Abstract
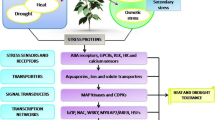
Mechanisms of drought tolerance have been studied by numerous groups, and a broad range of molecules have been identified to play important roles. A noteworthy response of stressed plants is the accumulation of novel protective proteins, including heat-shock proteins (HSPs) and late embryogenesis abundant (LEA) proteins. Identification of gene regulatory networks of these protective proteins in plants will allow a wide application of biotechnology for enhancement of drought tolerance and adaptation. Similarly, aquaporins are involved in the regulation of water transport, particularly under abiotic stresses. The molecular and functional characterization of protective proteins and aquaporins has revealed the significance of their regulation in response to abiotic stresses. Herein, we highlight new findings regarding the action mechanisms of these proteins. Finally, this review also surveys the current advances in engineering drought tolerant plants, particularly the engineering of protective proteins (sHSPs and LEA) and aquaporins for imparting drought stress tolerance in plants.
Similar content being viewed by others
Abbreviations
- ABA:
-
abscisic acid
- HSPs:
-
heat-shock proteins
- LEA:
-
late embryogenesis abundant
- MIPs:
-
major intrinsic proteins
- PIPs:
-
plasma membrane intrinsic proteins
- TIPs:
-
tonoplast intrinsic proteins
- WUE:
-
water use efficiency
References
Abdeen, A., Schnell, J., Miki, B.: Transcriptome analysis reveals absence of unintended effects in drought-tolerant transgenic plants overexpressing the transcription factor ABF3. — BMC Genomics 11: 69, 2010.
Abe, H., Urao, T., Ito, T., Seki, M., Shinozaki, K., Yamaguchi-Shinozaki, K.: Arabidopsis AtMYC2 (bHLH) and AtMYB2 (MYB) function as transcriptional activators in abscisic acid signaling. — Plant Cell 15: 63–78, 2003.
Agrawal, P.K., Jha, B.: Transcription factors in plants and ABA dependent and independent abiotic stress signaling. — Biol. Plant. 54: 201–212, 2010.
Aharon, R., Shahak, Y., Wininger, S., Bendov, R., Kapulnik, Y., Galili, G.: Overexpression of a plasma membrane aquaporin in transgenic tobacco improves plant vigor under favorable growth conditions but not under drought or salt stress. — Plant Cell 15: 439–447, 2003.
Ahmad, R., Kim, M.D., Back, K.H., Kim, H.S., Lee, H.S., Kwon, S.Y., Murata, N., Chung, W.I.I., Kwak, S.S.: Stressinduced expression of choline oxidase in potato plant chloroplasts confers enhanced tolerance to oxidative, salt, and drought stresses. — Plant Cell Rep. 27: 687–698, 2008.
Alamillo, J., Almoguera, C., Bartels, D., Jordano, J.: Constitutive expression of small heat shock proteins in vegetative tissues of the resurrection plant Craterostigma plantagineum. — Plant mol. Biol. 29: 1093–1099, 1995.
Almoguera, C., Coca, M.A., Jordano, J.: Tissue-specific expression of sunflower heat shock proteins in response to water stress. — Plant J. 4: 947–958, 1993.
Babu, R.C., Zhang, J., Blum, A., Ho, T.H.D., Wu, R., Nguyen, H.T.: HVA1, a LEA gene from barley confers dehydration tolerance in transgenic rice (Oryza sativa L.) via cell membrane protection. — Plant Sci. 166: 855–862, 2004.
Bahieldin, A., Mahfouz, H.T., Eissa, H.F., Saleh, O.M., Ramadan, A.M., Ahmed, I.A., Dyer, W.E., El-Itriby, H.A., Madkour, M.A.: Field evaluation of transgenic wheat plants stably expressing the HVA1 gene for drought tolerance. — Plant Physiol. 123: 421–427, 2005.
Baiges, I., Schaffner, A.R., Venzeller, M.J., Mas, A.: Plant aquaporins. — Physiol Plant. 115: 175–182, 2002.
Banzet, N., Richaud, C., Deveaux, Y., Kazmaier, M., Gagnon, J., Triantaphylides, C.: Accumulation of small heat shock proteins, including mitochondrial HSP22, induced by oxidative stress and adaptive response in tomato cells. — Plant J. 13: 519–527, 1998.
Bartels, D., Hussain, S.S.: Current status and implications of engineering drought tolerance in plants using transgenic approaches. — CAB Rev. 3: 20, 2008.
Bertrand, A., Paquin, R.: Influence of hardening temperature on frost tolerance of alfalfa and its sugar, starch and proline content. — Can. J. Plant Sci. 71: 737–747, 1991.
Bies-Etheve, N., Gaubier, P., Cooke, R., Raynal, M., Aspart, L., Delseny, M.: Late embryogenesis abundant (LEA) protein gene in Arabidopsis thaliana. — Seventh Int. Congr. Plant mol. Biol. 7: S03–S39, 2003.
Bohnert, H.J., Jensen R.G.: Strategies for engineering waterstress tolerance in plants. — Trends Biotechnol. 14: 89–97, 1996.
Bohnert, H.J., Shen B.: Transformation and compatible solutes. — Sci. Hort. 78: 237–260, 1999.
Bohnert, H.J., Sheveleva E.: Plant stress adaptations making metabolism move. — Curr. Opin. Plant Biol. 1: 267–274, 1998.
Boston, R.S., Viitanen, P.V., Vierling, E.: Molecular chaperones and protein folding in plants. — Plant mol. Biol. 32: 191–222, 1996.
Boursiac, Y., Chen, S., Luu, D.T., Sorieul, M., Van den Dries, N., Maurel, C.: Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. — Plant Physiol. 139: 790–805, 2005.
Boyer, J.S.: Plant productivity and environment. — Science 218: 443–448, 1982.
Bray, E.A., Bailey-Serres, J., Weretilnyk, E.: Responses to abiotic stresses. — In: Gruissem, W., Buchannan, B., Rockville, J.R. (ed.): Biochemistry and Molecular Biology of Plants. Pp. 1158–1249. American Society of Plant Physiologists, Rockville 2000.
Brini, F., Hanin, M., Lumbreras, V., Amara, I., Khoudi, H., Hassairi, A., Pages, M., Masmoudi, K.: Overexpression of wheat dehydrin DHN-5 enhances tolerance to salt and osmotic stress in Arabidopsis thaliana. — Plant Cell Rep. 26: 2017–2026, 2007.
Campbell, J.L., Klueva, N.Y., Zheng, H.G., Nieto-Sotelo, J, Ho, T.D., Nguyen, H.T.: Cloning of new members of heat shock protein HSP101 gene family in wheat (Triticum aestivum (L.) Moench) inducible by heat, dehydration, and ABA. — Biochim. biophys. Acta 1517: 270–277, 2001.
Chang, P.F.L., Jinn, T.L., Huang, W.K., Chen, Y., Chang, H.M., Wang, C.W.: Induction of a cDNA clone from rice encoding a class II small heat shock protein by heat stress, mechanical injury, and salicylic acid. — Plant Sci. 172: 64–75, 2007.
Chang, Y.Y., Liu, H.C., Liu, N.Y., Hsu, F.C., Ko, S.S.: Arabidopsis Hsa32, a novel heat shock protein, is essential for acquired thermotolerance during long recovery after acclimation. — Plant Physiol. 140: 1297–1305, 2006.
Chaumont, F., Barrieu, F., Jung, R., Chrispeels, M.J.: Plasma membrane intrinsic proteins from maize cluster in two sequence subgroups with differential aquaporin activity. — Plant Physiol. 122: 1025–1034, 2000.
Chaumont, F., Barrieu, F., Wojcik, E., Chrispeels, M.J., Jung, R.: Aquaporins constitute a large and highly divergent protein family in maize. — Plant Physiol. 125: 1206–1215, 2001.
Chen, J.B., Wang, S.M., Jing, R.L., Mao, X.G.: Cloning the PvP5CS gene from common bean (Phaseolus vulgaris) and its expression patterns under abiotic stresses. — J. Plant Physiol. 166: 12–19, 2009.
Chen, T.H., Murata, N.: Enhancement of tolerance of abiotic stress by metabolic engineering of betaines and other compatible solutes. — Curr. Opin. Plant Biol. 5: 250–257, 2002.
Cheng, W.H., Endo, A., Zhou, L., Penny, J., Chen, H.C., Arroyo, A., Leon, P., Nambara, E., Asami, T., Seo, M.: A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. — Plant Cell 14: 2723–2743, 2002.
Cho, E.K., Hong, C.B.: Over-expression of tobacco NtHSP70-1 contributes to drought-stress tolerance in plants. — Plant Cell Rep. 25: 349–358, 2006.
Chrispeels, M.J., Agre, P.: Aquaporins: water channel proteins of plant and animal cells. — Trends Biochem. Sci. 19: 421–425, 1994.
Close, T.J.: Dehydrins: emergence of a biochemical role of a family of plant dehydration proteins. — Plant Physiol. 97: 795–803, 1996.
Cortina, C., Culianez-Macia, F.: Tomato abiotic stress enhanced tolerance by trehalose synthesis. — Plant Sci. 169: 75–82, 2005.
Crowe, J.H., Carpenter, J.F., Crowe, L.M.: The role of vitrification in anhydrobiosis. — Annu. Rev. Plant Physiol. Plant mol.Biol. 60: 73–103, 1998.
Dalal, M., Tayal, D., Chinnusamy, V., Bansala, K.C.: Abiotic stress and ABA-inducible group 4 LEA from Brassica napus plays a key role in salt and drought tolerance. — J. Biotechnol. 139: 137–145, 2009.
Danielson, J.A.H., Johanson, U.: Unexpected complexity of the aquaporin gene family in the moss Physcomitrella patens. — BMC Plant Biol. 8: 45, 2008.
Delauney, A.J., Verma, D.P.S.: Proline biosynthesis and osmoregulation in plants. — Plant J. 4: 215–223, 1993.
Dubouzet, J.G., Sakuma, Y., Ito, Y., Kasuga, M., Dubouzet, E.G., Miura, S., Seki, M., Shinozaki, K., Yamaguchi-Shinozaki, K.: OsDREB genes in rice (Oryza sativa L.) encode transcription activators that function in drought, high salt and cold responsive gene expression. — Plant J. 33: 751–763, 2003.
Dure, L., III.: A repeating 11-mer amino acid motif and plant desiccation. — Plant J. 3: 363–369, 1993a.
Dure, L., III. Structural motifs in LEA proteins. — In: Close, T.J., Bray, E.A. (ed.): Plant Responses to Cellular Dehydration during Environmental Stress. Pp. 91–103. American Society of Plant Physiologists, location 1993b.
Ehrnsperger, M., Graber, S., Gaestel, M., Buchner, J.: Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. — EMBO J. 16: 221–229, 1997.
Ermawati, N., Liang, Y.S., Cha, J.Y., Shin, D., Jung, M.H., Lee, J.J., Lee, B.H., Han, D., Lee, K.H., Son, D.: A new tip homolog, ShTIP, from Salicornia shows a different involvement in salt stress compared to that of TIP from Arabidopsis. — Biol. Plant. 53: 271–277, 2009.
Figueras, M., Pujal, J., Saleh, A., Save, R., Pages, M., Goday, A.: Maize Rab17 overexpression in Arabidopsis plants promotes osmotic stress tolerance. — Ann. appl. Biol. 144: 251–257, 2004.
Flexas, J., Ribas-Carbo, M., Hanson, D.T., Bota, J., Otto, B., Cifre, J., McDowell, N., Medran, O.H., Kaldenhoff, R.: Tobacco aquaporin NtAQP1 is involved in mesophyll conductance to CO2 in vivo. — Plant J. 48: 427–439, 2006.
Fu, D., Huang, B., Xiao, Y., Muthukrishnan, S., Liang, G.H.: Overexpression of barley hva1 gene in creeping bentgrass for improving drought tolerance. — Plant Cell Rep. 26: 467–477, 2007.
Galau, G.A., Wang, H.Y.C., Hughes, D.W.: Cotton Lea5 and Lea14 encode atypical late embryogenesis abundant proteins. — Plant Physiol. 101: 695–696, 1993.
Garay-Arroyo, A., Colmenero-Flores, J.M., Garciarrubio, A., Covarrubias, A.A.: Highly hydrophilic proteins n prokaryotes and eukaryotes are common during conditions of water deficit. — J. biol. Chem. 275: 5668–5674, 2000.
Gilmour, S.J., Sebolt, A.M., Salazar, M.P., Everard, J.D., Thomashow, M.F.: Overexpression of the Arabidopsis CBF3 transcriptional activator mimics multiple biochemical changes associated with cold acclimation. — Plant Physiol. 124: 1854–1865, 2000.
Goddijn, O.J.M., Van Dun, K.: Trehalose metabolism in plants. -Trends Plant Sci. 4: 315–319, 1999.
Goyal, K., Walton, L.J., Tunnaclive, A.: LEA proteins prevent protein aggregation due to water stress. — J. Biochem. 388: 151–157, 2005.
Grelet, J., Benamar, A., Teyssier, E., Avelange-Macherel, M.H., Grunwald, D., Macherel, D.: Identification in pea seed mitochondria of a late-embryogenesis abundant protein able to protect enzymes from drying. — Plant Physiol. 137: 157–167, 2005.
Guan, J.C., Jinn, T.L., Yeh, C.H., Feng, S.P., Chen, Y.M., Lin, C.Y.: Characterization of the genomic structures and selective expression profiles of nine class I small heat shock protein genes clustered on two chromosomes in rice (Oryza sativa L.). — Plant mol. Biol. 56: 795–809, 2004.
Gubiš, J., Vaňková, R., Červená, V., Dragúňová, M.M., Hudcovicová, M., Lichtnerová, H.T., Dokupil, T., Jureková, Z.: Transformed tobacco plants with increased tolerance to drought. — South Afr. J. Bot. 73: 505–511, 2007.
Gupta, A.B., Sankararamakrishnan, R.: Genome-wide analysis of major intrinsic proteins in the tree plant Populus trichocarpa: characterization of XIP subfamily of aquaporins from evolutionary perspective. — BMC Plant Biol. 9: 134, 2009.
Hachez, C., Zelazny, E., Chaumont, F.: Modulating the expression of aquaporin genes in planta: a key to understand their physiological functions. — Biochim. biophys. Acta 1758: 1142–1156, 2006.
Hamilton, E.W., Heckathorn, S.A.: Mitochondrial adaptations to NaCl: complex I is protected by anti-oxidants and small heat shock proteins, whereas complex II is protected by proline and betaine. — Plant Physiol. 126: 1266–1274, 2001.
Hara, M., Terashima, S., Fukaya, T. Kuboi, T.: Enhancement of cold tolerance and inhibition of lipid peroxidation by citrus dehydrin in transgenic tobacco. — Planta 217: 290–298, 2003.
Harrington, H.M., Alm, D.M.: Interaction of heat and salt shock in cultured tobacco cells. — Plant Physiol. 88: 618–625, 1988.
Härndahl, U., Hall, R.B., Osteryoung, K.W., Vierling, E., Bornman, J.F., Sundby, C.: The chloroplast small heat shock protein undergoes oxidation-dependent conformational changes and may protect plants from oxidative stress. — Cell Stress Chaperones 4: 129–138, 1999.
Hartl, F.U.: Molecular chaperones in cellular protein folding. — Nature 381: 571–579, 1996.
Haslbeck, M., Walke, S., Stromer, T., Ehrnsperger, M., White, H.E., Chen, S., Saibil, H.R., Buchner, J.: Hsp26: a temperature regulated chaperone. — EMBO J. 18: 6744–6751, 1999.
Hendrick, J.P., Hartl, F.U.: Molecular chaperone functions of heat-shock proteins. — Annu. Rev. Biochem. 62: 349–384, 1993.
Hoekstra, F.A., Golovina, E.A., Buitink, J.: Mechanisms of plant desiccation tolerance. — Trends Plant Sci. 6: 431–438, 2001.
Hook, D.W.A., Harding, J.J.: Protection of enzymes by α-crystallin acting as a molecular chaperone. — Int. J. Biol. Macromol. 22: 295–306, 1998.
Horwitz, J.: α-Crystallin can function as a molecular chaperone. — Proc. nat. Acad. Sci. USA 89: 10449–10453, 1992.
Hsieh, T.H., Lee, J.T., Yang, P.T., Chiu, L.H., Charng, Y.Y., Wang, Y.C., Chan, M.T.: Heterology expression of the Arabidopsis c-repeat/dehydration response element binding factor 1 gene confers elevated tolerance to chilling and oxidative stresses in transgenic tomato. — Plant Physiol. 129: 1086–1094, 2002.
Hu, H., You, J., Fang, Y., Zhu, X., Qi, Z., Xiong, L.: Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. — Plant mol. Biol. 67:169–181, 2008.
Hu, W.H., Hu, G.C., Ban, B.: Genome-wide survey and expression profiling of heat shock proteins and heat shock factors revealed overlapped and stress specific response under abiotic stresses in rice. — Plant Sci. 176: 583–590, 2009.
Hu, X.J., Zhang, Z.B., Xu, P., Fu, Z.Y., Hu, S.B., Song, W.Y.: Multifunctional genes: the cross talk among the regulation networks of abiotic stress responses. — Biol. Plant. 54: 213–223, 2010.
Hussain, S.S., Ali, M., Maqbool, A., Siddique, K.H.M.: Polyamines: natural and engineered abiotic and biotic stress tolerance in plants. — Biotechnol. Adv. 29: 300–311, 2011a.
Hussain, S.S., Kayani, M.A., Amjad, M.: Transcription factors as tools to engineer enhanced drought stress tolerance in plants. — Biotechnol. Progr. 27: 297–306, 2011b.
Ingram, J., Bartels, D.: The molecular basis of dehydration tolerance in plants. — Annu. Rev. Plant Biol. 47: 377–403, 1996.
Ito, Y., Katsura, K., Maruyama, K., Taji, T., Kobayashi, M., Seki, S., Shinozaki, K., Yamaguchi-Shinozaki, K.: Functional analysis of rice DREB1/CBF type transcription factors involved in cold responsive gene expression in transgenic rice. — Plant Cell Physiol. 47: 141–153, 2006.
Iturriaga, G., Schneider, K., Salamini, F., Bartels, D.: Expression of desiccation-related proteins from the resurrection plant in transgenic tobacco. — Plant mol. Biol. 20: 555–558, 1992.
Iturriaga, G., Gaff, G.F., Zentella, R.: New desiccation tolerant plants, including a grass in the central highlands of Mexico, accumulate trehalose. — Aust. J. Bot. 48: 153–158, 2000.
Jain, R.K., Selvaraj, G.: Molecular genetic improvement of salt tolerance in plants. — Annu. Rev. Biotechnol. 3: 245–267, 1997.
Jang, J.Y., Lee, S.H., Rhee, J.Y., Chung, G.C., Ahn, S.J., Kang, H.: Transgenic Arabidopsis and tobacco plants overexpressing an aquaporin respond differently to various abiotic stresses. — Plant mol. Biol. 64: 621–632, 2007.
Javot, H., Lauvergeat, V., Santoni, V., Laurent, M.F., Guclu, J., Vinh, J., Heyes, J., Franck, K.I., Schaffner, A.R., Bouchez, D., Maurel, C.: Role of a single aquaporin isoform in root water uptake. — Plant Cell 15: 509–522, 2003.
Jiang, C., Xu, J., Zhang, H., Zhang, X., Shi, J., Li, M., Ming, F.: Acytosolic class I small heat shock protein, RcHSP17.8, of Rosa chinensis confers resistance to a variety of stresses to Escherichia coli, yeast and Arabidopsis thaliana. — Plant Cell Environ. 32: 1046–1059, 2009.
Jinn, T.L., Chen, Y.M., Lin, C.Y.: Characterization and physiological function of class 1 low molecular weight heat shock protein complexes in soybean. — Plant Physiol. 108: 693–701, 1995.
Johanson, U., Karlsson, M., Johansson, I., Gustavsson, S., Sjovall, S., Fraysse, L., Weig, A.R., Kjellbom, P.: The complete set of genes encoding major intrinsic proteins in Arabidopsis provides a framework for a new nomenclature for major intrinsic proteins in plants. — Plant Physiol. 126: 1358–1369, 2001.
Johnson, K.D., Höfte, H., Chrispeels, M.J.: An intrinsic tonoplast protein of protein storage vacuoles in seeds is structurally related to a bacterial solute transporter (GlpF). — Plant Cell 2: 525–532, 1990.
Jun, S.S., Choi, H.J., Lee, H.Y., Hong, Y.N.: Differential protection of photosynthetic capacity in trehalose and LEA protein producing transgenic plants under abiotic stresses. — J. Plant Biol. 51: 327–336, 2008.
Jung, C., Seo, J.S., Han, S.W., Koo, Y.J., Kim, C.H., Song, S.I., Nahm, B.H., Choi, Y.D., Cheong, J.J.: Overexpression of AtMYB44 enhances stomatal closure to confer abiotic stress tolerance in transgenic Arabidopsis. — Plant Physiol. 146: 623–635, 2008.
Jyothsnakumari, G., Thippeswamy, M., Veeranagamallaiah, G., Sudhakar, C.: Differential expression of LEA proteins in two genotypes of mulberry under salinity. — Biol. Plant. 53: 145–150, 2009.
Karim, S., Aronsson, H., Ericson, H., Pirhonen, M., Leyman, B., Welin, B., Mantyla, E., Palva, E.T., Van Dijck, P., Holstrom, K.O.: Improved drought tolerance without undesired side effects in transgenic plants producing trehalose. — Plant mol. Biol. 64: 371–386, 2007.
Kathuria, H., Giri, J., Tyagi, H., Tyagi, A.K.: Advances in transgenic rice biotechnology. — Crit. Rev. Plant Sci. 26: 65–103, 2007.
Katsuhara, M., Koshio, K., Shibasaka, M., Hayashi, Y., Hayakawa, T., Kasamo, K.: Over-expression of a barley aquaporin increased the shoot/root ratio and raised salt sensitivity in transgenic rice plants. — Plant Cell Physiol. 44: 1378–1383, 2003.
Kotak, S., Larkindale, J., Lee, U., Von Koskull-Doring, P., Vierling, E., Scharf, K.D.: Complexity of the heat stress response in plants. — Curr. Opin. Plant Biol. 10: 310–316, 2007.
Kumar, V., Shriram, V., Kavi-Kishor, P.B., Jawali, N., Shitole, M.G.: Enhanced praline accumulation and salt stress tolerance of transgenic indica rice by overexpressing P5CSF129A gene. — Plant Biotechnol. Rep. 4: 37–48, 2010.
Lal, S., Gulyani, V., Khurana, P.: Overexpression of HVA1 gene from barley generates tolerance to salinity and water stress in transgenic mulberry (Morus indica). — Transgenic Res. 17: 651–663, 2008.
Lee, B.H., Won, S.H., Lee, H.S., Miyao, M., Chung, W.I., Kim I.J., Jo, J.: Expression of the chloroplast-localized small heat shock protein by oxidative stress in rice. — Gene 245: 283–290, 2000.
Lee, G.J., Pokala, N., Vierling, E.: Structure and in vitro molecular chaperone activity of cytosolic small heat shock proteins from pea. — J. biol. Chem. 270: 10432–10438, 1995.
Lee, G.J., Roseman, A.M., Saibil, H.R., Vierling, E.: A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a foldingcompetent state. — EMBO J. 3: 659–671, 1997.
Lian, H.L., Yu, X., Ye, Q., Ding, X.S., Kitagawa, Y., Kwak, S.S., Su, W.A., Tang, Z.C.: The role of aquaporin RWC3 in drought avoidance in rice. — Plant Cell Physiol. 45: 481–489, 2004.
Liang, C.Y., Xi, Y., Shu, J., Li, J., Yang, J.L., Che, K.P., Jin, D.M., Liu, X.L., Weng, M.L., He, Y.K., Wang, B.: Construction of a BAC library of Physcomitrella patens and isolation of a LEA gene. — Plant Sci. 167: 491–498, 2004.
Liu, J.H., Nada, K., Honda, C., Kitashiba, H., Wen, X.P., Pang, X.M., Moriguchi, T.: Polyamine biosynthesis of apple callus under salt stress: importance of arginine decarboxylase pathway in stress response. — J. exp. Bot. 57: 2589–2599, 2006.
Liu, R., Liu, M., Liu, J., Chen, Y., Chen, Y., Lu, C.: Heterologus expression of a Ammopiptanthus mongolicus late embryogenesis abundant protein gene (AmLEA) enhances Escherichia coli viability under cold and heat stress. — Plant Growth Regul. 60: 163–168, 2010.
Liu, X., Wang, Z., Wang, L.L., Wu, R.H., Phillips, J., Deng, X.: LEA 4 group genes from the resurrection plant Boea hygrometrica confer dehydration tolerance in transgenic tobacco. — Plant Sci. 176: 90–98, 2009.
Livingston, D.P., III., Hincha, D.K., Heyer, A.G.: Fructan and its relationship to abiotic stress tolerance in plants. — Cell Mol. Life Sci. 66: 2007–2023, 2009.
Lopez-Matas, M.A., Nunez, P., Soto, A., Allona, I., Casado, R., Collada, C., Guevara, M.A., Aragoncillo, C., Gomez, L.: Protein cryoprotective activity of a cytosolic small heat shock protein that accumulates constitutively in chestnut stems and is up-regulated by low and high temperatures. — Plant Physiol. 134: 1708–1717, 2004.
Low, D., Brandle, K., Nover, L., Forreiter, C.: Cytosolic heatstress proteins Hsp17.7 class I and Hsp17.3 class II of tomato act as molecular chaperones in vivo. — Planta 211: 575–582, 2000.
Luu, D.T., Maurel, C.: Aquaporins in a challenging environment: molecular gears for adjusting plant water status. — Plant Cell Environ. 28: 85–96, 2005.
Lv, S., Yang, A., Zhang, K., Wang, L., Zhnag, J.: Increase of glycinebetaine synthesis improves drought tolerance in cotton. — Mol. Breed. 20: 233–248, 2007.
Ma, C., Haslbeck, M., Babujee, L., Jahn, O., Reumann, S.: Identification and characterization of a stress-inducible and a constitutive small heat-shock protein targeted to the matrix of plant peroxisomes. — Plant Physiol. 141: 47–60, 2006.
Malik, M.K., Slovin, J.P., Hwang, C.H., Zimmerman, J.L.: Modified expression of a carrot small heat shock protein gene, hsp17.7, results in increased or decreased thermo tolerance double danger. — Plant J. 20: 89–99, 1999.
Mamedov, T.G., Shono, M.: Molecular chaperone activity of tomato (Lycopersicon esculentum) endoplasmic reticulumlocated small heat shock protein. — J. Plant Res. 121: 235–243, 2008.
Mani, S., Van de Cotte, B., Montagu, M., Verbruggen, N.: Altered levels of proline dehydrogenase cause hypersensitivity to proline and its analogs in Arabidopsis. — Plant Physiol. 128: 73–83, 2002.
Maqbool, B., Zhong, H., El-Maghraby, Y., Ahmad, A., Chai, B., Wang, W., Sabzikar, R., Sticklen, B.: Competence of oat (Avena sativa L.) shoots apical meristems for integrative transformation, inherited expression, and osmotic tolerance of transgenic lines containing hva1. — Theor. appl. Genet. 105: 201–208, 2002.
Marini, I., Moschini, R., Corso, A.D., Mura, U.: Complete protection by α-crystallin of lens sorbitol dehydrogenase undergoing thermal stress. — J. biol. Chem. 275: 32559–32565, 2000.
Martre, P., Morillon, R., Barrieu, F., North, G.B., Nobel, P.S., Chrispeels, M.J.: Plasma membrane aquaporins play a significant role during recovery from water deficit. — Plant Physiol. 130: 2101–2110, 2002.
Maurel, C., Javot, H., Lauvergeat, V., Gerbeau, P., Tournaire, C., Santoni, V., Heyes, J.: Molecular physiology of aquaporins in plants. — Int. Rev. Cytol. 215: 105–148, 2002.
Maurel, C., Tacnet, F., Guclu, J., Guern, J., Ripoche, P.: Purified vesicles of tobacco cell vacuolar and plasma membranes exhibit dramatically different water permeability and water channel activity. — Proc. nat. Acad. Sci. USA 94: 7103–7108, 1997.
McCue, K.F., Hanson, A.D.: Drought and salt tolerance: toward understanding and application. — Trends Biotechnol. 8: 358–62, 1990.
McNeil, S.D., Nuccio, M.L., Hanson, A.D.: Betaines and related osmoprotectants. Targets for metabolic engineering of stress resistance. — Plant Physiol. 120: 945–949, 1999.
Miranda, J.A., Avonce, N., Suarez, R., Thevelein, J.M., Dijck, P.V., Iturriaga, G.: A bifunctional TPS-TPP enzyme from yeast confers tolerance to multiple and extreme abiotic conditions in transgenic Arabidopsis. — Planta 226: 1411–1421, 2007.
Moons, A., De Keyser, A., Van Montagu, M.: A group 3 LEA cDNA of rice, responsive to abscisic acid, but not to jasmonic acid, shows variety-specific differences in salt stress response. — Gene 191: 197–204, 1997.
Muchowski, P.J., Clark, J.I.: ATP-enhance molecular chaperone functions of the small heat shock protein human αB crystallin. — Biochemistry 95: 1004–1009, 1998.
Murakami, T., Matsuba, S., Funatsuki, H., Kawaguchi, K., Saruyama, H., Tanida, M., Sato, Y.: Over-expression of a small heat shock protein, sHSP17.7, confers both heat tolerance and UV-B resistance to rice plants. — Mol. Breed. 13: 165–175, 2004.
Murelli, C., Rizza, F., Marinone, A., Dulio, A., Terzi, V., Cattivelli, L.: Metabolic changes associated with coldacclimation in contrasting cultivars of barley. — Plant Physiol. 94: 87–93, 1995.
Naidu, B.P., Paleg, L.G., Aspinall, D., Jennings, A.C., Jones, G.P.: Amino acid and glycine betaine accumulation in coldstressed wheat seedlings. — Phytochemistry 30: 407–409, 1991.
Nakashima, K., Tran, L.S., Van Nyugen, D., Fujita, M., Maruyama, K., Todaka, D., Ito, Y., Hayashi, N., Shinozaki, K., Yamaguchi-Shinozaki, K.: Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. — Plant J. 51: 617–630, 2007.
Ndong, C., Danyluk, J., Wilson, K.E., Pocock, T., Huner, N.P.A., Sarhan, F.: Cold-regulated cereal chloroplast late embryogenesis abundant-like proteins. Molecular characterization and functional analyses. — Plant Physiol. 129: 1368–1381, 2002.
Neta-Sharir, I., Isaacson, T., Lurie, S., Weiss, D.: Dual role for tomato heat shock protein 21: protecting photosystem II from oxidative stress and promoting color changes during fruit maturation. — Plant Cell 17: 1829–1838, 2005.
Nomura, M., Muramoto, Y., Yasuda, S., Takabe, T., Kishitani S.: The accumulation of glycinebetaine during cold acclimation in early and late cultivars of barley. — Euphytica 83: 247–250, 1995.
Nuccio, M.L., Rhodes, D., McNeil, S.D., Hanson, A.D.: Metabolic engineering of plants for osmotic stress resistance. — Curr. Opin. Plant Biol. 2: 128–134, 1999.
Oh, S.J., Song, S.I., Kim, Y.S., Jang, H.J., Kim, M., Kim, Y.K.: Arabidopsis CBF3/DREB1A and ABF3 in transgenic rice increased tolerance to abiotic stress without stunting growth. — Plant Physiol. 138: 341–351, 2005.
Oraby, H.F., Ransom, C.B., Kravchenko, A.N., Sticklen, M.B.: Barley HVA1 gene confers salt tolerance in R3 transgenic oat. — Crop Sci. 45: 2218–2227, 2005.
Ouyang, S., Zhu, W., Hamilton, J., Lin, H., Campbell, M., Childs, K., Thibaud-Nissen, F., Malek, R.L., Lee, Y., Zheng, L., Orvis, J., Haas, B., Wortman, J., Buell, C.R.: The TIGR rice genome annotation resource: improvements and new features. — Nucl. Acids Res. 35 (Suppl.): D883-D887, 2007.
Pareek, A., Singla, S.L., Grover, A.: Evidence for accumulation of a 55kDa stress-related protein in rice and several other plant genera. — Plant Sci. 134: 191–197, 1998.
Park, B.J., Liu, Z., Kanno, A., Kameya, T.: Increased tolerance to salt- and water-deficit stress in transgenic lettuce (Lactuca sativa L.) by constitutive expression of LEA. — Plant Growth Regul. 45: 165–171, 2005a.
Park, B.J., Liu, Z., Kanno, A., Kameya, T.: Genetic improvement of Chinese cabbage for salt and drought tolerance by constitutive expression of a B. napus LEA gene. — Plant Sci. 169: 553–558, 2005b.
Park, E.J., Jeknic, Z., Pino, M.T., Murata, N., Chen, T.H.H.: Glycine betaine accumulation is more effective in chloroplasts than in the cytosol for protecting transgenic tomato plants against abiotic stress. — Plant Cell Environ. 30: 994–1005. 2007.
Park, E.J., Jeknic, Z., Sakamoto, A., Denoma, Y., Yawansiri, R., Murata, N., Chen, T.H.H.: Genetic engineering of glycine betaine synthesis in tomato protects seeds, plants, and flowers from chilling damage. — Plant J. 40: 474–487, 2004.
Park, S.H., Jun, S.S., An, G., Hong, Y.N., Park, M.C.: A comparative study on the protective role of trehalose and LEA proteins against abiotic stresses in transgenic Chinese cabbage (Brassica campestris) overexpressing CaLEA or OtsA. — J. Plant Biol. 46: 277–286, 2003.
Paul, J.M., Primavesi, L.F., Jhurreea, D., Zhang, Y.: Trehalose metabolism and signaling. — Annu. Rev. Plant Biol. 59: 417–441, 2008.
Pellegrineschi, A., Reynolds, M., Pacheco, M., Brito, R.M., Almeraya, R., Yamaguchi-Shinozaki, K., Hoisington, D.: Stress induced expression in wheat of the Arabidopsis thaliana DREB1A gene delays water stress symptoms under greenhouse conditions. — Genome 47: 493–500, 2004.
Peng, Y., Lin, W., Cai, W., Arora, R.: Overexpression of a Panax ginseng tonoplast aquaporin alters salt tolerance, drought tolerance and cold acclimation ability in transgenic Arabidopsis plants. — Planta 226: 729–740, 2007.
Pilon-Smits, E.A.H., Ebskamp, M.J.M., Paul, M.J., Jeuken, M.J.W., Weisbeek, P.J., Smeekens, S.C.M.: Improved performance of transgenic fructan-accumulating tobacco under drought stress. — Plant Physiol. 107: 125–130, 1995.
Porcel, R., Aroca, R., Rosario, A., Ruiz-Lozano, J.M.: PIP Aquaporin gene expression in arbuscular mycorrhizal Glycine max and Lactuca sativa plants in relation to drought stress tolerance. — Plant mol. Biol. 60: 389–404, 2006.
Puhakainen, T., Hess, M.W., Makela, P., Svensson, J., Heino, P., Palva, E.T.: overexpression of multiple dehydrin genes enhances tolerance to freezing stress in Arabidopsis. — Plant mol. Biol. 54: 743–753, 2004.
Qiu, Y., Yu, D.: Over-expression of the stress-induced OsWRKY45 enhances disease resistance and drought tolerance in Arabidopsis. — Environ. exp. Bot. 65: 35–47, 2009.
Quan, R., Shang, M., Zhang, H., Zhao, Y., Zhang, J.: Engineering of enhanced glycine betaine synthesis improves drought tolerance in maize. — Plant Biotechnol. J. 2: 477–486, 2004.
Quigley, F., Rosenberg, J.M., Shachar-Hill, Y., Bohnert, H.J.: From genome to function: the Arabidopsis aquaporins. — Genome Biol. 3: 1–17, 2002.
Raynal, M., Gaubier, P., Grellet, F., Delseny, M.: Nucleotide sequence of a radish cDNA clone coding for a late embryogenesis abundant (LEA) protein. — Nucl. Acids Res. 18: 6132, 1990.
Rizhsky, L., Liang, H., Shuman, J., Shulaev, V., Davletova, S., Mittler, R.: When defense pathways collide. The response of Arabidopsis to a combination of drought and heat stress. — Plant Physiol. 134: 1683–1696, 2004.
Rohilla, J.S., Jain, R.K., Wy, R.: Genetic improvement of Basmati rice for salt and drought tolerance by regulated expression of a barley Hva1 cDNA. — Plant Sci. 163: 525–532, 2002.
Roychoudhury, A., Roy, C., Sengupta, D.N.: Transgenic tobacco plants overexpressing the heterologous LEA gene Rab16A from rice during high salt and water deficit display enhanced tolerance to salinity stress. — Plant Cell Rep. 26: 1839–1859, 2007.
Sade, N., Gebretsadik, M., Seligmann, R., Schwartz, A., Wallach, R., Moshelion, M.: The role of tobacco aquaporin1 in improving water use efficiency, hydraulic conductivity and yield production under salt stress. — Plant Physiol. 152: 245–254, 2010.
Sade, N., Vinocur, B.J., Diber, A., Shatil, A., Nissan, H., Wallach, R., Karchi, H., Moshelion, M.: Improving plant stress tolerance and yield production: is the tonoplast aquaporin SITIP2;2 a key to isohydric to anisohydric conversion. — New Phytol. 181: 651–661, 2009.
Sakurai, J., Ishikawa, F., Yamaguchi, T., Uemura, M., Maeshima, M.: Identification of 33 rice aquaporin genes and analysis of their expression and function. — Plant Cell Physiol. 46: 1568–1577. 2005.
Sanmiya, K., Suzuki, K., Egawa, Y. Shono, M.: Mitochondrial small heat-shock protein enhances thermotolerance in tobacco plants. — FEBS Lett. 557: 265–268, 2004.
Sato, Y., Murakami, T., Funatsuki, H., Matsuba, S., Saruyama, H., Tanida, M.: Heat shock-mediated APX gene expression and protection against chilling injury in rice seedlings. — J. exp. Bot. 52: 145–151, 2001.
Sato, Y., Yokoya, S.: Enhanced tolerance to drought in transgenic rice plants overexpressing a small heat-shock protein, sHSP17.7. — Plant Cell Rep. 27: 329–334, 2008.
Schaeffner, A.R.: Aquaporin function, structure, and expression: are there more surprises to surface in water relations? — Planta 204: 131–139, 1998.
Schwechheimer, C., Zourelidou, M., Bevan, M.W.: Plant transcription factor studies. — Annu. Rev. Plant Physiol. Plant mol. Biol. 49: 127–150, 1998.
Sebehat, A., Lurie, S., Weiss, D.: Expression of small heatshock proteins at low temperatures. — Plant Physiol. 117: 651–658, 1998.
Sebehat, A., Weiss, D., Lurie, S.: The correlation between heat shock protein accumulation and persistence and chilling tolerance in tomato fruits. — Plant Physiol. 110: 531–537, 1996.
Seki, M., Narusaka, M., Abe, M., Kasuga, M., Yamaguchi-Shinozaki, K., Carninci, P., Hayashizaki, Y., Shinozaki, K.: Monitoring the expression pattern of 1300 Arabidopsis under drought and cold stresses by using a full length cDNA microarray. — Plant Cell 13: 62–72, 2001.
Seki, M., Umezawa, T., Urano, K., Shinozaki, K.: Regulatory metabolic networks in drought stress responses. — Curr. Opin. Plant Biol. 10: 296–302, 2007.
Serraj, R., Sinclair, T.R.: Osmolyte accumulation: can it really help increase crop yield under drought conditions? — Plant Cell Environ. 25: 333–341, 2002.
Sheveleva, E.V., Chmara, W., Bohnert, H.J., Jensen, R.G.: Increased salt and drought tolerance by D-ononitol production in transgenic Nicotiana tabacum L. — Plant Physiol. 115: 1211–1219, 1997.
Sheveleva, E.V., Marquez, S., Chmara, W., Zegeer, A., Jensen R.G, Bohnert, H.J.: Sorbitol-6-phosphate dehydrogenase expression in transgenic tobacco. High amounts of sorbitol lead to necrotic lesions. — Plant Physiol. 117: 831–839, 1998.
Shih, M.D., Lin, S.C., Hsieh, J.S., Tsou, C.H., Chow, T.Y., Lin, T.P., Hsing, Y.I.C.: Gene cloning and characterization of a soybean (Glycine max L.) LEA protein, GmPM16. — Plant mol. Biol. 56: 689–703, 2004.
Shinozaki, K., Yamaguchi-Shinozaki, K.: Molecular response to dehydration and low temperature: differences and cross-talk between two stress signaling pathways. — Curr. Opin. Plant Biol. 3: 217–223, 2000.
Shinozaki, K., Yamaguchi-Shinozaki, K., Seki, M.: Regulatory network of gene expression in the drought and cold stress responses. — Curr. Opin. Plant Biol. 6: 410–417, 2003.
Siddique, M., Gernhard, S., von Koskull-Doring, P., Vierling, E., Scharf, K.D.: The plant sHSP superfamily: five new members in Arabidopsis thaliana with unexpected properties. — Cell Stress Chaperones 13: 183–197, 2008.
Siefritz, F., Tyree, M.T., Lovisolo, C., Schubert, A., Kaldenho, V.R.: PIP1 plasma membrane aquaporins in tobacco: from cellular effects to function in plants. — Plant Cell 14: 869–876, 2002.
Simon-Sarkadi, L., Kocsy, G., Varhegyi, A., Galiba, G., De Ronde, J.A.: Genetic manipulation of proline accumulation influences the concentrations of other amino acids in soybean subjected to simultaneous drought and heat stress. — J. Agr. Food Chem. 53: 7512–7517, 2005.
Singh, K., Foley, R.C., Oñate-Sánchez, L.: Transcription factors in plant defense and stress responses. — Curr. Opin. Plant Biol. 5: 430–436, 2002.
Sivamani, E., Bahieldin, A., Wraith, J.M., Al-Niemi, T., Dyer, D.E., Ho, T.H.D., Qu, R.: Improved biomass productivity and water use efficiency under water deficit conditions in transgenic wheat constitutively expressing the barley HVA1 gene. — Plant Sci. 155: 1–9, 2000.
Smart, L.B., Moskal, W.A., Cameron, K.D., Bennett, A.B.: MIP genes are down-regulated under drought stress in Nicotiana glauca. — Plant Cell Physiol. 42: 686–693, 2001.
Smýkal, P., Mašín, J., Hardy, I., Konopásek, I., Žárský, V.: Chaperone activity of tobacco HSP18, a small heat-shock protein is inhibited by ATP. — Plant J. 23: 703–713, 2000.
Sørensen, J.G., Kristensen, T.N., Loeschcke, V.: The evolutionary and ecological role of heat shock proteins. — Ecol Lett. 6: 1025–37, 2003.
Sreenivasulu, N., Sopory, S.K., Kavi-Kishor, P.B.: Deciphering the regulatory mechanisms of abiotic stress tolerance in plants by genomic approaches. — Gene 388: 1–13, 2007.
Stiller, I., Dulai, S., Kondrák, M., Tarnai, R., Szabó, L., Toldó, O., Bánfalvi, Z.: Effects of drought on water content and photosynthetic parameters in potato plants expressing the trehalose-6-phosphate synthase gene of Saccharomyces cerevisiae. — Planta 227: 299–308, 2008.
Studer, S., Narberhaus, F.: Chaperone activity and homo- and hetero-oligomer formation of bacterial small heat shock proteins. — J. biol. Chem. 275: 37212–37218, 2000.
Suárez, R., Calderón, C., Iturriaga, G.: Enhanced tolerance to multiple abiotic stresses in transgenic alfalfa accumulating trehalose. — Crop Sci. 49: 1791–1799, 2009.
Sun, W., Bernard, C., Cotte, B.V., Montagu, M.V., Verbruggen, N.: At-HSP17.6A, encoding a small heat-shock protein in Arabidopsis, can enhance osmotolerance upon overexpression. — Plant J. 27: 407–415, 2001.
Sun, W., Van Montagu, M., Verbruggen, N.: Small heat shock proteins and stress tolerance in plants. — Biochim. biophys. Acta 1577: 1–9, 2002.
Suzuki, N., Bajad, S., Shuman, J., Shulaev, V., Mittler, R.: The transcriptional co-activator mbf1c is a key regulator of the thermotolerance in Arabidopsis thaliana. — J. biol. Chem. 283: 9269–9275, 2008.
Thomashow, M.F.: Role of cold-responsive genes in plant freezing tolerance. — Plant Physiol. 118: 1–7, 1998.
Thomashow, M.F.: Plant cold acclimation: freezing tolerance genes and regulatory mechanisms. — Annu. Rev. Plant. Biol. 50: 571–599, 1999.
Török, Z., Goloubinoff, P., Horvath, I., Tsvetkova, N.M., Glatz A., Balogh, G., Varvasovszki, V., Los, D.A., Vierling, E., Crowe, J.H., Vigh, L.: Synechocystis HSP17 is an amphitropic protein that stabilizes heat-stressed membranes and binds denatured proteins for subsequent chaperonemediated refolding. — Proc. nat. Acad. Sci. USA 98: 3098–3103, 2001.
Tran, L.S., Nakashima, K., Sakuma, Y., Simpson, S.D., Fujita, Y., Maruyama, K., Fujita, M., Seki, M., Shinozaki, K., Yamaguchi-Shinozaki, K.: Isolation and functional analysis of Arabidopsis stress inducible NAC transcription factors that bind to a drought responsive cis-element in the early responsive to dehydration stress 1 promoter. — Plant Cell 16: 2481–2498, 2004.
Trujillo, L., Menendez, C., Ochogavia, M.E., Hernandez, I., Borras, O., Rodriguez, R., Coll, Y., Arrieta, J.G., Banguela, A., Ramirez, R., Hernandez, L.: Engineering drought and salt tolerance in plants using SodERF3, a novel sugarcane ethylene responsive factor. — Biotechnol. Apl. 26: 168–171, 2009.
Trujillo, L., Sotolongo, M., Menendez, C., Ochogava, M.E., Coll, Y., Hernandez, I., Borras-Hidalgo, O., Thomma, B.P.H.J., Vera, P., Hernandez, L.: SodERF3, a novel sugarcane ethylene responsive factor (ERF), enhances salt and drought tolerance when over-expressed in tobacco plants. — Plant Cell Physiol. 49: 512–515, 2008.
Tunnacliffe, A., Wise, M.J.: The continuing conundrum of the LEA proteins. — Naturwissenschaften 94: 791–812, 2007.
Tyerman, S.D., Bohnert, H.J., Maurel, C., Steudle, E., Smith, J.A.: Plant aquaporins: their molecular biology, biophysics and significance for plant water relations. — J. exp. Bot. 50: 1055–1071, 1999.
Valliyodan, B., Nguyen, H.: Understanding regulatory networks and engineering for enhanced drought tolerance in plants. — Curr. Opin. Plant Biol. 9: 1–7, 2006.
Veinger, L., Diamant, L., Buchner, P., Goloubinoff, P.: The small heat-shock protein IbpB from Escherichia coli stabilizes stress denatured proteins for subsequent refolding by a multi-chaperone network. — J. biol. Chem. 273: 11032–11037, 1998.
Vendruscolo, E.C.G., Schuster, I., Pileggi, M., Scapim, C.A., Molinari, H.B.C., Marur, C.J., Vieira, L.G.E.: Stressinduced synthesis of proline confers tolerance to water deficit in transgenic wheat. — J. Plant Physiol. 164: 1367–1376, 2007.
Vierling, E.: The roles of heat-shock proteins in plants. — Ann. Rev. Plant Biol. 42: 579–620, 1991.
Vierling, E., Kimpel, J.A.: Plant responses to environmental stress. — Curr. Opin. Biotechnol. 3: 164–170, 1992.
Vij, S., Tyagi, A.K.: Emerging trends in the functional genomics of the abiotic stress response in crop plants. — Plant Biotechnol. J. 5: 361–380, 2007.
Vinocur, B., Altman, A.: Recent advances in engineering plant tolerance to abiotic stress: achievements and limitations. — Curr. Opin. Biotechnol. 16: 123–132, 2005.
Volkov, R.A., Panchuk, I.I., Mullineaux, P.M., Schoffl, F.: Heat stress-induced H2O2 is required for effective expression of heat shock genes in Arabidopsis. — Plant mol. Biol. 61: 733–746, 2006.
Wang, Q.Y., Guan, Y.C., Wu, Y.R., Chen, H.L., Chen, F., Chu, C.C.: Overexpression of a rice OsDREB1F gene increases salt, drought and low temperature tolerance in both Arabidopsis and rice. — Plant mol. Biol. 67: 589–602, 2008.
Wang, W.X., Vinocur, B., Altman, A.: Plant responses to drought, salinity and extreme temperatures: towards genetic engineering for stress tolerance. — Planta 219: 1–14, 2003.
Wang, Y., Jiang, J., Zhao, X., Liu G., Yang, C., Zhan, L.: A novel LEA gene from Tamarix rossowii confers drought tolerance in transgenic tobacco. — Plant Sci. 171: 655–662, 2006.
Wang, Y., Ying, J., Kuzma, M., Chalifoux, M., Sample, A., McArthur, C., Uchacz, T., Sarvas, C., Wan, J., Dennis, D.T., McCourt, P., Huang, Y.: Molecular tailoring of farnesylation for plant drought tolerance and yield protection. — Plant J. 43: 413–424, 2005.
Wanner, L.A., Junttila, O.: Cold-induced freezing tolerance in Arabidopsis. — Plant Physiol. 120: 391–400, 1999.
Waters, E.R., Lee, G.J., Vierling, E.: Evolution, structure and function of the small heat shock proteins in plants. — J. exp. Bot. 47: 325–338, 1996.
Weston, D.J., Gunter, L.E., Rogers, A., Wullschleger, S.D.: Connecting genes, coexpression modules, and molecular signatures to environmental stress phenotypes in plants. — BMC Syst. Biol. 2: 16, 2008.
Wise, M.J.: LEA ping to conclusions: a computational reanalysis of late embryogenesis abundant proteins and their possible roles. — BMC Bioinform. 4: 52, 2003.
Wise, M.J., Tunnacliffe, A.: POPP the question: what do LEA proteins do? — Trends Plant Sci. 9: 13–17, 2004.
Wollgiehn, R., Neumann, D.: Stress response of tomato cell cultures to toxic metals and heat shock: differences and similarities. — J. Plant Physiol. 146: 736–742, 1995.
Xiang, Y., Tang, N., Du, H., Ye, H., Xiong, L.: Charaterization of OsbZIP23 as a key player of the basic leucine zipper transcription factor family for conferring abscisic acid sensitivity and salinity and drought tolerance in rice. — Plant Physiol. 148: 1938–1952, 2008.
Xiao, B., Huang, Y., Tang, N., Xiong, L.: Over-expression of a LEA gene in rice improves drought resistance under the field conditions. — Theor. appl. Genet. 115: 35–46, 2007.
Xu, D., Duan X., Wang, B., Hong B., Ho, T.H.D., Ho, D., Wu, R.: Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. — Plant Physiol. 110: 249–257, 1996.
Yamada, M., Morishita H., Urano, K., Shiozaki N., Yamaguchi-Shinozaki, K., Shinozaki, K., Yoshiba, Y.: Effects of free proline accumulation in petunias under drought stress. — J. exp. Bot. 56: 1975–1981, 2005.
Yamaguchi-Shinozaki, K., Shinozaki, K.: Organization of cisacting regulatory elements in osmotic- and cold-stressresponsive promoters. — Trends Plant Sci. 10: 88–94, 2005.
Yoshida, T., Sakuma, Y., Todaka, D., Maruyama, K., Qin, F., Mizoi, J., Kidokoro, S., Fujita, Y., Shinozak, i K., Yamaguchi-Shinozak, i K.: Functional analysis of an Arabidopsis heat-shock transcription factor HsfA3 in the transcriptional cascade downstream of the DREB2A stressregulatory system. — Biochem. biophys. Res. Commun. 368: 515–521, 2008.
Yu, H., Chen, X., Hong, Y-Y., Wang, Y., Xu, P., Ke, S-D., Liu, H-Y., Zhu, J-K., Oliver, D.J., Xiang, C-B.: Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density. — Plant Cell 20: 1134–1151, 2008.
Yu, Q., Hu, Y., Li, J., Wu, Q., Lin, Z.: Sense and antisense expression of plasma membrane aquaporin BnPIP1 from Brassica napus in tobacco and its effects on plant drought resistance. — Plant Sci. 169: 647–656, 2005.
Zhang, G., Chen, M., Li, L., Xu, Z., Chen, X., Guo, J., Ma, Y.: Overexpression of the soybean GmERF3 gene, an AP2/ERF type transcription factor for increased tolerances to salt, drought and diseases in transgenic tobacco. — J. exp. Bot. 60: 3781–3796, 2009.
Zhu, J.K.: Salt and drought stress signal transduction in plants. — Annu. Rev. Plant Biol. 53: 247–273, 2002.
Zou, M., Guan, Y., Ren, H., Zhang, F., Chen, F.: A bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance. — Plant mol. Biol. 66: 675–683, 2008.
Acknowledgement
This work was supported by Higher Education Commission (HEC) of Pakistan by a project grant to SSH.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hussain, S.S., Iqbal, M.T., Arif, M.A. et al. Beyond osmolytes and transcription factors: drought tolerance in plants via protective proteins and aquaporins. Biol Plant 55, 401–413 (2011). https://doi.org/10.1007/s10535-011-0104-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10535-011-0104-9