Abstract
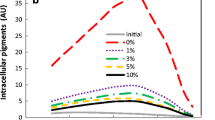
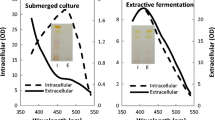
“Milking processing” describes the cultivation of microalgae in a water-organic solvent two-phase system that consists of simultaneous fermentation and secretion of intracellular product. It is usually limited by the conflict between the biocompatibility of the organic solvent to the microorganisms and the ability of the organic solvent to secret intracellular product into its extracellular broth. In the present work, submerged cultivation of Monascus in the nonionic surfactant Triton X-100 micelle aqueous solution for pigment production is exploited, in which the fungus Monascus remains actively growing. Permeabilization of intracellular pigments across the cell membrane and extraction of the pigments to the nonionic surfactant micelles of its fermentation broth occur simultaneously. “Milking” the intracellular pigments in the submerged cultivation of Monascus is a perstraction process. The perstractive fermentation of intracellular pigments has the advantage of submerged cultivation by secretion of the intracellular pigments to its extracellular broth and the benefit of extractive microbial fermentation by solubilizing the pigments into nonionic surfactant micelles. It is shown as the marked increase of the extracellular pigment concentration by the submerged cultivation of Monascus in the nonionic surfactant Triton X-100 micelle solution.







Similar content being viewed by others
References
Babitha S, Soccol CR, Pandey A (2007) Effect of stress on growth, pigment production and morphology of Monascus sp. in solid cultures. J Basic Microbiol 47:118–126
Cull SG, Holbrey JD, Vargas-Mora V, Seddon KR, Lye GJ (2000) Room-temperature ionic liquids as replacements for organic solvents in multiphase bioprocess operations. Biotechnol Bioeng 69(2):227–233
Daugulis AJ (2001) Two-phase partitioning bioreactors: a new technology platform for destroying xenobiotics. Trends Biotechnol 19(11):457–462
Evanst PJ, Wang HY (1984) Pigment production from immobilized Monascus sp. utilizing polymeric resin adsorption. Appl Environ Microbiol 47(6):1323–1326
Fernandes P, Prazeres DMF, Cabral JMS (2003) Membrane-assisted extractive bioconversions. Adv Biochem Eng/Biotechnol 80:115–148
Hajjaj H, Blanc P, Groussac E, Uribelarrea J-L, Goma G, Loubiere P (2000) Kinetic analysis of red pigment and citrinin production by Monascus ruber as a function of organic acid accumulation. Enzyme Microb Technol 27:619–625
Heipieper HJ, Neumann G, Cornelissen S, Meinhardt F (2007) Solvent-tolerant bacteria for biotransformation in two-phase fermentation systems. Appl Microbiol Biotechnol 74:961–973
Hejazi MA, Wijffels RH (2004) Milking of microalgae. Trends Biotechnol 22(4):189–194
Hejazi M, de Lamarliere C, Rocha JMS, Vermue M, Tramper J, Wijffels RH (2002) Selective extraction of carotenoids from the microalga Dunaliella salina with retention of viability. Biotechnol Bioeng 79(1):29–36
Hejazi MA, Holwerda E, Wijffels RH (2004) Milking microalga Dunaliella salina for β-carotene production in two-phase bioreactors. Biotechnol Bioeng 85(5):475–481
Hinze WL, Pramauro E (1993) A critical review of surfactant-mediated phase separations (cloud-point extraction): theory and applications. Criti Rev Anal Chem 24(2):133–177
Hsu F-L, Wang P-M, Lu S-Y, Wu W-T (2002) A combined solid-state and submerged cultivation integrated with adsorptive product extraction for production of Monascus red pigments. Bioprocess Biosyst Eng 25:165–168
Jung H, Kim C, Kim K, Shin CS (2003) Color characteristics of Monascus pigments derived by fermentation with various amino acids. J Agric Food Chem 51:1302–1306
Juzlova P, Martinkova L, Kren V (1996) Secondary metabolites of the fungus Monascus: review. J Ind Microbiol 16:163–170
Kang CD, Sim SJ (2008) Direct extraction of astaxanthin from Haematococcus culture using vegetable oils. Biotechnol Lett 30:441–444
Kleinegris DMM, Janssen M, Brandenburg WA, Wijffels RH (2011) Two-phase systems: potential for in situ extraction of microalgal products. Biotechnol Adv 29:502–507
Krairak S, Yamamura K, Irie R, Nakajima M, Shimizu H, Chim-Anage P, Yongsmith B, Shioya S (2000) Maximizing yellow pigment production in fed-batch culture of Monascus sp. J Biosci Bioeng 90(4):363–367
Kumn I (1980) Alcoholic fermentation in an aqueous two-phase system. Biotechnol Bioeng 12:2393–2398
Laouar L, Lowe KC, Muligan BJ (1996) Yeast responses to nonionic surfactants. Enzyme Microb Technol 18:433–438
Lin TF, Demain AL (1993) Resting cell studies on formation of water-soluble red pigments by Monascus sp. J Ind Microbiol 12:361–367
Lin TF, Demain AL (1995) Negative effect of ammonium nitrate as nitrogen source on the production of water-soluble red pigments by Monascus sp. Appl Microbial Biotechnol 43:701–705
Lin TF, Yakushijin K, Buchi GH, Demain AL (1992) Formation of water-soluble Monascus red pigments by biological and semi-synthetic processes. J Ind Microbiol 9:173–179
Lin Y-L, Wang T-H, Lee M-H, Su N-W (2008) Biologically active components and nutraceuticals in the Monascus-fermented rice: a review. Appl Microbiol Biotechnol 77:965–973
Liu J, Ren Y, Yao S (2010) Repeated-batch cultivation of encapsulated Monascus purpureus by polyelectrolyte complex for natural pigment production. Chin J Chem Eng 18(6):1013–1017
Malaviya A, Gomes J (2008) Enhanced biotransformation of sitosterol to androstenedione by Mycobacterium sp. using cell wall permeabilizing antibiotics. J Ind Microbiol Biotechnol 35:1235–1239
Malinowski JJ (2001) Two-phase partitioning bioreactors in fermentation technology. Biotechnol Adv 19:525–538
Mapari SAS, Thrane U, Meyer AS (2010) Fungal polyketide azaphilone pigments as future natural food colorants? Trends Biotechnol 28:300–307
Matsumura M, Markl H (1986) Elimination of ethanol inhibition by perstraction. Biotechnol Bioeng 28(4):534–541
Miozzari GF, Niederberger P, Hotter R (1978) Permeabilization of microorganisms by Triton X-100. Anal Chem 90:220–233
Qureshi N, Maddox IS (2005) Reduction in butanol inhibition by perstraction: utilization of concentrated lactose/whey permeate by Clostridium acetobutylicum to enhance butanol fermentation economics. Food Bioprod Process 83(1):43–52
Raya SK, Sawanta SB, Joshia JB, Pangarkar VG (1997) Perstraction of phenolic compounds from aqueous solution using a nonporous membrane. Separ Sci Technol 32(6):2669–2683
Shin CS, Kim HJ, Kim MJ, Ju JY (1998) Morphological change and enhanced pigment production of Monascus when cocultured with Saccharomyces cerevisiae or Aspergillus oryzae. Biotechnol Bioeng 59(5):576–581
Straathof AJJ (2003) Auxiliary phase guidelines for microbial biotransformations of toxic substrate into toxic product. Biotechnol Prog 19:755–762
Wang Z, Dai Z (2010) Extractive microbial fermentation in cloud point system. Enzyme Microb Technol 46:407–418
Wang Z, Zhao F, Hao X, Chen D, Li D (2004) Microbial transformation of hydrophobic compound in cloud point system. J Mol Catal B 27:147–153
Wei G, Li Y, Du G, Chen J (2003) Effect of surfactants on extracellular accumulation of glutathione by Saccharomyces cerevisiae. Process Biochem 38:1133–1138
Zhang F, Cheng L-H, Xu X-H, Zhang L, Chen H-L (2011) Screening of biocompatible organic solvents for enhancement of lipid milking from Nannochloropsis sp. Process Biochem 46:1934–1941
Zhao F, Yu J (2001) l-asparaginase released from Escherichia coli cells with K2HPO4 and 2 % (w/v) Triton X-100. Biotechnol Prog 17:490–494
Acknowledgment
The financial support from the National Natural Science Foundation of China (No: 21076123) is acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hu, Z., Zhang, X., Wu, Z. et al. Perstraction of intracellular pigments by submerged cultivation of Monascus in nonionic surfactant micelle aqueous solution. Appl Microbiol Biotechnol 94, 81–89 (2012). https://doi.org/10.1007/s00253-011-3851-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-011-3851-9