Abstract
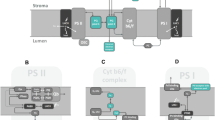
Theoretical modelling is often overlooked in photosynthesis research even if it can significantly help with understanding of explored system. A new model of light-induced photosynthetic reactions occurring in and around thylakoid membrane is introduced here and used for theoretical modelling of not only the light-induced chlorophyll (Chl) a fluorescence rise (FLR; the O-J-I-P transient), reflecting function of photosystem II (PSII), but also of the 820 nmtransmittance signal (I820), reflecting function of photosystem I (PSI) and plastocyanin (PC), paralleling the FLR. Correctness of the model was verified by successful simulations of the FLR and I820 signal as measured with the control (no treatment) sample but also as measured with 2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone- (inhibits electron transport in cytochrome b 6/f) and methylviologen- (accepts electrons from iron-sulphur cluster of PSI) treated samples and with the control sample upon different intensities of excitation light. From the simulations performed for the control sample, contribution of the oxidised donor of PSI, P700, and oxidised PC to the I820 signal minimum (reflects maximal accumulations of the two components) was estimated to be 75% and 25%, respectively. Further in silico experiments showed that PC must be reduced in the dark, cyclic electron transport around PSI must be considered in the model and activation of ferredoxin-NADP+-oxidoreductase (FNR) also affects the FLR. Correct simulations of the FLR and I820 signal demonstrate robustness of the model, confirm that the electron transport reactions occurring beyond PSII affect the shape of the FLR, and show usefulness and perspective of theoretical approach in studying of the light-induced photosynthetic reactions.
Similar content being viewed by others
Abbreviations
- bH or H:
-
high potential haem b of cyt b 6/f
- bL or L:
-
low potential haem b of cyt b 6/f
- c or C:
-
haem c of cyt b 6/f
- CET:
-
cyclic electron transport
- Chl:
-
chlorophyll
- cyt:
-
cytochrome
- DBMIB:
-
2,5-dibromo-3-methyl-6-isopropyl-p-benzoquinone
- F:
-
haem f of cyt b 6/f
- F0 :
-
minimal fluorescence
- FB or X:
-
iron-sulphur cluster of PSI
- FM :
-
maximal fluorescence
- Fd:
-
ferredoxin
- FLD:
-
fluorescence decrease
- FLI:
-
fluorescence induction
- FLR:
-
fluorescence rise
- FNR:
-
ferredoxin-NADP+-oxidoreductase
- I820 :
-
relative transmittance signal measured at 820 nm
- MV:
-
1,1′dimethyl-4,4′-bipyridinium-dichloride (methylviologen)
- NADP+ and NADPH:
-
oxidised and reduced nicotinamide adenine dinucleotide phosphate
- O, K, J, I, P, G, H:
-
particular steps of the FLR
- OEC:
-
oxygen evolving complex
- P680 or P:
-
electron donor of PSII
- P700 or R:
-
electron donor of PSI
- PC:
-
plastocyanin
- PQ and PQH2 :
-
oxidised and reduced plastoquinone
- PSI:
-
photosystem I
- PSII:
-
photosystem II
- QA or A:
-
first quinone electron acceptor of PSII
- QB or B:
-
second quinone electron acceptor of PSII
- RCII:
-
reaction centre of PSII
- RT:
-
room temperature
- Si (i = 0, 1, 2, 3):
-
redox states of OEC
- TM:
-
thylakoid membrane
References
Baake, E., Schlöder, J.P.: Modelling the fast fluorescence rise of photosynthesis. — Bull. Math. Biol. 54: 999–1021, 1992.
Bennoun, P.: Evidence for a respiratory chain in the chloroplast. — Proc. Natl. Acad. Sci. USA 79: 4352–4356, 1982.
Bukhov, N., Carpentier, R.: Alternative photosystem I-driven electron transport routes: mechanisms and functions. — Photosynth. Res. 82: 17–33, 2004.
Carrillo, N., Lucero, H.A., Vallejos, R.H.: Light modulation of chloroplast membrane-bound ferredoxin-NADP+ oxidoreductase. — J. Biol. Chem. 256: 1058–1059, 1981.
Cramer, W.A., Zhang, H.: Consequences of the structure of the cytochrome b 6 f complex for its charge transfer pathways. — Biochim. Biophys. Acta 1757: 339–345, 2006.
Crofts, A.R., Wraight, C.A.: The electrochemical domain of photosynthesis. — Biochim. Biophys. Acta 726: 149–185, 1983.
Dau, H.: Molecular mechanism and quantitative models of variable photosystem II fluorescence. — Photochem. Photobiol. 60: 1–23, 1994.
Dau, H., Windecker, R., Hansen, U.-P.: Effect of light-induced changes in thylakoid voltage on chlorophyll fluorescence of Aegopodium podagraria leaves. — Biochim. Biophys. Acta 1057: 337–345, 1991.
Duysens, L.N.M., Sweers, H.E.: Mechanism of the two photochemical reactions in algae as studied by means of fluorescence. — In: Japanese Society of Plant Physiologists (ed.): Studies on Microalgae and Photosynthetic Bacteria. Pp 353–372. Univ.Tokyo Press, Tokyo 1963.
Feild, T.S., Nedbal, L., Ort, D.R.: Nonphotochemical reduction of the plastoquinone pool in sunflower leaves originates from chlororespiration. — Plant Physiol. 116: 1209–1218, 1998.
Giersch, C.: Mathematical modelling of metabolism. — Curr. Opin. Plant Biol. 3: 249–253, 2000.
Govindjee: 63 years since Kautsky — chlorophyll a fluorescence. — Aust. J. Plant Physiol. 22: 131–160, 1995.
Guissé, B., Srivastava, A., Strasser, R.J.: The polyphasic rice of the chlorophyll a fluorescence (O-K-J-I-P) in heat-stressed leaves. — Arch. Sci. 48: 147–160, 1995.
Harbinson, J., Woodward, F.I.: The use of light-induced absorbance changes at 820 nm to monitor the oxidation state of P-700 in leaves. — Plant Cell Environ. 10: 131–140, 1987.
Heredia, P., De Las Rivas, J.: Fluorescence induction of Photosystem II membranes shows the steps till reduction and protonation of the quinone pool. — J. Plant Physiol. 160: 1499–1506, 2003.
Holtgrefe, S., Bader, K.P., Horton, P., Scheibe, R., von Schaewen, A., Backhausen, J.E.: Decreased content of leaf ferredoxin changes electron distribution and limits photosynthesis in transgenic potato plants. — Plant Physiol. 133: 1768–1778, 2003.
Ilík, P., Kouřil, R., Fiala, J., Nauš, J., Vácha, F.: Spectral characterization of chlorophyll fluorescence in barley leaves during linear heating. Analysis of high-temperature fluorescence rise around 60°C. — J. Photochem. Photobiol. 59: 103–114, 2000.
Ilík, P., Schansker, G., Kotabová, E., Váczi, P., Strasser, R.J., Barták, M.: A dip in the chlorophyll fluorescence induction at 0.2 — 2 s in Trebouxia-possessing lichens reflects a fast reoxidation of photosystem I. A comparison with higher plants. — Biochim. Biophys. Acta 1757: 12–20, 2006.
Jablonsky, J., Lazar, D.: Evidence for intermediate S-states as initial phase in the process of oxygen-evolving complex oxidation. — Biophys. J. 94: 2725–2736, 2008.
Jablonsky, J., Susila, P., Lazar, D.: Impact of dimeric organization of enzyme on its function: the case of photosynthetic water splitting. — Bioinformatics 24: 2755–2759, 2008.
Johnson, G.N.: Cyclic electron transport in C3 plants: fact or artefact? — J. Exp. Bot. 56: 407–416, 2005.
Joliot, A., Joliot, P.: Étude cinétique de la réaction photochimique libérant l’ oxygène au cours de la photosynthèse. — C. R. Acad. Sci. 258: 4622–4625, 1964.
Joliot, P., Joliot, A.: Mechanism of electron transfer in the cytochrome b/f complex of algae: Evidence for a semiquinone cycle. — Proc. Natl. Acad. Sci. USA 91: 1034–1038, 1994.
Joliot, P., Joliot, A.: Quantification of cyclic and linear flows in plants. — Proc. Natl. Acad. Sci. USA 102: 4913–4918, 2005.
Joly, D., Bigras, C., Harnois, J., Govindachary, S., Carpentier, R.: Kinetic analyses of the OJIP chlorophyll fluorescence rise in thylakoid membranes. — Photosynth. Res. 84: 107–112, 2005.
Kirchhoff, H., Schöttler, M.A., Maurer, J., Weis E.: Plastocyanin redox kinetics in spinach chloroplasts: evidence for disequilibrium in the high potential chain. — Biochim. Biophys. Acta 1659: 63–72, 2004.
Klughammer, C., Schreiber, U.: An improved method, using saturating light pulses, for the determination of photosystem I quantum yield via P700+-absorbance changes at 830 nm. — Planta 192: 261–268, 1994.
Kroon, B.M.A., Thoms, S.: From electron to biomass: A mechanistic model to describe phytoplankton photosynthesis and steady-state growth rates. — J. Phycol. 42: 593–609, 2006.
Kurreck, J., Schödel, R., Renger, G.: Investigation of the plastoquinone pool size and fluorescence quenching in thylakoid membranes and Photosystem II (PS II) membrane fragments. — Photosynth. Res. 63: 171–182, 2000.
Laisk, A., Eichelmann, H., Oja, V.: C3 photosynthesis in silico. — Photosynth. Res. 90: 45–66, 2006.
Lazár, D.: Chlorophyll a fluorescence induction. — Biochim. Biophys. Acta 1412: 1–28, 1999.
Lazár, D.: Chlorophyll a fluorescence rise induced by high light illumination of dark-adapted plant tissue studied by means of a model of photosystem II and considering photosystem II heterogeneity. — J. Theor. Biol. 220: 469–503, 2003.
Lazár, D.: The polyphasic chlorophyll a fluorescence rise measured under high intensity of exciting light. — Funct. Plant Biol. 33: 9–30, 2006.
Lazár, D., Ilík, P.: High-temperature induced chlorophyll fluorescence changes in barley leaves — Comparison of the critical temperatures determined from fluorescence induction and from fluorescence temperature curve. — Plant Sci. 124: 159–164, 1997.
Lazár, D., Jablonský, J.: On the approaches applied in formulation of a kinetic model of photosystem II: Different approaches lead to different simulations of the chlorophyll a fluorescence transients. — J. Theor. Biol. 257: 260–269, 2009.
Lazár, D., Kaňa, R., Klinkovský, T., Nauš, J.: Experimental and theoretical study on high temperature induced changes in chlorophyll a fluorescence oscillations in barley leaves upon 2 % CO2. — Photosynthetica 43: 13–27, 2005.
Lazár, D., Nauš, J., Matoušková, M., Flašarová, M.: Mathematical modeling of changes in chlorophyll fluorescence induction caused by herbicides. — Pestic. Biochem. Physiol. 57: 200–210, 1997.
Lazár, D., Pospíšil, P.: Mathematical simulation of chlorophyll a fluorescence rise measured with 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea-treated barley leaves at room and high temperatures. — Eur. Biophys. J. 28: 468–477, 1999.
Lazár, D., Schansker, G.: Models of chlorophyll a fluorescence transients. — In: Laisk, A., Nedbal, L., Govindjee (ed.): Photosynthesis in silico: Understanding Complexity from Molecules to Ecosystems. Pp 85–123. Springer, Dordrecht 2009.
Lebedeva, G.V., Belyaeva, N.E., Demin, O.V., Riznichenko, G.Y., Rubin, A.B.: A kinetic model of primary photosynthetic processes. Description of the fast phase of chlorophyll fluorescence induction under different light intensities. — Biofizika 47: 1044–1058, 2002.
McClendon, J.H., Fukshansky, L.: On the interpretation of absorption-spectra of leaves. 2. The nonabsorbed ray of the sieve effect and the mean optical pathlength in the remainder of the leaf. — Photochem. Photobiol. 51: 211–216, 1990.
Mendes, P.: GEPASI — a software package for modelling the dynamics, steady states and control of biochemical and other systems. — Comput. Appl. Biosci. 9: 563–571, 1993.
Oja, V., Bichele, I., Hüve, K., Rasulov, B., Laisk, A.: Reductive titration of photosystem I and differential extinction coefficient of P700+ at 810-950 nm in leaves. — Biochim. Biophys. Acta 1658: 225–234, 2004.
Pospíšil, P., Dau, H.: Chlorophyll fluorescence transients of Photosystem II membrane particles as a tool for studying photosynthetic oxygen evolution. — Photosynth. Res. 65: 41–52, 2000.
Pospíšil, P., Dau, H.: Valinomycin sensitivity proves that light-induced thylakoid voltages result in millisecond phase of chlorophyll fluorescence transients. — Biochim. Biophys. Acta 1554: 94–100, 2002.
Rios-Estepa, R., Lange, B.M.: Experimental and mathematical approaches to modeling plant metabolic network. — Phytochemistry 68: 2351–2374, 2007.
Rubin, A.B., Riznichenko, G.: Modeling of the primary processes in a photosynthetic membrane. — In: Laisk, A., Nedbal, L., Govindjee (ed.): Photosynthesis in silico: Understanding Complexity from Molecules to Ecosystems. Pp 151–176. Springer, Dordrecht 2009.
Santabarbara, S., Heathcote, P., Evans, M.C.V.: Modelling of the electron transfer reactions in Photosystem I by electron tunnelling theory: The phylloquinones bound to the PsaA and the PsaB reaction centre subunits of PS I are almost isoenergetic to the iron-sulfur cluster FX. — Biochim. Biophys. Acta 1708: 283–310, 2005.
Satoh, K.: Fluorescence induction and activity of ferredoxin-NADP+ reductase in Bryopsis chloroplasts. — Biochim. Biophys. Acta 638: 327–333, 1981.
Schansker, G., Srivastava, A., Govindjee, Strasser, R.J.: Characterization of the 820-nm transmission signal paralleling the chlorophyll a fluorescence rise (OJIP) in pea leaves. — Funct. Plant Biol. 30: 785–796, 2003.
Schansker, G., Tóth, S.Z., Strasser, R.J.: Methylviologen and dibromorhymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. — Biochim. Biophys. Acta 1706: 250–261, 2005.
Schreiber, U., Neubauer, C.: O2-dependent electron flow, membrane energization and the mechanism of nonphotochemical quenching of chlorophyll fluorescence. — Photosynth. Res. 25: 279–293, 1990.
Stirbet, A., Govindjee, Strasser, B.J., Strasser, R.J.: Chlorophyll a fluorescence induction in higher plants: Modelling and numerical simulation. — J. Theor. Biol. 193: 131–151, 1998.
Strasser, B.J.: Donor side capacity of Photosystem II probed by chlorophyll a fluorescence transients. — Photosynth. Res. 52: 147–155, 1997.
Strasser, R.J., Srivastava, A., Govindjee: Polyphasic chlorophyll a fluorescence transient in plants and cyanobacteria. — Photochem. Photobiol. 61: 32–42, 1995.
Strasser, R.J., Stirbet, A.D.: Estimation of the energetic connectivity of PS II centres in plants using the fluorescence rise O-J-I-P — Fitting of experimental data to three different PS II models. — Math. Comput. Simulat. 56: 451–461, 2001.
Sušila, P., Lazár, D., Ilík, P., Tomek, P., Nauš, J.: The gradient of exciting radiation within a sample affects relative heights of steps in the fast chlorophyll a fluorescence rise. — Photosynthetica 42: 161–172, 2004.
Tomek, P., Lazár, D., Ilík, P., Nauš, J.: On the intermediate steps between the O and P steps in chlorophyll alpha fluorescence rise measured at different intensities of exciting light. — Aust. J. Plant Physiol. 28: 1151–1160, 2001.
Tóth, S.Z., Schansker, G., Strasser, R.J.: In intact leaves, the maximum fluorescence level (F M) is independent of the redox state of the plastoquinone pool: A DCMU-inhibition study. — Biochim. Biophys. Acta 1708: 275–282, 2005.
Tsimilli-Michael, M., Pêcheux, M., Strasser, R.J.: Vitality and stress adaptation of the symbionts of coral reef and temperature foraminifers probed in hospite by the fluorescence kinetics OJIP. — Arch. Sci. 51: 205–240, 1998.
van Thor, J.J., Geerlings, T.H., Matthijs, H.C.P., Hellingwerf, K.J.: Kinetic evidence for the PsaE-dependent transient ternary complex photosystem I/ferredoxin/ferredoxin:NADP+ reductase in a cyanobacterium. — Biochemistry 38: 12735–12746, 1999.
Vernotte, C., Etienne, A.-L., Briantais, J.-M.: Quenching of the system II chlorophyll fluorescence by the plastoquinone pool. — Biochim. Biophys. Acta 545: 519–527, 1979.
Vredenberg, W.J.: A three-state model for energy trapping and chlorophyll fluorescence in photosystem II incorporating radical pair recombination. — Biophys. J. 79: 26–38, 2000.
Vredenberg, W.J., Bulychev, A.A.: Photo-electrochemical control of photosystem II chlorophyll fluorescence in vivo. — Bioelectrochem. 57: 123–128, 2002.
Vredenberg, W., Prášil, O.: Modeling of chlorophyll a fluorescence kinetics in plant cells: derivation of a descriptive algorithm. — In: Laisk, A., Nedbal, L., Govindjee (ed.): Photosynthesis in silico: Understanding Complexity from Molecules to Ecosystems. Pp 125–149. Springer, Dordrecht 2009.
Zhu, X.-G., Govindjee, Baker, N.R., deSturler, E., Ort, D.R., Long, S.P.: Chlorophyll a fluorescence induction kinetics in leaves predicted from a model describing each discrete step of excitation energy and electron transfer associated with Photosystem II. — Planta 223: 114–133, 2005.
Acknowledgements
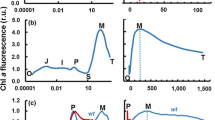
This work was supported by the Ministry of Education of the Czech Republic by a grant number MSM 6198959215 and through the Marie Curie Initial Training Network of the 7th Framework Programme of the European Union, contract number PITN-GA-2009-238017. The experimental data presented in Fig. 1 are courtesy of Gert Schansker and Petr Ilík.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lazár, D. Modelling of light-induced chlorophyll a fluorescence rise (O-J-I-P transient) and changes in 820 nm-transmittance signal of photosynthesis. Photosynthetica 47, 483–498 (2009). https://doi.org/10.1007/s11099-009-0074-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-009-0074-8