Abstract
Phytoplankton in karst lakes is characterized by co-occurrence of chrysophytes (Chrysophyceae), dinoflagellates (Dinophyceae), and diatoms (Bacillariophyta) as the most diverse and abundant group. Using Reynolds functional approach in ecological phytoplankton investigations makes karstic lakes comparable and facilitates interpretation of their responses to changing environmental conditions. Accurate taxonomic identification to species level, based on precise resolution of specific characteristics by electron microscopy, is essential in order to correctly associate species into coda. This paper defines the most abundant centric diatoms and coexisting species in the phytoplankton of karst lakes in Croatia as a contribution to better description of phytoplankton functional groups dominated by centric diatoms. Several representatives for diatom-based Reynolds functional groups of four lakes in Croatia were described in this paper: Pantocsekiella costei and Stephanocostis chantaica for group A, Pantocsekiella ocellata and Cyclotella distinguenda for group B, and Stephanodiscus neoastraea for group C, together with their ecological preferences which clearly correspond to pertaining functional classification. Selected and coexisting functional groups define natural, oligo- to mesotrophic karst deep lake systems.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Phytoplankton comprises extremely diverse, polyphyletic group of organisms, demonstrating a wide array of morphological, physiological, and behavioral traits (Litchman & Klausmeier, 2008). Due to the high phenotypic variability, phytoplankton species represent distinct ecological entities that respond to resource availability, environmental stimuli, predators, and intra- and inter-species interactions. Such high phytoplankton diversity accounts for different functions within the freshwater ecosystems, and thus its understanding in space and time is highly relevant and timely issue. Correct species identification is important because in many cases taxonomically similar planktonic species do not represent ecological equivalents. Changes in the species concept over the past few decades (Salmaso et al., 2014) resulted in many new species descriptions, creating taxonomic uncertainties and confusion. In addition, limnological studies have demonstrated that similar taxonomic groups can co-occur in different phytoplankton assemblages with same ecological preferences. These difficulties in species identification and their ecology have led to a development of new approaches, like the creation of phytoplankton functional groups. The main aim of the new functional approach in studying phytoplankton assemblages was to improve the traditional ecological and taxonomical species concept (Salmaso et al., 2012). During the past few decades, three morpho-functional classifications have been described and reported as the most useful to assess phytoplankton functionality and seasonality (Borics et al., 2012; Salmaso et al., 2012); Functional Groups (FG), Morpho-Functional Groups (MFG) and Morphologically Based Functional Groups (MBFG) (Reynolds et al., 2002; Salmaso & Padisák, 2007; Kruk et al., 2010). Reynolds (1980, 1984) proposed one of the first classifications based on the species physiological, morphological, and ecological features, also including the range of conditions within which different species co-occur. Reynolds FGs concept was based on the similarity of the species ecological niche and environmental preferences. Therefore, phytoplankton functional groups can be seen as groups of species with more or less precisely defined requirements for several different combinations of physical, chemical, and biological properties of the lake environment (Padisák et al., 2006). Subsequently, Reynolds et al. (2002) described 31 FGs or “coda” based on a large species database and their own expertise and knowledge. Using Reynolds functional approach makes environmental investigations comparable and facilitates evaluation of freshwater bodies’ responses to changing environmental conditions. During the past decade, data have been complemented to more than 40 coda (Padisák et al., 2009) and successfully applied to a wide range of different ecosystems: tropical coastal lagoons (Alves-de-Souza et al., 2006), tropical estuaries (Costa et al., 2009), subarctic lakes (Forsström, 2006), barrage lakes (Nabout & Nogueira, 2007), subtropical lakes (Kruk et al., 2002), subalpine lakes (Morabito et al., 2003), temporary limestone lakes (Pereira et al., 2011), shallow (Pasztaleniec & Poniewozik, 2010) and deep temperate zone lakes (Souza et al., 2008; Soylu & Gönülol, 2010), large rivers (Borics et al., 2007; Salmaso & Braioni, 2007; Abonyi et al., 2012, 2014; Stanković et al., 2012), and karst lakes (Gligora et al., 2007; Žutinić et al., 2014; Gligora Udovič et al., 2015).
In the array of freshwater ecosystems, karst lakes are unique by their geological, physical, and chemical properties (e.g., Mikac et al., 2011). In most oligo- and mesotrophic karst lakes, phytoplankton is characterized by the presence of different algal groups like chrysophytes (Chrysophyceae) and dinoflagellates (Dinophyceae), and diatoms (Bacillariophyta) as the most diverse and abundant group (Gligora Udovič et al., 2015). Within diatoms, centric species belonging to Mediophyceae (Medlin & Kaczmarska, 2004) are the most common descriptors of phytoplankton functional assemblages. When information on characters pertaining to populations is essential in addressing particular ecological questions or environmental issues, accurate identification of dominant species in one functional group is highly important. During ecological investigations of karst lakes (Gligora et al., 2007; Gligora Udovič et al., 2011, 2015; Žutinić et al., 2014), we found that the morphology of centric diatoms is extremely variable and that the inadequate taxonomic resolution may often result in incorrect placement of species into coda, or even prevents sorting into coda. Therefore, accurate taxonomic identification to a species level, based on a precise resolution of specific characteristics by electron microscopy, is essential in order to correctly associate species into coda. This paper defines the most abundant centric diatoms and coexisting species in the phytoplankton of karst lakes in Croatia as contribution to better description of phytoplankton functional groups dominated by centric diatoms. Therefore, specific aims of this work are as follows: (i) to address, using SEM micrographs, main characteristics necessary to describe each of the centric diatom species within FGs; (ii) to explore main environmental conditions controlling these functional groups and; and (iii) to clarify whether the karst habitat fits into environmental features of a given FG (codon).
Study sites
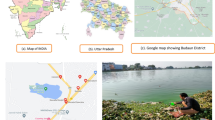
Croatian karstic lakes, including Vransko, Visovačko, Kozjak, and Prošće, are very heterogeneous by origin and type. The high proportion of carbonate bedrocks in the aquatic systems of the Croatian karst enabled formation of a specific tufa barrages, cascades, and/or sheets (e.g., Primc-Habdija et al., 2001; Pavlović et al., 2010). Tufa can be present in many forms, but probably the most famous are the travertine barriers that shape the waterfalls, like those of Plitvice Lakes and the Krka River (Kralj et al., 2006). Lake Prošće is the upper most of the sixteen cascading lakes forming Plitvice Lakes system with Matica River as an input of dissolved trace elements (e.g., As, Cr, Ni, Cu, Pb), of which concentrations sharply decrease downwards, thus indicating intensive deposition process. Calcium, a major element playing the key role in geochemistry of the Plitvice Lakes, also shows a decrease in the dissolved phase downwards due to intensive calcite precipitation (Miliša et al., 2006; Barešić et al., 2011; Dautović et al., 2013). As opposed, Lake Kozjak is the deepest and largest lake in the Plitvice Lakes system, with a longer water retention time, which ultimately results in a higher algal production in summer and autumn, contrary to spring algal maximum in Lake Prošće (Žutinić et al., 2014). Lake Visovačko, a lentic dilatation of the Krka River, also belongs to a group of karstic barrage lakes (Petrik, 1965; Gligora Udovič et al., 2011), while Vransko is an isolated, deep cryptodepression located on the Cres Island, Adriatic Sea (Petrik, 1965; Bonacci, 1993, 2014). Opposite to Plitvice Lakes, which are situated in continental part of the country with continental climate conditions, lakes Visovačko and Vransko are influenced by the mediterranean climate.
Materials and methods
Phytoplankton samples were collected monthly from April to September 2009 from lakes Visovačko and Kozjak, and 2010 from lakes Vransko and Prošće. Sampling was performed at 5 m intervals in the euphotic zone at the deepest part of each lake. Additional samples were taken in 2015 and used for detailed morphological analyses and comparison of the species. Samples used for water chemistry analysis were taken simultaneously and measured according to APHA (1995). Values of pH, conductivity, oxygen concentration, and saturation were measured on site with a WTW Multiline P4 portable meter. Secchi disk was used for measurements of water transparency. The euphotic zone (Z eu) was calculated as 2.5 times the Secchi depth (Mischke et al., 2012). The mixing zone (Z mix) was estimated from the temperature and dissolved oxygen profiles. Samples were fixed in 2% final concentration formaldehyde solution. Phytoplankton abundance was determined using inverted microscope (Axiovert 200, Carl Zeiss, Germany) and 50 ml chambers that were settled for 24 h (Utermöhl, 1958). At least 300 cells were counted at 400× magnification, corresponding to a counting error of 10% (Lund et al., 1958). Species cell size was measured with AxioVision LE 4.8.2 software (Carl Zeiss, Germany) using at least 20 individual cells, and cell volumes were calculated after approximation of geometric models following Hillebrand et al. (1999) and Sun & Liu (2003). Biomass was estimated from the product of the population and the mean cell volumes, and density of cells was assumed as 1 g ml−1 (Rott, 1981). The identified species were classified into functional groups (FGs) according to Reynolds et al. (2002) and Padisák et al. (2009). Biomass (phyla and FGs) and chemistry data were averaged monthly following WISER recommendation on sampling methodology for the water framework directive (Mischke et al., 2012): (i) When Z eu > Z mix data were averaged down to Z eu; (ii) When Z eu < Z mix data were averaged down to Z mix.
Non-metric multidimensional scaling (nMDS), Principal Component Analysis (PCA), SIMPER analysis, and Canonical analysis of principal coordinates (CAP) were calculated in PRIMER 6.1.10 (Clarke & Gorley, 2006). Phytoplankton biomass was transformed using the logarithm function prior to statistical analyses.
For examination of Pantocsekiella organic material, the formaldehyde preserved water sample was directly filtered on 3 µm Nucleopore polycarbonate filter (Nucleopore, Pleasanton, CA) and rinsed with distilled water. After dehydration in a series of ethanol solutions (25, 35, 50, 75, 80, 90%), the sample was prepared with distilled water and absolute ethanol, finishing with three rinses of 100% ethanol. For the drying method, the hexamethyldisilazane (HMDS) treatment was used (Bray et al., 1993). The sample was rinsed in a series of 100% ethanol: HMDS solutions (3:1, 1:1, 1:3), finishing with three changes of 100% HMDS, with a minimum of 5 min treatment at each step, allowing the last HMDS rinse to evaporate slowly at room temperature. For preparation of cleaned diatom frustules, subsamples were first washed with formaldehyde and subsequently treated with hydrochloric acid (HCl) to remove carbonates, sulphuric acid (H2SO4) and sodium nitrate (NaNO3) to clean the organic matter (2 ml of sample, 1:1, 3:1, 0, 5 g, respectively). Subsamples for scanning electron microscopy (SEM) were filtered onto membrane filters (Whatman Nucleopore 0.3 µm) and attached to aluminum stubs after air-drying. Stubs were sputter-coated with 30 nm of gold (Scancoat, Edwards Ltd., UK). Scanning electron microscopy was performed with a FEG Tescan MIRA3 (TESCAN, Czech Republic). Diatom species identification followed Krammer & Lange-Bertalot (1991), Håkansson (2002), Houk et al. (2010), Kiss et al. (2012), and Cvetkoska et al. (2014). Chrysophyte and dinoflagellate species identification followed Huber-Pestalozzi (1941), Taylor (1987), Popovský & Pfiester (1990), John et al. (2002, 2011) and Hansen & Flaim (2007).
Results
Environmental parameters
We studied four different karst lakes: Vransko (Cres Island), Kozjak and Prošće (Plitvice Lakes National Park), and Visovačko (Krka National Park) (Fig. 1). Extended details of the lakes’ physical and hydrological features are provided in Gligora Udovič et al. (2015). The main properties of each investigated lake (altitude, size, depth, geographical location) are presented in Table 1, while the physical and chemical measurements of their euphotic and/or mixing zones are listed in Table 2. Two groups were distinguished from the temperature profiles. The group comprising lakes Vransko and Visovačko was characterized by higher temperature minima (13.9 and 11.3°C, respectively) and maxima (20.0 and 22.1°C, respectively), while Kozjak and Prošće group had overall lower temperatures (Table 2) and mean values (12.9 and 12.3, respectively). Variations in pH were low in all lakes, ranging between 7.7 and 8.4 throughout the investigated period. The lowest alkalinity was measured at Lake Vransko, with a mean value below 120 mg l−1, which classifies it as a moderately hard lake. Alkalinity was much higher in the other lakes, with Lake Prošće having the highest values of 232–237 mg l−1, which classifies it as a very hard-type lake (Durfor & Becker, 1964). Electrical conductivity of the lakes was relatively high, ranging from 425 to 465 µS cm−1, except for Lake Kozjak, where the lowest values and widest ecological valence were detected (368–415 µS cm−1). The mean dissolved oxygen (DO) profile of all lakes showed high saturation level of the euphotic layer throughout the investigated period, while the chemical oxygen demand remained very low with the highest and lowest concentrations of DO noted in Lake Kozjak (Table 2). The concentrations of inorganic nitrogen compounds (NH4 + and NO2 −) showed only slight variations in all lakes throughout the investigated period. Ammonia (NH4 +) concentrations ranged from 1.0 to 35.0 μg l−1 for Lake Visovačko and Lake Prošće, respectively. The NO2 − concentration was from below the detection limit in Lake Vransko, up to 7.8 μg l−1 in Lake Kozjak. Only the nitrates (NO3 −) showed greater variation among the lakes; from below the detection limit to 664 μg l−1 (Lakes Vransko and Kozjak, respectively). Total nitrogen (TN) concentration also varied greatly from 155 (Lake Visovačko) to 805 μg l−1 (Lake Prošće). The mean soluble reactive phosphorus (SRP) and total phosphorus (TP) concentrations remained low in all the lakes (3.2–3.8 and 5.3–14.6 μg l−1, respectively), with exception of Lake Kozjak (0.8–79.8 μg l−1 SRP and 19.6–58.2 μg l−1 TP) (Table 2).
Eight environmental parameters for each lake were used for the principal component analysis (Fig. 2). First two axes accounted for 62.3% of the total variance, with eigenvalues of 3.49 and 1.49 for PCA axis 1 and PCA axix 2, respectively, comprising values for 5 most significant variables. TN and alkalinity were the most important parameters for the PCA axis 1 (intra-set correlations: −0.429 and −0.419, respectively). PCA axis 2 was described by SRP, TP, and DO (intra-set correlations: 0.562, 0.425 and −0.374, respectively). Samples were grouped spatially according to the lake representation. Lake Vransko samples were mainly singled out according to pH and temperature, while samples from Lake Visovačko were described by conductivity and temperature. The most important parameters for lakes Kozjak and Prošće were nutrients (TP, TN, SRP), DO and total alkalinity.
CAP results calculated from the phytoplankton FG biomass values and the environmental variables are shown in Fig. 3. In this case, both the eigenvalues (correlation coefficients) for the first two axes (0.9819 and 0.8619, respectively) and the squared environment-species correlations (0.9641 and 0.7429, respectively) were significantly positive. Permutation test showed that the ordination of both axes was statistically significant (P < 0.001). The first CAP axis was strongly positively correlated with NH4–N, pH, and TN (canonical eigenvectors: 0.482, 0.468, and 0.453, respectively) and negatively with temperature (canonical eigenvector −0.400), whereas the second CAP axis was mainly defined by total alkalinity and conductivity (canonical eigenvectors: 0.692 and 0.427, respectively). According to the results, three groupings were observed (with a selected threshold of 40% similarity). One group, comprising samples from Lake Vransko with an outlier from Lake Visovačko, positioned at the negative end of CAP2 axis and was constrained by conductivity and pH. Samples from Visovačko and Kozjak formed a separate cluster confined mainly by temperature and total alkalinity at the negative side of CAP1 axis. The third group, comprising the samples from Lake Prošće, was restrained by TN and NH4–N at the positive side of CAP2 axis.
Assemblage analyses
The nMDS analysis based on species biomass (Fig. 4) showed a clear separation between sampling sites and pointed out descriptor species for centric diatom-dominated functional groups (Fig. 5, 6, 7, 8). The phytoplankton community of Lake Vransko was characterized by a small (5–10 µm) centric diatom Pantocsekiella costei (Druart et F. Straub) Kiss & Ács (Fig. 9a–d) as a descriptor species of codon A. This species was constrained by the pH and conductivity, ranging between 8.2–8.4 and 429–442 µS cm−1, respectively (Table 2). Pantocsekiella costei was the dominant species in the phytoplankton assemblages of Vransko Lake throughout the entire study period, reaching maximum biomass of 0.10 mg l−1 in September 2010. Pantocsekiella ocellata (Pantocsek) Kiss & Ács (Fig. 10a–e), a decriptor species for codon B, dominated the phytoplankton communities in Lake Visovačko over the entire investigated period (Table 3), reaching a maximum biomass of 1.02 mg l−1 in June 2009. The species occurred within alkalinity values of 184–207 mg l−1 and temperature range of 11–22°C. Interestingly, P. ocellata from Lake Visovačko was often found forming chains of variable length (Fig. 10a) with cells interconnected with organic, mucilagenous material extruded from their central part (Fig. 10b). Lakes Vransko and Visovačko were also characterized by a range of other centric diatoms (Table 3). Stephanodiscus neoastraea Håkansson & Hickel (Fig. 7a–d), a representative of codon C, was the most dominant species in Lake Prošće from July to September 2010, with a maximum biomass in July 2010 (1.69 mg l−1), co-occurrring with codon B represetatives Cyclotella distinguenda Hustedt (Fig. 8a, c, e) (max biomass of 0.34 mg l−1, in September 2010) and Cyclotella plitvicensis Hustedt (Fig. 8b, d, f; max biomass of 0.14 mg l−1, in June 2010). For the entire study period, C. distinguenda was characteristic for the phytoplankton communities of Lake Kozjak, reaching a maximum biomass of 0.03 mg l−1 in July 2009. Both lakes Kozjak and Prošće showed much higher TN and TP concentrations and much lower temperature minima and maxima compared to lakes Vransko and Visovačko (Table 2). Stephanocostis chantaica Genkal & Kuzmina, a descriptor species for codon A, and Lindavia radiosa (Grunow) De Toni & Forti from codon B were also present in Lake Kozjak, but both were represented with low biomass. Diversity and distribution of diatom species identified in lakes Vransko, Visovačko, Kozjak, and Prošće are presented in Table 3.
a–e Pantocsekiella ocellata (Pantocsek) Kiss & Ács. f Pantocsekiella polymorpha (Meyer & Håkansson) Kiss & Ács. a–d, f External valve views. e Internal valve view. a P. ocellata chain of 15 cells. b Two cells of P. ocellata with extruded organic material in the central part of the frustules. a–d, f Lake Visovačko. e Lake Vransko. Scale bars 20 µm in (a); 5 µm in (c, f); 2 µm in (b, e)
Relative contribution (in %) of each FG to average resemblances between pairs of sample groups revealed dominance of different FGs within the plankton assemblages of the lakes (Primer, Simper-test, Table 4). Apart from codon A, Lake Vransko was represented by coda L O and E with Parvodinium inconspicuum (Lemmermann) S.Carty (Figs. 11, 12) and Dinobryon sociale (Ehrenberg) Ehrenberg/D. divergens O. E. Imhof (Figs. 15, 16) as the main descriptive species, respectively. The dominant codon B from Lake Visovačko, represented by the chain-forming P. ocellata (Fig. 10a–e), was accompanied by P. inconspicuum and Peridinium cinctum (O. F. Müller) Ehrenberg (Figs. 13, 14, 15, 16) that belong to group L O (Reynolds et al., 2002). Lake Prošće was characterized by centric diatoms from coda C and B, co-occurring with the following species: Dinobryon divergens/D. sociale/D. cylindricum O. E. Imhof (Fig. 17), Ulnaria sp. Ehrenberg, Fragilaria crotonensis Kitton, and Asterionella formosa Hassall from coda E, P, and D, respectively. Lake Kozjak was co-dominated by group B and E species (Dinobryon divergens/D. sociale/D. cylindricum), co-occuring with several descriptors from coda D and L O (Ulnaria acus (Kützing) Aboal and P. inconspicuum/P. cinctum, respectively).
Discussion
Environmental parameters
All investigated lakes Vransko, Visovačko, Prošće, and Kozjak have a naturally high content of bicarbonates derived from surface, and ground water inputs from the drainage basin are distinguished by having alkaline waters and can be classified as hard karst type waters (Vurnek et al., 2010). However, an altitudinal decrease of alkalinity is evident, as also noted by Iveković (1958) and Miliša et al. (2006), presumably as a consequence of intense calcite precipitation (Miliša et al., 2006; Vurnek et al., 2010). Total phosphorus concentrations in these nonpolluted investigated lakes extend over a wide range depending on the productivity of the system. The lowest and the highest TP values were noted in the euphotic zone during summer stratification, indicating both the bioavailable P and its consumption by the phytoplankton. Mean TP concentrations in lakes Visovačko, Kozjak, and Prošće indicated a slight shift toward a good status as defined by the Regulation on Quality Standards for Water (National Gazette, 73/13; National Gazette, 78/15; National Gazette, 151/14). Soluble reactive phosphorus, as an inorganic P form available for uptake by the phytoplankton (Overbeck, 1991), showed low values in all lakes, sometimes even bellow the range of detection as also noted in previous studies (Horvatinčić et al., 2006; Matoničkin Kepčija et al., 2006). The lowest recorded P concentrations usually occur during the summer period, when the productivity and P bioaccumulation rate are the highest. Moreover, increased pH values in lakes enhance the formation of calcium carbonate, thus coprecipitating available phosphate along with carbonates (Otsuki & Wetzel, 1972) and removing it from the water column. Calcareous hardwater lakes situated on limestone and dolomite waterbed are distinguished by having a strong carbonate buffering capacity with a relatively high pH values of water, as a consequence of dissolution of the substrate (Wetzel, 2001; Žutinić, 2014). Even slight changes in pH strongly affect phytoplankton assemblages, therefore higher pH noted in lakes during spring is a clear indication of plankton activity (Habdija et al., 2009). The phytoplankton community in Lake Vransko has access to less nutrients and bicarbonates, thus differing from other lakes and representing almost reference community for oligotrophic karst lakes exclusively described by centric diatoms, chrysophytes and dinoflagellates. The impact of direct precipitation input on the hydrological system of the lakes is visible through differences in conductivity. In barrage lake systems it is an indicator of calcium carbonate precipitation on tufa barriers between the systems (Horvatinčić et al., 2006; Biondić et al., 2010; Vurnek et al., 2010). The majority of the nitrogen in lakes usually comes from direct terrestrial runoff, but since it can be rapidly oxygenized, concentration of NO2 − is usually very low, as was the case in the investigated karst lakes. Contrary to NO2 − values, NO3 − levels can oscillate greatly depending on the season and surroundings. They were higher in lakes Prošće and Kozjak (Table 2), probably as a result of groundwater percolating through the humus layer of the wooded environment and transporting nutrient to the surface spring waters from which they are flushed in the Plitvice Lakes system (Žutinić, 2014). Increased N deposition has a particularly strong impact on karst lakes (Camacho et al., 2003; Gusev, 2008), therefore even small changes in N inputs can induce substantial changes in productivity and N cycling (Sheibley et al., 2014).
Assemblage analyses
Reynolds FG classification is based on the idea that, in order to be correctly associated into appropriate coda, all specimens from an assemblage should be identified to a species level (Reynolds et al., 2002). Diatom species identification, specifically from the centric genera like Cyclotella, Stephanodiscus and Cyclostephanos, has already been qualified as an arduous task, requiring long-term experience in taxonomy in combination with molecular techniques (Cox, 2014). Recent revisions and/or descriptions of new centric diatom genera like Stephanocostis (Genkal & Kuzmina, 1985), Discostella (Houk & Klee, 2004), Spicaticribra (Johansen et al., 2008), Lindavia (Nakov et al., 2015) and Pantocsekiella (Ács et al., 2016) have increased our knowledge about the diversity of freshwater centric diatoms (Karthick & Kociolek, 2011), but also stressed a further need for detailed phylogenetic analyses of this group of taxa (Kociolek & Khursevich, 2013). In particular, difficulties like barely discernible differences in the morphological structures under light and scanning electron microscopy (Kling & Håkansson, 1988; Abonyi et al., 2012; Kistenich et al., 2014), interspecific similarity (Sabater & Klee, 1990), and largely unknown species ecology (Sládeček, 1986) greatly account for erroneous or inaccurate species identifications (Håkansson & Kling, 1994) and related misplacements into FGs. Therefore, both taxonomical accuracy and precise functional characterization (Izaguirre et al., 2012; Žutinić et al., 2014) are fundamental for adequate ecological assessments (Cavalcante et al., 2013) and monitoring studies (Holzenthal et al., 2010; Martín & Reyes Fernández, 2012).
Reynolds classification assorts phytoplankton species with similar morphological, physiological and ecological traits into ecological categories—functional groups (FGs) (Reynolds, 1984; Reynolds et al., 2002). Centric diatoms from Reynolds codon A (e.g., Urosolenia, Cyclostephanos, Thalassiosira) are prominent members in the plankton of many clear, dilute, phosphorus-deficient, high-latitude medium-to-large lakes (Reynolds et al., 2002; Padisák et al., 2009). The diatom community of Lake Vransko, dominated by P. costei, and with Lindavia radiosa and S. chantaica as descriptor species, was restrained by conductivity and pH. Furthermore, physiological properties and ecotype placement (Gligora Udovič et al., 2015) of Lake Vransko is congruent with the placement of its dominant, typifying species into codon A. Pantocsekiella costei was originally described by Druart & Straub (1988) as Cyclotella costei during an ecological and palaeoecological study of a small alkaline, eutrophic lake Paladru (France). Pantocsekiella costei (Cyclotella costei) is closely related and probably conspecific with C. comensis and C. pseudocomensis (Kistenich et al., 2014; Duleba et al., 2015). Håkansson & Carter (1990) described Cyclotella cyclopuncta from Plitvice Lakes, but later taxonomic analyses of Houk et al. (2010) showed that both species share the same morphological features, and thus, C. cyclopuncta was considered a synonym of P. costei. This small sized species (4–17 µm in diameter) has been reported from the fossil and modern samples across a range of littoral and pelagic habitats from alkaline, oligo- to mesotrophic lakes (Houk et al., 2010). According to this study it is dominant in oligotrophic karst system, but also present in oligo- to mesotrophic conditions of all other investigated lakes. P. costei concurs with C. comensis in ecological preference toward oligotrophy, since C. comensis was inserted into codon A (Reynolds et al., 2002; Cellamare et al., 2016). Lindavia radiosa has been described from Lake Mondsee (Austria) as Cyclotella radiosa (Lemmermann, 1900), but latest taxonomic and phylogenetic analyses resulted with its transfer into genus Lindavia based on the position of rimoportula, striae structure, and presence of a complex alveolar structure in the central area (Nakov et al., 2015; Ács et al., 2016). Its biogeographic distribution, mainly in plankton assemblages from subalpine lakes like Mondsee, Staffelsee and Starnberger See, suggests oligo- to mesotrophic ecological preferences (Houk et al., 2010).
Community of Lake Vransko was strongly correlated with conductivity and pH values, constraints during which a small diatom S. chantaica remained a common species. Due to the size, S. chantaica did not participate in biomass significantly, but it clearly described the phytoplankton assemblage of Lake Vransko. Originally described from Lake Khantajskoe in Siberia (Genkal & Kuzmina, 1985), it is a rare species usually reported from circumpolar sites on the northern hemisphere (Scheffler & Padisák, 2000) and, because of its unclear status, even considered endangered (Kiss et al., 2012). In terms of ecology, it prefers deep, oligo- to mesotrophic, slightly alkaline lakes (Scheffler & Morabito, 2003). Functional classification places Stephanocostis into codon B, but due to unusual environmental conditions under which this and other species (e.g., Aulacoseira baicalensis (K. I. Meyer) Simonsen) grow, Padisák et al. (2009) proposed raising a separate codon. Based on aforementioned findings, the occurence of species in karstic systems, and congruous environmental settings of Lake Vransko, we consider S. chantaica to fit into codon A. Until now, there was no record of this cold-tolerant species from the Mediterranean climate zone.
Cyclotella species from codon B have a wide tolerance to various environmental parameters, such as adaptation to high lake stability (Beamud et al., 2015) and low light availability (Reynolds, 1997), allowing them to dominate in more mesotrophic water bodies (Hu et al., 2012). Light deficiency indicated by low Secchi disk depth (Gligora Udovič et al., 2015) and a strong thermal stratification were noted in Lake Visovačko throughout the investigated period, facilitating P. ocellata as the main descriptor of the diatom community, with accompanying Pantocsekiella polymorpha and Discostella pseudostelligera (Hustedt) Houk & Klee. Pantocsekiella ocellata is a diatom described from Lake Balaton, Hungary (Pantocsek, 1901). The latest phylogenetic analyses of the genus Cyclotella showed it can be split into several genera and allowed C. ocellata to be assigned a type species of a newly described diatom genus, Pantocsekiella (Ács et al., 2016). Pantocsekiella ocellata is a highly variable species in terms of its morphology and ecology, therefore a number of studies have been performed to delineate its phenotypic plasticity (Kiss et al., 1996; Edlund et al., 2003; Cremer et al., 2005; Genkal & Popovskaya, 2008; Winder et al., 2009; Duleba et al., 2015). Pantocsekiella ocellata (C. ocellata) is usually considered as a species complex (Edlund et al., 2003) rather than a single species (Duleba et al., 2015). It belongs in a complex together with Cyclotella krammeri Håkansson, Cyclotella rossii Håkansson, Cyclotella tripartita Håkansson, Cyclotella kuetzingiana Thwaites, C. polymorpha B. Meyer & Håkansson, and Cyclotella comensis Grunow (Edlund et al., 2003; Cherepanova et al., 2010; Duleba et al., 2015), with several species transferred into a genus Pantocsekiella K. T. Kiss et Ács, gen. nov.: P. ocellata, Pantocsekiella rossii (Håkansson) K. T. Kiss et Ács, comb. nov., Pantocsekiella tripartita (Håkansson) K. T. Kiss et Ács, comb. nov., P. polymorpha (B. Meyer et Håkansson) K.T. Kiss et Ács, comb. nov., and Pantocsekiella comensis (Grunow in van Heurck) K. T. Kiss et Ács, comb. nov. (Ács et al., 2016). Besides taxonomic uncertainties of the C. ocellata ‘group’, the Visovačko assemblage consisted of classical ‘ocellata’ morph, ‘trichonidea’ morph with slightly quadrangular contours and transitional forms (Duleba et al., 2015). In the case of Visovačko Lake, population partial sequences of 18S rDNA and rbcL showed no variation in samples with different morphs, and the difference was also low when compared to C. comensis, C. pseudocomensis, and C. costei and suggest that C. ocellata and C. comensis are two very closely related species that have only recently diverged from one another (Duleba et al., 2015). However, the life-form of P. ocellata occuring in chain formation as we observed in the Lake Visovačko has not been previously recorded in literature. Generally, in Cyclotella species, colony formation using organic material has been previously well described (Round et al., 1990) and already illustrated by Hustedt (1930). Cyclotella colonies can range from more or less amorphous aggregates embedded in mucilage such as in Lindavia socialis (in Hustedt 1930 as C. socialis) to C. planctonica forming wide and rigid gelatinous envelope enclosing the cells in a chain-like formation (Hustedt 1930). The most common Cyclotella life forms are the same as we observed in P. ocellata, and these include chain colonies of variable length formed by cells interconnected with chitin fibers as in C. cryptica (Round et al. 1990) or mucilage material as in C. quadriiuncta, C. glomerata, C. melosiroides (Hustedt, 1930), or C. choctawhatcheeana (Bosak, pers. obs.).
A range of centric diatoms in lakes Vransko and Visovačko were assorted into groups A and B attributed with a tolerance to low phosphorus conditions (Tilman & Kilham, 1976; Grigorszky et al., 2006) in stratified oligo- to mesotrophic lake types (Reynolds et al., 2002). Moreover, temperature profiles, mixing pattern, and trophic status of these lakes (Schmidt et al., 2000; Gligora Udovič et al., 2011, 2015) correspond both to the habitat template designated by Reynolds et al. (2002) and Padisák et al. (2009) and to the specified properties of selected species.
Actinocyclus normanii (Gregory ex Greville) Hustedt is a warm stenothermic species usually proliferating during summer. Although it had a very rare occurrence in Lake Visovačko during this study, it has been increasingly reported in the last decades from both lacustrine and riverine systems with different halobity, presumably as result of increased eutrophication, and is considered a potentially invasive species (Kaštovský et al., 2010; Abonyi et al., 2012; Kiss et al., 2012).
Cyclotella-dominated assemblage (Hutchinson, 1967) is favored in a thermally stratified, highly insolated water column (Miracle et al., 1992; Tolotti et al., 2007) with a relatively short retention time and deep mixing (Žutinić et al., 2014). In addition, typology and trophic status of lakes Kozjak and Prošće (Žutinić et al., 2014; Gligora Udovič et al., 2015), higher nutrient (TN and TP) values, lower temperature ranges, and high light conditions promote such diatom composition with no strict delimitation between associations B and C (Reynolds et al., 2002). Main descriptors of Lake Prošće were codon C species S. neoastraea, designated as Stephanodiscus sp. in previous studies by Žutinić et al. (2014) and Gligora Udovič et al. (2015), and codon B species C. distinguenda and C. plitvicensis. Relatively high biomass and dominance of large sized S. neoastraea recorded in Lake Prošće are likely sustained by higher nitrogen supply (Winder et al., 2009) and an indication of increased P concentration (Anneville et al., 2004; Kasperovičienė & Vaikutienė, 2007), although it was noted in oligotrophic systems (Üveges et al., 2012). Stephanodiscus neoastraea was described from Bornhoveder See (Germany) by Håkansson & Hickel (1986) and is easily distinguished from other similar species (e.g., S. rotula (Kützing) Hendey, S. niagarae Ehrenberg) by the absence of valve face fultoportula, an important morphological character for identification of Stephanodiscus taxa. On the other hand, S. minutulus (Kützing) Cleve & Möller and S. parvus Stoermer & Håkansson can be easily confused because of their small size. Although both species can co-occur in various aquatic ecosystems usually characterized by elevated ion concentrations (Krammer & Lange-Bertalot, 1991), they can be separated by the position of the valve face fultoportula (see Figs. 6, 7, 8, 9; 139–150 in Häkansson, 2002).
In compliance with its trophic status, Lake Kozjak was characterized by a codon B centric C. distinguenda, a widespread species characteristic for oligo- to mesotrophic lakes (Wunsam et al., 1995). It may be erroneously classified as Lindavia radiosa due to overlapping size and other morphological characters indiscernible under the light microscope (Žutinić et al., 2014; Gligora Udovič et al., 2015). C. distinguenda is closely related and morphologically similar to C. plitvicensis (e.g., diameter range and striae density; (Przybylowska-Lange, 1990; Huber, 2009), a species with highly restricted ecological niche. Although C. plitvicensis was not confirmed in Kozjak during this research, it was noted in lakes Prošće and Visovačko and placed accordingly into codon B. Stephanodiscus, a reliable indicator of more eutrophic conditions (Kilham et al., 1986; Hall & Smol, 1992), was represented by S. minutulus (codon B), S. neoastraea and S. parvus (codon C) in both lakes, although with substantially lower biomass in Lake Kozjak. Cyclotella distinguenda and the regionally endemic C. plitvicensis are similar in morphology, but nonetheless, both species can be separated by the shape of the central area (transversally undulated in C. distinguenda vs. flat in C. plitvicensis) and the number of rimportulae (at least one in C. distinguenda and one in C. plitvicensis). Latest phylogenetic analyses suggest both species should remain in the genus Cyclotella based on the characteristic position and shape of the rimoportula, as well as the presence of striae with equal length (Ács et al., 2016).
Alongside diatoms, chrysophytes frequently constitute a significant component of phytoplankton assemblages of karst lakes (Srdoč et al., 1992; Morata et al., 2003; Gligora et al., 2007; Žutinić et al., 2014; Gligora Udovič et al., 2015). The major representative, Dinobryon spp. complex, is assorted into functional group E (Reynolds et al., 2002; Padisák et al., 2009). Since codon E is designated for small, shallow, base poor lakes, or heterotrophic ponds, current habitat description does not fit into a description of typical karst lakes. Taxa belonging to group E are known to dominate spring-early summer plankton in oligotrophic–mesotrophic lakes (Žutinić et al., 2014), because they are mixotrophs and can supplement nutrient uptake by the phagotrophic ingestion of bacteria (Reynolds et al., 2002; Kamjunke et al., 2007). Studies addressing the rate of phagotrophy of Dinobryon in various environmental conditions showed contrasting results. Dinobryon cylindricum, often a dominant species in Croatian karst lakes, efficiently utilized phagotrophy in natural lakes (Laybourn-Parry & Marshall, 2003), but appeared rather inadequate when in culture environment (Caron et al., 1993). Olrik (1998) showed that Chrysophytes used bacteria more as a P substitute, than for C uptake. As supported by the mentioned studies, Dinobryon complex in karst lakes can be attributed to codon E if interpreted in the context of distinctive conditions prevailing in such ecosystems. Since no studies on the feeding preferences of Dinobryon were conducted in Croatian or similar karst lakes, these findings rise up unanswered questions that should be clarified by further investigations. Besides D. cylindricum, other regularly occurring species in Croatian karst lakes, like D. divergens, D. sociale, and D. sertularia Ehrenberg, all show similar ecological preferences with their simultaneous appearance. Besides centric diatoms and representatives of codon E, dinoflagellates belonging to the functional group L O often tend to appear in large abundance, as well as biomass, thus defining phytoplankton community in the habitat (Gligora Udovič et al., 2015). The features they share with genus Dinobryon include mixotrophy and motility (Popovsky & Pfiester, 1990). Codon L O covers a wide range of habitats comprising deep and shallow, oligo- to eutrophic, medium-to-large lakes. Based on ecological traits of its representative dinoflagellate species, group L O defines phytoplankton assemblages in the studied lakes and supports a concept of A–B and E–L O co-occurrence as suggested by Gligora Udovič et al. (2015) and herewith, can serve as a descriptor of natural communities in deep oligo-mesotrophic lakes of Dinaric karst.
Several representatives for diatom-based Reynolds FGs of four lakes in Croatia were described in this paper: P. costei and S. chantaica for group A, P. ocellata and C. distinguenda for group B, and S. neoastraea for group C, together with their ecological preferences which clearly correspond to pertaining functional classification. Selected and coexisting functional groups define natural, oligo- to mesotrophic karst deep lake systems. Every anthropogenic impact is clearly shown on these pristine ecosystems, therefore increased knowledge about keystone phytoplankton species in populations is crucial in order to understand and predict the response of environment to shifting conditions.
References
Abonyi, A., M. Leitão, A. M. Lançon & J. Padisák, 2012. Phytoplankton functional groups as indicators of human impacts along the River Loire (France). Hydrobiologia 698: 233–249.
Abonyi, A., M. Leitão, I. Stanković, G. Borics, G. Várbíró & J. Padisák, 2014. A large river (River Loire, France) survey to compare phytoplankton functional approaches: do they display river zones in similar ways? Ecological Indicators 46: 11–22.
Ács, É., E. Ari, M. Duleba, M. Dressler, S. I. Genkal, E. Jako, F. Rimet, L. Ector & K. T. Kiss, 2016. Pantocsekiella, a new centric diatom genus based on morphological and genetic studies. Fottea 16: 56–78.
Alves-de-Souza, C., M. Menezes & V. Huszar, 2006. Phytoplankton composition and functional groups in a tropical humic coastal lagoon, Brazil. Acta Botanica Brasilica 20: 701–708.
Anneville, O., S. Souissi, S. Gammeter & D. Straile, 2004. Seasonal and inter-annual scales of variability in phytoplankton assemblages: comparison of phytoplankton dynamics in three peri-alpine lakes over a period of 28 years. Freshwater Biology 49: 98–115.
APHA, 1995. Standard Methods for the Examination of Water and Wastewater. American Public Health Association, Washington, DC.
Barešić, J., N. Horvatinčić & Z. Roller–lutz, 2011. Spatial and seasonal variations in the stable C isotope composition of dissolved inorganic carbon and in physico-chemical water parameters in the Plitvice Lakes system. Isotopes in Environmental and Health Studies 47: 316–329.
Beamud, S. G., J. G. León, C. Kruk, F. L. Pedrozo & M. M. Diaz, 2015. Using trait-based approaches to study phytoplankton seasonal succession in a subtropical reservoir in arid central western Argentina. Environmental Monitoring and Assessment 187: 1–16.
Biondić, B., R. Biondić & H. Meaški, 2010. The conceptual hydrogeological model of the Plitvice Lakes. Geologia Croatica 63: 195–206.
Bonacci, O., 1993. The Vrana Lake hydrology (Island of Cres - Croatia). Journal of the American Water Resources Association 29: 407–417.
Bonacci, O., 2014. Analysis of the Vrana Lake (Island of Cres, Croatia) water level oscillations. Hrvatske vode: časopis za vodno gospodarstvo 22: 337–346.
Borics, G., B. Tóthmérész, B. Lukács & G. Várbíró, 2012. Functional groups of phytoplankton shaping diversity of shallow lake ecosystems. Hydrobiologia 698: 251–262.
Borics, G., G. Várbiró, I. Grigorszky, E. Krasznai, S. Szabó & K. K. Tihamer, 2007. A new evaluation technique of potamo-plankton for the assessment of the ecological status of rivers. Archiv für Hydrobiologie. Supplementband. Large rivers 17: 465–486.
Bray, D. F., J. Bagu & P. Koegler, 1993. Comparison of hexamethyldisilazane (HMDS), Peldri II, and critical-point drying methods for scanning electron microscopy of biological specimens. Microscopy Research and Technique 26: 489–495.
Camacho, A., W. A. Wurtsbaugh, M. R. Miracle, X. Armengol & E. Vicente, 2003. Nitrogen limitation of phytoplankton in a Spanish karst lake with a deep chlorophyll maximum: a nutrient enrichment bioassay approach. Journal of Plankton Research 25: 397–404.
Caron, D. A., R. W. Sanders, E. L. Lim, C. Marrasé, L. A. Amaral, S. Whitney, R. B. Aoki & K. G. Porters, 1993. Light-dependent phagotrophy in the freshwater mixotrophic chrysophyte Dinobryon cylindricum. Microbial Ecology 25: 93–111.
Cavalcante, K. P., P. I. Tremarin & T. A. V. Ludwig, 2013. Taxonomic studies of centric diatoms (Diatomeae): unusual nanoplanktonic forms and new records for Brazil. Acta Botanica Brasilica 27: 237–251.
Cherepanova, M. V., M. V. Usol’tseva, V. S. Pushkar & Y. F. Dubrovina, 2010. Morphogenesis in Cyclotella ocellata – complex from Lake El’gygytgyn (Chukchi Peninsula) during the Pleistocene-Holocene. Paleontological Journal 44: 1252–1261.
Cellamare, M., A. M. Lançon, M. Leitão, L. Cerasino, U. Obertegger & G. Flaim, 2016. Phytoplankton functional response to spatial and temporal differences in a cold and oligotrophic lake. Hydrobiologia 764: 199–209.
Clarke, K. R. & R. N. Gorley, 2006. PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth: 192.
Costa, L. S., V. L. M. de Huszar & A. R. Ovalle, 2009. Phytoplankton functional groups in a tropical estuary: hydrological control and nutrient limitation. Estuaries and Coasts 32: 508–521.
Cox, E. J., 2014. Diatom identification in the face of changing species concepts and evidence of phenotypic plasticity. Journal of Micropalaeontology 33: 111–120.
Cremer, H., B. Wagner, O. Juschus & M. Melles, 2005. A microscopical study of diatom phytoplankton in deep crater Lake El’gygytgyn, Northeast Siberia. Algological Studies 116: 147–169.
Cvetkoska, A., P. B. Hamilton, N. Ognjanova-Rumenova & Z. Levkov, 2014. Observations of the genus Cyclotella (Kützing) Brébisson in ancient lakes Ohrid and Prespa and a description of two new species Cyclotella paraocellata sp. nov. and Cyclotella prespanensis sp. nov. Nova Hedwigia 98: 313–340.
Dautović, J., Ž. Fiket, J. Barešić, M. Ahel & N. Mikac, 2013. Sources, distribution and behavior of major and trace elements in a complex karst lake system. Aquatic Geochemistry 20: 19–38.
Druart, J. C. & F. Straub, 1988. Description de deux nouvelles Cyclotelles (Bacillariophyceae) de milieux alcalins et eutrophes: Cyclotella costei nov. sp. et Cyclotella wuethrichiana nov. sp. Swiss Journal of Hydrology 50: 182–188.
Duleba, M., K. T. Kiss, A. Földi, J. Kovács, K. K. Borojević, L. F. Molnár, A. Plenković-Moraj, Z. Pohner, C. N. Solak, B. Tóth & É. Ács, 2015. Morphological and genetic variability of assemblages of Cyclotella ocellata Pantocsek/Cyclotella comensis Grunow complex (Bacillariophyta, Thalassiosirales). Diatom Research 30: 283–306.
Durfor, C. N., & E. Becker, 1964. Public water supplies of the 100 largest cities of the United States, 1962. US Government Printing Office, Washington, DC: 364 [available on internet at http://pubs.er.usgs.gov/publication/wsp1812].
Edlund, M. B., R. M. Williams & N. Soninkhishig, 2003. The planktonic diatom diversity of ancient Lake Hovsgol, Mongolia. Phycologia 42: 232–260.
Forsström, L., 2006. Phytoplankton Ecology of the Subarctic Lakes in Finnish Lapland. Department of Biological and Environmental Sciences, Aquatic Sciences and Kilpisjärvi Biological Station, Faculty of Biosciences, University of Helsinki, Helsinki.
Genkal, S. I. & A. E. Kuzmina, 1985. New genus and species Stephanocostis chantaicus Genkal et Kuzmina (Bailllariophyta). Biologia of inland waters 67: 8–10.
Genkal, S. I. & G. I. Popovskaya, 2008. Morphological variability of Cyclotella ocellata from Lake Khubsgul (Mongolia). Diatom Research 23: 75–91.
Gligora, M., A. Plenković-Moraj, K. Kralj, I. Grigorszky & D. Peroš-Pucar, 2007. The relationship between phytoplankton species dominance and environmental variables in a shallow lake (Lake Vrana, Croatia). Hydrobiologia 584: 337–346.
Gligora Udovič, M., K. Kralj Borojević, P. Žutinić, L. Šipoš & A. Plenković-Moraj, 2011. Net-phytoplankton species dominance in a travertine riverine Lake Visovac, NP Krka. Natura Croatica 20: 411–424.
Gligora Udovič, M., P. Žutinić, K. Kralj Borojević & A. Plenković-Moraj, 2015. Co-occurrence of functional groups in phytoplankton assemblages dominated by diatoms, chrysophytes and dinoflagellates. Fundamental and Applied Limnology 187: 101–111.
Grigorszky, I., K. T. Kiss, V. Béres, I. Bácsi, M. M-Hamvas, C. Máthé, G. Vasas, J. Padisák, G. Borics, M. Gligora & G. Borbély, 2006. The effects of temperature, nitrogen, and phosphorus on the encystment of Peridinium cinctum, Stein (Dinophyta). Hydrobiologia 563: 527–535.
Gusev, E. S., 2008. Phytoplankton primary production in several karst lakes in central Russia. Inland Water Biology 1: 356–361.
Habdija, I., M. Kerovec, M. Mrakovčić, A. Plenković-Moraj, & B. Primc-Habdija, 2009. Ekološko istraživanje površinskih kopnenih voda u Hrvatskoj prema kriterijima Okvirne direktive o vodama. Sveučilište u Zagrebu, Prirodoslovno-matematički fakultet, Biološki odsjek, Zagreb: 295.
Häkansson, H., 2002. A compilation and evaluation of species in the genera Stephanodiscus, Cyclostephanos and Cyclotella with a new genus in the family Stephanodiscaceae. Diatom Research 17: 1–139.
Håkansson, H. & J. R. Carter, 1990. An interpretation of Hustedt’s terms “Schattenlinie”, Perlenreihe“and”Hocker” using specimens of the Cyclotella radiosa-complex, Cyclotella distinguenda Hust., and Cyclotella cyclopuncta nov. sp. Journal of the Iowa Academy of Science 97: 153–156.
Håkansson, H. & B. Hickel, 1986. The morphology and taxonomy of the diatom Stephanodiscus neostraea sp. nov. British Phycological Journal 21: 39–43.
Håkansson, H. & H. Kling, 1994. Cyclotella agassizensis nov. sp. and its relationship to Cyclotella quillensis Bailey and other prairie Cyclotella species. Diatom Research 9: 289–301.
Hall, R. I. & J. P. Smol, 1992. A weighted - averaging regression and calibration model for inferring total phosphorus concentration from diatoms in British Columbia (Canada) lakes. Freshwater Biology 27: 417–434.
Hansen, G. & G. Flaim, 2007. Dinoflagellates of the Trentino Province, Italy. Journal of Limnology 66: 107.
Hillebrand, H., C. Dürselen, D. Kirschtel, U. Pollingher & T. Zohary, 1999. Biovolume calculation for pelagic and benthic microalgae. Journal of Phycology 35: 403–424.
Holzenthal, R. W., D. R. Robertson, S. U. Pauls & P. K. Mendez, 2010. Taxonomy and systematics: contributions to benthology and J-NABS. Journal of the North American Benthological Society 29: 147–169.
Horvatinčić, N., J. L. Briansó, B. Obelić, J. Barešić, & I. K. Bronić, 2006. Study of Pollution of the Plitvice Lakes by Water and Sediment Analyses. In Kronvang, B., J. Faganeli, & N. Ogrinc (eds), The Interactions Between Sediments and Water. Springer, Netherlands: 111–121 [available on internet at http://link.springer.com/chapter/10.1007/978-1-4020-5478-5_12].
Houk, V. & R. Klee, 2004. The stelligeroid taxa of the genus Cyclotella (Kutzing) Brebisson (Bacillariophyceae) and their transfer into the new genus Discostella gen. nov. Diatom Research 19: 203–228.
Houk, V., R. Klee & H. Tanaka, 2010. Atlas of freshwater centric diatoms, with a brief key and descriptions. Part 3: Stephanodiscaceae A: Cyclotella, Tertiarius, Discostella. Fottea 10: 1–498.
Hu, R., B. Han & L. Naselli-Flores, 2012. Comparing biological classifications of freshwater phytoplankton: a case study from South China. Hydrobiologia 701: 219–233.
Huber, K., 2009. Late glacial climatic and palaeoecological investigations of Längsee (Austria) using diatoms and chrysophyte cysts. PhD Thesis, University of Vienna, Vienna [available on internet at http://othes.univie.ac.at/5903/].
Huber-Pestalozzi, G., 1941. Das Phytoplankton des Süßwassers. Systematik und Biologie. 2. Teil, 1. Hälfte: Chrysophyceen. Farblose Flagellaten. Heterokonten. E. Schweizerbart’sche Verlagsbuchhandlung (Nagele u. Obermiller).
Hustedt, F., 1930. Bacillariophyta (Diatomeae) Zweite Auflage. Die Süsswasser-Flora Mitteleuropas. Heft 10. Jena: Verlag von Gustav Fischer: 466.
Hutchinson, G. E., 1967. A Treatise on Limnology. Introduction to Lake Biology and the Limnoplankton, Vol. 2. Wiley, New York.
Iveković, H., 1958. Mijenjanje kemijskog sastava vode Plitvičkih jezera. Nacionalni park Plitvička jezera: 227–275.
Izaguirre, I., L. Allende, R. Escaray, J. Bustingorry, G. Pérez & G. Tell, 2012. Comparison of morpho-functional phytoplankton classifications in human-impacted shallow lakes with different stable states. Hydrobiologia 698: 203–216.
Johansen, J., P. Kociolek & R. Lowe, 2008. Spicaticribra kingstonii, gen. nov. et sp. nov. (Thalassiosirales, Bacillariophyta) from Great Smoky Mountains National Park, USA. Diatom Research 23: 367–375.
John, D. M., B. A. Whitton & A. J. Brook, 2002. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae. Cambridge University Press, Cambridge.
John, D. M., B. A. Whitton & A. J. Brook (eds), 2011. The Freshwater Algal Flora of the British Isles: An Identification Guide to Freshwater and Terrestrial Algae. Cambridge University Press, Cambridge.
Kamjunke, N., T. Henrichs & U. Gaedke, 2007. Phosphorus gain by bacterivory promotes the mixotrophic flagellate Dinobryon spp. during re-oligotrophication. Journal of Plankton Research 29: 39–46.
Karthick, B. & J. P. Kociolek, 2011. Four new centric diatoms (Bacillariophyceae) from the Western Ghats, South India. Phytotaxa 22: 25–40.
Kasperovičienė, J. & G. Vaikutienė, 2007. Long–term changes in diatom communities of phytoplankton and the surface sediments in the Curonian Lagoon (Lithuanian part). Transitional Waters Bulletin 1: 27–37.
Kaštovský, J., T. Hauer, J. Mareš, M. Krautová, T. Bešta, J. Komárek, B. Desortová, J. Heteša, A. Hindáková, V. Houk, E. Janeček, R. Kopp, P. Marvan, P. Pumann, O. Skácelová & E. Zapomělová, 2010. A review of the alien and expansive species of freshwater cyanobacteria and algae in the Czech Republic. Biological Invasions 12: 3599–3625.
Kilham, P., S. S. Kilham & R. E. Hecky, 1986. Hypothesized resource relationships among African planktonic diatoms. Limnology and Oceanography 31: 1169–1181.
Kiss, K. T., C. Rojo & M. Alvarez Cobelas, 1996. Morphological variability of a Cyclotella ocellata (Bacillariophyceae) population in the Lake Las Madres (Spain). Algological Studies Supplement 82: 37–55.
Kiss, K. T., R. Klee, L. Ector & É. Ács, 2012. Centric diatoms of large rivers and tributaries in Hungary: morphology and biogeographic distribution. Acta Botanica Croatica 71: 311–363.
Kistenich, S., M. Dreßler, J. Zimmermann, T. Hübener, R. Bastrop & R. Jahn, 2014. An investigation into the morphology and genetics of Cyclotella comensis and closely related taxa. Diatom Research 29: 423–440.
Kling, H. & H. Håkansson, 1988. A light and electron microscope study of Cyclotella species (Bacillariophyceae) from central and northern Canadian lakes. Diatom Research 3: 55–82.
Kociolek, J. P. & G. K. Khursevich, 2013. Morphology of some fossil lacustrine centric species from the western United States assigned to the genus Cyclotella (Bacillariophyta), including four described as new. Phytotaxa 127: 81–99.
Kralj, K., A. Plenković-Moraj, M. Gligora, B. Primc-Habdija & L. Šipoš, 2006. Structure of periphytic community on artificial substrata: influence of depth, slide orientation and colonization time in karstic Lake Visovačko, Croatia. Hydrobiologia 560: 249–258.
Krammer, K., & H. Lange-Bertalot, 1991. Bacillariophyceae. 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In Ettl, H., Gerloff, J., Heynig, H., & Mollenhauer, D. (eds) Süsswasserflora von Mitteleuropa, Band 2/3. Gustav Fischer Verlag, Stuttgart.
Kruk, C., N. Mazzeo, G. Lacerot & C. S. Reynolds, 2002. Classification schemes for phytoplankton: a local validation of a functional approach to the analysis of species temporal replacement. Journal of Plankton Research 24: 901–912.
Kruk, C., V. L. M. de Huszar, E. T. H. M. Peeters, S. Bonilla, L. S. Costa, M. Lürling, C. S. Reynolds & M. Scheffer, 2010. A morphological classification capturing functional variation in phytoplankton. Freshwater Biology 55: 614–627.
Laybourn-Parry, J. & W. A. Marshall, 2003. Photosynthesis, mixotrophy and microbial plankton dynamics in two high Arctic lakes during summer. Polar Biology 26: 517–524.
Lemmermann, E., 1900. Beiträge zur Kenntnis der Planktonalgen. III. Neue Schwebalgen aus der Umgegend von Berlin. Berichte der deutsche botanischen Gesellschaft 18: 24–32.
Litchman, E. & C. A. Klausmeier, 2008. Trait-based community ecology of phytoplankton. Annual Review of Ecology, Evolution, and Systematics 39: 615–639.
Lund, J. W. G., C. Kipling & E. D. L. Cren, 1958. The inverted microscope method of estimating algal numbers and the statistical basis of estimations by counting. Hydrobiologia 11: 143–170.
Martín, G., & M. de los Reyes Fernández, 2012. Diatoms as indicators of water quality and ecological status: sampling, analysis and some ecological remarks In Voudouris, K. (ed.), Ecological Water Quality - Water Treatment and Reuse. InTech, Rijeka: 496 [available on internet at http://www.intechopen.com/books/ecological-water-quality-water-treatment-and-reuse/diatoms-as-indicators-of-water-quality-and-ecological-status-sampling-analysis-and-some-ecological-r].
Matoničkin Kepčija, R., I. Habdija, B. Primc-Habdija & M. Miliša, 2006. Simuliid silk pads enhance tufa deposition. Archiv für Hydrobiologie 166: 387–409.
Medlin, L. K. & I. Kaczmarska, 2004. Evolution of the diatoms: V. Morphological and cytological support for the major clades and a taxonomic revision. Phycologia 43: 245–270.
Mikac, I., Ž. Fiket, S. Terzić, J. Barešić, N. Mikac & M. Ahel, 2011. Chemical indicators of anthropogenic impacts in sediments of the pristine karst lakes. Chemosphere 84: 1140–1149.
Miliša, M., I. Habdija, B. Primc-Habdija, I. Radanović & R. Matoničkin Kepčija, 2006. The role of flow velocity in the vertical distribution of particulate organic matter on moss-covered travertine barriers of the Plitvice Lakes (Croatia). Hydrobiologia 553: 231–243.
Miracle, M. R., E. Vicente & C. Pedrós-Alió, 1992. Biological studies of Spanish meromictic and stratified karstic lakes. Limnetica 8: 59–77.
Mischke, U., S. J. Thackeray, M. J. Dunbar, C. M. McDonald, L. Carvalho, C. de Hoyos, M. Järvinen, C. Laplace-Treyture, G. Morabito, B. Skjelbred, A. Lyche Solheim, B. Brierley, & B. J. Dudley, 2012. WISER deliverable D3.1–4: guidance document on sampling, analysis and counting standards for phytoplankton in lakes [available on internet at http://www.wiser.eu/download/D3.1-4.pdf].
Morabito, G., A. Oggioni & P. Panzani, 2003. Phytoplankton assemblage at equilibrium in large and deep subalpine lakes: a case study from Lago Maggiore (N. Italy). Hydrobiologia 502: 37–48.
Morata, S. M., A. Camacho, M. R. Miracle & E. Vicente, 2003. Asociaciones fitoplanctónicas y su periodicidad en un lago marcadamente estratificado. Limnetica 22: 35–52.
Nabout, J. C. & I. S. Nogueira, 2007. Spatial and temporal dynamics of phytoplankton functional group in a blocked valley (Brazil). Acta Limnologica Brasiliensia 19: 305–314.
Nakov, T., W. Guillory, M. Julius, E. Theriot & A. Alverson, 2015. Towards a phylogenetic classification of species belonging to the diatom genus Cyclotella (Bacillariophyceae): Transfer of species formerly placed in Puncticulata, Handmannia, Pliocaenicus and Cyclotella to the genus Lindavia. Phytotaxa 217: 249–264.
National Gazette, 73/13: Uredba o standardu kakvoće voda (Regulation on Quality Standards for Water).
National Gazette, 78/15: Uredba o izmjenama i dopunama Uredbe o standardu kakvoće voda (Regulation of changes and updates of Regulation on Quality Standards for Water).
National Gazette, 151/14: Uredba o izmjenama i dopunama Uredbe o standardu kakvoće voda (Regulation of changes and updates of Regulation on Quality Standards for Water).
Olrik, K., 1998. Ecology of mixotrophic flagellates with special reference to Chrysophyceae in Danish lakes. Hydrobiologia 369–370: 329–338.
Otsuki, A. & R. G. Wetzel, 1972. Coprecipitation of phosphate with carbonates in a marl lake. Limnology and Oceanography 17: 763–767.
Overbeck, J., 1991. Early Studies on Ecto- and Extracellular Enzymes in Aquatic Environments. In Chróst, R. J. (ed.), Microbial Enzymes in Aquatic Environments. Springer, New York: 1–5 [available on internet on http://link.springer.com/chapter/10.1007/978-1-4612-3090-8_1].
Padisák, J., G. Borics, I. Grigorszky & É. Soróczki-Pintér, 2006. Use of phytoplankton assemblages for monitoring ecological status of lakes within the water framework directive: the assemblage index. Hydrobiologia 553: 1–14.
Padisák, J., L. Crossetti & L. Naselli-Flores, 2009. Use and misuse in the application of the phytoplankton functional classification: a critical review with updates. Hydrobiologia 621: 1–19.
Pantocsek, J., 1901. Die Kieselalgen oder Bacillarien des Balaton. Im Auftrage des ungarischen geographischen Gesellschaft auf Basis eigener Aufsammlungen Resultate der wissenschaftlichen Erforschung des Balatonsees. II. Band. Anhang zur II. Section des 2. Theiles. K. und K. Hofbuchdruckerei des Victor Hornyánszky, Budapest: 112.
Pasztaleniec, A. & M. Poniewozik, 2010. Phytoplankton based assessment of the ecological status of four shallow lakes (Eastern Poland) according to water framework directive – a comparison of approaches. Limnologica Ecology and Management of Inland Waters 40: 251–259.
Pavlović, G., J. Zupanič, E. Prohić & D. Tibljaš, 2010. Impressions of the biota associated with waterfalls and cascades from a Holocene tufa in the Zrmanja River Canyon, Croatia. Geologia Croatica 55: 25–37.
Pereira, H. C., N. Allott, C. Coxon, O. Naughton, P. Johnston & L. Gill, 2011. Phytoplankton of turloughs (seasonal karstic Irish lakes). Journal of Plankton Research 33: 385–403.
Petrik, M., 1965. Lakes in the Croatian limestone region. Hydrology of Fractured Rocks 2: 565–589.
Popovsky, J., & L. A. Pfiester, 1990. Süßwasserflora von Mitteleuropa. Band 6: Dinophyceae (Dinoflagellida). Jena & Stuttgart, Gustav Fischer.
Primc-Habdija, B., I. Habdija & A. Plenković-Moraj, 2001. Tufa deposition and periphyton overgrowth as factors affecting the ciliate community on travertine barriers in different current velocity conditions. Hydrobiologia 457: 87–96.
Przybylowska-Lange, W., 1990. Ultrastructure and morphological variability of fossil Cyclotella distinguenda Hust. (Bacillariophyceae) from Ferdynandów (eastern Poland). Acta Palaeobotanica 30: 59–63.
Reynolds, C. S., 1980. Phytoplankton assemblages and their periodicity in stratifying lake systems. Holarctic Ecology 3: 141–159.
Reynolds, C. S., 1984. Phytoplankton periodicity: the interactions of form, function and environmental variability. Freshwater Biology 14: 111–142.
Reynolds, C. S., 1997. Vegetation Processes in the Pelagic: A Model for Ecosystem Theory. Ecology Institute, Oldendorf.
Reynolds, C. S., V. L. M. de Huszar, C. Kruk, L. Naselli-Flores & S. Melo, 2002. Towards a functional classification of the freshwater phytoplankton. Journal of Plankton Research 24: 417–428.
Rott, E., 1981. Some results from phytoplankton counting intercalibrations. Schweizerische Zeitschrift für Hydrologie 43: 34–62.
Round, F. E., R. M. Crawford & D. G. Mann, 1990. The Diatoms: Biology & Morphology of the Genera. Cambridge University Press, Cambridge.
Sabater, S. & R. Klee, 1990. Observaciones-sobre diatomeas centrales del fitoplancton del Rio Ebro, con especial interés en algunas pequeñas Cyclotella/observations on centric diatoms of the River Ebro phytoplankton, with special interest on some small Cyclotella. Diatom Research 5: 141–154.
Salmaso, N. & M. G. Braioni, 2007. Factors controlling the seasonal development and distribution of the phytoplankton community in the lowland course of a large river in Northern Italy (River Adige). Aquatic Ecology 42: 533–545.
Salmaso, N. & J. Padisák, 2007. Morpho-functional groups and phytoplankton development in two deep lakes (Lake Garda, Italy and Lake Stechlin, Germany). Hydrobiologia 578: 97–112.
Salmaso, N., L. Naselli-Flores & J. Padisák, 2012. Impairing the largest and most productive forest on our planet: how do human activities impact phytoplankton? Hydrobiologia 698: 375–384.
Salmaso, N., F. Buzzi, L. Cerasino, L. Garibaldi, B. Leoni, G. Morabito, M. Rogora & M. Simona, 2014. Influence of atmospheric modes of variability on the limnological characteristics of large lakes south of the Alps: a new emerging paradigm. Hydrobiologia 731: 31–48.
Scheffler, W. & G. Morabito, 2003. Topical observations on centric diatoms (Bacillariophyceae, Centrales) of Lake Como (N. Italy). Journal of Limnology 62: 47.
Scheffler, W. & J. Padisák, 2000. Stephanocostis chantaicus (Bacillariophyceae): morphology and population dynamics of a rare centric diatom growing in winter under ice in the oligotrophic Lake Stechlin, Germany. Supplementband, Algological studies 133: 49–69.
Schmidt, R., J. Müller, R. Drescher-Schneider, R. Krisai, K. Szeroczyńska & A. Barić, 2000. Changes in lake level and trophy at Lake Vrana, a large karstic lake on the Island of Cres (Croatia), with respect to palaeoclimate and anthropogenic impacts during the last approx. 16,000 years. Journal of Limnology 59: 113–130.
Sheibley, R. W., M. Enache, P. W. Swarzenski, P. W. Moran & J. R. Foreman, 2014. Nitrogen deposition effects on diatom communities in lakes from three National Parks in Washington State. Water, Air, & Soil Pollution 225: 1–23.
Sládeček, V., 1986. Diatoms as indicators of organic pollution. Acta hydrochimica et hydrobiologica 14: 555–566.
Souza, M., C. Barros, F. Barbosa, É. Hajnal & J. Padisák, 2008. Role of atelomixis in replacement of phytoplankton assemblages in Dom Helvécio Lake, South-East Brazil. Hydrobiologia 607: 211–224.
Soylu, E. N. & A. Gönülol, 2010. Functional classification and composition of phytoplankton in Liman Lake. Turkish Journal of Fisheries and Aquatic Sciences 10: 53–60.
Srdoč, D., N. Horvatinčić, M. Ahel, W. Giger, C. Schaffner, I. K. Bronić, D. Petricioli, J. Pezdič, E. Marčenko & A. Plenković-Moraj, 1992. Anthropogenic influence on the 14C activity and other constituents of recent lake sediments: a case study. Radiocarbon 34: 585–592.
Stanković, I., T. Vlahović, M. G. Udovič, G. Várbíró, & G. Borics, 2012. Phytoplankton Functional and Morpho-Functional Approach in Large Floodplain Rivers. In Salmaso, D. N., L. Naselli-Flores, L. Cerasino, G. Flaim, M. Tolotti, & J. Padisák (eds), Phytoplankton Responses to Human Impacts at Different Scales. Springer, Netherlands: 217–231 [available on internet at http://link.springer.com/chapter/10.1007/978-94-007-5790-5_17].
Sun, J. & D. Liu, 2003. Geometric models for calculating cell biovolume and surface area for phytoplankton. Journal of Plankton Research 25: 1331–1346.
Taylor, F. J. R. (ed.), 1987. The Biology of Dinoflagellates. Blackwell Scientific Publications, Oxford.
Tilman, D. & S. S. Kilham, 1976. Phosphate and silicate growth and uptake kinetics of the diatoms Asterionella formosa and Cyclotella meneghiniana in batch and semicontinuous culture. Journal of Phycology 12: 375–383.
Tolotti, M., F. Corradini, A. Boscaini & D. Calliari, 2007. Weather-driven ecology of planktonic diatoms in Lake Tovel (Trentino, Italy). Hydrobiologia 578: 147–156.
Utermöhl, H., 1958. Zur vervollkomnung der quantitativen phytoplankton-methodik. Mitteilungen Internationale Vereiningung für Theoretische und Angewandte Limnologie 9: 1–38.
Üveges, V., K. Tapolczai, L. Krienitz & J. Padisák, 2012. Photosynthetic characteristics and physiological plasticity of an Aphanizomenon flos. Hydrobiologia 698: 263–272.
Vurnek, M., A. Brozinčević, G. Bušelić, G. Zwicker Kompar, & J. Rubinić, 2010. Intra-annual dynamics of water quality changes in Plitvice Lakes spring zone Balwois 2010/Morell, Marc (ed). Institut de Recherche pour le Developpement (France); Hydrometeorological Service of Republic of Macedonia; Hydrobiological Institute of Ohrid, Skopje, Makedonija: 463.
Wetzel, R. G., 2001. Limnology: Lake and River Ecosystems. Academic Press, San Diego.
Winder, M., J. E. Reuter & S. G. Schladow, 2009. Lake warming favours small-sized planktonic diatom species. Proceedings of the Royal Society B 276: 427–435.
Wunsam, S., R. Schmidt & R. Klee, 1995. Cyclotella-taxa (Bacillariophyceae) in lakes of the Alpine region and their relationship to environmental variables. Aquatic Sciences 57: 360–386.
Žutinić, P., 2014. Phytoplankton as a biological predictor in assessment of the ecological status of karstic lakes (case study – NP Plitvice lakes). PhD Thesis, University of Zagreb, Zagreb [available on internet at http://digre.pmf.unizg.hr/2533/].
Žutinić, P., M. Gligora Udovič, K. Kralj Borojević, A. Plenković-Moraj & J. Padisák, 2014. Morpho-functional classifications of phytoplankton assemblages of two deep karstic lakes. Hydrobiologia 740: 147–166.
Acknowledgments
This study has been co-financed by the European Union: the European Social Fund as part of the human Resources Development 2007–2013, as part of Project “HR.3.2.01-0342 Diatoms—descriptor of ecological status of Plitvice Lakes, Republic of Croatia.”
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling editor: Luigi Naselli-Flores
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gligora Udovič, M., Cvetkoska, A., Žutinić, P. et al. Defining centric diatoms of most relevant phytoplankton functional groups in deep karst lakes. Hydrobiologia 788, 169–191 (2017). https://doi.org/10.1007/s10750-016-2996-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-016-2996-z