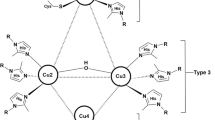
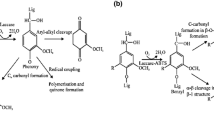
Abstract
The aim of this review is to determine the trends of state-of-art of laccase sources, properties, structure and recent application of fungal laccase in various fields. Laccases are biotechnologically important multi copper proteins that have broad substrate specificity towards aromatic and non-aromatic compounds. Fungi are the major laccase producers especially ascomycetes, deuteromycetes and basidiomycetes, and laccases have an average molecular weight between 50 and 130 kDa. Fungal laccases are used in biotechnological applications for preparation of anticancerous and anti-oxidant hormonal drugs, stabilization of food products, and laccase application is also extended to preparation of biosensors, DNA labeling, immunochemical assay, bioorganic compound synthesis etc. The environmental application of laccase is for biodegradation of dyes, phenols and pesticides, and the mechanism of degradation has been briefly explained. Analysis of the biodegraded dye sample by FT-IR and Mass (ESI)-spectrum has been discussed in a detailed manner. Modeling kinetics has been discussed with respect to degradation of wastes in order to understand the factors involved in the degradation process.
Similar content being viewed by others
References
Filazzola, M. T., F. Sannino, M. A. Rao, and L. Giangreda (1999) Bioremediation and biodegradation: Effect of various pollutants and soil-like constituents on laccase from Cerrena unicolor. J. Environ. Quality 28: 1929–1938.
Jolivalt, C., A. Raynal, E. Caminade, B. Kokel, F. Le Goffic, and C. Mougin (1999) Transformation of N’,N’-dimethyl-N-(hydroxyphenyl) ureas by laccase from the White-rot fungus Trametes versicolor. Appl. Microb. Biotechnol. 51: 676–681.
Arora, D. S. and R. Sharma (2010) Ligninolytic fungal laccase and their biotechnological applications. Appl. Biochem. Biotech. 160: 1760–1788.
Yaropolov, A. I., O. V. Skorobogatko, S. S. Vartanov, and S. D. Varfolomeyev (1994) Laccase: Properties, catalytic mechanism and applicability. Appl. Biochem. Biotech. 49: 257–280.
Call, H. P. and I. Mucke (1997) History, overview and applications of mediated lignolytic systems, especially laccase-mediator-systems (Lignozym®-process). J. Biotech. 53: 163–202.
Mayer, A. M. and R. C. Staples (2002) Laccase: New functions for an old enzyme. Phytochem. 60: 551–565.
Leonowicz, A., N. S. Cho, J. Luterek, A. Wilkolazka, M. Wotjas-Wasilewska, A. Matuszewska, M. Hofrichter, D. Wesenberg, and J. Rogalski (2001) Fungal laccase: Properties and activity on lignin. J. Basic Microb. 41: 185–227.
Thurston, C. (1994) The structure and function of fungal laccases. Microbiol. 140: 19–26.
Mukhopadhyay, M. and R. Banerjee (2014) Purification and biochemical characterization of a newly produced yellow laccase from Lentinus squarrosulus MR13. 3 Biotech 5: 227–236.
Germann, U. A., G. Muller, P. E. Hunziker, and K. Lerch (1988) Characterization of two allelic forms of Neurospora crassa laccase. The J. Biolog. Chem. 263: 885–896.
Mayer, A. M. (1987) Polyphenol oxidases in plant: Recent progress. Phytochem. 26: 11–20.
Walkerl, J. W. R. and R. F. McCallion (1980) The selective inhibition of ortho and paradiphenol oxidases. Phytochem. 19: 373–373.
Shleev, S. V., O. V. Morozova, O. V. Nikitina, E. S. Gorshina, T. V. Rusinova, V. A. Serezhenkov, D. S. Burbaev, I. G. Gazaryan, and A. I. Yaropolov (2004) Comparison of Physico-chemical characteristics of four laccases from different basidiomycetes. Biochem. 86: 693–703.
Palmer, A. E., D. W. Randall, F. Xu, and E. I. Solomon (1999) Spectroscopic studies and electronic structure description of the high potential type 1 copper site in fungal laccase: Insight into the effect of the axial ligand. J. Am. Chem. Soc. 121: 7138–7149.
Xu, F (1999) In: M. C. Flickinger and S. W. Drew (eds.). Encyclopedia of bioprocess technology: Fermentation, biocatalysis, bioseparation. John Wiley & Sons Inc., NY, USA. 1545–1554.
Baldrian, P. (2006) Fungal laccases occurrence and properties. FEMS Microb. Rev. 30: 215–242.
Benfield, G., S. M. Bocks, K. Bromley, and B. R. Brown (1964) Studies in fungal and plant laccases. Phytochem. 3: 79–88.
Prinz, A., K. Koch, A. Gorak, and T. Zeiner (2014) Multi-stage laccase extraction and separation using aqueous two-phase systems: Experiment and model. Proc. Biochem. 49: 1020–1031.
Rogalski, J. and A. Leonowicz (2004) “Laccase”. pp: 533–542. In: A. Pandey (ed.). Concise encyclopedia of bioresource technology. Food product press, Haworth Reference press, NY, USA.
Vasdev, K., S. Dhawan, R. K. Kapoor, and R. C. Kuhad (2005) Biochemical characterization and molecular evidence of a laccase from the birds nest fungus Cyathus bulleri. Fungal Gen. Biol. 42: 684–693.
Morozova, O. V., G. P. Shumakovich, M. A. Gorbacheva, S. V. Shleev, and A. I. Yaropolov (2007) Laccase–Mediator systems and their applications: A review. J. Biochem. (Moscow) 72: 1136–1150.
Zeng, X., Y. Cai, X. Liao, X. Zenga, S. Luo, and D. Zhang (2012) Anthraquinone dye assisted the decolourization of azo dyes by a novel Trametes trogii laccase. Proc. Biochem. 47: 160–163.
Stoilova, I., A. Krastanova, and V. Stanchev (2010) Properties of crude laccase from Trametes versicolor produced by solid substrate fermentation. Advan. Bioscie. Biotech. 1: 208–215.
Solomon, E. I., U. M. Sundaram, and T. E. Machonkin (1996) Multicopper oxidases and oxygenases. Chem. Rev. 96: 2563–2606.
Bourbonnais, R., M. G. Paice, I. D. Reid, P. Lanthier, and M. Yaguchi (1995) Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2’,2’-azinobis (3-ethylbenzthiazoline-6-sulphonate) in kraft pulp depolymerisation. Appl. Environ. Microb. 61: 1876–1880.
Leontievsky, A. A., T. Vares, P. Lankinen, J. K. Shergill, N. N. Pozdnyakova, N. M. Myasoedova, N. Kalkkinen, L. A. Golovleva, R. Cammack, C. F. Thurston, and A. Hatakka (1997) Blue and yellow laccases of ligninolytic fungi. FEMS Microb. Lett. 156: 9–14.
Mendoza, L (2011) Laccases from new fungal sources and new promising applications. Ph. D. Thesis. Department of Biotechnology, Lund University, Sweden.
Claus, H. (2004) Laccases: Structure, reactions, distribution. Micron. 35: 93–96.
Claus, H. (2003) Laccases and their occurrence in prokaryotes. Arch. Microb. 179: 145–150.
Givaudan, A., A. Effose, D. Faure, P. Potier, M. L. Bouillant, and R. Bally (1993) Polyphenol oxidase in Azospirillum lipoferum isolated from rice rhizosphere: Evidence for laccase activity in non-motile strains of Azospirillum lipoferum. FEMS Microb. Lett. 108: 205–210.
Alexandre, G. and I. B. Zhulin (2000) Laccases are wide spread in bacteria. Trends Biotech. 18: 41–42.
Galai, S., Y. Touhammi, and M. N. Marzouki (2012) Response surface methodology applied to laccase activities exhibited by Stenotrophomonas Maltophilia AAP56 in different growth conditions. Bio Res. 7: 706–726.
Octavio, L. C., P. P. M. C. Irma, B. R. J. Ricardo, and V. O. Francisco (2006) Laccases. Adv. Agricult. Food Biotech. 323–340.
Assavanig, A., B. Amornkitticharorn, N. Ekpaisal, V. Meevootisom, and T. W. Flegel (1992) Isolation, characterization and function of laccase from Trichoderma. Appl. Microb. Biotech. 38: 198–202.
Aisemberg, G. O., E. Grorewold, G. E. Taccioli, and N. Judewicz (1989) A major transcript in the response of Neurospora crassa to protein synthesis inhibition by cycloheximide. Exp. Mycol. 13: 121–128.
Levin, L. and F. Forchiassin (2001) Ligninolytic enzymes of the white-rot basidiomycete Trametes trogii. Acta Biotech. 21: 179–186.
Sadhasivam, S., S. Savitha, K. Swaminathan, and F.-H. Lin (2008) Production, purification and characterization of midredox potential laccase from a newly isolated Trichoderma harzianum WL1. Proc. Biochem. 43: 736–742.
Thakker, G. D., C. S. Evans, and K. K. Rao (1992) Purification and characterization of laccase from Monocillium indicum. Appl. Microb. Biotech. 37: 321–323.
Revankar, M. S. and S. S. Lele (2006) Enhanced production of laccase using a new isolate of white rot fungus WR-1. Proc. Biochem. 41: 581–588.
Dittmer, N. T., R. J. Suderman, H. Jiang, Y. C. Zhu, M. J. Gorman, K. J. Kramer, and M. R. Kanost (2004) Characterization of cDNA encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae. Insect Biochem. Mol. Biol. 34: 29–41.
Sharma, K. K. and R. C. Kuhad (2008) Laccase: Enzyme revisited and function refined. Ind. J. Microb. 48: 309–316.
Gochev, V. K. and A. I. Krastanov (2007) Isolation of laccase producing Trichoderma spp. Bulgarian J. Agricul. Sci. 13: 171–176.
Chefetz, B., Y. Chen, and Y. Hadar (1998) Purification and characterization of laccase from Chaemotomium thermophilum and its role in humification. Appl. Environ. Microb. 64: 3175–3179.
Levin, L., E. Melignani, and A. M. Ramos (2010) Effect of nitrogen sources and vitamins on lignolytic enzyme production by some white-rot fungi dye decolorization by selected culture filtrates. Biores. Tech. 101: 4554–4563.
Galhaup, C., H. Wagner, K. D. Kulbe, and D. Haltrich (2001) Efficient production of laccase activity by the white-rot fungus Trametes pubescens. Proceedings of the 8th international conference on biotechnology in the pulp and paper industry. June 4–8, Helsinki, Finland.
Mishra, A., S. Kumar, and A. K. Pandey (2011) Laccase production and simultaneous decolourization of synthetic dyes in unique expensive medium by new isolates of white-rot fungus. Internat. Biodeter. Biodeg. 65: 487–493.
Wang, Z.-X., Y.-J. Cai, X.-R. Liao, G.-J. Tao, Y.-Y. Li, F. Zhang, and D.-B. Zhang (2010) Purification and characterization of two thermostable laccases with high cold adapted characteristics from Pycnoporus sp. SYBC-L1. Proc. Biochem. 45: 1720–1729.
Liu, J., Y. Cai, X. Liao, Q. Huang, Z. Hao, M. Hu, D. Zhang, and Z. Li (2013) Efficiency of laccase production in a 65 L airlift reactor for potential green industrial and environmental application. J. Clean. Prod. 39: 154–160.
Patel, H., S. Gupte, M. Gahlout, and A. Gupte (2014) Purification and characterization of an extracellular laccase from solidstate culture of Pleurotus ostreatus HP-1. 3 Biotech 4: 77–84.
Chiranjeevi, P. V., M. R. Pandian, and T. Sathish (2014) Enhancement of laccase production from Pleurotus ostreatus PVCRSP-7 by altering the nutritional conditions using response surface methodology. BioRes. 9: 4212–4225.
Jordaan, J., B. I. Pletschke, and W. D. Leukes (2004) Purification and partial characterization of a thermostable laccase from an unidentified basidiomycetes. Enz. Microb. Technol. 34: 635–641.
Fonseca, M. I., E. Shimizu, P. D. Zapata, and L. L. Villalba (2010) Copper inducing effect on laccase production of white rot fungi native from Misiones (Argentina). Enz. Microb. Technol. 46: 534–539.
Boron, F. and O. Yesilada (2011) Enhanced production of laccase by fungi under solid substrate fermentation condition. Biores. 6: 4404–4416.
Mohammadian, M., M. F. Roudsari, N. Mollania, A. B. Dalfard, and K. Khajeh (2010) Enhanced expression of a recombinant bacterial laccase at low temperature and microaerobic conditions: Purification and biochemical characterization. J. Ind. Microb. Biotech. 5: 41–45.
Mohite, B. V., R. E. Jalgaonwala, S. Pawar, and A. Morankar (2010) Isolation and characterization of phenol degrading bacteria from oil contaminated soil. Innov. Roman. Food Biotech. 7: 61–65.
Kunamneni, A., S. Camarero, C. Garcia-Burgos, F. J. Plou, A. Ballesteros, and M. Alcalde (2008) Engineering and applications of fungal laccases for organic synthesis. Microb. Cell Fact. 7: 1–17.
Ardhaoui, M., S. Bhatt, M. Zheng, D. Dowling, C. Jolivalt, and F. A. Khonsari (2013) Biosensor based on laccase immobilized on plasma polymerized allylamine/ carbon electrode. Mat. Sci. Engin. C 33: 3197–3205.
Chhaya, U. and A. Gupte (2013) Possible role of laccase from Fusarium incarnatum UC-14 in bioremediation of Bisphenol A using reverse micelles system. J. Hazard. Mat. 254–255: 149–156.
Faramarzi, M. A. and H. Forootanfar (2011) Biosynthesis and characterization of gold nanoparticles produced by laccase from Paraconiothyrium variabile. Colloids Surf. B: Biointerfaces 87: 23–27.
Wong, K.-S., Q. Huang, C.-H. Au, J. Wang, and H.-S. Kwan (2012) Biodegradation of dyes and polyaromatic hydrocarbons by two allelic forms of Lentinula edodes laccase expressed from Pichia pastoris. Biores. Techn. 104: 157–164.
Lee, K. M., D. Kalyani, M. K. Tiwari, T. S. Kim, S. S. Dhiman, J. K. Lee, and I. W. Kima (2012) Enhanced enzymatic hydrolysis of rice straw by removal of Phenolic compounds using a novel laccase from yeast Yarrowia lipolytica. Biores. Technol. 123: 636–645.
Brondani, D., B. D. Souza, B. S. Souza, A. Neves, and L. C. Vieira (2013) PEI-coated gold nanoparticles decorated with laccase: A new platform for direct electrochemistry of enzymes and biosensing applications. Biosen. Bioelect. 42: 242–247.
Dai, Z., M. Q. Guo, X. J. Wang, H. F. Wang, and W. Y. Chen (2014) Development of amperometric laccase biosensor through immobilizing enzyme in magnesium-containing mesoporous silica sieve (Mg-MCM-41)/polyvinyl alcohol matrix. J. Nanomat. (Article ID 458245) 8.
Roy, J. J., T. E. Abraham, K. S. Abhijith, P. V. Kumar, and M. S. Thakur (2005) Biosensor for the determination of phenols based on cross-linked enzyme crystals (CLEC) of laccase. Biosen. Bioelect. 21: 206–211.
Luo, H., S. Jin, P. H. Fallgren, H. J. Park, and P. A. Johnson (2010) A novel laccase-catalyzed cathode for microbial fuel cells. Chem. Engin. J. 165: 524–528.
Chawla, S., R. Rawal, Shabnam, R. C. Kuhad, and C. S. Pundir (2011) An amperometric polyphenol biosensor based on laccase immobilized on epoxy resin membrane. Anal. Meth. 3: 709–714.
Morozova, O. V., G. P. Shumakovich, S. V. Shleev, and Y. I. Yaropolov, (2007) Laccase–Mediator systems and their applications: A review. Appl. Biochem. Microb. 43: 523–535.
Rosana, C., Y. Minussi, G. M. Pastore, and N. Durany (2002) Potential applications of laccase in the food industry. Trends Food Sci. Techn. 13: 205–216.
Minussi, R.C., G. M. Pastore, and N. Duran (2002) Potential applications of laccase in the food industry. Trends Food Sci. Techn. 13: 205–216.
Couto, S. R. and J. L. T. Herrera (2006) Industrial and biotechnological applications of laccases: A review. Biotech. Adv. 24: 500–513.
Rocasalbas, G., A. Francesko, S. Tourino, X. Fernandez-Francos, G. M. Guebitzc, and T. Tzanov (2013) Laccase-assisted formation of bioactive chitosan/gelatin hydrogel stabilized with plant polyphenols. Carbohyd. Polym. 92: 989–996.
Bourton, S. (2003) Laccases and phenol oxidases in organic synthesis. Curr. Organic Chem. 7: 1317–1331.
Pilz, R., E. Hammer, F. Schauer, and U. Krag (2003) Laccase catalysed synthesis of coupling products of Phenolic substrates in different reactors. Appl. Microb. Biotech. 60: 708–712.
Kurisawa, M., J. E. Chung, H. Uyama, and S. Kobayashi (2003) Laccase-catalyzed synthesis and antioxidant property of poly (catechin). Macromol. Biosci. 3: 758–764.
Forgacsa, E., T. Cserhatia, and G. Ores (2004) Removal of synthetic dyes from wastewaters: A review. Environ. Internat. 30: 953–971.
Erkurt, H. A (2010) Biodegradation of Azo Dyes. pp. 1–37. In: D. Barcelo and A. G. Kostianoy (eds.). The Hand Book of Environmental Chemistry. Springer Verlag Berlin Heidelberg.
Vandevivere, P. C., R. Bianchi, and W. S. Verstraete (1998) Treatment and reuse of wastewater from the textile wet-processing industry: Review of emerging technologies. J. Chem. Techn. Biotech. 72: 289–302.
Jaiswal, N., V. P. Pandey, and U. N. Dwivedi (2014) Purification of a thermostable laccase from Leucaena leucocephala using a copper alginate entrapment approach and the application of the laccase in dye decolorisation. Proc. Biochem. 49: 1196–1204.
Wesenberg, D., F. Buchon, and S. N. Agathos (2002) Degradation of dye-containing textile effluent by the agaric white-rot fungus Clitocybula dusenii. Biotech. Lett. 24: 989–993.
Bisschops, I. and H. Spanjers (2003) Literature review on textile waste water characterization. Environ. Techn. 24: 1399–1411.
Pereira, L., A. V. Coelho, C. A. Viegas, M. M. Santos, M. P. Robalo, and L. O. Martins (2009) Enzymatic biotransformation of the azo dye Sudan orange G with bacterial cot A-laccase. J. Biotech. 139: 68–77.
Wesenberg, D., I. Kyriakides, and S. N. Agathos (2003) Whiterot fungi and their enzymes for the treatment of industrial dye effluents. Biotech. Advan. 22: 161–187.
Eichlerova, I., L. Homolka, L. Lisa, and F. Nerud (2005) Orange G and Remazol Brilliant Blue R decolorisation by white rot fungi Dichomitus squalens, Ischnoderma resinosum and Pleurotus calyptratus. Chemosphere 60: 398–404.
Wong, Y. and J. Yu (1999) Laccase-catalyzed decolorisation of synthetic dyes. Water Res. 33: 3512–3520.
Yesilada, O., S. C. Yildirim, E. Birhanli, E. Apohan, D. Asma, and F. Kuru (2010) The evaluation of pre-grown mycelial pellets in decolorisation of textile dyes during repeated batch process. World J. Microb. Biotech. 26: 33–39.
Mouli, P. C., S. V. Mohan, and S. J. Reddy (2004) Electrochemical processes for the remediation of waste water and contaminated soil: emerging technology. J. Sci. Ind. Res. 63: 11–19.
Ozyurt, M. and H. Atacag (2003) Biodegradation of azo dyes: A review. Fresenius Environ. Bullet. 12: 1294–1302.
Khlifia, R., L. Belbaharia, S. Woodwarda, M. Ellouza, A. Dhouiba, S. Sayadia, and T. Mechichia (2010) Decolourization and detoxification of textile industry waste water by the laccasemediator system. J. Hazard. Mat. 175: 802–80.
Moilanen, U., J. F. Osma, E. Winquist, M. Leisola, and S. R. Couto (2010) Decolorization of simulated textile dye baths by crude laccases from Trametes hirsute and Cerrena unicolor. Eng. Life Sci. 10: 1–6.
Murugesan, K., I. H. Nam, Y. M. Kim, and Y. S. Chang (2007) Decolorization of reactive dyes by a thermostable laccase produced by Ganoderma lucidum in solid culture. Enz. Microb. Technol. 40: 1662–1672.
Rodriguez-Couto, S. (2014) Decolouration of industrial metalcomplex dyes in successive batches by active cultures of Trametes pubescens. Biotech. Reports 4: 156–160.
Casieri, L., G. C. Varese, A. Anastasi, V. Prigione, K. Svobodova, V. Filippelo Marchisio, and C. Novotny (2008) Decolorisation and detoxification of reactive industrial dyes by immobilized fungi Trametes pubescens and Pleurotus ostreatus. Folia Microbiol. 53: 44–52.
Robinson, T., G. McMullan, R. Marchant, and P. Nigam (2001) Remediation of dyes in textile effluent: A critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 77: 247–255.
Chen, C.-Y., Y.-C. Huang, C.-M. Wei, M. Meng, W.-H. Liu, and C.-H. Yang (2013) Properties of the newly isolated extracellular thermo-alkali-stable laccase from thermophilic actinomycetes, Thermobifida fusca and its application in dye intermediates oxi-dation. AMB Exp. 3: 49.
Cristova, R. O., A. P. M. Tavares, J. M. Loureiro, R. A. R. Boaventura, and E. A. Macedo (2008) Optimization of reactive dye degradation by laccase using Box-Bernken design. Environ. Tech. 29: 1357–1364.
Rodriguez, E., M. A. Pickard, and R. Vazquez-Duhalt (1999) Industrial dye decolorization by laccases from ligninolytic fungi. Curr. Microbiol. 38: 27–32.
Lu, L., M. Zhao, B. B. Zhang, S. Y. Yu, X. J. Bian, W. Wang, and Y. Wang (2007) Purification and characterization of laccase from Pycnoporus sanguineus and decolorization of an anthraquinone dye by the enzyme. Appl. Microbiol. Biotechnol. 74: 1232–1239.
Abadulla, E., T. Tzanov, S. Costa, K.-H. Robra, A. Cavaco-Paulo, and G. M. Gu Bitz (2000) Decolorization and detoxification of textile dyes with a laccase from Trametes hirsute. Appl. Environ. Microb. 66: 3357–3362.
Lorenzo, M., D. Moldes, and M. A. Sanroman (2006) Effect of heavy metals on the production of several laccase isoenzymes by Trametes versicolor and on their ability to decolourise dyes. Chemosphere 63: 912–917.
Kanagaraj, J., T. Senthilvelan, and R. C. Panda (2014) Biodegradation of azo dyes in industrial effluent: An eco-friendly way toward green technology. Clean Techn. Environ. Policy 17: 331–341.
Zhang, M., F. Wu, Z. Wei, Y. Xiao, and W. Gong (2006) Characterization and decolorization ability of a laccase from Panus rudis. Enz. Microb. Techn. 39: 92–97.
Champagne, P. P. and J. A. Ramsay (2005) Contribution of manganese peroxidase and laccase to dye decoloration by Trametes versicolor. Appl. Microb. Biotechnol. 69: 276–285.
Ramírez-Cavazosa, L. I., C. Junghannsb, N. Ornelas-Sotoa, D. L. Cardenas-Cháveza, C. Hernández-Lunac, P. Demarched, E. Enaudd, R. Garcia-Moralesa, S. N. Agathosd, and R. Parraa (2014) Purification and characterization of two thermostable laccases from Pycnoporus sanguineus and potential role in degradation of endocrine disrupting chemicals. J. Molecul. Cat. B: Enzym. 108: 32–42.
Pasti-Grigsby, M. B., A. Paszcynski, S. Goszczynski, D. L. Crawford, and R. L. Crawford (1992) Influence of aromatic substitution patterns on azo dye degradability by Streptomyces spp. and Phanerochaete chrysosporium. Appl. Environ. Microbiol. 58: 3605–3613.
Kandelbauer, A., O. Maute, R.W. Kessler, A. Erlacher, and G. M. Gubitz (2004) Study of dye decolorization in an immobilized laccase enzyme–Reactor using online spectroscopy. Biotechnol. Bioeng. 87: 552–563.
Rico, A., J. Rencoret, J. C. D. Rio, A. T. Martínez, and A. Gutierrez (2014) Pretreatment with laccase and a phenolic mediator degrade lignin and enhance saccharification of Eucalyptus feedstock. Biotech. Biofuels 7: 1–14.
Farragher, N. (2013) Degradation of pesticides by the ligninolytic enzyme Laccase-independent project in environmental science. MS Thesis. Sveriges lantbruksuniversitet Swedish University of Agricultural Sciences, Sweden.
Margot, J., C. Bennati-Granier, J. Maillard, P. Blánquez, D. A. Barry, and C. Holliger (2013) Bacterial versus fungal laccase: Potential for micropollutant degradation. AMB Exp. 3: 63.
Tisma, M., P. Nidarsie-Plazl, I. Plazl, B. Zelae, and D. Vasiae-Raekic (2008) Modelling of L-DOPA oxidation catalyzed by laccase. Chem. Biochem. Eng. Q 22: 307–313.
Ursula, F. and R. M. Blanca (2009) Effect of process parameters in laccase mediator system delignification of flax pulp. Part II: Impact on effluents properties. Chem. Eng. J. 152: 330–338.
Casas, N., P. Blanguez, T. Vicent, and M. Sarra (2013) Mathematical model for dye decoloration and laccase production by Trametes versicolor in fluidized bioreactor. Biochem. Eng. J. 1: 45–52.
Tavares, A. P. M., R. O. Cristovao, J. M. Loureiro, R. A. R. Boaventura, and E. A. Macedo (2009) Application of statistical experimental methodology to optimize reactive dye decolourization by commercial laccase. J. Hazard. Mat. 162: 1255–1260.
Paloma, G., S. Gurkan, G. Krist, and W. John (2010) Sensitivity analysis of a kinetic model describing the bi-enzymatic synthesis of Lactobionic acid. Computer-Aided Chem. Eng. Series 28: 1491–1496.
Vats, S., D. P. Maurya, A. Jain, V. Mall, and S. Negi (2013) Mathematical model-based optimization of physico-enzymatic hydrolysis of Pinus roxburghii needles for the production of reducing sugars. Ind. J. Exp. Biol. 51: 944–953.
Tusek, A. J., M. Tisma, V. Bregovi, A. Ptiar, Z. Kurtanjek, and B. Zelic (2013) Enhancement of phenolic compounds oxidation using laccase from Trametes versicolor in a microreactor. Biotech. Bioproc. Eng. 18: 686–696.
Senthilvelan, T., J. Kanagaraj, R. C. Panda, and A. B. Mandal (2014) Biodegradation of phenol by mixed microbial culture: an eco-friendly approach for the pollution reduction. Clean Techn. Environ. Policy 16: 113–126.
Sathishkumar, P., J.-C. Chae, A. R. Unnithan, T. Palvannan, H. Y. Kimb, K. J. Leea, M. Choa, S. Kamala-Kannana, and B.-T. Oha (2012) Laccase-poly (lactic-co-glycolic acid) (PLGA) nanofiber: Highly stable, reusable, and efficacious for the transformation of diclofenac. Enz. Microb. Technol. 51: 113–118.
Jang, M. Y., W. R. Ryu, and M. H. Cho (2002) Laccase production from repeated batch cultures using free mycelia of Trametes sp. Enz. Microb. Technol. 30: 741–746.
Saito, T., P. Hong, K. Kato, M. Okazaki, H. Inagaki, S. Maeda, and Y. Yokogawa (2003) Purification and characterization of an extracellular laccase of a fungus (family Chaetomiaceae) isolated from soil. Enz. Microb. Technol. 33: 520–526.
Ryan, S., W. Schnitzhofer, T. Tzanov, T. Cavaco-Paulo, and G. M. Gubitz (2003) An acid-stable laccase from Sclerotium rolfsii with potential for wool dye decolourization. Enz. Microb. Technol. 33: 766–774.
Baldrian, P. (2004) Increase of laccase activity during interspecific interactions of Whit-rot fungi. FEMS Microb. Ecol. 50: 245–253.
Tanaka, T., S. Eguchi, T. Aoki, T. Tamura, H. Saitoh, M. Taniguchi, H. Ohara, K. Nakanishi, and D. R. Lloyd (2007) Production of laccase by membrane-surface liquid culture of Trametes versicolor using a poly (L-lactic acid) membrane. Biochem. Eng. J. 33: 188–191.
Michniewicz, A., S. Ledakowicz, R. Ullrich, and M. Hofrichter (2008) Kinetics of the enzymatic decolorization of textile dyes by laccase from Cerrena unicolor. Dyes Pigments 77: 295–302.
Niladevi, K. N. and P. Prema (2008) Effect of inducers and process parameters on laccase production by Streptomyces psammoticus and its application in dye decolourization. Bioresour. Technol. 99: 4583–4589.
Ciullini, I., S. Tilli, A. Scozzafava, and F. Briganti (2008) Fungal laccase, cellobiose dehydrogenase, and chemical mediators: Combined actions for the decolorisation of different classes of textile dyes. Bioresour. Technol. 99: 7003–7010.
Rubia T, M. Lucas, and J. Martinez (2008) Controversial role of fungal laccases in decreasing the antibacterial effect of olive mill waste-waters. Biores. Technol. 99: 1018–1025.
Mishra, A. and S. Kumar (2009) Kinetic studies of laccase enzyme of Coriolus versicolor MTCC 138 in an inexpensive culture medium. Biochem. Eng. J. 46: 252–256.
Niebisch, C. H., A. K. Malinowski, R. Schadeck, D. A. Mitchell, V. Kava-Cordeiro, and J. Paba (2010) Decolorisation and biodegradation of reactive blue 220 textile dye by Lentinus crinitus extracellular extract. J. Hazard. Mat. 180: 316–322.
Shah, V., P. Dobiasova, P. Baldrian, F. Nerud, A. Kumar, and S. Seal (2010) Influence of iron and copper nanoparticle powder on the production of lignocellulose degrading enzymes in the fungus Trametes versicolor. J. Hazard. Mat. 178: 1141–1145.
Gassara, F., S. K. Brar, R. D. Tyagi, M. Verma, and R. Y. Surampalli (2010) Screening of agro-industrial wastes to produce ligninolytic enzymes by Phanerochaete chrysosporium. Biochem. Eng. J. 49: 388–394.
Tilli, S., I. Ciullini, A. Scozzafava, and F. Briganti (2011) Differential decolorisation of textile dyes in mixtures and the joint effect of laccase and cellobiose dehydrogenase activities present in extracellular extracts from Funalia trogii. Enz. Microb. Technol. 49: 465–471.
Sridhar, S., V. Chinnathambi, P. Arumugam, and P. K. Suresh (2012) Extracellular laccase enzyme production by Rigidoporous Sp. using the placket-burmann statistical design, spectral analysis and response surface methodology-based optimization of laccase-catalyzed decolorization of Acid blue 113-a prototype textile azo dye. American-Eurasian J. Agric. Environ. Sci. 12: 1617–1624.
Mathur, G., A. Mathur, B. M. Sharma, and R. S. Chauhan (2013) Enhanced production of laccase from Coriolus sp. using Plackett-Burman design. J. Pharm. Res. 6: 151–154.
Jadhav, S. B. and R. S. Singhal (2013) Polysaccharide conjugated laccase for the dye decolorization and reusability of effluent in textile industry. Internat. Biodeteri. Biodegrad. 85: 271–277.
Birhanli, E., S. Erdogan, O. Yesilada, and Y. Onal (2013) Laccase production by newly isolated white-rot fungus Funalia trogii: Effect of immobilization matrix on laccase production. Biochem. Eng. J. 71: 134–139.
Dhakar, K. and A. Pandey (2013) Laccase production from a temperature and pH tolerant fungal strain of Trametes hirsuta (MTCC 11397). Enz. Res. (Article ID 869062).
Manavalan, T., A. Manavalan, K. P. Thangavelu, and K. Heesed (2013) Characterization of optimized production, purification and application of laccase from Ganoderma lucidum. Biochem. Eng. J. 70: 106–114.
Nilsson, I., A. Moller, B. Mattiasson, M. S. T. Rubindamayugi, and U. Welander (2006) Decolorization of synthetic and real textile wastewater by the use of white-rot fungi. Enz. Microb. Technol. 38: 94–100.
Almansa, E., A. Kandelbauer, L. Pereira, A. Cavaco-Paulo, and G. M. Gubitz (2004) Influence of structure on dye degradation with laccase mediator systems. Biocatal. Biotrans. 22: 315–324.
Cristovao, R. O., A. P. M. Tavares, A. S. Ribeiro, J. M. Loureiro, R. A. R. Boaventura, and E. A. Macedo (2007) Kinetic modeling and simulation of laccase catalyzed degradation of reactive textile dyes. Biores. Techn. 99: 4768–4774.
Tauber, M. M., G. M. Gubitz, and A. Rehorek (2008) Degradation of azo dyes by oxidative processes-laccase and ultrasound treatment. Bioresour. Technol. 99: 4213–4220.
Telke, A. A., A. A. Kadam, S. S. Jagatap, J. P. Jadhav, and S. P. Govindwar (2010) Biochemical characterization and potential for textile dye degradation of blue laccase from Aspergillus ochraceus NCIM-1146. Biotechnol. Bioproc. Eng. 15: 696–703.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Senthivelan, T., Kanagaraj, J. & Panda, R.C. Recent trends in fungal laccase for various industrial applications: An eco-friendly approach - A review. Biotechnol Bioproc E 21, 19–38 (2016). https://doi.org/10.1007/s12257-015-0278-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12257-015-0278-7