Abstract
Background
β-D-xylosidase is a vital exoglycosidase with the ability to hydrolyze xylooligosaccharides to xylose and to biotransform some saponins by cleaving outer β-xylose. β-D-xylosidase is widely used as one of the xylanolytic enzymes in a diverse range of applications, such as fuel, food and the pharmaceutical industry; therefore, more and more studies have focused on the thermostable and xylose-tolerant β-D-xylosidases.
Results
A thermostable β-xylosidase gene (xln-DT) of 1509 bp was cloned from Dictyoglomus thermophilum and expressed in E.coli BL21. According to the amino acid and phylogeny analyses, the β-xylosidase Xln-DT is a novel β-xylosidase of the GH family 39. The recombinant β-xylosidase was purified, showing unique bands on SDS-PAGE, and had a protein molecular weight of 58.7 kDa. The β-xylosidase Xln-DT showed an optimal activity at pH 6.0 and 75 °C, with p-nitrophenyl-β-D-xylopyranoside (pNPX) as a substrate. Xln-DT displayed stability over a pH range of 4.0-7.5 for 24 h and displayed thermotolerance below 85 °C. The values of the kinetic parameters K m and V max for pNPX were 1.66 mM and 78.46 U/mg, respectively. In particular, Xln-DT displayed high tolerance to xylose, with 60% activity in the presence of 3 M xylose. Xln-DT showed significant effects on the hydrolyzation of xylobiose. After 3 h, all the xylobiose tested was degraded into xylose. Moreover, β-xylosidase Xln-DT had a high selectivity for cleaving the outer xylose moieties of natural saponins, such as notoginsenoside R1 and astragaloside IV, which produced the ginsenoside Rg1 with stronger anti-fatigue activity and produced cycloastragenol with stronger anti-aging activity, respectively.
Conclusion
This study provides a novel GH 39 β-xylosidase displaying extraordinary properties of highly catalytic activity at temperatures above 75 °C, remarkable hydrolyzing activity of xylooligosaccharides and rare saponins producing ability in the pharmaceutical and commercial industries.
Similar content being viewed by others
Background
Recently, more and more studies have focused on cellulose and hemicellulose hydrolysis in an effort to use lignocellulosic residues as feedstock for producing bioethanol [1, 2]. The plant cell wall mainly consists of 30-45% cellulose, 20-30% hemicellulose and 5-20% lignin [3]. Hemicelluloses, as the second most abundant renewable lignocellulosic biomass resource, are a series of heteropolysaccharides that contain xylans, arabinans, glucans and mannans, in which xylan is the major hemicellulosic polysaccharide that can be efficiently hydrolyzed into its sugar constituents [4,5,6]. The xylan backbone consists of β-(1,4)-linked xylopyranosyl units with side-chains, such as acetate, glucuronosyl and arabinofuranosyl depending on the source. Complete xylan degradation requires the concerted action of xylanolytic enzymes, owing to the complex structure. First, xylanase randomly cleaves the β-1,4 bonds in the xylan to yield xylooligosaccharides (XOs), xylobiose and xylose [7]. Second, α-arabinofuranosidase, acetylesterase, α-glucuronidase and ferulic acid esterase cleave the side-chain substituents [8]. In the last step, β-xylosidase, which is well known as the major hydrolytic enzyme that degrades the non-reducing ends of β-1,4-linked D-xylose residues to release xylose as the final product from short xylooligosaccharides [9, 10]. However, the roles of β-xylosidase include, but are not limited to, the degradation of xylooligosaccharide. β-xylosidase has large potential in many biotechnological applications, especially in the fuel and food industries as well as the bioconversion of saponins [11,12,13].
Saponin is a kind of bioactive substance with a range of physiological functions, such as anti-inflammatory, anti-cancer, immunoregulatory and anti-fatigue effects [14, 15]. Various saponins have disparate physiological activities as a result of diverse skeletons and sugar moieties. Thus, many studies have concentrated more on biotransforming glycosylated saponins to deglycosylated saponins by removing the sugar moieties with enzymes, such as β-glucosidase and α-rhamnosidase, to achieve better pharmacological effects [16, 17]. Furthermore, part of the saponins are needed to hydrolyze the xylose moiety by β-xylosidases. Unfortunately, until recently, it has been difficult to find a highly thermostable recombinant β-xylosidase with the ability to convert saponins. Ginsenoside Rg1 (Rg1) and cycloastragenol (CA) are the major bioactive constituents in the plants Panax notoginseng and Astragali radix, respectively [18, 19], which have been considered to be remarkable ingredients with pharmacological activities [20, 21]. Chinese Pharmacopoeia (Edition 2015) has used Rg1 as the major index component for the Quality Control (QC) of ginseng herbs [22]. However, it is quite difficult to improve the purity of the exact quantification of Rg1 because there are many homologous components with highly similar physicochemical properties from the target compound. Therefore, conversion from notoginsenoside R1 to Rg1 can greatly improve the purity of Rg1 [12]. CA, as the aglycone of astragaloside IV (ASI), can activate telomerase, which displays great potential for anti-aging activity [23]. Nevertheless, it is very difficult to isolate CA from Astragali radix due to its low concentration. Therefore, conversion from ASI to CA could increase the content of CA efficiently. Methods such as acid hydrolysis, heating treatment, steaming treatment, and microbial and enzymatic transformation, have been used to convert these saponins [24,25,26]. Among these methods, enzymatic transformation with β-xylosidase has exhibited potential because of its mild reaction condition and high specificity.
As is well known, β-xylosidases are largely found in GH families 1, 3, 30, 39, 43, 52, 54 and 120, according to the GH classification system CAZy (Carbonhydrate-Active Enzymes Database) [27]. Until recently, many β-xylosidases from different GH families have been described in a variety of microorganisms including bacteria, archaea, fungi and plant [28,29,30,31]. Generally, xylosidases from fungal sources have optimum pH values from 3 to 5 and an optimum temperature range from 40 °C to 60 °C, thus far, these xylosidases have only been grouped into GH 3, GH 43 and GH 54 [32, 33]. Literature about the extremely thermophilic and xylose-tolerant β-xylosidases is relatively scarce. Therefore, thermophilic bacteria are regarded as a remarkable source of thermotolerant β-xylosidases [34,35,36]. Usually, β-xylosidases with thermostability, greater specific activity and a greater tolerance to xylose are used in industrial processes, since high temperatures increase the solubility of substrates and reduce the risk of contamination, and the resistance to xylose can reduce the inhibition of substrate feedback [37]. As a result, the search for thermostable and xylose-tolerant β-xylosidases has increased considerably in recent years.
In this article, we cloned, expressed and characterized a novel thermostable GH 39 β-xylosidase from Dictyoglomus thermophilum DSM 3960. In particular, the high tolerance to xylose makes this β-xylosidase useful for various biotechnological applications. Moreover, this enzyme exhibited highly selective hydrolysis for outer xylose in notoginsenoside R1 and CA. These extraordinary properties make Xln-DT more suitable for producing rare saponins in the pharmaceutical and commercial industries.
Methods
Bacterial strains, plasmids and materials
Dictyoglomus thermophilum DSM 3960 was purchased from DSMZ (www.dsmz.de). The E. coli BL21 (DE3) and Top10F’ cells were grown in LB broth at 37 °C containing 100 mg/ml ampicillin. The vector pET-20b (Novagen, USA) was employed as a cloning and expression vector. The β-xylosidases XlnD, Tth XyB3, Tth Xyl and Tpe Xln3 and the β-glucosidase Tpebg3 were prepared by the Microbial Technology Research Laboratory, Nanjing Forestry University.
The substrates pNPX, pNP-β-D-glucopyranoside, pNP-β-D-galactopyranoside, pNPR, pNPAF and pNPAP were purchased from Sigma (USA). The sugar xylobiose was purchased from Aladdin (China). Notoginsenoside R1 (> 98% Purity, HPLC), ginsenoside Rg1 (> 98% Purity, HPLC), ASI (> 98% Purity, HPLC) and CA (> 98% Purity, HPLC) were purchased from the Chendu Institute of Biology, CAS (www.cdmust.com).
DNA manipulation
The DNA was manipulated following standard operating procedures. The Gel Extraction Kit and Plasmid Kit (Qiagen, USA) were used for purifying the PCR products and plasmids. The DNA restriction endonucleases BamHI and XhoI, the T4 DNA ligase and the Ex-Taq restriction polymerase were purchased from TaKaRa (China). The DNA transformation was manipulated by electroporation using a Bio-Rad Gene Pulser (USA).
Plasmid constructions
The β-xylosidase encoded gene xln-DT (accession number: KX449145) with a size of approximately 1500 bp was amplified from Dictyoglomus thermophilum DSM 3960 genomic DNA by PCR with the primers xln-DT-f (CGCGGATCCATGAACCATATAAAGATTGAAA) and xln-DT-r (CCGCTCGAGATATCCACCTGGTATTTTGCTATC). The restriction enzyme sites were the underlined sequences. The amplified PCR products were then digested with BamHI and XhoI endonucleases and subcloned into the pET-20b vector; finally, the expression plasmid pET-20b-xln-DT was obtained.
Nucleotide sequence analysis of xln-DT
The nucleotide sequences of xln-DT and the other known β-xylosidase genes were analyzed by the software Clustal 2.1. Databases were searched by using BLAST (http://www.blast.ncbi.nlm.nih.gov/Blast.cgi) at NCBI and against CAZy (www.cazy.org). Then, the neighbor-joining (NJ) evolutionary tree was constructed by the software mega 7.0.
Expression and purification of Xln-DT
The recombinant plasmid, pET-20b-xln-DT, was transformed into E.coli BL21 (DE3), grown in LB broth containing 100 mg/ml ampicillin at 37 °C and induced to express recombinant Xln-DT by adding IPTG to a final concentration of 0.1 mM and to an optical density, OD600, close to 0.8; the bacteria were further incubated at 28 °C for approximately 8 h.
The recombinant cells (one liter) were harvested by centrifugation at 8, 000×g (4 °C) for 20 min, washed with distilled water several times and resuspended in 50 mL 1 × binding buffer (pH 7.9). After sonication, the cell extracts were heat treated at 75 °C for 30 min, cooled in an ice-bath and centrifuged at 10, 000×g (4 °C) for 30 min. Finally, the supernatants were purified with an immobilized metal affinity column (Novagen, USA), and the enzyme protein was collected by eluting in 1 × binding buffer (pH 7.9). The purity of the target protein was canvassed by an SDS-PAGE gel, and the protein bands were analyzed by density scanning with an image analysis system (Bio-Rad, USA) [38]. The purified protein concentration was measured by the Bradford method using albumin from bovine serum as a standard.
β-Xylosidase assay
The substrate pNPX was used for the β-xylosidase activity assay. The reaction mixture was contained with 10 μL of 20 mM substrate pNPX, 180 μL of sodium phosphate buffer (50 mM, pH 6.0) and 10 μL of purified enzyme (0.64 μg). After 10 min (75 °C), the reaction was stopped by the addition of 600 μL Na2CO3 (1 M) [39]. The absorbance of the mixture was immediately measured at 405 nm. The definition of one unit of β-xylosidase activity (1 U) was consistent with the literature reference [36]. For every sample, the activity was measured three separate times.
The impact of temperature was measured by a standard assay over a range of temperatures (60-90 °C) in sodium phosphate buffer (50 mM, pH 6.0). The optimum pH was evaluated by incubation at 75 °C for 10 min in 50 mM sodium phosphate buffers with various pH values (pH 4.0-7.0). For the thermal stability analysis, the residual β-xylosidase activity was determined after the pre-incubation of enzymes (0.64 μg) at a range of temperatures from 65 to 95 °C for 2 h (every 30 min). The activity of the enzyme without pre-incubation was defined as 100%. The pH stability of the enzyme was measured by determining the remaining activity after incubating the enzyme (0.64 μg) in sodium phosphate buffer (50 mM) from pH 4.0 to 7.0 at 4 °C. After 24 h, the residual activity of the enzyme incubated at various pH values was measured. The purified enzyme (0.64 μg) was incubated at temperatures ranging from 75 to 95 °C in sodium phosphate buffer (50 mM, pH 6.0). The half-life was calculated from the first-order rate constants of inactivation, which were obtained from linear regressions in logarithmic coordinates [40]. The influence of various xylose concentrations (250, 500, 750, 1000, 1500, 2000, 2500 and 3000 mM) on the β-xylosidase activity was measured.
The effects of adding different ions and chemical reagents on the β-xylosidase activity of the purified enzyme (0.64 μg) were examined by adding different metal ions into the aforementioned buffer. Ni2+, Fe3+, Mn2+, Mg2+, Ca2+, K+, Zn2+, Al3+, Li2+, Na+, NH4+, Cu2+, Fe2+, Ba2+, Co2+, and Hg2+ as well as the chemical reagents EDTA and PMSF were assayed at 1 mM and 5 mM of the final concentrations in the reaction mixture.
The effects of organic solvents on the β-xylosidase activity of the purified enzyme (0.64 μg) were measured by adding 5, 10, 15, 20, 25 and 30% organic solvents (methanol, ethanol or DMSO) to the mixture. The β-xylosidase enzyme Xln-DT was mixed with each solvent for 30 min at 75 °C before adding pNPX to initiate the enzyme reaction. The activity of the enzyme without the metal ions, chemical reagents or organic solvents was defined as 100%. Every experiment was performed in triplicate.
The substrate specificity of the purified enzyme (0.64 μg) was tested by using pNPX, pNP-β-D-glucopyranoside, pNP-β-D-galactopyranoside, pNPR, pNPAF and pNPAP. The kinetic constant of the purified Xln-DT was measured by determining the initial rates at various pNPX ending concentrations (0.2, 0.4, 0.8, 1, 1.2, 1.4, 1.6, 1.8, 2, 3, 4, 5, 6 and 8 mM) under standard reaction conditions.
Xylobiose degradation
The hydrolysis products from xylobiose by the purified Xln-DT were analyzed by TLC. Then, 10% xylobiose (wt/vol) was dissolved in 100 μL of citrate buffer solution (50 mM, pH 6.0) and incubated at 75 °C for 3 h with the addition of 1 U Xln-DT β-xylosidase. The reaction was terminated by heating in boiling water for 10 min. The hydrolysis products were analyzed by TLC on a silica gel plate (G254, Qingdao, China) using the solvent system containing n-butanol, ethanol and water (5:3:2, v/v/v) and were visualized using the orcinol sulfuric acid reagent.
Enzymatic transformation of notoginsenoside R1 and ASI
For notoginsenoside R1 as a substrate, the reaction mixture contained 1 g/L notoginsenoside R1, 0.1 U Xln-DT and sodium phosphate buffer (50 mM, pH 6.0), and it was incubated at 75 °C for 30 min and then terminated by the addition of 400 μL methanol. For ASI as a substrate, the reaction mixture contained 1 g/L ASI, 0.5 U Xln-DT, 1 U Tpebg3 and sodium phosphate buffer (50 mM, pH 6.0), and it was incubated at 75 °C for 3 h and then terminated by the addition of 400 μL methanol. The methanol extract of this material was analyzed by HPLC.
The notoginsenoside R1 and ginsenoside Rg1 were analyzed using an Agilent HPLC 1260 system (USA) and a C18 column (4.6 × 250 mm; i.d., 5 μm; S.No. USNH017518, USA) with distilled water (A) and acetonitrile (B) as the mobile phase, with a gradient elution of 20% (B) from 0 to 3 min and 20-60% (B) from 3 to 28 min. The injection volume was 5 μL for each sample, the flow rate was 0.4 mL/min, and the detection was carried out by monitoring the absorbance at 203 nm. The ASI and CA were also analyzed using the same system and column described above, with distilled water (A) and acetonitrile (B) as the mobile phase, with A/B ratios of 40:60 from 0 to 16 min; the detection was performed by monitoring the ELSD. The injection volume was 10 μL for each sample, the flow rate was 1 mL/min, the gas flow rate was 2.1 L/min, and the ELSD drift tube temperature was 90 °C.
Results
Cloning and sequencing of the β-xylosidase gene xln-DT
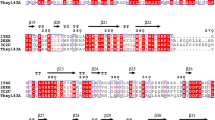
According to the analysis of the complete genome sequence of Dictyoglomus thermophilum DSM 3960, a protein with possible β-xylosidase activity (GenBank accession No. KX449145) encoded 502 amino acids with a full-length of 1509 bp and belonged to GH 39. To investigate the evolutionary relationship among the β-xylosidases, the phylogenetic trees were constructed by using the neighbor-joining method. The NJ trees revealed that there were seven clades, with each clade composed of a separated monophyletic group (Fig. 1). Clade I contained GH 3 β-xylosidases from bacteria, archaea and fungi, Clade II contained GH 39 β-xylosidases from several thermophilic bacteria, and Clade III to VII contained β-xylosidases that belonged to GH 43, 120, 30, 54 and 52, and were almost all from bacteria. Among the β-xylosidases from Clade II (GH 39), the members of the thermophilic genus Dictyoglomus had a distant relationship with Thermoanaerobacterium. Hence, it was posited that their enzymatic characterizations might be different. As is well known, GH 39 family β-xylosidases display a typical (α/β)8-barrel and perform hydrolysis in a two-stage reaction that consists of glycosylation and water-mediated deglycosylation steps. Based on the present database, it can be indicated that this protein could be a novel β-xylosidase that belongs to the GH 39 family.
The β-xylosidase gene xln-DT was cloned from the genomic DNA of D.thermophilum DSM 3960 and subcloned into the expression vector pET-20b to generate the plasmid pET-20b-xln-DT. Then the recombinant plasmid pET-20b-xln-DT was finally transformed into E. coli BL21 (DE3) and expressed with 0.1 mM IPTG. The activity of the recombinant β-xylosidase was approximately 2.3 U/mL in LB medium.
Purification and characterization of recombinant Xln-DT β-xylosidase
For the biochemical characterization of the recombinant β-xylosidase, the crude β-xylosidase was purified by all the purification preparations. The crude β-xylosidase was given a single band on a 12% SDS-PAGE gel, and the MW of the enzyme was slightly more than 55 kDa without the other bands (Fig. 2), which was similar to the theoretical MW of the monomer (58.7 kDa). The specific activity of the purified Xln-DT was 2.9-fold higher than that of the crude enzyme with a purification yield of 52.6% (Table 1).
SDS-PAGE analysis of recombinant Xln-DT β-xylosidase expressed in E.coli BL21 (DE3). Lane M: protein marker; Lane 1: the crude extract of E.coli BL21 (DE3) harboring pET-20b; Lane 2: the crude extracts of Xln-DT β-xylosidase; Lane 3: the cell extracts after sonication were heat treated at 75 °C for 30 min; Lane 4: purified Xln-DT β-xylosidase by Ni-NTA resin affinity chromatography
The enzymatic properties of the purified β-xylosidase Xln-DT were characterized. The optimal pH for Xln-DT was measured to be 6.0. The enzyme showed over 80% of the maximum activity at a pH from 5.0 to 6.5 (Fig. 3a). Moreover, the novel β-xylosidase showed good stability after being treated in sodium phosphate buffers at different pH levels for 24 h (Fig. 3a). The optimal temperature for Xln-DT was 75 °C. The enzyme showed more than 80% of the maximum activity at temperatures from 65 °C to 85 °C (Fig. 3b). Thermostability assays indicated that Xln-DT residual activity was above 60% of its initial activity at 65-85 °C when tested at pH 6.0 for 2 h (Fig. 3c). The kinetics of thermal inactivation were determined by incubating the enzyme at temperatures ranging from 75 °C to 95 °C for 3 h. As shown in Fig. 3d, the half-life of the recombinant β-xylosidase Xln-DT was approximately 29 h at 75 °C. At 95 °C, the half-life was approximately 1 h. In addition, Xln-DT had an unusually high tolerance to inhibition by xylose, up to 1 M xylose, which did not affect the Xln-DT activity, and 60% of the relative activity was reserved in 3 M xylose (Fig. 4).
Characterization of recombinant Xln-DT β-xylosidase. a Effects of optimum pH and pH stability of the enzyme Xln-DT; b Effect of temperature on Xln-DT activity; c The thermostability of the enzyme Xln-DT. The residual activity was monitored while the enzyme was incubated at 65 °C (filled diamonds), 75 °C (filled squares), 85 °C (flled triangles) and 95 °C (filled circles). The maximum activity was defined as 100%; d The kinetic of thermal inactivation of the enzyme Xln-DT at different temperatures ranging from 75 °C to 95 °C for several time intervals (75 °C (filled squares), 85 °C (filled circles), 95 °C (filled triangles))
The effects of the chemical reagents and metal ions on the enzyme activities of Xln-DT are shown in Table 2. The activity of Xln-DT was exceedingly inhibited by Hg2+ and Cu2+. Most of the other divalent metal cations had dissimilar effects on the activity of Xln-DT. In particular, the activity of Xln-DT, which was observably increased by Fe2+, Fe3+ and Mn2+, was similar to the alpha-mannosidase from the hen oviduct [41]. In addition, we incubated Xln-DT with Fe2+ at 75 °C for 3 h. The results showed that Fe2+ improved the thermostability of Xln-DT at 75 °C. The β-xylosidase activity of Xln-DT with 5 mM Fe2+ remained at over 300% after 3 h of incubation at 75 °C, while the Xln-DT without any metal ions only retained 80% activity. In addition, organic solvents such as methanol, ethanol and DMSO also affected the enzyme activity. The residual enzyme activity was over 100%, with 20% methanol or 10% ethanol. With the increasing concentrations of organic solvent, the enzyme activity was inhibited slowly.
The substrate specificity of Xln-DT was measured by using 1.0 mM substrates (Table 3). The results illustrated that Xln-DT has a high activity toward pNPX and a lower activity against pNP-β-D-glucopyranoside, while no other glycosidase activity was detected against pNP-β-D-galactopyranoside, pNPAF, pNPAP and pNPR. The values of K m and V max were 1.66 mM and 78.46 U/mg, respectively, with pNPX as the substrate. The kinetic parameters were measured from Lineweaver-Burk plots. These results suggest that Xln-DT has highly specific activity toward residual xylose.
Xylobiose hydrolyzation of Xln-DT
Xylobiose was incubated with purified Xln-DT β-xylosidase and examined using TLC (Fig. 5). After 3 h, all the xylobiose tested was completely degraded to xylose. This result indicates that this cloned enzyme is a true β-xylosidase with hydrolytic activity against short xylooligosaccharides.
Biotransformation of notoginsenoside R1 and ASI by Xln-DT
The biotransformation pathway from notoginsenoside R1 to ginsenoside Rg1 cleaved the outer xylose moiety at position C-6 of notoginsenoside R1 via β-xylosidase (Additional file 1: Figure S1). The biotransformation pathway from ASI to CA cleaved the outer glucose moiety at position C-6 and the xylose moiety at position C-3 of ASI via β-glucosidase and β-xylosidase, respectively (Additional file 1: Figure S2). As shown in Fig. 6, three GH 3 family β-xylosidases Tth XyB3, Tpe Xln3 and XlnD from Thermotoga thermarum DSM 5069, Thermotoga petrophila DSM 13995 and Aspergillus niger NL-1, respectively, one GH 120 family β-xylosidase Tth Xyl from Thermoanaerobacterium thermosaccharolyticum DSM 571 and Xln-DT (GH 39) were selected to biotransform notoginsenoside R1 and ASI. Only the β-xylosidase Xln-DT from GH 39 family had 100 and 88.9% of the biotransformation rate for notoginsenoside R1 and ASI (Additional file 1: Figures S3 and S4). A total of 1 g/L of notoginsenoside R1 was converted into 0.86 g/L of ginsenoside Rg1 after 30 min, with a corresponding molar conversion yield of 100% in total. A total of 1 g/L of ASI was converted into 0.36 g/L of CA after 3 h, with a corresponding molar conversion yield of 88.9% in total.
Comparison of enzymatic conversion efficiency of notoginsenoside R1 and ASI to ginsenoside Rg1 and CA by β-xylosidases from different sources. (Xln-DT was from Dictyoglomus thermophilum DSM3960, Tth XyB3 was from Thermotoga thermarum DSM 5069, Tth Xyl was from Thermoanaerobacterium thermosaccharolyticum DSM 571, Tpe Xln3 was from Thermotoga petrophila DSM 13995, XlnD was from Aspergillus niger NL-1)
Discussion
According to the database, more than 130 families were classified as glycoside hydrolases. Through the GenBank blast program, β-xylosidase Xln-DT belonged to the GH 39 family. This enzyme has the highest sequence similarity of 87% with β-xylosidase from Caldicellulosiruptor saccharolyticus (GenBank accession No. WP_049754849.1) and shares a 72% similarity with the β-xylosidase from Thermoanaerobacterium thermosaccharolyticum (GenBank accession No. WP_013297482.1). The phylogenetic analysis showed that Xln-DT had a distant relationship with other β-xylosidases belonging to the GH 39 family. This paper is the first report on the purification and biochemical characterization of Xln-DT β-xylosidase from Dictyoglomus thermophilum.
Generally, fungal β-xylosidases have an optimal pH under 5.0, while bacterial β-xylosidases are active up to pH 7.0. In this paper, the optimum pH of the recombinant Xln-DT from Dictyoglomus thermophilum was found to be nearly neutral (pH 6.0), which was similar to the β-xylosidase from Paecilomyces thermophila and GH 39 β-xylosidase from Caulobacter crescentus [26, 42]. The enzymatic characterization indicated that Xln-DT had a highly optimal temperature at 75 °C, which was higher than that observed for the β-xylosidases from Aspergillus niger, Sporotrichum thermophile and Caulobacter crescentus [43, 44]. Moreover, Xln-DT had a preferable temperature tolerance under 85 °C, which was similar to the thermostable GH 3 β-xylosidase from Thermotoga thermarum [36]. In practical applications, an enzyme with high heat resistance is desirable preferentially because of its longer half-life (29 h for 75 °C) implying lower a consumption of the enzyme. The recombinant β-xylosidase has satisfactory pH and temperature stability, which is the favorable factor for the potential applications of the enzyme, such as in the biotransformation field.
As is well known, highly xylose-tolerant β-xylosidase has great potential in biochemical application fields because xylose is a strong inhibitor of β-xylosidase. Unfortunately, the majority of microbial β-xylosidases are sensitive to xylose. Table 4 summarizes the xylose-resistant ability from different organisms. Most β-xylosidases from fungi, such as Neurospora crassa and Aspergillus niger B 03, display a K i for xylose below 2 mM. The bacterial β-xylosidases from Thermoanaerobacterium saccharolyticum, Thermotoga thermarum and Scytalidium thermophilum were insensitive to inhibition, ranging from 200 mM to 1000 mM xylose. In this study, it was surprising that Xln-DT had a very prominent tolerance to inhibition by xylose. There was no inhibition at 1 M xylose, and there was a 40% inhibition at 3 M xylose. Among the previously reported β-xylosidases, the K i for xylose of Xln-DT β-xylosidase was the highest among those reported. At present, there is no literature to analyze the xylose-tolerance mechanism of β-xylosidase, and only a few studies have reported the mechanism of glucose tolerance and stimulation of glucosidases [45]. The Xln-DT β-xylosidase with high xylose tolerance and stimulation suggested that some active sites at the entrance and middle of the substrate channel modulate the effects of xylose, which give the GH 39 β-xylosidase a more prominent application potential in industries. The purified enzyme activity was measured by the presence of the tested ions. A 1 mM (or 5 mM) concentration of Cu2+ and Hg2+ inhibited the enzyme activity significantly, which was relatively common for this enzyme from Paecilomyces thermophila [46]. The catalytic efficiency of the reaction system with Fe2+ and Fe3+ added at a 5 mM final concentration was nearly 3.2- and 1.9- times higher, respectively, than enzymes without pre-incubation of the metal ions, indicating that the Fe ion could be the element of the catalytic active center and a stable factor for the structure of the aim protein. A 1 mM or 5 mM concentration of EDTA had no significant effect on the enzyme activity, which suggests that the chelating agent EDTA did not affect the β-xylosidase activity and was not essential for the catalytic activity of this enzyme. The organic modulator, PMSF (1 mM or 5 mM), had slightly promoting role on enzyme activity. Moreover, Fe2+ and Fe3+ improved the thermostability of Xln-DT at 75 °C for 3 h, which indicated that this protein may have a metal-ion binding loop. By binding metal ions such as Fe2+ and Fe3+, the loop can keep the thermal stability of the protein structure. Since organic solvents have been used for solubilizing water-insoluble substrates in enzymatic reactions, the enzyme with organic solvent resistance has a higher application value. As shown in Table 5, low concentrations of organic solvents, such as methanol, ethanol and DMSO, promoted enzyme activity, which was similar to the β-glucosidase from Thermotoga petrophila. This enzyme seemed to be tolerant to methanol and ethanol, since it was almost uninhibited in 20% of methanol and retained over 80% of its residual activity in the presence of 30% methanol. This level of tolerance was higher than that previously reported of β-xylosidases from Geobacillus thermodenitrificans [35]. The results indicated that the enzyme could be used in industrial applications in the presence of some organic solvents.
β-xylosidase is an exoglycosidase with the ability to degrade the non-reducing ends of xylooligosaccharides into xylose, which is one kind of hemicellulolytic enzyme. Compared with the other β-xylosidases, Xln-DT possessed higher efficiency in xylobiose hydrolysis. Reported K m values of other β-xylosidases for bacterial enzymes ranged from 0.018 mM to 10 mM, while the K m values were normally over 1 mM for fungal β-xylosidases. The K m and V max values of Xln-DT for pNPX were 1.66 mM and 78.46 U/mg, respectively, which were higher than the β-xylosidase from S.thermophilum with 65 U/mg [47]. Moreover, the capacity of the β-xylosidase to hydrolyze xylobiose was studied by using 10% xylobiose incubated with the purified enzyme at 75 °C. After 3 h, the xylobiose was completely hydrolyzed into xylose, which revealed the obvious ability to convert the XOs into xylose.
As is well known, conventional β-xylosidases are frequently used to hydrolyze the end bone of β-1, 4-linked xylopyranosyl. However, for notoginsenoside R1 and ASI, it was the 1, 2-glycosidic bond. According to this paper, only Xln-DT could cleave the outer xylose moiety at position C-6 of the notoginsenoside R1 and ASI, which indicated that it had highly specificity in biotransforming notoginsenoside R1 and ASI to ginsenoside Rg1 and CA, without attacking the other glycosidic linkages. Although ginsenoside Rg1 and CA could be prepared by traditional methods, the yield was low, and it was time and money consuming. In addition, ginsenoside Rg1 and CA were accompanied with some by-products by traditional methods, which greatly impede later purification. Taken together, as a clean and green technology, biotransformation with specific enzymes showed the highest efficiency and specificity. All these results suggest that this recombinant Xln-DT has great potential for industrial applications, especially for bio-transformation to produce natural medicine.
Conclusions
In this paper, a novel β-xylosidase, Xln-DT, from Dictyoglomus thermophilum DSM 3960 was cloned and expressed in E. coli BL21. The phylogenetic analysis showed that Xln-DT had a distant relationship with other β-xylosidases belonging to the GH 39 family. The enzymatic characterization displayed that Xln-DT had a high optimal temperature and a partially neutral pH, and the thermostability was improved by the ion Fe. Most importantly, there was no inhibition at 1 M xylose, and there was a 40% inhibition at 3 M xylose. Compared with the other β-xylosidases, Xln-DT possessed higher efficiency in xylobiose hydrolysis and had efficiency for biotransforming notoginsenoside R1 and ASI by removing the 1, 2 glycosidic bond linked to the C-6 and C-3 carbons. This study indicates that recombinant Xln-DT would be suitable for producing natural medicine, such as ginsenoside Rg1 and CA.
Abbreviations
- ASI:
-
Astragaloside IV
- CA:
-
Cycloastragenol
- E.coli :
-
Escherichia coli
- EDTA:
-
Ethylenediaminetetraacetic acid
- ELSD:
-
Evaporative Light Scattering Detector
- GH:
-
Glycosyl hydrolase
- HPLC:
-
High performance liquid chromatography
- IPTG:
-
Isopropyl-β-D-thiogalactopyranoside
- LB:
-
Luria-Bertani
- MW:
-
Molecular weight
- NJ:
-
Neighbor-joining
- PCR:
-
Polymerase chain reaction
- pNPAF:
-
p-nitrophenyl-α-L-arabinofuranoside
- pNPAP:
-
p-nitrophenyl-α-L-arabinopyranoside
- pNPR:
-
p-nitrophenyl-α-L-rhamnopyranoside
- pNPX:
-
p-nitrophenyl-β-D-xylopyranoside
- QC:
-
Quality control
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- TLC:
-
Thin-layer chromatography
- XOs:
-
Xylooligosaccharides
References
Nigam PS, Singh A. Production of liquid biofuels from renewable resources. Prog Energy Combustion Sci. 2011;37:52–68.
Zimbardi AL, Sehn C, Meleiro LP, Souza FH, Masui DC, Nozawa MS, Guimarães LH, Jorge JA, Furriel RP. Optimization of β-glucosidase, β-xylosidase and xylanase production by Colletotrichum graminicola under solid-state fermentation and application in raw sugarcane trash saccharification. Int J Mol Sci. 2013;14:2875.
Barr CJ, Mertens JA, Schall CA. Critical cellulase and hemicellulase activities for hydrolysis of ionic liquid pretreated biomass. Bioresour Technol. 2012;104:480–5.
Sachslehner A, Haltrich D. Purification and some properties of a thermostable acidic endo-β-1,4-d-mannanase from Sclerotium (Athelia) rolfsii. FEMS Microbiol Lett. 1999;177:47–55.
Tabka MG, Herpoëlgimbert I, Monod F, Asther M, Sigoillot JC. Enzymatic saccharification of wheat straw for bioethanol production by a combined cellulase xylanase and feruloyl esterase treatment. Enzyme Microb Technol. 2006;39:897–902.
Ohta K, Fujimoto H, Fujii S. Cell-associated β-xylosidase from Aureobasidium pullulans ATCC 20524: purification, properties, and characterization of the encoding gene. J Biosci Bioeng. 2010;110:152–7.
Zhang J, Siikaaho M, Puranen T, Ming T, Tenkanen M, Viikari L. Thermostable recombinant xylanases from Nonomuraea flexuosa and Thermoascus aurantiacus show distinct properties in the hydrolysis of xylans and pretreated wheat straw. Biotechnol Biofuels. 2011;4:12.
Goldbeck R, Gonçalves TA, Damásio ARL, Brenelli LB, Wolf LD, Paixão DAA, Rocha GJM, Squina FM. Effect of hemicellulolytic enzymes to improve sugarcane bagasse saccharification and xylooligosaccharides production. J Mol Catalysis B Enzymatic. 2016;131:36–46.
Michelin M, Polizeli MDLTM, Ruzene DS, Silva DP, Ruiz HA, Vicente AA, Jorge JA, Terenzi HF, Teixeira JA. Production of xylanase and β-xylosidase from autohydrolysis liquor of corncob using two fungal strains. Bioprocess Biosyst Eng. 2012;35:1185–92.
Martins MP, Ventorim RZ, Coura RR, Maitan-Alfenas GP, Alfenas RF, Guimarães VM. The β-xylosidase from Ceratocystis fimbriata RM35 improves the saccharification of sugarcane bagasse. Biocatalysis Agric Biotechnol. 2018;13:291–8.
Jordan DB, Wagschal K. Properties and applications of microbial beta-D-xylosidases featuring the catalytically efficient enzyme from Selenomonas ruminantium. Appl Microbiol Biotechnol. 2010;86:1647–58.
Shin KC, Seo MJ, Oh DK. Characterization of β-xylosidase from Thermoanaerobacterium thermosaccharolyticum and its application to the production of ginsenosides Rg1 and Rh1 from notoginsenosides R1 and R2. Biotechnol Lett. 2014;36:2275–81.
Inoue H, Kitao C, Yano S, Sawayama S. Production of β-xylosidase from Trichoderma asperellum KIF125 and its application in efficient hydrolysis of pretreated rice straw with fungal cellulase. World J Microbiol Biotechnol. 2016;32:1–10.
Cheng W, Jing J, Wang Z, Wu D, Huang Y. Chondroprotective effects of Ginsenoside Rg1 in human osteoarthritis chondrocytes and a rat model of anterior cruciate ligament transection. Nutrients. 2017;9:263.
Zhao JH, Zhang QB, Liu B, Piao XH, Yan YL, Hu XG, Zhou K, Zhang YT, Feng NP. Enhanced immunization via dissolving microneedle array-based delivery system incorporating subunit vaccine and saponin adjuvant. Int J Nanomedicine. 2017;12:4763–72.
Xie J, Zhao D, Zhao L, Pei J, Xiao W, Ding G, Wang Z. Overexpression and characterization of a ca(2+) activated thermostable beta-glucosidase with high ginsenoside Rb1 to ginsenoside 20(S)-Rg3 bioconversion productivity. J Ind Microbiol Biotechnol. 2015;42:839–50.
Zhang R, Zhang BL, Xie T, Li GC, Tuo Y, Xiang YT. Biotransformation of rutin to isoquercitrin using recombinant α-L-rhamnosidase from Bifidobacterium breve. Biotechnol Lett. 2015;37:1257–64.
Sevimligür C, Onbaşılar I, Atilla P, Genç R, Cakar N, Deliloğlugürhan I, Bedir E. In vitro growth stimulatory and in vivo wound healing studies on cycloartane-type saponins of Astragalus genus. J Ethnopharmacol. 2011;134:844–50.
Chang HL, Kim JH. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J Ginseng Res. 2014;38:161.
Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685.
Valenzuela HF, Fuller T, Edwards J, Finger D, Molgora B. Cycloastragenol extends T cell proliferation by increasing telomerase activity. J Immunol. 2009;182.
Huang BM, Xiao SY, Chen TB, Xie Y, Luo P, Liu L, Zhou H. Purity assessment of ginsenoside Rg1 using quantitative 1H nuclear magnetic resonance. J Pharm Biomed Anal. 2017;139:193.
Ip FC, Ng YP, An HJ, Dai Y, Pang HH, Hu YQ, Chin AC, Harley CB, Wong YH, Ip NY. Cycloastragenol is a potent telomerase activator in neuronal cells: implications for depression management. Neurosignals. 2014;22:52–63.
Kim WY, Kim JM, Han SB, Lee SK, Kim ND, Park MK, Kim CK, Park JH. Steaming of ginseng at high temperature enhances biological activity. J Nat Prod. 2000;63:1702.
Bae EA, Han MJ, Kim EJ, Kim DH. Transformation of ginseng saponins to ginsenoside rh2 by acids and human intestinal bacteria and biological activities of their transformants. Arch Pharm Res. 2004;27:61–7.
Yang DC, Lin HQ, Liang Z, Ho-Bin K, Se-Hwa K, Se-Young K, Yeong-Deok N. Conversion of Ginsenoside Rd to compound K by crude enzymes from Lactobacillus brevis LH8. J Ginseng Res. 2008;32:226–31.
White A, Rose DR. Mechanism of catalysis by retaining beta-glycosyl hydrolases. Curr Opin Struct Biol. 1997;7:645.
Goujon T, Minic Z, El AA, Lerouxel O, Aletti E, Lapierre C, Joseleau JP, Jouanin L. AtBXL1, a novel higher plant (Arabidopsis thaliana) putative beta-xylosidase gene, is involved in secondary cell wall metabolism and plant development. Plant J. 2003;33:677–90.
Czjzek M, Ben DA, Bravman T, Shoham G, Henrissat B, Shoham Y. Enzyme-substrate complex structures of a GH39 beta-xylosidase from Geobacillus stearothermophilus. J Mol Biol. 2005;353:838–46.
Kirikyali N, Connerton IF. Heterologous expression and kinetic characterisation of Neurospora crassa β-xylosidase in Pichia pastoris. Enzyme Microb Technol. 2014;57:63–8.
Patel H, Kumar AK, Shah A. Purification and characterization of novel bi-functional GH3 family β-xylosidase/β-glucosidase from aspergillus Niger ADH-11. Int J Biol Macromol. 2018;109:1260–69.
Eneyskaya EV, Ivanen DR, Bobrov KS, Isaevaivanova LS, Shabalin KA, Savel'Ev AN, Golubev AM, Kulminskaya AA. Biochemical and kinetic analysis of the GH3 family beta-xylosidase from Aspergillus awamori X-100. Arch Biochem Biophys. 2007;457:225.
Mustafa G, Kousar S, Rajoka MI, Jamil A. Molecular cloning and comparative sequence analysis of fungal β-Xylosidases. AMB Express. 2016;6:30.
Lama L, Calandrelli V, Gambacorta A, Nicolaus B. Purification and characterization of thermostable xylanase and beta-xylosidase by the thermophilic bacterium Bacillus thermantarcticus. Res Microbiol. 2004;155:283–9.
Jain I, Kumar V, Satyanarayana T. Applicability of recombinant β-xylosidase from the extremely thermophilic bacterium Geobacillus thermodenitrificans in synthesizing alkylxylosides. Bioresour Technol. 2014;170:462–9.
Hao S, Xun L, Gu H, Yu Z, Huang Y, Wang L, Fei W. Biochemical properties of a novel thermostable and highly xylose-tolerant β-xylosidase/α-arabinosidase from Thermotoga thermarum. Biotechnol Biofuels. 2013;6:27.
Zhao L, Xie J, Zhang X, Cao F, Pei J. Overexpression and characterization of a glucose-tolerant β-glucosidase from Thermotoga thermarum DSM 5069T with high catalytic efficiency of ginsenoside Rb1 to Rd. J Mol Catalysis B Enzymatic. 2013;95:62–9.
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5.
Shao W, Wiegel J. Purification and characterization of a thermostable beta-xylosidase from Thermoanaerobacter ethanolicus. J Bacteriol. 1992;174:5848–53.
Ge L, Chen A, Pei J, Zhao L, Fang X, Ding G, Wang Z, Xiao W, Tang F. Enhancing the thermostability of α-L-rhamnosidase from Aspergillus terreus and the enzymatic conversion of rutin to isoquercitrin by adding sorbitol. BMC Biotechnol. 2017;17:21.
Yamashiro K, Itoh H, Yamagishi M, Natsuka S, Mega T, Hase S. Purification and characterization of neutral alpha-mannosidase from hen oviduct: studies on the activation mechanism of Co2+. J Biochem. 1997;122:1174–81.
Corrêa JM, Graciano L, Abrahão J, Loth EA, Gandra RF, Kadowaki MK, Henn C, Simão RDCG. Expression and characterization of a GH39 β-Xylosidase II from Caulobacter crescentus. Appl Biochem Biotechnol. 2012;168:2218–29.
Katapodis P, Nerinckx W, Claeyssens M, Christakopoulos P. Purification and characterization of a thermostable intracellular β-xylosidase from the thermophilic fungus Sporotrichum thermophile. Process Biochem. 2006;41:2402–9.
Delcheva G, Dobrev G, Pishtiyski I. Performance of Aspergillus niger B 03 β-xylosidase immobilized on polyamide membrane support. J Mol Catalysis B Enzymatic. 2008;54:109–15.
Yang Y, Zhang X, Yin Q, Fang W, Fang Z, Wang X, Zhang X, Xiao Y. A mechanism of glucose tolerance and stimulation of GH1 β-glucosidases. Sci Rep. 2015;5:17296.
Yan QJ, Wang L, Jiang ZQ, Yang SQ, Zhu HF, Li LT. A xylose-tolerant beta-xylosidase from Paecilomyces thermophila: characterization and its co-action with the endogenous xylanase. Bioresour Technol. 2008;99:5402.
Zanoelo FF, Terenzi HF, Jorge JA. Purification and biochemical properties of a thermostable xylose-tolerant β-D-xylosidase from Scytalidium thermophilum. J Ind Microbiol Biotechnol. 2004;31:170–6.
Shao W, Xue Y, Wu A, Kataeva I, Pei J, Wu H, Wiegel J. Characterization of a novel β-Xylosidase, XylC, from Thermoanaerobacterium saccharolyticum JW/SL-YS485. Appl Environ Microbiol. 2011;77:719–26.
Yang X, Shi P, Huang H, Luo H, Wang Y, Zhang W, Yao B. Two xylose-tolerant GH43 bifunctional β-xylosidase/α-arabinosidases and one GH11 xylanase from Humicola insolens and their synergy in the degradation of xylan. Food Chem. 2014;148:381–7.
Bankeeree W, Akada R, Lotrakul P, Punnapayak H, Prasongsuk S. Enzymatic hydrolysis of black liquor Xylan by a novel xylose-tolerant, thermostable β-Xylosidase from a tropical strain of Aureobasidium pullulans CBS 135684. Appl Biochem Biotechnol. 2018;184:919–34.
Funding
This study was funded by the National Key Research Development Program of China (Grant number 2017YFD0600805); the Forestry Achievements of Science and Technology to Promote Projects (Grant number [2017]10), the 11th Six Talents Peak Project of Jiangsu Province (Grant number 2014-JY-011) and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Availability of data and materials
The data set supporting the results of this article are included within the article.
Author information
Authors and Affiliations
Contributions
QL designed and performed the experiments and drafted the manuscript. TW and ZPQ helped to performed purification and characterization. JJP and FT helped to analyze the biotransformation of ginsenoside Rg1 and CA and revise the manuscript. LGZ directed the over-all study and had given final approval of the version to be published. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Additional file
Additional file 1:
Figure S1. Biotransformation pathway for production of ginsenoside Rg1 from notoginsenoside R1. Figure S2. Biotransformation pathway for production of CA from ASI. Figure S3. HPLC analysis of notoginsenoside R1 hydrolysis by Xln-DT. (a) Standards of notoginsenoside R1 and ginsenoside Rg1; (b-d) notoginsenoside R1 (1 g/L) incubated with Xln-DT for 0, 5 and 30 min, respectively. Figure S4. HPLC analysis of ASI hydrolysis by Xln-DT. (a) Standards of ASI and CA; (b-d) ASI (1 g/L) incubated with Xln-DT and Tpebg3 for 0, 1 and 3 h, respectively. (DOC 5520 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Li, Q., Wu, T., Qi, Z. et al. Characterization of a novel thermostable and xylose-tolerant GH 39 β-xylosidase from Dictyoglomus thermophilum. BMC Biotechnol 18, 29 (2018). https://doi.org/10.1186/s12896-018-0440-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12896-018-0440-3