Abstract
Nanotechnology mainly involves the fabrication, manipulation, and utilization of materials in nano size (materials having size less than a micron to that of individual atoms). However, nanoparticles can be synthesized by several chemical and physical approaches; now, it is also possible to integrate the use of biological entities. In recent years, mycogenesis of nanoparticles is considered as a prominent way where fungi can be used for the production of nanostructures with desirable shape and size intracellularly or extracellularly. Several researchers have reported that NADH-dependent nitrate reductase enzyme plays an important role in transformation of metal ions into metal nanoparticles. The size and shape of the nanoparticles depend on the microorganism utilized and experimental condition employed during synthesis process. Nanoparticles synthesized from microbes are safe, environmental benign and have several applications in agriculture, textile, medicine, drug delivery, biochemical sensors and allied areas. Future challenges may include large-scale production, enhancement of stability, reduced time to obtain desirable shape and size and their possible applications in several fields. In this review paper, we provide a brief overview on the emerging role of fungi in the synthesis of metal nanoparticles, possible mechanisms and their potential bio-prospective applications.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the past few decades, metal nanoparticles have attracted considerable attention because they exhibit unique physicochemical properties. Due to their unique characteristic features and importance of nanoparticles in several fields, many scientists, and researchers have worked on the synthesis of nanoparticles using various physical and chemical methods. However, these methods have successfully synthesized the nanoparticles with desirable shape and size but these processes are quite expensive and involve the use of hazardous chemical substances and perilous chemicals [1]. Presently, chemically synthesized silver nanoparticles used for the treatment of several diseases may cause toxic effects in human body. So, to reduce all these extreme conditions, there is a need to develop an environmentally benign approach with low toxicity. It was reported that both unicellular and multicellular organisms synthesize inorganic materials either intracellularly or extracellularly [2]. Synthesis of nanoparticles by green chemistry approaches using biological entities like plants, fungi, bacteria, algae, and actinomycetes provides additional advantages over other methods, as these methods are simple, cost effective, reliable, eco-friendly and can be easily scaled up for large-scale production. To obtain this goal, many researchers and scientists have already directed their interest to the use of biological synthesis approaches [3].
As a consequence, scientists have used biological processes for the nanoparticle synthesis which provides excellent control over particle size via quantum confinement [4], resulting in exploration of relatively new and rapidly growing area of nanotechnology using fungal biomass called “myconanotechnology” [5]. It mainly includes the synthesis protocols of nanoparticles using fungal biomass and their associated metabolites. Fungi contain variety of enzymes and proteins as reducing agents, so they can be invariably used for the synthesis of metal nanoparticles. Fungi usually grow faster than those of bacteria under the same conditions. Although synthesis of metal nanoparticles by bacteria is common, their synthesis by fungi is more advantageous because their mycelia offer a large surface area for interaction. Additionally, fungi secrete large amount of enzymes as compared to bacteria; thus, the conversion of metal salts into metal nanoparticles is very fast. Additionally, the bio-potential of the fungal cell wall is likely to play an important role in the absorption and reduction of metal ions as well as the formation of metal nanoparticles. There are several reports available on nanoparticle synthesis using fungi such as Aspergillus terreus, Aspergillus fumigatus, Aspergillus oryzae, Aspergillus niger, Aspergillus flavus, Aspergillus clavatus, Aspergillus conicus, Alternaria alternata, Agaricus bisporus, Acremonium diospyri, Amylomyces rouxii, Colletotrichum sp., Chrysosporium keratinophilum, Coriolus versicolor, Cladosporium cladosporioides, Chlamydomucor rouxii, Candida utilis, Cryptococcus humicola, Candida glabrata, Candida albicans, Chrysosporium, Epicoccum nigrum, Fusarium semitectum, Fusarium oxysporum, Fusarium solani, Gloeophyllum abietinum, Ganoderma sp., Neurospora intermedia, Phomosis sp., Pleurotus, Pleurotus florida, Pleurotus sajor-caju, Pleurotus ostreatus, Penicillium janthinellum, Penicillium verrucosum, Penicillium citrinum, Penicillium fellutanum, Penicillium chrysogenum, Paecilomyces lilacinus, Phoma glomerata, P. nodositatum, Phanerochaete sordid, Phytophthora, Pestalotia sp., Pycnoporus sanguineus, Phanerochaete chrysosporium, Schizosaccharomyces pombe, Sclerotium rolfsii, Shigella dysenteriae, Trichothecium, Trichoderma versicolor, Trichoderma harzianum, Trichoderma viride, Trichoderma Reesei, Verticillium sp., etc., [6].
Now the technological advance leads to the new good understanding of several physicochemical features, and the 1D, 2D and 3D superstructure knowledge has opened the door for new trend of research and applications of nanoparticles in several fields of science particularly in agriculture, medicine, textile, food industry and environment [7]. Metal nanoparticles exhibit several unique physicochemical properties that are remarkably different from their original bulk form which is due to their large fraction of surface atom, energy and spatial confinement. Due to these unique characteristic features of nanoparticles, they can be used in fields of catalysts, opticals, electronics, environment and biomedical analysis [8]. The possible mechanism behind the nanoparticle synthesis was hypothesized that it may be due to presence of ionic or electrostatic interactions between the metal ions and the functional groups present on the biological surfaces. However, the exact mechanism and the biological compounds in the synthesis of nanoparticles are not well known yet. These require further research in this field. The properties and the function of nanoparticles greatly depend on their size, shape, dispersity, crystallinity and the structure [9]. Biosynthesis of nanoparticles also depends on several experimental parameters during the synthesis process such as temperature, pH, reaction time, concentration of biomass and concentration of metal ions [10, 11]. To obtain desirable shape and size of the nanoparticle, it is necessary to establish the balance between all these factors. Table 1 shows an overview of various biological resources used for the synthesis of nanoparticles.
Nanoparticles have several applications in different fields such as medicine, environment, drug designing, drug delivery, cosmetics, textiles, food industry, optics and optical devices and more to count on. Nanoparticles also exhibit significant antimicrobial activity towards a diverse range of pathogens, and lead to considerable interest of researchers due to increasing resistance of microbes against the available antibiotics and development of resistant microbial strains. It has been found that nanoparticles-based drugs are easily suspended in liquids and are capable to penetrate into the cell, tissues or organs more specifically. Recently, nanoparticles are being synthesized with different chemical compositions and controlled monodispersity [12]. Nanoparticles have been used for the thousands of years for many purposes, e.g., gold nanoparticles which were used for the polishing of glasses also cured various diseases. Gold nanoparticles have been also found to be used for delivery of drugs like paclitaxel, methotrexate and doxorubicin to the specific cells or organs [13]. Silver nanoparticles have used in sensor technology, labelling, wounds healing, sterilising agents and several other biomedical applications. Platinum nanoparticles are also widely applicable as catalysts for many medical applications in combination with other nanoparticles as in alloy, core shell and bimetallic structures [14]. Nanoparticles synthesis by biological roots has several applications in biological fields like DNA sequencing and pharmaceuticals, biosensors, bimolecular detection and diagnostics, Tissue engineering, genetic engineering, photonics, therapeutics and catalysis, optics and optoelectronics, etc. Silver nanoparticles have broad range of applications, such as they are used in water filtering apparatus, catalysis, optical receptor for bio-labeling, antimicrobial activities, anticancer activities, antioxidants, anti-dermatophytic, anti-inflammatory, antitumor, hepatoprotective, cytotoxic, and immunomodulatory hypotensive activities, anti-HIV (human immunodeficiency virus), anti-diabetic, wound dressing, surgical masks, food packaging, paints, additives in bone cement, etc., [15].
In this review, we will mainly focus on the synthesis of metal nanoparticles using fungi and possible mechanism that could be involved in the reduction process as well as in stabilization process of the nanoparticles. Along with this, we also discuss about their potential bio-prospective applications.
Microorganisms used for biosynthesis of nanoparticle
Synthesis of nanoparticles using microbes is an alternative way that is mainly based on principle of green chemistry. Biomolecules like enzymes and proteins secreted by the microbial biomass can cat as reducing and capping agents during the synthesis process. Hence, these approaches are considered as green chemistry because it does not include any toxic chemicals [15]. Several microorganisms like bacteria, fungi, viruses and algae are well known for their potential to synthesize metal. Du et al. [16] reported that gold nanoparticles were synthesized by E. coli DH5α on the cell surface of these bacteria. Similarly, silica nanoparticles were synthesized by Fusarium oxysporum. Narayanan and Sakthivel [17] reported the synthesis of monodispersed peptide bound CdS nanoparticles by Candida glubrata. In 2010, they also reported the biosynthesis of iron oxide nanoparticles by Tobacco Mosaic Virus (TMV). Despite the numerous advantages of microbes-mediated nanoparticle synthesis, there are limitations too. One of the major problems is the difficulties to produce controlled shape, size and monodispersity of metal nanoparticles.
Usually, bacteria synthesize inorganic materials either extracellularly or intracellularly. In one of the studies, bacterial strain (Pseudomonas stutzari AG259) isolated from silver mines was reported for the synthesis of silver nanoparticle extracellularly [18]. Similarly, magnetotatic bacteria Magnetospirillium magneticum was also reported for the synthesis of magnetic nanoparticles [19]. They produce two types of nanoparticles, some of them produce magnetic nanoparticles (Fe3O4) in chains and others produce greigite (Fe3S4) nanoparticles, while some other produce both types of nanoparticles. Desulfovibrio desulfuricans NCIMB 8307 which is a sulfate-reducing bacteria reported for the synthesis of palladium nanoparticles in the presence of exogenous electron donor [20]. Lactobacillus species, commonly found in butter milk, was also reported for the synthesis of gold, silver and gold–silver alloy nano crystals of well-defined morphology. Husseiny et al. [21] reported the synthesis of gold nanoparticles using the bacterial cell supernatant of Pseudomonas aeruginosa. Cell filtrate of bacteria plays an important role in achieving better control over size, shape and polydispersity of nanoparticles. Later, it has been found that synthesis of nanoparticles using extracellular cell filtrate is more beneficial over intracellular metabolites. A filamentous cyanobacteria Plectonema boryanum UTEX485 were used for their reduction of gold ions into gold nanoparticles. It synthesized the gold nanoparticles with better morphological control of shape. When P. boryanum was treated with aqueous solution of Au(S2O3) −32 and AuCl4− at 25–100 °C for up to 1 month and for 1 day at 200 °C, resulted in the synthesis of cubic gold nanoparticles and octahedral gold nanoparticles, respectively [22]. Later, they explained the mechanism of gold bioaccumulation of cyanobacteria from Au(III) chloride solution. They reported that interaction of cyanobacteria with gold chloride solution initially promotes the synthesis of nanoparticles of amorphous gold (I) sulfide at the cell wall and finally gold nanoparticles were triggered in the form of octahedral platelet around the cell surface in solution. Further study also reveals that sulfate-reducing bacteria destabilize the gold (I) thiosulfate complex to metal gold and suggested that this could be achieved by three possible mechanisms involving iron sulfide localized reducing conditions and metabolism [22]. Some of the hyperthermophilic and mesophilic dissimilatory iron (Fe3+)-reducing bacteria and archaebacteria like Thermotoga maritime, Pyrobalaculum islandicum, Pyrococcus furiosus and Geobacter sulfurreducens have the capability to reduce metallic gold ions into gold nanoparticles in the presence of hydrogen as electron donor. Reduction occurs extracellularly due to the presence of Au(III) reductases near the outer cell surfaces of Fe3+ reducers [23].
As we know, algae are aquatic microorganisms and recently it has been found that some of them not only accumulate heavy metal ions but also synthesize metal nanoparticles. Chlorella vulgaris, a dried unicellular alga, is reported for the reduction of HAucl −4 ions into gold nanoparticles bound to the cell surface of the algal body. Accumulated gold nanoparticles were tetrahedral, decahedral and icosahedral in shapes [24]. Synthesis of silver nanoparticles using the extract of same algal species C. vulgaris at room temperature was reported by Xie et al. [25]. After detailed study, they concluded that the proteins present in the extract acted as reducing agent, shape controlling agent and stabilizing agent during biosynthesis process. Sargassum weightii which is a marine alga was also used for the extracellular synthesis of Au, Ag and Au/Ag bimetallic nanoparticles by Madhiyazhagan et al. [26]. Recently, it has been also reported that S. weightii could rapidly synthesize gold nanoparticles. They synthesize gold nanoparticles extracellularly and had size of range 8–12 nm. Mata et al. [27] reported the synthesis of gold nanoparticles using the biomass derived from brown alga Fucus vesiculosus. In contrast, Abdel-Raouf et al. [28] used the Kappaphycus alvarezii for extracellular synthesis of gold nanoparticles. Synthesis of gold and silver nanoparticles was recently reported by red and green alga Chondrus crispus and Spirogyra insignis, respectively [29]. Intracellular synthesis of gold nanoparticles using Tetraselnis Kochinensis was also reported by Iravani et al. [30].
Extremophilic actinomycetes like Thermomonospora sp. when treated with gold ions resulted in the production of gold metal nanoparticles extracellularly with a much improved polydispersity [31]. Ahmad et al. [32] carried out a study to elucidate the mechanism or condition which leads to the synthesis of metal nanoparticles. They carried out a study for the bio-reduction of AuCl4− ions by an extremophilic Thermomonospora sp. They found that reduction of metal ions and stabilization of the gold nanoparticles were described by an enzymatic process. When they compared their earlier reports of synthesis of gold nanoparticles using Fusarium oxysporum, they found that monodispersed gold nanoparticles were synthesized which might be due to extreme physiological conditions such as alkaline and slightly elevated temperature conditions. Similarly, an alkotolerant Rhodococcus sp. is reported for the synthesis of gold nanoparticles. During their study, they found that concentration of metal ions was higher on the cytoplasmic membrane and cell wall as compared to the cytosol, that is might be due to the reduction of metal ions by enzymes present on the cell wall and cytoplasmic membrane but not in cytosol. Apart from this, it has been found that these metal ions were not toxic to the synthesizing cells, and they were continued to multiply even after the biosynthesis of gold nanoparticles.
Synthesis of nanoparticles using virus is a novel technique that has been used to deliver the inorganic materials such as silicon dioxide (SiO2), Zinc sulfide (ZnS), iron dioxide (Fe2O3) and cadmium sulfide (CdS). Viruses have attractive features; they have dense surface covering of capsid proteins, which form a highly reactive surface capable of interacting with metallic ions [33]. A typical plant virus Tobacco mosaic virus (TMV) contains as many as 2130 capsid protein molecules on their covering surface. These arrays of protein molecules can act as attachment points for the dispersion of nano-sized materials. They can also be used to prepare 3D’ structure and vessels for pharmaceuticals [33]. In a study, when TMVs were added at a very low concentration to silver or gold salts before adding plant extracts of Nicotiana benthamiana, they found that the virus mediated solution not only decreased the size of the synthesized nanoparticles but also drastically increased the number of nanoparticles as compared to control. As a result it was concluded that TMV acted as a bio-template that converts metallic ions into nanowires. In addition, the potential of other viruses as a template for the synthesis of nanowires and nanotubes was also studied [34].
Mycogenesis of metal nanoparticles and their possible mechanism
Nature provides a good source of microorganisms as a biological factory to synthesize metal nanoparticles with potential bio-prospective applications [35]. Recently, the new synthetic pathway and applications of nano-biotechnology have attracted the peoples to work on this field. Synthesis of nanoparticles using microbes have great potential and huge benefits to human, because they eliminate the use of toxic chemicals and minimize the production cost involved in the synthesis. One of the main advantages of biosynthesis of metal nanoparticles is their immense role in the protection of environment which is also the ultimate target of other green technologies [36, 37]. Recently, mycogenesis of nanoparticles has attracted much attention of researchers; however, the exact mechanism related to the synthesis has not been understood yet. Fungi are a kingdom of usually multicellular eukaryotic organisms that are heterotrophs and have important role in nutrient cycling in an ecosystem. Fungi reproduce both sexually and asexually, and they also have symbiotic associations with plant and bacteria. Group of fungi majorly includes moulds, mildews, yeasts, rusts, and mushrooms [38]. As compared to bacteria, fungi produce large quantity of nanoparticles as they secreted large quantity of enzymes which directly involved in the bioreduction and stabilization process of nanoparticles. Enzymes secreted by these organisms, especially in case of fungi the reductases enzyme, might be involved in the mechanisms of nanoparticle production and stabilization. As the cell surface of the fungal biomass contains negative charge and contains sticky substances, the metal ions attach on the cell surface due to these adhesive substances and the electrostatic interaction. As we know, fungi synthesize nanoparticles both extracellularly and intracellularly. Intracellular synthesis method is suitable for formation of composite films. However, in extracellular synthesis method, the immobilization of metal ions in suitable carrier or support is achieved [39]. The key components, metabolites and the enzymes present in the fungi have great importance and they are secreted by the fungal cells to convert toxic matters into non-toxic matters; these substances might be also involved in the synthesis process of nanoparticles [40].
Trichoderma reesei is a fungus, which secreted proteins and enzymes outside the hyphae and plays a key role for synthesis of silver nanoparticles. It has been found that nanoparticle synthesized from fungi is mostly monodispersed with well-defined size and shape and also have different chemical compositions. As they secreted a number of enzymes inside and outside the cells, their handling is simple as compared to other microbes [41]. Additionally, the mycogenesis of nanoparticle is an emerging and new field of nano-biotechnology with significant potential because of wide spectrum diversity and availability of fungi [13]. As compared to bacteria, fungi have greater tolerance and uptake capacity for metals particularly in terms of binding capacity of metal salts; hence, fungal biomass is an advantage for large-scale production of nanoparticles. Including this, their downstream processing is quite easy so that it is easy to cultivate for the efficient low-cost synthesis of metal nanoparticles [42]. Various fungi have already known for the fabrication of several metal nanoparticles like Au, Ag, Pd, Cu, Zn, Ti, Fe and Pt [5]. Fungi show slower kinetics, so that they offer better manipulation and control over shape and size of nanoparticles along with their long-time stability [43]. Table 2 summarizes the different fungi that have used to synthesize a variety of nanoparticles including their composition, size, shape and applications.
Fungus-mediated synthesis of nanoparticles has been proposed by their possible mechanism, action of nitrate reductase, electron shuttle quinones or both. It has been reported that mainly nitrate reductase and α-NADPH-dependent reductases were found to be responsible for nanoparticle synthesis in both bacteria and fungi. Alghuthaymi et al. [44] found that fungus Verticillium sp. was used for the reduction of aqueous AuCl−4 ions leading to the synthesis of fairly well-desirable structure and good monodispersity of gold nanoparticles. Further detailed study in this has revealed that the metal ions AuCl−4 were trapped on the surface of fungal biomass by electrostatic interaction with their charged amino acid residues present in fungal enzymes in the cell wall of the fungal mycelia.
Nanoparticles synthesized extracellularly were stabilized by the proteins and enzymes secreted by the fungal biomass. In a research, it has been found that minimum four proteins which are high molecular weight have been found in association with nanoparticles synthesis. One of them was NADH-dependent reductase. The fluorescence spectra of these enzymes indicate the native form of these proteins present in the aqueous extract or solution as well as on the surface of nanoparticles in binding condition [45]. Further reports revealed that the reduction of metal ions and the surface binding molecule to the nanoparticles did not compromise the 3D structure of the proteins. For instance, an endophytic fungus Colletotrichum sp. isolated from Geranium leaf was used for the synthesis of gold nanoparticles; the synthesized nanoparticles was found to be highly stable and have different shape and size. The study concluded that fungus contains polypeptides and enzymes which might act as a reducing agent [46].
Hulkoti et al. [47] reported that protein isolated from the fungal biomass can also be successfully used for the synthesis of metal nanoparticles by cationic proteins nanocrystalline zirconium at room temperature. More studies on this confirmed that these proteins were similar in nature to silicatein, which is secreted by Fusarium oxysporum and they have the capability of hydrolyzing aqueous ZrF −26 ions extracellularly. In mycogenesis of nanoparticles, growth conditions play an important role during the synthesis of nanoparticles. Trichothecium sp. when incubated with gold ions under static conditions resulted in the formation of extracellular nanoparticles; while under shaking condition, they synthesized intracellular gold nanoparticles. The possible mechanism for this might be the enzymes and proteins that were released by fungal under stationary condition and did not secrete under shaking condition [48]. Synthesis of magnetic nanoparticles at room temperature was achieved by Fusarium oxysporum and Verticillium sp. [49]. The enzyme secreted by these fungi was capable of hydrolyzing the metal ions precursor (Fe3+) extracellularly to form metal oxide (Iron oxide) in the magnetite phase (Fe3O4).
Fusarium oxysporum also secreted a nitrate-dependent reductase and a shuttle quinone that were capable of synthesizing silver nanoparticles or silver hydrosols extracellularly. In a similar study, it was revealed that it was not true for all the species of Fusarium; one example of this is F. moniliforme that produces reductase enzyme but it could not be able to reduce silver ions into silver nanoparticles [50].
Shahi and Patra [51] reported that the lichenized fungi Usnea longissima have ability to synthesize bioactive nanoparticles (usnic acid) in respective medium. On the other hand, extracellular synthesis of silver nanoparticles was achieved by cell-free extract of Phoma sp. 3.28383 and Phoma glomerata. In another approach, synthesis of stable nanoparticles of Au–Ag alloys of several compounds has been achieved by controlling the amount of cofactor NADH [52]. Under in vitro condition, α-NADPH-dependent nitrate reductase and phytochelatin isolated from F. oxysporum have been applied for silver nanoparticle synthesis [53].
Bharde et al. [54] reported the extracellular synthesis of magnetic nanoparticles at room temperature by the cell filtrate of Vertillium sp. treated with aqueous solution of ferric chloride. Further investigation in this study suggested that hydrolysis of the anionic iron complexes occurs by cationic protein secreted by the fungi resulting in the formation of crystalline magnetic nanoparticles. TEM analysis showed a number of cubo-octahedrally shaped iron oxide particle ranging in size from 100 to 400 nm.
Mukherjee et al. [55] and Devi and Joshi et al. [56] reported the uses of whole fungus systems for the synthesis of gold and silver nanoparticles. They worked on Verticillium sp. for intracellular synthesis of gold and silver nanoparticles in the size between 2 and 20 nm. Synthesis of silver nanoparticles and gold crystals was achieved by intra- and extracellular processes by fungal system and actinomycetes, respectively. They found a number of silver nanoparticles on the surface of the mycelial wall of Verticillium sp. Mycelia of an individual Verticillium cell showed that silver nanoparticles closely bound to the surface of the cytoplasmic membrane, which confirms the intracellular synthesis of silver nanoparticles. TEM analysis revealed that the synthesised silver nanoparticles in Verticillium cells were 25 ± 12 nm in diameter. In another study, it has been found that when Thermomonospora sp. were exposed to aqueous gold solution, reduction takes place extracellularly. Synthesised gold nanoparticles have much improved shape, polydispersity and size (7–12 nm). Extracellular biosynthesis of nano-particulate magnetite at room temperature was achieved after treatment of cell filtrate of fungus Verticillium sp. with ferric and ferrous salts. They also studied the mechanical aspects. According to them, the cationic proteins and enzymes present on fungal cells hydrolyses the anionic iron complexes of the ferric and ferrous salts, and leads to the synthesis of crystalline magnetic nanoparticles. These magnetic nanoparticles contain the signature of a ferromagnetic transition which shows a small amount of spontaneous magnetization at low temperature. TEM analysis results showed a large number of cubo-octahedral shaped iron oxide nanoparticles of 100–400 nm size. Recently Volvariella sp., Penicillium sp., and other fungi have been reported for this purpose, yet microbial system like Fusarium sp. and Aspergillus sp. were used more prominently.
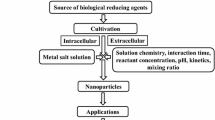
Aspergillus was most studied genus for the mycosynthesis of nanoparticles. For instance, Aspergillus fumigatus reported for the synthesis of silver nanoparticles extracellularly [57]. Gade et al. [58] synthesized silver nanoparticles from A. niger isolated from soil. They also suggested the mechanism for the antimicrobial action of silver nanoparticles on E. coli. Vigneshwaran et al. [59] reported the production of silver nanoparticles intracellularly by treating the aqueous solution of silver ions with the cell-free extract of fungi; they found that silver nanoparticles were synthesized in the fungal cell wall. In another study extracellular synthesis of silver nanoparticles was reported by using A. flavus isolated from soil of Ahar copper mines. Abdel-Hafez et al. [60] also reported the extracellular synthesis of silver nanoparticles using fungus Cladosporium cladosporioids. Results of TEM analysis showed polydispersed, spherical particles with the size ranging from 10 to 100 nm. Aspergillus fumigatus were found to be excellent source as they reduce silver ions into silver nanoparticles within 10 min [61]. Synthesized nanoparticles were fairly monodispersed and are highly stable. This was the first report of rapid synthesis of nanoparticles using fungi. The synthesis was also faster than that of chemical and physical approaches of synthesis. Therefore, the following method could be applicable for large scale production of metal nanoparticles. On the other hand, Bansal et al. [62] uses F. oxysporum for the production of tetragonal barium titanate (BaTio3) nanoparticles of approximately 10 nm dimensions, under ambient condition. The synthesized nanoparticles also exhibit ferroelectric properties that would transform the electronic industries by producing ultra-small capacitors and high density non-volatile ferromagnetic memory chips. Kumar et al. [63] also reported the synthesis of luminescent CdSe quantam dots using fungus F. oxysporum at room temperature with the mixture of CdCl2 and SeCl4. Figure 1 shows the general protocol for the mycogenesis of metal nanoparticles and their characterization.
Das et al. [64] investigated the synthesis of gold nanoparticles using fungus Rhizopus oryzae. In the study, they incubated the R. oryzae mycelia with HAuCl4 solution results in gradual color changes from pale white to purple. After 48 h of incubation, synthesized gold nanoparticles were characterized by UV–visible spectroscopy, showing a peak at 535 nm which confirms the presence of gold nanoparticles. Fungal proteins responsible for gold nanoparticles synthesis were particularly purified by separation of the membrane fraction from whole cell-free protein extract and then salted out using ammonium sulfate precipitation. Further study of this to evaluate that growing mycelia in the presence of gold ions shows the metabolism of gold ions by R. oryzae. Effects of Au(III) in the metabolism of R. oryzae was further studied to describe the bio-mineralization mechanism to investigate the role of Au(III) induced proteins in Au mineralization by the quantification of the total protein concentration in the mycelia. Results of this study suggested that R. oryzae anchorage various gold detoxification mechanism to reduce gold ions to gold nanoparticles. Biotransformation mechanisms of gold ions into gold nanoparticles by fungi can be described by the two reduction routes: (a) binding of Au(III) on the cell wall through electrostatic interaction followed by reduction to gold nanoparticles and second is (b) transportation of Au(III) into the cytoplasm and reduction to form Au nanoparticles. Negatively charged gold ions bind to the positively charged mycelia of R. oryzae through electrostatic interaction with phosphoproteins and then become reduced to Au(I) species as a result of the high redox potential of Au(III).
Chemical reaction mechanism based on the three reduction steps of gold ions into gold atoms and then precipitation into gold nanoparticles is shown below:
Bansal et al. [65] reported the synthesis of Silica and Titania nanoparticles by Fusarium oxysporum from their respective salts, i.e., K2SiF6 and K2TiF6. The size of synthesized nanoparticles was ranging from 5 to 15 nm with 9.8 ± 0.2 nm diameter. Similarly, Duran et al. [50] investigated the synthesis of metal nanoparticles by several species of Fusarium. They reported that when aqueous metal salt solution of silver ions was mixed with biomass filtrate of F. oxysporum, after few hours, reduction of silver ions into silver nanoparticles occurred. They also reported that biosynthesized nanoparticles were 20–50 nm in size. On the basis of their study, they proposed the mechanism that the reduction of metal ions occurs by a nitrate-dependent reductase enzyme and a shuttle of quinones extracellular process. In the year 2006, Riddin et al. [66] also reported that F. oxysporum, f. sp. lycopersici causes wilt diseases in tomato. They successfully isolated them and used for the synthesis of platinum nanoparticles. Another species F. ocuminatum isolated from infected ginger plants were also successfully used for the biosynthesis of silver nanoparticles [67]. They further explained that NADH-dependent nitrate reductase plays a crucial role in the synthesis of nanoparticles. They reported the formation of polydispersed spherical nanoparticles of 4–50 nm size and 13 nm diameter. Synthesis of magnetite nanoparticles from F. oxysporum strain was achieved by Bharde et al. [54]. Another species Fusarium, F. solani was isolated from infected fungi was also reported from synthesis of silver nanoparticles [68]. Synthesized silver nanoparticle were polydispersed, spherical and within the range of 5–35 nm. Figure 2 shows the possible mechanism of nanoparticle synthesis using fungi.
Fungi are eukaryotic organisms and due to this they have remarkable advantages in terms of metabolic flux and cellular level organizations. Including these fungi like Penicillium and Aspergillus sp. elaborates a large number of simple hydroxyl or methoxy derivatives of benzoquinones or toluquinones as a response of metallic stress. Jha and Prasad [69] found that the presence of these metabolites may generate a redox reaction due to tautomerization, leading to production of nanomaterials. Cell membrane of fungi contains small molecular mass metabolites like peptides and proteins, which play an important role during the detoxification process of metal or metal oxide nanoparticles and hence the significance of extracellular and cytoplasmic chelation reaction can not be underestimated [70, 71]. In this process, the membrane bound oxidoreductases and quinones might have played a crucial role in the process. Oxidoreductases enzymes are sensitive to pH and work in alternative manner. At a high pH reductase, they get inactivated while at low pH, they get activated [69]. Amino acids and amino-containing molecules like proline are frequently synthesized under heavy metal stress like Cd, Cu, Ni and Zn. Proline detoxifies the metal ions by following three ways specifically metal binding, antioxidant defence and signaling [72].
Yeast is also reported for the synthesis of metal nanoparticles. Yeast itself has been a good source of enzymes, proteins and vitamins. They have been also used as regular basis of media supplement in various culture protocols. In terms of culturing and handling they are nontoxic, handy and amenable. Saccharomyces cerevisiae also known as baker’s yeast has also been used to study their ability as putative candidate fungal genera for the production of metal and metal oxide nanoparticles, specifically due to their well-organized cellular structure and metabolic fluxes. Synthesis of nanoparticles using filamentous fungi has some additional advantages as compared to other organisms as they are easy to handle, have high wall binding affinity and require simple raw materials [73, 74]. In yeast under specific condition, glutathione (GSH) secretion is a very important element of the GSH homeostasis [75]. It has been found that GSH secretion under As (III) exposure initiates intracellular detoxification pathway. Clausen and Green [76] found that elaboration of inorganic metal chelators is seen among members of fungi especially white rot and brown rot fungi and this process is stimulated under Cu (III) and Cu (II) stress condition. Formation of water-insoluble metal-oxalate crystals is undoubtedly an efficient method to circumvent toxic metal ions which tend to enter fungal cells. As well as oxalate is important to maintain the lignolytic system of white rot basidiomycetes fungi [77].
Fungi produces metabolites like citric acid, peroxidases, homogenous proteins and heterogeneous proteins which made them effective source for bioreduction of heavy metals from industrial effluents. A large number of fungi have been reported for the extracellular secretion of mucilaginous materials (ECMM or emulsifier) which also comprises excellent toxic metal binding capabilities. Paraszkiewicz et al. [78] found that unlike other metal ions like (Cu II, Pb II and Zn II), Ni (II) did not stimulate ECMM production by Curvularia lunata. They also found that saturation of cellular fatty acids which is clearly characterized by Ni (II) triggered lipid peroxidation processes. Aureobasidium pullulans which produces pullulan was positively affected by Ni (II) and Cd (II) exposures and pullulan production increased the Cd (II) tolerance of this industrially important fungal species [79, 80]. High ratio of ECMM in the biomass of Trametes versicolor and Gloeophyllum trabeum was reported when biomass of these two was exposed to Cu (II) [81]. Tripathy et al. [82] also described that white rot fungus is ubiquitous in nature, and enzymes produced by them have ability to reduce the toxicity of metals by bio-sorption and decolorization, ultimately rendering the effluents eco-friendly.
Other fungi like Fusarium oxysporum, Aspergillus niger, A. fumigatus and Penicillum brevicompactum are being exhibited well-organized cellular systems as compared to bacteria. Jha et al. [83] showed that Fusarium oxysporum 10T5115 in MGYP media was able to convert silver nitrate into silver nanoparticles in the presence of enzyme NADH-dependent nitrate reductase under shaking condition. Volvariella volvacea and Agaricus bisporus (edible mushrooms) have also been reported for the synthesis of gold and silver nanoparticles which is achieved through various enzymes and amino acids [84].
Due to the cellular complexity of the fungi, further more research is necessary to explore the synthesis mechanism so that a better process can be designed. To obtain definite shape and size and to increase the rate of synthesis of nanoparticles, cellular and bio-chemical mechanism could be studied in detail. With the recent progress and the on-going work efforts in this field, they explored the several applications in various fields. However, the knowledge of exact mechanism for the synthesis of nanoparticles using fungi required more study and experimental trials.
Characterization techniques for nanoparticles
Characterization of nanoparticles includes the study of different forms of nanoparticles which comprise shape, size and structure, which is necessary for material research in general. According to Liu et al. [85] nanomaterials comprise unique structure and size which are expected to have various biological functions. Novel discovery of materials processes and phenomenon at the micro- and nano-scale will provide fresh opportunities for production of novel nanosystems and nanostructures. Characterization of metal nanoparticles is done by molecular techniques such as UV–visible spectroscopy, X-ray diffraction technique (XRD), Fourier transform infrared spectroscopy (FTIR), Atomic force microscopy (AFM), Scanning electron microscopy (SEM), Transmission electron microscopy (TEM), Energy Dispersive X-ray Spectroscopy (EDX), etc., (Table 3). Details of these techniques are discussed below:
UV–visible spectroscopy
UV–visible spectroscopy is a very reliable and useful technique for the primary characterization of synthesized nanoparticles which is also used to monitor the synthesis and stability of AgNPs. UV–visible spectroscopy is a useful technique to characterize noble metal nanoparticles, as they have bright color which is visible by naked eye. These nanoparticles have high extinction coefficient and their surface plasmon resonance is size and shape dependent. Hence, the qualitative information of the nanoparticles can be achieved by this technique. Absorption is mainly measured by Beers law. Absorption value (A) mainly depends on nanoparticle concentration, path length (l) of measuring cell and extinction coefficient of nanoparticles (ε):
Position of the absorption band mainly depends on particle size, shape, morphology, nature of stabilizing agent, pH, temperature, adsorbate that are present on the surface of nanoparticles and nature of the surrounding medium. The absorption band width increases with decreasing size of nanoparticle in the intrinsic size region and also increases with increase in the extrinsic size region [86, 87]. As the particle size increases, the SPR shows a red shift in the extrinsic region. With increase in the size, the gold nanoparticles show color change from ruby red to purple and finally blue. It was reported that when the space between the particles becomes less than their average diameter, the particles aggregated; hence, the plasmon resonance of each particle couple and their absorbance is red shifted. These optical properties of metal nanoparticles can be used for the development of sensors. In addition, UV–Vis spectroscopy is fast, easy, simple and sensitive for different types of nanoparticles, needs only a very short period for measurement and finally a calibration is not required for particle characterization of colloidal suspensions [88].
Fourier transform infrared spectroscopy (FTIR)
Fourier transform infrared spectroscopy is used to identify specific type of chemical bonds or functional groups based on their specific unique absorption signatures by measuring the chemical bond stretching and bending by absorption of energy through infrared spectroscopy. This energy is in the infra-red (IR) region of electromagnetic spectrum [89]. FTIR is an important technique as it gives several advantages over conventional IR spectroscopy. FTIR gives the information about surface chemistry of nanoparticles by identifying the functional groups attached to the metal nanoparticles surface as it shows different identical absorption pattern than corresponding to those of free groups.
X-ray diffraction technique (XRD)
X-ray diffraction technique is a non-destructive technique and used to identify crystalline phase of nanoparticles. Crystalline or powdered sample is placed over a sample holder and then illustrated with X-rays of a fixed wavelength and the intensity of the reflected radiation is recorded using a goniometer [87]. Data are then analyzed by Bragg’s equation, to calculate inter atomic spacing for the reflection angle:
where n is an integer, λ is wavelength, d is the distance between atomic planes and θ is the angle of incidence of the X-ray beam and the atomic planes. Metal nanoparticles give broad peaks on XRD. The broadening provides information about crystalline nature and size by Debye–Scherrer equation:
In this equation, к is the shape factor, λ is X-ray wavelength, β is the line broadening at half the maximum intensity (FWHM) in radius and θ is the Bragg’s angle.
Atomic force microscopy (AFM)
Atomic force microscopy is a useful analytical tool which gives information about the surface morphology and phase through production of a 3D map of the sample surface. The instrument has vertical resolution of less than 0.1 nm and lateral resolution of around 1 nm. A cantilever with a fine pointed tip which is mounted perpendicular to the longitudinal direction of the cantilever is placed on the sample. A laser beam is reflected from the back of the cantilever into a position sensitive to photodiode and records the deflection of the cantilever caused by Vander Waal’s force acting between the sample and tip. This interaction generates signals. These signals are further processed to achieve the topographical information of the surface.
Transmission electron microscopy (TEM)
Transmission electron microscopy is used to identify the shape, size and dispersity of the metal nanoparticles. Molecules having size less than 1 µm cannot be observed by optical microscopy because of diffraction effects. Information about a monograph depends on the applied resolution and the resolution mainly depends on the wavelength of the radiation beam selected on the imaging. High resolution is achieved by a short wavelength beam. TEM is most widely used technique to characterize the metal nanoparticles. The instrument is like a slide projector except that they shine a beam of electrons (around 100–300 kV) and the transmitted beam is projected on to a phosphor screen for final image formation. It generally provides information about topography, monodispersity, composition and the crystallinity of the sample.
Scanning electron microscopy (SEM)
Presently, the field of nanoscience and nanotechnology has provided very high-resolution microscopy techniques to learn and illustrate nanomaterials very deeply by a ray of high-energy electrons to probe. SEM is used to identify morphology and topology of the metal nanoparticles. In SEM, the image of surface of specimen is achieved by scanning the surface through electron beam of accelerated voltage. Backscattered and the secondary electrons are collected by the detector and analyzed for obtaining the pictures or image. SEM is basically a surface imaging method, fully capable of resolving different particle sizes, size distributions, nanomaterial shapes and the surface topology of the particles at the micro- or nanoscale [89]. The limitation of SEM is that, it only provides valuable information regarding the purity and the degree of particle aggregation and it is not able to resolve the internal structure. The SEM is applicable to identify the morphology of nanoparticles below the level of 10 nm.
High-resolution transmission electron microscopy (HRTEM)
High-resolution transmission electron microscopy is an imaging mode of the TEM which is used for the imaging of the crystallographic structure of a sample at an atomic scale [90]. Due to its high resolution, it is an important technique to study the properties of a nanocrystalline structure such as metals and semiconductors. HRTEM is used to investigate the highest resolution at 0.8 Å.
Brunauer–Emmett–Teller (BET)
Surface area of materials has been observed by BET. Experimental surface areas are most commonly obtained through the analysis of adsorption isotherms of nitrogen or some other gas. For this analysis, the BET method is used. The technique is mainly based on a well-defined adsorption model and it gives a monolayer capacity of the materials. This capacity is a well-defined quantity and can be used to compare experimental and simulated systems. To convert to a surface area, a value for the monolayer density is needed, which is obtained experimentally using a reference system of known surface area. The requirement of this method is due to the accuracy of the monolayer density which is transferable, i.e., it is not dependent on the surface curvature or pore structure and not strongly dependent on the chemistry of the underlying surface.
Electron energy loss spectroscopy (EELS)
Electron energy loss spectroscopy is useful for analysis of the elemental components of materials, in this the energy transferred in such an interaction which is directly related to the ionization potential of the atom, and therefore, the spectrum can be compared to that of known samples or standard sample. A material is firstly exposed to a beam of kinetic energies; due to this, some of the electrons lose energy by inelastic scattering, which is primarily an interaction of the sample. The inelastic scattering results in both a loss of energy and a change in momentum. These interactions may be plasmon excitations or inner shell ionization, phonon excitation, and inter- and intra-band transitions. It is useful for detecting the elemental components of a material, as the energy transferred in such an interaction is related to the ionization potential of the atom and thus, the spectrum can be compared to that of known samples [89].
Energy dispersive X-ray spectroscopy (EDS or EDX)
Energy dispersive X-ray spectroscopy (EDS or EDX) is an analytical tool that is specifically used for chemical characterizations of the nanoparticles. The technique is based on the fundamental properties that each element of the periodic table has a unique electronic structure, and hence has a unique response to electromagnetic waves. Its function depends on the investigation of a sample through interaction between matter and light, analyzing through X-rays penetration.
Thermo-gravimetric/differential thermal analyzer (TG/DTA)
The TG/DTA is an instrument combining TG, which performs a continuous measurement and uses a horizontal differential type balance beam, with the highly flexible DTA feature. The instrument is mainly used for the measurement of reaction velocity and acceleration degradation tests, as well as analysis of water contamination, heavy metal detection and ash content in reaction velocity and acceleration decomposition [91]. A horizontal differential type balance beam is utilized in this instrument. This light weight structure of the balance beam provides the stability in regard to temperature fluctuations and highly sensitive balance as well as stability of the differential balance to deal with disturbance such as oscillation. By the cooling unit, the instrument is automatically cooled to a set temperature after measurements, which raise the effectiveness of measurements.
Influence of various experimental parameters on mycogenic metal nanoparticles
Even with several advantages of biosynthesis approaches for nanoparticles, polydispersity of nanoparticles is always a major problem for researchers. Hence, the nanoparticle should be homogenous in size and morphology. Several studies have been carried out to obtain and establish a stable root for production of nanoparticles with definite size, shape and dispersity. Shape and size of the nanoparticle can be controlled by either constricting their environmental growth or calculating the functional molecule. Gurunathan et al. [92] reported the biosynthesis of 20 nm gold nanoparticles by Ganoderna sp. by altering the reaction conditions including temperature, pH, metal ion concentration, mixing ratio, aeration, incubation period and redox conditions. Similar study was carried out by Karbasian et al. [93]; they used response surface methodology to explore the effect of pH, temperature, incubation period, metal ion concentration, agitation rate and amount of enzyme secreted by the fungal biomass on the formation of metal nanoparticles. They synthesized spherical-shaped silver nanoparticles by addition of Fusarium oxysporum in 3 mM silver nitrate solution at pH 6 and incubated with 180 rpm for 96 h. The maximum optimal growth temperature for the growing microorganisms is suggested for the synthesis of metal nanoparticles because the enzymes and proteins responsible for the synthesis of nanoparticles are highly active at high temperature [94]. Dhillon et al. [95] reported that temperature plays an important role in controlling the fungal activities and the movement of metal ions. So, it is concluded that temperature has remarkable effect on the growth of fungus as well as metal uptake from the surrounding reaction mixture. Effect of metal ion concentration was studied by Kathiresan et al. [96]. They synthesized silver nanoparticles with fungus Penicillium fellutanum. They found that high concentration of metal ion would hamper the synthesis of nanoparticles. As the concentration of silver ions increases, particles size and the monodispersity of particles vary from desired nano-size range. When compared to chemical reactions, results are similar, the concentration of reactants greatly affects the particle size and monodispersity of the nanoparticles. Another study was carried out by Gericke and Pinches [97], they synthesized gold nanoparticles by Verticillium luteoalbum. The study concluded that when the AuCl4− concentration was below 500 mg/L, synthesized gold nanoparticles was uniform, narrow in the width with 20 nm in size and when the concentration increased, the size and shape of the nanoparticles increased from 50 to 100 nm. pH is also one of the most important factors that influences the important role during the biosynthesis of nanoparticles. On the other hand, different metal nanoparticles can be synthesized at different pH values. Isaria fumosorosea [98] showed synthesis of nanoparticles at alkaline pH while Fusarium acuminatum required acidic pH and Penicillium fellitanum required pH around 6.0. In case of bacteria usually basic pH is required to synthesize silver nanoparticles, E. coli synthesizes silver nanoparticles at pH 10 [92]. It has been concluded that acidic pH nanoparticles gets aggregated during synthesis process. The shape and size of nanoparticles can be manipulated by altering the pH of the reaction mixture. pH changes the electrical charges of biomolecules which might affect the reducing and stabilizing ability and frequent growth of nanoparticles. This might be also helpful in obtaining desirable shape and size of the nanoparticles. Gericke and Pinches [97] studied the role of pH in the synthesis of nanoparticles and their possible effect on shape along with crystal structure by varying the pH of the reaction mixture. On the basis of their experiment, they concluded that the nanoparticles synthesized by Verticillium luteoalbum at pH 3 were spherical in shape with 10 nm size while they were hexagonal, rod, triangle and spherical in shape at pH 5. However, at pH 7 and 9, the small particles synthesized are mostly spherical in shape and bigger particles are irregular and undefined shapes. It was also found that the number of particles synthesized was highest at pH 7 and lower at pH 9. Similar report was obtained by Sanghi and Verma [98]; they used Coriolus versicolor fungus for the synthesis of nanoparticles. In one study, Ahmad et al. [99] confirmed that silver nanoparticles were not synthesized in the presence of Fusarium moniliforme, while it was frequently synthesized with another strain of Fusarium, i.e., Fusarium oxysporum. Protein assay of these two fungi revealed that F. oxysporum contains a specific reductase enzyme (NADH-dependent reductase), while the other reductase enzymes are similar in both the fungal species. So, it was confirmed that NADH-dependent reductase is the possible reductase enzyme which plays a crucial role in the reduction process of nanoparticles. When F. oxysporum was used for the synthesis of titania nanoparticles from aqueous anionic complexes TiF −26 , extracellular protein-mediated hydrolysis of the anionic complexes results in the facile room temperature synthesis of crystalline titania nanoparticles [54]. Similar study was carried out by Bansal et al. [62], they reported that enzymes secreted by F. oxysporum were used for the synthesis of silica nanoparticles. F. oxysporum selectively bioleached the silicates present in the zircon sand, into silicic acid and finally to silica nanoparticles. On the basis of previously results of related studies, it was confirmed that the several experimental parameters like the growth medium, and the synthesis conditions will greatly affect the shape, size and dispersity of nanoparticles.
Bio-prospective applications of mycogenic nanoparticles
Nanoparticles due to their unique properties are used in daily human life. Nanoparticles have been found to be used in optical product formations, disinfectants, fabric cleaners, food packaging materials, biosensor and diagnostics, biology and medicine, cosmetics, textile, electronics, opticals, composites and energy, agricultural products, etc. (Fig. 3) [100]. However, the most applicable field of nanoparticles is pharmaceutical and biomedical science [31]. Nanoparticles formed by the noble metals like gold, silver, palladium and platinum are widely used in products of consumers goods such as creams, soaps, toothpastes, shampoos and shoes beside their applications in biomedical products [101]. Silver zeolites can be used in disinfection, food preservation and decontamination of products. Nanoparticles are also used as effective biofertilizers and biopesticides to control the plant diseases in crops [102] due to their eco-friendliness. FeO3/Fe3O4 and CeO2 nanoparticles were synthesized using several plant products and effectively used for solar energy applications [103]. These studies prove that metal nanoparticles are involved in the anti-inflammatory effects; the exact, precise mechanism of action remains to be identified. Metal nanoparticles have been extensively used for biomedical applications such as medicine, disease diagnostic, drug delivery systems, tissue engineering, genetic engineering, etc. Platinum nanoparticles are also majorly used as catalysts and in many biomedical applications in combination with other nanoparticles in alloy, core–shell, and bimetallic nanostructure. Similarly, palladium nanoparticles have large application in sensing, plasmonic wave guiding, catalysis and electrocatalysis [104]. Thostenson et al. [105] found that when nano-compounds are highly applicable for the production of food packaging materials, they are highly stable at high temperature and are able to tolerate mechanical stress during transportation and storage. Nanoparticles are also useful in environmental remediation. They are used for the treatment of hazardous waste materials by the greener approaches. Nanoparticles are widely used for the treatment of water sources and waste water contaminants by the metal ions, organic and inorganic solutes and microorganism [106]. This eco-friendly approach eliminates many cleaning chemicals used in regular manner. It has been found that Fe nanoparticles are considerably used for the removal of heavy metals from the soil and it is also used as disinfectant of contaminated water [107].
Some of the most recent literatures propose a large number of potential applications of the fungal-derived nanoparticles. Silver nanoparticles synthesized by fungi are reported for exhibiting excellent antimicrobial properties. Successful inhibition has been reported against some pathogenic bacteria such as Staphylococcus epidermidis, Klebsiella pneumoniae, Escherichia coli, Bacillus cereus, Proteus vulgaris, Micrococcus luteus, Staphylococcus aureus, Pseudomonas aeroginosa, Bacillus subtilis, Salmonella typhae, etc. Fungal-derived nanoparticles also reported to be a good antifungal agent against some pathogenic fungi including Candida sp., [108] Mucor sp., [109], Aspergillus sp., Fusarium sp., [6] some dermatophytic fungal pathogens Trichophyton sp. [110] and the main dandruff causing agent Malassezia furfur [111]. Gold nanoparticles synthesized from Trichoderma sp. were reported for good antibacterial activity against pathogenic bacterial strains. Some study also reported that silver nanoparticles show good action against some strains which were resistant to the common antifungal agent Fluconazole [112]. Furthermore, Birla et al. [113] reported that silver nanoparticles in combination with commercially available antimicrobial drugs shows higher activity as compare to drug alone.
Anticancer activity of metal nanoparticles also tested on human cell lines on epidermoid larynx carcinoma (HEP-2). The result showed 27.2 and 64% mortality at concentration of 10 and 100 µg/ml, respectively [114]. Yehia and Al-Sheikh [115] tested the anticancer potential of metal nanoparticles against MCF-7 (breast cancer) cell lines; they found a significant decrease in cell viability. Gold nanoparticles synthesized by Neurospora crassa showed good surface-enhanced Raman scattering (SERS) properties, which are useful in developing sensor technology, which is the first report of its kind [116].
Nanoparticles synthesized from fungi were also reported for their potential applications in mosquito control. Salunkhe et al. [117], Banu and Balasubramanian [118] successfully tested the silver and gold nanoparticles against dengue vector, Aedes aegypti and the mosquito vector of malaria Anopheles stephensi [118]. Silver nanoparticles also used in food preservation, the particles are incorporated in the sodium alginate film and used as fruits and vegetable preservatives [119]. All these reports confirm that fungal-derived silver nanoparticles could be considered as a potential antimicrobial agent and employed for their possible bio-medical applications. Table 4 shows the several bio-prospective applications of fungal derived metal nanoparticles.
One of the most prominent applications of nanoparticles in the field of environment is bioremediation and treatment of water through different mechanisms mainly by adsorption of toxic chemicals, heavy metals and other pollutants, removal of pathogens and transformation of toxic into nontoxic or less toxic form [120]. Scientists and researchers are succeeding towards making pollution-free environment via synthesizing special nanostructures. Using silver nano-catalysts leads to inhibit or decline the by-products generated in production of propylene oxide, a common compound used in plastics, paints, detergents, brake fluid, etc. It has been found that most of the iron and iron-containing nanostructures are used as catalyst for the removal of toxicants from organic dyes to clean the ground water through photo-degradation method. In this process, nanoparticles scatter in overall water and degrade the organic dyes. This procedure is cost effective for water purification which is then to be pumped out of the ground. At ambient temperature, nano-crystals can break down the volatile organic compound from air. These are mostly composed of manganese oxide doped with gold nanoparticles. Silver nanoparticles synthesized from Rhizopus oryzae fungal species have been used for waste water treatment and adsorption of pesticides [64]. Silver nanoparticles synthesized from fungi are also used to treat many environmental applications like air disinfection, waste water treatment, ground water treatment and surface disinfections [121]. In the year 2007, Duran and his co-workers [122] found that silver nanoparticles were synthesized by Fusarium oxysporum and can be used in textile fabrics to minimize the bacterial infection such as Staphylococcus aureus [122].
A large number of fungal species are reported as common plant pathogens and can be controlled by nano-based bio-formulations [123]. Antifungal activity of the silver nanoparticles on Colletotrichum gloesporioides was evaluated by Aguilar-Mendez et al. [124]. C. gloesporioides is a pathogen known to cause anthracnose in a wide range of fruits like papaya, mango, apple, etc. They found that in the presence of silver nanoparticles, C. gloesporioides showed significantly delayed growth. Hence, it could be concluded that nano-silver could be used as an alternate of chemical fungicide to manage plant diseases. Similarly, Kim et al. [125] reported the antifungal potential of double capsulated silver nanoparticles (1.5 nm) solution against rose powdery mildew. Rose powdery mildew is a common plant disease caused by Sphaerotheca pannosa var. rosae. They sprayed the nanosilver (1.5 nm) solution, diluted up to 10 ppm, to the infected area of the plant. They found that after 2 days of spray around 95% of the rose powdery mildew disappeared out and did not reoccur for a weak. Nanoparticles synthesized from fungus Aspergillus clavatus exhibited good antibacterial activity against some disease-causing pathogenic bacteria viz., E. coli and Pseudomonas flurorescens [126]. In another study Saha et al. [127] synthesizes the anisotropic silver nanoparticles by the phytopathogen Bipolaris nodulosa. They also reported that synthesized nanoparticles had great antimicrobial activities against some pathogenic bacterial and fungal species. Nanoparticles have also applications in biosensor development as they have unique optical and electronic features. Au–Ag alloy nanoparticles were reported for the synthesis of susceptible electrically chemical vanillin sensors by yeast cells [127]. Detailed study of this showed that they have the ability to increase the electro-chemical response of vanillin for at least minimum of five times by the vanillin sensor based on Au–Ag alloy nanoparticle-modified carbon electrode.
Spasova et al. [128] reported the development of nanofibrous mats, when they mix the chitosan and Trichoderma viride and kept the mixture at 28 °C; they observed the formation of nanofibrous mats which grows much faster and fights for space and nutrients against the fungi Alternaria sp. and Fusarium sp. Including this, T. viride also produces extracellular hydrolytic enzymes which attack on pathogens and destroy their cell walls. Spores incorporated into the fibrous mats are viable and the grown fungus T. viride preserves its ability to inhibit the growth of test pathogens. Aspergillus and Neurospora species are reported for synthesis of gold microwires [129]. When they exposed these fungal species to gold nanoparticles for a week, they were surface functionalized with glutamate, aspartate and polyethylene glycol.
Concluding remarks and future challenges
Microbes have an excellent potential for the synthesis of metal nanoparticles, with wide range of applications. In this concern, mycogenesis of nanoparticles has attracted a great interest in recent years. Mycogenesis of nanoparticles are found to be an efficient and suitable approach for the synthesis of various types of nanoparticles with huge potential and significant applications in several fields like agriculture, food, textile, medicine, cosmetics, opticals and electronics. Use of fungi to develop nanoparticles has advantages of easy handling and downstream processing. In recent years, nanoparticles of various compositions are restricted to metals, some metal sulfide and very less oxides only. Therefore, there is a need to develop a protocol to implement noble synthesis of nano-structures of other metal oxides, nitrides, carbides, etc.
Nanoparticle syntheses through biological processes are safe and relatively inexpensive process. Nanoparticles synthesis through fungi is found to be highly applicable for several fields. Developments of smart delivery system for the drugs to the specific site will be helpful for the disease diagnosis. Production of smart biosensor and detection system will be beneficial to protect agricultural crops against insects and pathogens. In brief, myconanotechnology is still under progress. The application of nanoparticles will continue to grow but still we need to study their toxicity effect and accumulation in the environment and their effect on human health and animals. There is also a new hope that nanoparticles can be used in treatment of various severe diseases and in future open the new avenue in the biomedical field. Also, these AgNPs would provide a potential solution for the present energy crisis by finding their use as energy-driven devices. Available literatures also revealed that there is considerable work carried out in in vitro applications of nanoparticles but less information available on in vivo applications. Further, more study is required to describe and elaborate the knowledge and functions of nanomaterials to achieve several milestones in the field of medicines, agriculture, cosmetics, electronics, environment etc. Despite a wide range of benefits of fungal-mediated synthesis of metal nanoparticles, still there are a number of limitations and challenges to overcome before it can be used practically.
As well more research is needed to optimize the various recation conditions to acheive better control over size, shape and monodispersity of the nanoparticles. Additionally, stability of nanoparticle is also an important parameter to consider. It is important to achieve all these limitations by focused research and development of new strategies in this field. The exact mechanisms of nanoparticle synthesis using fungi have not been clearly and deeply understood yet. Hence, more study and experimental trials are needed to elaborate the exact mechanism to identify the responsible biomolecules (enzymes and proteins) involved in reduction and stabilization of nanoparticles. In this way, formulation of low-cost recovery techniques to make production process commercially feasible is also needed.
References
Remya, V.R., Abitha, V.K., Rajput, P.S., Rane, A.V., Dutta, A.: Silver nanoparticles green synthesis: a mini review. Chem. Int. 3(2), 165–171 (2017)
Govindappa, M., Farheen, H., Chandrappa, C.P., Rai, R.V., Raghavendra, V.B.: Mycosynthesis of silver nanoparticles using extract of endophytic fungi, Penicillium species of Glycosmis mauritiana, and its antioxidant, antimicrobial, anti-inflammatory and tyrokinase inhibitory activity. Adv. Nat. Sci. Nanosci. Nanotech. 7(3), 035014 (2016)
Kumar, V., Yadav, S.K.: Plant-mediated synthesis of silver and gold nanoparticles and their applications. J. Chem. Technol. Biotechnol. 84(2), 151–157 (2009)
Singh, P., Kim, Y.J., Zhang, D., Yang, D.C.: Biological synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 34(7), 588–599 (2016)
Gade, A., Ingle, A., Whiteley, C., Rai, M.: Mycogenic metal nanoparticles: progress and applications. Biotechnol. Lett. 32(5), 593–600 (2010)
Khan, A.U., Malik, N., Khan, M., Cho, M.H., Khan, M.M.: Fungi-assisted silver nanoparticle synthesis and their applications. Bioprocess Biosyst. Eng. 41(1), 1–20 (2018)
Kashyap, P.L., Kumar, S., Srivastava, A.K., Sharma, A.K.: Myconanotechnology in agriculture: a perspective. World J. Microbiol. Biotechnol. 29(2), 191–207 (2013)
Wang, W., Chen, Q., Jiang, C., Yang, D., Liu, X., Xu, S.: One-step synthesis of biocompatible gold nanoparticles using gallic acid in the presence of poly-(N-vinyl-2-pyrrolidone). Coll. Surf. A Physicochem. Eng. Asp. 301, 73–79 (2007)
Srikar, S.K., Giri, D.D., Pal, D.B., Mishra, P.K., Upadhyay, S.N.: Green synthesis of silver nanoparticles: a review. Green Sustain. Chem. 6(01), 34 (2016)
Lukman, A.I., Gong, B., Marjo, C.E., Roessner, U., Harris, A.T.: Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J. Coll. Interface Sci. 353(2), 433–444 (2011)
Philip, D.: Green synthesis of gold and silver nanoparticles using Hibiscus rosa sinensis. Phys. E Low Dimens. Syst. Nanostruct. 42(5), 1417–1424 (2010)
Mohanpuria, P., Rana, N.K., Yadav, S.K.: Biosynthesis of nanoparticles: technological concepts and future applications. J. Nanopart. Res. 10(3), 507–517 (2008)
Rai, M.K., Deshmukh, S.D., Ingle, A.P., Gade, A.K.: Silver nanoparticles: the powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 112(5), 841–852 (2012)
Akhtar, M.S., Panwar, J., Yun, Y.S.: Biogenic synthesis of metallic nanoparticles by plant extracts. ACS Sustain. Chem. Eng. 1(6), 591–602 (2013)
Ahmad, N., Sharma, S., Alam, M.K., Singh, V.N., Shamsi, S.F., Mehta, B.R., Fatma, A.: Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Coll. Surf. B Biointerfaces 81(1), 81–86 (2010)
Du, L., Jiang, H., Liu, X., Wang, E.: Biosynthesis of gold nanoparticles assisted by Escherichia coli DH5α and its application on direct electrochemistry of hemoglobin. Electrochem. Commun. 9(5), 1165–1170 (2007)
Narayanan, K.B., Sakthivel, N.: Biological synthesis of metal nanoparticles by microbes. Adv. Coll. Interface Sci. 156(1–2), 1–13 (2010)
Rajora, N., Kaushik, S., Jyoti, A., Kothari, S.L.: Rapid synthesis of silver nanoparticles by Pseudomonas stutzeri isolated from textile soil under optimised conditions and evaluation of their antimicrobial and cytotoxicity properties. IET Nanobiotechnol. 10(6), 367–373 (2016)
Roh, Y., Lauf, R.J., McMillan, A.D., Zhang, C., Rawn, C.J., Bai, J., Phelps, T.J.: Microbial synthesis and the characterization of metal-substituted magnetites. Solid State Commun. 118, 529–534 (2001)
Omajali, J.B., Mikheenko, I.P., Merroun, M.L., Wood, J., Macaskie, L.E.: Characterization of intracellular palladium nanoparticles synthesized by Desulfovibrio desulfuricans and Bacillus benzeovorans. J. Nanopart. Res. 17(6), 264 (2015)
Husseiny, M.I., El-Aziz, M.A., Badr, Y., Mahmoud, M.A.: Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim. Acta A 67(3–4), 1003–1006 (2007)
Lengke, M., Fleet, M.E., Southam, G.: Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold (I)-thiosulfate and gold(III)-chloride complexes. Langmuir 22, 2780–2787 (2006)
Ahmed, S., Ikram, S.: Biosynthesis of gold nanoparticles: a green approach. J. Photochem. Photobiol. 161, 141–153 (2016)
Luangpipat, T., Beattie, I.R., Chisti, Y., Haverkamp, R.G.: Gold nanoparticles produced in a microalga. J. Nanopart. Res. 13(12), 6439–6445 (2011)
Xie, J., Lee, J.Y., Wang, D.I.: Seedless, surfactantless, high-yield synthesis of branched gold nanocrystals in HEPES buffer solution. Chem. Mater. 19(11), 2823–2830 (2007)
Madhiyazhagan, P., Murugan, K., Kumar, A.N., Nataraj, T., Dinesh, D., Panneerselvam, C., Nicoletti, M.: Sargassum muticum-synthesized silver nanoparticles: an effective control tool against mosquito vectors and bacterial pathogens. J. Parasitol. Res. 114(11), 4305–4317 (2015)
Mata, Y.N., Torres, E., Blazquez, M.L., Ballester, A., González, F.M.J.A., Munoz, J.A.: Gold (III) biosorption and bioreduction with the brown alga Fucus vesiculosus. J. Hazard. Mater. 166(2), 612–618 (2009)
Abdel-Raouf, N., Al-Enazi, N.M., Ibraheem, I.B.: Green biosynthesis of gold nanoparticles using Galaxaura elongata and characterization of their antibacterial activity. Arab. J. Chem. 10, S3029–S3039 (2017)
Castro, L., Blázquez, M.L., Muñoz, J.A., González, F., Ballester, A.: Biological synthesis of metallic nanoparticles using algae. IET Nanobiotechnol. 7(3), 109–116 (2013)
Iravani, S., Korbekandi, H., Mirmohammadi, S.V., Zolfaghari, B.: Synthesis of silver nanoparticles: chemical, physical and biological methods. Int. J. Res. Pharm. 9(6), 385 (2014)
Golinska, P., Wypij, M., Ingle, A.P., Gupta, I., Dahm, H., Rai, M.: Biogenic synthesis of metal nanoparticles from actinomycetes: biomedical applications and cytotoxicity. Appl. Microbiol. Biotechnol. 98(19), 8083–8097 (2014)
Ahmad, A., Senapati, S., Khan, M.I., Kumar, R., Sastry, M.: Extra-/intracellular biosynthesis of gold nanoparticles by an alkalotolerant fungus Trichothecium sp. J. Biomed. Nanotechnol. 1, 47–53 (2005)
Yildiz, I., Shukla, S., Steinmetz, N.F.: Applications of viral nanoparticles in medicine. Curr. Opin. Biotechnol. 22(6), 901–908 (2011)
Royston, E.S., Brown, A.D., Harris, M.T., Culver, J.N.: Preparation of silica stabilized Tobacco mosaic virus templates for the production of metal and layered nanoparticles. J. Coll. Interface Sci. 332(2), 402–407 (2009)
Azizi, M., Sedaghat, S., Tahvildari, K., Derakhshi, P., Ghaemi, A.: Synthesis of silver nanoparticles using Peganum harmala extract as a green route. Green Chem. Lett. Rev. 10(4), 420–427 (2017)
Ottoni, C.A., Simões, M.F., Fernandes, S., Dos Santos, J.G., Da Silva, E.S., de Souza, R.F.B., Maiorano, A.E.: Screening of filamentous fungi for antimicrobial silver nanoparticles synthesis. AMB Express 7(1), 31 (2017)
Omidi, S., Sedaghat, S., Tahvildari, K., Derakhshi, P., Motiee, F.: Biosynthesis of silver nanocomposite with Tarragon leaf extract and assessment of antibacterial activity. J. Nanostruct. Chem. 8(2), 171–178 (2018)
Duhan, J.S., Kumar, R., Kumar, N., Kaur, P., Nehra, K., Duhan, S.: Nanotechnology: the new perspective in precision agriculture. Biotechnol. Rep. 15, 11–23 (2017)
Afshar, P., Sedaghat, S.: Bio-synthesis of silver nanoparticles using water extracts of Satureja hortensis L and evaluation of the antibacterial properties. Curr. Nanosci. 12(1), 90–93 (2016)
Owaid, M.N., Ibraheem, I.J.: Mycosynthesis of nanoparticles using edible and medicinal mushrooms. Eur. J. Nanomed. 9(1), 5–23 (2017)
Velhal, S.G., Kulkarni, S.D., Latpate, R.V.: Fungal mediated silver nanoparticle synthesis using robust experimental design and its application in cotton fabric. Int. Nano. Lett. 6(4), 257–264 (2016)
Yadav, A., Kon, K., Kratosova, G., Duran, N., Ingle, A.P., Rai, M.: Fungi as an efficient mycosystem for the synthesis of metal nanoparticles: progress and key aspects of research. Biotechnol. Lett. 37(11), 2099–2120 (2015)
Zhao, X., Zhou, L., Riaz Rajoka, M.S., Yan, L., Jiang, C., Shao, D., Zhu, J., Shi, J., Huang, Q., Yang, H., Jin, M.: Fungal silver nanoparticles: synthesis, application and challenges. Crit. Rev. Biotechnol. 38(6), 817–835 (2018)
Alghuthaymi, M.A., Almoammar, H., Rai, M., Said-Galiev, E., Abd-Elsalam, K.A.: Myconanoparticles: synthesis and their role in phytopathogens management. Biotechnol. Biotechnol. Equip. 29(2), 221–236 (2015)
Shah, M., Fawcett, D., Sharma, S., Tripathy, S.K., Poinern, G.E.J.: Green synthesis of metallic nanoparticles via biological entities. Materials 8(11), 7278–7308 (2015)
Kitching, M., Ramani, M., Marsili, E.: Fungal biosynthesis of gold nanoparticles: mechanism and scale up. Microb. Biotechnol. 8(6), 904–917 (2015)
Hulkoti, N.I., Taranath, T.C.: Biosynthesis of nanoparticles using microbes—a review. Coll. Surf. B Biointerf. 121, 474–483 (2014)
Sowani, H., Mohite, P., Munot, H., Shouche, Y., Bapat, T., Kumar, A.R., Zinjarde, S.: Green synthesis of gold and silver nanoparticles by an actinomycete Gordonia amicalis HS-11: mechanistic aspects and biological application. Process Biochem. 51(3), 374–383 (2016)
Bansal, V., Rautaray, D., Ahmad, A., Sastry, M.: Biosynthesis of zirconia nanoparticles using the fungus Fusarium oxysporum. J. Mater. Chem. 14, 3303–3305 (2004)
Duran, N., Marcato, P.D., Alves, O.L., D’Souza, G., Esposito, E.: Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J. Nanobiotechnol. 3, 8–14 (2005)
Shahi, S.K., Patra, M.: Microbially synthesized bioactive nanoparticles and their formulation active against human pathogenic fungi. Rev. Adv. Mater. Sci. 5, 501–509 (2003)
Shivshankar, S., Ahmad, A., Pasricha, R., Sastry, M.: Bioreduction of chloroaurate ions by Geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J. Mater. Chem. 13, 1822–1826 (2003)
Senapati, S., Ahmad, A., Khan, M.I., Sastry, M., Kumar, R.: Extracellular biosynthesis of bimetallic Au–Ag alloy nanoparticles. Small 1, 517–520 (2005)
Bharde, A., Rautaray, D., Bansal, V., Ahmad, A., Sarkar, I., Yusuf, S.M., Sanyal, M., Sastry, M.: Extracellular biosynthesis of magnetite using fungi. Small 2(1), 135–141 (2006)
Mukherjee, P., Ahmad, A., Mandal, D., Senapati, S., Sainkar, S.R., Khan, M.I., Parishcha, R., Ajay, P.V., Alam, M., Kumar, R., Sastry, M.: Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 1, 515–519 (2001)
Devi, L.S., Joshi, S.R.: Antimicrobial and synergistic effects of silver nanoparticles synthesized using soil fungi of high altitudes of eastern Himalaya. Mycobiology 40(1), 27–34 (2012)
Bhainsa, K.C., D’Souza, S.F.: Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Coll. Surf. B: Biointerf. 47, 160–164 (2006)
Gade, A.K., Bonde, P., Ingle, A.P., Marcato, P.D., Duran, N., Rai, M.K.: Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Biobased Mater. Bioenergy. 2(3), 243–247 (2008)
Espinosa-Ortiz, E.J., Gonzalez-Gil, G., Saikaly, P.E., Van Hullebusch, E.D., Lens, P.N.: Effects of selenium oxyanions on the white-rot fungus Phanerochaete chrysosporium. Appl. Microbiol. Biotechnol. 99(5), 2405–2418 (2015)
Abdel-Hafez, S.I., Nafady, N.A., Abdel-Rahim, I.R., Shaltout, A.M., Mohamed, M.A.: Biogenesis and optimisation of silver nanoparticles by the endophytic fungus Cladosporium sphaerospermum. Int. J. Nano Chem. 2(1), 11–19 (2016)
Sarsar, V., Selwal, M.K., Selwal, K.K.: Biogenic synthesis, optimisation and antibacterial efficacy of extracellular silver nanoparticles using novel fungal isolate Aspergillus fumigatus MA. IET Nanobiotechnol. 10(4), 215–221 (2016)
Bansal, V., Poddar, P., Ahmad, A., Sastry, M.: Room-temperature biosynthesis of ferroelectric barium titanate nanoparticles. J. Am. Chem. Soc. 128, 11958–11963 (2006)
Kumar, S.A., Ayoobul, A.A., Absar, A., Khan, M.I.: Extracellular biosynthesis of CdSe quantum dots by the fungus Fusarium Oxysporum. J. Biomed. Nanotechnol. 3, 190–194 (2007)
Das, S.K., Dickinson, C., Lafir, F., Brougham, D.F., Marsili, E.: Synthesis, characterization and catalytic activity of gold nanoparticles biosynthesized with Rhizopus oryzae protein extract. Green Chem. 14(5), 1322–1334 (2012)
Bansal, V., Rautaray, D., Bharde, A., Ahire, K., Sanyal, A., Ahmad, A., Sastry, M.: Fungus-mediated biosynthesis of silica and titania particles. J. Mater. Chem. 15, 2583–2589 (2005)
Riddin, T.L., Gericke, M., Whiteley, C.G.: Analysis of the intra and extracellular formation of platinum nanoparticles by Fusarium oxysporum f. sp. Lycopersici using response surface methodology. Nanotechnology 17, 1–8 (2006)
Ingle, A., Gade, A., Pierrat, S., Sönnichsen, C., Rai, M.: Mycosynthesis of silver nanoparticles using the fungus Fusarium acuminatum and its activity against some human pathogenic bacteria. Curr. Nanosci. 4, 141–144 (2008)
Ingle, A., Gade, A., Bawaskar, M., Rai, M.: Fusarium solani: a novel biological agent for the extracellular synthesis of silver nanoparticles. J. Nanopart. Res. 11, 2079–2085 (2009)
Jha, A.K., Prasad, K.: Understanding biosynthesis of metallic/oxide nanoparticles: a biochemical perspective. In: Kumar, S.A., Thiagarajan, S., Wang, S.F. (eds.) Biocompatible nanomaterials synthesis, characterization and applications. NOVA Science Publishers Inc., Hauppauge, NY, USA (2010)
Tamás, M.J., Labarre, J., Toledano, M.B., Wysocki, R.: Mechanisms of toxic metal tolerance in yeast. In: Tamás, M.J., Martinoia, E. (eds.) Molecular biology of metal homeostasis and detoxification: from microbes to man. Springer, Heidelberg (2005)
Wysocki, R., Tamás, M.J.: How Saccharomyces cerevisiae copes with toxic metals and metalloids. FEMS Microbiol. Rev. 34, 925–951 (2010)
Shanti, S.S., Karl, J.D.: The significance of amino acids and amino acid derived molecules in plant responses and adaptation to heavy metal stress. J. Exp. Bot. 57, 711–726 (2006)
Agnihotri, M., Joshi, S., Kumar, A.R., Zinjarde, S., Kulkarni, S.: Biosynthesis of gold nanoparticles by the tropical marine yeast Yarrowia lipolytica NCIM 3589. Mater. Lett. 63(15), 1231–1234 (2009)
Haq, M., Rathod, V., Singh, D., Singh, A.K., Ninganagouda, S., Hiremath, J.: Dried mushroom Agaricus bisporus mediated synthesis of silver nanoparticles from Bandipora District (Jammu and Kashmir) and their efficacy against methicillin resistant Staphylococcus aureus (MRSA) strains. Nanosci. Nanotechnol. Int. J. 5, 1–8 (2015)
Perrone, G., Susca, A., Cozzi, G., Ehrlich, K., Varga, J., Frisvad, J.C., Meijer, M., Noonim, P., Mahakarnchanakul, W., Samson, R.A.: Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol. 59, 53–66 (2007)
Clausen, C.A., Green, F.: Oxalic acid overproduction by copper-tolerant brown-rot basidiomycetes on southern yellow pine treated with copper-based preservatives. Int. Biodeterior. Biodegrad. 51, 139–144 (2003)
Menon, S., Rajeshkumar, S., Kumar, V.: A review on biogenic synthesis of gold nanoparticles, characterization, and its applications. Resour. Effic. Technol. 3(2), 516–527 (2017)
Paraszkiewicz, K., Frycie, A., Słaba, M., Długoński, J.: Enhancement of emulsifier production by Curvularia lunata in cadmium, zinc and lead presence. Biometals 20, 797–805 (2007)
Keat, C.L., Aziz, A., Eid, A.M., Elmarzugi, N.A.: Biosynthesis of nanoparticles and silver nanoparticles. Bioresour. Bioprocess. 2(1), 47 (2015)
Athanassiou, C.G., Kavallieratos, N.G., Benelli, G., Losic, D., Rani, P.U., Desneux, N.: Nanoparticles for pest control: current status and future perspectives. J. Pest. Sci. 91(1), 1–15 (2018)
Vesentini, D., Dickinson, D.J., Murphy, R.J.: Fungicides affect the production of fungal extracellular mucilaginous material (ECMM) and the peripheral growth unit (PGU) in two wood rotting basidiomycetes. Mycol. Res. 110, 1207–1213 (2006)
Tripathi, A.K., Harsh, N.S.K., Gupta, N.: Fungal treatment of industrial effluents: a mini review. Life Sci. J 4, 78–81 (2007)
Jha, A.K., Prasad, K., Prasad, K.: A green low cost biosynthesis of Sb2O3 nanoparticles. Biochem. Eng. J. 43, 303–306 (2009)
Kalishwaralal, K., Deepak, V., Ramkumarpandian, S., Nellaiah, H., Sangiliyandi, G.: Extracellular biosynthesis of silver nanoparticles by the culture supernatant of Bacillus licheniformis. Mater. Lett. 62, 4411–4413 (2008)
Liu, T., Liu, J., Sonshine, D.A., Shervani, S., Hurt, R.H.: Controlled release of biologically active silver from nanosilver surfaces. ACS Nano 4, 6903–6913 (2010)
Sudha, S.S., Rajamanickam, K., Rengaramanujam, J.: Microalgae mediated synthesis of silver nanoparticles and their antibacterial activity against pathogenic bacteria. Indian J. Exp. Biol. 51(5), 393–399 (2013)
Aziz, N., Faraz, M., Pandey, R., Shakir, M., Fatma, T., Varma, A., Prasad, R.: Facile algae-derived route to biogenic silver nanoparticles: synthesis, antibacterial, and photocatalytic properties. Langmuir 31(42), 11605–11612 (2015)
Tomaszewska, E., Soliwoda, K., Kadziola, K., Celichowski, G., Cichomski, M., Szmaja, W., Grobelny, J.: Detection limits of DLS and UV-Vis spectroscopy in characterization of polydisperse nanoparticles colloids. J. Nanomater. 2013, 313081 (2013)
Wang, Z.L., Liu, Y., Zhang, Z. (eds.): Handbook of nanophase and nanostructured materials, Vol. I: synthesis, Vol. II: characterization, Vol. III: materials systems and applications I, Volume IV: materials systems and applications II. Springer, New York (2002). (ISBN: 978–0-306–47249-7)
Buseck, P., Cowley, J., Eyring, L. (eds.): High-resolution transmission electron microscopy. Springer, New York (1998). (ISBN 0–19-504275–1)
Fulias, A., Vlase, G., Grigorie, C., Ledeţi, I., Albu, P., Bilanin, M., Vlase, T.: Thermal behaviour studies of procaine and benzocaine. J. Therm. Anal. Calorim. 113(1), 265–271 (2013)
Gurunathan, S., Han, J.W., Kwon, D.N., Kim, J.H.: Enhanced antibacterial and anti-biofilm activities of silver nanoparticles against Gram-negative and Gram-positive bacteria. Nanoscale Res. Lett. 9(1), 373 (2014)
Karbasian, M., Atyabi, S.M., Siadat, S.D., Momen, S.B., Norouzian, D.: Optimizing nano-silver formation by Fusarium oxysporum PTCC 5115 employing response surface methodology. AJABS. 3(1), 433–437 (2008)
Gurunathan, S., Kalishwaralal, K., Vaidyanathan, R., Venkataraman, D., Pandian, S.R.K., Muniyandi, J., Eom, S.H.: Biosynthesis, purification and characterization of silver nanoparticles using Escherichia coli. Coll. Surf. B Biointerfaces 74(1), 328–335 (2009)
Dhillon, G.S., Brar, S.K., Kaur, S., Verma, M.: Green approach for nanoparticle biosynthesis by fungi: current trends and applications. Crit. Rev. Biotechnol. 32(1), 49–73 (2012)
Kathiresan, K., Manivannan, S., Nabeel, M.A., Dhivya, B.: Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Coll. Surf. B Biointerfaces 71(1), 133–137 (2009)
Gericke, M., Pinches, A.: Biological synthesis of metal nanoparticles. Hydrometallurgy 83, 132–140 (2006)
Sanghi, R., Verma, P.: pH dependant fungal proteins in the ‘green’ synthesis of gold nanoparticles. Adv. Mater. Lett. 1(3), 193–199 (2010)
Ahmad, A., Senapati, S., Khan, M.I., Kumar, R., Ramani, R., Srinivas, V., Sastry, M.: Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 14(7), 824 (2003)
Sedaghat, S., Agbolag, A.E.: Biosynthesis of silver nanoparticles using pennyroyal water extract as a green route. J. Nanostruct. Chem. 6(1), 25–27 (2016)
Ghiassi, S., Sedaghat, S., Mokhtary, M., Kefayati, H.: Plant-mediated bio-synthesis of silver–montmorillonite nanocomposite and antibacterial effects on gram-positive and-negative bacteria. J. Nanostruct. Chem. 8(3), 353–357 (2018)
Singh, N., Jenkins, G.J., Asadi, R., Doak, S.H.: Potential toxicity of super paramagnetic iron oxide nanoparticles (SPION). Nano Rev. 1(1), 5358 (2010)
Mittal, A.K., Chisti, Y., Banerjee, U.C.: Synthesis of metallic nanoparticles using plant extracts. Biotechnol. Adv. 31(2), 346–356 (2013)
Chen, G., Wang, S., Su, J., Zhang, H.: A novel green synthesis approach for polymer nanocomposites decorated with silver nanoparticles and their antibacterial activity. Analyst 139, 5793–5799 (2014)
Thostenson, E.T., Li, C., Chou, T.W.: Nanocomposites in context. Compos. Sci. Technol. 65(3–4), 491–516 (2005)
Kimizuka, N., Tanaka, M., Kunitake, T.: Spatially controlled synthesis of protein/inorganic nano-assembly: alternate molecular layers of Cyt C and TiO2 nanoparticles. Chem. Lett. 28(12), 1333–1334 (1999)
Lok, C.N., Ho, C.M., Chen, R., He, Q.Y., Yu, W.Y., Sun, H., Tam, P.K.H., Chiu, J.F., Che, C.M.: Silver nanoparticles: partial oxidation and antibacterial activities. J. Biol. Inorg. Chem. 12, 527–534 (2007)
Wang, H., Ma, H., Zheng, W., An, D., Na, C.: Multifunctional and recollectable carbon nanotube ponytails for water purification. ACS Appl. Mater. Interfaces 6(12), 9426–9434 (2014)
Musarrat, J., Dwivedi, S., Singh, B.R., Al-Khedhairy, A.A., Azam, A., Naqvi, A.: Production of antimicrobial silver nanoparticles in water extracts of the fungus Amylomyces rouxii strain KSU-09. Bioresour. Technol. 101(22), 8772–8776 (2010)
Arun, G., Eyini, M., Gunasekaran, P.: Green synthesis of silver nanoparticles using the mushroom fungus Schizophyllum commune and its biomedical applications. Biotechnol. Bioprocess. Eng. 19(6), 1083–1090 (2014)
Joshi, P.A., Bonde, S.R., Gaikwad, S.C., Gade, A.K., Abd-Elsalam, K., Rai, M.K.: Comparative studies on synthesis of silver nanoparticles by Fusarium oxysporum and Macrophomina phaseolina and its efficacy against bacteria and Malassezia furfur. J. Bionanosci. 7(4), 378–385 (2013)
Soshnikova, V., Kim, Y.J., Singh, P., Huo, Y., Markus, J., Ahn, S., Yang, D.C.: Cardamom fruits as a green resource for facile synthesis of gold and silver nanoparticles and their biological applications. Artif. Cells Nanomed. Biotechnol. 46(1), 108–117 (2018)
Birla, S.S., Tiwari, V.V., Gade, A.K., Ingle, A.P., Yadav, A.P., Rai, M.K.: Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett. Appl. Microbiol. 48(2), 173–179 (2009)
Nehra, P., Chauhan, R.P., Garg, N., Verma, K.: Antibacterial and antifungal activity of chitosan coated iron oxide nanoparticles. J. Biomed. Sci. 75(1), 13–18 (2018)
Yehia, R.S., Al-Sheikh, H.: Biosynthesis and characterization of silver nanoparticles produced by Pleurotus ostreatus and their anticandidal and anticancer activities. World J. Microbiol. Biotechnol. 30(11), 2797–2803 (2014)
Quester, K., Avalos-Borja, M., Vilchis-Nestor, A.R., Camacho-López, M.A., Castro-Longoria, E.: SERS properties of different sized and shaped gold nanoparticles biosynthesized under different environmental conditions by Neurospora crassa extract. PLoS One 8(10), e77486 (2013)
Salunkhe, R.B., Patil, S.V., Salunke, B.K., Patil, C.D., Sonawane, A.M.: Studies on silver accumulation and nanoparticle synthesis by Cochliobolus lunatus. Appl. Biochem. Biotechnol. 165(1), 221–234 (2011)
Banu, A.N., Balasubramanian, C.: Myco-synthesis of silver nanoparticles using Beauveria bassiana against dengue vector, Aedes aegypti (Diptera: Culicidae). J. Parasitol. Res. 113(8), 2869–2877 (2014)
Fayaz, A.M., Balaji, K., Kalaichelvan, P.T., Venkatesan, R.: Fungal based synthesis of silver nanoparticles—an effect of temperature on the size of particles. Coll. Surf. B 74(1), 123–126 (2009)
Gaur, N., Flora, G., Yadav, M., Tiwari, A.: A review with recent advancements on bioremediation-based abolition of heavy metals. Environ. Sci. Process. Impacts 16(2), 180–193 (2014)
Zhang, M., Zhang, K., De Gusseme, B., Verstraete, W., Field, R.: The antibacterial and anti-biofouling performance of biogenic silver nanoparticles by Lactobacillus fermentum. Biofouling 30(3), 347–357 (2014)
Durán, N., Marcato, P.D., De Souza, G.I., Alves, O.L., Esposito, E.: Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J. Biomed. Nanotechnol. 3(2), 203–208 (2007)
Singh, R., Arora, N.K.: Bacterial formulations and delivery systems against pests in sustainable agrofood production. Food Sci. 1, 1–11 (2016)
Aguilar-Méndez, M.A., San Martín-Martínez, E., Ortega-Arroyo, L., Cobián-Portillo, G., Sánchez-Espíndola, E.: Synthesis and characterization of silver nanoparticles: effect on phytopathogen Colletotrichum gloesporioides. J. Nanopart. Res. 13(6), 2525–2532 (2011)
Kim, K.J., Sung, W.S., Moon, S.K., Choi, J.S., Kim, J.G., Dong, G.L.: Antifungal effect of silver nanoparticles on dermatophytes. J. Microbiol. Biotechnol. 18, 1482–1484 (2008)
Verma, V.C., Kharwar, R.N., Gange, A.C.: Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine 5(1), 33–40 (2010)
Saha, S., Sarkar, J., Chattopadhyay, D., Patra, S., Chakraborty, A., Acharya, K.: Production of silver nanoparticles by a phytopathogenic fungus Bipolaris nodulosa and its antimicrobial activity. Dig. J. Nanomater. Biostruct. 5(4), 887–895 (2010)
Spasova, M., Manolova, N., Naydenov, M., Kuzmanova, J., Rashkov, I.: Electrospun biohybrid materials for plant biocontrol containing chitosan and Trichoderma viride spores. J. Bioact. Compat. Polym. 26(1), 48–55 (2011)
Sugunan, A., Melin, P., Schnürer, J., Hilborn, J.G., Dutta, J.: Nutrition-driven assembly of colloidal nanoparticles: growing fungi assemble gold nanoparticles as microwires. Adv. Mater. 19(1), 77–81 (2007)
Singh, B.R., Dwivedi, S., Al-Khedhairy, A.A., Musarrat, J.: Synthesis of stable cadmium sulfide nanoparticles using surfactin produced by Bacillus amyloliquifaciens strain KSU-109. Coll. Surf. B 85(2), 207–213 (2011)
Elbeshehy, E.K., Elazzazy, A.M., Aggelis, G.: Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against Bean yellow mosaic virus and human pathogens. Front. Microbiol. 6, 453 (2015)
Wang, C., Kim, Y.J., Singh, P., Mathiyalagan, R., Jin, Y., Yang, D.C.: Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif. Cells Nanomed. Biotechnol. 44(4), 1127–1132 (2016)
Soni, N., Prakash, S.: Antimicrobial and mosquitocidal activity of microbial synthesized silver nanoparticles. J. Parasitol. Res. 114(3), 1023–1030 (2015)
Singh, P., Kim, Y.J., Singh, H., Mathiyalagan, R., Wang, C., Yang, D.C.: Biosynthesis of anisotropic silver nanoparticles by Bhargavaea indica and their synergistic effect with antibiotics against pathogenic microorganisms. J. Nanomater. 2015(4), 1–10 (2015)
Singh, P., Kim, Y.J., Singh, H., Wang, C., Hwang, K.H., Farh, M.E.A., Yang, D.C.: Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int. J. Nanomed. 10, 2567 (2015)
Jo, J.H., Singh, P., Kim, Y.J., Wang, C., Mathiyalagan, R., Jin, C.G., Yang, D.C.: Pseudomonas deceptionensis DC5-mediated synthesis of extracellular silver nanoparticles. Artif. Cells Nanomed. Biotechnol. 44(6), 1576–1581 (2016)
Singh, P., Kim, Y.J., Wang, C., Mathiyalagan, R., Yang, D.C.: Weissella oryzae DC6-facilitated green synthesis of silver nanoparticles and their antimicrobial potential. Artif. Cells Nanomed. Biotechnol. 44(6), 1569–1575 (2016)
Lengke, M.F., Fleet, M.E., Southam, G.: Morphology of gold nanoparticles synthesized by filamentous cyanobacteria from gold (I)-thiosulfate and gold (III)-chloride complexes. Langmuir 22(6), 2780–2787 (2006)
Lengke, M.F., Fleet, M.E., Southam, G.: Synthesis of palladium nanoparticles by reaction of filamentous cyanobacterial biomass with a palladium (II) chloride complex. Langmuir 23(17), 8982–8987 (2007)
Ahmad, A., Mukherjee, P., Senapati, S., Mandal, D., Khan, M.I., Kumar, R., Sastry, M.: Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Coll. Surf. B 28, 313–318 (2003)
Kumar, C.G., Poornachandra, Y., Chandrasekhar, C.: Green synthesis of bacterial mediated anti-proliferative gold nanoparticles: inducing mitotic arrest (G2/M phase) and apoptosis (intrinsic pathway). Nanoscale 7(44), 18738–18750 (2015)
Sedaghat, S., Afshar, P.: Green bio-synthesis of silver nanoparticles using Ziziphora tenuior L water extract. J. Appli. Chem. Res. 10(1), 103–109 (2016)
Soltani, N.M., Shahidi, B.G.H., Khaleghi, N.: Biosynthesis of gold nanoparticles using Streptomyces fulvissimus isolate. Nanomed. J. 2(2), 153–159 (2015)
Ahmad, A., Senapati, S., Khan, M.I., Kumar, R., Ramani, R., Srinivas, V., Sastry, M.: Intracellular synthesis of gold nanoparticles by a novel alkalotolerant actinomycete, Rhodococcus species. Nanotechnology 14, 824–828 (2003)
Gajbhiye, M., Kesharwani, J., Ingle, A., Gade, A., Rai, M.: Fungus-mediated synthesis of silver nanoparticles and their activity against pathogenic fungi in combination with fluconazole. Nanomedicine 5(4), 382–386 (2009)
Verma, V.C., Singh, S.K., Solanki, R., Prakash, S.: Biofabrication of anisotropic gold nanotriangles using extract of endophytic Aspergillus clavatus as a dual functional reductant and stabilizer. Nanoscale Res. Lett. 6(1), 16 (2011)
Moharrer, S., Mohammadi, B., Gharamohammadi, R.A., Yargoli, M.: Biological synthesis of silver nanoparticles by Aspergillus flavus, isolated from soil of Ahar copper mine. Indian J. Sci. Technol. 5(S3), 2443–2444 (2012)
Raliya, R., Tarafdar, J.C., Gulecha, K., Choudhary, K., Ram, R., Mal, P.: Review article; scope of nanoscience and nanotechnology in agriculture. J. Appl. Biol. Biotechnol. 1, 041–044 (2013)
Kowshik, M., Ashtaputre, S., Kharrazi, S., Vogel, W., Urban, J., Kulkarni, S.K., Paknikar, K.M.: Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology 14, 95–100 (2003)
Binupriya, A.R., Sathishkumar, M., Vijayaraghavan, K., Yun, S.I.: Bioreduction of trivalent aurum to nano-crystalline gold particles by active and inactive cells and cell-free extract of Aspergillus oryzae var. viridis. J. Hazard. Mater. 177(1-3), 539–545 (2010)
Li, G., He, D., Qian, Y., Guan, B., Gao, S., Cui, Y., Wang, L.: Fungus-mediated green synthesis of silver nanoparticles using Aspergillus terreus. Int. J. Mol. Sci. 13(1), 466–476 (2011)
Kelly, K.L., Coronado, E., Zhao, L.L., Schatz, G.C.: The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J. Phys. Chem. 107(3), 668–677 (2003)
Abdullaeva, Z., Omurzak, E., Iwamoto, C., Ganapathy, H.S., Sulaimankulova, S., Liliang, C., Mashimo, T.: Onion-like carbon-encapsulated Co, Ni, and Fe magnetic nanoparticles with low cytotoxicity synthesized by a pulsed plasma in a liquid. Carbon 50(5), 1776–1785 (2012)
Qian, Y., Yu, H., He, D., Yang, H., Wang, W., Wan, X., Wang, L.: Biosynthesis of silver nanoparticles by the endophytic fungus Epicoccum nigrum and their activity against pathogenic fungi. Bioprocess. Biosyst. Eng. 36, 1613–1619 (2013)
Balaji, D.S., Basavaraja, S., Deshpande, R., Mahesh, D.B., Prabhakar, B.K., Venkataraman, A.: Extracellular biosynthesis of functionalized silver nanoparticles by strains of Cladosporium cladosporioides fungus. Coll. Surf. B Biointerfaces 68, 88–92 (2009)
Gurunathan, S., Raman, J., Malek, S.N.A., John, P.A., Vikineswary, S.: Green synthesis of silver nanoparticles using Ganoderma neo-japonicum Imazeki: a potential cytotoxic agent against breast cancer cells. Int. J. Nanomed. 8, 4399 (2013)
Gurunathan, S., Lee, K.J., Kalishwaralal, K., Sheikpranbabu, S., Vaidyanathan, R., Eom, S.H.: Antiangiogenic properties of silver nanoparticles. Biomaterials 30(31), 6341–6350 (2009)
Balakumaran, M.D., Ramachandran, R., Kalaichelvan, P.T.: Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol. Res. 178, 9–17 (2015)
Amutha, R., Muruganandham, M., Lee, G.J., Wu, J.J.: Facile microwave-combustion synthesis of wurtzite CdS nanoparticles. J. Nanosci. Nanotechnol. 11(9), 7940–7944 (2011)
Kumar, A., Vemula, P.K., Ajayan, P.M., John, G.: Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat. Mater. 7(3), 236 (2008)
Varshney, R., Mishra, A.N., Bhadauria, S., Gaura, M.S.: Novel microbial route to synthesize silver nanoparticles using fungus Hormoconis resinae. Dig. J. Nanomater. Biostruct. 4(2), 349–355 (2009)
Salvadori, M.R., Lepre, L.F., Ando, R.A., do Nascimento, C.A.O., Correa, B.: Biosynthesis and uptake of copper nanoparticles by dead biomass of Hypocrea lixii isolated from the metal mine in the Brazilian Amazon region. PLoS One. 8(11), 1–8 (2013)
Mondal, N.K., Chowdhury, A., Dey, U., Mukhopadhya, P., Chatterjee, S., Das, K., Datta, J.K.: Green synthesis of silver nanoparticles and its application for mosquito control. Asian Pac. J. Trop. 4, S204–S210 (2014)
El-Baz, A.F., El-Batal, A.I., Abomosalam, F.M., Tayel, A.A., Shetaia, Y.M., Yang, S.T.: Extracellular biosynthesis of anti-Candida silver nanoparticles using Monascus purpureus. J. Basic Microbiol. 56(5), 531–540 (2016)
Castro-Longoria, E., Vilchis-Nestor, A.R., Avalos-Borja, M.: Biosynthesis of silver, gold and bimetallic nanoparticles using the filamentous fungus Neurospora crassa. Coll. Surf. B 83, 42–48 (2011)
Owaid, M.N., Raman, J., Lakshmanan, H., Al-Saeedi, S.S.S., Sabaratnam, V., Abed, I.A.: Mycosynthesis of silver nanoparticles by Pleurotus cornucopiae var. citrinopileatus and its inhibitory effects against Candida sp. Mater. Lett. 153, 186–190 (2015)
Raman, J., Reddy, G.R., Lakshmanan, H., Selvaraj, V., Gajendran, B., Nanjian, R., Sabaratnam, V.: Mycosynthesis and characterization of silver nanoparticles from Pleurotus djamor var. roseus and their in vitro cytotoxicity effect on PC3 cells. Process. Biochem. 50(1), 140–147 (2015)
Borovaya, M., Pirko, Y., Krupodorova, T., Naumenko, A., Blume, Y., Yemets, A.: Biosynthesis of cadmium sulphide quantum dots by using Pleurotus ostreatus (Jacq.) P. kumm. Biotechnol. Biotechnol. Equip. 29(6), 1156–1163 (2015)
Shaligram, N.S., Bule, M., Bhambure, R., Singhal, R.S., Singh, S.K., Szakacs, G., Pandey, A.: Biosynthesis of silver nanoparticles using aqueous extract from the compactin producing fungal strain. Process. Biochem. 44(8), 939–943 (2009)
Zhang, X., He, X., Wang, K., Wang, Y., Li, H., Tan, W.: Biosynthesis of size-controlled gold nanoparticles using fungus, Penicillium sp. J. Nanosci. Nanotechnol. 9(10), 5738–5744 (2009)
Raheman, F., Deshmukh, S., Ingle, A., Gade, A., Rai, M.: Silver nanoparticles: novel antimicrobial agent synthesized from an endophytic fungus Pestalotia sp. isolated from leaves of Syzygium cumini (L). Nano Biomed. Eng. 3(3), 174–178 (2011)
Thirumurugan, G., Shaheedha, S.M., Dhanaraju, M.D.: In vitro evaluation of anti-bacterial activity of silver nanoparticles synthesized by using Phytophthora infestans. Int. J. Chem. Tech. Res. 1(3), 714–716 (2009)
Rajput, K., Raghuvanshi, S., Bhatt, A., Rai, S.K., Agrawal, P.K.: A review on synthesis silver nano-particles. Int. J. Curr. Microbiol. App. Sci. 6(7), 1513–1528 (2017)
Ge, L., Li, Q., Wang, M., Ouyang, J., Li, X., Xing, M.M.: Nanosilver particles in medical applications: synthesis, performance, and toxicity. Int. J. Nanomed. 9, 2399 (2014)
Bhat, R., Deshpande, R., Ganachari, S.V., Huh, D.S., Venkataraman, A.: Photo-irradiated biosynthesis of silver nanoparticles using edible mushroom Pleurotus florida and their antibacterial activity studies. Bioinorg. Chem. Appl. (2011)
Mazumdar, H., Haloi, N.: A study on biosynthesis of iron nanoparticles by Pleurotus sp. Res. Rev. J. Microbiol. Biotechnol. 1(3), 39–49 (2017)
Shi, C., Zhu, N., Cao, Y., Wu, P.: Biosynthesis of gold nanoparticles assisted by the intracellular protein extract of Pycnoporus sanguineus and its catalysis in degradation of 4-nitroaniline. Nanoscale Res. Lett. 10(1), 147 (2015)
Das, R., Nath, S.S., Chakdar, D., Gope, G.R., Bhattacharjee, H.: Preparation of silver nanoparticles and their characterization. J. Nanotech. 5, 1–6 (2009)
Afreen, R.V., Ranganath, E.: Synthesis of monodispersed silver nanoparticles by Rhizopus Stolonifer and its antibacterial activity against MDR strains of Pseudomonas Aeruginosa from burnt patients. Int. J. Environ. Sci. 1(7), 1582–1592 (2011)
Cuevas, R., Durán, N., Diez, M.C., Tortella, G.R., Rubilar, O.: Extracellular biosynthesis of copper and copper oxide nanoparticles by Stereum hirsutum, a native white-rot fungus from chilean forests. J. Nanomater. 16(1), 1–7 (2015)
Kowshik, M., Vogel, W., Urban, J., Kulkarni, S.K., Paknikar, K.M.: Microbial synthesis of semiconductor PbS nanocrystallites. Adv. Mater. 14(11), 815 (2002)
Cuevas, R., Durán, N., Diez, M.C., Tortella, G.R., Rubilar, O.: Extracellular biosynthesis of copper and copper oxide nanoparticles by Stereum hirsutum, a native white-rot fungus from chilean forests. J. Nanomater. 16(1), 57 (2015)
Philip, D.: Biosynthesis of Au, Ag and Au–Ag nanoparticles using edible mushroom extract. Spectrochim. Acta A 73(2), 374–381 (2009)
El-Rafie, M.H., Mohamed, A.A., Shaheen, T.I., Hebeish, A.: Antimicrobial effect of silver nanoparticles produced by fungal process on cotton fabrics. Carbohydr. Polym. 80(3), 779–782 (2010)
Sundaramoorthi, C., Kalaivani, M., Mathews, D.M., Palanisamy, S., Kalaiselvan, V., Rajasekaran, A.: Biosynthesis of silver nanoparticles from Aspergillus niger and evaluation of its wound healing activity in experimental rat model. Int. J. Pharm. Tech. Res. 1, 1523–1529 (2009)
Kumar, S.A., Ansary, A.A., Ahmad, A., Khan, M.I.: Extracellular biosynthesis of CdSe quantum dots by the fungus, Fusarium oxysporum. J. Biomed. Nanotechnol. 3(2), 190–194 (2007)
Ray, S., Sarkar, S., Kundu, S.: Extracellular biosynthesis of silver nanoparticles using the mycorrhizal mushroom Tricholoma crassum (BERK.) SACC: its antimicrobial activity against pathogenic bacteria and fungus, including multidrug resistant plant and human bacteria. Digest. J. Nanomater. Biostruct. 6, 1289–1299 (2011)
Salunke, B.K., Sawant, S.S., Lee, S.I., Kim, B.S.: Microorganisms as efficient biosystem for the synthesis of metal nanoparticles: current scenario and future possibilities. World J. Microbiol. Biotechnol. 32(5), 88 (2016)
Amerasan, D., Nataraj, T., Murugan, K., Panneerselvam, C., Madhiyazhagan, P., Nicoletti, M., Benelli, G.: Myco-synthesis of silver nanoparticles using Metarhizium anisopliae against the rural malaria vector Anopheles culicifacies Giles (Diptera: Culicidae). J. Pest. Sci. 89(1), 249–256 (2016)
Acknowledgements
The authors are thankful to Head, Department of Botany, GGV, Bilaspur, Chhattisgarh, India for providing facilities to carry out the study and Guru Ghasidas Vishwavidyalaya, Bilaspur (CG) for providing financial assistance (Vishwavidyalaya VRET fellowship 145081604).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Khandel, P., Shahi, S.K. Mycogenic nanoparticles and their bio-prospective applications: current status and future challenges. J Nanostruct Chem 8, 369–391 (2018). https://doi.org/10.1007/s40097-018-0285-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40097-018-0285-2