Abstract
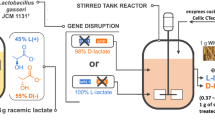
To develop cost-effective systems for d-lactate production, here, the effect of high-cell density cultivation of metabolically engineered Lactobacillus plantarum on d-lactate production was evaluated. A xylose-assimilating strain of L. plantarum was anaerobically cultured with mixed sugars (glucose and xylose) as substrates. Compared to undiluted nutrient-rich de Man, Rogosa, and Sharpe (MRS) medium, d-lactate production by cultivating in 10-fold diluted MRS (0.1 MRS) medium or normal saline solution was 89.7 and 81.3 %, respectively. Notably, the xylose consumption rate was comparable in the three cultures, whereas the glucose consumption rate decreased by 18.3 and 26.1 % in 0.1 MRS medium and normal saline solution, respectively, resulting in a reduction of the d-lactate production rate. The d-lactate productivity in high-cell density cultivation was proportional to the initial cell concentrations. The use of a two-step cultivation process involving growing and resting cells in a single bioreactor revealed that the ratio of the glucose and xylose consumption rates (based on grams consumed) in resting cell conditions was 1.88, whereas that in growing conditions was 2.58. Cultivation of L. plantarum in growing conditions for 24 h produced 73.2 g/l d-lactate with the yield of 0.90 g/g, whereas cells cultivation under resting cell conditions in a saline solution for 24 h produced 68.7 g/l d-lactate with the yield of 0.93 g/g. In total, 141.9 g/l d-lactate was produced after 48 h cultivation, a value that represents the highest reported concentration of d-lactate produced from mixed sugars to date. Our findings contribute to the cost-effective, large-scale production of d-lactate.





Similar content being viewed by others
References
Abdel-Rahman MA, Tashiro Y, Sonomoto K (2013) Recent advances in lactic acid production by microbial fermentation processes. Biotechnol Adv 31:877–902
Adsul MG, Singhvi MS, Gaikaiwari SA, Gokhale DV (2011) Development of biocatalysts for production of commodity chemicals from lignocellulosic biomass. Bioresour Technol 102:4304–4312
Aristidou A, Penttilä M (2000) Metabolic engineering applications to renewable resource utilization. Curr Opin Biotechnol 11:187–198
Bae D, Liu C, Zhang T, Jones M, Peterson SN, Wang C (2012) Global gene expression of Listeria monocytogenes to salt stress. J Food Prot 75:906–912
Bustos G, Moldes AB, Cruz JM, Domínguez JM (2005) Influence of the metabolism pathway on lactic acid production from hemi-cellulosic trimming vine shoots hydrolyzates using Lactobacillus pentosus. Biotechnol Prog 21:793–798
Chaillou S, Bor YC, Batt CA, Postma PW, Pouwels PH (1998) Molecular cloning and functional expression in Lactobacillus plantarum 80 of xylT, encoding the d-xylose-H+ symporter of Lactobacillus brevis. Appl Environ Microbiol 64:4720–4728
Deutscher J, Francke C, Postma PW (2006) How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev 70:939–1031
Doleyres Y, Beck P, Vollenweider S, Lacroix C (2005) Production of 3-hydroxypropionaldehyde using a two-step process with Lactobacillus reuteri. Appl Microbiol Biotechnol 68:467–474
Fukushima K, Chang YH, Kimura Y (2007) Enhanced stereocomplex formation of poly (l-lactic acid) and poly (d-lactic acid) in the presence of stereoblock poly(lactic acid). Macromol Biosci 7:829–835
Gonçalves LM, Xavier AM, Almeida J, Carrondo MJ (1991) Concomitant substrate and product inhibition kinetics in lactic acid production. Enzym Microb Technol 13:314–319
Görke B, Stülke J (2008) Carbon catabolite repression in bacteria: many ways to make the most out of nutrients. Nat Rev Microbiol 6:613–624
Gourdon P, Raherimandimby M, Dominguez H, Cocaign-Bousquet M, Lindley ND (2003) Osmotic stress, glucose transport capacity and consequences for glutamate overproduction in Corynebacterium glutamicum. J Biotechnol 104:77–85
Helanto M, Kiviharju K, Leisola M, Nyyssölä A (2007) Metabolic engineering of Lactobacillus plantarum for production of l-ribulose. Appl Environ Microbiol 73:7083–7091
Hujanen M, Linko S, Linko YY, Leisola M (2001) Optimisation of media and cultivation conditions for L(+)(S)-lactic acid production by Lactobacillus casei NRRL B-441. Appl Microbiol Biotechnol 56:126–130
Ikada Y, Jamshidi K, Tsuji H, Hyon S (1987) Stereocomplex formation between enantiomeric poly lactides. Macromolecules 20:904–906
Karjomaa S, Suortti T, Lempiainen R, Selin J, Itavaara M (1998) Microbial degradation of poly-(l-lactic acid) oligomers. Polym Degrad Stab 59:333–336
Ladero V, Ramos A, Wiersma A, Goffin P, Schanck A, Kleerebezem M, Hugenholtz J, Smid EJ, Hols P (2007) High-level production of the low-calorie sugar sorbitol by Lactobacillus plantarum through metabolic engineering. Appl Environ Microbiol 73:1864–1872
Lüthi-Peng Q, Schärer S, Puhan Z (2002) Production and stability of 3-hydroxypropionaldehyde in Lactobacillus reuteri. Appl Microbiol Biotechnol 60:73–80
Okano K, Yoshida S, Tanaka T, Ogino C, Fukuda H, Kondo A (2009a) Homo-d-lactic acid fermentation from arabinose by redirection of the phosphoketolase pathway to the pentose phosphate pathway in l-lactate dehydrogenase gene-deficient Lactobacillus plantarum. Appl Environ Microbiol 75:5175–5178
Okano K, Yoshida S, Yamada R, Tanaka T, Ogino C, Fukuda H, Kondo A (2009b) Improved production of homo-d-lactic acid via xylose fermentation by introduction of xylose assimilation genes and redirection of the phosphoketolase pathway to the pentose phosphate pathway in l-lactate dehydrogenase gene-deficient Lactobacillus plantarum. Appl Environ Microbiol 75:7858–7861
Tejayadi S, Cheryan M (1995) Lactic acid from cheese whey permeate. Productivity and economics of a continuous membrane bioreactor. Appl Microbiol Biotechnol 43:242–248
Titgemeyer F, Hillen W (2002) Global control of sugar metabolism: a gram-positive solution. Antonie Van Leeuwenhoek 82:59–71
Yamada R, Hasunuma T, Kondo A (2013) Endowing non-cellulolytic microorganisms with cellulolytic activity aiming for consolidated bioprocessing. Biotechnol Adv 31:754–763
Yoshida S, Okano K, Tanaka T, Ogino C, Kondo A (2011) Homo-d-lactic acid production from mixed sugars using xylose-assimilating operon-integrated Lactobacillus plantarum. Appl Microbiol Biotechnol 92:67–76
Zhang Y, Song L, Gao Q, Yu SM, Li L, Gao NF (2012) The two-step biotransformation of monosodium glutamate to GABA by Lactobacillus brevis growing and resting cells. Appl Microbiol Biotechnol 94:1619–1627
Acknowledgments
We are grateful to Yumiko Yoshihara and Shoko Miyazaki for technical assistance. This work was supported by Special Coordination Funds for Promoting Science and Technology, Creation of Innovation Centers for Advanced Interdisciplinary Research Areas (Innovative Bioproduction, Kobe).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tsuge, Y., Kawaguchi, H., Sasaki, K. et al. Two-step production of d-lactate from mixed sugars by growing and resting cells of metabolically engineered Lactobacillus plantarum . Appl Microbiol Biotechnol 98, 4911–4918 (2014). https://doi.org/10.1007/s00253-014-5594-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-014-5594-x