Abstract
To verify the hypothesis that cell redox status regulates the process of microspore embryogenesis (ME), reactive oxygen species (ROS) generation and the activity of enzymatic and non-enzymatic antioxidants were analyzed in eight doubled haploid lines of triticale with significant differences in embryogenic potential. The analyses were performed in anthers excised from freshly cut tillers (control) and from low temperature (LT) pre-treated tillers (3 weeks at 4 °C) in which ME has been initiated. Significant associations between ME effectiveness and the variables studied were found. In control cultures, high superoxide dismutase (SOD) activity appeared crucial for microspore viability. On the other hand, positive though non-linear correlation between ME effectiveness and H2O2 generation, and negative correlation with catalase (CAT) activity suggest that some threshold level of H2O2 is important for successful ME initiation. LT tillers pre-treatment significantly increased H2O2 accumulation, which had a negative effect on ME effectiveness. However, even high level of H2O2 did not endanger cell viability as long as the cells exhibited high activity of ROS-decomposing enzymes (SOD, CAT and POX). The ability to sustain antioxidative enzyme activity under cold stress in the dark was another important requirement for high effectiveness of ME, allowing for the generation of the signal initiating microspore reprogramming and simultaneously protecting the cells from the toxic effects of ROS production. The role of antioxidative enzymes cannot be replaced even by high activity of non-enzymatic antioxidants. In conclusion, genetically controlled but environmentally modified cell tolerance to oxidative stress seems to play an important role in triticale ME.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The process of in vitro microspore embryogenesis (ME) imitates embryo development in planta but takes place in immature cells of male gametophyte and results in haploid plant formation. Due to the fact that haploid plants can quite easily, or even spontaneously, double the chromosome set and become so-called ‘doubled haploids’ (DH), it can be seen as the shortest way to total homozygosity, highly valued in breeding programmes and research areas such as gene mapping, sequencing, and genome engineering (Kasha and Maluszynski 2003).
Microspore embryogenesis is under strict genotypic control and large variation in embryogenic potential has been observed among various plant species, cultivars, lines, or even plants. It has been revealed that the initiation of ME is triggered by stress, and alongside the genetic factor, the parameters of stress treatment significantly modulate the efficiency of DHs production (Touraev et al. 1997; Zoriniants et al. 2005). Increased stress intensity enhances the frequency of ME initiation but on the other hand, it can negatively affect cells viability and induce recombination in nuclear or chloroplast genomes leading to a decreased regeneration ability, albino plants formation or somaclonal variation (Shariatpanahi et al. 2006a).
It has been hypothesized that such dual effect can be associated with reactive oxygen species (ROS) generation and its influence on cell integrity and functioning. These partially reduced or activated derivatives of oxygen, like singlet oxygen (1O2), superoxide anion (\({\text{O}}_{2}^{ \cdot - }\)), hydrogen peroxide (H2O2), and hydroxyl radical (HO∙) are unavoidable byproducts of all aerobic metabolic processes taking place in a living cell (Mittler 2002). At low concentrations ROS act as secondary messengers involved in the control and regulation of many physiological processes such as photosynthesis, photorespiration, cell cycle, growth, development, and senescence (reviewed by Gill and Tuteja 2010). Among others, ROS have been identified as signalling molecules in several hormone signalling pathways (review by Vranová et al. 2002) e.g. in somatic embryogenesis. However, many stress factors like chilling, heat shock, mechanical stress, and starvation—used as ME triggers or accompanying ME initiation procedures—highly intensify ROS accumulation (Mittler 2002). Excessive ROS production results in so-called ‘oxidative stress’, which can lead to cell death via oxidative destruction or by induction of programmed cell death (PCD). Several transcriptomic analyses of ME initiation in mannitol-treated barley microspores revealed an up-regulation of genes involved in defence reactions against oxidative stress (Maraschin et al. 2006; Muñoz-Amatriain et al. 2009; Jacquard et al. 2009). Recent report by Rodríguez-Serrano et al. (2012) showed an association between ROS production in mannitol-treated cultures of barley microspores and PCD initiation via increase of caspase-3-like activity.
Thus, a hypothesis has been put forward, claiming that the effectiveness of ME depends on both cell tolerance to/defence ability against oxidative stress and ROS-induced signal transduction associated with the stress treatment used for microspore reprogramming.
In plant cells, the precise equilibrium between ROS generation and decomposition depends on the activity of enzymatic and non-enzymatic components of the antioxidative system. Major ROS-scavenging enzymes include superoxide dismutase (SOD), peroxidase (POX), and catalase (CAT), whereas ascorbic acid, glutathione, proline, α-tocopherols, caretonoids, and flavonoids belong to the most important non-enzymatic antioxidants (Gill and Tuteja 2010).
In order to gain a better understanding of the mechanisms that control ME in triticale, ROS generation and the activity of SOD, CAT, POX, and non-enzymatic antioxidants were analyzed in anthers of triticale DH lines with significant differences in ME effectiveness. The selection of four highly ‘responsive’ and four ‘recalcitrant’ DH lines from the population of 102 DH lines ‘Saka 3006’ × ‘Modus’ was based on the results of preliminary experiments conducted with anther culture method (Wędzony 2003; Krzewska et al. 2012). The analyses were performed in anthers excised from freshly cut tillers at the developmental phase optimal for ME initiation and then after low temperature (LT) pre-treatment (3 weeks at 4 °C) used as a standard trigger for triticale microspore reprogramming. At the same time, ME effectiveness was evaluated based on several parameters that described the frequency of androgenic structure formation and plant regeneration. Statistical analyses allowed for the identification of the factors that significantly influence ME induction and determine the effectiveness of the process. To the best of our knowledge, this is the first comprehensive and detailed examination of LT-induced modulations in cell ROS tolerance and antioxidative system activity and its effect on embryogenic potential of microspores.
Materials and methods
Plant material
Eight winter triticale DH lines derived from the F1 generation of a cross between German inbred line ‘Saka 3006’ and Polish cv. ‘Modus’ (four responsive: DH44, DH18, DH28, DH101 and four recalcitrant: DH72, DH119, DH2 and DH144) were under examination. Germinating triticale kernels were placed in perlite pre-soaked with Hoagland’s salt solution and vernalized for 7 weeks at 4 °C and 8 h/16 h (day/night) photoperiod. Vernalized seedlings were planted into pots containing a mixture of soil, de-acidified substrate peat, and sand (2/2/1; v/v/v), and grown in a greenhouse at 25 °C and 16 h/8 h photoperiod.
Anther culture protocol
The evaluation of androgenic responsiveness was performed by anther culture method according to Wędzony (2003) with some modifications described by Krzewska et al. (2012).
Tillers from donor plants were collected when the majority of microspores were at mid- to late uni-nucleate stage of development. The tillers were placed in Hoagland’s salt solution and stored at 4 °C in the dark for 3 weeks. Then the spikes were sterilized with 70 % ethanol and the anthers were excised and transferred to modified C17 medium (Wang and Chen 1983) containing 1 mg dm−3 Dicamba, 1 mg dm−3 Picloram, 0.5 mg dm−3 Kinetin, 90 g dm−3 maltose, and 0.6 % agar, with pH of 5.8.
Each 60 × 15 mm Petri dish seen as one replication contained about 100 anthers from one spike and about 20 immature ovaries isolated from the same spike. The cultures were kept at 28 °C in the dark. The cultures of anthers isolated from freshly cut tillers without low temperature pre-treatment were used as the control.
Starting from the sixth week of culture, the androgenic structures (AS) of size >1 mm were transferred to regeneration medium 190-2 (Zhuang and Xu 1983) supplemented with 0.5 mg dm−3 kinetin, 0.5 mg dm−3 NAA, 30 g dm−3 sucrose, and 0.6 % agar, with pH of 6.0. The regeneration phase took place at 26 °C, with 16 h/8 h (day/night) photoperiod and 80–100 μmol m−2s−1 light intensity.
The efficiency of ME was expressed by several parameters: AS/100A—the number of androgenic structures produced per 100 anthers; R/100AS—the number of regenerated plants per 100 androgenic structures; GR/100AS—the number of green regenerated plants per 100 androgenic structures; R/100A—the total number of regenerated plants per 100 anthers; GR/100A—the number of green regenerated plants per 100 anthers. The parameters were calculated as the mean from ten replications.
Viability test
Microspore viability was determined by fluorochromatic reaction to fluorescein diacetate (FDA; λEx = 465 nm, λEm = 515 nm, green fluorescence) according to Heslop-Harrison and Heslop-Harrison (1970). FDA was prepared as stock solution in acetone at 5 mg·cm−3. Immediately before use, 0.01 % dilution was prepared by adding 20 µl of the stock to 1 ml 0.3 mol dm−3 mannitol.
Microspore viability was determined both in anthers isolated from freshly cut and LT-treated tillers, squashed on microscopic slides in the drop of 0.01 % FDA solution.
Sample preparation
The anthers were isolated from freshly cut tillers (control) at the phase of development optimal for ME initiation when the majority of microspores were at late uni-nucleated phase of development, and from low temperature-treated tillers (3 weeks at 4 °C; LT). ROS generation assays were performed on the freshly collected anthers immediately after isolation. For other analysis plant material was frozen in liquid nitrogen and stored at −60 °C.
Superoxide anion generation assay
Histochemical staining for \({\text{O}}_{2}^{ \cdot - }\) production in the anther tissue was based on the ability of cells to reduce nitroblue tetrazolium (NBT), according to the method described by Doke and Ohashi (1988). Gently excised anthers were immediately immersed in potassium phosphate buffer (PPB, pH 7.0) at 4 °C. Then the anthers were vacuum-infiltrated with 10 mmol dm−3 PPB (pH 7.0) containing 0.5 % (w/v) NBT and 0.005 % (w/v) Triton X-100. After 15 min of staining at room temperature (RT) in the light, the anthers were placed in a chloral hydrate solution for the next 15 min to remove chlorophyll (Chl) and preserve tissue integrity. Then the anthers were treated with 96 % ethanol to remove the rest of Chl and to visualize the accumulation of formazan blue, the product of NBT reduction. Densitometric determinations were performed by the analysis of blue levels along the length of the anther. The areas of the tissue accumulating forazan blue were calculated using WinDIAS3 software (Delta-T Devices Ltd, Cambridge, UK) and expressed as the percentage of the whole anther area (% aa).
Hydrogen peroxide analysis
Hydrogen peroxide was determined according to the method of Ishikawa et al. (1993). Plant material (anthers with total fresh weight (FW) of 0.3 g) were homogenized in 50 mmol dm−3 PPB (pH 7.0) supplemented with 1 mmol dm−3 EDTA at 4 °C. The homogenate was centrifuged at 14,000 rpm for 3 min. Reaction mixture contained 2.5 ml homovanilic acid, 2.5 µl peroxidase 1380 U mg−1, and 20 µl sample. Absorbance was determined at 315 nm (λEm 400–450 nm) by spectrofluorotometer PERKIN ELMER LS 50B (Norwalk, USA). Hydrogen peroxide concentration was calculated through comparison to the standard curve.
Non-enzymatic antioxidants activity
Plant material (anthers with total FW of 0.3 g) was freeze-dried and ground with ball mill MM400 (Retsch, Germany), and after adding 1 ml of 50 % ethanol, shaken for 2 h at RT. The extracts were then centrifuged for 20 min in a refrigerated centrifuge at 18,000×g (MPW-350R, Poland) and the supernatant was used for the measurements. The total content of antioxidants (free radical-scavenging activity) was measured by DPPH method according to Brand-Williams et al. (1995) with some modifications adapting the protocol to 96-well microtitre plates and to the measurement of absorbance by microtitre plate reader. 0.5 mmol dm−3 solution of stable free radical 1,1-diphenyl-2-picrylhydrazyl (DPPH, SIGMA) in methanol was used. After 30 min of reaction at 37 °C, absorbance at 515 nm was determined using reader Model 680 (Bio-Rad Laboratories, USA). The results were expressed as µmol dm−3 of Trolox equivalents. For each DH line and treatment at least four measurements were made on two independent samples each collected from five different plants.
Sample preparation for analysis of antioxidative enzymes activity
Plant material (anthers with total FW of 0.3 g) was homogenized in 50 mmol dm−3 PPB (pH 7.8) supplemented with 1 % polyvinylpyrrolidone (PVPP) for SOD activity measurements or in 50 mmol dm−3 PPB (pH 7.0 or pH 7.8) supplemented with 1 mmol dm−3 EDTA at 4 °C for POX and CAT measurements, respectively. The homogenate was centrifuged at 14,000 rpm for 3 min.
Protein content was determined according to Bradford (1976) using bovine serum albumin (BSA) as a standard.
Assay of superoxide dismutase activity (SOD)
SOD activity was determined according to the method described by Droillard et al. (1987). A sample of tissue extract (20 µl) was added to 1 ml of assay buffer containing 56 mmol dm−3 NBT, 0.1 mmol dm−3 xanthine, and 1 mmol dm−3 EDTA in 50 mmol dm−3 PPB (pH 7.8). The reaction was started by adding 10 µl xanthine oxidase (0.03 U). Absorbance at 560 nm was recorded for 2 min by spectrofluorotometer PERKIN ELMER Lambda Bio29 (UK). SOD activity was calculated as the percentage of the inhibition of NBT reduction. The measurements were performed in at least four replicates.
Visualization of SOD isoforms
Electrophoresis was performed at 4 °C and 180 V in 13 % polyacrylamide gel (Laemmli 1970). Identification of individual SOD isoforms was carried out according to the procedure described by Beauchamp and Fridovich (1971). After electrophoresis the gels were incubated for 7 min in the dark at RT in the staining buffer consisting of 50 mmol dm−3 PPB (pH 7.8), 1 mmol dm−3 EDTA, 28 mmol dm−3 TEMED, 0.03 mmol dm−3 riboflavin, and 2.45 mmol dm−3 NBT. Afterwards, the gels were exposed to daylight until SOD activity bands became visible. To inhibit Cu/ZnSOD and FeSOD isoforms, the gels were stained in the buffer containing 5 mmol dm−3 H2O2. Selective inhibition of Cu/ZnSOD was executed by gel incubation in the buffer containing 3 mmol dm−3 KCN. MnSOD isoform is resistant to both H2O2 and KCN.
Assay of non-specific peroxidase activity (POX)
Non-specific peroxidase activity (POX) was measured spectrophotometrically (460 nm) with the method of Bergmeyer (1965) using p-phenylenediamine (pPD) as the substrate. The reaction mixture contained 2 ml of 50 mmol dm−3 PPB (pH 7.8) with 1 mmol dm−3 EDTA, 12 μl pPD, and 12 μl of plant extract. The reaction was started by adding 12 μl of 30 % H2O2. One unit of POX activity (ΔA) is defined as the change in absorbance per minute and expressed in terms of units per mg of protein. The measurements were performed in three replicates.
Assay of catalase activity (CAT)
Catalase activity was measured spectrophotometrically with the modified method of Aebi (1984) based on the rate of H2O2 decomposition, which is proportional to the reduction of absorbance (240 nm). Plant extract (50–100 μl) was added to the reaction mixture consisting of 0.3 ml of 30 % H2O2 in 50 mmol dm−3 PPB (pH 7.8) and 1 mmol dm−3 EDTA. One unit of CAT activity is defined as the amount of the enzyme that decomposes 1 µmol H2O2 per min and is expressed in terms of units per mg of protein. The measurements were performed in three replicates.
Statistical analysis
The evaluation of data started with descriptive statistical analysis (mean, standard error). The normal distribution of scores was verified by Shapiro–Wilk test to validate the use of parametric tests. The effect of normally distributed variables was examined by one-factor analysis of variance (ANOVA), after which post hoc comparison was conducted using Duncan’s multiple range test (p ≤ 0.05). Variables with non-normal distributed data were analyzed with non-parametric Kolmogorov–Smirnov tests (p ≤ 0.001). Non-parametric Spearman’s Rank-Order Correlation coefficients (R) were used to analyze the association between variables with non-normal distribution of scores. The parameters that contributed significantly to the final score were determined by stepwise multiple regression analysis. Principal component analysis (PCA) was employed for the simultaneous visualization of the effects of studied genotypes and traits. All statistical analyses were performed using STATISTICA version 10.0 (Stat Soft Inc., USA, 2011) package.
Results
The efficiency of microspore embryogenesis
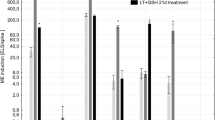
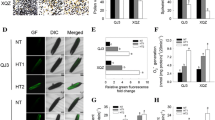
It has been revealed that for the studied triticale DH lines LT stress was not a prerequisite for ME initiation, though it significantly enhanced the effectiveness of the process (Kolmogorov–Smirnov test, p ≤ 0.001; Fig. 1). Its effect could be seen in higher viability of microspores and in the emergence of microspores with morphological features typical for initiation of embryogenic development like increased size, ‘star-like structures’ (SLS) or symmetrically divided microspores (Fig. 2). FDA staining revealed that as a result of LT treatment, the mean number of viable microspores increased from 16 to 56 % and from 6 to 38 %, respectively for ‘responsive’ and ‘recalcitrant’ DH lines. ME induction in control cultures ranged from 2.5 to 3.6 AS/100A for ‘recalcitrant’ and from 20.8 to 56 AS/100A for ‘responsive’ DH lines. Due to very low ME effectiveness in the group of ‘recalcitrant’ DH lines, the effect of LT pre-treatment was not significant though the mean number of produced AS increased from 3.2 to 10.9 per 100A. For the majority of ‘responsive’ DH lines LT strongly stimulated AS development, raising mean AS/100A from 38.8 to 78.3. The only exception was DH44, for which the effect of LT was not significant (Fig. 1). Significant variation (Kolmogorov–Smirnov test, p ≤ 0.001) in ME efficiency was observed between the groups of DH lines identified as ‘responsive’ and ‘recalcitrant’ to ME induction treatment (Table 1; Fig. 2). Depending on tillers treatment, mean ME induction efficiency was from 7- (LT-treated) to 13-fold (control) higher for ‘responsive’ DH lines in comparison with ‘recalcitrant’ ones.
The effect of low temperature tillers pre-treatment (3 weeks at 4 °C) on the effectiveness of microspore embryogenesis in anther culture of selected DH lines of winter triticale (×Triticosecale Wittm.). Control means anther cultures initiated without low temperature pre-treatment. Presented data are the means of ten biological replications. Mean values marked with the same letter within the group do not differ significantly according to Duncan’s multiple range test (p ≤ 0.05). AS/100A—the number of androgenic structures produced per 100 anthers
The effect of low temperature (LT) treatment on androgenic structure production in triticale DH lines (×Triticosecale Wittm.) with different responsiveness to androgenesis induction. a1–3 Anther culture of the responsive DH line after LT treatment. b1–3 Anther culture of the responsive DH line without LT treatment. c1–3 Anther culture of the recalcitrant DH line after LT treatment. d1–3 Anther culture of the recalcitrant DH line without LT treatment. a1, b1, c1, d1 Viability of microspores isolated from anthers. Intensive green fluorescence marks viable microspores. a2, b2, c2, d2 Microspores’ morphology on the initial day of in vitro culture (arrows on a2 indicate embryogenic star-like structures). a3, b3, c3, d3 Androgenic structures after 6 weeks of in vitro anther culture. a1, b1, c1, d1 Green fluorescence (FITC) caused by FDA staining demonstrates vital microspores. Bar 50 µm (a1–2, b1–2, c1–2, d1–2)
From 0.6 to 10.6 % of AS regenerated plantlets, and among them the number of green plants varied from 0 to 9.0 %. LT treatment stimulated regeneration ability of produced AS but this positive effect was observed also in green plants production only in the case of ‘responsive’ genotypes. So regardless of tillers pre-treatment, the final and total ME effectiveness (R/100A) was significantly higher for ‘responsive’ genotypes (Table 1), whereas the efficiency of green plant formation (GR/100A) was influenced by LT. Without LT pre-treatment, the yield of green plants was similar for both groups of triticale lines (Table 1), whereas after LT stress green plant production in ‘responsive’ DH lines was much more effective.
Generation of reactive oxygen species
Formazan blue, the product of NBT reduction, was detected both in control (33.3–55.6 % aa) and in LT-treated (21.4–50 % aa) anthers of all studied DH lines (Fig. 3). Generation of \({\text{O}}_{2}^{ \cdot - }\) was detected mainly in anther filament and in connective tissue layer, sometimes also in anther wall (Fig. 4). Densitometric analysis of \({\text{O}}_{2}^{ \cdot - }\) accumulation in anthers isolated from freshly cut tillers revealed significant variation among studied DH lines but without any relation to its embryogenic potential (ANOVA/MANOVA, p ≤ 0.05; Table 2). Generally, LT pre-treatment had no effect or increased \({\text{O}}_{2}^{ \cdot - }\) generation in ‘recalcitrant’ DH lines (Fig. 3). Simultaneously, it significantly decreased \({\text{O}}_{2}^{ \cdot - }\) accumulation in majority of ‘responsive’ DH lines. As a result, after LT treatment, the mean amount of \({\text{O}}_{2}^{ \cdot - }\) was significantly lower in anthers of ‘responsive’ DH lines in comparison with ‘recalcitrant’ ones (Table 2).
The effect of low temperature tillers pre-treatment (3 weeks at 4 °C) on \({\text{O}}_{2}^{ \cdot - }\) generation in anthers of selected DH lines of winter triticale (×Triticosecale Wittm.) expressed as the percentage of the anther area (% aa) stained by the coloured reaction with nitroblue tetrazolium (NBT). Control means anthers isolated from freshly cut tillers. Presented data are the means of three replications. Mean values marked with the same letter within the group do not differ significantly according to Duncan’s multiple range test (p ≤ 0.05)
Histochemical localisation of superoxide anion (\({\text{O}}_{2}^{ \cdot - }\)) production in anthers of DH lines of winter triticale (×Triticosecale Wittm.) by nitroblue tetrazolium (NBT) staining. a–h Non-stained anthers collected from freshly cut tillers. a1–h1 NBT-stained anthers collected from freshly cut tillers (dark blue). a2–h2 NBT-stained anthers collected from low temperature pre-treated tillers (dark blue). Excised anthers are ordered from top to bottom according to the increasing intensity of \({\text{O}}_{2}^{ \cdot - }\) staining. In the individual figures staining is visible in anther wall (***), connective (**), and filament (*); bar 1,000 µm
A relatively high, genotype-dependent (ANOVA/MANOVA, p ≤ 0.05) level of H2O2 was detected in the anthers collected from freshly cut tillers (16.6–26.7 µmol g−1 FW; Fig. 5). LT treatment enhanced H2O2 generation by about 12 % for ‘responsive’ and 23 % for ‘recalcitrant’ DH lines (Table 2). No linear correlation between H2O2 generation and ME effectiveness was detected when the anthers used for in vitro culture were collected from freshly cut tillers. However, after LT tillers pre-treatment the level of H2O2 was negatively correlated with the effectiveness of ME (R = −0.71).
The effect of low temperature tillers pre-treatment (3 weeks at 4 °C) on H2O2 generation in anthers of selected DH lines of winter triticale (×Triticosecale Wittm.). Control means anthers isolated from freshly cut tillers. Presented data are the means of three replications. Mean values marked with the same letter within the group do not differ significantly according to Duncan’s multiple range test (p ≤ 0.05)
The total activity of low molecular weight (LMW) antioxidants
In the anthers collected from freshly cut tillers, the total activity of non-enzymatic antioxidants ranged between 23.7 and 32.7 mmol Trolox g−1 DW (Fig. 6). LT pre-treatment enhanced mean antioxidative activity from 29.8 to 31.7 mmol Trolox g−1 DW. Both with and without LT pre-treatment the mean activity of non-enzymatic (LMW) antioxidants was higher in ‘recalcitrant’ DH lines in comparison with ‘responsive’ ones (Table 2). A significant negative correlation (R = −0.71) between the activity of LMW antioxidants in anthers and ME effectiveness was detected but only in the case of control cultures started without LT pre-treatment.
The effect of low temperature tillers pre-treatment (3 weeks at 4 °C) on non-enzymatic (low molecular weight, LMW) antioxidants activity in anthers of selected DH lines of winter triticale (×Triticosecale Wittm.). Control means anthers isolated from freshly cut tillers. Presented data are the means of four biological replications. Mean values marked with the same letter within the group do not differ significantly according to Duncan’s multiple range test (p ≤ 0.05)
The activity of antioxidative enzymes
In anthers excised from freshly cut tillers total activity of SOD varied from 0.48 to 1.60 U g−1 protein (Fig. 7) and was significantly lower for the ‘recalcitrant’ DH lines of triticale (Table 2). The activity of this enzyme was enhanced by LT tiller pre-treatment to the range 1.07–3.07 U g−1 protein and in this case no significant difference between ‘responsive’ and ‘recalcitrant’ groups of DH lines was detected (ANOVA, p ≤ 0.05; Table 2). However, Spearman Rank Correlation analysis revealed significant positive correlation (R = 0.61, p ≤ 0.05) between the activity of this enzyme and ME effectiveness. Three various isoforms, two Cu/ZnSOD isozymes of various molecular mass and one MnSOD, were identified, whereas FeSOD isoform was not detected (Fig. 8). LT pre-treatment did not induce the synthesis of any new SOD isoform.
The effect of low temperature tillers pre-treatment (3 weeks at 4 °C) on superoxide dismutase activity [U g−1 protein] in the anthers of selected DH lines of winter triticale (×Triticosecale Wittm.). Control means anthers isolated from freshly cut tillers. Presented data are the means of four biological replications. Mean values marked with the same letter within the group do not differ significantly according to Duncan’s multiple range test (p ≤ 0.05)
CAT activities for individual DH lines are presented in Fig. 9. Contrary to SOD, the activity of this enzyme was significantly lower in the anthers of ‘responsive’ DH lines excised from freshly cut tillers in comparison to ‘recalcitrant’ DH lines (Table 2). Moreover, the effect induced by LT pre-treatment was significantly different for DH lines with different embryogenic potential. In anthers of ‘responsive’ DH lines, LT pre-treatment enhanced CAT activity by almost 45 %, whereas in anthers of ‘recalcitrant’ DH lines CAT activity decreased to less than 50 % of its initial value (Table 2).
The effect of low temperature tillers pre-treatment (3 weeks at 4 °C) on catalase activity [µmol H2O2 min−1 mg−1 protein] in the anthers of selected DH lines of winter triticale (×Triticosecale Wittm.). Control means anthers isolated from freshly cut tillers. Presented data are the means of four biological replications. Mean values marked with the same letter within the group do not differ significantly according to Duncan’s multiple range test (p ≤ 0.05)
Both studied groups of DH lines started from almost the same level of POX activity in anthers isolated from freshly cut tillers. Similar to CAT, the effect of LT was different for DH lines of various embryogenic potential (Table 2). The changes induced by LT, although not very pronounced, resulted in significantly higher POX activity in anthers of ‘responsive’ genotypes as compared to ‘recalcitrant’ ones. Data for individual DH lines are presented in Fig. 10.
The effect of low temperature tillers pre-treatment (3 weeks at 4 °C) on peroxidase activity [ΔA min−1 mg−1 protein] in the anthers of selected DH lines of winter triticale (×Triticosecale Wittm.). Control means anthers isolated from freshly cut tillers. Presented data are the means of four biological replications. Mean values marked with the same letter within the group do not differ significantly according to Duncan’s multiple range test (p ≤ 0.05)
Selecting key parameters linked to high ME responsiveness by statistical approaches
Multiple regression examines the simultaneous influence of many independent variables on one dependent trait, which in this study was the effectiveness of ME (AS/100A). Without LT pre-treatment, a significant negative effect on ME was exerted by the activity of LMW antioxidants: AS/100A = 209.1 − 0.84 × LMW activity (with multiple R-square = 0.70). When ME initiation was preceded by LT tillers treatment the strongest predictors of the effectiveness of the process included H2O2 level and CAT activity: AS/100A = 322.6 − 0.81 × H2O2 concentration + 0.48 × CAT activity. The effect of these variables contributed 85 % of the variation in ME effectiveness.
PCA was used separately for various tillers treatments (control, LT; Fig. 9a, b) to visualize similarities of the interrelationships among studied traits and genotypes. The analysis was based on the first two principal components (PC) and explained a high proportion of the total variation (72 and 65 %, respectively). The analysis confirmed that LT treatment radically changed the relation pattern among tested variables. According to PCA for anthers isolated from control, non-stressed tillers, the concentration of H2O2 was positively correlated with ME effectiveness as indicated by the acute angle between their vectors (Fig. 11a). In this case, high activity of LMW antioxidants and CAT had a negative effect on ME, as indicated by the obtuse and straight angles between their vectors. The second PC cumulated the effects of SOD and POX activity and was positively correlated with ME effectiveness. In contrast, after LT tillers pre-treatment H2O2 generation had a negative effect on ME (obtuse angle), whereas the activity of PEX, CAT and SOD was positively correlated with the process (acute angles; Fig. 11b). For both treatments the value of PC1 discriminated triticale genotypes ‘responsive’ toward ME induction treatment from ‘recalcitrant’ ones.
Vector view of genotype-by-trait principal component analysis showing interrelationships among studied variables in anthers excised from freshly cut (a) and low temperature treated tillers (b). AS/100A—the number of androgenic structures produced per 100 anthers, CAT—catalase activity, H 2 O 2 —hydrogen peroxide generation, LMW—activity of low molecular weight antioxidants; POX—non-specific peroxidase activity, \({\text{O}}_{2}^{ \cdot - }\)—superoxide anion generation, SOD—superoxide dismutase activity
Discussion
The presented study was conducted in order to verify the hypothesis that ROS generation, cell tolerance to ROS accumulation and antioxidative defense efficiency influence the effectiveness of ME in in vitro anther cultures of triticale. Eight winter triticale DH lines used in the study had been selected on the base of preliminary experiments from the mapping population ‘Saka 3006’×’Modus’ as significantly different in their embryogenic potential. The evaluation of their responsiveness was conducted through a 3-year-long set of anther culture experiments according to the method described by Wędzony (2003) with some modifications (Krzewska et al. 2013). The characterization of selected DH lines in respect of their embryogenic potential was fully confirmed in this study. Moreover, additional information was obtained, concerning the effect of LT tiller pre-treatment, used in triticale as a standard trigger for ME initiation. Cold shock belongs to stressors widely used for ME initiation and its positive effect was often reported in various plant species (review by Zoriniants et al. 2005; Shariatpanahi et al. 2006a; Islam and Tuteja 2012). It was observed also in anther (Ponitka et al. 1999; Immonen and Robinson 2000) and isolated microspore cultures of triticale (Pauk et al. 2000, 2003; Żur et al. 2008, 2009). Several authors described the effects of LT on microspore viability, metabolism, and gene expression pattern (reviewed by Zoriniants et al. 2005 and Shariatpanahi et al. 2006a). However, the role and the mechanism of the observed changes still remain unclear (Żur et al. 2012).
The results of this study revealed that LT pre-treatment (3 weeks at 4 °C) increased microspores viability and the frequency of microspores with morphological features typical for embryogenesis initiation. It has been observed that upon stress treatment, embryogenic microspores swell and rearrange their cytoskeleton forming so-called SLSs, with the nucleus moved to a central position, fragmented vacuole and cytoplasmic strands connecting the perinuclear cytoplasm with the subcortical cytoplasm (Touraev et al. 1997). Such changes are characteristic for various plant species, including tobacco, oilseed rape, wheat, rice and triticale (Touraev et al. 1997; Dubas et al. 2010). Although LT treatment increased the efficiency of ME in almost all studied triticale lines, it was not a prerequisite for ME initiation. Even in ‘recalcitrant’ genotypes some AS were produced from the anthers excised from freshly cut tillers. Similarly, a method for producing DH wheat plants without any apparent stress treatment was described by Shariatpanahi et al. (2006b). It could be supposed that for some genotypes accumulation of various types of stress factors (mechanical injuring, disinfection, transfer to in vitro culture conditions) during in vitro culture set-up procedure generates a sufficient signal to induce microspore reprogramming. Although the physiological/molecular nature of this signal has not been identified yet, the involvement of ROS in ME initiation has been proposed (Jacquard et al. 2006, 2009; Żur et al. 2008, 2009). Regardless of embryogenic potential of DH line, LT pre-treatment also stimulated regeneration ability of androgenic structures, however it increased the frequency of green plant production only in the group of ‘responsive’ lines.
Enhanced ROS accumulation is typical for cells under stress-inducing conditions. An elevated level of ROS imposes a real threat to cell integrity and functioning but on the other hand, ROS play an important role in the regulation of various physiological processes including growth, development, hormone signalling, defence reaction, and PCD (Mittler 2002; Mittler et al. 2004; Rodríguez-Serrano et al. 2012). Among others, the involvement of ROS as second messengers in somatic embryogenesis of many plants species has been revealed (reviewed by Zavattieri et al. 2010). In the presented study, the evaluation of oxidative stress intensity and activity of antioxidative system revealed some interesting associations with ME initiated in triticale anther cultures. All measurements were performed on the whole organ level (anther) as, despite the fact that microspores comprise only a minor part of the anther cell population, the sporophytic tissues that surround them influence significantly microspore development and differentiation. In vivo, the tapetum functions as a source of nutrients for developing pollen grains. It also secretes into the locule several enzymes that release the microspores from tetrads and provides the components of the outer pollen surface (Taylor et al. 1997). The importance of the tapetum for viable pollen formation has recently been shown both in dicots and monocots (Zhang et al. 2011). In vitro, in mannitol treated anthers of barley the death of tapetum cells was supposed to release signals triggering microspore embryogenic development (Wang et al. 2000). This hypothesis seems to be confirmed by the results of this study.
A single electron O2 reduction generates \({\text{O}}_{2}^{ \cdot - }\), a moderately reactive and short-lived radical, with a half-life of approx. 1–4 µs (Gill and Tuteja 2010; Karuppanapandian et al. 2011). As a negatively charged molecule, it theoretically cannot pass through cell membranes so its effects are restricted to the areas next to the site of its generation (Bhattacharjee 2011). Its direct role in the signal transduction has not been fully confirmed although some reports suggest that \({\text{O}}_{2}^{ \cdot - }\) can activate genes involved in plant defence signalling pathways (Jabs et al. 1996; Vranová et al. 2002; Scarpeci et al. 2008). Our results proved significant variation among studied DH lines with respect to \({\text{O}}_{2}^{ \cdot - }\) production, which was related to its embryogenic potential. High level of \({\text{O}}_{2}^{ \cdot - }\) was detected in anthers excised from freshly cut tillers of both ‘recalcitrant’ and ‘responsive’ DH lines, but only in the latter did it decrease significantly after LT pre-treatment. It could be supposed that an important role in this phenomenon was played by SOD, as higher activity of this enzyme was detected in the anthers of ‘responsive’ DH lines just after cutting tillers. As the enzyme catalyses the reaction of superoxide radical dismutation to H2O2 and oxygen, it probably protects the microspores from oxidative stress induced by high level of \({\text{O}}_{2}^{ \cdot - }\) and from the formation of the most toxic and highly reactive oxidant, hydroxyl radical (Gill and Tuteja 2010). It could be the reason for higher viability of microspores isolated from freshly cut tillers of ‘responsive’ DH lines and for the positive effect induced by LT, which was associated with increased SOD activity in all studied genotypes. Significant positive correlation (R = 0.61, p ≤ 0.05) between the activity of this enzyme and ME effectiveness confirmed the important role played by this enzyme in ME induction. Increased SOD activity is usually the first response to ROS (particularly \({\text{O}}_{2}^{ \cdot - }\)) accumulation (Alscher et al. 2002; Kayihan et al. 2012) and has been reported in plants exposed to various environmental stresses, where it was often correlated with increased stress tolerance (reviewed by Sharma et al. 2012). In all studied DH lines of triticale, both copper/zinc and manganese isoforms of SOD were detected and accumulated under LT pre-treatment, which suggests that ROS were produced in almost all cellular compartments including mitochondria, chloroplasts, peroxisomes, cytosol, and apoplast. Similarly, increased SOD activity was often observed after cold acclimation of plants e.g. in wheat (Scebba et al. 1999) and tomato seedlings (Zhou et al. 2012). The lack of FeSOD was not surprising, as despite extensive research, the absence of this isozym was often reported e.g. in rice, maize (Alscher et al. 2002) or barley cultivars (Kayihan et al. 2012). Similar to our study, new isoforms of SOD were not detected in cold acclimated wheat seedlings, either (Scebba et al. 1998).
For effective cell protection, SOD must cooperate with enzymes that remove H2O2 accumulated as the product of \({\text{O}}_{2}^{ \cdot - }\) dismutation. H2O2 is a moderately reactive and relatively long-lived molecule with a half-life of 1 ms. The lack of unpaired electrons allows for its diffusion through aquaporins of cell membranes and transport over some distances from the site of its formation (Gill and Tuteja 2010). All these features predestined this radical—at low/moderate concentration—to be a part of the signal transduction pathway involved in the regulation of several biological processes such as stomata movement, photorespiration and photosynthesis, growth, development, and senescence (Gill and Tuteja 2010; Karuppanapandian et al. 2011). ROS signal can be transduced and translated using several elements/mechanisms: redox-sensitive proteins, altered cytosolic calcium concentrations, protein phosphorylation, changes in the activity of protein phosphatases, protein kinases, and transcription factors, and finally modulation of gene expression profile (reviewed in Sharma et al. 2012). However, high concentration of H2O2 that exceeds defence system efficiency can oxidize thiol groups of enzymes and transcription factors inhibiting or decreasing their activity. Moreover, in the presence of transition metals it becomes the source of the most toxic hydroxyl radical through the Fenton reaction, which can ultimately lead to cell death via oxidative destruction or by induction of PCD. According to the results of Cheeseman (2006) analysis on several field-grown plant species, the concentrations between 1 and 5 μmol g FW of H2O2 can be assumed as ‘normal’ for plant tissue. In this context, the level of H2O2 detected in the anthers excised from freshly cut tillers of triticale (about 20 µmol g−1 FW) can be appraised as relatively high. Such high level of H2O2 was possibly the result of the stress connected with anthers isolation and was not associated with embryogenic potential evaluated by in vitro anther culture method. Similar level of H2O2 was reported by Tewari et al. (2004) in maize plants subjected to macronutrients deficiency. Further H2O2 accumulation was observed after LT treatment in triticale anthers, as it was observed in many other studies concerning cold acclimation of wheat (Okuda et al. 1991), maize (Prasad et al., 1994) and tomato (Zhou et al. 2012). Interestingly, the effect of H2O2 depends on the anthers’ pre-treatment: a positive although non-linear association with ME effectiveness was revealed in non-stressed anthers whereas a linear negative correlation was detected after LT pre-treatment. It could be supposed that some threshold level of H2O2 in control cultures was important for the initiation of defense reactions and/or signal transduction pathways, which positively influenced microspore stress tolerance and reprogramming. It was earlier demonstrated that H2O2 can induce expression of several antioxidative enzymes like SOD, CAT, ascorbate and glutathione peroxidases, alternative oxidase and glutathione-S-transferase (reviewed in Mylona and Polidoros 2011). Increased endogenous level of H2O2 preceded somatic embryogenesis initiation, embryo differentiation and plant regeneration in in vitro cultures of various plant species (Kairong et al. 1999; Libik et al. 2005; Li et al. 2007; Agrawal and Purohit 2012). A certain level of this molecule was required to initiate rhizogenesis in mud bean seedlings (Li et al. 2009a, b) and hypocotyl explants of Mesembryanthemum crystallinum (Konieczny et al. 2014).
The obtained results also suggest that although a further increase in H2O2 level induced by LT treatment was negatively correlated with the effectiveness of ME, it did not have detrimental effects as long as it was accompanied by high activity of H2O2-decomposing enzymes, CAT and POX. The activity of CAT seems to be particularly important, differentiating ‘recalcitrant’ from ‘responsive’ DH lines of triticale. Significantly higher activity of this enzyme was detected in anthers collected from freshly cut tillers of ‘recalcitrant’ DH lines in comparison with ‘responsive’ ones. However, in ‘recalcitrant’ genotypes LT pre-treatment significantly decreased CAT activity, as it was reported by Taşgin et al. (2006) and Janda et al. (2003) in several frost-tolerant and frost-sensitive cereal species. According to these authors, it confirmed high cold sensitivity of CAT. This feature is evidently genotype-dependent as in the case of studied triticale DH lines no change or an increase in CAT activity was observed in ‘responsive’ genotypes. This seems to be confirmed by Prasad et al. (1994), who reported the induction of CAT in chilled maize seedlings and considered CAT as an essential enzyme protecting mitochondria against LT stress. The ability to sustain CAT activity under darkness and LT conditions could be another factor ensuring the success of ME initiation. Most catalase is localised in peroxisomes in order to detoxify H2O2 produced by oxidase enzymes involved in β-oxidation of fatty acids, photorespiration, and purine catabolism. H2O2 produced in mitochondria and chloroplasts is removed by peroxidase, though in this case the efficiency of detoxification depends upon the availability of reducing co-substrates (Bhattacharjee 2011). Although mean POX activity was significantly higher for ‘responsive’ DH lines in comparison with ‘recalcitrant’ ones, strong genotypic variation suggests no specific function of this enzyme in ME initiation. In the majority of cases, LT decreased enzyme activity, which confirmed earlier results obtained for two other triticale cultivars (Żur et al. 2008).
Many various LMW antioxidants are extracted by 50 % aqueous-ethanolic solution e.g. glutathione, ascorbic acid, phenols, polyphenols, flavonoids, anthocyanins, polysaccharides, tannins, etc. Their functions in plant responses to stress have been extensively studied and reviewed among others by Hong-bo et al. (2008). Higher activity of these antioxidative molecules in ‘recalcitrant’ DH lines of triticale suggests an existence of a compensatory relation between both (enzymatic and non-enzymatic) antioxidative systems. However, negative correlation between LMW antioxidants activity and ME effectiveness detected in anthers collected from freshly cut tillers proved this compensatory effort to be unsuccessful.
In conclusion, genetically controlled but environmentally modified cell tolerance to oxidative stress seems to play an important role in triticale ME. In control cultures, with ME initiated without additional stress treatment, high SOD activity protecting the cells against dangerous reactivity of superoxide anion (\({\text{O}}_{2}^{ \cdot - }\)) appeared crucial for microspore viability. On the other hand, a positive although non-linear correlation between ME effectiveness and H2O2 generation, and a negative correlation with CAT activity suggest that some threshold level of this ROS is important for successful ME initiation. LT tillers pre-treatment significantly increased H2O2 accumulation, which had a negative effect on ME effectiveness. However, even high level of H2O2 did not endanger cell viability as long as cells exhibited high activity of ROS-decomposing enzymes (SOD, CAT and POX). The ability to sustain antioxidative enzyme activity under cold stress in the dark was possibly one of the most important reasons for high effectiveness of ME, allowing for generation and transduction of the signal initiating microspore reprogramming and simultaneously protecting the cells from the toxic effects of ROS production. The role of antioxidative enzymes cannot be replaced even by high activity of non-enzymatic antioxidants.
To the best of our knowledge, this is the first such a comprehensive and detailed examination of LT-induced modulations in cell ROS tolerance and antioxidative system activity, and their effect on embryogenic potential of in vitro cultured triticale anthers. However, although the results obtained increase our understanding of the physiological and biochemical aspects of efficient ME initiation, further examination of this complex phenomenon is still necessary.
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agrawal M, Purohit S (2012) Changes in antioxidant enzymes activity during in vitro morphogenesis of carnation and the effect of antioxidants on plant regeneration. World J Sci Tech 2:87–92
Alscher RG, Erturk N, Heath LS (2002) Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J Exp Bot 53(372):1331–1341
Beauchamp C, Fridovich I (1971) Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal Biochem 44:276–287
Bergmeyer HU (1965) Methods of enzymatic analysis. Academic Press, New York and London, Verlag Chemie GMBH Weinheim/Bergstr.
Bhattacharjee S (2011) Sites of generation and physicochemical basis of formation of reactive oxygen species in plant cell. In: Gupta SD (ed) Reactive oxygen species and antioxidants in higher plants. Sci Publ, Enfield, pp 1–29
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem 72:248–254
Brand-Williams W, Cuvelier ME, Berset C (1995) Use of a free-radical method to evaluate antioxidant activity. LWT Food Sci Technol 28:25–30
Cheeseman JM (2006) Hydrogen peroxide concentrations in leaves under natural conditions. J Exp Bot 57(10):2435–2444
Doke N, Ohashi Y (1988) Involvement of and O ·2 generating system in the induction of necrotic lesions on tobacco leaves infected with tobacco mosaic virus. Physiol Mol Plant Pathol 32:165–175
Droillard MJ, Paulin A, Massot JC (1987) Free radical production, catalase and superoxide dismutase activities and membrane integrity during senescence of petals of cut carnations (Dianthus caryophyllus). Physiol Plantarum 71:197–202
Dubas E, Wędzony M, Petrowska B, Salaj J, Żur I (2010) Cell cell structural reorganization during induction of androgenesis in isolated microspore cultures of triticale (×Triticosecale Wittm.). Acta Biologica Cracoviensia Series Botanica 52(1):73–86
Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Bioch 48:909–930
Heslop-Harrison J, Heslop-Harrison Y (1970) Evaluation of pollen viability by enzymatically induced fluorescence: intracellular hydrolysis of fluorescein diacetate. Stain Technol 45:115–120
Hong-bo S, Chu L-Y, Ming-an S, Jaleel CA, Hong-mei M (2008) Higher plant antioxidants and redox signalling under environmental stresses CR. Biol 331:433–441
Immonen S, Robinson J (2000) Stress treatments and ficoll for improving green plant regeneration in triticale anther culture. Plant Sci 150:77–84
Ishikawa T, Takeda T, Shigeoka S, Hirayama O, Mitsunaga T (1993) Hydrogen peroxide generation in organells of Euglena gracilis. Phytochemistry 33:1297–1299
Islam SMS, Tuteja N (2012) Enhancement of androgenesis by abiotic stress and other pretreatments in major crop species. Plant Sci 182:134–144
Jabs T, Dietrich RA, Dangl JL (1996) Initiation of runaway cell death in an Arabidopsis mutant by extracellular superoxide. Sci 273:1853–1856
Jacquard C, Mazeyrat-Gourbeyre F, Devaux P, Baillieul F, Clément C (2006) Plant defence mechanisms are triggered in the anther during the pre-treatment process. In: The international conference ‘Haploids in Higher Plants III’ Vienna, 12–15 February, 2006, Book of Abstracts, p 29
Jacquard C, Nolin F, Hécart C, Grauda D, Rashal I, Dhondt-Cordelier S, Sangwan RS, Devaux P, Mazeyrat-Gourbeyre F, Clément C (2009) Microspore embryogenesis and programmed cell death in barley: effects of copper on albinism in recalcitrant cultivars. Plant Cell Rep 28:1329–1339
Janda T, Szalai G, Rios-Gonzalez K, Veisz O, Páldi E (2003) Comparative study of frost tolerance and antioxidant activity in cereals. Plant Sci 164:301–306
Kairong C, Gengsheng X, Xinmin L, Gengmei X, Wang Gafu W (1999) Effect of hydrogen peroxide on somatic embryogenesis of Lycium barbarum L. Plant Sci 146:9–16
Karuppanapandian T, Moon J-C, Kim C, Manoharan K, Kim W (2011) Reactive oxygen species in plants: their generation, signal transduction, and scavenging mechanisms. Aust J Crop Sci 5(6):709–725
Kasha KJ, Maluszynski M (2003) Production of doubled haploids in crop plants. An introduction. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants. A manual. Kluwer, Dordrecht, pp 1–4
Kayihan C, Eyidogan F, Afsar N, Oktem HA, Yucel M (2012) Cu/Zn superoxide dismutase activity and respective gene expression during cold acclimation and freezing stress in barley cultivars. Biol Plant 56(4):693–698
Konieczny R, Banaś AK, Surówka E, Michalec Z, Miszalski Z, Libik-Konieczny M (2014) Pattern of antioxidant enzyme activities and hydrogen peroxide content during developmental stages of rhizogenesis from hypocotyl explants of Mesembryanthemum crystallinum L. Plant Cell Rep 33:165–177
Krzewska M, Czyczyło-Mysza I, Dubas E, Gołębiowska-Pikania G, Golemiec E, Stojałowski S, Chrupek M, Żur I (2012) Quantitative trait loci associated with androgenic responsiveness in triticale (×Triticosecale Wittm.) anther culture. Plant Cell Rep 31:2099–2108
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–687
Li S, Xue L, Xu S, Feng H, An L (2007) Hydrogen peroxide involvement in formation and development of adventitious roots in cucumber. Plant Growth Regul 52:173–180
Li S, Xue L, Xu S, Feng H, An L (2009a) Hydrogen peroxide acts as a signal molecule in the adventitious root formation of mung bean seedlings. Environ Exp Bot 65:63–71
Li S, Xue L, Xu S, Feng H, An L (2009b) IBA-induced changes in antioxidant enzymes during adventitious rooting in mung bean seedlings: the role of H2O2. Environ Exp Bot 66:442–445
Libik M, Konieczny R, Pater B, Ślesak I, Miszalski Z (2005) Differences in the activities of some antioxidant enzymes and H2O2 content during rhizogenesis and somatic embryogenesis in callus cultures of the ice plant. Plant Cell Rep 23:834–841
Maraschin SF, Caspers M, Potokina E, Wülfert F, Graner A, Spaink HP, Wang M (2006) cDNA array analysis of stress-induced gene expression in barley androgenesis. Physiol Plantarum 127:535–550
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7(9):405–410
Mittler R, Vanderauwera S, Gollery M, Van Breusegem F (2004) Reactive oxygen gene network of plants. Trends Plant Sci 9(10):490–498
Muñoz-Amatriain M, Svensson JT, Castillo AM, Close TJ, Vallés MP (2009) Microspore embryogenesis: assignment of genes to embryo formation and green vs. albino plant production. Funct Integr Genomics 9:311–323
Mylona PV, Polidoros AN (2011). ROS regulation of antioxidant genes. In: Dutta Gupta S (ed) Reactive oxygen species and antioxidants in higher plants. Sci Publ, CRC Press, pp 101–128, ISBN 978-1-57808-686-3
Okuda T, Matsuda Y, Yamanaka A, Sagisaka S (1991) Abrupt increase in the level of hydrogen peroxide in leaves of winter wheat is caused by cold treatment. Plant Physiol 97(3):1265–1267
Pauk J, Puolimatka M, Tók L, Monostori T (2000) In vitro androgenesis of triticale in isolated microspore culture. Plant Cell Tiss Org Cult 61:221–229
Pauk J, Mhaly R, Monostori T, Puolimatka M (2003) Protocol for triticale (×Triticosecale Wittmack) microspore culture. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants. A manual. Kluwer, Dordrecht, pp 129–134
Ponitka A, Ślusarkiewicz-Jarzina A, Wędzony M, Marcińska I, Woźna J (1999) The influence of various in vitro culture conditions on androgenetic embryo induction and plant regeneration from hexaploid triticale (×Triticosecale Wittm.). J Appl Genet 40:165–174
Prasad TK, Anderson MD, Martin BA, Stewart CR (1994) Physiological evidence for chilling-induced oxidative stress in maize seedlings and a regulatory role for hydrogen peroxide. Plant Cell 6:65–74
Rodríguez-Serrano M, Bárány I, Prem D, Coronado M-J, Risueño MC, Testillano PS (2012) NO, ROS, and cell death associated with caspase-like activity increase in stress-induced microspore embryogenesis of barley. J Exp Bot 63(5):2007–2024
Scarpeci TE, Zanor MI, Carrillo N, Mueller-Roeber B, Valle EM (2008) Generation of superoxide anion in chloroplasts of Arabidopsis thaliana during active photosynthesis: a focus on rapidly induced genes. Plant Mol Biol 66:361–378
Scebba F, Sebastiani L, Vitagliano C (1998) Changes in activity of antioxidative enzymes in wheat (Triticum aestivum) seedlings under cold acclimation. Physiol Plantarum 104:747–752
Scebba F, Sebastiani L, Vitagliano C (1999) Protective enzymes against oxygen species in wheat (Triticum aestivum L.) seedlings: responses to cold acclimation. J Plant Physiol 155:762–768
Shariatpanahi ME, Bala U, Heberle-Bors E, Touraev A (2006a) Stresses applied for the re-programming of plant microspores towards in vitro embryogenesis. Physiol Plantarum 127:519–534
Shariatpanahi ME, Belogradova K, Hessamvaziri L, Heberle-Bors E, Touraev A (2006b) Efficient embryogenesis and regeneration in freshly isolated and cultured wheat (Triticum aestivum L.) microspores without stress pretreatment. Plant Cell Rep 25:1294–1299
Sharma P, Jha AB, Dubey RS, Pessarakli M (2012) Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J Bot. doi:10.1155/2012/217037
Taylor AA, Horsch A, Rzepczyk A, Hasenkampf CA, Riggs CD (1997) Maturation and secretion of a serine proteinase is associated with events of late microsporogenesis. Plant J 12(6):1261–1271
Taşgin E, Atici Ö, Nalbantoğlu B, Popova LP (2006) Effects of salicylic acid and cold treatments on protein levels and on the activities of antioxidant enzymes in the apoplast of winter wheat leaves. Phytochemistry 67:710–715
Tewari R, Kumar P, Tewari N, Srivastava S, Sharma P (2004) Macronutrient deficiences and differential antioxidant responses: influence on the activity and expression of superoxide dismutase in maize. Plant Sci 166:687–694
Touraev A, Vicente O, Heberle-Bors E (1997) Initiation of microspore embryogenesis by stress. Trends Plant Sci 2:285–303
Vranová E, Inzé D, Van-Breusegen F (2002) Signal transduction during oxidative stress. J Exp Bot 53(372):1227–1236
Wang P, Chen Y (1983) Preliminary study on prediction of height of pollen H2 generation in winter wheat grown in the field. Acta Agron Sin 9:283–284
Wang M, van Bergen S, van Duijn B (2000) Insights into key developmental switch and its importance for efficient plant breeding. Plant Physiol 124:523–530
Wędzony M (2003) Protocol for doubled haploid production in hexaploid triticale (×Triticosecale Wittm.) by crosses with maize. In: Maluszynski M, Kasha KJ, Forster BP, Szarejko I (eds) Doubled haploid production in crop plants. A manual. Kluwer, Dordrecht, pp 135–140
Zavattieri MA, Frederico AM, Lima M, Sabino R, Schmitt BA (2010) Induction of somatic embryogenesis as an example of stress-related plant reactions. Electronc J Biotech 13(1). ISSN: 0717-3458
Zhang D, Luo X, Zhu L (2011) Cytological analysis and genetic control of rice anther development. J Genet Genomics 38:379–390
Zhou J, Wang J, Shi K, Xia XJ, Zhou YH, Yu JQ (2012) Hydrogen peroxide is involved in the cold acclimation-induced chilling tolerance of tomato plants. Plant Physiol Biochem 60:141–149
Zhuang JJ, Xu J (1983) Increasing differentiation frequencies in wheat pollen callus. In: Hu H, Vega MR (eds) Cell and tissue culture techniques for cereal crop improvement. Science Press, Beijing, p 431
Zoriniants S, Tashpulatov AS, Heberle-Bors E, Touraev A (2005) The role of stress in the induction of haploid microspore embryogenesis. In: Palmer CE, Keller WA, Kasha KJ (eds) Haploids in crop improvement II. Springer, Berlin, pp 35–52
Żur I, Dubas E, Golemiec E, Szechyńska-Hebda M, Janowiak F, Wędzony M (2008) Stress-induced changes important for effective androgenic induction in isolated microspore culture of triticale (×Triticosecale Wittm.). Plant Cell Tiss Org Cult 94:319–328
Żur I, Dubas E, Golemiec E, Szechyńska-Hebda M, Golebiowska G, Wędzony M (2009) Stress-related variation in antioxidative enzymes activity and cell metabolism efficiency associated with embryogenesis induction in isolated microspore culture of triticale (×Triticosecale Wittm.). Plant Cell Rep 28:1279–1287
Żur I, Krzewska M, Dubas E, Gołębiowska-Pikania G, Janowiak F, Stojałowski S (2012) Molecular mapping of loci associated with abscisic acid accumulation in triticale (×Triticosecale Wittm.) anthers in response to low temperature stress inducing androgenic development. Plant Growth Regul 68:483–492
Acknowledgments
The seeds of triticale DH lines were kindly provided by Dr Eva Bauer from the State Plant Breeding Institute, Hohenheim University, Germany. The work was supported by Polish National Project NN310 452 638 financed by Polish Ministry of Science and Higher Education.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Żur, I., Dubas, E., Krzewska, M. et al. Antioxidant activity and ROS tolerance in triticale (×Triticosecale Wittm.) anthers affect the efficiency of microspore embryogenesis. Plant Cell Tiss Organ Cult 119, 79–94 (2014). https://doi.org/10.1007/s11240-014-0515-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-014-0515-3