research leading to these results received funding from Vinayaka Mission’s Research Foundation (Deemed to be University), Salem-636 308, Tamilnadu, India, under Grant Agreement No VMRF/SeedMoney/2020/VMCP-Salem/4/dated 3 Feb 2020 for the proposal “Biosynthetic fucoidan derivatives as novel biomolecules against dengue virus: An investigational study”.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
No potential conflict of interest was reported by the authors.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kothai, R., Arul, B. & Anbazhagan, V. Anti-Dengue Activity of ZnO Nanoparticles of Crude Fucoidan from Brown Seaweed S.marginatum. Appl Biochem Biotechnol 195, 3747–3763 (2023). https://doi.org/10.1007/s12010-022-03966-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03966-w