Abstract
Unequivocal international guidelines regarding the diagnosis and management of patients with acute appendicitis are lacking. The aim of the consensus meeting 2015 of the EAES was to generate a European guideline based on best available evidence and expert opinions of a panel of EAES members. After a systematic review of the literature by an international group of surgical research fellows, an expert panel with extensive clinical experience in the management of appendicitis discussed statements and recommendations. Statements and recommendations with more than 70 % agreement by the experts were selected for a web survey and the consensus meeting of the EAES in Bucharest in June 2015. EAES members and attendees at the EAES meeting in Bucharest could vote on these statements and recommendations. In the case of more than 70 % agreement, the statement or recommendation was defined as supported by the scientific community. Results from both the web survey and the consensus meeting in Bucharest are presented as percentages. In total, 46 statements and recommendations were selected for the web survey and consensus meeting. More than 232 members and attendees voted on them. In 41 of 46 statements and recommendations, more than 70 % agreement was reached. All 46 statements and recommendations are presented in this paper. They comprise topics regarding the diagnostic work-up, treatment indications, procedural aspects and post-operative care. The consensus meeting produced 46 statements and recommendations on the diagnostic work-up and management of appendicitis. The majority of the EAES members supported these statements. These consensus proceedings provide additional guidance to surgeons and surgical residents providing care to patients with appendicitis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acute appendicitis is a common gastrointestinal disease affecting 5.7–57/per 100.000 individuals each year with the highest incidence in children and adolescents [1–6]. The variation of incidence is due to variations in ethnicity, sex, age, obesity and season of the year [3, 6–11]. Based upon the entrenched idea that appendicitis is an irreversible progressive disease eventually leading to perforation, removal of the appendix is the gold standard of treatment. The medical profession has gained much experience in managing patients with acute appendicitis ever since Fitz’s first report in 1886 [12]. Large heterogeneity exists, however, between existing intercontinental, European and national guidelines regarding diagnosing and managing acute appendicitis. For instance, in the Netherlands, pre-operative imaging studies are promoted and considered mandatory in order to prevent negative appendectomies according to national guidelines, whereas in guidelines of other countries, it is not promoted nor considered mandatory [13]. Another example is the inconsistency regarding the management of an unexpected “normal appendix” during diagnostic laparoscopy [13, 14]. This heterogeneity prompted the need for an European consensus development conference for the diagnosis and management of acute appendicitis.
The European Association of Endoscopic Surgery (EAES) initiated a consensus development conference meeting on the management of acute appendicitis for its 2015 meeting in Bucharest. The aim of this consensus meeting was to develop practical guidelines based on the available evidence combined with the expertise of a selected panel of EAES surgeons. The findings are reported in this manuscript.
Materials and methods
The coordinating team (HJB, RG, HE and MGS) invited ten surgeons from nine European countries to serve as experts in this consensus development conference. An international research team of 16 young surgical researchers across 11 European countries was formed to evaluate and process the existing literature on the management of acute appendicitis. The coordinators generated a list of topics on acute appendicitis to be addressed (Appendix 1). An exploratory literature search was conducted in order to identify any additional topics of interest. All topics were approved by the experts and subsequently divided into three main parts: pre-operative care, operative care and after care. Based upon the topics, research questions were formulated, reviewed and approved by the panel of experts.
Literature search and processing of the literature
Research questions were used as guidance to conduct literature searches. The searches were conducted in cooperation with a medical information specialist of the Vrije Universiteit. Searches were performed in the following databases: PubMed, Web of science and the Cochrane library from inception up to 31 December 2014. No limitation was used regarding year of publication. Searches have been attached in Appendix 2. All papers published in European languages, and all study types with the exception of case reports were included in the search.
All articles were screened and reviewed by teams of two research fellows for eligibility, based on title and abstract. If eligible for inclusion, full text articles were obtained. If no full text was available, the article was excluded. In case of disagreement between the two research fellows, the coordinator dedicated to the topic acted as referee. Full text articles were summarized, evaluated and discussed at research meetings to assess their eligibility for inclusion in the review process. All included studies were evaluated according to the GRADE system [15–18]. The GRADE system systematically evaluates the available literature and focuses on the level of evidence based upon the types of studies included. The level of evidence can be marked as high, moderate, low or very low. This could be either downgraded in case of significant bias or upgraded when multiple high-quality studies showed consistent results. The highest levels of evidence (systematic reviews) were assessed first. If the systematic review was of sufficient quality, it was used to answer the research question. If no systematic review of sufficient quality was found, randomized controlled trials (RCTs) and cohort studies were evaluated. All selected studies were uploaded to a Mendeley database that was accessible to all research fellows, coordinators and experts.
After the literature search, an expert was assigned to every couple of researchers. This threesome was assigned research questions from the pre-operative care, operative care and after care. Hereafter they were responsible for formulating a statement/conclusion and, if possible, a recommendation on the assigned research questions. Again, the quality of the evidence was evaluated according to the GRADE/SIGN system [15–19]. The strength of the recommendation was based on the level of evidence and qualified as weak or strong. This was reflected in terms, using “recommend” in case of a strong recommendation and “suggest” in case of a weak recommendation.
A face-to-face consensus meeting among the experts was held in Amsterdam on the 1 May 2015 to discuss the final statements and recommendations. The coordinating team all experts and members of the international research team attended the meeting. A modified Delphi method was used. The Delphi method is a structured process, commonly used to develop healthcare quality indicator and consists of four key components; iteration, controlled acquisition of feedback, aggregation of responses and anonymity. As anonymity was not applicable in our situation, we used the term modified [20–22]. All statements and recommendations were shared with proposed levels of evidence with the entire group. After displaying the statements and recommendations, the experts casted their votes of agreement or disagreement. Refrain from voting was not allowed. No discussion was allowed between the experts at this point of time. In case of 100 % consensus, the statement and recommendation were accepted without further voting or discussion. In case of lack of consensus, the research team responsible for the statement presented the underlying considerations. After discussion between the experts, a second voting round was conducted. The statement or recommendation was accepted in case of at least 70 % consensus. Those statements and recommendations with less than 70 % consensus in the expert meeting were not included in the web survey or in the 2015 Bucharest meeting.
All finalized recommendations and statements with levels of evidence were entered into a web survey and distributed to all EAES members by e-mail. The web survey was open from 27 May until 3 July 2015. The recommendations or statements as well as the levels of evidence were open to several voting options: “agree”, “partly agree”, “disagree” or “don’t know”. The option “partly agree” meant that the voter agreed with the recommendation, but did not agree with the strength of recommendation.
All finalized recommendations and statements from the Amsterdam meeting with levels of evidence were presented at a plenary session of the 23rd annual meeting of the EAES on the 5 June 2015 in Bucharest. Live voting was performed using a digital voting system. Voting options were the same as the abovementioned.
Both results from the web survey and the Bucharest meeting are presented in the “Results” section.
Results
The literature search yielded 13,132 articles. The title, abstract and full text were reviewed. In total, 675 articles were selected and reviewed in detail to define 75 statements and recommendations, which were subsequently discussed at the Amsterdam meeting. (Appendix 1) During this meeting, the following statements and recommendations were excluded: on incidence and prevalence of appendicitis (n = 4), on the place of NOTES in acute appendicitis (n = 1), on the learning curve of appendectomy (n = 1), on day surgery for acute appendicitis (n = 1) and on the skeletonizing technique of the meso-appendix (n = 1). Twenty-one statements were combined leaving a total of 46 statement and recommendations; 8 statements and 14 recommendations for pre-operative care, 1 statement and 15 recommendations for operative care and 2 statements and 6 recommendations for aftercare (Fig. 1). Of the 675 articles, 100 were excluded due to the fact that statements and recommendations were excluded or were combined, rendering 575 articles (Fig. 1; Appendix 3).
Web survey
In total, 317 EAES members responded to the web survey; 90 % were surgeons and 10 % surgical residents.
Bucharest meeting
The 2015 EAES congress in Bucharest was attended by 1166 delegates. During the plenary consensus meeting, 232 delegates voted. Sixty-eight per cent were surgeons, 26 % surgical residents and 6 % scientists, physician assistants and others.
Pre-operative care
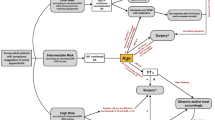
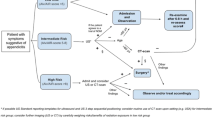
Establishing the diagnosis of acute appendicitis remains challenging. The clinical presentation of acute appendicitis can vary from mild symptoms to signs of generalized peritonitis and sepsis. Hence, the value of individual clinical variables to determine the likelihood of acute appendicitis in a patient is low [23, 24]. Biochemical testing is performed routinely in most patients. Its value in confirming acute appendicitis is debatable. A recent systematic review showed that elevated C-reactive protein levels render the highest diagnostic accuracy followed by increased numbers of leucocytes with an area under the curve of 0.75 [95 % CI 0.71–0.78] and 0.72 [95 % CI 0.68–0.76], respectively [24]. The area under the curve represents the ability of a test to correctly classify patients. In case the score is between the 0.7 and 0.8, it represents a fair test. Both clinical and biochemical variables have been combined into clinical predicting rules (CPR) such as the Alvarado score and paediatric appendicitis score (PAS) [25, 26]. This was done to increase the value of the individual variables. Ohle et al. [27] demonstrated that the Alvarado score was good at “ruling-out” appendicitis with an overall sensitivity and specificity of 96 and 81 %, respectively. In children, however, it has been shown that the PAS outperforms the Alvarado score [28]. To increase the predictive value of these two tests Ebell et al. [29] identified new cut-off values for the Alvarado score and PAS, which improved sensitivity and specificity rates. Based upon the Alvarado score, patients can now be categorised into low risk (Score < 4), intermediate (4–8) and high risk (≥9). The use of such CPRs appears useful to determine the likelihood of acute appendicitis. Distinguishing between low, intermediate and high risk provides guidance whether imaging studies are necessary.
Imaging studies in patients with a clinical suspicion of acute appendicitis can reduce the negative appendectomy rate, which has been reported to be as high as 15 %. Ultrasonography, abdominal computed tomography (CT) and magnetic resonance imaging (MRI) are most commonly used. Ultrasonography is non-invasive, avoids radiation and is associated with a sensitivity rate between 71 and 94 % and a specificity rate between 81 and 98 %. The positive likelihood ratio of ultrasonography is high at values between 6 and 46, while the negative likelihood ratio is moderate (0.08–0.30) [30–39]. Ultrasonography is therefore reliable to confirm presence of appendicitis but unreliable to exclude appendicitis. Furthermore, one should bear in mind that ultrasonography is highly operator dependent. Inconclusive ultrasonography findings, mostly due to failure visualizing the appendix, mandate additional imaging studies.
Abdominal computed tomography (CT) for suspected appendicitis has sensitivity and specificity rates between 76–100 % and 83–100 %, respectively, and, therefore, is superior to ultrasonography. Lower values of sensitivity and specificity can be explained by the use of enteral contrast [32, 33, 35–44]. However, the radiation exposure of abdominal CT is a concern particularly in children and during pregnancy. The estimated lifetime cancer-related mortality risk of developing a radiation-induced malignancy is approximately 0.18 % for a 1-year-old child and 0.11 % in a 15-year-old child if an abdominal CT is performed [45, 46]. Computed tomographies employing only a quarter of the standard radiation dose (low-dose CTs) provide similar imaging results as standard CTs and are, hence, an excellent alternative [47]. Regarding the administration of oral contrast, Andersson et al. [48] concluded in their meta-analysis that a CT scan without oral contrast was superior to CTs with oral contrast in terms of sensitivity and specificity. Therefore, low-dose CTs without oral contrast are preferable in patients with suspected appendicitis [48].
Magnetic resonance imaging (MRI) is used in pregnant patients and children with inconclusive findings at ultrasonography [49]. A recent meta-analysis on MRI in 363 patients with appendicitis, yielded a sensitivity rate of 97 % [95 % CI 92–99 %], a specificity rate of 95 % [95 % CI 94–99 %], a positive likelihood ratio of 16.3 [95 % CI 9.10–29.10] and a negative likelihood ratio of 0.09 [95 % CI 0.04–0.20] [50]. These rates are comparable to those of CT imaging, although these findings should be interpreted with care as most studies have been performed in a selected group of patients. MRI is associated with significant costs, and interpreting the images requires experience. Therefore, at the present time, use of MRI appears limited to pregnant women and children.
The algorithm associated with the Alvarado score (recommendation 4) is shown in Fig. 2.
Algorithm. *The cut-off values are based upon the study by Ebell et al. [29]. **One could consider performing additional imaging studies in patients with high probability based upon the Alvarado score in order to reduce the negative appendectomy rate. ***Ultrasound should be performed as a first level diagnostic imaging study, although in specific patient groups (such as the obese) an immediate CT scan might be considered. ****In case of an inconclusive result from the ultrasound, we recommend that additional imaging studies should be performed. Either a CT or MRI is preferred although it is recommended to perform an MRI in children and pregnant patients. It is therefore obligated to rule out pregnancy before a CT scan is obtained in a woman of reproductive age suspected of appendicitis. *****In case all the imaging studies are inconclusive, patients should be observed and reassessed. Diagnostic laparoscopy should be reserved for those patients with a continuous high index of suspicion after reassessment. ******In case of low probability based upon the Alvarado score, other diagnoses should be excluded and the patient can be either discharged with good instruction (with an optional reassessment the next day) or admitted for observation if the clinical condition mandates this. In case appendicitis is excluded, patients should be treated for the set diagnosis according to the local protocols
In obese patients (definition depends on the reference study), the diagnostic accuracy of ultrasound is diminished due to an increase of the subcutaneous and intra-abdominal fat. Anderson et al. [51] demonstrated that the body mass index (BMI) does not alter the diagnostic accuracy of a CT scan. CT appears therefore more reliable than ultrasonography in obese patients with the exception of children and pregnancy.
Patients with appendicitis are classified as uncomplicated or complicated appendicitis based upon pre-operative, intra-operative and/or histopathological findings. In this report, uncomplicated appendicitis has been defined as an inflamed appendix without signs of gangrene, perforation, intra-peritoneal purulent fluid, contained phlegmon or intra-abdominal abscess (IAA). Complicated appendicitis applies to all patients with either a gangrenous inflamed appendix with or without perforation, intra-abdominal abscess, peri-appendicular contained phlegmon or purulent free fluid. Classification is necessary as treatment strategies may differ.
Uncomplicated appendicitis
Appendectomy is still considered to be the gold standard for uncomplicated appendicitis. Two main approaches to remove an inflamed appendix are available; the open approach (OA) or the laparoscopic approach (LA). In 2010, a large Cochrane review on 67 studies showed that LA significantly reduced the rate of surgical site infection (SSI) (OR 0.43; 95 % CI 0.34–0.54) but significantly increased the risk of an intra-abdominal abscess (IAA) (OR 1.77; 95 % CI 1.14–2.76) compared to the open approach [52]. It was stated that LA was associated with fewer superficial wound infections, less post-operative pain, shorter hospital stay and earlier return to work, but the higher rate of IAA raised concerns [52]. Ever since, inconsistent results have been reported regarding the potential higher incidence of IAA after LA [53–61]. Benefits of LA over OA reported in meta-analyses are: reduced incidence of SSI, post-operative and long-term bowel obstruction with better outcome in terms of shorter hospital stay, its diagnostic value, less pain, earlier return to work, earlier start of oral intake, improved scar and body satisfaction and fewer incisional hernias [54, 55, 58, 61–66]. Disadvantages besides the possible higher incidence of IAA are longer operating time and possibly increased costs [58, 63].
To reduce the surgical trauma even more, new treatment strategies have been introduced such as single-incision laparoscopic surgery (SILS) first reported by Pelosi et al. [67]. Since then, numerous studies (RCTs and SR) have been published on the potential advantages and disadvantages of the SILS technique. It can be concluded that SILS is associated with comparable post-operative morbidity rates compared to conventional LA [68–70]. The disadvantage is the fact that SILS is a more difficult technique as is reflected by the higher technical failure rate, longer operating time and conversion rate [71–78]. Main advantages of SILS would be less post-operative pain and better cosmetic outcomes, although inconsistent results have been reported [71, 75, 76, 79–81]. At the present time, evidence is lacking that SILS is superior to conventional LA [79, 82, 83]. SILS is, however, a safe and feasible alternative.
Recently, initial non-operative management of appendicitis has been investigated in the adult population. Five RCTs reported an effectiveness of 41–85 % at 1-year follow-up [84–88]. Meta-analyses of these studies revealed that non-operative treatment of acute appendicitis is less effective but could avoid surgery in 60–85 % of patients [89–94]. Opponents of this strategy raise concerns such as recurrent appendicitis, missing an underlying malignancy and progression of uncomplicated into complicated appendicitis. Due to the possible avoidance of surgery with an initial non-operative treatment strategy, morbidity was diminished [91, 93, 95]. However, both RCTs and meta-analyses showed significant heterogeneity of methodological quality, studies included and definitions of outcome parameters. Until higher qualitative evidence has been obtained regarding the potential benefits of initial non-operative management of acute appendicitis and the potential long-term effects have been investigated appropriately, appendectomy remains the gold standard in acute uncomplicated appendicitis.
Complicated appendicitis
Due to the heterogeneity of the definitions used in the literature, it is difficult to draw firm conclusions regarding the treatment of complicated appendicitis. In 2013, Dimitriou published a retrospective cohort study on 150 patients with complicated appendicitis (defined as perforated with an abscess or peritonitis). They showed that LA reduced the incidence of SSI, number of reoperations and length of hospital stay as compared to OA with no difference in IAA rate [96]. A RCT encompassing 81 patients with clinically and histopathologically confirmed complicated appendicitis showed similar outcomes after OA and LA [97]. It should be noted, however, that the incidence of IAA after LA for patients with complicated appendicitis was reported to be higher in some studies. Tuggle and colleagues reported that LA in patients with complicated appendicitis was associated with an incidence of IAA of 6.7 versus 3.7 % in patients who underwent an open appendectomy [98]. The incidence of small bowel obstructions after LA is lower compared to OA (pooled odds ratio 0.44 [95 % CI 0.26–0.74] with large heterogeneity regarding follow-up period) [65].
In case of a contained phlegmon or abscess (peri-appendicular mass), some authors opt for non-operative treatment while others advocate aggressive operative treatment. In 2007, Andersson et al. [99] demonstrated that immediate surgical treatment of patient with an abscess or phlegmon was associated with higher morbidity compared to initial non-operative treatment (OR 3.3 95 % CI 1.9–5.6). Similis et al. showed in their meta-analysis of 17 studies regarding this specific patient group that non-operative treatment was associated with fewer complications (SSI, IAA and bowel obstructions). It must be mentioned that this meta-analysis was subject to large heterogeneity [100]. Recent cohort studies draw opposite conclusions [101, 102]. They opt for a more aggressive surgical approach at time of presentation in case of an appendicular mass or appendicular abscess, based upon the idea that there is a relative high failure rate for non-surgical treatment [101, 102]. In our opinion, with this new evidence, a new systematic review should be performed. Until then, initial non-operative treatment of an appendicular mass of appendicular abscess is the preferred treatment of choice. Although not covered in this consensus guideline, the value of interval appendectomy after initial non-operative treatment of an appendicular mass is still subject of debate. Some opt for an interval appendectomy based upon the chance of missing an underlying and untreated malignancy (incidence 6 %) and the chance of developing recurrent appendicitis (incidence 5–44 %) [101–103]. Both can be avoided with an interval appendectomy, although data are lacking on its benefits.
Specific patient groups
Obese patients
Abdominal surgery in obese patients is challenging for both the anaesthesiologist and surgeon due to higher incidence of respiratory dysfunction, difficult access to the abdominal cavity, blurred anatomical landmarks and reduced working space in the abdominal cavity. Clarke et al. [104] performed a subgroup analysis among 37 patients (14 LA and 23 OA) with a BMI higher than 30 kg/m2 and reported similar morbidity after LA and OA [104]. This was confirmed by a meta-analysis, although a reduced length of hospital stay was noted after LA [105]. More recently, two recent meta-analyses showed a reduction of mortality and morbidity rates after LA [106, 107].
Pregnancy
Pregnancy induces anatomical and physiological changes that challenge the surgeon. The potential effects of carbon dioxide and increased abdominal pressure during LA on the foetus remain unclear. Loss of the foetus is most feared. In 2008, Walsh et al. [108] published a systematic review of 637 laparoscopic appendectomies in pregnant patients and noted foetal loss in approximately 6 % of the patients, with the highest incidence in patients with complicated appendicitis. Another review confirmed these findings and reported a nearly twofold increase of foetal loss in the LA group [109]. Both reviews, however, are mainly dominated by one study and based on low-grade evidence (retrospective studies with small numbers of patients) [108–110]. Recently, a review suggested that based upon the little available evidence no recommendation can be made regarding the preferred approach in pregnant patients [111]. More studies are necessary to ascertain the role of laparoscopic surgery during pregnancy. Until more evidence comes available, the surgical approach should be at the surgeon’s discretion. Based upon expert opinion, we recommend laparoscopy in case of sufficient experience. Although not supported by the literature, we strongly advise a multi-disciplinary approach to the pregnant patient with appendicitis [13, 54, 82, 111, 112].
Children
One meta-analysis included 107,624 children with both uncomplicated and complicated appendicitis [113]. Laparoscopic appendectomy in children with uncomplicated appendicitis LA was associated with a significant reduction of hospital stay with similar morbidity compared to open surgery. In children with complicated appendicitis, LA was associated with lower rates of morbidity, SSI, length of hospital admission and bowel obstruction. However, laparoscopic surgeries lasted longer and were followed by more intra-abdominal abscesses [113]. In more recent prospective cohort studies in children below 5 years of age, LA was associated with fewer complications [114]. Non-operative treatment of acute non-complicated appendicitis appears more promising in children than in adults [115, 116]
Elderly
Elderly patients have higher morbidity, reduced physiological reserves and impaired inflammatory responses, which increases their peri-operative risks. All studies of laparoscopic appendectomy in elderly support the use of laparoscopic surgery [117–121]. One meta-analysis, comprising more than 15,000 patients reported that LA reduced post-operative mortality (0.24; 95 % CI 0.15–0.37), post-operative complications (0.61; 95 % CI 0.50–0.73) and length of hospital stay (−0.51; 95 % CI −0.64 to −0.37) compared to OA (Tables 1, 2, 3, 4) [119].
Timing
Determining the best moment to perform surgery in case of acute appendicitis is of crucial importance [122, 123]. Acute appendicitis has been considered to be an irreversible progressive disease although recent studies have questioned this dogma [84, 89, 124]. Nowadays, the idea is endorsed that two types of appendicitis exist: uncomplicated (non-perforating) and complicated (perforating) appendicitis. The aetiology and pathogenesis of acute appendicitis remain largely unknown. Predicting a mild or fulminant course of appendicitis is not possible. Delaying an appendectomy increases the risk of perforated appendicitis, which is associated with higher incidence of short and long-term morbidity [125–127]. Hence, it is recommended to perform appendectomy as soon as possible. Although it should be noted that some studies have revealed that the clinical outcome was not affected by time to surgery (when surgery was performed within 12 h after presentation at the emergency department) [128, 129].
Antibiotic prophylaxis
Antibiotic prophylaxis has been proven effective in prevention of superficial surgical site infections and intra-abdominal abscesses in patients with appendicitis [130–132]. Prophylaxis should be commenced at the time of establishing the diagnosis of acute appendicitis. The choice of antibiotics is dependent on the local microbiome and drug resistance pattern and is not influenced by age.
Technique
Open access to the abdominal cavity as well as closed access using the Veress needle are accepted techniques to perform laparoscopy [133–135]. The debate on the preferred technique continues. However, in children, the majority of surgeons employs open establishment of a pneumoperitoneum.
The placement of the camera port and the work ports depend on the anatomy of the patient and preference of the operating surgeon. Primary principle of trocar placement in laparoscopy is that a triangular working space should be pursued.
Intra-operative procedure
Increased employment of pre-operative radiologic testing (e.g. ultrasound, CT or MRI) in cases of suspected appendicitis has significantly reduced the incidence of a normal appearing appendix encountered during surgery [136]. Macroscopic distinction between a normal appendix and appendicitis during surgery can be difficult [137, 138]. The “gold standard” for defining appendicitis is histopathology. In some studies, histopathological assessment revealed abnormal findings in up to 26 % of macroscopically normal appearing appendices [139, 140]. Therefore, it is recommended to perform an appendectomy in case of a normal appearing appendix during surgery for suspected appendicitis.
Several studies have investigated the safety of different methods of securing the appendicular stump [82, 141–143]. None of the different closure methods has a clear advantage in case of a healthy appendix base. Stapler devices provide the most standardized and patent closure of the appendix base. Suturing of the appendix base provides sufficient closure as well, but is technically more demanding than other techniques [142]. In case of perforation of the appendicular base, clips or endoloops do not provide secure closure and staple devices or laparoscopic suturing is required [82].
Reduction of bacterial load by meticulous suction of intra-peritoneal fluids is advised [144–146]. The right paracolic and pelvic area should be inspected to leave no fluid collections behind. Irrigation of the intra-peritoneal space in case of perforated appendicitis seems to be contra-productive leading to a higher number of abscesses [144, 145]. It is believed that irrigation of the intra-peritoneal space leads to spreading of bacteria. Routine use of drains does not reduce the incidence of abscesses [145, 147]. Necessity of a drain for special indications is left to the discretion of the surgeon.
Intra-operative unexpected findings
When an appendicular mass is encountered during surgery, one should restrain from continuing the operation. Continuation of the operation can necessitate bowel resection. Antibiotic treatment of phlegmon and drainage of any abscess should be performed [99, 148, 149].
The extent of surgical resection in case of suspected malignancy depends on the location and size of the appendicular mass [150–154]. Routine inclusion of the meso-appendix with the appendectomy is advised. Definitive histological findings determine whether an additional resection after total appendectomy is indicated. In cases of small neuroendocrine tumours (NET) or low-grade appendicular mucinous neoplasms (LAMN), a total meso-appendicular resection can be sufficient. In cases of a NET > 1 cm, LAMN grade 3–4 or an adenocarcinoma of the appendix, a formal right hemicolectomy is indicated to provide an oncologically sufficient resection. It is advised to perform a total meso-appendicular resection at the primary operation and an additional hemicolectomy at a later stage when indicated (Tables 5, 6, 7, 8) [150–154].
Post-operative antibiotics
The incidence of SSI after appendectomy has been reported to range from 0 to 11 % [155–164]. The severity of appendicitis strongly influences the risk of developing post-operative complications resulting in a substantially higher complication rate (up to 2–4 times) in patients with complicated appendicitis. In this specific group, post-operative administration of antibiotics significantly reduces the rate of SSI. In addition, to reduce bacteraemia and sepsis, these patients are uniformly treated with a course of post-operative antibiotics [155–158, 163]. In uncomplicated appendicitis, there is no evidence supporting routine administration of post-operative antibiotics. Therefore, only one pre-operative dose is advised [155–158].
Advice on type of antibiotics depends on local microbiome and resistance patterns and therefore should be left up to the discretion of the surgeon [159, 160]. Available evidence on duration of treatment is limited and mainly focused on children. However, there is no firm evidence on the duration (3, 5, 7, 10 days) and route of administration (usually intravenous administration for 48 h, then oral administration) [156, 157, 159, 161, 162].
Post-operative complications
The incidence of post-operative complications ranges from 3.0 to 28.7 % [164–174]. Complications include small bowel obstruction (0–1.9 %.), SSI (1.2–12.0 %), IAA (1.6–8 %), stump leakage and stump appendicitis [164–174]. Literature suggests a higher rate of complications in complicated appendicitis [166, 167, 171, 175].
Literature on stump leakage and stump appendicitis is limited, and no exact incidences have been reported in the literature, although it is assumed that it is more common in patients with complicated appendicitis and after OA [176]. A recommendation to avoid stump leakage or stump appendicitis is to resect the appendix as a whole [176]. Therefore, the stump should be no longer than 0.5 cm and caecal taenia should be followed onto the appendix at removal to ensure complete resection. Stump appendicitis is significantly more associated with perforation, as diagnosis is delayed by misled attention. This is caused by the assumption that the appendix as a whole is resected. Prevention is crucial. In case of timely diagnosis, stump resection with laparoscopic or open approach is feasible. In case of perforation, extended bowel resection is usually required [176].
In the initial management of IAA after appendectomy conservative measures (i.e. non-operative with antibiotics) are effective in most patients. However, in case of lack of improvement or deterioration, a more invasive strategy should be applied (percutaneous drainage or surgical (laparoscopic) drainage) [177–179].
Post-operative care
The use of prophylactic anti-emetics diminishes the incidence of post-operative nausea and vomiting. Increasing the diet is best determined by the patient’s ability to tolerate oral intake. There is no evidence that a liberal diet causes complications in the post-operative period [164, 180].
Post-operative pain management should follow local protocol for pain management after abdominal surgery. Post-operative analgesia with PCA provides effective and safe pain relief in children and adults and is less time costly [181]. Recently positive results have been published regarding the pre-emptive incision site infiltration with a local anaesthetic. Studies demonstrated that this decreases the total opioid consumption and lowers pain score experienced by patients in the first 24 h after surgery [182–184].
Pathology
Carcinoid is the most commonly found neoplasm in appendectomy specimens at an incidence between 0.13 and 2.4 % [185–190]. Other unexpected findings can be encountered in 1.4–2.4 % of patients, including: diverticulitis (1.2 %), tuberculosis appendix 0.08 %, endometriosis (3.6 %), adenocarcinoma (<1 %) and mucinous cystadenoma (0.2–0.6 %) [191–194].
Treatment of unexpected findings ranges from no further surgical treatment, to right hemi colectomy and even hyperthermic intra-peritoneal chemotherapy (HIPEC) in some cases [195–197]. Even though the incidence of unexpected findings seems low, the actual number of patients is significant and correct diagnosis is crucial for adequate treatment (Tables 9, 10, 11, 12) [198–202].
Discussion
This EAES consensus development conference regarding the diagnosis and management of acute appendicitis resulted in 46 statements and recommendations based upon the available evidence. Results from this meeting led to this paper, which can be used as a guideline for surgeons treating patients with appendicitis. Local guidelines, national guidelines and guidelines from scientific communities regarding appendicitis were available but showed great heterogeneity [13, 14, 203]. With this consensus meeting, we managed to gather experts from different European nations to compare and debate management of patients with acute appendicitis. This led to a consensus meeting in which 41 of the 46 statements and the majority of the members of the EAES supported recommendations. The transfer of knowledge between the member countries, the opportunity to discuss views and above all, the creation of a widely supported paper appears valuable.
Our list of topics was created by the coordinating team and expert panel and was thought to cover the most important topics in the field of acute appendicitis. Despite local differences, the general idea within the consensus group on the management of patients with acute appendicitis was comparable. In some cases, differences of treatment strategies between members of the expert panel were due to available surgical supplies and finances. This is reflected for instance on the statements and recommendations regarding SILS and MRI. However, we want to emphasize that in defining statements we refrained from stating specific procedures. We rather stated the general principles to follow. In this way, the results from this consensus guideline can also be applied in areas with limited resources.
The methodology of a consensus guideline is always subject to discussion. In the literature, there are several ways to conduct consensus conferences [20, 204–206]. However, not one was suited for our situation. It was therefore decided to modify the Delphi method, as described in the method section, in order to systematically evaluate each statement and recommendation [20–22]. We decided to finalize only those statements and recommendation with 70 % or more consensus, which is the arbitrary cut-off value we selected. The results of both the web-based survey and the live voting at the EAES conference in Bucharest are presented independently rather than combined to rule out any bias. As expected, small differences were noted between the several voting rounds. Although supported by the experts, some statements and recommendations were not supported by the scientific community in both the web survey as in the Bucharest meeting. The topics that were not supported were on accuracy of MRI compared to CT, the application of SILS, extensive work-up in the elderly and treatment strategy for immune compromised patients and the open access to the peritoneal cavity. Explanations for these discrepancies might be related to local habits, experience and financial situation. Of more interest are the discrepancies noted between the outcome in the web survey and during the Bucharest meeting. Discrepancies were noted on the topic of MRI application in children, the preferred approach in pregnant patients and the use of local anaesthetics prior to incision. This can again be explained by the fact that local habits, experience, composition of the voting public and financial situation might influence the outcome. The question was raised if the web survey alone would be sufficient to reach a consensus for future meetings. Limiting a consensus meeting to only the web survey would limit the time as well as the costs involved. Moreover, a higher percentage of surgeons participated in the web survey. In our opinion, however, the integration of an actual face-to-face meeting in the consensus methodology raises more awareness, provides an opportunity to discuss views and encourages the transfer of knowledge eventually leading to the creation of a widely supported paper.
The literature review was ended in December 2014. No studies after that were integrated for the consensus meeting as this was decided in our methodology. Therefore, new studies might have been conducted on some topics. Future research should be focused on the laparoscopic appendectomy in pregnant patients, elucidating the value of MRI in specific patient groups, evaluating the outcomes of initial non-operative treatment for both uncomplicated and complicated appendicitis, specific patient groups and the need for interval appendectomy. We therefore propose that these statements are updated on a regular basis.
Although some limitations can be identified in our methodology, we have integrated a new systematic method for a consensus meeting. In our opinion, this is the way forward and we need to efflorescence this method. Reproducibility, involving members of the scientific community and applicability are key components of a consensus meeting. We believe that only after evaluation of the general opinion within the EAES such guidelines should be put into order.
In conclusion, the consensus meeting of the EAES resulted in several statements and recommendations regarding the diagnosis and management of appendicitis based upon available evidence and expert opinion and was supported by the European surgical community. It provides guidance to surgeons and surgical residents facing patients with acute appendicitis.
References
Andersson R, Hugander A, Thulin A, Nyström PO, Olaison G (1994) Indications for operation in suspected appendicitis and incidence of perforation. BMJ 308:107–110
Basoli A, Zarba Meli E, Salvio A, Crovaro M, Scopelliti G, Mazzocchi P, Lomanto D, Fiocca F, Speranza V (1993) Trends in the incidence of acute appendicitis in Italy during the past 30 years. Minerva Chir 48:127–132
Elangovan S, Knapp DP, Kallail KJ (1997) Incidence of acute appendicitis confirmed by histopathologic diagnosis. Kans Med 98:10–13
Ilves I, Fagerström A, Herzig KH, Juvonen P, Miettinen P, Paajanen H (2014) Seasonal variations of acute appendicitis and nonspecific abdominal pain in Finland. World J Gastroenterol 20:4037–4042
Viniol A, Keunecke C, Biroga T, Stadje R, Dornieden K, Bösner S, Donner-Banzhoff N, Haasenritter J, Becker A (2014) Studies of the symptom abdominal pain—a systematic review and meta-analysis. Fam Pract 31:517–529
Wei PL, Chen CS, Keller JJ, Lin HC (2012) Monthly variation in acute appendicitis incidence: a 10-year nationwide population-based study. J Surg Res 178:670–676
Oguntola AS, Adeoti ML, Oyemolade TA (2010) Appendicitis: trends in incidence, age, sex, and seasonal variations in South-Western Nigeria. Ann Afr Med 9:213–217
Petroianu A, Oliveira-Neto JE, Alberti LR (2004) Comparative incidence of acute appendicitis in a mixed population, related to skin color. Arq Gastroenterol 41:24–26
Brink CF, Prinsloo H, van der Poel JS (1985) The seasonal incidence of acute appendicitis. S Afr Med J 68:156–158
Blanco FC, Sandler AD, Nadler EP (2012) Increased incidence of perforated appendicitis in children with obesity. Clin Pediatr (Phila) 51:928–932
Walker AR, Shipton E, Walker BF, Manetsi B, Van Rensburg PS, Vorster HH (1989) Appendicectomy incidence in black and white children aged 0 to 14 years with a discussion on the disease’s causation. Trop Gastroenterol 10:96–101
Fitz RH (1886) Perforating inflammation of the vermiform appendix. Am J Med Sci 92:321–346
Bakker OJ, Go PM, Puylaert JB, Kazemier G, Heij HA, Werkgroep richtlijn Diagnostiek en behandeling van acute appendicitis (2010) Guideline on diagnosis and treatment of acute appendicitis: imaging prior to appendectomy is recommended. Ned Tijdschr Geneeskd 54:A303
Korndorffer JR Jr, Fellinger E, Reed W (2010) SAGES guideline for laparoscopic appendectomy. Surg Endosc 24:757–761
Howick J (2009) Oxford centre for evidence-based medicine–levels of evidence. http://www.cebm.net/oxfors-centre-evidence-based-medicine-levels-evidence-march-2009. Accessed on the first of November 2014
Guyatt GH, Oxman AD, Vist G, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ (2008) Rating quality of evidence and strength of recommendations GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336:924–926
Goldet G, Howick J (2013) Understanding GRADE: an introduction. J Evid Based Med 6:50–54
Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, Flottorp S, Guyatt GH, Harbour RT, Haugh MC, Henry D, Hill S, Jaeschke R, Leng G, Liberati A, Magrini N, Mason J, Middleton P, Mrukowicz J, O’Connell D, Oxman AD, Phillips B, Schünemann HJ, Edejer T, Varonen H, Vist GE, Williams JW Jr, Zaza S, GRADE Working Group (2004) Grading quality of evidence and strength of recommendations. BMJ 328:1490
Scottish Intercollegiate Guidelines Network (SIGN) (1993). Sign methodology. http://www.sign.ac.uk/methodology/index.html. Accessed first of November 2014
Boulkedid R, Abdoul H, Loustau M, Sibony O, Alberti C (2011) Using and reporting the Delphi method for selecting healthcare quality indicators: a systematic review. PLoS ONE. doi:10.1371/journal.pone.0020476
Dalkey NC, Helmer O (1963) An experimental application of the Delphi method to the use of experts. Manage Sci 9:458–467
Hasson F, Keeney S, McKenna H (2000) Research guidelines for the Delphi survey technique. J Adv Nurs 32:1008–1015
Bundy DG, Byerley JS, Liles EA, Perrin EM, Katznelson J, Rice HE (2007) Does this child have appendicitis? JAMA 298:438–451
Andersson RE (2004) Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg 91:28–37
Alvarado A (1986) A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med 15:557–564
Samuel M (2002) Pediatric appendicitis score. J Pediatr Surg 37:877–881
Ohle R, O’Reilly F, O’Brien KK, Fahey T, Dimitrov BD (2011) The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. doi:10.1186/1741-7015-9-139
Kulik DM, Uleryk EM, Maguire JL (2013) Does this child have appendicitis? A systematic review of clinical prediction rules for children with acute abdominal pain. J Clin Epidemiol 66:95–104
Ebell MH, Shinholser J (2014) What are the most clinically useful cutoffs for the Alvarado and pediatric appendicitis scores? A systematic review. Ann Emerg Med 64:365–372
Carroll PJ, Gibson D, El-Faedy O, Dunne C, Coffey C, Hannigan A, Walsh SR (2013) Surgeon-performed ultrasound at the bedside for the detection of appendicitis and gallstones: systematic review and meta-analysis. Am J Surg 205:102–108
Douglas CD, Macpherson NE, Davidson PM, Gani JS (2000) Randomised controlled trial of ultrasonography in diagnosis of acute appendicitis, incorporating the Alvarado score. BMJ 321:919–922
Parker L, Nazarian LN, Gingold EL, Palit CD, Hoey CL, Frangos AJ (2014) Cost and radiation savings of partial substitution of ultrasound for CT in appendicitis evaluation: a national projection. AJR Am J Roentgenol 202:124–135
Doria AS, Moineddin R, Kellenberger CJ, Epelman M, Beyene J, Schuh S, Babyn PS, Dick PT (2006) US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology 241:83–94
Horton MD, Counter SF, Florence MG, Hart MJ (2000) A prospective trial of computed tomography and ultrasonography for diagnosing appendicitis in the atypical patient. Am J Surg 179:379–381
Kwok MY, Kim MK, Gorelick MH (2004) Evidence-based approach to the diagnosis of appendicitis in children. Pediatr Emerg Care 20:690–698
van Randen A, Bipat S, Zwinderman AH, Ubbink DT, Stoker J, Boermeester MA (2008) Acute appendicitis: meta-analysis of diagnostic performance of CT and graded compression US related to prevalence of disease. Radiology 249:97–106
van Randen A, Laméris W, van Es HW, van Heesewijk HP, van Ramshorst B, Ten Hove W, Bouma WH, van Leeuwen MS, van Keulen EM, Bossuyt PM, Stoker J, Boermeester MA, OPTIMA Study Group (2011) A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur Radiol 21:1535–1545
Weston AR, Jackson TJ, Blamey S (2005) Diagnosis of appendicitis in adults by ultrasonography or computed tomography: a systematic review and meta-analysis. Int J Technol Assess Health Care 21:368–379
Terasawa T, Blackmore CC, Bent S, Kohlwes RJ (2004) Systematic review: computed tomography and ultrasonography to detect acute appendicitis in adults and adolescents. Ann Intern Med 141:537–546
Hlibczuk V, Dattaro JA, Jin Z, Falzon L, Brown MD (2010) Diagnostic accuracy of noncontrast computed tomography for appendicitis in adults: a systematic review. Ann Emerg Med 55:51–59
Howell JM, Eddy OL, Lukens TW, Thiessen ME, Weingart SD, Decker WW, American College of Emergency Physicians (2010) Clinical policy: critical issues in the evaluation and management of emergency department patients with suspected appendicitis. Ann Emerg Med 55:71–116
Kaiser S, Frenckner B, Jorulf HK (2002) Suspected appendicitis in children: US and CT–a prospective randomized study. Radiology 223:633–638
Neumayer L, Kennedy A (2003) Imaging in appendicitis: a review with special emphasis on the treatment of women. Obstet Gynecol 102:1404–1409
Stephen AE, Segev DL, Ryan DP, Mullins ME, Kim SH, Schnitzer JJ, Doody DP (2003) The diagnosis of acute appendicitis in a pediatric population: to CT or not to CT. J Pediatr Surg 38:367–371
Brenner D, Elliston C, Hall E, Berdon W (2001) Estimated risks of radiation-induced fatal cancer from pediatric CT. AJR Am J Roentgenol 176:289–296
Brenner DJ, Elliston CD, Hall EJ, Berdon WE (2001) Estimates of the cancer risks from pediatric CT radiation are not merely theoretical: comment on “point/counterpoint: in X-ray computed tomography, technique factors should be selected appropriate to patient size against the proposition”. Med Phys 28:2387–2388
Kim K, Kim YH, Kim SY, Kim S, Lee YJ, Kim KP, Lee HS, Ahn S, Kim T, Hwang SS, Song KJ, Kang SB, Kim DW, Park SH, Lee KH (2012) Low-dose abdominal CT for evaluating suspected appendicitis. N Engl J Med 366:1596–1605
Anderson BA, Salem L, Flum DR (2005) A systematic review of whether oral contrast is necessary for the computed tomography diagnosis of appendicitis in adults. Am J Surg 190:474–478
Fonseca AL, Schuster KM, Kaplan LJ, Maung AA, Lui FY, Davis KA (2014) The use of magnetic resonance imaging in the diagnosis of suspected appendicitis in pregnancy: shortened length of stay without increase in hospital charges. JAMA Surg 149:687–693
Barger RL Jr, Nandalur KR (2010) Diagnostic performance of magnetic resonance imaging in the detection of appendicitis in adults: a meta-analysis. Acad Radiol 17:1211–1216
Anderson SW, Rhea JT, Milch HN, Ozonoff A, Lucey BC, Soto JA (2010) Influence of body habitus and use of oral contrast on reader confidence in patients with suspected acute appendicitis using 64 MDCT. Emerg Radiol 17:445–453
Sauerland S, Jaschinki T, Neugebauer EA (2010) Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. doi:10.1002/14651858.CD001546
Sohn M, Hoffmann M, Hochrein A, Buhr HJ, Lehmann KS (2015) Laparoscopic appendectomy is safe: influence of appendectomy technique on surgical-site infections and intra-abdominal abscesses. Surg Laparosc Endosc Percutan Tech 25:e90–e94
Massoomi H, Mills S, Dolich MO, Ketana N, Carmichael JC, Nguyen NT, Stamos MJ (2011) Comparison of outcomes of laparoscopic versus open appendectomy in adults: data from the nationwide inpatient sample (NIS), 2006–2008. J Gastrointest Surg 15:2226–2231
Xiao Y, Shi G, Zhang J, Cao JG, Liu LJ, Chen TH, Li ZZ, Wang H, Zhang H, Lin ZF, Lu JH, Yang T (2014) Surgical site infection after laparoscopic and open appendectomy: a multicenter large consecutive cohort study. Surg Endosc 29:1384–1393
Kocataş A, Gönenç M, Bozkurt MA, Karabulut M, Gemici E, Alış H (2013) Comparison of open and laparoscopic appendectomy in uncomplicated appendicitis: a prospective randomized clinical trial. Ulus Travma Acil Cerrahi Derg 19:200–204
Asarias JR, Schlussel AT, Cafasso DE, Carlson TL, Kasprenski MC, Washington EN, Lustik MB, Yamamura MS, Matayoshi EZ, Zagorski SM (2011) Incidence of postoperative intraabdominal abscesses in open versus laparoscopic appendectomies. Surg Endosc 25:2678–2683
Othani H, Tamamori Y, Arimoto Y, Nishiguchi Y, Maeda K, Hirakawa K (2012) Meta-analysis of the results of randomized controlled trials that compared laparoscopic and open surgery for acute appendicitis. J Gastrointest Surg 16:1929–1939
Wilson DG, Bond AK, Ladwa N, Sajid MS, Baig MK, Sains P (2013) Intra-abdominal collections following laparoscopic versus open appendicectomy: an experience of 516 consecutive cases at a district general hospital. Surg Endosc 27:2351–2356
Swank HA, Eshuis EJ, van Berge Henegouwen MI, Bemelman WA (2011) Short- and long-term results of open versus laparoscopic appendectomy. World J Surg 35:1221–1226
Li X, Zhang J, Sang L, Zhang W, Chu Z, Li X, Liu Y (2010) Laparoscopic versus conventional appendectomy–a meta-analysis of randomized controlled trials. BMC Gastroenterol. doi:10.1186/1471-230X-10-129
Liu Z, Zhang P, Ma Y, Chen H, Zhou Y, Zhang M, Chu Z, Qin H (2010) Laparoscopy or not: a meta analysis of the surgical effects of laparoscopic versus open appendicectomy. Surg Laparosc Endosc Percutan Tech 20:362–370
Wei B, Qi CL, Chen TF, Zheng ZH, Huang JL, Hu BG, Wei HB (2011) Laparoscopic versus open appendectomy for acute appendicitis: a metaanalysis. Surg Endosc 25:1199–1208
Wei HB, Huang JL, Zheng ZH, Wei B, Zheng F, Qiu WS, Guo WP, Chen TF, Wang TB (2010) Laparoscopic versus open appendectomy: a prospective randomized comparison. Surg Endosc 24:266–269
Markar SR, Penna M, Harris A (2014) Laparoscopic approach to appendectomy reduces the incidence of short- and long-term post-operative bowel obstruction: systematic review and pooled analysis. J Gastrointest Surg 18:1683–1692
Kapischke M, Friedrich F, Hedderich J, Schulz T, Caliebe A (2011) Laparoscopic versus open appendectomy quality of life 7 years after surgery. Langenbecks Arch Surg 396:69–75
Pelosi MA, Pelosi MA 3rd (1992) Laparoscopic appendectomy using a single umbilical puncture (minilaparoscopy). J Reprod Med 37:588–594
Antoniou SA, Koch OO, Antoniou GA, Lasithiotakis K, Chalkiadakis GE, Pointner R, Granderath FA (2014) Meta-analysis of randomized trials on single-incision laparoscopic versus conventional laparoscopic appendectomy. Am J Surg 207:613–622
Frutos D, Abrisqueta J, Lujan J, Abellan I, Parrilla P (2013) Randomized prospective study to compare laparoscopic appendectomy versus umbilical single incision appendectomy. Ann Surg 257:413–418
Gill RS, Shi X, Al-adra DP, Birch DW, Kamali S (2012) Single-incision appendectomy is comparable to conventional laparoscopic appendectomy: a systematic review and pooled analysis. Surg Laparosc Endosc Percutan Tech 22:319–327
Cai YL, Xiong XZ, Wu SJ, Cheng Y, Lu J, Zhang J, Lin YX, Cheng NS (2013) Single-incision laparoscopic appendectomy vs conventional laparoscopic appendectomy: systematic review and meta-analysis. World J Gastroenterol 19:5165–5173
Clerveus M, Morandeira-Rivas A, Moreno-Sanz C, Herrero-Bogajo ML, Picazo-Yeste JS, Tadeo-Ruiz G (2014) Systematic review and meta-analysis of randomized controlled trials comparing single incision versus conventional laparoscopic appendectomy. World J Surg 38:1937–1946
Markar SR, Karthikesalingam A, Di Franco F, Harris AM (2013) Systematic review and meta-analysis of single-incision versus conventional multiport appendicectomy. Br J Surg 13:1709–1718
Xu AM, Huang L, Li TJ (2015) Single-incision versus three-port laparoscopic appendectomy for acute appendicitis: systematic review and meta-analysis of randomized controlled trials. Surg Endosc 29:822–843
Xue C, Lin B, Huang Z, Chen Z (2015) Single-incision laparoscopic appendectomy versus conventional 3-port laparoscopic appendectomy for appendicitis: an updated meta-analysis of randomized controlled trials. Surg Today 45:1179–1186
Zhou H, Jin K, Zhang J, Wang W, Sun Y, Ruan C, Hu Z (2014) Single incision versus conventional multiport laparoscopic appendectomy: a systematic review and meta-analysis of randomized controlled trials. Dig Surg 31:384–391
Li P, Chen ZH, Li QG, Qiao T, Tian YY, Wang DR (2013) Safety and efficacy of single-incision laparoscopic surgery for appendectomies: a meta-analysis. World J Gastroenterol 19:4072–4082
Liang HH, Hung CS, Wang W, Tam KW, Chang CC, Liu HH, Yen KL, Wei PL (2014) Single-incision versus conventional laparoscopic appendectomy in 688 patients: a retrospective comparative analysis. Can J Surg 57:E89–E97
Gao J, Li P, Li Q, Tang D, Wang DR (2013) Comparison between single-incision and conventional three-port laparoscopic appendectomy: a meta-analysis from eight RCTs. Int J Colorectal Dis 28:1319–1327
Teoh AYB, Chiu PWY, Wong TCL, Poon MCM, Wong SKH, Leong HT, Lai PB, Ng EK (2012) A double blinded randomized controlled trial of laparoendoscopic single site access versus conventional 3-port appendectomy. Ann Surg 256:909–914
Lee WS, Choi ST, Lee JN, Kim KK, Park YH, Lee WK, Baek JH, Lee TH (2013) Single-Port laparoscopic appendectomy versus conventional laparoscopic appendectomy. A prospective randomized controlled study. Ann Surg 257:214–218
Gorter RR, Heij HA, Eker HH, Kazemier G (2014) Laparoscopic appendectomy: state of the art. Tailored approach to the application of laparoscopic appendectomy? Best Pract Res Clin Gastroenterol 28:211–224
Hua J, Gong J, Xu B, Yang T, Song Z (2014) Single-incision versus conventional laparoscopic appendectomy: a meta-analysis of randomized controlled trials. J Gastrointest Surg 18:426–436
Hansson J, Körner U, Khorram-Manesh A, Solberg A, Lundholm K (2009) Randomized clinical trial of antibiotic therapy versus appendectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg 96:473–481
Eriksson S, Granstrom L (1995) Randomized clinical trial of appendectomy versus antibiotic therapy for acute appendicitis. Br J Surg 82:166–169
Styrud J, Eriksson S, Nilsson I, Ahlberg G, Haapaniemi S, Neovius G, Rex L, Badume I, Granstrom L (2006) Appendectomy versus antibiotic treatment in acute appendicitis. A prospective multicentre randomized controlled trial. World J Surg 30:1033–1037
Malik AA, Bari S (2009) Conservative management of acute appendicitis. J Gastrointest Surg 13:966–970
Vons C, Barry C, Maitre S, Pautrat K, Leconte M, Costaglioli B, Karoui M, Alves A, Dousset B, Valleus P, Falissard B, Franco D (2011) Amoxicillin plus clavulanic acid versus appendicectomy for treatment of acute uncomplicated appendicitis. An open label, non-inferiority, randomised controlled trial. Lancet 377:1573–1579
Wilms IMHA, de Hoog DENM, de Visser DC, Janzing HMJ (2011) Appendectomy versus antibiotic treatment for acute appendicitis (review). Cochrane Database Syst Rev. doi:10.1002/14651858.CD008359.pub2
Svensson JF, Hall NJ, Eaton S, Pierro A, Wester T (2012) A review of conservative treatment of acute appendicitis. Eur J Pediatr Surg 22:185–194
Mason RJ, Moazzez A, Sohn H, Katkhouda N (2012) Meta-analysis of randomized trials comparing antibiotic therapy with appendectomy for acute uncomplicated (no abscess or phlegmon) appendicitis. Surg Infect (Larchmt) 13:74–84
Kirby A, Hobson RP, Burke D, Cleveland V, Ford G, West RM (2015) Appendicectomy for suspected uncomplicated appendicitis is associated with fewer complications than conservative antibiotic management: a meta-analysis of post-intervention complications. J Infect 70:105–110
Varadhan KK, Neal KR, Lobo DN (2012) Safety and efficacy of antibiotics compared with appendicectomy for treatment of uncomplicated acute appendicitis: meta analysis of randomised controlled trials. BMJ. doi:10.1136/bmj.e2156
Ansaloni L, Catena F, Coccolini F, Ercolani G, Gazzotti F, Pasqualini E, Pinna AD (2011) Surgery versus conservative antibiotic treatment in acute appendicitis: a systematic review and meta-analysis of randomized controlled trials. Dig Surg 28:210–221
Liu K, Fogg L (2011) Use of antibiotics alone for treatment of uncomplicated acute appendicitis: a systematic review and meta-analysis. Surgery 150:673–683
Dimitriou I, Reckmann B, Nephuth O, Betzler M (2013) Single institution’s experience in laparoscopic appendectomy as a suitable therapy for complicated appendicitis. Langenbecks Arch Surg 398:147–152
Thomson JE, Kruger D, Jann-Kruger C, Kiss A, Omoshoro-Jones JA, Luvhengo T, Brand M (2015) Laparoscopic versus open surgery for complicated appendicitis: a randomized controlled trial to prove safety. Surg Endosc 29:2027–2032
Tuggle KR, Ortega G, Bolorunduro OB, Oyetunji TA, Alexander R, Turner PL, Chang DC, Cornwell EE 3rd, Fullum TM (2010) Laparoscopic versus open appendectomy in complicated appendicitis: a review of the NSQIP database. J Surg Res 163:225–228
Andersson RE, Petzold MG (2007) Non-surgical treatment of appendiceal abscess or phlegmon. A systematic review and meta-analysis. Ann Surg 246:741–748
Simillis C, Symeonides P, Shorthouse AJ, Tekkis PP (2010) A meta-analysis comparing conservative treatment versus acute appendectomy for complicated appendicitis (abscess or phlegmon). Surgery 147:818–829
Kim JK, Ryoo S, Oh HK, Kim JS, Shin R, Choe EK, Jeong SY, Park KJ (2010) Management of appendicitis presenting with abscess or mass. J Korean Soc Coloproctol 26:413–419
Deelder JD, Richir MC, Schoorl T, Schreurs WH (2014) How to treat an appendiceal inflammatory mass: operatively or nonoperatively? J Gastrointest Surg 18:641–645
Hall NJ, Jones CE, Eaton S, Stanton MP, Burge DM (2011) Is interval appendicectomy justified after successful nonoperative treatment of an appendix mass in children? A systematic review. J Pediatr Surg 46:767–771
Clarke T, Katkhouda N, Mason RJ, Cheng BC, Olasky J, Sohn HJ, Moazzez A, Algra J, Chaghouri E, Berne TV (2011) Laparoscopic versus open appendectomy for the obese patient: a subset analysis from a prospective, randomized, double-blind study. Surg Endosc 25:1276–1280
Markar SR, Venkat-Raman V, Ho A, Karthikesalingam A, Kinross J, Evans J, Bloom I (2011) Laparoscopic versus open appendicectomy in obese patients. Int J Surg 9:451–455
Woodham BL, Cox MR, Eslick GD (2012) Evidence to support the use of laparoscopic over open appendicectomy for obese individuals: a meta-analysis. Surg Endosc 26:2566–2570
Dasari BV, Baker J, Markar S, Gardiner K (2015) Laparoscopic appendicectomy in obese is associated with improvements in clinical outcome: systematic review. Int J Surg 13:250–256
Walsh CA, Tang T, Walsh SR (2008) Laparoscopic versus open appendicectomy in pregnancy: a systematic review. Int J Surg 6:339–344
Wilasrusmee C, Sukrat B, McEvoy M, Attia J, Thakkinstian A (2012) Systematic review and meta-analysis of safety of laparoscopic versus open appendicectomy for suspected appendicitis in pregnancy. Br J Surg 99:1470–1478
McGory ML, Zingmond DS, Tillou A, Hiatt JR, Ko CY, Cryer HM (2007) Negative appendectomy in pregnant women is associated with substantial risk of fetal loss. J Am Coll Surg 205:534–540
Walker HG, Al Samaraee A, Mills SJ, Kalbassi MR (2014) Laparoscopic appendicectomy in pregnancy: a systematic review of the published evidence. Int J Surg 12:1235–1241
Chung JC, Cho GS, Shin EJ, Kim HC, Song OP (2013) Clinical outcomes compared between laparoscopic and open appendectomy in pregnant women. Can J Surg 56:341–346
Markar SR, Blackburn S, Cobb R, Karthikesalingam A, Evans J, Kinross J, Faiz O (2012) Laparoscopic versus open appendectomy for complicated and uncomplicated appendicitis in children. J Gastrointest Surg 16:1993–2004
Zwintscher NP, Johnson EK, Martin MJ, Newton CR (2013) Laparoscopy utilization and outcomes for appendicitis in small children. J Pediatr Surg 48:1941–1945
Armstrong J, Merritt N, Jones S, Scott L, Bütter A (2014) Non-operative management of early, acute appendicitis in children: Is it safe and effective? J Pediatr Surg 49:782–785
Svensson JF, Patkova B, Almström M, Naji H, Hall NJ, Eaton S, Pierro A, Wester T (2015) Nonoperative treatment with antibiotics versus surgery for acute nonperforated appendicitis in children: a pilot randomized controlled trial. Ann Surg 261:67–71
Yeh CC, Wu SC, Liao CC, Su LT, Hsieh CH, Li TC (2011) Laparoscopic appendectomy for acute appendicitis is more favorable for patients with comorbidities, the elderly, and those with complicated appendicitis: a nationwide population-based study. Surg Endosc 25:2932–2942
Masoomi H, Mills S, Dolich MO, Ketana N, Carmichael JC, Nguyen NT, Stamos MJ (2012) Does laparoscopic appendectomy impart an advantage over open appendectomy in elderly patients? World J Surg 36:1534–1539
Southgate E, Vousden N, Karthikesalingam A, Markar SR, Black S, Zaidi A (2012) Laparoscopic vs open appendectomy in older patients. Arch Surg 147:557–562
Wang YC, Yang HR, Chung PK, Jeng LB, Chen RJ (2006) Laparoscopic appendectomy in the elderly. Surg Endosc 20:887–889
Kim MJ, Fleming FJ, Gunzler DD, Messing S, Salloum RM, Monson JR (2011) Laparoscopic appendectomy is safe and efficacious for the elderly: an analysis using the National Surgical Quality Improvement Project database. Surg Endosc 25:1802–1807
McCartan DP, Fleming FJ, Grace PA (2010) The management of right iliac fossa pain—Is timing everything? Surgeon 8:211–217
Fair BA, Kubasiak JC, Janssen I, Myers JM, Millikan KW, Deziel DJ (2015) The impact of operative timing on outcomes of appendicitis: a National Surgical Quality Improvement Project analysis. Am J Surg 209:498–502
Salminen P, Paajanen H, Rautio T, Nordström P, Aarnio M, Rantanen T, Tuominen R, Hurme S, Virtanen J, Mecklin JP, Sand J, Jartti A, Rinta-Kiikka I, Grönroos JM (2015) Antibiotic therapy vs appendectomy for treatment of uncomplicated acute appendicitis: the APPAC randomized clinical trial. JAMA 313:2340–2348
Cappendijk VC, Hazebroek FW (2000) The impact of diagnostic delay on the course of acute appendicitis. Arch Dis Child 83:64–66
Papandria D, Goldstein SD, Rhee D, Salazar JH, Arlikar J, Gorgy A, Ortega G, Zhang Y, Abdullah F (2013) Risk of perforation increases with delay in recognition and surgery for acute appendicitis. J Surg Res 184:723–729
United Kingdom National Surgical Research Collaborative, Bhangu A (2014) Safety of short, In-Hospital delays before surgery for acute appendicitis: multicentre Cohort Study, systematic review, and meta-analysis. Ann Surg 259:894–903
Shin CS, Roh YN, Kim JL (2014) Delayed appendectomy versus early appendectomy in the treatment of acute appendicitis: a retrospective study. World J Emerg Surg. doi:10.1186/1749-7922-9-8
Ingraham AM, Cohen ME, Bilimoria KY, Ko CY, Hall BL, Russel TR, Nathens AB (2010) Effect of delay of operation on outcomes in adults with acute appendicitis. Arch Surg 145:886–892
Andersen BR, Kallehave FL, Andersen HK (2005) Antibiotics versus placebo for prevention of postoperative infection after appendicectomy. Cochrane Database Syst Rev. doi:10.1002/14651858.CD001439.pub2
Daskalakis K, Juhlin C, Påhlman L (2014) The use of pre- or postoperative antibiotics in surgery for appendicitis: a systematic review. Scand J Surg 103:14–20
Kasatpibal N, Nørgaard M, Sørensen HT, Schønheyder HC, Jamulitrat S, Chongsuvivatwong V (2006) Risk of surgical site infection and efficacy of antibiotic prophylaxis: a cohort study of appendectomy patients in Thailand. BMC Infect Dis. doi:10.1186/1471-2334-6-111
Angioli R, Terranova C, De Cicco Nardone C, Cafà EV, Damiani P, Portuesi R, Muzii L, Plotti F, Zullo MA, Panici PB (2013) A comparison of three different entry techniques in gynecological laparoscopic surgery: a randomized prospective trial. Eur J Obstet Gynecol Reprod Biol 171:339–342
Agresta F, De Simone P, Ciardo LF, Bedin N (2004) Direct trocar insertion vs Veress needle in nonobese patients undergoing laparoscopic procedures: a randomized prospective single-center study. Surg Endosc 18:1778–1781
Bemelman WA, Dunker MS, Busch OR, Den Boer KT, de Wit LT, Gouma DJ (2000) Efficacy of establishment of pneumoperitoneum with the Veress needle, Hasson trocar, and modified blunt trocar (TrocDoc): a randomized study. J Laparoendosc Adv Surg Tech A 10:325–330
van Rossem CC, Bolmers MD, Schreinemacher MH, van Geloven AA, Bemelman WA, Snapshot Appendicitis Collaborative Study Group (2016) Prospective nationwide outcome audit of surgery for suspected acute appendicitis. Br J Surg 103:144–151
Hamminga JT, Hofker HS, Broens PM, Kluin PM, Heineman E, Haveman JW (2013) Evaluation of the appendix during diagnostic laparoscopy, the laparoscopic appendicitis score: a pilot study. Surg Endosc 27:1594–1600
Strong S, Blencowe N, Bhangu A, National Surgical Research Collaborative (2015) How good are surgeons at identifying appendicitis? Results from a multi-centre cohort study. Int J Surg 15:107–112
Champault G, Rizk N, Ziol M, Taffinder N, Catheline JM (1996) Can we recognize the pathological character of the appendix during laparoscopy? Prospective study: 81 cases. J Chir (Paris) 133:320–323
Grunewald B, Keating J (1993) Should the ‘normal’ appendix be removed at operation for appendicitis? J R Coll Surg Edinb 38:158–160
Sajid MS, Rimple J, Cheek E, Baig MK (2009) Use of endo-GIA versus endo-loop for securing the appendicular stump in laparoscopic appendicectomy: a systematic review. Surg Laparosc Endosc Percutan Tech 19:11–15
Ates M, Dirican A, Ince V, Ara C, Isik B, Yilmaz S (2012) Comparison of intracorporeal knot-tying suture (Polyglactin) and titanium endoclips in laparoscopic appendiceal stump closure. Surg Laparosc Endosc Percutan Tech 22:226–231
Kazemier G, In’t Hof KH, Saad S, Bonjer HJ, Sauerland S (2006) Securing the appendiceal stump in laparoscopic appendectomy: evidence for routine stapling? Surg Endosc 20:1473–1476
St Peter SD, Adibe OO, Iqbal CW, Fike FB, Sharp SW, Juang D, Lanning D, Murphy JP, Andrews WS, Sharp RJ, Snyder CL, Holcomb GW, Ostlie DJ (2012) Irrigation versus suction alone during laparoscopic appendectomy for perforated appendicitis. Ann Surg 256:581–585
Akkoyun I, Tuna AT (2012) Advantages of abandoning abdominal cavity irrigation and drainage in operations performed on children with perforated appendicitis. J Pediatr Surg 47:1886–1890
Hartwich JE, Carter RF, Wolfe L, Goretsky M, Heath K, Peter SDS (2013) The effects of irrigation on outcomes in cases of perforated appendicitis in children. J Surg Res 180:222–225
Allemann P, Probst H, Demartines N, Schäfer M (2011) Prevention of infectious complications after laparoscopic appendectomy for complicated acute appendicitis-the role of routine abdominal drainage. Langenbecks Arch Surg 396:63–68
St Peter SD, Aguayo P, Fraser JD, Keckler SC, Sharp SW, Leys CM, Murphy JP, Snyder CL, Sharp RJ, Andrews WS, Holcomb GW, Ostlie DJ (2010) Initial laparoscopic appendectomy versus initial nonoperative management and interval appendectomy for perforated appendicitis with abscess: a prospective, randomized trial. J Pediatr Surg 45:236–240
Olsen J, Skovdal J, Qvist N, Bisgaard T (2014) Treatment of appendiceal mass—a qualitative systematic review. Dan Med J 61:A4881
Misdraji J, Yantiss RK, Graeme-Cook FM, Balis UJ, Young RH (2003) Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol 27:1089–1103
McDonald JR, O’Dwyer ST, Rout S, Chakrabarty B, Sikand K, Fulford PE, Wilson MS, Renehan AG (2012) Classification of and cytoreductive surgery for low-grade appendiceal mucinous neoplasms. Br J Surg 99:987–992
Arnason T, Kamionek M, Yang M, Yantiss RK, Misdraji J (2015) Significance of proximal margin involvement in low-grade appendiceal mucinous neoplasms. Arch Pathol Lab Med 139:518–521
Henderson L, Fehily C, Folaranmi S, Kelsey A, McPartland J, Jawaid WB, Craigie R, Losty PD (2014) Management and outcome of neuroendocrine tumours of the appendix—a two centre UK experience. J Pediatr Surg 49:1513–1517
Kim SS, Kays DW, Larson SD, Islam S (2014) Appendiceal carcinoids in children—management and outcomes. J Surg Res 192:250–253
Coakley B, Sussman E, Wolfson T, Bhagavath A, Choi J, Ranasinghe N, Lynn E, Divino C (2011) Postoperative antibiotics correlate with worse outcomes after appendectomy for nonperforated appendicitis. J Am Coll Surg 213:778–783
van Rossem CC, Schreinemacher M, Treskes K, Hogezand RM, Geloven AAW (2014) Duration of antibiotic treatment after appendicectomy for acute complicated appendicitis. Br J Surg 101:715–719
Pinto DJ, Sanderson PJ (1980) Rational use of antibiotic therapy after appendicectomy. Br J Surg 280:275–277
Ambrose NS, Donovan IA, Wise R, Lowe P (1983) Metronidazole and ticarcillin in the prevention of sepsis after appendicectomy. Am J Surg 146:346–348
Campbell WB (1980) Prophylaxis of infection after appendicectomy: a survey of current surgical practice. BMJ 281:1597–1600
Buckels JA, Brookstein R, Bonser R, Bullen B, Alexander-Williams J (1985) A comparison of the prophylactic value of cefotetan and metronidazole appendectomy. World J Surg 9:814–818
Nadler E, Reblock KK, Ford HR, Gaines BA (2003) Monotherapy versus multi-drug therapy for the treatment of perforated appendicitis in children. Surg Infect (Larchmt) 4:327–333
Yu TC, Hamill JKM, Evans SM, Price NR, Morreau PN, Upadhyay VA, Ferguson RS, Best EJ, Hill AG (2013) Duration of postoperative intravenous antibiotics in childhood complicated appendicitis: a propensity score-matched comparison study. Eur J Pediatr Surg 24:341–349
Lau WY, Fan ST, Yiu TF, Wong SH (1983) Prophylaxis of post-appendicectomy sepsis by metronidazole and ampicillin: a randomized, prospective and double-blind trial. Br J Surg 70:155–157
Lau DHW, Yau KKK, Chung CC, Leung FCS, Tai YP, Li MKW (2005) Comparison of needlescopic appendectomy versus conventional laparoscopic appendectomy: a randomized controlled trial. Surg Laparosc Endosc Percutan Techn 15:75–79
Andersson RE (2001) Small bowel obstruction after appendicectomy. Br J Surg 88:1387–1391
Emil S, Elkady S, Shbat L, Youssef F, Baird R, Laberge JM, Puligandla P, Shaw K (2014) Determinants of postoperative abscess occurrence and percutaneous drainage in children with perforated appendicitis. Pediatr Surg Int 30:1265–1271
Fleming FJ, Kim MJ, Messing S, Gunzler D, Salloum R, Monson JR (2010) Balancing the risk of postoperative surgical infections: a multivariate analysis of factors associated with laparoscopic appendectomy from the NSQIP database. Ann Surg 252:895–900
Bahar MM, Jangjoo A, Amouzeshi A, Kavianifar K (2010) Wound infection incidence in patients with simple and gangrenous or perforated appendicitis. Arch Iran Med 13:13–16
Guller U, Hervey S, Purves H, Muhlbaier LH, Peterson ED, Eubanks S, Pietrobon R (2004) Laparoscopic versus open appendectomy: outcomes comparison based on a large administrative database. Ann Surg 239:43–52
Isaksson K, Montgomery A, Moberg AC, Andersson R, Tingstedt B (2014) Long-term follow-up for adhesive small bowel obstruction after open versus laparoscopic surgery for suspected appendicitis. Ann Surg 259:1173–1177
Boomer LA, Cooper JN, Deans KJ, Minneci PC, Leonhart K, Diefenbach KA, Kenney BD, Besner GE (2014) Does delay in appendectomy affect surgical site infection in children with appendicitis? J Pediatr Surg 49:1026–1029
Graat LJ, Bosma E, Roukema JA, Heisterkamp J (2012) Appendectomy by residents is safe and not associated with a higher incidence of complications. Ann Surg 255:715–719
Gandaglia G, Ghani KR, Sood A, Meyers JR, Sammon JD, Schmid M, Varda B, Briganti A, Montorsi F, Sun M, Menon M, Kibel AS, Trinh QD (2014) Effect of minimally invasive surgery on the risk for surgical site infections: results from the National Surgical Quality Improvement Program (NSQIP) Database. JAMA Surg 149:1039–1044
Advani V, Ahad S, Gonczy C, Markwell S, Hassan I (2012) Does resident involvement effect surgical times and complication rates during laparoscopic appendectomy for uncomplicated appendicitis? An analysis of 16,849 cases from the ACS-NSQIP. Am J Surg 203:347–351
Galli R, Banz V, Fenner H, Metzger J (2013) Laparoscopic approach in perforated appendicitis: increased incidence of surgical site infection? Surg Endosc 27:2928–2933
Kanona H, Al Samaraee A, Nice C, Bhattacharya V (2012) Stump appendicitis: a review. Int J surg 10:425–428
Clark JJ, Johnson SM (2011) Laparoscopic drainage of intraabdominal abscess after appendectomy: an alternative to laparotomy in cases not amenable to percutaneous drainage. J Pediatr Surg 46:1385–1389
Ben Dhaou M, Ghorbel S, Chouikh T, Charieg A, Nouiare F, Ben Khalifa S, Khemekhem R, Jlidi S, Chaouachi B (2010) Conservative management of post-appendectomy intra-abdominal abscesses. Ital J Pediatr. doi:10.1186/1824-7288-36-68
Forgues D, Habbig S, Dillo AF, Kalfa N, Lopez M, Allal H, Guibal MP, Sabatier-Laval E, Galifer RB (2007) Post-appendectomy intra-abdominal abscesses; Can they successfully be managed with the sole use of antibiotic therapy? Eur J Pediatr Surg 17:104–109
Contreras-Dominguez V, Carbonell-Bellilio C (2008) Profylactic antiemetic therapy for acute abdominal surgery. A comparative study of Droperidol, Metoclopramide, Tropisetron, granisetron and Dexamethasone. Rev Bras Anesthesiol 58:35–44
Morton NS, O’Brien K (1999) Analgesic efficacy of paracetamol and diclofenac in children receiving PCA morphine. Br J Anaesth 82:715–717
Jalil RMA, Yahya N, Sulaimn O, Mat WRW, Teo R, Izaham A, Rahman RA (2013) Comparing the effectiveness of ropivacaine 0.5 % versus ropivacaine 0.2 % for transabdominis plane block in providing postoperative analgesia after appendectomy. Acta Anaesthesiol Taiwan 52:49–53
Ahn SR, Kang DB, Lee C, Park WC, Lee JK (2013) Postoperative pain relief using wound infiltration with 0.5 % bupivacaine in single-incision laparoscopic surgery for an appendectomy. Ann Coloproctol 29:238–242
Cho S, Kim YJ, Kim DY, Chung SS (2013) Postoperative analgesic effects of ultrasound-guided transversus abdominis plane block for open appendectomy. J Korean Surg Soc 85:128–133
Taal BG (2005) Diagnosis and treatment in intestinal carcinoid tumours. Curr Gastroenterol Rep 7:1–3
Sieren LM, Collins JN, Weireter LJ, Britt RC, Reed SF, Novosel TJ (2010) The incidence of benign and malignant neoplasia presenting as acute appendicitis. Am Surg 76:808–811
Benedix F, Reimer A, Gastinger I, Mroczkowski P, Lippert H, Kube R (2010) Primary appendiceal carcinoma–epidemiology, surgery and survival: results of a German multi-center study. Eur J Surg Oncol 36:763–771
Charfi S, Sellami A, Affes A, Yaïch K, Mzali R, Boudawara TS (2014) Histopathological findings in appendectomy specimens: a study of 24,697 cases. Int J Colorectal Dis 29:1009–1012
Anwar K, Desai M, Al-Bloushi N, Alam F, Cyprian FS (2014) Prevalence and clinicopathological characteristics of appendiceal carcinoids in Sharjah (United Arab Emirates). World J Gastrointest Oncol 6:253–256
In’t Hof K, Wal HC, Kazemier G, Lange JF (2008) Carcinoid tumour of the appendix: an analysis of 1,485 consecutive emergency appendectomies. J Gastrointest Surg 12:1436–1438
Chandrasegaram MD, Rothwell LA, An EI, Miller RJ (2012) Pathologies of the appendix: a 10-year review of 4670 appendicectomy specimens. ANZ J Surg 82:844–847
Ramezani MA, Dehghani MR (2007) Relationship between Enterobius vermicularis and the incidence of acute appendicitis. Southeast Asian J Trop Med Public Health 38:20–23
Chong VH, Telisinghe PU, Yapp KS, Chong CF (2011) Tuberculous appendix: a review of clinical presentations and outcomes. Singapore Med J 52:90–93
Khan SA, Khokhar HA, Nasr AR, Carton E (2013) Incidence of right-sided colonic tumours (non-appendiceal) in patient’s 40 years of age presenting with features of acute appendicitis. Int J Surg 11:301–304
González-Moreno S, Sugarbaker PH (2004) Right hemicolectomy does not confer a survival advantage in patients with mucinous carcinoma of the appendix and peritoneal seeding. Br J Surg 91:304–311
Amini A, Masoumi-Moghaddam S, Ehteda A, Morris DL (2014) Secreted mucins in pseudomyxoma peritonei: pathophysiological significance and potential therapeutic prospects. Orphanet J Rare Dis. doi:10.1186/1750-1172-9-71
Shapiro R, Eldar S, Sadot E, Papa MZ, Zippel DB (2011) Appendiceal carcinoid at a large tertiary center: pathologic findings and long-term follow-up evaluation. Am J Surg 201:805–808
Marudanayagam R, Williams GT, Rees BI (2006) Review of the pathological results of 2660 appendicectomy specimens. J Gastroenterol 41:745–749
Connor SJ, Hanna GB, Frizelle FA (1997) Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumours from 7,970 Appendectomies. Dis Colon Rectum 41:75–80
Alemayehu H, Snyder CL, St Peter SD, Ostlie DJ (2014) Incidence and outcomes of unexpected pathology findings after appendectomy. J Pediatr Surg 49:1390–1393
Lobo-machín I, Delgado-Plasencia L, Hernández-González I, Brito-García A, Burillo-Putze G, Bravo-Gutiérrez A (2014) Appendiceal diverticulitis and acute appendicitis: differences and similarities. Rev Esp Enferm Dig 106:452–458
Lohsiriwat V, Vongjirad A, Lohsiriwat D (2009) Incidence of synchronous appendiceal neoplasm in patients with colorectal cancer and its clinical significance. World J Surg Oncol. doi:10.1186/1477-7819-7-51
Vettoretto N, Gobbi S, Corradi A, Belli F, Piccolo D, Pernazza G, Mannino L, Italian Association of Hospital Surgeons (Associazione dei Chirurghi Ospedalieri Italiani) (2011) Consensus conference on laparoscopic appendectomy: development of guidelines. Colorectal Dis 13:748–754
Nair R, Aggarwal R, Khanna D (2011) Methods of formal consensus in classification/diagnostic criteria and guideline development. Semin Arthritis Rheum 41:95–105
Rycroft-Malone J (2001) Formal consensus: the development of a national clinical guideline. Qual Health Care 10:238–244
Loblaw DA, Prestrud AA, Somerfield MR, Oliver TK, Brouwers MC, Nam RK, Lyman GH, Basch E, American Society of Clinical Oncology Clinical Practice Guidelines (2012) American Society of Clinical Oncology Clinical Practice Guidelines: formal systematic review-based consensus methodology. J Clin Oncol 30:3136–3140
Funding
This project was supported by a grant from the EAES.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Disclosures
Dr. Antoniou received personal fees from the EAES (including Journal and Publication Committee) and from the European Hernia Society. Dr. Muysoms received grants personal fees and non-financial support from Medtronic and Johnson & Johnson. He received grants and personal fees from B. Braun and Dynamesh. He received personal fees from BARD davol, Cousin Biotech, WL Gore@ass and Dansac outside the submitted work. Prof. Dr. Bonjer received grants and personal fees from Johnson & Johnson, Applied Medical, Medtronic and Olympus. He received personal fees from Cook. All outside the submitted work. Drs. Defoort, Dr. Go, Prof. Dr. Ozmen, Dr. Rhodes, Drs. Gorter, Dr. Eker, Drs Gorter-Stam, Drs. Abis, Drs. Acharya, Drs. Ankersmit, Drs. Arolfo, Drs. Babic, Prof. Dr. Boni, Drs. Bruntink, Drs. Van Dam, Drs. Deijen, Drs. DeLacy, Drs. Harmsen, Drs. Van den Helder, Dr. Iordache, Drs. Ket, Drs. Papoulas, Drs. Straatman, Drs. Tenhagen, Drs. Turrado, Dr. Vereczkei, Prof. Dr. Vilallonga and Drs. Deelder have no conflict of interest or financial ties to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Gorter, R.R., Eker, H.H., Gorter-Stam, M.A.W. et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc 30, 4668–4690 (2016). https://doi.org/10.1007/s00464-016-5245-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00464-016-5245-7