Abstract
Cell suspension culture using mycelia as whole cell biocatalyst for production of orange Monascus pigments has been carried out successfully in a nonionic surfactant micelle aqueous solution. Thus, selection of mycelia as whole cell biocatalyst and the corresponding enzymatic kinetics for production of orange Monascus pigments can be optimized independently. Mycelia selected from submerged culture in a nonionic surfactant micelle aqueous solution with low pH 2.5 exhibits robust bioactivity. At the same time, enzymatic kinetic study shows that the bioactivity of mycelia as whole cell biocatalyst is sensitive to high product concentration. Segregation of product from mycelia by cell suspension culture in a nonionic surfactant micelle aqueous solution or peanut oil–water two-phase system is not only necessary for studying the enzymatic kinetics but also beneficial to industrial application of mycelia as whole cell biocatalyst.
Similar content being viewed by others
Introduction
Whole cell biocatalyst has been applied for production of valuable compound involving multi-step enzymatic biosynthesis as well as bio-redox reaction involving cofactor regeneration. In the case that product formation and cell growth are coupled, such as production of intracellular products, microbial fermentation using growing cells is applied, in which process optimization aiming at maximizing product usually defines biomass as objective function (Chen et al. 2015). On the contrary, when product formation is decoupled from cell growth, cell suspension culture using resting cells, i.e., the cells in no-growth or limited growth state by depletion of a compulsory nutrient element, is preferable. Cell suspension culture using resting cells exhibits some advantages in comparison with microbial fermentation using growing cells. Firstly, non-sterilization operation is possible due to the fact that the resting cell operation mode is carried out under nutrient limited condition (Sun et al. 2015). Secondly, high cell density is usually adopted during cell suspension culture, with which high product productivity can be achieved (Tsuge et al. 2014). Thirdly, high product yield based on energy source is also possible due to the avoidance of energy for biomass formation (Julsing et al. 2012). Furthermore, selection of whole cells as robust biocatalyst and optimization of enzymatic kinetics of whole cell biocatalyst can be carried out independently due to the decoupling of product formation from cell growth (Wang et al. 2005; Willrodt et al. 2016).
The bioactivity of whole cell biocatalyst is related to microbial physiology (Cornelissen et al. 2011). The microbial physiology is related to cultivation condition. There are many reports on the effect of cultivation conditions on the bioactivity of whole cell biocatalyst (Chen et al. 2011; Olaofe et al. 2013; Ramesh et al. 2016). On the other hand, there are few reports on the effect of mycelia culture period on bioactivity of whole cell biocatalyst (Mascotti et al. 2012). Whole cells utilized as biocatalyst are usually harvested at the late logarithm phase during growing cell submerged culture (Oremland et al. 1999). In addition, even robust biocatalyst may exhibit very low bioactivity under an unfavorable enzymatic kinetic condition. For example, product inhibition (Julsing et al. 2012; Kuhn et al. 2013) as well as product degradation (Winter et al. 2014) usually leads to low apparent bioactivity. Segregation of product from the whole cell biocatalyst is an efficient strategy for prevention of product inhibition on the biocatalyst or elimination of product degradation, which can be achieved by process engineering. In-situ product removal, such as addition of solid-state adsorbent (Evanst and Wang 1984), nonaqueous two-phase extraction (Wang and Dai 2010), is the most common strategy for elimination of product inhibition/degradation. Cascade conversion of instable/toxic product into stable/non-toxic compound by enzymatic reaction (Willrodt et al. 2015) or non-enzymatic reaction (Xiong et al. 2015; Domaille et al. 2016; Wallace and Balskus 2016) is also developed for bioprocess optimization.
Monascus is an ascomycete fungi widely utilized as a microbial source for production of natural pigments, including three major groups of Monascus pigments (red ones, monascorubramine and rubropunctamine; yellow ones, monascin and ankaflavin; and orange ones, rubropunctatin and monascorubrin) (Feng et al. 2012). The pigment profile as well as other secondary metabolites is strongly affected by the cultivation condition. Microbial fermentation at low pH leads to the accumulation of intracellular orange Monascus pigments (Kang et al. 2013), inhibition of citrinin production (Kang et al. 2014), and high lipid content in biomass (Wang et al. 2015). On the contrary, nearly neutral pH usually leads to accumulation of extracellular red Monascus pigments, production of toxic citrinin, and very low lipid content. Furthermore, nitrogen source in the cultivation medium strongly affects the pH of fermentation medium during fermentation process without pH control. Thus, nitrogen sources in the defined media (Kang et al. 2013, 2014) as well as complex media (Xiong et al. 2014) also influences the profile of Monascus pigments and citrinin accumulation. The influence of nitrogen concentration on profiling of Monascus proteome is also reported (Lin et al. 2008).
Submerged culture of Monascus sp. usually accumulates intracellular orange Monascus pigments under low pH condition. Microbial fermentation with high cell density can be applied for intensified production of intracellular Monascus pigments (Chen et al. 2015). In our previous work, extractive fermentation in a nonionic surfactant micelle aqueous solution has successfully released the intracellular Monascus pigments into its extracellular broth, meanwhile mycelia with very limited of intracellular Monascus pigments are achieved (Hu et al. 2012). Utilizing those mycelia as whole cell biocatalyst, cell suspension culture in a nonionic surfactant micelle aqueous solution (Wang et al. 2016) as well as plant oil–water two-phase system (Hu et al. 2016) is also carried out successfully. Thus, selection of mycelia as whole cell biocatalyst and optimization of enzymatic kinetics during biocatalytic process can be carried out independently.
In the present work, the relationship between cultivation condition, microbial physiology, and bioactivity of mycelia as whole cell biocatalyst for production of orange Monascus pigments was studied systemically. Firstly, the influence of cultivation conditions, such as culture period, carbon/nitrogen concentration, and pH, on microbial physiology, such as lipid content in biomass and intracellular Monascus pigment concentration, was examined. Then, the bioactivity of various mycelia as whole cell biocatalyst for production of orange Monascus pigments was determined in a nonionic surfactant micelle aqueous solution. At the same time, the corresponding enzymatic kinetic was further checked in a nonionic surfactant micelle aqueous solution and a plant oil–water two-phase system, respectively.
Materials and methods
Strain and culture media
Monascus anka (China Center of Industrial Culture Collection, CICC 5013) was used in this study. The strain was maintained on potato dextrose agar (PDA) medium (potato 200 g, glucose 20 g, and agar 15–20 g, per liter of tap water) at 4 °C.
The seed culture medium consisted of glucose 20 g, (NH4)2SO4 4 g, peptone 10 g, KCl 0.5 g, KH2PO4 4 g, and FeSO4∙7H2O 0.01 g, per liter of tap water. Inoculum culture was carried out in a 250 ml Erlenmeyer flask with working volume 50 ml at 30 °C and 200 rpm for 30 h.
Collection of mycelia as whole cell biocatalyst
Submerged culture of Monascus sp. was carried out in a fermentation medium in the presence of nonionic surfactant (Hu et al. 2012). The fermentation medium consisted of nonionic surfactant Triton X-100 70 g, KH2PO4 2.4 g, K2HPO4 2.4 g, FeSO4∙7H2O 0.01 g, and ZnSO4∙7H2O 0.01 g, per liter of tap water while glucose, monosodium glutamate (MSG), as well as the initial pH [adjustment with 10% (V/V) hydrochloric acid] were listed in Table 1. After inoculum culture, 2 ml of inoculum culture were added into 250 ml Erlenmeyer flask with 50 ml of every entry of fermentation medium (Table 1), which was incubated at 30 °C and run 200 rpm. At a specified culture period, such as the 6th day, mycelia in the culture medium were collected by filtration and rinsed with pH 2 water (50 ml) to scour off the residual nutrients as well as nonionic surfactants. Dry cell weight (DCW), lipid content, and the concentration of intracellular Monascus pigments in mycelia were determined.
Determination of mycelia bioactivity
The bioactivity of mycelia as whole cell biocatalyst was determined by cell suspension culture in a nonionic surfactant micelle aqueous solution. The nonionic surfactant micelle aqueous solution consisted of nonionic surfactant Triton X-100 70 g, glucose 50 g, KH2PO4 2.4 g, K2HPO4 2.4 g, FeSO4∙7H2O 0.01 g, and ZnSO4∙7H2O 0.01 g, per liter of tap water with an initial pH 5.5. A certain amount of wet mycelia (1.8 g wet mycelia, corresponding to approximately 10 g lipid-free DCW per liter) was added into 25 ml of the nonionic surfactant micelle aqueous solution in a 100 ml flask. The cell suspension aqueous solution was incubated at 30 °C and run 200 rpm for 42 h. The final pH reached to approximately 3.5. Then, DCW, lipid content, intracellular Monascus pigment concentration, and extracellular one, were determined.
Time course of cell suspension culture
Time course of cell suspension culture using mycelia as whole cell biocatalyst was carried out in a nonionic surfactant micelle aqueous solution and peanut oil–water two-phase system, respectively. Cell suspension culture in a nonionic surfactant micelle aqueous solution was conducted under the same condition as the determination of mycelia bioactivity. For cell suspension culture in peanut oil–water two-phase system, Triton X-100 was replaced by peanut oil as extractant with volume ratio of peanut oil to aqueous solution equaling 1:1. The above micelle aqueous solution (25 ml) or oil–water two-phase system (25 ml aqueous phase) containing mycelia (1.8 g wet mycelia, corresponding to approximately 10 g lipid-free DCW per liter) was filled into 100 ml flask. A series of flasks were incubated at 30 °C and run 200 rpm under the same condition. At a certain time interval, three flasks were fetched for analysis of DCW, lipid content, intracellular Monascus pigment concentration, and extracellular ones.
Analysis methods
DCW and lipid content were determined as detailed in our previous work (Wang et al. 2015). Mycelia after filtration from the culture broth were washed three times with equal volume of water [adjustment of pH to 2 with 10% (V/V) hydrochloric acid]. Wet mycelia were dried at 110 °C for at least overnight until constant mycelia weight reached. Datum of DCW was determined by gravity. Lipid weight in the dry mycelia was determined following the standard method (Bligh and Dyer 1959). For lipids might also be considered as a kind of secondary metabolite (Ratledge 2004), biomass was represented by lipid-free DCW (deduction of lipid weight from DCW) and lipid content was represented by ratio of lipid weight to lipid-free DCW.
Intracellular Monascus pigment concentration was determined as detailed in our previous work (Kang et al. 2013). Briefly, qualitative wet mycelia (such as 0.3 g) were incubated in 10 ml ethanol aqueous solution (70%, V/V, pH 2) at 30 °C and run at 200 rpm for 1 h. The intracellular Monascus pigments had been extracted completely into the ethanol aqueous solution. The absorbance of Monascus pigments in the ethanol aqueous solution was determined at 470 nm and represented as absorbance unit (AU, multiplication of the absorbance with its dilution ratio for a certain sample). Based on the datum of the corresponding lipid-free DCW, the concentration of intracellular Monascus pigments was achieved. There were small differences among the initial biomass loading in every entry of cell suspension culture. In order to eliminate the influence of those small differences in every entry, the concentrations of intracellular Monascus pigments before and after cell suspension culture were normalized to initial biomass concentration of 10 g lipid-free DCW per liter.
After removal of mycelia from the culture broth by filtration, the supernatant of filtrate was used to analyze extracellular Monascus pigment concentration. The supernatant (1 ml) was diluted properly with ethanol aqueous solution (70%, V/V, pH 2). The absorbance of Monascus pigments in the ethanol aqueous solution was determined at 470 nm and represented as absorbance unit (AU, multiplication of the absorbance with its dilution ratio for a certain sample). In order to compare with the data of intracellular Monascus pigment concentration, the concentration of extracellular Monascus pigments was also normalized to initial biomass concentration of 10 g lipid-free DCW per liter. In the case of cultivation in peanut oil–water two-phase system, intracellular Monascus pigments were exported near completely into the peanut oil phase. The peanut oil phase (0.9 g, corresponding to 1 ml) was washed with ethanol aqueous solution (70%, V/V, pH 2) until nearly no pigment was observed in the oil phase. Pigment concentration in the ethanol aqueous solution was determined, which was also normalized to the volume of water phase with initial biomass concentration of 10 g lipid-free DCW per liter.
In addition, supernatant (1 ml) of the nonionic surfactant micelle aqueous solution or water phase (1 ml) of peanut oil–water two-phase system was also diluted directly with water to determine the residual glucose concentration. The residual glucose concentration was determined by the standard 3,5-dinitrosalicylic acid method (DNS).
Results
Effect of cultivation condition on mycelia physiology
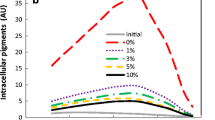
The cultivation media for extractive fermentation was divided into three groups (Table 1), i.e., different MSG concentration between entry 1 and entry 2, different pH between entry 3 and entry 4, and different glucose concentration between entry 5 and entry 6. The effect of cultivation medium as well as culture period on mycelia physiology, lipid content in mycelia (Fig. 1a) and intracellular Monascus pigment concentration (Fig. 1b), was examined. In most cases, the lipid content was kept below 20%. However, high lipid content was maintained at the early stage in the case of pH 4.5 and glucose 20 g/l (entry 1 and entry 2) or at the later stage in the case of pH 2.5, MSG 5 g/l, and glucose 50 g/l (entry 4) (Fig. 1a). On the other hand, the concentration of intracellular Monascus pigments was very low, as the presence of Triton X-100 in the media had exported the intracellular Monascus pigments into its extracellular broth (Hu et al. 2012). In the case of low pH and relatively high glucose concentration (entry 5 and entry 4), a relatively higher intracellular Monascus pigment concentration was observed at the later stage (Fig. 1b).
Effect of cultivation medium on microbial physiology. a Lipid content, defined as the lipid weight per 100 g lipid-free DCW; b intracellular Monascus pigments, normalized to biomass concentration of 10 g lipid-free DCW per liter. The data are average of triplicates. For clear vision, no error bar was represented
Bioactivity of whole cell biocatalyst
The bioactivity of mycelia was determined by cell suspension culture using mycelia as whole cell biocatalyst. Cell suspension culture under the depletion of a compulsory nutrient element (MSG) condition, biomass (lipid-free DCW) increase was very limited. When mycelia were collected at the later stage of culture period, the increase of lipid-free DCW was no more than 20% (Fig. 2a). This result is consistent with the mycelia maintaining resting cell state under the depletion of a compulsory nutrient element condition (Willrodt et al. 2016). On the contrary, lipid content increased during the cell suspension culture (Fig. 2b). In comparison with the initial lipid content in the mycelia (Fig. 1b), it was found that high initial lipid content in the mycelia (such as the early stage of entry 1 and entry 2 as well as the later stage of entry 4) maintained nearly no increase of lipid content. On the other hand, mycelia with low initial lipid content, such as the early stage of culture period, lipid content increased very remarkable. In other words, relatively high lipid content in mycelia was achieved during cell suspension culture under the depletion of MSG condition.
Cell suspension culture with mycelia collected from different cultivation conditions. a Change of biomass, defined as the ratio of lipid-free DCW after and before determination of mycelia bioactivity; b change of lipid content, defined as the ratio of lipid content after and before determination of mycelia bioactivity; c intracellular Monascus pigments concentration, normalized to biomass concentration of 10 g lipid-free DCW per liter; d extracellular Monascus pigments concentration, normalized to biomass concentration of 10 g lipid-free DCW per liter. The data are average of triplicates. For clear vision, no error bar was represented
Furthermore, orange Monascus pigments were also produced during cell suspension culture. The extracellular Monascus pigment concentration as well as the intracellular one was normalized to initial biomass concentration of 10 g lipid-free DCW per liter. In most cases, the concentration of intracellular Monascus pigments before (Fig. 1b) and after (Fig. 2c) cell suspension culture was neglectable. Thus, the concentration of extracellular Monascus pigments could be used to index the bioactivity of mycelia as whole cell biocatalyst for production of orange Monascus pigments (Fig. 2d). It was found that the accumulation of orange Monascus pigments depended not only on the cultivation media but also on cultivation period. The influence of MSG concentration on production of extracellular Monascus pigments was very limited (entry 1 and entry 2). However, the effect of pH on concentration of extracellular Monascus pigments was remarkable that low pH 2.5 led to high concentration of extracellular Monascus pigments (entry 3 and entry 4). The influence of pH on concentration of extracellular Monascus pigments was further confirmed under the relatively lower glucose concentration condition (entry 5 and entry 6). Thus, mycelia collected from cultivation condition with very low pH 2.5 (entry 4, 5, and 6) and at a middle culture period (approximately the 6th day) exhibited high bioactivity for production of high concentration of Monascus pigments. It should be pointed out that submerged culture with high glucose concentration 50 g/l and low pH 2.5 led to relatively higher intracellular Monascus pigment concentration at the later culture period (the 11th and 12th day of entry 4 in Fig. 1b). However, the corresponding extracellular Monascus pigment concentration was low after cell suspension culture of those mycelia as whole cell biocatalyst (Fig. 2d).
Monascus pigment degradation/inhibition
Submerged culture was carried out in a medium with glucose 60 g/l, MSG 5 g/l, and pH 2.5 to the 10th day. Then mycelia were collected to further confirm the influence of intracellular Monascus pigments on mycelia bioactivity for production of orange Monascus pigments. Some of the mycelia were subjected to releasing their intracellular Monascus pigments by being washed with Triton X-100 (70 g/l) micelle aqueous solution. Both washed mycelia and no washed mycelia were used as whole cell biocatalyst for cell suspension culture for 42 h (Table 2). The lipid content of washed mycelia were almost unaffected while most of intracellular Monascus pigments were exported. After cell suspension culture, washed mycelia consumed glucose more rapidly. Defining accumulation of Monascus pigments as the sum of both intracellular Monascus pigments and extracellular ones after cell suspension culture and subtracting the initial intracellular Monascus pigments before cell suspension culture, relatively higher concentration of extracellular orange Monascus pigments confirmed the bioactivity of washed mycelia. On the contrary, a negative accumulation of Monascus pigments was observed during the cell suspension culture of no washed mycelia. The result indicated the pigment degradation rate under the experimental condition was faster than the pigment biosynthesis rate by using no washed mycelia as whole cell biocatalyst. No doubt, it is also possible that the relatively higher concentration of intracellular Monascus pigments has an inhibitory effect on bioactivity of no washed mycelia for production of orange Monascus pigments.
Time course of cell suspension culture
The time course of cell suspension culture using mycelia as whole cell biocatalyst in a nonionic surfactant micelle aqueous solution was further examined (Fig. 3a). The nonionic surfactant micelle aqueous solution had exported the intracellular Monascus pigments into its extracellular broth and then the Monascus pigments were solubilized in the micelles. At early stage (from beginning to the 40th h), glucose concentration decreased rapidly. The corresponding Monascus pigments were solubilized in micelles and the concentration of extracellular Monascus pigments increased linearly. Then (from the 40th to 66th h), the increase of extracellular Monascus pigment concentration stagnated while the concentration of intracellular Monascus pigments increased slowly with the consumption of glucose. Finally, glucose was degraded completely and both intracellular Monascus pigment concentration and extracellular one maintained stable.
Time course of cell suspension culture. a In nonionic surfactant micelle aqueous solution with Triton X-100 70 g. Initial mycelia loading was 10 g lipid-free DCW per liter of the basic medium; b in peanut oil–water two-phase system with volume ratio of oil to water was 1:1. Initial mycelia loading was 12 g lipid-free DCW per liter of the basic medium. Mycelia were collected from extractive fermentation with glucose 35 g/l, MSG 5 g/l, and pH 2.5 (entry 5 in Table 1) at the 6th day
Replacing nonionic surfactant with peanut oil as extractant, intracellular Monascus pigments were exported near completely into the peanut oil phase, while both intracellular Monascus pigment concentration and pigment concentration in the water phase were neglectable in the whole cultivation process (data not shown). Residual glucose concentration and the concentration of extracellular Monascus pigments were presented (Fig. 3b). At the beginning stage, glucose was consumed and extracellular Monascus pigments were produced. However, even without glucose consumption, the increase of pigment concentration was still observed with the further prolonged cell suspension culture time. Interestingly, a very high concentration of extracellular Monascus pigments (approximately 110 AU at 470 nm) was achieved while the corresponding intracellular Monascus pigments maintained neglectable.
Discussion
The cultivation condition affects the microbial physiology and then the bioactivity of mycelia as whole cell biocatalyst (Chen et al. 2011; Olaofe et al. 2013; Ramesh et al. 2016). In the present work, mycelia were collected after submerged culture under different conditions. The bioactivity of using those mycelia as whole cell biocatalyst was determined by cell suspension culture in nitrogen (MSG)-free culture medium. The mycelia exhibited limited biomass (lipid-free DCW) growth (Fig. 2a), which indicated that mycelia were kept in resting cell state during cell suspension culture. The resting cells as whole cell biocatalyst showed bioactivity, such as production of lipids (Fig. 2b), biosynthesis of orange Monascus pigments (Fig. 2c, d) as well as consumption of glucose (Fig. 3a). The bioactivity for production of orange Monascus pigments was found to be related to the culture period. It consists with the fact that whole cell biocatalyst is usually harvested at the late logarithm phase during growing cell submerged culture (Oremland et al. 1999). It was also observed that low pH cultivation condition was a key factor for mycelia to exhibit high bioactivity for production of orange Monascus pigments (Fig. 2d). In our previous work, accumulation of intracellular orange Monascus pigments (Kang et al. 2013), inhibition of citrinin production (Kang et al. 2014), and high lipid content in biomass (Wang et al. 2015) at low pH are reported. It is attributed to the fact that nitrogen source is assimilated by microorganism via the way of controlled release of extracellular nitrogen source and maintenance of low intracellular nitrogen concentration under low pH condition (Kang et al. 2013). It is even reported that enzyme activity is regulated by nutritional requirements and is nitrogen-dependent (Hebert et al. 2000). However, no correlation between microbial physiology (lipid content or intracellular Monascus pigments as shown in Fig. 1) and bioactivity for production of orange Monascus pigments (Fig. 2d) was observed.
The bioactivity of mycelia as whole cell biocatalyst was very sensitive to high concentration of intracellular Monascus pigments (Table 2). This fact should be attributed to the instability of orange Monascus pigments under the experimental condition. At the same time, the inhibitory effect of product on the bioactivity of whole cell biocatalyst should also not be excluded. Bioactivity is an intrinsic character of mycelia as whole cell biocatalyst. However, it is usually underestimated or even neglected due to the measurement under an unfavorable experimental condition (Winter et al. 2014). Traditionally, biosynthesis of Monascus pigments is regarded as cell-growth dependence and production of Monascus pigments is usually carried out by microbial fermentation with growing cells (Chen et al. 2015). Benefiting from extractive fermentation in a nonionic surfactant micelle aqueous solution, intracellular Monascus pigments are exported out of cell interior and the extracellular Monascus pigments are solubilized in the nonionic surfactant micelles (Hu et al. 2012). And then, enzymatic kinetic study could be carried out by keeping biocatalyst in an environment with low product concentration (Fig. 2d; Table 2). Thus, production of orange Monascus pigments by mycelia as whole cell biocatalyst has also been recognized (Wang et al. 2016; Hu et al. 2016).
Segregation of product from biocatalyst for elimination of product degradation/inhibition is not only necessary for study of enzymatic kinetics but also beneficial to industrial application of mycelia as whole cell biocatalyst. Time course of cell suspension culture in a nonionic surfactant micelle aqueous solution indicated that product degradation as well as inhibition was eliminated at early stage (before reaching the extraction capacity of nonionic surfactant micelles) and then intracellular Monascus pigments were accumulated at the later stage (after reaching the extraction capacity of nonionic surfactant micelles, which is similar to traditional fermentation in an aqueous solution) (Fig. 3a). Thus, extraction capacity of nonionic surfactant micelles was related to elimination of product degradation/inhibition and then related to product accumulation, which had been further confirmed by replacing nonionic surfactant with peanut oil as extractant (Fig. 3b). Accumulation of high concentration of extracellular Monascus pigments was realized due to the plant oil phase with high extractive capacity. Furthermore, instable/toxic extracellular product can also be converted into relatively stable/nontoxic compound by enzymatic reaction (Willrodt et al. 2015) or non-enzymatic reaction (Xiong et al. 2015; Domaille et al. 2016; Wallace and Balskus 2016), which may be a more efficient strategy to make whole cell biocatalyst play its potential.
In conclusion, collection of optimized mycelia as robust biocatalyst for production of orange Monascus pigments has been achieved by submerged culture in a nonionic surfactant micelle aqueous solution at low pH 2.5. The bioactivity of mycelia as whole cell biocatalyst is sensitive to high product concentration, which is attributed to the product degradation/inhibition. Segregation of product from biocatalyst by cell suspension culture in a nonionic surfactant micelle aqueous solution or peanut oil–water two-phase system is not only necessary for studying the enzymatic kinetics but also beneficial to industrial application of mycelia as whole cell biocatalyst.
Abbreviations
- MSG:
-
monosodium glutamate
- DCW:
-
dry cell weight
References
Bligh EG, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Chen XH, Lou WY, Zong MH, Smith TJ (2011) Optimization of culture conditions to produce high yields of active Acetobacter sp. CCTCC M209061 cells for anti-Prelog reduction of prochiral ketones. BMC Biotechnol. doi:10.1186/1472-6750-11-110
Chen G, Shi K, Song D, Quan L, Wu Z (2015) The pigment characteristics and productivity shifting in high cell density culture of Monascus anka mycelia. BMC Biotechnol. doi:10.1186/s12896-015-0183-3
Cornelissen S, Liu S, Deshmukh AT, Schmid A, Buhler B (2011) Cell physiology rather than enzyme kinetics can determine the efficiency of cytochrome P450-catalyzed C–H-oxyfunctionalization. J Ind Microbiol Biotechnol 38(9):1359–1370
Domaille DW, Hafenstine GR, Greer MA, Goodwin AP, Cha JN (2016) Catalytic upgrading in bacteria-compatible conditions via a biocompatible aldol condensation. ACS Sustain Chem Eng 4:671–675
Evanst PJ, Wang HY (1984) Pigment production from immobilized Monascus sp. utilizing polymeric resin adsorption. Appl Environ Microbiol 47(6):1323–1326
Feng Y, Shao Y, Chen F (2012) Monascus pigments. Appl Microbiol Biotechnol 96(6):1421–1440
Hebert EM, Raya RR, De Giori GS (2000) Nutritional requirements and nitrogen-dependent regulation of proteinase activity of Lactobacillus helveticus CRL 1062. Appl Environ Microbiol 66(12):5316–5321
Hu Z, Zhang X, Wu Z, Qi H, Wang Z (2012) Perstraction of intracellular pigments by submerged cultivation of Monascus in nonionic surfactant micelle aqueous solution. Appl Microbiol Biotechnol 94:81–89
Hu M, Zhang X, Wang Z (2016) Releasing intracellular product to prepare whole cell biocatalyst for biosynthesis of Monascus pigments in water-edible oil two-phase system. Bioprocess Biosyst Eng 39(11):1785–1791
Julsing MK, Kuhn D, Schmid A, Buhler B (2012) Resting cells of recombinant E. coli show high epoxidation yields on energy source and high sensitivity to product inhibition. Biotechnol Bioeng 109(5):1109–1119
Kang B, Zhang X, Wu Z, Qi H, Wang Z (2013) Effect of pH and nonionic surfactant on profile of intracellular and extracellular Monascus pigments. Process Biochem 48:759–767
Kang B, Zhang X, Wu Z, Qi H, Wang Z, Park S (2014) Production of citrinin-free Monascus pigments by submerged culture at low pH. Enzym Microb Technol 55:50–57
Kuhn D, Fritzsch FSO, Zhang XM, Wendisch VF, Blank LM, Buhler B, Schmid A (2013) Subtoxic product levels limit the epoxidation capacity of recombinant E. coli by increasing microbial energy demands. J Biotechnol 163(2):194–203
Lin W-Y, Chang J-Y, Hish C-H, Pan T-M (2008) Profiling the Monascus pilosus proteome during nitrogen limitation. J Agric Food Chem 56:433–441
Mascotti ML, Orden AA, Bisogno FR, de Gonzalo G, Kurina-Sanz M (2012) Aspergillus genus as a source of new catalysts for sulfide oxidation. J Mol Catal B 82:32–36
Olaofe OA, Fenner CJ, Gudiminchi RK, Smit MS, Harrison STL (2013) The influence of microbial physiology on biocatalyst activity and efficiency in the terminal hydroxylation of n-octane using Escherichia coli expressing the alkane hydroxylase, CYP153A6. Microb Cell Fact. doi:10.1186/1475-2859-12-8
Oremland RS, Blum JS, Bindi AB, Dowdle PR, Herbel M, Stolz JF (1999) Simultaneous reduction of nitrate and selenate by cell suspensions of selenium-respiring bacteria. Appl Environ Microbiol 65(10):4385–4392
Ramesh H, Zajkoska P, Rebros M, Woodley JM (2016) The effect of cultivation media and washing whole-cell biocatalysts on monoamine oxidase catalyzed oxidative desymmetrization of 3-azabicyclo 3,3,0 octane. Enzym Microb Technol 83:7–13
Ratledge C (2004) Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochemie 86:807–815
Sun W, Xiao F, Wei Z, Cui F, Yu L, Yu S, Zhou Q (2015) Non-sterile and buffer-free bioconversion of glucose to 2-keto-gluconic acid by using Pseudomonas fluorescens AR4 free resting cells. Process Biochem 50:493–499
Tsuge Y, Kawaguchi H, Sasaki K, Tanaka T, Kondo A (2014) Two-step production of d-lactate from mixed sugars by growing and resting cells of metabolically engineered Lactobacillus plantarum. Appl Microbiol Biotechnol 98:4911–4918
Wallace S, Balskus EP (2016) Designer micelles accelerate flux through engineered metabolism in E. coli and support biocompatible chemistry. Angew Chem Int Ed 55:6023–6027
Wang Z, Dai Z (2010) Extractive microbial fermentation in cloud point system. Enzyme Microb Technol 46:407–418
Wang Z, Zhao F, Chen D, Li D (2005) Cloud point system as a tool to improve the efficiency of biotransformation. Enzym Microb Technol 36:589–594
Wang B, Zhang X, Wu Z, Wang Z (2015) Investigation of relationship between lipid and Monascus pigment accumulation by extractive fermentation. J Biotechnol 212:167–173
Wang B, Zhang X, Wu Z, Wang Z (2016) Biosynthesis of Monascus pigments by resting cell submerged culture in nonionic surfactant micelle aqueous solution. Appl Microbiol Biotechnol 100(16):7083–7089
Willrodt C, Hoschek A, Buhler B, Schmid A, Julsing MK (2015) Coupling limonene formation and oxyfunctionalization by mixed-culture resting cell fermentation. Biotechnol Bioeng 112(9):1738–1750
Willrodt C, Hoschek A, Buhler B, Schmid A, Julsing MK (2016) Decoupling production from growth by magnesium sulfate limitation boosts de novo limonene production. Biotechnol Bioeng 113(6):1305–1314
Winter G, Averesch NJH, Nunez-Bernal D, Krömer JO (2014) In vivo instability of chorismate causes substrate loss during fermentative production of aromatics. Yeast 31:333–341
Xiong X, Zhang X, Wu Z, Wang Z (2014) Optimal selection of agricultural products to inhibit citrinin production during submerged culture of Monascus anka. Biotechnol Bioprocess Eng 19:1005–1013
Xiong X, Zhang X, Wu Z, Wang Z (2015) Coupled aminophilic reaction and directed metabolic channeling to red Monascus pigments by extractive fermentation in nonionic surfactant micelle aqueous solution. Process Biochem 50(2):180–187
Authors’ contributions
FL planned and carried out the experiments, analyzed the data and wrote the manuscript; YH assisted to carry out experiments; XZ reviewed the manuscript; ZW participated in the data analysis and finalized the manuscript. All authors read and approved the final manuscript.
Acknowledgements
This study was funded by National Natural Science Foundation of China (No: 21276155).
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
We conducted experiments and data generated. All data are shown in figures and tables.
Ethics approval and consent to participate
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Funding
This study was funded by National Natural Science Foundation of China (No: 21276155).
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lu, F., Huang, Y., Zhang, X. et al. Biocatalytic activity of Monascus mycelia depending on physiology and high sensitivity to product concentration. AMB Expr 7, 88 (2017). https://doi.org/10.1186/s13568-017-0391-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s13568-017-0391-4