Abstract
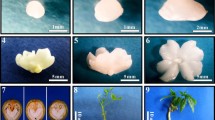
Accumulation of proline, activities of peroxidase (POX), catalase (CAT), phenylalanine ammonia lyase (PAL) and malate dehydrogenase (MDH) were studied during different developmental stages of somatic embryos in chickpea. Callus cultures that did not form somatic embryos served as control. While increased levels of proline and POX activity were noticed in globular stages of embryos, CAT activity increased during early and late heart-shaped embryo formation indicating tissue-specific activation of these enzymes. The activity of PAL reached a peak during torpedo and cotyledonary stages of embryo development. On the other hand, MDH activity enhanced during the germination of somatic embryos inferring more requirement of energy during this stage. Electrophoretic (sodium dodecyl sulfate polyacrylamide gel electrophoresis) pattern of proteins revealed that ten bands are associated with non-embryogenic tissues, whereas 11 bands with globular, heart, torpedo and cotyledonary stages of embryo development and nine bands during the germination stage of embryos. Two extra stage-specific protein bands with molecular masses of 16 and 18 kDa appeared during globular, heart, torpedo, and cotyledonary stages. But, these bands disappeared during germination of embryos and are absent in non-embryogenic cultures. This study thus may help in the identification of proteins and the role of above enzymes during different developmental stages of somatic embryo induction and their maturation in a recalcitrant leguminous crop plant chickpea.


Similar content being viewed by others
References
Bagnoli F.; Capuana M.; Racchi M. L. Developmental changes of catalase and superoxide dismutase isoenzymes in zygotic and somatic embryos of horse chestnut. Aust. J. Plant Physiol. 25: 909–913; 1998.
Bates L. S.; Waldren R. P.; Teare I. D. Rapid determination of free proline for water stress studies. Plant Soil. 39: 205–207; 1973. doi:10.1007/BF00018060.
Bewley J. D.; Black M. Seeds physiology of development and germination. 2nd ed. Plenum Press, New York1994.
Brueske C. H. Phenylalanine ammonia lyase activity in tomato roots infected and resistant to the root-knot nematode (Meloidogyne incogni). Physiol. Plant Pathol. 16: 409–414; 1980.
Chengalrayan K.; Hazra S.; Gallo-Meagher M. Histological analysis of somatic embryogenesis and organogenesis induced from mature zygotic embryo-derived leaflets of peanut (Arachis hypogaea L.). Plant Sci. 161: 415–421; 2001. doi:10.1016/S0168-9452(01)00439-3.
Cordewener J.; Booij H.; Vander Zett H.; Van Engelen F.; VanKammen A.; deVries S. C. Tunicamycin-inhibited carrot somatic embryogenesis can be restored by secreted cationic peroxidase isoenzymes. Planta. 184: 478–486; 1991. doi:10.1007/BF00197895.
Cvikova M.; Hrubcova M.; Vagner M.; Machackova I.; Eder J. Phenolic acids and peroxdase activity in alfalfa (Medicago sativa L.) embrogenic culture after ethphone treatment. Physiol. Plant. 91: 226–233; 1994. doi:10.1111/j.1399-3054.1994.tb00423.x.
Dhindsa P. L. P.; Dhindsa R. S.; Thrope T. A. Non-autotrophic CO2 fixation during shoot formation in tobacco callus. J. Exp. Bot. 30: 759–776; 1979. doi:10.1093/jxb/30.4.759.
Dhindsa R. S.; Beasley C. A.; Ting I. P. Osmoregulation in cotton fiber. Plant Physiol. 56: 394–398; 1975.
Dhindsa R. S.; Dhindsa P.; Thorpe T. S. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation and decreased levels of SOD and catalase. J. Exp. Bot. 32: 93–101; 1981. doi:10.1093/jxb/32.1.93.
Dodeman V. L.; Ducreux G. Total protein expression during induction and development of carrot somatic embryos. Plant Sci. 120: 57–69; 1996. doi:10.1016/S0168-9452(96)04487-1.
Evans J. J. Peroxidase from extreme dwarf tomatoes, identification, isolation and partial purification. Plant Physiol. 43: 1037–1041; 1968.
Fellers J. P.; Guenzi A. C.; Porter D. R. Marker proteins associated with somatic embryogenesis of wheat callus cultures. J. Plant Physiol. 151: 201–208; 1997.
Fowler M. W. Role of the malic enzymes reaction in plant roots. Utilization of (2, 3-14 C) malate and (1-14 C) pyruvate by pea root apices and measurement of enzyme activity. Biochem. Biophy. Acta. 372: 245–254; 1974.
Goldberg R.; Imberty A.; Liberman M.; Prat R. Relationships between peroxidatic activities and cell wall plasticity. In: Greppin H.; Penel C.; Gaspar T. (eds) Molecular and physiological aspects of plant peroxidases. University of Geneva Press, Geneva, Switzerland, pp 208–220; 1986.
Gupta, Y. P. Nutritive value of food legumes. In: Arora S. K. (ed) Chemistry and Biochemistry of Legumes Oxford and IBH Publishing Co. New Delhi, pp 287–327; 1982.
Ibrahim R. K.; Edgar D. Phenolic synthesis in Perilla cell suspension cultures. Phytochemistry 15: 129–131; 1976. doi:10.1016/S0031-9422(00)89067-6.
Kavi Kishor P. B. Activities of phenylalanine and tyrosine-ammonia lyases and amino transferases during organogenesis in callus cultures of rice. Plant Cell Physiol. 30: 25–29; 1989.
Kavi Kishor P. B.; Mehta A. R. Changes in some enzyme activities during growth and organogenesis in dark grown tobacco callus culture. Plant Cell Physiol. 29: 255–259; 1988.
Kiran G.; Kaviraj C. P.; Jogeswar G.; Kavi Kishor P. B.; Rao, S. Direct and high frequency somatic embryogenesis and plant regeneration from hypocotyls of chickpea (Cicer arietinum L.), a grain legume. Curr. Sci. 89: 1012–1018; 2005.
Kormutak A.; Salaj T.; Matusova R.; Vookova B. Biochemistry of zygotic and somatic embryogenesis in silver fir (Abies alba Mill.). Acta Biologica. 45: 59–62; 2003.
Kumar V. D.; Kirti P. B.; Sachan J. K. S.; Chopra V. L. Picloram induced somatic embryogenesis in chickpea (Cicer arietinum L.). Plant Sci. 109: 207–213; 1995. doi:10.1016/0168-9452(95)04167-S.
Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage. Nature 227: 680–685; 1970. doi:10.1038/227680a0.
Murashige T.; Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue culture. Plant Physiol. 15: 473–497; 1962. doi:10.1111/j.1399-3054.1962.tb08052.x.
Murthy B. N. S.; Jerrin V.; Rana P. S.; Filetcher R. A.; Praveen K. S. In virtro regeneration of chickpea (Cicer arietinum L.) stimulation of direct organogenesis and somatic embryogenesis, by thidiazron. Plant Growth Reg. 19: 233–240; 1996. doi:10.1007/BF00037796.
Ochoa S. Malic dehydrogenase from pig heart. Methods Enzymol. 1: 735–739; 1955. doi:10.1016/0076-6879(55)01128-2.
Roja Rani A.; Reddy V. D.; Prakash Babu P.; Padmaja G. Changes in protein profiles associated with somatic embryogenesis in peanut. Biol. Plant. 49: 347–354; 2005. doi:10.1007/s10535-005-0006-9.
Sagare A. P.; Suhasini K.; Krishnamurthy K. V. Plant regeneration via somatic embryogenesis in chickpea (Cicer arietinum). Plant Cell Rep. 12: 652–655; 1993. doi:10.1007/BF00232818.
Stirn S.; Jacobsen H. J. Marker proteins for embryogenic differentiation patterns in pea callus. Plant Cell Rep. 6: 50–54; 1987. doi:10.1007/BF00269738.
Venkatachalam P.; Kavi Kishor P. B.; Jayabalan N. High frequency somatic embryogenesis and efficient plant regeneration from hypocotyl explants of groundnut (Arachis hypogaea. L.). Curr. Sci. 72: 271–275; 1997.
Whetten R. W.; MacKay J. J.; Sederoff R. R. Recent advances in understanding lignin biosynthesis. Ann. Rev. Plant Physiol. Mol. Biol. 49: 585–609; 1998. doi:10.1146/annurev.arplant.49.1.585.
Willekens H.; Inze D.; Van Montagu M.; Van Camp W. Catalases in plants. Mol. Breed. 1: 207–228; 1995. doi:10.1007/BF02277422.
Author information
Authors and Affiliations
Corresponding author
Additional information
Editor: Rida A. Shibli
Rights and permissions
About this article
Cite this article
Kiran Ghanti, S., Sujata, K.G., Rao, S. et al. Role of enzymes and identification of stage-specific proteins in developing somatic embryos of chickpea (Cicer arietinum L.). In Vitro Cell.Dev.Biol.-Plant 45, 667–672 (2009). https://doi.org/10.1007/s11627-009-9197-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11627-009-9197-7