Abstract
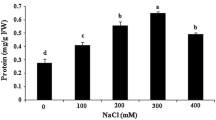
Salinity is a major abiotic stress that limits plant productivity. Plants respond to salinity by switching on a coordinated set of physiological and molecular responses that can result in acclimation. Medicago truncatula is an important model legume species, thus understanding salt stress responses and acclimation in this species is of both fundamental and applied interest. The aim of this work was to test whether acclimation could enhance NaCl tolerance in calli of M. truncatula. A new protocol is described incorporating multi-step up acclimation over 0–350 mM exogenous NaCl. By the end of the experiment, calli were tolerant to 150 mM and competent for embryogenesis at 100 mM NaCl. Positive and negative linear relationships between Na+ and K+ uptake and exogenous NaCl concentration intercepted at 160 mM suggesting a Na+/K+ homeostasis. Proline level peaked at 100/150 mM whilst highest osmolarity and lowest water content occurred at 250/350 mM NaCl. The concentration of water soluble sugars was positively related to 0–250 mM NaCl whilst callus growth and embryogenesis occurred regardless of endoreduplication. Expression of genes linked to growth (WEE1), in vitro embryogenesis (SERK), salt tolerance (SOS1), proline synthesis (P5CS) and ploidy level (CCS52 and WEE1) peaked at 100/150 mM NaCl. Hence, these genes and various physiological traits except sugar levels, served as useful markers of NaCl tolerance. To our knowledge, this is the first report of a multi-step acclimation conferring tolerance to 150 mM NaCl in leaf-derived calli of M. truncatula.





Similar content being viewed by others
Abbreviations
- 2,4-D,:
-
2,4-Dichlorophenoxyacetic acid
- AGR:
-
Absolute growth rate(s)
- CCS52 :
-
Cell cycle switch52
- ECR:
-
Embryo conversion and rooting medium
- EDM:
-
Embryo development medium
- EID:
-
Embryo induction development
- FW:
-
Fresh weight
- MS:
-
Murashige and Skoog (1962)
- P5CS :
-
γ-Glutamyl kinase
- RGR:
-
Relative growth rate
- SEM:
-
Somatic embryogenesis
- SERK :
-
Somatic embryogenesis receptor-like kinase
- SOS1 :
-
Salt overly sensitive
References
Ashraf M, Fatima H (1995) Responses of some salt tolerant and salt sensitive lines of safflower (Carthamus tinctorius L.). Acta Physiol Plant 17:61–71
Barker DG, Bianchi S, London F, Dattee Y, Duc G, Essad S, Flament P, Gallusci P, Genier G, Guy P et al (1990) Medicago truncatula, a model plant for studying the molecular genetics of the Rhizobiumlegume symbiosis. Plant Mol Biol 8:40–49
Boukel M, Houassine D (1997) Adaptation au stress hydrique de quelques variétés de blé dur (Triticum durum), thèse magistère, INA, Algérie: 90
Bourdon M, Frange N, Nafati M, Mathieu E, Cheniclet C, Renaudin J-P, Chevalier C (2010) Endoreduplication and growth of fleshy fruits. Heidelberg Germany, In, Progress in Botany (eds Lütgge U, Beyschlag W, Büdel B, Francis D) Springer, Springer, pp 101–132
Cebolla A, Vinardell JM, Kiss E, Ola-h B, Roudier F, Kondorosi A, Kondorosi E (1999) The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO J 18:4476–4484
Chai M, Jia Y, Chen S, Gao Z, Wang H, Liu L, Wang P, Hou D (2011) Callus induction, plant regeneration, and long-term maintenance of embryogenic cultures in Zoysia matrella [L.] Merr. Plant Cell Tissue Organ Cult 104:187–192
Chen Z, Pottosin II, Cuin TA, Fuglsang AT, Tester M, Jha D, Zepeda-Jazo I, Zhou M, Palmgren MG, Newman IA, Shabala S (2007) Root plasma membrane transporters controlling K+/Na+ homeostasis in salt stressed barley. Plant Physiol 145:1714–1725
Chen J-B, Wang S-M, Jing R-L, Mao X-G (2009) Cloning the PvP5CS gene from common bean (Phaseolus vulgaris) and its expression patterns under abiotic stresses. J Plant Physiol 166:12–19
Chen S, Chai M, Jia Y, Gao Z, Li Z, Gu M (2011) In vitro selection of salt tolerant variants following 60Co gamma irradiation of long-term callus cultures of Zoysia matrella [L.] Merr. Plant Cell Tiss Organ Cult 107:493–500
Davenport SB, Gallego SM, Benavides MP, Tomaro ML (2003) Behaviour of antioxidant defense system in the adaptive response to salt stress in Helianthus annuus L. cells. Plant Growth Regul 40:81–88
De Schutter K, Joubes J, Cools T, Verkest A, Corellou F, Babiychuk E, Der Schueren E-V, Beeckman T, Kushnir S, Inze D, De Veylder L (2007) Arabidopsis WEE1 kinase controls cell cycle arrest in response to activation of the DNA integrity checkpoint. Plant Cell 19:211–225
Elmaghrabi AM, Ochatt SJ (2006) Isoenzymes and flow cytometry for the assessment of true-to-typeness of calluses and cell suspension of barrel medic prior to regeneration. Plant Cell Tissue Org Cult 85:31–43
Gonzalez N, Hernould M, Delmas F, Gevaudant F, Duffe P, Causse M, Mouras A, Chevalier C (2004) Molecular characterization of a WEE1 gene homologue in tomato (Lycopersicon esculentum Mill.). Plant Mol Biol 56:849–861
Gonzalez N, Gevaudant F, Hernould M, Chevalier C, Mouras A (2007) The cell cycle associated protein kinase WEE1 regulates cell size in relation to endoreduplication in developing tomato fruit. Plant J 51:642–655
Gossett DR, Banks SW, Millhollon EP, Lucas MC (1996) Antioxidant response to NaCl stress in a control and a NaCl-tolerant cotton cell line grown in the presence of paraquat, buthionine sulfoximine and exogenous glutathione. Plant Physiol 112:803–809
Hariadi Y, Marandon K, Tian Y, Jacobsen S-E, Shabala S (2011) Ionic and osmotic relations in quinoa (Chenopodium quinoa Willd.) plants grown at various salinity levels. J Exp Bot 62:1–9
Hu H, Xiong L, Yang Y (2005) Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta 222:107–117
Iantcheva A, Vlahova M, Atanassov A (2006) Somatic embryogenesis from leaf explants of Medicago truncatula cv. Jemalong genotype 2HA. Medicago truncatula handbook, pp 1–9
Larson-Rabin Z, Li Z, Masson PH, Day CD (2009) FZR2/CCS52A1 Expression is a determinant of endoreduplication and cell expansion in Arabidopsis. Plant Physiol 149:874–884
Leone A, Costa A, Tucci M, Grillo S (1994) Adaptation versus shock response to polyethylene glycol-induced low water potential in cultured potato cells. Physiol Plant 92:21–30
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔC method. Methods 25:402–408
Merchan F, de Lorenzo L, Rizzos SG, Niebel A, Manyani H, Frugier F, Sousa C, Crespi M (2007) Identification of regulatory pathways involved in the reacquisition of root growth after salt stress in Medicago truncatula. Plant J 51:1–17
Miki Y, Hashiba M, Hisajima S (2001) Establishment of salt stress tolerant rice plant through set up NaCl treatment in vitro. Plant Biol 44:391–395
Misic D, Siler B, Zivkovic NJ, Simonovic A, Maksimovic V, Budimir S, Janosevic D, Ðurickovic M, Nikolic M (2012) Contribution of inorganic cations and organic compounds to osmotic adjustment in root cultures of two Centaurium species differing in tolerance to salt stress. Plant Cell Tissue Organ Cult 108:389–400
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Nolan KE, Rose RJ, Gorst JG (1989) Regeneration Medicago truncatula from tissue culture: increased somatic embryogenesis using explants from regenerated plants. Plant Cell Rep 8:278–281
Nolan KE, Kurdyukov S, Rose RJ (2009) Expression of the somatic embryogenesis receptor like kinase1 (SERK1) gene is associated with developmental change in the life cycle of model legume Medicago truncatula. J Exp Bot 60:1759–1771
Ochatt SJ (2008) Flow cytometry in plant breeding. Cytometry A 73:581–598
Ochatt SJ, Marconi PL, Radice S, Arnozis PA, Caso OH (1999) In vitro recurrent selection of potato: production and characterization of salt tolerant cell lines and plants. Plant Cell Tissue Org Cult 55:1–8
Ochatt SJ, Pontecaille C, Rancillac M (2000) The growth regulators used for bud regeneration and shoot rooting affect the competence for flowering and seed set in regenerated plants of protein pea. In Vitro Cell Dev Biol Plants 36:188–193
Ochatt S, Jacas L, Patat-Ochatt EM, Djennane S (2013) Flow cytometric analysis and molecular characterization of Agrobacterium tumefaciens-mediated transformants of Medicago truncatula. Plant Cell Tissue Organ Cult. doi:10.1007/s11240-012-0263-1
Oldroyd GED, Geurts R (2001) Medicago truncatula, going where no plant has gone before. Trends Plant Sci 6:552–554
Plummer DT (1987) Introduction to Practical Biochemistry, 3rd edn. McGraw Hill Book Company Ltd, London, pp 179–180
Queiros F, Fidalgo F, Santos I, Salema R (2007) In vitro selection of salt tolerant cell lines in Solanum tuberosum L. Plant Biol 51:728–734
Raven JA (1985) Regulation of pH and generation of osmolarity in vascular plants: a cost-benefit analysis in relation to efficiency of use of energy, nitrogen and water. New Phytol 101:25–77
Rhind N, Russell P (2000) Chk1 and Cds1: linchpins of the DNA damage and replication checkpoint pathways. J Cell Sci 113:3896–3899
Rubio MC, González EM, Minchin FR, Webb KJ, Arrese-lgor C, Ramos J, Becana M (2002) Effects of water stress on antioxidant enzymes of leaves and nodules of transgenic alfalfa overexpressing superoxide dismutases. Plant Physiol 115:531–540
Shankhdhar D, Shankhdhar SC, Mani SC (2000) In vitro selection for salt tolerance in rice. Plant Biol 43:477–480
Shi H, Quintero FJ, Pardo JM, Zhu JK (2002) The putative plasma membrane Na+/H+ antiporter SOS1 controls long-distance Na+ transport in plants. Plant Cell 14:465–477
Shi HZ, Lee B-H, Wu S-J, Zhu J-K (2003) Overexpression of a plasma membrane Na+/H+ antiporter gene improves salt tolerance in Arabidopsis thaliana. Nat Biotechnol 21:81–85
Shinozaki K, Yamaguchi-Shinozaki K (2007) Gene networks involved in drought stress response and tolerance. J Exp Bot 58:221–227
Silva-Ortega CO, Ochoa-Alfaro AE, Reyes-Agüero JA, Aguado-Santacruz GA, Jiménez-Bremont JF (2008) Salt stress increases the expression of p5cs gene and induces proline accumulation in cactus pear. Plant Physiol Biochem 46:82–92
Sivanesan I, Lim YM, Jeong RB (2011) Somatic embryogenesis and plant regeneration from leaf and petiole explants of Campanula punctata Lam. var. rubriflora Makino. Plant Cell Tiss Organ Cult 107:365–369
Skirycz A, Claeys H, De Bodt S, Oikawa A, Shinoda S, Andriankaja M, Maleux K, Nubia BE, Eloy B, Coppens F, Yoo S-D, Saito K, Inzé D (2011) Pause-and-stop: the effects of osmotic stress on cell proliferation during early leaf development in Arabidopsis and a role for ethylene signaling in cell cycle arrest. Plant Cell 23:1876–1888
Sorrell DA, Marchbank A, McMahon K, Dickinson JR, Rogers HJ, Francis D (2002) A WEE1 homologue from Arabidopsis thaliana. Planta 215:518–522
Spadafora ND, Doonan JH, Herbert RJ, Bitonti NB, Wallace E, Rogers HJ, Francis D (2011) Arabidopsis T-DNA insertional lines for CDC25 are hypersensitive to hydroxyurea but not to zeocin or salt stress. Ann Bot 107:1183–1192
Spadafora ND, Parfitt D, Li S, Bruno L, Vaughan R, Buchanan-Wollaston V, Herbert RJ, Bitonti MB, Doonan J, Albani D, Prinsen E, Francis D, Rogers HJ (2012) Perturbation of cytokinin and ethylene-signalling pathways explain the strong rooting phenotype exhibited by Arabidopsis expressing the Schizosaccharomyces pombe mitotic inducer, cdc25. BMC Plant Biol 12:45
Sun Y, Dilkes BP, Zhang C, Dante RA, Carneiro NP, Lowe KS, Jug R, Gordon-Kamm WJ, Larkins BA (1999) Characterization of maize (Zea mays L.) Wee1 and its activity in developing endosperm. Proc Natl Acad Sci USA 96:4180–4185
Svetoslavova G, Vlahova M, Iantcheva A, Atanassov A (2005) High frequency plant regeneration of diploid Medicago coerulea through somatic embryogenesis. Biotechnol Biotechnol Equip 19:57–61
Trinh TH, Ratet P, Kondorosi E, Durand P, Kamate K, Bauer P, Kondorosi A (1998) Rapid and efficient transformation of diploid Medicago truncatula and Medicago sativa ssp. Falcata lines improved in somatic embryogenesis. Plant Cell Rep 17:345–355
Troll W, Lindsley J (1955) A photometric method for the determination of proline. J Biol Chem 215:655–660
Türkan I, Demiral T (2009) Recent developments in understanding salinity tolerance. Environ Exp Bot 67:2–9
Vandepoele K, Raes J, De Veylder L, Rouzé PS, Inzé D (2002) Genome-wide analysis of core cell cycle genes in Arabidopsis. Plant Cell 14:903–916
Vinayak H, Lokhande TD, Nikam VY, Patade ML, Ahire PS (2011) Effects of optimal and supra-optimal salinity stress on antioxidative defence, osmolytes and in vitro growth responses in Sesuvium portulacastrum L. Plant Cell Tiss Organ Cult 104:41–49
West G, Inzé D, Beemster GTS (2004) Cell cycle modulation in the response of the primary root of arabidopsis to salt stress. Plant Physiol 135:1050–1058
Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci 12:615–620
Young DY, Udvardi M (2009) Translating Medicago truncatula genomics to crop legumes. Curr Opin Plant Biol 12:193–201
Zhu J-K (2002) Salt and drought stress signal transduction in plants. Ann Rev Plant Biol 53:2273–2467
Acknowledgments
AME thanks the Libyan Government for financial support, and we thank Mike O’Reilly for assistance with analytical work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Elmaghrabi, A.M., Ochatt, S., Rogers, H.J. et al. Enhanced tolerance to salinity following cellular acclimation to increasing NaCl levels in Medicago truncatula . Plant Cell Tiss Organ Cult 114, 61–70 (2013). https://doi.org/10.1007/s11240-013-0306-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-013-0306-2