Abstract
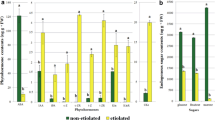
Cotyledons of peach (Prunus persica L. Batsch cv. ZiseMay®) were cultured in vitro on medium deprived of plant growth regulators. Two different lines varying in their embryogenic capacity were studied after 90 days in culture media. Endogenous levels of abscisic acid (ABA), indole-3-acetic acid (IAA), trans-zeatin (Z), trans-zeatin riboside (ZR), the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC), salicylic acid (SA), and jasmonic acid (JA) were analyzed in embryogenic and non-embryogenic cotyledons. No significant differences were observed in total ABA, IAA, ZR, SA and JA concentrations between the embryogenic and non-embryogenic cotyledons. On the contrary, lower Z and ACC contents, and also a reduced balance between Z and IAA levels were related with the embryogenic capacity of the cotyledons. These results suggest that the difference in somatic embryo formation capacity observed between embryogenic and non-embryogenic cotyledons is related to their endogenous Z contents, and that the endogenous hormonal balance between Z and IAA is an important index defining the embryogenic potential in peach cotyledons.



Similar content being viewed by others
References
Albacete A, Ghanem ME, Martínez-Andújar C, Acosta M, Sánchez-Bravo J, Martínez V, Lutts S, Dodd IC, Pérez-Alfocea F (2008) Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.) plants. J Exp Bot 59:4119–4131
Bhansali RR, Driver JA, Durzan DJ (1990) Rapid multiplication of adventitious somatic embryos in peach and nectarine by secondary embryogenesis. Plant Cell Rep 9:280–284
Bhansali RR, Driver JA, Durzan DJ (1991) Adventitious embryogenesis and plant regeneration from rescued embryos of peach, Prunus persica (L.). Indian J Exp Biol 29:334–337
Buddendorf-Joosten JMC, Woltering EJ (1994) Component of the gaseous environment and their effects on plant growth and development in vitro. Plant Growth Regul 15:1–16
Centeno ML, Rodríguez R, Berros B, Rodríguez A (1997) Endogenous hormonal content and somatic embryogenesis capacity of Corylus avellana L. cotyledons. Plant Cell Rep 17:139–144
Dobrev PI, Kaminek M (2002) Fast and efficient separation of cytokinins from auxin and abscisic acid and their purification using mixed-mode solid-phase extraction. J Chromatogr 950:21–29
Dudits D, Gyorgyey J, Bögre L, Bako L (1995) Molecular biology of somatic embryogenesis. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer, Dordrecht, pp 264–309
Etienne H, Sotta B, Montoro P, Miginiac E, Carron MP (1993) Relation between exogenous growth regulators and endogenous indole-3-acetic acid and abscisic acid in the expression of somatic embryogenesis in Hevea brasiliensis (Mull. Arg). Plant Sci 88:91–96
George EF (1993a) Auxin-cytokinin interaction. In: Plant propagation by tissue culture. Part 1. The technology, 2nd edn. Exegetics Limited, England, pp 445–446
George EF (1993b) Plant growth regulators. In: Plant propagation by tissue culture. Part 1. The technology, 2nd edn. Exegetics Limited, England, pp 420–479
Guiderdoni E, Mérot B, Eksomtramge T, Paulet F, Feldmann P, Glaszmann JC (1995) Somatic embryogenesis in sugarcane (Saccharum species). In: Bajaj YPS (ed) Somatic embryogenesis and synthesis seed I. Biotechnology in agriculture and forestry, vol 31. Springer, Berlin, pp 92–113
Hammerschlag FA, Bauchan G, Scorza R (1985) Regeneration of peach plants from callus derived from immature embryos. Theor Appl Genet 70:248–251
Huang XL, Li XJ, Li Y, Huang LZ (2001) The effect of AOA on ethylene and polyamide metabolism during early phases of somatic embryogenesis in Medicago sativa. Physiol Plant 113:424–429
Jiménez VM (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regul 47:91–110
Jiménez VM, Bangerth F (2000) Relationship between endogenous hormone levels in grapevine callus cultures and their morphogenetic behavior. Vitis 39:151–157
Kępczyńska E, Ruduś I, Kępczyński J (2009) Endogenous ethylene in indirect somatic embryogenesis of Medicago sativa L. Plant Growth Regul 59:63–73
Lakshmanan P, Taji A (2000) Somatic embryogenesis in leguminous plants. Plant Biol 2:136–148
Liang WS, Wen JQ, Liang HG (1997) Stimulation of ethylene production in aged potato tuber slices by salicylic acid. Phytochem 44:221–223
Lloyd G, McCown B (1980) Commercially-feasible micropropagation of mountain laurel, Kalmia latifolia, by use of shoot tip culture. Proc Int Plant Prop Soc 30:421–427
Meng X, Zhou W (1981) Induction of embryoid and production of plantlets in vitro from endosperm of peach. Acta Agric Univ Peking 7:95–98
Molassiotis A, Diamantidis G, Therios I, Dimassi K (2005) Effects of salicylic acid on ethylene induction and antioxidant activity in peach rootstock regenerants. Biol Plant 49(4):609–613
Nagaty MA (2012) Establishment of regeneration system for Taif peach (Prunus persica L. Batsch) cultivar (balady cultivar) in Taif, KSA. J Am Sci 8(4):232–239
Niessen P (1994) Stimulation of somatic embryogenesis in carrot by ethylene–effects of modulators of ethylene biosynthesis and action. Physiol Plant 92:397–403
Padilla IMG, Golis A, Gentile A, Damiano C, Scorza R (2006) Evaluation of transformation in peach Prunus persica explants using green fluorescent protein (GFP) and beta-glucuronidase (GUS) reporter genes. Plant Cell, Tissue Organ Cult 84:309–314
Pérez-Clemente R, Pérez-Sanjuán A, García-Férriz L, Beltrán JP, Cañas LA (2004) Transgenic peach plants (Prunus persica L.) produced by genetic transformation of embryo sections using the green fluorescent protein (GFP) as an in vivo marker. Mol Breed 14:419–427
Piaggesi A, Perata P, Vitagliano C, Alpi A (1991) Level of abscisic acid in integuments, nucellus, endosperm, and embryo of peach seeds (Prunus persica L. cv. Springcrest) during development. Plant Physiol 97:793–797
Pooler MR, Scorza R (1995) Regeneration of peach (Prunus persica (L.) Batsch) rootstock cultivars from cotyledons of mature stored seed. Hortscience 30(2):355–356
Prewein C, Vagner M, Wilhelm E (2004) Changes in water status and proline and abscisic acid concentrations in developing somatic embryos of pedunculate oak (Quercus robur) during maturation and germination. Tree Physiol 24:1251–1257
Reidiboym-Talleux L, Diemer F, Sourdiox M, Chapelain K, Grenier-De March G (1999) Improvement of somatic embryogenesis in wild cherry (Prunus avium). Effect of maltose and ABA supplements. Plant Cell, Tissue Organ Cult 55:199–209
Ruduś I, Kępczyńska E, Kępczyński J (2006) Comparative efficacy of abscisic acid and methyl jasmonate for indirect somatic embryogenesis in Medicago sativa L. Plant Growth Regul 48:1–11
Ruduś I, Kępczyńska E, Weiler EW (2009) Do stress-related phytohormones, abscisic acid and jasmonic acid play a role in the regulation of Medicago sativa L. somatic embryogenesis? Plant Growth Regul 59:63–73
Sáez L, Azpeitia A, Oropeza C, Jones LH, Fuchsova K, Spichal L, Strnad M (2010) Endogenous cytokinins in Cocos nucifera L. in vitro cultures obtained from plumular explants. Plant Cell Rep 29:1227–1234
Schneider KE, Speranzani D, Riggs AR (1992) Ontogeny of shoot regenerants on excised immature peach embryos. Can J Plant Sci 72:497–506
Scorza R, Morgens PH, Cordts JM, Mantem S, Callahan AM (1990) Agrobacterium-mediated transformation of peach (Prunus persica L. Batch) leaf segments, immature embryos and long term embryogenic callus. In Vitro Cell Dev Biol Plant 26:829–834
Smigocki AC, Freddi A, Hammerschlag A (1991) Regeneration of plants from peach embryo cells infected with a shooty mutant strain of Agrobacterium. J Am Soc Hort Sci 116(6):1092–1097
Srivastava LM (2001) Apical dominance and some other phenomena illustrating correlative effects of hormones. Section II. Auxins and embryo development. In: Plant growth and development. Hormones and environment. London Academic Press, pp 305–307
Svircev AM, Biggs AR, Miles NW (1993) Peach regeneration from callus derived from embryos of selected cultivars. Fruit Var J 47(1):13–16
Trewavas A (1981) How do plant growth substances work? Plant, Cell Environ 4:203–228
Ye X, Brown SK, Scorza R, Cordts JM, Sanford JC (1994) Genetic transformation of peach tissues by particle bombardment. J Am Soc Hort Sci 119:367–373
Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5:1411–1423
Acknowledgments
The authors gratefully acknowledge Dr Francisco Pérez Alfocea for his thorough review and suggestions to improve the manuscript and his generosity. This report was supported by the Instituto Nacional de Investigaciones Agrarias (INIA) (RTA2008-00121-00-00) and by a fellowship also provided by INIA to Margarita Pérez-Jiménez.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pérez-Jiménez, M., Cantero-Navarro, E., Acosta, M. et al. Relationships between endogenous hormonal content and direct somatic embryogenesis in Prunus persica L. Batsch cotyledons. Plant Growth Regul 71, 219–224 (2013). https://doi.org/10.1007/s10725-013-9822-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-013-9822-7