Abstract
The endogenous level of cytokinins (CKs) and auxins (Auxs) was analyzed in isolated microspores and ovaries of two doubled haploid (DH) lines of triticale (× Triticosecale Wittm.) to better understand the mechanism of microspore embryogenesis (ME)—the most efficient and widely applied method of producing DHs. The responsiveness of the studied lines to ME significantly varied. ME was induced by pre-treating tillers with low temperature (4 °C for 3 weeks) alone or in combination with synthetic auxin (2,4-D), auxin inhibitor (PCIB) or melatonin (MEL) applied for 4 days before microspore isolation. Hormonal profile analyses, accompanied by ME effectiveness evaluation confirmed the multi-level crosstalk of Auxs/CKs and the specific hormonal homeostasis required for effective microspore reprogramming. It was found that triticale microspores contained mainly cis zeatin derivatives: cis-zeatin-O-glucoside (cZOG), cis-zeatin riboside (cZR) and cis-zeatin (cZ), as well as indole-3-acetic acid (IAA) and IAA-aspartate (IAAsp). Increased ME efficiency was associated with higher contents of most of the identified CKs and Auxs, as well as the higher active Aux/active CK ratio. Trans CK isoforms were detected only in ovaries, confirming their importance as a source of bioactive molecules stimulating embryogenic development. Two of the pre-treatments tested: 12.5 μM PCIB and 50 μM MEL decreased the active Aux/active CK ratio, which was accompanied by an increase in the regeneration efficiency.
Key message
Effective embryogenic development of isolated triticale microspores requires high concentration of auxins and moderate levels of cytokinins, among which active trans isomers released by co-cultured ovaries are the most important. However, high regeneration efficiency is favored by a significantly lower ratio of active Auxs/active CKs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acceleration of plant breeding can be successfully achieved using advanced biotechnological tools, such as genome editing and genome selection (Watson et al. 2018). However, more classical methods can also be beneficial, provided that efficient procedures are developed. One such example, frequently implemented into breeding programs, is DH technology, known as the fastest route to complete plant homozygosity (Wędzony et al. 2009). Among the various methods used for DH production, the process of microspore embryogenesis (ME), defined as the reproduction of an individual with genetically exclusive male origin, is potentially the most effective (Segui-Simarro 2010). This technique requires a complete re-programming of the microspore developmental fate into an embryo rather than pollen grain formation, and is known to be accompanied by many physiological, metabolic and molecular changes. Despite extensive studies, knowledge concerning the molecular and physiological basis of this switch is still fragmentary (Żur et al., 2014 and citations therein) and very often strong genotypic control and a significant influence of environmental factors limit the implementation of DH technology into breeding practice.
Triticale (× Triticosecale Wittm.)—a hybrid developed to combine the high yield potential and quality of wheat with the adaptability, stress tolerance and unique nutritional composition of rye—is among plant species extensively tested for ME. Unfortunately, many triticale materials with high breeding value are rather disappointing in terms of DH production efficiency. Nevertheless, over the years, significant progress has been observed in the frequency of green plant regeneration per single donor spike (GR/spike) as the most accurate parameter indicative of the protocol success. The highest efficiency (55 GR/spike) was obtained by Oleszczuk et al. (2004) in the highly responsive triticale cultivar ‘Bogo’, while the general ME effectiveness was shown to range from 5 to 12 GR/spike (Asif et al. 2013a, 2014a, 2014b, 2013b; Lantos et al. 2014; Pauk et al. 2003; Würschum et al. 2014; Żur et al. 2009, 2008, 2019).
Stress treatment applied before or after microspore isolation is one of the important factors that trigger microspore reprogramming (Touraev et al. 2000, 1997). To date, various stresses have been examined as potential ME-inducing factors, including high/low temperature, carbohydrate/nitrogen starvation, osmotic stress or colchicine (Islam and Tuteja 2012). Among them, long-term (2–4 weeks) tiller pre-treatment with low temperature is the most successfully and most often applied (Krzewska et al. 2012; Żur et al. 2014). Several authors have described the effects of low temperature on microspore viability, metabolism and gene expression pattern (reviewed by Shariatpanahi et al. 2006; Zoriniants et al. 2005). However, the role and mechanism of the observed changes still remain unclear (Żur et al. 2012). It should be noted that the applied stress pre-treatment may negatively affect cell viability and induce recombination in nuclear or chloroplast genomes, leading to reduced regeneration capacity, albino plant formation or somaclonal variations (Shariatpanahi et al. 2006). Therefore, further progress can be expected with a better understanding of the physiological and molecular mechanisms involved in this process.
The role of plant growth regulators (PGRs) is one of the most thoroughly studied aspects of ME. These key signaling molecules control plant growth and development and initiate signal transduction pathways in response to various environmental stimuli (Kohli et al. 2013). Among PGRs, auxins (Auxs) and cytokinins (CKs) play the most important role and can act synergistically or antagonistically, depending on the context and their respective levels (Jones and Ljung 2011). Moreover, their effect can be modified by the plant genome, stage of plant development and tissue type (Moubayidin et al. 2009). Auxs regulate or influence diverse responses both at the whole-plant and cellular levels, such as cell enlargement, division and differentiation (Hagen and Guilfoyle 2002). These PGRs have been used in in vitro cultures to induce cell divisions and cyto-, organo- and embryogenic differentiation (Moller and Weijers 2009). At low concentrations, they promote root initiation (Overvoorde et al. 2010), whereas at higher levels, they induce callus formation. In turn, CK incorporation in culture media triggers cell divisions, chloroplast development, induces differentiation of adventitious shoots from callus and organs, as well as stimulates shoot proliferation by releasing axillary buds from apical dominance or by inhibiting root growth (Riefler et al. 2006). Many authors have examined and characterized the role of PGRs in ME (review in Wędzony et al. (2009); Żur et al. (2015a)). However, most studies focused on the effect of exogenous PGR application to media, and only a few described the influence of endogenous PGRs on ME effectiveness (Delalonde and Coumans 1998; Gorbunova et al. 2001; Lulsdorf et al. 2012). The analysis of triticale anthers confirmed significant changes in PGR homoeostasis associated with ME induction (Żur et al. 2015b). Pre-treatment of tillers with low temperature (3 weeks at 4 °C; LT) resulted in a higher accumulation of indole-3-acetic acid (IAA), indole-3-butyric acid (IBA), cis-zeatin (cZ) and cis-zeatin riboside (cZR), with a simultaneous decrease in trans-zeatin (tZ) levels. The amplitude of changes was determined by the plant genotype and associated with anther-culture responsiveness. A distinctly lower Aux/CK ratio was observed in anthers of responsive triticale cultivars in comparison to recalcitrant ones both before and after ME-inducing treatment, which was consistent with the fact that endogenous hormonal balance between Auxs and CKs determines embryogenic potential (Pérez-Jiménez et al. 2013; Żur et al. 2015b).
It should be noted that proper embryogenic development of isolated microspores of some cereals, including triticale, requires co-culture with ovaries. However, the nature of the conditioning factors or growth enhancers released by the ovaries to the medium has not yet been recognized (Li and Devaux 2001; Zheng et al. 2002). For several years, the biggest interest has been focused on arabinogalactan proteins (AGPs), which had been identified as molecules which promote embryogenic development of microspores (Paire et al. 2003; Borderies et al. 2004; Letarte et al. 2006; Tang et al. 2006). However, data published by Lippmann et al. (2015) demonstrated that the molecule(s) sought should have a molecular weight below 3 kDa. This excluded a number of potential candidates (e.g. AGPs or chitinases) and focused the attention on compounds like PGRs, peptides, polyamines or sugar derivatives.
As a continuation of our research aimed at better understanding the mechanisms of triticale ME and its regulation, the present study analyzed endogenous levels of two groups of phytohormones (Auxs and CKs) in two triticale DH lines highly differentiated in their ME responsiveness. Hormonal profiles were determined in isolated microspores and immature ovaries after LT tiller pre-treatment, which induced triticale microspore reprogramming. Moreover, the influence of exogenously applied substances, i.e. synthetic auxin (2,4-dichlorophenoxyacetic acid; 2,4-D), anti-auxin (p-chlorophenoxyisobutyric acid; PCIB) and melatonin (N-acetyl-5-methoxytryptamine; MEL) on hormonal homeostasis and ME effectiveness was also studied.
Materials and methods
Plant material
Two triticale DH lines used in the study were derived from the F1 generation of a cross between the German inbred line ‘Saka 3006’ and the Polish cv. ‘Modus’, identified earlier as ‘responsive’ (DH28) and ‘recalcitrant’ (DH19) to ME induction (Nowicka et al. 2019; Żur et al. 2015b, 2019).
Germinating triticale seeds were placed in perlite pre-soaked with Hoagland’s liquid medium (as described by Wędzony (2003)) and vernalized for 7 weeks at 4 °C and 8/16 h (day/night) photoperiod. Vernalized seedlings were planted in pots containing a mixture of soil, deacidified substrate peat and sand (2/2/1; v/v/v), and grown in a greenhouse at 25 °C with 16/8 h photoperiod. Tillers from donor plants were collected when the majority of microspores were at the mid- to late-uninucleate stage of development.
Tiller pre-treatment
Collected tillers were pre-treated with low temperature (3 weeks at 4 °C, in the dark; LT). During pre-treatment, the tillers were kept in Hoagland’s liquid nutrient solution (control). Additionally, the effect of various PGRs and PGR inhibitors was tested, namely: 2,4-D (2,4-dichlorophenoxyacetic acid; D70724, Sigma-Aldrich, Darmstadt, Germany), PCIB (p-chlorophenoxyisobutyric acid; 197777, Sigma-Aldrich, Darmstadt, Germany) and MEL (melatonin; N-acetyl-5-methoxytryptamine M5250, Sigma-Aldrich, Darmstadt, Germany). These compounds were added to liquid Hoagland’s medium for 4 days before the scheduled date of microspore isolation at different concentration: 5 or 12.5 µM for 2,4-D and PCIB, and 50 or 100 µM for MEL. The experimental design is presented in Fig. 1.
Scheme of the experiment. PLANT MATERIAL: two DH lines of winter triticale (resposive DH28 and recalcitrant DH19); TILLERS PRE-TREATMENTS: Control—low temperature (LT) treatment (21 days (21d) at 4 °C); 2,4-D—LT treatment combined with the application of 5 µM or 12.5 µM 2,4-dichlorophenoxyacetic acid for 4 days (4d) before microspore isolation; PCIB—LT treatment combined with the application of 5 µM or 12.5 µM p-chlorophenoxyisobutyric acid for 4d before microspore isolation; MEL—LT treatment combined with the application of 50 µM or 100 µM melatonin for 4d before microspore isolation. ANALYSES: the estimation of (1) ME induction effectiveness: EM [%]—the mean percentage of embryogenic microspores observed in the whole population of isolated cells at the day of isolation); ELS [%]—the mean percentage of embryo-like structures observed in the whole population of isolated cells after 8 days of in vitro culture; (2) regeneration effectiveness observed after 6 weeks of in vitro culture: GR/100 ELS—the number of green regenerants per 100 ELS; AR/100 ELS—the number of albino regenerants per 100 ELS, and (3) the analysis of endogenous cytokinins (CK) and auxins (Aux) in isolated microspores and ovaries with HPLC–MS/MS method
Microspore culture protocol
After 3 weeks of the above-characterized pre-treatments, tillers were homogenized in cold 0.3 M mannitol, filtrated through a metal sieve (74 µm, Tissue Grinder Homogenizer Kit, Sigma-Aldrich) and centrifuged (100×g, 10 min). The pellet was resuspended in 0.3 M mannitol and viable microspores were separated from dead microspores and debris by density gradient centrifugation (0.3 M mannitol/0.58 M maltose, 80×g, 10 min). Viable microspores were collected from the band at the mannitol/maltose interphase, washed in 0.3 M mannitol and centrifuged again (100×g, 10 min). Subsequently, pelleted microspores were resuspended in 190–2 medium (Zhuang and Xu 1983), modified according to Pauk et al. (2000), with a final culture density of 80,000 microspores per mL. Microspore suspensions were co-cultured with immature ovaries (10 per 1 mL of suspension) and incubated in the dark at 26 °C. Embryo-like structures (ELSs), with a diameter over 1 mm, were transferred onto 190–2 regeneration medium (Zhuang and Xu 1983), modified by Pauk et al. (2000), supplemented with 0.5 mg/L NAA (α-naphthaleneacetic acid, N0640, Sigma-Aldrich) and 0.5 mg/L KIN (kinetin, 48,130; Sigma-Aldrich). The cultures were kept at 26 °C, in dim light [80–100 µM (hν) m−2 s−1 (PAR)] with 16/8 h (day/night) photoperiod.
Analysis of cell viability and microspore developmental stage
Microspore viability was checked by fluorescein diacetate (FDA; green fluorescence) staining according to the procedure described by Heslop-Harrison and Heslop-Harrison (1970).
The developmental stage of microspores was determined by 4,6-diamidino-2-phenylindole staining (DAPI; blue fluorescence). Observations were performed on the day of isolation (0d). At least 500 microspores from 10 fields of view (magnification ×10) were evaluated for each sample.
Microscopic observations were conducted using a Nikon Eclipse E 600-microscope, equipped with a differential interference contrast (DIC) and a digital camera DS-Ri1 (Nikon). Fluorescence was tested using an EX 510-560/DM 580 BA/590 EF (PI) filter. Images were acquired and processed using NIS Elements (BR, AR 2.10 Laboratory Imaging System Ltd.) software package.
Assessment of the effectiveness of microspore embryogenesis induction and plant regeneration
The effectiveness of ME was estimated on the basis of the following parameters: (i) EM [%]—the mean percentage of embryogenic microspores (EM) observed in the whole population of isolated cells at the day of isolation (0d). This class includes microspores with star-like morphology (star-like structures, SLS) and after the first symmetrical nuclear division—features characteristic of ME initiation in some plant species (Touraev et al. 2000) and citations therein, Indrianto et al. (2001)), including isolated microspore cultures of triticale (Dubas et al. 2010); (ii) ELS [%]—the mean percentage of multicellular structures still closed within the sporoderm and ELS released after sporoderm wall rupture observed after 8 days of in vitro culture (8d). Both parameters were calculated as the mean of three biological replications, where each 35 × 15 mm Petri dish, containing approx. 80,000 microspores excised from eight spikes, was considered as one replication; (iii) GR/100 ELS—number of green plants (GR) regenerated per 100 ELS; (iv) AR/100 ELS—number of albino plants (AR) regenerated per 100 ELS.
Sample preparation for endogenous hormone analysis
Plant materials used for the determination of CKs and Auxs: (i) viable microspores collected from the mannitol/maltose interphase band after density gradient centrifugation, (ii) ovaries from LT pre-treated tillers, and (iii) ovaries collected from suspension cultures in which multicellular structures were observed. The collected samples were frozen in liquid nitrogen, lyophilized and purified by in-tip µSPE according to previously described protocols for CKs by Svačinová et al. (2012) and for Auxs by Pěnčík et al. (2018).
Extraction, purification and quantification of cytokinins and auxins
To determine endogenous CK levels, approximately 0.4 mg DW of lyophilized samples was used for the extraction with 1 mL of Bieleski buffer (Novák et al. 2008). A mix of isotope-labeled CK internal standards (0.25 pmol for B and 7G samples, and 0.5 pmol for OG and NT samples) was added to each sample to check for recovery during the purification step and to validate the determination. Cytokinin StageTips contained 3 layers of individual stationary phases: C18, SDB and Cation (Empore™, 3 M™,) and the purification procedure was performed according to Svačinová et al. (2012). Eluates were collected and evaporated to dryness. The samples were subsequently dissolved in 30 µL of 10% MeOH and 10 µL of each sample was injected and analyzed by ultraperformance liquid chromatography (Acquity UPLC® I-class system; Waters, Milford, MA, USA), coupled to a triple quadrupole mass spectrometer equipped with an electrospray interface (Xevo TQ-S, Waters, Manchester, UK), using a method described by Svačinová et al. (2012). Multiple reaction monitoring of [M + H]+ and the appropriate product ion were used to quantify peptides. Conditions, dwell time, cone voltage and collision energy in the collision cell were optimized for each cytokinin metabolite. Quantification was performed with Masslynx software using the standard isotope dilution method.
Lyophilized samples (1 mg DW) for Aux determination were extracted using 1 mL 50 mM Na-phosphate buffer (pH 7.0; 4 °C) containing 0.1% diethyldithiocarbamic acid salt. A mix of isotope-labeled Aux internal standards [13C6]IAAsp, [13C6]IAGlu, [13C6]IAA, [13C6]oxIAA, (all at 5 pmol per sample) was added to each sample. The extraction and purification protocol was carried out according to Pěnčík et al. (2018) using multi-StageTip columns with a combination of 3 layers of two stationary phases: C18 and SDB-XC (Empore™, 3 M™). Sample eluates were evaporated to dryness and subsequently dissolved in 30 µL of 10% MeOH, injected onto a reversed-phase column (Kinetex 1.7 um C18 100A, 50 × 2.1 mm; Phenomenex) and analyzed using Acquity UPLC® I-class system (Waters, Milford, MA, USA), coupled to Xevo™ TQ-S (Waters, Manchester, UK) equipped with an electrospray interface (ESI) by the method described by Pěnčík et al. (2009).
Statistical analysis
Data evaluation began with descriptive statistical analysis (mean, standard deviation). Variables with normal distribution were tested using one-way analysis of variance (ANOVA), followed by post hoc comparison using Duncan’s multiple range test (p ≤ 0.05). All statistical analyses were performed using STATISTICA version 13.0 (Stat Soft Inc., Tulsa, OK USA).
Results
Effectiveness of ME induction and plant regeneration in two DH lines of triticale as the effect of standard and modified tiller pre-treatments
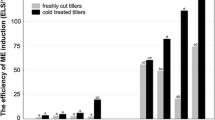
The present study confirmed the previously observed variability of two triticale DH lines in response to ME induction treatment (Żur et al. 2015b, 2019). Our study also revealed that this variation was not determined by microspore viability, as after LT pre-treatment of tillers, the frequency of viable microspores was similar, from nearly 70% for the recalcitrant line DH19 to 80% for the responsive line DH28 (Fig. 2). Moreover, most of the tiller pre-treatment modifications had no effect on microspore viability. The only exception was the pre-treatment with 5 µM 2,4-D, which reduced the viability of DH28 microspores by nearly 50% compared to the control treatment.
Microspore viability estimated on the day of isolation (0d) after various microspore embryogenesis-inducing tillers pre-treatments for two DH lines of winter triticale. All modifications (2,4-D, PCIB, and MEL) were applied for 4 days before microspore isolation. Data represent means from 3 biological replications (isolations) ± SD. Statistical analysis was performed separately for each DH line of triticale. Data marked with different letters are significantly different at p ≤ 0.05 according to Duncan’s multiple range test. Control—low temperature (LT) treatment (21 days at 4 °C); 2,4-D—LT treatment combined with the application of 5 µM or 12.5 µM 2,4-dichlorophenoxyacetic acid; PCIB—LT treatment combined with the application of 5 µM or 12.5 µM p-chlorophenoxyisobutyric acid; MEL—LT treatment combined with the application of 50 µM or 100 µM melatonin
Microscopic observation on the day of isolation (0d) showed a high number of EMs in both DH28 and DH19 lines (Fig. 3). LT pre-treatment of tillers resulted in embryogenesis induction in 40 and 24% of microspores in DH28 and DH19, respectively. Among the modified pre-treatments, application of PCIB in DH19 and 2,4-D in DH28 significantly altered the effectiveness of ME induction, reducing the number of EM by 30–50%. The number of microspores continuing embryogenic development decreased after eight days (8d) of in vitro culture, and most microspores (76–93%, depending on the treatment and triticale genotype) began to shrink, accumulate starch or die.
Progressive stages of embryogenesis in isolated microspore cultures of two DH lines of triticale (DH28 and DH19), on the example of cultures isolated after control and tiller pre-treatments with 12.5 µM 2,4-D, 12.5 µM PCIB and 50 µM MEL. First two columns represent microspore suspensions on the day of isolation (0d) and on the 8th day (8d) of in vitro culture. Included are the mean percentage of embryogenic microspores (EM; microspores with star-like morphology (arrows) and after first symmetrical nuclear division) and the mean percentage of embryo-like structures (ELSs) in isolated microspore suspensions. The third column shows the efficiency of plant regeneration after six weeks of in vitro culture. Included is the frequency of green (GR/100 ELS) and albino (AR/100 ELS) regenerants formation. Aborted—microspore suspensions with inhibited ELS development. Control—low temperature (LT) treatment (21 days at 4 °C); 2,4-D—LT treatment combined with the application of 12.5 µM 2,4-dichlorophenoxyacetic acid; PCIB—LT treatment combined with the application of 12.5 µM p-chlorophenoxyisobutyric acid; MEL—LT treatment combined with the application of 50 µM melatonin. All modifications (2,4-D, PCIB, and MEL) were applied for 4 days before microspore isolation
Despite the relatively high frequency of ME initiation, the DH19 line confirmed its low embryogenic potential, as SLS development was arrested and most of them died. Regardless of the tiller pre-treatment applied, only a few ELSs developed, but aborted before they reached a size suitable for transfer to the regeneration medium. As a result, plant regeneration was observed only in isolated microspore cultures of the responsive DH28 line (Fig. 3). After LT pre-treatment of tillers, about 20% of the produced ELSs regenerated into green plants. Two of the selected pre-treatments (12.5 µM PCIB and 50 µM MEL) induced a positive effect on green plant regeneration, increasing the number of green regenerants to 27 and 34 GR/100 ELS, respectively.
Hormonal profile of microspores and ovaries after standard, LT tiller pre-treatment used for ME induction
The analyses of Auxs in both studied DH triticale lines showed the presence of indole-3-acetic acid (IAA) and its two conjugates: IAA-aspartate (IAAsp) and IAA-glutamate (IAGlu) (Fig. 4A, B). In microspores of DH28, IAA accounted for 34% of the total Aux pool, with 59% of IAAsp and 2% of IAGlu. Small fraction (5%) was identified as Aux catabolite (2-oxindole-3-acetic acid, oxIAA). Microspores of the DH19 line had a higher proportion of IAA (51%), with 45% of IAAsp and 4% of IAGlu. Moreover, the comparison of hormone content showed a higher accumulation of both active (almost threefold) and conjugated Aux forms (5.5-fold and over twofold higher for IAAsp and IAGlu, respectively) in DH28 compared to DH19 (Fig. 4B).
A Auxin (Aux) profile in microspores of two doubled haploid (DH) lines of triticale varied in microspore embryogenesis (ME) effectiveness (recalcitrant DH19 and responsive DH28) after control low temperature (LT) tiller pre-treatment. B Content of auxins (Auxs) [pmol/g DW] in microspores of two doubled haploid (DH) lines of triticale varied in microspore embryogenesis (ME) effectiveness (recalcitrant DH19 and responsive DH28) after various ME inducing tiller pre-treatments. All modifications (2,4-D, PCIB, and MEL) were applied for 4 days before microspore isolation. Data represent mean values (n = 3) ± SD. Data marked with different letters are significantly different at p ≤ 0.05 according to Duncan’s multiple range test. Control—low temperature (LT) treatment (21 days at 4 °C); 2,4-D—LT treatment combined with the application of 5 µM or 12.5 µM 2,4-dichlorophenoxyacetic acid; PCIB—LT treatment combined with the application of 5 µM or 12.5 µM p-chlorophenoxyisobutyric acid; MEL—LT treatment combined with the application of 50 µM or 100 µM melatonin. IAA—indole-3-acetic acid; oxIAA—2-oxindole-3-acetic acid; IAAsp—IAA-aspartate; IAGlu—IAA-glutamate
Several types of CKs and their conjugates were identified in triticale microspores collected after density gradient centrifugation: cZ, cZR, cis-zeatin-O-glucoside (cZOG), cis-zeatin riboside-O-glucoside (cZROG), N6-isopentenyladenine (iP) and N6-isopentenyladenosine (iPR) (Fig. 5A, B). Among them, three CK forms (cZOG, cZR, and cZ) were dominant, yielding about 40%, above 20% and about 15% of total CK pool in examined DH lines, respectively (Fig. 5A). After the control (LT) tiller pre-treatment, DH28 was characterized by a 1.5-fold higher content of cZOG and iPR and a twofold higher content of cZR compared to DH19 (Fig. 5B).
A Cytokin (CK) profile in microspores of two doubled haploid (DH) lines of triticale varied in microspore embryogenesis (ME) effectiveness: recalcitrant DH19 and responsive DH28 after control low temperature (LT) tiller pre-treatment. B Content of cytokinins (CKs) [pmol/g DW] in microspores of two doubled haploid (DH) lines of triticale varied in microspore embryogenesis (ME) effectiveness (recalcitrant DH19 and responsive DH28) after various ME inducing tiller pre-treatments. All modifications (2,4-D, PCIB, and MEL) were applied for 4 days before microspore isolation. Data represent mean values (n = 3) ± SD. Data marked with different letters are significantly different at p ≤ 0.05 according to Duncan’s multiple range test. Control—low temperature (LT) treatment (21 days at 4 °C); 2,4-D—LT treatment combined with the application of 5 µM or 12.5 µM 2,4-dichlorophenoxyacetic acid; PCIB—LT treatment combined with the application of 5 µM or 12.5 µM p-chlorophenoxyisobutyric acid; MEL—LT treatment combined with the application of 50 µM or 100 µM melatonin. cZ—cis-zeatin; cZOG—cis-zeatin-O-glucoside; cZR—cis-zeatin riboside; cZROG—cis-zeatin riboside-O-glucoside; iP—N6-isopentenyladenine; iPR—N6-isopentenyladenosine
The determination of phytohormones was also performed in triticale ovaries (Fig. 6A–C). Triticale ovaries collected from LT pre-treated tillers were characterized by different Aux contents compared to microspores, i.e. lower concentrations of IAA and IAAsp and higher levels of oxIAA (Fig. 4B, 6B). In ovaries of both tested DH lines, oxIAA was dominant and constituted 80% and 95% of the total Aux pool in DH28 and DH19, respectively (Fig. 6A). The comparison between the two lines demonstrated that DH28 had a threefold higher IAA content compared to DH19 (Fig. 6B). In contrast, this responsive line had 3 times less oxIAA and 4 times less IAAsp.
A Composition of auxins (Aux) and cytokinins (CK) in ovaries of two doubled haploid (DH) lines of triticale varied in microspore embryogenesis (ME) effectiveness (recalcitrant DH19 and responsive DH28) after control low temperature (LT) tiller pre-treatment. B–C The content of auxins and cytokinins [pmol/g DW] in ovaries of two doubled haploid (DH) lines of triticale varied in microspore embryogenesis (ME) effectiveness (recalcitrant DH19 and responsive DH28) after various ME inducing tiller pre-treatments. All modifications (2,4-D, PCIB, and MEL) were applied for 4 days before microspore isolation. Data represent mean values (n = 3) ± SD. Data marked with different letters are significantly different at p ≤ 0.05 according to Duncan’s multiple range test. Control—low temperature (LT) treatment (21 days at 4 °C); 2,4-D—LT treatment combined with the application of 5 µM or 12.5 µM 2,4-dichlorophenoxyacetic acid; PCIB—LT treatment combined with the application of 5 µM or 12.5 µM p-chlorophenoxyisobutyric acid; MEL—LT treatment combined with the application of 50 µM or 100 µM melatonin. IAA—indole-3-acetic acid; IAAsp—IAA-aspartate; oxIAA—2-oxindole-3-acetic acid; cZ—cis-zeatin; cZOG—cis-zeatin-O-glucoside; cZR—cis-zeatin riboside; cZROG—cis-zeatin riboside-O-glucoside; iP—N6-isopentenyladenine; iPR—N6-isopentenyladenosine; tZ—trans-zeatin; tZR—trans-zeatin riboside; tZ9G—trans-zeatin N9-glucoside; tZR5’MP—trans-zeatin riboside-5-monophosphate
Interestingly, in contrast to the analyzed microspores, ovaries contained relatively high levels of trans CK isomers (Fig. 6A, C). A significant effect of plant genotype was also observed. In DH28 ovaries, trans-zeatin N9-glucoside (tZ9G) was the dominant form (30% of total CK pool), with lower amounts of cZR and cZOG (approximately 23% and 12%, respectively). DH19 ovaries were characterized by a high proportion of cZR and tZ9G, constituting approx. 39% and 29% of the CK pool, respectively. Other detected CK forms did not exceed 10% of total CK content. In response to the LT tiller pre-treatment, the ovaries of highly embryogenic DH28 accumulated higher quantities (by 46%) of cZOG and lower amounts (by 30%) of cZR in comparison to DH19 (Fig. 6C). Among trans CK isomers identified only in ovaries, the DH28 line had a higher content of tZR and trans-zeatin riboside-5-monophosphate (tZR5’MP), as well as dihydrozeatin-O-glucoside riboside (DHZROG) compared to DH19 (Fig. 6C).
Ovaries collected from microspore suspensions of DH28, which continued embryogenic development, were characterized by a quite different hormonal profile as compared to ovaries isolated from LT pre-treated tillers (Fig. 6A, 7A). After 8 days of in vitro culture, IAA level fell below the detection limit, which was associated with the dramatic increase in IAAsp share (from 2 to 70%) and a decrease in oxIAA share (from 80 to 17%) in the total Aux pool. About 13% of total Auxs was identified as IAGlu, which was not detected previously, in the ovaries isolated from LT pre-treated tillers. Co-cultured ovaries were also characterized by a different set of CKs, as trans isomers of zeatin and its derivatives (tZ9G, tZ, tZR) were not detected after 8 days of in vitro culture (Fig. 7A, C). Moreover, the ovaries contained much lower level of cis isomers (Fig. 7C) and different proportion of CKs and its derivatives: 36% of cis-zeatin riboside-5-monophosphate (cZR5’MP), 17% of cZOG and cZROG and 5% of cZR, as well as a relatively high level (25%) of glucoside KIN derivative (kinetin-9-glucoside; K9G) (Fig. 7A).
A Composition of auxins (Auxs) and cytokinins (CKs) in ovaries of DH28 collected after various tiller pre-treatments and followed by 8-day in vitro microspore/ovary co-culture. B–C The content of auxins (Auxs) and cytokinins (CKs) [pmol/g DW] in ovaries of DH28 collected after various tiller pre-treatments and followed by 8-day in vitro microspore/ovary co-culture. All modifications were applied for 4 days before microspore isolation. Showed data concerns the concentration of 2,4-D, PCIB and MEL identified as more effective in microspore reprogramming. Data represent the mean values (n = 3) ± SD. Data marked with different letters are significantly different within each identified phytohormone at p ≤ 0.05 according to Duncan’s multiple range test. Control—low temperature (LT) treatment (21 days at 4 °C); 2,4-D—LT treatment combined with the application of 12.5 µM 2,4-dichlorophenoxyacetic acid; PCIB—LT treatment combined with the application of 12.5 µM p-chlorophenoxyisobutyric acid; MEL—LT treatment combined with the application of 50 µM melatonin. IAA—indole-3-acetic acid; oxIAA—2-oxindole-3-acetic acid; IAAsp—IAA-aspartate; IAGlu—IAA-glutamate; cZOG—cis-zeatin-O-glucoside; cZR—cis-zeatin riboside; cZROG—cis-zeatin riboside-O-glucoside; cZR5’MP—cis-zeatin riboside-5-monophosphate; KIN—kinetin; K9G—kinetin-9-glucoside
Disturbances in hormonal homeostasis induced by Aux/Aux inhibitor pre-treatment in microspores and ovaries of triticale
Both the direction and extent of changes induced by tiller pre-treatments with Aux (2,4-D) and Aux inhibitor (PCIB) depended on plant genotype.
Pre-treatment of tillers with 2,4-D caused a significant change in Aux contents. It decreased the accumulation of IAA, IAGlu and IAAsp in DH28 microspores in the range from 13 to 56% (Fig. 4B). Interestingly, a stronger effect was induced by a lower concentration (5 μM) of this synthetic Aux analogue, which simultaneously decreased the content of cZOG, cZR and iPR by about 30% (Fig. 5B). In turn, an increase in oxIAA content by 24% and 47% was observed after 5 μM and 12.5 μM 2,4-D pre-treatments, respectively, compared to control (Fig. 4B). This was associated with reduced viability of DH28 microspores (after 5 μM 2,4-D pre-treatment) and a decrease in the number of EM (after 12.5 μM 2,4-D pre-treatment) (Fig. 2, 3).
In DH19 microspores, synthetic Aux, applied at 5 μM, increased IAA content by 17% and IAAsp by 38%, while at 12.5 μM, it decreased IAGlu content by 17% and iP by 44% compared to the control pre-treatment (Fig. 4B, 5B). However, all these changes had no effect either on microspore viability or ELS development.
Decreased IAA content (by 15% and 25%) and increased level of oxIAA (by 26% and 55%) compared to the control were observed in DH28 microspores isolated after PCIB application at 5 μM and 12.5 μM, respectively (Fig. 4B). At a lower concentration, PCIB increased IAAsp and IAGlu by 21% and 9%, respectively. Its higher concentration had positive influence on the effectiveness of green plant regeneration, which was associated with a decrease in the concentration of IAAsp and IAGlu (by 9% and 34%, respectively) and an increase in the content of cZ and cZR (by 47% and 27%, respectively) as compared to control (Fig. 4B, 5B). In DH19, only the higher concentration (12.5 μM) of PCIB significantly affected Aux content, reducing IAA, IAGlu and IAAsp accumulation by 42%, 27% and 10%, respectively (Fig. 4B). It was associated with a decrease in cZR concentration by 20% (Fig. 5B). The only effect induced in DH19 microspores by pre-treatment with 5 µM PCIB was an 25% increase in the level of cZROG.
Pre-treatment of tillers with lower dose of synthetic Aux (5 μM) led to 55–100% decrease in IAA and 57–100% decrease in oxIAA contents in the ovaries of both DH lines of triticale compared to control (Fig. 6B). This pre-treatment also decreased IAAsp content more than threefold compared to control, but only in ovaries of recalcitrant line (DH19). Higher concentration of 2,4-D (12.5 μM) decreased IAA level by 38% in microspores of DH28 which was associated with an increased content of IAAsp by 63%. The same pre-treatment decreased dramatically IAAsp and oxIAA contents in microspores of DH19 to 28% and to 11% of control, respectively.
A decline in the amount of most of identified CKs was also noted in the ovaries of this recalcitrant line (DH19) at a lower concentration of 2,4-D (Fig. 6C). In contrast, the content of cZOG and cZROG in the ovaries of DH28 excised from tillers pre-treated with 5 μM 2,4-D was about 40% higher compared to control (Fig. 6C), while the content of tZ and tZR’5MP decreased by 42%, and 66%, respectively. Application of 12.5 μM 2,4-D resulted in increased level of cZR by 24% and decreased content of tZ by 36% in microspores of DH28, whereas an increase in the content of cZOG by 46% and accumulation of new CKs derivative (DHZROG) was detected in microspores of DH19.
The content of IAA and oxIAA in DH28 ovaries isolated from PCIB pre-treated tillers was lower in comparison to the LT-treated control, from 20 to 40% to approx. 70%, respectively (Fig. 6B). Simultaneously, PCIB pre-treatment at 5 μM, significantly increased the content of IAAsp by 50%. Interestingly, no Aux forms were detected in the ovaries of DH19 after PCIB pre-treatment (Fig. 6B).
PCIB at 5 μM, significantly increased the content of some cis CK isoforms in microspores of DH28: cZOG, cZR and cZROG (by 52%, 32% and 45%, respectively), while it decreased the level of trans isoforms: tZ by 80% and tZR by 70% (Fig. 6C). Application of PCIB at 12.5 μM concentration had smaller effect, decreasing the level of tZ by 44% and tZR’5MP by 73%.
A similar, accumulation inhibiting effect was observed for most of trans CK isoforms in microspores of DH19 isolated after tiller pre-treatment with 5 μM PCIB. The same pre-treatment decreased the level of cZ, cZR and iP by 59%, 41% and 100% but increased the content of cZOG and cZROG by 51%, and 45%, respectively (Fig. 6C). Similarly, higher concentration of PCIB (12.5 μM) was less effective, decreasing the level of cZ, tZ and iP by 45%, 47% and 100% respectively.
DH28 ovaries, collected from the suspension culture continuing embryogenic development and isolated from tillers pre-treated with 12.5 µM 2,4-D, showed lower proportion and a significantly lower level of both Aux conjugates (IAGlu and IAAsp) compared to control (Fig. 7A, B). The highest share, 63% of total pool of Aux, was represented by oxIAA. Like in control, the level of IAA was below the detection limit. Also, the concentration of cZR and cZROG fell below the detection limit (Fig. 7C). On the contrary, pre-treatment with high concentration of 2,4-D increased the level of K9G and its share from 25% to over 56% of total CK pool (Fig. 7A, C). Only after this pre-treatment, co-cultured ovaries accumulated KIN (Fig. 7C). Similarly, a lower levels of IAA derivatives (IAAsp, IAGlu) and no oxIAA were detected in ovaries collected from PCIB pre-treated tillers after 8 days of in vitro culture in comparison to control (Fig. 7B). In contrast, PCIB pre-treatment induced slight accumulation of IAA (Fig. 7A, B) and increased the levels of cZOG (by 37%), cZR (by 39%) cZROG (by 65%) and K9G (by 68%) (Fig. 7C).
Disturbances in hormonal homeostasis induced by MEL pre-treatment in microspores and ovaries of triticale
MEL pre-treatments resulted in similar changes in respect of Aux content in microspores of both tested DH lines (Fig. 4B). Exogenous MEL, especially at 50 μM, reduced the level of active and conjugated Auxs compared to the control (LT) pre-treatment. The greatest decline in DH28 microspores was recorded for IAA, whose content decreased to approx. 55%. It was accompanied with threefold increase in oxIAA level, almost 30% increase in cZ and 16% decrease in cZOG contents (Fig. 4B, 5B). Importantly, the changes induced by this pre-treatment improve the ability for green plant regeneration (Fig. 3). Generally, higher MEL concentration (100 μM) induced similar effects on Aux accumulation in DH28 microspores, with much stronger stimulation of IAA catabolism, in result of which the concentration of oxIAA increased dramatically (Fig. 4B). This pre-treatment also increased the level of cZ (by 38%) and decreased the level of cZR and iPR (by 30% and 25%, respectively).
In DH19 microspores, MEL reduced IAA and IAGlu levels at both concentrations applied (Fig. 4B). Additionally, only at lower concertation (50 μM) MEL reduced the level of IAAsp and induce slight accumulation of oxIAA. Its effect on CK contents was visible particularly at the higher concentration, reducing the content of cZ, cZOG, iP and iPR by 33%-68% compared to control (Fig. 5B).
The content of many PGRs in DH28 ovaries, excised from tillers pre-treated with 100 μM MEL, was higher in comparison to the LT pre-treated control: oxIAA by 65%, IAAsp by 58%, cZ by 42%, cZR by 38%, tZR by 25%, tZ9G by 52% and iPR by 13% (Fig. 6B, 6C). In turn, IAA content decreased on average by 28% after MEL pre-treatment, regardless of the concentration applied. On the contrary, both tested MEL pre-treatments reduced oxIAA and IAAsp contents in DH19 ovaries. The greatest reduction (about 98%) was observed in oxIAA after 100 μM MEL treatment. The content of most of the identified CKs in DH19 ovaries drastically decreased after treatment with 50 μM MEL. The greatest reduction was observed for cZROG and iP, as their contents dropped below the level of detection, followed by the level of cZOG (by 86%) and tZ (by 75%).
Ovaries collected from embryogenic suspensions (DH28) isolated from MEL-pre-treated tillers were characterized by low amount of IAA (not detected in ovaries collected from the LT pre-treated control) and decreased contents of IAAsp and IAGlu (by over 90%; Fig. 7B). The concentration of oxIAA fell below the detection limit. The level of the majority of CKs increased: cZR by 81%, cZROG by 83%, cZR5’MP by 62% and K9G by 66%, compared to control (Fig. 7C).
Comparison of triticale DH lines with different embryogenic potential in respect of their hormonal profile
The summary presented in Fig. 8 shows that effective ME is associated with a higher content of PGRs. Highly embryogenic DH28 microspores after LT pre-treatment—a standard used as a trigger for triticale microspore reprogramming—contained over fourfold more of total Auxs and 1.4-fold more of total CKs in relation to recalcitrant DH19 microspores (Fig. 8A, B). The composition of the Aux pool was also different, as embryogenic microspores accumulated less IAA, more conjugated Aux forms (IAAsp, IAGlu) and a low level of Aux catabolite (oxIAA), which was not detected in DH19 microspores (Fig. 8A). Such variation was not observed in the case of CKs (Fig. 8B).
Quantitative composition of auxins (Auxs) and cytokinins (CKs) in microspores of two DH lines of triticale varied in microspore embryogenesis (ME) effectiveness (recalcitrant DH19 and responsive DH28) after various ME inducing tiller pre-treatments. The absolute amount of total phytohormones for each treatment is shown inside of the charts. Control—low temperature (LT) treatment (21 days at 4 °C); 2,4-D—LT treatment combined with the application of 12.5 µM 2,4-dichlorophenoxyacetic acid; PCIB—LT treatment combined with the application of 12.5 µM p-chlorophenoxyisobutyric acid; MEL—LT treatment combined with the application of 50 µM melatonin. All modifications (PCIB, and MEL) were applied for 4 days before microspore isolation. CK nucleobases: cis-zeatin (cZ), N6-isopentenyladenine (iP); ribosides: cis-zeatin riboside (cZR), N6-isopentenyladenosine (iPR); glucosides: cis-zeatin-O-glucoside (cZOG), cis-zeatin riboside-O-glucoside (cZROG)). Auxs: IAA, conjugates: IAA-aspartate (IAAsp), IAA-glutamate (IAGlu); catabolites: 2-oxindole-3-acetic acid (oxIAA)
It was shown that 2,4-D pre-treatment slightly declined the total amount of Auxs in microspores of DH28 line probably due to increased intensity of IAA oxidation (Fig. 8A). Similarly, 2,4-D pre-treatment declined the total amount of CKs, but only very subtle changes in CK profile were detected (Fig. 8B). Total amount of CKs decreased also in microspores of DH19 and it was the only one more pronounced effect of 2,4-D pre-treatment.
Tillers pre-treatments with 12.5 µM PCIB and 50 µM MEL—the most promising as modulators of plant regeneration effectiveness—reduced both the content of total and active Aux(s) in DH28 microspores (Fig. 8A). A similar effect was observed in DH19 microspores after MEL pre-treatment, whereas PCIB increased the amount of IAA compared to control.
Quite similar composition of CKs was found in triticale microspores after PCIB and MEL tiller pre-treatments. Only in microspores of DH28, a slightly higher proportion of nucleobases at the expense of other forms was observed compared to control (Fig. 8B).
It was observed that the effective ME was not related only to high amounts of PGRs, but rather to some specific balance between their active and inactive forms and the ratio of active Auxs to CKs. The ratio of the most active (among those detected) forms of both PGR groups (cZ, iP and IAA in microspores, and tZ, cZ, iP and IAA in ovaries) was over 3 times higher compared to DH19 (Table 1). All pre-treatments generally lowered the AuxA/CKA ratio in DH28 microspores. The greatest, threefold decrease, was observed after MEL application, which also positively affect ELS regeneration capacity. Such a uniform effect was not detected in DH19 microspores, as both increases and decreases in the AuxA/CKA ratio were observed.
Similarly to microspores, the ovaries of responsive DH28 were characterized by a higher AuxA/CKA ratio compared to recalcitrant DH19. The applied pre-treatments also reduced the AuxA/CKA ratio, but the amplitude of changes was not that wide. The AuxA/CKA ratio in DH19 ovaries was visibly lower in comparison to both DH28 ovaries and DH19 microspores. The effects of treatments with PCIB and 5 µM 2,4-D were most pronounced, as no Aux forms could be detected.
Discussion
Questions about the cause of variation in responsiveness to ME-induction treatment have been posed for many years, and many factors affecting ME efficiency have been already studied (Nowicka et al. 2019; Pérez-Pérez et al. 2019; Rivas-Sendra et al. 2019; Testillano 2018; Żur et al. 2015a). However, progress in understanding the mechanisms that control microspore reprogramming has been very slow, largely due to the complex interplay of genetic, physiological and environmental factors regulating this process. Among them, hormonal background is one of the most important, especially the homeostasis between Auxs and CKs, which control cell divisions and morphogenesis in in vitro cultures (Wędzony et al. 2009; Żur et al. 2015a). It has been clearly documented that these two groups of hormones control each other’s synthesis and signaling, showing synergistic, antagonistic and additive effects, depending on the context and their respective levels (Jones and Ljung 2011; Nordstrom et al. 2004). The complexity of Aux/CK interactions has been widely studied, for example using several mutants and transgenic lines of Arabidopsis thaliana (Kurepa et al. 2019). It was found that Aux at low concentrations inhibited CK signaling and limited their activity; on the other hand, elevated CK levels inhibited Aux signaling, whereas high concentrations of both hormone groups abolished these antagonistic interactions.
Precise analysis of PGR effects is very difficult also due to the fact that their levels depend on the processes of biosynthesis, transport, (ir)reversible conversion to various conjugates and degradation. Many uncertainties are associated with the synthesis and hydrolysis of conjugates, usually much less active than the free forms. The high number and variety of these connections make it difficult to study their biosynthetic pathways and limit the possibility of determining which of them play an important signaling function and which are secondary non-specific metabolites (Weyers and Paterson 2001).
The effect of low temperature tiller pre-treatment on microspore hormonal homeostasis and the effectiveness of ME
The physiological status of microspores can be partially described by PGR composition, especially Auxs and CKs, whose homeostasis is disturbed under stress treatment (Kohli et al. 2013), which is used to trigger microspore reprogramming. It was previously confirmed that LT pre-treatment of tillers, although not a critical prerequisite for ME induction, significantly enhanced tolerance to stress associated with microspore isolation procedure (Żur et al. 2008) and increased ME efficiency in triticale anther cultures (Żur et al. 2014). It was also shown that hormonal balance of LT-treated triticale anthers was disturbed and significantly associated with embryogenic competency (Żur et al. 2015b). The present study is a continuation of research with the use of isolated microspores, which, due to the exclusion of anther somatic tissue, allows for a more in-depth and detailed analysis of changes in PGR balance related to ME induction and the effectiveness of haploid/DH plant production.
Among Auxs detected in triticale microspores and ovaries, IAA is the only one considered as a true bioactive form, and both IAA amino acid conjugates (IAAsp and IAGlu) are usually assigned a backup function (Slovin et al. 1999), whereas oxIAA is the major catabolite of IAA (Jones and Ljung 2011). It was found that the effective induction of ME in isolated DH28 microspore cultures was associated with a high level of all detected Auxs and high IAA content in microspores and freshly isolated ovaries. The importance of IAA and its dynamic utilization was confirmed by the fact that its content in ovaries drastically decreased (below the detection limit) after a few days of in vitro microspore/ovaries co-culture, likely due to secretion and/or transformation into less active conjugates. Moreover, IAA conjugate with aspartate and its downstream metabolites appeared to play some function, as they were predominant among Auxs identified in microspores of responsive DH28. Their role could be associated with stress tolerance, because a mutant cell line (XIIB2) of Egyptian henbane (Hyoscyamus muticus L.), with IAAsp biosynthesis impairment, died rapidly at 33 °C, a temperature tolerated by wild-type cell cultures (Oetiker and Aeschbacher 1997). The study of Shibasaki et al. (2009) demonstrated that temperature can effectively influence Aux transport. Their experiments testing the effect of cold stress (8 to 12 h at 4 °C) on the growth of Arabidopsis thaliana roots revealed that Aux transport inhibition could be related to either polar targeting or intracellular trafficking of PIN2, which belongs to the major family of auxin efflux carriers. The inhibitory effect of cold stress was also confirmed in our previous work, where 21 days of tiller pre-treatment with 4 °C led to lower IAA accumulation in triticale anthers (Żur et al. 2015b).
Recent studies on the biological activity of CK conjugates (Lomin et al. 2015) have proven that free CK bases are the most active forms, but ribosides and cis-zeatin can also bind to its membrane (AHK3) receptors (Spíchal et al. 2004). Among CKs, tZ is the most abundant and plays the most important physiological role. Our results demonstrated, however, that the isolated triticale microspores contained only cis CK isoforms, differing in the position of the terminal hydroxyl group on the isoprenoid side chain. Similarly, in our earlier study (Żur et al. 2015b), the content of tZ and tZR detected in triticale anthers was significantly lower in comparison to cis isoforms. The active role of cis CK isoforms in the regulation of plant development has been questioned for many years. Only a few reports have suggested that cZ and its derivatives may play a more important role than previously recognized (Martin et al. 2001; Veach et al. 2003), and only the last decade has provided evidence confirming their involvement in the regulation of plant development (Gajdošová et al. 2011; Kudo et al. 2012; Stirk et al. 2012) and stress response (Silva-Navas et al. 2019). However, even these reports suggested a moderate activity of cZ isomer or its derivatives, and its function in plants was limited to developmental phases accompanied by a restricted growth rate. On the other hand, relatively high levels of reversibly conjugated CK forms (ribosides, nucleotides and O-glucosides) detected in microspores of both DH lines, mainly used for transport and storage purposes, have suggested that CK activity is tightly controlled and can be rapidly modified also in vitro, according to developmental requirements or environmental changes (Ooi et al. 2013).
This study was the first to find the presence of tZ and tZR in triticale ovaries, and despite their low levels, this presence seemed to be important. Especially that the ovaries of the responsive DH28 line were characterized by their higher contents and the presence of additional forms of their derivatives (tZR5’MP and DHZROG), absent in microspores of the recalcitrant DH19 line. Furthermore, the applied 2,4-D tiller pre-treatment, which led to a decrease in the amount of some trans CK isoforms, especially in the responsive DH28 line, was associated with lower microspore viability and ELS productivity. Taken together, these data likely explain why the co-culture with isolated immature ovaries is crucial for the proper continuation of EM development of several plant species, including triticale. The hypothesis that ovaries secrete active CKs into the medium seemed to be confirmed by the fact that ovaries collected after 8 days of in vitro culture from suspensions continuing embryogenic development did not contain any trans CK isoforms, and cis form levels were orders of magnitude lower than in ovaries excised directly from tillers. Similarly, Lippmann et al. (2015) reported that pistil-preconditioned medium was effective in stimulating ME in barley only when refreshed at least every 4 days, which suggested that the effective compound(s) was either labile or consumed. Surprisingly, a glucoside derivative of KIN (K9G), considered a KIN breakdown product was identified only in these ovaries cultured in vitro. Its presence could be a symptom of tissue aging as it is the first stable secondary DNA damage product with well-defined antioxidant properties (Barciszewski et al. 2007).
The effect of synthetic Aux analogue (2,4-D) and Aux inhibitor (PCIB) on microspore hormonal homeostasis and the effectiveness of ME
In addition to naturally occurring Auxs, some more stable synthetic analogues are used in in vitro culture systems. Among them, 2,4-D is one of the most frequently applied culture media supplements, demonstrating high effectiveness in maintaining callus and suspension cultures from somatic tissue of both dicotyledonous and monocotyledonous plants (Raghavan 2004). It is also used for haploid/DH production through wide hybridization technology. Applied post-pollination with alien pollen, 2,4-D induces ovary enlargement and enhances the growth of the haploid embryos (Juzoń et al. 2022 and references therein).
In our experiment, application of 2,4-D during LT tiller pre-treatment had a detrimental effect, decreasing microspores viability or ME induction effectiveness and ELS formation in the responsive DH28 line. According to the literature, this synthetic hormone may act as a stress factor (Fehér 2006), causing stress hormone accumulation and inhibition of further development (Zheng and Konzak 1999). Zhang et al. (1995) suggested that exogenous Auxs could downregulate endogenous CKs, possibly by increasing CK oxidase activity. Such an effect is observed in our experiment after application of lower dose of 2,4-D in microspores of the responsive DH28 line, in which the concertation of some active (iPR) and conjugated CKs (cZR, cZOG) decreased significantly. However, in the case of microspores, 2,4-D had even a stronger effect on endogenous Auxs, significantly reducing the accumulation of most of the identified forms (IAA, IAAsp, IAGlu) and increasing the accumulation of Aux catabolite (oxIAA).
Stressogenic effect of this pre-treatment seems to be confirmed by the fact that ovaries from embryogenic cultures contained relatively high level of KIN only after 12.5 μM 2,4-D pre-treatment. A similar result was obtained by Dziurka et al. (2019), who observed KIN accumulation in oat ovaries following maize pollination. For many years, this hormone has been regarded as a substance that does not occur naturally in plants, until Barciszewski et al. (1996) identified it in dried coconut. However, knowledge of its role and mechanism of action is still insufficient. The identification of KIN in plant cell extracts suggests its excision from DNA by repair mechanisms (Barciszewski et al. 2007). The presence of this modification can induce DNA conformational changes, causing mispairing and mutations (Wyszko et al. 2003). It also can affect chloroplast differentiation and chlorophyll biosynthesis by stimulating the synthesis of its precursor—5-aminolevulinc acid (Mok and Mok 2001). However, high KIN levels can contribute to the process of programmed cell death (Kunikowska et al. 2013).
In addition to Auxs, certain anti-auxins or auxin antagonists like PCIB can be used as a supplement to the culture medium. Due to structural similarity, PCIB can compete with IAA for binding sites of its receptors (McRae and Bonner 1953), inhibit Aux action (Heupel and Stange 1995; Kim et al. 2000; Xie et al. 2000) and influence embryo development through auxin homeostasis disruption (Lankova et al. 2010). Recent studies revealed that PCIB, at a concentration of 20 μM, enhanced the development of microspore-derived embryos of Brassica juncea and B. napus, while it favored abnormal embryo formation at higher concentrations (Agarwal et al. 2006; Ahmadi et al. 2012). This effect could be due to overcoming the inhibitory influence of high Aux concentration in multicellular structures. A positive impact of PCIB was also observed in the present study for responsive DH28, as tiller pre-treatment with 12.5 μM PCIB stimulated green plant regeneration effectiveness. It was probably the effect of lowered concentration of IAA and increased level of some CKs (cZ and cZR) what resulted in reduced AuxA/CKA ratio. Also, the level of IAA in DH28 ovaries isolated from PCIB pre-treated tillers was reduced in comparison to control. PCIB has had also prolonged effect on some CKs accumulation, as the concertation of cZR, cZOG and cZROG was significantly higher in ovaries after 8 days of in vitro co-culture. The use of anti-auxin as an approach to suppress or block the inhibitory effect of high endogenous auxin concentrations on regeneration was postulated by Cistué et al. (1998), Pal et al. (2012) and Żur et al. (2015b).
The effect of MEL on microspore hormonal homeostasis and the effectiveness of ME
MEL is a pineal hormone, whose presence in plants was confirmed in the 1990s (Dubbels et al. 1995; Hattori et al. 1995), and since then there has been a steady growing number of studies reporting the detection of this indoleamine in various plant species (Reiter et al. 2007). Plant MEL, also called ‘phytomelatonin’ (Blask et al. 2004), can act as a dual agent: cell protector against oxidative stress and plant morphogenic regulator in in vitro applications. The possible protective role of MEL in plants against abiotic stressors was postulated based on the initial studies in carrot culture cells (Daucus carota L.), where the presence of exogenous MEL attenuated cold-induced apoptosis (Lei et al. 2004). Positive effect of MEL on green plant regeneration observed in the current study could be connected with MEL ability to scavenge hazardous reactive oxygen and nitrogen species (ROS, RNS) (Arnao and Hernandez-Ruiz 2014), generated under ME-inducing stress treatment, microspore isolation and transfer to in vitro culture (Żur et al. 2014, 2021). MEL regulates antioxidant-related genes, coding antioxidative enzymes (e.g. superoxide dismutase, peroxidases, catalase), as well as osmoprotective elements by regulating proline biosynthesis genes (reviewed in Moustafa-Farag et al. (2020). Increased tolerance and survival of plants in the presence of exogenous MEL under abiotic stress have been postulated by many authors (Lei et al. 2004; Moustafa-Farag et al. 2020; Posmyk et al. 2008; Szafrańska et al. 2013; Tan et al. 2007). MEL is also known to participate in the regulation of phytohormone levels, as it interacts with gibberellins (GAs) via GA-signaling, which further controls Aux biosynthesis. Overexpression of genes involved in MEL biosynthesis led to decreased IAA levels, possibly due to competition for the same precursor, tryptophan (Yang et al. 2019). Moreover, Lei et al. (2004) applied the TUNEL procedure and reported an inhibitory effect of MEL on DNA fragmentation, one of the hallmarks of apoptosis. Nevertheless, the potential of MEL to strengthen plants subjected to stressors has opened up an interesting area of research on this natural substance, especially in the area of crop improvement and pathogen protection (Arnao and Hernandez-Ruiz 2014). Our results demonstrated that MEL treatment stimulated green plant regeneration in isolated microspore cultures of the responsive DH28 line. The increased regeneration efficiency was accompanied by a marked decrease in Aux content in microspores, leading to an almost threefold decrease in the AuxA/CKA ratio. It also decreased IAA concentration in ovaries isolated from MEL pre-treated tillers. Whereas MEL effect on CKs profile in microspores was highly limited, ovaries isolated from MEL pre-treated tillers contained significantly higher amount of almost all detected CKs after 8 days of in vitro co-culture in comparison with control. All this findings confirm that proper ELS development, guaranteeing high plant regeneration efficiency, was promoted by a moderate Aux/CK ratio (Żur et al. 2015b).
Summary and Conclusions
ME is a complex process, comprehensively regulated by a composite network of molecular and physiological factors, among which hormone signaling is one of the most important. Taking benefit of the advanced UPLC-MS/MS method, our study revealed a wide range of endogenous phytohormones and its derivatives accumulated in triticale microspores and ovaries in response to ME-inducing treatment. Our study confirmed that triticale microspores contained mainly cis CKs, but co-cultured triticale ovaries were shown for the first time to be a source of highly active trans CK isoforms, possibly involved in subsequent stages of embryogenic development. Furthermore, it was demonstrated that cold tiller pre-treatment (3 weeks at 4 °C), which induced efficient microspore reprogramming in the responsive DH28 triticale line, was associated with a higher content of most of the identified CKs and Auxs and a higher ratio of AuxA/CKA in comparison to recalcitrant DH19. Of the applied pre-treatments, 12.5 μM PCIB and 50 μM MEL could be used as potential factors stimulating green plant regeneration. Their positive effect was associated with higher CKA accumulation and concomitant lower AuxA levels detected in isolated microspores, resulting in a 2.4–threefold lower AuxA/CKA ratio.
To the best of our knowledge, this study currently represents the most comprehensive overview of plant hormones involved in triticale ME. The presented data not only broaden our knowledge about the hormonal basis of ME, but also point to the possibility of its practical application to support embryogenic development in isolated microspore cultures.
Data availability
Additional data associated with this research work are provided as Supplementary materials.
Abbreviations
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- AR:
-
Albino regenerant
- Aux:
-
Auxin
- CK:
-
Cytokinins
- cZ:
-
cis-Zeatin
- cZOG:
-
cis-Zeatin-O-glucoside
- cZR:
-
cis-Zeatin riboside
- cZROG:
-
cis-Zeatin riboside-O-glucoside
- cZR5’MP:
-
cis-Zeatin riboside-5-monophosphate
- DAPI:
-
4,6-Diamidino-2-phenylindole
- DH:
-
Doubled haploid
- DHZR:
-
Dihydrozeatin riboside
- DHZROG:
-
Dihydrozeatin-O-glucoside riboside
- ELS:
-
Embryo-like structure
- EM:
-
Embryogenic microspore
- FDA:
-
Fluorescein diacetate
- GR:
-
Green regenerant
- IAA:
-
Indole-3-acetic acid
- IAAsp:
-
IAA-aspartate
- IAGlu:
-
IAA-glutamate
- IBA:
-
Indole-3-butyric acid
- iP:
-
N6-isopentenyladenine
- iPR:
-
N6-isopentenyladenosine
- K9G:
-
Kinetin-9-glucoside
- KIN:
-
Kinetin
- LT:
-
Low temperature
- ME:
-
Microspore embryogenesis
- MEL:
-
Melatonin (N-acetyl-5-methoxytryptamine)
- oxIAA:
-
2-Oxindole-3-acetic acid
- PCIB:
-
P-chlorophenoxyisobutyric acid
- PGR:
-
Plant growth regulator
- SLS:
-
Star-like structure
- tZ:
-
trans-Zeatin
- tZ9G:
-
trans-Zeatin N9-glucoside
- tZR:
-
trans-Zeatin riboside
- tZR5’MP:
-
trans-Zeatin riboside-5-monophosphate
- UPLC-MS/MS:
-
Ultra-performance liquid chromatography with a tandem mass spectrometer
References
Agarwal PK, Agarwal P, Custers JBM, Liu C-M, Bhojwani SS (2006) PCIB an antiauxin enhances microspore embryogenesis in microspore culture of Brassica juncea. Plant Cell Tissue Organ Cult 86(2):201–210. https://doi.org/10.1007/s11240-006-9108-0
Ahmadi B, Alizadeh K, Teixeira da Silva JA (2012) Enhanced regeneration of haploid plantlets from microspores of Brassica napus L. using bleomycin, PCIB, and phytohormones. Plant Cell Tissue Organ Cult 109(3):525–533. https://doi.org/10.1007/s11240-012-0119-8
Arnao MB, Hernandez-Ruiz J (2014) Melatonin: plant growth regulator and/or biostimulator during stress? Trends Plant Sci 19(12):789–797. https://doi.org/10.1016/j.tplants.2014.07.006
Asif M, Eudes F, Goyal A, Amundsen E, Randhawa H, Spaner D (2013a) Organelle antioxidants improve microspore embryogenesis in wheat and triticale. In Vitro Cell Dev Biol Plant 49(5):489–497. https://doi.org/10.1007/s11627-013-9514-z
Asif M, Eudes F, Randhawa H, Amundsen E, Yanke J, Spaner D (2013b) Cefotaxime prevents microbial contamination and improves microspore embryogenesis in wheat and triticale. Plant Cell Rep 32(10):1637–1646. https://doi.org/10.1007/s00299-013-1476-4
Asif M, Eudes F, Randhawa H, Amundsen E, Spaner D (2014a) Induction medium osmolality improves microspore embryogenesis in wheat and triticale. In Vitro Cell Dev Biol Plant 50(1):121–126. https://doi.org/10.1007/s11627-013-9545-5
Asif M, Eudes F, Randhawa H, Amundsen E, Spaner D (2014b) Phytosulfokine alpha enhances microspore embryogenesis in both triticale and wheat. Plant Cell Tissue Organ Cult 116(1):125–130. https://doi.org/10.1007/s11240-013-0379-y
Barciszewski J, Siboska GE, Pedersen BO, Clark BFC, Rattan SIS (1996) Evidence for the presence of kinetin in DNA and cell extracts. FEBS Lett 393(2–3):197–200. https://doi.org/10.1016/0014-5793(96)00884-8
Barciszewski J, Massino F, Clark BFC (2007) Kinetin—a multiactive molecule. Int J Biol Macromol 40(3):182–192. https://doi.org/10.1016/j.ijbiomac.2006.06.024
Blask DE, Dauchy RT, Sauer LA, Krause JA (2004) Melatonin uptake and growth prevention in rat hepatoma 7288CTC in response to dietary melatonin: melatonin receptor-mediated inhibition of tumor linoleic acid metabolism to the growth signaling molecule 13-hydroxyoctadecadienoic acid and the potential role of phytomelatonin. Carcinogenesis 25(6):951–960. https://doi.org/10.1093/carcin/bgh090
Borderies G, le Béchec M, Rossignol M, Lafitte C, Le Deunff E, Beckert M, Dumas C, Matthys-Rochon E (2004) Characterization of proteins secreted during maize microspore culture: arabinogalactan proteins (AGPs) stimulate embryo development. Eur J Cell Biol 83(5):205–212. https://doi.org/10.1078/0171-9335-00378
Cistué L, Ramos A, Castillo AM (1998) Influence of anther pretreatment and culture medium composition on the production of barley doubled haploids from model and low responding cultivars. Plant Cell Tissue Organ Cult 55(3):159–166. https://doi.org/10.1023/a:1006130028396
Delalonde M, Coumans MP (1998) Effect of IAA content modulators on peroxidase activity and on endogenous IAA during cold pretreatment of maize anthers prior to androgenesis. Plant Growth Regul 26(2):123–130. https://doi.org/10.1023/a:1006131620264
Dubas E, Wędzony M, Petrovska B, Salaj J, Żur I (2010) Cell structural reorganization during induction of androgenesis in isolated microspores cultures of triticale (× Triticosecale Wittm.). Acta Biol Crac Ser Bot 52(1):73–86. https://doi.org/10.2478/v10182-010-0010-z
Dubbels R, Reiter RJ, Klenke E, Goebel A, Schnakenberg E, Ehlers C, Schiwara HW, Schloot W (1995) Role melatonin in edible plants identified by radioimmunoassay and high performance liquid chromathography-mass spectrometry. J Pineal Res 18(1):28–31. https://doi.org/10.1111/j.1600-079X.1995.tb00136.x
Dziurka K, Dziurka M, Warchoł M, Czyczyło-Mysza I, Marcińska I, Noga A, Kapłoniak K, Skrzypek E (2019) Endogenous phytohormone profile during oat (Avena sativa L.) haploid embryo development. In Vitro Cell Dev Biol Plant 55(2):221–229. https://doi.org/10.1007/s11627-019-09967-5
Fehér A (2006) Why somatic plant cells start to form embryos? Somat Embryog 2:85–101. https://doi.org/10.1007/7089_019
Gajdošová S, Spíchal L, Kamínek M, Hoyerová K, Novák O, Dobrev PI, Galuszka P, Klíma P, Gaudinová A, Žižková E, Hanuš J, Dančák M, Trávníček B, Pešek B, Krupička M, Vaňková R, Strnad M, Motyka V (2011) Distribution, biological activities, metabolism, and the conceivable function of cis-zeatin-type cytokinins in plants. J Exp Bot 62(8):2827–2840. https://doi.org/10.1093/jxb/erq457
Gorbunova VY, Kruglova NN, Abramov SN (2001) The induction of androgenesis in vitro in spring soft wheat. Balance of exogenous and endogenous phytohormones. Biol Bull 28(1):25–30. https://doi.org/10.1023/a:1026602603527
Hagen G, Guilfoyle T (2002) Auxin-responsive gene expression: genes, promoters and regulatory factors. Plant Mol Biol 49(3–4):373–385. https://doi.org/10.1023/a:1015207114117
Hattori A, Migitaka H, Iigo M, Itoh M, Yamamoto K, Ohtanikaneko R, Hara M, Suzuki T, Reiter RJ (1995) Identification of melatonin in plants and its effect on plasma melatonin levels and binding to melatonin receptors in vertebrates. Biochem Mol Biol Educ 35(3):627–634
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Technol 45(3):115. https://doi.org/10.3109/10520297009085351
Heupel T, Stange L (1995) The auxin antagonist p-chlororphenoxyisobutyric acid abolishes polar distribution of DNA synthesizing cells within the meristem of Riella helicophylla. J Plant Physiol 146(5–6):757–759. https://doi.org/10.1016/S0176-1617(11)81946-2
Indrianto A, Barinova I, Touraev A, Heberle-Bors E (2001) Tracking individual wheat microspores in vitro: Identification of embryogenic microspores and body axis formation in the embryo. Planta 212(2):163–174. https://doi.org/10.1007/s004250000375
Islam SMS, Tuteja N (2012) Enhancement of androgenesis by abiotic stress and other pretreatments in major crop species. Plant Sci 182:134–144. https://doi.org/10.1016/j.plantsci.2011.10.001
Jones B, Ljung K (2011) Auxin and cytokinin regulate each other’s levels via a metabolic feedback loop. Plant Signal Behav 6(6):901–904. https://doi.org/10.4161/psb.6.6.15323
Juzoń K, Warchoł M, Dziurka K, Czyczyło-Mysza IM, Marcińska I, Skrzypek E (2022) The effect of 2,4-dichlorophenoxyacetic acid on the production of oat (Avena sativa L.) doubled haploid lines through wide hybridization. PeerJ 10:e12854. https://doi.org/10.7717/peerj.12854
Kim SK, Chang SC, Lee EJ, Chung WS, Kim YS, Hwang S, Lee JS (2000) Involvement of brassinosteroids in the gravitropic response of primary root of maize. Plant Physiol 123(3):997–1004. https://doi.org/10.1104/pp.123.3.997
Kohli A, Sreenivasulu N, Lakshmanan P, Kumar PP (2013) The phytohormone crosstalk paradigm takes center stage in understanding how plants respond to abiotic stresses. Plant Cell Rep 32(7):945–957. https://doi.org/10.1007/s00299-013-1461-y
Krzewska M, Czyczyło-Mysza I, Dubas E, Gołębiowska-Pikania G, Golemiec E, Stojalowski S, Chrupek M, Żur I (2012) Quantitative trait loci associated with androgenic responsiveness in triticale (× Triticosecale Wittm.) anther culture. Plant Cell Rep 31(11):2099–2108. https://doi.org/10.1007/s00299-012-1320-2
Kudo T, Makita N, Kojima M, Tokunaga H, Sakakibara H (2012) Cytokinin activity of cis-zeatin and phenotypic alterations induced by overexpression of putative cis-zeatin-o-glucosyltransferase in rice. Plant Physiol 160(1):319–331. https://doi.org/10.1104/pp.112.196733
Kunikowska A, Byczkowska A, Doniak M, Kazmierczak A (2013) Cytokinins résumé: their signaling and role in programmed cell death in plants. Plant Cell Rep 32(6):771–780. https://doi.org/10.1007/s00299-013-1436-z
Kurepa J, Shull TE, Smalle JA (2019) Antagonistic activity of auxin and cytokinin in shoot and root organs. Plant Direct. https://doi.org/10.1002/pld3.121
Lankova M, Smith RS, Pesek B, Kubes M, Zazimalova E, Petrasek J, Hoyerova K (2010) Auxin influx inhibitors 1-NOA, 2-NOA, and CHPAA interfere with membrane dynamics in tobacco cells. J Exp Bot 61(13):3589–3598. https://doi.org/10.1093/jxb/erq172
Lantos C, Bona L, Boda K, Pauk J (2014) Comparative analysis of in vitro anther- and isolated microspore culture in hexaploid Triticale (× Triticosecale Wittmack) for androgenic parameters. Euphytica 197(1):27–37. https://doi.org/10.1007/s10681-013-1031-y
Lei XY, Zhu RY, Zhang GY, Dai YR (2004) Attenuation of cold-induced apoptosis by exogenous melatonin in carrot suspension cells: the possible involvement of polyamines. J Pineal Res 36(2):126–131. https://doi.org/10.1046/j.1600-079X.2003.00106.x
Letarte J, Simion E, Miner M, Kasha JK (2006) Arabinogalactans and arabinogalactan-proteins induce embryogenesis in wheat (Triticum aestivum L.) microspore culture. Plant Cell Rep 24:691. https://doi.org/10.1007/s00299-005-0013-5
Li H, Devaux P (2001) Enhancement of microspore culture efficiency of recalcitrant barley genotypes. Plant Cell Rep 20(6):475–481. https://doi.org/10.1007/s002990100368
Lippmann R, Friedel S, Mock H-P, Kumlehn J (2015) The low molecular weight fraction of compounds released from immature embryogenesis. Front Plant Sci. https://doi.org/10.3389/fpls.2015.00498
Lomin SN, Krivosheev DM, Steklov MY, Arkhipov DV, Osolodkin DI, Schmuelling T, Romanov GA (2015) Plant membrane assays with cytokinin receptors underpin the unique role of free cytokinin bases as biologically active ligands. J Exp Bot 66(7):1851–1863. https://doi.org/10.1093/jxb/eru522
Lulsdorf M, Yuan HY, Slater S, Vandenberg A, Han X, Zaharia LI (2012) Androgenesis-inducing stress treatments change phytohormone levels in anthers of three legume species (Fabaceae). Plant Cell Rep 31(7):1255–1267. https://doi.org/10.1007/s00299-012-1246-8
Martin RC, Mok MC, Habben JE, Mok DWS (2001) A maize cytokinin gene encoding an O-glucosyltransferase specific to cis-zeatin. Proc Natl Acad Sci USA 98(10):5922–5926. https://doi.org/10.1073/pnas.101128798
McRae DH, Bonner J (1953) Chemical structure and antiauxin activity. Physiol Plant 6(3):485–510. https://doi.org/10.1111/j.1399-3054.1953.tb08406.x
Mok DWS, Mok MC (2001) Cytokinin metabolism and action. Annu Rev Plant Physiol Plant Mol Biol 52:89–118. https://doi.org/10.1146/annurev.arplant.52.1.89
Moller B, Weijers D (2009) Auxin control of embryo patterning. Cold Spring Harb Perspect Biol. https://doi.org/10.1101/cshperspect.a001545
Moubayidin L, Di Mambro R, Sabatini S (2009) Cytokinin-auxin crosstalk. Trends Plant Sci 14(10):557–562. https://doi.org/10.1016/j.tplants.2009.06.010
Moustafa-Farag M, Mahmoud A, Arnao MB, Sheteiwy MS, Dafea M, Soltan M, Elkelish A, Hasanuzzaman M, Ai S (2020) Melatonin-Induced water stress tolerance in plants: recent advances. Antioxidants. https://doi.org/10.3390/antiox9090809
Nordstrom A, Tarkowski P, Tarkowska D, Norbaek R, Astot C, Dolezal K, Sandberg G (2004) Auxin regulation of cytokinin biosynthesis in Arabidopsis thaliana: a factor of potential importance for auxin-cytokinin-regulated development. Proc Natl Acad Sci USA 101(21):8039–8044. https://doi.org/10.1073/pnas.0402504101
Novák O, Hauserová E, Amakorová P, Doležal K, Miroslav Strnad M (2008) Cytokinin profiling in plant tissues using ultra-performance liquid chromatography–electrospray tandem mass spectrometry. Phytochemistry 69:2214–2224. https://doi.org/10.1016/j.phytochem.2008.04.022
Nowicka A, Juzon K, Krzewska M, Dziurka M, Dubas E, Kopec P, Zielinski K, Zur I (2019) Chemically-induced DNA de-methylation alters the effectiveness of microspore embryogenesis in triticale. Plant Sci. https://doi.org/10.1016/j.plantsci.2019.110189
Oetiker JH, Aeschbacher G (1997) Temperature-sensitive plant cells with shunted indole-3-acetic acid conjugation. Plant Physiol 114(4):1385–1395. https://doi.org/10.1104/pp.114.4.1385
Oleszczuk S, Sowa S, Zimny J (2004) Direct embryogenesis and green plant regeneration from isolated microspores of hexaploid triticale (× Triticosecale Wittmack) cv. Bogo. Plant Cell Rep 22(12):885–893. https://doi.org/10.1007/s00299-004-0796-9
Ooi S-E, Novak O, Dolezal K, Ishak Z, Ong-Abdullah M (2013) Cytokinin differences in in vitro cultures and inflorescences from normal and mantled oil palm (Elaeis guineensis Jacq.). J Plant Growth Regul 32(4):865–874. https://doi.org/10.1007/s00344-013-9352-6
Overvoorde P, Fukaki H, Beeckman T (2010) Auxin control of root development. Cold Spring Harb Perspect Biol 2(6):a001537. https://doi.org/10.1101/cshperspect.a001537
Paire A, Devaux P, Lafitte C, Dumas C, Matthys-Rochon E (2003) Proteins produced by barley microspores and their derived androgenic structures promote in vitro zygotic maize embryo formation. Plant Cell Tiss Org Cult 73:167–176. https://doi.org/10.1023/A:1022805623167
Pal AK, Acharya K, Ahuja PS (2012) Endogenous auxin level is a critical determinant for in vitro adventitious shoot regeneration in potato (Solanum tuberosum L.). J Plant Biochem Biotechnol 21(2):205–212. https://doi.org/10.1007/s13562-011-0092-z
Pauk J, Puolimatka M, Tók L, Monostori T (2000) In vitro androgenesis of triticale in isolated microspore culture. Plant Cell Tissue Organ Cult 61:221–229. https://doi.org/10.1023/A:1006416116366
Pauk J, Mhaly R, Monostori T, Puolimatka M (2003) Protocol for triticale (× Triticosecale Wittmack) microspore culture. In: Maluszynski M, Kasha K, Forster B, Szarejko I (eds) Doubled haploid production in crop plants. A manual. Kluwer, Dordrecht, pp 129–134
Pěnčík A, Rolčík J, Novák O, Magnus V, Barták P, Buchtik R, Salopek-Sondi B, Strnad M (2009) Isolation of novel indole-3-acetic acid conjugates by immunoaffinity extraction. Talanta 80(2):651–655. https://doi.org/10.1016/j.talanta.2009.07.043
Pěnčík A, Casanova-Sáez R, Pilařová V, Žukauskaité A, Pinto R, Micol JL, Ljung K, Novák O (2018) Ultra-rapid auxin metabolite profiling for high-throughput mutant screening in Arabidopsis. J Exp Bot 69(10):2569–2579. https://doi.org/10.1093/jxb/ery084
Pérez-Jiménez M, Cantero-Navarro E, Acosta M, Cos-Terrer J (2013) Relationships between endogenous hormonal content and direct somatic embryogenesis in Prunus persica L. Batsch cotyledons. Plant Growth Regul 71(3):219–224. https://doi.org/10.1007/s10725-013-9822-7
Pérez-Pérez Y, El-Tantawy A-A, Teresa Solis M, Risueño MC, Testillano PS (2019) Stress-induced microspore embryogenesis requires endogenous auxin synthesis and polar transport in barley. Front Plant Sci. https://doi.org/10.3389/fpls.2019.01200
Posmyk MM, Kuran H, Marciniak K, Janas KM (2008) Presowing seed treatment with melatonin protects red cabbage seedlings against toxic copper ion concentrations. J Pineal Res 45(1):24–31. https://doi.org/10.1111/j.1600-079X.2007.00552.x
Raghavan V (2004) Role of 2,4-dichlorophenoxyacetic acid (2,4-D) in somatic embryogenesis on cultured zygotic embryos of Arabidopsis: cell expansion, cell cycling, and morphogenesis during continuous exposure of embryos to 2,4-D. Am J Bot 91(11):1743–1756. https://doi.org/10.3732/ajb.91.11.1743
Reiter RJ, Tan D-X, Manchester LC, Simopoulos AP, Maldonado MD, Flores LJ, Terron MP (2007) Melatonin in edible plants (phytomelatonin): identification, concentrations, bioavailability and proposed functions. World Rev Nutr Diet 97:211–230. https://doi.org/10.1159/000097917
Riefler M, Novak O, Strnad M, Schmulling T (2006) Arabidopsis cytokinin receptor mutants reveal functions in shoot growth, leaf senescence, seed size, germination, root development, and cytokinin metabolism. Plant Cell 18(1):40–54. https://doi.org/10.1105/tpc.105.037796
Rivas-Sendra A, Corral-Martinez P, Porcel R, Camacho-Fernandez C, Calabuig-Serna A, Segui-Simarro JM (2019) Embryogenic competence of microspores is associated with their ability to form a callosic, osmoprotective subintinal layer. J Exp Bot 70(4):1267–1281. https://doi.org/10.1093/jxb/ery458
Segui-Simarro JM (2010) Androgenesis revisited. Bot Rev 76(3):377–404. https://doi.org/10.1007/s12229-010-9056-6
Shariatpanahi ME, Bal U, Heberle-Bors E, Touraev A (2006) Stresses applied for the re-programming of plant microspores towards in vitro embryogenesis. Physiol Plant 127(4):519–534. https://doi.org/10.1111/j.1399-3054.2006.00675.x
Shibasaki K, Uemura M, Tsurumi S, Rahman A (2009) Auxin response in arabidopsis under cold stress: underlying molecular mechanisms. Plant Cell 21(12):3823–3838. https://doi.org/10.1105/tpc.109.069906
Silva-Navas A, Conesa CM, Saez A, Navarro-Neila S, Garcia-Mina JM, Zamarreno AM, Baigorri R, Swarup R, del Pozo JC (2019) Role of cis-zeatin in root responses to phosphate starvation. New Phytol 224(1):242–257. https://doi.org/10.1111/nph.16020
Slovin JP, Bandurski RS, Cohen JD (1999) Auxin. In: Hooykaas PJJ, Hall MA, Libbenga KR (eds) Biochemistry and molecular biology of plant hormones. Elsevier Science, London, pp 115–140
Spíchal L, Rakova NY, Riefler M, Mizuno T, Romanov GA, Strnad M, Schmulling T (2004) Two cytokinin receptors of Arabidopsis thaliana, CRE1/AHK4 and AHK3, differ in their ligand specificity in a bacterial assay. Plant Cell Physiol 45(9):1299–1305. https://doi.org/10.1093/pcp/pch132
Stirk WA, Vaclavikova K, Novak O, Gajdosova S, Kotland O, Motyka V, Strnad M, van Staden J (2012) Involvement of cis-zeatin, dihydrozeatin, and aromatic cytokinins in germination and seedling establishment of maize, oats, and lucerne. J Plant Growth Regul 31(3):392–405. https://doi.org/10.1007/s00344-011-9249-1
Svačinová J, Novák O, Plačková L, Lenobel R, Holík J, Strnad M, Doležal K (2012) A new approach for cytokinin isolation from Arabidopsis tissues using miniaturized purification: pipette tip solid-phase extraction. Plant Methods. https://doi.org/10.1186/1746-4811-8-17
Szafrańska K, Glińska S, Janas KM (2013) Ameliorative effect of melatonin on meristematic cells of chilled and re-warmed Vigna radiata roots. Biol Plant 57(1):91–96. https://doi.org/10.1007/s10535-012-0253-5
Tan D-X, Manchester LC, Terron MP, Flores LJ, Reiter RJ (2007) One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res 42(1):28–42. https://doi.org/10.1111/j.1600-079X.2006.00407.x
Tang X-C, He Y-Q, Wang Y, Sun M-X (2006) The role of arabinogalactan proteins binding to Yariv reagents in the initiation, cell developmental fate, and maintenance of microspore embryogenesis in Brassica napus L. cv. Topas. J Exp Bot 57(11):2639–2650. https://doi.org/10.1093/jxb/erl027
Testillano PS (2018) Stress-induced microspore embryogenesis in crop plants: cell totipotency acquisition and embryo development. In: Cánovas F, Lüttge U, Leuschner C, Risueño M (eds) Progress in botany, vol 81. Springer, Cham, pp 227–241
Touraev A, Vicente O, HeberleBors E (1997) Initiation of microspore embryogenesis by stress. Trends Plant Sci 2(8):297–302. https://doi.org/10.1016/s1360-1385(97)89951-7
Touraev A, Tashpulatov AI, Ndrianto A, Barinova J, Katholnigg H, Akimcheva S, Ribarits A, Voronin V, Zhexsembekova M, Heberle-Bors E (2000) Fundamental aspects of microspore embryogenesis. In: COST Action 824, ‘Biotechnological approaches for utilisation of gametic cells’, Bled, 1–5 July 2000. pp 205–214
Veach YK, Martin RC, Mok DWS, Malbeck J, Vankova R, Mok MC (2003) O-glucosylation of cis-zeatin in maize. Characterization of genes, enzymes, and endogenous cytokinins. Plant Physiol 131(3):1374–1380. https://doi.org/10.1104/pp.017210
Watson A, Ghosh S, Williams MJ, Cuddy WS, Simmonds J, Rey M-D, Hatta MAM, Hinchliffe A, Steed A, Reynolds D, Adamski NM, Breakspear A, Korolev A, Rayner T, Dixon LE, Riaz A, Martin W, Ryan M, Edwards D, Batley J, Raman H, Carter J, Rogers C, Domoney C, Moore G, Harwood W, Nicholson P, Dieters MJ, DeLacy IH, Zhou J, Uauy C, Boden SA, Park RF, Wulff BBH, Hickey LT (2018) Speed breeding is a powerful tool to accelerate crop research and breeding. Nature Plants 4(1):23–29. https://doi.org/10.1038/s41477-017-0083-8
Wędzony M (2003) Protocol for anther culture in hexaploid triticale (× Triticosecale Wittm.). In: Maluszynski M, Kasha K, Forster B, Szarejko I (eds) Doubled haploid production in crop plants. Kluwer Academic Publishers, Dordrecht/Boston/London, pp 123–129
Wędzony M, Forster BP, Żur I, Golemiec E, Szechyńska-Hebda M, Dubas E, Gotębiowska G (2009) Progress in doubled haploid technology in higher plants. In: Touraev A, Forster B, Jain S (eds) Advances in haploid production in higher plants. Springer, Cham, pp 1–33
Weyers JDB, Paterson NW (2001) Plant hormones and the control of physiological processes. New Phytol 152:375–407. https://doi.org/10.1046/j.0028-646X.2001.00281.x
Würschum T, Tucker M, Maurer H (2014) Stress treatments influence efficiency of microspore embryogenesis and green plant regeneration in hexaploidy triticale (× Triticosecale Wittmack L.). In Vitro Cell Dev Biol Plant 50:143–148. https://doi.org/10.1007/s11627-013-9539-3
Wyszko E, Barciszewska MZ, Markiewicz M, Szymanski M, Markiewicz WT, Clark BFC, Barciszewski J (2003) “Action-at-a distance” of a new DNA oxidative damage product 6-furfuryl-adenine (kinetin) on template properties of modified DNA. Biochim Biophys Acta 1625(3):239–245. https://doi.org/10.1016/s0167-4781(02)00622-x
Xie Q, Frugis G, Colgan D, Chua NH (2000) Arabidopsis NAC1 transduces auxin signal downstream of TIR1 to promote lateral root development. Genes Dev 14(23):3024–3036. https://doi.org/10.1101/gad.852200
Yang W-J, Du Y-T, Zhou Y-B, Chen J, Xu Z-S, Ma Y-Z, Chen M, Min D-H (2019) Overexpression of TaCOMT improves melatonin production and enhances drought tolerance in transgenic Arabidopsis. Int J Mol Sci. https://doi.org/10.3390/ijms20030652
Zhang R, Zhang X, Wang J, Letham DS, McKinney SA, Higgins TJV (1995) The effect of auxin on cytokinin levels and metabolism in transgenic tobacco tissue expressing an ipt gene. Planta 196(1):84–94
Zheng MY, Konzak CF (1999) Effect of 2,4-dichlorophenoxyacetic acid on callus induction and plant regeneration in anther culture of wheat (Triticum aestivum L.). Plant Cell Rep 19(1):69–73. https://doi.org/10.1007/s002990050712
Zheng MY, Weng Y, Liu W, Konzak CF (2002) The effect of ovary-conditioned medium on microspore embryogenesis in common wheat (Triticum aestivum L.). Plant Cell Rep 20(9):802–807. https://doi.org/10.1007/s00299-001-0411-2
Zhuang J, Xu J (1983) Increasing differentiation frequencies in wheat pollen callus. In: Hu H, Vega M (eds) Cell and tissue culture techniques for cereal crop improvement, vol 431. Science Press, Beijing
Zoriniants S, Tashpulatov AS, Heberle-Bors E, Touraev A (2005) The role of stress in the induction of haploid microspore embryogenesis. In: Palmer C, Keller W, Kasha K (eds) Haploids in crop improvement II. Springer, Berlin, pp 35–52
Żur I, Dubas E, Golemiec E, Szechyńska-Hebda M, Janowiak F, Wędzony M (2008) Stress-induced changes important for effective androgenic induction in isolated microspore culture of triticale (× Triticosecale Wittm.). Plant Cell Tissue Organ Cult 94(3):319–328. https://doi.org/10.1007/s11240-008-9360-6
Żur I, Dubas E, Golemiec E, Szechyńska-Hebda M, Gołębiowska G, Wędzony M (2009) Stress-related variation in antioxidative enzymes activity and cell metabolism efficiency associated with embryogenesis induction in isolated microspore culture of triticale (× Triticosecale Wittm.). Plant Cell Rep 28(8):1279–1287. https://doi.org/10.1007/s00299-009-0730-2
Żur I, Krzewska M, Dubas E, Gołębiowska-Pikania G, Janowiak F, Stojałowski S (2012) Molecular mapping of loci associated with abscisic acid accumulation in triticale (× Triticosecale Wittm.) anthers in response to low temperature stress inducing androgenic development. Plant Growth Regul 68(3):483–492. https://doi.org/10.1007/s10725-012-9738-7
Żur I, Dubas E, Krzewska M, Janowiak F, Hura K, Pociecha E, Bączek-Kwinta R, Plażek A (2014) Antioxidant activity and ROS tolerance in triticale (× Triticosecale Wittm.) anthers affect the efficiency of microspore embryogenesis. Plant Cell Tissue Organ Cult 119(1):79–94. https://doi.org/10.1007/s11240-014-0515-3
Żur I, Dubas E, Krzewska M, Janowiak F (2015a) Current insights into hormonal regulation of microspore embryogenesis. Front Plant Sci 6:424. https://doi.org/10.3389/fpls.2015.00424
Żur I, Dubas E, Krzewska M, Waligórski P, Dziurka M, Janowiak F (2015b) Hormonal requirements for effective induction of microspore embryogenesis in triticale (× Triticosecale Wittm.) anther cultures. Plant Cell Rep 34(1):47–62. https://doi.org/10.1007/s00299-014-1686-4
Żur I, Dubas E, Krzewska M, Zieliński K, Fodor J, Janowiak F (2019) Glutathione provides antioxidative defence and promotes microspore-derived embryo development in isolated microspore cultures of triticale (× Triticosecale Wittm.). Plant Cell Rep 38(2):195–209. https://doi.org/10.1007/s00299-018-2362-x
Żur I, Dubas E, Krzewska M, Kopeć P, Nowicka A, Surówka E, Gawrońska K, Gołębiowska G, Juzoń K, Malaga S (2021) Triticale and barley microspore embryogenesis induction requires both reactive oxygen species generation and efficient system of antioxidative defence. Plant Cell Tissue Organ Cult 145(2):347–366. https://doi.org/10.1007/s11240-021-02012-7
Funding
The work was supported by the International Visegrad Scholarship under the International Visegrad Fund (application No: 51700770) and by the National Science Centre (NCN) 2015/18/M/NZ3/00348 financed by the Polish Ministry of Science and Higher Education. The work of K.D. and L.P. was supported by the Ministry of Education, Youth and Sports of CR from the European Regional Development Fund-Project “Centre for Experimental Plant Biology”: No. CZ.02.1.01/0.0/0.0/16_019/0000738.
Author information
Authors and Affiliations
Contributions
KJS conceived and managed the project, performed the experiments and wrote the manuscript. IŻ supervised the concept and design of the study, contributed to the interpretation of the results and critically reviewed the manuscript. LP conducted the analysis of phytohormones. AN participated in microspore isolation and in vitro culturing, reviewed the manuscript and performed graphic processing of photos. KD provided critical feedback and helped shape the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors declare that there is no conflict of interest.
Consent to participate
All authors consent to participate in the publication.
Consent for publication
All the authors have read, agreed with the content and issued consent to publish the manuscript.
Additional information
Communicated by Jose M. Segui-Simarro.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Juzoń-Sikora, K., Nowicka, A., Plačková, L. et al. Hormonal homeostasis associated with effective induction of triticale microspore embryogenesis. Plant Cell Tiss Organ Cult 152, 583–604 (2023). https://doi.org/10.1007/s11240-022-02433-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-022-02433-y