Abstract
The dark-to-light transitions enable energization of the thylakoid membrane (TM), which is reflected in fast and slow (OJIPSMT or OABCDE) stages of fluorescence induction (FI) and P700 oxidoreduction changes (ΔA810). A Thylakoid Membrane model (T-M model), in which special emphasis has been placed on ferredoxin-NADP+-oxidoreductase (FNR) activation and energy-dependent qE quenching, was applied for quantifying the kinetics of FI and ΔA810. Pea leaves were kept in darkness for 15 min and then the FI and ΔA810 signals were measured upon actinic illumination, applied either directly or after a 10-s light pulse coupled with a subsequent 10-s dark interval. On the time scale from 40 µs to 30 s, the parallel T-M model fittings to both FI and ΔA810 signals were obtained. The parameters of FNR activation and the buildup of qE quenching were found to differ for dark-adapted and preilluminated leaves. At the onset of actinic light, photosystem II (PSII) acceptors were oxidized (neutral) after dark adaptation, while the redox states with closed and/or semiquinone QA(−)QB(−) forms were supposedly generated after preillumination, and did not relax within the 10 s dark interval. In qE simulations, a pH-dependent Hill relationship was used. The rate constant of heat losses in PSII antenna kD(t) was found to increase from the basic value kDconst, at the onset of illumination, to its maximal level kDvar due to lumenal acidification. In dark-adapted leaves, a low value of kDconst of ∼ 2 × 106 s−1 was found. Simulations on the microsecond to 30 s time scale revealed that the slow P-S-M-T phases of the fluorescence induction were sensitive to light-induced FNR activation and high-energy qE quenching. Thus, the corresponding time-dependent rate constants kD(t) and kFNR(t) change substantially upon the release of electron transport on the acceptor side of PSI and during the NPQ development. The transitions between the cyclic and linear electron transport modes have also been quantified in this paper.









Similar content being viewed by others
Abbreviations
- Chl:
-
Chlorophyll
- Cyt b 6 f :
-
Cytochrome b6f complex
- CEF:
-
Cyclic electron flow (around PSI)
- ET:
-
Electron transfer
- ETC:
-
Electron transport chain
- Fd, Fdr :
-
Ferredoxin
- FL:
-
Fluorescence
- FNR:
-
Ferredoxin-NADP+-oxidoreductase
- F 0 :
-
Minimal chlorophyll a fluorescence yield
- F m :
-
Maximal chlorophyll a fluorescence yield (induced by multiturnover light pulses)
- H L +, H S + :
-
Protons in lumen (L), protons in stroma (S)
- NADP+ :
-
Nicotinamide adenine dinucleotide phosphate, oxidized form
- NPQ:
-
Non-photochemical quenching (of the excited state of Chl a)
- PFD:
-
Photon flux density
- Phe, Ph:
-
Primary PSII electron acceptor, pheophytin
- pHL, pHS :
-
pH in lumen, in stroma
- Pc:
-
Plastocyanin
- PQ:
-
Plastoquinone
- PQH2 :
-
Plastoquinol
- PS II, PS I:
-
Photosystem II, Photosystem I
- PT:
-
Proton transfer
- P680, P680 :
-
Chlorophyll a acting as electron donor in PSII
- P700:
-
Chlorophyll a acting as electron donor in PSI
- Q A and Q B :
-
Primary and secondary plastoquinone electron acceptors of PSII
- qE:
-
Energy-dependent quenching
- RC:
-
Reaction center (of PS II, or of PS I)
- WOC:
-
Water-oxidizing complex
- YZ :
-
Tyrosine 161 of the PS II D1 polypeptide
- ΔΨ:
-
Electrical potential across the thylakoid membrane
References
Baake E, Schlöder JP (1992) Modelling the fast fluorescence rise of photosynthesis. Bull Math Biol 54:999–1021
Baker NR (2008) Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol 59:659–668
Baker NR, Harbinson J, Kramer DM (2007) Determining the limitations and regulation of photosynthetic energy transduction in leaves. Plant Cell Environ 30:1107–1125
Belyaeva NE, Lebedeva GV, Riznichenko GY (2003) Kinetic model of primary photosynthetic processes in chloroplasts. Modeling of thylakoid membranes electric potential. In: Riznichenko GY (eds) Mathematics computer education, vol 10. Progress-Traditsiya, Moscow, pp 263–276 (in Russian)
Belyaeva NE, Schmitt F-J, Steffen R, Paschenko VZ, Riznichenko GY Chemeris YK, Renger G, Rubin AB (2008) PS II model-based simulations of single turnover flash-induced transients of fluorescence yield monitored within the time domain of 100 ns–10 s on dark-adapted Chlorella pyrenoidosa cells. Photosynth Res 98:105–119
Belyaeva NE, Bulychev AA, Riznichenko GY, Rubin AB (2011a) A model of photosystem II for the analysis of fast fluorescence rise in plant leaves. Biophysics 56(3):464–477
Belyaeva NE, Schmitt F-J, Paschenko VZ, Riznichenko GY, Rubin AB, Renger G (2011b) PS II model based analysis of transient fluorescence yield measured on whole leaves of Arabidopsis thaliana after excitation with light flashes of different energies. BioSystems 103(2):188–195
Belyaeva NE, Schmitt F-J, Paschenko VZ, Riznichenko GY, Rubin AB, Renger G (2014) Model based analysis of transient fluorescence yield induced by actinic laser flashes in spinach leaves and cells of green alga Chlorella pyrenoidosa Chick. Plant Physiol Biochem 77:49–59
Belyaeva NE, Schmitt F-J, Paschenko VZ, Riznichenko GY, Rubin AB (2015) Modelling of the redox state dynamics in photosystem II of Chlorella pyrenoidosa Chick cells and leaves of spinach and Arabidopsis thaliana from single flash induced fluorescence quantum yield changes on the 100 ns–10 s time scale. Photosynth Res 125:123–140
Belyaeva NE, Bulychev AA, Riznichenko GY, Rubin AB (2016) Thylakoid membrane model of the Chl a fluorescence transient and P700 induction kinetics in plant leaves. Photosynth Res 130:491–515
Bernát G, Steinbach G, Kaňa R, Govindjee, Misra AN, Prášil O (2017) On the origin of the slow M–T chlorophyll a fluorescence decline in cyanobacteria: interplay of short-term light-responses. Photosynth Res. https://doi.org/10.1007/s11120-017-0458-8
Brettel K (1997) Electron transfer and arrangement of the redox cofactors in photosystem I. Biochim Biophys Acta 1318(3):322–373
Bulychev AA (2011) Induction changes in photosystems I and II in plant leaves upon modulation of membrane ion transport. Biochem (Mosc) Suppl Ser A Membr Cell Biol 5:335–342
Bulychev AA, Vredenberg WJ (1999) Light-triggered electrical events in the thylakoid membrane of plant chloroplast. Physiol Plantarum 105:577–584
Bulychev AA, Vredenberg WJ (2010) Induction kinetics of photosystem I—activated P700 oxidation in plant leaves and their dependence on pre-energization. Russ J Plant Physiol 57(5):599–608
Bulychev AA, Cherkashin AA, Rubin AB (2010) Dependence of chlorophyll P700 redox transients during the induction period on the transmembrane distribution of protons in chloroplasts of pea leaves. Russ J Plant Physiol 57(1):20–27
Bulychev AA, Osipov VA, Matorin DN, Vredenberg WJ (2013) Effects of far-red light on fluorescence induction in infiltrated pea leaves under diminished ∆pH and ∆φ components of the proton motive force. J Bioenerg Biomembr 45:37–45
Carrillo N, Ceccarelli EA (2003) Open questions in ferredoxin-NADP+ reductase catalytic mechanism. Eur J Biochem 270:1900–1915
Carrillo N, Lucero H, Vallejos RH (1981) Light modulation of chloroplast membrane-bound ferredoxin-NADP+ oxidoreductase. J Biol Chem 256:1058–1059
Cruz JA, Sacksteder CA, Kanazawa A, Kramer DM (2001) Contribution of electric field (∆ψ) to steady-state transthylakoid proton motive force (pmf) in vivo and in vitro. Control of pmf parsing into ∆ψ and ∆pH by ionic strength. Biochemistry 40:1226–1237
Cruz JA, Kanazawa A, Treff N, Kramer DM (2005) Storage of light-driven transthylakoid proton motive force as an electric field (∆ψ) under steady-state conditions in intact cells of Chlamydomonas reinhardtii. Photosynth Res 85:221–233
Dau H (1994) Molecular mechanism and quantitative models of variable photosystem II fluorescence. Photochem Photobiol 60:1–23
Demin OV, Westerhoff HV, Kholodenko BN (1998) Mathematical modeling of superoxide generation with the bc 1 complex of mitochondria. Biochemistry (Moscow) (6):634–649
Ebenhöh O, Houwaart T, Lokstein H, Schlede S, Tirok K (2011) A minimal mathematical model of nonphotochemical quenching of chlorophyll fluorescence. Biosystems 103(2):196–204
Ebenhöh O, Fucile G, Finazzi G, Rochaix JD, Goldschmidt-Clermont M (2014) Short-term acclimation of the photosynthetic electron transfer chain to changing light: a mathematical model. Philos Trans R Soc B 369:20130223
Foyer CH, Lelandais M, Harbinson J (1992) Control of the quantum efficiencies of photosystems I and II, electron flow, and enzyme activation following dark-to-light transitions in pea leaves. Plant Physiol 99:979–986
Foyer CH, Neukermans J, Queval G, Noctor G, Harbinson J (2012) Photosynthetic control of electron transport and the regulation of gene expression. J Exp Bot 63:1637–1661
Gizzatkulov N, Klimov A, Lebedeva G, Demin O (2004) DBsolve7: new update version to develop and analyze models of complex biological systems. In: ISMB/ECCB conference, Glasgow, Scotland, UK, 31 July–5 August 2004. http://www.insysbio.ru
Govindjee (ed) (1982) Photosynthesis, vol 2. Academic Press, New York
Harbinson J, Hedley CL (1993) Changes in P-700 oxidation during the early stages of the induction of photosynthesis. Plant Physiol 103:649–660
Heldt HW, Werdan K, Milovancev M, Geller G (1973) Alkalinization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid lumen. Biochim Biophys Acta 314:224–241
Hope AB (1993) The chloroplast cytochrome bf complex: a critical focus on function. Biochim Biophys Acta 1143:1–22
Joliot P, Joliot A (2002) Cyclic electron transfer in plant leaf. PNAS 99:10209–10214
Junge W, Auslander W, McGeer A, Runge T (1979) The buffering capacity of the internal phase of thylakoids and the magnitude of the pH changes inside under flashing light. Biochim Biophys Acta 546:121–141
Kamali MJ, Lebedeva GV, Demin OV, Beljaeva NE, Riznichenko GY, Rubin AB (2004) A kinetic model of the cytochrome bf complex with fitted parameters. Biophysics 49:1061–1068
Klughammer C, Schreiber U (2016) Deconvolution of ferredoxin, plastocyanin, and P700 transmittance changes in intact leaves with a new type of kinetic LED array spectrophotometer. Photosynth Res 128:195–214. https://doi.org/10.1007/s11120-016-0219-0
Kodru S, Malavath T, Devadasu E, Nellaepalli S, Stirbet A, Subramanyam R, Govindjee (2015) The slow S to M rise of chlorophyll a fluorescence induction reflects transition from state 2 to state 1 in the green alga Chlamydomonas reinhardtii. Photosynth Res 125(1–2):219–231
Krause GH, Jahns P (2004) Non-photochemical energy dissipation determined by chlorophyll fluorescence quenching: characterization and function. In: Papageorgiou GC, Govindjee (ed) Chlorophyll a fluorescence: a signature of photosynthesis. Springer, Dordrecht, pp 463–495
Kuvykin IV, Ptushenko VV, Vershubskii AV, Tikhonov AN (2011) Regulation of electron transport in C(3) plant chloroplasts in situ and in silico: short-term effects of atmospheric CO(2) and O(2). Biochim Biophys Acta 1807(3):336–347
Laisk A, Walker DA (1989) A mathematical model of electron transport. Thermodynamic necessity for photosystem II regulation. Proc R Soc Lond B 237:417–444
Laisk A, Eichelmann H, Oja V (2006) C3 photosynthesis in silico. Photosynth Res 90:45–66
Lazár D (2003) Chlorophyll a fluorescence rise induced by high light illumination of dark-adapted plant tissue studied by means of a model of Photosystem II and considering Photosystem II heterogeneity. J Theor Biol 220:469–503
Lazár D (2009) Modelling of light-induced chlorophyll a fluorescence rise (O-J-I-P transient) and changes in 820 nm-transmittance signal of photosynthesis. Photosynthetica 47(4):483–498
Lebedeva GV, Belyaeva NE, Riznichenko GY, Rubin AB, Demin OV (2000) Kinetic model of photosystem II of higher green plants. Russ J Phys Chem 74:1702–1710
Lebedeva GV, Belyaeva NE, Demin OV, Riznichenko GY, Rubin AB (2002) Kinetic model of primary photosynthetic processes in chloroplasts. Description of the fast phase of chlorophyll fluorescence induction under different light intensities. Biophysics 47:968–980
Lyu H, Lazár D (2017) Modeling the light-induced electric potential difference (∆Ψ), the pH difference (∆pH) and the proton motive force across the thylakoid membrane in C3 leaves. J Theor Biol 413:11–23
McDonald AE, Ivanov AG, Bode R, Maxwell DP, Rodermel SR, Huner NPA (2011) Flexibility in photosynthetic electron transport: the physiological role of plastoquinol terminal oxidase (PTOX). Biochim Biophys Acta 1807:954–967
Mulo P, Medina M (2017) Interaction and electron transfer between ferredoxin–NADP+ oxidoreductase and its partners: structural, functional, and physiological implications. Photosynth Res 134:265–280
Papageorgiou GC, Govindjee (eds) (2004) Chlorophyll a fluorescence: a signature of photosynthesis. Advances in photosynthesis and respiration, vol 19. Springer, Dordrecht
Papageorgiou GC, Govindjee (2011) Photosystem II fluorescence: slow changes—scaling from the past. J Photochem Photobiol B 104:258–270
Papageorgiou GC, Tsimilli-Michael M, Stamatakis K (2007) The fast and slow kinetics of chlorophyll a fluorescence induction in plants, algae and cyanobacteria: a viewpoint. Photosynth Res 94:275–290
Ptushenko VV, Zhigalova TV, Avercheva OV, Tikhonov AN (2018) Three phases of energy-dependent induction of P700 + and Chl a fluorescence in Tradescantia fluminensis leaves. Photosynth Res. https://doi.org/10.1007/s11120-018-0494-z
Renger G (2004) Coupling of electron and proton transfer in oxidative water cleavage in photosynthesis. Biochim Biophys Acta 1655:195–204
Renger G (2012) Mechanism of light induced water splitting. In Photosystem II of oxygen evolving photosynthetic organisms. Biochim Biophys Acta 1817:1164–1176
Renger G, Schulze A (1985) Quantitative analysis of fluorescence induction curves in isolated spinach chloroplasts. Photobiochem Photobiophys 9:79–87
Renger G, Eckert HJ, Bergmann A, Bernarding J, Liu B, Napiwotzki A, Reifarth F, Eichler HJ (1995) Fluorescence and spectroscopic studies on exciton trapping and electron transfer in photosystem II of higher plants. Aust J Plant Physiol 22:167–181
Reynolds IA, Johnson EA, Tanford C (1985) Incorporation of membrane potential into theoretical analysis of electrogenic ion pumps. Proc Natl Acad Sci USA 82:6869–6873
Rich PR (1988) A critical examination of the supposed variable proton stoichiometry of the chloroplast cytochrome b/f complex. Biochim Biophys Acta 932:33–42
Roelofs TA, Lee CH, Holzwarth AR (1992) Global target analysis of picosecond chlorophyll fluorescence kinetic from pea chloroplasts. Biophys J 61:1147–1163
Rubin A, Riznichenko GY (2009) Modeling of the primary processes in a photosynthetic membrane. In: Laisk A, Nedbal L, Govindjee (eds) Photosynthesis in silico: understanding complexity from molecules to ecosystems, advances in photosynthesis and respiration, vol 29. Springer, Dordrecht, pp 151–176
Rutherford AW, Govindjee, Inoue Y (1984) Charge accumulation and photochemistry in leaves studied by thermoluminescence and delayed light emission. Proc Natl Acad Sci USA 81:1107–1111
Satoh K (1982) Mechanism of photoactivation of electron transport in intact Bryopsis chloroplasts. Plant Physiol 70:1413–1416
Schansker G, Strasser RJ (2005) Quantification of non-Q B-reducing centers in leaves using a far-red pre-illumination. Photosynth Res 84:145–151
Schansker G, Srivastava A, Govindjee, Strasser RJ (2003) Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. Funct Plant Biol 30:785–796
Schansker G, Tóth SZ, Strasser RJ (2006) Dark-recovery of the Chl a fluorescence transient (OJIP) after light adaptation: the qT component of non-photochemical quenching is related to an activated photosystem I acceptor side. Biochim Biophys Acta 1757:787–797
Schatz GH, Brock H, Holzwarth AR (1988) Kinetic and energetic model for the primary processes in photosystem II. Biophys J 54:397–405
Semenov AYu, Cherepanov DA, Mamedov MD (2008) Electrogenic reactions and dielectric properties of photosystem II. Photosynth Res 98:121–130
Steffen R, Eckert H-J, Kelly AA, Dörmann PG, Renger G (2005) Investigations on the reaction pattern of photosystem II in leaves from Arabidopsis thaliana by time-resolved fluorometric analysis. Biochemistry 44:3123–3132
Stirbet A, Govindjee (2012) Chlorophyll a fluorescence induction: a personal perspective of the thermal phase, the J-I-P rise. Photosynth Res 113:15–61
Stirbet A, Govindjee (2016) The slow phase of chlorophyll a fluorescence induction in silico: origin of the S-M fluorescence rise. Photosynth Res 130:193–213. https://doi.org/10.1007/s11120-016-0243-0
Stirbet A, Govindjee, Strasser BJ, Strasser RJ (1998) Chlorophyll a fluorescence induction in higher plants: modeling and numerical simulation. J Theor Biol 193:131–151
Stirbet A, Riznichenko GY, Rubin AB, Govindjee (2014) Modeling chlorophyll a fluorescence transient: relation to photosynthesis. Biochemistry 79:291–323
Stirbet A, Lazár D, Kromdijk J, Govindjee (2018) Chlorophyll a fluorescence induction: can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 56(1):86–104
Takizawa K, Cruz JA, Kanazawa A, Kramer DM (2007) The thylakoid proton motive force in vivo. Quantitative, non-invasive probes, energetics, and regulatory consequences of light-induced pmf. Biochem Biophys Acta 1767:1233–1244. https://doi.org/10.1016/j.bbabio.2007.07.006
Talts E, Oja V, Rämma H, Rasulov B, Laisk AA (2007) Dark inactivation of ferredoxin-NADP reductase and cyclic electron flow under far-red light in sunflower leaves. Photosynth Res 94:109–120
Tikhonov AN (2014) The cytochrome b6f complex at the crossroad of photosynthetic electron transport pathways. Plant Physiol Biochem 81:163–183
Tikhonov AN (2015) Induction events and short-term regulation of electron transport in chloroplasts: an overview. Photosynth Res 125:65–94
Tikhonov AN, Vershubskii AV (2017) Connectivity between electron transport complexes and modulation of photosystem II activity in chloroplasts. Photosynth Res 133:103–114
Trouillard M, Shahbazi M, Moyet L, Rappaport F, Joliot P, Kuntz M, Finazzi G (2012) Kinetic properties and physiological role of the plastoquinone terminal oxidase (PTOX) in a vascular plant. Biochim Biophys Acta 1817:2140–2148. https://doi.org/10.1016/j.bbabio.2012.08.006
Tsimilli-Michael M, Stamatakis K, Papageorgiou GC (2009) Dark-to-light transition in Synechococcus sp. PCC 7942 cells studied by fluorescence kinetics assesses plastoquinone redox poise in the dark and photosystem II fluorescence component and dynamics during state 2 to state 1 transition. Photosynth Res 99(3):243–255
van Kooten O, Snel JFH, Vredenberg WJ (1986) Photosynthetic free energy transduction to the electric potential changes across the thylakoid membrane. Photosynth Res 9:211–227
Vredenberg WJ (2000) A 3-state model for energy trapping and fluorescence in PS II incorporating radical pair recombination. Biophys J 79:26–38
Vredenberg WJ (2011) Kinetic analysis and mathematical modeling of primary photochemical and photoelectrochemical processes in plant photosystems. BioSystems 103:139–151
Vredenberg WJ, Bulychev AA (2010) Photoelectrochemical control of the balance between cyclic- and linear electron transport in photosystem I. Algorithm for P700+ induction kinetics, Biochim. Biophys Acta 1797:1521–1532
Walz D, Goldstein L, Avron M (1974) Determination and analysis of the buffer capacity of isolated chloroplasts in the light and in the dark. Eur J Biochem 47:403–407
Zaks J, Amarnath K, Kramer DM, Niyogi KK, Fleming GR (2012) A kinetic model of rapidly reversible nonphotochemical quenching. PNAS 109:15757–15762
Zhu XG, Wang Y, Ort DR, Long SP (2013) e-photosynthesis: a comprehensive dynamic mechanistic model of C3 photosynthesis: from light capture to sucrose synthesis. Plant Cell Environ 36:1711–1727
Acknowledgements
This work was supported by the RFBR, Project No. 16-04-00318. We wish to thank Professor Wim J. Vredenberg (Wageningen University, The Netherlands) for thorough consideration of the manuscript and stimulating discussion. We are grateful to Reviewer 2 for critical reading and careful editing the manuscript. We thank Professor V. Z. Paschenko and Ph.D. I. V. Konyukhov (Biophysics Department, Moscow State University) for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
The Thylakoid model components
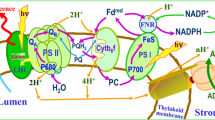
The interacting components of ETC comprise various redox states of electron carriers in a membrane protein complex (PSI, PSII, b6f) or mobile electron carriers (Pc, Fd, PQ). The model dynamic variable Xi(t), calculated using the ODE system (1), describes (Belyaeva et al. 2016) the time-dependent relative fraction (population) of the ith component multiplied by the total concentration of the ETC complex/mobile carrier in the system. The lumenal and stromal concentrations of H+, K+ and Cl− are also model dynamic variables. The stoichiometry of the system constituents in the thylakoid membrane (Fig. 2), for PSI, PSII, Cyt b6f complexes and PQ, Pc, Fd electron carriers was assumed to be 1:1:1:10:3:4.
Modeling catalytic cycles of PSII, Cyt b6f and PS I (see Fig. 10).
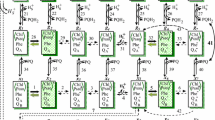
Scheme of the catalytic cycle of the photosystem II. Each rectangle denotes a certain state of PSII, determined by the redox states of the PSII cofactors: \(\left\langle {\begin{array}{*{20}{c}} {{\text{Chl}}} \\ {{\text{P680}}} \end{array}} \right\rangle\)—chlorophyll pigments of antenna and RC P680 (singlet excited states 1Chl* being delocalized on all pigments); Phe—pheophytin, the primary electron acceptor of PSII; QA and QB—primary and secondary quinone acceptors; PQ—plastoquinone; PQH2—plastoquinol; HL+ or HS+—protons in lumen or stroma. The model variables (xi, yi, zi, gi, i = 1,…,7) are defined above the rectangles. Reaction numbers are denoted above the arrows. Dashed arcs show irreversible reactions of non-radiative recombination of Phe− with P680+· (42–45) and QA− with P680+· (46–49). States capable of emitting fluorescence quanta are shaded. The gray backward arrows (see details in Fig. 3) specify the sum of all deactivation processes of 1Chl* (except the photochemical quenching) given by Eqs. (2)–(5)
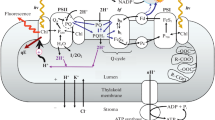
Scheme of the measuring system. Induction curves of chlorophyll fluorescence and differential absorbance changes at 810–870 nm were measured simultaneously using a PEA fluorometer (Plant Efficiency Analyzer, Hansatech, UK) in combination with a PAM-101 measuring system. 1—multi-branch fiber-optic light guide 101-F5 (Walz); 2—ED-P700DW dual-wavelength emitter-detector unit (Walz); 3—dual-wavelength P700 control unit (ED-P700DW, Walz); 4—computer-controlled LED source of white light; 5—leaf sample placed on a mirror support; 6—output from PAM101 amplifier to an analog-digital converter (PCI-6024E A/D, National Instruments, USA); 7—a sensor unit of Plant Efficiency Analyzer with an array of six light-emitting diodes (providing red light with a peak wavelength of 650 nm); 8—control unit of Plant Efficiency Analyzer; 9—output from a two-channel AD converter to computer
PSII model description
The electron carriers of PSII are modeled in reduced/neutral states for the cofactors Phe−/Phe and QA−/QA. In addition, the reduced/excited/ oxidized states of the primary electron donor P680 coupled to antenna, Chl-P680/(Chl-P680)*/Chl-P680+, respectively, are taken into account. The QB -site can exist in four redox states (in Fig. 10: neutral, reduced, protonated, double reduced, empty). Hence, the PSII model network has 48 hypothetical states whereas the model scheme in Fig. 10 contains 28 redox states of the PSII RC. Together with two states of PQ in the PQ pool, a set of 30 Xi(t) components is described when forward/backward transfer steps are given with rate constants kn/k−n (n = 1–49). Reduction of model forms was explained (Lebedeva et al. 2000; Belyaeva et al. 2015, 2016) with respect to information about the primary photosynthetic reactions (Govindjee 1982; Renger and Schulze 1985; Baake and Schlöder 1992; Dau 1994; Renger et al. 1995; Stirbet et al. 1998; Lazár 2003).
Thus the concentrations \({{X_i}(t)}\) for the different PSII states:
are related to the population probabilities with \({\sum {({x_i}(t)} +{g_i}(t)+{y_i}(t)+{z_i}(t))=1},\quad i=1, \ldots ,7.\)
Figure 10 includes the light-induced reactions of the PSII model pattern: charge separation (2, 9, 16, 29), charge stabilization (3, 10, 17, 30), electron transfer from QA−QB(−) to QAQB(H−) (7, 14), PQH2 release (21–27) and refilling of the empty QB-site (34–40). The WOC operation (reactions 4, 11, 18, 31) was assumed with one proton released into the lumen for one electron, transferred from WOC via YZ to P680++ averaging the Si cycles stoichiometry (refs. in Renger 2004, 2012).
For reactions with numbers n = 1, 5, 8, 12, 15, 19, 28, 32 the text in “Updating the Thylakoid Membrane model” introduces Eqs. (2)–(5) explaining the entity of gray backward arrows in Figs. 3 and 10 that define the sum of deactivation processes of 1Chl* via FL emission and heat dissipation. Moreover, dissipative energy losses are defined for closed RCs. The irreversible reactions of non-radiative recombination are shown (Fig. 10) by dashed arcs as transitions 42–45 from the 7th (with Phe−P680+) to the 5th forms (x7, g7, y7, z7) → (x5, g5, y5, z5) and transitions 46–49 from the 4th (with QA−P680+) to the 1st forms (x4, g4, y4, z4) → (x1, g1, y1, z1).
The Cyt b6f model was shown earlier in details (Lebedeva et al. 2002; Kamali et al. 2004; Rubin and Riznichenko 2009), including the set of redox reactions known as the Q cycle (Hope 1993; Tikhonov 2014). The reduced scheme in Fig. 4a shows transitions in two catalytic centers at the lumenal (p) Qo or stromal (n) Qi sites that provide proton coupled electron transfer (PCET) reactions that can oxidize/reduce PQ/PQH2 molecules.
Light-induced electron flow via PQH2 diffusion delivers electrons into the Q cycle (step 41, Figs. 4c, 10) while mobile Pc and bL heme are reduced in parallel (Fig. 4a, steps 50, 51 for the transitions f1 → f2 and f3 → f4). Step 52, f2 → f3 across the membrane reduces the heme bH and PQ reduction in the catalytic stromal center becomes possible (f4 → f2 and f3 → f1) to produce the semiquinone (steps 53, 54) and plastoquinone (steps 55, 56) species that are protonated by two protons consumed from the stroma.
The PSI model is shown in Fig. 4d. Because of the very rapid (10− 12–10− 9 s) ET along the A0–A1–FX chain (Brettel 1997), only the final electron acceptor in PSI, shown as FeS (uniting clusters FX, FA, and FB) in Fig. 4d, is considered. Under light, states with P700+FeSr are generated while two ways are active via steps 61, 62 or vice versa by steps 64, 63 giving rise to P700+ reduction from Pc and ET from reduced FeS complex to Fd. Subsequently, at the PSI acceptor side, the ET outflow is supplied by Fd mobile carriers that participate in both LEF and CEF fluxes: step 65, and steps 58–59, respectively (Fig. 4b).
Rights and permissions
About this article
Cite this article
Belyaeva, N.E., Bulychev, A.A., Riznichenko, G.Y. et al. Analyzing both the fast and the slow phases of chlorophyll a fluorescence and P700 absorbance changes in dark-adapted and preilluminated pea leaves using a Thylakoid Membrane model. Photosynth Res 140, 1–19 (2019). https://doi.org/10.1007/s11120-019-00627-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-019-00627-8