Abstract
Laccases are multi-copper containing oxidases (EC 1.10.3.2), widely distributed in fungi, higher plants and bacteria. Laccase catalyses the oxidation of phenols, polyphenols and anilines by one-electron abstraction, with the concomitant reduction of oxygen to water in a four-electron transfer process. In the presence of small redox mediators, laccase offers a broader repertory of oxidations including non-phenolic substrates. Hence, fungal laccases are considered as ideal green catalysts of great biotechnological impact due to their few requirements (they only require air, and they produce water as the only by-product) and their broad substrate specificity, including direct bioelectrocatalysis.
Thus, laccases and/or laccase-mediator systems find potential applications in bioremediation, paper pulp bleaching, finishing of textiles, bio-fuel cells and more. Significantly, laccases can be used in organic synthesis, as they can perform exquisite transformations ranging from the oxidation of functional groups to the heteromolecular coupling for production of new antibiotics derivatives, or the catalysis of key steps in the synthesis of complex natural products. In this review, the application of fungal laccases and their engineering by rational design and directed evolution for organic synthesis purposes are discussed.
Similar content being viewed by others
Laccases: general features
Distribution
Laccases (benzenediol:oxygen oxidoreductase, EC 1.10.3.2) belong to the multicopper oxidase family, along with such different proteins as plant ascorbic oxidase, mammalian ceruloplasmin or Fet3p ferroxidase from Saccharomyces cerevisiae, among others [1]. These copper-containing enzymes catalyze the oxidation of various substrates with the simultaneous reduction of molecular oxygen to water [2]. Yoshida first discovered laccases in 1883 after observing that latex from the Japanese lacquer tree (Rhus vernicifera) hardened in the presence of air [3, 4]. This makes laccase as one of the oldest enzymes ever described. Since then, laccase activity has been found in plants, some insects [5, 6], and few bacteria [7]. However, most biotechnologically useful laccases (i.e. those with high redox potentials) are of fungi origin. Over 60 fungal strains belonging to Ascomycetes, Deuteromycetes and especially Basidiomycetes show laccase activities. Among the latter group, white-rot fungi are the highest producers of laccases but also litter-decomposing and ectomycorrhizal fungi secret laccases [8].
Biochemical features
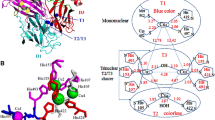
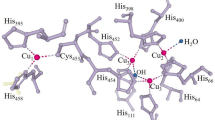
Laccases are typically monomeric extracellular enzymes containing four copper atoms bound to 3 redox sites (T1, T2 and T3). The termed "blue copper" at the T1 site-because of its greenish-blue colour in its oxidized resting state-is responsible of the oxidation of the reducing substrate. The trinuclear cluster (containing one Cu T2 and two Cu T3) is located approx. 12 Å away from the T1 site, and it is the place where molecular oxygen is reduced to water [1]. Laccases catalyze one-electron substrate oxidation coupled to the four-electron reduction of O2. It is assumed that laccases operate as a battery, storing electrons from the four individual oxidation reactions of four molecules of substrate, in order to reduce molecular oxygen to two molecules of water.
Fungal laccases often occur as multiple isoenzymes expressed under different cultivation conditions (e.g. inducible or constitutive isoforms). Most are monomeric proteins, although laccases formed by several units have been also described [9, 10]. They are glycoproteins with average molecular mass of 60–70 kDa, and carbohydrate contents of 10–20% which may contribute to the high stability of laccases. The covalently linked carbohydrate moiety of the enzyme is typically formed by mannose, N-acetylglucosamine and galactose. The amino acid chain contains about 520–550 amino acids including a N-terminal secretion peptide [4].
Biological functions and industrial applications
Biological functions attributed to laccases include spore resistance and pigmentation [11, 12], lignification of plant cell walls [13], lignin biodegradation, humus turnover and detoxification processes [8], virulence factors [12], and copper and iron homeostasis [14].
Laccases exhibit an extraordinary natural substrate range (phenols, polyphenols, anilines, aryl diamines, methoxy-substituted phenols, hydroxyindols, benzenethiols, inorganic/organic metal compounds and many others) which is the major reason for their attractiveness for dozens of biotechnological applications [15–17]. Moreover, in the presence of small molecules, known as redox mediators, laccases enhance their substrate specificity. Indeed, laccase oxidizes the mediator and the generated radical oxidizes the substrate by mechanisms different from the enzymatic one, enabling the oxidative transformation of substrates with high redox potentials-otherwise not oxidized by the enzyme-, Figure 1A. The industrial applicability of laccase may therefore be extended by the use of a laccase-mediator system (LMS). Thus, laccase and LMS find potential application in delignification and biobleaching of pulp [18–21]; treatment of wastewater from industrial plants [22, 23]; enzymatic modification of fibers and dye-bleaching in the textile and dye industries [24, 25]; enzymatic crosslinking of lignin-based materials to produce medium density fiberboards [26]; detoxification of pollutants and bioremediation [27–31]; detoxification of lignocellulose hydrolysates for ethanol production by yeast [32, 33]; enzymatic removal of phenolic compounds in beverages-wine and beer stabilization, fruit juice processing [34–36]-; and construction of biosensors and biofuel cells [37].
In organic synthesis, laccases have been employed for the oxidation of functional groups [38–42], the coupling of phenols and steroids [43–45], the construction of carbon-nitrogen bonds [46] and in the synthesis of complex natural products [47] and more.
As mentioned above, many of these applications require the use of redox mediators opening a big window for new biotransformations of non-natural substrates towards which laccase alone hardly shows activity. On the other hand, in most of the cases large quantities of enzymes are required, which makes the efficient expression of laccase in heterologous systems an important issue. Moreover, the protein engineering of fungal laccases with the aim of improving several enzymatic features (such as activity towards new substrates, stability under harsh operating conditions -e.g. presence of organic cosolvents, extreme pH values-, thermostability, and others) is a critical point in the successful application of this remarkable biocatalyst. All these issues are addressed in the following lines, paying special attention to their application in organic synthesis.
Laccase-mediator system (LMS)
The combination of the laccase with low molecular weight molecules such as 2,2'-azino-bis-(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) or 1-hydroxybenzotriazole (HBT) not only lead to higher rates and yields in the transformation of laccase substrates but also add new oxidative reactions to the laccase repertory towards substrates in which the enzyme alone had no or only marginal activity, Figure 1A, B. Thus, LMS enlarges substrate range being able to oxidize compounds with redox potential (E°) higher than that of laccase (typically, laccase E° at the T1 site is in the range +475 to +790 mV but the LMS allows to oxidize molecules with E° above +1100 mV) [48, 49]. Besides, the mediator acts as a diffusible electron carrier enabling the oxidation of high molecular weight biopolymers such as lignin, cellulose or starch [1]. Hence, the steric issues that hinder the direct interaction between enzyme and polymer are overcome by the action of the redox mediator.
LMS has resulted highly efficient in many biotechnological and environmental applications as regards the numerous research articles and invention patents published [50, 51]. Many artificial mediators have been widely studied, from ABTS the first described laccase mediator [52], to the use of synthetic mediators of the type -NOH- (such as HBT, violuric acid (VIO), N-hydroxyphtalimide (HPI) and N-hydroxyacetanilide (NHA), the stable 2,2,6,6-tetramethyl-1-piperidinyloxy free radical (TEMPO), or the use of phenothiazines and other heterocycles (e.g. promazine or 1-nitroso-naphthol-3,6-disulfonic acid), Figure 2[18, 38, 53]. More recently, complexes of transition elements (polyoxometalates) have been also demonstrated to mediate lignin degradation catalyzed by laccase [54, 55].
Chemical structures of some representative artificial (ABTS, HBT, violuric acid -VIO-, TEMPO, promazine -PZ- and 1-nitroso-naphthol-3,6-disulfonic acid -NNDS-) and lignin-derived natural mediators (acetosyringone, syringaldehyde, vanillin, acetovanillone, p -coumaric acid, ferulic acid and sinapic acid).
The choice of a proper mediator (over 100 redox mediators have been described [56]) represents a key consideration for a given biotransformation. The use of different mediators may yield different final products when using the same precursors. This is basically due to the fact that substrate oxidation in laccase-mediator reactions occurs via different mechanisms. The mediator radicals preferentially perform a specific oxidation reaction based on its chemical structure and effective redox potential (or dissociation bond energy) [43, 38, 53, 57]. For example, ABTS and HBT follow two different radical pathways: i) electron transfer (ET) in the case of ABTS radicals (ABTS•+or ABTS2+) and ii) hydrogen atom transfer (HAT) for nitroxyl radicals (N-O•) of HBT, Figure 3. On the contrary, the stable radical TEMPO follows an ionic oxidation mechanism [38, 39], Figure 4.
Diagram showing the differences between the oxidation mechanisms followed by ABTS radicals (Electron Transfer route, ET) and HBT radicals (Hydrogen Atom Transfer route, HAT) in LMS for oxidation of non-phenolic substrates (according to Galli and Gentili[52]).
Mechanisms of the laccase-TEMPO oxidation of hydroxymethyl groups to aldehyde groups by TEMPO according to d'Acunzo et al. [43].
Despite all the associated advantages of LMS, there are two major drawbacks hindering the use of mediators: they are expensive and they can generate toxic derivatives. Moreover, in some cases, while oxidizing the mediator, laccase is inactivated by the mediator radicals, or the latter can be transformed into inactive compounds with no more mediating capability (e.g. generation of benzotriazol from HBT by losing the hydroxyl group). Last trends are focusing in the use of low-cost and eco-friendly alternative mediators; in this sense, several naturally occurring mediators produced by fungi (phenol, aniline, 4-hydroxybenzoic acid and 4-hydroxybenzyl alcohol) have been identified [49]. More recently, phenolic compounds derived from lignin degradation (such as acetosyringone, syringaldehyde, vanillin, acetovanillone, ferulic acid or p-coumaric acid) have been demonstrated to be highly-efficient laccase mediators of natural origin (even better than the powerful artificial ones) for dye decolorization, removal of polycyclic aromatic hydrocarbons, pulp bleaching and pitch removal [58–61], Figure 2. These natural compounds can be obtained at low cost due to their abundance in nature and also in industrial paper pulp wastes, smoothing the progress to a more environmental-friendly and sustainable white biotechnology processes.
Heterologous expression of fungal laccases
Biotechnological and environmental applications require large amounts of enzymes. Laccases secreted from wild-type fungal organisms may not be suitable for commercial purposes mainly because the low yields and undesirable preparation procedures (such as presence of toxic inducers) are not economically advantageous; however recent advances in bioreactor design and culture conditions have significantly increased the production yields [62].
Heterologous expression should be better suited for large-scale production, because of the potential of expressing different laccases in one selected optimised host. Laccases, like other oxidative enzymes, are difficult to express in non-fungal systems. The heterologous expression of active laccases has been reported mainly in filamentous fungi (Aspergillus oryzae, Aspergillus niger, Aspergillus sojae and Trichoderma reseei) and yeasts (Saccharomyces cerevisiae, Pichia pastoris, Pichia methalonica, Yarrowia lipolytica and Kluyveromyces lactis), Table 1. There is one remarkable exception of homologous expression, in which the basidiomycete fungus Pycnoporus cinabarinus was used as host to overexpress the active laccase (up to 1.2 g l-1) [63]. Unfortunately, the functional expression of fungal laccases in bacteria (Escherichia coli) has not been yet accomplished (perhaps due to the requirement of glycosylation, missing chaperones, and different codon usage, among other shortcomings).
Laccase engineering
Crystallographic structure determination is an essential tool for structure-function relationships studies (i.e. rational design). However, since the crystallization of the first (but inactive) laccase from Coprinus cinereus in 1998 by Ducros et al.[107], few crystal structures of active laccases have been published: one from the ascomycete Melanocarpus albomyces [108], two from basidomycetes Trametes versicolor [109] and Rigidosporus lignosun [110] and another from Bacillus subtilis [111]. Based on these laccase structures, over the last decade several residues in the neighbourhood of the catalytic copper ions have been subjected to site-directed mutagenesis to determine the parameters that define the catalytic activity and the E° of fungal laccases [112, 113]. One consequence of these comprehensive structure-function studies has been the generation of a collection of mutants with structural perturbations at the T1 copper center.
To overcome many of the limitations of the rational design, and in the absence of enough structural information, directed molecular evolution represents a promising alternative. This methodology recreates in the laboratory the key events of natural evolution (mutation, recombination and selection) doing in such a manner those more efficient enzymes-even with novel functions-can be tailored. Diversity is mimicked by inducing mutations and/or recombination in the gene encoding a specific protein. Afterwards, the best performers in each generation are selected and further used as the parental types for a new round of evolution. The process is repeated as many times as necessary enhancing exponentially the targeted features, until a biocatalyst with the desired traits is obtained: stability at high temperature or in organic solvents; improved catalytic activities; higher specificity; etc.
A thorough understanding of efficient and reliable high-throughput screening methodologies is a prerequisite for the design and validation of this type of experiments [114]. A key query result of smart laboratory evolution is the improvement of several enzymatic properties at the same time (e.g. stability and activity). The first successful example of directed laccase evolution reported came from Arnold group [68]. They carried out the functional expression of a thermophilic laccase in S. cerevisiae by directed evolution: after ten rounds of laboratory evolution and screening, the total enzymatic activity was improved 170-fold along with better performances at high temperatures.
It is well known that most of the laccase catalysed transformations for organic syntheses (from the oxidation of steroid hormones to the enzymatic polymerisation required for the synthesis of phenolic-based resins such as poly-α-naphtol, poly-pyrogallol and poly-catechol [1, 115]., as well as conductive water-soluble polymers [116]) must be carried out in the presence of organic solvents. However, at high concentrations of organic co-solvents laccases undergo unfolding, therefore losing their catalytic activity. Recently, our group generated a thermostable laccase-the genetic product of five rounds of directed evolution expressed in S. cerevisiae [117, 118]-that tolerates high concentrations of co-solvents. This evolved laccase mutant is capable of resisting a wide array of biotechnologically relevant miscible co-solvents at concentrations as high as 50% (v/v). Indeed, in 40% (v/v) ethanol or in 30% (v/v) acetonitrile the performance of the laccase mutant was comparable to that of the parental enzyme in aqueous solution, a capacity that has not been acquired in nature. Intrinsic electrochemical laccase features such as the redox potential at the T1 and T2/T3 sites and the geometry and electronic structure of the catalytic coppers varied slightly during the course of the in vitro evolution. Indeed, some mutations at the protein surface stabilized the evolved laccase by allowing additional electrostatic and hydrogen-bonding to occur [117]. Additionally, the protein folding in the post-translational maturation steps seemed to be modified by mutations in processing regions [119].
Besides methods that involve iterative steps of random mutagenesis and/or DNA recombination, semi-rational studies-which take advantage from both protein structure and combinatorial libraries constructed by saturation mutagenesis- are being employed successfully. This approach involves the mutation of any single amino acid codon to all the other codons that will generate the 20 naturally occurring amino acids coupled to screen for the desire function. This technique is commonly employed to improve the characteristics of enzymes at "hot-spot" residues already identified by conventional random mutagenesis. In addition, it can be employed to simultaneously mutate several codons (combinatorial saturation mutagenesis), which will enable all possible combinations of interesting residues to be evaluated in order to identify their optimal interactions and synergies.
In a recent study [120] of the evolved Myceliophthora thermophila laccase variant T2 (MtLT2) expressed in S. cerevisiae [68], we applied combinatorial saturation mutagenesis to residues L513 (the axial non-coordinating ligand supposedly essential for the E° at the T1 site) and S510 (belonging to the tripeptide 509VSG511 that is common to the low-medium E° laccases). A mutant with 3-fold higher turnover rates than the parent type, contained one beneficial mutation (TCGS510GGGG) that could not be achieved by conventional error-prone PCR techniques, since it was dependent on the two consecutive nucleotide changes. In a more exhaustive study [119], several regions of the same variant were investigated by combinatorial saturation mutagenesis. After exploring over 180,000 clones, the S510G mutant revealed a direct interaction between the conserved 509VSG511 tripeptide located in the neighbourhood of the T1 site and the C-terminal plug.
Applications of laccases in organic synthesis
Organic synthesis of chemicals suffers from several drawbacks, including the high cost of chemicals, cumbersome multi-step reactions and toxicity of reagents [2, 17]. Laccases might prove to be very useful in synthetic chemistry, where they have been proposed to be applicable for production of complex polymers and medical agents [16, 121]. Indeed, the application of laccase in organic synthesis has arisen due to its broad substrate range, and the conversion of substrates to unstable free (cation) radicals that may undergo further non-enzymatic reactions such as polymerization or hydration. The list of laccases used for organic synthesis is presented in Table 2.
Laccases for enzymatic polymerization and polymer functionalization
Enzymatic polymerization using laccases has drawn considerable attention recently since laccase or LMS are capable of generating straightforwardly polymers that are impossible to produce through conventional chemical synthesis [127].
For example, the polymerization ability of laccase has been applied to catechol monomers for the production of polycatechol [127]. Polycatechol is considered a valuable redox polymer; among its applications are included chromatographic resins and the formation of thin films for biosensors. Former methods for the production of polycatechol used soybean peroxidase or horseradish peroxidase (HRP), which suffer from the common "suicide H2O2 inactivation". The main limitation of all heme-containing peroxidases is their low operational stability, mostly due to their rapid deactivation by H2O2-with half-lifes in the order of minutes in the presence of 1 mM H2O2 [127, 137].
Inert phenolic polymers, for example poly(1-napthol), may also be produced by laccase-catalyzed reactions [125, 138–140]. These polymers have application in wood composites, fiber bonding, laminates, foundry resins, abrasives, friction and molding materials, coatings and adhesives [125, 141].
The enzymatic preparation of polymeric polyphenols by the action of laccases has been investigated extensively in the past decades as a viable and non-toxic alternative to the usual formaldehyde-based chemical production of these compounds [142–144]. Poly(2,6-dimethyl-1,4-oxyphenylene)-"poly(phenylene oxide)", PPO-, is widely used as high-performance engineering plastic, since the polymer has excellent chemical and physico-mechanical properties. PPO was first prepared from 2,6-dimethylphenol monomer using a copper/amine catalyst system. 2,6-Dimethylphenol was also polymerized through HRP catalysis to give a polymer consisting of exclusively 1,4-oxyphenylene units [145]. On the other hand, a small amount of Mannich-base and 3,5,3'5'-tetramethyl-4,4'-diphenoquinone units are contained in the commercially available PPO. The polymerization also proceeded under air in the presence of laccase derived from Pycnoporus coccineus without the addition of H2O2 [123, 146].
It has been also reported that laccase induced a new type of oxidative polymerization of 4-hydroxybenzoic acid derivatives, 3,5-dimethoxy-4-hydroxybenzoic acid (syringic acid) and 3,5-dimethyl-4-hydroxybenzoic acid. The polymerization involved elimination of CO2 and H2 from the monomer to give PPO derivatives with molecular weight up to 1.8 × 104 (Figure 5A) [145, 147].
A novel system of enzymatic polymerization, i.e. a laccase-catalyzed cross-linking reaction of new "urushiol analogues" for the preparation of "artificial urushi" polymeric films (Japanese traditional coating) has been demonstrated (Figure 5B) [148–151]. Flavonoids have been also polymerized by polyphenol oxidase and laccase. The flavonoid-containing polymers showed good antioxidant properties and enzyme inhibitory effect [152].
It has been reported that laccase induced radical polymerization of acrylamide with or without mediator [146]. Laccase has been also used for the chemo-enzymatic synthesis of lignin graft-copolymers [153]. Along these lines, the potential of this enzyme for crosslinking and functionalizing lignocellulose compounds is also reported [154]. Laccases can be used in the enzymatic adhesion of fibers in the manufacturing of lignocellulose-based composite materials, such as fiber boards. In particular, laccase has been proposed to activate the fiberbound lignin during manufacturing of the composites, and boards with good mechanical properties without toxic synthetic adhesives have been obtained by using laccases [155, 156]. Another possibility is to functionalize lignocellulosic fibers by laccases in order to improve the chemical or physical properties of the fiber products. Preliminary results have shown that laccases are able to graft various phenolic acid derivatives onto kraft pulp fibers [157, 158]. This ability could be used in the future to attach chemically versatile compounds to the fiber surfaces, possibly resulting in fiber materials with completely novel properties, such as hydrophobicity or charge.
Finally, laccase-TEMPO mediated system has been also used to catalyze the regioselective oxidation of the primary hydroxyl groups of sugar derivatives or even starch, pullulan and cellulose allowing the polymer functionalization [132, 159]. The efficiency of this system was initially tested with mono- and disaccharides (i.e., phenyl β-D-glucopyranoside), and the corresponding glucopyranosiduronates were isolated and characterized (Figure 6A). Subsequently, this chemo-enzymatic approach has been exploited to achieve the partial oxidation of a water soluble cellulose sample. Also, the same approach has been applied for the mild oxidation of the glycosylated saponin, asiaticoside [160] (Figure 6B), and a series of natural glycosides [133].
Oxidative transformation of organic compounds by laccase
Laccases have been used to synthesize products of pharmaceutical importance. The first chemical that comes to mind is actinocin, synthesized via a laccase-catalyzed reaction from 4-methyl-3-hydroxyanthranilic acid as shown in Figure 7A. This pharmaceutical product has proven effective in the fight against cancer as it blocks transcription of tumor cell DNA [161, 162].
Other examples of the potential application of laccases for organic syntheses include the oxidative coupling of katarantine and vindoline to yield vinblastine. Vinblastine is an important anti-cancer drug, especially useful in the treatment of leukemia. Vinblastine is a natural product that may be extracted from the plant Catharanthus roseus. The compound is however only produced in small quantity in the plant, whereas the precursors-namely katarantine and vindoline- are at much higher concentrations, and thus are relatively inexpensive to obtain and purify. A method of synthesis has been developed through the use of laccase with preliminary results reaching 40% conversion of the precursors to vinblastine [2]. Laccase coupling has also resulted in the production of several other novel compounds that exhibit beneficial properties, e.g. antibiotic properties [163].
The study of new synthetic routes to aminoquinones is of great interest because a number of antineoplast drugs in use, like mitomycin, or under development, like nakijiquinone-derivatives [164] or herbamycin-derivatives [165], contain an aminoquinone moiety. Several simple aminoquinones possess activity against a number of cancer cell-lines [166–168] as well as antiallergic or 5-lipoxygenase inhibiting activity [168, 169].
Laccases have also been employed to synthesize new cyclosporin derivatives [170]. Cyclosporin A was converted to cyclosporin A Methyl vinyl ketone [R1 = (E)-2-butenyl to R1 = (E)-3-oxo-1-butenyl] by HBT-mediated laccase oxidation [170], (Figure 7B).
Laccases are also able to oxidize catechins. These molecules are the condensed structural units of tannins, which are considered important antioxidants found in herbs, vegetables and teas. Catechins ability to scavenge free radicals makes them important in preventing cancer, inflammatory and cardiovascular diseases. Oxidation of catechin by laccase has yielded products (Figure 7C) with enhanced antioxidant capability [136, 171].
Last but not least, laccase finds applications in the synthesis of hormone derivatives (generating dimers or oligomers by the coupling of the reactive radical intermediates). Intra et al. [172] and Nicotra et al. [44] have recently exploited the laccase capabilities to isolate new dimeric derivatives of the hormone β-estradiol (Figure 8A) and of the phytoalexin resveratrol (Figure 8B), respectively. Similarly, laccase oxidation of totarol, and of isoeugenol or coniferyl alcohol gave novel dimeric derivatives [134] and a mixture of dimeric and tetrameric derivatives [173] respectively, whereas an even more complex mixture of products was observed in the oxidation of substituted imidazole (Figure 9A) [126]. These novel substituted imidazoles or oligomerization products (2–4) are applicable for pharmacological purposes. In another study, derivatization of the natural compound 3-(3,4-dihydroxyphenyl)-propionic acid can be achieved by laccase-catalyzed N-coupling of aromatic and aliphatic amines (Figure 9B). The derivatives of this antiviral natural compound 3-(3,4-dihydroxyphenyl)-propionic acid may have interesting pharmaceutical uses. More recently, nuclear amination of p-hydroquinones with primary aromatic amines catalyzed by laccases in the presence of O2resulted in the formation of the corresponding monoaminated or diaminated quinones [174, 175], (Figure 9C).
(A) N -[2-alkylamino-4-phenylimidazol-1-yl]-acetamide (substrate 1) and products 2–4 formed during incubation with T. versicolor laccase, (B) The natural compound 3-(3,4-dihydroxyphenyl)-propionic acid derivative can be synthesized by laccase-catalyzed N-coupling of aromatic and aliphatic amines, and (C) the coupling of p -hydroquinones with primary aromatic amines by laccases.
Conclusion
The use of laccases in organic synthesis does show as a promising green alternative to the classical chemical oxidation with a wide range of substrates. In the near future, the practical use of fungal laccases for troublesome transformations (digestion of lignocellulose to use as a carbon source; modifications of lignosulfonates for production of emulsifiers, surfactants and adhesives; synthesis of polymers with properties as redox films for bioelectronic devices; synthesis of antibiotics and much more) will expand the need for this biocatalyst. Meanwhile, the development of more robust fungal laccases tailored by protein engineering and the search for environment-friendly mediators along with further research on heterologous expression are significant hurdles that must be overcome.
References
Alcalde M: Laccase: biological functions, molecular structure and industrial applications. Industrial Enzymes: structure, function and applications. Edited by: J Polaina J, MacCabe AP. 2007, 459-474. New York, Springer
Yaropolov AI, Skorobogat'ko OV, Vartanov SS, Varfolomeyev SD: Laccase: Properties, catalytic mechanism, and applicability. Appl Biochem Biotechnol. 1994, 49: 257-280.
Call HP, Mücke I: History, overview and applications of mediated ligninolytic systems, especially laccase-mediator-systems (Lignozyme®-process). J Biotechnol. 1997, 53: 163-202.
Gianfreda L, Xu F, Bollag JM: Laccases: a useful group of oxidoreductive enzymes. Bioremed J. 1999, 3: 1-25.
Dittmer NT, Suderman RJ, Jiang H, Zhu YC, Gorman MJ, Kramer KJ, Kanost MR: Characterization of cDNAs encoding putative laccase-like multicopper oxidases and developmental expression in the tobacco hornworm, Manduca sexta, and the malaria mosquito, Anopheles gambiae. Insect Biochem Mol Biol. 2004, 34: 29-41.
Kramer KJ, Kanost MR, Hopkins TL, Jing H, Zhu YC, Xhu R, Kerwin JL, Turecek F: Oxidative conjugation of catechols with proteins in insect skeletal systems. Tetrahedron. 2001, 57: 385-392.
Claus H: Laccases and their occurrence in prokaryotes. Arch Microbiol. 2003, 179: 145-150.
Baldrian P: Fungal laccases – occurrence and properties. FEMS Microbiol Rev. 2006, 30: 215-242.
Yaver DS, Xu F, Golightly EJ, Brown KM, Brown SH, Rey MW, Schneider P, Halkier T, Mondorf K, Dalboge H: Purification, characterization, molecular cloning, and expression of two laccase genes from the white rot basidiomycete Trametes villosa. Appl Environ Microbiol. 1996, 62: 834-841.
Marques De Souza CG, Peralta RM: Purification and characterization of the main laccase produced by the white-rot fungus Pleurotus pulmonarius on wheat bran solid state medium. J Basic Microbiol. 2003, 43 (4): 278-286.
Aramayo R, Timberlake WE: The Aspergillus nidulans yA gene is regulated by abaA. EMBO J. 1993, 12: 2039-2048.
Williamson PR, Wakamatsu K, Ito S: Melanin biosynthesis in Cryptococcus neoformans. J Bacteriol. 1998, 180: 1570-1572.
O' Malley DM, Whetten R, Bao W, Chen CL, Seedorf RR: The role of laccase in lignification. Plant J. 1993, 4: 751-757.
Stoj C, Kosman DJ: Cuprous oxidase activity of yeast Fet3p and human ceruloplasmin: implication for function. FEBS Lett. 2003, 554: 422-426.
Nyanhongo GS, Gomes J, Gubitz GM, Zvauya R, Read JS, Steiner W: Decolorization of textile dyes by laccases from a newly isolated strain of Trametes modesta. Water Res. 2002, 36: 1449-1456.
Xu F: Applications of oxidoreductases: recent progress. Industrial Biotechnol. 2005, 1: 38-50.
Riva S: Laccases: blue enzyme for green chemistry. Trends Biotechnol. 2006, 24: 219-226.
Bourbonnais R, Paice MG, Freiermuth B, Bodie E, Borneman S: Reactivities of various mediators and laccases with kraft pulp and lignin model compounds. Appl Environ Microbiol. 1997, 63 (12): 4627-4632.
Smith M, Thurston CF, Wood DA: Fungal laccases: role in delignification and possible industrial applications. Multi-copper oxidases. Edited by: Messerschmidt A. 1997, 201-224. Singapore, World Scientific
Camarero S, García O, Vidal T, Colom J, del Río JC, Gutiérrez A, Gras JM, Monje R, Martínez MJ, Martínez AT: Efficient bleaching of non-wood high-quality paper pulp using laccase-mediator system. Enzyme Microb Technol. 2004, 35: 113-120.
Ibarra D, Camarero S, Romero J, Martínez MJ, Martínez AT: Integrating laccase-mediator treatment into an industrial-type sequence for totally chlorine free bleaching eucalypt kraft pulp. J Chem Technol Biotechnol. 2006, 81: 1159-1165.
Bergbauer M, Eggert C, Kraepelin G: Degradation of chlorinated lignin compounds in a bleach plant effluent by the white-rot fungus Trametes versicolor. Appl Microbiol Biotechnol. 1991, 35: 105-109.
Berrio J, Plou FJ, Ballesteros A, Martinez AT, Martinez MJ: Immobilization of Pycnoporus coccineus laccase on Eupergit C: Stabilization and treatment of olive oil mill wastewaters. Biocatal Biotransform. 2007, 25: 130-134.
Abadulla E, Tzanov T, Costa S, Robra KH, Cavaco-Paulo A, Gübitz GM: Decolourisation and detoxification of textile dyes with laccase from Trametes hirsuta. Appl Environ Microbiol. 2000, 66: 3357-3362.
Kunamneni A, Ghazi I, Camarero S, Ballesteros A, Plou FJ, Alcalde M: Decolorization of synthetic dyes by laccase immobilized on epoxy-activated carriers. Process Biochem. 2008, 43: 169-178.
Widsten P, Tuominen S, Qvintus-Leino P, Laine JE: The influence of high defibration temperature on the properties of medium-density fiberboard (MDF) made from laccase-treated softwood fibers. Wood Sci Technol. 2004, 38: 521-528.
Keum YS, Li QX: Reduction of nitroaromatic pesticides with zero-valent iron. Chemosphere. 2004, 54 (3): 255-263.
Bollag JM, Chu HL, Rao MA, Gianfreda L: Enzymatic oxidative transformation of chlorophenol mixtures. J Environ Qual. 2003, 32: 63-69.
Gianfreda L, Rao MA: Potential of extracellular enzymes in remediation of polluted soils: a review. Enzyme Microb Technol. 2004, 35: 339-354.
Alcalde M, Ferrer M, Plou FJ, Ballesteros A: Environmental biocatalysis: from remediation with enzymes to novel green processes. Trends Biotechnol. 2006, 24 (6): 281-287.
Zumarraga M, Plou FJ, Garcia-Arellano H, Ballesteros A, Alcalde M: Bioremediation of polycyclic aromatic hydrocarbons by fungal laccases engineered by directed evolution. Biocatal Biotrans. 2007, 25: 219-228.
Jonsson LJ, Palmqvist E, Nilvebrant NO, Hahn Hagerdal B: Detoxification of wood hydrolysates with laccase and peroxidase from the White-rot fungus Trametes versicolor. Appl Microbiol Biotechnol. 1998, 49: 691-697.
Larsson S, Reimann A, Nilvebrant NO, Jonsson LJ: Comparison of different methods for the detoxification of lignocellulose hydrolysates of spruce. Appl Biochem Biotechnol. 1999, 77–79: 91-103.
Cantarelli C, Brenna O, Giovanelli G, Rossi M: Beverage stabilization through enzymatic removal of phenolics. Food Biotechnol. 1989, 3: 203-214.
Servili M, De Stefano G, Piacquadio P, Sciancalepore V: A novel method for removing phenols from grape must. Am J Enol Viticul. 2000, 51: 357-361.
Minussi RC, Pastore GM, Duran N: Potential applications of laccase in the food industry. Trends Food Sci Technol. 2002, 13: 205-216.
Ghindilis A: Direct electron transfer catalysed by enzymes: application for biosensor development. Biochem Soc Trans. 2000, 28: 84-89.
Baiocco P, Barreca AN, Fabbrini M, Galli C, Gentili P: Promoting laccase activity towards non-phenolic substrates: A mechanistic investigation with some laccase-mediator systems. Org Biomol Chem. 2003, 1: 191-197.
Fabbrini M, Galli C, Gentili P: Comparing the efficiency of some mediators of laccase. J Mol Catal B Enzym. 2002, 16: 231-240.
Fritz-Langhals E, Kunath B: Synthesis of aromatic aldehydes by laccase mediator assisted oxidation. Tetrahedron Lett. 1998, 39: 5955-5956.
Potthast A, Rosenau T, Chen CL, Gratzl JS: A novel method for the conversion of benzyl alcohols to benzaldehydes by laccase-catalyzed oxidation. J Mol Catal A Chem. 1996, 108: 5-9.
Fabbrini M, Galli C, Gentili P, Macchitella D: An oxidation of alcohols by oxygen with the enzyme laccase, and mediation by TEMPO. Tetrahedron Lett. 2001, 42: 7551-7553.
d'Acunzo F, Galli C, Masci B: Oxidation of phenols by laccase and laccase-mediator systems: Solubility and steric issues. Eur J Biochem. 2002, 269: 5330-5335.
Nicotra S, Cramarossa MR, Mucci A, Pagnoni UM, Riva S, Forti L: Biotransformation of resveratrol: synthesis of trans-dehydrodimers catalyzed by laccases from Myceliophtora thermophyla and from Trametes pubescens. Tetrahedron. 2004, 60: 595-600.
Ponzoni C, Beneventi E, Cramarossa MR, Raimondi S, Trevisi G, Pagnoni UM, Riva S, Forti L: Laccase-catalyzed dimerization of hydroxystilbenes. Adv Synth Catal. 2007, 349: 1497-1506.
Mikolasch A, Hammer E, Jonas U, Popowski K, Stielow A, Schauer F: Synthesis of 3-(3, 4-dihydroxyphenyl)-propionic acid derivatives by N-coupling of amines using laccase. Tetrahedron. 2002, 58: 7589-7593.
Barilli A, Belinghieri F, Passarella D, Lesma G, Riva S, Silvani A, Danieli B: Enzyme assisted enantioselective synthesis of the alkaloid (+)-aloperine. Tetrahedron Asym. 2004, 15: 2921-2925.
Fernández-Sánchez C, Tzanov T, Gübitz GM, Cavaco-Paulo A: Voltametric monitoring of laccase-catalysed mediated reactions. Bioelectrochemistry. 2002, 58 (2): 149-156.
Johannes C, Majcherczyk A: Laccase activity tests and laccase inhibitors. J Biotechnol. 2000, 78: 193-199.
Morozova OV, Shumakovich GP, Shleev SV, Iaropolov YI: Laccase-mediator systems and their applications: A review. Prikl Biokhim Mikrobiol. 2007, 43 (5): 583-597.
Kunamneni A, Plou FJ, Alcalde M, Ballesteros A: Laccases and their applications: A patent review. Recent Patent Biotechnol. 2008, 2: 10-24.
Bourbonnais R, Paice MG: Oxidation of non-phenolic substrates. An expanded role for laccase in lignin biodegradation. FEBS Lett. 1990, 267: 99-102.
Galli C, Gentili P: Chemical messengers: mediated oxidations with the enzyme laccase. J Phys Org Chem. 2004, 17: 973-977.
Gamelas JAF, Tavares APM, Evtuguin DV, Xavier AMB: Oxygen bleaching of kraft pulp with polyoxometalates and laccase applying a novel multi-stage process. J Mol Catal B: Enzym. 2005, 33: 57-64.
Gamelas JAF, Pontes ASN, Evtuguin DV, Xavier AMRB, Esculcas AP: New polyoxometalate-laccase integrated system for kraft pulp delignification. Biochem Eng J. 2007, 33: 141-147.
Call HP: Mehrkomponentensystem zum Verändern, Abbau oder Bleichen von Lignin, ligninhaltigen Materialien oder ähnlichen Stoffen sowie Verfahren zu seiner Anwendung. EP 0717143A1. 1996
d'Acunzo F, Galli C, Gentili P, Sergi F: Mechanistic and steric issues in the oxidation of phenolic and non-phenolic compounds by laccase or laccase-mediator systems. The case of bifunctional substrates. New J Chem. 2006, 30: 583-591.
Camarero S, Ibarra D, Martínez MJ, Martínez AT: Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol. 2005, 71: 1775-1784.
Cañas A, Alcalde M, Plou FJ, Martínez MJ, Martínez AT, Camarero S: Transformation of polycyclic aromatic hydrocarbons by laccase is strongly enhanced by phenolic compounds present in soil. Environ Sci Technol. 2007, 41: 2964-2971.
Camarero S, Ibarra D, Martínez AT, Romero J, Gutiérrez A, del Río JC: Paper pulp delignification using laccase and natural mediators. Enzyme Microb Technol. 2007, 40: 1264-1271.
Gutiérrez A, Rencores J, Ibarra D, Molina S, Camarero S, Romero J, del Río JC, Martínez AT: Removal of lipophilic extractives from paper pulp by laccase and lignin-derived phenols as natural mediators. Environ Sci Technol. 2007, 41: 4124-4129.
Couto SR, Toca-Herrera JL: Laccase production at reactor scale by filamentous fungi. Biotechnol Adv. 2007, 25: 558-569.
Alves AMCR, Record E, Lomascolo A, Scholtmeijer K, Asther M, Wessels JGH, Wosten HAB: Highly efficient production of laccase by the basidiomycete Pycnoporus cinnabarinus. Appl Environ Microbiol. 2004, 70: 6379-6384.
Kojima Y, Tsukuda Y, Hawai Y, Tsukamoto A, Sugiura J, Sakaino M, Kita Y: Cloning, sequence analysis, and expression of ligninolytic phenoloxidase genes of the white-rot basidiomycete Coriolus hirsutus. J Biol Chem. 1990, 265: 15224-15230.
Saloheimo M, Niku-Paavola ML: Heterologous production of a ligninolytic enzyme: expression of the Phlebia radiata laccase gene in Trichoderma reesei. Biotechnol. 1991, 9: 987-990.
Wahleithner JA, Xu F, Brown SH, Golightly EJ, Halkier T, Kauppinen S, Pederson A, Schneider P: The identification and characterization of four laccases from the plant pathogenic fungus Rhizoctonia solani. Curr Genet. 1996, 29: 395-403.
Berka RMP, Schneider EJ, Golightly SH, Brown M, Madden KM, Brown T, Halkier K, Mondorf , Xu F: Characterization of the gene encoding an extracellular laccase of Myceliophtora thermophila and analysis of the recombinant enzyme expressed in Aspergillus oryzae. Appl Environ Microbiol. 1997, 63: 3151-3157.
Bulter T, Alcalde M, Sieber V, Meinhold P, Schlachtbauer C, Arnold FH: Functional expression of a fungal laccase in Saccharomyces cerevisiae by directed evolution. Appl Environ Microbiol. 2003, 69: 987-995.
Jönsson LJ, Saloheimo M, Penttila M: Laccase from the white-rot fungus Trametes versicolor: cDNA cloning of lcc1 and expression in Pichia pastoris. Curr Genet. 1997, 32: 425-430.
O'Callaghan J, O'Brien MM, McClean K, Dobson ADW: Optimisation of the expression of Trametes versicolor laccase gene in Pichia pastoris. J Ind Microbiol Biotechnol. 2002, 29: 55-59.
Hong F, Meinander QM, Jönsson LF: Fermentation strategies for improved heterologous expression of laccase in Pichia pastoris. Biotechnol Bioeng. 2002, 79 (4): 438-449.
Cassland P, Jönsson LJ: Characterization of a gene encoding Trametes versicolor laccase A and improved heterologous expression in Saccharomyces cerevisiae by decreased cultivation temperature. Appl Microbiol Biotechnol. 1999, 52: 393-400.
Larsson S, Cassland P, Jönsson LJ: Development of a Saccharomyces cerevisiae strain with enhanced resistance to phenolic fermentation inhibitors in lignocellulose hydrolysates by heterologous expression of laccase. Appl Environ Microbiol. 2001, 67: 1163-1170.
Gelo-Pujic M, Kim HH, Butlin NG, Palmore GT: Electrochemical studies of a truncated laccase produced in Pichia pastoris. Appl Environ Microbiol. 1999, 65: 5515-5521.
Brown MA, Zhao Z, Mauk AG: Expression and characterization of a recombinant multi-copper oxidase: laccase IV from Trametes versicolor. Inorg Chim Acta. 2002, 331: 232-238.
Hood EE, Bailey MR, Beifuss K, Magallanes-Lundback M, Horn ME, Callaway E, Drees C, Delaney DE, Clough R, Howard JA: Criteria for high-level expression of a fungal laccase gene in transgenic maize. Plant Biotechnol J. 2003, 1: 129-140.
Guo M, Lu FP, Du LX, Pu J, Bai DQ: Optimization of the expression of a laccase gene from Trametes versicolor in Pichia methanolica. Appl Microbiol Biotechnol. 2006, 71: 848-852.
Jolivalt C, Madzak C, Brault A, Caminade E, Malosse C, Mougin C: Expression of laccase IIIb from the white-rot fungus Trametes versicolor in the yeast Yarrowia lipolytica for environmental applications. Appl Microbiol Biotechnol. 2005, 66: 450-456.
Necochea R, Valderrama B, Diaz-Sandoval S, Folch-Mallol JL, Vazquez-Duhalt R, Iturriaga G: Phylogenetic and biochemical characterisation of a recombinant laccase from Trametes versicolor. FEMS Microbiol Lett. 2005, 244: 235-241.
Bohlin C, Jönsson LJ, Roth R, vanZyl WH: Heterologous expression of Trametes versicolor laccase in Pichia pastoris and Aspergillus niger. Appl Biochem Biotechnol. 2006, 129: 195-214.
Téllez-Jurado A, Arana-Cuenca A, González Becerra AE, Viniegra-González G, Loera O: Expression of a heterologous laccase by Aspergillus niger cultured by solid-state and submerged fermentations. Enzyme Microb Technol. 2006, 38: 665-669.
Hatamoto O, Sekine H, Nakano E, Abe K: Cloning and expression of a cDNA encoding the laccase from Schizophyllum commune. Biosci Biotechnol Biochem. 1999, 63: 58-64.
Yaver DS, Overjero MD, Xu F, Nelson BA, Brown KM, Halkier T, Bernauer S, Brown SH, Kauppinen S: Molecular characterization of laccase genes from the basidiomycete Coprinus cinereus and heterologous expression of the laccase lcc 1. Appl Environ Microbiol. 1999, 65: 4943-4948.
Kilaru S, Hoegger PJ, Majcherczyk A, Burns C, Shishido K, Bailey A, Foster GD, Kues U: Expression of laccase gene lcc1 in Coprinopsis cinerea under control of various basidiomycetous promoters. Appl Microbiol Biotechnol. 2006, 71 (2): 200-210.
LaFayette PR, Eriksson KE, Dean JF: Characterization and heterologous expression of laccase cDNAs from xylem tissues of yellow-poplar (Liriodendron tulipifera). Plant Mol Biol. 1999, 40: 23-35.
Otterbein L, Record E, Longhi S, Asther M, Moukha S: Molecular cloning of the cDNA encoding laccase from Pycnoporus cinnabarinus I-937 and expression in Pichia pastoris. Eur J Biochem. 2000, 267: 1619-1625.
Record E, Punt PJ, Chamkha M, Labat M, Hondel van Den CAMJJ, Esther M: Expression of the Pycnoporus cinnabarinus laccase gene in Aspergillus niger and characterization of the recombinant enzyme. Eur J Biochem. 2002, 269: 602-609.
Sigoillot C, Record E, Belle V, Robert JL, Levasseur A, Punt PJ, Hondel Van Den CA, Fournel A, Sigoillot JC, Asther M: Natural and recombinant fungal laccases for paper pulp bleaching. Appl Microbiol Biotechnol. 2004, 64: 346-352.
Madzak C, Otterbein L, Chamkha M, Moukha S, Asther M, Gaillardin C, Beckerich JM: Heterologous production of a laccase from the basidiomycete Pycnoporus cinnabarinus in the dimorphic yeast Yarrowia lipolytica. FEMS Yeast Res. 2005, 5: 635-646.
Sato Y, Wuli B, Sederoff R, Whetten R: Molecular cloning and expression of eight cDNAs in loblolly pine (Pinus taeda). J Plant Res. 2001, 114: 147-155.
Sanchez-Amat A, Lucas-Elio P, Fernandez E, Garcia-Borron JC, Solano F: Molecular cloning and functional characterisation of a unique multipotent polyphenol oxidase from Marinomonas mediterranea. Biochim Biophys Acta. 2001, 1547: 104-116.
Soden DM, O'Callaghan J, Dobson AD: Molecular cloning of a laccase isozyme gene from Pleurotus sajor-caju and expression in the heterologous Pichia pastoris host. Microbiology. 2002, 148 (Pt 12): 4003-4014.
Li L, Steffens JC: Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta. 2002, 215: 239-247.
Kiiskinen LL, Kruus K, Bailey M, Ylosmaki E, Siika-Aho M, Saloheimo M: Expression of Melanocarpus albomyces laccase in Trichoderma reesei and characterisation of the purified. Microbiol. 2004, 150: 3065-3074.
Klonowska A, Gaudin C, Asso M, Fournel A, Reglier M, Tron T: LAC3, a new low redox potential laccase from Trametes sp. strain C30 obtained as a recombinant protein in yeast. Enzyme Microb Technol. 2005, 36: 34-41.
Piscitelli A, Giardina P, Mazzoni C, Sannia G: Recombinant expression of Pleurotus ostreatus laccases in Kluyveromyces lactis and Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2005, 69: 428-439.
Festa G, Autore F, Fraternali F, Giardina P, Sannia G: Development of new laccases by directed evolution: Functional and computational analyses. Proteins. 2008, 72 (1): 25-34.
Faraco V, Ercole C, Festa G, Piscitelli A, Sannia G: Heterologous expression of heterodimeric laccase from Pleurotus ostreatus in Kluyveromyces lactis. Appl Microbiol Biotechnol. 2008, 77: 1329-1335.
Hoshida H, Fujita T, Murata K, Kubo K, Akada R: Copper-dependent production of a Pycnoporus coccineus extracellular laccase in Aspergillus oryzae and Saccharomyces cerevisiae. Biosci Biotechnol Biochem. 2005, 69: 1090-1097.
Colao MC, Lupino S, Garzillo AM, Buonocore V, Ruzzi M: Heterologous expression of lccl gene from Trametes trogii in Pichia pastoris and characterization of the recombinant enzyme. Microb Cell Fact. 2006, 5: doi
Camattari A, Bianchi MM, Branduardi P, Porro D, Brambilla L: Induction by hypoxia of heterologous-protein production with the Kl PDC1 promoter in yeasts. Appl Environ Microbiol. 2007, 73: 922-929.
Li F, Hong YZ, Xiao YZ, Xu YH, Fang W: High production of laccase B from Trametes sp. in Pichia pastoris. World J Microbiol Biotechnol. 2007, 23: 741-745.
Hong YZ, Zhou HM, Tu XM, Li JF, Xiao YZ: Cloning of a laccase gene from a novel basidiomycete Trametes sp 420 and its heterologous expression in Pichia pastoris. Curr Microbiol. 2007, 54: 260-265.
Rodríguez E, Ruiz-Dueñas FJ, Kooistra R, Ram A, Martínez AT, Martínez MJ: Isolation of two laccase genes from the white-rot fungus Pleurotus eryngii and heterologous expression of the pel 3 encoded protein. J Biotechnol. 2008, 134: 9-19.
Bleve G, Lezzi C, Mita G, Rampino P, Perrotta C, Villanova L, Grieco F: Molecular cloning and heterologous expression of a laccase gene from Pleurotus eryngii in free and immobilized Saccharomyces cerevisiae cells. Appl Microbiol Biotechnol. 2008, 79: 731-741.
Hu MR, Chao YP, Zhang GQ, Yang XQ, Xue ZQ, Qian SJ: Molecular evolution of Fome lignosus laccase by ethyl methane sulfonate-based random mutagenesis in vitro. Biomol Eng. 2007, 24: 619-624.
Ducros V, Brzozowski AM, Wilson KS, Brown SH, Ostergaard P, Schneider P, Yaver DS, Pedersen AH, Davies GJ: Crystal structure of the type-2 Cu depleted laccase from Coprinus cinereus at 2.2 angstrom resolution. Nat Struct Biol. 1998, 5: 310-316.
Bertrand TC, Jolivalt C, Briozzo P, Caminade E, Joly N, Madzak C, Mougin C: Crystal structure of four-copper laccase complexed with an arylamin: insights into substrate recognition and correlation with kinetics. Biochemistry. 2002, 41 (23): 7325-7333.
Piontek K, Antorini M, Choinowski T: Crystal structure of a laccase from the fungus Trametes versicolor at 1.90-Å resolution containing a full complement of coppers. J Biol Chem. 2002, 277: 37663-37669.
Garavaglia S, Cambria MT, Miglio M, Ragusa S, Lacobazzi V, Palmieri F, D'Ambrosio C, Scaloni A, Rizzi M: The structure of Rigidoporus lignosus laccase containing a full complement of copper ions, reveals an asymmetrical arrangement for the T3 copper pair. J Mol Biol. 2004, 342: 1519-1531.
Enguita FJ, Martins LO, Henriques AO, Larrondo MA: Crystal structure of a bacterial endospore coat component: A laccase with enhanced thermostability properties. J Biol Chem. 2003, 278: 19416-19425.
Xu F, Berka RM, Wahleithner JA, Nelson BA, Shuster JR, Brown SH, Palmer AE, Solomon EI: Site-directed mutations in fungal laccase: effect on redox potential, activity and pH profile. Biochem J. 1998, 334: 63-70.
Palmer AE, Szilagyi RK, Cherry JR, Jones A, Xu F, Solomon EI: Spectroscopic characterization of the Leu513His variant of fungal laccase: Effect of increased axial ligand interaction on the geometric and electronic structure of the type 1 Cu site. Inorg Chem. 2003, 42: 4006-4017.
Alcalde M, Bulter T, Arnold FH: Colorimetric assays for biodegradation of polycyclic aromatic hydrocarbons by fungal laccases. J Biomol Screen. 2002, 7 (6): 547-553.
Torres E, Bustos-Jaimes I, Le Borgne S: Potential use of oxidative enzymes for the detoxification of organic pollutants. Appl Catal B-Environ. 2003, 46: 1-15.
Karamyshev AV, Shleev SC, Koroleva OV, Yaropolov AI, Sakharov IY: Laccase-catalyzed synthesis of conducting polyaniline. Enzyme Microb Tech. 2003, 33: 556-564.
Zumarraga M, Bulter T, Shleev S, Polaina J, Plou FJ, Ballesteros A, Alcalde M: In vitro evolution of a fungal laccase in high concentrations of organic cosolvents. Chem Biol. 2007, 14: 1052-1064.
Zumarraga M, Camarero S, Martinez-Arias A, Ballesteros A, Plou FJ, Alcalde M: Altering the laccase functionality by in vivo assembly of mutant libraries with different mutational spectra. Proteins. 2008, 71 (1): 250-260.
Zumarraga M, Vaz Domínguez C, Camarero S, Shleev S, Polaina J, Martínez-Arias A, Ferrer M, de Lacey A, Fernández V, Ballesteros A, Plou FJ, Alcalde M: Combinatorial saturation mutagenesis of the Myceliophthora thermophila laccase T2 mutant: the connection between the C-terminal plug and the conserved 509VSG511tripeptide. Comb Chem High-Throughput Scr. 2008
Alcalde M, Zumarraga M, Polaina J, Ballesteros A, Plou FJ: Combinatorial saturation mutagenesis by in vivo overlap extension for the engineering of fungal laccases. Comb Chem High Throughput Screen. 2006, 9 (10): 719-727.
Xu F: Recent progress in laccase study: properties, enzymology, production, and applications. The encyclopedia of bioprocessing technology: fermentation, biocatalysis and bioseparation. Edited by: Flickinger MC, Drew SW. 1999, 1545-1554. NY, John Wiley & Sons
Baker WL, Sabapathy K, Vibat M, Lonergan G: Laccase catalyzes formation of an indamine dye between 3-methyl-2-benzothiazolinone hydrazone and 3-dimethylaminobenzoic acid. Enzyme Microb Technol. 1996, 18: 90-94.
Uyama H, Kobayashi S: Enzyme-catalyzed polymerization to functional polymers. J Mol Catal B: Enzym. 2002, 19–20: 117-127.
Setti L, Giuliani S, Spinozzi G, Pifferi PG: Laccase catalyzed oxidative coupling of 3-methyl 2-benzothiazolinone hydrazone and methoxyphenols. Enzyme Microb Technol. 1999, 25: 285-289.
Aktaş N, Çiçek H, Ünal AT, Kibarer G, Kolankaya N, Tanyolaç A: Reaction kinetics for laccase-catalyzed polymerization of 1-napthol. Bioresour Technol. 2001, 80: 29-36.
Schäfer A, Specht M, Hetzheim A, Francke W, Schauer F: Synthesis of substituted imidazoles and dimerization products using cells and laccase from Trametes versicolor. Tetrahedron. 2001, 57: 7693-7699.
Aktas N, Tanyolac A: Reaction conditions for laccase catalyzed polymerization of catechol. Bioresour Technol. 2003, 87: 209-214.
Boumans JWL, Nagtegaal RMA, Dunnewind A, Happe RP, Bos MA, Faegemand M, Degn P: A method for enzymatic cross-linking of a protein, cross-linked protein thus obtained and use thereof. WO Patent 2006/016809. 2006
Hajdok S, Leutbecher H, Greiner G, Conrad J, Beifuss U: Laccase initiated oxidative domino reactions for the efficient synthesis of 3, 4-dihydro-7, 8-dihydroxy-2 H-dibenzofuran-1-ones. Tetrahedr Lett. 2007, 48: 5073-5076.
Uchida H, Fukuda T, Miyamoto H, Kawabata T, Suzuki M, Uwajima T: Polymerization of bisphenol A by purified laccase from Trametes villosa. Biochem Biophys Res Commun. 2001, 287: 355-358.
Mattinen ML, Hellman M, Permi P, Autio K, Kalkkinen N, Buchert J: Effect of protein structure on laccase-catalyzed protein oligomerization. J Agric Food Chem. 2006, 54: 8883-8890.
Marzorati M, Danieli B, Haltrich D, Riva S: Selective laccase-mediated oxidation of sugars derivatives. Green Chem. 2005, 7: 310-315.
Baratto L, Candido A, Marzorati M, Sagui F, Riva S, Danieli B: Laccase-mediated oxidation of natural glycosides. J Mol Cat B: Enzym. 2006, 39: 3-8.
Ncanana S, Baratto L, Roncaglia L, Riva S, Burton SG: Laccase-mediated oxidation of totarol. Adv Synth Catal. 2007, 349: 1507-1513.
Suderman RJ, Dittmer NT, Kanost MR, Kramer KJ: Model reactions for insect cuticle sclerotization: Crosslinking of recombinant proteins upon their laccase-catalyzed oxidative conjugation with catechols. Insect Biochem Mol Biol. 2006, 36: 353-365.
Kurisawa M, Chung JE, Uyama H, Kobayashi S: Laccase-catalyzed synthesis and antioxidant property of poly(catechin). Macromol Biosci. 2003, 3: 758-764.
Aktas N, Kibarer G, Tanyolaç A: Effects of reaction conditions on laccase-catalysed 1-naphthol polymerisation. J Chem Technol Biotechnol. 2000, 75: 840-846.
Aktas N, Tanyolaç A: Kinetics of laccase-catalyzed oxidative polymerization of catechol. J Mol Catal B: Enzym. 2003, 22: 61-69.
Ceylan H, Kubilay S, Aktas N, Sahiner N: An approach for prediction of optimum reaction conditions for laccase-catalyzed bio-transformation of 1-naphthol by response surface methodology (RSM). Bioresour Technol. 2008, 99: 2025-2031.
Intra A, Nicotra S, Riva S, Danieli B: Significant and unexpected solvent influence on the selectivity of laccase-catalyzed coupling of tetrahydro-2-naphthol derivatives. Adv Synth Catal. 2005, 347: 973-977.
Dodrick JS, Marletta MA, Klibanov AM: Polymerization of phenols catalyzed by peroxidase in nonaqueous media. Biotechnol Bioeng. 1987, 30: 31-36.
Wariishi H, Nonaka D, Nishihashi S, Hirahashi T, Ito K: Enzymic preparation of polyphenylne oxides. Patent JP2006280259 A2. 2006
An ES, Kim SC, Kim YH, Park SY, Ryu JY, Song BK, Song JK: Production of phenolic polymers using bio-catalysts for polymerization. Patent KR2005011958A. 2005
Takahara J: Enzymic manufacture of polyphenylene oxide (PPO). Patent JP2004313057 A2. 2004
Ikeda R, Sugihara J, Uyama H, Kobayashi S: Enzymatic oxidative polymerization of 2, 6-dimethylphenol[J]. Macromol. 1996, 29: 8702-8705.
Ikeda R, Tanaka H, Uyama H, Kobayashi S: Laccase-catalyzed polymerization of acrylamide. Macromol Rapid Commun. 1998, 19: 423-425.
Ikeda R, Sugihara J, Uyama H, Kobayashi S: Enzymatic oxidative polymerization of 4-hydroxybenzoic acid derivatives to poly(phenylene oxide)s[J]. Polym Int. 1998, 47: 295-301.
Kobayashi S, Ikeda R, Oyabu H, Tanaka H, Kobayashi S: Artificial Urushi: Design, synthesis, and enzymatic curing of new urushiol analogues. Chem Lett. 2000, 29: 1214-1215.
Kobayashi S, Uyama H, Ikeda R: Artificial urushi. Chemistry. 2001, 7 (22): 4754-4760.
Ikeda R, Tsujimoto T, Tanaka H, Oyabu H, Uyama H, Kobayashi S: Man-made Urushi. Preparation of crosslinked polymeric films from renewable resources via air-oxidation processes. Proc Jpn Acad. 2000, 76B: 155-160.
Ikeda R, Tanaka H, Oyabu H, Uyama H, Kobayashi S: Preparation of artificial urushi via an environmentally benign process. Bull Chem Soc Jpn. 2001, 74: 1067-1073.
Uyama H, Kobayashi S: Enzymatic synthesis of polyesters via polycondensation. Adv Polym Sci. 2006, 194: 133-158.
Gübitz GM, Paulo AC: New substrates for reliable enzymes: enzymatic modification of polymers. Curr Opin Biotechnol. 2003, 14 (6): 577-582.
Gronqvist S, Rantanen K, Alen R, Mattinen ML, Buchert J, Viikari L: Laccase-catalysed functionalisation of TMP with tyramine. Holzforschung. 2006, 60: 503-508.
Felby C, Pedersen LS, Nielsen BR: Enhanced autoadhesion of wood fibers using phenol oxidases. Holzforschung. 1997, 51: 281-286.
Huttermann A, Mai C, Kharazipour A: Modification of lignin for the production of new compound and materials. Appl Microbiol Biotechnol. 2001, 55: 387-394.
Lund M, Ragauskas AJ: Enzymatic modification of kraft lignin through oxidative coupling with water-soluble phenols. Appl Microbiol Biotechnol. 2001, 55: 699-703.
Chandra RP, Ragauskas AJ: Evaluating laccase-facilitated coupling of phenolic acids to high-yield kraft pulps. Enzyme Microb Technol. 2002, 30: 855-861.
Bragd PL, van Bekkum H, Besemer AC: TEMPO-mediated oxidation of polysaccharides: survey of methods and applications. Topics in Catal. 2004, 27: 49-66.
Monti D, Candido D, Manuel Cruz Silva M, Køen V, Riva S, Danieli B: Biocatalyzed generation of molecular diversity: selective modification of the saponin asiaticoside. Adv Synth Catal. 2005, 347: 1168-1174.
Burton S: Laccases and phenol oxidases in organic synthesis. Curr Org Chem. 2003, 7: 1317-1331.
Ossiadacz J, Al-Adhami AJH, Bajraszewska D, Fischer P, Peczynska-Czoch W: On the use of Trametes versicolor laccase for the conversion of 4-methyl-3-hydroxyanthranilic acid to actinocin chromophore. J Biotechnol. 1999, 72: 141-149.
Pilz R, Hammer E, Schauer F, Kragl U: Laccase-catalyzed synthesis of coupling products of phenolic substrates in different reactors. Appl Microbiol Biotechnol. 2003, 60: 708-712.
Stahl P, Kissau L, Mazitschek R, Giannis A, Waldmann H: Natural product derived receptor tyrosin kinase inhibitors: Identification of IGF1R-, Tie-2 and VEGFR3 inhibitors. Angew Chem Int Ed Engl. 2002, 41 (7): 1174-1178.
Honma Y, Kasukabe T, Hozumi M, Shibata K, Omura S: Effects of herbimycin A derivatives on growth and differentiation of K562 human leukemic cells. Anticancer Res. 1992, 12: 189-192.
Mathew AE, Zee-Cheng KY, Cheng CC: Amino-substituted p-benzoquinones. J Med Chem. 1986, 29: 1792-1795.
Zee-Cheng KY, Cheng CC: Preparation and the results of antitumor screening of some substituted amino-, azido-, halogeno- and hydroxy-p-benzoquinones. J Med Chem. 1970, 13: 264-268.
Pöckel D, Niedermeyer THJ, Pham HTL, Mikolasch A, Mundt S, Lindequist U, Lalk M, Werz O: Inhibition of human 5-lipoxygenase and anti-neoplastic effects by 2-amino-1, 4-benzoquinones. Med Chem. 2006, 2: 591-595.
Timo HJN, Michael L: Nuclear amination catalyzed by fungal laccases: Comparison of laccase catalyzed amination with known chemical routes to aminoquinones. J Mol Catal B: Enzym. 2007, 45: 113-117.
Molino BF, Haydar SN, Yang Z, Michels PC, Hemenway MS, Rich JO, Khmelnitsky Y: Preparation of novel cyclosporins. Patent WO2004082629 A2. 2004
Hosny M, Rosazza JPN: Novel oxidations of (+)-catechin by horseradish peroxidase and laccase. J Agric Food Chem. 2002, 50: 5539-5545.
Intra A: Ossidazione di derivati fenolici ad opera di laccasi. Tesi di laurea, Universita' di Milano. 2003
Shiba T, Xiao L, Miyakoshi T, Chen C-L: Oxidation of isoeugenol and coniferyl alcohol catalyzed by laccase isolated from Rhus vernicifera Stokes and Pycnoporus coccineus. J Mol Catal B: Enzym. 2000, 10: 605-615.
Niedermeyer THJ, Mikolasch A, Lalk M: Nuclear amination catalyzed by fungal laccases: reaction products of p-hydroquinones and primary aromatic amines. J Org Chem. 2005, 70: 2002-2008.
Manda K, Hammer E, Mikolasch A, Niedermeyer T, Dec J, Daniel Jones A, Benesi AJ, Schauer F, Bollag JM: Laccase-induced cross-coupling of 4-aminobenzoic acid with para-dihydroxylated compounds 2, 5-dihydroxy-N-(2-hydroxyethyl)-benzamide and 2, 5-dihydroxybenzoic acid methyl esters. J Mol Catal B: Enzym. 2005, 35: 86-92.
Acknowledgements
This material is based upon work funded by the Spanish Ministry of Education and Science (projects VEM2004-08559, CTQ2005-08925-C02-02/PPQ, PIE200880I033); EU project NMP2-CT-2006-026456; CSIC project 200580M121 and Ramon y Cajal Programme. Dr. A. Kunamneni is the recipient of a Marie Curie fellowship (MIF1-CT-2006-040163) of EU's FP6.
Author information
Authors and Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AB suggested the topic and got the approval from the Editor. AK wrote the first draft. MA wrote the second draft, which was revised critically and contributed additional content throughout by AK, AB, FJP, SC and CGB. MA coordinated the final version of the review, which was read and approved by all authors.
Authors’ original submitted files for images
Below are the links to the authors’ original submitted files for images.
Rights and permissions
Open Access This article is published under license to BioMed Central Ltd. This is an Open Access article is distributed under the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
About this article
Cite this article
Kunamneni, A., Camarero, S., García-Burgos, C. et al. Engineering and Applications of fungal laccases for organic synthesis. Microb Cell Fact 7, 32 (2008). https://doi.org/10.1186/1475-2859-7-32
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/1475-2859-7-32