Abstract
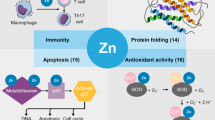
Zinc ions are essential cofactors of a wide range of enzymes, transcription factors, and other regulatory proteins. Moreover, zinc is also involved in cellular signaling and enzymes inhibition. Zinc dysregulation, deficiency, over-supply, and imbalance in zinc ion transporters regulation are connected with various diseases including cancer. A zinc ion pool is maintained by two types of proteins: (i) zinc-binding proteins, which act as a buffer and intracellular donors of zinc and (ii) zinc transporters responsible for zinc fluxes into/from cells and organelles. The decreased serum zinc ion levels have been identified in patients suffering from various cancer diseases, including head and neck tumors and breast, prostate, liver, and lung cancer. On the contrary, increased zinc ion levels have been found in breast cancer and other malignant tissues. Zinc metalloproteomes of a majority of tumors including brain ones are still not yet fully understood. Current knowledge show that zinc ion levels and detection of certain zinc-containing proteins may be utilized for diagnostic and prognostic purposes. In addition, these proteins can also be promising therapeutic targets. The aim of the present work is an overview of the importance of zinc ions, zinc transporters, and zinc-containing proteins in brain tumors, which are, after leukemia, the second most common type of childhood cancer and the second leading cause of death in children after accidents.




Similar content being viewed by others
References
Fleming AJ, Chi SN (2012) Brain tumors in children. Curr Probl Pediatr Adolesc Health Care 42:80–103
Mueller S, Chang S (2009) Pediatric brain tumors: current treatment strategies and future therapeutic approaches. Neurotherapeutics 6:570–586
Qi ZX, Cai JJ, Chen LC, Yue Q, Gong Y, Yao Y, Mao Y (2016) TRIM28 as an independent prognostic marker plays critical roles in glioma progression. J Neuro-Oncol 126:19–26
Mehrian-Shai R, Yalon M, Simon AJ, Eyal E, Pismenyuk T, Moshe I, Constantini S, Toren A (2015) High metallothionein predicts poor survival in glioblastoma multiforme. BMC Med Genet 8:1–9
Wrensch M, Minn Y, Chew T, Bondy M, Berger MS (2002) Epidemiology of primary brain tumors: current concepts and review of the literature. Neuro-Oncology 4:278–299
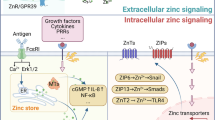
Murakami M, Hirano T (2008) Intracellular zinc homeostasis and zinc signaling. Cancer Sci 99:1515–1522
Klug A (2010) The discovery of zinc fingers and their applications in gene regulation and genome manipulation. In: Raetz CRH, Rothman JE, Thorner JW (eds) Kornberg RD. Annual Review of Biochemistry Annual Reviews, Palo Alto, pp. 213–231
Lipkowitz S, Weissman AM (2011) RINGs of good and evil: RING finger ubiquitin ligases at the crossroads of tumour suppression and oncogenesis. Nat Rev Cancer 11:629–643
Maret W (2013) Inhibitory zinc sites in enzymes. Biometals 26:197–204
Fukada T, Yamasaki S, Nishida K, Murakami M, Hirano T (2011) Zinc homeostasis and signaling in health and diseases. J Biol Inorg Chem 16:1123–1134
Gumulec J, Masarik M, Krizkova S, Adam V, Hubalek J, Hrabeta J, Eckschlager T, Stiborova M, Kizek R (2011) Insight to physiology and pathology of zinc(II) ions and their actions in breast and prostate carcinoma. Curr Med Chem 18:5041–5051
Lichten LA, Cousins RJ (2009) Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29:153–176
Qi ST, Song Y, Peng YP, Wang H, Long H, Yu XL, Li ZY, Fang LX, Wu AB, Luo WR, et al. (2012) ZEB2 mediates multiple pathways regulating cell proliferation, migration, invasion, and apoptosis in glioma. PLoS One 7:1–12
Maret W (2013) Zinc biochemistry: from a single zinc enzyme to a key element of life. Adv Nutr 4:82–91
Beyersmann D, Haase H (2001) Functions of zinc in signaling, proliferation and differentiation of mammalian cells. Biometals 14:331–341
Andreini C, Bertini I, Cavallaro G, Holliday GL, Thornton JM (2008) Metal ions in biological catalysis: from enzyme databases to general principles. J Biol Inorg Chem 13:1205–1218
Waldron KJ, Rutherford JC, Ford D, Robinson NJ (2009) Metalloproteins and metal sensing. Nature 460:823–830
Nagel WW, Vallee BL (1995) Cell-cycle regulation of metallothionein in human colonic-cancer cells. Proc Natl Acad Sci U S A 92:579–583
Pedersen MO, Larsen A, Pedersen DS, Stoltenberg M, Penkowa M (2009) Metallic gold treatment reduces proliferation of inflammatory cells, increases expression of VEGF and FGF, and stimulates cell proliferation in the subventricular zone following experimental traumatic brain injury. Histol Histopath 24:573–586
Takeda A, Fujii H, Minamino T, Tamano H (2014) Intracellular Zn2+ signaling in cognition. J Neurosci Res 92:819–824
Takeda A, Nakamura M, Fujii H, Tamano H (2013) Synaptic Zn2+ homeostasis and its significance. Metallomics 5:417–423
Frederickson CJ, Suh SW, Silva D, Thompson RB (2000) Importance of zinc in the central nervous system: the zinc-containing neuron. J Nutr 130:1471S–1483S
Sindreu C, Storm DR (2011) Modulation of neuronal signal transduction and memory formation by synaptic zinc. Front Behav Neurosci 5:1–14
Miles AT, Hawksworth GM, Beattie JH, Rodilla V (2000) Induction, regulation, degradation, and biological significance of mammalian metallothioneins. Crit Rev Biochem Mol Biol 35:35–70
Eckschlager T, Adam V, Hrabeta J, Figova K, Kizek R (2009) Metallothioneins and cancer. Curr Protein Pept Sci 10:360–375
Colvin RA, Holmes WR, Fontaine CP, Maret W (2010) Cytosolic zinc buffering and muffling: their role in intracellular zinc homeostasis. Metallomics 2:306–317
Krizkova S, Fabrik I, Adam V, Hrabeta P, Eckschlager T, Kizek R (2009) Metallothionein—a promising tool for cancer diagnostics. Bratisl Med J 110:93–97
Lindeque JZ, Levanets O, Louw R, van der Westhuizen FH (2010) The involvement of metallothioneins in mitochondrial function and disease. Curr Protein Pept Sci 11:292–309
Kadota Y, Suzuki S, Ideta S, Fukinbara Y, Kawakami T, Imai H, Nakagawa Y, Sato M (2010) Enhanced metallothionein gene expression induced by mitochondrial oxidative stress is reduced in phospholipid hydroperoxide glutathione peroxidase-overexpressed cells. Eur J Pharmacol 626:166–170
Verrax J, Pedrosa RC, Beck R, Dejeans N, Taper H, Calderon PB (2009) In situ modulation of oxidative stress: a novel and efficient strategy to kill cancer cells. Curr Med Chem 16:1821–1830
Ostrakhovitch EA, Olsson PE, Jiang S, Cherian MG (2006) Interaction of metallothionein with tumor suppressor p53 protein. FEBS Lett 580:1235–1238
Krizkova S, Fabrik I, Huska D, Adam V, Babula P, Hrabeta J, Eckschlager T, Pochop P, Darsova D, Kukacka J, et al. (2010) An adsorptive transfer technique coupled with Brdicka reaction to reveal the importance of metallothionein in chemotherapy with platinum based cytostatics. Int J Mol Sci 11:4826–4842
Krizkova S, Masarik M, Majzlik P, Kukacka J, Kruseova J, Adam V, Prusa R, Eckschlager T, Stiborova M, Kizek R (2010) Serum metallothionein in newly diagnosed patients with childhood solid tumours. Acta Biochim Pol 57:561–566
Kruseova J, Hynek D, Adam V, Kizek R, Prusa R, Hrabeta J, Eckschlager T (2013) Serum metallothioneins in childhood tumours—a potential prognostic marker. Int J Mol Sci 14:12170–12185
Prusa R, Kukacka J, Vajtr D, Huska D, Alba J, Adam V, Kizek R (2008) New technique for quantitative elelectrochemical determination of total plasma mRNA. Clin Chem 54:A156–A157
Krizkova S, Fabrik I, Adam V, Kukacka J, Prusa R, Chavis GJ, Trnkova L, Strnadel J, Horak V, Kizek R (2008) Utilizing of adsorptive transfer stripping technique Brdicka reaction for determination of metallothioneins level in melanoma cells, blood serum and tissues. Sensors 8:3106–3122
Fabrik I, Krizkova S, Huska D, Adam V, Hubalek J, Trnkova L, Eckschlager T, Kukacka J, Prusa R, Kizek R (2008) Employment of electrochemical techniques for metallothionein determination in tumor cell lines and patients with a tumor disease. Electroanalysis 20:1521–1532
Krizkova S, Ryvolova M, Hrabeta J, Adam V, Stiborova M, Eckschlager T, Kizek R (2012) Metallothioneins and zinc in cancer diagnosis and therapy. Drug Metab Rev 44:287–301
Dziegiel P, Pula B, Kobierzycki C, Stasiolek M, PodhorskaOkolow M (2016) Metallothioneins in normal and cancer cells. Cancer Cells:1–117
Bonaventura P, Benedetti G, Albarede F, Miossec P (2015) Zinc and its role in immunity and inflammation. Autoimmun Rev 14:277–285
Tubek S (2007) Zinc supplementation or regulation of its homeostasis: advantages and threats. Biol Trace Elem Res 119:1–9
Leone N, Courbon D, Ducimetiere P, Zureik M (2006) Zinc, copper, and magnesium and risks for all-cause, cancer, and cardiovascular mortality. Epidemiology 17:308–314
Cousins RJ (1986) Toward a molecular understanding of zinc-metabolism. Clin Physiol Biochem 4:20–30
Wu TJ, Sempos CT, Freudenheim JL, Muti P, Smith E (2004) Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults. Ann Epidemiol 14:195–201
Arslan M, Demir H, Arslan H, Gokalp AS, Demir C (2011) Trace elements, heavy metals and other biochemical parameters in malignant glioma patients. Asian Pac J Cancer Prev 12:447–451
Kensova R, Hynek D, Kynicky J, Konecna M, Eckschlager T, Adam V, Hubalek J, Kizek R (2014) Determination of metal ions in the plasma of children with tumour diseases by differential pulse voltammetry. Int J Electrochem Sci 9:4675–4691
Lu H, Cai L, Mu LN, Lu QY, Zhao JK, Cui Y, Sul JH, Zhou XF, Ding BG, Elashoff RM, et al. (2006) Dietary mineral and trace element intake and squamous cell carcinoma of the esophagus in a Chinese population. Nutr Cancer 55:63–70
Zhou W, Park S, Liu G, Miller DP, Wang LI, Pothier L, Wain JC, Lynch TJ, Giovannucci E, Christiani DC (2005) Dietary iron, zinc, and calcium and the risk of lung cancer. Epidemiology 16:772–779
Lee DH, Anderson KE, Folsom AR, Jacobs DR (2005) Heme iron, zinc and upper digestive tract cancer: the Iowa Women’s Health Study. Int J Cancer 117:643–647
Gallus S, Foschi R, Negri E, Talamini R, Franceschi S, Montella M, Ramazzotti V, Tavani A, Dal Maso L, La Vecchia C (2007) Dietany zinc and prostate cancer risk: a case-control study from Italy. Eur Urol 52:1052–1057
Dimitropoulou P, Nayee S, Liu JF, Demetriou L, van Tongeren M, Hepworth SJ, Muir KR (2008) Dietary zinc intake and brain cancer in adults: a case-control study. Br J Nutr 99:667–673
Qin ZY, Caruso JA, Lai B, Matusch A, Becker JS (2011) Trace metal imaging with high spatial resolution: applications in biomedicine. Metallomics 3:28–37
Zoriy MV, Dehnhardt M, Matusch A, Becker JS (2008) Comparative imaging of P, S, Fe, Cu, Zn and C in thin sections of rat brain tumor as well as control tissues by laser ablation inductively coupled plasma mass spectrometry. Spectroc Acta Pt B-Atom Spectr 63:375–382
Takeda A, Tamano H, Oku N (2003) Alteration of zinc concentrations in the brain implanted with C6 glioma. Brain Res 965:170–173
Yoshida D, Ikeda Y, Nakazawa S (1993) Quantitative-analysis of copper, zinc and copper-zinc ratio in selected human brain-tumors. J Neuro-Oncol 16:109–115
Zhuang GS, Wang YS, Tan MG, Zhi M, Wang YG, Zhang FL (1991) Preliminary-study of trace-elements in human brain-tumor tissues by instrumental neutron-activation analysis. J Radioanal Nucl Chem-Artic 151:327–335
Wandzilak A, Czyzycki M, Wrobel P, Szczerbowska-Boruchowska M, Radwanska E, Adamek D, Lankosz M (2013) The oxidation states and chemical environments of iron and zinc as potential indicators of brain tumour malignancy grade—preliminary results. Metallomics 5:1547–1553
Wandzilak A, Czyzycki M, Radwanska E, Adamek CD, Geraki K, Lankosz M (2015) X-ray fluorescence study of the concentration of selected trace and minor elements in human brain tumours. Spectroc Acta Pt B-Atom Spectr 114:52–57
Zaichick VY, Sviridova TV, Zaichick SV (1997) Zinc in human prostate gland: normal, hyperplastic and cancerous. J Radioanal Nucl Chem 217:157–161
Hogstrand C, Kille P, Nicholson RI, Taylor KM (2009) Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol Med 15:101–111
Plum LM, Rink L, Haase H (2010) The essential toxin: impact of zinc on human health. Int J Environ Res Public Health 7:1342–1365
Cousins RJ, Liuzzi JP, Lichten LA (2006) Mammalian zinc transport, trafficking, and signals. J Biol Chem 281:24085–24089
Kambe T, Hashimoto A, Fujimoto S (2014) Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell Mol Life Sci 71:3281–3295
Lin Y, Chen Y, Wang YZ, Yang JX, Zhu VF, Liu YL, Cui XB, Chen L, Yan W, Jiang T, et al. (2013) ZIP4 is a novel molecular marker for glioma. Neuro-Oncology 15:1008–1016
Kang X, Chen R, Zhang J, Li G, Dai PG, Chen C, Wang HJ (2015) Expression profile analysis of zinc transporters (ZIP4, ZIP9, ZIP11, ZnT9) in gliomas and their correlation with IDH1 mutation status. Asian Pac J Cancer Prev 16:3355–3360
Yan W, Imanishi M, Futaki S, Sugiura Y (2007) Alpha-helical linker of an artificial 6-zinc finger peptide contributes to selective DNA binding to a discontinuous recognition sequence. Biochemistry 46:8517–8524
Zhou YX, Su ZP, Huang YL, Sun T, Chen SS, Wu TF, Chen GL, Xie XS, Li B, Du ZW (2011) The Zfx gene is expressed in human gliomas and is important in the proliferation and apoptosis of the human malignant glioma cell line U251. J Exp Clin Cancer Res 30:1–10
Song G, Ouyang GL, Bao SD (2005) The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med 9:59–71
Zhu ZC, Li K, Xu DF, Liu YJ, Tang HL, Xie Q, Xie LQ, Liu JW, Wang HT, Gong Y, et al. (2013) ZFX regulates glioma cell proliferation and survival in vitro and in vivo. J Neuro-Oncol 112:17–25
DeSouza R-M, Jones BRT, Lowis SP, Kurian KM (2014) Pediatric medulloblastoma—update on molecular classification driving targeted therapies. Front Oncol 4:176–176
Kurisaka M, Mori K (1996) Immunohistochemical study of medulloblastoma with a monoclonal antibody against human copper and zinc-superoxide dismutase. Neurol Med-Chir 36:220–223
Palmer CJ, Galan-Caridad JM, Weisberg SP, Lei L, Esquilin JM, Croft GF, Wainwright B, Canoll P, Owens DM, Reizis B (2014) Zfx facilitates tumorigenesis caused by activation of the hedgehog pathway. Cancer Res 74:5914–5924
Santoni M, Burattini L, Nabissi M, Morelli MB, Berardi R, Santoni G, Cascinu S (2013) Essential role of Gli proteins in glioblastoma multiforme. Curr Protein Pept Sci 14:133–140
Dey J, Ditzler S, Knoblaugh SE, Hatton BA, Schelter JM, Cleary MA, Mecham B, Rorke-Adams LB, Olson JM (2012) A distinct smoothened mutation causes severe cerebellar developmental defects and medulloblastoma in a novel transgenic mouse model. Mol Cell Biol 32:4104–4115
Vriend J, Ghavami S, Marzban H (2015) The role of the ubiquitin proteasome system in cerebellar development and medulloblastoma. Mol Brain 8:1–14
He QW, Xia YP, Chen SC, Wang Y, Huang M, Huang Y, Li JY, Li YN, Gao Y, Mao L, et al. (2013) Astrocyte-derived sonic hedgehog contributes to angiogenesis in brain microvascular endothelial cells via RhoA/ROCK pathway after oxygen-glucose deprivation. Mol Neurobiol 47:976–987
Ellison DW, Dalton J, Kocak M, Nicholson SL, Fraga C, Neale G, Kenney AM, Brat DJ, Perry A, Yong WH, et al. (2011) Medulloblastoma: clinicopathological correlates of SHH, WNT, and non-SHH/WNT molecular subgroups. Acta Neuropathol 121:381–396
Hui CC, Angers S (2011) Gli proteins in development and disease. In: Goldstein L, Lehmann R (eds) Schekman R. Annual review of cell and developmental biology annual reviews, Palo Alto, pp. 513–537
Srivastava VK, Nalbantoglu J (2010) The cellular and developmental biology of medulloblastoma current perspectives on experimental therapeutics. Cancer Biol Ther 9:843–852
Buczkowicz P, Ma J, Hawkins C (2011) GLI2 is a potential therapeutic target in pediatric medulloblastoma. J Neuropathol Exp Neurol 70:430–437
Bar EE, Chaudhry A, Farah MH, Eberhart CG (2007) Hedgehog signaling promotes medulloblastoma survival via Bc/II. Am J Pathol 170:347–355
Fan HR, Oro AE, Scott MP, Khavari PA (1997) Induction of basal cell carcinoma features in transgenic human skin expressing sonic hedgehog. Nat Med 3:788–792
Sakakini N, Turchi L, Bergon A, Holota H, Rekima S, Lopez F, Paquis P, Almairac F, Fontaine D, Baeza-Kallee N, et al. (2016) A positive feed-forward loop associating EGR1 and PDGFA promotes proliferation and self-renewal in glioblastoma stem cells. J Biol Chem in press
Zhang CR, Zhu QB, He H, Jiang L, Qiang Q, Hu LH, Hu GH, Jiang Y, Ding XH, Lu YC (2016) RIZ1: a potential tumor suppressor in glioma (vol 15, 990, 2015). BMC Cancer 16:1–10
Spina R, Filocamo G, Iaccino E, Scicchitano S, Lupia M, Chiarella E, Mega T, Bernaudo F, Pelaggi D, Mesuraca M, et al. (2013) Critical role of zinc finger protein 521 in the control of growth, clonogenicity and tumorigenic potential of medulloblastoma cells. Oncotarget 4:1280–1292
Rauscher J, Beschorner R, Gierke M, Bisdas S, Braun C, Ebner FH, Schittenhelm J (2014) WT1 expression increases with malignancy and indicates unfavourable outcome in astrocytoma. J Clin Pathol 67:556–561
Ryu HH, Jung S, Jung TY, Moon KS, Kim IY, Jeong YI, Jin SG, Pei J, Wen M, Jang WY (2012) Role of metallothionein 1E in the migration and invasion of human glioma cell lines. Int J Oncol 41:1305–1313
Berghoff AS, Hainfellner JA, Marosi C, Preusser M (2015) Assessing MGMT methylation status and its current impact on treatment in glioblastoma. CNS Oncol 4:47–52
Meyer MA (2014) Highly expressed genes in human high grade gliomas: immunohistochemical analysis of data from the human protein atlas. Neurol Int 6:5348–5348
Sahab ZJ, Hall MD, Sung YM, Dakshanamurthy S, Ji Y, Kumar D, Byers SW (2011) Tumor suppressor RARRES1 interacts with cytoplasmic carboxypeptidase AGBL2 to regulate the alpha-tubulin tyrosination cycle. Cancer Res 71:1219–1228
Kandoth C, McLellan MD, Vandin F, Ye K, Niu BF, Lu C, Xie MC, Zhang QY, McMichael JF, Wyczalkowski MA, et al. (2013) Mutational landscape and significance across 12 major cancer types. Nature 502:333–339
Suzuki K, Matsubara H (2011) Recent advances in p53 research and cancer treatment. J Biomed Biotechnol 2011:1–7
Loh SN (2010) The missing zinc: p53 misfolding and cancer. Metallomics 2:442–449
Merino D, Malkin D (2014) p53 and hereditary cancer. Subcell Biochem 85:1–16
Krutilkova V, Trkova M, Fleitz J, Gregor V, Novotna K, Krepelova A, Sumerauer D, Kodet R, Siruckova S, Plevova P, et al. (2005) Identification of five new families strengthens the link between childhood choroid plexus carcinoma and germline TP53 mutations. Eur J Cancer 41:1597–1603
Azmi AS, Philip PA, Beck FWJ, Wang Z, Banerjee S, Wang S, Yang D, Sarkar FH, Mohammad RM (2011) MI-219-zinc combination: a new paradigm in MDM2 inhibitor-based therapy. Oncogene 30:117–126
Rasheed BKA, McLendon RE, Herndon JE, Friedman HS, Friedman AH, Bigner DD, Bigner SH (1994) Alterations of the tp53 gene in human gliomas. Cancer Res 54:1324–1330
James CD, Carlbom E, Nordenskjold M, Collins VP, Cavenee WK (1989) Mitotic recombination of chromosome-17 in astrocytomas. Proc Natl Acad Sci U S A 86:2858–2862
Ohgaki H, Eibl RH, Schwab M, Reichel MB, Mariani L, Gehring M, Petersen I, Holl T, Wiestler OD, Kleihues P (1993) Mutations of the p53 tumor-suppressor gene in neoplasms of the human nervous-system. Mol Carcinog 8:74–80
Ichimura K, Bolin MB, Goike HM, Schmidt EE, Moshref A, Collins VP (2000) Deregulation of the p14(ARF)/MDM2/p53 pathway is a prerequisite for human astrocytic gliomas with G(1)-S transition control gene abnormalities. Cancer Res 60:417–424
Ohgaki H, Dessen P, Jourde B, Horstmann S, Nishikawa T, Di Patre PL, Burkhard C, Schuler D, Probst-Hensch NM, Maiorka PC, et al. (2004) Genetic pathways to glioblastoma: a population-based study. Cancer Res 64:6892–6899
Artells E, Palacios O, Capdevila M, Atrian S (2014) In vivo-folded metal-metallothionein 3 complexes reveal the Cu-thionein rather than Zn-thionein character of this brain-specific mammalian metallothionein. FEBS J 281:1659–1678
Lee SJ, Park MH, Kim HJ, Koh JY (2010) Metallothionein-3 regulates lysosomal function in cultured astrocytes under both normal and oxidative conditions. Glia 58:1186–1196
Bacolod MD, Fehdrau R, Johnson SP, Bullock NS, Bigner DD, Colvin M, Friedman HS (2009) BCNU-sequestration by metallothioneins may contribute to resistance in a medulloblastoma cell line. Cancer Chemother Pharmacol 63:753–758
Florianczyk B, Osuchowski J, Kaczmarczyk R, Trojanowski T, Stryjecka-Zimmer M (2003) Influence of metallothioneins on zinc and copper distribution in brain tumours. Folia Neuropathol 41:11–14
Maier H, Jones C, Jasani B, Ofner D, Zelger B, Schmid KW, Budka H (1997) Metallothionein overexpression in human brain tumours. Acta Neuropathol 94:599–604
Peyre M, Commo F, Dantas-Barbosa C, Andreiuolo F, Puget S, Lacroix L, Drusch F, Scott V, Varlet P, Mauguen A, et al. (2010) Portrait of ependymoma recurrence in children: biomarkers of tumor progression identified by dual-color microarray-based gene expression analysis. PLoS One 5:1–15
Quail DF, Joyce JA (2013) Microenvironmental regulation of tumor progression and metastasis. Nat Med 19:1423–1437
Chabottaux V, Noel A (2007) Breast cancer progression: insights into multifaceted matrix metalloproteinases. Clin Exp Metastasis 24:647–656
Xu YM, Zhong ZW, Yuan J, Zhang ZH, Wei QT, Song WZ, Chen HW (2013) Collaborative overexpression of matrix metalloproteinase-1 and vascular endothelial growth factor-C predicts adverse prognosis in patients with gliomas. Cancer Epidemiol 37:697–702
Ulasov I, Yi RY, Guo D, Sarvaiya P, Cobbs C (2014) The emerging role of MMP14 in brain tumorigenesis and future therapeutics. Biochim Biophys Acta-Rev Cancer 1846:113–120
Wang L, Yuan J, Tu YY, Mao XG, He SM, Fu GQ, Zong JH, Zhang YS (2013) Co-expression of MMP-14 and MMP-19 predicts poor survival in human glioma. Clin Transl Oncol 15:139–145
Xie TX, Huang FJ, Aldape KD, Kang SH, Liu AG, Gershenwald JE, Xie KP, Sawaya R, Huang SY (2006) Activation of Stat3 in human melanoma promotes brain metastasis. Cancer Res 66:3188–3196
Stark AM, Anuszkiewicz B, Mentlein R, Yoneda T, Mehdorn HM, Held-Feindt J (2007) Differential expression of matrix metalloproteinases in brain- and bone-seeking clones of metastatic MDA-MB-231 breast cancer cells. J Neuro-Oncol 81:39–48
Mendes O, Kim HT, Stoica G (2005) Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin Exp Metastasis 22:237–246
Hu L, Zhang JQ, Zhu HB, Min J, Feng YM, Zhang HL (2010) Biological characteristics of a specific brain metastatic cell line derived from human lung adenocarcinoma. Med Oncol 27:708–714
Liu H, Kato Y, Erzinger SA, Kiriakova GM, Qian YZ, Palmieri D, Steeg PS, Price JE (2012) The role of MMP-1 in breast cancer growth and metastasis to the brain in a xenograft model. BMC Cancer 12:1–11
Rooprai HK, Rucklidge GJ, Panou C, Pilkington GJ (2000) The effects of exogenous growth factors on matrix metalloproteinase secretion by human brain tumour cells. Br J Cancer 82:52–55
Bodey B, Siegel SE, Kaiser HE (2000) Matrix metalloproteinase expression in childhood astrocytomas. Anticancer Res 20:3287–3292
Snuderl M, Chi SN, De Santis SM, Stemmer-Rachamimov AO, Betensky RA, De Girolami U, Kieran MW (2008) Prognostic value of tumor microinvasion and metalloproteinases expression in intracranial pediatric ependymomas. J Neuropathol Exp Neurol 67:911–920
Xia ZQ, Liu WQ, Li SD, Jia G, Zhang YQ, Li CD, Ma ZY, Tian JH, Gong J (2011) Expression of matrix metalloproteinase-9, type IV collagen and vascular endothelial growth factor in adamantinous craniopharyngioma. Neurochem Res 36:2346–2351
Nardinocchi L, Puca R, Sacchi A, Rechavi G, Givol D, D'Orazi G (2009) Targeting hypoxia in cancer cells by restoring homeodomain interacting protein-kinase 2 and p53 activity and suppressing HIF-1 alpha. PLoS One 4:1–12
Babula P, Masarik M, Adam V, Eckschlager T, Stiborova M, Trnkova L, Skutkova H, Provaznik I, Hubalek J, Kizek R (2012) Mammalians’ metallothioneins and their properties and functions. Metallomics 4:739–750
Zhu J, Wan H, Xue CQ, Jiang T, Qian C, Zhang YQ (2013) Histone deacetylase 3 implicated in the pathogenesis of children glioma by promoting glioma cell proliferation and migration. Brain Res 1520:15–22
Campos B, Bermejo JL, Han L, Felsberg J, Ahmadi R, Grabe N, Reifenberger G, Unterberg A, Herold-Mende C (2011) Expression of nuclear receptor corepressors and class I histone deacetylases in astrocytic gliomas. Cancer Sci 102:387–392
Chen CH, Chang YJ, Ku MSB, Chung KT, Yang JT (2011) Enhancement of temozolomide-induced apoptosis by valproic acid in human glioma cell lines through redox regulation. J Mol Med 89:303–315
Mirlohi S, Duncan SE, Harmon M, Case D, Lesser G, Dietrich AM (2015) Analysis of salivary fluid and chemosensory functions in patients treated for primary malignant brain tumors. Clin Oral Investig 19:127–137
el-Yazigi A, Al-Saleh I, Al-Mefty O (1986) Concentrations of zinc, iron, molybdenum, arsenic, and lithium in cerebrospinal fluid of patients with brain tumors. Clin Chem 32:2187–2190
Palm R, Hallmans G (1982) Zinc concentrations in the cerebrospinal-fluid of normal adults and patients with neurological diseases. J Neurol Neurosurg Psychiatry 45:685–690
Vyslouzilova L, Krizkova S, Anyz J, Hynek D, Hrabeta J, Kruseova J, Eckschlager T, Adam V, Stepankova O, Kizek R (2013) Use of brightness wavelet transformation for automated analysis of serum metallothioneins- and zinc-containing proteins by Western blots to subclassify childhood solid tumours. Electrophoresis 34:1637–1648
Krezel A, Maret WG (2008) Thionein/metallothionein control Zn(II) availability and the activity of enzymes. J Biol Inorg Chem 13:401–409
Cherian MG, Kang YJ (2006) Metallothionein and liver cell regeneration. Exp Biol Med 231:138–144
Feng W, Cai J, Pierce WM, Franklin RB, Maret W, Benz FW, Kang YJ (2005) Metallothionein transfers zinc to mitochondrial aconitase through a direct interaction in mouse hearts. Biochem Biophys Res Commun 332:853–858
Haga A, Nagase H, Kito H, Sato T (1996) Effect of metallothioneins on transformation of gelatinase A from human fibroblast WI-38 cells. Cancer Lett 105:175–180
Haga A, Nagase H, Kito H, Sato T (1996) Effect of metallothioneins on collagenolytic activity of tumor gelatinase B. Cancer Res Ther Control 5:17–22
Haga A, Nagase H, Kito H, Sato T (1996) Enhanced invasiveness of tumour cells after host exposure to heavy metals. Eur J Cancer 32A:2342–2347
Scaruffi P, Morandi F, Gallo F, Stigliani S, Parodi S, Moretti S, Bonassi S, Fardin P, Garaventa A, Zanazzo G, et al. (2012) Bone marrow of neuroblastoma patients shows downregulation of CXCL12 expression and presence of IFN signature. Pediatr Blood Cancer 59:44–51
Sandoval JA, Hoelz DJ, Woodruff HA, Powell RL, Jay CL, Grosfeld JL, HickeyD RJ, Malkas LH (2006) Novel peptides secreted from human neuroblastoma: useful clinical tools? J Pediatr Surg 41:245–251
Bautista F, Paci A, Minard-Colin V, Dufour C, Grill J, Lacroix L, Varlet P, Valteau-Couanet D, Geoerger B (2014) Vemurafenib in pediatric patients with BRAFV600E mutated high-grade gliomas. Pediatr Blood Cancer 61:1101–1103
Romer JT, Kimura H, Magdaleno S, Sasai K, Fuller C, Baines H, Connelly M, Stewart CF, Gould S, Rubin LL, et al. (2004) Suppression of the Shh pathway using a small molecule inhibitor eliminates medulloblastoma in Ptc1(+/−) p53(−/−) mice. Cancer Cell 6:229–240
Raju GP (2011) Arsenic: a potentially useful poison for hedgehog-driven cancers. J Clin Invest 121:14–16
Roosen N, Doz F, Yeomans KL, Dougherty DV, Rosenblum ML (1994) Effect of pharmacological doses of zinc on the therapeutic index of brain-tumor chemotherapy with carmustine. Cancer Chemother Pharmacol 34:385–392
Yamagata T, Nakamura Y, Yamagata Y, Nakanishi M, Matsunaga K, Nakanishi H, Nishimoto T, Minakata Y, Mune M, Yukawa S (2003) The pilot trial of the prevention of the increase in electrical taste thresholds by zinc containing fluid infusion during chemotherapy to treat primary lung cancer. J Exp Clin Cancer Res 22:557–563
Yao YL, Ma J, Xue YX, Wang P, Li Z, Li ZQ, Hu Y, Shang XL, Liu YH (2015) MiR-449a exerts tumor-suppressive functions in human glioblastoma by targeting Myc-associated zinc-finger protein. Mol Oncol 9:640–656
Ma J, Yao YL, Wang P, Liu YH, Zhao LN, Li Z, Li ZQ, Xue YX (2014) MiR-181a regulates blood-tumor barrier permeability by targeting Kruppel-like factor 6. J Cereb Blood Flow Metab 34:1826–1836
Zhao LN, Wang P, Liu YH, Ma J, Xue YX (2015) miR-34c regulates the permeability of blood-tumor barrier via MAZ-mediated expression changes of ZO-1, occludin, and claudin-5. J Cell Physiol 230:716–731
Kennette W, Collins OM, Zalups RK, Koropatnick J (2005) Basal and zinc-induced metallothionein in resistance to cadmium, cisplatin, zinc, and tertButyl hydroperoxide: studies using MT knockout and antisense-downregulated MT in mammalian cells. Toxicol Sci 88:602–613
Anzellotti AI, Farrell NP (2008) Zinc metalloproteins as medicinal targets. Chem Soc Rev 37:1629–1651
Jin YL, Xiao WZ, Song TT, Feng GJ, Dai ZS (2016) Expression and prognostic significance of p53 in glioma patients: a meta-analysis. Neurochem Res 41:1723–1731
Tashakori M, Zhang Y, Xiong SB, You MJ, Lozano G (2016) p53 activity dominates that of p73 upon Mdm4 loss in development and tumorigenesis. Mol Cancer Res 14:56–65
Waye S, Naeem A, Choudhry MU, Parasido E, Tricoli L, Sivakumar A, Mikhaiel JP, Yenugonda V, Rodriguez OC, Karam SD, et al. (2015) The p53 tumor suppressor protein protects against chemotherapeutic stress and apoptosis in human medulloblastoma cells. Aging-Us 7:854–868
Pei J, Park IH, Ryu HH, Li SY, Li CH, Lim SH, Wen M, Jang WY, Jung S (2015) Sublethal dose of irradiation enhances invasion of malignant glioma cells through p53-MMP 2 pathway in U87MG mouse brain tumor model. Radiat Oncol 10
Bartesaghi S, Graziano V, Galavotti S, Henriquez NV, Betts J, Saxena J, Deli A, Karlsson A, Martins LM, Capasso M, et al. (2015) Inhibition of oxidative metabolism leads to p53 genetic inactivation and transformation in neural stem cells. Proc Natl Acad Sci U S A 112:1059–1064
Cohen AL, Colman H (2015) Glioma biology and molecular markers. In: Parsa A (ed) Raizer J. Current understanding and treatment of gliomas Springer, Dordrecht, pp. 15–30
Checler F, da Costa CA (2014) p53 in neurodegenerative diseases and brain cancers. Pharmacol Ther 142:99–113
Narla S, Uppin MS, Saradhi MV, Sahu BP, Purohit AK, Sundaram C (2014) Assessment of expression of epidermal growth factor receptor and p53 in meningiomas. Neurol India 62:37–41
England B, Huang TG, Karsy M (2013) Current understanding of the role and targeting of tumor suppressor p53 in glioblastoma multiforme. Tumor Biol 34:2063–2074
Takahashi R, Giannini C, Sarkaria JN, Schroeder M, Rogers J, Mastroeni D, Scrable H (2013) p53 isoform profiling in glioblastoma and injured brain. Oncogene 32:3165–3174
Louis DN (1994) The p53 gene and protein in human brain-tumors. J Neuropathol Exp Neurol 53:11–21
Tomkova K, Tomka M, Zajac V (2008) Contribution of p53, p63, and p73 to the developmental diseases and cancer minireview. Neoplasma 55:177–181
Fatt MP, Cancino GI, Miller FD, Kaplan DR (2014) p63 and p73 coordinate p53 function to determine the balance between survival, cell death, and senescence in adult neural precursor cells. Cell Death Differ 21:1546–1559
Rushing EJ, Olsen C, Man YG (2008) Correlation of p63 immunoreactivity with tumor grade in meningiomas. Int J Surg Pathol 16:38–42
Antonelli A, Lenzi L, Nakagawara A, Osaki T, Chiaretti A, Aloe L (2007) Tumor suppressor proteins are differentially affected in human ependymoblastoma and medulloblastoma cells exposed to nerve growth factor. Cancer Investig 25:94–101
Kamiya M, Nakazato Y (2002) The expression of p73, p21 and MDM2 proteins in gliomas. J Neuro-Oncol 59:143–149
Loiseau H, Arsaut J, Demotes-Mainard J (1999) p73 gene transcripts in human brain tumors: overexpression and altered splicing in ependymomas. Neurosci Lett 263:173–176
Kirsch M, Zhu JJ, Black PM (1997) Analysis of the BRCA1 and BRCA2 genes in sporadic meningiomas. Genes Chromosomes & Cancer 20:53–59
Bencokova Z, Pauron L, Devic C, Joubert A, Gastaldo J, Massart C, Balosso J, Foray N (2008) Molecular and cellular response of the most extensively used rodent glioma models to radiation and/or cisplatin. J Neuro-Oncol 86:13–21
Dodgshun AJ, Sexton-Oates A, Saffery R, Sullivan MJ (2016) Biallelic FANCD1/BRCA2 mutations predisposing to glioblastoma multiforme with multiple oncogenic amplifications. Cancer Genetics 209:53–56
Lampert K, Machein U, Machein MR, Conca W, Peter HH, Volk B (1998) Expression of matrix metalloproteinases and their tissue inhibitors in human brain tumors. Am J Pathol 153:429–437
Nakagawa T, Kubota T, Kabuto M, Sato K, Kawano H, Hayakawa T, Okada Y (1994) Production of matrix metalloproteinases and tissue inhibitor of metalloproteinases-1 by human brain-tumors. J Neurosurg 81:69–77
Forsyth PA, Wong H, Laing TD, Rewcastle NB, Morris DG, Muzik H, Leco KJ, Johnston RN, Brasher PMA, Sutherland G, et al. (1999) Gelatinase-A (MMP-2), gelatinase-B (MMP-9) and membrane type matrix metalloproteinase-1 (MT1-MMP) are involved in different aspects of the pathophysiology of malignant gliomas. Br J Cancer 79:1828–1835
Yamamoto M, Mohanam S, Sawaya R, Fuller GN, Seiki M, Sato H, Gokaslan ZL, Liotta LA, Nicolson GL, Rao JS (1996) Differential expression of membrane-type matrix metalloproteinase and its correlation with gelatinase A activation in human malignant brain tumors in vivo and in vitro. Cancer Res 56:384–392
Ulasov I, Yi RY, Guo D, Sarvaiya P, Cobbs C (2014) The emerging role of MMP14 in brain tumorigenesis and future therapeutics. Biochimica Et Biophysica Acta-Reviews on Cancer 1846:113–120
Liu MF, Hu YY, Jin T, Xu K, Wang SH, Du GZ, Wu BL, Li LY, Xu LY, Li EM, et al. (2015) Matrix metalloproteinase-9/neutrophil gelatinase-associated lipocalin complex activity in human glioma samples predicts tumor presence and clinical prognosis. Dis Markers. doi:10.1155/2015/138974
Musumeci G, Magro G, Cardile V, Coco M, Marzagalli R, Castrogiovanni P, Imbesi R, Graziano ACE, Barone F, Di Rosa M, et al. (2015) Characterization of matrix metalloproteinase-2 and-9, ADAM-10 and N-cadherin expression in human glioblastoma multiforme. Cell Tissue Res 362:45–60
Li QB, Chen BS, Cai JQ, Sun Y, Wang GZ, Li YL, Li RY, Feng Y, Han B, Li JL, et al. (2016) Comparative analysis of matrix metalloproteinase family members reveals that MMP9 predicts survival and response to temozolomide in patients with primary glioblastoma. PLoS One 11
Sakr M, Takino T, Sabit H, Nakada M, Li ZC, Sato H (2016) miR-150-5p and miR-133a suppress glioma cell proliferation and migration through targeting membrane-type-1 matrix metalloproteinase. Gene 587:155–162
Chung HJ, Choi YE, Kim ES, Han YH, Park MJ, Bae IH (2015) miR-29b attenuates tumorigenicity and stemness maintenance in human glioblastoma multiforme by directly targeting BCL2L2. Oncotarget 6:18429–18444
Somasundaram A, Ardanowski N, Opalak CF, Fillmore HL, Chidambaram A, Broaddus WC (2014) Wilms tumor 1 gene, CD97, and the emerging biogenetic profile of glioblastoma. Neurosurg Focus 37
Kijima N, Hashimoto N, Chiba Y, Fujimoto Y, Sugiyama H, Yoshimine T (2016) Functional roles of Wilms’ tumor 1 (WT1) in malignant brain tumors. Wilms Tumor DOI:261–272
Dennis SL, Manji SSM, Carrington DP, Scarcella DL, Ashley DM, Smith PJ, Algar EM (2002) Expression and mutation analysis of the Wilms’ tumor 1 gene in human neural tumors. Int J Cancer 97:713–715
Mehrian-Shai R, Yalon M, Simon AJ, Eyal E, Pismenyuk T, Moshe I, Constantini S, Toren A (2015) High metallothionein predicts poor survival in glioblastoma multiforme. BMC Med Genet 8
Hiura T, Khalid H, Yamashita H, Tokunaga Y, Yasunaga A, Shibata S (1998) Immunohistochemical analysis of metallothionein in astrocytic tumors in relation to tumor grade, proliferative potential, and survival. Cancer 83:2361–2369
Florianczyk B, Osuchowski J, Kaczmarczyk R, Staroslawska E, Trojanowski T (2005) Distribution of metallothioneins in the brain neoplastic cells. Folia Neuropathol 43:91–96
Dasari VR, Kaur K, Velpula KK, Gujrati M, Fassett D, Klopfenstein JD, Dinh DH, Rao JS (2010) Upregulation of PTEN in glioma cells by cord blood mesenchymal stem cells inhibits migration via downregulation of the PI3K/Akt pathway. PLoS One 5
Zhang JH, Wu G, Miller CP, Tatevossian RG, Dalton JD, Tang B, Orisme W, Punchihewa C, Parker M, Qaddoumi I, et al. (2013) Whole-genome sequencing identifies genetic alterations in pediatric low-grade gliomas. Nat Genet 45:602-+
Rodriguez FJ, Lim KS, Bowers D, Eberhart CG (2013) Pathological and molecular advances in pediatric low-grade astrocytoma. In: Abbas AK, Galli SJ, Howley PM (eds) Annual review of pathology: mechanisms of disease, Vol 8 Annual Reviews, Palo Alto, pp. 361–379
Liu ZG, Liu YG, Li LL, Xu ZK, Bi BB, Wang YY, Li JY (2014) MiR-7-5p is frequently downregulated in glioblastoma microvasculature and inhibits vascular endothelial cell proliferation by targeting RAF1. Tumor Biol 35:10177–10184
Verreault M, Schmitt C, Goldwirt L, Pelton K, Haidar S, Levasseur C, Guehennec J, Knoff D, Labussiere M, Marie Y, et al. (2016) Preclinical efficacy of the MDM2 inhibitor RG7112 in MDM2-amplified and TP53 wild-type glioblastomas. Clin Cancer Res 22:1185–1196
Reifenberger G, Liu L, Ichimura K, Schmidt EE, Collins VP (1993) Amplification and overexpression of the MDM2 gene in a subset of human-malignant gliomas without p53 mutations. Cancer Res 53:2736–2739
Suzuki SO, Iwaki T (2000) Amplification and overexpression of mdm2 gene in ependymomas. Mod Pathol 13:548–553
Markert JM, Fuller CM, Gillespie GY, Bubien JK, McLean LA, Hong RL, Lee K, Gullans SR, Mapstone TB, Benos DJ (2001) Differential gene expression profiling in human brain tumors. Physiol Genomics 5:21–33
Kraus JA, Felsberg J, Tonn JC, Reifenberger G, Pietsch T (2002) Molecular genetic analysis of the TP53, PTEN, CDKN2A, EGFR, CDK4 and MDM2 tumour-associated genes in supratentorial primitive neuroectodermal tumours and glioblastomas of childhood. Neuropathol Appl Neurobiol 28:325–333
Ranuncolo SM, Varela M, Morandi A, Lastiri J, Christiansen S, Joffe EBD, Pallotta MG, Puricelli L (2004) Prognostic value of Mdm2, p53 and p16 in patients with astrocytomas. J Neuro-Oncol 68:113–121
Kafadar A, Kucukhuseyin O, Turan S, Yenilmez EN, Tunoglu S, Zeybek U, Kaynar MY, Kemerdere R, Yaylim I (2015) Distribution and effects of CDKN2 p16 540 C > G and 580 C > T, and MDM2 SNP309 T > G polymorphisms in patients with primary brain tumors. Anticancer Res 35:3933–3942
Wang CL, Wang JY, Liu ZY, Ma XM, Wang XW, Jin H, Zhang XP, Fu D, Hou LJ, Lu YC (2014) Ubiquitin-specific protease 2a stabilizes MDM4 and facilitates the p53-mediated intrinsic apoptotic pathway in glioblastoma. Carcinogenesis 35:1500–1509
Sampieri K, Mencarelli MA, Epistolato MC, Toti P, Lazzi S, Bruttini M, De Francesco S, Longo I, Meloni I, Mari F, et al. (2008) Genomic differences between retinoma and retinoblastoma. Acta Oncol 47:1483–1492
Rao SK, Edwards J, Joshi AD, Siu IM, Riggins GJ (2010) A survey of glioblastoma genomic amplifications and deletions. J Neuro-Oncol 96:169–179
Perry C, Sklan EH, Soreq H (2004) CREB regulates AChE-R-induced proliferation of human glioblastoma cells. Neoplasia 6:279–286
Mantamadiotis T, Papalexis N, Dworkin S (2012) CREB signalling in neural stem/progenitor cells: recent developments and the implications for brain tumour biology. BioEssays 34:293–300
Daniel P, Filiz G, Brown DV, Hollande F, Gonzales M, D'Abaco G, Papalexis N, Phillips WA, Malaterre J, Ramsay RG et al (2014) Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation. Oncogenesis 3: 1–10
Zhang JQ, Yao QH, Kuang YQ, Ma Y, Yang LB, Huang HD, Cheng JM, Yang T, Liu EY, Liang L, et al. (2014) Prognostic value of coexistence of abnormal expression of micro-RNA-200b and cyclic adenosine monophosphate-responsive element-binding protein 1 in human astrocytoma. Hum Pathol 45:2154–2161
Ajeawung NF, Maltais R, Jones C, Poirier D, Kamnasaran D (2013) Viability screen on pediatric low grade glioma cell lines unveils a novel anti-cancer drug of the steroid biosynthesis inhibitor family. Cancer Lett 330:96–105
Zhao RJ, Zhang XL, Chu SG, Zhang M, Kong LF, Wang Y (2016) Clinicopathologic and neuroradiologic studies of papillary glioneuronal tumors. Acta Neurochir 158:695–702
Lin T, Wang M, Jiang HS, Liu EZ (2015) The expression of P53, MGMT and EGFR in brain glioma and clinical significance. J Biol Regul Homeost Agents 29:143–149
Oka H, Utsuki S, Tanizaki Y, Hagiwara H, Miyajima Y, Sato K, Kusumi M, Kijima C, Fujii K (2013) Clinicopathological features of human brainstem gliomas. Brain Tumor Pathology 30:1–7
Chin L, Meyerson M, Aldape K, Bigner D, Mikkelsen T, VandenBerg S, Kahn A, Penny R, Ferguson ML, Gerhard DS, et al. (2008) Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455:1061–1068
Brandes AA, Franceschi E, Tosoni A, Blatt V, Pession A, Tallini G, Bertorelle R, Bartolini S, Calbucci F, Andreoli A, et al. (2008) MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol 26:2192–2197
Silber JR, Mueller BA, Ewers TG, Berger MS (1993) Comparison of O6-methylguanine-DNA methyltransferase activity in brain-tumors and adjacent normal brain. Cancer Res 53:3416–3420
Kolodziej MA, Weischer C, Reinges MHT, Uhl E, Weigand MA, Schwarm FP, Schanzer A, Acker T, Quint K, Uhle F, et al. (2016) NDRG2 and NDRG4 expression is altered in glioblastoma and influences survival in patients with MGMT-methylated tumors. Anticancer Res 36:887–897
Horing E, Harter PN, Seznec J, Schittenhelm J, Buhring HJ, Bhattacharyya S, von Hattingen E, Zachskorn C, Mittelbronn M, Naumann U (2012) The “go or grow” potential of gliomas is linked to the neuropeptide processing enzyme carboxypeptidase E and mediated by metabolic stress. Acta Neuropathol 124:83–97
Denis CJ, Lambeir AM (2013) The potential of carboxypeptidase M as a therapeutic target in cancer. Expert Opin Ther Targets 17:265–279
Verbovsek U, Motaln H, Rotter A, Atai NA, Gruden K, Van Noorden CJF, Lah TT (2014) Expression analysis of all protease genes reveals cathepsin K to be overexpressed in glioblastoma Plos One:9
Haapasalo JA, Nordfors KM, Hilvo M, Rantala IJ, Soini Y, Parkkila AK, Pastorekova S, Pastorek J, Parkkila SM, Haapasalo HK (2006) Expression of carbonic anhydrase IX in astrocytic tumors predicts poor prognosis. Clin Cancer Res 12:473–477
Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KSC, Chapman JD, Eckelman WC, Fyles AW, Giaccia AJ, et al. (2006) Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol 82:699–757
Said HM, Supuran CT, Hageman C, Staab A, Polat B, Katzer A, Scozzafava A, Anacker J, Flentje M, Vordermark D (2010) Modulation of carbonic anhydrase 9 (CA9) in human brain cancer. Curr Pharm Des 16:3288–3299
Nordfors K, Haapasalo J, Korja M, Niemela A, Laine J, Parkkila AK, Pastorekova S, Pastorek J, Waheed A, Sly WS et al (2010) The tumour-associated carbonic anhydrases CA II, CA IX and CA XII in a group of medulloblastomas and supratentorial primitive neuroectodermal tumours: an association of CA IX with poor prognosis. Bmc Cancer 10: 1–10
Proescholdt MA, Merrill MJ, Stoerr EM, Lohmeier A, Pohl F, Brawanski A (2012) Function of carbonic anhydrase IX in glioblastoma multiforme. Neuro-Oncology 14:1357–1366
Li XN, Shu Q, Su JMF, Perlaky L, Blaney SM, Lau CC (2005) Valproic acid induces growth arrest, apoptosis, and senescence in medulloblastomas by increasing histone hyperacetylation and regulating expression of p21Cip1, CDK4, and CMYC. Mol Cancer Ther 4:1912–1922
Lee SJ, Lindsey S, Graves B, Yoo S, Olson JM, Langhans SA (2013) Sonic hedgehog-induced histone deacetylase activation is required for cerebellar granule precursor hyperplasia in medulloblastoma Plos One:8
Ecker J, Oehme I, Mazitschek R, Korshunov A, Kool M, Hielscher T, Kiss J, Selt F, Konrad C, Lodrini M et al (2015) Targeting class I histone deacetylase 2 in MYC amplified group 3 medulloblastoma. Acta Neuropathologica Communications 3: 1–12
Kesari S (2011) Understanding glioblastoma tumor biology: the potential to improve current diagnosis and treatments. Semin Oncol 38:S2–S10
Acknowledgments
This research was carried out under the project CEITEC 2020 (LQ1601) with financial support from the Ministry of Education, Youth and Sports of the Czech Republic under the National Sustainability Programme II. The financial support from the project AZV CR 15-28334A and from the Ministry of Health of the Czech Republic for conceptual development of research organization 00064203 (University Hospital Motol, Prague, Czech Republic) is highly acknowledged.
Author contributions
J.H., T.E., and M.S. wrote and discussed the chapter “The roles of zinc ions and their transporter proteins in cell and tissue development” and conceived the study. Z.H. prepared and critically reviewed “Zinc ions and zinc containing proteins as possible therapeutic targets”. S.K. and V.A. prepared “Zinc, zinc-containing biomolecules and childhood brain tumors” and “Utilization of zinc and zinc containing proteins for cancer diagnostics” and conceived the study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hrabeta, J., Eckschlager, T., Stiborova, M. et al. Zinc and zinc-containing biomolecules in childhood brain tumors. J Mol Med 94, 1199–1215 (2016). https://doi.org/10.1007/s00109-016-1454-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00109-016-1454-8