Abstract
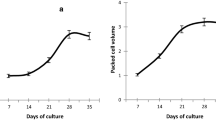
The influence of fungus elicitor Aspergillus flavus on alkaloid yield was investigated in Catharanthus roseus. The study reveals increased yield of vinblastine and vincristine in cultivated tissues. Different concentrations of extract applied to solid MS medium were: 0.05 % (T1), 0.15 % (T2), 0.25 % (T3), and 0.35 % (T4) along with control (T0). The callus biomass, embryo formation and plant regeneration were studied in response to elicitor treatments. The embryogenic callus was induced from hypocotyls of in vitro germinated seeds and various tissues were exposed to fungal elicitation. The use of A. flavus fungal elicitation improved callus biomass growth, which later differentiated into embryos, maximum somatic embryo induction being in T2 (106.53/callus mass). Biochemical analysis revealed more accumulation of sugar, protein and proline in growing tissues especially amended with elicitor. The somatic embryos germinated into plantlets on 2.24 µM BA added MS medium. The percent germination, shoot-, root length of germinated somatic embryos were high in low doses of elicitation (T1/T2). The quantitative analysis of vinblastine and vincristine yield was conducted in different elicitor treated tissues by the use of HPTLC. Vinblastine yield was maximum in germinating embryos (0.837 µg gm−1 dry weight), A. flavus elicitation at T2 improved vinblastine yield further (0.903 µg gm−1 dry weight). Compared to vinblastine, the yield of vincristine was low and on A. flavus addition, maximum vincristine yield was noted (0.216 µg gm−1 dry weight). The highest 7.88 and 15.50 % increased yield of vinblastine and vincristine respectively was noted on A. flavus elicitated tissues. In order to understand the role of elicitor on plant defense responses various antioxidant enzymes activity were investigated as the addition of elicitor induced cellular stress on tissues. Maturated and germinating somatic embryos had high SOD activity and on elicitation the activity of enzymes was further increased, indicating extra cellular stress on tissues, which yielded enriched level of vinblastine and vincristine at T2/T1.






Similar content being viewed by others
References
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Andrade SAL, Malik S, Sawaya ACHF, Bottcher A, Mazzafera P (2013) Association with arbuscular mycorrhizal fungi influences alkaloid synthesis and accumulation in Catharanthus roseus and Nicotiana tabacum plants. Acta Physiol Plant 35:867–880
Bates L, Waldren PP, Teare JD (1973) Rapid determination of free proline of water stress studies. Plant Soil 39:205–207
Benkirane H, Sabounji K, Chlyah A, Chlyah H (2000) Somatic embryogenesis and plant regeneration from fragments of immature inflorescences and coleoptiles of durum wheat. Plant Cell Tissue Org Cult 61:107–113
Bilgin DD, Zavala JA, Zhu JIN, Clough SJ, Ort DR, DeLucia E (2010) Biotic stress globally downregulates photosynthesis genes. Plant, Cell Environ 33(10):1597–1613
Binder BY, Peebles CA, Shanks JV, San KY (2009) The effects of UV-B stress on the production of terpenoidindole alkaloids in Catharanthus roseus hairy roots. Biotechnol Prog 25:861–865
Bradford MM (1976) A rapid and sensitive method for quantification of microgram quantities of protein, utilizing the principle of protein dye binding. Anal Biochem 72:248–541
Cai Z, Kastell A, Mewis I, Knorr D, Smetanska I (2012) Polysaccharide elicitors enhance anthocyanin and phenolic acid accumulation in cell suspension cultures of Vitis vinifera. Plant Cell Tissue Org Cult 108:401–409
Chen J-B, Wang S-M, Jing R-L, Mao X-G (2009) Cloning the PvP5CS gene from common bean (Phaseolus vulgaris) and its expression patterns under abiotic stresses. J Plant Physiol 166:12–19
Cheng DM, Yousef GG, Grace MH, Rogers RB, Gorelick-Feldman J, Raskin I, Lila MA (2008) In vitro production of metabolism enhancing phytoecdysteroids from Ajuga turkestanica. Plant Cell Tissue Org Cult 93(1):73–83
Coste A, Vlase L, Halmagyi A, Deliu C, Coldea G (2011) Effects of plant growth regulators and elicitors on production of secondary metabolites in shoot cultures of Hypericum hirsutum and Hypericum maculatum. Plant Cell Tissue Org Cult 106(2):279–288
D’Souza MR, Devaraj VR (2010) Biochemical responses of Hyacinth bean (Lablab purpureus) to salinity stress. Acta Physiol Plant 32:341–353
De Schutter K, Joubes J, Cools T, Verkest A, Corellou F, Babiychuk E, Der Schueren E-V, Beeckman T, Kushnir S, Inze D, De Veylder L (2007) Arabidopsis WEE1 kinase controls cell cycle arrest in response to activation of the DNA integrity checkpoint. Plant Cell 19:211–225
Dey PM (1990) Methods in plant biochemistry. Carbohydarates, vol 2. Academic Press, London
Dhindsa RH, Plumb-Dhindsa R, Thorpe TA (1981) Leaf senescence correlated with increased level of membrane permeability, lipid peroxidation and decreased level of SOD and CAT. J Exp Bot 32:93–101
Elkahoui S, Hernandez JA, Abdelly C, Ghrir R, Limam F (2005) Effects of salt on lipid peroxidation and antioxidant enzyme activities of Catharanthus roseus suspension cells. Plant Sci 168:607–613
Elmaghrabi AM, Ochatt S, Rogers HJ, Francis D (2013) Enhanced tolerance to salinity following cellular acclimation to increasing NaCl levels in Medicago truncatula. Plant Cell Tissue Org Cult 114:61–70
El-Sayed M, Verpoorte R (2007) Catharanthus terpenoid indole alkaloids: biosynthesis and regulation. Phytochem Rev 6:277–305
Facchini PJ, De Luca VD (2008) Opium poppy and Madagascar periwinkle: model non-model systems to investigate alkaloid biosynthesis in plants. Plant J 54:763–784
Feher A (2015) Somatic embryogenesis—Stress-induced remodeling of plant cell fate. Biochim Biophys Acta 1849(4):385–402
Fei L, Weathers PJ (2014) From cells to embryos to rooted plantlets in a mist bioreactor. Plant Cell Tissue Org Cult 116:37–46
Huttner C, Beuerle T, Scharnhop H, Ernst L, Beerhues L (2010) Differential effect of elicitors on biphenyl and dibenzofuran formation in Sorbus aucuparia cell cultures. J Agric Food Chem 58(22):11977–11984
Junaid A, Mujib A, Bhat MA, Sharma MP (2006) Somatic embryo proliferation, maturation and germination in Catharanthus roseus. Plant Cell Tissue Org Cult 84:325–332
Junaid A, Mujib A, Sharma MP (2010) Variations in vinblastine production at different stages of somatic embryogenesis, embryo and field grown plantlets of Catharanthus roseus L. (G) Don, as revealed by HPLC. In Vitro Cell Dev Biol-Plant 46:348–353
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plant Res 3:1222–1239
Kawana Y, Sasamoto H (2008) Stimulation effects of salts on growth in suspension culture of a mangrove plant, Sonneratia alba, compared with another mangrove, Bruguiera sexangula and nonmangrove tobacco BY-2 cells. Plant Biotechnol 25:151–155
Kiran Ghanti S, Sujata KG, Rao S, Udayakumar M, KaviKishor PB (2009) Role of enzymes and identification of stage-specific proteins in developing somatic embryos of chickpea (Cicer arietinum L.) In Vitro Cell. Dev Biol-Plant 45:667–672
Kornfeld A, Kaufman PB, Lu CR, Gibson DM, Bolling SF, Warber SL, Chang SC, Kirakosyan A (2007) The production of hypericins in two selected Hypericum perforatum shoot cultures is related to differences in black gland culture. Plant Physiol Biochem 45:24–32
Kundu S, Chakraborty D, Kundu A, Pal A (2013) Proteomics approach combined with biochemical attributes to elucidate compatible and incompatible plant-virus interactions between Vigna mungo and Mungbean Yellow Mosaic India Virus. Proteome Sci 11:15. doi:10.1186/1477-5956-11-15
Lovkova MY, Buzuk GN, Sokolova SM, Buzuk LN (2005) Role of elements and physiologically active compounds in the regulation of synthesis and accumulation of indole alkaloids in Catharanthus roseus L. Appl Biochem Microbiol 41:299–305
Martin EK, Vishal G, Susan CR (2008) Pharmaceutically active natural product synthesis and supply via plant cell culture technology. Mol Pharm 5:243–256
Memelink J, Verpoorte R, Kijne JW (2001) Orcanization of jasmonate-responsive gene expression in alkaloid metabolism. Trends Plant Sci 6:212–219
Misic D, Siler B, Zivkovic NJ, Simonovic A, Maksimovic V, Budimir S, Janosevic D, Durickovic M, Nikolic M (2012) Contribution of inorganic cations and organic compounds to osmotic adjustment in root cultures of two Centaurium species differing in tolerance to salt stress. Plant Cell Tissue Org Cult 108:389–400
Miura Y, Hirata K, Miyamoto K, Uchida K (1988) Formation of vinblastine from multiple shoot culture of Catharanthus roseus. Planta Med 54:8–20
Moreno PRH (1995) Influence of stress factors on the secondary metabolism in suspension cultured Catharanthus roseus cells. Ph.D. thesis, Leiden University, Leiden
Mujib A, Samaj J (2006) Somatic embryogenesis. Springer, Berlin
Mujib A, Ilah A, Gandotra N, Abdin MZ (2003) In vitro application to improve alkaloid yield in Catharanthus roseus. In: Govil JN, Kumar PA, Singh VK (eds) Biotechnology and genetic engineering. Recent progress in medicinal plants, vol IV. Sci. Tech., Houston, pp 415–440
Mujib A, Ilah A, Aslam J, Fatima S, Siddiqui ZH, Maqsood M (2012) Catharanthus roseus alkaloids: application of biotechnology for improving yield. Plant Growth Reg 68:111–127
Mukherjee AK, Basu S, Sarkar N, Ghosh AC (2001) Advances in cancer therapy with plant based natural products. Curr Med Chem 8:1467–1486
Mulabagal V, Tsay HS (2004) Plant cell cultures- an alternative and efficient source for the production of biologically important secondary metabolites. Int J Appl Sci Eng 2:29–48
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol Plant 15:473–497
Murthy HN, Lee EJ, Paek KY (2014) Production of secondary metabolites from cell and organ cultures: strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Org Cult 118:1–16
Mustafa NR, Kim HK, Choi YH, Erkelens C, Lefeber AW, Spijksma G, van der Heijden R, Verpoorte R (2009) Biosynthesis of salicylic acid in fungus elicited Catharanthus roseus cells. Phytochemistry 70:532–539
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Pasternak TP, Prinsen E, Ayaydin F, Miskolezi P, Potters G, Asard H, Van Onckelen A, Dudits D, Feher A (2002) The role of auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast derived cells of alfalfa. Plant Physiol 129:1807–1819
Pawar KD, Yadav AV, Shouche YS, Thengane SR (2011) Influence of endophytic fungal elicitation on production of inophyllum in suspension cultures of Calophyllum inophyllum L. Plant Cell Tissue Org Cult 106(2):345–352
Prasad A, Mathur A, Kalra A, Gupta MM, Lal RK, Mathur AK (2013) Fungal elicitor-mediated enhancement in growth and asiaticoside content of Centella asiatica L. shoot cultures. Plant Growth Reg 69:265–273
Radman R, Saez T, Bucke C, Keshavarz T (2003) Elicitation of plants and microbial cell systems. Biotechnol Appl Biochem 37:91–102
Ramani S, Jayabaskaran C (2008) Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. J Mol Signal 25(3):9
Rhee HS, Cho HY, Son SY, Yoon SYH, Park JM (2010) Enhanced accumulation of decursin and decursinol angelate in root cultures and intact roots of Angelica gigas Nakai following elicitation. Plant Cell Tissue Org Cult 101(3):295–302
Roja Rani A, Reddy VD, PrakashBabu P, Padmaja G (2005) Changes in protein profiles associated with somatic embryogenesis in peanut. Biol Plant 49:347–354
Saiman MZ, Mustafa NR, Pomahocova B, Verberne M, Verpoorte R, Choi YH, Schulte AE (2014) Analysis of metabolites in the terpenoid pathway of Catharanthus roseus cell suspensions. Plant Cell Tissue Org Cult 117:225–239
Samar F, Mujib A, Samaj J (2011) Anti-oxidant enzyme responses during in vitro embryogenesis in Catharanthus roseus. J Hort Sci Biotechnol 86(6):569–574
Samar F, Mujib A, Dipti T (2015) NaCl amendment improves vinblastine and vincristine synthesis in Catharanthus roseus: a case of stress signalling as evidenced by antioxidant enzymes activities. Plant Cell Tissue Org. Cult. doi:10.1007/s11240-015-0715-5
Shibli RA, Kushad M, Yousef GG, Lila MA (2007) Physiological and biochemical responses of tomato microshoots to induced salinity stress with associated ethylene accumulation. Plant Growth Reg 51:159–169
Siahsar B, Rahimi M, Tavasssoli A, Raissi AS (2011) Application of biotechnology in production of medicinal plants. Am-Euras J Agric Environ Sci 11:439–444
Singh G, Gavrieli J, Oakey JS, Curtis WR (1998) Interaction of methyl jasmonate, wounding and fungal elicitation during sesquiterpene induction in Hyoscyamus muticus in root cultures. Plant Cell Rep 17:391–395
Song Y (2013) Insight into the mode of action of 2,4-Dichlorophenoxyacetic acid (2,4-D) as an herbicide. J Integr Plant Biol 56:106–113
Sorrell DA, Marchbank A, McMahon K, Dickinson JR, Rogers HJ, Francis D (2002) A WEE1 homologue from Arabidopsis thaliana. Planta 215:518–522
Staniszewska I, Krolicka A, Malinski E, Jojkowska E, Szafranek JZ (2003) Elicitation of secondary metabolites is in vitro cultures of Ammi majus L. Enzy Microbial Technol 33:565–568
Valluri JV (2009) Bioreactor production of secondary metabolites from cell cultures of periwinkle and sandalwood. Methods Mol Biol 547:325–335
Van der Heijden R, Jacobs DT, Snoeijer W, Hallard D, Verpoorte R (2004) The Catharanthus alkaloids: pharamacognosy and biochemistry. Curr Med Chem 11:607–628
Verma P, Mathur AK, Srivastava A, Mathur A (2012) Emerging trends in research on spatial and temporal organization of terpenoid indole alkaloid pathway in Catharanthus roseus. Protoplasma 249:255–268
Vinterhalter B, Jankovic T, Sovikin L, Nikolic R, Vinterhalter D (2008) Propagation and xanthone content of Gentianella austriaca shoot cultures. Plant Cell Tissue Org Cult 94:329–335
Winkelmann T, Muβmann V, Serek M (2004) Cryopreservation of embryogenic cell suspension cultures of Cyclamen persicum Mill. Plant Cell Rep 23:1–8
Xu M, Dong J (2005) Elicitor-induced nitric oxide burst is essential for triggering catharanthine synthesis in Catharanthus roseus suspension cells. Appl Microbiol Biotechnol 67:40–44
Zahid SH, Mujib A (2012) Accumulation of vincristine in calcium chloride elicitated Catharanthus roseus cultures. Nat Prod J 2(9):307–315
Zahid SH, Mujib A, Maqsood M (2011) Liquid overlaying improves somatic embryogenesis in Catharanthus roseus. Plant Cell Tissue Org Cult 104:247–256
Zhao J, Hu Q, Guo YQ, Zhu WH (2001) Effects of stress factors, bioregulators, and synthetic precursors on indole alkaloid production in compact callus clusters cultures of Catharanthus roseus. Appl Microbiol Biotechnol 55:693–698
Zizhen L, Tiantian Z, Xiaoqian Z, Jia Z, Changqi Z (2015) An alkaloid and a steroid from the endophytic fungus Aspergillus fumigatus. Molecules 20:1424–1433
Zubek S, Mielcarek S, Turnau K (2012) Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 22:149–156
Acknowledgments
The authors are highly thankful to University Grant Commission (UGC) and Department of Botany, Hamdard University (Jamia Hamdard) for receiving financial assistance and other research facilities.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Tonk, D., Mujib, A., Maqsood, M. et al. Aspergillus flavus fungus elicitation improves vincristine and vinblastine yield by augmenting callus biomass growth in Catharanthus roseus . Plant Cell Tiss Organ Cult 126, 291–303 (2016). https://doi.org/10.1007/s11240-016-0998-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-016-0998-1