Abstract
Biosorption is an effective way of extracting heavy metal ions from aqueous solutions of various compositions. The brown algae, Cystoseria indica, when treated with sodium chloride, demonstrates significant capacity to extract cadmium and nickel, simultaneously, from aqueous solutions. The batch system was running over wide ranges of initial metal ion concentrations (5–150 mg/L), pH (2–6), adsorbent mass (1–4 g/L), and contact times (20–300 min), at a temperature of 25 °C. The results obtained when applying the system in these conditions exhibit higher removal capacities for cadmium than nickel. The optimal conditions of the biosorption process were found as the adsorbent mass of 1 g/L, initial concentration of adsorbates of 100 mg/L and pH of 6. The equilibrium data obtained are better described by the extended-Freundlich isotherm for nickel and cadmium. The maximum biosorption of nickel and cadmium in binary-metal-component system were 18.17 and 55.34 mg/g, respectively. The kinetic data derived from these experiments were evaluated with pseudo-first-order, pseudo-second-order and intra-particle-diffusion kinetic models. Kinetic examination of the equilibrium data derived from these models suggest that the adsorption of nickel and cadmium both follow the intra-particle-diffusion kinetic model.
Similar content being viewed by others
Introduction
Heavy metals constitute significant contaminants in the oceans, in terrestrial water courses and industrial wastewaters. Typically, pollution related to heavy metals is associated with various industries such as batteries, mining, nuclear reactors, oil refineries, and agriculture. Nickel and cadmium are two of the most common heavy-metal contaminants, derived particularly from oil refining, textiles manufacture, batteries, paints, and coatings [1]. The presence of heavy metals in the environment causes damage to many species in the biosphere, including adverse human health impacts and ecosystem disruption [2, 3]. Soluble heavy metal components are not biodegradable and they tend to accumulate in living organisms as well as oceans, lakes, and rivers [4]. Common methods used to separate heavy metals from industrial wastewaters include chemical precipitation, filtration, reverse osmosis, membranes, ion exchange and adsorption [5]. There are a variety of treatment processes deployed to remove heavy metal ions from the environment, but these, typically, are associated with expensive running costs [6]. Consequently, there is an urgent requirement to develop more-effective and low-cost processes to remove heavy metals from aqueous solutions [7].
Several biomass-based heavy-metal extraction methods have been proposed. A large quantity of materials has been investigated as biosorbents for the removal of metals or organics extensively. The tested biosorbents can be basically classified into the following categories: bacteria (Bacillus subtilis), fungi (Rhizopus arrhizus), yeast (Saccharomyces cerevisiae), algae, industrial wastes, agricultural wastes and other polysaccharide materials [3]. Biosorption technology is well known for providing practically-feasible methods for removing heavy metal from the environment [8].
A number of studies have demonstrated that among biosorbents, marine algae, especially the brown algae, are excellent biosorbent for heavy metals [9]. The carboxylic group is known to be the key acidic functional group in the brown algae [10]. This is because of the carboxylic groups constitute the highest percentage of titratable sites in dried brown algae biomass. It is the existence of these active sites that provides the capacity of brown algae to adsorb heavy metals [11]. As brown algae occur in abundance in the biosphere there is the potential to exploit them as cost-effective biosorbents [12]. Despite their valuable biosorbent properties for extracting and recovering heavy metals, brown algae have the potential to cause secondary pollution [13]. Organic substances (e.g., alginate) released as the brown algae biosorption processes proceed are potential pollutants that can hinder their use industrial applications. Moreover, there is the potential for leaching to occur as biosorption proceeds, thereby removing key adsorptive components of brown algae causing degradation their biosorption capacity [14].
The Gulf of Oman is considered as one of the main ecological capitals with the highest biological diversity. Macroalgae is found in the northern coast of the Gulf of Oman is known as the wealthiest sources of macroalgae. Brown algae (Cystoseria indica) are vital groups of bioactive complexes in the northern coast of the Gulf of Oman. These algae (C. indica) are reputation due to their high density and richness in the region, growth in large sizes and valuable combinations such as alginic acid, iodine vitamins, and minerals. The Gulf of Oman is used for fisheries, aquaculture activities, import and export, economy, urbanization, and industries Heavy metals discharging into the marine ecosystem of the Gulf of Oman have possible to negatively affect marine organism [15].
Here, the surface of brown algae (Cystoseria indica), derived from the Oman Sea is treated with sodium chloride to increase the stability of its biosorbent components. The effects of the initial concentrations of nickel and cadmium on the biosorbent performance of Cystoseria indica are established and the optimal conditions of the biosorption process were found. Biosorption equilibrium data are evaluated using extended-Langmuir and extended-Freundlich isotherms. The biosorption mechanism is also investigated in terms of its kinetic reaction characteristics at optimum concentrations over a range of contact times.
Methods
Materials and preparations
A set of aqueous metal solutions displaying various concentrations of cadmium (Cd) and Nickel (Ni) were established by diluting cadmium nitrate (Cd (NO3)2·4H2O) and nickel nitrate (Ni (NO3)2·6H2O) with distilled water. The pH of the solutions was adjusted using HCl (0.1 N) and NaOH (0.1 N) to achieve the desired values.
Cystoseria indica samples were gathered from the coastal area of the Oman Sea, Iran. The algal samples were initially washed with deionized water to remove surface contaminants and impurities (e.g. sand and salt). Clean algal samples were then dried by exposure to the sun for 24 h. Dried algal samples were then crushed and subsequently sieved to a particle size of 0.5–1.0 mm. The sieved samples were then washed with deionized water and desiccated in an oven at 70 °C for 24 h. The samples were then subjected to surface alkaline treatment with sodium chloride (NaCl), which, as demonstrated by Brierley [16], disrupts the cell walls of algae potentially exposing additional functional groups to which metal ions are able to bind (Brierley protocol) [16, 17].
Batch biosorption
Bioadsorption experiments were conducted over wide ranges of initial metal ion concentrations (5–150 mg/L), pH (2–6), adsorbent mass (1–4 g/L), and contact times (10–180 min), at a temperature of 25 °C, with the algae added to a series of Erlenmeyer flasks. Previous research has shown that the cadmium and nickel biosorption ability was excellent using brown algae (Cystoseria indica) at the pH of 6 [10]. The flasks contained a 100-mL solution of nickel and cadmium simultaneously. The flasks were agitated at 175 rpm in an incubator shaker at 25 °C for 5 h. Algae were separated from the metal solutions by filtration. After each experiment, the remaining metal ions concentrations in solutions after the adsorption experiments were determined using atomic absorption spectroscopy (PinAcle 500, Perkin Elmer, USA). The concentrations of the metals adsorbed (mg/L) by the treated algae was calculated using Eq. (1):
where: Ci and Cf (mg/L) are the initial and final metal-ion concentrations in the solution, respectively; V is the solution volume in liters; and, m is the mass of biosorbent.
Unlike continuous system, the batch adsorption process is running without input and output.
Biosorption isotherm
Industrial effluents typically contain a combination of several metal contaminants [18]. Consequently, it is usually necessary to remove more than one metal contaminant during bioadsorption. It is for this reason that we investigate here the efficiency of Cystoseria indica in simultaneously removing for nickel and cadmium from aqueous solutions. Published experiments suggest that many adsorbents are less effective in systems contaminated with multiple-metal components than those contaminated with single-metal components [19]. This effect is likely to be caused by metal ions hindering each other during the adsorption process in multi-component systems. The mathematical models considered to best describe biosorption phenomena, are the Langmuir and Freundlich isotherms [20, 21]. The Langmuir isotherm is based on three basic theoretical assumptions:
Adsorption is monolayer;
All the active sites are equivalent and the adsorption process is uniform; and,
The adsorption of a molecule by a free site does not depend on the occupied neighboring sites.
The Langmuir equation is applicable to homogenous adsorption while the Freundlich equation is employed to describe heterogeneous systems and reversible adsorption and is not restricted to the formation of monolayers. The mathematic expression of the extended-Langmuir isotherm can be expressed by Eqs. (2) and (3):
where: KL1, KL2, qm1, qm2 are the Langmuir isotherm constants; Ce1 (mg/L) and Ce2 (mg/L) are the unadsorbed concentration of nickel and cadmium, respectively; and qe1 (mg/L) and qe2 (mg/L) are the equilibrium adsorption values for nickel and cadmium, respectively [22, 23].
On the other hand, for multi-component systems, experimental equilibrium data were difficult to obtain. Therefore, several methods were proposed to estimate multi-component adsorption equilibrium in a mixture on the basis of single-component adsorption isotherms [24]. For example, Freundlich extended model was proposed to predict binary component adsorption systems using known single adsorption isotherms the Freundlich isotherm assumes that the stronger binding sites are occupied first. The mathematic expression of extended-Freundlich isotherm can be expressed as Eqs. (4) and (5):
where: x1, y1, z1, x2, y2, z2 are the Freundlich isotherm constants; Ce1 and Ce2 are the unadsorbed concentrations of nickel and cadmium, respectively; the unit of Ce1 and Ce2 are mg/L. qe1 and qe2 are the equilibrium adsorption values of nickel and cadmium, respectively; qe1 and qe2 are equal to mg/L. Suffix 1 indicates nickel and suffix 2 signifies cadmium; and, KF1, KF2, n1 and n2 are the Freundlich isotherm constants [25]. These are obtained from the equilibrium data of the single metal components and are listed in Table 1 [26, 27]. These data come from experiments in which nickel and cadmium were adsorbed by the Cystoseria indica separately. All the model parameters were evaluated by non-linear regression using MATLAB software. Coefficient of determination (R2) is used to measure the goodness of fit between the measured data and the theoretical models.
The coefficient of determination (R2) is defined by Eq. (6):
A higher R2 (i.e. closer to 1.0) indicates a better fitting curve.
Biosorption kinetics
Experimental observations indicate that the adsorption processes for both cadmium and nickel using Cystoseria indica reached equilibrium after approximately 180 min of contact. For batch systems, the adsorption process kinetics can be described by a number of models [28, 29]. These models include: pseudo-first-order (Eq. 7), pseudo-second-order (Eq. 8), and intra-particle diffusion (Eq. 9) [30]. The linearized pseudo-first-order, pseudo-second-order, and intra-particle diffusion kinetic models are defined by Eqs. (7), (8) and (9), respectively [30, 31]:
where: qt (mg/L) and qe (mg/L) are the metal adsorbed after specific exposure times and at equilibrium conditions, respectively; and K1 (min−1) and K2 (g/mg min) are the first-order reaction rate equilibrium constant and the second-order reaction rate equilibrium constant, respectively; and, in Eq. 9, C (mg/g) is adsorption constant and Kp (mg/g min0.5) is the rate of intra-particle diffusion.
Results and discussion
Characterization of C. indica
SEM micrographs were obtained using a scanning electron microscope (Hitachi SU-70, Japan). Figure 1 shows SEM images of the C. indica at the different magnifications. C. indica had a honeycomb structure. Pores greater than 200 mm were observed at surface of C. indica. SEM image of C. indica showed no uniform pore distribution and high roughness.
Oxygen-containing acidic surface functional groups (hydroxyl, phenolic, aldehydic, ketonic, and carboxylic) on the surface of biochar, which are active binding sites for the adsorption process, were derived from macromolecules in macroalgae [32]. The FTIR spectra of C. Indica (Fig. 2) showed the intense bonds at ~ 3400 1/cm (O–H stretching vibration), which can be allocated to hydroxyl functional groups [32]. The absorbance bonds at ~ 2900 1/cm, ~ 1640 1/cm, and ~ 1100 1/cm were assigned to vibration modes of C–H, aromatic CC and, COC= bonds, respectively [33, 34]. Furthermore, the intense peak at 1037 1/cm (Si-OS-i stretching vibration) is due to high silica content of the biomasses and consequently the ash content of the biochars [35]. The following indices obtained from the integration of absorbance peak regions were utilized to evaluate the chemical properties of biochars, aromaticity, the degree of the aromatic ring, aliphatic chain length, oxygen function groups, and ‘C’ factor. The ratio of the C=O to CC= stretching group (‘C’ factor) was used as a criterion for describing oxygen-containing groups in samples [33, 35].
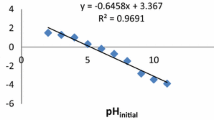
Effect of pH
One of the most important factors affecting the biosorption of metal ions is acidity of solution. Solution pH affects the cell wall metal binding sites and the metal ion chemistry in water. In the present study, the effect of pH and biosorbent pretreatment on two metals biosorption was understandable (Fig. 3). At low pH, metal cations and protons contested for binding sites, resulting in low adsorption of metal ions. Moreover, the number of positively charged surface sites increased, which did not favor the adsorption of positively charged metal cations due to electrostatic repulsion. With an increase in pH, the number of negatively charged active sites also increased, which would attract more positively charged metal ions for binding. The pH of 6 is the best pH in which both metals reach their highest adsorption capacity. Consequently, this pH (6) was selected for further investigations.
Effect of biosorbent dosage
The biosorbent dosage is an important factor because it can determine the capacity of biosorbent for a given initial metal ion concentration. The effect of biomass dosage in the range of 1–4 g/L on the Ni and Ca uptake by NaCl-pretreated C. indica in the initial metal concentration of 100 mg/L and temperature of 25 °C at pH 6 is shown in Fig. 4. According to this Fig. 4, the biosorption capacity of Ni(II) and Ca(II) from an aqueous solution decreased with the increase of the biosorbent dosage, because some of the active sites stayed unsaturated during the biosorption process while the number of sites available on the NaCl-pretreated C. indica biomass increased by increasing the biosorbent dosage. Consequently, the dosage of 1 g/L was selected for further investigations.
Effect of contact time
The rate of adsorption is of high importance in modeling and designing the adsorption process in industry. Therefore, the effect of contact time on nickel and cadmium sorption onto the C. indica is revealed in (Fig. 5) in the initial concentration of 100 mg/L and 25 °C at pH 6 with the contact time varies from 10 to 180 min. According to Fig. 3, biosorption capacity increased with increasing contact time. More than 95% of the total adsorption of nickel and cadmium occurs within the first 60 min. This behavior might be due to the fact that initially all active sites on the adsorbents surface are vacant and the solution concentration is high. After that period, few surface active sites will be available, so only a very low increase in the metal uptake is observed. Therefore, a contact time of 180 min was selected for all the equilibrium tests. Yinghui and Fang studied the biosorption of Cu(II), Cd(II), Ni(II) and Zn(II) onto pretreated biomass of brown algae [34] and Ibrahim, investigate the biosorption of Co(II), Cd(II), Cr(II) and Pb(II) by red macro-algae [35] and reported similar contact time behavior for Cd(II) and Ni(II) metal ions as observed in current study.
Effect of temperature
The biosorption studies were carried out for both heavy metal at four different temperatures 20, 25, 30 and 35 °C in binary component systems, and the obtained results were presented in Fig. 6. It can be observed that the biosorption capacity of cadmium increased with an increase of temperature from 20 to 25 °C, over 180 min of contact time. Also, the biosorption capacity of cadmium was constant after 25 °C. On the other hand the biosorption capacity of nickel was constant with increasing of temperature.
Effect of initial concentration
Published research [27, 28, 36] indicates that nickel and cadmium achieve their highest capacities of algal adsorption in aqueous solutions with concentrations ranging from 5 to 130 mg/L when they are in the two-component system. The effects of interfering metal ions on the adsorption of cadmium and nickel ions by Cystoseria indica are illustrated in Figs. 7 and 8.
Tests were conducted to compare the adsorption performance of Cystoseria indica with respect to nickel and cadmium. An initial concentration of nickel of 60 mg/L was set in three containers. In one of those containers, the initial concentration of cadmium was set at 30 mg/L, in the second container it was set at 60 mg/L, and in the third container it was set at 130 mg/L. The measured adsorption of nickel by Cystoseria indica was 17.5, 9.6 and 5 mg/L, respectively in the first, second and third containers. For comparison purposes, an initial concentration of cadmium of 60 mg/L was set in another three containers. In one of those containers the initial concentration of nickel was set at 30 mg/L, in the second container was set at 60 mg/L, and in the third container it was set at 130 mg/L. The measured adsorption of cadmium by Cystoseria indica was 35, 20.6 and 10.1 mg/L, respectively in the first, second and third container. These observations suggest that Cystoseria indica clearly has a tendency to adsorb significantly more cadmium than nickel. Adsorption is believed to occur at several functional groups within algae, including carboxyl, sulfonic acid and alginate [37]. There may also be other functional groups in Cystoseria indica that are involved in the adsorption of both metal ions. Figures 7 and 8 display the synergistic effect in the binary system by which cadmium is more successful adsorbed at active sites by Cystoseria indica. The alginic acid component, present in alginate within the cell walls of Cystoseria indica and other brown algae, has been suggested as the reason for the higher cadmium adsorption potential [29, 38]. Metallic behavior in multi-component systems is believed to strongly depend on the physical and chemical characteristics of the adsorbent that, in turn, affects the equilibrium behavior of the biosorption processes. The number and type of ions, the concentration of each component ion, pH, and the isotherm models considered will determine the equilibrium constants [39,40,41].
Adsorption isotherms
The isotherm constants and coefficient of determination (R2) calculated from the tests for the Cystoseria indica tests with the extended-Langmuir and extended-Freundlich isotherms are listed in Table 2. The optimized conditions were obtained from above section following: Initial concentration 100 mg/L, pH 6, biosorbent dosage 1 g/L. Also, all experiments were lasted 180 min at 25 °C. The appropriate parameter values for the isotherm models considered for Cystoseria indica adsorption of nickel and cadmium are derived from the experimental data by maximizing the objective function (i.e., R2). These values are listed in Table 1 for single-metal-component systems and Table 2 for binary-metal-component system. For the nickel and cadmium binary system, the biosorption equilibrium data for Cystoseria indica are better described (i.e., higher R2) using the extended- Freundlich isotherm for nickel. On the other hand, the biosorption equilibrium data are better described (i.e., higher R2) using the extended-Freundlich isotherm for cadmium.
Alginate is considered to play a pivotal role in the ability of different brown algae species to adsorb heavy metal ions [42]. Alginate has a non-uniform macromolecular structure providing it with the ability to form a selective bond with cadmium ions. Because of its orientation, shape and the space between dents, glucuronic acid create better complex with cadmium than mannuronic acid in terms of allowing ions to permeate in the structure [31]. The molecular structure of alginate is illustrated in Fig. 9.
The molecular structure of alginic acid including glucuronic acid (G) mannuronic acid (M) as it exists within alginate [43]
Figures 10, 11, 12 and 13 show curve-fitting for the extended-Freundlich and extended-Langmuir isotherms based on the experimental data. The extended-Freundlich isotherm data demonstrates positive correlations between experimental data (q exp) and calculated-adsorption (q cal) for both cadmium and nickel. The extended-Langmuir isotherm data shows low-confident correlation between experimental data (q exp) and calculated-adsorption (q cal) for nickel and cadmium data because of the low value of R2. It is apparent that the extended-Freundlich isotherm was a better fit than the extended-Langmuir isotherm equation for nickel and cadmium sorption according to the values of R2 and, the highest value of R2 was 0.97 for cadmium in extended-Freundlich isotherm. Also, the highest value of R2 was 0.94 for nickel in extended-Freundlich isotherm. Two best values suggested that the extended-Freundlich is the best model for nickel and cadmium sorption. It indicates that the sorption of nickel and cadmium ions are similar a multilayer sorption.
Adsorption process kinetics
Table 3 shows that the experimental data using Cystoseria indica conform more closely to the intra-particle diffusion model for both nickel and cadmium. However, the fit with that model is only approximate (i.e. R2 ≤ 0.93), with the lower-exposure-time data responsible for much of the data spread. On the other hand, the data fit for the pseudo-first-order and pseudo-second-order models is very poor, R2 ≤ 0.741 and ≤ 0.811, respectively. Table 3 lists the coefficient of determination (R2) values of 0.90 for nickel and 0.93 for cadmium, for the intra-particle diffusion model, values that are significantly higher than those achieved by pseudo-first-order and pseudo-second-order models.
The consequences achieved in this study can be related with to some others reported in the works Comparing uptake capacities of brown marine algae obtained for metal concentrations in the liquid phase similar to that used in the present study, have shown that In spite of some differences in the experimental conditions, cadmium, and nickel uptake capacities are close to those obtained by other researchers. The accumulation profiles obtained for the different brown algae species show that the metal uptake is rather fast, and more than 75% of the total uptake occurs within the first 20 min and then no further significant adsorption is observed [31, 44].
Availability of data and materials
Not applicable.
References
Kim DJ, Shin HJ, Ahn BK, Lee Jh (2016) Competitive adsorption of thallium in different soils as influenced by selected counter heavy metals. J Appl Biol Chem 59(5):695–701. https://doi.org/10.1007/s13765-016-0215-2
Luo Z, Wang Z, Yan Y, Li J, Yan C, Xing B (2018) Titanium dioxide nanoparticles enhance inorganic arsenic bioavailability and methylation in two freshwater algae species. J Environ Pollut 238(5):631–637
Xu L, Shen J, Marinova D, Guo X, Sun F, Zhu F (2013) Changes of public environmental awareness in response to the Taihu blue-green algae bloom incident in China. J Environ Dev Sustain 15(5):1281–1302
Park JH, Ok YS, Kim SH et al (2015) Characteristics of biochars derived from fruit tree pruning wastes and their effects on lead adsorption. J Korean Soc Appl Biol Chem 58(5):751–760. https://doi.org/10.1007/s13765-015-0103-1
Erdoğdular AO, Apar DK (2019) Bioremoval of reactive dye Remazol Navy by kefir grains. J Appl Biol Chem 62:22. https://doi.org/10.1186/s13765-019-0429-1
Kim SU, Owens VN, Kim SY, Hong CO (2017) Effect of different way of bottom ash and compost application on phytoextractability of cadmium in contaminated arable soil. Appl Biol Chem 60(4):353–362. https://doi.org/10.1007/s13765-017-0287-7
Lee JH, Hur HG (2014) Microbially facilitated incorporation of As(III) into bioreduced Fe-(hydr)oxide minerals. J Appl Biol Chem 57(1):123–128. https://doi.org/10.1007/s13765-014-4026-z
Li J, Chen J, Chen S (2018) Supercritical water treatment of heavy metal and arsenic metalloid-bioaccumulating-biomass. J Ecotoxicol Environ Saf 157:102–110
Zhimiao Z, Xinshan S, Wei W, Yanping X, Zhijie G, Yuhui W, Yufeng Z, Yu C, Mengyuan M (2016) Influences of iron and calcium carbonate on wastewater treatment performances of algae based reactors. J Bioresour Technol 216:1–11
Akbari M, Hallajisani A, Keshtkar AR, Shahbeig H, Ghorbanian SA (2015) Equilibrium and kinetic study and modeling of Cu(II) and Co(II) synergistic biosorption from Cu(II)-Co(II) single and binary mixtures on brown algae C. indica. J Environ Chem Eng 3:140–149
Chu WL, Dang NL, Kok YY, Yap KSI, Phang SM, Convey P (2018) Heavy metal pollution in Antarctica and its potential impacts on algae. J Polar Sci 20(1):75–83. https://doi.org/10.1016/j.polar.2018.10.004
Shanura Fernandoa IP, Nahb JW, Jeona YJ (2016) Potential anti-inflammatory natural products from marine algae. J Environm Toxicol Pharmacol 48:22–30
Montazer-Rahmatia MM, Rabbania P, Abdolalia A, Keshtkarb AR (2011) Kinetics and equilibrium studies on biosorption of cadmium, lead, and nickel ions from aqueous solutions by intact and chemically modified brown algae. J Hazard Mater 185(1):401–407. https://doi.org/10.1016/j.jhazmat.2010.09.047
Davis TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal biosorption by brown algae. Water Res 37:4311–4330
Sinaei M, Loghmani M, Bolouki M (2011) Application of biomarkers in brown algae (Cystoseria indica) to assess heavy metals (Cd, Cu, Zn, Pb, Hg, Ni, Cr) pollution in the northern coasts of the Gulf of Oman. J Ecotoxicol Environ Saf 164:675–680
Pinnola A, Formighieri C, Bassi R (2019) Algae: a new biomass resource. In: Kaltschmitt M (ed) Energy from organic materials (biomass), pp 165–197 https://doi.org/10.1007/978-1-4939-7813-7_436
Sari A, Tuzen M (2008) Biosorption of cadmium (II) from aqueous solution by red argue (Ceramium virgatum): equilibrium, kinetic and thermodynamic studies. J Hazard Mater 157(2–3):448–454
Hadi P, Barford J, McKay GM (2013) Synergistic effect in the simultaneous removal of binary cobalt–nickel heavy metals from effluents by a novel e-waste-derived material. J Chem Eng 228(2013):140–146
Villar da Gama BM, do Nascimento GE, Cesar D, Sales S, Rodríguez-Díaz JM, Bezerra de Menezes Barbosa CMB, Duarte MMMB (2018) Mono and binary component adsorption of phenol and cadmium using adsorbent derived from peanut shells. J Clean Prod 201:219–228
Negm NA, Wahed MGAbdE, Hassan ARA, Abou Kana MTH (2018) Feasibility of metal adsorption using brown algae and fungi: effect of biosorbents structure on adsorption isotherm and kinetics. J Mol liq 264:292–306. https://doi.org/10.1016/j.molliq.2018.05.027
Gustafsson JP, Akram M, Tiberg C (2015) predicting sulphate adsorption/desorption in forest soils: evaluation of an extended Freundlich equation. J Chemosphere 119:83–89
Putro JN, Santoso SP, Ismadji S, Ju YH (2017) Investigation of heavy metal adsorption in binary system by nanocrystalline cellulose e Bentonite nanocomposite: improvement on extended Langmuir isotherm model. J Microporous Mesoporous Mater 246:166–177
Gaikwad MS, Balomajumder C (2017) Simultaneous electrosorptive removal of chromium(VI) and fluoride ions by capacitive deionization (CDI): multicomponent isotherm modeling and kinetic study. J Sep Purif Technol 182:272–281. https://doi.org/10.1016/j.seppur.2017.06.017
Wang C, Boithias L, Ning Z, Hana Y, Sauvage S, Sanchez-Perez JM, Kuramochi K, Hatano R (2016) Comparison of Langmuir and Freundlich adsorption equations within the SWAT-K model for assessing potassium environmental losses at basin scale. J Agric Water Manag 180(PartB):205–211. https://doi.org/10.1016/j.agwat.2016.08.001
Fan Huan HJ, Tsai YS, Furuya E (2008) Prediction of individual Freundlich isotherms from binary and ternary phenolic compounds mixtures. Chemosphere 71:886–893
Chen Z, Ma W, Han M (2008) Biosorption of nickel and copper and to treated algae (Undria pinnatifida): application of isotherm and kinetics study. J Hazard Mater 155:327–333
Yalçın S, Sezer S, Apak R (2012) Characterization and lead(II), cadmium(II), nickel(II) biosorption of dried marine brown macro algae Cystoseira barbata. Environ Sci Pollut Res 19(18):3118–3125. https://doi.org/10.1007/s11356-012-0807-2
Nuhoglu Y, Malkoc E, Gurses A, Canpolat N (2002) The removal of cu (II) from aqueous solutions by Ulothrix zonata. J Bioresour Technol 85:331–333
Mudhoo A, Garg VK, Wang S (2012) Heavy metals: toxicity and removal by biosorption. In: Lichtfouse E, Schwarzbauer J, Robert D (eds) Environmental chemistry for a sustainable world, pp 379–442
Volesky B (2001) Detoxification of metal-bearing effluents: biosorption for the next century. J Hydrometall 59:203–216
Hamdy A (2000) Biosorption of heavy metals by marine algae. Curr Microbiol 4:232. https://doi.org/10.1007/s002840010126
Leng L, Yuan X, Huang H, Shao J, Wang H, Chen X, Zeng G (2015) Bio-char derived from sewage sludge by liquefaction: characterization and application for dye adsorption. J Appl Surf Sci 346:223–231
Xiao B, Dai Q, Yu X, Yu P, Zhai S, Liu R, Guo X, Liu J, Chen H (2018) Effects of sludge thermal-alkaline pretreatment on cationic red X-GRL adsorption onto pyrolysis biochar of sewage sludge. J Hazard Mater 343:347–355
Liu Y, Cao Q, Luo F, Chen J (2009) Biosorption of Cd2+, Cu2+, Ni2+ and Zn2+ ions from aqueous solutions by pretreated biomass of brown algae. J Hazard Mater 163:931–938. https://doi.org/10.1016/j.jhazmat.2008.07.046.18755544
Abdulrazzaq H, Jol H, Husni A, Abu-Bakr R (2014) Characterization and stabilisation of biochars obtained from empty fruit bunch, wood, and rice husk. J Bioresour 9:2888–2898
De France FP, Tavares APM, Da Costa ACA (2002) Calcium interference with continuous biosorption of zinc by Sargassum sp. (Phaeophyta) in tubular laboratory reactors. J Bioresour Technol 83:159–163
Emami Z, Ehsani M, Zandi M, Foudazi R (2018) Controlling alginate oxidation conditions for making alginate-gelatin hydrogels. J Carbohydr Polym 198:509–517
Xiong Y, Xu J, Shan W, Lou Z, Fang D, Zang S, Han G (2013) A new approach for rhenium(VII) recovery by using modified brownalgae Laminaria japonica adsorbent. J Bioresour Technol 127:464–472
Volesky B, Naja GM (2011) Biosorption for industrial application. Biosorption process fundamentals and a pilot design. Biosorption Ind Appl 6:25. https://doi.org/10.1016/B978-0-08-088504-9.00399-8
Davisa TA, Volesky B, Mucci A (2003) A review of the biochemistry of heavy metal bio sorption by brown algae. J Water Res 37:4311–4330
Senthilkumar R, Vijayaraghavan K, Thilakavathi M, Iyer PVR, Velan M (2006) Seaweeds for the remediation of waste waters contaminated with zinc(II) ions. J Hazard Mater 136:791–799
Kumar M, Singh AK, Sikandar M (2018) Study of sorption and desorption of Cd (II) from aqueous solution using isolated green algae Chlorella vulgaris. J Appl Water Sci 8:225. https://doi.org/10.1007/s13201-018-0871-y
Pereira RF, Sousa A, Barrias CC, Bayat A, Granja PL, Bártolo PJ (2017) Advances in bioprinted cell-laden hydrogels for skin tissue engineering. J Biomanufacturing Rev 2:1. https://doi.org/10.1007/s40898-017-0003-8
Freitas OMM, Martins RJE, Delerue-Matos CM, Boaventura RAR (2008) Removal of Cd(II), Zn(II) and Pb(II) from aqueous solutions by brown marine macro algae: kinetic modelling. J Hazard Mater 153:493–501
Acknowledgements
This research was supported by Islamic Azad University branch of North Tehran (2018–2019).
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
MK carried out heavy metal sampling, heavy metal solution analyses, and data organization. AH, MK, DAW and NM participated in interpreting the obtained results and organizing the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Khajavian, M., Wood, D.A., Hallajsani, A. et al. Simultaneous biosorption of nickel and cadmium by the brown algae Cystoseria indica characterized by isotherm and kinetic models. Appl Biol Chem 62, 69 (2019). https://doi.org/10.1186/s13765-019-0477-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13765-019-0477-6