Abstract
The use of materials of natural origin for the adsorption of heavy metal ions from aqueous solutions has gained attention in recent years among the scientific community. This is explained by the fact that nickel compounds, due to severe health consequences, are considered to be among the most dangerous to the environment. This article reviews the results of studies on the use of biosorbents for purification of aqueous solutions from nickel ions, and then attempts to classify them according to their origin. The characteristics of materials and their sorption capacity have been compared, and the removal mechanisms identified of which chemisorption and ion exchange are considered to be the most common. From the analyses, a major trend is the use of biomass; however, biosorbents from other groups also continue to attract the interest of researchers. Conducting laboratory studies can help select materials with high efficiency. The highest sorption capacity values for the materials in each group were: for waste products 56 mg Ni·g−1 (olive stone), for peat 61 mg Ni·g−1, for miscellaneous 225 mg Ni·g−1 (microbial flocculant GA1), for biomass 286 mg Ni·g−1 (Plantanus orientalis bark) and for composites/modified materials calcinated eggshells 769 mg Ni·g−1 (calcinated eggshells). However, for some materials the sorption phenomenon may be accompanied by precipitation in the presence of hydroxides, which significantly affects the sorption capacity achieved. There is a need to transfer these experiments to an industrial scale so as to verify their applicability. In such industrial scale applications, attention should be paid not only to the effectiveness of the material, but also to its availability, price, and ease of use, as well as the effect of the biosorbent in terms of changing the quality parameters of the aquatic environment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
With the advance of civilization, human activities and progressive industrialization, the level of nickel pollution is intensifying. This phenomenon is particularly evident in surface water, soil water, and shallow groundwater. Water pollution is of particular importance due to the role played by water in ecosystem services (Liu et al. 2021).
Nickel can originate from both natural sources and anthropogenic activities. It is the 6th most abundant element by mass in the Earth's crust after iron, oxygen, silicon, magnesium, and sulfur. Due to toxicity and carcinogenicity, nickel compounds are considered to be among the most hazardous to the environment. Many studies have proven that long and constant exposure to this metal can cause skin allergies lung fibrosis, cardiovascular disease, respiratory tract cancer, and asthma (Tchounwou et al. 2012; Jan et al. 2015; Uter et al. 2018). In combination with oxygen and sulfur as oxides and sulfides, nickel may be naturally emitted from volcanoes. An important source of nickel pollution to the environment is its emission to the atmosphere as a result of the combustion process of coal and liquid fuels. Thanks to its peculiar physicochemical properties (resistance to very high temperatures, oxidation and corrosion, big ductile and readily in processing), nickel is used in many industrial sectors, including the paper industry, steel mills, refineries, and fertilizer factories. Increased nickel ion concentrations are also noticed as a result of the production of galvanic coatings and pickling as well as the electropolishing of stainless steels, electroforming and production of nickel–cadmium batteries and electronic equipment (Lochynski et al. 2016; Lochyński et al. 2019). This is particularly important issue because nickel is readily soluble at pH < 6.5 in dilute oxidizing acids. This pH is often found in effluent from this kind of industrial. Moreover, nickel salts of strong acids (chloride, nitrate and sulphate) and organic salts are easily water-soluble. In terms of chemical speciation, the main form of nickel is Ni(II) form. The divalent nickel forms strong complexes with organic ligands such as carboxylates, fulvates and humates. Nickel forms soluble chloride, sulphate and nitrate salts, whereas nickel hydroxides, sulphides, arsenides and silicates are almost insoluble. In contrast, nickel oxide is soluble only under acidic conditions. Due to the chemical similarity between Ni2+ ions and Fe2+ and Co2+ ions, they can be substituted by nickel in many compounds. The widespread industrial use and peculiarities of nickel means that significant amounts of this metal can be found in aquatic environments. From these sources, and along with other metals, nickel gets dispersed into the environment and directly or through plants enters animals and the human body (Harasim and Filipek 2015; Das et al. 2019). Nickel is a metal that bioaccumulates easily. It is observed not only in water and wastewater but also in sewage sludge (Tytła 2019). The bioavailability of nickel in the form of hydrated nickel(II) contributes to its toxic effects, hence its removal from aqueous solutions is essential.
The growing public awareness in recent years as to the contamination of the environment with heavy metals of anthropogenic origin is reflected in the tightening of legal regulations in the European Union and individual countries in terms of permissible concentrations of emissions of heavy metals and other hazardous substances into the environment (The European Parliament and the Council of the European Union 2020). According to WHO guidelines, the maximum allowable concentration of Ni(II) in drinking water is a mere 0.02 mg·L−1. Therefore, removal of Ni(II) from water and wastewater to reduce its concentration to anacceptable range is an absolute necessity. The need to take action in this area in support of the European Green Deal and Sustainable Development Goals, especially SDG 6: "Ensure the availability and sustainable management of water and sanitation for all", is just as important. The implementation of the provisions of the above-mentioned documents involves an intensification of the search for alternative solutions to wastewater and water treatment, reduction of generated waste, or the use of readily available natural materials (Paweska and Bawiec 2017; Domańska et al. 2019; Garbowski et al. 2020; Burszta-Adamiak and Spychalski 2021). Because of the significant problem posed by heavy metals to the environment and humans, this issue has been intensively studied in recent years.
To date, many methods have been developed to remove metals from aqueous solutions. In wastewater treatment processes, chemical and electrochemical precipitation, ion exchange, membrane filtration, reverse osmosis, electrolysis and solvent extraction, among others, have been used to remove heavy metals (Kurniawan et al. 2006; Bodzek and Konieczny 2011; Fu and Wang 2011; Raval et al. 2016). In addition, various combination methods like complexation/ultrafiltration, complexation/membrane filtration, coagulation/flocculation, electrochemical reduction/oxidation, electroflocculation/filtration hybridization have recently been used (Yaqub and Lee 2019; Zhang et al. 2019). Despite the many advantages and treatment efficiencies achieved, the main disadvantage of these processes is the production of secondary chemical or biological sludge which requires additional treatment. Some of these methods are not economically beneficial. In addition, commercial ionites are most often products derived from a non-renewable energy source (from crude oil processing), and once used they are difficult to dispose. For these reasons, in recent years, one method of water and wastewater treatment that is gaining attentionis adsorption. Commercial materials such as activated carbon or synthetic resins are very effective adsorbents due to their structural characteristics. Reusability and availability from renewable sources are other advantages highlighted in a recent study (Ani et al. 2020). However, its wide application is limited due to the high capital and regeneration cost (Erto et al. 2013). This situation has encouraged researchers to search for alternative adsorbents. The development of biodegradable adsorbents that are low-cost, abundant in nature, and available in large quantities is currently an area of extensive research due to their potential applications in biosorption processes for the removal of nickel (II) ions from water and wastewater. Among them, natural materials like bacteria (Mardiyono et al. 2019), fungi biomass (Silah and Gül 2017), macroalgae (Eka Putri 2019), peat (Charazińska et al. 2021), old newspaper fibers (Ossman et al. 2016), lemon peel waste (Villen-Guzman et al. 2021), peanut hulls (Tapia et al. 2018), sugarcane (Rico et al. 2018) and palm bagasse (Candelaria et al. 2019), surface-engineered yeast (Li et al. 2019) have been developed and tested for the removal of nickel metal ions.
There is an ongoing need to identify and develop different, easily available yet highly effective adsorbents that are harmless to the environment for the efficient removal of heavy metal ions. However, the adsorption efficiency depends on the type of adsorbents, contact time, solution pH, etc. The large number of biosorbents studied and the broad variations in the results from analysis of their performance were among the reasons for the authors to undertake a review of the studies carried out in the last five years. The main goal of this paper is to review and assess current knowledge on the topic of using biosorbents in the aquatic environment. In the proposed review, the authors focused on the most recent achievements of researchers in the topic of nickel ion sorption. The analysis covered the last 5 years and a wide range of materials used. Based on the research results, the most effective materials for nickel removal were indicated, while future perspectives for conducting research were also determined. For the analyses, it was necessary to introduce our own classification of adsorbents so as to identify research trends in recent years.
2 Methodology
The study of recent trends in the area of nickel biosorption covered the last 5 years (I 2016-IV 2021). This study follows a process whereby publications indexed in the Web of Science and Scopus databases were collected and analyzed. Both databases used are among the largest and contain collections of the most reputable and influential journals, thereby providing a comprehensive overview of the global scientific output in engineering areas.
Due to the wide variety of nomenclature used in papers related to the topic of biosorption, the challenge was to select keywords that are specific enough to allow access to reliable research results, while being general enough not to omit publications relevant to the topic. The search strategy for scientific articles published in English included essential and additional terms. “Sorption”, "biosorption", "heavy metals", "nickel" or "Ni" were selected as essential terms. Additional terms were "organic material", “non-conventional adsorbents”, “waste material”, “plant”, “algae”, and “peat”. In the search criteria, the essential terms had to be included initially in the title or keywords of the article, followed by the essential and/or additional terms in the title, abstract or keywords. A preliminary list of over 1000 bibliographic records were gathered. Then, these records were checked, and the articles relevant to research into the use of natural materials for sorption of nickel ions were archived for further analysis. In this review, only journal articles were used because they present a higher quality and more comprehensive information than other types of publications. Finally, 150 scientific papers were selected for a deeper analysis, the results of which are presented in this article.
In this paper, an authored classification of adsorbents was developed, which enabled the analysis of diverse literature reports. The materials were divided into the following main groups: biomass, composites and modified materials, waste, peat, and miscellaneous. The last category included all other natural materials that could not be clearly classified into the other created groups. According to the definition provided by Hougton “Biomass refers to the mass of living organisms, including plants, animals, and microorganisms, or, from a biochemical perspective, cellulose, lignin, sugars, fats, and proteins. Biomass includes both the above- and belowground tissues of plants, for example, leaves, twigs, branches, boles, as well as roots of trees and rhizomes of grasses” (Houghton 2008). Due to the large variety and number of materials classified as biomass as well as the rather general nature of this term, it was decided to make this category more specific by dividing it further into algae, fruits, plants, organisms, and others.
3 Trends in biosorbent research
The first literature reports on the application of biosorption focused on metal removal (Tsezos and Volesky 1982; Volesky and Holan 1995) due to the fact that metals have been a major environmental problem as a result of human activities for decades (Tchounwou et al. 2012; Jaishankar et al. 2014; Fisher and Gupta 2020). For this reason, the development of techniques to remove them has become a priority. The results of ongoing research confirm that biosorption is a very effective, economical and environment-friendly removal technique (Paweska and Bawiec 2017; Garbowski et al. 2020b).
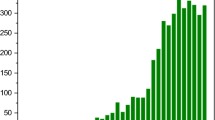
A review of scientific articles published in the last 5 years clearly indicates that the study of nickel sorption using materials of natural origin is of interest not only to academics and scientists, but also to industrial units due to the potential for applying this technique in various fields. The most frequently used materials for nickel removal are relatively cheap and naturally available. Figure 1 presents the cumulative number of papers on the topic of nickel ion sorption in the last 5 years (from beginning of 2016). In this period, there is a general increase of interest in the use of various materials of natural origin. The largest number of studies focused on the use of biomass, for which the number of studies was almost three times higher than for the other categories (composites/modified, waste, peat, miscellaneous). There were over 10 new publications that focused on the topic of nickel sorption by biomass every year, while for the other groups there were 2–7 publications per year. A more detailed analysis allowed us to divide the "biomass" group into subcategories, as illustrated in Fig. 1b). Plant materials are the dominant group in the entire body of publications on nickel sorption using biomass. The authors have used both whole plants (e.g. Lemna gibba (Morales-Barrera et al. 2020), Azolla filiculoides (Naghipour et al. 2018), Pistia stratiotes or Nymphaea lotus (Ugya et al. 2019)) and various plant fragments in their studies. For nickel sorption, both plant parts such as bark (Cancelo-González et al. 2017; Akar et al. 2019) or cones (Oguz 2020), and more fragmented parts such as fibers (Boudaoud et al. 2017), bagasse (Rico et al. 2018; Candelaria et al. 2019) and sawdust (Richard et al. 2020) were used. Researchers have also used algae (Vetrivel et al. 2017; Kipigroch 2018; El-Naggar and Rabei 2020) and fruits (Pertile et al. 2020) including their seeds (Aravind et al. 2017), shells (Cruz-Lopes et al. 2021) or peels (Priyantha and Kotabewatta 2019) to remove metals from aqueous solutions. The least amount of research was observed for the organisms category containing materials from invertebrates, insects, and their shells (Gürel and Güneş 2018; Foroutan et al. 2019; Marzuki et al. 2019; Złotko et al. 2021). Based on the trends observed in Fig. 1a) and b), it can be concluded that interest in biosorption with respect to metal ion removal is still an ongoing phenomenon, such that further development of research in this area can be expected in the coming years.
In several of these studies, the use of biomass as a sorbent is not limited to the removal of nickel ions, but of other metals as well (Table 1). In laboratory studies, the authors mostly used Ni(NO3)2 × 6H2O, NiCl2 × 6H2O, and NiSO4 × 6H2O to prepare nickel stock solutions. Apart from nickel, researchers have mostly undertaken the task of testing the possibility of removing cadmium and lead ions. These elements appear in many publications probably due to the significant harm they pose to both humans and the environment, rendering their effective removal an important issue. Other metals that have been studied include copper, chromium, zinc, cobalt, and arsenic which can pose a threat when present in excessive quantities.
4 Sorption capacity and removal percentage of nickel ions
In many papers, the authors reported high efficiencies of the tested materials, even exceeding 80%. However, considering the other process parameters, especially the initial concentrations of the solutions and the mass of the material used, the obtained removal percentage results cannot always be compared with each other due to the different initial conditions.
Sivakumar et al. (2018), using bamboo-activated carbon, showed a nickel ion removal percentage of 98.7%. Similar values were also achieved by Davarnejad et al. (2018) using regenerated cellulose and Nithya et al. (2017) doing experiments with acid-treated Lantana camara fruit. However, in analyzing these results, it is important to note the initial concentration, which varied considerably in each of the works mentioned. The reduction achieved for bamboo-activated carbon from an initial concentration of 99 mg·L−1 indicates that over 97 mg·L−1 of nickel was successfully removed from the solution. For the other materials, these values were much lower due to the lower initial concentrations used. For regenerated cellulose, more than 32 mg·L−1 of nickel was removed from the solution (starting concentration of 32.5 mg·L−1), and for acid-treated Lantana camara fruit 24.25 mg·L−1 (starting concentration of 25 mg·L−1). When analyzing these types of results, the weight of the material used must also be taken into account. In most of the studies, the authors observed an increase in effectiveness as the mass of the material increased, indicating that this parameter is extremely important. To illustrate the importance of the mass used, two materials with a similar removal percentage and initial concentration can be compared. For the aforementioned bamboo-activated carbon, a 98.7% reduction percentage was obtained using an initial concentration of 99 mg Ni·L−1 and 1.5 g·L−1 of material. For eggshells (Mashangwa et al. 2017) at an initial concentration of 100 mg·L−1, it was necessary to use a much larger material mass of 7 g to achieve a reduction in nickel concentration of 94%. The removal percentage of nickel ions for the selected materials is shown in Table 2.
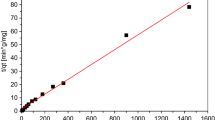
With such a variety of test results, and despite the high removal rate shown, it is difficult and sometimes impossible to compare the performance of different materials among themselves. In order to demonstrate the obtained results, a comparison of the nickel ion removal efficiency to the analyzed materials along with division into the adopted categories is presented in Fig. 2. The use of a uniform unit describing a given sorbent in terms of its sorption capacity qm (mg·g−1).Indicating the maximum amount of the adsorbate (heavy metal) per unit weight of the adsorbent allows even very different materials to be compared. Thanks to the results being presented in this way, within each category one can notice materials characterized by higher values of sorption capacity and those characterized by lower values.
The highest variability in the results was observed for the group composites/modified materials., For this group the values reached by the materials ranged from 1 to 769 mg Ni·g−1, containing natural materials subjected to modification, i.e. biochar produced from natural materials, copolymers or composites. The modifications of materials are aimed at increasing their efficiency. At the same time, there is one material in this group which showed the highest sorption capacity, several times exceeding even the other most effective materials. In their work, Kristianto et al. (2019) presented a method of eggshell powder modification to increase the efficiency of nickel ion removal. Dried chicken eggshells were subjected to calcination at 850 °C for 4 h using an electrical muffle furnace. This process resulted in changing CaCO3, present in the shells, into CaO while increasing the porosity of the material. The resulting calcined eggshell powder had a sorption capacity of 769.23 mg·g−1, which is 60 times higher than the material without the above modifications. The authors speculate that this large increase in sorption capacity may be due to the increased porosity of the material after the calcination process. The treatment of eggshells proposed by Kristianto et al. (2019) is found to be extremely effective when compared to other literature reports on the use of eggshells. However, the lower eggshell efficiencies described by the researchers were often associated with the use of material that had not been subjected to the calcination process as in the results presented by Kristianto et al. Vinegar-treated eggshell waste biomass was also used by Stevens and Batlokwa (2017) to remove nickel ions. For the tested wastewater with an initial concentration of 25 mg Ni·L−1 at a sorbent dosage of 81 mg and contact time of 73 min, the authors obtained a nickel(II) percentage removal of 79%. Mashangwa et al. (2017), using dried and ground chicken eggshells, determined an optimal sorption process parameters of pH 7, adsorbent dosage of 7 g, and contact time of 360 min (for the removal of 100 mg·L−1 metal ions). Using these parameters allowed for the removal of 97% lead, 95% copper, 94% nickel, and 80% zinc from the solution. Sankaran et al. (2020) investigating the sorption of zinc ions using calcined eggshells determined that the ions were adsorbed onto the surface of the material through electrostatic interactions and/or cation exchange process. CaCO3, constituting more than 90% of the eggshell composition, would undergo displacement reaction in aqueous media. The calcination process at high temperature (above 800 °C) changes CaCO3 into CaO. The shells are likely to dissolve and partially release Ca2+, CO32−, HCO3¬, and OH− ions, which can be adsorbed on the surface of the material rendering the negative charge properties (Zulfikar et al. 2012). At the same time calcium ions can be exchanged with other metal ions from the solution and thus can be adsorbed onto the negatively charged surface of the shells. The dissolution process of calcite in the eggshell can simultaneously increase the alkalinity of the solution, which together with the dependence of metal uptake on pH can significantly affect the sorption results achieved. Given the increased pH of the solution it is likely that in addition to sorption some metal ions are removed by heterogeneous and/or homogeneous precipitation of metals in the form of hydroxides, carbonates and/or hydroxycarbonate composites (Mashangawa et al. 2017). Another material within this group with high value of sorption capacity was novel flower globular magnesium hydroxide material (FGMH), which was prepared by way of gentle method using trisodium citrate as a crystal modifier (Jiang et al. 2019). The resulting material was characterized by a high surface area. Using optimal parameters (30 mg of FGMH, a 50 min contact time, pH values between 6.07 and 7.71 for the Ni(II) solution, and adsorption at room temperature for 50 mL of 80 mg·L−1 Ni(II) solution), the sorption capacity obtained was 287.11 mg·g−1. The analyses performed by the authors indicate that nickel ions were chemisorbed on the surface of the material.
Similar performance results were obtained by the best of the materials classified into groups of biomass or miscellaneous. For the biomass materials, the best was presented by Akar et al. (2019). The authors used Platanus orientalis bark with and without nitric acid activation as a biosorbent to remove Cr(VI) and Ni from nickel–chromium plating wastewater. Based on isotherm models, the maximum amount of nickel ions adsorbed was 126.58 mg·g−1 for material without activation. After acid activation with HNO3 maximum capacity increased, the value of qm was 285.7 mg Ni·g−1. Acid treatment increased the sorption capacity of the material twofold, but the results obtained for the material without treatment still rank among the best in this category.
For the group miscellaneous containing all other natural materials that could not be clearly classified into any of the created groups, the best results were obtained by microbial flocculant. The microbial flocculant GA1, used in Zhou et al. (2017) and obtained from the fermentation fluid of Paenibacillus polymyxa GA1, was the most effective in that group. This is an extracellular polymeric substance (EPS) with variable molecular weight and structural properties, containing many polysaccharides, several proteins, and nucleic acids. The researchers were able to achieve, under optimal conditions, a 99.35% removal rate for nickel ions, while the sorption capacity of the test material was 225.1 mg·g−1. Analysis of the process mechanism showed that bridging with precipitation enmeshment plays an important role in the flocculation and biosorption process.
Considering the best sorption capacity results for the groups, waste materials and peat had the lowest sorption capacity values. For peat, qm values oscillated within a range of 16–61 mg·g−1. Even modification or treatment of this material did not sufficiently increase its effectiveness for nickel ion removal. For NaOH and HCl-treated peat, Leiviskä et al. (2018) recorded sorption capacities of about 35 and 23 mg·g−1, respectively. At the same time, for peat without any modification, Bartczak et al. (2018) managed to achieve a value of 61.27 mg Ni·g−1.
In the case of waste materials, their undoubted advantage is the fact that they are easily accessible and cheap, while their further use in accordance with the principles of a closed-cycle economy, which is an alternative to utilization, positively affects the environment. Unfortunately, despite these many advantages, waste materials presented in the literature did not show a high sorption capacity. In this category, the best results were obtained using olive stone waste, which is waste emanating from an oil extraction plant. Bobadilla et al. (2020) obtained maximum sorption capacity for the analyzed material at the level of 56 mg·g−1. At the same time, they emphasized in their work that the proposed solutions may prove to be effective in the case of nickel ion removal from wastewater in a safe, economical and environmental-friendly manner, while allowing to solve the problem of olive stone waste accumulation.
5 Identification
Considering the great variety of materials used, their characterization in terms of physical properties as well as elemental composition is also presented in studies in a very diverse manner. Scanning electron microscopy (SEM) is a technique that is often used to study the surface morphology of a biosorbent before and after the biosorption of metal ions, particularly the changes in cell wall structure after metal ion binding. Moreover, the combination of SEM and EDX (energy dispersive X-ray) techniques provides valuable information on the distribution of different elements on the surface of biomass.
Guarín-Romero et al. (2019) imaged the biomass surface of Brown Algae Durvillaea Antarctica through SEM studies. In the raw state, an irregular and rough surface was observed with cavities providing space for interaction with ions in solution. In contrast, the biomass after sorption of Ni(II) and Cr(III) ions was characterized by the presence of homogeneous open cavities of about 20 μm in diameter. It was indicated that the interaction of the material surface with metal ions may cause a change in the structure and opening of the cavities. The analysis of EDX spectrum confirmed the increased presence of nickel and chromium after the biosorption process.
In the SEM images of Tectona grandis biochar, presented by Vilvanathan and Shanthakumar (2018), a clear pattern of the distribution of pores on the adsorbent surface can be observed. The material’s open, exposed and patterned pores can favorably accommodate the metal ions. The authors indicated that the pore-occupying particles visible after the sorption process could be Ni2+ and Co2+ ions, which was further confirmed by EDX analysis. The researchers concluded that Tectona grandis biochar has good adsorption potential with evenly distributed exposed pores, which can reduce mass transfer resistance and can facilitate metal ion diffusion, thereby improving the adsorption capacity of the adsorbent.To study the mechanisms of biosorption, it is necessary to identify the sorbent functional groups that are involved in this process. Fourier-transform infrared spectroscopy (FTIR) provides important information related to the nature of the bonds and allows for the identification of different functional groups in the cell wall structure. The shifts of bands in contaminant-loaded biomass compared to the natural state indicate the degree of interaction between functional groups and a given contaminant (Murphy et al. 2007). There are several characteristic areas observed in the FTIR spectra of biomaterials, but it should be kept in mind that due to the wide variety of these types of materials, there may be differences among them. Here are examples of stretching frequencies observed in the spectra. Bonded –OH groups were observed at 3130–3300 cm−1 (Sharma and Shukla 2021), and –OH and –NH 3370–3410 cm−1 (Sheng et al. 2004). At 2925 cm−1 authors noted asymmetric and at 2854 cm−1 symmetric stretching band of aliphatic chains (Pons et al. 2004). The absorbance peaks at 1738 cm−1 correspond to the C=O stretch of COOH, and at 1.630 cm−1 asymmetric stretching C=O (Fourest and Volesky 1996; Figueira et al. 1999). At 1550–1530 cm−1 N–H in plane (amide-II) (Ibarra et al. 1996; Zaccheo et al. 2002; Sheng et al. 2004), and at 1,450 cm−1: symmetric stretching C = O (Fourest and Volesky 1996). Authors reported also C–H deformations at 1450 cm−1, 1370 cm−1 (Parker 1971; Solomon et al. 2005), and combination of C–O stretching and O–H deformation at 1080–1030 cm−1 (Grube et al. 2006).
Changes in the adsorbent’s FTIR frequencies before and after adsorption can indicate which functional groups present are involved in the process. Aravind et al. (2015) observed that the peak absorbance in pigeon pea under biosorbent loaded with Ni(II) is subsequently lower compared to the starting material. The paper reported that the FTIR frequencies after the adsorption process were 3395 cm−1 for O–H stretch, 1631 cm−1 for C = O bond, 1473 cm−1 for C=C bond, 1424 cm−1 for C–O stretch, and 850 cm−1 for C–H bond. According to the authors, this indicates that in the presence of nickel, bond stretching occurs to a lesser extent and consequently peak absorption is attenuated. The main functional groups involved in effective nickel removal are attributed to the presence of C=O, C–O, O–H, and C-H bonds, which can coordinate with nickel. In another work on nickel sorption using Indian gooseberry seeds (Aravind et al. 2017), the authors observed a similar relationship of a decrease in the absorbance of peaks after the adsorption process. The peak shifts showed that the stretching of O–H group, bending of C–H groups, stretching of C–Br, C–N, C=O, C=C, and C–C bonds were responsible for the adsorption of Ni(II). Guarín-Romero et al. (2019) noted that the spectrum of Brown Algae biomass, consumed after the sorption process, revealed several changes in the intensity and frequency of some peaks which may suggest that these functional groups are involved in the interactions and adsorption mechanism. For example, the peak at 3510 cm−1 shifted to lower frequencies in both cases (Ni(II) and Cr(III) biosorption), suggesting that -OH and -NH groups are involved in the absorption of metal ions.
Several authors have complemented the characterisation of the materials tested with determination of the pH of point zero charge (pHpzc). For a mixture of three nonliving algae classes the authors determined that pHpzc was approximately 5.8, which indicates that beyond this pH value the surface of this biomass will be negatively charged (Mohammed et al. 2019). pH above 8 was excluded from effect study to prevent removal of Ni(II), by other mechanisms like precipitation. The maximum biosorption efficiency of 75% was observed at pH 7. Singh and Shukla (2017) observed that pHpzc of Citrus limetta peels was 4.85. Authors noted higher hydrogen ions sorption at pH < pHpzc as compared to Ni(II), but on the other hand at pH > pHpzc, the uptake of Ni(II) was higher. The maximum adsorption capacity was observed at pH 6, hence it was considered as optimum pH.
6 Effect of adsorption parameters
6.1 Initial pH
Solution pH is one of the important factors in the removal of heavy metals from aqueous solutions (Akpomie and Conradie 2020). Biosorption is mainly based on the ion exchange mechanism between metal ions and existing counter-ions on negatively charged ionic groups. The maximum adsorption capacity of the material can be reduced at low pH values owing to increased competition between protons and metal ions for binding sites. On the other hand, at high pH, metal ions can be precipitated in the form of hydroxides and this is a significant risk affecting the adsorption capacity. Based on nickel stability diagrams, it can be observed that in acidic, neutral and fairly basic solutions, at pH below 8.0, Ni2+ ions are the dominant form of nickel. When the pH exceeds 8, insoluble nickel(II) hydroxides precipitate (Beverskog and Puigdomenech 1997). Therefore, the reduction of Ni(II) concentration in the presence of an adsorbent at pH > 8 depends not only on its adsorption itself, but also on the chemical precipitation of Ni(OH)2. However, experimental results clearly indicate that acidic pH is not conducive to nickel ion adsorption, and maximum adsorption capacities are obtained by researchers at pH in the range of 4 to 8. In studies conducted by Cruz-Lopes et al. (2021), the pH range of 2.5 to 7.5 was investigated. Finally, the effect of pH value on nickel removal was clearly determined using four different biosorbents. It was found that the best nickel removal efficiencies were achieved at pH 5 for all biosorbents tested. The highest adsorption was obtained with chestnut shell, reaching 75% followed by walnut shell (71%), burnt wood (64%) and wood (45%). In another nickel, cadmium and lead biosorption study conducted using chitosan waste shrimp extract, the optimum pH for nickel removal from aqueous solutions was 7 (Marzuki et al. 2019). As the pH of the medium increases, the adsorption capacity of the biosorbent increases to a maximum at pH 7. Beyond this point, the adsorption capacity decreases. Guo et al. (2018) examined removal of Ni2+ and Cd2+ from wastewater using a green longan hull adsorbent. For the initial nickel ions concentrations of 50 mg·L−1 and the absorbent quantity of 0.5 g the maximum adsorption occurred at pH 4.5–5.5. The adsorption capacity of Ni2+ and Cd2+ increased in the pH range from 1 to 5. At pH above 7.0, the cadmium and nickel hydroxides became insoluble, and the adsorption capacity of metal ions decreased dramatically. Authors explained nickel adsorption capabilities at pH 1–5.5 by the fact that the metal ions compete with hydrogen ions for the binding sites on the surface of the dried longan hull. In addition, when the pH increases, there is an increase in the negative surface charge, which results in a lower electrostatic repulsion between the positively charged metal ions and the surface of the longan hull, which favors the adsorption process.
6.2 Initial metal concentration
The initial metal ion concentration is a key driving force for metal ions to overcome the mass transfer resistance between the aqueous and bulk phases. Therefore, the change in the initial nickel ion concentration is an important factor that should be investigated in the biosorption tests carried out. Aravind et al. (2017) examined the effect of initial Ni (II) concentration on the adsorption system involving gooseberry seed powder as biosorbent. Initial nickel ions concetrations ranged from 20 to100 mg·L−1. Authors observed that the increase in initial Ni (II) concentration decreased the removal efficiency of the adsorbent what may be attributed to the saturation of metal binding sites in the adsorbent. Nickel adsorption using yeast Saccharomyces cerevisiae was carried out by Zinicovscaia et al. (2021). Increase of the Ni(II) concentration from 10 to 100 mg·L−1 resulted in raising of its sorption on yeast biomass from 0.6 to 3.95 mg·g−1 in Ni(II) system. The authors concluded that at lower concentrations of metal ions in solution high metal biosorption is associated with the large number of the available sorption sites. At higher concentrations, the available sites of biosorption became fewer and saturation of the sorption sites is observed, resulting in the decrease of metal ion removal.
6.3 Temperature
The temperature of the sorption reaction is usually of little importance due to the fact that most reactions are exothermic and temperature independent. However, exceptions can also be found in the literature. In some cases, temperature can affect the interaction between metal ions and biomass.
For the adsorption of Ni(II) ions on Citrus Limettioides peel and seed carbon authors investigated correlation between temperature and the removal rate of Ni(II) ions (Sudha and Srinivasan 2015). In a batch adsorption studies were performed at different temperatures (27, 37, 47 °C) for the initial Ni(II) ions concentration of 10 mg·L−1 at constant adsorbent dose of 1 g·L−1 and an optimum pH value of 5 maximum removal was obtained at 27 °C. The percentage removal of Ni(II) ions decreases with the increase in temperature suggesting that the adsorption between Ni(II) and CLPC, CLSC is an exothermic process. Gupta et al. (2019) studied effect of temperature on sorption using A. barbadensis Miller waste leaves powder in the temperature range 20–60 °C at pH 7. Simmilar to other papers, it was found that uptake capacity decreased with an increase in temperature. Authors noted that the value of sorption rate constant slightly decreased between 40 and 60 °C and above 40° very much decreased. The observed relationship was associated with a reduced surface activity, suggesting that the process occurring is exothermic. This variation in nickel removal appears to be caused by a reduction in boundary layer thickness due to enhancement of relative escaping tendencies of the metal ions from aqueous phase to the bulk. Furthermore, the decrease in removal at increasing temperatures may also be attributed to the increased solubility of the metal at relatively higher temperatures. In the work presented by Aranda-García et al. (2018), the effect of temperature on Ni(II) biosorption by Acorn Shell of Quercus crassipes was examined in the temperature range from 20 to 60 °C. Results shows that the material biosorption capacity and rate increased with the increasing temperature of the biosorption system. These results suggest that the Ni(II) biosorption process is endothermic in nature and that a chemical reaction between Ni(II) ions and the Acorn Shell of Quercus crassipes surface is involved in the biosorption. The observed phenomenon was explained by the authors as possibly resulting from several factors such as a rise in the kinetic energy, an increase in the meterial surface activity, improved desolvation of Ni(II) ions; a decrease in the thickness of the boundary layer surrounding the biosorbent, or an increase in the material active sites. As can be seen from the literature data, the effect of temperature for the different types of biosorbents is very different. Some biomasses show a positive effect of increasing temperature on the sorption capacity, while others show the opposite.
6.4 Contact time
The results of kinetic studies show that the adsorption rate of Ni(II) ions on different types of adsorbents can be highly variable and difficult to predict. Modyfied adsorbents and composites usually require a longer time to reach equilibrium than for example waste materials. This difference may be related to structural and physicochemical properties (density, surface area, pore size, accessibility of adsorption sites etc.) and more complex structure. In the case of waste materials, a biosorbent is a waste product from some production process which can affect its properties. The shortest equilibrium times of 15 min among analysed materials were achieved by peat (Bartczak et al. 2018). The next shortest equilibrium times (20 min) were obtained by pistachio hull waste and waste powdered activated sludge biomass from waste category and The Curtobacterium sp. FM01 from category Miscalaneous. On te other hand, an equilibrium time of more than 180 min was achieved for the brown macroalgae Fucus vesiculosus biomass and several materials in the composites/modified category. The longest equilibrium time recorded by the researchers among the analysed materials was 960 and was achieved for Meerchaum (Nodular Sepiolite) by Kipcak et al. (2020). In addition, other process parameters such as solution pH, temperature and mixing conditions can also affect the adsorption rate.
7 Kinetics
Kinetic and isothermal models are used for the mathematical modeling of biosorption processes. In the case of kinetic models as described in papers on biosorption, the authors usually focus on pseudo-first and pseudo-second order models. There is an advantage towards one of them due to the fact that most of the researchers reported results that followed the pseudo-second order kinetic model (Table 3). However, there are also examples in the literature for which the pseudo-first order model provides a better fit for the results obtained (Chandra Joshi et al. 2019; Priyantha and Kotabewatta 2019). Another of the frequently used models is the Elovich model which in the work of Richard et al. (2020) obtained the best fit for all the materials analyzed.
Traditionally, the most commonly used kinetic models are the pseudo-first order and pseudo-second order. From these models, various parameters are derived to characterize the process rate, time to equilibrium, and even to determine which process steps may be limiting. The determination of the pseudo-first order and pseudo-second order kinetic models follows the equation, respectively (1) and (2).
where: qe [mg·g–1] is the adsorbent equilibrium adsorption capacity and qt [mg·g–1] is the adsorbent adsorption capacity at time t, ks1 [min–1] is the first-order rate constant of this adsorption model.
where: qe [mg·g–1] is the adsorbent equilibrium adsorption capacity and qt [mg·g–1] is the adsorbent adsorption capacity at time t, k2 [g·(mg·s)–1] is the second-order rate constant of this adsorption model.
Adsorption isotherms indicate the distribution of molecules between the liquid and solid phases when the adsorption process reaches equilibrium. It is used to determine the maximum sorption capacity of adsorbents and is expressed as the amount of adsorbed metal per unit mass of the adsorbent used. Of the isotherm models, the ones most often used are the Langmuir (Marzuki et al. 2019) and Freundlich (Foroutan et al. 2019) isotherms.
The Langmuir model assumes that the surface is homogeneous. This model clearly indicates that adsorption sites have equal sorbate affinity. Furthermore, adsorption at one site does not affect sorption at a adjacent site. This model explains well the formation of a monolayer coverage of the adsorbate on the outer surface of the adsorbent (Eq. 3).
where: bL is the Langmuir constant related to adsorption capacity [mg·g–1], which correlates the variation of the suitable area with the porosity of the adsorbent.
The basic properties of the Langmuir isotherm can be explained by a dimensionless constant called the Langmuir separation factor RL, which is calculated according to Eq. (4):
where the RL values indicate if adsorption is just unfavorable for RL > 1, linear for RL = 1, favorable for 0 < RL < 1, and irreversible for RL = 0.
The Freundlich adsorption isotherm model determines the degree of heterogeneity of the adsorbent surface. The adsorption sites consist of small heterogeneous adsorption sites, each of which is homogeneous (Kurniawan et al. 2006). This model is presented in Eq. (5):
where: aF is Freundlich adsorption capacity [L·mg–1], and nF is adsorption intensity.
The adsorption capacity also indicates the relative energy distribution and heterogeneity of adsorbate adsorption sites. The larger the value of adsorption capacity aF, the greater the adsorption capacity. The magnitude of 1/nF ranges from 0 to 1, and is an indicator of favorable adsorption, becoming more heterogeneous as its value tends towards zero (Yaqub and Lee 2019; Zhang et al. 2019).
Most of the literature reports focus on the models presented, determining the one whose fit parameters are better and which one is more favorable. Depending on the material used and the way the tests were conducted, researchers often note a good fit for the Freundlich and Langmuir models (Table 4). However, it is not clear which model appears more often in the literature as being the one with a better fit for sorption using natural materials. Despite the very high popularity of these models, there are also studies whose results were obtained from an attempt to fit less popular models or to ensure that a better fit was obtained for them. In a study on heavy metal removal using heavy metal-resistance bacterium, Zhang et al. (2017a) used the Langmuir–Freundlich dual model which is a modification of the Langmuir and Freundlich models. The experimental kinetic data obtained provided a good fit for the model used (R2 > 0.97). The data also follows the pseudo-second order model which indicates the simultaneous occurrence of both physical and chemical sorption. A combination of the characteristics of both Langmuir and Freundlich models into the Sips model was used by Guarín-Romero et al. (2019). Studies on sorption at the surface of brown algae showed that the Sips model presented the best fit to the data obtained for single system and competitive adsorption of the Ni(II) ion with Cr(III) and/or Cr(VI). In their work on Ni2+ and Zn2+ biosorption onto Lemna gibba, Morales-Barrera et al. (2020) presented results for different models. They found the Freundlich and Redlich-Peterson models to best represent the isotherm of Ni2+ ion biosorption by L. gibba, while the Langmuir and Redlich-Peterson models best described the equilibrium pattern of Zn2+ biosorption. In a study on nickel removal from wastewater using gooseberry seeds, Aravind et al. (2017) tested the data obtained from studies on their applicability to different isotherm models: Langmuir, Freundlich, Temkin and Dubinin–Radushkevich, Harkins–Jura and Frumkin isotherm. The best fitting coefficient value was obtained for the Harkins–Jura model, which accounts for multilayer adsorption and can be explained by the existence of a heterogeneous pore distribution. In a study on the removal of divalent nickel from aqueous solution using blue-green marine algae, Ramadoss and Subramaniam (2019) focused on the adsorption modeling and applicability of various isotherm models. The results obtained under laboratory conditions were tested to fit both popular isotherm models and those rarely used. The wide range of analyses performed will allow the authors to determine whether the Fritz-Schlunder-V isotherm model is highly significant in establishing the mechanism of adsorption of Ni(II) under the conditions employed in their investigation followed by Freundlich model.
8 Biosorption mechanisms
The complexity of the structures of natural materials suggests that there are many possible ways for metal uptake by their cells. Because of this, the mechanisms of biosorption are diverse and, in some cases, still not very well understood. Vegliò and Beolchini (Veglio’ and Beolchini 1997) proposed, among others, a classification of mechanisms based on the location where the removed metal is found after sorption (Fig. 3). In the case of physical adsorption, ion exchange and complexation being the physicochemical interactions between the metal and functional groups of the cell surface, we are dealing with surface sorption which does not depend on metabolism. Intracellular accumulation occurs due to metal transport across the cell membrane. This type of biosorption is dependent on cell metabolism and can only occur in living cells. Metal precipitation can actually occur both in solution and at the cell’s surface (Garbowski et al. 2020a). If, in the presence of metals, microorganisms produce compounds that promote the precipitation process, the process will be dependent on the cell's metabolism. Conversely, if it occurs after a chemical interaction between the metal and the cell surface, the process may be independent of cell metabolism.
Biosorption mechanisms (own study based on (Veglio’ and Beolchini 1997))
Different functional groups are involved in the biosorption of metals (Rico et al. 2018), therefore the pH affecting their charge, and thus the amount of biosorbed metal, is very important. Given that cationic forms are usually among the predominant forms of metals in an aqueous solution, the more negative the charge of the biosorbent, the greater the amount of biosorbed metal. For this reason, the most suitable pH range for metal biosorption is 7.0–8.0. At lower pH values hydrogen ions and metal ions compete for binding sites, while at higher pH values metal ions precipitate in the form of hydroxides, thereby reducing the amount of biosorbed metal. However, this behavior is different for some metals whose predominant forms are anionic, e.g. chromium, arsenic, molybdenum, among others. In this case, acidic pH (2.0–4.0) is the most favorable for enhancing biosorption because for these pH values, the biomass has a higher number of positive charges that allows anions to be attracted.
Although pH is considered a key factor in the sorption process, temperature is also important because it affects the reaction rate. Higher temperatures tend to augment the rate of biosorption due to the increase in surface activity and kinetic energy of the sorbent (Sedlakova-Kadukova et al. 2019). However, the effect of temperature on the maximum amount of metal biosorbed is debatable. It is generally accepted that an increase in temperature raises the maximum amount of metal that can be absorbed. This phenomenon occurs when the process is endothermic and is caused by various factors such as structural changes in the sorbent or the breakdown of bonds between sorbent molecules, but exothermic processes are an exception. In this case, there is a decrease in biosorption capacity with increasing temperature, probably due to damage to the biosorbent surface (Moreira et al. 2019; Kocaman 2020; Libatique et al. 2020). The effect of temperature on the biosorption process is particularly evident in the case of living biomass, wherein the amount of biosorbed metal increases significantly as temperature increases (Dai et al. 2019; Hu et al. 2020). A higher temperature results in a higher metabolic activity of the living cells, leading to the incorporation of more metal into their interiors. However, this relationship is observed only in the optimal temperature range. Above this range, the living material is destroyed and the biosorption efficiency decreases to a greater extent than in the case of dead biomass (Ahmad et al. 2019).
Another important factor affecting sorption is the ionic strength, the increase of which reduces the amount of biosorbed metal due to competition from other cations for binding sites on functional groups (Barquilha et al. 2019). This is a major drawback when using biosorbents in real aqueous solutions, e.g. wastewater which often has complex concentrations of different cations.
In the literature, one can find various ways of noting down reactions illustrating the sorption phenomenon. Depending on the functional groups responsible in any given case of the process, the authors use notations taken from the literature or their own, so that in this paper they are presented according to the notation proposed in the original works. Sulyman (2017) described the process of adsorption of adsorbate molecules from the liquid phase to solid phase (adsorbent) in four steps. The first is the mass transfer of adsorbate molecules through the outer boundary layer towards the solid molecule. Secondly, the adsorbate molecules are then transported from the surface of the particle to the active sites by diffusion in the pore-filling liquid and migration along the solid pore surface. Thirdly, the adsorption of solute molecules onto active sites on the inner surfaces of the pores. Finally, the molecule approaches the interior of the adsorbent’s pores.
The authors often describe the sorption mechanism as complex, indicating ion exchange and complexation at the same time. Arslan et al. (2007) described the sorption mechanism of Cu(II) and Ni(II) using lignite-based humic acids as ion exchange (Eq. 6,7) and hydrogen bonding (Eq. 8):
With increasing amounts of H+ ions in the solution, the neutralization of negative surface charge increases, which enhances metal ion removal efficiency in the pH range of 4.0–5.0 (Brown et al. 2000; Bayat 2002; Arslan et al. 2007). At lower pH, H+ ions compete with metal cations for exchange sites on the material surface, resulting in the partial release of metal ions which, under extremely acidic conditions, may even be released completely. With increasing pH, metal ions can be adsorbed in a hydrogen bonding mechanism along with ion exchange. For example, the exchange of divalent metal ions with monovalent Na+ ions bound with a phosphoryl group is presented below (Eq. 9) (El-Naggar and Rabei 2020):

The formation of cation surface complexes may involve the coordination of metal ions with oxygen atoms and the release of protons (Eqs. 10,11) (Gadd 2009):

The cation can bind to the surface as an inner-sphere or outer-sphere complex depending on whether a chemical bond, mainly covalent, is formed between the metal and the oxygen ion donating electrons in this case (inner-sphere complex). It may also depend on whether the cation approaches the surface negative groups at a critical distance, even though the cation and base are separated by at least one water molecule.
Bartczak et al. (2018) presented the sorption of nickel and lead ions using peat as a phenomenon caused by various factors. One of them could be electrostatic attraction between the negative charge on the biomass and the positive metal cations. Another likelihood, which may also occur simultaneously with the previous one, is ion-exchange adsorption in which Ni2+ and Pb2+ ions both replace a proton from the surface of the adsorbent (substitution of protons in the surface groups on metal ions), as described below (Eqs. 12,13):
As a consequence, the positive remaining charge can be located on the surface which renders the zeta potential value more positive. The electrokinetic analysis and FTIR spectra conducted by researchers have confirmed the validity of the proposed mechanism of interaction between the analyzed metal ions and peat.
Mineral-rich biochar can be produced from various biomass wastes including grasses, animal manures and crop straws. The salts of alkali and alkali-earth metals contained therein are converted to carbonates during pyrolysis making biochar alkaline (Yuan et al. 2011). Mineral-rich biochar can facilitate the precipitation of heavy metal cations. Xu et al. (2014) showed that inorganic minerals played a dominant role in Pb2+ removal by means of biochars prepared from rice straw and diary manure, while the proportion of organic fractions was less than 1%. Produced from celery biomass, biochar containing a high content of alkaline minerals was used for lead sorption by Zhang et al. (2017b). Based on X-ray Powder Diffraction patterns of the Pb2+ laden biochars, the authors determined the significant contribution of precipitation to the total sorption. The formation of hydrocerussite (2PbCO3·Pb(OH)2) and leadhillite (Pb4(CO3)2(SO4)(OH)2) precipitates was noted to be associated with the high alkalinity of carbonates that existed in the celery-derived biochars. The effectiveness of the test material was related to the sorption mechanism involving precipitation and cation exchange as well as surface complexation.
Chen et al. (2015) also studied biochar as a material for metal ion removal. They noted that it could adsorb Cr(III) quite effectively. They determined that the mechanism of Cr(III) adsorption by biochar mainly involves dissolving Ca and Mg and pH rising (Eqs. 14–17), the formation of Cr(OH)3 precipitation (Eq. 18), along with the cation exchange between soluble Cr3+ and cations fixed on the biochar matrix (Eqs. 19,20).
A biosorption mechanism based on intracellular bioaccumulation was presented in their work by Lin et al. (2012). They investigated the possibility of using Streptomyces zinciresistens to remove zinc and cadmium. They used FTIR, SEM, TEM, and energy-dispersive X-ray scanning (EDXS) techniques to characterize the biosorbent. Their study showed the predominance of electrostatic interactions in the bioaccumulation of metals on Streptomyces zinciresistens, with the authors proposing that S. zinciresistens accumulated Zn2+i Cd2+ mainly on the cell wall followed by intracellular accumulation. After analyzing the results based on the Langmuir equation for chromium sorption, Hlihor et al. (2017) concluded that biosorption of a living biosorbent may follow a heterogeneous model and surface binding, while other mechanisms such as intracellular bioaccumulation may contribute to chromium ion binding.
Ion exchange is the main mechanism of metal biosorption along with surface complexation and microprecipitation (Wang et al. 2019b; Dinh et al. 2020; Giese 2020). The identification of processes behind the efficiency of biosorption is an important issue for further research on a given material. It may allow for predicting whether, by influencing the parameters of the sorption process, its efficiency or material yield can be increased. An additional aspect is the possibility of appropriate regeneration of used material, as well as determining the possibility of its reuse or utilization. This can help in planning the process on an industrial scale.
9 Biosorbents’ regeneration
One important industrial application of biosorption processes is the recovery of metals, especially precious metals, while regenerating the biosorbent for subsequent reuse (Park et al. 2010). Biomass regeneration is usually important when the costs associated with biosorbent preparation are high and biomass is not available in large quantities. The success of metal desorption depends on how the biosorption mechanisms work and the mechanical stability of the biosorbent (Vijayaraghavan and Balasubramanian 2015). The use of a particular biomass as a metal biosorbent depends not only on its biosorption capacity, but also on the ease with which the biosorbent can be regenerated and reused (Bishnoi and Garima 2005). However, most of the published works have been concerned with evaluating the biosorption capacity of materials and the parameters affecting biosorption, without considering the regeneration capacity necessary for the industrial application of the process (Vijayaraghavan and Yun 2008).
The efficiency of the desorption process can be expressed by the concentration ratio, which determines the total ability of the biosorbent to hold up metal. The high values of the ratio are desirable and indicate a good efficiency of the sorption process, making metal recovery a more rational solution given the increasing concentration of metal in the eluent volume (Volesky 2001). The methods used to remove metal bound on the surface of a material can be divided, depending on the condition of the material after the process and the potential for further use. If the biomass used is cheap, then destructive methods can be an alternative, especially for the recovery of valuable metals. However, this type of recovery increases the cost of sludge disposal (Gadd 1990). Destructive methods include dissolution in strong acids or incineration with subsequent recovery of metals from the ash (Gadd 2009; Park et al. 2010). Non-destructive methods are of greater interest to researchers because of the potential for biosorbent reuse. These methods can be divided into physical (heating or microwaving) or chemical (using acids, bases and organic solvents). Chemical desorption is the most commonly used method by researchers, even though the selection of an appropriate eluent plays an important role therein. Not only the type of material used but also the mechanism of metal adsorption should be considered. Moreover, the eluent must be inexpensive, environment-friendly, efficient, and not damaging to the surface of the biosorbent (Vijayaraghavan and Yun 2008). Acids (HNO3, HCl, H2SO4), hydroxides (NaOH), salts (CaCl2, KNO3, NaNO3, NaCl) or chelators (EDTA, Na2EDTA) as well as water are commonly used eluents for the recovery of Ni(II) ions. Of these, acidic agents (HCl and HNO3) are the most effective and widely used.
The efficiency of conducting the desorption process under acidic conditions can be explained by the ion-exchange equilibrium shift. At low pH, a high concentration of hydrogen ions causes a shift of the active sites in the functional groups towards the protonated form. Furthermore, H+ has a higher diffusivity coefficient than Ni2+ ions due to its smaller radius. Cation exchange between H+ and Ni2+ ions, protonation of functional groups and exchange of Ni(II) ions for H+ in adsorbent-adsorbate complexes are the three phenomena that cause the release of adsorbed nickel ions into the desorption solution (Vakili et al. 2021). Recycling of used adsorbent is an important aspect from the point of view of its applicability in industrial conditions, therefore many studies on the efficiency of biosorbents have carried out desorption tests as well.
Vilvanathan and Shanthakumar (2018) carried out desorption tests of metals adsorbed by Tectona grandis biochar using distilled water, 0.1 M HCl and 0.1 M NaOH. The one with the highest potential to desorb was HCl, which after 120 min was able to achieve up to 90.1% and 89.3% for Ni2+ and Co2+, respectively. Further studies have determined that the tested material can be effectively used in three to four adsorption–desorption cycles.
Kulkarni et al. (2019) used brewery sludge containing a yeast biomass of Saccharomyces carlsbergensis to study the biosorption potential of Ni(II) and Cd(II). After sopping, the metal-loaded biomass was subjected to three consecutive cycles of biosorption-desorption in a batch system using 0.1 N HCl eluent and regeneration using distilled water. This allowed desorption from the spent brewery sludge to exceed 90% in three cycles, while the recorded decrease in sorption was 12.7% for Ni(II) and 5.6% for Cd(II).
Barquilha et al. (2019) used an alginate-based biosorbent produced from seaweed Sargassum sp. for Ni2+ i Cu 2+ ions sorption. An acid solution of HCl (pH 1.0) and acidified CaCl2 solution (0.5 M and pH 3.0) were used for desorption, but the results were not good enough. The decrease in biosorption capacity after the first cycle was reported to be 15% for nickel when using acid eluent and 40% for acidified saline eluent. The authors concluded that the described decrease in the material’s sorption capacity can be attributed to the amount of residual metal ions, the acidification of the biosorbent after the desorption process, the carbonation of the biosorbent with Ca2+ ions that can compete with metal ions, and the decrease in biosorbent mass due to the desorption process.
10 Discussion
Current research on the use of natural materials to remove nickel ions from aqueous solutions covers quite a wide range of substrates. Most research has focused on the use of biomass as a sorbent, and the trend of increasing interest in these biosorbents has continued for well over five years now. This may be due to the low cost, ease of acquisition, and the large variety of materials including waste materials. The additional use of waste material is consistent with the circular economy, one of the most important global trends in responsible business. According to this trend, the development of innovative solutions is based on optimizing the usefulness of products, recovering energy, increasing environmental protection, and reducing the use of natural resources. In addition to environmental considerations, one of the biggest advantages of waste materials is the cost of obtaining them, which is always very low. In some cases, such materials are free or else their continued use generates profit for the manufacturer by not requiring disposal. On the other hand, the desire to use a material that is readily available may be associated with its low effectiveness. It should also be taken into account that natural materials, after the sorption process, may cause the release of soluble organic compounds contained in plant materials (Rico et al. 2018; Tapia et al. 2018), and thus increase the values of some of the outflowing wastewater’s parameters.
Candelaria et al. (2019) used agroindustrial by-products to remove nickel and chromium ions. The agroindustrial source palm bagasse and by-products of the process of obtaining starch (yam and plantain) had high adsorption efficiency of well over 70%. Palm bagasse was the most effective material and showed a 92% concentration reduction. For sugarcane bagasse, Ezeonuegbu et al. (2021) obtained a maximum sorption capacity of 123.46 mg·g−1and 1.61 mg·g−1for nickel and lead, respectively.
Gill et al. (2013) used vegetable waste, composed of 1:1 mixture of potato and carrot peels for the removal of Ni(II). Under optimal conditions (pH = 4, contact time 75 min, temperature 30 °C) for an initial concentration of 50 mg·L−1, the use of 3 g of biosorbent made it possible to achieve the maximum removal of nickel at 79%. Miralles et al. (2008) studied the sorption capacity of wine grape stalk wastes for which the maximum uptake was 0.181 mmol Ni·g−1. In the case of tapioca peels, the maximum sorption capacity for nickel ions was 20.37 mg·g−1 (Panumas and Pinthita 2015). On the basis of the conducted research, the authors determined that hydroxyl and carboxyl groups present in tapioca peel were mainly responsible for the biosorption of nickel. During attempts to regenerate the used absorbent, a slight decrease in biosorption capacity of 8.8% for five cycles was observed, which in combination with the effectiveness of the tested material may be the basis for its use as an alternative to the traditional method.
Gupta and Kumar (2019) used A. barbadensis Miller leaves, sourced from a nearby herbal industry, to remove nickel ions. A maximum biosorption of 60.2% was achieved for an initial Ni(II) ion concentration of 100 mg·L−1. Another type of waste material was used by Stevens and Batlokwa (2017). After treatment with acetic acid (vinegar), the eggshell biomass was tested as a sorbent for nickel and cobalt ions. The material proved to be effective and the achieved removal percentages of nickel (II) and cobalt (II) were 79% and 77%, respectively.
The high sorption capacity value compared to other waste materials was characterized by wasted black tea, which Malakahmad et al. (2016) used to remove nickel and zinc ions from aqueous solution. Due to its high concentrations of carbon and calcium and the high availability of functional groups, not to mention its porous surface, this material was identified as a potentially effective sorbent. This was confirmed by an evaluation of the isotherms, which determined the maximum capacity of wasted black tea to be 90.91 mg·g−1 for nickel ions and as high as 166.67 mg·g−1 for zinc ions. Research on waste tea was also conducted by Shah et al. (2015) who worked on the sorption of nickel ions by the material after treatment. Treatment of waste tea leaves using formaldehyde allowed for a sorption capacity value of 120.50 mg·g−1 for nickel ions. The authors also investigated the applicability of the modified material using tap water and samples collected from Khyber Pakhtunkhwa (Pakistan). It was observed that the presence of some common alkali and alkaline earth metal ions in solution does not affect the sorption of nickel ions, thus formaldehyde-treated waste tea leaves can potentially be used as a low-cost alternative to conventional sorbents.
In many countries around the world even today, much of the waste from fruits and other plant products still end up in landfills. It not only increases the amount of waste that needs to be stored or processed, but can also release many toxic pollutants as well as carbon dioxide into the environment. Reusing such waste as sorbents or raw materials for activated carbon, among other things, may be a better option for minimizing the amount of waste in landfills. At the same time, it can contribute to solving environmental pollution by means of agricultural by-products in a manner that is economically viable (Lochynski et al. 2016).
From the literature review performed, it appears that there is a lack of research relating to the use of natural materials in real wastewater and scale-up approaches. Conducting processes under industrial conditions involves quality requirements, e.g. for effluent, which have to be met, as well as limitations due to the technical aspects. Because of the economics of the process, the product used under industrial conditions should be cheap and easy to use or dispose of. The technical parameters of the process should also be taken into account, including the amount of material used which is associated with the need for storage as well as the amount of waste generated. Under industrial conditions, it is not always possible to precisely control quantities, concentrations, and time or pH during the process. Because of the significant differences between sorption processes conducted under laboratory and industrial conditions, researchers often opt for laboratory tests using real wastewater. This type of testing allows for cost savings, as all tests for the selection of optimal parameters are carried out in small volumes without interrupting production. The results obtained in this way mark a step forward towards achieving satisfactory results under industrial conditions. Particularly important, in the context of practical applications, are studies on the treatment of real wastewaters characterized by a high content of nickel and other metals, such as post-coalvanic and steel processing wastewaters.
Akar et al. (2019) used Platanus orientalis bark to adsorb Cr (VI) and Ni ions from a nickel–chromium plating industrial solution. The results show that the adsorption of nickel ions occurs faster than for chromium. Better results were obtained for acidic conditions than for alkaline conditions, which, due to the low pH of the studied wastewater, is a positive outcome as it improves the efficiency of the process. For nickel ions, the unmodified material with a removal percentage of 65.75% was more effective, while for Cr (VI) acid activation with HNO3 of the Platanus orientalis bark the result was 90.15%. The authors concluded that as a biosorbent, P. orientalis bark can be considered as a cheap, natural and readily available yet effective adsorbent for the removal of Cr(VI) and Ni(II) from plating wastewater and can thus be offered as a decent substitute for the commercial adsorbent.
To remove heavy metals from real nickel-containing effluent obtained from the electroplating company Zinicovscaia et al. (2021) used the yeast Saccharomyces cerevisiae. For the real effluent tests, it was necessary to run the process in two steps to increase efficiency. The best results of Ni(II) ion removal of 82% were obtained when 30 g·L−1 of biomass was applied in the first stage, and 10 g·L−1 of biomass in the second stage. The authors determined that the tested material (Saccharomyces cerevisiae) can be considered an effective, cheap and efficient sorbent for the treatment of complex effluents, particularly nickel ion removal.
Pertile et al. (2020) investigated the possibility of using a biosorbent which is a mixture of cones from coniferous trees to remove the residual concentration of metals from hazardous waste at a neutralization station. In their work, they presented both preliminary studies performed in laboratory conditions and tests from the operation of the neutralization station. The laboratory tests for selected elements showed the effectiveness of the material and managed to reduce the concentration of nickel by 96% and zinc by 19% in the case of NaOH activated material, while for the inactivated biosorbent, 93% of nickel and 31% of zinc were removed. For the operational tests, inactivated biosorbent was applied for cost reduction. A significant reduction in metal concentrations occurred when the material was used as a pre-treatment, even though the values obtained still exceeded the emission limits. After 72 h, reductions were obtained for nickel from 4056 to 10 mg·L−1, for copper 2252 to 1 mg·L−1, for zinc 4020 to 1 mg·L−1, and for iron from 1853 to 7 mg·L−1. As insufficiently low values were reached, the biosorbent was therefore also used in the filtration unit. After passing through the filtration unit, the concentrations of all monitored parameters were reduced to a minimum while their values met the regulatory emission limits. The used biosorbent was then applied to thicken the residual sludge. This contributed to a significant reduction in the total cost of residual hazardous waste disposal.
Yan et al. (2020) proposed a simple and feasible process for the purification of Ni-electroplating wastewater using biochars obtained through the pyrolysis of Lemna minor. The obtained material showed a high adsorption capacity of nickel in wastewater as well as other metals. Due to the properties of the studied wastewater, the adsorption of biochar without pretreatment did not meet the discharge requirements for wastewaters. The extremely complex and low pH of the actual wastewater would reduce complexation between metal ions and oxygen-containing functional groups while increasing the competition between H+ and Ni2+ for the number of active sites. Further studies showed that Ca(OH)2 can be used as a pH regulator and optimal precipitant, as its use sharply reduced the concentrations of heavy metals and NH4+. The authors emphasized that the determination and use of the optimum Ca(OH)2 dose is crucial to ensuring an appropriate balance between the results obtained and the cost of treating the reagent and chemical effluent. A dosage of 5.0 g·L−1 Ca(OH)2 was selected as the optimum which achieved a nickel residual concentration of 7.79 mg·L−1, a pH value of 7.01, and an acceptable amount of chemical sludge.
11 Future perspective
Based on the research results and their presentation in the articles that were analysed, two lines of action can be identified that should be taken in to consideration the near future. The first one is the scientific aspect and the second is the practical approach. It should be noted that the adsorption capacities of the studied materials vary considerably due to differences in the properties of individual adsorbents, the degree of surface modification, and the initial adsorbate concentration. In view of the wide variety of materials, there is an urgent need in future papers to pay more attention to the possibilities of their actual use, and then to try to standardise the results presented by the researchers. Only studies defining both the effectiveness of the materials and all the process conditions together with the concentrations used allowed for a full evaluation of the biosorbent under study. The use of standardized units such as sorption capacity to determine the effectiveness would make it possible to compare different materials and their preliminary selection.
Despite increasingly rigorous environmental regulations at the same time as the need to remove heavy metals including nickel ions from industrial wastewater is growing, assessments of the feasibility of using biosorbents are mainly limited to laboratory-scale studies. For these reasons a transition from lab scale to full scale is necessary in the future. Testing with real industrial wastewater would allow to verify the effectiveness of biomaterials in practice and real verification with other traditional technologies like reverse osmosis, ion exchange, precipitation, etc. One of the reasons for limiting full scale testing, is the difficulty in regeneration and reuse of the biomass as well as the negative effects of co-existing pollutions on biosorptive capacity. Industrial wastewater usually contains many organic compounds and metal ions that can inhibit the biosorption process. Therefore, before using biosorbents in industry, it is necessary to evaluate their performance in multi-pollutant solution system. From the point of view of practical applications, it is essential to select the right material for the type of solution, taking into account not only the contaminants present but also their initial concentration, pH and other initial parameters (Fig. 4). For solutions with low initial concentrations, the cost, availability and ease of use will be more important than high performance. Often, due to excessively high initial solution concentrations, the biosorption process can also be used as a further, additional treatment step in combination with conventional technology. The pH value should also be taken into account in order to select a material that will be most effective at a given pH range. Laboratory studies can help identify high performance materials that may potentially prove effective even under industrial conditions.
In industrial applications, many other factors are important but often neglected in laboratory studies such as cost or time. The costs should take into account not only the price of the materials, but also the preparation and disposal of by-products costs. As evident from reviewed papers, surface modifications carried out on selected biosorbents helped improve their nickel binding properties but that leads to higher the overall cost of the process.
After analysing a wide range of literature reports on sorption using natural materials, the authors proposed a selection scheme to determine the application potential of biosorbents (Fig. 4). It presents the proposed process input parameters to be considered in the further evaluation of the sorption process. An assessment consisting mainly of data analysis for the application of kinetic and sorption models can identify the sorption mechanism for a given material. The authors also identified additional recommended parameters and factors that should be considered in the process of assessing the suitability of the material for further industrial applications.
The recycling and reuse of the biosorbent increases, by definition, the economic viability of the biosorption process, However, in practice, it would be worthwhile to analyse the impact of the desorption agent on the total investment costs of the process as well as on maintaining the original biosorption efficiency of the regenerated biosorbent. In addition to costs, the availability and reusability of a biosorbent determine its potential for use in practice. Future research should be particularly directed towards the search for new waste materials with maximum nickel sorption capacity. No less important is the development of cost-effective methods to modify biowastes in order to promote the large-scale use of biosorbents.
Biosorbents can be considered as two-functional materials due to their ability to both biosorb and desorb in a single aqueous solution treatment process. Regenerability and reusability largely determine their economic value and the profitability of their use, especially in real-scale. However, it is worth noting that in the literature biosorption is subjected to in-deep analyses, but only few authors deal with the determination of the regenerability of biosorbents. Hence, in the future studies attention should be given to regeneration studies to understand the worth of low-cost biosorbents. It is important that after the sorption process the used biosorbents are separated from the medium, regenerated and recycled. Hence, besides desorption, regeneration capacity is very important in selecting an appropriate biosorbent for the biosorption process. Regeneration capacity usually decreases with increasing number of cycles, but even after several adsorption desorption cycles, the adsorbent still exhibited good adsorption capacity for Ni2+ and maintained high stability. However, some of the biosorbents are not easily regenerable (regenerated), which makes their use in practice questionable as they create additional waste after one or two cycles. This in turn can lead to environmental pollution problems. Therefore, in the case of nickel-loaded biomass, other technologies can be considered for use as an alternative to desorption. Spent biosorbents, which consist primarily of degradable organic matter, can be converted to energy fuels through fermentation processes or heat energy through incineration. The use of biosorbent as compost would only be justified in this case where there has been complete removal of adsorbed metals from the biosorbents.
It would also be worth paying more attention to the need to test in the future the effectiveness of hybrid technologies, which combine alterative processes like biosorption, bioreduction with traditional methods like electrochemical processes, membrane technology etc. The results of these studies could be helpful for treating industrial wastewater in a full-scale manner.
12 Conclusion
In the literature, there are indeed studies that deal with the sorption of nickel from aqueous solutions using many different materials of natural origin. To illustrate trends in biosorbent research, the materials were divided into the following main groups: biomass, composites and modified materials, waste, peat, and miscellaneous. The last category included all other natural materials that could not be clearly classified into the other created groups. Researchers have been working mainly with biomass materials, but in recent years there has also been interest in various modifications of natural materials in order to improve their efficiency. Calcined eggshells (769 mg Ni·g−1) had a sorption capacity several times higher than the rest of the materials and were the most effective in the modified materials category. However, for this material the sorption phenomenon may be accompanied by precipitation in the presence of hydroxides, which significantly affects the sorption capacity achieved. In the next two groups, the best classified materials had similar values, which were Platanus orientalis bark (285 mg Ni·g−1) and microbacterial flocculant GA1 (225 mg Ni·g−1) for the biomass and miscellaneous groups, respectively. Among the waste materials, olive stone waste proved to be the most effective, with a sorption capacity similar to peat of about 55–60 mg Ni·g−1. Despite achieving the best result in the groups, it had a lower effectiveness in comparison with every other material.
In order to determine why some materials are better sorbents than others, it is helpful to know the mechanism behind this process. From a review of adsorption isotherms, kinetic and thermodynamic data of different adsorbents, it was found that Langmuir and Freundlich adsorption isotherm models were the ones most commonly used to evaluate the adsorption capacity of different adsorbents that had good agreement with experimental data. The kinetic data for the adsorption of Ni(II) ions usually followed the pseudo-second-order kinetic model. The authors often emphasized that the mechanisms responsible for the biosorption process are several and may occur jointly, while ion exchange and complexation were identified as the most dominant.
The future perspectives of biomaterials research were analysed. Often in laboratory studies, researchers compile poor characteristics or omit important parameters, which hinders the proper and critical evaluation of these materials, and scale-up reports are still insufficient. Finally, the authors of this paper proposed an original parameters and assessments diagram recommended to determine the application potential of biosorbents. The scheme includes three interrelated steps: imput data (material type, mass, pretreatment, concentration, pH, temperature, time), assessment (qm, equlibrium time, desorpion potential, mechanism, ΔH° and ΔS°) and applicability potential (biosorption capacity, price, availability, reusability, regenerability, biodegrability, leaching potential). We believe that each time a thorough and reliable analysis can help to evaluate and give the right perspective for further research.
References
Aboli E, Jafari D, Esmaeili H (2020) Heavy metal ions (lead, cobalt, and nickel) biosorption from aqueous solution onto activated carbon prepared from Citrus limetta leaves. Carbon Lett 30:683–698. https://doi.org/10.1007/s42823-020-00141-1
Ahmad A, Bhat AH, Buang A (2019) Enhanced biosorption of transition metals by living Chlorella vulgaris immobilized in Ca-alginate beads. Environ Technol (united Kingdom) 40:1793–1809. https://doi.org/10.1080/09593330.2018.1430171
Akar S, Lorestani B, Sobhanardakani S et al (2019) Surveying the efficiency of Platanus orientalis bark as biosorbent for Ni and Cr(VI) removal from plating wastewater as a real sample. Environ Monit Assess. https://doi.org/10.1007/s10661-019-7479-z
Akpomie KG, Conradie J (2020) Banana peel as a biosorbent for the decontamination of water pollutants. A review. Environ Chem Lett 18:1085–1112. https://doi.org/10.1007/s10311-020-00995-x
Ani JU, Akpomie KG, Okoro UC et al (2020) Potentials of activated carbon produced from biomass materials for sequestration of dyes, heavy metals, and crude oil components from aqueous environment. Appl Water Sci 10:1–11. https://doi.org/10.1007/s13201-020-1149-8
Anitha D, Ramadevi A, Seetharaman R (2021) Biosorptive removal of Nickel(II) from aqueous solution by Mangosteen shell activated carbon. Mater Today Proc 45:718–722. https://doi.org/10.1016/j.matpr.2020.02.748
Aranda-García E, Cristiani-Urbina E (2018) Kinetic, equilibrium, and thermodynamic analyses of Ni(II) Biosorption from aqueous solution by acorn shell of quercus crassipes. Water Air Soil Pollut. https://doi.org/10.1007/s11270-018-3775-4
Aravind J, Bhattacharya G, Keerthana B, Saud MHA, Nachammai SS (2017) Removal of nickel from synthetic waste water using gooseberry seeds as biosorbent. In: Prashanthi M, Sundaram R, Jeyaseelan A, Kaliannan T (eds) Bioremediation and sustainable technologies for cleaner environment. Environmental Science and Engineering. Springer, Cham. https://doi.org/10.1007/978-3-319-48439-6_10
Aravind J, Lenin C, Nancyflavia C et al (2015) Response surface methodology optimization of nickel (II) removal using pigeon pea pod biosorbent. Int J Environ Sci Technol 12:105–114. https://doi.org/10.1007/s13762-013-0391-0
Arslan G, Cetin S, Pehlivan E (2007) Removal of Cu(II) and Ni(II) from aqueous solution by lignite-based humic acids. Energy Sour Part A Recover Util Environ Eff 29:619–630. https://doi.org/10.1080/009083190957711
Aslan S, Yildiz S, Ozturk M (2018) Biosorption of Cu2+ and Ni2+ Ions from aqueous solutions using waste dried activated sludge biomass. Polish J Chem Technol 20:20–28. https://doi.org/10.2478/pjct-2018-0034
Barquilha CER, Cossich ES, Tavares CRG, da Silva EA (2019) Biosorption of nickel(II) and copper(II) ions from synthetic and real effluents by alginate-based biosorbent produced from seaweed Sargassum sp. Environ Sci Pollut Res 26:11100–11112. https://doi.org/10.1007/s11356-019-04552-0
Bartczak P, Klapiszewski Ł, Wysokowski M et al (2017) Treatment of model solutions and wastewater containing selected hazardous metal ions using a chitin/lignin hybrid material as an effective sorbent. J Environ Manage 204:300–310. https://doi.org/10.1016/j.jenvman.2017.08.059
Bartczak P, Norman M, Klapiszewski Ł et al (2018) Removal of nickel(II) and lead(II) ions from aqueous solution using peat as a low-cost adsorbent: a kinetic and equilibrium study. Arab J Chem 11:1209–1222. https://doi.org/10.1016/j.arabjc.2015.07.018
Bayat B (2002) Comparative study of adsorption properties of Turkish fly ashes: I. The case of nickel(II), copper(II) and zinc(II). J Hazard Mater 95:251–273. https://doi.org/10.1016/S0304-3894(02)00140-1
Beverskog B, Puigdomenech I (1997) Revised Pourbaix diagrams for nickel at 25–300 °C. Corros Sci 39:969–980. https://doi.org/10.1016/S0010-938X(97)00002-4
Bishnoi NR, Garima A (2005) Fungus—an alternative for bioremediation of heavy metal containing wastewater: a review. J Sci Ind Res (india) 64:93–100
Bobadilla MC, Lorza RL, Gómez FS, García RE (2020) Adsorptive of Nickel in wastewater by olive stone waste: optimization through multi-response surface methodology using desirability functions. Water (switzerland). https://doi.org/10.3390/W12051320
Bodzek M, Konieczny K (2011) Membrane techniques in the removal of inorganic anionic micropollutants from water environment state of the art. Arch Environ Prot 37:15–19
Boudaoud A, Djedid M, Benalia M, Ad C, Bouzar N, Elmsellem H (2017) Removal of nickel (II) and cadmium (II) ions from wastewater by palm fibers. Chem Chem Eng Biotechnol Food Ind 18:391–406
Brown PA, Gill SA, Allen SJ (2000) Metal removal from wastewater using peat. Water Res 34:3907–3916. https://doi.org/10.1016/S0043-1354(00)00152-4
Burszta-Adamiak E, Spychalski P (2021) Water savings and reduction of costs through the use of a dual water supply system in a sports facility. Sustain Cities Soc. https://doi.org/10.1016/j.scs.2020.102620
Cancelo-González J, Martiñá-Prieto D, Hernández-Huerta D, Barral MT (2017) Metal removal by pine bark compost using a permeable reactive barrier device at laboratory scale. Environ Chem 14:310–318. https://doi.org/10.1071/EN17028
Candelaria TT, Angel VO, Erika RP et al (2019) Characterization and use of agroindustrial by-products in the removal of metal ions in aqueous solution. J Teknol 81:151–158. https://doi.org/10.11113/jt.v81.13644
Chandra Joshi N, Rangar V, Sati R et al (2019) Adsorption behavior of waste leaves of quercus leucotrichophora for the removal of Ni2+ and Cd2+ ions from waste water. Orient J Chem 35:591–596. https://doi.org/10.13005/ojc/350212
Charazińska S, Lochyński P, Burszta-Adamiak E (2021) Removal of heavy metal ions form acidic electrolyte for stainless steel electropolishing via adsorption using Polish peats. J Water Process Eng J. https://doi.org/10.1016/j.jwpe.2021.102169
Chen T, Zhou Z, Xu S et al (2015) Adsorption behavior comparison of trivalent and hexavalent chromium on biochar derived from municipal sludge. Bioresour Technol 190:388–394. https://doi.org/10.1016/j.biortech.2015.04.115
Cruz-Lopes LP, Macena M, Esteves B, Guiné RPF (2021) Ideal pH for the adsorption of metal ions Cr6+, Ni2+, Pb2+in aqueous solution with different adsorbent materials. Open Agric 6:115–123. https://doi.org/10.1515/opag-2021-0225
Dai QH, Bian XY, Li R et al (2019) Biosorption of lead(II) from aqueous solution by lactic acid bacteria. Water Sci Technol 79:627–634. https://doi.org/10.2166/wst.2019.082
Das KK, Reddy RC, Bagoji IB et al (2019) Primary concept of nickel toxicity—an overview. J Basic Clin Physiol Pharmacol 30:141–152. https://doi.org/10.1515/jbcpp-2017-0171
Davarnejad R, Moraveji MK, Havaie M (2018) Integral technique for evaluation and optimization of Ni (II) ions adsorption onto regenerated cellulose using response surface methodology. Arab J Chem 11:370–379. https://doi.org/10.1016/j.arabjc.2015.05.022
Dinh VP, Xuan TD, Hung NQ et al (2020) Primary biosorption mechanism of lead (II) and cadmium (II) cations from aqueous solution by pomelo (Citrus maxima) fruit peels. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-020-10176-6
Dobrowolski RSA, Czemierska M, Jarosz-Wikołazka A (2017) Studies of cadmium(II), lead(II), nickel(II), cobalt(II) and chromium(VI) sorption on extracellular polymeric substances produced by Rhodococcus opacus and Rhodococcus rhodochrous. Bioresour Technol 225:113–120. https://doi.org/10.1016/j.biortech.2016.11.040
Domańska M, Boral A, Hamal K et al (2019) Efficiency of municipal wastewater treatment with membrane bioreactor. J Water L Dev 41:47–54. https://doi.org/10.2478/jwld-2019-0026
Eka Putri LS (2019) The adsorption of heavy metals from industrial wastewater using sargassum crassifolium. Int J Geomate 17:21–27. https://doi.org/10.21660/2019.59.4603
El-Naggar NEA, Rabei NH (2020) Bioprocessing optimization for efficient simultaneous removal of methylene blue and nickel by Gracilaria seaweed biomass. Sci Rep 10:1–21. https://doi.org/10.1038/s41598-020-74389-y
Erto A, Giraldo L, Lancia A, Moreno-Piraján JC (2013) A comparison between a low-cost sorbent and an activated carbon for the adsorption of heavy metals from water. Water Air Soil Pollut. https://doi.org/10.1007/s11270-013-1531-3
Esmaeili A, Khoshnevisan N (2016) Optimization of process parameters for removal of heavy metals by biomass of Cu and Co-doped alginate-coated chitosan nanoparticles. Bioresour Technol 218:650–658. https://doi.org/10.1016/j.biortech.2016.07.005
Ezeonuegbu BA, Machido DA, Whong CMZ et al (2021) Agricultural waste of sugarcane bagasse as efficient adsorbent for lead and nickel removal from untreated wastewater: Biosorption, equilibrium isotherms, kinetics and desorption studies. Biotechnol Rep 30:e00614. https://doi.org/10.1016/j.btre.2021.e00614
Fernández-González R, Martín-Lara MA, Iáñez-Rodríguez I, Calero M (2018) Removal of heavy metals from acid mining effluents by hydrolyzed olive cake. Bioresour Technol 268:169–175. https://doi.org/10.1016/j.biortech.2018.07.124
Figueira MM, Volesky B, Mathieu HJ (1999) Instrumental analysis study of iron species biosorption by Sargassum biomass. Environ Sci Technol 33:1840–1846. https://doi.org/10.1021/es981111p
Fisher RM, Gupta V (2020) Heavy metals. StatPearls Publishing, Treasure Island
Foroutan R, Esmaeili H, Sanati AM et al (2018) Adsorptive removal of Pb(II), Ni(II), and Cd(II) from aqueous media and leather wastewater using Padinasanctae-crucis biomass. Desalin Water Treat 135:236–246. https://doi.org/10.5004/dwt.2018.23179
Foroutan R, Khoo FS, Ramavandi B, Abbasi S (2017) Heavy metals removal from synthetic and shipyard wastewater using phoenix dactylifera activated carbon. Desalin Water Treat 82:146–156. https://doi.org/10.5004/dwt.2017.20908
Foroutan R, Mohammadi R, Farjadfard S et al (2019) Characteristics and performance of Cd, Ni, and Pb bio-adsorption using Callinectes sapidus biomass: real wastewater treatment. Environ Sci Pollut Res 26:6336–6347. https://doi.org/10.1007/s11356-018-04108-8
Fourest E, Volesky B (1996) Contribution of sulfonate groups and alginate to heavy metal biosorption by the dry biomass of Sargassum fluitans. Environ Sci Technol 30:277–282. https://doi.org/10.1021/es950315s
Fu F, Wang Q (2011) Removal of heavy metal ions from wastewaters: a review. J Environ Manage 92:407–418. https://doi.org/10.1016/j.jenvman.2010.11.011
Gadd GM (1990) Heavy metal accumulation by bacteria and other microorganisms. Experientia 46:834–840. https://doi.org/10.1007/BF01935534
Gadd GM (2009) Biosorption: Critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28. https://doi.org/10.1002/jctb.1999
Garbowski T, Charazińska S, Pulikowski K, Wiercik P (2020a) Application of microalgae cultivated on pine bark for the treatment of municipal wastewater in cylindrical photobioreactors. Water Environ J 34:949–959. https://doi.org/10.1111/wej.12606
Garbowski T, Pietryka M, Pulikowski K, Richter D (2020b) The use of a natural substrate for immobilization of microalgae cultivated in wastewater. Sci Rep 10:1–9. https://doi.org/10.1038/s41598-020-64656-3
Georgieva VG, Gonsalvesh L, Tavlieva MP (2020) Thermodynamics and kinetics of the removal of nickel (II) ions from aqueous solutions by biochar adsorbent made from agro-waste walnut shells. J Mol Liq 312:112788. https://doi.org/10.1016/j.molliq.2020.112788
Giese EC (2020) Biosorption as green technology for the recovery and separation of rare earth elements. World J Microbiol Biotechnol 36:1–11. https://doi.org/10.1007/s11274-020-02821-6
Gill R, Mahmood A, Nazir R (2013) Biosorption potential and kinetic studies of vegetable waste mixture for the removal of Nickel(II). J Mater Cycles Waste Manage 15:115–121. https://doi.org/10.1007/s10163-012-0079-4
Grube M, Lin JG, Lee PH, Kokorevicha S (2006) Evaluation of sewage sludge-based compost by FT-IR spectroscopy. Geoderma 130:324–333. https://doi.org/10.1016/j.geoderma.2005.02.005
Guarín-Romero JR, Rodríguez-Estupiñán P, Giraldo L, Moreno-Piraján JC (2019) Simple and competitive adsorption study of nickel(II) and chromium(III) on the surface of the brown algae durvillaea antarctica Biomass. ACS Omega 4:18147–18158. https://doi.org/10.1021/acsomega.9b02061
Guo X, Tang S, Song Y, Nan J (2018) Adsorptive removal of Ni2+ and Cd2+ from wastewater using a green longan hull adsorbent. Adsorpt Sci Technol 36:762–773. https://doi.org/10.1177/0263617417722254
Gupta S, Kumar A (2019) Removal of nickel (II) from aqueous solution by biosorption on A. barbadensis Miller waste leaves powder. Appl Water Sci 9:1–11. https://doi.org/10.1007/s13201-019-0973-1
Gürel L, Güneş S (2018) Determination of heavy metal removal potential of the Samsun Coast mussel shells. Pamukkale Univ J Eng Sci 24:1135–1140. https://doi.org/10.5505/pajes.2017.68984
Harasim P, Filipek T (2015) Nickel in the Environment. J Elem 20:525–534. https://doi.org/10.5601/jelem.2014.19.3.651
Hlihor RM, Figueiredo H, Tavares T, Gavrilescu M (2017) Biosorption potential of dead and living Arthrobacter viscosus biomass in the removal of Cr(VI): Batch and column studies. Process Saf Environ Prot 108:44–56. https://doi.org/10.1016/j.psep.2016.06.016
Hoseinzadeh S, Shahabivand S, Aliloo AA (2017) Toxic metals accumulation in Trichoderma asperellum and T harzianum. Microbiol Russ Fed 86:728–736. https://doi.org/10.1134/S0026261717060066
Houghton RA (2008) Biomass. In: Jorgensen SE, Fath BD (eds) Encyclopedia of ecology, 1st ed. Academic Press, pp 448–453
Hu X, Cao J, Yang H et al (2020) Pb2+ biosorption from aqueous solutions by live and dead biosorbents of the hydrocarbon-degrading strain Rhodococcus sp HX-2. PLoS ONE 1:5. https://doi.org/10.1371/journal.pone.0226557
Ibarra JV, Muñoz E, Moliner R (1996) FTIR study of the evolution of coal structure during the coalification process. Org Geochem 24:725–735. https://doi.org/10.1016/0146-6380(96)00063-0
Ibrahim AG, Saleh AS, Elsharma EM et al (2019) Chitosan-g-maleic acid for effective removal of copper and nickel ions from their solutions. Int J Biol Macromol 121:1287–1294. https://doi.org/10.1016/j.ijbiomac.2018.10.107
Jaishankar M, Tseten T, Anbalagan N et al (2014) Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol 7:60–72. https://doi.org/10.2478/intox-2014-0009
Jan AT, Azam M, Siddiqui K et al (2015) Heavy metals and human health: mechanistic insight into toxicity and counter defense system of antioxidants. Int J Mol Sci 16:29592–29630. https://doi.org/10.3390/ijms161226183
Jiang D, Yang Y, Huang C et al (2019) Removal of the heavy metal ion nickel (II) via an adsorption method using flower globular magnesium hydroxide. J Hazard Mater 373:131–140. https://doi.org/10.1016/j.jhazmat.2019.01.096
Keshvardoostchokami M, Babaei L, Zamani AA et al (2017) Synthesized chitosan/iron oxide nanocomposite and shrimp shell in removal of nickel, cadmium and lead from aqueous solution. Glob J Environ Sci Manag 3:267–278. https://doi.org/10.22034/gjesm.2017.03.03.004
Khajavian M, Wood DA, Hallajsani A, Majidian N (2019) Simultaneous biosorption of nickel and cadmium by the brown algae Cystoseria indica characterized by isotherm and kinetic models. Appl Biol Chem. https://doi.org/10.1186/s13765-019-0477-6
Khan Rao RA, Khatoon A (2017) Aluminate treated Casuarina equisetifolia leaves as potential adsorbent for sequestering Cu(II), Pb(II) and Ni(II) from aqueous solution. J Clean Prod 165:1280–1295. https://doi.org/10.1016/j.jclepro.2017.07.160
Kipçak İ, Ersal EK, Özdemir M (2020) Adsorptive removal of Ni2+ IONS from aqueous solutions by nodular sepiolite (meerschaum) and industrial Sepiolite samples from Eskişehir, Turkey. Clays Clay Miner 68:220–236. https://doi.org/10.1007/s42860-020-00077-7
Kipigroch K (2018) The use of algae in the process of heavy metal ions removal from wastewater. Desalin Water Treat 134:289–295. https://doi.org/10.5004/dwt.2018.23222
Kocaman S (2020) Synthesis and cationic dye biosorption properties of a novel low-cost adsorbent: coconut waste modified with acrylic and polyacrylic acids. Int J Phytoremediation 22:551–566. https://doi.org/10.1080/15226514.2020.1741509
Komarabathina S, Pulipati K, Pujari M, Kodavaty J (2020) Biosorption of nickel from aqueous solution onto Liagora viscida: kinetics, isotherm, and thermodynamics. Environ Prog Sustain Energy 39:1–9. https://doi.org/10.1002/ep.13330
Kristianto H, Daulay N, Arie AA (2019) Adsorption of Ni(II) Ion onto calcined eggshells: a study of equilibrium adsorption Isotherm. Indones J Chem 19:143–150. https://doi.org/10.22146/ijc.29200
Kulkarni RM, Vidya Shetty K, Srinikethan G (2019) Kinetic and equilibrium modeling of biosorption of nickel (II) and cadmium (II) on brewery sludge. Water Sci Technol 79:888–894. https://doi.org/10.2166/wst.2019.090
Kumar R, Sharma RK (2019) Synthesis and characterization of cellulose based adsorbents for removal of Ni(II), Cu(II) and Pb(II) ions from aqueous solutions. React Funct Polym 140:82–92. https://doi.org/10.1016/j.reactfunctpolym.2019.04.014
Kumar R, Sharma RK, Singh AP (2019) Grafting of cellulose with N-isopropylacrylamide and glycidyl methacrylate for efficient removal of Ni(II), Cu(II) and Pd(II) ions from aqueous solution. Sep Purif Technol 219:249–259. https://doi.org/10.1016/j.seppur.2019.03.035
Kurniawan TA, Chan GYS, Lo WH, Babel S (2006) Physico-chemical treatment techniques for wastewater laden with heavy metals. Chem Eng J 118:83–98. https://doi.org/10.1016/j.cej.2006.01.015
Leiviskä T, Khalid MK, Gogoi H, Tanskanen J (2018) Enhancing peat metal sorption and settling characteristics. Ecotoxicol Environ Saf 148:346–351. https://doi.org/10.1016/j.ecoenv.2017.10.053
Li H, Dong W, Liu Y et al (2019) Enhanced biosorption of nickel ions on immobilized surface-engineered yeast using nickel-binding peptides. Front Microbiol. https://doi.org/10.3389/fmicb.2019.01254
Libatique MJH, Lee MC, Yeh HY, Jhang FJ (2020) Total and inorganic arsenic biosorption by Sarcodia suiae (Rhodophyta), as affected by controlled environmental conditions. Chemosphere 248:2020–2022. https://doi.org/10.1016/j.chemosphere.2020.126084
Lin Y, Wang X, Wang B et al (2012) Bioaccumulation characterization of zinc and cadmium by Streptomyces zinciresistens, a novel actinomycete. Ecotoxicol Environ Saf 77:7–17. https://doi.org/10.1016/j.ecoenv.2011.09.016
Liu S, Lei Y, Zhao J et al (2021) Research on ecosystem services of water conservation and soil retention: a bibliometric analysis. Environ Sci Pollut Res 28:2995–3007. https://doi.org/10.1007/s11356-020-10712-4
Lochyński P, Charazińska S, Łyczkowska-Widłak E, Sikora A (2019) Electropolishing of stainless steel in laboratory and industrial scale. Metals (basel) 9:854. https://doi.org/10.3390/met9080854
Lochynski P, Kowalski M, Szczygiel B, Kuczewski K (2016) Improvement of the stainless steel electropolishing process by organic additives. Polish J Chem Technol 18:76–81. https://doi.org/10.1515/pjct-2016-0074
Malakahmad A, Tan S, Yavari S (2016) Valorization of wasted black tea as a low-cost adsorbent for nickel and zinc removal from aqueous solution. J Chem. https://doi.org/10.1155/2016/5680983
Mardiyono S, Masykuri M, Setyono P (2019) Bioremediation of nickel heavy metals in electroplating industrial liquid waste with Bacillus subtilis. AIP Conf Proc. https://doi.org/10.1063/1.5141697
Marzuki I, Bachtiar E, Alwi RS, Iryani AS (2019) Chitosan performance of shrimp shells in the biosorption ion metal of cadmium, lead and nickel based on variations pH interaction. 5–11. https://doi.org/10.31219/osf.io/kmr5h
Mashangwa TD, Tekere M, Sibanda T (2017) Determination of the efficacy of eggshell as a low-cost adsorbent for the treatment of metal laden effluents. Int J Environ Res 11:175–188. https://doi.org/10.1007/s41742-017-0017-3
Masoumi F, Khadivinia E, Alidoust L et al (2016) Nickel and lead biosorption by curtobacterium sp. FM01, an indigenous bacterium isolated from farmland soils of northeast Iran. J Environ Chem Eng 4:950–957. https://doi.org/10.1016/j.jece.2015.12.025
Miralles N, Martínez M, Florido A et al (2008) Grape stalks waste as low cost biosorbents: an alternative for metal removal from aqueous solutions. Solvent Extr Ion Exch 26:261–270. https://doi.org/10.1080/07366290802053660
Moawad MN, El-Sayed AAM, El-Naggar NA (2020) Biosorption of cadmium and nickel ions using marine macrophyte, Cymodocea nodosa. Chem Ecol 36:458–474. https://doi.org/10.1080/02757540.2020.1752199
Mohamad N, Abustan I, Mohamad M, Samuding K (2018) Metal removal from municipal landfill leachate using mixture of laterite soil, peat soil and rice husk. Mater Today Proc 5:21832–21840. https://doi.org/10.1016/j.matpr.2018.07.039
Mohammed AA, Najim AA, Al-Musawi TJ, Alwared AI (2019) Adsorptive performance of a mixture of three nonliving algae classes for nickel remediation in synthesized wastewater. J Environ Heal Sci Eng 17:529–538. https://doi.org/10.1007/s40201-019-00367-w
Morales-Barrera L, Flores-Ortiz CM, Cristiani-Urbina E (2020) Single and binary equilibrium studies for Ni2+ and Zn2+ biosorption onto lemna gibba from aqueous solutions. Processes 8:1–25. https://doi.org/10.3390/pr8091089
Moreira VR, Lebron YAR, Lange LC, Santos LVS (2019) Simultaneous biosorption of Cd(II), Ni(II) and Pb(II) onto a brown macroalgae Fucus vesiculosus: Mono- and multi-component isotherms, kinetics and thermodynamics. J Environ Manage 251:109587. https://doi.org/10.1016/j.jenvman.2019.109587
Murphy V, Hughes H, McLoughlin P (2007) Cu(II) binding by dried biomass of red, green and brown macroalgae. Water Res 41:731–740. https://doi.org/10.1016/j.watres.2006.11.032
Naghipour D, Ashrafi SD, Gholamzadeh M et al (2018) Phytoremediation of heavy metals (Ni, Cd, Pb) by Azolla filiculoides from aqueous solution: a dataset. Data Br 21:1409–1414. https://doi.org/10.1016/j.dib.2018.10.111
Naik BR, Suresh C, Kumar NVS et al (2017) Biosorption of Pb(II) and Ni(II) ions by chemically modified Eclipta alba stem powder: kinetics and equilibrium studies. Sep Sci Technol 52:1717–1732. https://doi.org/10.1080/01496395.2017.1298614
Naskar A, Bera D (2018) Mechanistic exploration of Ni (II) removal by immobilized bacterial biomass and interactive influence of coexisting surfactants. Environ Prog Sustain Energy 37:342–354. https://doi.org/10.1002/ep.12685
Nithya K, Sathish A, Kumar PS, Ramachandran T (2017) An insight into the prediction of biosorption mechanism, and isotherm, kinetic and thermodynamic studies for Ni(II) ions removal from aqueous solution using acid treated biosorbent: The Lantana camara fruit. Desalin Water Treat 80:276–287. https://doi.org/10.5004/dwt.2017.20912
Nnaji CC, Agim AE, Mama CN et al (2021) Equilibrium and thermodynamic investigation of biosorption of nickel from water by activated carbon made from palm kernel chaff. Sci Rep 11:1–20. https://doi.org/10.1038/s41598-021-86932-6
Noormohamadi HR, Fat’hi MR, Ghaedi M, Ghezelbash GR (2019) Potentiality of white-rot fungi in biosorption of nickel and cadmium: modeling optimization and kinetics study. Chemosphere 216:124–130. https://doi.org/10.1016/j.chemosphere.2018.10.113
Oguz E (2020) Simultaneous removal of lead, copper, cadmium, nickel, and cobalt heavy metal ions from the quinary system by Abies bornmulleriana cones. Water Sci Technol 82:3032–3046. https://doi.org/10.2166/wst.2020.547
Ossman ME, Abdelfatah M, Kiros Y (2016) Preparation, characterization and adsorption evaluation of old newspaper fibres using basket reactor (Nickel removal by adsorption). Int J Environ Res 10:119–130. https://doi.org/10.22059/IJER.2016.56894
Panumas P, Pinthita M (2015) Biosorption of nickel (II) ions from aqueous solutions by tapioca peel. African J Environ Sci Technol 9:662–670. https://doi.org/10.5897/ajest2015.1963
Park D, Yun YS, Park JM (2010) The past, present, and future trends of biosorption. Biotechnol Bioprocess Eng 15:86–102. https://doi.org/10.1007/s12257-009-0199-4
Parker FS (1971) Applications of spectroscopy infrared in biochemistry, biology, and medicine. Plenum Press, New York
Paweska K, Bawiec A (2017) Activated sludge technology combined with hydroponic lagoon as a technology suitable for treatment of wastewater delivered by slurry tanks. J Ecol Eng 18:29–37. https://doi.org/10.12911/22998993/67505
Pertile E, Vaclavik V, Dvorsky T, Heviankova S (2020) The removal of residual concentration of hazardous metals in wastewater from a neutralization station using biosorbent—a case study company gutra, czech republic. Int J Environ Res Public Health 17:1–14. https://doi.org/10.3390/ijerph17197225
Pons MN, Le BS, Potier O (2004) Spectral analysis and fingerprinting for biomedia characterisation. J Biotechnol 113:211–230. https://doi.org/10.1016/j.jbiotec.2004.03.028
Priyantha N, Kotabewatta PA (2019) Biosorption of heavy metal ions on peel of Artocarpus nobilis fruit: 1—Ni(II) sorption under static and dynamic conditions. Appl Water Sci 9:1–10. https://doi.org/10.1007/s13201-019-0911-2
Pundir R, Chary GHVC, Dastidar MG (2018) Application of Taguchi method for optimizing the process parameters for the removal of copper and nickel by growing Aspergillus sp. Water Resour Ind 20:83–92. https://doi.org/10.1016/j.wri.2016.05.001
Ramadoss R, Subramaniam D (2019) Removal of divalent nickel from aqueous solution using blue-green marine algae: adsorption modeling and applicability of various isotherm models. Sep Sci Technol 54:943–961. https://doi.org/10.1080/01496395.2018.1526194
Raval NP, Shah PU, Shah NK (2016) Adsorptive removal of nickel(II) ions from aqueous environment: a review. J Environ Manage 179:1–20. https://doi.org/10.1016/j.jenvman.2016.04.045
Richard D, Mucci A, Neculita CM, Zagury GJ (2020) Comparison of organic materials for the passive treatment of synthetic neutral mine drainage contaminated by nickel: short- and medium-term batch experiments. Appl Geochem. https://doi.org/10.1016/j.apgeochem.2020.104772
Rico ILR, Carrazana RJC, Karna NK et al (2018) Modeling the mass transfer in biosorption of Cr (VI) y Ni (II) by natural sugarcane bagasse. Appl Water Sci 8:1–8. https://doi.org/10.1007/s13201-018-0692-z
Romar-Gasalla A, Coelho GF, Nóvoa-Muñoz JC et al (2017) Wheat straw as a bio-sorbent for arsenate, chromate, fluoride, and nickel. Water (sitzerland) 9:1–11. https://doi.org/10.3390/w9090690
Sankaran R, Show PL, Ooi CW et al (2020) Feasibility assessment of removal of heavy metals and soluble microbial products from aqueous solutions using eggshell wastes. Clean Technol Environ Policy 22:773–786. https://doi.org/10.1007/s10098-019-01792-z
Sedlakova-Kadukova J, Kopcakova A, Gresakova L et al (2019) Bioaccumulation and biosorption of zinc by a novel Streptomyces K11 strain isolated from highly alkaline aluminium brown mud disposal site. Ecotoxicol Environ Saf 167:204–211. https://doi.org/10.1016/j.ecoenv.2018.09.123
Shah J, Jan MR, Ul Haq A, Zeeshan M (2015) Equilibrium, kinetic and thermodynamic studies for sorption of Ni (II) from aqueous solution using formaldehyde treated waste tea leaves. J Saudi Chem Soc 19:301–310. https://doi.org/10.1016/j.jscs.2012.04.004
Shaikh RB, Saifullah B, ur Rehman F, Shaikh RI, (2018) Greener method for the removal of toxic metal ions from the wastewater by application of agricultural waste as an adsorbent. Water (switzerland). https://doi.org/10.3390/w10101316
Sharma B, Shukla P (2021) A comparative analysis of heavy metal bioaccumulation and functional gene annotation towards multiple metal resistant potential by Ochrobactrum intermedium BPS-20 and Ochrobactrum ciceri BPS-26. Bioresour Technol 320:124330. https://doi.org/10.1016/j.biortech.2020.124330
Sharma RK, Kumar R, Singh AP (2019) Metal ions and organic dyes sorption applications of cellulose grafted with binary vinyl monomers. Sep Purif Technol 209:684–697. https://doi.org/10.1016/j.seppur.2018.09.011
Sheng PX, Ting YP, Chen JP, Hong L (2004) Sorption of lead, copper, cadmium, zinc, and nickel by marine algal biomass: Characterization of biosorptive capacity and investigation of mechanisms. J Colloid Interface Sci 275:131–141. https://doi.org/10.1016/j.jcis.2004.01.036
Shin Seok W (2017) Adsorption characteristics of phenol and heavy metals on biochar from Hizikia fusiformis. Environ Earth Sci 76:1–9. https://doi.org/10.1007/s12665-017-7125-4
Silah H, Gül ÜD (2017) Comparison of nickel biosorption properties of living and dead Rhizopus arrhizus biosorbent. AIP Conf Proc. https://doi.org/10.1063/1.4981753
Singh S, Shukla SR (2017) Theoretical studies on adsorption of Ni(II) from aqueous solution using citrus limetta peels. Environ Prog Sustain Energy 36:864–872. https://doi.org/10.1002/ep.12526
Sivakumar D, Nouri J, Modhini TM, Deepalakshmi K (2018) Nickel removal from electroplating industry wastewater: A bamboo activated carbon. Glob J Environ Sci Manag 4:325–338. https://doi.org/10.22034/GJESM.2018.03.006
Solomon D, Lehmann J, Kinyangi J et al (2005) Carbon K-Edge NEXAFS and FTIR-ATR spectroscopic investigation of organic carbon speciation in soils. Soil Sci Soc Am J 69:107–119. https://doi.org/10.2136/sssaj2005.0107dup
Stevens M, Batlokwa B (2017) Removal of Nickel (II) and cobalt (II) from wastewater using vinegar-treated eggshell waste biomass. J Water Resour Prot 09:931–944. https://doi.org/10.4236/jwarp.2017.98062
Sudha R, Srinivasan K (2015) Equilibrium and kinetic studies on Ni(II) removal from aqueous solution by Citrus Limettioides peel and its carbon adsorbent. Indian J Environ Prot 35:300–311
Sulyman M, Namiesnik J, Gierak A (2017) Low-cost adsorbents derived from agricultural by-products/wastes for enhancing contaminant uptakes from wastewater: a review. Polish J Environ Stud 26:479–510. https://doi.org/10.15244/pjoes/66769
Tapia P, Pavez O, Garrido N, Sepúlveda B (2018) Removal of cooper and nickel ions with peanut shell. Holos 3:57–69. https://doi.org/10.15628/holos.2018.7064
Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ (2012) Volume 3: Environmental Toxicology
The European Parliament and the Council of the European Union (2020) Directive (EU) 2020/2184, EU (revised) Drinking Water Directive. Annex 1. Part B. Off J Eur Communities 2019:35
Torres-Caban R, Vega-Olivencia CA, Mina-Camilde N (2019) Adsorption of Ni2+ and Cd2+ from water by calcium Alginate/Spent coffee grounds composite beads. Appl Sci. https://doi.org/10.3390/app9214531
Tsezos M, Volesky B (1982) The mechanism of thorium biosorption by Rhizopus arrhizus. Biotechnol Bioeng 24:955–969. https://doi.org/10.1002/bit.260240415
Tytła M (2019) Assessment of heavy metal pollution and potential ecological risk in sewage sludge from municipal wastewater treatment plant located in the most industrialized region in poland—case study. Int J Environ Res Public Health 16:1–16. https://doi.org/10.3390/ijerph16132430
Ugya AY, Imam TS, Hua X, Ma J (2019) Efficacy of Eichhornia crassipes, pistia stratiotes and Nymphaea lotus in the biosorption of nickel from refinery wastewater. Appl Ecol Environ Res 17:13075–13087. https://doi.org/10.15666/aeer/1706_1307513087
Uter W, Werfel T, White IR, Johansen JD (2018) Contact allergy: a review of current problems from a clinical perspective. Int J Environ Res Public Health. https://doi.org/10.3390/ijerph15061108
Vakili M, Rafatullah M, Yuan J, Zwain HM, Mojiri A, Gholami Z, Gholami F, Wang W, Giwa AS, Yu Y, Cagnetta G, Yu G (2021) Nickel ion removal from aqueous solutions through the adsorption process: a review. Rev Chem Eng 37:755–778. https://doi.org/10.1515/revce-2019-0047
Veglio’ F, Beolchini F (1997) Removal of metals by biosorption: a review. Hydrometallurgy 44:301–316. https://doi.org/10.1016/s0304-386x(96)00059-x
Veneu DM, Yokoyama L, Cunha OGC et al (2019) Nickel sorption using Bioclastic Granules as a sorbent material: equilibrium, kinetic and characterization studies. J Mater Res Technol 8:840–852. https://doi.org/10.1016/j.jmrt.2018.05.020
Vetrivel SA, Diptanghu M, Ebhin MR et al (2017) Green algae of the genus Spirogyra: a potential absorbent for heavy metal from coal mine water. Remediation 27:81–90. https://doi.org/10.1002/rem.21522
Vijayaraghavan K, Balasubramanian R (2015) Is biosorption suitable for decontamination of metal-bearing wastewaters? A critical review on the state-of-the-art of biosorption processes and future directions. J Environ Manage 160:283–296. https://doi.org/10.1016/j.jenvman.2015.06.030
Vijayaraghavan K, Yun YS (2008) Bacterial biosorbents and biosorption. Biotechnol Adv 26:266–291. https://doi.org/10.1016/j.biotechadv.2008.02.002
Villen-Guzman M, Cerrillo-Gonzalez MM, Paz-Garcia JM et al (2021) Valorization of lemon peel waste as biosorbent for the simultaneous removal of nickel and cadmium from industrial effluents. Environ Technol Innov 21:3–5. https://doi.org/10.1016/j.eti.2021.101380
Vilvanathan S, Shanthakumar S (2018) Ni2+ and Co2+ adsorption using Tectona grandis biochar: kinetics, equilibrium and desorption studies. Environ Technol (united Kingdom) 39:464–478. https://doi.org/10.1080/09593330.2017.1304454
Volesky B (2001) Detoxification of metal-bearing effluents: Biosorption for the next century. Hydrometallurgy 59:203–216. https://doi.org/10.1016/S0304-386X(00)00160-2
Volesky B, Holan ZR (1995) Biosorption of Heavy Metals. Biotechnol Prog 11:235–250. https://doi.org/10.1021/bp00033a001
Wang J, Liu M, Duan C et al (2019a) Preparation and characterization of cellulose-based adsorbent and its application in heavy metal ions removal. Carbohydr Polym 206:837–843. https://doi.org/10.1016/j.carbpol.2018.11.059
Wang N, Qiu Y, Xiao T, Wang J, Chen Y, Xu X, Kang Z, Fan L, Yu H (2019b) Comparative studies on Pb(II) biosorption with three spongy microbe-based biosorbents: high performance, selectivity and application. J Hazard Mater 373:39–49. https://doi.org/10.1016/j.jhazmat.2019.03.056
Wierzba S (2017) Biosorption of nickel (II) and zinc (II) from aqueous solutions by the biomass of yeast Yarrowia lipolytica. Polish J Chem Technol 19:1–10. https://doi.org/10.1515/pjct-2017-0001
Winters C, Guéguen C, Noble A (2017) Equilibrium and kinetic studies of Cu(II) and Ni(II) sorption on living Euglena gracilis. J Appl Phycol 29:1391–1398. https://doi.org/10.1007/s10811-016-1040-z
Xu X, Cao X, Zhao L et al (2014) Interaction of organic and inorganic fractions of biochar with Pb(II) ion: further elucidation of mechanisms for Pb(II) removal by biochar. RSC Adv. https://doi.org/10.1039/C4RA07303G
Yan FL, Wang Y, Wang WH et al (2020) Application of biochars obtained through the pyrolysis of Lemna minor in the treatment of Ni-electroplating wastewater. J Water Process Eng 37:101464. https://doi.org/10.1016/j.jwpe.2020.101464
Yaqub M, Lee SH (2019) Heavy metals removal from aqueous solution through micellar enhanced ultrafiltration: a review. Environ Eng Res 24:363–375. https://doi.org/10.4491/EER.2018.249
Yous R, Mohellebi F, Cherifi H, Amrane A (2018) Competitive biosorption of heavy metals from aqueous solutions onto Streptomyces rimosus. Korean J Chem Eng 35:890–899. https://doi.org/10.1007/s11814-018-0004-1
Yuan JH, Xu RK, Zhang H (2011) The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour Technol 102:3488–3497. https://doi.org/10.1016/j.biortech.2010.11.018
Zaccheo P, Cabassi G, Ricca G, Crippa L (2002) Decomposition of organic residues in soil: experimental technique and spectroscopic approach. Org Geochem 33:327–345. https://doi.org/10.1016/S0146-6380(01)00164-4
ZamaniBeidokhti M, OmidNaeeni ST, AbdiGhahroudi MS (2019) Biosorption of Nickel (II) from aqueous solutions onto pistachio hull waste as a low-cost biosorbent. Civ Eng J 5:447. https://doi.org/10.28991/cej-2019-03091259
Zdujić A, Trivunac K, Pejić B et al (2021) A comparative study of Ni (II) removal from aqueous solutions on Ca-alginate beads and alginate-impregnated hemp fibers. Fibers Polym 22:9–18. https://doi.org/10.1007/s12221-021-9814-6
Zhang H, Hu X, Lu H (2017a) Ni(II) and Cu(II) removal from aqueous solution by a heavy metal-resistance bacterium: kinetic, isotherm and mechanism studies. Water Sci Technol 76:859–868. https://doi.org/10.2166/wst.2017.275
Zhang Q, Gao J, Qiu YR (2019) Removal of Ni (II) and Cr (III) by complexation-ultrafiltration using rotating disk membrane and the selective separation by shear induced dissociation. Chem Eng Process Process Intensif 135:236–244. https://doi.org/10.1016/j.cep.2018.12.005
Zhang T, Zhu X, Shi L et al (2017b) Efficient removal of lead from solution by celery-derived biochars rich in alkaline minerals. Bioresour Technol 235:185–192. https://doi.org/10.1016/j.biortech.2017.03.109
Zhou Y, YangHui Z, Huang J et al (2017) Ni(II) removal from aqueous solution by biosorption and flocculation using microbial flocculant GA1. Res Chem Intermed 43:3939–3959. https://doi.org/10.1007/s11164-016-2845-8
Zinicovscaia I, Yushin N, Grozdov D et al (2021) Removal of metals from synthetic and real galvanic nickel-containing effluents by Saccharomyces cerevisiae. Chem Ecol 37:83–103. https://doi.org/10.1080/02757540.2020.1817404
Złotko K, Waśko A, Kamiński DM et al (2021) Isolation of chitin from black soldier fly (Hermetia illucens) and its usage to metal sorption. Polymers (basel) 13:1–16. https://doi.org/10.3390/polym13050818
Zulfikar MA, Mariske ED, Djajanti SD (2012) Adsorption of lignosulfonate compounds using powdered eggshell. Songklanakarin J Sci Technol 34:309–316
Funding
The publication stage was supported by the Leading Research Groups support project from the subsidy increased for the period 2020–2025 in the amount of 2% of the subsidy referred to Art. 387 (3) of the Law of 20 July 2018 on Higher Education and Science, obtained in 2019.
Author information
Authors and Affiliations
Contributions
S.C.: Conceptualization, Investigation, Writing-Original draft preparation; E.B.-A.: Writing—Original draft preparation and Reviewing and Editing, Supervision; P.L.: Resources, Writing—Reviewing and Editing, Supervision. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
The authors of manuscript “Recent trends in Ni(II) sorption from aqueous solutions using natural materials”: Sylwia Charazińska, Ewa Burszta-Adamiak and declare Paweł Lochyński that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Charazińska, S., Burszta-Adamiak, E. & Lochyński, P. Recent trends in Ni(II) sorption from aqueous solutions using natural materials. Rev Environ Sci Biotechnol 21, 105–138 (2022). https://doi.org/10.1007/s11157-021-09599-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11157-021-09599-5