Abstract
Legumes are recalcitrant to androgenesis and induction protocols were only recently developed for pea (Pisum sativum L.) and chickpea (Cicer arietinum L.), albeit with low regeneration frequencies. Androgenesis is thought to be mediated through abscisic acid (ABA) but other phytohormones, such as auxins, cytokinins, and gibberellins, have also been implicated. In view of improving induction protocols, the hormone content of pea, chickpea, and lentil anthers was measured after exposure to cold, centrifugation, electroporation, sonication, osmotic shock, or various combinations thereof using an analytical mass spectrometer. Indole-3-acetic acid (IAA) had a key function during the induction process. In pea, high concentrations of IAA-asparagine (IAA-Asp), a putative IAA metabolite, accumulated during the application of the different stresses. In chickpea, the IAA-Asp concentration increased 30-fold compared to pea but only during the osmotic shock treatment and likely as a result of the presence of exogenous IAA in the medium. In contrast, no treatment in lentil (Lens culinaris) invoked such an increase in IAA-Asp content. Of the various cytokinins monitored, only cis zeatin riboside increased after centrifugation and electroporation in pea and possibly chickpea. No bioactive gibberellins were detected in any species investigated, indicating that this hormone group is likely not linked to androgenesis in legumes. In contrast to the other stresses, osmotic shock treatment caused a reduction in the levels of all hormones analyzed, with the exception of IAA-Asp in chickpea. A short period of low hormone content might be a necessary transition phase for androgenesis induction of legumes.
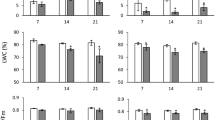
Key message Five androgenesis-inducing stress treatments changed content of ABA, auxin and cytokinin in anthers of three legumes. Osmotic shock treatment differed because it reduced hormone content to very low levels.
Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- ABA-GE:
-
ABA-glucose ester
- 7′OH-ABA:
-
7′-Hydroxy ABA
- BAP:
-
Benzyl amino purine
- C:
-
Centrifugation
- 4-Cl-IAA:
-
4-Chloroindole-3-acetic acid
- 2,4-D:
-
2,4-Dichlorophenoxyacetic acid
- DH:
-
Doubled-haploid
- DPA:
-
Dihydrophaseic acid
- E:
-
Electroporation
- GA1–53 :
-
Gibberellins 1–53
- IAA:
-
Indole-3-acetic acid
- IAA-Ala:
-
N-(Indole-3-yl-acetyl)-alanine
- IAA-Asp:
-
N-(Indole-3-yl-acetyl)-aspartic acid
- IAA-Glu:
-
N-(Indole-3-yl-acetyl)-glutamic acid
- IAA-Leu:
-
N-(Indole-3-yl-acetyl)-leucine
- IBA:
-
Indole-3-butyric acid
- 2iP:
-
Isopentenyladenine
- iPA:
-
Isopentenyladenosine
- NAA:
-
1-Naphthaleneacetic acid
- O:
-
Osmotic shock
- PA:
-
Phaseic acid
- S:
-
Sonication
- Z:
-
Zeatin
- ZOG:
-
Zeatin-O-glucoside
- dhZ:
-
Dihydro-zeatin
- ZR:
-
Zeatin riboside
References
Abrams SR, Nelson K, Ambrose SJ (2003) Deuterated abscisic acid analogs for mass spectrometry and metabolism studies. J Label Compd Radiopharmaceut 46:273–283
Alabadi D, Blázquez MA, Carbonell J, Ferrándiz C, Pérez-Amador MA (2009) Instructive roles for hormones in plant development. Int J Dev Biol 53:1597–1608
Aloni R, Aloni E, Langhans M, Ullrich CI (2006) Role of auxin in regulating Arabidosis flower development. Planta 223:315–328
Appleford NEJ, Evans DJ, Lenton PG, Croker SJ, Devos KM, Phillips AL, Hedden P (2006) Function and transcript analysis of gibberellin-biosynthetic enzymes in wheat. Planta 223:568–582
Böttcher C, Keyzers RA, Boss PK, Davies C (2010) Sequestration of auxin by the indole-3-acetic acid-amido synthetase GH3-1 in grape berry (Vitis vinifera L.) and the proposed role of auxin conjugation during ripening. J Exp Bot 61:3615–3625
Brugiere N, Jiao S, Hantke S, Zinselmeier C, Roessler JA, Niu X, Jones RJ, Habben JE (2003) Cytokinin oxidase gene expression in maize is localized to the vasculature, and is induced by cytokinins, abscisic acid, and abiotic stress. Plant Physiol 132:1228–1240
Chiwocha SDS, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross ARS, Kermode AR (2003) A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: analysis of hormone regulation of thermodormancy of lettuce (Lactuca sativa L.) seeds. Plant J 35:405–417
Chiwocha SDS, Cutler AJ, Abrams SR, Ambrose SJ, Yang J, Ross ARS, Kermode AR (2005) The etr1-2 mutation in Arabidopsis thaliana affects the abscisic acid, auxin, cytokinin and gibberellin metabolic pathways during maintenance of seed dormancy, moist-chilling and germination. Plant J 42:35–48
Croser JS, Lulsdorf MM, Davies PA, Clarke HJ, Bayliss KL, Mallikarjuna N, Siddique KHM (2006) Toward doubled haploid production in the Fabaceae: progress, constraints, and opportunities. Crit Rev Plant Sci 25:139–157
DeMason DA, Polowick PL (2009) Patterns of DR5:GUS expression in organs of pea (Pisum sativum). Int J Plant Sci 170:1–11
Dorcey E, Urbez C, Blazquez MA, Carbonell J, Perez-Amador MA (2009) Fertilization-dependent auxin response in ovules triggers fruit development through the modulation of gibberellin metabolism in Arabidopsis. Plant J 58:318–332
Dunwell JM, Thurling N (1985) Role of sucrose in microspore embryo production in Brassica napus ssp. oleifera. J Exp Bot 36:1478–1491
Emery RJN, Leport L, Barton JE, Turner NC, Atkins CA (1998) cis-Isomers of cytokinins predominate in chickpea seeds throughout their development. Plant Physiol 117:1515–1523
Engvild KC, Egsgaard H, Larsen E (1981) Determination of 4-chloroindoleacetic acid methyl ester in Viciae species by gas chromatography–mass spectrometry. Physiol Plant 53:79–81
Ferrie AMR, Palmer CE, Keller WA (1995) Haploid embryogenesis. In: Thorpe TA (ed) In vitro embryogenesis in plants. Kluwer, Dordrecht, pp 309–344
Ferrie AMR, Taylor DC, MacKenzie SL, Keller WA (1999) Microspore embryogenesis of high sn-2 erucic acid Brassica oleracea germplasm. Plant Cell Tiss Organ Cult 57:79–84
Fizzell LA (1988) Biological effects of acoustic cavitation. In: Suslick KS (ed) Ultrasound. Its chemical, physical and biological effects. VCH, Weinheim, pp 287–304
Fleet CM, Sun T (2005) A delicate balance: the role of gibberellin in plant morphogenesis. Curr Opin Plant Biol 8:77–85
Gao Y, Li T, Liu Y, Ren C, Zhao Y, Wang M (2010) Isolation and characterization of gene encoding G protein α subunit protein responsive to plant hormones and abiotic stresses in Brassica napus. Mol Biol Rep 37:3957–3965
Ghanashyam C, Jain M (2009) Role of auxin-responsive genes in biotic stress responses. Plant Signal Behav 4:846–848
Grewal RK, Lulsdorf M, Croser J, Ochatt S, Vandenberg A, Warkentin TD (2009) Doubled-haploid production in chickpea (Cicer arietinum L.): role of stress treatments. Plant Cell Rep 28:1289–1299
Hirano K, Aya K, Hobo T, Sakakibara H, Kojima M, Shim RA, Hasegawa Y, Ueguchi-Tanaka M, Matsuoka M (2008) Comprehensive transcriptome analysis of phytohormone biosynthesis and signaling genes in microspore/pollen and tapetum of rice. Plant Cell Physiol 49:1429–1450
Hoekstra S, van Zijderveld MH, Heidekamp F, van der Mark F (1993) Microspore culture of Hordeum vulgare L.: the influence of density and osmolality. Plant Cell Rep 2:661–665
Imamura J, Harada H (1980) Effects of abscisic acid and water stress on the embryo and plantlet production in anther culture of Nicotiana tabacum cv Samsun. Zeitschrift Pflanzenphysiol 100:285–289
Jain M, Khurana JP (2009) Transcript profiling reveals diverse roles of auxin-responsive genes during reproductive development and abiotic stress in rice. FEBS J 276:3148–3162
Jardinaud MF, Souvre A, Alibert G (1993) Transient GUS gene expression in Brassica napus electroporated microspores. Plant Sci 93:177–184
Joersbo M, Brunstedt J (1992) Sonication: a new method for gene transfer to plants. Physiol Plant 85:230–234
Kaltchuk-Santos E, Mariath JE, Mundstock E, Hu C, Bodanese-Zanettini MH (1997) Cytological analysis of early microspore divisions and embryo formation in cultured soybean anthers. Plant Cell Tiss Organ Cult 49:107–115
Kikuchi A, Sanuki N, Higashi K, Koshiba T, Kamada H (2006) Abscisic acid and stress treatment are essential for the acquisition of embryogenic competence by carrot somatic cells. Planta 223:637–645
Laurain D, Tremouillaux-Guiller J, Chenieux JC (1993) Embryogenesis from micropsores of Gingko biloba L., a medicinal woody species. Plant Cell Rep 12:501–505
Lionneton E, Beuret W, Delaitre C, Ochatt S, Rancillac M (2001) Improved microspore culture and doubled haploid plant regeneration in the brown condiment mustard (Brassica juncea). Plant Cell Rep 20:126–130
Maraschin SF, de Priester W, Spaink HP, Wang M (2005) Androgenic switch: an example of plant embryogenesis from the male gametophyte perspective. J Exp Bot 56:1711–1726
Maraschin SF, Caspers M, Potokina E, Wulfert F, Graner A, Spaink HP, Wang M (2006) cDNA array analysis of stress-induced gene expression in barley androgenesis. Physiol Plant 127:535–550
Normanly J, Slovin JP, Cohen JD (1995) Rethinking auxin biosynthesis and metabolism. Plant Physiol 107:323–329
Ochatt S, Chand P, Rech EL, Davey MR, Power JB (1988) Electroporation-mediated improvement of plant regeneration from Colt cherry (Prunus avium × pseudocerasus) protoplast. Plant Sci 54:165–169
Ochatt S, Pech C, Grewal R, Conreux C, Lulsdorf M, Jacas L (2009) Abiotic stress enhances androgenesis from isolated microspores of some legume species (Fabaceae). J Plant Physiol 166:1314–1328
Oetiker JH, Aeschbacher G (1997) Temperature-sensitive plant cells with shunted indole-3-acetic acid conjugation. Plant Physiol 114:1385–1395
Ostrowski M, Jakubowska A (2011) Purification and biochemical characterization of indole-3-acetyl-aspartic acid synthetase from immature seeds of pea (Pisum sativum). J Plant Growth Regul 30:30–40
Park J-E, Park J-Y, Kim Y-S, Staswick PE, Jeon J, Yun J, Kim S-Y, Kim J, Lee Y-H, Park C-M (2007) GH3-mediated auxin homeostasis links growth regulation with stress adaptation response in Arabidopsis. J Biol Chem 282(13):10036–10046
Park S, Ozga JA, Cohen JD, Reinecke DM (2010) Evidence of 4-Cl-IAA and IAA bound to proteins in pea fruit. J Plant Growth Regul 29:184–193
Quesnelle E, Emery RJN (2007) Cis-cytokinins that predominate in Pisum sativum during early embryogenesis will accelerate embryo growth in vitro. Can J Bot 85:91–103
Rashid A, Street HE (1974) Segmentations in microspores of Nicotiana tabacum which lead to embryoid formation in anther cultures. Protoplasma 80:323–324
Rech EL, Ochatt S, Chand P, Power JB, Davey MR (1987) Electro-enhancement of division of plant protoplast-derived cells. Protoplasma 141:169–176
Reinecke DM (1999) 4-Chloroindole-3-acetic acid and plant growth. Plant Growth Regul 27:3–13
Ribalta FM, Croser JS, Ochatt SJ (2012) Flow cytometry enables identification of sporophytic eliciting stress treatments in gametic cells. J Plant Physiol 169:104–110
Ross ARS, Ambrose SJ, Cutler AJ, Feurtado JA, Kermode AR, Nelson K, Zhou R, Abrams SR (2004) Determination of endogenous and supplied deuterated abscisic acid in plant tissues by high performance liquid chromatography-electrospray ionization tandem mass spectrometry with multiple reaction monitoring. Anal Biochem 329:324–333
Sáenz L, Azpeitia A, Oropeza C, Jones LH, Fuchsova K, Spichal L, Strnad M (2010) Endogenous cytokinins in Cocos nucifera L. in vitro cultures obtained from plumular explants. Plant Cell Rep 29:1227–1234
Sakakibara H (2006) Cytokinins: activity, biosynthesis, and translocation. Ann Rev Plant Biol 57:431–449
Sangwan-Norreel BS (1977) Androgenic stimulating factors in anther and isolated pollen grain culture of Datura innoxia Mill. J Exp Bot 28:843–852
Sasaki K, Shimomura K, Kamada H, Harada H (1994) IAA metabolism in embryogenic and non-embryogenic carrot cells. Plant Cell Physiol 35(8):1159–1164
Segui-Simarro JM, Nuez F (2008) How microspores transform into haploid embryos: changes associated with embryogenesis induction and microspore-derived embryogenesis. Physiol Plant 134:1–12
Shariatpanahi ME, Bal U, Heberle-Bors E, Touraev A (2006) Stresses applied for the re-programming of plant microspores towards in vitro embryogenesis. Physiol Plant 127:519–534
Simon S, Petrášek J (2011) Why plants need more than one type of auxin. Plant Sci 180:454–460
Smykal P (2000) Pollen embryogenesis—the stress mediated switch from gametophytic to sporophytic development. Current status and future prospects. Biol Plant 4:481–489
Swain SM, Singh DP (2005) Tall tales from sly dwarves: novel functions of gibberellins in plant development. Trends Plant Sci 10:123–129
Tanaka M (1973) The effect of centrifugal treatment on the emergence of plantlets from cultured anthers of tobacco. Jpn J Breed 23:171–174
Tret’yakova IN, Ivanitskaya AC, Ivanova AN, Barsukova AV (2009) Phytohormone content in microstrobiles and androgenetic callus of Siberian larch. Russ J Plant Physiol 56:647–653
Tromas A, Perrot-Rechenmann C (2010) Recent progress in auxin biology. Comptes Rendus Biol 333:297–306
van Bergen S, Kottenhagen MJ, van der Meulen RM, Wang M (1999) The role of abscisic acid in induction of androgenesis: a comparative study between Hordeum vulgare L. cvs. Igri and Digger. J Plant Growth Regul 18:135–143
Wang M, Hoekstra S, van Bergen S, Lamers GE, Oppedijk BJ, van der Heijden MW, de Priester W, Schilperoort RA (1999) Apoptosis in developing anthers and the role of ABA in this process during androgenesis in Hordeum vulgare L. Plant Mol Biol 39:489–501
Wedzony M, Forster BP, Żur I, Golemiec E, Szechynska-Hebda M, Dubas E, Gotebiowska G (2009) Progress in doubled haploid technology in higher plants. In: Touraev A, Forster BP, Jain SM (eds) Advances in haploid production in higher plants. Springer Science, New York, pp 1–33
Werner T, Schmülling T (2009) Cytokinin action in plant development. Curr Opin Plant Biol 12:527–538
Zaharia LI, Galka MM, Ambrose SJ, Abrams SR (2005) Preparation of deuterated abscisic acid metabolites for use in mass spectrometry and feeding studies. J Labelled Compd Radiopharmaceut 48:435–445
Zaki MAM, Dickinson HG (1990) Structural changes during the first divisions of embryos resulting from anther and free microspore culture in Brassica napus. Protoplasma 56:149–162
Zavattieri MA, Frederico AM, Lima M, Sabino R, Arnholdt-Schmitt B (2010) Induction of somatic embryogenesis as an example of stress-related plant reactions. Electron J Biotechn 13(1). ISSN 0717-3458. http://www.ejbiotechnology.cl/content/vol13/issue1/full/4/index.html
Zhao Y (2010) Auxin biosynthesis and its role in plant development. Ann Rev Plant Biol 61:49–64
Żur I, Dubas E, Golemiec E, Szechyńska-Hebda M, Janowiak F, Wędzony M (2008) Stress-induced changes important for effective androgenic induction of isolated microspore culture of triticale (× Triticosecale Wittm.). Plant Cell Tiss Organ Cult 94:319–328
Acknowledgments
Financial support for this project was provided by the Saskatchewan Pulse Growers Association. We thank T. Ament, J. Chow, R. Grewal, S.B. Mudiyanselage, S. Tschirren, and J. Walsh for their endless hours of dissection. We also thank V. Cekic and M. Lafond (Plant Biotechnology Institute) for hormone profiling sample preparation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Q. Zhao.
Rights and permissions
About this article
Cite this article
Lulsdorf, M., Yuan, H.Y., Slater, S. et al. Androgenesis-inducing stress treatments change phytohormone levels in anthers of three legume species (Fabaceae). Plant Cell Rep 31, 1255–1267 (2012). https://doi.org/10.1007/s00299-012-1246-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-012-1246-8