Abstract
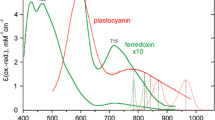
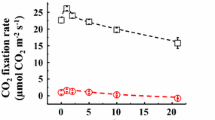
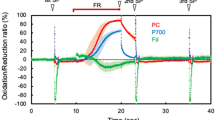
The oxidation kinetics under far-red light (FRL) of photosystem I (PSI) high potential donors P700, plastocyanin (PC), and cytochrome f (Cyt f) were investigated in sunflower leaves with the help of a new high-sensitivity photometer at 810 nm. The slopes of the 810 nm signal were measured immediately before and after FRL was turned on or off. The same derivatives (slopes) were calculated from a mathematical model based on redox equilibrium between P700, PC and Cyt f and the parameters of the model were varied to fit the model to the measurements. Typical best-fit pool sizes were 1.0–1.5 μmol m−2 of P700, 3 PC/P700 and 1 Cyt f/P700, apparent equilibrium constants were 15 between P700 and PC and 3 between PC and Cyt f. Cyclic electron flow (CET) was calculated from the slope of the signal after FRL was turned off. CET activated as soon as electrons accumulated on the PSI acceptor side. The quantum yield of CET was close to unity. Consequently, all PSI in the leaf were able to perform in cycle, questioning the model of compartmentation of photosynthetic functions between the stroma and grana thylakoids. The induction of CET was very fast, showing that it was directly redox-controlled. After longer dark exposures CET dominated, because linear e− transport was temporarily hindered by the dark inactivation of ferredoxin-NADP reductase.









Similar content being viewed by others
Abbreviations
- CET:
-
Cyclic electron transport
- Cyt:
-
Cytochrome
- Fd:
-
Ferredoxin
- FNR:
-
Ferredoxin-NADP reductase
- FRL:
-
Far-red light
- PC:
-
Plastocyanin
- PFD, PAD:
-
Photon flux density, incident and absorbed
- PQ:
-
Plastoquinone
- PSI, PSII:
-
Photosystem I and photosystem II
- P700:
-
Donor pigment of PSI
- WL:
-
White light
References
Albertsson P-A (1995) The structure and function of the chloroplast photosynthetic membrane – a model for the domain organization. Photosynth Res 46:141–149
Albertsson P-A (2001) A quantitative model of the domain structure of the photosynthetic membrane. Trends Plant Sci 6:349–354
Anderson JM, Chow WS, Park Y-I (1995) The grand design of photosynthesis: acclimation of the photosynthetic apparatus to environmental cues. Photosynth Res 46:129–139
Asada K, Heber U, Schreiber U (1992) Pool size of electrons that can be donated to P700+, as determined in intact leaves: donation to P700+ from stromal components via the intersystem chain. Plant Cell Physiol 33:927–932
Asada K, Heber U, Schreiber U (1993) Electron flow to the intersystem chain from stromal components and cyclic electron flow in maize chloroplasts, as detected in intact leaves by monitoring redox change of P700 and chlorophyll fluorescence. Plant Cell Physiol 34:39–50
Breyton C, Nandha B, Johnson G, Joliot P, Finazzi G (2006) Redox modulation of cyclic electron flow around photosystem I in C3 plants. Biochemistry 45:13465–13475
Böhme H (1978) Quantitative determination of ferredoxin, Ferredoxin-NADP reductase and plastocyanin in spinach chloroplasts. Eur J Biochem 83:137–141
Clarke JE, Johnson GN (2001) In vivo temperature dependence of cyclic and pseudocyclic electron transport in barley. Planta 212:808–816
Gerst U, Schönknecht G, Heber U (1994) ATP and NADPH as the driving force of carbon reduction in leaves in relation to thylakoid ennergization by light. Planta 193:421–429
Golding AJ, Johnson GN (2003) Down-regulation of linear and activation of cyclic electron transport during drought. Planta 218:107–114
Harbinson J, Hedley C (1993) Changes in P-700 oxidation during the early stages of the induction of photosynthesis. Plant Physiol 103:649–660
Harbinson J, Woodward FI (1987) The use of light-induced absorbance changes at 820 nm to monitor the oxidation state of P-700 in leaves. Plant Cell Environ 10:131–140
Hiyama T (1985) Quantum yield and requirement for the photooxidation of P700. Physiol Veg 23:605–610
Joët T, Cournac L, Peltier G, Havaux M (2002) Cyclic electron flow around photosystem I in C3 plants. In vivo control by the redox state of chloroplasts and involvement of the NADH-dehydrogenase complex. Plant Physiol 128:760–769
Joliot P, Joliot A (2002) Cyclic electron transfer in plant leaf. PNAS 99:10209–10214
Joliot P, Joliot A (2005) Quantification of cyclic and linear flows in plants. PNAS 102:4913–4918
Joliot P, Béal D, Joliot A (2004) Cyclic electron flow under saturating excitation of dark-adapted Arabidopsis leaves. Biochim Biophys Acta 1656:166–176
Kirchhoff H, Schöttler MA, Maurer J, Weis E (2004) Plastocyanin redox kinetics in spinach chloroplasts: evidence for disequilibrium in the high potential chain. Biochim Biophys Acta 1659:63–72
Klughammer C, Schreiber U (1991) Analysis of light-induced absorbance changes in the near-infrared spectral region. I. Characterization of various components in isolated chloroplasts. Z Naturforsch 46c:233–244
Laisk A, Oja V (1998) Dynamic gas exchange of leaf photosynthesis. Measurement and interpretation. CSIRO, Canberra
Laisk A, Oja V, Rasulov B, Rämma H, Eichelmann H, Kasparova I, Pettai H, Padu E, Vapaavuori E (2002) A computer-operated routine of gas exchange and optical measurements to diagnose photosynthetic apparatus in leaves. Plant Cell Environ 25:923–943
Miyake C, Horiguchi S, Makino A, Shinzaki Y, Yamamoto H, Tomizawa KI (2005a) Effects of light intensity on cyclic electron flow around PSI and its relationship to non-photochemical quenching of Chl fluorescence in tobacco leaves. Plant Cell Physiol 46:1819–1830
Miyake C, Miyata M, Shinzaki Y, Tomizawa K (2005b) CO2 response of cyclic electron flow around PSI (CEF-PSI) in tobacco leaves – relative electron fluxes through PSI and PSII determine the magnitude of non-photochemical quenching (NPQ) of Chl fluorescence. Plant Cell Physiol 46:629–637
Oja V, Bichele I, Hüve K, Rasulov B, Laisk A (2004) Reductive titration of photosystem I and differential extinction coefficient of P700+ at 810–950 nm in leaves. Biochim Biophys Acta 1658:225–234
Oja V, Eichelmann H, Peterson RB, Rasulov B, Laisk A (2003) Deciphering the 820 nm signal: redox state of donor side and quantum yield of photosystem I in leaves. Photosynth Res 78:1–15
Pettai H, Oja V, Freiberg A, Laisk A (2005) Photosynthetic activity of far-red light in green plants. Biochim Biophys Acta 1708:311–321
Ruhle W, Pschorn R, Wild A (1987) Regulation of the photosynthetic electron transport during dark-light transitions by activation of the ferrodoxin-NADP+-oxidoreductase in higher plants. Photosynth Res 11:161–171
Acknowledgments
This work was funded by targeted financing theme 180517s98 from Estonian Ministry of Education and supported by grants 6607 and 6611 from Estonian Science Foundation.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Redox equilibrium-based modeling of the 810 nm signal
For convenience, we defined the following denotations:
where g denote the reciprocal redox equilibrium constants between the specified compound and P700. The system of the redox-equilibrated compounds is resolved with the help of an assistant variable x, reflecting the oxidation state of P700:
Since the optical signal is generated by oxidized fractions, the model is based on the oxidized fractions of the redox-equilibrated compounds. Using these denotations, the sum of all redox-equilibrated oxidized fractions is
Here P700T denotes the only involved total pool in μmol m−2, other donor side electron carriers PC and Cyt f are expressed relative to P700T. The 810 nm optical signal T is generated by oxidized P700 and PC as follows:
where extP700 is the absolute extinction coefficient of P700, the fractional transmittance change per μmol m−2 of oxidized P700. The maximum signal is expressed from (2) by letting x → ∞, as follows
Under FRL the rate of oxidation of all equilibrated PSI donors is
where η is the quantum efficiency of PSI electron transport under FRL (assumed to be unity), FRL is the flux density absorbed by PSI and P700 is now denoting the reduced fraction, \( P700\; = \;1/(1\; + \;x) \).
Before we can express the speed of signal change versus time, we express the change versus the P700 oxidation parameter x by differentiating (1) and (2) with respect to x:
The change of the signal per change of electrons in the equilibrated compounds is expressed as
where the numerator is from Eq. 5 and denominator from Eq. 4. Considering that
we obtain, considering Eq. 3
In Eq. 8 extP700 is presented as a fraction of transmittance change per μmol m−2 of P700, FRL is the absorbed flux density, μmol quanta m−2 s−1 and η is dimensionless net efficiency of PSI electron transport. For the oxidative titration, x was varied and the corresponding abscissa value of Fig. 3 was calculated as the signal normalized to the maximum signal:
The corresponding ordinate was calculated from Eq. 8. The whole modeled curve was obtained as a series of pairs obtained from Eqs. 8 and 9 by varying x from 0 to ∞. Model parameters P700, PC and Cyt f, as well as equilibrium constants g PC and g f were varied until the calculated curve best fitted to the data points. Later, when the corresponding number of electrons was calculated from a certain optical signal, the value of x corresponding to the particular measured T/T max value was expressed from Eq. 9 and the corresponding number of electrons in PSI donors was calculated from Eq. 1, separately in Cyt f, PC and P700, as well as their sum.
The solution of Eq. 9 with respect to x results in a quadratic equation
where
where \( a = T/T_{{\max }} \; \cdot \;(1\; + \;(PC/\varepsilon _{{{\text{PC}}}})). \)
Rights and permissions
About this article
Cite this article
Talts, E., Oja, V., Rämma, H. et al. Dark inactivation of ferredoxin-NADP reductase and cyclic electron flow under far-red light in sunflower leaves. Photosynth Res 94, 109–120 (2007). https://doi.org/10.1007/s11120-007-9224-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-007-9224-7