Abstract
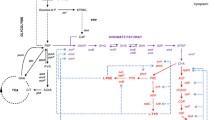
In this review, the metabolic engineering approaches including those used by the authors in creating phenylalanine producers based on Escherichia coli were systematized. Optimization of the amino acid biosynthesis was conducted in order to obtain significant quantities of phenylalanine and to ensure the availability of its direct precursors in cell metabolism—erythrose 4-phosphate and phosphoenolpyruvic acid. The possibility of altering global regulation mechanisms was investigated for a full reorientation of the metabolism to phenylalanine synthesis with the use of Csr (carbon storage regulator). The identification of the aromatic amino acids exporter YddG is associated with the use of the phenylalanine producer as a test-system. Novel approaches to phenylalanine producer construction (use of the “leaky” allele tyrA—ssrA, promoters of the phosphate regulon), as well as new methods of obtaining producers of similar amino acid tyrosine, were discussed. Examples of the synthesis of useful aromatic compounds from phenylalanine or its precursor, phenylpyruvic acid, with E. coli as the ecipient for foreign gene expression were examined.
Similar content being viewed by others
Abbreviations
- CB:
-
culture broth
- PCR:
-
polymerase chain reaction
- ATP:
-
adenosine triphosphate
- FBA:
-
flux balance analysis
- CSR:
-
carbon storage regulator
- DAHP-synthase:
-
3-Deoxy-D-arabinoheptulosonate 7-phosphate synthase
- E-4-P:
-
erythrose 4-phosphate
- MFA:
-
metabolic flux analysis
- NADPH:
-
nicotinamide adenine dinucleotide phosphate
- PEP:
-
phosphoenolpyruvate
- Phe:
-
phenylalanine
- Pi:
-
inorganic phosphate
- PTS:
-
phosphotransferase system
- Pyr:
-
pyruvate
- TCA cycle:
-
tricarboxylic acid cycle.
References
Bongaerts, J., Kramer, M., Muller, U., Raeven, L., and Wubbolts, M., Metabolic engineering for microbial production of aromatic amino acids and derived compounds, Metab. Eng., 2001, vol. 3, no. 4, pp. 289–300.
Ikeda, M., Towards bacterial strains overproducing l-tryptophan and other aromatics by metabolic engineering, Appl. Microbiol. Biotechnol., 2006, vol. 69, no. 6, pp. 615–626.
Sprenger, G.A., From scratch to value: engineering Escherichia coli wild type cells to the production of L-phenylalanine and other fine chemicals derived from chorismate, Appl. Microbiol. Biotechnol., 2007, vol. 75, no. 4, pp. 739–749.
Alternative Sweeteners—US Industry Study with Forecasts for 2013–2018. http://www.freddoniagroup.com
Becker, J. and Wittmann, C., Systems and synthetic metabolic engineering for amino acid production—the heartbeat of industrial strain development, Curr. Opin. Biotechnol., 2012, vol. 23, no. 5, pp. 718–726.
Backman, K., M.J. O’Connor, Maruyan, A., Rudd, E., Mckay, D., Balakrishnan, R., Radjai, M., Dipasquantonio, V., Shoda. D., Hatch, R., and Venkatasubramanian, K., Genetic engineering of metabolic pathways applied to the production of phenylalanine, Ann. N. Y. Acad. Sci., 1990, vol. 589, pp. 16–24.
Grinter, N.J., Developing an L-phenylalanine process, Chem. Tec., 1997, vol. 28, no. 7, pp. 33–37.
Bailey, J.E., Towards a science of metabolic engineering, Science, 1991, vol. 252, no. 5013, pp. 1668–1674.
Stephanopoulos, G., Metabolic fluxes and metabolic engineering, Metab. Eng., 1999, vol. 1, no. 1, pp. 1–11.
Park, J.H. and Lee, S.Y., Towards systems metabolic engineering of microorganisms for amino acid production, Curr. Opin. Biotechnol., 2008, vol. 19, no. 5, pp. 454–460.
Esvelt, K.M. and Wang, H.H., Genome-scale engineering for systems and synthetic biology, Mol. Syst. Biol., 2013, vol. 9, no. 641. doi: 10.1038/msb.2012.66
Kern, A., Tilley, E., Hunter, I.S., Legisa, M., and Glieder, A., Engineering primary metabolic pathways of industrial micro-organisms, J. Biotechnol., 2007, vol. 129, no. 1, pp. 6–29.
Buschke, N., Schafer. R., Becker, J., and Wittmann, C., Metabolic engineering of industrial platform microorganisms for biorefinery applications—optimization of substrate spectrum and process robustness by rational and evolutive strategies, Bioresour. Technol., 2013, vol. 135, pp. 544–554.
Leuchtenerger, W., Huthmacher, K., and Drauz, K., Biotechnological production of amino acids and derivatives: current status and prospects, Appl. Microbiol. Biotechnol., 2005, vol. 69, no. 1, pp. 1–8.
Khamduang, M., Packdibamrung, K., Chutmanop, J., Chisti, Y., and Srinophakun, P., Production of L-phenylalanine from glycerol by a recombinant Escherichia coli, J. Ind. Microbiol. Biotechnol., 2009, vol. 36, no. 10, pp. 1267–1274.
Weiner, M., Albermann, C., Gottlieb, K., Sprenger, G.A., and Weuster-Botz, D., Fed-batch production of L-phenylalanine from glycerol and ammonia with recombinant Escherichia coli, Biochem. Eng. J., 2014, vol. 83, pp. 62–69.
Ahn, J.O., Lee, H.W., Saha, R., Park, M.S., Jung, J.K., and Lee, D.Y., Exploring the effects of carbon sources on the metabolic capacity for shikimic acid production in Escherichia coli using in silico metabolic predictions, J. Microbiol. Biotechnol., 2008, vol. 18, no. 11, pp. 1773–1784.
Pittard, J. and Yang, J., Biosynthesis of aromatic amino acids, in Escherichia coli and Salmonella: Cellular and Molecular Biology, 3rd ed., Hatfield, W., Section Ed., Washington: American Soc. Microbiol. Press, 2008. doi: 10.1128/ecosal.3.6.1.8
Postma, P.W., Lengeler, J.W., and Jacobson, G.R., Phosphoenolpyravate: carbohydrate phosphotranferase systems, in Escherichia coli and Salmonella: Cellular and Molecular Biology, 2nd edn. [Ed. F.C. Neidhardt]. Wasthington, DC: American Soc. Microbiol. Press, 1996. p. 1149–1174.
Forberg, C., Eliaeson, T., and Haggstrom, L., Correlation of theoretical and experimental yields of phenylalanine from non-growing cells of a rec Escherichia coli strain, J. Biotechnol., 1988, vol. 7, no. 4, pp. 319–332.
Chen, R., Hatzimanikatis, V., Yap, W.M.G.J., Postma, P.W., and Bailey, J.E., Metabolic consequences of phosphotransferase (pts) mutation in a phenylalanine-producing recombinant Escherichia coli, Biotechnol. Prog., 1997, vol. 13, no. 6, pp. 768–775.
Keseler, I.M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Hama-Casto, S., Miniz-Racado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A.G., Mackie, A., Paulsen, I., Gunsalus, R.P., and Karp, P.D., EcoCyc: a comprehensive database of Escherichia coli biology, Nucleic Acid Res., 2011, vol. 39, pp. 583–590.
Varma, A., Boesch, B.W., and Palsson, B.O., Biochemical production capabilities of Escherichia coli, Biotechnol. Bioeng., 1993, vol. 42, no. 1, pp. 59–73.
Klamt, S., Stelling, J., Ginkel, M., and Gilles, D.E., Flux analyzer: exploring structure, pathways, and flux distribution in metabolic networks on interactive flux maps, Bioinformatics, 2003, vol. 19, no. 2, pp. 261–269.
Toya, Y., Kono, N., Kazuharu, A., and Tomita, M., Metabolic flux analysis and visualization, J. Proteome Res., 2011, vol. 10, no. 8, pp. 3313–3323.
Flores, S., Gosset, G., Flores, N., de Graff, A.A. and Bolivar, F., Analysis of carbon metabolism in Escherichia coli strains with an inactive phosphotransferase system by 13C labeling and NMR spectroscopy, Metab. Eng., 2002, vol. 4, no. 2, pp. 124–137.
Gosset, G., Improvement of Escherichia coli production strains by modification of the phosphoenolpyruvate; sugar phosphotransferase system, Microb. Cell Fact., 2005, vol. 4, no. 1. doi: 10.1186/1475-2859-4-14
Rodriquez, A., Martinez, J.A., Baez-Viveros, J.L., Flores, N., Hernandez-Chavez, G., Ramirez, O.T., Gosset, G., and Bolivar, F., Constitutive expression of selected genes from the pentose phosphate and aromatic pathways increases the shikimic acid yield in high-glucose batch cultures of an Escherichia coli strain lacking PTS and pykF, Microb. Cell Fact., 2013, vol. 12, no. 86. doi: 10.1186/1475-2859-12-86
Barker, J.L. and Frost, J.W., Microbial synthesis of p-hydroxybenzoic acid from glucose, Biotechnol. Bioeng., 2001, vol. 76, no. 4, pp. 376–390.
Dell, K.A. and Frost, J.W., Identification and removal of impediments to biocatalytic synthesis of aromatics from d-glucose: rate-limiting enzymes in the common pathway of aromatic amino acid biosynthesis, J. Am. Chem. Soc., 1993, vol. 115, no. 24, pp. 11581–11589.
Stephanopoulos, G.N., Aristidou, A.A., and Nielsen, J., Metabolic Engineering: Principles and Methodologies, New York: Acad. Press, 1998.
Liu, S.P., Xiao, M.E., Zhang, I., Xu, J., Ding, Z.Y., Gu, Z.H., and Shi, G.Y., Production of L-phenylalanine from glucose by metabolic engineering of wild type Escherichia coli W3110, Process Biochem., 2013, vol. 48, no. 3, pp. 413–419.
Nelms, J., Edwards, R.M., Warwick, J., and Fotheringham, I., Novel mutations in the pheA gene of Escherichia coli K-12 which result in highly feedback inhibition-resistant variants of chorismate mutase/prephenate dehydratase, Appl. Environ. Microbiol., 1992, vol. 58, no. 8, pp. 2592–2598.
Kikuchi, Y., Tsujimoto, K., and Kurahashi, O., Mutational analysis of the feedback sites of phenylalaninesensitive 3-deoxy-D-arabino-heptulosonate-7-phosphate synthase of Escherichia coli, Appl. Environ. Microbiol., 1997, vol. 63, pp. 761–762.
Choi, Y.J. and Tribe, D.E., Continuous production of phenylalanine using an Escherichia coli regulatory mutant, Biotechnol. Lett., 1982, vol. 4, no. 4, pp. 223–228.
Polen, Y., Kramer, M., Bongaerts, J., Wubbolts, M., and Wendisch, V.F., The global gene expression response of Escherichia coli to L-phenylalanine, J. Biotechnol., 2005, vol. 115, no. 3, pp. 221–237.
Konstantinov, K.B. and Yoshida, T., On-line monitoring of representative structural variables in fed-batch cultivation of recombinant Escherichia coli for phenylalanine production, J. Ferm. Bioeng., 1990, vol. 70, no. 6, pp. 420–426.
Konstantinov, K.B., Nishio, T.S., and Yoshida, T., Physiologically motivated strategies for control of the fed-batch cultivation of recombinant Escherichia coli for phenylalanine production, J. Bioeng., 1991, vol. 71, no. 5, pp. 350–355.
Patnaik, R. and Liao, J.C., Engineering of Escherichia coli central metabolism for aromatic production with near theoretical yield, Appl. Environ. Microbiol., 1994, vol. 60, no. 11, pp. 3903–3908.
Liao, J.C., Hou, S.Y., and Chao, Y.P., Pathway analysis, engineering, and physiological considerations for redirecting central metabolism, Biotechnol. Bioeng., 1996, vol. 52, no. 1, pp. 129–140.
Frost, J.W., Snell, K.D., and Frost, K.M., Deblocking the common pathway of aromatic amino acid synthesis, EU Patent no. EP0763127, 1995.
Juinaga, D., Baidoo, E.E., Reding-Johanson, A.M., Batth, T.S., Burd, H., Mikhopadhyay, A., Petzold, C.J., and Keasling, J.D., Modular engineering of Ltyrosine production in Escherichia coli, Appl. Environ. Microbiol., 2012, vol. 78, no. 1, pp. 89–98.
Chao, Y.P., Lai, Z.J., Chen, P., and Chern, J.T., Enhanced conversion rate of L-phenylalanine by coupling reactions of aminotransferases and phosphoenolpyruvate carboxykinase in Escherichia coli K-12, Biotechnol. Prog., 1999, vol. 15, no. 3, pp. 453–458.
Thonhchuang, M., Pongsawasdi, P., Chisti, Y., and Packdibamrung, K., Design of a recombinant Escherichia coli for producing L-phenylalanine from glycerol, J. Microbiol. Biotechnol., 2012, vol. 28, no. 10, pp. 2937–2943.
Wahl, A., Massaoudi, M.E., Schipper, D., Wiechert, W., and Takors, R., Serial 13C-based flux analysis of an L-phenylalanine producing E. coli strain using the sensor reactor, Biotechnol. Prog, 2004, vol. 20, no. 3, pp. 706–714.
Baez-Viveros, J.L, Osuna, J., Hernandez-Chavez, G., Soberon, X., Bolivar, F., and Gosset, G., Metabolic engineering and protein directed evolution increase the yield of L-phenylalanine synthesized from glucose in Escherichia coli, Biotehcnol. Bioeng., 2004, vol. 87, no. 4, pp. 516–524.
Baez-Viveros, J.L, Flores, N., Juarez, K., Castillo-Wspana, P., f. bolivar, g. gosset, Metabolic transcription analysis of engineered Escherichia coli strains that overproduce L-phenylalanine, Microb. Cell Fact., 2007, vol. 19, no. 13. doi: 10.1186/1475-2859-6-30
Doroshenko, V.G., Shakulov, R.S., Kazakova, S.M., Kivero, A.D., Yampolskaya, T.A., and Mashko, S.V., Construction of an L-phenylalanine-producing tyrosine-prototrophic Escherichia coli strain using tyrA ssrA-like tagged alleles, Biotechnol. Lett., 2010, vol. 32, no. 8, pp. 1117–1121.
Takagi, M., Nishio, Y., Oh, G., and Yoshida, T., Control of L-phenylalanine production by dual feeding of glucose and L-tyrosine, Biotechnol. Bioeng., 1996, vol. 52, no. 6, pp. 653–660.
Wang, J., Liu, L., Zhou, H., Li, J., Du, G., and Chen, J., Comparative study of L-phenylalanine production in the growing and stationary phases during high cell density cultivation of an ausotrophic Escherichia coli, Biotechnol. Bioprocess Eng., 2011, vol. 16, no. 5, pp. 916–922.
Shpanchenko, O.V., Bugaeva, E.Yu., Golovin, A.V., and Dontsova, O.A., Trans-translation: findings and hypotheses, Mol. Biol. (Moscow), 2010, vol. 44, no. 4, pp. 495–502.
Eggeling, L. and Sahm, H., New ubiquitous translocators: amino acid export by corynebacterium glutamicum and Escherichia coli, Arch. Microbiol., 2003, vol. 180, no. 3, pp. 155–160.
Livshits, V.A., Zakataeva, N.P., Aleshin, V.V., and Vitushkina, M.V., Identification and characterization of the new gene rhtA in threonine and homoserine efflux in Escherichia coli, Res. Microbiol., 2003, vol. 154, pp. 123–135.
Brown, K.D., Maintenance and exchange of the aromatic amino acid pool in Escherichia coli, J. Bacteriol., 1971, vol. 106, no. 1, pp. 70–81.
Koyanagi, T., Katayama, T., Suzuki, H., and Kumagai, H., Identification of the LIV-I/LS system as the third phenylalanine trasporter in Escherichia coli K-12, J. Bacteriol., 2004, vol. 186, no. 2, pp. 343–350.
Wang, P., Yang, J., Ishihama, A., and Pittard, J., Demonstration that the TyrR protein and RNA polymerase complex formed at the divergent P3 promoter inhibits binding of RNA polymerase to the major promoter, P1, of the aroP gene of Escherichia coli, J. Bacteriol., 1998, vol. 180, no. 20, pp. 5466–5472.
Tribe, D.E., Novel microorganism and method, US Patent No. US4681852, 1987.
Kramer, R., Secretion of amino acids by bacteria: physiology and mechanism, FEMS Microbiol. Rev., 1994, vol. 13, no. 1, pp. 75–94.
Livshits, V.A., Vitushkina, M.V., Mashko, S.V., and Doroshenko, V.G., A method for obtaining L-amino acids: an Escherichia coli strain producing the amino acid, RF Patent no. 2222596, 2002.
Doroshenko, V., Airich, L., Vitushkina, M., Kolokolova, A., Livshits, V., and Mashko, S., YddG from Escherichia coli promotes export of aromatic amino acids, FEMS Microbiol. Letts., 2007, vol. 275, no. 2, pp. 312–318.
Airich, L.G., Tsyrenzhapova, I.S., Vorontsova, O.V., Feofanov, A.V., Doroshenko, V.G., and Mashko, S.V., Membrane topology analysis of the Escherichia coli aromatic amino acid efflux protein YddG, J. Mol. Microbiol. Biotechnol., 2010, vol. 19, no. 4, pp. 189–197.
Saier, M.H., ffixJr., Yen, M.R., Noto, K., Tamang, D.G., and Elkan, C., The transporter classification database: recent advances, Nucleic Acid Res., 2009, vol. 37, pp. D274–278.
Tsyrenzhapova, I.S., Doroshenko, V.G., Airikh, L.G., Mironov, A.S., and Mashko, S.V., Gene yddG of Escherichia coli encoding the putative exporter of aromatic amino acids: constitutive transcription and dependence of the expression on the cell growth rate, Genetika, 2009, vol. 45, no. 5, pp. 525–532.
Hori, H., Yoneyama, H., Tobe, R., Ando, T., Isogai, E., and Katsumata, R., Inducible L-alanine exporter encoded by the novel gene ygaW (alaE) in Escherichia coli, Appl. Environ. Microbiol., 2011, vol. 77, no. 12, pp. 4027–4034.
Gosset, G., Production of aromatic compounds in bacteria, Curr. Opin. Biotechnol., 2009, vol. 20, no. 6, pp. 651–658.
Wang, J., Cheng, L.K., Wang, J., Liu, Q., Shen, T., and Chen, N., Genetic engineering of Escherichia coli to enhance production of L-tryptophan, Appl. Microbiol Biotechnol., 2013, vol. 97, no. 17, pp. 7587–7596.
Takumi, K. and Nonaka, G., L-amino acid-producing bacterium and a method for producing an L-amino acid, EU Patent No. EP2218729, 2010.
Airich, L.G., Doroshenko, V.G., and Tsyrenzhapova, I.S., A mutant protein encoded by the yddG gene, and a method for producing aromatic L-amino acids using a bacterium of the genus Escherichia, International Patent Application No. WO2013129432, 2013.
Akhverdyan, V.Z., Savrasova, E.A., Kaplan, A.M., Lobanov, A.O., Vavilova, E.Yu., and Kozlov, Yu.I., Development of a mini-Mu system providing efficient integration of genetic material into the chromosome of the bacterium Escherichia coli and its amplification, Biotekhnologiya, 2007, no. 3, pp. 3–20.
Savrasova, E.A., Creation of a mini-Mu system devoid of selective markers for gene integration into the chromosome of the bacterium Escherichia coli, Akhverdyan, V.Z., Lobanov, A.O., Kaplan, A.M., and Kozlov, Yu.I., Biotekhnologiya, 2007, no. 4, pp. 3–17.
Martinez-Morales, F., Borges, A.C., Martinez, A., Shanmugam, K.T., and Ingram, L.O., Chromosomal integration of heterologous DNA in Escherichia coli with precise removal of markers and replicons used during construction, J. Bacteriol., 1999, vol. 181, no. 22, pp. 7143–7148.
Haldimann, A. and Wanner, B.L., Conditional-replication, integration, excision, and retrieval plasmid-host systems for gene structure-function studies of bacteria, J. Bacteriol., 2001, vol. 183, no. 21, pp. 6384–6393.
Minaeva, N.I., Gak, E.R., Zimenkov, D.V., Skorokhodova, A.Y., Biryukova, I.V., and Mashko, S.V., Dual in/out strategy for genes integration into bacterial chromosome: a novel approach to step-by-step construction of plasmid-less marker-less recombinant e. coli strains with predesigned genome structure, BMC Biotechnol., 2008, vol. 8, no. 63. doi: 10.1186//14726750-8-63
Chiang, C.J., Chen, P.T., and Chao, Y.P., Repliconfree and markerless methods for genomic insertion of DNAs in phage attachment sites and controlled expression of chromosomal genes in Escherichia coli, Biotechnol. Bioeng., 2008, vol. 101, no. 5, pp. 985–995.
Katashkina Zh.I., Skorokhodova, A.Yu., Zimenkov, D.V., Gulevich, A.Yu., Minaeva, N.I., Doroshenko, V.G., Biryukova, I.V., and Mashko, S.V., Tuning the expression level of a gene located on a bacterial chromosome, Mol. Biol. (Moscow), 2005, vol. 39, no. 5, pp. 719–726.
Park, N.H. and Rogers, P.L., L-phenylalanine production in continuous culture using a hyperproducing mutant of Escherichia coli K-12, Chem. Eng. Commun., 1986, vol. 45, no. 16, pp. 185–196.
Wanner, B.L., Phosphorus assimilation and control of the phosphate regulon, in Escherichia coli and Salmonella: Cellular and Molecular Biology, 2nd ed., Neidhardt, F.C., Ed., Washington, DC: ASM Press, 1996, pp. 1357–1381.
Doroshenko, V.G., Tsyrenzhapova, I.S., Krylov, A.A., Kiseleva, E.M., Erishev, V.Y., Kazakova, S.M., Biryukova, I.V., and Mashko, S.V., Pho regulon promoter-mediated transcription of the key pathway gene aroGFbr improves the performance of an L-phenylalanine-producing Escherichia coli strain, Appl. Microbiol. Biotechnol., 2010, vol. 8, no. 6, pp. 1287–1295.
Nogueira, T. and Springer, M., Pst-transcriptional control by global regulators of gene expression in bacteria, Curr. Opin. Microbiol., 2000, vol. 3, no. 2, pp. 154–158.
Martínez-Antonio, A. and Collado-Vides, J., Identifying global regulators in transcriptional regulatory networks in bacteria, Curr. Opin. Microbiol., 2003, vol. 6, no. 5, pp. 482–489.
Alper, H. and Stephanopoulos, G., Global transcription machinery engineering: a new approach for improving cellular phenotype, Metab. Eng., 2007, vol. 9, no. 3, pp. 258–267.
McKee, A.E., Rutherford, B.J., Chivian, D.C., Baidoo, E.K., Juminaga, D., Kuo, D., Benke, P.I., Dietrich, J.A., Ma, S.M., Arkin, A.P., Petzold, C.J., Adas, P.D., Keasling, J.D., and Chhabra, S.R., Manipulation of the carbon storage regulator system for metabolite remodeling and biofuel production in Escherichia coli, Microb. Cell Fact., 2012, vol. 11, no. 79.
Tatarko, M. and Romeo, T., Disruption of a global regulatory gene to enhance central carbon flux into phenylalanine biosynthesis in Escherichia coli, Curr. Microbiol., 2001, vol. 43, no. 1, pp. 26–32.
Yakandawala, N., Romeo, T., Friesen, A.D., and Madhyastha, S., Metabolic engineering of Escherichia coli to enhance phenylalanine production, Appl. Microbiol. Biotechnol., 2008, vol. 78, no. 2, pp. 283–291.
Romeo, T., Gong, M., Liu, M.Y., and Brun-Zinkernagel, A.M., Identification and molecular characterization of csrA, a pleiotropic gene form Escherichia coli that affects glycogen biosynthesis, gluconeogenesis, cell size, and surface properties, J. Bacteriol., 1993, vol. 175, no. 15, pp. 4744–4755.
Sabnis, N.A., Ynag, H., and Romeo, T., Pleiotropic regulation of central carbohydrate metabolism in Escherichia coli via the gene csrA, J. Biol. Chem., 1995, vol. 270, no. 49, pp. 29096–29104.
Romeo, T., Vakulskas, C.A., and Babitzke, P., Posttranscriptional regulation on a global scale: form and function of Csr/Rsm systems, Environ. Microbiol., 2013, vol. 15, no. 2, pp. 313–324.
Lutke-Evesloh, T., Santos, C.N., and Stephanopoulos, G., Perspectives of biotechnological production of L-tyrosine and its applications, Appl. Microbiol. Biotechnol., 2007, vol. 77, no. 4, pp. 751–762.
Olson, M.M., Templeton, L.JH., W. Su, Youderian, P., Sariaslani, F.S., Gatenby, A.A., and Van Dyk, T.K., Production of L-tyrosine from sucrose or glucose achieved by rapid genetic changes to phenylalanine-producing Escherichia coli strains, Appl. Microbiol. Biotechnol., 2007, vol. 74, no. 5, pp. 1031–1040.
Gatenby, A.A., Patnaik, R., Sariaslani, F.S., Suh, W., and Van Dyk, T.K., Method of producing an L-tyrosine over-producing bacterial strain, US Patent no. 7700328 B2, 2010.
Santos, C.N. and Stephanopoulos, G., Melanin-based high-throughput screen for L-tyrosine production in Escherichia coli, Appl. Environ, Microbiol., 2008, vol. 74, no. 4, pp. 1190–1197.
Santos, C.N., Xiao, W., and Stephanopoulos, G., Rational, combinatorial, and genomic approaches for engineering L-tyrosine production, Proc. Natl. Acad. Sci. USA, 2012, vol. 109, no. 34, pp. 13538–13543.
Deutscher, J., Francke, C., and Postma, P.W., How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria, Microbiol. Mol. Biol. Rev., 2006, vol. 70, no. 4, pp. 939–1031.
Tsujioto, N., Suzuki, T., and Ito, H., Method for producing target substance using microorganisms with reduced interactions between MalK and IIAGlc, US Patent US0070979999, 2006.
Takeshita, R., Kadotani, N., and Abe, K., Method for producing a target substance by fermentation, US Patent Application No. US20110003347, 2011.
Martinez, K., de Anda, R., Hernandez, G., Escalante, A., Gosset, G., Ramirez, O.T., and Bolivar, F.G., Coutilization of glucose and glycerol enhances the production of aromatic compounds in an Escherichia coli strain lacking the phosphoenolpyruvate: carbohydrate phosphotransferase system, Microb. Cell Fact., 2008, vol. 7, no. 1. doi: 101186/1475-2859-7-1
Sabrl, S., Nielsen, L.K., and Vickers, C.E., Molecular control of sucrose utilization in Escherichia coli W, an efficient sucrose-utilizing strain, Appl. Environ. Microbiol., 2013, vol. 79, no. 2, pp. 478–487.
Schmid, K., Ebner, R., Altenbuchner, J., Schmitt, R., and Lengler, J.W., Plasmid-mediated sucrose metabolism in Escherichia coli K12: mapping of the scr genes of pUR400, Mol. Microbiol., 1988, vol. 2, no. 1, pp. 1–8.
Doroshenko, V.G., Danilevich, V.N., Karataev, G.I., and Livshits, V.A., Structural and functional organization of the transposon Tn2555 carrying saccharose utilization genes, Mol. Biol., 1988, vol. 22, no. 3, pp..
Schmid, K., Ebner, R., Jahreis, K., Lengeler, J.W., and Titgemeyer, F., A sugar-specific porin, SerY, is involved in sucrose uptake in enteric bacteria, Mol. Microbiol., 1991, vol. 5, no. 4, pp. 941–950.
Bockman, J., Heuel, H., and Lengheler, J.W., Characterization of a chromosomally encoded, non-pts metabolic pathway for sucrose utilization in Escherichia coli EC3132, Mol. Gen. Genet., 1992, vol. 325, no. 1, pp. 22–32.
Hochhut, B., Jahreis, K., Lengler, J.W., and Schmid, K., CTranscr94, a conjugative transposon found in enterobacteria, J. Bacteriol., 1997, vol. 179, no. 7, pp. 2097–2102a.
Jahreis, K., Bentler, L., Bockmann, J., Hans, S., Meyer, A., Siepelmeyer, J., and Lengeler, J.W., Adaptation of sucrose metabolism in the V wild-type strain EC3132, J. Bacteriol., 2002, vol. 184, no. 19, pp. 5307–5316.
Doroshenko, V.G. and Livshits, V.A., Structure and mode of transposition of tn2555 carrying sucrose utilization genes, FEMS Microbiol. Letts., 2004, vol. 233, no. 2, pp. 353–359.
Livshits, V.A., Doroshenko, V.G., Mashko, S.V., Akhverdian, V.Z., and Kozlov, Yu.I., Method of producing amino acids using e. coli transformed with sucrose PTS genes, US Patent No. US717623, C07H21/04, C07K1/00, C12N1/20, C12N1/21, C12N15/09, C12N15/52, C12N9/00, C12N9/12, C12P13/04, C12P13/06, C12P13/08, C12P13/22, C12R1/19, 2007.
Zimenkov, D.V., E. coli chromosome regions preferred for gene insertion when using the integration system based on phage Mu transposon, Skorokhodova, A.Yu., Katashkina, Zh.I., Minaeva, N.I., Savrasova, E.A., Biryukova, I.V., Doroshenko, V.G., Akhverdyan, V.Z., and Mashko, S.V., Biotekhnologiya, 2004, no. 6, pp. 3–18.
Gomez, K., New insights into Escherichia coli metabolites: carbon scavenging, acetate metabolism and carbon recycling responses during growth on glycerol, Martínez-Gomez, K., Flores, N., Castaneda, H.M., Martínez-Batalar, G., Hernandez-Chavez, G., Rairez, O.T., Gosset, G., Encarnacion, S., and Bolivar, F., Microb. Cell Fact., 2012, vol. 11, no. 46. doi: 10.1186/1475-2859-11-46
Da Silva, G.P., Mack, M., and Contiero, J., Glycerol: a promising and abundant carbon source for industrial microbiology, Biotechnol. Adv., 2009, vol. 27, no. 1, pp. 30–39.
Chatzifragkou, A. and Papankikolaou, S., Effect of impurities in biodiesel-derived waste glycerol on the performance and feasibility of biotechnological processes, Appl. Microbiol. Biotechnol., 2012, vol. 95, no. 1, pp. 13–27.
Srinophakum, P., Reakasame, S., Khamduang, M., Packdibamrung, K., and Thanapimmetha, A., Potential of L-phenylalanine production form raw glycerol of palm biodiesel process by a recombinant Escherichia coli, Chiang Mai J., 2012, vol. 39, no. 1, pp. 59–68.
Taylor, P.P., Grinter, N.J., McCarthy, S.L., Pantaleone, D.P., Ton, J.L., Yoshida, R.K., and Fotheringham, I.G., D-phenolalanine biosynthesis using Escherichia coli: creation of a new metabolic pathway, in Applied Biocatalysis in Specialty Chemicals and Pharmaceuticals, Saha, B.C. and Demirjian, D.C., Eds., (ACS Symposium Series, vol. 776), Washington, DC: American Chem. Soc., 2001, pp. 65–75.
Fotheringhan, I.G., Bledig, S.A., and Taylor, P.P., Characterization of the genes encoding d-amino acid transaminase and glutamate racemase, two D-glutamate biosynthetic enzymes of Bacillus sphaericus ATCC 10207, J. Bacteriol., 1998, vol. 180, no. 16, pp. 4319–4323.
Sun, Z., Ning, Y., Liu, L., Liu, Y., Sun, B., Jiang, W., Yang, C., and Yang, S., Metabolic engineering of the L-phenylalanine pathway in Escherichia coli for the production of Sor R-andelic acid, Microb. Cell Fact., 2011, vol. 10, no. 1, pp. 1–13.
Koma, D., Yamanaka, H., Moriyoshi, K., Ohmoto, T., and Sakai, K., Production of aromatic compounds by metabolically engineered Escherichia coli with an expanded shikimate pathway, Appl. Environ. Microbiol., 2012, vol. 78, no. 17, pp. 6203–6216.
Koma, D., Yamanaka, H., Moriyoshi, K., Ohmoto, T., and Sakai, K., A convenient method for multiple insertions of desired genes into target loci on the Escherichia coli chromosome, Appl. Microbiol. Biotechnol., 2012, vol. 93, no. 2, pp. 815–829.
McKenna, R. and Nielsen, D.R., Styrene biosynthesis form glucose by engineered E. coli, Metab. Eng., 2011, vol. 13, no. 5, pp. 544–554.
Koketsu, K., Mitsuhashi, S., and Tabata, K., Identification of homophenylalanine biosynthetic genes from the cyanobacterium Nostoc punctiforme PCC73102 and application to its microbial production by Escherichia coli, Appl. Environ. Microbiol., 2013, vol. 78, no. 17, pp. 2201–2208.
Nijakmp, K., van Luijk, N., de Bont, J.A., and Wery, J., The solvent-tolerant Pseudomonas putida S12 as host for the production of cinnamic acid from glucose, Appl. Microbiol. Biotechnol., 2005, vol. 69, no. 2, pp. 170–177.
Klein-Marcuschamer, D., Santos, C.N., Yu, H., and Stephanopoulos, G., Mutagenesis of the bacterial RNA polymerase alpha subunit for improvement of complex phenotypes, Appl. Environ. Microbiol., 2009, vol. 75, no. 4, pp. 2705–2711.
Author information
Authors and Affiliations
Corresponding author
Additional information
Original Russian Text © V.G. Doroshenko, V.A. Livshits, L.G. Airich, I.S. Shmagina, E.A. Savrasova, M.V. Ovsienko, S.V. Mashko, 2014, published in Biotekhnologiya, 2014, No. 4, pp. 8–27.
Rights and permissions
About this article
Cite this article
Doroshenko, V.G., Livshits, V.A., Airich, L.G. et al. Metabolic engineering of Escherichia coli for the production of phenylalanine and related compounds. Appl Biochem Microbiol 51, 733–750 (2015). https://doi.org/10.1134/S0003683815070017
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0003683815070017