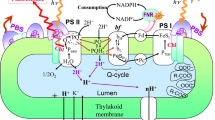
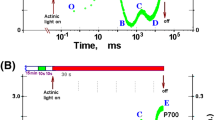
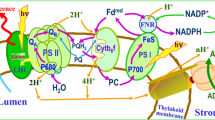
Abstract
The OJIPSMT pattern of chlorophyll (Chl) a fluorescence induction (FI) was obtained using Scenedesmus obliquus (Scenedesmus) microalgal cells exposed, after dark adaptation, to moderate and high light intensities (1200 and 1800 μmol photons m–2 s–1) in a time range from the microseconds to 10 minutes. The fast and, particularly, slow OJIPS(M)T stages of FI extending up to 100 s were quantitatively described by fitting a thylakoid membrane (TM) model. The calculations adequately reproduced the dynamics of P700 oxidoreduction transients. Parameters of TM processes were determined, and the transients of the TM model variables are shown in calculations. Dynamic stages of redox reactions in reaction centers (RCs) of photosystem II (PSII), the PQ/PQH2 quinone pool, the cytochrome b6 f complex (Cyt bf), and P700 of photosystem I (PSI) RCs were found to accompany the adaptation of the TM system to light. In silico analysis revealed the role that Cyt bf plays in regulating electron fluxes when bL/H hemes remain in a more reduced state at a high light intensity than at moderate illumination in an interval from 100 ms to 3 s. A dependence of the rate constants k42–49 on the level of \({\text{Q}}_{{\text{B}}}^{{(2) - }}\) reduction was revealed for non-radiative recombination of separated charges in the RC Phe–P680+ and Q\(_{{\text{A}}}^{ - }\)P680+, together with a dependence of k42–49 on chloroplast illumination. Setting the dynamic rate constants kFNR(t) and kD-qE(t) made it possible to compare the generation ΔpHo-i(t) = pHS(t) – pHL(t), initiation of qE quenching at pHL|trigg = 6.7, activation of FNR reductase in the light, and achievement of stationary charge flows within 1–2 minutes of light induction. Fitting the model of isolated PSII to phytoplankton FI signals showed similar electron transfer parameters of PSII RCs for Scenedesmus and Chlorella monocultures and phytoplankton samples. Markers of the phytoplankton community state were an exception, differing between degrading and ecologically functional water bodies. The differences in markers included a reduced size of the antennas, an increased energy dissipation, and a decrease in pH in the lumen.








Similar content being viewed by others
REFERENCES
N. E. Belyaeva, F.-J. Schmitt, R. Steffen, et al., Photosynth. Res., 98, 105 (2008).
N. E. Belyaeva, F.-J. Schmitt, V. Z. Paschenko, et al., Photosynth. Res. 125, 123 (2015).
N. E. Belyaeva, A. A. Bulychev, K. E. Klementiev, et al., Photosynth. Res. 146 (1), 259 (2020).
A. Stirbet and Govindjee, Photosynth. Res. 113, 15 (2012).
A. Stirbet D. Lazar, G. C. Papageorgiou, and Govindjee, in Cyanobacteria: From Basic Science to Applications, Ed. by A. K. Mishra, D. N. Tiwari, and A. N. Rai (Acad. Press, Elsevier, 2019), pp. 79–130.
A. N. Tikhonov, Photosynth. Res. 125, 65 (2015). https://doi.org/10.1007/s11120-015-0094-0
Chlorophyll a Fluorescence: a Signature of Photosynthesis, Vol. 19: Advances in Photosynthesis and Respiration, Ed. by G. C. Papageorgiou and Govindjee (Springer-Verlag, Dordrecht, 2004).
A. N. Sharov, Doctoral Dissertation in Biology (St. Petersburg Sci. Res. Centre Ecol. Saf. Russ. Acad. Sci., St. Petersburg, 2020).
N. F. Reimers, Ecology. Theories, Laws, Rules, Principles and Hypotheses (Rossiya molodaya, Moscow, 1994) [in Russian].
C. R. Ireland, S. P. Long, and N. R. Baker, Planta 160, 550 (1984).
G. H. Schatz, H. Brock, and A. R. Holzwarth, Biophys. J. 54, 397 (1988).
T. A. Roelofs, C. H. Lee, and A. R. Holzwarth, Biophys. J. 61, 1147 (1992).
J. M. Bowes and A. R. Crofts, Biochim. Biophys. Acta 590, 373 (1980).
R. J. Strasser, A. Srivastava, and Govindgee, Photochem. Photobiol. 61, 32 (1995).
G. Renger and A. Schulze, Photobiochem. Photobiophys. 9. 79 (1985).
E. Baake and J. P. Schloder, Bull. Math. Biol. 54, 999 (1992).
A. Stirbet, Govindjee, B. J. Strasser, and R. J. Strasser, J. Theor. Biol. 193, 131 (1998).
D. Lazar, J. Theor. Biol. 220, 469 (2003).
D. Lazar, Photosynthetica 47 (4), 483 (2009).
G. V. Lebedeva, N. E. Belyaeva, O. V. Demin, et al., Biofizika 47, 1044 (2002).
R. J. Strasser, M. Tsimilli-Michael, and A. Srivastava, in Chlorophyll a Fluorescence: a Signature of Photosynthesis, Vol. 19: Advances in Photosynthesis and Respiration, Ed. by G. C. Papageorgiou and Govindjee (Springer-Verlag, Dordrecht, 2004), pp. 321–362.
A. A. Volgusheva, O. V. Yakovleva, G. P. Kukapckix, et al., Biofizika 56 (1), 105 (2011).
A. Stirbet, D. Lazar, J. Kromdijk, and Govindjee, Photosynthetica 56, 86 (2018).
N. E. Belyaeva, A. A. Bulychev, G. Yu. Riznichenko, and A. B. Rubin, Photosynth. Res. 130, 491 (2016).
N. Belyaeva, A. Bulychev, G. Riznichenko, and A. Rubin, Photosynth. Res. 140, 1 (2019).
G. Riznichenko, G. Lebedeva, O. Demin, and A. Rubin, J. Biol. Phys. 25, 177 (1999).
G. V. Lebedeva, N. E. Beljaeva, G. Yu. Riznichenko, and O. V. Demin, in BioThermoKinetics in the Post Genomic Era, Ed. by C. Larsson, I. Pahlman, and L. Gustafsson (Chalmers Reproservice, Goteborg, 1998), pp. 196–199.
N. E. Belyaeva, G. V. Lebedeva, G. Yu. Riznichenko, et al., in Mathematics. Computer. Education (Moscow, 2000), vol. 7, pp. 606–614.
N. E. Belyaeva, G. V. Lebedeva, G. Yu. Riznichenko, et al., in Mathematics. Computer. Education (Moscow, 2003), vol. 10, pp. 263–276.
N. E. Belyaeva, Candidate’s Dissertation in Mathematics and Physics (Moscow State Univ., Moscow, 2004).
N. E. Belyaeva, V. Z. Pashchenko, G. Renger, et al., Biofizika 51 (6) 976 (2006).
N. E. Belyaeva, A. A. Bulychev, G. Yu. Riznichenko, and A. B. Rubin, Biophysics 56 (3) 464 (2011a).
N. E. Belyaeva, F.-J. Schmitt, V. Z. Paschenko, et al., BioSystems 103 (2), 188 (2011b).
N. E. Belyaeva, F.-J. Schmitt, V. Z. Paschenko, et al., Plant Physiol. Biochem. 77, 49 (2014).
O. Ebenhoh, G. Fucile, G. Finazzi, et al., Philos. Trans. R. Soc., B 369, 20130223 (2014).
A. Stirbet and Govindjee, Photosynth. Res. 130, 193 (2016).
A. Laisk, H. Eichelmann, and V. Oja, Photosynth. Res. 90, 45 (2006).
A. A. Bulychev, A. A. Cherkashin, E. M. Muronets, and I. V. Elanskaya, Biochim. Biophys. Acta, Bioenerg. 1859, 1086 (2018).
V. V. Ptushenko, T. V. Zhigalova, O. V. Avercheva, and A. N. Tikhonov, Photosynth. Res. 139, 509 (2019).
I. V. Elanskaya, A. A. Bulychev, E. P. Lukashev, and E. M. Muronets, Biochim. Biophys. Acta, Bioenerg. 1862, 148318 (2021).
G. C. Papageorgiou, M. Tsimilli-Michael, and K. Stamatakis, Photosynth. Res. 94, 275 (2007).
G. Schansker, S. Z. Toth, and R. J. Strasser, Biochim. Biophys. Acta 1757, 787 (2006).
S. Kodru, T. Malavath, E. Devadasu, et al., Photosynth. Res. 125, 219 (2015).
A. Morales, X. Yin, J. Harbinson, et al., Plant Physiol. 176, 1247 (2018).
A. Stirbet, D. Lazar, Y. Guo, and Govindjee, Ann. Bot. 126, 511 (2020).
P. V. Fursova, E. N. Voronova, A. P. Levich, et al., Moscow Univ. Biol. Sci. Bull. 72 (4), 184 (2017).
S. I. Pogosyan, S. V. Gal’chuk, Yu. V. Kazimirko, et al., Voda: Khim. Ekol. 6, 34 (2009).
G. E. Milanovsky, A. A. Petrova, D. A. Cherepanov, and A. Y. Semenov, Photosynth. Res. 133, 185 (2017).
M. J. Kamali, G. V. Lebedeva, O. V. Demin, et al., Biophysics 49, 1061 (2004).
I. A. Reynolds, E. A. Johnson, and C. Tanford, Proc. Natl. Acad. Sci. U. S. A. 82, 6869 (1985).
A. A. Bulychev, M. M. Niyazova, and A. B. Rubin, Biol. Membr. 4, 262 (1987).
G. Schansker, S. Z. Toth, A. R. Holzwarth, and G. Garab, Photosynth. Res. 120, 43 (2014).
A. Laisk and V. Oja, Photosynth. Res. 143, 335 (2020).
ACKNOWLEDGMENTS
We are grateful to Cand. Sci. (Biol.) E.V. Voronova (Department of Biophysics, Biological Faculty, Moscow State University) for Scenedesmus obliquus microalgal cell samples, Prof. S.I. Pogosyan for fruitful discussion, and Cand. Sci. (Biol.) S.S. Khrushchev for technical support.
Funding
This work was supported by a state contract with the Moscow State University (project no. 121032500060-0), the Russian Foundation for Basic Research (project no. 20-04-00465), and the Russian Science Foundation (project no. 22-11-00009).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest. The authors declare that they have no conflicts of interest. This article does not contain any studies involving animals or human subjects performed by any of the authors.
Additional information
Translated by T. Tkacheva
Abbreviations: TM, thylakoid membrane; PSI, photosystem I; PSII, photosystem II; Cyt bf, cytochrome b6 f complex; RC, reaction center; QA and QB, primary and secondary quinone acceptors of PSII; PQ, plastoquinone; Phe, pheophytin; OEC, oxygen-evolving complex; Chl and Chl a, chlorophylls of antennae and RCs; ETC, electron transport chain; P680, chlorophyll of PSII RCs; FI, fluorescence induction; F0 and Fm, minimal and maximal fluorescence yields, respectively; LEF, linear electron flow; CEF, cyclic electron flow; PFD, photon flux density.
Rights and permissions
About this article
Cite this article
Belyaeva, N.E., Bulychev, A.A., Paschenko, V.Z. et al. Dynamics of In Vivo Membrane Processes in Algal Thylakoids as Analyzed from Chlorophyll Fluorescence Induction using the Photosystem II and Thylakoid Models. BIOPHYSICS 67, 708–725 (2022). https://doi.org/10.1134/S0006350922050050
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1134/S0006350922050050