Abstract
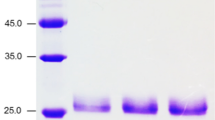
In the present work, the gene xynB2, encoding a β-xylosidase II of the Glycoside Hydrolase 39 (GH39) family, of Caulobacter crescentus was cloned and successfully overexpressed in Escherichia coli DH10B. The recombinant protein (CcXynB2) was purified using nickel-Sepharose affinity chromatography, with a recovery yield of 75.5 %. CcXynB2 appeared as a single band of 60 kDa on a sodium dodecyl sulfate polyacrylamide gel and was recognized by a specific polyclonal antiserum. The predicted CcXynB2 protein showed a high homology with GH39 β-xylosidases of the genus Xanthomonas. CcXynB2 exhibited an optimal activity at 55 °C and a pH of 6. CcXynB2 displayed stability at pH values of 4.5–7.5 for 24 h and thermotolerance up to 50 °C. The K M and V Max values were 9.3 ± 0.45 mM and 402 ± 19 μmol min−1 for ρ-nitrophenyl-β-d-xylopyranoside, respectively. The purified recombinant enzyme efficiently produced reducing sugars from birchwood xylan and sugarcane bagasse fibers pre-treated with a purified xylanase. As few bacterial GH39 family β-xylosidases have been characterized, this work provides a good contribution to this group of enzymes.




Similar content being viewed by others
References
Ahmed, S., Riaz, S., & Jamil, A. (2009). Molecular cloning of fungal xylanases: an overview. Applied Microbiology and Biotechnology, 84, 19–35.
Cantarel, B. L., Coutinho, P. M., Rancurel, C., Bernard, T., Lombard, V., & Henrissat, B. (2009). The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics. Nucleic Acids Research, 37, D233–D238.
Czjzek, M., David, A. B., Bravman, T., Shoham, G., Henrissat, B., & Shoham, Y. (2005). Enzyme-substrate complex structures of a GH39 β-xylosidase from Geobacillus stearothermophillus. Journal of Molecular Biology, 353, 838–846.
White, A., & Rose, D. R. (1997). Mechanism of catalysis by retaining β-glycosyl hydrolases. Current Opinion in Structural Biology, 7, 645–651.
Whiters, S. G. (2001). Mechanisms of glycosyl transferases and hydrolases. Carbohydrate Polymers, 44, 325–337.
Smaali, I., Rémond, C., & O’Donohue, M. J. (2006). Expression in Escherichia coli and characterization of β-xylosidases GH39 and GH-43 from Bacillus halodurans C-125. Applied Microbiology and Biotechnology, 73, 582–590.
Marks, M. E., Castro-Rojas, C. M., Teiling, C. D. U. L., Kapatral, V., Walunas, T. L., & Crosson, S. (2010). The genetics basis of laboratory adaptation in Caulobacter crescentus. Journal of Bacteriology, 192, 3678–3688.
Juturu, V., & Wu, J. C. (2011). Microbial xylanases: engineering, production and industrial applications. Biotechnology Advances. doi:10.1016/j.biotechadv.2011.11.006.
Santos, C. R., Polo, C. C., Corrêa, J. M., Simão, R. C. G., Seixas, F. A. V., & Murakami, M. T. (2012). Accessory domain changes accessibility and molecular topography of the catalytic interface in monomeric GH39 Beta-xylosidases. Acta Crystallographica Section D, 68, 1339–1345.
Graciano, L., Corrêa, J. M., Gandra, R. F., Seixas, F. A. V., Kadowaki, M. K., Sampaio, S. C., et al. (2012). The cloning, expression, purification, characterization and modeled structure of Caulobacter crescentus β-xylosidase I. World Journal of Microbiology and Biotechnology, 28(9), 2879–2888.
Poindexter, J. S. (1964). Biological properties and classification of the Caulobacter group. Bacteriological Reviews, 28, 231–295.
Sambrook, J., Fritsch, E. F., & Maniatis, T. (1989). Molecular cloning: a laboratory manual (2nd ed.). Cold Spring Harbor: Cold Spring Harbor Laboratory.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head bacteriophage T4. Nature, 226, 680–685.
Towbin, H., Staehelin, T., & Gordon, J. (1979). Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proceedings of the National Academy of Sciences of the United States of America, 76, 4350–4354.
McIlvaine, T. C. (1921). A buffer solution for colorimetric comparison. Journal of Biological Chemistry, 49, 183–186.
Miller, G. L. (1959). Use of dinitrosalicylic acid reagent for determination of reducing sugars. Analytical Chemistry, 31(3), 426–428.
Tuncer, M. (2000). Characterization of β-xylosidase and α-L-arabinofuranosidase activities from Thermomonospora fusca BD25. Turkish Journal of Biology, 24, 753–767.
Mai, J., Juergen, W., & Lorenz, W. W. (2000). Cloning, sequencing, and characterization of the bifunctional xylosidase–arabinosidase from the anaerobic thermophile Thermoanaerobacter ethanolicus. Gene, 247, 137–143.
Yang, J. K., Yoon, H. J., Ahn, H. J., Lee, B. I., Pedelacq, J. D., Liong, E. C., et al. (2004). Crystal structure of β-D-xylosidase from Thermoanaerobacterium saccharolyticum, a family 39 glycoside hydrolase. Journal of Molecular Biology, 335, 155–165.
Wagschal, K., Franqui-Espiet, D., Lee, C. C., Robertson, G. H., & Wong, D. W. (2005). Enzyme-coupled assay for β-xylosidase hydrolysis of natural substrates. Applied and Environmental Microbiology, 71, 5318–5323.
Wagschal, K., Franqui-Espiet, D., Lee, C. C., & Wong, D. W. (2008). Cloning, expression and characterization of a glycoside hydrolase family 39 xylosidase from Bacillus halodurans C-125. Applied Biochemistry and Biotechnology, 146, 69–78.
Evinger, M., & Agabian, N. (1977). Envelope-associated nucleoid from Caulobacter crescentus stalked and swarmer cells. Journal of Bacteriology, 132, 294–301.
Gottschalk, L. M. F., Oliveira, R. A., & Bom, E. P. S. (2010). Cellulases, xylanases, β-glucosidase and ferulic acid esterase produced by Trichoderma and Aspergillus act synergistically in the hydrolysis of sugarcane bagasse. Biochemical Engineering Journal, 51, 72–78.
Acknowledgments
This work was supported by grants from the Fundação Araucária (convênio 893/2012), Fundo Paraná SETI, CNPq, and Fundação Parque Tecnológico Itaipu (PTI C&T/FPTI-BR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Corrêa, J.M., Graciano, L., Abrahão, J. et al. Expression and Characterization of a GH39 β-Xylosidase II from Caulobacter crescentus . Appl Biochem Biotechnol 168, 2218–2229 (2012). https://doi.org/10.1007/s12010-012-9931-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-012-9931-1