Abstract
Immunotherapies have revolutionized the treatment paradigms of various types of cancers. However, most of these immunomodulatory strategies focus on harnessing adaptive immunity, mainly by inhibiting immunosuppressive signaling with immune checkpoint blockade, or enhancing immunostimulatory signaling with bispecific T cell engager and chimeric antigen receptor (CAR)-T cell. Although these agents have already achieved great success, only a tiny percentage of patients could benefit from immunotherapies. Actually, immunotherapy efficacy is determined by multiple components in the tumor microenvironment beyond adaptive immunity. Cells from the innate arm of the immune system, such as macrophages, dendritic cells, myeloid-derived suppressor cells, neutrophils, natural killer cells, and unconventional T cells, also participate in cancer immune evasion and surveillance. Considering that the innate arm is the cornerstone of the antitumor immune response, utilizing innate immunity provides potential therapeutic options for cancer control. Up to now, strategies exploiting innate immunity, such as agonists of stimulator of interferon genes, CAR-macrophage or -natural killer cell therapies, metabolic regulators, and novel immune checkpoint blockade, have exhibited potent antitumor activities in preclinical and clinical studies. Here, we summarize the latest insights into the potential roles of innate cells in antitumor immunity and discuss the advances in innate arm-targeted therapeutic strategies.
Similar content being viewed by others
Background
During cancer evolution, accumulating point mutations and structural alterations drive malignant transformation and contribute to the immunogenicity of cancer cells [1]. Tumor antigens expressed by mutated genes could be recognized by host immunity as non-self and initiate immune elimination [2]. In immune-mediated elimination, innate immunity cooperates with adaptive immunity to orchestrate a cascade multi-step process, which begins with tumor antigen capture and ends with immune killing [3,4,5,6]. Innate immunity serves as the first front line of host defense, consisting of physical and chemical barriers and various types of immune cells with pattern-recognition receptors (PRRs). Innate immune components, involving dendritic cells (DCs), macrophages, monocytes, neutrophils, eosinophils, basophils, mast cells, natural killer (NK) cells, natural killer T (NKT) cells, γδ T cells, mucosa-associated invariant T (MAIT) cells, retard tumor growth mainly by nonspecifically killing malignant cells or mobilizing adaptive immune response [7]. In contrast with the innate arm, the adaptive arm of host immunity specifically eradicates cancer cells by T and B cells [8].
Ideally, all transformed cells are recognized and eliminated by host immunity. However, cancer is a heterogeneous disease, and a large scale of genetic and epigenetic alterations are unevenly distributed in several parallel subclones [9,10,11]. Under the selective pressure of adaptive immunity, tumor subclones with weak immunogenicity become the predominant subclones that escape immune-mediated tumor clearance [12]. The poor immunogenicity, coupled with multiple immunosuppressive factors such as immune checkpoint pathways, metabolite reprogramming, and dysregulated cytokine repertoire, support selected subclones to develop into clinically apparent lesions [13,14,15,16,17,18]. Besides, immunosuppressive cell populations in the tumor microenvironment (TME), including tumor-associated macrophages (TAMs), regulatory T (Treg) cells, regulatory B (Breg) cells, myeloid-derived suppressor cells (MDSCs), tumor-associated neutrophils (TANs), and cancer-associated fibroblasts (CAFs), also promote immune evasion and cancer progression [19,20,21,22,23].
Antitumor immunotherapies, including immune checkpoint blockade [24] and adoptive cell transfer [25,26,27], have been widely validated and clinically approved for various cancers. These strategies aim to eradicate cancer cells by enabling T cell-mediated antitumor responses. Immune checkpoint molecules are commonly upregulated in the TME, which hamper T cell activation by counteracting T cell receptor (TCR) signaling or attenuating the costimulatory pathway [28,29,30]. Immune checkpoint antibodies disturb immunosuppressive pathways in T cells, especially programmed cell death protein 1 (PD-1)-programmed cell death ligand 1 (PD-L1) and cytotoxic T lymphocyte-associated protein 4 (CTLA-4)-CD80/CD86 signaling [31, 32]. Up to now, more than ten anti-PD-1/PD-L1 antibodies have been approved for cancer treatment.
Meanwhile, adoptive cell transfer strategies, mainly chimeric antigen receptor (CAR)-T cell therapy, make a breakthrough in hematological malignancies [33, 34]. CAR-T cells are prepared by transducing genetically engineered receptors into autologous T cells [35]. These engineered TCRs contain extracellular domains recognizing tumor antigens and intracellular domains mimicking TCR activation signaling [36, 37]. At the present stage, six CAR-T cell products have been clinically approved: Yescarta (anti-CD19), Kymriah (anti-CD19), Tecartus (anti-CD19), Breyanzi (anti-CD19), Abecma (anti-BCMA), and Carvykti (anti-BCMA) for B cell malignancies and multiple myeloma [38,39,40,41]. Also, DC-targeted adoptive cell transfer strategies have made substantial headway. Provenge, autologous DC loaded with the fusion protein of granulocyte–macrophage colony-stimulating factor and prostatic acid phosphatase, has been approved for prostate cancer [42].
Although these immunotherapies have achieved tremendous success in advanced cancers, some thorny issues remain to be resolved, including the unsatisfactory response rate and lack of accurate predictors. It was estimated that 43.63% of all cancer patients were eligible for immune checkpoint blockade, and the overall response rate was below 13% in the US [43]. Besides, CAR-T cell therapy indications are limited to hematologic malignancies, without significant antitumor activity in solid tumors [44,45,46,47]. Generally, most clinically approved immunotherapies are T cell-centered. However, the effector functions of T cells are non-autonomous. The initiation and sustainability of T cell response and the maintenance of T cell memory depend on innate immunity [48]. Innate immunity detects, captures, and processes cancer antigens and then triggers adaptive immunity. At the same time, innate immune cells directly eradicate tumors by mounting their effector responses, such as the cytotoxicity of NK cells and the phagocytosis of macrophages [48]. Besides, due to the expression of Fc receptor (FcR) on macrophages and NK cells, innate immunity could participate in adaptive immunity by launching antibody-dependent cell cytotoxicity and phagocytosis (ADCC and ADCP) [49]. As the essential role of the innate immune arm in the onset, propagation, and maintenance of the cancer-immunity cycle, it is rationale to harness innate response to improve the current immunotherapy performance and relieve treatment resistance. In this work, we review the roles of innate immune components in antitumor immunity and summarize the advances in innate immunity-targeted immunotherapies.
The role of DC in antitumor response and DC-targeted therapy
DCs are a heterogeneous group of myeloid-derived populations. According to the developmental origin, DCs are commonly classified into several subsets: conventional DC (including cDC1 and cDC2), plasmacytoid DC (pDC), monocyte-derived DC (MoDC), and tumor-infiltrating DC3 [50]. Among these subsets, cDC1 is functionally specialized in the cross-presentation of cancer antigens [51, 52], while pDC is the specialized producer of IFN-I [53]. Besides, based on tissue-specific compartmentalization, DCs could be classified as migratory DC (migDC, trafficking from peripheral tissues to draining lymph nodes) and resident DC (resDC, residing in peripheral lymphoid organs). Notably, the omics technique, especially single-cell RNA sequencing, provides a high-resolution landscape of DC differentiation and ontogeny [54]. To trigger and maintain robust antitumor response, DCs orchestrate a cascade of events: antigen capture and process, trafficking to tumor-associated draining lymph nodes (tdLNs), priming naïve T cells, recruiting primed T cells into the TME by secreting chemokines, and interacting with effector T cells in the TME [55].
Innate sensing and cancer antigen presentation
The presence and accumulation of DCs are the prerequisites of innate immune sensing. The recruitment and expansion of DC in the TME are dependent on several cytokines and chemokines, such as NK cell-derived FLT3L [56], XCL1, CCL5 [57], as well as tumor-derived CCL4 [58]. In the presence of damage-associated molecular patterns (DAMPs) from stressed or injured cancer cells, these immature DCs are activated by various PRR pathways [59]. Additionally, chemotherapy and radiotherapy could promote DC maturation by inducing the immunogenic cell death (ICD) of cancer cells [60]. DAMPs released during ICD stimulate DC maturation and improve DC functions: adenosine triphosphate (ATP) facilitating DC recruitment and activation, calreticulin (CRT) enhancing cancer antigen engulfment, and high-mobility group box 1 (HMGB1) improving antigen presentation of DCs [60]. Moreover, genomic instability, mitochondrial dysfunction, oxidative stress, and conventional antitumor regimens could support DC maturation by inducing DNA damage and activating cytosolic DNA sensing signaling, such as cGAS/STING/IFN-I pathway (Fig. 1a) [61].
DC-targeted cancer therapies. a The maturation of DCs. In the TME, genomic instability, mitochondrial dysfunction, oxidative stress, and conventional antitumor regimens could support DC maturation by inducing DNA damage and activating cytosolic DNA sensing signaling, such as cGAS/STING/IFN-I pathway. Besides, In the presence of damage-associated molecular patterns from stressed or injured cancer cells, these immature DCs are activated by various PRR pathways. Additionally, chemotherapy and radiotherapy could promote DC maturation by inducing the ICD of cancer cells. DAMPs released during ICD stimulate DC maturation and improve DC functions: ATP facilitates DC recruitment and activation, CRT enhances cancer antigen engulfment, and HMGB1 improves antigen presentation of DCs. b DC-targeted cancer therapies. DC-targeted strategies mainly consist of agonists for DC differentiation, expansion, and activation, blockade of immunoinhibitory signals, and DC vaccines. Abbreviations: DC, dendritic cell; ICD, immunogenic cell death; ATP, adenosine triphosphate; CRT, calreticulin; HMGB1, high-mobility group box 1. Adapted from Yi et al. 2022 [62].
Once upon cancer antigen capture, DCs undergo maturation with the licensing stimuli such as IFN-I. In this process, DCs alter their morphology, upregulate costimulatory molecules such as CD40, CD80, and CD86, enhance antigen presentation capability, and secret proinflammatory cytokines [63]. Then, mature DCs migrate to the T cell-rich zone of tdLNs in the CCR7/CCL21-dependent manner [64, 65]. In tdLNs, mature DCs (primarily cDC1s) cross-prime naïve CD8+ T cells by DC-T cell immune synapses. Also, cDC1s could prime naïve CD4+ T cells by MHC-II, while activated CD4+ T cells license cDC1s to trigger cancer-specific CD8+ T cell response in turn [66].
Apart from tdLNs, DCs could continue interacting with T cells in the TME to support cancer-specific immunity. Tumor-infiltrating cDC1 promotes T cell infiltration by secreting CXCL9 and CXCL10 (ligands of CXCR3) to guide T cell homing [67]. Beyond de novo T cell priming, tumor-infiltrating CD103+ DCs maintain T cell response by restimulating previously activated or memory CD8+ T cells [68, 69]. Recent studies demonstrate a positive feedback loop between cDC1s and T cells. After primed and activated by cDC1s, CD8+ T cells could secret IFN-γ to promote cDC1s to product IL-12 in a non-canonical NF-κB-dependent manner [70].
Dysregulated DC functions in the TME
The functions of DCs are disturbed by various immunosuppressive factors in the TME, hampering immune surveillance and supporting tumor progression [71]. Some tumor and stroma-derived cytokines regulate the survival, differentiation, maturation, and antigen presentation of DCs. For example, transforming growth factor-β (TGF-β) is a crucial component in maintaining host immune homeostasis [72]. Deleting Tgfbr2 in DCs by CD11c-Cre murine models leads to multiorgan inflammation [73]. On the one hand, TGF-β inhibits the antigen-presentation of DCs by downregulating MHC-II expression [74]. Also, the TGF-β-inhibitor of differentiation 1 (ID1) axis induces DC differentiation toward an immunosuppressive myeloid cell phenotype [75]. In murine melanoma and breast cancer models, activated TGF-β signaling increases enzyme indoleamine 2,3-dioxygenase (IDO) in pDCs and CCL22 in myeloid DCs, promoting Treg infiltration as well as immune escape [76]. On the other hand, these tolerogenic DCs contribute to cancer immune evasion by TGF-β secretion. Tumor cells educate DCs to generate TGF-β, which in turn facilitates Treg differentiation [77].
Along with TGF-β, other immunoinhibitory molecules also limit the functions of DCs. IL-10 drives the transformation of immature DCs towards the tolerogenic phenotype [78]. IL-6 undermines DC maturation by STAT3-mediated downregulation of MHC-II and CCR7 [79]. Besides, IL-6 cooperates with prostaglandin E2 (PGE2) to convert cDC2 to the CD14+ immunosuppressive phenotype [80]. PGE2 alone could disturb NK cell-stimulated cDC1 recruitment by suppressing NK cell survival and chemokine receptor expression of cDC1 [57]. Moreover, IL-10 inhibits IL-12 production of CD103+ cDC1s [81, 82]. Vascular endothelial-derived growth factor (VEGF) is identified as another cytokine hampering the differentiation and antigen presentation of DCs [83,84,85]. Increased VEGF is associated with decreased circulating and tumor-infiltrating DCs [86]. Some tumor-derived metabolites, such as oxysterols and lactic acid, restrain the CCR7-mediated migration and antigen presentation capability of DCs [87, 88]. Further investigations showed the activation of lactate receptor GPR81 specifically downregulated MHC-II expression [89]. Generally, the functions of DCs are dampened, and antigen presentation machinery is disorganized in the TME [90]. Therefore, reinvigorating DC from abnormal status is feasible to boost antitumor immunity and overcome immunotherapy resistance [91, 92].
Harnessing DC for cancer treatment
As the core component bridging innate immunity and adaptive immunity, DC is a valuable target for immunotherapy, especially for patients resistant to T cell-based therapies. At present, DC-targeted strategies mainly consist of agonists for DC differentiation, expansion, and activation, blockade of immunoinhibitory signals, and DC vaccines (Fig. 1b) (Table 1) [93].
Agonists for DC, differentiation, expansion, and activation
The cGAS/STING signaling is a well-known innate immune sensing mechanism responding to infection, senescence, DNA damage, and dysregulated cell cycle [94]. cGAS recognizes cytoplasmic double-stranded DNA and then catalyzes the formation of secondary messenger cyclic GMP-AMP (cGAMP). Stimulated by cGAMP, STING undergoes conformation changes and then translocates from endoplasmic reticulum to Golgi body, triggering downstream TBK1/IRF3/IFN-I or TBK1/NF-κB cascades [61, 95]. STING-dependent TBK1/IRF3/IFN-I axis licenses DCs to cross-present cancer antigens to CD8+ T cells with MHC-I molecules. At the same time, STING-dependent NF-κB activation enables DCs to generate proinflammatory cytokines. Notably, in some tumor-associated myeloid cells, STING-dependent NF-κB signaling could also be initialized by inhibitor of kB kinase ε (IKK-ε) in a TBK1-independent manner [96]. Based on the immunostimulatory effects of STING-dependent IFN-I production, pharmacological activation of STING by intratumorally injecting cGAMP retards tumor growth in multiple murine colon carcinoma and melanoma models [97,98,99,100,101]. However, the applications of cGAMP and synthetic cyclic dinucleotides (CDNs) are limited by poor bioavailability and intratumoral delivery [102]. Relatively, non-CDN small-molecule STING agonists overcome these shortcomings that could be systemically delivered. Despite the failure of DMXAA [103], some novel STING agonists, such as di-ABZI, MSA-2, and manganese, exhibit potent antitumor activity in murine tumor models, which are undergoing clinical evaluations (Table 2) [104,105,106,107,108]. These STING agonists effectively upregulate costimulatory molecules (e.g., CD40, CD80, CD83, and CD86) and MHC on DCs. Besides, STING agonists improve the antigen presentation of DCs, especially the tumor-specific antigen cross-presentation to CD8+ T cells [95]. As a result, STING agonist administration enhances the expression of IFN-β and other proinflammatory cytokines (e.g., IL-6 and TNF-α) or chemokines (e.g., CCL2/3/4/5 and CXCL9/10), the maturation and functions of DCs, and the expansion of tumor-infiltrating CD8+ T cells [106]. Besides, some STING agonists, such as manganese, could strengthen NK cell activation and NK cell-mediated cytotoxicity in the TME [107]. STING agonists are a promising strategy for cancer immunotherapy, mobilizing the innate defensive sensor for immunological surveillance and promoting cancer-specific T cell priming.
Besides cGAS/STING, Toll-like receptors (TLRs) are also damage- or pathogen-sensing pathways contributing to DC activation [109]. Up to now, more than ten functional TLRs (TLR1-10) have been identified in humans [110]. Human DC subsets have different TLR expression patterns: TLR3/8 in cDC1 and TLR7/9 in pDC [93, 111]. TLR3 agonist such as Poly(I:C) enhances cDC1 maturation and cytokine production such as IL-12 and IFN-I [112]. Additionally, TLR8 agonist, such as Motolimod, promotes cDC1 maturation, with encouraging antitumor activity and tolerable toxicity profiles in squamous cell head and neck cancer [113,114,115]. Moreover, TLR7 and TLR9 are widely explored due to their capability to induce IFN-I generation in pDCs. The immunostimulatory effects and antitumor activity of TLR7 agonists such as Imiquimod have been confirmed in various types of cancer [116,117,118,119]. TLR9 agonists also promote cytokine production and pDC maturation [120, 121]. Also, other novel agents such as granulocyte macrophage-colony stimulating factor (GM-CSF), Flt3L agonist, and RIG-I agonist improve DC-mediated T cell response by expanding DC population, promoting DC activation or phagocytic potential [122,123,124,125].
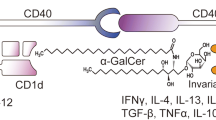
In contrast with the co-inhibitory signaling pathway, costimulatory pathways such as CD40/CD40L enhance the cross-priming capability of antigen-presenting cells [13]. CD40 on DCs is activated by CD40L on CD4+ T cells, leading to the upregulation of MHC, costimulatory molecules, and various TNF superfamily ligands (CD137L, GITRL, and OX40L). Furthermore, CD40-activated DCs generate more IL-12 to support CD8+ T cell activation and skew the following adaptive immunity toward Th1 polarization [126]. Overwhelming evidence demonstrates that agonistic CD40 antibodies expand cancer antigen-specific CD8+ T cells and provide robust immune protection by cross-presenting DCs [127]. In some murine tumor models, the antitumor activity of agonistic CD40 antibodies is T cell-dependent [128,129,130]. However, some current studies showed that CD40 activation-mediated tumor regression was independent of T cells. On the contrary, agonistic CD40 antibodies activate macrophages (also highly expressing CD40), causing stroma depletion and tumor regressions [131]. This effect is attributed to systemically released IFNγ and CCL2, which redirect Ly6C+CCR2+ monocytes and macrophages to infiltrate into the TME and degenerate fibrosis [132]. To date, multiple CD40-targeted monoclonal antibodies have been developed and tested in clinical trials [133]. Generally, agonistic CD40 antibodies have a minimal response rate in cancer patients, except for selicrelumab [134]. In the phase 1 study of selicrelumab, 27% of melanoma patients achieved partial responses [134, 135]. For most types of cancers with low immunogenicity, it is hard to effectively destroy tumors by agonistic CD40 antibody monotherapy. Combination therapies with chemotherapy, radiotherapy, or other immunotherapies might be worth exploring in the future [126].
Blockade of immunoinhibitory signals
As mentioned above, various immunosuppressive factors like TGF-β, IL-10, IDO, PGE2, and VEGF hamper the functions of DCs, hindering immune surveillance and promoting tumor advancement [93, 136]. Therefore, neutralizing these immunoinhibitory factors enhances the recruitment, survival, activation, and antigen presentation capability of DCs [137]. Anti-VEGF antibodies improve the functions of DCs of spleen and lymph node, synergizing with peptide-pulsed DCs to prolong the survival of tumor-bearing mice [138]. In a phase 1 study of VEGF-Trap, VEGF inhibition significantly increased the ratio of mature DCs, without alterations in populations of total DCs [139]. Besides, in the MMTV-PyMT tumor model, blocking IL-10 signaling by anti-IL-10 receptor antibody enhanced treatment response to carboplatin and paclitaxel. This improved efficacy is attributed to the strengthened IL-12 production of DC and CD8+ T cell response [82]. Also, neutralizing TGF-β by conventional or bispecific antibodies increases the number of functional DCs in the TME [140,141,142]. Furthermore, IDO, functioning as an intracellular enzyme within the cytosol, transforms tryptophan into kynurenine. This conversion disrupts the activities of cytotoxic T cells, elevates the presence of Tregs and TAMs, and impedes the maturation of DCs [143,144,145]. Consequently, IDO contributes to rendering the TME more immunosuppressive, facilitating cancer escape from immune surveillance. Pharmacologic inhibition of IDO or deletion of Ido1 gene induces differentiation of inflammatory Ly6c+CD103+ DCs in mice, promoting anti-tumor T-cell response and inhibiting tumor growth [146]. The application of anti-IDO siRNA therapy enhances cytokine production and the antigen presentation capabilities of DCs [147]. Tumor vaccines that incorporate IDO inhibitors effectively enhance the uptake of tumor antigens and the maturation of DCs, ultimately inducing a robust tumor-specific T-cell response [148]. Currently, numerous clinical trials are underway to assess the effectiveness of immunotherapies involving IDO inhibitors [144].
Recent data demonstrate that PD-L1 on DCs dampens T cell activation and antitumor immune response. PD-L1 blockade enhances de novo T cell priming in tdLNs and reactivates dysfunctional T cells in the TME [149]. The antitumor activity of anti-PD-L1 therapy is more dependent on the renaissance of dysfunctional T cells inside tumors rather than newly activated T cell response in tdLNs [149]. Moreover, DCs could simultaneously overexpress PD-1, PD-L1, and CD80 [150, 151]. When DCs express a large amount of CD80, the cis-CD80/PD-L1 interactions on DCs prevent PD-L1 binding to PD-1 on T cells, contributing to the optimal T cell response [152]. However, for patients with cancers, the expression level of PD-L1 is significantly higher than CD80 on tumor-associated and peripheral DCs [153]. In this situation, anti-PD-L1 antibodies dissociate cis-CD80/PD-L1 binding, allowing CD80/CD28 interaction to provide costimulatory signaling for T cell activation [153]. Apart from PD-L1, T-cell immunoglobulin and mucin domain 3 (TIM-3) expressed on tumor-infiltrating DCs suppresses HMGB1-mediated activation of the innate sensing system [154]. Further explorations reveal that TIM-3 limits HMGB1-dependent DNA uptake, while TIM-3 blockade promotes the activation of the cGAS-STING pathway and CXCL9 expression of cDC1 [155]. Extensive preclinical evidence has demonstrated the advantages of anti-TIM-3 antibodies, especially in combination with anti-PD-1/PD-L1 therapies [156]. The therapeutic potential of TIM-3 blockade is currently being evaluated in multiple types of cancers.
Cancer vaccines and other strategies
The administration of cancer antigens, which could be captured and presented by endogenous DCs, is a promising immunotherapy approach [157]. These cancer antigen vaccines contain synthetic peptides, recombinant cancer antigen-expressing viruses, or tumor lysates [55, 158]. Fuelled by next-generation sequencing and prediction algorithms in silico, the identification of neoantigens increases the specificity of cancer antigen vaccines [159,160,161]. Considering that antigen presentation by DCs is the cornerstone for cancer antigen vaccines, antigens and adjuvants are usually encapsulated in degradable biomaterial or nanoparticles [162, 163]. To date, YS-ON-001 (rabies virus-based vaccine) has been approved for pancreatic cancer and hepatocellular carcinoma in the US [164]. Currently, advances have been made in targeted delivery to specific DC subsets [165]. DEC205, langerin, and CLEC9A are commonly used to target cDC1s. In vitro experiments confirm that the fusion protein of anti-DEC205 single-chain fragment variable and peptides of cancer antigen MAGE-A3 is presented more efficiently than direct peptide pulse [166]. Fusion antibody of anti-DEC205 and cancer antigen NY-ESO-1 effectively mobilizes CD8+ T cell response [167], showing encouraging antitumor activity in phase 1 studies [168]. Besides, more DC-targeted cancer antigen vaccines, such as CD209/DC-SIGN-fusion protein, are still under evaluation [169,170,171].
In addition to cancer antigen vaccines, the application of DC vaccines is extensively explored as well (Table 3) [157]. Such vaccines consist of manipulated autologous DCs isolated from cancer patients and expanded in vitro [172]. cDC precursors or monocyte-derived DCs are loaded with cancer antigens, activated with cytokine cocktails, and then reinfused into patients [173]. In various types of cancers, including non-small cell lung cancer (NSCLC), ovarian cancer, prostate cancer, melanoma, renal cell carcinoma, and glioblastoma, DC vaccines exhibit potent antitumor activity with a manageable safety profile [174,175,176,177,178,179,180,181,182,183,184]. In the latest phase 3 study of tumor lysate-loaded DC vaccine (DCVax-L), the combination of DCVax-L and standard of care (temozolomide) significantly extended the survival of patients with recurrent (HR = 0.58; P < 0.001) or newly diagnosed (HR = 0.80; P = 0.002) glioblastoma, compared to patients receiving temozolomide treatment alone [182]. At present, the DC vaccine sipuleucel-T (consisting of autologous DCs pulsed with the recombinant fusion protein containing GM-CSF and prostatic acid phosphatase) has been approved for prostate cancer [185]. In the phase 3 study NCT00065442, sipuleucel-T prolonged the survival of patients with castration-resistant prostate cancer (HR = 0.77; P = 0.02) [186]. However, immunosuppressive TME is a great obstacle to DC vaccination. Thus, the combination of DC vaccination with other therapies, such as immune checkpoint inhibitors, appears ideal for fostering de novo cancer-specific T-cell response [187].
Other DC-targeted strategies, such as agents improving DC migration by the CCR7-CCL19/CCL21 axis, have been adopted for cancer immunotherapy. When DCs encounter foreign stimuli, they undergo a mature process, with the upregulation of costimulatory molecules, MHC, and CCR7. The increased CCR7 expression on DCs drives their migration toward lymph nodes under the guide of the CCL19/CCL21 concentration gradient. Then, the CCR7-CCL19/CCL21 signaling directs DCs to distribute in the T-cell zone, where they prime and activate naïve T cells by antigen presentation [188]. Theoretically, CCL19 or CCL21 therapy could potentiate antitumor immunity by improving the trafficking of cytotoxic T cells and DCs. In multiple murine tumor models, intratumoral injection of CCL19 or CCL21 increases the numbers of tumor-infiltrating DCs and T cells, retards tumor growth, and prolongs the survival of tumor-bearing mice [189,190,191,192,193]. Besides, inducing tumor cells to overexpress CCL19 or CCL21 by transfection also enhances the functions of DCs and tumor control [194,195,196,197]. Also, autologous DCs engineered to overexpress CCR7 exhibit stronger migration capability and antitumor properties in murine tumors [198]. Besides immune response, CCR7 signaling also contributes to tumor progression, especially metastasis to the lymph nodes [199]. As a result, approaches inhibiting lymph node metastasis through CCR7 antagonism might unintentionally hinder the immune response to cancer. Conversely, strategies enhancing CCR7 expression or introducing CCL19/CCL21 into the TME could inadvertently promote metastasis. Therefore, several unresolved questions remain, necessitating answers before maximizing the therapeutic potential of the CCL19/CCL21-CCR7 axis. The initial pivotal question stems from the paradox between CCR7’s roles in enhancing the immune response to tumors and facilitating lymph node migration and metastasis.
Besides, IL-12 is a proinflammatory cytokine that activates both the innate and adaptive arms of the host immune system. In preclinical investigations, recombinant IL-12 has demonstrated strong antitumor effects [200]. It has been observed that the success of anti-PD-1 therapy relies on the presence of IL-12-producing DCs [70]. To address the challenges associated with the toxicity of systemic IL-12 administration, various localized delivery methods for IL-12 have been developed. These approaches include immunocytokine fusion, cell-based delivery, nucleic acid-based delivery, and virus-based delivery [201,202,203,204]. Clinical studies have confirmed the safety of intratumoral injections involving an adenoviral vector encoding IL-12 or DCs transfected with an adenovirus encoding IL-12 [205, 206]. Additionally, virotherapy through the intratumoral injection of a Semliki Forest virus encoding IL-12 (SFV-IL-12) has been shown to induce an inflammatory response and synergize effectively with anti-PD-1 therapy in tumor models [207]. Furthermore, SFV-IL-12 has been found to enhance the therapeutic effects of a 4-1BB agonist antibody [208]. In multiple preclinical investigations, the adoptive transfer of tumor-specific CD8+ T cells transiently expressing IL-12 has also demonstrated significant antitumor activity [209, 210]. Together, the outcomes of localized IL-12 immunotherapies, particularly in preclinical studies, have shown significant potential, meriting further investigation in clinical studies.
Macrophage-targeted cancer immunotherapy
Macrophages are a heterogeneous population of cells with high plasticity, showing diverse phenotypes under different stimuli [211]. Historically, macrophages are classified into two phenotypes, commonly referred to as M1 (classically activation, stimulated by IFN-γ and TLR ligands) and M2 (alternatively activation, stimulated by IL-4 and IL-13) [212]. M1 phenotype contributes to macrophage-mediated inflammatory tissue injury and tumor cell clearance, while M2 phenotype participates in damage repair and remodeling, as well as defense against parasites [213]. In the process of inflammation, the activation and polarization of macrophages are dynamically changed: M1 cells in triggering and propagating immune response, M2 or M2-like populations in inflammation resolution, or smouldering chronic inflammation [214,215,216]. However, with the development of omics technology, more and more novel macrophage subsets have been identified, and mixed expression of M1 and M2 biomarkers is also observed in tumor-infiltrating macrophages [217,218,219,220]. It is realized that the M1-M2 classification system is too simplistic to present complex phenotypes of macrophages.
Tumor-associated macrophage (TAM)
Tumor-infiltrating macrophage (termed TAM) is an important player in antitumor immune response and cancer progression [221]. Although some studies have opposite results [222], high infiltration of TAM is generally considered a risk factor in most preclinical and clinical studies [223]. Notably, signals regulating the polarization and education of TAMs change in different tumors and even in different stages or spatial locations of the same tumor, leading to various phenotypes of TAMs [224,225,226]. Therefore, TAM subsets should be preciously redefined to elaborate on the distinct roles of TAMs under specific circumstances.
Macrophages are recruited and educated by multiple factors in the TME, including colony-stimulating factor-1 (CSF1), GM-CSF, TGF-β, IL-1, IL-4, CCL2, CCL5, immune complexes, complement, histamine, tumor-derived non-coding RNAs [213, 227,228,229,230]. Besides, increased TNF-α and IL-1β in the TME could induce IL-8 expression, which recruits immunosuppressive myeloid leukocytes including macrophages and predicts poor outcomes in patients treated with immune checkpoint inhibitors [231, 232]. As a result, TAMs are commonly set in the protumor M2-like phenotype [233]. It has been validated that TAMs have substantial influences on tumor initiation and progression, especially by enhancing immune escape [234,235,236,237,238,239]. TAM-derived soluble molecules such as IL-10, IL-23, TGF-β, IDO, PGE2, and arginase 1 (ARG1) directly suppress the functions of tumor-infiltrating T and NK cells (Fig. 2a) [240,241,242,243,244,245]. Besides, autocrine IL-10 and TNF-α stimulate PD-L1 upregulation on TAMs [246]. These increased immune checkpoint ligands, such as B7-H4 and PD-L1/2, induce T cell exhaustion [247, 248]. Furthermore, TAMs inhibit the functions of T cells and NK cells by HLA-G/ILT2 and HLA-E/CD94 pathways [249]. Also, TAMs directly suppress the antitumor immune response by recruiting Tregs and supporting their differentiation [250]. Chemokines produced by TAMs, including CCL5, CCL20, and CCL22, recruit Treg into TME, while TGF-β and IL-10 induce Treg differentiation [249, 250].
The protumor activities of TAMs and TAM-targeted cancer therapies. a The protumor properties of TAMs. TAMs are commonly set in the protumor M2-like phenotype and have substantial influences on tumor initiation and progression. On the one hand, TAM-derived soluble molecules directly suppress the functions of tumor-infiltrating T cells and NK cells. Besides, autocrine IL-10 and TNF-α stimulate PD-L1 upregulation on TAMs. Also, TAMs directly suppress the antitumor immune response by recruiting Tregs and supporting their differentiation. On the other hand, TAMs also promote tumor progression in immune-independent ways, including tumor initiation and growth, angiogenesis, stemness, EMT, and distant metastasis. b TAM-targeted therapies. TAMs could be harnessed by targeting their recruitment, activation, immune checkpoint pathways, and metabolism. Besides, macrophage-based cell therapies, such as nanoparticle-loaded monocytes, CAR-M, and genetically engineered hematopoietic progenitors, also show potent antitumor activities. Abbreviations: TAM, tumor-associated macrophage; EMT, epithelial-mesenchymal transition; CSF1, colony-stimulating factor 1; CSF1R, CSF1 receptor; TLR, Toll-like receptor; STING, Stimulator of interferon genes; LILRB, Leukocyte immunoglobulin-like receptor B; SIRPα, Signal regulatory protein-α; IDO, Indoleamine 2,3-dioxygenase; CAR, Chimeric antigen receptor
In parallel with the immunosuppressive effects, TAMs also promote tumor progression in immune-independent ways, including angiogenesis, stemness, treatment resistance, and distant metastasis [251]. In gastric and colon cancers, chronic inflammation and oncogenic signals enhance the activities of multiple inflammation-associated transcription factors such as NF-κB, STAT3, and HIF-1α, recruiting macrophages into the TME [211]. Subsequently, these recruited macrophages generate a panel of molecules (e.g., EGF, proinflammatory cytokines, and ROS) to reshape the microenvironment and facilitate tumor initiation [252,253,254,255,256]. Also, TAMs induce epithelial-mesenchymal transition (EMT) of cancer cells by secreting CCL2, CCL5, CCL18, COX-2, MMP9, EGF, TGF-β, and IL-6 [254, 257,258,259,260,261,262,263]. These paracrine cytokines from TAMs endow cancers with greater invasive and metastatic capacities [264]. Furthermore, TAM-derived soluble molecules and TAM-tumor interactions maintain the stemness of cancer cells [265,266,267,268]. Moreover, TAMs support tumor growth by producing proangiogenic factors, including VEGFA, EGF, and TGF-β1 [269,270,271]. Given the pivotal roles of TAMs in cancer development, intensive attempts have been made to delete TAMs or reprogram TAM behaviors.
TAM-targeted therapies
Numerous studies have confirmed the protumor roles of TAM in the majority of human tumors. As a result, targeting TAMs has emerged as a promising therapeutic strategy for cancer patients (Table 4). In the ensuing paragraphs, we summarize several TAM-based therapeutic strategies, including targeting TAM recruitment, activation, and metabolism (Fig. 2b). Besides, myeloid checkpoint inhibitors and macrophage cell therapies are promising, especially with present immune checkpoint blockade.
Inhibiting TAM recruitment and expansion
As mentioned above, TAM recruitment is driven by chemokines and CSF1. Although therapeutic antibodies or inhibitors targeting attractants such as CCL2-CCR2 (e.g., Lenalidomide and Trabectedin) have exhibited antitumor activities in preclinical studies, there are rare clinical trials with positive data [272]. Relatively, clinical trials of CSF1-CSF1R inhibitors (e.g., Cabiralizumab and Pexidartinib) are experiencing improved efficiency and progress. CSF1-CSF1R blockade deletes the TAM population, retards tumor growth, and increases treatment sensitivity [273,274,275]. Besides, the CSF1R inhibitor BLZ945 could reprogram TAM from a tumor-promoting toward a tumor-suppressing phenotype, enhancing antigen presentation and T or NK cell activation [273]. Moreover, in the phase 1 study of diffuse-type tenosynovial giant-cell tumor (NCT01494688), anti-CSF1R antibody emactuzumab decreased tumor-infiltrating CD68/CD163+ macrophages and achieved pronounced activity (response rate: 71%) [276]. At present, more clinical studies of CSF1R inhibitors combined with other therapies are still ongoing. Some novel TAM depletion strategies, such as CAR-T cells recognizing folate receptor-β, eliminate M2-like TAM subsets and promote tumor-specific T-cell response [277]. Furthermore, Lurbinectedin, which is a synthetic alkaloid, remodels the TME by prompting apoptosis in TAMs and diminishing the expression of CCL2. Lurbinectedin has received approval for the treatment of small-cell lung cancer [278]. Also, there have been recent advancements in the use of M2 macrophage-targeting peptides (M2peps) to specifically target and deliver pro-apoptotic agents to M2-like TAMs in preclinical tumor models [279]. These therapeutic agents associated with M2peps demonstrate preferential toxicity towards M2-like TAMs and exhibit potent anti-tumor effects, holding promise for potential clinical applications in TAM-focused immunomodulation [280].
Regulating TAM activation
Classical activation endows macrophages with antitumor properties. Agents enhancing classical activation pathways, including CD40, STING, and TLR, reset TAMs in the antitumor M1-like phenotype. As described above, CD40L-CD40 is the core pathway to activate antigen-presentation cells [281]. Preclinical studies demonstrate that agonistic CD40 antibodies effectively arm macrophages with cytostatic activity against tumor cells, stimulating antitumor response and slowing tumor growth [282, 283]. Furthermore, agonistic CD40 antibodies improve the antigen presentation capability of TAMs by upregulating costimulatory molecules and MHC expression [213]. Besides CD40, agents targeting TLR exert immunostimulatory effects by enhancing the cytotoxic activity and chemokine production of TAMs [284, 285]. The TLR4 agonist monophosphoryl lipid A combined with IFN-γ drives the transformation from CD206+ TAMs to iNOS+ macrophages, activating T cells by inducing macrophages to secret IL-12 and TNF-α [285]. Additionally, STING agonists promote IFN-I secretion and macrophage polarization toward the M1-like phenotype. In murine tumor models, STING agonists increase the ratio of M1/M2 ratio and synergize with anti-PD-1/PD-L1 therapies [105, 106, 286].
Targeting immune checkpoints
The phagocytosis and cross-presentation capabilities of TAMs are constrained by immune checkpoints such as signal regulatory protein-α (SIRPα), SLAM family receptors (SFRs), sialic acid-binding immunoglobulin-like lectin (Siglec), and leukocyte immunoglobulin-like receptor B (LILRB) families [287,288,289]. CD47 is the ligand of SIRPα, also known as the “not eat me” signal. In the TME, overexpressed CD47 on cancer cells bind to SIRPα on myeloid cells, especially macrophages, monocytes, granulocytes, and CD4+ DCs, limiting phagocytosis and intracellular degradation [290]. Agents blocking the CD47-SIRPα axis improve macrophage phagocytosis, enhance programmed cell death of cancer cells, and promote macrophage-mediated ADCP or ADCC effects [291,292,293,294,295,296]. Besides, anti-CD47 antibody-mediated phagocytosis facilitates antigen presentation and cross-priming of CD8+ T cells [297]. In the phase 1 study of non-Hodgkin’s lymphoma NCT02953509, the anti-CD47 antibody Hu5F9-G4 combined with rituximab showed promising activity (response rate: 50%; complete response rate: 36%) [298]. Besides, more anti-CD47 antibody-involved strategies achieve encouraging results in solid and hematological malignancies [299,300,301].
Besides the CD47-SIRPα axis, other immune checkpoints, such as Siglec receptors, are also vital targets for cancer immunotherapy [302]. Similar to PD-1 signaling, sialoglycan ligands bind to inhibitory Siglec receptors (e.g., Siglec-7 and Siglec-9), suppressing intracellular immune signaling by recruiting SHP1/2 phosphatases [303]. Innate immune cells, especially TAMs, highly express Siglec receptors [304]. In various cancers, tumor-derived ligands (e.g., CD24 and sialoglycans) induce monocyte differentiation toward protumor TAM phenotype by Siglec-7, Siglec-9, Siglec-10, Siglec-15, and Siglec-E [287, 305,306,307,308,309,310,311]. Actually, Siglec signaling undermines the functions of multiple immune cells, including but not limited to DCs, NK cells, and T cells. Degenerating sialic acid residues by sialidase improves lymphocyte phagocytosis [312]. Preclinical studies have demonstrated that Siglec-15 blockade boosts antitumor immunity and inhibits tumor growth [310, 313]. Interrupting CD24-Siglec-10 interaction by anti-CD24 antibody improves phagocytic clearance of cancer cells by macrophages [287]. Moreover, other immune checkpoints and scavenger receptors are also identified as important regulators for TAM polarization and functions, such as LILRB, PD-1, and P-selectin glycoprotein ligand 1 (PSGL1) [314,315,316,317,318,319]. At present, most agents targeting these pathways are in clinical evaluation except for anti-PD-1/PD-L1 antibodies.
TAM metabolism regulators or other novel agents reprogramming TAM
Driven by nutrient deprivation and hypoxia, dysregulated metabolic conditions in the TME promote the accumulation of TAMs [320]. The by-product of glycolysis is lactic acid, which could promote the polarization of macrophages toward the M2-like phenotype [321]. Agents targeting glycolysis, such as 2-deoxy-D-glucose (2-DG), reverse M2 polarization [322]. Moreover, the respiratory complex I inhibitor metformin reprograms the TME: increasing immunoinhibitory CD11c+ but decreasing immunosupportive CD163+ TAMs, and strengthening macrophage phagocytosis against cancer cells [323]. Inhibiting tumor-derived retinoic acid induces the differentiation of monocytes toward immunostimulatory DCs rather than TAMs [324]. Also, glutamine metabolism inhibitors retard tumor growth by rewiring TAMs toward the M1-like phenotype [325]. Furthermore, IDO1-mediated tryptophan metabolism, tumor-derived PGE2, and oxysterol receptor LXR transcription factor also maintain the immunoinhibitory functions of TAMs [213, 326, 327]. Agents blocking these molecules have immense potential and broad prospects. Apart from regulating tumor metabolites, other novel agents, such as anti-IL-1 antibodies and nanoparticles containing mRNAs encoding IRF5-IKKβ or miRNA-155, effectively reprogram TAMs toward antitumor effectors [328,329,330].
Macrophage-based cell therapy
The TAM pool is dynamically replenished by peripheral circulating monocytes, which are constantly trafficked into the TME. Therefore, monocytes could be used as Trojan horses to delivery agents into tumors [331,332,333]. Nanoparticle-loaded monocytes exhibit superior antitumor activity to free nanoparticles [334]. Also, genetically engineered hematopoietic progenitors with high expression of Tie-2 and IFN-α effectively migrate to tumors and reshape the TME by releasing IFN-α [335]. Genetically engineered myeloid cells highly expressing IL-12 improve T cell response and inhibit tumor growth [336]. Furthermore, engineered particles (containing cytokines such as IFN-α) adhering to macrophage surfaces could facilitate TAMs to maintain their antitumor phenotype in the hostile TME [337].
Apart from engineering macrophages for drug delivery, macrophage engineered with CAR (CAR-M) therapy is also a promising manner to mobilize antitumor immune response [338, 339]. Similar to CAR-T cells, CAR-M contains extracellular antigen-recognizing, transmembrane, and intracellular domains. However, ZAP-70, a kinase for T cell activation, is not available in macrophages. Instead, CAR-M transduces phagocytic signals by another kinase Syk, which contains tSH2 domain and binds to CD3ζ [340]. Besides CD3ζ, other domains with immunoreceptor tyrosine-based activation motifs (ITAMs), such as multiple epidermal growth factor-like domains protein 10 (Megf10) and Fc receptor (FcRγ), also elicit phagocytosis of macrophages [341, 342]. CD3, CD147, FcR, and Megf10 are commonly utilized intracellular signaling domains in CAR-M products [343].
The first CAR-M product was developed in 2018, initially referred to as CAR-phagocytes (CAR-Ps), by employing a lentiviral vector to introduce a CAR with either Megf10 or FcRγ as the cytosolic domain into mouse macrophages [342]. These CAR-Ps displayed specific engulfment of entire human cancer cells, particularly when a tandem PI3K p85 subunit was integrated into the CAR. Although this study primarily focused on the impact of CAR on phagocytosis while excluding other essential anti-tumor functions carried out by macrophages, it marked a significant milestone in CAR-based immunotherapy [342]. Moreover, CAR-M cells possess the capacity to stimulate the transformation of M2 macrophages into M1 and release proinflammatory cytokines in the TME. It was reported that anti-HER2 CAR-M cells not only displayed tumor-killing capabilities but also induced a proinflammatory TME. Additionally, CAR-M products could enhance the activity of tumor-specific T cells by generating proinflammatory chemokines and cytokines, reprogramming M2-like into M1-like macrophages, and increasing the expression of antigen presentation machinery [341]. Furthermore, the extracellular matrix (ECM) hampers immune cell infiltration into the TME, limiting the efficacy of immunotherapy. In contrast, macrophages are naturally attracted to the TME, break down the ECM, and consequently represent the most abundant immune cell population in tumors by secreting MMPs. Shen et al. engineered CAR-M cells utilizing CD147 as the intracellular signaling domain (referred to as CAR-147 M). They observed that when these CAR-147 M cells were co-cultured with target cells, there was a significant increase in MMP expression. Although this boost in MMPs did not affect tumor cell proliferation in vitro, CAR-147 M cells rapidly accumulated at the tumor site when administered in vivo. This led to a reduction in tumor collagen deposition and promoted the infiltration of immune cells, ultimately resulting in significant tumor suppression [344]. Generally, CAR-M has some advantages over CAR-T cells in solid tumors, especially enhanced trafficking and infiltration into the TME [345, 346]. At present, most CAR-M products are at the preclinical stage, and only one autologous CAR-M targeting Her-2 is in clinical evaluation (CT-0508, NCT04660929, Phase 1) [339, 347].
In addition to Trojan horse strategies and CAR-M, there exist combinations that merge elements from both strategies. Nanocomplexes comprised of nanocarriers designed for macrophage targeting and plasmid DNA encoding CAR-interferon-γ, when administered in vivo, induce the development of CAR-M1 macrophages. These specialized macrophages exhibit the ability to engage in CAR-mediated cancer cell phagocytosis, orchestrate anti-tumor immunomodulatory responses, and effectively impede the growth of solid tumors [348].
Harnessing MDSC for cancer therapy
MDSCs are a heterogeneous population of cells with immunosuppressive effects [349]. Under normal physiological conditions, bone marrow cells differentiate into mature subsets, including DCs, macrophages, and granulocytes (also termed terminally differentiated cells) [350]. However, the differentiation process of MDSC is disturbed by the TME, arresting it in an immature state [351]. The immunosuppressive nature of MDSCs contributes to cancer progression by promoting immune evasion and treatment resistance. For several solid tumors and hematologic malignancies, elevated levels of MDSCs have been associated with poor prognosis and treatment response [352,353,354,355,356,357,358,359,360]. Understanding the role of MDSCs in cancer is crucial for developing effective therapeutic strategies. Targeting MDSCs and modulating their immunosuppressive functions may hold promise in enhancing antitumor immune responses and improving patient outcomes.
MDSCs and their protumor effects
MDSCs could be mainly classified into two cell subsets named polymorphonuclear MDSC (PMN-MDSC, similar to neutrophils in phenotype and morphology) and monocytic MDSC (M-MDSC, similar to monocytes in phenotype and morphology) [361]. PMN-MDSCs typically account for more than 80% of all MDSCs in various cancers [361]. Besides, within the overall population of MDSCs, there is a small subset comprising less than 3% of cells that possess myeloid colony-forming capability [361]. In mice, MDSCs are distributed in peripheral blood, bone marrow, spleen, lung, liver, and tumors. Murine PMN-MDSC is commonly defined as CD11b+Ly6G+Ly6Clo, while murine M-MDSC is defined as CD11b+Ly6G−Ly6Chi [362]. In humans, MDSCs are distributed in peripheral blood and tumors. Predominantly, human PMN-MDSC is defined as CD11b+CD15+HLA-DRloCD66b+, while human M-MDSC is defined as CD11b+CD14+CD33+HLA-DRlo/− [363]. Moreover, Lin−HLA-DR−CD33+ cells (early-stage MDSC or e-MDSC) are a mixture of MDSCs containing more immature progenitors [364].
The primary characteristic of MDSCs is immune suppression. Although MDSCs have been implicated in undermining the functions of multiple immune cells, their main targets are T cells. MDSCs cause immune suppression by upregulating TGF-β, IL-10, IDO, iNOS, ARG1, PEG2, reactive oxygen species (ROS), PD-L1, and depleting cystine and cysteine in the TME (Fig. 3a) [21, 365, 366]. Besides, the ADAM17 on MDSCs exerts immunosuppressive effects by downregulating L-selectin (T cell homing receptor) on naïve T cells [367, 368]. It has been confirmed that PMN-MDSCs and M-MDSCs prefer different manners to inhibit T cell response. PMN-MDSCs preferentially produce ARG1, ROS, peroxynitrite, and PGE2, while M-MDSCs preferentially generate NO, TGF-β, and IL-10 [351, 369, 370]. Apart from cytotoxic T cells, MDSCs impair other tumoricidal immune cells, including DCs, B cells, and NK cells [371,372,373]. Furthermore, MDSCs weaken antitumor immunity by inducing the differentiation or enhancing the functions of immunosuppressive cells such as TAMs and Tregs [374,375,376].
MDSC-mediated T cell suppression and MDSC-targeted therapies. a MDSC-mediated T cell suppression. Although MDSCs have been implicated in undermining the functions of multiple immune cells, their main targets are T cells. MDSCs cause immune suppression by upregulating TGF-β, IL-10, IDO, iNOS, ARG1, ROS, PD-L1, and depleting cystine and cysteine in the TME. Besides, the ADAM17 on MDSCs exerts immunosuppressive effects by downregulating L-selectin (T cell homing receptor) on naïve T cells. b MDSC-targeted therapies can be categorized into four groups: suppressing the recruitment and expansion of MDSCs; facilitating the differentiation of MDSCs into mature myeloid cells; counteracting the functions of MDSCs; and directly depleting MDSCs. Abbreviations: MDSC, myeloid-derived suppressor cell; ASC, asctype amino acid transporter; CAT-2B, cationic amino acid transporter 2B; Xc−, cystine-glutamate transporter; IDO, indole-2,3 dioxygenase; NO, nitric oxide; iNOS, inducible nitric oxide synthase; TCR, T cell receptor; ROS, reactive oxygen species
In addition to exerting immunosuppressive effects, MDSCs contribute to tumor progression by promoting tumor angiogenesis, maintaining cancer stemness, inducing EMT, and facilitating premetastatic niche formation [377]. On the one hand, MDSCs support vascularization by generating VEGF and MMP-9 [378]. On the other hand, some MDSCs have the potential to differentiate toward endothelial-like cells, directly incorporating into tumor endothelium [379]. Moreover, exosomal S100A9 released by MDSCs increases the stemness of colorectal cancer in a HIF-1α-dependent manner [380]. MDSC-endowed stemness qualities are also observed by triggering STAT3-NOTCH crosstalk and inducing miRNA-101 in breast and ovarian cancer cells [381, 382]. Besides, in murine colorectal cancer models, increased CXCL1 in premetastatic tissues attracts CXCR2+ MDSCs, which support cancer cell survival and promote metastatic niche formation [383].
MDSC-targeted therapies
The significant involvement of MDSCs in tumor development has sparked the exploration of MDSC-targeted therapies. These strategies can be categorized into four groups: (1) suppressing the recruitment and expansion of MDSCs; (2) facilitating the differentiation of MDSCs into mature myeloid cells; (3) counteracting the functions of MDSCs; and (4) directly depleting MDSCs (Table 5) (Fig. 3b) [21, 384].
Suppressing the recruitment and expansion of MDSCs
MDSCs migrate to tumors under the guidance of chemokine pathways such as CXCLs-CXCR1/2 and CCL2-CCR2 [385, 386]. CXCLs-CXCR1/2 blockade improves the antitumor activities of immunotherapies in various murine models by preventing the trafficking of PMN-MDSCs into the TME [387,388,389]. So far, CXCR1/2 inhibitors (e.g., AZD5069, Reparixin, Navarixin, and SX-682) and anti-CXCL8 antibodies neutralizing IL-8 (also termed CXCL8 in humans) (e.g., HuMax-IL8 and ABX-IL8) are undergoing clinical evaluation [390, 391]. Besides, IL-1β contributes to the recruitment and expansion of MDSCs and modulates their immunoinhibitory functions in the TME [392]. Inhibiting IL-1β or NLRP3 inflammasome (a key component for IL-1β maturation) reduces MDSCs and enhances antitumor immunity in head and neck squamous cell carcinoma models [393,394,395,396,397]. Additionally, GM-CSF leads to MDSC accumulation and weakens cancer antigen-specific T-cell response [398]. At the same time, G-CSF initiates MDSC mobilization and promotes tumor angiogenesis [399]. GM-CSF/G-CSF blockade with antibodies reduces MDSC accumulation and overcomes cancer immune escape [400, 401]. Moreover, MDSCs simultaneously express S100A8/A9 and their receptors RAGE, forming a positive feedback loop that promotes the recruitment of MDSCs and amplifies their immunosuppressive capabilities. S100A8/A9 inhibitors disturb this positive feedback loop, diminish MDSC accumulation, and retard tumor growth in various murine models [402,403,404]. Furthermore, anti-VEGF-VEGFR therapies also inhibit MDSC recruitment by blocking VEGFR1 signaling of MDSCs [405, 406].
Facilitating the differentiation of MDSCs into mature myeloid cells
All-trans retinoic acid (ATRA) regulates cell differentiation, proliferation, and apoptosis by nuclear retinoid receptors [407]. Differentiation therapy with ATRA has altered the therapeutic paradigm of acute promyelocytic leukemia and significantly improved patient outcomes [408]. Similarly, ATRA could promote the differentiation of immature MDSCs toward terminated differentiated myeloid cells (DCs, macrophages, and granulocytes) [409]. In patients with metastatic renal cell carcinoma, ATRA treatment substantially reduces MDSC in peripheral blood, increases the cDC/pDC ratio, and enhances antigen presentation and antigen-specific T-cell response [410]. In multiple clinical trials of lung cancer (NCT00617409) and melanoma (NCT02403778), additional ATRA treatment significantly augments immunotherapy and chemotherapy [411,412,413]. Moreover, constitutive STAT3 activation prevents the differentiation of immature myeloid cells and maintains their immunosuppressive properties [414, 415]. In patients with advanced lung cancers, Cucurbitacin B (JAK2/STAT3 inhibitor) decreases the ratio of immature-to-mature myeloid cells in peripheral blood [416]. In patients with diffuse large B-cell lymphomas, AZD9150 (antisense oligonucleotide of STAT3) reduces peripheral PMN-MDSCs as well [417]. The synergistic effects between STAT3 inhibitors and immunotherapies have been validated in a series of preclinical and clinical studies [418,419,420,421,422].
TLRs also play an important role in the maturation and differentiation of MDSCs. CpG oligodeoxynucleotides (termed CpG ODN, TLR9 agonist) stimulates antitumor immunity by activating CD8+T/NK cells, inducing the differentiation of M-MDSC toward M1-like macrophages [423,424,425]. In vivo experiments demonstrate that CpG effectively promotes the maturation of MDSC and abrogates MDSC-mediated T-cell suppression by triggering IFN-α production of pDCs [426]. Also, TLR7/8 and TLR3 agonists, such as resiquimod, motolimod, and Poly (I: C), relieve MDSC-induced immune evasion and revive antitumor immune response [114, 427,428,429]. Furthermore, some novel agents, such as curcumin, β-glucans, and icariin, drive the differentiation of MDSCs into DCs and macrophages and undermine their suppressive functions [430,431,432].
Counteracting the functions of MDSC
The COX-2-PGE2 axis is the key pathway to maintain the immunosuppressive functions of MDSCs [433,434,435]. On the one hand, PGE2 in the TME attracts MDSCs by CXCL12-CXCR4 [436]. On the other hand, PGE2 from tumor cells triggers the nuclear p50/NF-κB signaling in M-MDSCs, which reprograms their response to IFN-γ and decreases TNF-α generation [437]. Besides, paracrine PGE2 induces MDSCs to upregulate COX-2 expression, which could stimulate autocrine PGE2 production, forming a positive feedback loop [438]. This PGE2-COX-2 positive feedback loop facilitates to stabilize MDSC phenotype [438]. Agents targeting COX-2-PGE2 signaling hamper the immunoinhibitory functions of MDSCs and improve the sensitivity to immunotherapies [439, 440]. For example, celecoxib (COX-2 inhibitor) decreases the production of ROS and NO in MDSCs and reverses T-cell tolerance [441]. Besides, celecoxib combined with CD40 agonist therapy effectively increases CXCL10 but reduces ARG1 in MDSCs. As a result, antitumor immunity is restored, and tumor growth is suppressed in glioma-bearing mice [442].
Additionally, phosphodiesterase 5 (PDE5) inhibitors such as tadalafil and sildenafil reduce the levels of ARG1, iNOS, and IL-4Rα (myeloid suppressor cell suppressive marker) [443, 444]. In clinical studies of melanoma (EudraCT: 2011–003273-28) and head and neck squamous cell carcinoma (NCT00843635 and NCT00894413), tadalafil reduces MDSC frequency, hampers the immunoinhibitory properties of MDSCs, and augments cancer-specific immunity [445,446,447]. Moreover, epigenetic regulators such as histone deacetylase inhibitors (HDACis) have substantial influences on the functions of MDSCs. In murine tumor models, HDACi treatment significantly downregulates the expression of COX-2, ARG1, and iNOS in MDSCs [448, 449]. The class I HDACi entinostat mainly modulates the functions of PMN-MDSCs, while class II HDAC6 inhibitor ricolinostat primarily regulates the functions of M-MDSCs [450]. Moreover, other novel agents such as Nrf2 activator (CDDO-Me), vitamin D3, and nitroaspirin (the derivative of aspirin with nitro moiety) are identified as negative regulators for MDSC-mediated immunosuppression [451,452,453].
The functions of MDSCs could be suppressed by disturbing their metabolism. Due to the high consumption and active fatty acid oxidation (FAO) of MDSC, inhibiting some key molecules in FAO impedes MDSC-mediated immune suppression [454]. Agents targeting FAO rate-limiting enzymes such as etomoxir (targeting enzyme CPT1) and lipofermata (targeting enzyme FATP2) remarkably abrogate the immunosuppressive activities of MDSCs in the TME [455, 456]. In addition to fatty acid metabolism, glycolysis also has positive effects on the survival and activity of MDSCs. In murine tumor models, tumor-infiltrating MDSCs have more active glycolysis and mTOR signaling [457]. Rapamycin (mTOR inhibitor) downregulates the quantity and activity of M-MDSCs in mice [458]. Also, a glycolysis modulator (metformin) counteracts the inhibitory functions of MDSCs by impeding the expression and enzymatic activity of CD39/CD73 [459]. Furthermore, targeting other metabolic enzymes or metabolites such as IDO (converting tryptophan to kynurenine) inhibitors and CD39/CD73 (converting ATP to adenosine) inhibitors also reprograms MDSCs and contributes to the renaissance of antitumor response [384, 460, 461].
It is important to note that certain agents affecting metabolism can also impact immune cells within the TME apart from MDSCs. For instance, the activation of STAT3 signaling leads to a metabolism biased toward FAO in CD8+ T cells, which impairs their functionality and contributes to the development of obesity-related breast cancer. On the other hand, inhibiting FAO enhances the performance of CD8+ T effector cells and inhibits tumor growth [462]. Additionally, the peroxisome proliferator-activated receptor agonist Bezafibrate stimulates mitochondria, enhancing oxidative phosphorylation, glycolysis, and FAO, ultimately leading to improved functionality in T cells infiltrating tumors [463]. Furthermore, the costimulatory signal 4-1BB enhances the glucose and fatty acid metabolism in T cells to meet their growing energy demands. The effects on the T cell cycle and anti-apoptotic activity mediated by 4-1BB signaling are entirely nullified by the FAO inhibitor etomoxir [464]. Moreover, there is evidence that Metformin therapy restores the impaired metabolic function of hepatic CD8+ T cells in non-alcoholic steatohepatitis (NASH) and enhances the efficacy of anti-PD-1 treatment in liver tumors associated with NASH [465]. Furthermore, the impact of immunometabolism on other immune cells, such as DCs and macrophages, has been confirmed. The anabolic and catabolic processes substantially influence the immunogenicity and tolerogenicity of DCs, while succinate and citrate directly regulate macrophage functions [466]. Hence, it is essential to comprehensively consider the effects of metabolism-modulating agents on various components of the TME beyond MDSCs to achieve optimal immunotherapy efficacy.
Directly depleting MDSCs
Some chemotherapeutic agents could selectively eradicate regulatory immune cells, especially MDSC, and alleviate immune suppression [467]. For example, 5-fluorouracil and gemcitabine induce the MDSC apoptosis and restore tumor-specific CD8+ T cell response [468]. Carboplatin and paclitaxel cause MDSC depletion and boost therapeutic vaccination-mediated immune response [469]. Besides, low-dose capecitabine reduces circulating MDSCs and increases cytotoxic immune infiltration in the TME [470]. It is notable that some cytotoxic agents might also have positive effects on MDSCs, such as cyclophosphamide (CTX). The difference could be attributed to agents, administration schedules and doses, and heterogeneity of sampling [471]. Generally, these agents are not MDSC-specific, with cytotoxic effects on all rapidly proliferating, even lymphocytes in the TME. Relatively, therapies targeting CD33 have better specificity for MDSCs [472]. Fc-engineered anti-CD33 antibodies (BI 836858) and anti-CD33 antibody-conjugated drug (gemtuzumab ozogamicin) could specifically eliminate MDSCs [472, 473]. Additionally, agonists of TNF-related apoptosis-induced ligand (TRAIL) receptors and anti-angiogenesis tyrosine kinase inhibitor sunitinib are regarded as MDSC eliminators as well [474, 475].
Collectively, MDSCs play a crucial role in tumor development, leading to the exploration of four main categories of MDSC-targeted therapies. These approaches include (1) suppressing MDSC recruitment and expansion through blockade of chemokine pathways and cytokines, (2) promoting MDSC differentiation into mature myeloid cells using agents like ATRA, STAT3 inhibitors, and TLR agonists, (3) countering MDSC functions by targeting the COX-2-PGE2 axis and metabolic pathways, and (4) directly depleting MDSCs, often through chemotherapeutic agents like 5-fluorouracil and gemcitabine or specific MDSC-targeting therapies like anti-CD33 antibodies. Notably, some metabolic modulators can affect other immune cells in the TME. These strategies offer potential in enhancing cancer immunotherapy by either reducing MDSC numbers or neutralizing their suppressive functions, but their broader effects on immune cells need to be considered for optimal outcomes.
Targeting NK for cancer immunotherapy
NK cells are a type of immune cell that make up the innate lymphoid cellular defense and surveillance system [476, 477]. When encountering tumor cells, it serves as the primary sentinel in safeguarding organismal health. In humans, NK cells lack membranal TCR and CD3 molecules but have neural cell adhesion molecule (NCAM, also known as CD56), along with activating and inhibitory receptors [478, 479]. Particularly, unlike other surface biomarkers only found in the bloodstream, CD335 is an activating receptor that can also identify NK cells in formalin-fixed paraffin-embedded tissue specimens [480]. Commonly, NK cells take up approximately 5%-20% of circulating lymphocytes in humans [481]. NK cells can be activated and exert cytotoxic effects independent of specific antigen recognition, as they recognize foreign organisms and malignancies through the aforementioned stimulatory and inhibitory receptors [476].
The biology of human NK cells
It is well established that human NK cells proceed through five discrete stages in lineage derivation and development [482]. Human NK cells, together with other kinds of innate lymphoid cells (ILCs), are derived from multipotent CD34+ hematopoietic progenitors [483, 484]. A subset of these progenitors is committed into α-lymphoid precursor (αLP) cells by expressing integrin α4β7 [485, 486]. Subsequently, αLP cells expressing CXCR6 are able to develop into precursor NK and ILC-3 subtypes [487]. The symbolic event of precursor NK occurrence is the IL-1R1 expression on αLP cells [488, 489]. Predominant precursor NK cells undergo further development and maturation in bone marrow, while a minority of cells undergo maturation in peripheral lymphoid organs [490, 491]. In these sites, NK cells gradually express specific surface receptors driven by multiple transcription factors, including T-bet, Id2, E4BP4, and Eomesodermin (Eomes) [492,493,494,495]. In brief, NK cell development involves dynamic changes in lineage-specific biomarkers, with a gradual decrease in progenitor and precursor markers and an increase in bioactive receptors.
The trafficking, homing, and activation of NK cells are mutually reinforcing processes that complement the maturation of these cells. Specifically, immature NK cells require certain factors to facilitate their trafficking during maturation. Most NK cells undergo maturation within a specialized niche located in bone marrow, where they are surrounded and nourished by parenchymal sinusoidal vessels [496]. Only after CXCR4 is downregulated, NK cells migrate from bone marrow into the sinusoidal vessel and subsequently into peripheral blood [497]. Additionally, various chemokines and integrins, along with their corresponding receptors and ligands, are involved in this process. Therefore, distinct types of chemokines and integrins can be identified as biomarkers for circulating or tissue-resident NK cells [498, 499]. The migration of NK cells from sinusoidal vessels into circulation also needs the participation of various factors, particularly CX3CR1, S1P5, and CXCR6 [500,501,502,503,504,505]. Additionally, CX3CR1 boosts the infiltration of NK cells into the central nervous system, which is traditionally considered the forbidden zone for immune cells [504, 506].
The mature NK cells can be further classified into two major subtypes: CD56brightCD16dim/− and CD56dimCD16+ [490], while rare cells differentiate into memory NK cells under specific stimuli [507, 508]. The CD56brightCD16dim/− subset possesses poor cytotoxicity and minor circulating proportion, which could further differentiate into CD56dimCD16+ cells [481]. The CD56brightCD16dim/− subset is more commonly observed in lymph nodes, the gastrointestinal tract, and tonsils, where the overall proportion of NK cells is lower [478]. In these sites, they exert more secretory biologic function rather than cell lysed function [509,510,511]. On the contrary, the CD56dimCD16+ subset is regarded as cytotoxic NK cells, which could directly eradicate tumor cells by death receptor signaling or cytotoxic effector molecules [512].
After licensing, NK cells are directly activated, equipped with a diverse array of inhibitory and activating surface receptors, independent of MHC-restricted antigen recognition when encountering detrimental factors [492]. In the TME, NK cells are activated by the construction changes or expression downregulation of MHC-I molecules [513]. Also, NK cells can be activated by stimulatory receptors such as NKp30, NKp44, and NKp46 [514]. Additionally, the CD16 receptor on NK cells exerts separate activating functions after being engaged by the immunoglobulin-opsonized cells. This process elicits the phosphorylation of the ITAM domain of FcεRIγ and CD3ζ on the surface of NK cells, ultimately culminating in ADCC [515, 516].
The roles of NK cells in antitumor immunity
However, the antitumor activity of NK cells is limited by multiple factors, such as insufficient NK cell infiltration and the hostile TME [517, 518]. It has been validated that cancer-derived exosomes and hypoxia could blunt NK cell activity [519, 520]. Besides, some modulatory immune cells and cytokines, such as TGF-β, activin-A, and adenosine, also contribute to the immunosuppression of tumor-infiltrating NK cells [521,522,523,524,525].
High NK cell abundance in the TME predicts a favorable prognosis in a myriad of cancers [241, 526,527,528], and NK cells suppress tumorigenesis by executing immunosurveillance [529, 530]. In the TME, NK cell activation is determined by activating and inhibiting signals, such as NKG2D, 2B4, DNAM1, LFA1, CD28H, IL-12, IFN-α and TGF-β (Fig. 4) [531,532,533]. Notably, HLA-E exerts dualistic immunoregulatory effects on NK cells when binding to different receptors [534,535,536,537,538]. Activated NK cells can eliminate tumor cells by releasing perforin and granzymes, as well as by inducing apoptosis via ADCC, FasL, or TRAIL [531, 539]. Besides, NK cells secrete cytokines, including IFN-γ and TNF-α, which lead to tumor growth arrest [540]. The ruptured tumor cells will unleash neoantigens, subsequently prompting the adaptive immune response [541]. As the communicative bridge between innate and adaptive immunity, DC plays crucial and intricate roles in antitumor immune responses. Additionally, NK cells promote the recruitment of cDCs into the TME [57]. A novel type of NK cells, termed induced pluripotent stem cells (iPSCs)-derived NK cells, is reported to recruit T cells into the TME and augment the therapeutic effect of immune checkpoint inhibitors [542]. Apart from executing immune surveillance and elimination functions by tissue-resident NK cells, circulating NK cells can also prevent tumor metastasis by activating the NKp46/NCR1 signaling [478, 543]. The mechanisms underlying the recognition of tumor cells by NK cells highlight the perspectives for NK cell-targeted strategies, particularly in cold tumors lacking neoantigens.
Interaction between NK cell and the TME. Schematic diagram depicting primary receptors expressed by NK cells and their corresponding ligands on tumor cells or cytokines in the TME. Activating stimulative receptors triggers an intracellular signaling cascade that activates NK cells and vice versa. The two factors dynamically modulate the behavioral pattern of NK cells, whose disequilibrium may lead to immune evasion or clearance. Abbreviations: NK cell, natural killer cell; TME, tumor microenvironment; Ecto-CRT, ecto-calreticulin; A2AR, A2a adenosine receptor; ACVR1, activin receptor type 1
Developing NK cell-targeted therapies
The antitumor activity of NK cells has been unequivocally demonstrated through in vitro experiments and animal models, providing a solid rationale for investigating their potential as anticancer agents [544,545,546]. Generally, NK cell-based therapeutic strategies can be categorized into five distinct groups based on the sequential processes of NK cells, including trafficking, activation, effector function execution, and secondary adaptive immune priming, which synergistically reinforce each other (Fig. 5).
NK cell-based therapeutic strategies. a NK cell-based therapeutic strategies could enhance multiple biological processes, including trafficking, activation, tumor-killing activity, and NK cell-mediated secondary adaptive immune priming. b NK cell-based therapeutic strategies. The green box denotes the biological process targeted by the corresponding strategy, while the red box denotes the biological process not involved in the corresponding strategy. Abbreviations: ADCC, natural killer cell-mediated antibody-dependent cellular cytotoxicity; CAR, chimeric antigen receptor
NK cell adoptive transfer
The most primitive conception of the employment of NK cells for cancer treatment is the transfer of healthy allogenous NK cells in patients with malignancies. In the 1980s, NK cell adoptive transfer technology was used to conquer hematological malignancies [544]. However, the antitumor activity of autogenous natural NK cells is moderate. To overcome the limitations of natural NK cells, engineered NK cells are developed by augmenting stimulating receptors and dampening inhibitory receptors [547, 548]. The innovation has revitalized the application of adoptive NK cell transfer. Since then, it has become a dominant therapeutic strategy [549,550,551], with superior safety relative to other adoptive cellular transfer therapies [552]. Despite the rosy perspective, more efforts are needed to improve NK cell infiltration and tumor specificity [553]. Furthermore, autologous activated and expanded natural killer cells, referred to as NKAE, offer a highly effective and low-toxicity strategy for multiple myeloma. In a phase 1 clinical trial, two out of five patients achieved a clinical objective response after receiving two infusions of NKAE [554]. The growing body of preclinical and clinical research in multiple myeloma has positioned NK adoptive cell therapy as a comparable treatment approach to CAR-T [551, 555, 556].
NK cell-stimulating lymphokine regimen
The cytokine regimen, which could enhance the cytotoxic activity of killer cells, was originally clinically used for renal cell carcinoma [557]. In this clinical study, NK cells became the predominant lymphocyte subset of peripheral blood mononuclear cells (PBMC) after the IL-2 combined IFN-γ treatment, indicating the promising application perspective of NK cell-stimulating lymphokine strategies [557]. Further investigations have been conducted to explore the efficacies of NK cell-stimulating cytokines, particularly IL-15, IL-2, and IFN-α [558,559,560,561,562,563,564,565]. These lymphokines enhance the tumor-killing activity of NK cells. Administrating exogenous cytokines might be a promising complement to other NK cell-based therapies.
Harnessing ADCC of NK cells
Antibodies targeting molecules on the surface of NK cells were developed at the end of the last century [566]. The classical antitumor agents, such as trastuzumab, cetuximab, and rituximab, are capable of eliciting the ADCC of NK cells [540]. Mechanically, these antibodies act as a physical bridge, linking the NK and tumor cells. For example, trastuzumab binds to CD16 on NK cells via its immunoglobulin G1 (IgG1) Fc portion and binds to HER2 on tumor cells via its Fab portion, mediating ADCC either synchronously or subsequently [567,568,569]. Besides, some immune checkpoint inhibitors like avelumab could trigger ADCC as well [570]. For tumor cells with high PD-L1 expression, avelumab directly guides NK cells to execute immune clearance, independent of the PD-1/PD-L1 signaling [570].
Immune checkpoint blockade of NK cells
Moreover, immune checkpoint inhibitors targeting NK cells, which could boost the activation and cytotoxic functions of NK cells, have emerged as a promising approach in cancer immunotherapy. Several receptors on NK cells have been recognized as immune checkpoints, including NKG2A/CD94, KIR family, LIR1, TIGIT/CD96, B7H3, PD-1, CTLA-4, LAG-3, TIM-3, CD200R, and SIRPα [532, 571]. The anti-inhibitory KIR antibody IPH2101 (1-7F9) effectively triggers NK cell-mediated killing of multiple myeloma in murine tumor models [572, 573]. Besides, anti-NKG2A antibodies could simultaneously enhance the cytotoxicity of NK and T cells against tumor cells [538, 574,575,576]. A recent clinical trial indicated that monalizumab (anti-NKG2A antibody) or oleclumab (anti-CD73 antibody, inhibiting adenosine production) synergized with PD-L1 blockade in advanced NSCLC patients [577]. Also, blocking TIGIT/CD96 can prevent NK cell exhaustion and trigger a potent NK cell-dependent tumor-specific T cell response [578]. Two early-stage clinical trials utilizing anti-TIGIT antibodies (vibostolimab or etigilimab), either as a monotherapy or in combination with the anti-PD-1 antibodies, presented encouraging activities in refractory solid tumors [579, 580]. Furthermore, another anti-TIGIT monoclonal antibody (tiragolumab) combined with anti-PD-L1 antibody (atezolizumab) showed a significant advantage over atezolizumab monotherapy in progression-free survival (5.4 vs. 3.6 months, HR = 0.57, P = 0.015) [581]. Moreover, the poor prognosis associated with B7H3 overexpression in multiple types of cancers, coupled with enhanced functions of NK cells resulting from B7H3 inhibition, is evidential for targeting B7H3 to enhance NK cell-mediated immune protection [582, 583]. ChT-1A5, a human-mouse chimeric monoclonal antibody of B7H3, can effectively trigger ADCC of NK cells against leukemic cells while sparing normal hematopoietic cells. Other immune checkpoints, such as PD-1, CTLA-4, LAG-3 [584], TIM-3 [585], CD200R [586], and SIRPα [587], are predominantly expressed in other immune cells and will not be expounded upon within this section. The major corresponding checkpoint inhibitors associated with NK cells are presented in Table 6.
CAR-NK therapy
Theoretically, chimeric antigen receptor-engineered natural killer cells (CAR-NK) technology represents the latest generation of NK cell adoptive cellular transfer (ADT) [541, 588, 589]. As mentioned earlier, allogeneic NK cell ADT provides notable safety advantages over allogeneic T cell ADT treatment in terms of minimizing the risk of developing graft-versus-host disease (GVHD) or a cytokine storm and neurotoxicity [590,591,592]. The off-the-shelf CAR-NK products, readily available for preparation in advance, hold immense potential in the battle against cancer [541]. CAR-NK cells can be prepared based on a diverse array of donor cells, including the NK-92 cell line, PBMCs, umbilical cord blood (UCB), hematopoietic progenitor cells (HPCs), and iPSCs [593]. Among these various options, the irradiated NK-92 cell line is the most commonly employed in clinical trials due to its characteristics of immortality, rapid proliferation rate, and commercial availability [594, 595]. In addition to the diverse origins of cell components, CAR-NK could be constructed to target different cancer-specific antigens, such as CD19, CD5, CD123, GFR, GD2, and Mesothelin [592]. Engineering CAR-NK cells commonly depends on viral vehicles [596,597,598,599,600,601]. Besides, exogenous CAR fragments are introduced by electroporation and liposome, with lower genetic toxicity and shorter initiation time for gene expression [601]. Afterward, the reformative transposon system is developed and exploited in clinical trials, which possesses increased safety, decreased expenditure, and enhanced editable flexibility [602, 603].
Notably, NKG2D plays a crucial role in the detection and elimination of cancer cells [604]. Typically, therapeutic approaches targeting NKG2D primarily revolve around CAR technology. Preclinical investigations have illustrated that the utilization of NKG2D-CAR-engineered NK cells, known as NKAE, effectively hindered the progression of tumors in MM models [556]. Clinical findings have shown that the application of NKG2D-CAR-NK cells, created through RNA electroporation, not only reduced the formation of ascites but also led to tumor regression in metastatic lesions among patients with colorectal cancer [605]. Furthermore, the combination of NKG2D-CAR-NK cell therapy with an anti-HER2/NKG2D bispecific antibody exhibited remarkable anti-cancer effectiveness, even in cases of HER2-positive tumors lacking NKG2DL expression [606].
Thus far, numerous clinical trials involving CAR-NK cells have been implemented for various cancer types. In the phase 1/2 clinical study of anti-CD19 CAR-NK therapy for lymphoid tumors, the administration of CAR-NK cells achieved a response rate of 73%, without cytokine release syndrome, neurotoxicity, or GVHD [599]. In order to improve readability, we have compiled a comprehensive list of ongoing or completed clinical trials, excluding those withdrawn or terminated (Table 7). The findings of multiple studies have demonstrated that CAR-NK represents a promising therapeutic approach for both hematological malignancies and solid tumors [607,608,609,610]. In conclusion, abundant evidence indicates that NK cell-based therapeutic strategies for tumor treatment occupy a prominent and substantial position in cancer immunotherapy.
Targeting granulocytes or other innate immune cells for cancer treatment
Granulocytes encompass a diverse group of leukocytes, namely neutrophils, basophils, eosinophils, and mast cells [611]. These cells are part of the innate immune system and, upon activation, release molecules that stimulate the immune response to defend against infections [612]. Besides, granulocytes are implicated in various conditions such as asthma, allergies, autoimmune diseases, and cancers [613]. Among the granulocytes, neutrophils are the most abundant (50–70% of circulating leukocytes in humans), followed by eosinophils [614]. Basophils are the least common, constituting less than 1% of circulating leukocytes [615]. Mast cells, on the other hand, predominantly reside in tissues [616].
Neutrophil-targeted therapies
Neutrophils play a crucial role as the first line of defense against microbial infections and are also implicated in various inflammatory diseases [617,618,619]. Recently, there has been growing interest in understanding the versatile roles of neutrophils in cancer initiation and progression [620]. Specifically, tumor-associated neutrophils (TANs) exhibit diverse behaviors influenced by external stimuli from the TME [621]. These TANs can switch antitumor (N1) and protumor (N2) phenotypes [622]. N1 neutrophils could eliminate tumor cells by direct cytotoxic activities and indirectly stimulating adaptive immune responses. Contrarily, N2 neutrophils promote cancer cell proliferation, angiogenesis, and immune evasion [623, 624]. It has been confirmed that IFN-I polarizes neutrophils toward the antitumor N1 phenotype, while TGF-β drives the polarization toward the protumor N2 phenotype [625,626,627,628,629]. The N1/N2 nomenclature for TAN is inspired by the classification of activation states observed in TAM. However, specific surface markers reliably distinguishing between N1 and N2 TANs are unavailable, unlike TAM [630]. The plasticity of neutrophil polarization underscores the dynamic alterations of their functions in the TME. Neutrophils can adopt different activation states and functions depending on the specific cues and signals they encounter [631,632,633]. Understanding the precise mechanisms and factors that drive neutrophil polarization in the TME is a research hotspot, that provides insights into potential therapeutic strategies by modulating neutrophil functions [628]. Recent studies have suggested that targeting neutrophils could be a potential strategy in cancer therapy, mainly by inhibiting their protumoral capabilities [634, 635].
The roles of TANs in cancer development
TAN is generally regarded as a protumor factor in multiple types of cancers [636]. Numerous studies have demonstrated that the high neutrophil-to-lymphocyte ratio correlates with poor outcomes [637,638,639,640]. TAN-derived molecules, including ROS, protumor cytokines/chemokines, and enzymes, contribute to cancer initiation, progression, and metastasis [633]. ROS released by neutrophils leads to DNA damage and mutations, which are important to carcinogenesis [641, 642]. Besides, ROS from neutrophils promotes HIF-1α-VEGF axis-mediated angiogenesis and triggers oncogenic pathways in cancer cells such as MAPK, PI3K-AKT, and IKK/NF-κB [643]. Additionally, ROS is associated with immune escape, cancer-related inflammation, EMT, and treatment resistance in multiple types of cancers [643,644,645]. Apart from ROS, neutrophils secret other protumor cytokines and chemokines such as TGF-β, oncostatin M (IL-6 superfamily member), CCL4, CXCL8, BV8, and HGF to facilitate the malignant properties of cancer cells [646,647,648,649,650,651,652]. Moreover, some enzymes in neutrophil granules, including neutrophil elastase (NE), cathepsin G (CG), and MMP8/9, participate in extracellular matrix remodeling, EMT, and activation of oncogenic pathways such as EGFR-MAPK and PI3K-AKT signaling [653,654,655,656].
Significantly, extracellular traps (NETs) released by neutrophils have emerged as a pro-tumor factor. On the one hand, NETs assist in tumor growth and distant metastasis by entrapping circulating tumor cells [657,658,659,660,661]. On the other hand, NETs facilitate immune evasion and shield tumor cells from immune cytotoxicity [662]. Research has shown that tumor-produced CXCR1/2 ligands stimulate the generation of NETs, which envelop tumor cells, impeding their interactions with NK cells and CD8+ T cells. Consequently, tumor cells wrapped in NETs evade immune attacks. Disrupting NET formation with inhibitors of protein arginine deiminase 4 counteracts NET-mediated immune evasion and synergizes with immune checkpoint inhibitors in mouse models of cancer [663]. Similarly, in pancreatic ductal adenocarcinoma, IL-17 promotes neutrophil recruitment, NET formation, and the exclusion of CD8+ T cells. Blocking NET formation through IL-17 inhibition enhances the efficacy of immune checkpoint inhibitors [664].
Although TAN is identified as a risk factor for cancer patients in a majority of studies, TAN plays antitumor roles in certain circumstances. TANs could directly eradicate tumor cells by H2O2-mediated lethal Ca2+ influx, FasL-Fas interaction, and nitric oxide release [665,666,667]. Besides, TANs enhance the antitumor immune response by triggering ADCC, recruiting and activating T cells, and acquiring antigen presentation capabilities in some subsets [668,669,670]. TANs support T cell activation and priming not only by secreting proinflammatory factors such as TNF-α and CathG but also by NET-mediated downregulation of T cell activation threshold [671]. Collectively, TANs play complex roles in tumorigenesis and tumor progression. Further research is needed to fully understand the precise contributions and potential therapeutic targeting of TANs in cancer.
Manipulating TANs for cancer therapies
Various strategies for targeting TANs have been developed, including inhibiting their recruitment, inhibiting their functions, and reprogramming them toward the antitumor phenotype. These strategies aim to either eliminate or reprogram TANs to exert beneficial effects in cancer therapy (Table 8). Similar to PMN-MDSCs, the recruitment of TANs into the TME is mainly driven by CXCR2/CXCR4 signaling [672,673,674]. Agents blocking the CXCLs/CXCR2 axis effectively retard tumor progression by abrogating TAN-mediated protumor effects in preclinical models [675,676,677]. CXCR2 selective antagonists such as Navarixin and SCH527123 decrease neutrophil levels in patients [678,679,680]. Besides, CXCR4 silence in myeloid cells enhances NK cell-mediated immune surveillance against tumor cells, and systemic CXCR4 antagonist administration effectively suppresses tumor growth in melanoma models [681]. In the phase 2 study of pancreatic ductal adenocarcinoma (NCT02826486), CXCR4 antagonist BL-8040 combined with pembrolizumab and chemotherapy significantly reduces PMN-MDSC/TAN but increases T cell infiltration in the TME [682]. Also, suppressing TAN accumulation by lorlatinib treatment improves anti-PD-1 therapy in murine tumor models [683]. Moreover, tumor-derived oxysterols, the IL-23/IL-17/G-CSF axis, and the complement component 5-a (C5a) are also identified as neutrophil attractants [684,685,686]. Therefore, therapies blocking oxysterols, G-CSF, and C5a might be promising TAN-targeting strategies in the future [687].
Besides, some therapies increase the antitumor activity of TANs but undermine their protumor capabilities. In murine colon tumor models, PD-L1+ TANs dampen the cytotoxic activities of PD1+ NK and T cells, leading to cancer immune escape [683, 688, 689]. Blocking the PD-1/PD-L1 axis relieves the immunosuppressive functions of PD-L1+ TANs and strengthens the tumor-killing activities of TANs [690]. Parallelly, anti-CD47/SIRPα immunotherapy magnifies TAN-mediated ADCC and inhibits tumor growth [691,692,693]. Moreover, S100A9+ neutrophils propel M2 polarization in a COX-2-dependent manner [694]. Nuclear S100A9 binds to C/EBPβ, which cooperatively activates Cox-2 promoter and initiates the expression of PGE2, leading to M2 polarization [694]. In patients with advanced solid tumors, COX inhibitor combined with immune checkpoint inhibitor shows superior antitumor activity to immune checkpoint inhibitor monotherapy [695]. Theoretically, strategies targeting S100A9 or COX could prevent TAN-mediated immunosuppression, needing further clinical validations.
As we mentioned above, TGF-β is the core component stimulating TAN polarization toward the protumor N2 phenotype. Hence, neutralizing TGF-β in the TME reprograms the TAN phenotype and promotes immune clearance against tumor cells [629]. At present, several TGF-β blockade therapies have been undergoing clinical evaluation, especially anti-PD-L1/TGF-β bispecific or bifunctional antibodies [696, 697]. In the preclinical and clinical studies, anti-PD-L1/TGF-β bispecific antibodies such as M7824, YM101, and BiTP exhibit potent activities and achieve higher response rates in multiple types of cancers, relative to historical data [698,699,700]. Besides, nicotinamide phosphoribosyltransferase (NAMPT) contributes to the switch toward N2 TAN, while NAMPT inhibitors impair TAN-mediated tumorigenesis in murine tumor models [701]. In summary, by understanding the intricate interactions between TANs and the TME, novel therapeutic approaches can be developed to harness the antitumor potential of neutrophils while mitigating their protumoral effects. Targeting TANs holds promise for enhancing the efficacy of cancer treatments and improving patient outcomes. Future studies and clinical trials will be instrumental in translating these findings into practical and effective therapeutic strategies for cancer patients.
Additionally, neutrophils have shown the potential as carriers for drug delivery [702]. As the most abundant white blood cells, neutrophils can effectively traverse formidable barriers like the blood–brain barrier, facilitating the transport of drugs or nanoparticles to inflamed tissues such as tumors [703]. Preclinical research has demonstrated that neutrophils loaded with liposomes containing paclitaxel can effectively infiltrate the murine brain and suppress glioma recurrence following tumor resection. Enhanced inflammatory signals in the brain post-surgery promote the release of liposomal paclitaxel from neutrophils, enabling the effective delivery of paclitaxel to the remaining tumors [704]. Besides, Chang and colleagues have devised anti-glioblastoma CAR-neutrophils derived from human pluripotent stem cells, which can load and transport glioblastoma-targeted nanodrugs without necessitating the induction of additional inflammation in tumors, such as that resulting from surgery [705]. Collectively, these systems for drug and particle delivery utilizing neutrophils exhibit potent antitumor activity and a reduced risk of off-target effects, holding significant promise for clinical translation.
Eosinophil-targeted therapies
Eosinophils are originally believed to play a vital role in parasitic infection and allergic diseases [706, 707]. Although tumor-infiltrating eosinophils (termed tumor-associated tissue eosinophils, TATEs) were observed a century ago, their roles in cancer development are still unclear and controversial [708, 709]. For instance, TATEs are a favorable prognosis predictor for head and neck cancer and colon cancer [710, 711] but a risk factor for Hodgkin’s lymphoma [712]. This controversy could partly be explained by insufficient patient quantity and technical differences, especially staining methods for TATEs [713, 714]. Besides, the heterogeneity and plasticity of the eosinophils also lead to opposing functions in response to diverse stimuli [715, 716].
The mechanisms of TATE recruitment are still not fully understood, which might be mediated by IL-5-CCR3 signaling and chemokines such as eotaxin [717,718,719]. Once eosinophils infiltrate into the TME, they could exert cytotoxic activities by secreting granule proteins, including major basic protein (MBP), eosinophil-derived neurotoxin, peroxidase, and cationic protein [720]. Besides, the co-culture experiments using eosinophils and colon cancer cells demonstrate that TNF-α and granzyme-A also participate in eosinophil-mediated tumor killing [721]. Further explorations indicate that IL-18 facilitates the antitumor effects of eosinophils by increasing the expression of adhesion molecules [722]. Eosinophils express functional natural killer cell-associated killing receptors such as CD244, and eosinophil activation by CD244 cross-linking induces cytotoxicity against tumor cells [723, 724]. Besides, IL-12 and IL-10 from eosinophils downregulate the migration and enhance the adhesion of tumor cells by increasing their E-cadherin expression [725]. Furthermore, eosinophils could mediate antitumor response in indirect manners. TATEs attract CD8+ T cells into the TME by secreting CCL5, CXCL9, and CXCL10 [726]. Additionally, activated TATEs promote macrophage polarization toward the antitumor phenotype [726]. Also, the antitumor properties of TATEs are associated with TATE-orchestrated vasculature normalization [727].
On the contrary, TATEs possess protumor capabilities in some cancer contexts. TATEs increase Treg accumulation by secreting CCL22 and undermine T cell response by generating IDO in the TME [728, 729]. Moreover, thymic stromal lymphopoietin generated by tumor cells could induce TATEs to produce multiple cytokines (e.g., IL-10, IL-4, IL-5, and IL-13), promoting cancer cell proliferation and inducing macrophage polarization toward the protumor M2-like phenotype [730, 731]. Thymic stromal lymphopoietin also promotes TATEs to secret VEGFA, improving tumor angiogenesis [732]. TATE-derived molecules such as EGF, FGF, and PDGF directly support tumor growth [733]. TATEs also accelerate tumor metastasis and metastatic seeding by TGF-β-induced EMT and MMP2/9-mediated matrix remodeling [734, 735].
Eosinophil level has been identified as a potential biomarker for cancer immunotherapies. Increased eosinophil abundance (absolute eosinophil count) is associated with higher response rates and more prolonged survival in patients treated with ipilimumab [736,737,738]. Besides, eosinophilia is positively correlated to the efficacy of anti-PD-1 treatment in patients with advanced melanoma and Hodgkin’s lymphoma [739,740,741,742]. Mechanistically, immune checkpoint inhibitors stimulate CD4+ T cells to produce IL-5, promoting systemic eosinophil proliferation [743]. Then, treatment-induced IL-33 improves eosinophil infiltration into the TME and CD8+ T cell activity in an eosinophil-dependent manner [743, 744].
While the current understanding of eosinophils in the TME is limited, there is an urgent need to delve into their roles to develop effective strategies for cancer treatment. Due to the heterogeneity and plasticity of the eosinophils in different types of cancers, eosinophil-targeted therapies might need to be carried out individually. For tumors where eosinophils with protumor properties, targeting them becomes an attractive avenue. In this circumstance, eosinophil-depleting agents such as anti-IL-5 and anti-eotaxin antibodies might be an optional strategy. However, targeting eosinophils becomes more complex when they exhibit antitumor activities, as extensive antigen-independent degranulation may result in severe adverse effects. It is crucial to design drugs that selectively target tumor cells while sparing normal cells [708, 727, 745].
Targeting basophils and mast cells
Basophils and mast cells share certain features, such as the presence of basophilic granules in the cytoplasm, the expression of the high-affinity IgE receptor (FcεRI), and the release of proinflammatory substances like cysteinyl leukotrienes and histamine [746, 747]. These similarities initially led to the mistaken notion that basophils were the circulating counterparts or precursors of tissue-resident mast cells. However, extensive evidence now demonstrates clear disparities between human basophils and mast cells in terms of their morphology, ultrastructure, immunological characteristics, biochemical composition, and pharmacological responses [615]. As a result, the previous concept that basophils serve as the precursor or counterpart to tissue mast cells is no longer accepted [748, 749]. Recent studies demonstrate that these cells not only participate in allergic diseases, chronic or autoimmune inflammation, and defense against infections, but also play a vital role in cancer development [750, 751].
In specific human solid tumors, alterations in the count of circulating basophils are associated with disease progression. Basophilia, an increase in basophil count, is linked to improved prognosis of patients with NSCLC, melanoma, ovarian cancer, and glioblastoma [752,753,754,755,756]. On the contrary, basopenia, a decrease in basophil count, is associated with an unfavorable prognosis of colorectal cancer [757,758,759]. Indeed, the effects of basophils are diverse in different tumor settings: either in protumor or antitumor roles [760]. Basophils and their mediators may exhibit antitumor effects in specific contexts. Basophil recruitment is facilitated by factors like VEGF and IL-3 released by cancer and immune cells in the TME by VEGFR2 and IL-3Rα pathways [761, 762]. Intratumoral basophils release CCL3 and CCL4, which recruits CD8+ T cells to the TME, resulting in tumor regression in murine melanoma models [763]. Tumor-derived IL-33 activates basophils and enhances their ability to kill cancer cells [764, 765]. In ovarian cancer patients, the presence of an activated basophil signature is associated with better outcomes [755].
In contrast, basophils have been identified as protumor factors under certain circumstances. A key player in this process is Galectin-3 (Gal-3), a protein highly expressed by cancer cells and linked to poor prognosis. Gal-3 promotes immunosuppression within the TME [766]. Laboratory studies have demonstrated that Gal-3 on cancer cells can activate basophils, leading to the release of significant amounts of IL-4 and IL-13 [767, 768]. These cytokines, in turn, stimulate the polarization of M2-like TAMs, further undermining antitumor immune response [769]. Besides, IL-4-producing basophils accumulate in tumor-draining lymph nodes, regulating the TME and promoting the protumor Th2 inflammation [770]. Additionally, basophils promote tumor angiopoiesis by secreting VEGF-A [771]. Developing a comprehensive framework of the molecular mechanisms controlled by basophils within the TME may pave the way for the creation of innovative pharmacological and immunological approaches. These strategies could be utilized to regulate basophil activities, potentially impeding cancer development. So far, some basophil-targeted therapies, such as anti-IL-3Rα/CD123 antibodies, show promising activities in hematologic malignancies [762].
Similar to basophil, mast cell is a double-edged sword in cancer development as well [772,773,774]. Although tumor-infiltrated mast cells were reported a hundred years ago, it is still unclear whether these innate cells contribute to tumor progression or regression [775,776,777,778,779]. Recent studies have demonstrated that mast cells act as a protumor or antitumor factor depending on cancer types, tumor stages, and TME statuses [780]. On the one hand, mast cells exert protumor activity through secreting proangiogenic factors, releasing growth factors, reshaping the extracellular matrix, and suppressing antitumor immune response [616, 781]. Specifically, accumulated mast cells in the TME generate multiple proangiogenic molecules (such as VEGF-A/B, heparin, FGF, histamine, and stem cell factor) and lymphangiogenic cytokines (VEGF-C/D), promoting tumor angiogenesis and metastasis [782,783,784,785,786,787]. Besides acting as an important source of proangiogenic cytokines, mast cells also participate in cancer immune evasion. Mast cells secret anti-inflammatory cytokines like IL-10 and TGF-β and mobilize Tregs and MDSCs [788, 789]. On the other hand, mast cells possess antitumor properties under certain conditions. They not only induce cytotoxic effects on tumor cells but also attract immune effector cells [772, 790, 791]. Mast cells selectively recruit other immune cells by regulating cell adhesion and vascular permeability and releasing chemokines. CCL3, CCL5, CXCL10, and LTB4 from mast cells guide T-cell infiltration into the TME [792, 793]. Also, mast cells induce the chemotaxis of neutrophils and NK cells by secreting IL-8 [794, 795].
Hereto, manipulating the recruitment, activation, and status of mast cells would be valuable in controlling tumor growth [796, 797]. UV radiation induces the migration of skin mast cells by CXCR4-CXCL12 signaling while interrupting the CXCR4-CXCL12 pathway prevents sunlight-caused skin cancers [798]. Besides, mast cell-stabilizing drugs such as infliximab (anti-TNF antibody) suppress colorectal tumor progression [799]. SCF-c-kit pathway is the core signaling regulating mast cell development, and the c-kit inhibitor imatinib mesylate abrogates the influences of mast cells on tumor progression [800]. However, the role of mast cells changes along with cancer types, tumor stages, and mast cell statuses. Therefore, inhibiting the accumulation or functions of mast cells might not benefit all types of cancers.
Exploiting other innate immune cells
Recently, the importance of other innate immune cells in tumor progression is beginning to come into focus, especially unconventional T cell subsets γδ T cells, NKT cells, and MAIT cells [801]. Relative to conventional T cells, these innate T cells possess limited or semi-invariant TCR repertoires [802,803,804]. The unconventional T cells activate, mediate, and regulate antitumor response, becoming promising targets for cancer immunotherapy [805].
Human γδ T cells commonly exert antitumor properties upon activation [806]. Activated γδ T cells directly kill tumor cells by releasing cytolytic granules or expressing ligands of death receptors such as FASL and TRAIL [807,808,809]. Besides, γδ T cells improve the recruitment and functions of other immune cells, including αβ T cells, B cells, NKs, and antigen-presentation cells [810,811,812,813,814,815]. However, in some specific conditions, γδ T cells possess protumor activities [816]. For example, γδ T cell-derived IL-17 induces the formation of immunoinhibitory TME, supports angiogenesis, and promotes tumor progression [817,818,819,820]. Most clinical studies demonstrate that γδ T cell is a favorable biomarker for the prognosis and treatment response of cancer patients [821,822,823,824,825]. Considering their potent antitumor activity, manageable safety profile, and potential in allogeneic adoptive cell therapy, γδ T cells have become promising candidates for cancer immunotherapy [826]. At present, the development of CAR-γδ T cell, TCRγδ-transduced T cell, and γδ T cell-specific engagers has substantially innovated the blueprint for cancer immunotherapy. Multiple bispecific antibodies, such as TRGV9/CD40, TRGV9/CD1d, TRGV9/CD123, TRGV9/EGFR, and TRGV9/HER-2, exhibit potent activity in preclinical hematological and solid malignancy models [827,828,829,830,831]. Besides, anti-butyrophilin 3A (BTN3A) antibody could activate γ9Vδ2 T cells to eradicate tumor cells, and the preliminary data demonstrate that anti-BTN3A therapy is well-tolerated in patients with advanced solid tumors [832]. Moreover, adoptive cell therapies with expanded γδ T cells, CAR-γδ T cells, and γδTCR-engineered T cells also show encouraging activities in preclinical and clinical studies (Table 9) [833,834,835,836,837,838,839,840,841,842]. For instance, allogeneic Vδ1 T cells, genetically engineered to express anti-GPC-3 CAR and soluble IL-15, could effectively sustain self-proliferation and inhibit antitumor activity, representing a promising antitumor agent warranting clinical evaluation [843].
MAIT cells are a cluster of evolutionarily conserved unconventional T cells, with enormous potential in cancer immunotherapy [844]. MAIT cells could kill tumor cells by MHC-related molecule 1 (MR1)-TCR or NK cell-activating receptors [845, 846]. Apart from direct antitumor activity after activation, some basic studies indicate that MAIT cells also possess immunomodulatory functions, especially enhancing the functions of NK cells [847]. Also, accumulated MAIT cells are closely associated with improved response to anti-PD-1 treatment [848,849,850]. Due to their potent antitumor ability, high safety profile, and the ability to undergo genetic modification, MAIT and CAR-MAIT cells have emerged as encouraging options for cancer immunotherapy [844]. The high abundance of MAIT cells in the gastrointestinal tract, lung, and cervix suggests that cancers originating in these mucosal-associated peripheral tissues might be more likely to benefit from MAIT cell-based treatment [844].
NKT cells are a group of unconventional T cells that recognize glycolipids presented by CD1d [851]. Type I NKT cells, also termed invariant natural killer T cells (iNKTs), express invariant TCR α chain (Vα24-Jα18), with antigen specificity to synthetic glycolipid alpha-galactosylceramide (αGalCer) [852]. On the contrary, type II NKT cells have diverse TCR repertoires with poorly defined antigen specificity [853]. Commonly, most NKT-involved immunotherapies are based on iNKT cells [854]. Upregulated functions or levels of iNKTs are positively correlated with improved outcomes in lung cancer, colon cancer, neuroblastoma, and multiple myeloma [855,856,857,858]. iNKT cells could directly kill CD1d+ cancer cells [859,860,861,862,863,864]. Besides, iNKT cells boost antitumor response by regulating other immune cells. For example, tumor-infiltrating iNKT cells induce the polarization of CD1d+ TAMs toward antitumor M1-like or directly deplete them [865, 866]. Moreover, iNKT cells promote the maturation of DCs and convert MDSC to immunostimulatory antigen-presentation cells [867,868,869]. At present, unmodified and engineered NKT therapies have been developed for cancer immunotherapy (Table 10). Redirected NKT therapies endow NKT cells with cancer specificity and antitumor capability by CARs, cancer-specific TCRs, and anti-CD1d antibody fusion proteins [854]. NKT cells expressing CARs recognizing cancer-associated antigens exhibit potent activity in several murine tumor models [870,871,872,873,874,875]. In the phase 1 clinical study of neuroblastoma (NCT03294954), anti-GD2 CAR-NKT cells achieve encouraging efficacy with a tolerable safety profile [876]. Moreover, the efficacies of TCR-modified NKT and anti-CD1d antibody fusion proteins have been validated in a series of preclinical tumor models, needing further validation in clinical studies [877,878,879,880,881]. Generally, the powerful antitumor properties of unconventional T cells have been well-accepted, and regulating these components might provide an effective immunoprotection against cancers [882].
Perspective and conclusion
Immunotherapies have revolutionized cancer treatment, offering promising outcomes and prolonged survival for patients across various cancer types. Current immunomodulatory strategies predominantly focus on harnessing adaptive immunity, utilizing approaches such as immune checkpoint blockade and CAR-T cell therapy. While these approaches have shown remarkable success in some cases, the overall response rates remain limited, highlighting the need for novel therapeutic avenues. In recent years, accumulating evidence has emphasized the crucial role of the innate immune system in orchestrating antitumor immune responses. By recognizing and eliminating cancer cells, as well as modulating adaptive immunity, innate immune cells present a fertile ground for innovative immunotherapeutic interventions.
Beyond their well-established roles in immune surveillance and clearance of pathogens, innate immune cells actively participate in cancer immune evasion and surveillance. Macrophages, DCs, MDSCs, neutrophils, and NK cells are key components of the innate immune arm that influence the TME and shape antitumor immune responses (Fig. 6). Understanding the intricate interplay between innate immune cells and tumor progression is crucial for developing effective therapeutic interventions. Moreover, exploiting the potential of innate immunity opens new avenues for cancer immunotherapy. Several strategies have emerged that focus on modulating innate immune cells to enhance antitumor responses. STING agonists have shown promising preclinical results by enhancing antitumor immunity and triggering the production of IFN-I. Another promising avenue is the genetically engineered innate cells, such as CAR-macrophages or CAR-NK cells, which have demonstrated potent antitumor activities in preclinical models. Additionally, TLR agonists have been explored to induce the maturation of antigen-presenting cells, augmenting their ability to present tumor antigens to T cells and promote antitumor responses.
Harnessing innate immunity to improve antitumor immune response. The involvement of innate immunity is crucial for initiating and sustaining adaptive immunity, and it plays a significant role in the overall cancer-immunity cycle. When a tumor is detected, innate immune cells are activated, leading to the enhancement of their effector functions and the destruction of tumor cells. Apart from directly killing tumor cells, innate immune cells participate in priming, expanding, and infiltrating tumor-specific T-cells. Manipulating innate immunity by therapeutic strategies could effectively stimulate antitumor immune response and overcome immune evasion. Abbreviations: DC, dendritic cell; TAM, tumor-associated macrophage; NK cell, natural killer cell; MDSC, myeloid-derived suppressor cell; TME, tumor microenvironment; TCR, T cell receptor; MHC, major histocompatibility complex
Recognizing the interconnectedness of innate and adaptive immunity, combination therapies that simultaneously target both arms of the immune system hold great promise. Immune checkpoint blockade, a mainstay of current immunotherapies, primarily focuses on reversing T cell exhaustion and reinvigorating adaptive immune responses. However, the effectiveness of immune checkpoint inhibitors can be enhanced by incorporating strategies that activate innate immune cells. For instance, combining immune checkpoint blockade with STING agonists can amplify both innate and adaptive immune responses, resulting in synergistic antitumor effects [62]. Similarly, STING agonists can improve CAR-T cell trafficking and persistence in the TME, effectively enhancing the efficacy of CAR-T cells in solid tumors [883,884,885].
While harnessing innate immunity presents exciting opportunities, several challenges need to be addressed to fully unleash its potential. A comprehensive understanding of the intricate crosstalk between innate immune cells and the TME is crucial for designing effective therapies. Furthermore, strategies targeting innate immunity should carefully consider potential off-target effects and avoid excessive systemic inflammation. Developing robust biomarkers to predict patient response to innate immune-based therapies and selecting optimal combination regimens are additional challenges that warrant attention.
In conclusion, the advent of cancer immunotherapies has revolutionized cancer treatment, but the full potential of the immune system in eradicating tumors is yet to be realized. Exploiting the power of innate immunity offers a promising approach to overcoming current limitations. Innate immune cells play multifaceted roles in modulating antitumor immune responses and can be harnessed through various approaches, including but not limited to STING agonists, CAR-macrophage or -NK cell therapies, metabolic regulators, and innate immune checkpoint blockade. Synergistic combination therapies that simultaneously activate innate and adaptive immunity hold great promise for future advancements in cancer immunotherapy. By expanding our focus beyond adaptive immunity and embracing the potential of the innate immune system, we can develop more effective and personalized treatments for cancer patients. Unrevealing the multifaceted contributions of innate immune cells and exploring their therapeutic potential will propel the field of cancer immunotherapy forward.
Availability of data and materials
Not applicable.
Abbreviations
- 2-DG:
-
2-Deoxy-D-glucose
- αLP:
-
α-Lymphoid precursor
- αGalCer:
-
Alpha-galactosylceramide
- ADCC:
-
Antibody-dependent cell cytotoxicity
- ADCP:
-
Antibody-dependent cell phagocytosis
- ARG1:
-
Arginase 1
- ATP:
-
Adenosine triphosphate
- ATRA:
-
All-trans retinoic acid
- Breg:
-
Regulatory B
- BM:
-
Bone marrow
- BTN3A:
-
Butyrophilin 3A
- C5a:
-
Complement 5a
- CAF:
-
Cancer-associated fibroblast
- CAR:
-
Chimeric antigen receptor
- CRT:
-
Calreticulin
- cDC:
-
Conventional DC
- CDN:
-
Cyclic dinucleotide
- cGAMP:
-
Cyclic GMP-AMP
- CSF1:
-
Colony-stimulating factor-1
- CTLA-4:
-
Cytotoxic T lymphocyte-associated protein 4
- CTX:
-
Cyclophosphamide
- DC:
-
Dendritic cell
- DAMP:
-
Damage-associated molecular pattern
- ECM:
-
Extracellular matrix
- EGF:
-
Epidermal growth factor
- EMT:
-
Epithelial-mesenchymal transition
- Eomes:
-
Eomesodermin
- FAO:
-
Fatty acid oxidation
- FcR:
-
Fc receptor
- FcRγ:
-
Fc receptor
- FGF:
-
Fibroblast growth factor
- Gal-3:
-
Galectin-3
- GM-CSF:
-
Granulocyte macrophage-colony stimulating factor
- HDACi:
-
Histone deacetylase inhibitor
- HMGB1:
-
High-mobility group box 1
- HPC:
-
Hematopoietic progenitor cell
- ICD:
-
Immunogenic cell death
- IDO1:
-
Indoleamine2,3-dioxygenase1
- IgG1:
-
Immunoglobulin G1
- IKK-ε:
-
Inhibitor of kB kinase ε
- ILC:
-
Innate lymphoid cell
- iNOS:
-
Inducible nitric oxide synthase
- iNKT:
-
Invariant natural killer T cell
- iPSC:
-
Induced pluripotent stem cell
- ITAM:
-
Immunoreceptor tyrosine-based activation motif
- LILRB:
-
Leukocyte immunoglobulin-like receptor B
- MAIT:
-
Mucosa-associated invariant T
- MBP:
-
Major basic protein
- MDSC:
-
Myeloid-derived suppressor cell
- MR1:
-
MHC-related molecule 1
- M-MDSC:
-
Monocytic MDSC
- MMP:
-
Matrix metalloproteinase
- Megf10:
-
Multiple epidermal growth factor-like domains protein 10
- MoDC:
-
Monocyte-derived DC
- NAMPT:
-
Nicotinamide phosphoribosyltransferase
- NCAM:
-
Neural cell adhesion molecule
- NET:
-
Neutrophil extracellular trap
- NK:
-
Natural killer
- NKT:
-
Natural killer T
- NSCLC:
-
Non-small cell lung cancer
- ODN:
-
Oligodeoxynucleotides
- PBMC:
-
Peripheral blood mononuclear cell
- PD-1:
-
Programmed cell death protein 1
- PD-L1:
-
Programmed cell death ligand 1
- pDC:
-
Plasmacytoid DC
- PDE5:
-
Phosphodiesterase 5
- PGE2:
-
Prostaglandin E2
- PMN-MDSC:
-
Polymorphonuclear MDSC
- PRR:
-
Pattern-recognition receptor
- ROS:
-
Reactive oxygen species
- TAM:
-
Tumor-associated macrophage
- TAN:
-
Tumor-associated neutrophil
- TCR:
-
T cell receptor
- tdLN:
-
Tumor-associated draining lymph node
- TRAIL:
-
TNF-related apoptosis-induced ligand
- Siglec:
-
Sialic acid-binding immunoglobulin-like lectin
- SIRPα:
-
Signal regulatory protein-α
- SFR:
-
SLAM family receptor
- TAN:
-
Tumor-associated neutrophil
- TATE:
-
Tumor-associated tissue eosinophil
- TIM-3:
-
T-cell immunoglobulin and mucin domain 3
- TLR:
-
Toll-like receptor
- TME:
-
Tumor microenvironment
- Treg:
-
Regulatory T
- UCB:
-
Umbilical cord blood
- VEGF:
-
Vascular endothelial-derived growth factor
References
Negrini S, Gorgoulis VG, Halazonetis TD. Genomic instability–an evolving hallmark of cancer. Nat Rev Mol Cell Biol. 2010;11:220–8.
Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17:807–21.
Zuo B, Zhang Y, Zhao K, Wu L, Qi H, Yang R, et al. Universal immunotherapeutic strategy for hepatocellular carcinoma with exosome vaccines that engage adaptive and innate immune responses. J Hematol Oncol. 2022;15:46.
van Duijn A, Van der Burg SH, Scheeren FA. CD47/SIRPα axis: bridging innate and adaptive immunity. J Immunother Cancer. 2022;10:e004589.
Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22.
Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity. 2013;39:1–10.
Maiorino L, Daßler-Plenker J, Sun L, Egeblad M. Innate Immunity and Cancer Pathophysiology. Annu Rev Pathol. 2022;17:425–57.
van der Leun AM, Thommen DS, Schumacher TN. CD8(+) T cell states in human cancer: insights from single-cell analysis. Nat Rev Cancer. 2020;20:218–32.
Tsimberidou AM, Fountzilas E, Nikanjam M, Kurzrock R. Review of precision cancer medicine: Evolution of the treatment paradigm. Cancer Treat Rev. 2020;86:102019.
Haffner MC, Zwart W, Roudier MP, True LD, Nelson WG, Epstein JI, et al. Genomic and phenotypic heterogeneity in prostate cancer. Nat Rev Urol. 2021;18:79–92.
Lim ZF, Ma PC. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J Hematol Oncol. 2019;12:134.
O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol. 2019;16:151–67.
Qin S, Xu L, Yi M, Yu S, Wu K, Luo S. Novel immune checkpoint targets: moving beyond PD-1 and CTLA-4. Mol Cancer. 2019;18:155.
Xia L, Oyang L, Lin J, Tan S, Han Y, Wu N, et al. The cancer metabolic reprogramming and immune response. Mol Cancer. 2021;20:28.
Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting TGF-β signal transduction for fibrosis and cancer therapy. Mol Cancer. 2022;21:104.
Yi M, Li T, Niu M, Wu Y, Zhao Z, Wu K. TGF-β: A novel predictor and target for anti-PD-1/PD-L1 therapy. Front Immunol. 2022;13:1061394.
Kim BG, Malek E, Choi SH, Ignatz-Hoover JJ, Driscoll JJ. Novel therapies emerging in oncology to target the TGF-β pathway. J Hematol Oncol. 2021;14:55.
Shi X, Yang J, Deng S, Xu H, Wu D, Zeng Q, et al. TGF-β signaling in the tumor metabolic microenvironment and targeted therapies. J Hematol Oncol. 2022;15:135.
Muenst S, Läubli H, Soysal SD, Zippelius A, Tzankov A, Hoeller S. The immune system and cancer evasion strategies: therapeutic concepts. J Intern Med. 2016;279:541–62.
Yan Y, Huang L, Liu Y, Yi M, Chu Q, Jiao D, et al. Metabolic profiles of regulatory T cells and their adaptations to the tumor microenvironment: implications for antitumor immunity. J Hematol Oncol. 2022;15:104.
Wu Y, Yi M, Niu M, Mei Q, Wu K. Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy. Mol Cancer. 2022;21:184.
Liu T, Han C, Wang S, Fang P, Ma Z, Xu L, et al. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12:86.
Jia Q, Wang A, Yuan Y, Zhu B, Long H. Heterogeneity of the tumor immune microenvironment and its clinical relevance. Exp Hematol Oncol. 2022;11:24.
Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54.
Kirtane K, Elmariah H, Chung CH, Abate-Daga D. Adoptive cellular therapy in solid tumor malignancies: review of the literature and challenges ahead. J Immunother Cancer. 2021;9:e002723.
Abbott RC, Hughes-Parry HE, Jenkins MR. To go or not to go? Biological logic gating engineered T cells. J Immunother Cancer. 2022;10:e004185.
Lemoine J, Ruella M, Houot R. Born to survive: how cancer cells resist CAR T cell therapy. J Hematol Oncol. 2021;14:199.
Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7–H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8:793–800.
Lingel H, Brunner-Weinzierl MC. CTLA-4 (CD152): A versatile receptor for immune-based therapy. Semin Immunol. 2019;42:101298.
Liu Z, Yu X, Xu L, Li Y, Zeng C. Current insight into the regulation of PD-L1 in cancer. Exp Hematol Oncol. 2022;11:44.
Herbst RS, Giaccone G, de Marinis F, Reinmuth N, Vergnenegre A, Barrios CH, et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N Engl J Med. 2020;383:1328–39.
Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Cowey CL, Lao CD, et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373:23–34.
Liu H, Pan C, Song W, Liu D, Li Z, Zheng L. Novel strategies for immuno-oncology breakthroughs with cell therapy. Biomark Res. 2021;9:62.
Zhou W, Yu J, Li Y, Wang K. Neoantigen-specific TCR-T cell-based immunotherapy for acute myeloid leukemia. Exp Hematol Oncol. 2022;11:100.
Safarzadeh Kozani P, Naseri A, Mirarefin SMJ, Salem F, Nikbakht M, Evazi Bakhshi S, et al. Nanobody-based CAR-T cells for cancer immunotherapy. Biomark Res. 2022;10:24.
Edeline J, Houot R, Marabelle A, Alcantara M. CAR-T cells and BiTEs in solid tumors: challenges and perspectives. J Hematol Oncol. 2021;14:65.
Liu Y, An L, Huang R, Xiong J, Yang H, Wang X, et al. Strategies to enhance CAR-T persistence. Biomark Res. 2022;10:86.
Sterner RC, Sterner RM. CAR-T cell therapy: current limitations and potential strategies. Blood Cancer J. 2021;11:69.
Amini L, Silbert SK, Maude SL, Nastoupil LJ, Ramos CA, Brentjens RJ, et al. Preparing for CAR T cell therapy: patient selection, bridging therapies and lymphodepletion. Nat Rev Clin Oncol. 2022;19:342–55.
Su CT, Ye JC. Emerging therapies for relapsed/refractory multiple myeloma: CAR-T and beyond. J Hematol Oncol. 2021;14:115.
Wang JN, Gu T, Hu Y, Huang H. Novel cellular immunotherapies for hematological malignancies: recent updates from the 2021 ASH annual meeting. Exp Hematol Oncol. 2022;11:61.
Bilusic M, Madan RA, Gulley JL. Immunotherapy of Prostate Cancer: Facts and Hopes. Clin Cancer Res. 2017;23:6764–70.
Haslam A, Prasad V. Estimation of the Percentage of US Patients With Cancer Who Are Eligible for and Respond to Checkpoint Inhibitor Immunotherapy Drugs. JAMA Netw Open. 2019;2:e192535.
Newick K, O’Brien S, Moon E, Albelda SM. CAR T Cell Therapy for Solid Tumors. Annu Rev Med. 2017;68:139–52.
Yu S, Li A, Liu Q, Li T, Yuan X, Han X, et al. Chimeric antigen receptor T cells: a novel therapy for solid tumors. J Hematol Oncol. 2017;10:78.
Zhang Y, Liu Z, Wei W, Li Y. TCR engineered T cells for solid tumor immunotherapy. Exp Hematol Oncol. 2022;11:38.
Cao X, Dai H, Cui Q, Li Z, Shen W, Pan J, et al. CD7-directed CAR T-cell therapy: a potential immunotherapy strategy for relapsed/refractory acute myeloid leukemia. Exp Hematol Oncol. 2022;11:67.
Demaria O, Cornen S, Daëron M, Morel Y, Medzhitov R, Vivier E. Harnessing innate immunity in cancer therapy. Nature. 2019;574:45–56.
Chen W, Yuan Y, Jiang X. Antibody and antibody fragments for cancer immunotherapy. J Control Release. 2020;328:395–406.
Marciscano AE, Anandasabapathy N. The role of dendritic cells in cancer and anti-tumor immunity. Semin Immunol. 2021;52:101481.
Zhang M, Yang W, Wang P, Deng Y, Dong YT, Liu FF, et al. CCL7 recruits cDC1 to promote antitumor immunity and facilitate checkpoint immunotherapy to non-small cell lung cancer. Nat Commun. 2020;11:6119.
Sánchez-Paulete AR, Teijeira A, Cueto FJ, Garasa S, Pérez-Gracia JL, Sánchez-Arráez A, et al. Antigen cross-presentation and T-cell cross-priming in cancer immunology and immunotherapy. Ann Oncol. 2017;28:xii44-xii55.
Greene TT, Jo YR, Zuniga EI. Infection and cancer suppress pDC derived IFN-I. Curr Opin Immunol. 2020;66:114–22.
Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, et al. Landscape and Dynamics of Single Immune Cells in Hepatocellular Carcinoma. Cell. 2019;179:829-45.e20.
Wculek SK, Cueto FJ, Mujal AM, Melero I, Krummel MF, Sancho D. Dendritic cells in cancer immunology and immunotherapy. Nat Rev Immunol. 2020;20:7–24.
Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. 2018;24:1178–91.
Böttcher JP, Bonavita E, Chakravarty P, Blees H, Cabeza-Cabrerizo M, Sammicheli S, et al. NK Cells Stimulate Recruitment of cDC1 into the Tumor Microenvironment Promoting Cancer Immune Control. Cell. 2018;172:1022-37.e14.
Spranger S, Bao R, Gajewski TF. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–5.
Hernandez C, Huebener P, Schwabe RF. Damage-associated molecular patterns in cancer: a double-edged sword. Oncogene. 2016;35:5931–41.
Kroemer G, Galluzzi L, Kepp O, Zitvogel L. Immunogenic cell death in cancer therapy. Annu Rev Immunol. 2013;31:51–72.
Li A, Yi M, Qin S, Song Y, Chu Q, Wu K. Activating cGAS-STING pathway for the optimal effect of cancer immunotherapy. J Hematol Oncol. 2019;12:35.
Yi M, Zheng X, Niu M, Zhu S, Ge H, Wu K. Combination strategies with PD-1/PD-L1 blockade: current advances and future directions. Mol Cancer. 2022;21:28.
Montoya M, Schiavoni G, Mattei F, Gresser I, Belardelli F, Borrow P, et al. Type I interferons produced by dendritic cells promote their phenotypic and functional activation. Blood. 2002;99:3263–71.
Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, et al. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity. 2004;21:279–88.
Johnson LA, Jackson DG. Inflammation-induced secretion of CCL21 in lymphatic endothelium is a key regulator of integrin-mediated dendritic cell transmigration. Int Immunol. 2010;22:839–49.
Ferris ST, Durai V, Wu R, Theisen DJ, Ward JP, Bern MD, et al. cDC1 prime and are licensed by CD4(+) T cells to induce anti-tumour immunity. Nature. 2020;584:624–9.
Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Commun. 2015;6:7458.
Broz ML, Binnewies M, Boldajipour B, Nelson AE, Pollack JL, Erle DJ, et al. Dissecting the tumor myeloid compartment reveals rare activating antigen-presenting cells critical for T cell immunity. Cancer Cell. 2014;26:638–52.
Enamorado M, Iborra S, Priego E, Cueto FJ, Quintana JA, Martínez-Cano S, et al. Enhanced anti-tumour immunity requires the interplay between resident and circulating memory CD8(+) T cells. Nat Commun. 2017;8:16073.
Garris CS, Arlauckas SP, Kohler RH, Trefny MP, Garren S, Piot C, et al. Successful Anti-PD-1 Cancer Immunotherapy Requires T Cell-Dendritic Cell Crosstalk Involving the Cytokines IFN-γ and IL-12. Immunity. 2018;49:1148-61.e7.
Del Prete A, Salvi V, Soriani A, Laffranchi M, Sozio F, Bosisio D, et al. Dendritic cell subsets in cancer immunity and tumor antigen sensing. Cell Mol Immunol. 2023;20:432–47.
Batlle E, Massagué J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity. 2019;50:924–40.
Ramalingam R, Larmonier CB, Thurston RD, Midura-Kiela MT, Zheng SG, Ghishan FK, et al. Dendritic cell-specific disruption of TGF-β receptor II leads to altered regulatory T cell phenotype and spontaneous multiorgan autoimmunity. J Immunol. 2012;189:3878–93.
Piskurich JF, Wang Y, Linhoff MW, White LC, Ting JP. Identification of distinct regions of 5’ flanking DNA that mediate constitutive, IFN-gamma, STAT1, and TGF-beta-regulated expression of the class II transactivator gene. J Immunol. 1998;160:233–40.
Papaspyridonos M, Matei I, Huang Y, do Rosario Andre M, Brazier-Mitouart H, Waite JC, et al. Id1 suppresses anti-tumour immune responses and promotes tumour progression by impairing myeloid cell maturation. Nat Commun. 2015;6:6840.
Hanks BA, Holtzhausen A, Evans KS, Jamieson R, Gimpel P, Campbell OM, et al. Type III TGF-β receptor downregulation generates an immunotolerant tumor microenvironment. J Clin Invest. 2013;123:3925–40.
Dumitriu IE, Dunbar DR, Howie SE, Sethi T, Gregory CD. Human dendritic cells produce TGF-beta 1 under the influence of lung carcinoma cells and prime the differentiation of CD4+CD25+Foxp3+ regulatory T cells. J Immunol. 2009;182:2795–807.
Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–83.
Kitamura H, Kamon H, Sawa S, Park SJ, Katunuma N, Ishihara K, et al. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23:491–502.
Di Blasio S, van Wigcheren GF, Becker A, van Duffelen A, Gorris M, Verrijp K, et al. The tumour microenvironment shapes dendritic cell plasticity in a human organotypic melanoma culture. Nat Commun. 2020;11:2749.
Moore KW, de Waal MR, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annu Rev Immunol. 2001;19:683–765.
Ruffell B, Chang-Strachan D, Chan V, Rosenbusch A, Ho CM, Pryer N, et al. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell. 2014;26:623–37.
Gabrilovich D, Ishida T, Oyama T, Ran S, Kravtsov V, Nadaf S, et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood. 1998;92:4150–66.
Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18:60.
Qin S, Li A, Yi M, Yu S, Zhang M, Wu K. Recent advances on anti-angiogenesis receptor tyrosine kinase inhibitors in cancer therapy. J Hematol Oncol. 2019;12:27.
Takahashi A, Kono K, Ichihara F, Sugai H, Fujii H, Matsumoto Y. Vascular endothelial growth factor inhibits maturation of dendritic cells induced by lipopolysaccharide, but not by proinflammatory cytokines. Cancer Immunol Immunother. 2004;53:543–50.
Fontana R, Raccosta L, Rovati L, Steffensen KR, Paniccia A, Jakobsson T, et al. Nuclear receptor ligands induce TREM-1 expression on dendritic cells: analysis of their role in tumors. Oncoimmunology. 2019;8:1554967.
Caronni N, Simoncello F, Stafetta F, Guarnaccia C, Ruiz-Moreno JS, Opitz B, et al. Downregulation of Membrane Trafficking Proteins and Lactate Conditioning Determine Loss of Dendritic Cell Function in Lung Cancer. Cancer Res. 2018;78:1685–99.
Brown TP, Bhattacharjee P, Ramachandran S, Sivaprakasam S, Ristic B, Sikder MOF, et al. The lactate receptor GPR81 promotes breast cancer growth via a paracrine mechanism involving antigen-presenting cells in the tumor microenvironment. Oncogene. 2020;39:3292–304.
Xiao Y, Ma D, Zhao S, Suo C, Shi J, Xue MZ, et al. Multi-Omics Profiling Reveals Distinct Microenvironment Characterization and Suggests Immune Escape Mechanisms of Triple-Negative Breast Cancer. Clin Cancer Res. 2019;25:5002–14.
Smyth MJ, Ngiow SF, Ribas A, Teng MW. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat Rev Clin Oncol. 2016;13:143–58.
Du Z, Huang Z, Chen X, Jiang G, Peng Y, Feng W, et al. Modified dendritic cell-derived exosomes activate both NK cells and T cells through the NKG2D/NKG2D-L pathway to kill CML cells with or without T315I mutation. Exp Hematol Oncol. 2022;11:36.
Gardner A, de Mingo PÁ, Ruffell B. Dendritic Cells and Their Role in Immunotherapy. Front Immunol. 2020;11:924.
Decout A, Katz JD, Venkatraman S, Ablasser A. The cGAS-STING pathway as a therapeutic target in inflammatory diseases. Nat Rev Immunol. 2021;21:548–69.
Chin EN, Sulpizio A, Lairson LL. Targeting STING to promote antitumor immunity. Trends Cell Biol. 2023;33:189–203.
Balka KR, Louis C, Saunders TL, Smith AM, Calleja DJ, D’Silva DB, et al. TBK1 and IKKε Act Redundantly to Mediate STING-Induced NF-κB Responses in Myeloid Cells. Cell Rep. 2020;31: 107492.
Wang H, Hu S, Chen X, Shi H, Chen C, Sun L, et al. cGAS is essential for the antitumor effect of immune checkpoint blockade. Proc Natl Acad Sci U S A. 2017;114:1637–42.
Deng L, Liang H, Xu M, Yang X, Burnette B, Arina A, et al. STING-Dependent Cytosolic DNA Sensing Promotes Radiation-Induced Type I Interferon-Dependent Antitumor Immunity in Immunogenic Tumors. Immunity. 2014;41:843–52.
Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–16.
Fu J, Kanne DB, Leong M, Glickman LH, McWhirter SM, Lemmens E, et al. STING agonist formulated cancer vaccines can cure established tumors resistant to PD-1 blockade. Sci Transl Med. 2015;7:283ra52.
Jiang M, Chen P, Wang L, Li W, Chen B, Liu Y, et al. cGAS-STING, an important pathway in cancer immunotherapy. J Hematol Oncol. 2020;13:81.
Shae D, Becker KW, Christov P, Yun DS, Lytton-Jean AKR, Sevimli S, et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat Nanotechnol. 2019;14:269–78.
Conlon J, Burdette DL, Sharma S, Bhat N, Thompson M, Jiang Z, et al. Mouse, but not human STING, binds and signals in response to the vascular disrupting agent 5,6-dimethylxanthenone-4-acetic acid. J Immunol. 2013;190:5216–25.
Ramanjulu JM, Pesiridis GS, Yang J, Concha N, Singhaus R, Zhang SY, et al. Design of amidobenzimidazole STING receptor agonists with systemic activity. Nature. 2018;564:439–43.
Pan BS, Perera SA, Piesvaux JA, Presland JP, Schroeder GK, Cumming JN, et al. An orally available non-nucleotide STING agonist with antitumor activity. Science. 2020;369:eaba6098.
Yi M, Niu M, Wu Y, Ge H, Jiao D, Zhu S, et al. Combination of oral STING agonist MSA-2 and anti-TGF-β/PD-L1 bispecific antibody YM101: a novel immune cocktail therapy for non-inflamed tumors. J Hematol Oncol. 2022;15:142.
Lv M, Chen M, Zhang R, Zhang W, Wang C, Zhang Y, et al. Manganese is critical for antitumor immune responses via cGAS-STING and improves the efficacy of clinical immunotherapy. Cell Res. 2020;30:966–79.
Yi M, Niu M, Zhang J, Li S, Zhu S, Yan Y, et al. Combine and conquer: manganese synergizing anti-TGF-β/PD-L1 bispecific antibody YM101 to overcome immunotherapy resistance in non-inflamed cancers. J Hematol Oncol. 2021;14:146.
Fitzgerald KA, Kagan JC. Toll-like Receptors and the Control of Immunity. Cell. 2020;180:1044–66.
Wang X, Smith C, Yin H. Targeting Toll-like receptors with small molecule agents. Chem Soc Rev. 2013;42:4859–66.
Chiang CL, Kandalaft LE. In vivo cancer vaccination: Which dendritic cells to target and how? Cancer Treat Rev. 2018;71:88–101.
Le Naour J, Galluzzi L, Zitvogel L, Kroemer G, Vacchelli E. Trial watch: TLR3 agonists in cancer therapy. Oncoimmunology. 2020;9:1771143.
Lu H, Dietsch GN, Matthews MA, Yang Y, Ghanekar S, Inokuma M, et al. VTX-2337 is a novel TLR8 agonist that activates NK cells and augments ADCC. Clin Cancer Res. 2012;18:499–509.
Chow LQM, Morishima C, Eaton KD, Baik CS, Goulart BH, Anderson LN, et al. Phase Ib Trial of the Toll-like Receptor 8 Agonist, Motolimod (VTX-2337), Combined with Cetuximab in Patients with Recurrent or Metastatic SCCHN. Clin Cancer Res. 2017;23:2442–50.
He M, Soni B, Schwalie PC, Hüsser T, Waltzinger C, De Silva D, et al. Combinations of Toll-like receptor 8 agonist TL8-506 activate human tumor-derived dendritic cells. J Immunother Cancer. 2022;10:e004268.
Adams S, Kozhaya L, Martiniuk F, Meng TC, Chiriboga L, Liebes L, et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin Cancer Res. 2012;18:6748–57.
Salazar LG, Lu H, Reichow JL, Childs JS, Coveler AL, Higgins DM, et al. Topical Imiquimod Plus Nab-paclitaxel for Breast Cancer Cutaneous Metastases: A Phase 2 Clinical Trial. JAMA Oncol. 2017;3:969–73.
Adams S, O’Neill DW, Nonaka D, Hardin E, Chiriboga L, Siu K, et al. Immunization of malignant melanoma patients with full-length NY-ESO-1 protein using TLR7 agonist imiquimod as vaccine adjuvant. J Immunol. 2008;181:776–84.
Trutnovsky G, Reich O, Joura EA, Holter M, Ciresa-König A, Widschwendter A, et al. Topical imiquimod versus surgery for vulvar intraepithelial neoplasia: a multicentre, randomised, phase 3, non-inferiority trial. Lancet. 2022;399:1790–8.
Sato-Kaneko F, Yao S, Ahmadi A, Zhang SS, Hosoya T, Kaneda MM, et al. Combination immunotherapy with TLR agonists and checkpoint inhibitors suppresses head and neck cancer. JCI Insight. 2017;2:e93397.
Jin Y, Zhuang Y, Dong X, Liu M. Development of CpG oligodeoxynucleotide TLR9 agonists in anti-cancer therapy. Expert Rev Anticancer Ther. 2021;21:841–51.
Hegde S, Krisnawan VE, Herzog BH, Zuo C, Breden MA, Knolhoff BL, et al. Dendritic Cell Paucity Leads to Dysfunctional Immune Surveillance in Pancreatic Cancer. Cancer Cell. 2020;37:289-307.e9.
Jiang Y, Zhang H, Wang J, Chen J, Guo Z, Liu Y, et al. Exploiting RIG-I-like receptor pathway for cancer immunotherapy. J Hematol Oncol. 2023;16:8.
Castiello L, Zevini A, Vulpis E, Muscolini M, Ferrari M, Palermo E, et al. An optimized retinoic acid-inducible gene I agonist M8 induces immunogenic cell death markers in human cancer cells and dendritic cell activation. Cancer Immunol Immunother. 2019;68:1479–92.
Martinez M, Ono N, Planutiene M, Planutis K, Nelson EL, Holcombe RF. Granulocyte-macrophage stimulating factor (GM-CSF) increases circulating dendritic cells but does not abrogate suppression of adaptive cellular immunity in patients with metastatic colorectal cancer receiving chemotherapy. Cancer Cell Int. 2012;12:2.
Vonderheide RH. CD40 Agonist Antibodies in Cancer Immunotherapy. Annu Rev Med. 2020;71:47–58.
Vonderheide RH, Glennie MJ. Agonistic CD40 antibodies and cancer therapy. Clin Cancer Res. 2013;19:1035–43.
van Mierlo GJ, den Boer AT, Medema JP, van der Voort EI, Fransen MF, Offringa R, et al. CD40 stimulation leads to effective therapy of CD40(-) tumors through induction of strong systemic cytotoxic T lymphocyte immunity. Proc Natl Acad Sci U S A. 2002;99:5561–6.
Mangsbo SM, Broos S, Fletcher E, Veitonmäki N, Furebring C, Dahlén E, et al. The human agonistic CD40 antibody ADC-1013 eradicates bladder tumors and generates T-cell-dependent tumor immunity. Clin Cancer Res. 2015;21:1115–26.
Sandin LC, Orlova A, Gustafsson E, Ellmark P, Tolmachev V, Tötterman TH, et al. Locally delivered CD40 agonist antibody accumulates in secondary lymphoid organs and eradicates experimental disseminated bladder cancer. Cancer Immunol Res. 2014;2:80–90.
Beatty GL, Chiorean EG, Fishman MP, Saboury B, Teitelbaum UR, Sun W, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–6.
Long KB, Gladney WL, Tooker GM, Graham K, Fraietta JA, Beatty GL. IFNγ and CCL2 Cooperate to Redirect Tumor-Infiltrating Monocytes to Degrade Fibrosis and Enhance Chemotherapy Efficacy in Pancreatic Carcinoma. Cancer Discov. 2016;6:400–13.
Tang T, Cheng X, Truong B, Sun L, Yang X, Wang H. Molecular basis and therapeutic implications of CD40/CD40L immune checkpoint. Pharmacol Ther. 2021;219:107709.
Vonderheide RH, Flaherty KT, Khalil M, Stumacher MS, Bajor DL, Hutnick NA, et al. Clinical activity and immune modulation in cancer patients treated with CP-870,893, a novel CD40 agonist monoclonal antibody. J Clin Oncol. 2007;25:876–83.
Bajor DL, Xu X, Torigian DA, Mick R, Garcia LR, Richman LP, et al. Immune activation and a 9-year ongoing complete remission following CD40 antibody therapy and metastasectomy in a patient with metastatic melanoma. Cancer Immunol Res. 2014;2:1051–8.
Niu M, Yi M, Wu Y, Lyu L, He Q, Yang R, et al. Synergistic efficacy of simultaneous anti-TGF-β/VEGF bispecific antibody and PD-1 blockade in cancer therapy. J Hematol Oncol. 2023;16:94.
Zhu S, Yang N, Wu J, Wang X, Wang W, Liu YJ, et al. Tumor microenvironment-related dendritic cell deficiency: a target to enhance tumor immunotherapy. Pharmacol Res. 2020;159:104980.
Gabrilovich DI, Ishida T, Nadaf S, Ohm JE, Carbone DP. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin Cancer Res. 1999;5:2963–70.
Fricke I, Mirza N, Dupont J, Lockhart C, Jackson A, Lee JH, et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin Cancer Res. 2007;13:4840–8.
Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. Sci Transl Med. 2018;10:eaan5488.
Yi M, Zhang J, Li A, Niu M, Yan Y, Jiao Y, et al. The construction, expression, and enhanced anti-tumor activity of YM101: a bispecific antibody simultaneously targeting TGF-β and PD-L1. J Hematol Oncol. 2021;14:27.
Seeger P, Musso T, Sozzani S. The TGF-β superfamily in dendritic cell biology. Cytokine Growth Factor Rev. 2015;26:647–57.
Heidari F, Ramezani A, Erfani N, Razmkhah M. Indoleamine 2, 3-Dioxygenase: A Professional Immunomodulator and Its Potential Functions in Immune Related Diseases. Int Rev Immunol. 2022;41:346–63.
Fujiwara Y, Kato S, Nesline MK, Conroy JM, DePietro P, Pabla S, et al. Indoleamine 2,3-dioxygenase (IDO) inhibitors and cancer immunotherapy. Cancer Treat Rev. 2022;110:102461.
Hou DY, Muller AJ, Sharma MD, DuHadaway J, Banerjee T, Johnson M, et al. Inhibition of indoleamine 2,3-dioxygenase in dendritic cells by stereoisomers of 1-methyl-tryptophan correlates with antitumor responses. Cancer Res. 2007;67:792–801.
Sharma MD, Pacholczyk R, Shi H, Berrong ZJ, Zakharia Y, Greco A, et al. Inhibition of the BTK-IDO-mTOR axis promotes differentiation of monocyte-lineage dendritic cells and enhances anti-tumor T cell immunity. Immunity. 2021;54:2354-71.e8.
Flatekval GF, Sioud M. Modulation of dendritic cell maturation and function with mono- and bifunctional small interfering RNAs targeting indoleamine 2,3-dioxygenase. Immunology. 2009;128:e837–48.
Su Q, Wang C, Song H, Zhang C, Liu J, Huang P, et al. Co-delivery of anionic epitope/CpG vaccine and IDO inhibitor by self-assembled cationic liposomes for combination melanoma immunotherapy. J Mater Chem B. 2021;9:3892–9.
Peng Q, Qiu X, Zhang Z, Zhang S, Zhang Y, Liang Y, et al. PD-L1 on dendritic cells attenuates T cell activation and regulates response to immune checkpoint blockade. Nat Commun. 2020;11:4835.
Zhao Y, Harrison DL, Song Y, Ji J, Huang J, Hui E. Antigen-Presenting Cell-Intrinsic PD-1 Neutralizes PD-L1 in cis to Attenuate PD-1 Signaling in T Cells. Cell Rep. 2018;24:379-90.e6.
Oh SA, Wu DC, Cheung J, Navarro A, Xiong H, Cubas R, et al. PD-L1 expression by dendritic cells is a key regulator of T-cell immunity in cancer. Nat Cancer. 2020;1:681–91.
Sugiura D, Maruhashi T, Okazaki IM, Shimizu K, Maeda TK, Takemoto T, et al. Restriction of PD-1 function by cis-PD-L1/CD80 interactions is required for optimal T cell responses. Science. 2019;364:558–66.
Mayoux M, Roller A, Pulko V, Sammicheli S, Chen S, Sum E, et al. Dendritic cells dictate responses to PD-L1 blockade cancer immunotherapy. Sci Transl Med. 2020;12:eaav7431.
Chiba S, Baghdadi M, Akiba H, Yoshiyama H, Kinoshita I, Dosaka-Akita H, et al. Tumor-infiltrating DCs suppress nucleic acid-mediated innate immune responses through interactions between the receptor TIM-3 and the alarmin HMGB1. Nat Immunol. 2012;13:832–42.
de Mingo PÁ, Hänggi K, Celias DP, Gardner A, Li J, Batista-Bittencourt B, et al. The inhibitory receptor TIM-3 limits activation of the cGAS-STING pathway in intra-tumoral dendritic cells by suppressing extracellular DNA uptake. Immunity. 2021;54:1154-67.e7.
Acharya N, Sabatos-Peyton C, Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer. 2020;8:e000911.
Yu J, Sun H, Cao W, Song Y, Jiang Z. Research progress on dendritic cell vaccines in cancer immunotherapy. Exp Hematol Oncol. 2022;11:3.
Finn OJ. Human Tumor Antigens Yesterday, Today, and Tomorrow. Cancer Immunol Res. 2017;5:347–54.
Yi M, Qin S, Zhao W, Yu S, Chu Q, Wu K. The role of neoantigen in immune checkpoint blockade therapy. Exp Hematol Oncol. 2018;7:28.
Yi M, Dong B, Chu Q, Wu K. Immune pressures drive the promoter hypermethylation of neoantigen genes. Exp Hematol Oncol. 2019;8:32.
Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359:1355–60.
Moyer TJ, Zmolek AC, Irvine DJ. Beyond antigens and adjuvants: formulating future vaccines. J Clin Invest. 2016;126:799–808.
Chesson CB, Zloza A. Nanoparticles: augmenting tumor antigen presentation for vaccine and immunotherapy treatments of cancer. Nanomedicine (Lond). 2017;12:2693–706.
Goyvaerts C, Breckpot K. The Journey of in vivo Virus Engineered Dendritic Cells From Bench to Bedside: A Bumpy Road. Front Immunol. 2018;9:2052.
Kreutz M, Tacken PJ, Figdor CG. Targeting dendritic cells–why bother? Blood. 2013;121:2836–44.
Birkholz K, Schwenkert M, Kellner C, Gross S, Fey G, Schuler-Thurner B, et al. Targeting of DEC-205 on human dendritic cells results in efficient MHC class II-restricted antigen presentation. Blood. 2010;116:2277–85.
Tsuji T, Matsuzaki J, Kelly MP, Ramakrishna V, Vitale L, He LZ, et al. Antibody-targeted NY-ESO-1 to mannose receptor or DEC-205 in vitro elicits dual human CD8+ and CD4+ T cell responses with broad antigen specificity. J Immunol. 2011;186:1218–27.
Dhodapkar MV, Sznol M, Zhao B, Wang D, Carvajal RD, Keohan ML, et al. Induction of antigen-specific immunity with a vaccine targeting NY-ESO-1 to the dendritic cell receptor DEC-205. Sci Transl Med. 2014;6:232ra51.
Tacken PJ, de Vries IJ, Gijzen K, Joosten B, Wu D, Rother RP, et al. Effective induction of naive and recall T-cell responses by targeting antigen to human dendritic cells via a humanized anti-DC-SIGN antibody. Blood. 2005;106:1278–85.
Hutten TJ, Thordardottir S, Fredrix H, Janssen L, Woestenenk R, Tel J, et al. CLEC12A-Mediated Antigen Uptake and Cross-Presentation by Human Dendritic Cell Subsets Efficiently Boost Tumor-Reactive T Cell Responses. J Immunol. 2016;197:2715–25.
Chatterjee B, Smed-Sörensen A, Cohn L, Chalouni C, Vandlen R, Lee BC, et al. Internalization and endosomal degradation of receptor-bound antigens regulate the efficiency of cross presentation by human dendritic cells. Blood. 2012;120:2011–20.
Bol KF, Schreibelt G, Gerritsen WR, de Vries IJ, Figdor CG. Dendritic Cell-Based Immunotherapy: State of the Art and Beyond. Clin Cancer Res. 2016;22:1897–906.
Massa C, Thomas C, Wang E, Marincola F, Seliger B. Different maturation cocktails provide dendritic cells with different chemoattractive properties. J Transl Med. 2015;13:175.
Ding Z, Li Q, Zhang R, Xie L, Shu Y, Gao S, et al. Personalized neoantigen pulsed dendritic cell vaccine for advanced lung cancer. Signal Transduct Target Ther. 2021;6:26.
Fucikova J, Hensler M, Kasikova L, Lanickova T, Pasulka J, Rakova J, et al. An Autologous Dendritic Cell Vaccine Promotes Anticancer Immunity in Patients with Ovarian Cancer with Low Mutational Burden and Cold Tumors. Clin Cancer Res. 2022;28:3053–65.
Mitchell DA, Batich KA, Gunn MD, Huang MN, Sanchez-Perez L, Nair SK, et al. Tetanus toxoid and CCL3 improve dendritic cell vaccines in mice and glioblastoma patients. Nature. 2015;519:366–9.
Santos PM, Adamik J, Howes TR, Du S, Vujanovic L, Warren S, et al. Impact of checkpoint blockade on cancer vaccine-activated CD8+ T cell responses. J Exp Med. 2020;217:e20191369.
Sonpavde G, McMannis JD, Bai Y, Seethammagari MR, Bull JMC, Hawkins V, et al. Phase I trial of antigen-targeted autologous dendritic cell-based vaccine with in vivo activation of inducible CD40 for advanced prostate cancer. Cancer Immunol Immunother. 2017;66:1345–57.
Butterfield LH, Vujanovic L, Santos PM, Maurer DM, Gambotto A, Lohr J, et al. Multiple antigen-engineered DC vaccines with or without IFNα to promote antitumor immunity in melanoma. J Immunother Cancer. 2019;7:113.
Berntsen A, Trepiakas R, Wenandy L, Geertsen PF, thor Straten P, Andersen MH, et al. Therapeutic dendritic cell vaccination of patients with metastatic renal cell carcinoma: a clinical phase 1/2 trial. J Immunother. 2008;31:771–80.
Liau LM, Ashkan K, Tran DD, Campian JL, Trusheim JE, Cobbs CS, et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J Transl Med. 2018;16:142.
Liau LM, Ashkan K, Brem S, Campian JL, Trusheim JE, Iwamoto FM, et al. Association of Autologous Tumor Lysate-Loaded Dendritic Cell Vaccination With Extension of Survival Among Patients With Newly Diagnosed and Recurrent Glioblastoma: A Phase 3 Prospective Externally Controlled Cohort Trial. JAMA Oncol. 2023;9:112–21.
Lau SP, Klaase L, Vink M, Dumas J, Bezemer K, van Krimpen A, et al. Autologous dendritic cells pulsed with allogeneic tumour cell lysate induce tumour-reactive T-cell responses in patients with pancreatic cancer: A phase I study. Eur J Cancer. 2022;169:20–31.
Wen PY, Reardon DA, Armstrong TS, Phuphanich S, Aiken RD, Landolfi JC, et al. A Randomized Double-Blind Placebo-Controlled Phase II Trial of Dendritic Cell Vaccine ICT-107 in Newly Diagnosed Patients with Glioblastoma. Clin Cancer Res. 2019;25:5799–807.
Mitsogiannis I, Tzelves L, Dellis A, Issa H, Papatsoris A, Moussa M. Prostate cancer immunotherapy. Expert Opin Biol Ther. 2022;22:577–90.
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363:411–22.
Garg AD, Coulie PG, Van den Eynde BJ, Agostinis P. Integrating Next-Generation Dendritic Cell Vaccines into the Current Cancer Immunotherapy Landscape. Trends Immunol. 2017;38:577–93.
Salem A, Alotaibi M, Mroueh R, Basheer HA, Afarinkia K. CCR7 as a therapeutic target in Cancer. Biochim Biophys Acta Rev Cancer. 2021;1875: 188499.
Hillinger S, Yang SC, Zhu L, Huang M, Duckett R, Atianzar K, et al. EBV-induced molecule 1 ligand chemokine (ELC/CCL19) promotes IFN-gamma-dependent antitumor responses in a lung cancer model. J Immunol. 2003;171:6457–65.
Lu J, Ma J, Cai W, Wangpu X, Feng H, Zhao J, et al. CC motif chemokine ligand 19 suppressed colorectal cancer in vivo accompanied by an increase in IL-12 and IFN-γ. Biomed Pharmacother. 2015;69:374–9.
Hillinger S, Yang SC, Batra RK, Strieter RM, Weder W, Dubinett SM, et al. CCL19 reduces tumour burden in a model of advanced lung cancer. Br J Cancer. 2006;94:1029–34.
Ashour AE, Lin X, Wang X, Turnquist HR, Burns NM, Tuli A, et al. CCL21 is an effective surgical neoadjuvant for treatment of mammary tumors. Cancer Biol Ther. 2007;6:1206–10.
Phan-Lai V, Kievit FM, Florczyk SJ, Wang K, Disis ML, Zhang M. CCL21 and IFNγ recruit and activate tumor specific T cells in 3D scaffold model of breast cancer. Anticancer Agents Med Chem. 2014;14:204–10.
Wu S, Xing W, Peng J, Yuan X, Zhao X, Lei P, et al. Tumor transfected with CCL21 enhanced reactivity and apoptosis resistance of human monocyte-derived dendritic cells. Immunobiology. 2008;213:417–26.
Hisada M, Yoshimoto T, Kamiya S, Magami Y, Miyaji H, Yoneto T, et al. Synergistic antitumor effect by coexpression of chemokine CCL21/SLC and costimulatory molecule LIGHT. Cancer Gene Ther. 2004;11:280–8.
Gao JQ, Sugita T, Kanagawa N, Iida K, Okada N, Mizuguchi H, et al. Anti-tumor responses induced by chemokine CCL19 transfected into an ovarian carcinoma model via fiber-mutant adenovirus vector. Biol Pharm Bull. 2005;28:1066–70.
Okada N, Sasaki A, Niwa M, Okada Y, Hatanaka Y, Tani Y, et al. Tumor suppressive efficacy through augmentation of tumor-infiltrating immune cells by intratumoral injection of chemokine-expressing adenoviral vector. Cancer Gene Ther. 2006;13:393–405.
Okada N, Mori N, Koretomo R, Okada Y, Nakayama T, Yoshie O, et al. Augmentation of the migratory ability of DC-based vaccine into regional lymph nodes by efficient CCR7 gene transduction. Gene Ther. 2005;12:129–39.
Cunningham HD, Shannon LA, Calloway PA, Fassold BC, Dunwiddie I, Vielhauer G, et al. Expression of the C-C chemokine receptor 7 mediates metastasis of breast cancer to the lymph nodes in mice. Transl Oncol. 2010;3:354–61.
Cirella A, Luri-Rey C, Di Trani CA, Teijeira A, Olivera I, Bolaños E, et al. Novel strategies exploiting interleukin-12 in cancer immunotherapy. Pharmacol Ther. 2022;239: 108189.
Nguyen KG, Vrabel MR, Mantooth SM, Hopkins JJ, Wagner ES, Gabaldon TA, et al. Localized Interleukin-12 for Cancer Immunotherapy. Front Immunol. 2020;11: 575597.
Di Trani CA, Cirella A, Arrizabalaga L, Alvarez M, Bella Á, Fernandez-Sendin M, et al. Intratumoral injection of IL-12-encoding mRNA targeted to CSFR1 and PD-L1 exerts potent anti-tumor effects without substantial systemic exposure. Mol Ther Nucleic Acids. 2023;33:599–616.
Olivera I, Bolaños E, Gonzalez-Gomariz J, Hervas-Stubbs S, Mariño KV, Luri-Rey C, et al. mRNAs encoding IL-12 and a decoy-resistant variant of IL-18 synergize to engineer T cells for efficacious intratumoral adoptive immunotherapy. Cell Rep Med. 2023;4: 100978.
Cirella A, Bolaños E, Di Trani CA, de Andrea CE, Sánchez-Gregorio S, Etxeberria I, et al. Intratumoral Gene Transfer of mRNAs Encoding IL12 in Combination with Decoy-Resistant IL18 Improves Local and Systemic Antitumor Immunity. Cancer Immunol Res. 2023;11:184–98.
Sangro B, Mazzolini G, Ruiz J, Herraiz M, Quiroga J, Herrero I, et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J Clin Oncol. 2004;22:1389–97.
Mazzolini G, Alfaro C, Sangro B, Feijoó E, Ruiz J, Benito A, et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J Clin Oncol. 2005;23:999–1010.
Quetglas JI, Labiano S, Aznar M, Bolaños E, Azpilikueta A, Rodriguez I, et al. Virotherapy with a Semliki Forest Virus-Based Vector Encoding IL12 Synergizes with PD-1/PD-L1 Blockade. Cancer Immunol Res. 2015;3:449–54.
Quetglas JI, Dubrot J, Bezunartea J, Sanmamed MF, Hervas-Stubbs S, Smerdou C, et al. Immunotherapeutic synergy between anti-CD137 mAb and intratumoral administration of a cytopathic Semliki Forest virus encoding IL-12. Mol Ther. 2012;20:1664–75.
Etxeberria I, Bolaños E, Quetglas JI, Gros A, Villanueva A, Palomero J, et al. Intratumor Adoptive Transfer of IL-12 mRNA Transiently Engineered Antitumor CD8(+) T Cells. Cancer Cell. 2019;36:613-29.e7.
Di Trani CA, Cirella A, Arrizabalaga L, Bella Á, Fernandez-Sendin M, Russo-Cabrera JS, et al. Intracavitary adoptive transfer of IL-12 mRNA-engineered tumor-specific CD8(+) T cells eradicates peritoneal metastases in mouse models. Oncoimmunology. 2023;12:2147317.
Zhu S, Yi M, Wu Y, Dong B, Wu K. Roles of tumor-associated macrophages in tumor progression: implications on therapeutic strategies. Exp Hematol Oncol. 2021;10:60.
Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95.
Mantovani A, Allavena P, Marchesi F, Garlanda C. Macrophages as tools and targets in cancer therapy. Nat Rev Drug Discov. 2022;21:799–820.
Locati M, Curtale G, Mantovani A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu Rev Pathol. 2020;15:123–47.
Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83.
Schett G, Neurath MF. Resolution of chronic inflammatory disease: universal and tissue-specific concepts. Nat Commun. 2018;9:3261.
Zhang L, Li Z, Skrzypczynska KM, Fang Q, Zhang W, O’Brien SA, et al. Single-Cell Analyses Inform Mechanisms of Myeloid-Targeted Therapies in Colon Cancer. Cell. 2020;181:442-59.e29.
Ren X, Zhang L, Zhang Y, Li Z, Siemers N, Zhang Z. Insights Gained from Single-Cell Analysis of Immune Cells in the Tumor Microenvironment. Annu Rev Immunol. 2021;39:583–609.
Cheng S, Li Z, Gao R, Xing B, Gao Y, Yang Y, et al. A pan-cancer single-cell transcriptional atlas of tumor infiltrating myeloid cells. Cell. 2021;184:792-809.e23.
Li MO, Wolf N, Raulet DH, Akkari L, Pittet MJ, Rodriguez PC, et al. Innate immune cells in the tumor microenvironment. Cancer Cell. 2021;39:725–9.
Xiang X, Wang J, Lu D, Xu X. Targeting tumor-associated macrophages to synergize tumor immunotherapy. Signal Transduct Target Ther. 2021;6:75.
Edin S, Wikberg ML, Oldenborg PA, Palmqvist R. Macrophages: Good guys in colorectal cancer. Oncoimmunology. 2013;2:e23038.
Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ, et al. Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS ONE. 2012;7:e50946.
El Kasmi KC, Stenmark KR. Contribution of metabolic reprogramming to macrophage plasticity and function. Semin Immunol. 2015;27:267–75.
Vitale I, Manic G, Coussens LM, Kroemer G, Galluzzi L. Macrophages and Metabolism in the Tumor Microenvironment. Cell Metab. 2019;30:36–50.
Wu K, Lin K, Li X, Yuan X, Xu P, Ni P, et al. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front Immunol. 2020;11:1731.
Zhao S, Mi Y, Guan B, Zheng B, Wei P, Gu Y, et al. Tumor-derived exosomal miR-934 induces macrophage M2 polarization to promote liver metastasis of colorectal cancer. J Hematol Oncol. 2020;13:156.
Franklin RA, Liao W, Sarkar A, Kim MV, Bivona MR, Liu K, et al. The cellular and molecular origin of tumor-associated macrophages. Science. 2014;344:921–5.
Datar I, Qiu X, Ma HZ, Yeung M, Aras S, de la Serna I, et al. RKIP regulates CCL5 expression to inhibit breast cancer invasion and metastasis by controlling macrophage infiltration. Oncotarget. 2015;6:39050–61.
Ruffell B, Affara NI, Coussens LM. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 2012;33:119–26.
Olivera I, Sanz-Pamplona R, Bolaños E, Rodriguez I, Etxeberria I, Cirella A, et al. A Therapeutically Actionable Protumoral Axis of Cytokines Involving IL-8, TNFα, and IL-1β. Cancer Discov. 2022;12:2140–57.
Schalper KA, Carleton M, Zhou M, Chen T, Feng Y, Huang SP, et al. Elevated serum interleukin-8 is associated with enhanced intratumor neutrophils and reduced clinical benefit of immune-checkpoint inhibitors. Nat Med. 2020;26:688–92.
Pathria P, Louis TL, Varner JA. Targeting Tumor-Associated Macrophages in Cancer. Trends Immunol. 2019;40:310–27.
Lin Y, Xu J, Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J Hematol Oncol. 2019;12:76.
Nowak M, Klink M. The Role of Tumor-Associated Macrophages in the Progression and Chemoresistance of Ovarian Cancer. Cells. 2020;9:1299.
Li C, Xu X, Wei S, Jiang P, Xue L, Wang J. Tumor-associated macrophages: potential therapeutic strategies and future prospects in cancer. J Immunother Cancer. 2021;9:1299.
Cheng N, Bai X, Shu Y, Ahmad O, Shen P. Targeting tumor-associated macrophages as an antitumor strategy. Biochem Pharmacol. 2021;183:114354.
Liu Y, Xu R, Gu H, Zhang E, Qu J, Cao W, et al. Metabolic reprogramming in macrophage responses. Biomark Res. 2021;9:1.
Wang L, He T, Liu J, Tai J, Wang B, Chen Z, et al. Pan-cancer analysis reveals tumor-associated macrophage communication in the tumor microenvironment. Exp Hematol Oncol. 2021;10:31.
Arlauckas SP, Garren SB, Garris CS, Kohler RH, Oh J, Pittet MJ, et al. Arg1 expression defines immunosuppressive subsets of tumor-associated macrophages. Theranostics. 2018;8:5842–54.
Xu Y, Zeng H, Jin K, Liu Z, Zhu Y, Xu L, et al. Immunosuppressive tumor-associated macrophages expressing interlukin-10 conferred poor prognosis and therapeutic vulnerability in patients with muscle-invasive bladder cancer. J Immunother Cancer. 2022;10:e003416.
Fu Q, Xu L, Wang Y, Jiang Q, Liu Z, Zhang J, et al. Tumor-associated Macrophage-derived Interleukin-23 Interlinks Kidney Cancer Glutamine Addiction with Immune Evasion. Eur Urol. 2019;75:752–63.
Peng P, Zhu H, Liu D, Chen Z, Zhang X, Guo Z, et al. TGFBI secreted by tumor-associated macrophages promotes glioblastoma stem cell-driven tumor growth via integrin αvβ5-Src-Stat3 signaling. Theranostics. 2022;12:4221–36.
Dancsok AR, Gao D, Lee AF, Steigen SE, Blay JY, Thomas DM, et al. Tumor-associated macrophages and macrophage-related immune checkpoint expression in sarcomas. Oncoimmunology. 2020;9:1747340.
Zhang J, Zhang H, Ding X, Hu J, Li Y, Zhang J, et al. Crosstalk between macrophage-derived PGE(2) and tumor UHRF1 drives hepatocellular carcinoma progression. Theranostics. 2022;12:3776–93.
Kuang DM, Zhao Q, Peng C, Xu J, Zhang JP, Wu C, et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J Exp Med. 2009;206:1327–37.
Podojil JR, Miller SD. Potential targeting of B7–H4 for the treatment of cancer. Immunol Rev. 2017;276:40–51.
Li W, Wu F, Zhao S, Shi P, Wang S, Cui D. Correlation between PD-1/PD-L1 expression and polarization in tumor-associated macrophages: A key player in tumor immunotherapy. Cytokine Growth Factor Rev. 2022;67:49–57.
Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9.
Noy R, Pollard JW. Tumor-associated macrophages: from mechanisms to therapy. Immunity. 2014;41:49–61.
Chen Y, Song Y, Du W, Gong L, Chang H, Zou Z. Tumor-associated macrophages: an accomplice in solid tumor progression. J Biomed Sci. 2019;26:78.
Canli Ö, Nicolas AM, Gupta J, Finkelmeier F, Goncharova O, Pesic M, et al. Myeloid Cell-Derived Reactive Oxygen Species Induce Epithelial Mutagenesis. Cancer Cell. 2017;32:869-83.e5.
Zhang J, Li H, Wu Q, Chen Y, Deng Y, Yang Z, et al. Tumoral NOX4 recruits M2 tumor-associated macrophages via ROS/PI3K signaling-dependent various cytokine production to promote NSCLC growth. Redox Biol. 2019;22:101116.
Zeng XY, Xie H, Yuan J, Jiang XY, Yong JH, Zeng D, et al. M2-like tumor-associated macrophages-secreted EGF promotes epithelial ovarian cancer metastasis via activating EGFR-ERK signaling and suppressing lncRNA LIMT expression. Cancer Biol Ther. 2019;20:956–66.
Wan S, Zhao E, Kryczek I, Vatan L, Sadovskaya A, Ludema G, et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology. 2014;147:1393–404.
Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci U S A. 2011;108:12425–30.
Lee S, Lee E, Ko E, Ham M, Lee HM, Kim ES, et al. Tumor-associated macrophages secrete CCL2 and induce the invasive phenotype of human breast epithelial cells through upregulation of ERO1-α and MMP-9. Cancer Lett. 2018;437:25–34.
Tu W, Gong J, Zhou Z, Tian D, Wang Z. TCF4 enhances hepatic metastasis of colorectal cancer by regulating tumor-associated macrophage via CCL2/CCR2 signaling. Cell Death Dis. 2021;12:882.
Huang R, Wang S, Wang N, Zheng Y, Zhou J, Yang B, et al. CCL5 derived from tumor-associated macrophages promotes prostate cancer stem cells and metastasis via activating β-catenin/STAT3 signaling. Cell Death Dis. 2020;11:234.
Chen J, Yao Y, Gong C, Yu F, Su S, Chen J, et al. CCL18 from tumor-associated macrophages promotes breast cancer metastasis via PITPNM3. Cancer Cell. 2011;19:541–55.
Han Y, Guo W, Ren T, Huang Y, Wang S, Liu K, et al. Tumor-associated macrophages promote lung metastasis and induce epithelial-mesenchymal transition in osteosarcoma by activating the COX-2/STAT3 axis. Cancer Lett. 2019;440–441:116–25.
Zhong Q, Fang Y, Lai Q, Wang S, He C, Li A, et al. CPEB3 inhibits epithelial-mesenchymal transition by disrupting the crosstalk between colorectal cancer cells and tumor-associated macrophages via IL-6R/STAT3 signaling. J Exp Clin Cancer Res. 2020;39:132.
Cai J, Xia L, Li J, Ni S, Song H, Wu X. Tumor-Associated Macrophages Derived TGF-β–Induced Epithelial to Mesenchymal Transition in Colorectal Cancer Cells through Smad 2,3–4/Snail Signaling Pathway. Cancer Res Treat. 2019;51:252–66.
Gazzillo A, Polidoro MA, Soldani C, Franceschini B, Lleo A, Donadon M. Relationship between Epithelial-to-Mesenchymal Transition and Tumor-Associated Macrophages in Colorectal Liver Metastases. Int J Mol Sci. 2022;23:16197.
Chen P, Hsu WH, Han J, Xia Y, DePinho RA. Cancer Stemness Meets Immunity: From Mechanism to Therapy. Cell Rep. 2021;34:108597.
Fang M, Li Y, Huang K, Qi S, Zhang J, Zgodzinski W, et al. IL33 Promotes Colon Cancer Cell Stemness via JNK Activation and Macrophage Recruitment. Cancer Res. 2017;77:2735–45.
Valeta-Magara A, Gadi A, Volta V, Walters B, Arju R, Giashuddin S, et al. Inflammatory Breast Cancer Promotes Development of M2 Tumor-Associated Macrophages and Cancer Mesenchymal Cells through a Complex Chemokine Network. Cancer Res. 2019;79:3360–71.
Zhang B, Ye H, Ren X, Zheng S, Zhou Q, Chen C, et al. Macrophage-expressed CD51 promotes cancer stem cell properties via the TGF-β1/smad2/3 axis in pancreatic cancer. Cancer Lett. 2019;459:204–15.
Fu LQ, Du WL, Cai MH, Yao JY, Zhao YY, Mou XZ. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell Immunol. 2020;353:104119.
Wenes M, Shang M, Di Matteo M, Goveia J, Martín-Pérez R, Serneels J, et al. Macrophage Metabolism Controls Tumor Blood Vessel Morphogenesis and Metastasis. Cell Metab. 2016;24:701–15.
Shu Y, Cheng P. Targeting tumor-associated macrophages for cancer immunotherapy. Biochim Biophys Acta Rev Cancer. 2020;1874:188434.
Argyle D, Kitamura T. Targeting Macrophage-Recruiting Chemokines as a Novel Therapeutic Strategy to Prevent the Progression of Solid Tumors. Front Immunol. 2018;9:2629.
Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19:1264–72.
Ries CH, Cannarile MA, Hoves S, Benz J, Wartha K, Runza V, et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell. 2014;25:846–59.
Cassetta L, Pollard JW. Targeting macrophages: therapeutic approaches in cancer. Nat Rev Drug Discov. 2018;17:887–904.
Cassier PA, Italiano A, Gomez-Roca C, Le Tourneau C, Toulmonde M, D’Angelo SP, et al. Long-term clinical activity, safety and patient-reported quality of life for emactuzumab-treated patients with diffuse-type tenosynovial giant-cell tumour. Eur J Cancer. 2020;141:162–70.
Rodriguez-Garcia A, Lynn RC, Poussin M, Eiva MA, Shaw LC, O’Connor RS, et al. CAR-T cell-mediated depletion of immunosuppressive tumor-associated macrophages promotes endogenous antitumor immunity and augments adoptive immunotherapy. Nat Commun. 2021;12:877.
Patel S, Petty WJ, Sands JM. An overview of lurbinectedin as a new second-line treatment option for small cell lung cancer. Ther Adv Med Oncol. 2021;13:17588359211020528.
Ngambenjawong C, Gustafson HH, Pineda JM, Kacherovsky NA, Cieslewicz M, Pun SH. Serum Stability and Affinity Optimization of an M2 Macrophage-Targeting Peptide (M2pep). Theranostics. 2016;6:1403–14.
Ngambenjawong C, Cieslewicz M, Schellinger JG, Pun SH. Synthesis and evaluation of multivalent M2pep peptides for targeting alternatively activated M2 macrophages. J Control Release. 2016;224:103–11.
Buhtoiarov IN, Lum HD, Berke G, Sondel PM, Rakhmilevich AL. Synergistic activation of macrophages via CD40 and TLR9 results in T cell independent antitumor effects. J Immunol. 2006;176:309–18.
Buhtoiarov IN, Lum H, Berke G, Paulnock DM, Sondel PM, Rakhmilevich AL. CD40 ligation activates murine macrophages via an IFN-gamma-dependent mechanism resulting in tumor cell destruction in vitro. J Immunol. 2005;174:6013–22.
Weiss JM, Back TC, Scarzello AJ, Subleski JJ, Hall VL, Stauffer JK, et al. Successful immunotherapy with IL-2/anti-CD40 induces the chemokine-mediated mitigation of an immunosuppressive tumor microenvironment. Proc Natl Acad Sci U S A. 2009;106:19455–60.
Li W, Wang F, Guo R, Bian Z, Song Y. Targeting macrophages in hematological malignancies: recent advances and future directions. J Hematol Oncol. 2022;15:110.
Sun L, Kees T, Almeida AS, Liu B, He XY, Ng D, et al. Activating a collaborative innate-adaptive immune response to control metastasis. Cancer Cell. 2021;39:1361-74.e9.
Jing W, McAllister D, Vonderhaar EP, Palen K, Riese MJ, Gershan J, et al. STING agonist inflames the pancreatic cancer immune microenvironment and reduces tumor burden in mouse models. J Immunother Cancer. 2019;7:115.
Barkal AA, Brewer RE, Markovic M, Kowarsky M, Barkal SA, Zaro BW, et al. CD24 signalling through macrophage Siglec-10 is a target for cancer immunotherapy. Nature. 2019;572:392–6.
Etzerodt A, Tsalkitzi K, Maniecki M, Damsky W, Delfini M, Baudoin E, et al. Specific targeting of CD163(+) TAMs mobilizes inflammatory monocytes and promotes T cell-mediated tumor regression. J Exp Med. 2019;216:2394–411.
Li D, Xiong W, Wang Y, Feng J, He Y, Du J, et al. SLAMF3 and SLAMF4 are immune checkpoints that constrain macrophage phagocytosis of hematopoietic tumors. Sci Immunol. 2022;7:eabj5501.
Matlung HL, Szilagyi K, Barclay NA, van den Berg TK. The CD47-SIRPα signaling axis as an innate immune checkpoint in cancer. Immunol Rev. 2017;276:145–64.
Majeti R, Chao MP, Alizadeh AA, Pang WW, Jaiswal S, Gibbs KD Jr, et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell. 2009;138:286–99.
Chan KS, Espinosa I, Chao M, Wong D, Ailles L, Diehn M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–21.
Zhang M, Hutter G, Kahn SA, Azad TD, Gholamin S, Xu CY, et al. Anti-CD47 Treatment Stimulates Phagocytosis of Glioblastoma by M1 and M2 Polarized Macrophages and Promotes M1 Polarized Macrophages In Vivo. PLoS ONE. 2016;11:e0153550.
Zhao XW, van Beek EM, Schornagel K, Van der Maaden H, Van Houdt M, Otten MA, et al. CD47-signal regulatory protein-α (SIRPα) interactions form a barrier for antibody-mediated tumor cell destruction. Proc Natl Acad Sci U S A. 2011;108:18342–7.
Chao MP, Alizadeh AA, Tang C, Myklebust JH, Varghese B, Gill S, et al. Anti-CD47 antibody synergizes with rituximab to promote phagocytosis and eradicate non-Hodgkin lymphoma. Cell. 2010;142:699–713.
Martinez-Torres AC, Quiney C, Attout T, Boullet H, Herbi L, Vela L, et al. CD47 agonist peptides induce programmed cell death in refractory chronic lymphocytic leukemia B cells via PLCγ1 activation: evidence from mice and humans. PLoS Med. 2015;12:e1001796.
Tseng D, Volkmer JP, Willingham SB, Contreras-Trujillo H, Fathman JW, Fernhoff NB, et al. Anti-CD47 antibody-mediated phagocytosis of cancer by macrophages primes an effective antitumor T-cell response. Proc Natl Acad Sci U S A. 2013;110:11103–8.
Advani R, Flinn I, Popplewell L, Forero A, Bartlett NL, Ghosh N, et al. CD47 Blockade by Hu5F9-G4 and Rituximab in Non-Hodgkin’s Lymphoma. N Engl J Med. 2018;379:1711–21.
Ansell SM, Maris MB, Lesokhin AM, Chen RW, Flinn IW, Sawas A, et al. Phase I Study of the CD47 Blocker TTI-621 in Patients with Relapsed or Refractory Hematologic Malignancies. Clin Cancer Res. 2021;27:2190–9.
Querfeld C, Thompson JA, Taylor MH, DeSimone JA, Zain JM, Shustov AR, et al. Intralesional TTI-621, a novel biologic targeting the innate immune checkpoint CD47, in patients with relapsed or refractory mycosis fungoides or Sézary syndrome: a multicentre, phase 1 study. Lancet Haematol. 2021;8:e808–17.
Lakhani NJ, Chow LQM, Gainor JF, LoRusso P, Lee KW, Chung HC, et al. Evorpacept alone and in combination with pembrolizumab or trastuzumab in patients with advanced solid tumours (ASPEN-01): a first-in-human, open-label, multicentre, phase 1 dose-escalation and dose-expansion study. Lancet Oncol. 2021;22:1740–51.
Stanczak MA, Läubli H. Siglec receptors as new immune checkpoints in cancer. Mol Aspects Med. 2023;90:101112.
Adams OJ, Stanczak MA, von Gunten S, Läubli H. Targeting sialic acid-Siglec interactions to reverse immune suppression in cancer. Glycobiology. 2018;28:640–7.
Bärenwaldt A, Läubli H. The sialoglycan-Siglec glyco-immune checkpoint - a target for improving innate and adaptive anti-cancer immunity. Expert Opin Ther Targets. 2019;23:839–53.
Rodriguez E, Boelaars K, Brown K, Eveline Li RJ, Kruijssen L, Bruijns SCM, et al. Sialic acids in pancreatic cancer cells drive tumour-associated macrophage differentiation via the Siglec receptors Siglec-7 and Siglec-9. Nat Commun. 2021;12:1270.
Beatson R, Tajadura-Ortega V, Achkova D, Picco G, Tsourouktsoglou TD, Klausing S, et al. The mucin MUC1 modulates the tumor immunological microenvironment through engagement of the lectin Siglec-9. Nat Immunol. 2016;17:1273–81.
Ibarlucea-Benitez I, Weitzenfeld P, Smith P, Ravetch JV. Siglecs-7/9 function as inhibitory immune checkpoints in vivo and can be targeted to enhance therapeutic antitumor immunity. Proc Natl Acad Sci U S A. 2021;118:e2107424118.
Läubli H, Pearce OM, Schwarz F, Siddiqui SS, Deng L, Stanczak MA, et al. Engagement of myelomonocytic Siglecs by tumor-associated ligands modulates the innate immune response to cancer. Proc Natl Acad Sci U S A. 2014;111:14211–6.
Stanczak MA, Rodrigues Mantuano N, Kirchhammer N, Sanin DE, Jacob F, Coelho R, et al. Targeting cancer glycosylation repolarizes tumor-associated macrophages allowing effective immune checkpoint blockade. Sci Transl Med. 2022;14:eabj1270.
Wang J, Sun J, Liu LN, Flies DB, Nie X, Toki M, et al. Siglec-15 as an immune suppressor and potential target for normalization cancer immunotherapy. Nat Med. 2019;25:656–66.
Xiao N, Zhu X, Li K, Chen Y, Liu X, Xu B, et al. Blocking siglec-10(hi) tumor-associated macrophages improves anti-tumor immunity and enhances immunotherapy for hepatocellular carcinoma. Exp Hematol Oncol. 2021;10:36.
Meesmann HM, Fehr EM, Kierschke S, Herrmann M, Bilyy R, Heyder P, et al. Decrease of sialic acid residues as an eat-me signal on the surface of apoptotic lymphocytes. J Cell Sci. 2010;123:3347–56.
Sun J, Lu Q, Sanmamed MF, Wang J. Siglec-15 as an Emerging Target for Next-generation Cancer Immunotherapy. Clin Cancer Res. 2021;27:680–8.
Sharma N, Atolagbe OT, Ge Z, Allison JP. LILRB4 suppresses immunity in solid tumors and is a potential target for immunotherapy. J Exp Med. 2021;218:e20201811.
Viitala M, Virtakoivu R, Tadayon S, Rannikko J, Jalkanen S, Hollmén M. Immunotherapeutic Blockade of Macrophage Clever-1 Reactivates the CD8(+) T-cell Response against Immunosuppressive Tumors. Clin Cancer Res. 2019;25:3289–303.
Gordon SR, Maute RL, Dulken BW, Hutter G, George BM, McCracken MN, et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature. 2017;545:495–9.
Binnewies M, Pollack JL, Rudolph J, Dash S, Abushawish M, Lee T, et al. Targeting TREM2 on tumor-associated macrophages enhances immunotherapy. Cell Rep. 2021;37:109844.
Yuan L, Tatineni J, Mahoney KM, Freeman GJ. VISTA: A Mediator of Quiescence and a Promising Target in Cancer Immunotherapy. Trends Immunol. 2021;42:209–27.
Nguyen P, Phennicie R, Kauffman K, Nowakowska D, Zafari M, Komoroski V, et al. 862 Targeting PSGL-1, a novel macrophage checkpoint, repolarizes suppressive macrophages, induces an inflammatory tumor microenvironment, and suppresses tumor growth. J Immunother Cancer. 2020;8:A513.
Mehla K, Singh PK. Metabolic Regulation of Macrophage Polarization in Cancer. Trends Cancer. 2019;5:822–34.
Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–63.
Zhao Q, Chu Z, Zhu L, Yang T, Wang P, Liu F, et al. 2-Deoxy-d-Glucose Treatment Decreases Anti-inflammatory M2 Macrophage Polarization in Mice with Tumor and Allergic Airway Inflammation. Front Immunol. 2017;8:637.
Wang S, Lin Y, Xiong X, Wang L, Guo Y, Chen Y, et al. Low-Dose Metformin Reprograms the Tumor Immune Microenvironment in Human Esophageal Cancer: Results of a Phase II Clinical Trial. Clin Cancer Res. 2020;26:4921–32.
Devalaraja S, To TKJ, Folkert IW, Natesan R, Alam MZ, Li M, et al. Tumor-Derived Retinoic Acid Regulates Intratumoral Monocyte Differentiation to Promote Immune Suppression. Cell. 2020;180:1098-114.e16.
Menga A, Serra M, Todisco S, Riera-Domingo C, Ammarah U, Ehling M, et al. Glufosinate constrains synchronous and metachronous metastasis by promoting anti-tumor macrophages. EMBO Mol Med. 2020;12:e11210.
Bonavita E, Bromley CP, Jonsson G, Pelly VS, Sahoo S, Walwyn-Brown K, et al. Antagonistic Inflammatory Phenotypes Dictate Tumor Fate and Response to Immune Checkpoint Blockade. Immunity. 2020;53:1215-29.e8.
Hong C, Tontonoz P. Liver X receptors in lipid metabolism: opportunities for drug discovery. Nat Rev Drug Discov. 2014;13:433–44.
Aggen DH, Ager CR, Obradovic AZ, Chowdhury N, Ghasemzadeh A, Mao W, et al. Blocking IL1 Beta Promotes Tumor Regression and Remodeling of the Myeloid Compartment in a Renal Cell Carcinoma Model: Multidimensional Analyses. Clin Cancer Res. 2021;27:608–21.
Zhang F, Parayath NN, Ene CI, Stephan SB, Koehne AL, Coon ME, et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat Commun. 2019;10:3974.
Zang X, Zhang X, Zhao X, Hu H, Qiao M, Deng Y, et al. Targeted Delivery of miRNA 155 to Tumor Associated Macrophages for Tumor Immunotherapy. Mol Pharm. 2019;16:1714–22.
Xia Y, Rao L, Yao H, Wang Z, Ning P, Chen X. Engineering Macrophages for Cancer Immunotherapy and Drug Delivery. Adv Mater. 2020;32:e2002054.
Khatoon N, Zhang Z, Zhou C, Chu M. Macrophage membrane coated nanoparticles: a biomimetic approach for enhanced and targeted delivery. Biomater Sci. 2022;10:1193–208.
Liang T, Zhang R, Liu X, Ding Q, Wu S, Li C, et al. Recent Advances in Macrophage-Mediated Drug Delivery Systems. Int J Nanomedicine. 2021;16:2703–14.
Allavena P, Palmioli A, Avigni R, Sironi M, La Ferla B, Maeda A. PLGA Based Nanoparticles for the Monocyte-Mediated Anti-Tumor Drug Delivery System. J Biomed Nanotechnol. 2020;16:212–23.
De Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, et al. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 2008;14:299–311.
Kaczanowska S, Beury DW, Gopalan V, Tycko AK, Qin H, Clements ME, et al. Genetically engineered myeloid cells rebalance the core immune suppression program in metastasis. Cell. 2021;184:2033-52.e21.
Shields CWt, Evans MA, Wang LL, Baugh N, Iyer S, Wu D, et al. Cellular backpacks for macrophage immunotherapy. Sci Adv. 2020;6:eaaz6579.
Maalej KM, Merhi M, Inchakalody VP, Mestiri S, Alam M, Maccalli C, et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer. 2023;22:20.
Sly LM, McKay DM. Macrophage immunotherapy: overcoming impediments to realize promise. Trends Immunol. 2022;43:959–68.
Pan K, Farrukh H, Chittepu V, Xu H, Pan CX, Zhu Z. CAR race to cancer immunotherapy: from CAR T, CAR NK to CAR macrophage therapy. J Exp Clin Cancer Res. 2022;41:119.
Klichinsky M, Ruella M, Shestova O, Lu XM, Best A, Zeeman M, et al. Human chimeric antigen receptor macrophages for cancer immunotherapy. Nat Biotechnol. 2020;38:947–53.
Morrissey MA, Williamson AP, Steinbach AM, Roberts EW, Kern N, Headley MB, et al. Chimeric antigen receptors that trigger phagocytosis. Elife. 2018;7:e36688.
Zhang P, Zhang G, Wan X. Challenges and new technologies in adoptive cell therapy. J Hematol Oncol. 2023;16:97.
Zhang W, Liu L, Su H, Liu Q, Shen J, Dai H, et al. Chimeric antigen receptor macrophage therapy for breast tumours mediated by targeting the tumour extracellular matrix. Br J Cancer. 2019;121:837–45.
Wang S, Yang Y, Ma P, Zha Y, Zhang J, Lei A, et al. CAR-macrophage: An extensive immune enhancer to fight cancer. EBioMedicine. 2022;76:103873.
Chocarro L, Blanco E, Fernández-Rubio L, Arasanz H, Bocanegra A, Echaide M, et al. Cutting-Edge CAR Engineering: Beyond T Cells. Biomedicines. 2022;10:3035.
Zhang L, Tian L, Dai X, Yu H, Wang J, Lei A, et al. Pluripotent stem cell-derived CAR-macrophage cells with antigen-dependent anti-cancer cell functions. J Hematol Oncol. 2020;13:153.
Kang M, Lee SH, Kwon M, Byun J, Kim D, Kim C, et al. Nanocomplex-Mediated In Vivo Programming to Chimeric Antigen Receptor-M1 Macrophages for Cancer Therapy. Adv Mater. 2021;33:e2103258.
Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–506.
Schulz C, Gomez Perdiguero E, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science. 2012;336:86–90.
Gabrilovich DI, Ostrand-Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12:253–68.
Solito S, Falisi E, Diaz-Montero CM, Doni A, Pinton L, Rosato A, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood. 2011;118:2254–65.
Hao Z, Li R, Wang Y, Li S, Hong Z, Han Z. Landscape of Myeloid-derived Suppressor Cell in Tumor Immunotherapy. Biomark Res. 2021;9:77.
Yu S, Ren X, Li L. Myeloid-derived suppressor cells in hematologic malignancies: two sides of the same coin. Exp Hematol Oncol. 2022;11:43.
Yang Z, Guo J, Weng L, Tang W, Jin S, Ma W. Myeloid-derived suppressor cells-new and exciting players in lung cancer. J Hematol Oncol. 2020;13:10.
Arihara F, Mizukoshi E, Kitahara M, Takata Y, Arai K, Yamashita T, et al. Increase in CD14+HLA-DR -/low myeloid-derived suppressor cells in hepatocellular carcinoma patients and its impact on prognosis. Cancer Immunol Immunother. 2013;62:1421–30.
Diaz-Montero CM, Salem ML, Nishimura MI, Garrett-Mayer E, Cole DJ, Montero AJ. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol Immunother. 2009;58:49–59.
Angell TE, Lechner MG, Smith AM, Martin SE, Groshen SG, Maceri DR, et al. Circulating Myeloid-Derived Suppressor Cells Predict Differentiated Thyroid Cancer Diagnosis and Extent. Thyroid. 2016;26:381–9.
Tada K, Kitano S, Shoji H, Nishimura T, Shimada Y, Nagashima K, et al. Pretreatment Immune Status Correlates with Progression-Free Survival in Chemotherapy-Treated Metastatic Colorectal Cancer Patients. Cancer Immunol Res. 2016;4:592–9.
Butterfield LH, Zhao F, Lee S, Tarhini AA, Margolin KA, White RL, et al. Immune Correlates of GM-CSF and Melanoma Peptide Vaccination in a Randomized Trial for the Adjuvant Therapy of Resected High-Risk Melanoma (E4697). Clin Cancer Res. 2017;23:5034–43.
Gabrilovich DI. Myeloid-Derived Suppressor Cells. Cancer. Immunol Res. 2017;5:3–8.
Veglia F, Sanseviero E, Gabrilovich DI. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat Rev Immunol. 2021;21:485–98.
Hegde S, Leader AM, Merad M. MDSC: Markers, development, states, and unaddressed complexity. Immunity. 2021;54:875–84.
Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. 2016;7:12150.
Kumar V, Patel S, Tcyganov E, Gabrilovich DI. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016;37:208–20.
Dysthe M, Parihar R. Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1224:117–40.
Parker KH, Sinha P, Horn LA, Clements VK, Yang H, Li J, et al. HMGB1 enhances immune suppression by facilitating the differentiation and suppressive activity of myeloid-derived suppressor cells. Cancer Res. 2014;74:5723–33.
Hanson EM, Clements VK, Sinha P, Ilkovitch D, Ostrand-Rosenberg S. Myeloid-derived suppressor cells down-regulate L-selectin expression on CD4+ and CD8+ T cells. J Immunol. 2009;183:937–44.
Movahedi K, Guilliams M, Van den Bossche J, Van den Bergh R, Gysemans C, Beschin A, et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–44.
Condamine T, Mastio J, Gabrilovich DI. Transcriptional regulation of myeloid-derived suppressor cells. J Leukoc Biol. 2015;98:913–22.
Li H, Han Y, Guo Q, Zhang M, Cao X. Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol. 2009;182:240–9.
Wang Y, Schafer CC, Hough KP, Tousif S, Duncan SR, Kearney JF, et al. Myeloid-Derived Suppressor Cells Impair B Cell Responses in Lung Cancer through IL-7 and STAT5. J Immunol. 2018;201:278–95.
Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother. 2012;61:827–38.
Schlecker E, Stojanovic A, Eisen C, Quack C, Falk CS, Umansky V, et al. Tumor-infiltrating monocytic myeloid-derived suppressor cells mediate CCR5-dependent recruitment of regulatory T cells favoring tumor growth. J Immunol. 2012;189:5602–11.
Haist M, Stege H, Grabbe S, Bros M. The Functional Crosstalk between Myeloid-Derived Suppressor Cells and Regulatory T Cells within the Immunosuppressive Tumor Microenvironment. Cancers (Basel). 2021;13:210.
Ostrand-Rosenberg S, Sinha P, Beury DW, Clements VK. Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol. 2012;22:275–81.
Safarzadeh E, Orangi M, Mohammadi H, Babaie F, Baradaran B. Myeloid-derived suppressor cells: Important contributors to tumor progression and metastasis. J Cell Physiol. 2018;233:3024–36.
Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31.
Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–21.
Wang Y, Yin K, Tian J, Xia X, Ma J, Tang X, et al. Granulocytic Myeloid-Derived Suppressor Cells Promote the Stemness of Colorectal Cancer Cells through Exosomal S100A9. Adv Sci (Weinh). 2019;6:1901278.
Peng D, Tanikawa T, Li W, Zhao L, Vatan L, Szeliga W, et al. Myeloid-Derived Suppressor Cells Endow Stem-like Qualities to Breast Cancer Cells through IL6/STAT3 and NO/NOTCH Cross-talk Signaling. Cancer Res. 2016;76:3156–65.
Cui TX, Kryczek I, Zhao L, Zhao E, Kuick R, Roh MH, et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity. 2013;39:611–21.
Wang D, Sun H, Wei J, Cen B, DuBois RN. CXCL1 Is Critical for Premetastatic Niche Formation and Metastasis in Colorectal Cancer. Cancer Res. 2017;77:3655–65.
Li K, Shi H, Zhang B, Ou X, Ma Q, Chen Y, et al. Myeloid-derived suppressor cells as immunosuppressive regulators and therapeutic targets in cancer. Signal Transduct Target Ther. 2021;6:362.
Bian Z, Shi L, Venkataramani M, Abdelaal AM, Culpepper C, Kidder K, et al. Tumor conditions induce bone marrow expansion of granulocytic, but not monocytic, immunosuppressive leukocytes with increased CXCR2 expression in mice. Eur J Immunol. 2018;48:532–42.
Alfaro C, Teijeira A, Oñate C, Pérez G, Sanmamed MF, Andueza MP, et al. Tumor-Produced Interleukin-8 Attracts Human Myeloid-Derived Suppressor Cells and Elicits Extrusion of Neutrophil Extracellular Traps (NETs). Clin Cancer Res. 2016;22:3924–36.
Sun L, Clavijo PE, Robbins Y, Patel P, Friedman J, Greene S, et al. Inhibiting myeloid-derived suppressor cell trafficking enhances T cell immunotherapy. JCI Insight. 2019;4: e126853.
Greene S, Robbins Y, Mydlarz WK, Huynh AP, Schmitt NC, Friedman J, et al. Inhibition of MDSC Trafficking with SX-682, a CXCR1/2 Inhibitor, Enhances NK-Cell Immunotherapy in Head and Neck Cancer Models. Clin Cancer Res. 2020;26:1420–31.
Steele CW, Karim SA, Leach JDG, Bailey P, Upstill-Goddard R, Rishi L, et al. CXCR2 Inhibition Profoundly Suppresses Metastases and Augments Immunotherapy in Pancreatic Ductal Adenocarcinoma. Cancer Cell. 2016;29:832–45.
Schott AF, Goldstein LJ, Cristofanilli M, Ruffini PA, McCanna S, Reuben JM, et al. Phase Ib Pilot Study to Evaluate Reparixin in Combination with Weekly Paclitaxel in Patients with HER-2-Negative Metastatic Breast Cancer. Clin Cancer Res. 2017;23:5358–65.
Alfaro C, Sanmamed MF, Rodríguez-Ruiz ME, Teijeira Á, Oñate C, González Á, et al. Interleukin-8 in cancer pathogenesis, treatment and follow-up. Cancer Treat Rev. 2017;60:24–31.
Tannenbaum CS, Rayman PA, Pavicic PG, Kim JS, Wei W, Polefko A, et al. Mediators of Inflammation-Driven Expansion, Trafficking, and Function of Tumor-Infiltrating MDSCs. Cancer Immunol Res. 2019;7:1687–99.
Chen L, Huang CF, Li YC, Deng WW, Mao L, Wu L, et al. Blockage of the NLRP3 inflammasome by MCC950 improves anti-tumor immune responses in head and neck squamous cell carcinoma. Cell Mol Life Sci. 2018;75:2045–58.
Mangan MSJ, Olhava EJ, Roush WR, Seidel HM, Glick GD, Latz E. Targeting the NLRP3 inflammasome in inflammatory diseases. Nat Rev Drug Discov. 2018;17:588–606.
Sota J, Vitale A, Insalaco A, Sfriso P, Lopalco G, Emmi G, et al. Safety profile of the interleukin-1 inhibitors anakinra and canakinumab in real-life clinical practice: a nationwide multicenter retrospective observational study. Clin Rheumatol. 2018;37:2233–40.
Kaplanov I, Carmi Y, Kornetsky R, Shemesh A, Shurin GV, Shurin MR, et al. Blocking IL-1β reverses the immunosuppression in mouse breast cancer and synergizes with anti-PD-1 for tumor abrogation. Proc Natl Acad Sci U S A. 2019;116:1361–9.
Yuan B, Clowers MJ, Velasco WV, Peng S, Peng Q, Shi Y, et al. Targeting IL-1β as an immunopreventive and therapeutic modality for K-ras-mutant lung cancer. JCI Insight. 2022;7:e157788.
Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 2004;64:6337–43.
Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, et al. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A. 2009;106:6742–7.
Li W, Zhang X, Chen Y, Xie Y, Liu J, Feng Q, et al. G-CSF is a key modulator of MDSC and could be a potential therapeutic target in colitis-associated colorectal cancers. Protein Cell. 2016;7:130–40.
Horikawa N, Abiko K, Matsumura N, Baba T, Hamanishi J, Yamaguchi K, et al. Anti-VEGF therapy resistance in ovarian cancer is caused by GM-CSF-induced myeloid-derived suppressor cell recruitment. Br J Cancer. 2020;122:778–88.
Kinoshita R, Sato H, Yamauchi A, Takahashi Y, Inoue Y, Sumardika IW, et al. Newly developed anti-S100A8/A9 monoclonal antibody efficiently prevents lung tropic cancer metastasis. Int J Cancer. 2019;145:569–75.
Qin H, Lerman B, Sakamaki I, Wei G, Cha SC, Rao SS, et al. Generation of a new therapeutic peptide that depletes myeloid-derived suppressor cells in tumor-bearing mice. Nat Med. 2014;20:676–81.
Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–49.
Rivera LB, Bergers G. Intertwined regulation of angiogenesis and immunity by myeloid cells. Trends Immunol. 2015;36:240–9.
Horikawa N, Abiko K, Matsumura N, Hamanishi J, Baba T, Yamaguchi K, et al. Expression of Vascular Endothelial Growth Factor in Ovarian Cancer Inhibits Tumor Immunity through the Accumulation of Myeloid-Derived Suppressor Cells. Clin Cancer Res. 2017;23:587–99.
Borges GSM, Lima FA, Carneiro G, Goulart GAC, Ferreira LAM. All-trans retinoic acid in anticancer therapy: how nanotechnology can enhance its efficacy and resolve its drawbacks. Expert Opin Drug Deliv. 2021;18:1335–54.
Ni X, Hu G, Cai X. The success and the challenge of all-trans retinoic acid in the treatment of cancer. Crit Rev Food Sci Nutr. 2019;59:S71-s80.
Kusmartsev S, Su Z, Heiser A, Dannull J, Eruslanov E, Kübler H, et al. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2008;14:8270–8.
Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–307.
Iclozan C, Antonia S, Chiappori A, Chen DT, Gabrilovich D. Therapeutic regulation of myeloid-derived suppressor cells and immune response to cancer vaccine in patients with extensive stage small cell lung cancer. Cancer Immunol Immunother. 2013;62:909–18.
Tobin RP, Jordan KR, Robinson WA, Davis D, Borges VF, Gonzalez R, et al. Targeting myeloid-derived suppressor cells using all-trans retinoic acid in melanoma patients treated with Ipilimumab. Int Immunopharmacol. 2018;63:282–91.
Arrieta O, González-De la Rosa CH, Aréchaga-Ocampo E, Villanueva-Rodríguez G, Cerón-Lizárraga TL, Martínez-Barrera L, et al. Randomized phase II trial of All-trans-retinoic acid with chemotherapy based on paclitaxel and cisplatin as first-line treatment in patients with advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:3463–71.
Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 2005;65:9525–35.
Trovato R, Fiore A, Sartori S, Canè S, Giugno R, Cascione L, et al. Immunosuppression by monocytic myeloid-derived suppressor cells in patients with pancreatic ductal carcinoma is orchestrated by STAT3. J Immunother Cancer. 2019;7:255.
Lu P, Yu B, Xu J. Cucurbitacin B regulates immature myeloid cell differentiation and enhances antitumor immunity in patients with lung cancer. Cancer Biother Radiopharm. 2012;27:495–503.
Reilley MJ, McCoon P, Cook C, Lyne P, Kurzrock R, Kim Y, et al. STAT3 antisense oligonucleotide AZD9150 in a subset of patients with heavily pretreated lymphoma: results of a phase 1b trial. J Immunother Cancer. 2018;6:119.
Guha P, Gardell J, Darpolor J, Cunetta M, Lima M, Miller G, et al. STAT3 inhibition induces Bax-dependent apoptosis in liver tumor myeloid-derived suppressor cells. Oncogene. 2019;38:533–48.
Datta J, Dai X, Bianchi A, De Castro SI, Mehra S, Garrido VT, et al. Combined MEK and STAT3 Inhibition Uncovers Stromal Plasticity by Enriching for Cancer-Associated Fibroblasts With Mesenchymal Stem Cell-Like Features to Overcome Immunotherapy Resistance in Pancreatic Cancer. Gastroenterology. 2022;163:1593–612.
Bitsch R, Kurzay A, Özbay Kurt F, De La Torre C, Lasser S, Lepper A, et al. STAT3 inhibitor Napabucasin abrogates MDSC immunosuppressive capacity and prolongs survival of melanoma-bearing mice. J Immunother Cancer. 2022;10:e004384.
Zhang L, Huang J, Chen X, Pan C, He Y, Su R, et al. Self-assembly nanovaccine containing TLR7/8 agonist and STAT3 inhibitor enhances tumor immunotherapy by augmenting tumor-specific immune response. J Immunother Cancer. 2021;9:e003132.
Kim TW, Kim Y, Keum H, Jung W, Kang M, Jon S. Combination of a STAT3 inhibitor with anti-PD-1 immunotherapy is an effective treatment regimen for a vemurafenib-resistant melanoma. Mol Ther Oncolytics. 2022;26:1–14.
Shirota H, Klinman DM. Effect of CpG ODN on monocytic myeloid derived suppressor cells. Oncoimmunology. 2012;1:780–2.
Shirota Y, Shirota H, Klinman DM. Intratumoral injection of CpG oligonucleotides induces the differentiation and reduces the immunosuppressive activity of myeloid-derived suppressor cells. J Immunol. 2012;188:1592–9.
Vollmer J, Krieg AM. Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev. 2009;61:195–204.
Zoglmeier C, Bauer H, Noerenberg D, Wedekind G, Bittner P, Sandholzer N, et al. CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice. Clin Cancer Res. 2011;17:1765–75.
Lee M, Park CS, Lee YR, Im SA, Song S, Lee CK. Resiquimod, a TLR7/8 agonist, promotes differentiation of myeloid-derived suppressor cells into macrophages and dendritic cells. Arch Pharm Res. 2014;37:1234–40.
Cresswell GM, Wang B, Kischuk EM, Broman MM, Alfar RA, Vickman RE, et al. Folate Receptor Beta Designates Immunosuppressive Tumor-Associated Myeloid Cells That Can Be Reprogrammed with Folate-Targeted Drugs. Cancer Res. 2021;81:671–84.
Forghani P, Waller EK. Poly (I: C) modulates the immunosuppressive activity of myeloid-derived suppressor cells in a murine model of breast cancer. Breast Cancer Res Treat. 2015;153:21–30.
Liu D, You M, Xu Y, Li F, Zhang D, Li X, et al. Inhibition of curcumin on myeloid-derived suppressor cells is requisite for controlling lung cancer. Int Immunopharmacol. 2016;39:265–72.
Rui K, Tian J, Tang X, Ma J, Xu P, Tian X, et al. Curdlan blocks the immune suppression by myeloid-derived suppressor cells and reduces tumor burden. Immunol Res. 2016;64:931–9.
Zhou J, Wu J, Chen X, Fortenbery N, Eksioglu E, Kodumudi KN, et al. Icariin and its derivative, ICT, exert anti-inflammatory, anti-tumor effects, and modulate myeloid derived suppressive cells (MDSCs) functions. Int Immunopharmacol. 2011;11:890–8.
Mao Y, Sarhan D, Steven A, Seliger B, Kiessling R, Lundqvist A. Inhibition of tumor-derived prostaglandin-e2 blocks the induction of myeloid-derived suppressor cells and recovers natural killer cell activity. Clin Cancer Res. 2014;20:4096–106.
Rodríguez-Ubreva J, Català-Moll F, Obermajer N, Álvarez-Errico D, Ramirez RN, Company C, et al. Prostaglandin E2 Leads to the Acquisition of DNMT3A-Dependent Tolerogenic Functions in Human Myeloid-Derived Suppressor Cells. Cell Rep. 2017;21:154–67.
Prima V, Kaliberova LN, Kaliberov S, Curiel DT, Kusmartsev S. COX2/mPGES1/PGE2 pathway regulates PD-L1 expression in tumor-associated macrophages and myeloid-derived suppressor cells. Proc Natl Acad Sci U S A. 2017;114:1117–22.
Obermajer N, Muthuswamy R, Odunsi K, Edwards RP, Kalinski P. PGE(2)-induced CXCL12 production and CXCR4 expression controls the accumulation of human MDSCs in ovarian cancer environment. Cancer Res. 2011;71:7463–70.
Porta C, Consonni FM, Morlacchi S, Sangaletti S, Bleve A, Totaro MG, et al. Tumor-Derived Prostaglandin E2 Promotes p50 NF-κB-Dependent Differentiation of Monocytic MDSCs. Cancer Res. 2020;80:2874–88.
Obermajer N, Wong JL, Edwards RP, Odunsi K, Moysich K, Kalinski P. PGE(2)-driven induction and maintenance of cancer-associated myeloid-derived suppressor cells. Immunol Invest. 2012;41:635–57.
Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, et al. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–74.
Hou W, Sampath P, Rojas JJ, Thorne SH. Oncolytic Virus-Mediated Targeting of PGE2 in the Tumor Alters the Immune Status and Sensitizes Established and Resistant Tumors to Immunotherapy. Cancer Cell. 2016;30:108–19.
Veltman JD, Lambers ME, van Nimwegen M, Hendriks RW, Hoogsteden HC, Aerts JG, et al. COX-2 inhibition improves immunotherapy and is associated with decreased numbers of myeloid-derived suppressor cells in mesothelioma Celecoxib influences MDSC function. BMC Cancer. 2010;10:464.
Kosaka A, Ohkuri T, Okada H. Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells. Cancer Immunol Immunother. 2014;63:847–57.
Noonan KA, Ghosh N, Rudraraju L, Bui M, Borrello I. Targeting immune suppression with PDE5 inhibition in end-stage multiple myeloma. Cancer Immunol Res. 2014;2:725–31.
Serafini P, Meckel K, Kelso M, Noonan K, Califano J, Koch W, et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J Exp Med. 2006;203:2691–702.
Hassel JC, Jiang H, Bender C, Winkler J, Sevko A, Shevchenko I, et al. Tadalafil has biologic activity in human melanoma. Results of a pilot trial with Tadalafil in patients with metastatic Melanoma (TaMe). Oncoimmunology. 2017;6:e1326440.
Weed DT, Vella JL, Reis IM, De la Fuente AC, Gomez C, Sargi Z, et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:39–48.
Califano JA, Khan Z, Noonan KA, Rudraraju L, Zhang Z, Wang H, et al. Tadalafil augments tumor specific immunity in patients with head and neck squamous cell carcinoma. Clin Cancer Res. 2015;21:30–8.
Orillion A, Hashimoto A, Damayanti N, Shen L, Adelaiye-Ogala R, Arisa S, et al. Entinostat Neutralizes Myeloid-Derived Suppressor Cells and Enhances the Antitumor Effect of PD-1 Inhibition in Murine Models of Lung and Renal Cell Carcinoma. Clin Cancer Res. 2017;23:5187–201.
Christmas BJ, Rafie CI, Hopkins AC, Scott BA, Ma HS, Cruz KA, et al. Entinostat Converts Immune-Resistant Breast and Pancreatic Cancers into Checkpoint-Responsive Tumors by Reprogramming Tumor-Infiltrating MDSCs. Cancer Immunol Res. 2018;6:1561–77.
Hashimoto A, Fukumoto T, Zhang R, Gabrilovich D. Selective targeting of different populations of myeloid-derived suppressor cells by histone deacetylase inhibitors. Cancer Immunol Immunother. 2020;69:1929–36.
Hiramoto K, Satoh H, Suzuki T, Moriguchi T, Pi J, Shimosegawa T, et al. Myeloid lineage-specific deletion of antioxidant system enhances tumor metastasis. Cancer Prev Res (Phila). 2014;7:835–44.
Fleet JC, Burcham GN, Calvert RD, Elzey BD, Ratliff TL. 1α, 25 Dihydroxyvitamin D (1,25(OH)(2)D) inhibits the T cell suppressive function of myeloid derived suppressor cells (MDSC). J Steroid Biochem Mol Biol. 2020;198:105557.
De Santo C, Serafini P, Marigo I, Dolcetti L, Bolla M, Del Soldato P, et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc Natl Acad Sci U S A. 2005;102:4185–90.
Al-Khami AA, Rodriguez PC, Ochoa AC. Metabolic reprogramming of myeloid-derived suppressor cells (MDSC) in cancer. Oncoimmunology. 2016;5:e1200771.
Hossain F, Al-Khami AA, Wyczechowska D, Hernandez C, Zheng L, Reiss K, et al. Inhibition of Fatty Acid Oxidation Modulates Immunosuppressive Functions of Myeloid-Derived Suppressor Cells and Enhances Cancer Therapies. Cancer Immunol Res. 2015;3:1236–47.
Veglia F, Tyurin VA, Blasi M, De Leo A, Kossenkov AV, Donthireddy L, et al. Fatty acid transport protein 2 reprograms neutrophils in cancer. Nature. 2019;569:73–8.
Wu T, Zhao Y, Wang H, Li Y, Shao L, Wang R, et al. mTOR masters monocytic myeloid-derived suppressor cells in mice with allografts or tumors. Sci Rep. 2016;6:20250.
Deng Y, Yang J, Luo F, Qian J, Liu R, Zhang D, et al. mTOR-mediated glycolysis contributes to the enhanced suppressive function of murine tumor-infiltrating monocytic myeloid-derived suppressor cells. Cancer Immunol Immunother. 2018;67:1355–64.
Li L, Wang L, Li J, Fan Z, Yang L, Zhang Z, et al. Metformin-Induced Reduction of CD39 and CD73 Blocks Myeloid-Derived Suppressor Cell Activity in Patients with Ovarian Cancer. Cancer Res. 2018;78:1779–91.
Meireson A, Devos M, Brochez L. IDO Expression in Cancer: Different Compartment, Different Functionality? Front Immunol. 2020;11:531491.
Antonioli L, Pacher P, Vizi ES, Haskó G. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–67.
Zhang C, Yue C, Herrmann A, Song J, Egelston C, Wang T, et al. STAT3 Activation-Induced Fatty Acid Oxidation in CD8(+) T Effector Cells Is Critical for Obesity-Promoted Breast Tumor Growth. Cell Metab. 2020;31:148-61.e5.
Chowdhury PS, Chamoto K, Kumar A, Honjo T. PPAR-Induced Fatty Acid Oxidation in T Cells Increases the Number of Tumor-Reactive CD8(+) T Cells and Facilitates Anti-PD-1 Therapy. Cancer Immunol Res. 2018;6:1375–87.
Choi BK, Lee DY, Lee DG, Kim YH, Kim SH, Oh HS, et al. 4–1BB signaling activates glucose and fatty acid metabolism to enhance CD8(+) T cell proliferation. Cell Mol Immunol. 2017;14:748–57.
Wabitsch S, McCallen JD, Kamenyeva O, Ruf B, McVey JC, Kabat J, et al. Metformin treatment rescues CD8(+) T-cell response to immune checkpoint inhibitor therapy in mice with NAFLD. J Hepatol. 2022;77:748–60.
O’Neill LA, Pearce EJ. Immunometabolism governs dendritic cell and macrophage function. J Exp Med. 2016;213:15–23.
Rivera Vargas T, Apetoh L. Can Immunogenic Chemotherapies Relieve Cancer Cell Resistance to Immune Checkpoint Inhibitors? Front Immunol. 2019;10:1181.
Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. 2010;70:3052–61.
Welters MJ, van der Sluis TC, van Meir H, Loof NM, van Ham VJ, van Duikeren S, et al. Vaccination during myeloid cell depletion by cancer chemotherapy fosters robust T cell responses. Sci Transl Med. 2016;8:334ra52.
Peereboom DM, Alban TJ, Grabowski MM, Alvarado AG, Otvos B, Bayik D, et al. Metronomic capecitabine as an immune modulator in glioblastoma patients reduces myeloid-derived suppressor cells. JCI Insight. 2019;4:e130748.
Ding ZC, Munn DH, Zhou G. Chemotherapy-induced myeloid suppressor cells and antitumor immunity: The Janus face of chemotherapy in immunomodulation. Oncoimmunology. 2014;3:e954471.
Fultang L, Panetti S, Ng M, Collins P, Graef S, Rizkalla N, et al. MDSC targeting with Gemtuzumab ozogamicin restores T cell immunity and immunotherapy against cancers. EBioMedicine. 2019;47:235–46.
Eksioglu EA, Chen X, Heider KH, Rueter B, McGraw KL, Basiorka AA, et al. Novel therapeutic approach to improve hematopoiesis in low risk MDS by targeting MDSCs with the Fc-engineered CD33 antibody BI 836858. Leukemia. 2017;31:2172–80.
Tazzari M, Negri T, Rini F, Vergani B, Huber V, Villa A, et al. Adaptive immune contexture at the tumour site and downmodulation of circulating myeloid-derived suppressor cells in the response of solitary fibrous tumour patients to anti-angiogenic therapy. Br J Cancer. 2014;111:1350–62.
Condamine T, Kumar V, Ramachandran IR, Youn JI, Celis E, Finnberg N, et al. ER stress regulates myeloid-derived suppressor cell fate through TRAIL-R-mediated apoptosis. J Clin Invest. 2014;124:2626–39.
Ruf B, Greten TF, Korangy F. Innate lymphoid cells and innate-like T cells in cancer - at the crossroads of innate and adaptive immunity. Nat Rev Cancer. 2023;23:351–71.
Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18:671–88.
Dogra P, Rancan C, Ma W, Toth M, Senda T, Carpenter DJ, et al. Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell. 2020;180:749-63.e13.
Spits H, Bernink JH, Lanier L. NK cells and type 1 innate lymphoid cells: partners in host defense. Nat Immunol. 2016;17:758–64.
Freud AG, Zhao S, Wei S, Gitana GM, Molina-Kirsch HF, Atwater SK, et al. Expression of the activating receptor, NKp46 (CD335), in human natural killer and T-cell neoplasia. Am J Clin Pathol. 2013;140:853–66.
Freud AG, Mundy-Bosse BL, Yu J, Caligiuri MA. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity. 2017;47:820–33.
Freud AG, Yokohama A, Becknell B, Lee MT, Mao HC, Ferketich AK, et al. Evidence for discrete stages of human natural killer cell differentiation in vivo. J Exp Med. 2006;203:1033–43.
Mrózek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–40.
Freud AG, Becknell B, Roychowdhury S, Mao HC, Ferketich AK, Nuovo GJ, et al. A human CD34(+) subset resides in lymph nodes and differentiates into CD56bright natural killer cells. Immunity. 2005;22:295–304.
Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. A committed precursor to innate lymphoid cells. Nature. 2014;508:397–401.
Klose CSN, Flach M, Möhle L, Rogell L, Hoyler T, Ebert K, et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell. 2014;157:340–56.
Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, et al. Notch signaling is necessary for adult, but not fetal, development of RORγt(+) innate lymphoid cells. Nat Immunol. 2011;12:949–58.
Ran GH, Lin YQ, Tian L, Zhang T, Yan DM, Yu JH, et al. Natural killer cell homing and trafficking in tissues and tumors: from biology to application. Signal Transduct Target Ther. 2022;7:205.
Hughes T, Becknell B, Freud AG, McClory S, Briercheck E, Yu J, et al. Interleukin-1beta selectively expands and sustains interleukin-22+ immature human natural killer cells in secondary lymphoid tissue. Immunity. 2010;32:803–14.
Yu J, Freud AG, Caligiuri MA. Location and cellular stages of natural killer cell development. Trends Immunol. 2013;34:573–82.
Renoux VM, Zriwil A, Peitzsch C, Michaëlsson J, Friberg D, Soneji S, et al. Identification of a Human Natural Killer Cell Lineage-Restricted Progenitor in Fetal and Adult Tissues. Immunity. 2015;43:394–407.
Sivori S, Vacca P, Del Zotto G, Munari E, Mingari MC, Moretta L. Human NK cells: surface receptors, inhibitory checkpoints, and translational applications. Cell Mol Immunol. 2019;16:430–41.
Yang R, Mele F, Worley L, Langlais D, Rosain J, Benhsaien I, et al. Human T-bet Governs Innate and Innate-like Adaptive IFN-γ Immunity against Mycobacteria. Cell. 2020;183:1826-47.e31.
Artis D, Spits H. The biology of innate lymphoid cells. Nature. 2015;517:293–301.
Luevano M, Madrigal A, Saudemont A. Transcription factors involved in the regulation of natural killer cell development and function: an update. Front Immunol. 2012;3:319.
Peng H, Tian Z. NK cell trafficking in health and autoimmunity:a comprehensive review. Clin Rev Allergy Immunol. 2014;47:119–27.
Mayol K, Biajoux V, Marvel J, Balabanian K, Walzer T. Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood. 2011;118:4863–71.
Shannon MJ, Mace EM. Natural Killer Cell Integrins and Their Functions in Tissue Residency. Front Immunol. 2021;12:647358.
Yao X, Matosevic S. Chemokine networks modulating natural killer cell trafficking to solid tumors. Cytokine Growth Factor Rev. 2021;59:36–45.
Walzer T, Chiossone L, Chaix J, Calver A, Carozzo C, Garrigue-Antar L, et al. Natural killer cell trafficking in vivo requires a dedicated sphingosine 1-phosphate receptor. Nat Immunol. 2007;8:1337–44.
Jenne CN, Enders A, Rivera R, Watson SR, Bankovich AJ, Pereira JP, et al. T-bet-dependent S1P5 expression in NK cells promotes egress from lymph nodes and bone marrow. J Exp Med. 2009;206:2469–81.
Ponzetta A, Sciumè G, Benigni G, Antonangeli F, Morrone S, Santoni A, et al. CX3CR1 regulates the maintenance of KLRG1+ NK cells into the bone marrow by promoting their entry into circulation. J Immunol. 2013;191:5684–94.
Sciumè G, De Angelis G, Benigni G, Ponzetta A, Morrone S, Santoni A, et al. CX3CR1 expression defines 2 KLRG1+ mouse NK-cell subsets with distinct functional properties and positioning in the bone marrow. Blood. 2011;117:4467–75.
Hamann I, Unterwalder N, Cardona AE, Meisel C, Zipp F, Ransohoff RM, et al. Analyses of phenotypic and functional characteristics of CX3CR1-expressing natural killer cells. Immunology. 2011;133:62–73.
Cuff AO, Perchet T, Dertschnig S, Golub R, Male V. Tbet promotes CXCR6 expression in immature natural killer cells and natural killer cell egress from the bone marrow. Immunology. 2020;161:28–38.
Hertwig L, Hamann I, Romero-Suarez S, Millward JM, Pietrek R, Chanvillard C, et al. CX3CR1-dependent recruitment of mature NK cells into the central nervous system contributes to control autoimmune neuroinflammation. Eur J Immunol. 2016;46:1984–96.
Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16:112–23.
Lopez-Vergès S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, et al. Expansion of a unique CD57+NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci U S A. 2011;108:14725–32.
Cooper MA, Fehniger TA, Turner SC, Chen KS, Ghaheri BA, Ghayur T, et al. Human natural killer cells: a unique innate immunoregulatory role for the CD56(bright) subset. Blood. 2001;97:3146–51.
Yu J, Mao HC, Wei M, Hughes T, Zhang J, Park IK, et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood. 2010;115:274–81.
Cichocki F, Grzywacz B, Miller JS. Human NK Cell Development: One Road or Many? Front Immunol. 2019;10:2078.
Shevtsov M, Multhoff G. Immunological and Translational Aspects of NK Cell-Based Antitumor Immunotherapies. Front Immunol. 2016;7:492.
Elliott JM, Yokoyama WM. Unifying concepts of MHC-dependent natural killer cell education. Trends Immunol. 2011;32:364–72.
Tong L, Jiménez-Cortegana C, Tay AHM, Wickström S, Galluzzi L, Lundqvist A. NK cells and solid tumors: therapeutic potential and persisting obstacles. Mol Cancer. 2022;21:206.
Bournazos S, Wang TT, Dahan R, Maamary J, Ravetch JV. Signaling by Antibodies: Recent Progress. Annu Rev Immunol. 2017;35:285–311.
Zhu H, Blum RH, Bjordahl R, Gaidarova S, Rogers P, Lee TT, et al. Pluripotent stem cell-derived NK cells with high-affinity noncleavable CD16a mediate improved antitumor activity. Blood. 2020;135:399–410.
Russick J, Torset C, Hemery E, Cremer I. NK cells in the tumor microenvironment: Prognostic and theranostic impact Recent advances and trends. Semin Immunol. 2020;48:101407.
Cózar B, Greppi M, Carpentier S, Narni-Mancinelli E, Chiossone L, Vivier E. Tumor-Infiltrating Natural Killer Cells. Cancer Discov. 2021;11:34–44.
Ni J, Wang X, Stojanovic A, Zhang Q, Wincher M, Bühler L, et al. Single-Cell RNA Sequencing of Tumor-Infiltrating NK Cells Reveals that Inhibition of Transcription Factor HIF-1α Unleashes NK Cell Activity. Immunity. 2020;52:1075-87.e8.
Hosseini R, Sarvnaz H, Arabpour M, Ramshe SM, Asef-Kabiri L, Yousefi H, et al. Cancer exosomes and natural killer cells dysfunction: biological roles, clinical significance and implications for immunotherapy. Mol Cancer. 2022;21:15.
Dong W, Wu X, Ma S, Wang Y, Nalin AP, Zhu Z, et al. The Mechanism of Anti-PD-L1 Antibody Efficacy against PD-L1-Negative Tumors Identifies NK Cells Expressing PD-L1 as a Cytolytic Effector. Cancer Discov. 2019;9:1422–37.
Benson DM Jr, Bakan CE, Mishra A, Hofmeister CC, Efebera Y, Becknell B, et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood. 2010;116:2286–94.
Melaiu O, Lucarini V, Cifaldi L, Fruci D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front Immunol. 2019;10:3038.
Robson NC, Wei H, McAlpine T, Kirkpatrick N, Cebon J, Maraskovsky E. Activin-A attenuates several human natural killer cell functions. Blood. 2009;113:3218–25.
Cekic C, Day YJ, Sag D, Linden J. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74:7250–9.
Smyth MJ, Crowe NY, Godfrey DI. NK cells and NKT cells collaborate in host protection from methylcholanthrene-induced fibrosarcoma. Int Immunol. 2001;13:459–63.
Smyth MJ, Thia KY, Street SE, Cretney E, Trapani JA, Taniguchi M, et al. Differential tumor surveillance by natural killer (NK) and NKT cells. J Exp Med. 2000;191:661–8.
Lee H, Quek C, Silva I, Tasker A, Batten M, Rizos H, et al. Integrated molecular and immunophenotypic analysis of NK cells in anti-PD-1 treated metastatic melanoma patients. Oncoimmunology. 2019;8:e1537581.
Guerra N, Tan YX, Joncker NT, Choy A, Gallardo F, Xiong N, et al. NKG2D-deficient mice are defective in tumor surveillance in models of spontaneous malignancy. Immunity. 2008;28:571–80.
Takeda K, Hayakawa Y, Smyth MJ, Kayagaki N, Yamaguchi N, Kakuta S, et al. Involvement of tumor necrosis factor-related apoptosis-inducing ligand in surveillance of tumor metastasis by liver natural killer cells. Nat Med. 2001;7:94–100.
Huntington ND, Cursons J, Rautela J. The cancer-natural killer cell immunity cycle. Nat Rev Cancer. 2020;20:437–54.
Khan M, Arooj S, Wang H. NK Cell-Based Immune Checkpoint Inhibition. Front Immunol. 2020;11:167.
Hansen ML, Woetmann A, Krejsgaard T, Kopp KL, Søkilde R, Litman T, et al. IFN-α primes T- and NK-cells for IL-15-mediated signaling and cytotoxicity. Mol Immunol. 2011;48:2087–93.
Rölle A, Pollmann J, Ewen EM, Le VT, Halenius A, Hengel H, et al. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. J Clin Invest. 2014;124:5305–16.
Rölle A, Meyer M, Calderazzo S, Jäger D, Momburg F. Distinct HLA-E Peptide Complexes Modify Antibody-Driven Effector Functions of Adaptive NK Cells. Cell Rep. 2018;24:1967-76.e4.
Rölle A, Jäger D, Momburg F. HLA-E Peptide Repertoire and Dimorphism-Centerpieces in the Adaptive NK Cell Puzzle? Front Immunol. 2018;9:2410.
Gornalusse GG, Hirata RK, Funk SE, Riolobos L, Lopes VS, Manske G, et al. HLA-E-expressing pluripotent stem cells escape allogeneic responses and lysis by NK cells. Nat Biotechnol. 2017;35:765–72.
Liu X, Song J, Zhang H, Liu X, Zuo F, Zhao Y, et al. Immune checkpoint HLA-E:CD94-NKG2A mediates evasion of circulating tumor cells from NK cell surveillance. Cancer Cell. 2023;41:272-87.e9.
Zhang C, Hu Y, Xiao W, Tian Z. Chimeric antigen receptor- and natural killer cell receptor-engineered innate killer cells in cancer immunotherapy. Cell Mol Immunol. 2021;18:2083–100.
Bald T, Krummel MF, Smyth MJ, Barry KC. The NK cell-cancer cycle: advances and new challenges in NK cell-based immunotherapies. Nat Immunol. 2020;21:835–47.
Yilmaz A, Cui H, Caligiuri MA, Yu J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J Hematol Oncol. 2020;13:168.
Cichocki F, Bjordahl R, Gaidarova S, Mahmood S, Abujarour R, Wang H, et al. iPSC-derived NK cells maintain high cytotoxicity and enhance in vivo tumor control in concert with T cells and anti-PD-1 therapy. Sci Transl Med. 2020;12:eaaz5618.
Glasner A, Ghadially H, Gur C, Stanietsky N, Tsukerman P, Enk J, et al. Recognition and prevention of tumor metastasis by the NK receptor NKp46/NCR1. J Immunol. 2012;188:2509–15.
Takvorian T, Canellos GP, Ritz J, Freedman AS, Anderson KC, Mauch P, et al. Prolonged disease-free survival after autologous bone marrow transplantation in patients with non-Hodgkin’s lymphoma with a poor prognosis. N Engl J Med. 1987;316:1499–505.
Dolstra H, Roeven MWH, Spanholtz J, Hangalapura BN, Tordoir M, Maas F, et al. Successful Transfer of Umbilical Cord Blood CD34(+) Hematopoietic Stem and Progenitor-derived NK Cells in Older Acute Myeloid Leukemia Patients. Clin Cancer Res. 2017;23:4107–18.
Attal M, Richardson PG, Rajkumar SV, San-Miguel J, Beksac M, Spicka I, et al. Isatuximab plus pomalidomide and low-dose dexamethasone versus pomalidomide and low-dose dexamethasone in patients with relapsed and refractory multiple myeloma (ICARIA-MM): a randomised, multicentre, open-label, phase 3 study. Lancet. 2019;394:2096–107.
Whalen KA, Rakhra K, Mehta NK, Steinle A, Michaelson JS, Baeuerle PA. Engaging natural killer cells for cancer therapy via NKG2D, CD16A and other receptors. MAbs. 2023;15:2208697.
Kamiya T, Seow SV, Wong D, Robinson M, Campana D. Blocking expression of inhibitory receptor NKG2A overcomes tumor resistance to NK cells. J Clin Invest. 2019;129:2094–106.
Lee JH, Lee JH, Lim YS, Yeon JE, Song TJ, Yu SJ, et al. Adjuvant immunotherapy with autologous cytokine-induced killer cells for hepatocellular carcinoma. Gastroenterology. 2015;148:1383-91.e6.
Björklund AT, Carlsten M, Sohlberg E, Liu LL, Clancy T, Karimi M, et al. Complete Remission with Reduction of High-Risk Clones following Haploidentical NK-Cell Therapy against MDS and AML. Clin Cancer Res. 2018;24:1834–44.
Sakamoto N, Ishikawa T, Kokura S, Okayama T, Oka K, Ideno M, et al. Phase I clinical trial of autologous NK cell therapy using novel expansion method in patients with advanced digestive cancer. J Transl Med. 2015;13:277.
Shimasaki N, Jain A, Campana D. NK cells for cancer immunotherapy. Nat Rev Drug Discov. 2020;19:200–18.
Zhuang X, Long EO. NK Cells Equipped With a Chimeric Antigen Receptor That Overcomes Inhibition by HLA Class I for Adoptive Transfer of CAR-NK Cells. Front Immunol. 2022;13:840844.
Leivas A, Perez-Martinez A, Blanchard MJ, Martín-Clavero E, Fernández L, Lahuerta JJ, et al. Novel treatment strategy with autologous activated and expanded natural killer cells plus anti-myeloma drugs for multiple myeloma. Oncoimmunology. 2016;5:e1250051.
Thangaraj JL, Ahn SY, Jung SH, Vo MC, Chu TH, Thi Phan MT, et al. Expanded natural killer cells augment the antimyeloma effect of daratumumab, bortezomib, and dexamethasone in a mouse model. Cell Mol Immunol. 2021;18:1652–61.
Leivas A, Valeri A, Córdoba L, García-Ortiz A, Ortiz A, Sánchez-Vega L, et al. NKG2D-CAR-transduced natural killer cells efficiently target multiple myeloma. Blood Cancer J. 2021;11:146.
Escudier B, Farace F, Angevin E, Triebel F, Antoun S, Leclercq B, et al. Combination of interleukin-2 and gamma interferon in metastatic renal cell carcinoma. Eur J Cancer. 1993;29a:724–8.
Margolin K, Morishima C, Velcheti V, Miller JS, Lee SM, Silk AW, et al. Phase I Trial of ALT-803, A Novel Recombinant IL15 Complex, in Patients with Advanced Solid Tumors. Clin Cancer Res. 2018;24:5552–61.
Conlon KC, Lugli E, Welles HC, Rosenberg SA, Fojo AT, Morris JC, et al. Redistribution, hyperproliferation, activation of natural killer cells and CD8 T cells, and cytokine production during first-in-human clinical trial of recombinant human interleukin-15 in patients with cancer. J Clin Oncol. 2015;33:74–82.
Romee R, Cooley S, Berrien-Elliott MM, Westervelt P, Verneris MR, Wagner JE, et al. First-in-human phase 1 clinical study of the IL-15 superagonist complex ALT-803 to treat relapse after transplantation. Blood. 2018;131:2515–27.
Chauvin JM, Ka M, Pagliano O, Menna C, Ding Q, DeBlasio R, et al. IL15 Stimulation with TIGIT Blockade Reverses CD155-mediated NK-Cell Dysfunction in Melanoma. Clin Cancer Res. 2020;26:5520–33.
Sim GC, Liu C, Wang E, Liu H, Creasy C, Dai Z, et al. IL2 Variant Circumvents ICOS+ Regulatory T-cell Expansion and Promotes NK Cell Activation. Cancer Immunol Res. 2016;4:983–94.
Rekers NH, Zegers CM, Yaromina A, Lieuwes NG, Biemans R, Senden-Gijsbers BL, et al. Combination of radiotherapy with the immunocytokine L19-IL2: Additive effect in a NK cell dependent tumour model. Radiother Oncol. 2015;116:438–42.
Smits EL, Anguille S, Berneman ZN. Interferon α may be back on track to treat acute myeloid leukemia. Oncoimmunology. 2013;2:e23619.
Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. IFN-α Is Effective for Treatment of Minimal Residual Disease in Patients with Acute Leukemia after Allogeneic Hematopoietic Stem Cell Transplantation: Results of a Registry Study. Biol Blood Marrow Transplant. 2017;23:1303–10.
Ferrini S, Prigione I, Miotti S, Ciccone E, Cantoni C, Chen Q, et al. Bispecific monoclonal antibodies directed to CD16 and to a tumor-associated antigen induce target-cell lysis by resting NK cells and by a subset of NK clones. Int J Cancer. 1991;48:227–33.
Bianchini G, Gianni L. The immune system and response to HER2-targeted treatment in breast cancer. Lancet Oncol. 2014;15:e58-68.
Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–6.
Carter P, Presta L, Gorman CM, Ridgway JB, Henner D, Wong WL, et al. Humanization of an anti-p185HER2 antibody for human cancer therapy. Proc Natl Acad Sci U S A. 1992;89:4285–9.
Juliá EP, Amante A, Pampena MB, Mordoh J, Levy EM. Avelumab, an IgG1 anti-PD-L1 Immune Checkpoint Inhibitor, Triggers NK Cell-Mediated Cytotoxicity and Cytokine Production Against Triple Negative Breast Cancer Cells. Front Immunol. 2018;9:2140.
Deuse T, Hu X, Agbor-Enoh S, Jang MK, Alawi M, Saygi C, et al. The SIRPα-CD47 immune checkpoint in NK cells. J Exp Med. 2021;218:e20200839.
Romagné F, André P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1–7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood. 2009;114:2667–77.
Korde N, Carlsten M, Lee MJ, Minter A, Tan E, Kwok M, et al. A phase II trial of pan-KIR2D blockade with IPH2101 in smoldering multiple myeloma. Haematologica. 2014;99:e81–3.
Salomé B, Sfakianos JP, Ranti D, Daza J, Bieber C, Charap A, et al. NKG2A and HLA-E define an alternative immune checkpoint axis in bladder cancer. Cancer Cell. 2022;40:1027-43.e9.
van Montfoort N, Borst L, Korrer MJ, Sluijter M, Marijt KA, Santegoets SJ, et al. NKG2A Blockade Potentiates CD8 T Cell Immunity Induced by Cancer Vaccines. Cell. 2018;175:1744-55.e15.
André P, Denis C, Soulas C, Bourbon-Caillet C, Lopez J, Arnoux T, et al. Anti-NKG2A mAb Is a Checkpoint Inhibitor that Promotes Anti-tumor Immunity by Unleashing Both T and NK Cells. Cell. 2018;175:1731-43.e13.
Herbst RS, Majem M, Barlesi F, Carcereny E, Chu Q, Monnet I, et al. COAST: An Open-Label, Phase II, Multidrug Platform Study of Durvalumab Alone or in Combination With Oleclumab or Monalizumab in Patients With Unresectable, Stage III Non-Small-Cell Lung Cancer. J Clin Oncol. 2022;40:3383–93.
Zhang Q, Bi J, Zheng X, Chen Y, Wang H, Wu W, et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat Immunol. 2018;19:723–32.
Niu J, Maurice-Dror C, Lee DH, Kim DW, Nagrial A, Voskoboynik M, et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer. Ann Oncol. 2022;33:169–80.
Mettu NB, Ulahannan SV, Bendell JC, Garrido-Laguna I, Strickler JH, Moore KN, et al. A Phase 1a/b Open-Label, Dose-Escalation Study of Etigilimab Alone or in Combination with Nivolumab in Patients with Locally Advanced or Metastatic Solid Tumors. Clin Cancer Res. 2022;28:882–92.
Cho BC, Abreu DR, Hussein M, Cobo M, Patel AJ, Secen N, et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022;23:781–92.
Lee YH, Martin-Orozco N, Zheng P, Li J, Zhang P, Tan H, et al. Inhibition of the B7–H3 immune checkpoint limits tumor growth by enhancing cytotoxic lymphocyte function. Cell Res. 2017;27:1034–45.
Tyagi A, Ly S, El-Dana F, Yuan B, Jaggupilli A, Grimm S, et al. Evidence supporting a role for the immune checkpoint protein B7–H3 in NK cell-mediated cytotoxicity against AML. Blood. 2022;139:2782–96.
Petersen J, Rossjohn J. Overcoming the LAG3 phase problem. Nat Immunol. 2022;23:993–5.
Wolf Y, Anderson AC, Kuchroo VK. TIM3 comes of age as an inhibitory receptor. Nat Rev Immunol. 2020;20:173–85.
Kotwica-Mojzych K, Jodłowska-Jędrych B, Mojzych M. CD200:CD200R Interactions and Their Importance in Immunoregulation. Int J Mol Sci. 2021;22:1602.
Bian HT, Shen YW, Zhou YD, Nagle DG, Guan YY, Zhang WD, et al. CD47: Beyond an immune checkpoint in cancer treatment. Biochim Biophys Acta Rev Cancer. 2022;1877:188771.
Gong Y, Klein Wolterink RGJ, Wang J, Bos GMJ, Germeraad WTV. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J Hematol Oncol. 2021;14:73.
Wang X, Yang X, Yuan X, Wang W, Wang Y. Chimeric antigen receptor-engineered NK cells: new weapons of cancer immunotherapy with great potential. Exp Hematol Oncol. 2022;11:85.
Hunter BD, Jacobson CA. CAR T-Cell Associated Neurotoxicity: Mechanisms, Clinicopathologic Correlates, and Future Directions. J Natl Cancer Inst. 2019;111:646–54.
Laskowski TJ, Biederstädt A, Rezvani K. Natural killer cells in antitumour adoptive cell immunotherapy. Nat Rev Cancer. 2022;22:557–75.
Xie G, Dong H, Liang Y, Ham JD, Rizwan R, Chen J. CAR-NK cells: A promising cellular immunotherapy for cancer. EBioMedicine. 2020;59:102975.
Heipertz EL, Zynda ER, Stav-Noraas TE, Hungler AD, Boucher SE, Kaur N, et al. Current Perspectives on “Off-The-Shelf” Allogeneic NK and CAR-NK Cell Therapies. Front Immunol. 2021;12:732135.
Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, et al. NK-92: an “off-the-shelf therapeutic” for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother. 2016;65:485–92.
Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, et al. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy. 2013;15:1563–70.
Kruschinski A, Moosmann A, Poschke I, Norell H, Chmielewski M, Seliger B, et al. Engineering antigen-specific primary human NK cells against HER-2 positive carcinomas. Proc Natl Acad Sci U S A. 2008;105:17481–6.
Altvater B, Landmeier S, Pscherer S, Temme J, Schweer K, Kailayangiri S, et al. 2B4 (CD244) signaling by recombinant antigen-specific chimeric receptors costimulates natural killer cell activation to leukemia and neuroblastoma cells. Clin Cancer Res. 2009;15:4857–66.
Liu E, Tong Y, Dotti G, Shaim H, Savoldo B, Mukherjee M, et al. Cord blood NK cells engineered to express IL-15 and a CD19-targeted CAR show long-term persistence and potent antitumor activity. Leukemia. 2018;32:520–31.
Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med. 2020;382:545–53.
Soldierer M, Bister A, Haist C, Thivakaran A, Cengiz SC, Sendker S, et al. Genetic Engineering and Enrichment of Human NK Cells for CAR-Enhanced Immunotherapy of Hematological Malignancies. Front Immunol. 2022;13:847008.
Carlsten M, Childs RW. Genetic Manipulation of NK Cells for Cancer Immunotherapy: Techniques and Clinical Implications. Front Immunol. 2015;6:266.
Vargas JE, Chicaybam L, Stein RT, Tanuri A, Delgado-Cañedo A, Bonamino MH. Retroviral vectors and transposons for stable gene therapy: advances, current challenges and perspectives. J Transl Med. 2016;14:288.
Rostovskaya M, Fu J, Obst M, Baer I, Weidlich S, Wang H, et al. Transposon-mediated BAC transgenesis in human ES cells. Nucleic Acids Res. 2012;40:e150.
Lanier LL. NKG2D Receptor and Its Ligands in Host Defense. Cancer Immunol Res. 2015;3:575–82.
Xiao L, Cen D, Gan H, Sun Y, Huang N, Xiong H, et al. Adoptive Transfer of NKG2D CAR mRNA-Engineered Natural Killer Cells in Colorectal Cancer Patients. Mol Ther. 2019;27:1114–25.
Zhang C, Röder J, Scherer A, Bodden M, Pfeifer Serrahima J, Bhatti A, et al. Bispecific antibody-mediated redirection of NKG2D-CAR natural killer cells facilitates dual targeting and enhances antitumor activity. J Immunother Cancer. 2021;9:e002980.
Zhang C, Burger MC, Jennewein L, Genßler S, Schönfeld K, Zeiner P, et al. ErbB2/HER2-Specific NK Cells for Targeted Therapy of Glioblastoma. J Natl Cancer Inst. 2015;108. https://doi.org/10.1093/jnci/djv375.
Dhakal B, Berdeja JG, Gregory T, Ly T, Bickers C, Zong X, et al. Interim Phase I Clinical Data of FT576 As Monotherapy and in Combination with Daratumumab in Subjects with Relapsed/Refractory Multiple Myeloma. Blood. 2022;140:4586–7.
Dickinson M, Hamad N, Bryant CE, Borthakur G, Hosing C, Shook D, et al. A Phase 1 Study of NKX019, a CD19 Chimeric Antigen Receptor Natural Killer (CAR NK) Cell Therapy, in Subjects with B-Cell Malignancies. Blood. 2021;138:3868.
Liu M, Huang W, Guo Y, Zhou Y, Zhi C, Chen J, et al. CAR NK-92 cells targeting DLL3 kill effectively small cell lung cancer cells in vitro and in vivo. J Leukoc Biol. 2022;112:901–11.
Breedveld A, Groot Kormelink T, van Egmond M, de Jong EC. Granulocytes as modulators of dendritic cell function. J Leukoc Biol. 2017;102:1003–16.
Rivera A, Siracusa MC, Yap GS, Gause WC. Innate cell communication kick-starts pathogen-specific immunity. Nat Immunol. 2016;17:356–63.
Tavares LP, Peh HY, Tan WSD, Pahima H, Maffia P, Tiligada E, et al. Granulocyte-targeted therapies for airway diseases. Pharmacol Res. 2020;157:104881.
Liew PX, Kubes P. The Neutrophil’s Role During Health and Disease. Physiol Rev. 2019;99:1223–48.
Marone G, Schroeder JT, Mattei F, Loffredo S, Gambardella AR, Poto R, et al. Is There a Role for Basophils in Cancer? Front Immunol. 2020;11:2103.
Aponte-López A, Muñoz-Cruz S. Mast Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2020;1273:159–73.
Herrero-Cervera A, Soehnlein O, Kenne E. Neutrophils in chronic inflammatory diseases. Cell Mol Immunol. 2022;19:177–91.
Burn GL, Foti A, Marsman G, Patel DF, Zychlinsky A. The Neutrophil. Immunity. 2021;54:1377–91.
Mutua V, Gershwin LJ. A Review of Neutrophil Extracellular Traps (NETs) in Disease: Potential Anti-NETs Therapeutics. Clin Rev Allergy Immunol. 2021;61:194–211.
Xiong S, Dong L, Cheng L. Neutrophils in cancer carcinogenesis and metastasis. J Hematol Oncol. 2021;14:173.
Coffelt SB, Wellenstein MD, de Visser KE. Neutrophils in cancer: neutral no more. Nat Rev Cancer. 2016;16:431–46.
Schernberg A, Blanchard P, Chargari C, Deutsch E. Neutrophils, a candidate biomarker and target for radiation therapy? Acta Oncol. 2017;56:1522–30.
Antuamwine BB, Bosnjakovic R, Hofmann-Vega F, Wang X, Theodosiou T, Iliopoulos I, et al. N1 versus N2 and PMN-MDSC: A critical appraisal of current concepts on tumor-associated neutrophils and new directions for human oncology. Immunol Rev. 2023;314:250–79.
Zhang Y, Guoqiang L, Sun M, Lu X. Targeting and exploitation of tumor-associated neutrophils to enhance immunotherapy and drug delivery for cancer treatment. Cancer Biol Med. 2020;17:32–43.
de Los Reyes AA, Kim Y. Optimal regulation of tumour-associated neutrophils in cancer progression. R Soc Open Sci. 2022;9:210705.
Pylaeva E, Lang S, Jablonska J. The Essential Role of Type I Interferons in Differentiation and Activation of Tumor-Associated Neutrophils. Front Immunol. 2016;7:629.
Andzinski L, Kasnitz N, Stahnke S, Wu CF, Gereke M, von Köckritz-Blickwede M, et al. Type I IFNs induce anti-tumor polarization of tumor associated neutrophils in mice and human. Int J Cancer. 2016;138:1982–93.
Raftopoulou S, Valadez-Cosmes P, Mihalic ZN, Schicho R, Kargl J. Tumor-Mediated Neutrophil Polarization and Therapeutic Implications. Int J Mol Sci. 2022;23:3218.
Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–94.
Giese MA, Hind LE, Huttenlocher A. Neutrophil plasticity in the tumor microenvironment. Blood. 2019;133:2159–67.
Hajizadeh F, Aghebati Maleki L, Alexander M, Mikhailova MV, Masjedi A, Ahmadpour M, et al. Tumor-associated neutrophils as new players in immunosuppressive process of the tumor microenvironment in breast cancer. Life Sci. 2021;264:118699.
Zhou Z, Wang P, Sun R, Li J, Hu Z, Xin H, et al. Tumor-associated neutrophils and macrophages interaction contributes to intrahepatic cholangiocarcinoma progression by activating STAT3. J Immunother Cancer. 2021;9:e001946.
Wu L, Saxena S, Awaji M, Singh RK. Tumor-Associated Neutrophils in Cancer: Going Pro. Cancers (Basel). 2019;11:564.
Masucci MT, Minopoli M, Carriero MV. Tumor Associated Neutrophils. Their Role in Tumorigenesis, Metastasis, Prognosis and Therapy. Front Oncol. 2019;9:1146.
Fridlender ZG, Albelda SM. Tumor-associated neutrophils: friend or foe? Carcinogenesis. 2012;33:949–55.
Shaul ME, Fridlender ZG. Neutrophils as active regulators of the immune system in the tumor microenvironment. J Leukoc Biol. 2017;102:343–9.
Cupp MA, Cariolou M, Tzoulaki I, Aune D, Evangelou E, Berlanga-Taylor AJ. Neutrophil to lymphocyte ratio and cancer prognosis: an umbrella review of systematic reviews and meta-analyses of observational studies. BMC Med. 2020;18:360.
Templeton AJ, McNamara MG, Šeruga B, Vera-Badillo FE, Aneja P, Ocaña A, et al. Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst. 2014;106:dju124.
Diem S, Schmid S, Krapf M, Flatz L, Born D, Jochum W, et al. Neutrophil-to-Lymphocyte ratio (NLR) and Platelet-to-Lymphocyte ratio (PLR) as prognostic markers in patients with non-small cell lung cancer (NSCLC) treated with nivolumab. Lung Cancer. 2017;111:176–81.
Lin N, Li J, Yao X, Zhang X, Liu G, Zhang Z, et al. Prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer liver metastasis: A meta-analysis of results from multivariate analysis. Int J Surg. 2022;107:106959.
Cadet J, Wagner JR. DNA base damage by reactive oxygen species, oxidizing agents, and UV radiation. Cold Spring Harb Perspect Biol. 2013;5:a012559.
Cooke MS, Evans MD, Dizdaroglu M, Lunec J. Oxidative DNA damage: mechanisms, mutation, and disease. Faseb j. 2003;17:1195–214.
Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–96.
Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, et al. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–35.
Parekh A, Das S, Parida S, Das CK, Dutta D, Mallick SK, et al. Multi-nucleated cells use ROS to induce breast cancer chemo-resistance in vitro and in vivo. Oncogene. 2018;37:4546–61.
Aoyagi Y, Oda T, Kinoshita T, Nakahashi C, Hasebe T, Ohkohchi N, et al. Overexpression of TGF-beta by infiltrated granulocytes correlates with the expression of collagen mRNA in pancreatic cancer. Br J Cancer. 2004;91:1316–26.
Queen MM, Ryan RE, Holzer RG, Keller-Peck CR, Jorcyk CL. Breast cancer cells stimulate neutrophils to produce oncostatin M: potential implications for tumor progression. Cancer Res. 2005;65:8896–904.
Wislez M, Rabbe N, Marchal J, Milleron B, Crestani B, Mayaud C, et al. Hepatocyte growth factor production by neutrophils infiltrating bronchioloalveolar subtype pulmonary adenocarcinoma: role in tumor progression and death. Cancer Res. 2003;63:1405–12.
Dumitru CA, Fechner MK, Hoffmann TK, Lang S, Brandau S. A novel p38-MAPK signaling axis modulates neutrophil biology in head and neck cancer. J Leukoc Biol. 2012;91:591–8.
Zhong C, Qu X, Tan M, Meng YG, Ferrara N. Characterization and regulation of bv8 in human blood cells. Clin Cancer Res. 2009;15:2675–84.
Galdiero MR, Varricchi G, Loffredo S, Bellevicine C, Lansione T, Ferrara AL, et al. Potential involvement of neutrophils in human thyroid cancer. PLoS ONE. 2018;13:e0199740.
Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, et al. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. 2012;130:1109–19.
Felix K, Gaida MM. Neutrophil-Derived Proteases in the Microenvironment of Pancreatic Cancer -Active Players in Tumor Progression. Int J Biol Sci. 2016;12:302–13.
Yui S, Osawa Y, Ichisugi T, Morimoto-Kamata R. Neutrophil cathepsin G, but not elastase, induces aggregation of MCF-7 mammary carcinoma cells by a protease activity-dependent cell-oriented mechanism. Mediators Inflamm. 2014;2014:971409.
Yang R, Zhong L, Yang XQ, Jiang KL, Li L, Song H, et al. Neutrophil elastase enhances the proliferation and decreases apoptosis of leukemia cells via activation of PI3K/Akt signaling. Mol Med Rep. 2016;13:4175–82.
Gregory AD, Houghton AM. Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res. 2011;71:2411–6.
Cools-Lartigue J, Spicer J, McDonald B, Gowing S, Chow S, Giannias B, et al. Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J Clin Invest. 2013;123:3446–58.
Najmeh S, Cools-Lartigue J, Rayes RF, Gowing S, Vourtzoumis P, Bourdeau F, et al. Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int J Cancer. 2017;140:2321–30.
Tohme S, Yazdani HO, Al-Khafaji AB, Chidi AP, Loughran P, Mowen K, et al. Neutrophil Extracellular Traps Promote the Development and Progression of Liver Metastases after Surgical Stress. Cancer Res. 2016;76:1367–80.
Yang LY, Luo Q, Lu L, Zhu WW, Sun HT, Wei R, et al. Increased neutrophil extracellular traps promote metastasis potential of hepatocellular carcinoma via provoking tumorous inflammatory response. J Hematol Oncol. 2020;13:3.
Chen Y, Hu H, Tan S, Dong Q, Fan X, Wang Y, et al. The role of neutrophil extracellular traps in cancer progression, metastasis and therapy. Exp Hematol Oncol. 2022;11:99.
Teijeira A, Garasa S, Ochoa MC, Villalba M, Olivera I, Cirella A, et al. IL8, Neutrophils, and NETs in a Collusion against Cancer Immunity and Immunotherapy. Clin Cancer Res. 2021;27:2383–93.
Teijeira Á, Garasa S, Gato M, Alfaro C, Migueliz I, Cirella A, et al. CXCR1 and CXCR2 Chemokine Receptor Agonists Produced by Tumors Induce Neutrophil Extracellular Traps that Interfere with Immune Cytotoxicity. Immunity. 2020;52:856-71.e8.
Zhang Y, Chandra V, Riquelme Sanchez E, Dutta P, Quesada PR, Rakoski A, et al. Interleukin-17-induced neutrophil extracellular traps mediate resistance to checkpoint blockade in pancreatic cancer. J Exp Med. 2020;217:e20190354.
Gershkovitz M, Fainsod-Levi T, Zelter T, Sionov RV, Granot Z. TRPM2 modulates neutrophil attraction to murine tumor cells by regulating CXCL2 expression. Cancer Immunol Immunother. 2019;68:33–43.
Finisguerra V, Di Conza G, Di Matteo M, Serneels J, Costa S, Thompson AA, et al. MET is required for the recruitment of anti-tumoural neutrophils. Nature. 2015;522:349–53.
Sun B, Qin W, Song M, Liu L, Yu Y, Qi X, et al. Neutrophil Suppresses Tumor Cell Proliferation via Fas /Fas Ligand Pathway Mediated Cell Cycle Arrested. Int J Biol Sci. 2018;14:2103–13.
Hagerling C, Gonzalez H, Salari K, Wang CY, Lin C, Robles I, et al. Immune effector monocyte-neutrophil cooperation induced by the primary tumor prevents metastatic progression of breast cancer. Proc Natl Acad Sci U S A. 2019;116:21704–14.
Vono M, Lin A, Norrby-Teglund A, Koup RA, Liang F, Loré K. Neutrophils acquire the capacity for antigen presentation to memory CD4(+) T cells in vitro and ex vivo. Blood. 2017;129:1991–2001.
Hubert P, Heitzmann A, Viel S, Nicolas A, Sastre-Garau X, Oppezzo P, et al. Antibody-dependent cell cytotoxicity synapses form in mice during tumor-specific antibody immunotherapy. Cancer Res. 2011;71:5134–43.
Tillack K, Breiden P, Martin R, Sospedra M. T lymphocyte priming by neutrophil extracellular traps links innate and adaptive immune responses. J Immunol. 2012;188:3150–9.
Li Y, He Y, Butler W, Xu L, Chang Y, Lei K, et al. Targeting cellular heterogeneity with CXCR2 blockade for the treatment of therapy-resistant prostate cancer. Sci Transl Med. 2019;11:eaax0428.
Liu Q, Li A, Tian Y, Wu JD, Liu Y, Li T, et al. The CXCL8-CXCR1/2 pathways in cancer. Cytokine Growth Factor Rev. 2016;31:61–71.
Faget J, Peters S, Quantin X, Meylan E, Bonnefoy N. Neutrophils in the era of immune checkpoint blockade. J Immunother Cancer. 2021;9:e002242.
Nie M, Yang L, Bi X, Wang Y, Sun P, Yang H, et al. Neutrophil Extracellular Traps Induced by IL8 Promote Diffuse Large B-cell Lymphoma Progression via the TLR9 Signaling. Clin Cancer Res. 2019;25:1867–79.
Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, et al. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. 2018;67:1112–23.
Gong L, Cumpian AM, Caetano MS, Ochoa CE, De la Garza MM, Lapid DJ, et al. Promoting effect of neutrophils on lung tumorigenesis is mediated by CXCR2 and neutrophil elastase. Mol Cancer. 2013;12:154.
Holz O, Khalilieh S, Ludwig-Sengpiel A, Watz H, Stryszak P, Soni P, et al. SCH527123, a novel CXCR2 antagonist, inhibits ozone-induced neutrophilia in healthy subjects. Eur Respir J. 2010;35:564–70.
Rennard SI, Dale DC, Donohue JF, Kanniess F, Magnussen H, Sutherland ER, et al. CXCR2 Antagonist MK-7123. A Phase 2 Proof-of-Concept Trial for Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2015;191:1001–11.
Ocana A, Nieto-Jiménez C, Pandiella A, Templeton AJ. Neutrophils in cancer: prognostic role and therapeutic strategies. Mol Cancer. 2017;16:137.
Yang J, Kumar A, Vilgelm AE, Chen SC, Ayers GD, Novitskiy SV, et al. Loss of CXCR4 in Myeloid Cells Enhances Antitumor Immunity and Reduces Melanoma Growth through NK Cell and FASL Mechanisms. Cancer Immunol Res. 2018;6:1186–98.
Bockorny B, Semenisty V, Macarulla T, Borazanci E, Wolpin BM, Stemmer SM, et al. BL-8040, a CXCR4 antagonist, in combination with pembrolizumab and chemotherapy for pancreatic cancer: the COMBAT trial. Nat Med. 2020;26:878–85.
Nielsen SR, Strøbech JE, Horton ER, Jackstadt R, Laitala A, Bravo MC, et al. Suppression of tumor-associated neutrophils by lorlatinib attenuates pancreatic cancer growth and improves treatment with immune checkpoint blockade. Nat Commun. 2021;12:3414.
Raccosta L, Fontana R, Maggioni D, Lanterna C, Villablanca EJ, Paniccia A, et al. The oxysterol-CXCR2 axis plays a key role in the recruitment of tumor-promoting neutrophils. J Exp Med. 2013;210:1711–28.
Wu L, Awaji M, Saxena S, Varney ML, Sharma B, Singh RK. IL-17-CXC Chemokine Receptor 2 Axis Facilitates Breast Cancer Progression by Up-Regulating Neutrophil Recruitment. Am J Pathol. 2020;190:222–33.
Corrales L, Ajona D, Rafail S, Lasarte JJ, Riezu-Boj JI, Lambris JD, et al. Anaphylatoxin C5a creates a favorable microenvironment for lung cancer progression. J Immunol. 2012;189:4674–83.
Tie Y, Tang F, Wei YQ, Wei XW. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J Hematol Oncol. 2022;15:61.
Wang TT, Zhao YL, Peng LS, Chen N, Chen W, Lv YP, et al. Tumour-activated neutrophils in gastric cancer foster immune suppression and disease progression through GM-CSF-PD-L1 pathway. Gut. 2017;66:1900–11.
Deng H, Kan A, Lyu N, He M, Huang X, Qiao S, et al. Tumor-derived lactate inhibit the efficacy of lenvatinib through regulating PD-L1 expression on neutrophil in hepatocellular carcinoma. J Immunother Cancer. 2021;9:e002305.
Yajuk O, Baron M, Toker S, Zelter T, Fainsod-Levi T, Granot Z. The PD-L1/PD-1 Axis Blocks Neutrophil Cytotoxicity in Cancer. Cells. 2021;10:1510.
Ring NG, Herndler-Brandstetter D, Weiskopf K, Shan L, Volkmer JP, George BM, et al. Anti-SIRPα antibody immunotherapy enhances neutrophil and macrophage antitumor activity. Proc Natl Acad Sci U S A. 2017;114:E10578–85.
Treffers LW, Ten Broeke T, Rösner T, Jansen JHM, van Houdt M, Kahle S, et al. IgA-Mediated Killing of Tumor Cells by Neutrophils Is Enhanced by CD47-SIRPα Checkpoint Inhibition. Cancer Immunol Res. 2020;8:120–30.
Matlung HL, Babes L, Zhao XW, van Houdt M, Treffers LW, van Rees DJ, et al. Neutrophils Kill Antibody-Opsonized Cancer Cells by Trogoptosis. Cell Rep. 2018;23:3946-59.e6.
Mizutani T, Ano T, Yoshioka Y, Mizuta S, Takemoto K, Ouchi Y, et al. Neutrophil S100A9 supports M2 macrophage niche formation in granulomas. iScience. 2023;26:106081.
Wang SJ, Khullar K, Kim S, Yegya-Raman N, Malhotra J, Groisberg R, et al. Effect of cyclo-oxygenase inhibitor use during checkpoint blockade immunotherapy in patients with metastatic melanoma and non-small cell lung cancer. J Immunother Cancer. 2020;8:e000889.
Liu S, Ren J, Ten Dijke P. Targeting TGFβ signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6:8.
Li T, Wang X, Niu M, Wang M, Zhou J, Wu K, et al. Bispecific antibody targeting TGF-β and PD-L1 for synergistic cancer immunotherapy. Front Immunol. 2023;14:1196970.
Strauss J, Heery CR, Schlom J, Madan RA, Cao L, Kang Z, et al. Phase I Trial of M7824 (MSB0011359C), a Bifunctional Fusion Protein Targeting PD-L1 and TGFβ, in Advanced Solid Tumors. Clin Cancer Res. 2018;24:1287–95.
Paz-Ares L, Kim TM, Vicente D, Felip E, Lee DH, Lee KH, et al. Bintrafusp Alfa, a Bifunctional Fusion Protein Targeting TGF-β and PD-L1, in Second-Line Treatment of Patients With NSCLC: Results From an Expansion Cohort of a Phase 1 Trial. J Thorac Oncol. 2020;15:1210–22.
Yi M, Wu Y, Niu M, Zhu S, Zhang J, Yan Y, et al. Anti-TGF-β/PD-L1 bispecific antibody promotes T cell infiltration and exhibits enhanced antitumor activity in triple-negative breast cancer. J Immunother Cancer. 2022;10:e005543.
Pylaeva E, Harati MD, Spyra I, Bordbari S, Strachan S, Thakur BK, et al. NAMPT signaling is critical for the proangiogenic activity of tumor-associated neutrophils. Int J Cancer. 2019;144:136–49.
Chu D, Dong X, Shi X, Zhang C, Wang Z. Neutrophil-Based Drug Delivery Systems. Adv Mater. 2018;30:e1706245.
Hosseinalizadeh H, Mahmoodpour M, Razaghi Bahabadi Z, Hamblin MR, Mirzaei H. Neutrophil mediated drug delivery for targeted glioblastoma therapy: A comprehensive review. Biomed Pharmacother. 2022;156:113841.
Xue J, Zhao Z, Zhang L, Xue L, Shen S, Wen Y, et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat Nanotechnol. 2017;12:692–700.
Chang Y, Cai X, Syahirah R, Yao Y, Xu Y, Jin G, et al. CAR-neutrophil mediated delivery of tumor-microenvironment responsive nanodrugs for glioblastoma chemo-immunotherapy. Nat Commun. 2023;14:2266.
Chusid MJ. Eosinophils: Friends or Foes? J Allergy Clin Immunol Pract. 2018;6:1439–44.
Valent P, Degenfeld-Schonburg L, Sadovnik I, Horny HP, Arock M, Simon HU, et al. Eosinophils and eosinophil-associated disorders: immunological, clinical, and molecular complexity. Semin Immunopathol. 2021;43:423–38.
Reichman H, Karo-Atar D, Munitz A. Emerging Roles for Eosinophils in the Tumor Microenvironment. Trends Cancer. 2016;2:664–75.
Sakkal S, Miller S, Apostolopoulos V, Nurgali K. Eosinophils in Cancer: Favourable or Unfavourable? Curr Med Chem. 2016;23:650–66.
Goldsmith MM, Belchis DA, Cresson DH, Merritt WD 3rd, Askin FB. The importance of the eosinophil in head and neck cancer. Otolaryngol Head Neck Surg. 1992;106:27–33.
Pretlow TP, Keith EF, Cryar AK, Bartolucci AA, Pitts AM, Pretlow TG 2nd, et al. Eosinophil infiltration of human colonic carcinomas as a prognostic indicator. Cancer Res. 1983;43:2997–3000.
von Wasielewski R, Seth S, Franklin J, Fischer R, Hübner K, Hansmann ML, et al. Tissue eosinophilia correlates strongly with poor prognosis in nodular sclerosing Hodgkin’s disease, allowing for known prognostic factors. Blood. 2000;95:1207–13.
Gatault S, Legrand F, Delbeke M, Loiseau S, Capron M. Involvement of eosinophils in the anti-tumor response. Cancer Immunol Immunother. 2012;61:1527–34.
Davis BP, Rothenberg ME. Eosinophils and cancer. Cancer. Immunol Res. 2014;2:1–8.
Lee JJ, Jacobsen EA, McGarry MP, Schleimer RP, Lee NA. Eosinophils in health and disease: the LIAR hypothesis. Clin Exp Allergy. 2010;40:563–75.
Rosenberg HF, Dyer KD, Foster PS. Eosinophils: changing perspectives in health and disease. Nat Rev Immunol. 2013;13:9–22.
Ellyard JI, Simson L, Bezos A, Johnston K, Freeman C, Parish CR. Eotaxin selectively binds heparin. An interaction that protects eotaxin from proteolysis and potentiates chemotactic activity in vivo. J Biol Chem. 2007;282:15238–47.
Simson L, Ellyard JI, Dent LA, Matthaei KI, Rothenberg ME, Foster PS, et al. Regulation of carcinogenesis by IL-5 and CCL11: a potential role for eosinophils in tumor immune surveillance. J Immunol. 2007;178:4222–9.
Cormier SA, Taranova AG, Bedient C, Nguyen T, Protheroe C, Pero R, et al. Pivotal Advance: eosinophil infiltration of solid tumors is an early and persistent inflammatory host response. J Leukoc Biol. 2006;79:1131–9.
Gleich GJ. Mechanisms of eosinophil-associated inflammation. J Allergy Clin Immunol. 2000;105:651–63.
Legrand F, Driss V, Delbeke M, Loiseau S, Hermann E, Dombrowicz D, et al. Human eosinophils exert TNF-α and granzyme A-mediated tumoricidal activity toward colon carcinoma cells. J Immunol. 2010;185:7443–51.
Gatault S, Delbeke M, Driss V, Sarazin A, Dendooven A, Kahn JE, et al. IL-18 Is Involved in Eosinophil-Mediated Tumoricidal Activity against a Colon Carcinoma Cell Line by Upregulating LFA-1 and ICAM-1. J Immunol. 2015;195:2483–92.
Munitz A, Bachelet I, Fraenkel S, Katz G, Mandelboim O, Simon HU, et al. 2B4 (CD244) is expressed and functional on human eosinophils. J Immunol. 2005;174:110–8.
Kataoka S, Konishi Y, Nishio Y, Fujikawa-Adachi K, Tominaga A. Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol. 2004;23:549–60.
Furbert-Harris PM, Parish-Gause D, Hunter KA, Vaughn TR, Howland C, Okomo-Awich J, et al. Activated eosinophils upregulate the metastasis suppressor molecule E-cadherin on prostate tumor cells. Cell Mol Biol (Noisy-le-grand). 2003;49:1009–16.
Carretero R, Sektioglu IM, Garbi N, Salgado OC, Beckhove P, Hämmerling GJ. Eosinophils orchestrate cancer rejection by normalizing tumor vessels and enhancing infiltration of CD8(+) T cells. Nat Immunol. 2015;16:609–17.
Grisaru-Tal S, Itan M, Klion AD, Munitz A. A new dawn for eosinophils in the tumour microenvironment. Nat Rev Cancer. 2020;20:594–607.
Zaynagetdinov R, Sherrill TP, Gleaves LA, McLoed AG, Saxon JA, Habermann AC, et al. Interleukin-5 facilitates lung metastasis by modulating the immune microenvironment. Cancer Res. 2015;75:1624–34.
Odemuyiwa SO, Ghahary A, Li Y, Puttagunta L, Lee JE, Musat-Marcu S, et al. Cutting edge: human eosinophils regulate T cell subset selection through indoleamine 2,3-dioxygenase. J Immunol. 2004;173:5909–13.
Xie F, Liu LB, Shang WQ, Chang KK, Meng YH, Mei J, et al. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 2015;364:106–17.
Kratochvill F, Neale G, Haverkamp JM, Van de Velde LA, Smith AM, Kawauchi D, et al. TNF Counterbalances the Emergence of M2 Tumor Macrophages. Cell Rep. 2015;12:1902–14.
Zhang B, Wei CY, Chang KK, Yu JJ, Zhou WJ, Yang HL, et al. TSLP promotes angiogenesis of human umbilical vein endothelial cells by strengthening the crosstalk between cervical cancer cells and eosinophils. Oncol Lett. 2017;14:7483–8.
Rothenberg ME, Hogan SP. The eosinophil. Annu Rev Immunol. 2006;24:147–74.
Yasukawa A, Hosoki K, Toda M, Miyake Y, Matsushima Y, Matsumoto T, et al. Eosinophils promote epithelial to mesenchymal transition of bronchial epithelial cells. PLoS ONE. 2013;8:e64281.
da Silva JM, Moreira Dos Santos TP, Sobral LM, Queiroz-Junior CM, Rachid MA, Proudfoot AEI, et al. Relevance of CCL3/CCR5 axis in oral carcinogenesis. Oncotarget. 2017;8:51024–36.
Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, et al. Baseline Peripheral Blood Biomarkers Associated with Clinical Outcome of Advanced Melanoma Patients Treated with Ipilimumab. Clin Cancer Res. 2016;22:2908–18.
Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al. Myeloid Cells and Related Chronic Inflammatory Factors as Novel Predictive Markers in Melanoma Treatment with Ipilimumab. Clin Cancer Res. 2015;21:5453–9.
Lang BM, Peveling-Oberhag A, Faidt D, Hötker AM, Weyer-Elberich V, Grabbe S, et al. Long-term survival with modern therapeutic agents against metastatic melanoma-vemurafenib and ipilimumab in a daily life setting. Med Oncol. 2018;35:24.
Moreira A, Leisgang W, Schuler G, Heinzerling L. Eosinophilic count as a biomarker for prognosis of melanoma patients and its importance in the response to immunotherapy. Immunotherapy. 2017;9:115–21.
Hude I, Sasse S, Bröckelmann PJ, von Tresckow B, Momotow J, Engert A, et al. Leucocyte and eosinophil counts predict progression-free survival in relapsed or refractory classical Hodgkin Lymphoma patients treated with PD1 inhibition. Br J Haematol. 2018;181:837–40.
Weide B, Martens A, Hassel JC, Berking C, Postow MA, Bisschop K, et al. Baseline Biomarkers for Outcome of Melanoma Patients Treated with Pembrolizumab. Clin Cancer Res. 2016;22:5487–96.
Rafei-Shamsabadi D, Lehr S, von Bubnoff D, Meiss F. Successful combination therapy of systemic checkpoint inhibitors and intralesional interleukin-2 in patients with metastatic melanoma with primary therapeutic resistance to checkpoint inhibitors alone. Cancer Immunol Immunother. 2019;68:1417–28.
Blomberg OS, Spagnuolo L, Garner H, Voorwerk L, Isaeva OI, van Dyk E, et al. IL-5-producing CD4(+) T cells and eosinophils cooperate to enhance response to immune checkpoint blockade in breast cancer. Cancer Cell. 2023;41:106-23.e10.
Grisaru-Tal S, Munitz A. T cell-eosinophil crosstalk-A new road for effective immune checkpoint blockade in breast cancer? Cancer Cell. 2023;41:9–11.
Grisaru-Tal S, Rothenberg ME, Munitz A. Eosinophil-lymphocyte interactions in the tumor microenvironment and cancer immunotherapy. Nat Immunol. 2022;23:1309–16.
Marone G, Borriello F, Varricchi G, Genovese A, Granata F. Basophils: historical reflections and perspectives. Chem Immunol Allergy. 2014;100:172–92.
Varricchi G, Raap U, Rivellese F, Marone G, Gibbs BF. Human mast cells and basophils-How are they similar how are they different? Immunol Rev. 2018;282:8–34.
Galli SJ, Tsai M, Marichal T, Tchougounova E, Reber LL, Pejler G. Approaches for analyzing the roles of mast cells and their proteases in vivo. Adv Immunol. 2015;126:45–127.
Metcalfe DD, Pawankar R, Ackerman SJ, Akin C, Clayton F, Falcone FH, et al. Biomarkers of the involvement of mast cells, basophils and eosinophils in asthma and allergic diseases. World Allergy Organ J. 2016;9:7.
Rigoni A, Colombo MP, Pucillo C. Mast cells, basophils and eosinophils: From allergy to cancer. Semin Immunol. 2018;35:29–34.
Chauhan J, Stavraka C, Grandits M, Palhares L, Josephs DH, Lacy KE, et al. Clinical and Translational Significance of Basophils in Patients with Cancer. Cells. 2022;11:438.
Hiltbrunner S, Spohn ML, Wechsler R, Akhoundova D, Bankel L, Kasser S, et al. Comprehensive Statistical Exploration of Prognostic (Bio-)Markers for Responses to Immune Checkpoint Inhibitor in Patients with Non-Small Cell Lung Cancer. Cancers (Basel). 2021;14:75.
Wagner NB, Luttermann F, Gassenmaier M, Forschner A, Leiter U, Garbe C, et al. Absolute and relative differential blood count predicts survival of AJCC stage I-II melanoma patients scheduled for sentinel lymph node biopsy. Australas J Dermatol. 2020;61:e310–8.
Rosner S, Kwong E, Shoushtari AN, Friedman CF, Betof AS, Brady MS, et al. Peripheral blood clinical laboratory variables associated with outcomes following combination nivolumab and ipilimumab immunotherapy in melanoma. Cancer Med. 2018;7:690–7.
Bax HJ, Chauhan J, Stavraka C, Khiabany A, Nakamura M, Pellizzari G, et al. Basophils from Cancer Patients Respond to Immune Stimuli and Predict Clinical Outcome. Cells. 2020;9:1631.
Zheng L, Yu M, Zhang S. Prognostic value of pretreatment circulating basophils in patients with glioblastoma. Neurosurg Rev. 2021;44:3471–8.
Liu Q, Luo D, Cai S, Li Q, Li X. Circulating basophil count as a prognostic marker of tumor aggressiveness and survival outcomes in colorectal cancer. Clin Transl Med. 2020;9:6.
Wei Y, Zhang X, Wang G, Zhou Y, Luo M, Wang S, et al. The impacts of pretreatment circulating eosinophils and basophils on prognosis of stage I-III colorectal cancer. Asia Pac J Clin Oncol. 2018;14:e243–51.
Wu J, Ge XX, Zhu W, Zhi Q, Xu MD, Duan W, et al. Values of applying white blood cell counts in the prognostic evaluation of resectable colorectal cancer. Mol Med Rep. 2019;19:2330–40.
Zhang J, Yin H, Chen Q, Zhao G, Lou W, Wu W, et al. Basophils as a potential therapeutic target in cancer. J Zhejiang Univ Sci B. 2021;22:971–84.
Detoraki A, Staiano RI, Granata F, Giannattasio G, Prevete N, de Paulis A, et al. Vascular endothelial growth factors synthesized by human lung mast cells exert angiogenic effects. J Allergy Clin Immunol. 2009;123(1142–9):9.e1-5.
Varricchi G, Poto R, Marone G, Schroeder JT. IL-3 in the development and function of basophils. Semin Immunol. 2021;54:101510.
Sektioglu IM, Carretero R, Bulbuc N, Bald T, Tüting T, Rudensky AY, et al. Basophils Promote Tumor Rejection via Chemotaxis and Infiltration of CD8+ T Cells. Cancer Res. 2017;77:291–302.
Marone G, Gambardella AR, Mattei F, Mancini J, Schiavoni G, Varricchi G. Basophils in Tumor Microenvironment and Surroundings. Adv Exp Med Biol. 2020;1224:21–34.
Poto R, Gambardella AR, Marone G, Schroeder JT, Mattei F, Schiavoni G, et al. Basophils from allergy to cancer. Front Immunol. 2022;13:1056838.
Curti BD, Koguchi Y, Leidner RS, Rolig AS, Sturgill ER, Sun Z, et al. Enhancing clinical and immunological effects of anti-PD-1 with belapectin, a galectin-3 inhibitor. J Immunother Cancer. 2021;9:e002371.
Schroeder JT, Bieneman AP. Activation of Human Basophils by A549 Lung Epithelial Cells Reveals a Novel IgE-Dependent Response Independent of Allergen. J Immunol. 2017;199:855–65.
Schroeder JT, Adeosun AA, Do D, Bieneman AP. Galectin-3 is essential for IgE-dependent activation of human basophils by A549 lung epithelial cells. J Allergy Clin Immunol. 2019;144:312-5.e1.
Egawa M, Mukai K, Yoshikawa S, Iki M, Mukaida N, Kawano Y, et al. Inflammatory monocytes recruited to allergic skin acquire an anti-inflammatory M2 phenotype via basophil-derived interleukin-4. Immunity. 2013;38:570–80.
De Monte L, Wörmann S, Brunetto E, Heltai S, Magliacane G, Reni M, et al. Basophil Recruitment into Tumor-Draining Lymph Nodes Correlates with Th2 Inflammation and Reduced Survival in Pancreatic Cancer Patients. Cancer Res. 2016;76:1792–803.
de Paulis A, Prevete N, Fiorentino I, Rossi FW, Staibano S, Montuori N, et al. Expression and functions of the vascular endothelial growth factors and their receptors in human basophils. J Immunol. 2006;177:7322–31.
Baran J, Sobiepanek A, Mazurkiewicz-Pisarek A, Rogalska M, Gryciuk A, Kuryk L, et al. Mast Cells as a Target-A Comprehensive Review of Recent Therapeutic Approaches. Cells. 2023;12:1187.
Dawicki W, Marshall JS. New and emerging roles for mast cells in host defence. Curr Opin Immunol. 2007;19:31–8.
Weller CL, Collington SJ, Williams T, Lamb JR. Mast cells in health and disease. Clin Sci (Lond). 2011;120:473–84.
Abdel-Majid RM, Marshall JS. Prostaglandin E2 induces degranulation-independent production of vascular endothelial growth factor by human mast cells. J Immunol. 2004;172:1227–36.
Melillo RM, Guarino V, Avilla E, Galdiero MR, Liotti F, Prevete N, et al. Mast cells have a protumorigenic role in human thyroid cancer. Oncogene. 2010;29:6203–15.
Nakae S, Suto H, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cells enhance T cell activation: Importance of mast cell-derived TNF. Proc Natl Acad Sci U S A. 2005;102:6467–72.
Prevete N, Staiano RI, Granata F, Detoraki A, Necchi V, Ricci V, et al. Expression and function of Angiopoietins and their tie receptors in human basophils and mast cells. J Biol Regul Homeost Agents. 2013;27:827–39.
Ribatti D, Vacca A, Marzullo A, Nico B, Ria R, Roncali L, et al. Angiogenesis and mast cell density with tryptase activity increase simultaneously with pathological progression in B-cell non-Hodgkin’s lymphomas. Int J Cancer. 2000;85:171–5.
Derakhshani A, Vahidian F, Alihasanzadeh M, Mokhtarzadeh A, Lotfi Nezhad P, Baradaran B. Mast cells: A double-edged sword in cancer. Immunol Lett. 2019;209:28–35.
Segura-Villalobos D, Ramírez-Moreno IG, Martínez-Aguilar M, Ibarra-Sánchez A, Muñoz-Bello JO, Anaya-Rubio I, et al. Mast Cell-Tumor Interactions: Molecular Mechanisms of Recruitment, Intratumoral Communication and Potential Therapeutic Targets for Tumor Growth. Cells. 2022;11:349.
Kolset SO, Pejler G. Serglycin: a structural and functional chameleon with wide impact on immune cells. J Immunol. 2011;187:4927–33.
Beer TW, Ng LB, Murray K. Mast cells have prognostic value in Merkel cell carcinoma. Am J Dermatopathol. 2008;30:27–30.
Maccarana M, Jia J, Li H, Zhang X, Vlodavsky I, Li JP. Implications of Heparanase on Heparin Synthesis and Metabolism in Mast Cells. Int J Mol Sci. 2022;23:4821.
Vizio B, Biasi F, Scirelli T, Novarino A, Prati A, Ciuffreda L, et al. Pancreatic-carcinoma-cell-derived pro-angiogenic factors can induce endothelial-cell differentiation of a subset of circulating CD34+ progenitors. J Transl Med. 2013;11:314.
Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature. 2011;473:298–307.
Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Marone G, et al. Are Mast Cells MASTers in Cancer? Front Immunol. 2017;8:424.
Taipale J, Lohi J, Saarinen J, Kovanen PT, Keski-Oja J. Human mast cell chymase and leukocyte elastase release latent transforming growth factor-beta 1 from the extracellular matrix of cultured human epithelial and endothelial cells. J Biol Chem. 1995;270:4689–96.
Yang Z, Zhang B, Li D, Lv M, Huang C, Shen GX, et al. Mast cells mobilize myeloid-derived suppressor cells and Treg cells in tumor microenvironment via IL-17 pathway in murine hepatocarcinoma model. PLoS ONE. 2010;5:e8922.
Oldford SA, Marshall JS. Mast cells as targets for immunotherapy of solid tumors. Mol Immunol. 2015;63:113–24.
Sobiepanek A, Kuryk Ł, Garofalo M, Kumar S, Baran J, Musolf P, et al. The Multifaceted Roles of Mast Cells in Immune Homeostasis, Infections and Cancers. Int J Mol Sci. 2022;23:2249.
Oldford SA, Haidl ID, Howatt MA, Leiva CA, Johnston B, Marshall JS. A critical role for mast cells and mast cell-derived IL-6 in TLR2-mediated inhibition of tumor growth. J Immunol. 2010;185:7067–76.
Ott VL, Cambier JC, Kappler J, Marrack P, Swanson BJ. Mast cell-dependent migration of effector CD8+ T cells through production of leukotriene B4. Nat Immunol. 2003;4:974–81.
Salamon P, Shoham NG, Gavrieli R, Wolach B, Mekori YA. Human mast cells release Interleukin-8 and induce neutrophil chemotaxis on contact with activated T cells. Allergy. 2005;60:1316–9.
Burke SM, Issekutz TB, Mohan K, Lee PW, Shmulevitz M, Marshall JS. Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism. Blood. 2008;111:5467–76.
Groot Kormelink T, Abudukelimu A, Redegeld FA. Mast cells as target in cancer therapy. Curr Pharm Des. 2009;15:1868–78.
Rao Q, Chen Y, Yeh CR, Ding J, Li L, Chang C, et al. Recruited mast cells in the tumor microenvironment enhance bladder cancer metastasis via modulation of ERβ/CCL2/CCR2 EMT/MMP9 signals. Oncotarget. 2016;7:7842–55.
Sarchio SNE, Scolyer RA, Beaugie C, McDonald D, Marsh-Wakefield F, Halliday GM, et al. Pharmacologically antagonizing the CXCR4-CXCL12 chemokine pathway with AMD3100 inhibits sunlight-induced skin cancer. J Invest Dermatol. 2014;134:1091–100.
Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer Prev Res (Phila). 2010;3:1314–33.
Takeuchi K, Koike K, Kamijo T, Ishida S, Nakazawa Y, Kurokawa Y, et al. STI571 inhibits growth and adhesion of human mast cells in culture. J Leukoc Biol. 2003;74:1026–34.
Stolk D, van der Vliet HJ, de Gruijl TD, van Kooyk Y, Exley MA. Positive & Negative Roles of Innate Effector Cells in Controlling Cancer Progression. Front Immunol. 2018;9:1990.
Cogswell DT, Gapin L, Tobin HM, McCarter MD, Tobin RP. MAIT Cells: Partners or Enemies in Cancer Immunotherapy? Cancers (Basel). 2021;13:1502.
Bayatipoor H, Mehdizadeh S, Jafarpour R, Shojaei Z, Pashangzadeh S, Motallebnezhad M. Role of NKT cells in cancer immunotherapy-from bench to bed. Med Oncol. 2022;40:29.
Kabelitz D, Serrano R, Kouakanou L, Peters C, Kalyan S. Cancer immunotherapy with γδ T cells: many paths ahead of us. Cell Mol Immunol. 2020;17:925–39.
Godfrey DI, Le Nours J, Andrews DM, Uldrich AP, Rossjohn J. Unconventional T Cell Targets for Cancer Immunotherapy. Immunity. 2018;48:453–73.
Mensurado S, Blanco-Domínguez R, Silva-Santos B. The emerging roles of γδ T cells in cancer immunotherapy. Nat Rev Clin Oncol. 2023;20:178–91.
Viey E, Fromont G, Escudier B, Morel Y, Da Rocha S, Chouaib S, et al. Phosphostim-activated gamma delta T cells kill autologous metastatic renal cell carcinoma. J Immunol. 2005;174:1338–47.
Mattarollo SR, Kenna T, Nieda M, Nicol AJ. Chemotherapy and zoledronate sensitize solid tumour cells to Vgamma9Vdelta2 T cell cytotoxicity. Cancer Immunol Immunother. 2007;56:1285–97.
D’Asaro M, La Mendola C, Di Liberto D, Orlando V, Todaro M, Spina M, et al. V gamma 9V delta 2 T lymphocytes efficiently recognize and kill zoledronate-sensitized, imatinib-sensitive, and imatinib-resistant chronic myelogenous leukemia cells. J Immunol. 2010;184:3260–8.
Gao Y, Yang W, Pan M, Scully E, Girardi M, Augenlicht LH, et al. Gamma delta T cells provide an early source of interferon gamma in tumor immunity. J Exp Med. 2003;198:433–42.
Rampoldi F, Ullrich L, Prinz I. Revisiting the Interaction of γδ T-Cells and B-Cells. Cells. 2020;9:743.
Maniar A, Zhang X, Lin W, Gastman BR, Pauza CD, Strome SE, et al. Human gammadelta T lymphocytes induce robust NK cell-mediated antitumor cytotoxicity through CD137 engagement. Blood. 2010;116:1726–33.
Brandes M, Willimann K, Moser B. Professional antigen-presentation function by human gammadelta T Cells. Science. 2005;309:264–8.
Altvater B, Pscherer S, Landmeier S, Kailayangiri S, Savoldo B, Juergens H, et al. Activated human γδ T cells induce peptide-specific CD8+ T-cell responses to tumor-associated self-antigens. Cancer Immunol Immunother. 2012;61:385–96.
Holmen Olofsson G, Idorn M, Carnaz Simões AM, Aehnlich P, Skadborg SK, Noessner E, et al. Vγ9Vδ2 T Cells Concurrently Kill Cancer Cells and Cross-Present Tumor Antigens. Front Immunol. 2021;12:645131.
Silva-Santos B, Mensurado S, Coffelt SB. γδ T cells: pleiotropic immune effectors with therapeutic potential in cancer. Nat Rev Cancer. 2019;19:392–404.
Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–8.
Rei M, Gonçalves-Sousa N, Lança T, Thompson RG, Mensurado S, Balkwill FR, et al. Murine CD27(-) Vγ6(+) γδ T cells producing IL-17A promote ovarian cancer growth via mobilization of protumor small peritoneal macrophages. Proc Natl Acad Sci U S A. 2014;111:E3562–70.
Rei M, Pennington DJ, Silva-Santos B. The emerging Protumor role of γδ T lymphocytes: implications for cancer immunotherapy. Cancer Res. 2015;75:798–802.
Patil RS, Shah SU, Shrikhande SV, Goel M, Dikshit RP, Chiplunkar SV. IL17 producing γδT cells induce angiogenesis and are associated with poor survival in gallbladder cancer patients. Int J Cancer. 2016;139:869–81.
Wang J, Lin C, Li H, Li R, Wu Y, Liu H, et al. Tumor-infiltrating γδT cells predict prognosis and adjuvant chemotherapeutic benefit in patients with gastric cancer. Oncoimmunology. 2017;6:e1353858.
Lu H, Dai W, Guo J, Wang D, Wen S, Yang L, et al. High Abundance of Intratumoral γδ T Cells Favors a Better Prognosis in Head and Neck Squamous Cell Carcinoma: A Bioinformatic Analysis. Front Immunol. 2020;11:573920.
Nguyen S, Chevalier MF, Benmerzoug S, Cesson V, Schneider AK, Rodrigues-Dias SC, et al. Vδ2 T cells are associated with favorable clinical outcomes in patients with bladder cancer and their tumor reactivity can be boosted by BCG and zoledronate treatments. J Immunother Cancer. 2022;10:e004880.
Gherardin NA, Waldeck K, Caneborg A, Martelotto LG, Balachander S, Zethoven M, et al. γδ T Cells in Merkel Cell Carcinomas Have a Proinflammatory Profile Prognostic of Patient Survival. Cancer Immunol Res. 2021;9:612–23.
Wu Y, Biswas D, Usaite I, Angelova M, Boeing S, Karasaki T, et al. A local human Vδ1 T cell population is associated with survival in nonsmall-cell lung cancer. Nat Cancer. 2022;3:696–709.
Buccheri S, Guggino G, Caccamo N, Li Donni P, Dieli F. Efficacy and safety of γδT cell-based tumor immunotherapy: a meta-analysis. J Biol Regul Homeost Agents. 2014;28:81–90.
de Weerdt I, Lameris R, Scheffer GL, Vree J, de Boer R, Stam AG, et al. A Bispecific Antibody Antagonizes Prosurvival CD40 Signaling and Promotes Vγ9Vδ2 T cell-Mediated Antitumor Responses in Human B-cell Malignancies. Cancer Immunol Res. 2021;9:50–61.
de Weerdt I, Lameris R, Ruben JM, de Boer R, Kloosterman J, King LA, et al. A Bispecific Single-Domain Antibody Boosts Autologous Vγ9Vδ2-T Cell Responses Toward CD1d in Chronic Lymphocytic Leukemia. Clin Cancer Res. 2021;27:1744–55.
Ganesan R, Chennupati V, Ramachandran B, Hansen MR, Singh S, Grewal IS. Selective recruitment of γδ T cells by a bispecific antibody for the treatment of acute myeloid leukemia. Leukemia. 2021;35:2274–84.
de Bruin RCG, Veluchamy JP, Lougheed SM, Schneiders FL, Lopez-Lastra S, Lameris R, et al. A bispecific nanobody approach to leverage the potent and widely applicable tumor cytolytic capacity of Vγ9Vδ2-T cells. Oncoimmunology. 2017;7:e1375641.
Oberg HH, Peipp M, Kellner C, Sebens S, Krause S, Petrick D, et al. Novel bispecific antibodies increase γδ T-cell cytotoxicity against pancreatic cancer cells. Cancer Res. 2014;74:1349–60.
De Gassart A, Le KS, Brune P, Agaugué S, Sims J, Goubard A, et al. Development of ICT01, a first-in-class, anti-BTN3A antibody for activating Vγ9Vδ2 T cell-mediated antitumor immune response. Sci Transl Med. 2021;13:eabj0835.
Schiller CB, Braciak TA, Fenn NC, Seidel UJ, Roskopf CC, Wildenhain S, et al. CD19-specific triplebody SPM-1 engages NK and γδ T cells for rapid and efficient lysis of malignant B-lymphoid cells. Oncotarget. 2016;7:83392–408.
Lamb LS, Pereboeva L, Youngblood S, Gillespie GY, Nabors LB, Markert JM, et al. A combined treatment regimen of MGMT-modified γδ T cells and temozolomide chemotherapy is effective against primary high grade gliomas. Sci Rep. 2021;11:21133.
Deniger DC, Switzer K, Mi T, Maiti S, Hurton L, Singh H, et al. Bispecific T-cells expressing polyclonal repertoire of endogenous γδ T-cell receptors and introduced CD19-specific chimeric antigen receptor. Mol Ther. 2013;21:638–47.
Zhai X, You F, Xiang S, Jiang L, Chen D, Li Y, et al. MUC1-Tn-targeting chimeric antigen receptor-modified Vγ9Vδ2 T cells with enhanced antigen-specific anti-tumor activity. Am J Cancer Res. 2021;11:79–91.
Ang WX, Ng YY, Xiao L, Chen C, Li Z, Chi Z, et al. Electroporation of NKG2D RNA CAR Improves Vγ9Vδ2 T Cell Responses against Human Solid Tumor Xenografts. Mol Ther Oncolytics. 2020;17:421–30.
Nishimoto KP, Barca T, Azameera A, Makkouk A, Romero JM, Bai L, et al. Allogeneic CD20-targeted γδ T cells exhibit innate and adaptive antitumor activities in preclinical B-cell lymphoma models. Clin Transl Immunology. 2022;11:e1373.
Marcu-Malina V, Heijhuurs S, van Buuren M, Hartkamp L, Strand S, Sebestyen Z, et al. Redirecting αβ T cells against cancer cells by transfer of a broadly tumor-reactive γδT-cell receptor. Blood. 2011;118:50–9.
Xu Y, Yang Z, Horan LH, Zhang P, Liu L, Zimdahl B, et al. A novel antibody-TCR (AbTCR) platform combines Fab-based antigen recognition with gamma/delta-TCR signaling to facilitate T-cell cytotoxicity with low cytokine release. Cell Discov. 2018;4:62.
Liu C, Liu H, Dasgupta M, Hellman LM, Zhang X, Qu K, et al. Validation and promise of a TCR mimic antibody for cancer immunotherapy of hepatocellular carcinoma. Sci Rep. 2022;12:12068.
Sánchez Martínez D, Tirado N, Mensurado S, Martínez-Moreno A, Romecín P, Gutiérrez Agüera F, et al. Generation and proof-of-concept for allogeneic CD123 CAR-Delta One T (DOT) cells in acute myeloid leukemia. J Immunother Cancer. 2022;10:e005400.
Makkouk A, Yang XC, Barca T, Lucas A, Turkoz M, Wong JTS, et al. Off-the-shelf Vδ1 gamma delta T cells engineered with glypican-3 (GPC-3)-specific chimeric antigen receptor (CAR) and soluble IL-15 display robust antitumor efficacy against hepatocellular carcinoma. J Immunother Cancer. 2021;9:e003441.
Li YR, Zhou K, Wilson M, Kramer A, Zhu Y, Dawson N, et al. Mucosal-associated invariant T cells for cancer immunotherapy. Mol Ther. 2023;31:631–46.
Flores-Villanueva P, Sobhani N, Wang X, Li Y. MR1-Restricted T Cells in Cancer Immunotherapy. Cancers (Basel). 2020;12:2145.
Rudak PT, Choi J, Haeryfar SMM. MAIT cell-mediated cytotoxicity: Roles in host defense and therapeutic potentials in infectious diseases and cancer. J Leukoc Biol. 2018;104:473–86.
Petley EV, Koay HF, Henderson MA, Sek K, Todd KL, Keam SP, et al. MAIT cells regulate NK cell-mediated tumor immunity. Nat Commun. 2021;12:4746.
Favreau M, Venken K, Faict S, Maes K, De Veirman K, De Bruyne E, et al. Both mucosal-associated invariant and natural killer T-cell deficiency in multiple myeloma can be countered by PD-1 inhibition. Haematologica. 2017;102:e266–70.
De Biasi S, Gibellini L, Lo Tartaro D, Puccio S, Rabacchi C, Mazza EMC, et al. Circulating mucosal-associated invariant T cells identify patients responding to anti-PD-1 therapy. Nat Commun. 2021;12:1669.
Vorwald VM, Davis DM, Van Gulick RJ, Torphy RJ, Borgers JS, Klarquist J, et al. Circulating CD8(+) mucosal-associated invariant T cells correlate with improved treatment responses and overall survival in anti-PD-1-treated melanoma patients. Clin Transl Immunology. 2022;11:e1367.
Kronenberg M, Gapin L. The unconventional lifestyle of NKT cells. Nat Rev Immunol. 2002;2:557–68.
Porcelli S, Yockey CE, Brenner MB, Balk SP. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J Exp Med. 1993;178:1–16.
Singh AK, Tripathi P, Cardell SL. Type II NKT Cells: An Elusive Population With Immunoregulatory Properties. Front Immunol. 2018;9:1969.
Courtney AN, Tian G, Metelitsa LS. Natural killer T cells and other innate-like T lymphocytes as emerging platforms for allogeneic cancer cell therapy. Blood. 2023;141:869–76.
Motohashi S, Kobayashi S, Ito T, Magara KK, Mikuni O, Kamada N, et al. Preserved IFN-alpha production of circulating Valpha24 NKT cells in primary lung cancer patients. Int J Cancer. 2002;102:159–65.
Tachibana T, Onodera H, Tsuruyama T, Mori A, Nagayama S, Hiai H, et al. Increased intratumor Valpha24-positive natural killer T cells: a prognostic factor for primary colorectal carcinomas. Clin Cancer Res. 2005;11:7322–7.
Metelitsa LS, Wu HW, Wang H, Yang Y, Warsi Z, Asgharzadeh S, et al. Natural killer T cells infiltrate neuroblastomas expressing the chemokine CCL2. J Exp Med. 2004;199:1213–21.
Dhodapkar MV, Geller MD, Chang DH, Shimizu K, Fujii S, Dhodapkar KM, et al. A reversible defect in natural killer T cell function characterizes the progression of premalignant to malignant multiple myeloma. J Exp Med. 2003;197:1667–76.
Metelitsa LS, Weinberg KI, Emanuel PD, Seeger RC. Expression of CD1d by myelomonocytic leukemias provides a target for cytotoxic NKT cells. Leukemia. 2003;17:1068–77.
Spanoudakis E, Hu M, Naresh K, Terpos E, Melo V, Reid A, et al. Regulation of multiple myeloma survival and progression by CD1d. Blood. 2009;113:2498–507.
Fais F, Tenca C, Cimino G, Coletti V, Zanardi S, Bagnara D, et al. CD1d expression on B-precursor acute lymphoblastic leukemia subsets with poor prognosis. Leukemia. 2005;19:551–6.
Fais F, Morabito F, Stelitano C, Callea V, Zanardi S, Scudeletti M, et al. CD1d is expressed on B-chronic lymphocytic leukemia cells and mediates alpha-galactosylceramide presentation to natural killer T lymphocytes. Int J Cancer. 2004;109:402–11.
Dhodapkar KM, Cirignano B, Chamian F, Zagzag D, Miller DC, Finlay JL, et al. Invariant natural killer T cells are preserved in patients with glioma and exhibit antitumor lytic activity following dendritic cell-mediated expansion. Int J Cancer. 2004;109:893–9.
Liu D, Song L, Brawley VS, Robison N, Wei J, Gao X, et al. Medulloblastoma expresses CD1d and can be targeted for immunotherapy with NKT cells. Clin Immunol. 2013;149:55–64.
Cortesi F, Delfanti G, Grilli A, Calcinotto A, Gorini F, Pucci F, et al. Bimodal CD40/Fas-Dependent Crosstalk between iNKT Cells and Tumor-Associated Macrophages Impairs Prostate Cancer Progression. Cell Rep. 2018;22:3006–20.
Song L, Asgharzadeh S, Salo J, Engell K, Wu HW, Sposto R, et al. Valpha24-invariant NKT cells mediate antitumor activity via killing of tumor-associated macrophages. J Clin Invest. 2009;119:1524–36.
Cui J, Shin T, Kawano T, Sato H, Kondo E, Toura I, et al. Requirement for Valpha14 NKT cells in IL-12-mediated rejection of tumors. Science. 1997;278:1623–6.
Takeda K, Hayakawa Y, Atsuta M, Hong S, Van Kaer L, Kobayashi K, et al. Relative contribution of NK and NKT cells to the anti-metastatic activities of IL-12. Int Immunol. 2000;12:909–14.
Ko HJ, Lee JM, Kim YJ, Kim YS, Lee KA, Kang CY. Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol. 2009;182:1818–28.
Simon B, Wiesinger M, März J, Wistuba-Hamprecht K, Weide B, Schuler-Thurner B, et al. The Generation of CAR-Transfected Natural Killer T Cells for the Immunotherapy of Melanoma. Int J Mol Sci. 2018;19:2365.
Poels R, Drent E, Lameris R, Katsarou A, Themeli M, van der Vliet HJ, et al. Preclinical Evaluation of Invariant Natural Killer T Cells Modified with CD38 or BCMA Chimeric Antigen Receptors for Multiple Myeloma. Int J Mol Sci. 2021;22:1096.
Rotolo A, Caputo VS, Holubova M, Baxan N, Dubois O, Chaudhry MS, et al. Enhanced Anti-lymphoma Activity of CAR19-iNKT Cells Underpinned by Dual CD19 and CD1d Targeting. Cancer Cell. 2018;34:596-610.e11.
Xu X, Huang W, Heczey A, Liu D, Guo L, Wood M, et al. NKT Cells Coexpressing a GD2-Specific Chimeric Antigen Receptor and IL15 Show Enhanced In Vivo Persistence and Antitumor Activity against Neuroblastoma. Clin Cancer Res. 2019;25:7126–38.
Heczey A, Liu D, Tian G, Courtney AN, Wei J, Marinova E, et al. Invariant NKT cells with chimeric antigen receptor provide a novel platform for safe and effective cancer immunotherapy. Blood. 2014;124:2824–33.
Delfanti G, Dellabona P, Casorati G, Fedeli M. Adoptive Immunotherapy With Engineered iNKT Cells to Target Cancer Cells and the Suppressive Microenvironment. Front Med (Lausanne). 2022;9:897750.
Heczey A, Courtney AN, Montalbano A, Robinson S, Liu K, Li M, et al. Anti-GD2 CAR-NKT cells in patients with relapsed or refractory neuroblastoma: an interim analysis. Nat Med. 2020;26:1686–90.
Landoni E, Smith CC, Fucá G, Chen Y, Sun C, Vincent BG, et al. A High-Avidity T-cell Receptor Redirects Natural Killer T-cell Specificity and Outcompetes the Endogenous Invariant T-cell Receptor. Cancer Immunol Res. 2020;8:57–69.
Delfanti G, Cortesi F, Perini A, Antonini G, Azzimonti L, de Lalla C, et al. TCR-engineered iNKT cells induce robust antitumor response by dual targeting cancer and suppressive myeloid cells. Sci Immunol. 2022;7:eabn6563.
Corgnac S, Perret R, Derré L, Zhang L, Stirnemann K, Zauderer M, et al. CD1d-antibody fusion proteins target iNKT cells to the tumor and trigger long-term therapeutic responses. Cancer Immunol Immunother. 2013;62:747–60.
Das R, Guan P, Wiener SJ, Patel NP, Gohl TG, Evans E, et al. Enhancing the antitumor functions of invariant natural killer T cells using a soluble CD1d-CD19 fusion protein. Blood Adv. 2019;3:813–24.
Kharkwal SS, Johndrow CT, Veerapen N, Kharkwal H, Saavedra-Avila NA, Carreño LJ, et al. Serial Stimulation of Invariant Natural Killer T Cells with Covalently Stabilized Bispecific T-cell Engagers Generates Antitumor Immunity While Avoiding Anergy. Cancer Res. 2021;81:1788–801.
Catafal-Tardos E, Baglioni MV, Bekiaris V. Inhibiting the Unconventionals: Importance of Immune Checkpoint Receptors in γδ T, MAIT, and NKT Cells. Cancers (Basel). 2021;13:4647.
Xu N, Palmer DC, Robeson AC, Shou P, Bommiasamy H, Laurie SJ, et al. STING agonist promotes CAR T cell trafficking and persistence in breast cancer. J Exp Med. 2021;218:e20200844.
Ji F, Zhang F, Zhang M, Long K, Xia M, Lu F, et al. Targeting the DNA damage response enhances CD70 CAR-T cell therapy for renal carcinoma by activating the cGAS-STING pathway. J Hematol Oncol. 2021;14:152.
Smith TT, Moffett HF, Stephan SB, Opel CF, Dumigan AG, Jiang X, et al. Biopolymers codelivering engineered T cells and STING agonists can eliminate heterogeneous tumors. J Clin Invest. 2017;127:2176–91.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural Science Foundation of China (Nos. 82073370, 82272794, 81874120, 82072597 and 62131009) and China Postdoctoral Science Foundation (No. 2022M722766).
Author information
Authors and Affiliations
Contributions
MY and TL drafted the manuscript and prepared the figures. MN, QM and BZ collected the related references and participated in discussion. QC, ZD and KW designed this review and revised the manuscript. All authors contributed to this manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Yi, M., Li, T., Niu, M. et al. Exploiting innate immunity for cancer immunotherapy. Mol Cancer 22, 187 (2023). https://doi.org/10.1186/s12943-023-01885-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s12943-023-01885-w