Abstract
Zinc transporters, the Zrt-, Irt-like protein (ZIP) family and the Zn transporter (ZnT) family transporters, are found in all aspects of life. Increasing evidence has clarified the molecular mechanism, in which both transporters play critical roles in cellular and physiological functions via mobilizing zinc across the cellular membrane. In the last decade, mutations in ZIP and ZnT transporter genes have been shown to be implicated in a number of inherited human diseases. Moreover, dysregulation of expression and activity of both transporters has been suggested to be involved in the pathogenesis and progression of chronic diseases including cancer, immunological impairment, and neurodegenerative diseases, although comprehensive understanding is far from complete. The diverse phenotypes of diseases related to ZIP and ZnT transporters reflect the multifarious biological functions of both transporters. The present review summarizes the current understanding of ZIP and ZnT transporter functions from the standpoint of human health and diseases. The study of zinc transporters is currently of great clinical interest.


Similar content being viewed by others
Abbreviations
- ZIP:
-
Zrt, Irt-like protein
- ZnT:
-
Zinc transporter
- SLC:
-
Solute carrier
- TMD:
-
Transmembrane domain
- AE:
-
Acrodermatitis enteropathica
- EDS:
-
Ehlers–Danlos syndrome
- SCD-EDS:
-
Spondylocheiro dysplastic form of EDS
- TNZD:
-
Transient neonatal zinc deficiency
- T1DM:
-
Type 1 diabetes
- T2DM:
-
Type 2 diabetes
- SNP:
-
Single-nucleotide polymorphism
- EV:
-
Epidermodysplasia verruciformis
References
Jackson MJ (1989) Physiology of zinc: general aspects. In: Mills CF (ed) Zinc in human biology. Springer, Berlin Heidelberg New York, pp 1–14
Maret W, Sandstead HH (2006) Zinc requirements and the risks and benefits of zinc supplementation. J Trace Elem Med Biol 20:3–18
Vallee BL, Falchuk KH (1993) The biochemical basis of zinc physiology. Physiol Rev 73:79–118
Maret W, Li Y (2009) Coordination dynamics of zinc in proteins. Chem Rev 109:4682–4707
Fukada T, Kambe T (2011) Molecular and genetic features of zinc transporters in physiology and pathogenesis. Metallomics 3:662–674
Prasad AS (1991) Discovery of human zinc deficiency and studies in an experimental human model. Am J Clin Nutr 53:403–412
Hambidge M (2000) Human zinc deficiency. J Nutr 130(5S Suppl):1344S–1349S
Fraker PJ, King LE (2004) Reprogramming of the immune system during zinc deficiency. Annu Rev Nutr 24:277–298
Devirgiliis C, Zalewski PD, Perozzi G, Murgia C (2007) Zinc fluxes and zinc transporter genes in chronic diseases. Mutat Res 622:84–93
Krebs NF (2000) Overview of zinc absorption and excretion in the human gastrointestinal tract. J Nutr 130(5S Suppl):1374S–1377S
Liuzzi JP, Bobo JA, Lichten LA, Samuelson DA, Cousins RJ (2004) Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis. Proc Natl Acad Sci USA 101:14355–14360
Kambe T, Yamaguchi-Iwai Y, Sasaki R, Nagao M (2004) Overview of mammalian zinc transporters. Cell Mol Life Sci 61:49–68
Soybel DI, Kohler JE (2011) Zinc and the gastrointestinal tract. In: Rink L (ed) Zinc in human health. IOS Press, Amsterdam, pp 448–472
Hambidge M, Krebs NF (2001) Interrelationships of key variables of human zinc homeostasis: relevance to dietary zinc requirements. Annu Rev Nutr 21:429–452
King JC, Shames DM, Woodhouse LR (2000) Zinc homeostasis in humans. J Nutr 130:1360S–1366S
Reyes JG (1996) Zinc transport in mammalian cells. Am J Physiol 270:C401–C410
Barnett JP, Blindauer CA, Kassaar O, Khazaipoul S, Martin EM, Sadler PJ, Stewart AJ (2013) Allosteric modulation of zinc speciation by fatty acids. Biochim Biophys Acta 1830:5456–5464
Thiers RE, Vallee BL (1957) Distribution of metals in subcellular fractions of rat liver. J Biol Chem 226:911–920
Haase H, Rink L (2014) Zinc signals and immune function. Biofactors 40:27–40
Coyle P, Philcox JC, Carey LC, Rofe AM (2002) Metallothionein: the multipurpose protein. Cell Mol Life Sci 59:627–647
Andreini C, Bertini I, Cavallaro G (2011) Minimal functional sites allow a classification of zinc sites in proteins. PLoS ONE 6:e26325
Hopfner KP, Craig L, Moncalian G, Zinkel RA, Usui T, Owen BA, Karcher A, Henderson B, Bodmer JL, McMurray CT, Carney JP, Petrini JH, Tainer JA (2002) The Rad50 zinc-hook is a structure joining Mre11 complexes in DNA recombination and repair. Nature 418:562–566
Prasad AS, Halsted JA, Nadimi M (1961) Syndrome of iron deficiency anemia, hepatosplenomegaly, hypogonadism, dwarfism and geophagia. Am J Med 31:532–546
Prasad AS (1985) Clinical manifestations of zinc deficiency. Annu Rev Nutr 5:341–363
Prasad AS (2003) Zinc deficiency. BMJ 326:409–410
Weaver BP, Andrews GK (2012) Zinc transporter mutations and human growth. In: Preedy VR (ed) Handbook of growth and growth monitoring in health and disease. Springer Science + Business Media, LLC, Berlin, pp 2319–2336
Besecker BY, Exline MC, Hollyfield J, Phillips G, Disilvestro RA, Wewers MD, Knoell DL (2011) A comparison of zinc metabolism, inflammation, and disease severity in critically ill infected and noninfected adults early after intensive care unit admission. Am J Clin Nutr 93:1356–1364
Uciechowski P, Kahmann L, Plumakers B, Malavolta M, Mocchegiani E, Dedoussis G, Herbein G, Jajte J, Fulop T, Rink L (2008) TH1 and TH2 cell polarization increases with aging and is modulated by zinc supplementation. Exp Gerontol 43:493–498
Mocchegiani E, Romeo J, Malavolta M, Costarelli L, Giacconi R, Diaz LE, Marcos A (2013) Zinc: dietary intake and impact of supplementation on immune function in elderly. Age (Dordr) 35:839–860
Haase H, Mocchegiani E, Rink L (2006) Correlation between zinc status and immune function in the elderly. Biogerontology 7:421–428
Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, Cardozo LJ (2007) Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr 85:837–844
A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8 (2001) Arch Ophthalmol 119:1417–1436
Brown KH, Peerson JM, Rivera J, Allen LH (2002) Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: a meta-analysis of randomized controlled trials. Am J Clin Nutr 75:1062–1071
Wardlaw T, Salama P, Brocklehurst C, Chopra M, Mason E (2010) Diarrhoea: why children are still dying and what can be done. Lancet 375:870–872
Penny ME (2013) Zinc supplementation in public health. Ann Nutr Metab 62(Suppl 1):31–42
Kambe T, Weaver BP, Andrews GK (2008) The genetics of essential metal homeostasis during development. Genesis 46:214–228
Lichten LA, Cousins RJ (2009) Mammalian zinc transporters: nutritional and physiologic regulation. Annu Rev Nutr 29:153–176
Bin BH, Fukada T, Hosaka T, Yamasaki S, Ohashi W, Hojyo S, Miyai T, Nishida K, Yokoyama S, Hirano T (2011) Biochemical characterization of human ZIP13 protein: a homo-dimerized zinc transporter involved in the spondylocheiro dysplastic Ehlers–Danlos syndrome. J Biol Chem 286:40255–40265
Taylor KM, Morgan HE, Smart K, Zahari NM, Pumford S, Ellis IO, Robertson JF, Nicholson RI (2007) The emerging role of the LIV-1 subfamily of zinc transporters in breast cancer. Mol Med 13:396–406
Kambe T (2013) Regulation of zinc transport. In: Culotta V, Scott RA (eds) Encyclopedia of inorganic and bioinorganic chemistry-metals in cells. Wiley, Chichester, pp 301–309
Girijashanker K, He L, Soleimani M, Reed JM, Li H, Liu Z, Wang B, Dalton TP, Nebert DW (2008) Slc39a14 gene encodes ZIP14, a metal/bicarbonate symporter: similarities to the ZIP8 transporter. Mol Pharmacol 73:1413–1423
Fujishiro H, Yano Y, Takada Y, Tanihara M, Himeno S (2012) Roles of ZIP8, ZIP14, and DMT1 in transport of cadmium and manganese in mouse kidney proximal tubule cells. Metallomics 4:700–708
Liuzzi JP, Aydemir F, Nam H, Knutson MD, Cousins RJ (2006) Zip14 (Slc39a14) mediates non-transferrin-bound iron uptake into cells. Proc Natl Acad Sci USA 103:13612–13617
Fukunaka A, Suzuki T, Kurokawa Y, Yamazaki T, Fujiwara N, Ishihara K, Migaki H, Okumura K, Masuda S, Yamaguchi-Iwai Y, Nagao M, Kambe T (2009) Demonstration and characterization of the heterodimerization of ZnT5 and ZnT6 in the early secretory pathway. J Biol Chem 284:30798–30806
Kambe T (2012) Molecular architecture and function of ZnT transporters. Curr Top Membr 69:199–220
Suzuki T, Ishihara K, Migaki H, Nagao M, Yamaguchi-Iwai Y, Kambe T (2005) Two different zinc transport complexes of cation diffusion facilitator proteins localized in the secretory pathway operate to activate alkaline phosphatases in vertebrate cells. J Biol Chem 280:30956–30962
Lu M, Fu D (2007) Structure of the zinc transporter YiiP. Science 317:1746–1748
Lu M, Chai J, Fu D (2009) Structural basis for autoregulation of the zinc transporter YiiP. Nat Struct Mol Biol 16:1063–1067
Coudray N, Valvo S, Hu M, Lasala R, Kim C, Vink M, Zhou M, Provasi D, Filizola M, Tao J, Fang J, Penczek PA, Ubarretxena-Belandia I, Stokes DL (2013) Inward-facing conformation of the zinc transporter YiiP revealed by cryoelectron microscopy. Proc Natl Acad Sci USA 110:2140–2145
Podar D, Scherer J, Noordally Z, Herzyk P, Nies D, Sanders D (2012) Metal selectivity determinants in a family of transition metal transporters. J Biol Chem 287:3185–3196
Tanaka N, Kawachi M, Fujiwara T, Maeshima M (2013) Zinc-binding and structural properties of the histidine-rich loop of Arabidopsis thaliana vacuolar membrane zinc transporter MTP1. FEBS Open Bio 3:218–224
Ohana E, Hoch E, Keasar C, Kambe T, Yifrach O, Hershfinkel M, Sekler I (2009) Identification of the Zn2+ binding site and mode of operation of a mammalian Zn2+ transporter. J Biol Chem 284:17677–17686
Quadri M, Federico A, Zhao T, Breedveld GJ, Battisti C, Delnooz C, Severijnen LA, Di Toro Mammarella L, Mignarri A, Monti L, Sanna A, Lu P, Punzo F, Cossu G, Willemsen R, Rasi F, Oostra BA, van de Warrenburg BP, Bonifati V (2012) Mutations in SLC30A10 cause Parkinsonism and dystonia with hypermanganesemia, polycythemia, and chronic liver disease. Am J Hum Genet 90:467–477
Tuschl K, Clayton PT, Gospe SM Jr, Gulab S, Ibrahim S, Singhi P, Aulakh R, Ribeiro RT, Barsottini OG, Zaki MS, Del Rosario ML, Dyack S, Price V, Rideout A, Gordon K, Wevers RA, Chong WK, Mills PB (2012) Syndrome of hepatic cirrhosis, dystonia, polycythemia, and hypermanganesemia caused by mutations in SLC30A10, a manganese transporter in man. Am J Hum Genet 90:457–466
Jeong J, Eide DJ (2013) The SLC39 family of zinc transporters. Mol Aspects Med 34:612–619
Huang L, Tepaamorndech S (2013) The SLC30 family of zinc transporters—a review of current understanding of their biological and pathophysiological roles. Mol Aspects Med 34:548–560
Maverakis E, Fung MA, Lynch PJ, Draznin M, Michael DJ, Ruben B, Fazel N (2007) Acrodermatitis enteropathica and an overview of zinc metabolism. J Am Acad Dermatol 56:116–124
Schmitt S, Kury S, Giraud M, Dreno B, Kharfi M, Bezieau S (2009) An update on mutations of the SLC39A4 gene in acrodermatitis enteropathica. Hum Mutat 30:926–933
Lombeck T, Schnippering HG, Ritzl F, Feinendegen LE, Bremer HJ (1975) Letter: absorption of zinc in acrodermatitis enteropathica. Lancet 1:855
Kury S, Dreno B, Bezieau S, Giraudet S, Kharfi M, Kamoun R, Moisan JP (2002) Identification of SLC39A4, a gene involved in acrodermatitis enteropathica. Nat Genet 31:239–240
Wang K, Zhou B, Kuo YM, Zemansky J, Gitschier J (2002) A novel member of a zinc transporter family is defective in acrodermatitis enteropathica. Am J Hum Genet 71:66–73
Andrews GK (2008) Regulation and function of Zip4, the acrodermatitis enteropathica gene. Biochem Soc Trans 36:1242–1246
Li CR, Yan SM, Shen DB, Li Q, Shao JP, Xue CY, Cao YH (2010) One novel homozygous mutation of SLC39A4 gene in a Chinese patient with acrodermatitis enteropathica. Arch Dermatol Res 302:315–317
Kawamura T, Ogawa Y, Nakamura Y, Nakamizo S, Ohta Y, Nakano H, Kabashima K, Katayama I, Koizumi S, Kodama T, Nakao A, Shimada S (2012) Severe dermatitis with loss of epidermal Langerhans cells in human and mouse zinc deficiency. J Clin Investig 122:722–732
Dufner-Beattie J, Weaver BP, Geiser J, Bilgen M, Larson M, Xu W, Andrews GK (2007) The mouse acrodermatitis enteropathica gene Slc39a4 (Zip4) is essential for early development and heterozygosity causes hypersensitivity to zinc deficiency. Hum Mol Genet 16:1391–1399
Geiser J, Venken KJ, De Lisle RC, Andrews GK (2012) A mouse model of acrodermatitis enteropathica: loss of intestine zinc transporter ZIP4 (Slc39a4) disrupts the stem cell niche and intestine integrity. PLoS Genet 8:e1002766
Dufner-Beattie J, Wang F, Kuo YM, Gitschier J, Eide D, Andrews GK (2003) The acrodermatitis enteropathica gene ZIP4 encodes a tissue-specific, zinc-regulated zinc transporter in mice. J Biol Chem 278:33474–33481
Wang F, Kim BE, Dufner-Beattie J, Petris MJ, Andrews G, Eide DJ (2004) Acrodermatitis enteropathica mutations affect transport activity, localization and zinc-responsive trafficking of the mouse ZIP4 zinc transporter. Hum Mol Genet 13:563–571
Dufner-Beattie J, Kuo YM, Gitschier J, Andrews GK (2004) The adaptive response to dietary zinc in mice involves the differential cellular localization and zinc regulation of the zinc transporters ZIP4 and ZIP5. J Biol Chem 279:49082–49090
Kim BE, Wang F, Dufner-Beattie J, Andrews GK, Eide DJ, Petris MJ (2004) Zn2+-stimulated endocytosis of the mZIP4 zinc transporter regulates its location at the plasma membrane. J Biol Chem 279:4523–4530
Mao X, Kim BE, Wang F, Eide DJ, Petris MJ (2007) A histidine-rich cluster mediates the ubiquitination and degradation of the human zinc transporter, hZIP4, and protects against zinc cytotoxicity. J Biol Chem 282:6992–7000
Weaver BP, Dufner-Beattie J, Kambe T, Andrews GK (2007) Novel zinc-responsive post-transcriptional mechanisms reciprocally regulate expression of the mouse Slc39a4 and Slc39a5 zinc transporters (Zip4 and Zip5). Biol Chem 388:1301–1312
Kambe T, Andrews GK (2009) Novel proteolytic processing of the ectodomain of the zinc transporter ZIP4 (SLC39A4) during zinc deficiency is inhibited by acrodermatitis enteropathica mutations. Mol Cell Biol 29:129–139
Liuzzi JP, Guo L, Chang SM, Cousins RJ (2009) Kruppel-like factor 4 regulates adaptive expression of the zinc transporter Zip4 in mouse small intestine. Am J Physiol Gastrointest Liver Physiol 296:G517–G523
Antala S, Dempski RE (2012) The human ZIP4 transporter has two distinct binding affinities and mediates transport of multiple transition metals. Biochemistry 51:963–973
Wang K, Pugh EW, Griffen S, Doheny KF, Mostafa WZ, al-Aboosi MM, el-Shanti H, Gitschier J (2001) Homozygosity mapping places the acrodermatitis enteropathica gene on chromosomal region 8q24.3. Am J Hum Genet 68:1055–1060
Li M, Zhang Y, Liu Z, Bharadwaj U, Wang H, Wang X, Zhang S, Liuzzi JP, Chang SM, Cousins RJ, Fisher WE, Brunicardi FC, Logsdon CD, Chen C, Yao Q (2007) Aberrant expression of zinc transporter ZIP4 (SLC39A4) significantly contributes to human pancreatic cancer pathogenesis and progression. Proc Natl Acad Sci USA 104:18636–18641
Zhang Y, Chen C, Yao Q, Li M (2010) ZIP4 upregulates the expression of neuropilin-1, vascular endothelial growth factor, and matrix metalloproteases in pancreatic cancer cell lines and xenografts. Cancer Biol Ther 9:236–242
Weaver BP, Zhang Y, Hiscox S, Guo GL, Apte U, Taylor KM, Sheline CT, Wang L, Andrews GK (2010) Zip4 (Slc39a4) expression is activated in hepatocellular carcinomas and functions to repress apoptosis, enhance cell cycle and increase migration. PLoS ONE 5:e13158
Lin Y, Chen Y, Wang Y, Yang J, Zhu VF, Liu Y, Cui X, Chen L, Yan W, Jiang T, Hergenroeder GW, Fletcher SA, Levine JM, Kim DH, Tandon N, Zhu JJ, Li M (2013) ZIP4 is a novel molecular marker for glioma. Neuro Oncol 15:1008–1016
Beighton P, De Paepe A, Steinmann B, Tsipouras P, Wenstrup RJ (1998) Ehlers–Danlos syndromes: revised nosology, Villefranche, 1997. Ehlers–Danlos National Foundation (USA) and Ehlers–Danlos Support Group (UK). Am J Med Genet 77:31–37
Parapia LA, Jackson C (2008) Ehlers–Danlos syndrome—a historical review. Br J Haematol 141:32–35
Giunta C, Elcioglu NH, Albrecht B, Eich G, Chambaz C, Janecke AR, Yeowell H, Weis M, Eyre DR, Kraenzlin M, Steinmann B (2008) Spondylocheiro dysplastic form of the Ehlers–Danlos syndrome—an autosomal-recessive entity caused by mutations in the zinc transporter gene SLC39A13. Am J Hum Genet 82:1290–1305
Fukada T, Civic N, Furuichi T, Shimoda S, Mishima K, Higashiyama H, Idaira Y, Asada Y, Kitamura H, Yamasaki S, Hojyo S, Nakayama M, Ohara O, Koseki H, Dos Santos HG, Bonafe L, Ha-Vinh R, Zankl A, Unger S, Kraenzlin ME, Beckmann JS, Saito I, Rivolta C, Ikegawa S, Superti-Furga A, Hirano T (2008) The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS ONE 3:e3642
Jeong J, Walker JM, Wang F, Park JG, Palmer AE, Giunta C, Rohrbach M, Steinmann B, Eide DJ (2012) Promotion of vesicular zinc efflux by ZIP13 and its implications for spondylocheiro dysplastic Ehlers–Danlos syndrome. Proc Natl Acad Sci USA 109:E3530–E3538
Dupuis J, Langenberg C, Prokopenko I, Saxena R, Soranzo N, Jackson AU, Wheeler E, Glazer NL, Bouatia-Naji N, Gloyn AL, Lindgren CM, Magi R, Morris AP, Randall J, Johnson T, Elliott P, Rybin D, Thorleifsson G, Steinthorsdottir V, Henneman P, Grallert H, Dehghan A, Hottenga JJ, Franklin CS, Navarro P, Song K, Goel A, Perry JR, Egan JM, Lajunen T, Grarup N, Sparso T, Doney A, Voight BF, Stringham HM, Li M, Kanoni S, Shrader P, Cavalcanti-Proenca C, Kumari M, Qi L, Timpson NJ, Gieger C, Zabena C, Rocheleau G, Ingelsson E, An P, O’Connell J, Luan J, Elliott A, McCarroll SA, Payne F, Roccasecca RM, Pattou F, Sethupathy P, Ardlie K, Ariyurek Y, Balkau B, Barter P, Beilby JP, Ben-Shlomo Y, Benediktsson R, Bennett AJ, Bergmann S, Bochud M, Boerwinkle E, Bonnefond A, Bonnycastle LL, Borch-Johnsen K, Bottcher Y, Brunner E, Bumpstead SJ, Charpentier G, Chen YD, Chines P, Clarke R, Coin LJ, Cooper MN, Cornelis M, Crawford G, Crisponi L, Day IN, de Geus EJ, Delplanque J, Dina C, Erdos MR, Fedson AC, Fischer-Rosinsky A, Forouhi NG, Fox CS, Frants R, Franzosi MG, Galan P, Goodarzi MO, Graessler J, Groves CJ, Grundy S, Gwilliam R, Gyllensten U, Hadjadj S, Hallmans G, Hammond N, Han X, Hartikainen AL, Hassanali N, Hayward C, Heath SC, Hercberg S, Herder C, Hicks AA, Hillman DR, Hingorani AD, Hofman A, Hui J, Hung J, Isomaa B, Johnson PR, Jorgensen T, Jula A, Kaakinen M, Kaprio J, Kesaniemi YA, Kivimaki M, Knight B, Koskinen S, Kovacs P, Kyvik KO, Lathrop GM, Lawlor DA, Le Bacquer O, Lecoeur C, Li Y, Lyssenko V, Mahley R, Mangino M, Manning AK, Martinez-Larrad MT, McAteer JB, McCulloch LJ, McPherson R, Meisinger C, Melzer D, Meyre D, Mitchell BD, Morken MA, Mukherjee S, Naitza S, Narisu N, Neville MJ, Oostra BA, Orru M, Pakyz R, Palmer CN, Paolisso G, Pattaro C, Pearson D, Peden JF, Pedersen NL, Perola M, Pfeiffer AF, Pichler I, Polasek O, Posthuma D, Potter SC, Pouta A, Province MA, Psaty BM, Rathmann W, Rayner NW, Rice K, Ripatti S, Rivadeneira F, Roden M, Rolandsson O, Sandbaek A, Sandhu M, Sanna S, Sayer AA, Scheet P, Scott LJ, Seedorf U, Sharp SJ, Shields B, Sigurethsson G, Sijbrands EJ, Silveira A, Simpson L, Singleton A, Smith NL, Sovio U, Swift A, Syddall H, Syvanen AC, Tanaka T, Thorand B, Tichet J, Tonjes A, Tuomi T, Uitterlinden AG, van Dijk KW, van Hoek M, Varma D, Visvikis-Siest S, Vitart V, Vogelzangs N, Waeber G, Wagner PJ, Walley A, Walters GB, Ward KL, Watkins H, Weedon MN, Wild SH, Willemsen G, Witteman JC, Yarnell JW, Zeggini E, Zelenika D, Zethelius B, Zhai G, Zhao JH, Zillikens MC, Borecki IB, Loos RJ, Meneton P, Magnusson PK, Nathan DM, Williams GH, Hattersley AT, Silander K, Salomaa V, Smith GD, Bornstein SR, Schwarz P, Spranger J, Karpe F, Shuldiner AR, Cooper C, Dedoussis GV, Serrano-Rios M, Morris AD, Lind L, Palmer LJ, Hu FB, Franks PW, Ebrahim S, Marmot M, Kao WH, Pankow JS, Sampson MJ, Kuusisto J, Laakso M, Hansen T, Pedersen O, Pramstaller PP, Wichmann HE, Illig T, Rudan I, Wright AF, Stumvoll M, Campbell H, Wilson JF, Bergman RN, Buchanan TA, Collins FS, Mohlke KL, Tuomilehto J, Valle TT, Altshuler D, Rotter JI, Siscovick DS, Penninx BW, Boomsma DI, Deloukas P, Spector TD, Frayling TM, Ferrucci L, Kong A, Thorsteinsdottir U, Stefansson K, van Duijn CM, Aulchenko YS, Cao A, Scuteri A, Schlessinger D, Uda M, Ruokonen A, Jarvelin MR, Waterworth DM, Vollenweider P, Peltonen L, Mooser V, Abecasis GR, Wareham NJ, Sladek R, Froguel P, Watanabe RM, Meigs JB, Groop L, Boehnke M, McCarthy MI, Florez JC, Barroso I (2010) New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat Genet 42:105–116
Potocki S, Rowinska-Zyrek M, Valensin D, Krzywoszynska K, Witkowska D, Luczkowski M, Kozlowski H (2011) Metal binding ability of cysteine-rich peptide domain of ZIP13 Zn2+ ions transporter. Inorg Chem 50:6135–6145
Ackland ML, Michalczyk A (2006) Zinc deficiency and its inherited disorders-a review. Genes Nutr 1:41–49
Roberts LJ, Shadwick CF, Bergstresser PR (1987) Zinc deficiency in two full-term breast-fed infants. J Am Acad Dermatol 16:301–304
Chowanadisai W, Lonnerdal B, Kelleher SL (2006) Identification of a mutation in SLC30A2 (ZnT-2) in women with low milk zinc concentration that results in transient neonatal zinc deficiency. J Biol Chem 281:39699–39707
Murthy SC, Udagani MM, Badakali AV, Yelameli BC (2010) Symptomatic zinc deficiency in a full-term breast-fed infant. Dermatol Online J 16:3
Lasry I, Seo YA, Ityel H, Shalva N, Pode-Shakked B, Glaser F, Berman B, Berezovsky I, Goncearenco A, Klar A, Levy J, Anikster Y, Kelleher SL, Assaraf YG (2012) A dominant negative heterozygous G87R mutation in the zinc transporter, ZnT-2 (SLC30A2), results in transient neonatal zinc deficiency. J Biol Chem 287:29348–29361
Itsumura N, Inamo Y, Okazaki F, Teranishi F, Narita H, Kambe T, Kodama H (2013) Compound heterozygous mutations in SLC30A2/ZnT2 results in low milk zinc concentrations: a novel mechanism for zinc deficiency in a breast-fed infant. PLoS ONE 8:e64045
Miletta MC, Bieri A, Kernland K, Schoni MH, Petkovic V, Fluck CE, Eble A, Mullis PE (2013) Transient neonatal zinc deficiency caused by a heterozygous G87R mutation in the zinc transporter ZnT-2 (SLC30A2) gene in the mother highlighting the importance of Zn (2+) for Normal growth and development. Int J Endocrinol 2013:259189
Qian L, Wang B, Tang N, Zhang W, Cai W (2012) Polymorphisms of SLC30A2 and selected perinatal factors associated with low milk zinc in Chinese breastfeeding women. Early Hum Dev 88:663–668
Seo YA, Kelleher SL (2010) Functional analysis of two single-nucleotide polymorphisms in SLC30A2 (ZnT2): implications for mammary gland function and breast disease in women. Physiol Genomics 42A:219–227
Piletz JE, Ganschow RE (1978) Zinc deficiency in murine milk underlies expression of the lethal milk (lm) mutation. Science 199:181–183
Huang L, Gitschier J (1997) A novel gene involved in zinc transport is deficient in the lethal milk mouse. Nat Genet 17:292–297
Lonnerdal B (2007) Trace element transport in the mammary gland. Annu Rev Nutr 27:165–177
Yamawaki N, Yamada M, Kan-no T, Kojima T, Kaneko T, Yonekubo A (2005) Macronutrient, mineral and trace element composition of breast milk from Japanese women. J Trace Elem Med Biol 19:171–181
Yasuda H, Yoshida K, Yasuda Y, Tsutsui T (2011) Infantile zinc deficiency: association with autism spectrum disorders. Sci Rep 1:129
Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, Lin JK, Farzadfar F, Khang YH, Stevens GA, Rao M, Ali MK, Riley LM, Robinson CA, Ezzati M (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378:31–40
Stancakova A, Kuulasmaa T, Paananen J, Jackson AU, Bonnycastle LL, Collins FS, Boehnke M, Kuusisto J, Laakso M (2009) Association of 18 confirmed susceptibility loci for type 2 diabetes with indices of insulin release, proinsulin conversion, and insulin sensitivity in 5,327 nondiabetic Finnish men. Diabetes 58:2129–2136
Fu J, Festen EA, Wijmenga C (2011) Multi-ethnic studies in complex traits. Hum Mol Genet 20:R206–R213
Sladek R, Rocheleau G, Rung J, Dina C, Shen L, Serre D, Boutin P, Vincent D, Belisle A, Hadjadj S, Balkau B, Heude B, Charpentier G, Hudson TJ, Montpetit A, Pshezhetsky AV, Prentki M, Posner BI, Balding DJ, Meyre D, Polychronakos C, Froguel P (2007) A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature 445:881–885
Saxena R, Voight BF, Lyssenko V, Burtt NP, de Bakker PI, Chen H, Roix JJ, Kathiresan S, Hirschhorn JN, Daly MJ, Hughes TE, Groop L, Altshuler D, Almgren P, Florez JC, Meyer J, Ardlie K, Bengtsson Bostrom K, Isomaa B, Lettre G, Lindblad U, Lyon HN, Melander O, Newton-Cheh C, Nilsson P, Orho-Melander M, Rastam L, Speliotes EK, Taskinen MR, Tuomi T, Guiducci C, Berglund A, Carlson J, Gianniny L, Hackett R, Hall L, Holmkvist J, Laurila E, Sjogren M, Sterner M, Surti A, Svensson M, Tewhey R, Blumenstiel B, Parkin M, Defelice M, Barry R, Brodeur W, Camarata J, Chia N, Fava M, Gibbons J, Handsaker B, Healy C, Nguyen K, Gates C, Sougnez C, Gage D, Nizzari M, Gabriel SB, Chirn GW, Ma Q, Parikh H, Richardson D, Ricke D, Purcell S (2007) Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science 316:1331–1336
Scott LJ, Mohlke KL, Bonnycastle LL, Willer CJ, Li Y, Duren WL, Erdos MR, Stringham HM, Chines PS, Jackson AU, Prokunina-Olsson L, Ding CJ, Swift AJ, Narisu N, Hu T, Pruim R, Xiao R, Li XY, Conneely KN, Riebow NL, Sprau AG, Tong M, White PP, Hetrick KN, Barnhart MW, Bark CW, Goldstein JL, Watkins L, Xiang F, Saramies J, Buchanan TA, Watanabe RM, Valle TT, Kinnunen L, Abecasis GR, Pugh EW, Doheny KF, Bergman RN, Tuomilehto J, Collins FS, Boehnke M (2007) A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science 316:1341–1345
Zeggini E, Weedon MN, Lindgren CM, Frayling TM, Elliott KS, Lango H, Timpson NJ, Perry JR, Rayner NW, Freathy RM, Barrett JC, Shields B, Morris AP, Ellard S, Groves CJ, Harries LW, Marchini JL, Owen KR, Knight B, Cardon LR, Walker M, Hitman GA, Morris AD, Doney AS, McCarthy MI, Hattersley AT (2007) Replication of genome-wide association signals in UK samples reveals risk loci for type 2 diabetes. Science 316:1336–1341
Cauchi S, Del Guerra S, Choquet H, D’Aleo V, Groves CJ, Lupi R, McCarthy MI, Froguel P, Marchetti P (2010) Meta-analysis and functional effects of the SLC30A8 rs13266634 polymorphism on isolated human pancreatic islets. Mol Genet Metab 100:77–82
Nicolson TJ, Bellomo EA, Wijesekara N, Loder MK, Baldwin JM, Gyulkhandanyan AV, Koshkin V, Tarasov AI, Carzaniga R, Kronenberger K, Taneja TK, da Silva Xavier G, Libert S, Froguel P, Scharfmann R, Stetsyuk V, Ravassard P, Parker H, Gribble FM, Reimann F, Sladek R, Hughes SJ, Johnson PR, Masseboeuf M, Burcelin R, Baldwin SA, Liu M, Lara-Lemus R, Arvan P, Schuit FC, Wheeler MB, Chimienti F, Rutter GA (2009) Insulin storage and glucose homeostasis in mice null for the granule zinc transporter ZnT8 and studies of the type 2 diabetes-associated variants. Diabetes 58:2070–2083
Boesgaard TW, Zilinskaite J, Vanttinen M, Laakso M, Jansson PA, Hammarstedt A, Smith U, Stefan N, Fritsche A, Haring H, Hribal M, Sesti G, Zobel DP, Pedersen O, Hansen T (2008) The common SLC30A8 Arg325Trp variant is associated with reduced first-phase insulin release in 846 non-diabetic offspring of type 2 diabetes patients–the EUGENE2 study. Diabetologia 51:816–820
Kirchhoff K, Machicao F, Haupt A, Schafer SA, Tschritter O, Staiger H, Stefan N, Haring HU, Fritsche A (2008) Polymorphisms in the TCF7L2, CDKAL1 and SLC30A8 genes are associated with impaired proinsulin conversion. Diabetologia 51:597–601
Tamaki M, Fujitani Y, Hara A, Uchida T, Tamura Y, Takeno K, Kawaguchi M, Watanabe T, Ogihara T, Fukunaka A, Shimizu T, Mita T, Kanazawa A, Imaizumi MO, Abe T, Kiyonari H, Hojyo S, Fukada T, Kawauchi T, Nagamatsu S, Hirano T, Kawamori R, Watada H (2013) The diabetes-susceptible gene SLC30A8/ZnT8 regulates hepatic insulin clearance. J Clin Investig 123:4513–4524
Wenzlau JM, Juhl K, Yu L, Moua O, Sarkar SA, Gottlieb P, Rewers M, Eisenbarth GS, Jensen J, Davidson HW, Hutton JC (2007) The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA 104:17040–17045
Delli AJ, Vaziri-Sani F, Lindblad B, Elding-Larsson H, Carlsson A, Forsander G, Ivarsson SA, Ludvigsson J, Kockum I, Marcus C, Samuelsson U, Ortqvist E, Groop L, Bondinas GP, Papadopoulos GK, Lernmark A (2012) Zinc transporter 8 autoantibodies and their association with SLC30A8 and HLA-DQ genes differ between immigrant and Swedish patients with newly diagnosed type 1 diabetes in the Better Diabetes Diagnosis study. Diabetes 61:2556–2564
Dang M, Rockell J, Wagner R, Wenzlau JM, Yu L, Hutton JC, Gottlieb PA, Davidson HW (2011) Human type 1 diabetes is associated with T cell autoimmunity to zinc transporter 8. J Immunol 186:6056–6063
Lampasona V, Petrone A, Tiberti C, Capizzi M, Spoletini M, di Pietro S, Songini M, Bonicchio S, Giorgino F, Bonifacio E, Bosi E, Buzzetti R (2010) Zinc transporter 8 antibodies complement GAD and IA-2 antibodies in the identification and characterization of adult-onset autoimmune diabetes: Non Insulin Requiring Autoimmune Diabetes (NIRAD) 4. Diabetes Care 33:104–108
Wenzlau JM, Liu Y, Yu L, Moua O, Fowler KT, Rangasamy S, Walters J, Eisenbarth GS, Davidson HW, Hutton JC (2008) A common nonsynonymous single-nucleotide polymorphism in the SLC30A8 gene determines ZnT8 autoantibody specificity in type 1 diabetes. Diabetes 57:2693–2697
Kawasaki E, Uga M, Nakamura K, Kuriya G, Satoh T, Fujishima K, Ozaki M, Abiru N, Yamasaki H, Wenzlau JM, Davidson HW, Hutton JC, Eguchi K (2008) Association between anti-ZnT8 autoantibody specificities and SLC30A8 Arg325Trp variant in Japanese patients with type 1 diabetes. Diabetologia 51:2299–2302
Rungby J (2010) Zinc, zinc transporters and diabetes. Diabetologia 53:1549–1551
Kambe T, Narita H, Yamaguchi-Iwai Y, Hirose J, Amano T, Sugiura N, Sasaki R, Mori K, Iwanaga T, Nagao M (2002) Cloning and characterization of a novel mammalian zinc transporter, zinc transporter 5, abundantly expressed in pancreatic beta cells. J Biol Chem 277:19049–19055
Kambe T (2011) An overview of a wide range of functions of ZnT and Zip zinc transporters in the secretory pathway. Biosci Biotechnol Biochem 75:1036–1043
Pound LD, Sarkar SA, Benninger RK, Wang Y, Suwanichkul A, Shadoan MK, Printz RL, Oeser JK, Lee CE, Piston DW, McGuinness OP, Hutton JC, Powell DR, O’Brien RM (2009) Deletion of the mouse Slc30a8 gene encoding zinc transporter-8 results in impaired insulin secretion. Biochem J 421:371–376
Lemaire K, Ravier MA, Schraenen A, Creemers JW, Van de Plas R, Granvik M, Van Lommel L, Waelkens E, Chimienti F, Rutter GA, Gilon P, in’t Veld PA, Schuit FC (2009) Insulin crystallization depends on zinc transporter ZnT8 expression, but is not required for normal glucose homeostasis in mice. Proc Natl Acad Sci USA 106:14872–14877
Wijesekara N, Dai FF, Hardy AB, Giglou PR, Bhattacharjee A, Koshkin V, Chimienti F, Gaisano HY, Rutter GA, Wheeler MB (2010) Beta cell-specific Znt8 deletion in mice causes marked defects in insulin processing, crystallisation and secretion. Diabetologia 53:1656–1668
Pound LD, Sarkar SA, Ustione A, Dadi PK, Shadoan MK, Lee CE, Walters JA, Shiota M, McGuinness OP, Jacobson DA, Piston DW, Hutton JC, Powell DR, O’Brien RM (2012) The physiological effects of deleting the mouse SLC30A8 gene encoding zinc transporter-8 are influenced by gender and genetic background. PLoS ONE 7:e40972
Hardy A, Wijesekara N, Genkin I, Prentice KJ, Bhattacharjee A, Kong D, Chimienti F, Wheeler M (2012) Effects of high fat diet feeding on zinc transporter 8 (Znt8) null mice: differences between beta cell and global knockout of Znt8. Am J Physiol Endocrinol Metab 302:E1084–E1096
Hoch E, Lin W, Chai J, Hershfinkel M, Fu D, Sekler I (2012) Histidine pairing at the metal transport site of mammalian ZnT transporters controls Zn2+ over Cd2+ selectivity. Proc Natl Acad Sci USA 109:7202–7207
Montanini B, Blaudez D, Jeandroz S, Sanders D, Chalot M (2007) Phylogenetic and functional analysis of the cation diffusion facilitator (CDF) family: improved signature and prediction of substrate specificity. BMC Genomics 8:107
Bosomworth HJ, Adlard PA, Ford D, Valentine RA (2013) Altered expression of ZnT10 in Alzheimer’s disease brain. PLoS ONE 8:e65475
Lazarczyk M, Cassonnet P, Pons C, Jacob Y, Favre M (2009) The EVER proteins as a natural barrier against papillomaviruses: a new insight into the pathogenesis of human papillomavirus infections. Microbiol Mol Biol Rev 73:348–370
Lazarczyk M, Pons C, Mendoza JA, Cassonnet P, Jacob Y, Favre M (2008) Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J Exp Med 205:35–42
Palmiter RD, Findley SD (1995) Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J 14:639–649
Costello LC, Franklin RB (2011) Zinc is decreased in prostate cancer: an established relationship of prostate cancer! J Biol Inorg Chem 16:3–8
Kolenko V, Teper E, Kutikov A, Uzzo R (2013) Zinc and zinc transporters in prostate carcinogenesis. Nat Rev Urol 10:219–226
Hennigar SR, Kelleher SL (2012) Zinc networks: the cell-specific compartmentalization of zinc for specialized functions. Biol Chem 393:565–578
Gumulec J, Masarik M, Krizkova S, Adam V, Hubalek J, Hrabeta J, Eckschlager T, Stiborova M, Kizek R (2011) Insight to physiology and pathology of zinc(II) ions and their actions in breast and prostate carcinoma. Curr Med Chem 18:5041–5051
Kagara N, Tanaka N, Noguchi S, Hirano T (2007) Zinc and its transporter ZIP10 are involved in invasive behavior of breast cancer cells. Cancer Sci 98:692–697
Shen R, Xie F, Shen H, liu Q, Zheng T, Kou X, Wang D, Yang J (2013) Negative correlation of LIV-1 and E-cadherin expression in hepatocellular carcinoma cells. PLoS ONE 8:e56542
Hogstrand C, Kille P, Ackland ML, Hiscox S, Taylor KM (2013) A mechanism for epithelial-mesenchymal transition and anoikis resistance in breast cancer triggered by zinc channel ZIP6 and STAT3 (signal transducer and activator of transcription 3). Biochem J 455:229–237
Taylor KM, Vichova P, Jordan N, Hiscox S, Hendley R, Nicholson RI (2008) ZIP7-mediated intracellular zinc transport contributes to aberrant growth factor signaling in antihormone-resistant breast cancer Cells. Endocrinology 149:4912–4920
Hogstrand C, Kille P, Nicholson RI, Taylor KM (2009) Zinc transporters and cancer: a potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol Med 15:101–111
Franklin RB, Feng P, Milon B, Desouki MM, Singh KK, Kajdacsy-Balla A, Bagasra O, Costello LC (2005) hZIP1 zinc uptake transporter down regulation and zinc depletion in prostate cancer. Mol Cancer 4:32
Desouki MM, Geradts J, Milon B, Franklin RB, Costello LC (2007) hZip2 and hZip3 zinc transporters are down regulated in human prostate adenocarcinomatous glands. Mol Cancer 6:37
Chen QG, Zhang Z, Yang Q, Shan GY, Yu XY, Kong CZ (2012) The role of zinc transporter ZIP4 in prostate carcinoma. Urol Oncol 30:906–911
Overall CM, Lopez-Otin C (2002) Strategies for MMP inhibition in cancer: innovations for the post-trial era. Nat Rev Cancer 2:657–672
Suzuki T, Ishihara K, Migaki H, Matsuura W, Kohda A, Okumura K, Nagao M, Yamaguchi-Iwai Y, Kambe T (2005) Zinc transporters, ZnT5 and ZnT7, are required for the activation of alkaline phosphatases, zinc-requiring enzymes that are glycosylphosphatidylinositol-anchored to the cytoplasmic membrane. J Biol Chem 280:637–643
Fukunaka A, Kurokawa Y, Teranishi F, Sekler I, Oda K, Ackland ML, Faundez V, Hiromura M, Masuda S, Nagao M, Enomoto S, Kambe T (2011) Tissue nonspecific alkaline phosphatase is activated via a two-step mechanism by zinc transport complexes in the early secretory pathway. J Biol Chem 286:16363–16373
Franz MC, Anderle P, Burzle M, Suzuki Y, Freeman MR, Hediger MA, Kovacs G (2013) Zinc transporters in prostate cancer. Mol Aspects Med 34:735–741
Sandstead HH, Henriksen LK, Greger JL, Prasad AS, Good RA (1982) Zinc nutriture in the elderly in relation to taste acuity, immune response, and wound healing. Am J Clin Nutr 36:1046–1059
Prasad AS (1985) Clinical and biochemical manifestations of zinc deficiency in human subjects. J Am Coll Nutr 4:65–72
Prasad AS (1995) Zinc: an overview. Nutrition 11(1 Suppl):93–99
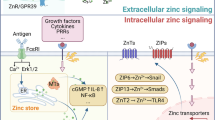
Hirano T, Murakami M, Fukada T, Nishida K, Yamasaki S, Suzuki T (2008) Roles of zinc and zinc signaling in immunity: zinc as an intracellular signaling molecule. Adv Immunol 97:149–176
Murakami M, Hirano T (2008) Intracellular zinc homeostasis and zinc signaling. Cancer Sci 99:1515–1522
Haase H, Rink L (2009) Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr 29:133–152
Liu MJ, Bao S, Galvez-Peralta M, Pyle CJ, Rudawsky AC, Pavlovicz RE, Killilea DW, Li C, Nebert DW, Wewers MD, Knoell DL (2013) ZIP8 regulates host defense through zinc-mediated inhibition of NF-kappaB. Cell Rep 3:386–400
Yu M, Lee WW, Tomar D, Pryshchep S, Czesnikiewicz-Guzik M, Lamar DL, Li G, Singh K, Tian L, Weyand CM, Goronzy JJ (2011) Regulation of T cell receptor signaling by activation-induced zinc influx. J Exp Med 208:775–785
Kitamura H, Morikawa H, Kamon H, Iguchi M, Hojyo S, Fukada T, Yamashita S, Kaisho T, Akira S, Murakami M, Hirano T (2006) Toll-like receptor-mediated regulation of zinc homeostasis influences dendritic cell function. Nat Immunol 7:971–977
Aydemir TB, Liuzzi JP, McClellan S, Cousins RJ (2009) Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells. J Leukoc Biol 86:337–348
Matsuura W, Yamazaki T, Yamaguchi-Iwai Y, Masuda S, Nagao M, Andrews GK, Kambe T (2009) SLC39A9 (ZIP9) regulates zinc homeostasis in the secretory pathway: characterization of the ZIP subfamily I protein in vertebrate cells. Biosci Biotechnol Biochem 73:1142–1148
Taniguchi M, Fukunaka A, Hagihara M, Watanabe K, Kamino S, Kambe T, Enomoto S, Hiromura M (2013) Essential role of the zinc transporter ZIP9/SLC39A9 in regulating the activations of Akt and Erk in B-cell receptor signaling pathway in DT40 cells. PLoS ONE 8:e58022
Hood MI, Skaar EP (2012) Nutritional immunity: transition metals at the pathogen-host interface. Nat Rev Microbiol 10:525–537
Corbin BD, Seeley EH, Raab A, Feldmann J, Miller MR, Torres VJ, Anderson KL, Dattilo BM, Dunman PM, Gerads R, Caprioli RM, Nacken W, Chazin WJ, Skaar EP (2008) Metal chelation and inhibition of bacterial growth in tissue abscesses. Science 319:962–965
Subramanian Vignesh K, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS, Jr (2013) Granulocyte macrophage-colony stimulating factor induced Zn sequestration enhances macrophage superoxide and limits intracellular pathogen survival. Immunity 39:697–710
Vignesh KS, Landero Figueroa JA, Porollo A, Caruso JA, Deepe GS, Jr (2013) Zinc sequestration: arming phagocyte defense against fungal attack. PLoS Pathog 9:e1003815
Sensi SL, Paoletti P, Bush AI, Sekler I (2009) Zinc in the physiology and pathology of the CNS. Nat Rev Neurosci 10:780–791
Sensi SL, Paoletti P, Koh JY, Aizenman E, Bush AI, Hershfinkel M (2011) The neurophysiology and pathology of brain zinc. J Neurosci 31:16076–16085
Takeda A, Nakamura M, Fujii H, Tamano H (2013) Synaptic Zn(2+) homeostasis and its significance. Metallomics 5:417–423
Szewczyk B (2013) Zinc homeostasis and neurodegenerative disorders. Front Aging Neurosci 5:33
Hozumi I, Hasegawa T, Honda A, Ozawa K, Hayashi Y, Hashimoto K, Yamada M, Koumura A, Sakurai T, Kimura A, Tanaka Y, Satoh M, Inuzuka T (2011) Patterns of levels of biological metals in CSF differ among neurodegenerative diseases. J Neurol Sci 303:95–99
Beyer N, Coulson DT, Heggarty S, Ravid R, Hellemans J, Irvine GB, Johnston JA (2012) Zinc transporter mRNA levels in Alzheimer’s disease postmortem brain. J Alzheimers Dis 29:863–873
Smith JL, Xiong S, Markesbery WR, Lovell MA (2006) Altered expression of zinc transporters-4 and -6 in mild cognitive impairment, early and late Alzheimer’s disease brain. Neuroscience 140:879–888
Palmiter RD, Cole TB, Quaife CJ, Findley SD (1996) ZnT-3, a putative transporter of zinc into synaptic vesicles. Proc Natl Acad Sci USA 93:14934–14939
Beyer N, Coulson DT, Heggarty S, Ravid R, Irvine GB, Hellemans J, Johnston JA (2009) ZnT3 mRNA levels are reduced in Alzheimer’s disease post-mortem brain. Mol Neurodegener 4:53
Adlard PA, Parncutt JM, Finkelstein DI, Bush AI (2010) Cognitive loss in zinc transporter-3 knock-out mice: a phenocopy for the synaptic and memory deficits of Alzheimer’s disease? J Neurosci 30:1631–1636
Rovelet-Lecrux A, Legallic S, Wallon D, Flaman JM, Martinaud O, Bombois S, Rollin-Sillaire A, Michon A, Le Ber I, Pariente J, Puel M, Paquet C, Croisile B, Thomas-Anterion C, Vercelletto M, Levy R, Frebourg T, Hannequin D, Campion D (2012) A genome-wide study reveals rare CNVs exclusive to extreme phenotypes of Alzheimer disease. Eur J Hum Genet 20:613–617
Fujimoto S, Itsumura N, Tsuji T, Anan Y, Tsuji N, Ogra Y, Kimura T, Miyamae Y, Masuda S, Nagao M, Kambe T (2013) Cooperative functions of ZnT1, metallothionein and ZnT4 in the cytoplasm are required for full activation of TNAP in the early secretory pathway. PLoS ONE 8:e77445
Gallaher DD, Johnson PE, Hunt JR, Lykken GI, Marchello MJ (1988) Bioavailability in humans of zinc from beef: intrinsic vs extrinsic labels. Am J Clin Nutr 48:350–354
Faa G, Nurchi VM, Ravarino A, Fanni D, Nemolato S, Gerosa C, Eyken PV, Geboes K (2008) Zinc in gastrointestinal and liver disease. Coord Chem Rev 252:1257–1269
Patrushev N, Seidel-Rogol B, Salazar G (2012) Angiotensin II requires zinc and downregulation of the zinc transporters ZnT3 and ZnT10 to induce senescence of vascular smooth muscle cells. PLoS ONE 7:e33211
Bosomworth HJ, Thornton JK, Coneyworth LJ, Ford D, Valentine RA (2012) Efflux function, tissue-specific expression and intracellular trafficking of the Zn transporter ZnT10 indicate roles in adult Zn homeostasis. Metallomics 4:771–779
Acknowledgments
This work was supported by grants-in-aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan and by the Takeda Science Foundation (to T.K.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kambe, T., Hashimoto, A. & Fujimoto, S. Current understanding of ZIP and ZnT zinc transporters in human health and diseases. Cell. Mol. Life Sci. 71, 3281–3295 (2014). https://doi.org/10.1007/s00018-014-1617-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-014-1617-0