Abstract
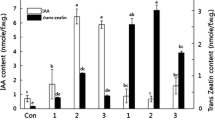
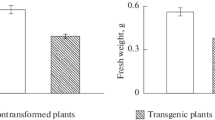
The ipt gene from the T-DNA of Agrobacterium tumefaciens was transferred to tobacco (Nicotiana tabacum L.) in order to study the control which auxin appears to exert over levels of cytokinin generated by expression of this gene. The transgenic tissues contained elevated levels of cytokinins, exhibited cytokinin and auxin autonomy and grew as shooty calli on hormone-free media. Addition of 1-naphthylacetic acid to this culture medium reduced the total level of cytokinins by 84% while 6-benzylaminopurine elevated the cytokinin level when added to media containing auxin. The cytokinins in the transgenic tissue were labelled with 3H and auxin was found to promote conversion of zeatin-type cytokinins to 3H-labelled adenine derivatives. When the very rapid metabolism of exogenous [3H]zeatin riboside was suppressed by a phenylurea derivative, a noncompetitive inhibitor of cytokinin oxidase, auxin promoted metabolism to adenine-type compounds. Since these results indicated that auxin promoted cytokinin oxidase activity in the transformed tissue, this enzyme was purified from the tobacco tissue cultures. Auxin did not increase the level of the enzyme per unit tissue protein, but did enhance the activity of the enzyme in vitro and promoted the activity of both glycosylated and non-glycosylated forms. This enhancement could contribute to the decrease in cytokinin level induced by auxin. Studies of cytokinin biosynthesis in the transgenic tissues indicated that trans-hydroxylation of isopentenyladenine-type cytokinins to yield zeatin-type cytokinins occurred principally at the nucleotide level.
Similar content being viewed by others
Abbreviations
- Ade:
-
adenine
- Ados:
-
adenosine
- BA:
-
6-benzylaminopurine
- C:
-
control
- Con A:
-
concanavallin A
- CP:
-
cellulose phosphate
- IPT:
-
isopentenyl transferase
- NAA:
-
1-naphthylacetic acid
- NP:
-
normal phase
- NPPU:
-
N-(3-nitrophenyl)-N′-phenylurea
- RIA:
-
radioimmunoassay
- RP:
-
reversed phase
References
Akiyoshi, D.E., Morris, R.O., Hinz, R., Mischke, B.S., Kosuge, T., Garfinkel, D.J., Gordon, M.P., Nester, E.W. (1983) Cytokinin/auxin balance in crown gall tumors is regulated by specific loci in the T-DNA. Proc. Natl. Acad. Sci. USA 80, 407–411
An, G. (1986) Development of plant promoter expression vectors and their use for analysis of differential activity of the nopaline synthase promoter in transformed tobacco cells. Plant Physiol. 81, 86–91
Badenoch-Jones, J., Letham, D.S., Parker, C.W., Rolfe, B.G. (1984) Quantitation of cytokinin in biological samples using antibodies against zeatin riboside. Plant. Physiol. 75, 1117–1125
Badenoch-Jones, J., Parker, C.W., Letham, D.S. (1987) Use of isopentenyladenosine and dihydrozeatin riboside antibodies for the quantification of cytokinins. J. Plant Growth Regul. 6, 159–182
Beinsberger, S.E.I., Valcke, R.L.M., Deblaere, R.Y., Clijsters, H.M.M., De Greef, J.A., Van Onckelen, H.A. (1991) Effects of the introduction of Agrobacterium tumefaciens T-DNA ipt gene in Nicotiana tabacum L. cv. Petit Havana SR1 plant cells. Plant Cell Physiol. 32, 489–496
Binns, A.N., Labriola, J., Black, R.C. (1987) Initiation of auxin autonomy in Nicotiana glutinosa cells by the cytokinin-biosynthesis gene from Agrobacterium tumefaciens. Planta 171, 539–548
Bruce, M.I., Zwar, J.A. (1966) Cytokinin activity of some substituted ureas and thioureas. Proc. Roy. Soc. London Ser. B 165, 245–265
Burch, L.R., Horgan, R. (1989) The purification of cytokinin oxidase from Zea mays kernels. Phytochemistry 28, 1313–1319
Chatfield, J.M., Armstrong, D.J. (1986) Regulation of cytokinin oxidase activity in callus tissues of Phaseolus vulgaris L. cv. Great Northern. Plant Physiol. 80, 493–499
Chatfield, J.M., Armstrong, D.J. (1987) Cytokinin oxidase from Phaseolus vulgaris callus tissues-enhanced in vitro activity of the enzyme in the presence of copper-imidazole complexes. Plant Physiol. 84, 726–731
Chatfield, J.M., Armstrong, D.J. (1988) Cytokinin oxidase from Phaseolus vulgaris callus cultures — affinity for concanavalin A. Plant Physiol. 88, 245–247
Chen, C.M., Leisner, S.M. (1984) Modification of cytokinins by cauliflower microsomal enzymes. Plant Physiol. 75, 442–446
Hall, P.J., Badenoch-Jones, J., Parker, C.W., Letham, D.S., Barlow, B.A. (1987) Identification and quantification of cytokinins in the xylem sap of mistletoes and their hosts in relation to leaf mimicry. Aust. J. Plant Physiol. 14, 429–438
Hansen, C.E., Meins, F., Aebi, R. (1987) Hormonal regulation of zeatin-riboside accumulation by cultured tobacco cells. Planta 172, 520–525
Heidekamp, F., Dirkse, W.G., Hille, J., van Ormond, H. (1983) Nucleotide sequence of the Agrobacterium tumefaciens octopine Ti plas-mid-encoded tmr gene. Nucleic Acid Res. 11, 6211–6223
Higgins, T.J.V., Newbigin, E.J., Spencer, D., Llewellyn, D.J., Craig, S. (1988) The sequence of a pea vicilin gene and its expression in transgenic tobacco plants. Plant Mol. Biol. 11, 683–695
Ishikawa, K., Kamada, H., Yamaguchi, I., Takahashi, N., Harada, H. (1988) Morphology and hormone levels of tobacco and carrot tissues transformed by Agrobacterium tumefaciens. I. Auxin and cytokinin contents of cultured tissues transformed with wild-type and mutant Ti plasmids. Plant Cell Physiol. 29, 461–466
Korber, H., Strizhov, N., Staiger, D., Feldwisch, J., Olsson, O., Sandberg., G., Palme, K., Schell, J., Koncz, C. (1991) T-DNA gene 5 of Agrobacterium modulates auxin response by autoregulated synthesis of a growth hormone antagonist in plants. EMBO J. 10, 3983–3991
Laloue, M., Fox, J.E. (1989) Cytokinin oxidase from wheat: partial purification and general properties. Plant Physiol. 90, 899–906
Leopold, A.C., Nooden, L.D. (1984) Hormonal regulatory systems in plants. In: Encyclopaedia of plant physiol. vol. 10: pp. 4–22, Scott, T.K. ed. Springer-Verlag, New York
Letham, D.S., Palni, L.M.S. (1983) The biosynthesis and metabolism of cytokinins. Annu. Rev. Plant Physiol. 34, 163–197
Letham, D.S., Singh, S., Willcocks, D.A. (1992) Reversed phase thin layer, chromatographic methods for separation of cytokinins. Phytochemical Analysis 3, 218–222
Martin, R.O., Mok, M.C., Shaw, G., Mok, D.W.S. (1989) An enzyme mediating the conversion of zeatin to dihydrozeatin in Phaseolus embryos. Plant Physiol. 90, 1630–1635
McGaw, B.A., Horgan, R., Heald, J.K., Wullems, G.J., Schilperoort, R.A. (1988) Mass-spectrometric quantitation of cytokinins in tobacco crown-gall tumours induced by mutated octopine Ti plasmids of Agrobacterium tumefaciens. Planta 176, 230–234
Morris, R.O. (1986) Genes specifying auxin and cytokinin biosynthesis in phytopathogens. Annu. Rev. Plant Physiol. 37, 509–538
Motyka, V., Kaminek, M. (1992) Characterization of cytokinin oxidase from tobacco and poplar callus cultures. In: Physiology and biochemistry of cytokinins in plants, pp. 33–39, Kaminek, M., Mok, D.W.S., Zazimalova, E., eds. SPB Academic Publishing, The Hague
Murashige, T., Skoog, F. (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plant. 15, 473–497
Neuman, D.S., Smit, B.A. (1990) Interference from xylem sap in an enzyme-linked immunosorbent assay for zeatin riboside. Physiol. Plant. 78, 548–553
Palmer, M.V., Letham, D.S., Gunning, B.E.S. (1984) Cytokinin metabolism in nondividing and auxin-induced dividing explants of Helianthus tuberosa tuber tissue. J. Plant Growth Regul. 2, 289–298
Palni, L.M.S., Burch, L., Horgan, R. (1988) The effect of auxin concentration on cytokinin stability and metabolism. Planta 174, 231–234
Palni, L.M.S., Horgan, R., Darrall, N.M., Stuchbury, T., Wareing, P.F. (1983) Cytokinin biosynthesis in crown-gall tissue of Vinca rosea: the significance of nucleotides. Planta 159, 50–59
Rudelsheim, P., Prinsen, E., Van Lijsebettens, M., Inze, D., Van Montagu, M., De Greef, J., Van Onckelen, H. (1987) The effect of mutations in the T-DNA encoded auxin pathway on the endogenous phytohormone content in cloned Nicotiana tabacum crown gall tissues. Plant Cell Physiol. 28, 475–484
Singh, S., Letham, D.S., Jameson, P.E., Zhang, R., Parker, C.W., Bade- noch-Jones, J., Nooden, L.D. (1988) Cytokinin biochemistry in relation to leaf senescence IV. Cytokinin metabolism in soybean ex-plants. Plant Physiol. 88, 788–794
Singh, S., Letham, D.S., Zhang, X., Palni, L.M.S. (1992) Cytokinin biochemistry in relation to leaf senescence VI. Effect of nitrogenous nutrients on cytokinin levels and senescence of tobacco leaves. Physiol. Plant. 84, 262–268
Smart, C.M., Scofield, S.R., Bevan, M.W., Dyer, T.A. (1991) Delayed leaf senescence in tobacco plants transformed with tmr, a gene for cytokinin production in Agrobacterium. Plant Cell 3, 647–656
Spanier, K., Schell, J., Schreier, P.H. (1989) A functional analysis of T- DNA gene 6b: The fine tuning of cytokinin effects on shoot development. Mol. Gen. Genet. 219, 209–216
Tinland, B., Fournier, P., Heckel, T., Otten, L. (1992) Expression of a chimaeric heat-shock-inducible Agrobacterium 6b oncogene in Nicotiana rustica. Plant Mol. Biol. 18, 921–930
Van Slogteren, G.M.S., Hoge, J.H., Hoogkaas, P.J.J., Schilperoort, R.A. (1983) Clonal analysis of heterogenous crown gall tumor tissues induced by wild-type and shooter mutant strains of Agrobacterium tumefaciens — expression of T-DNA genes. Plant Mol. Biol. 2, 321–333
Vankova, R., Kaminek, M., Eder, J., Vanek, T. (1987) Dynamics of production of trans-zeatin and trans-zeatin riboside by immobilized cytokinin-autonomous and cytokinin-dependent tobacco cells. J. Plant Growth Regul. 6, 147–157
Zhang, X.D. (1992) Biosynthesis, metabolism and function of cytokinin in transgenic and normal tobacco plants. Ph.D. thesis, Australian National University, Canberra, Australia
Author information
Authors and Affiliations
Additional information
We wish to thank Dr. J. Zwar for supplying phenylurea derivitives.
Rights and permissions
About this article
Cite this article
Zhang, R., Zhang, X., Wang, J. et al. The effect of auxin on cytokinin levels and metabolism in transgenic tobacco tissue expressing an ipt gene. Planta 196, 84–94 (1995). https://doi.org/10.1007/BF00193221
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00193221