Abstract
Carbon adsorbents derived from biomass (agricultural and household residues) have been widely used in the sequestration of hazardous substances from the environment due to their distinctive qualities of large internal surface area, mechanical integrity, and regeneration. The need for carbon adsorbents for sequestration of dyes, heavy metals, and crude oil components has increased because of environmental concerns. This has led to studies of carbon adsorbents derived from agricultural and household biomass residues. These adsorbents have been used to remove pollutants. Although numerous reviews have been published before, analogy of results obtained using different adsorbents is hard due to dissimilarities in research data. Against this backdrop, the purpose of the research survey was to review the contemporary publications regarding the production of activated carbon from biomass sources highlighting specifically its utilization in removing toxic wastes from water solution such as oil spill, dyes, and sundry hazardous substances. Also the work focuses on the methods for the restoration of the spent adsorbents and their end use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Carbon adsorbents are highly porous, made up of mostly sp2-hybridized carbon, and have large internal surfaces. Also, the other properties of a good adsorbent include having sufficient pores sizeable to pass the molecules, be regenerable, not degrade rapidly from constant usage, and withstand vibration from industrial units (Gupta and Tai 2016). There are, however, other adsorbents in commercial use.
The adsorbents in commercial use in the industry with their uses are silica gel for drying of gases, activated alumina for HCl removal, carbons for decolorization of syrups and (waste) water, and zeolites for the separation of normal paraffins from branched paraffins. Other adsorbents in use include polymers and resins for water purification and clays for edible oil treatment (Richardson et al. 2010):
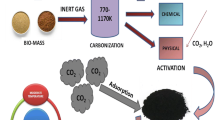
There are two fundamental steps for producing activated carbon adsorbents (Alaya et al. 2000; Zondlo and Velez 2007): “the pyrolysis of the raw material below 800 °C, and physical or chemical activation of the carbonized material.”
There have been studies in some treatment methods. These include coagulation, ultrafiltration, electrochemical adsorption, photo-oxidation, and ion exchange. The adsorption technique was found to be most suitable because it removes pollutants at very low concentrations (Sharma et al. 2012). Furthermore, adsorption requires less land area (half to quarter of what is needed in biological method). When a solid surface makes contact with a liquid or gas medium, the molecules from the mass of the fluid phase have the likelihood to adsorb on the solid (Otowa et al. 1997; Bansal et al. 1988). “An adsorption process depends on solid–liquid equilibrium and the rate of mass transfer” (Gupta et al. 2007).
Oil pollutants were formerly removed from water using synthetic materials. This practice went on until it was discovered that dispersants like Corexit could contaminate water, leading to the search for alternative material. Converting agricultural by-products into activated carbon provides another disposal method which minimizes environmental pollutions. A country generates its agricultural byproduct based on its agriculture (Ezechi et al. 2017). “In Malaysia about 1.2 million tons of agricultural waste is generated yearly” (Billy et al. 2013). Agro-waste is incinerated which can pollute the atmosphere. The common waste management technology in India is land filling (70–90%). “In Berlin the waste management technology is incineration and land filling which can lead to methane emissions.” The Nigerian agriculture produces huge amount of corn, rice, and groundnut residues, most of which remain unused (Rosenkranz et al. 2011).
Table 1 presents some review articles on wastewater decontamination by economical adsorbents, published in 2013–2019.
Bhatnagar et al. (2015) explored the use of agro-waste peels (e.g., orange, pomelo, grapefruit, lemon, banana, cassava, and jackfruit) as sorbents for the decontamination of pollutants from contaminated water. The sorption capacities of waste peel biosorbents for organic and inorganic pollutants were reviewed. Different modification methods which have been employed to develop modified peel-based adsorbents were also presented to highlight and discuss the key advancements in the preparation of novel adsorbents using agro-waste peels. Adsorption mechanisms responsible for pollutant removal by peel-based adsorbents have also been discussed. Future research needs in this field were proposed. Several gaps which need more attention were enumerated by the authors, such as enhancement of biosorption capacity through modification of biosorbent, assessment of biosorbents under multicomponent pollutants, investigation of these materials with real industrial effluents, recovery of metal ions, regeneration studies, and continuous flow studies.
Although numerous reviews have been published before, Gautam et al. (2014) carried out analysis of results obtained using a wide range of locally available non-conventional low-cost biosorbents for the removal of heavy metals as metal ions, viz Cd, Ni, Cr, Zn(II), Se(IV), and organometallic compounds from industrial effluents. Various adsorption models used to study the mechanism of adsorption such as isotherm, kinetics, and thermodynamic models and biosorption technologies (e.g., batch, column, and reactor) were also discussed. Further the authors cited that a direct comparison of data obtained using different biosorbents is difficult because of inconsistencies in the data presentation.
Thus, taking this situation into account the aim of this study was to analyze the contemporary research reports regarding the beneficiation of agricultural and household wastes for the production of efficient adsorbent using uncomplicated and economical methods. The objectives of the paper were to: compare and contrast the production and characterization of activated carbon from various sources, review the properties and adsorption capabilities of different biomass waste sorbents in oil spill recovery, highlight the major properties and sorption capabilities of different biomass waste sorbents for dyes recovery from wastewater, provide an overview of the major properties and adsorption capabilities of different biomass waste sorbents for removing sundry pollutants, and carefully highlight the reusability of activated carbon adsorbents and their end of life.
Production and characterization of activated carbon from different sources
As was presented earlier, the two main steps used to produce activated carbon adsorbents were: the pyrolysis of a carbon-rich raw material below 800 °C and activation of the carbonized material by physical or chemical method (Zhang et al. 2004; Zondlo and Venez 2007; Ani et al. 2019). The carbon-rich raw materials for producing activated carbon are cleaned, washed, and dried before physical or chemical activation is applied.
Physical activation also called thermal activation occurs in two steps (Jiazhen et al. 2018). It involves carbonization at the temperature range of 500–600 °C of a carbon-rich product with the activation of the charcoal between 800 and 1100 °C in the presence of oxidizing agents such as CO2, steam, air, or their mixtures. Kumar and Namasivayam (2009) prepared activated carbon by steam activation. In their work, washed, dried, and pulverized jatropha husk (450 g) in 100 ml of water in a tightly covered steel container was carbonized in a muffle furnace for 1 h at 800 °C. Ioannidou and Zabaniotou (2007) had reported in a review paper that corn cob, oak, corn husks, corn stover, rice straw, rice husks, pecan shells, peanut husks, and almond shells have been used to prepare activated carbon by physical method. Sulaiman et al. (2011) investigated the differences in ash content between oil palm wastes namely shell, frond and trunk; by soaking 100.0 g of precursor in 5.0 l tap water then leaving it for 10 min which is now with the unwashed biomass. Their findings show that the ash content in palm shell, frond and trunk declined to approximately 43.16, 52.18, and 7.42%, respectively upon water-washing.
For the chemical process, the two steps involving carbonization and activation are performed at the same time, with the raw material being mixed with some chemicals, as dehydrating agents and oxidants (Gupta et al. 2008; Mittal et al. 2008; Hai et al. 2017). Also Kumar and Namasivayam (2009) used concentrated acids such as H2SO4, HCl, HNO3, and H3PO4 for chemical activation. One hundred grams (100 g) of dried jatropha husk was combined with 2.8 parts by mass of concentrated acid and left in the oven at 185 ± 5 °C for 24 h. The carbonized product was washed with 500 ml distilled water four times and with 1% NaHCO3 solution. The final product was soaked overnight in 1% NaHCO3 solution to remove excess acid. The product was washed with water to pH 7, dried at 105 ± 5 °C for 8 h and sieved to 250–500 μm size. Rios et al. (2003) described a simple and versatile method for the introduction of heteroatoms such as sulphur and nitrogen containing molecules onto carbon surface which therefore improved its adsorption capability of Pb+2, Cu+2 and Ni+2.
The scanning electron micrograph (SEM) showed that when the jatropha husk was treated with activating chemicals, the pores in the surface of the char were wider, due to the removal of volatile compounds by chemical activation. The resulting morphology was not the same for all activating reagents, because the chemicals possess different properties. Furthermore, the acid-activated carbons produced higher yields than the steam-, ZnCl2-, and NaOH-activated carbons.
The properties and adsorption capabilities of different biomass waste sorbents in oil spill recovery
The influence of acetylation on oil sorption capability of corncobs, found around Enugu, Nigeria, was investigated by Nwadiogbu et al. (2016). In the study, the corncobs were treated with acetic anhydride to enhance the hydrophobic properties and also improve the adsorption in water. It was observed that acetylated corncobs were suitable for the sorption of crude oil from water.
“A good source of non-toxic magnetic sorbent that has potential in oil pollution treatment is coco peat powder (CPD) which is a byproduct of coconut palm. CPD is abundant in many countries, including China, Malaysia, Philipines and Thailand” (Owolabi et al. 1985; Keerthika et al. 2016). Yang et al. (2017) used mussel-inspired polydopamine to immobilize Fe3O4 nanoparticles on coco peat powder which was chemically modified by low-surface-energy octadecylamine to prepare a novel magnetic coco peat powder (MCPD) for selective oil–water separation. Table 2 presents the major properties and adsorption capabilities of different biomass wastes in oil spill recovery.
Some characteristics of biomass waste sorbents for removing dyes pollutants from wastewater
Some industries including textiles, rubber, plastics, printing, leather, cosmetics, etc., use large quantities of dyes to color their products. Dye-polluted wastewater is therefore generated. “Dye-bearing wastewater in natural streams and rivers causes severe pollution problems to the aquatic life, food web and also causes damage to the aesthetic nature of the environment” (Bharati and Ramesh 2013).
Some biomasses are being studied for removal of different dyes pollution by adsorption. Table 3 gives the properties and other qualities of biomass sorbent for removing dye from water. Garg et al. (2003) used rosewood sawdust to remove malachite green dye from water solution. Also to improve its efficiency, the adsorbent was treated with formaldehyde and sulfuric acid.
Further, Stavropoulos and Zabaniotu (2005) studied the potential of olive waste residue as adsorbent for removal of methylene blue pollution from water solution. It showed that the biomass has good potential.
Some adsorption characteristics of biomass waste sorbents for removing sundry pollutants
The biomass waste from rice husk ash (Mbui et al. 2002), olive seed waste (Stavropoulos and Zabaniotou 2005), cassava peels (Owamah 2014), waste bamboo (Ademiluyi et al. 2009), sugarcane bagasse and corn husk (Billy et al. 2013), cashew nut shell (Ponnusamy et al. 2011), corncob (El-Sayed et al. 2014), groundnut shell (Malik et al. 2007), walnut shell (Aygun et al. 2003), kenaf core fiber (Shamsudinn et al. 2016), rice shell (Aydin et al. 2008), rice husk (Ding et al. 2014; Xu et al. 2015; Yadav et al. 2015), Typha orientalis (cattail) leaves (Anisuzzaman et al. 2015), activated bone and wood (Okafor and Aneke 2006), wood derived biochar (Kizito et al. 2015), potato peel (Aman et al. 2008), and microwave-activated carbon coconut shells (Pillai et al. 2014) have been studied for removing pollutants from wastewater.
Stavropoulos and Zabaniotou (2005) studied the production of activated carbon from olive seed waste using KOH. The product was initially pulverized and sieved to 125–160 μm and subsequently dried at 110 °C for 24 h. To prepare the charcoal, pyrolysis of the olive seed waste was carried out at 800 °C for 1 h in inert atmosphere using a tubular fixed bed reactor.
Ademiluyi et al. (2009) performed batch and column tests for removing organic compounds from petroleum refinery effluent using granular activated carbon from Nigerian waste bamboo. The produced activated carbon was characterized by bulk density, porosity, pore volume, ash content, average particle size, moisture content, and pH. Granulated activated carbon from the biomass was used for the removal of organic pollutants from a refinery wastewater sample, after mechanical and biological treatment.
Anisuzzaman et al. (2015) investigated the production of activated carbon from Typha orientalis (cattail) leaf using muffle furnace. The work also evaluated the adsorption capability of the produced activated carbon by adsorbing Pb(II) in aqueous solution. The maximum BET surface area of the Typha orientalis (cattail) leaf was 1, 238 m2/g. The morphology showed that the produced activated carbon had clear burnout pore that had honeycomb-like structure. “The functional groups found in the activated carbon included C–O, C=O, O–H, C= C.” The Langmuir and Freundlich isotherms were used to model the equilibrium data of Pb(II) adsorption on Typha orientalis (cattail) leaf. The Langmuir isotherm having a correlation coefficient (R2) = 0.9999 described the adsorption process better than the Freundlich isotherm. The kinetic data gave a pseudo-second-order rate equation (R2 = 0.9999) better than the pseudo-first-order model.
Reusability and end of life of sorbent material
Exhausted activated carbon can be recovered by various techniques with the objective to desorb accumulated adsorbates and restore the original porous structure with little or no damage to the carbon (Salil and Ralph 1998). Apart from being restored for reuse the regenerated carbon is also safe for disposal due to stringent environmental regulations. The ease of regeneration depends on if the adsorption is physical or chemical. In physical adsorption, the shift of equilibrium from adsorption to desorption is accomplished by heating, lowering the pressure. In the case of chemical adsorption, a supply of energy greater than the adsorptive force is required to break the strong ionic or covalent bonds. The regeneration of spent activated carbons includes thermal process, wet air oxidation, and chemical and solvent regeneration. Furthermore, the recovery of used activated carbon is dependent on the characteristics of the base material, the activation process, and the type or types of adsorbates (Girgis and El-Hendawy 2002).
Thermal regeneration of granular activated carbon involves three steps: drying (200 °C), pyrolysis of adsorbates (400–600 °C), and reactivation (oxidation of the residue from the adsorbate which is carried out at 870–1000 °C. Thermal desorption has an advantage that it can be used for carbon which contain a heterogeneous mixture of adsorbates. The demerits of thermal regeneration include loss of adsorption capabilities due to changes in the pore structure of the carbon, high energy requirements, air pollution problems associated with off gases, and incompatibility of some adsorbates with high-temperature operations.
Wet air oxidation is an aqueous phase oxidation process that uses molecular oxygen as an oxidant. Mundale et al. (1991) studied the regeneration of activated carbon with phenol. Applying a wet air oxidation process, experiments were conducted at temperatures of 150–185 °C and an oxygen partial pressure of 0.5 MPa. They reported 5–10% losses, respectively, at temperatures of 185 and 150 °C. The loss of adsorption capacity was attributed to surface oxidation and the formation of carbon–oxygen complexes on the surface of the carbon.
Two types of reagents are used in chemical regeneration: those with oxidizing ability and those with solubilizing property. Subsequent washing with water is required to remove the regenerating agent. Chemical regeneration exhibits a number of advantages over thermal regeneration. The process can be done in situ which eliminates losses due to pumping, transport, and repacking. Also, carbon loss due to burn-off is eliminated, and recovery of adsorbate is achievable by using subsequent treatment methods such as distillation. The disadvantages associated with chemical regeneration include the high cost of reagents, danger of pollution from hazardous chemicals, and incomplete regeneration. The extent of regeneration depends on the solubility of the adsorbate in the regenerant solution. Furthermore, since most industrial wastewater contains a heterogeneous mixture of adsorbates, multiple regenerants are required.
In solvent regeneration process, a solvent is passed through a bed of spent carbon material to extract the adsorbate. Salvador and Sanchez (1996) studied supercritical water regeneration (300 °C and 12.2 MPa) of three types of carbon exhausted with phenols, textile dyes, and pesticides. They reported that phenol desorbs at low temperature (155 °C), dyes (sirrus red) at medium temperature (263 °C), and carbofuran, a pesticide, at temperature range of 100–250 °C. Sanchez-Montero et al. (2018) suggested the regeneration of exhausted activated carbon using supercritical carbon dioxide as an alternative to conventional thermal regeneration at critical temperature 400 °C and critical pressure 225 bar.
Salman et al. (2011) studied the desorption of bentazon from spent activated carbon prepared from branches of pomegranate trees using 100 ml of 95 vol% ethanol. The desorption was repeated for four cycles while regeneration efficiency was found to be 92–96% indicating that the exhausted adsorbent can be reused.
El-din et al. (2017) studied the recovery of used banana peel-based adsorbent to separate gas oil, 1-day weathered crude oil, and 7-day weathered crude oil from aqueous solution. Mechanical pressing and chemical reagent (n-hexane) were used to recover the exhausted adsorbent. The investigation revealed that for gas oil, about 90% of the initial sorption capacity remained after 10 cycles of applying mechanical action 20 times.
Yang et al. (2017) studied the desorption of oil from coco peat powder and found out that the percent removal of adsorption for cotton seed oil, paraffin oil, machine oil, and silicone oil were 11.98%, 11.63%, 10.63%, and 15.32%, respectively, after 11 cycles.
According to Vlaev et al. (2011), black rice husk ash saturated with crude oil, diesel fuel, or different hydrocarbons characterized by high calorific value can be burnt in incinerators, industrial ovens, or steam generators, after cycles of regeneration. Therefore, the rice husk ash is useful. “Grafted bagasse material containing oil can be used as fuel in the production of sugar cane or other industrial heating processes” (Said et al. 2009).
Conclusions
Based on the far-reaching literature studied, the following are the conclusions:
Activated carbon prepared from biomass wastes has been found to be useful in removing pollutants from aqueous media. Data presentations were found to be dissimilar which makes it difficult to compare the results. The work confirmed the regeneration of the novel adsorbents and also their end of life.
References
Abdelkarim S, Mohammed H, Nouredine B (2017) Sorption of methylene blue dye from aqueous solution using an agricultural waste. Trends Green Chem 3:4. https://doi.org/10.21767/2471-9889.100017
Ademiluyi T, Amadi SA, Amakama NJ (2009) Adsorption and treatment of organic contaminants using activated carbon from waste Nigerian Bamboo. J Appl Sci Environ Manage 13:39–47
Alaya MN, Girgis BS, Mourad WE (2000) Activated carbon from some agricultural wastes under action of one-step steam pyrolysis. J Porous Mater 7:509–517
Aljeboree AM, Alshirifi AN, Alkaim AF (2017) Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab J Chem 10:S3381–S3393
Aman T, Kazi AA, Sabri MU, Bano Q (2008) Potato peels as solid waste for the removal of heavy metal copper(II) from waste water/industrial effluent. Colloid Surf B 63:116–121
Amaya A, Medero N, Tancredi N, Silva H, Deina C (2007) Activated carbon briquettes from biomass materials. Bioresour Technol 98:1635–1641
Amirza MAR, Adib MMR, Hamdan R (2017) Application of agricultural wastes activated carbon for dye removal-an overview. MATEC Web Conf. https://doi.org/10.1051/mateconnf/201710306013
Anastopolous I, Pashalidis I, Hoseini-Bandigharaei A et al (2019) Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. J Mol Liq 295:111684
Ani JU, Ochonogor AE, Akpomie KG, Olikagu CS, Igboanugo CC (2019) Abstraction of arsenic (III) on activated carbon prepared from dialium guineese seed shell: kinetics, isotherms and thermodynamic studies. SN Appl Sci 1:1304. https://doi.org/10.1007/s42452-019-1335-1
Anisuzzaman SM, Joseph CG, Daud W, Krishnaiah D, Ye HS (2015) Preparation and characterization of activated carbon from Typha orientalis Leaves. Int J Ind Chem 6:9–21
Aydin H, Bulut Y, Yerlikaya C (2008) Removal of copper(II) from aqueous solution by adsorption onto low-cost adsorbents. J Environ Manage 87:37–45
Aygun A, Yenisoy-Karakas S, Duman I (2003) Production of granular activated carbon from fruit stones and nutshells and evaluation of their physical, chemical and adsorption properties. Microporous Mesoporous Mater 66:189–195
Bansal RC, Donnet JB, Stoeckli F (1988) Active carbon. Markel Dekker, New York
Bayat A, Aghamiri SF, Moheb A, Vakili-Nezhaad GR (2005) Oil spill cleanup from sea water by sorbent materials. Chem Eng Technol 28:1525–1528
Bharathi KS, Ramesh ST (2013) Removal of dyes using agricultural waste as low-cost adsorbents: a review. Appl Water Sci 3:773–790
Bhatnagar A, Sillanpaa M, Witek-Krowaik A (2015) Agricultural waste peels as versatile biomass for water purification: a review. Chem Eng J 270:244–271
Billy THG, Puziah AL, Taufiq YHY (2013) Physical preparation of activated carbon from sugar cane bagasse and corn husk and its physical and chemical characteristics. Int J Engg Res Sci Tech 3:1–14
Ding L, Zou B, Gao W, Liu Q, Wang Z, Guo Y, Wang X, Liu Y (2014) Adsorption of rhodamine-b from aqueous solution using treated rice-husk based` activated carbon. Colloids Surf A Physicochem Eng Asp 446:1–7
Doshi B, Sillanpaa M, Kalliola S (2018) A review of bio-based materials for oil spill treatment. Water Res 135:262–277
El-Changhaby GA, Ramis ES, Ahmad AF (2018) Rice straw and rice straw ash for the removal of brilliant green dye from wastewater. AJACR 1:1–9
El-Din GA, Amer AA, Malsh G, Hussein M (2017) Study on the use of banana peels for oil spill removal. Alex Eng J. https://doi.org/10.1016/j.acj.2017.05.020
El-sayeed GO, Yehia MH, Asaad AA (2014) Assessment of activated carbon prepared from corncob by chemical activation with phosphoric acid. Water Resour Ind 7–8:66–75
Ezechi EH, Nwabuko CG, Enyinnaya OC, Babington CJ (2017) Municipal solid waste management in Aba, Nigeria: challenges and prospects. Environ Eng Res 22:231–236
Garg VK, Gupta R, Yadav RB, Kumar R (2003) Dye removal from aqueous solutions by adsorption on treated sawdust. Bioresour Technol 89:121–124
Gautam RK, Mudhoo A, Lofrano G, Chattophyaya MC (2014) Biomass derived biosorbents for metals ions sequestration: adsorbent modification and activation methods and adsorbent regeneration. J Environ Chem Eng 2:239–259
Girgis BS, El-Hendawy ANA (2002) Porosity development in activated carbons obtained from date pits under chemical activation with phosphoric acid. Microporous Mesoporous Mater 52:105–117
Gupta S, Tai N (2016) Carbon materials as oil sorbents: a review on the synthesis and performance. J Mater Chem A 4:1550–1565
Gupta VK, Mittal A, Jain R, Mathur M, Sikarwar S (2006) Adsorption of Safranin-T from wastewater using waste materials-activated carbon and activated rice husks. J Colloid Interface Sci 303:80–86
Gupta VK, Jain R, Varshney S, Saini VK (2007) Removal of Reactofix Navy Blue 2 GFN from aqueous solutions using adsorption techniques. J Colloid Interface Sci 307:326–332
Gupta VK, Mittal A, Gajbe V, Mittal J (2008) Adsorption of basic fuchsin using waste materials-bottom ash and deoiled soya as adsorbents. J Colloid Interface Sci 319:30–39
Haddad ME, Slimani R, Mamouni R, ElAntri S, Lazar S (2013) Removal of two textile dyes from aqueous solutions onto calcined bones. J Assoc Arab Univ Basic Appl Sci 14:51–59
Hai NT, Huan-Ping C, Sheng-Jie Y (2017) Activated carbons from golden shower upon different chemical activation methods: synthesis and characterizations. Adsorpt Sci Technol https://doi.org/10.1177/0263617416684837
Ioannidou O, Zabaniotu A (2007) Agricultural residues as precursors for activated carbon production: a review. Renew Sust Energy Rev 11:1966–2005
Jiazhen Z, Anran L, Youcai Z (2018) Preparation and characterization of activated carbon from waste tea by physical activation using steam. J Air Waste Manage Assoc. https://doi.org/10.1080/10962247.2018.1460282
Keerthika B, Umayavalli M, Jeyalalitha T, Krishnaveni N (2016) Coconut shell powder as cost effective filler in copolymer of acrylonitrile and butadiene rubber. Ecotoxicol Environ Saf 130:1–3
Kizito S, Wu S, Kirui WK, Lei M, Lu Q, Bah H et al (2015) Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci Total Environ 505:102–112
Kumar R, Namasivayam C (2009) Development and characteristics of activated carbons from jatropha husk, an agro industrial solid waste by chemical activation methods. J Environ Eng Manage 19:173–178
Malik R, Ramteke DS, Wate SR (2007) Adsorption of malachite green on groundnut shell waste based powdered activated carbon. Waste Manage 27:1129–1138
Manera C, Tonello AP, Perondi D, Godhino M (2018) Adsorption of leather dyes on activated carbon from leather shaving wastes: kinetics equilibrium and thermodynamics studies. Environ Technol. https://doi.org/10.1080/09593330.2018.1452984
Mbui DN, Shiundu PM, Ndonye RM, Kamau GN (2002) Adsorption and detection of some phenolic compounds by rice husk ash of Kenyan origin. J Environ Monit 4:978–984
Michael M, Nicholas PX, Dionissios M, Evan D (2008) Complete treatment of olive pomace leachate by coagulation, activated carbon adsorption and electrochemical oxidation. Water Res 42:2883–2888
Mittal A, Gupta VK, Malviya A, Mittal J (2008) Process development for the batch and bulk removal and recovery of a hazardous, water soluble azo dye (Metanil Yellow) by adsorption over waste materials (Bottom Ash and De-Oiled Soya). J Hazard Mater 151:821–832
Mundale V, Joglekar A, Josi J (1991) Regeneration of spent activated carbon by wet air oxidation. Can J Chem Eng 69:1149–1159
Nguyen TAH, Ngo HH, Guo WS, Zhang J, Liang S, Yue QY, Liu Q, Nguyen TV (2013) Applicability of agricultural Waste and By-products for Adsorptive removal of heavy metals from wastewater. Bioresour Technol 148:574–585
Nwadiogbu JO, Ajiwe VIE, Okoye PAC (2016) Removal of crude oil from aqueous medium by sorption on hydrophobic corncobs: equilibrium and kinetic studies. J Taibah Univ Sci 10:56–63
Okafor JO, Aneke NAG (2006) Characterization of adsorbents for the purification of coca-cola effluent. J Niger Soc Chem Eng 21:19–24
Otowa T, Nojiyama Y, Miyazaki T (1997) Development of KOH activated-high surface area-carbon and its application to drinking water purification. Carbon 35:1315–1319
Owamah HI (2014) Biosorptive removal of Pb(II) and Cu(II) from wastewater using activated carbon from cassava peels. J Mater Cycles Waste Manage 16:347–358
Owolabi O, Czvikovszky T, Kovacs I (1985) Coconut-fiber-reinforced thermosetting plastics. J Appl Polym Sci 30:1827–1836
Pillai MG, Simha P, Gugalia A (2014) Recovering urea from human urine by bio-sorption onto microwave activated carbonized coconut shells: equilibrium, kinetics, optimization and field studies. J Environ Chem Eng 2:46–55
Ponnusamy SK, Subramaniam R, Kirupha SD, Arukkani M, Thangaraj V, Sivanesan S (2011) Adsorption behaviour of nickel(II) onto cashew nut shell: equilibrium, thermodynamics, kinetics, mechanism and process design. Chem Eng J 167:122–131
Ratan JK, Kaur M, Adiraju B (2018) Synthesis of activated carbon from agricultural waste using a simple method: characterization, parametric and isotherms study. Mater Today Proc 5:3334–3345
Richardson JF, Harker JH, Backhurst JR (2010) Coulson and Richardson’s chemical engineering. Butterworth-Heinemann, Oxford
Rios RRA, Alves DE, Dalmazio I, Bento SFV, Donnici CL, Lago RM (2003) Tailoring activated carbon by surface chemical modification with O, S, and N containing molecules. Mater Res. https://doi.org/10.1590/S1516-14392003000200004
Rosenkranz A, Pichelin F, Lehmann M, Job C, Kimeng HT, Muastapha S, Nduka OE, Mgbemene CA (2011) Nigerian agricultural waste products for the production of particle board. Italic 6—Science and Technology of Biomass: Advances and Challenges, September 5–8
Said AA, Ludwick AG, Aglain HA (2009) Usefulness of raw bagasse for oil absorption: a comparison of raw and acylated bagasse and their components. Bioresour Technol 100:2219–2222
Salil UR, Ralph TY (1998) Desorption by ultrasound: phenol on activated carbon and polymeric resin. AIChE J 44:1519–1528
Salman JM, Njoku VO, Hameed BH (2011) Bentazon and carbofuran adsorption onto date-seed activated carbon: kinetics and equilibrium. Chem Eng J 173:361–368
Salvador F, Sanchez C (1996) A new method for regenerating activated carbon by thermal desorption with liquid water under subcritical conditions. Carbon 34:511–516
Sanchez-Montero MJ, Pelaz J, Martin-Sanchez N, Izquierdo C, Salvador F (2018) Supercritical regeneration of an activated carbon fiber exhausted with phenol. Appl Sci 8:1–14
Shamsudinn MS, Yusoff NRN, Sulaimann MA (2016) Synthesis and characterization of activated carbon produced from Kenaf core fiber using H3PO4 activation. Procedia Chem 19:558–565
Sharma N, Tiwari DP, Singh SK (2012) Declourisation of synthetic dyes by agricultural waste: a review. Int J Sci Eng Res 3:1–10
Sidik SM, Jalil AA, Triwahyono S, Adam SH, Satar MAH, Hameed BH (2012) Modified oil palm leaves adsorbent with enhanced hydrophobicity for crude oil removal. Chem Eng J 203:9–18
Sokker HH, El-Sawy NM, Hassan MA, Anadouli BE (2011) Adsorption of crude oil from aqueous solution by hydrogel of chitosan based polyacrylamide prepared by radiation induced graft polymerization. J Hazard Mater 190:359–365
Srinivasan A, Viraraghavan T (2010) Oil removal from water using biomaterials. Bioresour Technol 101:6594–6600
Stavropolous GG, Zabaniotou AA (2005) Production and characterization of activated carbons from olive-seed waste residue. Microporous Mesoporous Mater 82:79–85
Subramaniam R, Ponussamy SK (2015) Novel adsorbent from agricultural waste (cashew nut shell) for methylene blue dye removal: optimization by response surface methodology. Water Res Ind 11:64–70
Sulaiman F, Abdullah N, Rahman AA (2011) Basic properties of washed and unwashed oil palm wastes. In: Proceedings of the 3rd CUTSE international conference, Miri, Sarawak, 8–9 November 2011, pp 307–311
Vlaev L, Petkov P, Dimitrov A, Genieva S (2011) Cleanup of water polluted with crude oil or diesel fuel using rice husks ash. J Taiwan Insti Chem Eng 42:957–964
Wei X, Zhang S, Han Y, Wolfe FA (2019) Treatment of petrochemical wastewater and produced water from oil and gas. Water Environ Res. https://doi.org/10.1002/Wer.1172
Wong S, Ngadi N, Inuwa IM, Hassan O (2018) Recent advances in applications of activated carbon from biowaste for wastewater treatment: a short review. J Clean Prod 175:361–375
Xu M, Yin P, Liu X, Tang Q, Qu R, Xu Q (2015) Utilization of rice husks modified by organomultiphosphonic acids as low-cost biosorbents for enhanced adsorption of heavy metal ions. Bioresour Technol 149:420–424
Yadav D, Kapur M, Kumar P, Mondal MK (2015) Adsorptive removal of phosphate from aqueous solution using rice husk and fruit juice residue. Process Saf Environ 94:402–409
Yang L, Ziru W, Yang L, Li X, Zhang Y, Lu C (2017) Coco peat powder as a source of magnetic sorbent for selective oil-water separation. Ind Crop Prod 101:1–10
Zhang T, Walawender WP, Fan LT, Fan M, Daugaard D, Brown RC (2004) Preparation of activated carbon from forest and agricultural residues through CO2 activation. Chem Eng J 105:53–59
Zondlo JW, Velez MR (2007) Development of surface area and pore structure for activation of anthracite coal. Fuel Process Technol 88:369–374
Acknowledgements
The technical assistance of Dr. L.N. Obasi and Mr. I.O. Obi, of the Department of Pure and Industrial Chemistry, University of Nigeria, Nsukka, is acknowledged.
Author information
Authors and Affiliations
Contributions
JA prepared the initial draft of the article. UO, LA, OO, and JA contributed to conception and design of the study. KA and OTU contributed to revising the work critically for important intellectual content.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted without any conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ani, J.U., Akpomie, K.G., Okoro, U.C. et al. Potentials of activated carbon produced from biomass materials for sequestration of dyes, heavy metals, and crude oil components from aqueous environment. Appl Water Sci 10, 69 (2020). https://doi.org/10.1007/s13201-020-1149-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13201-020-1149-8