Abstract
Climate change is rapidly changing how we live, what we eat and produce, the crops we breed and the target traits. Previously underutilized orphan crops that are climate resilient are receiving much attention from the crops research community, as they are often the only crops left in the field after periods of extreme weather conditions. There are several orphan crops with incredible resilience to biotic and abiotic stresses. Some are nutritious, while others provide good sources of biofuel, medicine and other industrial raw materials. Despite these benefits, orphan crops are still lacking in important genetic and genomic resources that could be used to fast track their improvement and make their production profitable. Progress has been made in generating draft genomes of at least 28 orphan crops over the last decade, thanks to the reducing cost of sequencing. The implementation of a structured breeding program that takes advantage of additional modern crop improvement tools such as genomic selection, speed breeding, genome editing, high throughput phenotyping and breeding digitization would make rapid improvement of these orphan crops possible, but would require coordinated research investment. Other production challenges such as lack of adequate germplasm conservation, poor/non-existent seed systems and agricultural extension services, as well as poor marketing channels will also need to be improved if orphan crops were to be profitable. We review the importance of breeding orphan crops under the increasing effects of climate change, highlight existing gaps that need to be addressed and share some lessons to be learned from major crops.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Background
Climate change is predicted to bring about increased temperatures across the world in the range of 1.6–6 °C, and an increase in average precipitations above 2% by 2050 (Jarvis et al. 2009), triggering a host of extreme weather events including drought, flooding and heat waves (Feulner 2017). These predicted changes in climate are expected to have worldwide impacts on agriculture, with the most vulnerable areas being Africa, Asia and Latin America (Jarvis et al. 2009; Ayanlade et al. 2018). There is increasing evidence that climate change is impacting total precipitation and its temporal dynamics with significant effects on crop yields (Shortridge 2019) and biodiversity (Jarvis et al. 2009; Bálint et al. 2011; FAO 2015). One of the options for better adaptation to climate change includes the management of biodiversity for ecosystem resilience (Dauber and Miyake 2016; UNCCD 2017). A prerequisite for the use of adapted plant genetic resources in increasing the resilience of future production systems is improved knowledge of these plant resources, their origin and characterization in terms of valuable traits for climate change adaptation (FAO 2015). Unfortunately, many locally adapted varieties and plant species are orphan crops that are neglected and underutilized with a high risk of extinction before their potential roles in climate change adaptation are fully exploited (FAO 2015).
Orphan crops, which are also referred to as ‘underutilized’ (Dawson and Jaenicke 2006), ‘minor’ (Umesh et al. 2019), ‘neglected’ (Hendre et al. 2019; Tadele 2019; Popoola et al. 2019), ‘promising’ (for emerging markets, or because of previously unrecognized valuable traits), ‘niche’ (of marginal importance in production systems and economies) and/or ‘traditional’ (used for centuries or even millennia) crops (Gregory et al. 2019), are crops with important attributes, not globally known, have the potential to be grown for profit or subsistence, have been under-researched in the past and, therefore, have inadequate or total lack of genetic and genomic resources. Despite their neglect in research and investment, orphan crops have the potential to address multiple UN Sustainable Development Goals in the low-income nations of Africa (Hendre et al. 2019), Asia (Gregory et al. 2019) and Latin America, as well as in the growing western consumers’ interests in new healthier foods (Dawson et al. 2019).
Most orphan crops are generally more adapted to the extreme soil and climatic conditions as they contain the relevant alleles and mechanisms for growth in poor environments and for resilience under stress (Oibiokpa et al. 2014; Tátrai et al. 2016) that have potentially been lost from major crops (Ellstrand et al. 2010; Cullis and Kunert 2017). Orphan crops have been recognized as potential sources of resilience traits (Chiurugwi et al. 2019) that can be used to improve major crops and also play a role in improving sustainability of food systems (Mabhaudhi et al. 2019; Borelli et al. 2020; Dawson et al. 2019). This increasing recognition of the important role of orphan crops has resulted in the launch of advanced research and development initiatives (Tadele and Bartels 2019). Other traits of importance include nutrition (Dawson et al. 2019), medicinal value (Tlili et al. 2011), biofuel (King et al. 2015), cosmetics (Saikia and Konwar 2012) and for feed/fodder (Tolera and Sundstøl 2000). A summary of select orphan crops and their importance is presented in Supplementary Table 1. We further discuss the key contributions of orphan crops globally under four sub-topics below.
Resilience to biotic stresses
A recent study estimated significant economic losses in major crops as a result of pests and diseases and recommended the prioritization of plant health to improve the sustainability of agro-ecosystems (Savary et al. 2019). Part of the solution lies in the diversification of agro-ecosystems using orphan crops, majority of which have been reported to show tolerance to some of the pests and diseases (Hendre et al. 2019). Forage legumes in the genus Desmodium, mainly D. uncinatum and D. tortum, have been used to suppress one of the most devastating parasitic weeds in Africa, Striga hermonthica (Midega et al. 2017). Striga is a parasitic weed to most cereals including maize (Zea mays L.), sorghum [Sorghum bicolor (L.) Moench] and rice (Oryza sativa L.). Desmodium spp., an orphan crop, suppresses Striga when intercropped with cereals (Midega et al. 2010) through a combination of different mechanisms including the production of an allelochemical that inhibits the radicle growth of Striga (Hooper et al. 2010). Spider plant (Gynandropsis gynandra (L.) Briq.) has been reported to significantly reduce the incidence of thrip species Megalurothrips and Frankliniella occidentalis (Waiganjo et al. 2007) when used as a companion crop with snap bean (Phaseolus vulgaris), while finger millet (Eleusine coracana subsp. coracana) is effective in suppressing weed growth (Samarajeewa et al. 2006).
Other orphan crops have been used as donors of resistance genes that were successfully introgressed into major crops. Within the Solanaceae family, the African eggplant (Solanum aethiopicum), which is used as a vegetable in Africa, is a source of resistance to Fusarium oxysporium f. sp. melongenae (Rizza et al. 2002; Toppino et al. 2008) and Ralstonia solanacearum (Collonnier et al. 2001) for the improvement of other Solanaceae crops. S. aethiopicum rootstocks have been reported to improve disease resistance in tomato (Solanum lycopersium L.) (Nkansah et al. 2013), while Solanum torvum, also an orphan vegetable, is the preferred rootstock for improved resistance to diseases in brinjal eggplant (Solanum melongena) (Sakata et al. 1989; Ramesh et al. 2016). Watermelon (Cucumis melo) grafted onto the rootstock of the little known bottle gourd (Lagenaria siceraria), conferred resistance to Fusarium spp. (Davis et al. 2008).
Resilience to abiotic stresses
Many orphan crops have been identified as climate-smart and able to adapt to the ever-changing climate of their respective agro-ecological regions. Drought is one of the major abiotic constraints limiting agricultural production worldwide alongside low temperatures, soil salinity, nutrient deficiencies and toxic metals (Shinozaki et al. 2015). There are several orphan crops that have been reported to exhibit high levels of tolerance to drought stress, although the stability of their yields, in most cases, has not been established. Such crops include finger millet (Neshamba 2010; Krishnamurthy et al. 2016), foxtail millet (Setaria italica) (Puranik et al. 2011), fonio (Digitaria spp.) (Vietnameyer et al. 1996), grass pea (Lathyrus sativa L.) (Hanbury et al. 2000) and quinoa (Chenopodium quinoa Willd.) (Hinojosa et al. 2018). The drought mechanisms reported in these orphan crops include efficient antioxidant potential (Puranik et al. 2011; Bhatt et al. 2012; Jiang et al. 2013), association with arbuscular mycorrhiza (Tyagi et al. 2017), osmotic adjustment (Jiang et al. 2013; Tyagi et al. 2017), reduction in the green leaf area and stomatal conductance (Cullis and Kunert 2017). Caper (Capparis spinosa L.), an orphan shrub cultivated for its flower buds and fruits in the Mediterranean, shows remarkable resilience to heat stress (Levizou et al. 2004).
Other orphan crops have developed genetic and molecular mechanisms to survive in poor soils of low fertility where most of the major plants do not grow (Naluwairo 2011; Takada et al. 2017; Cullis et al. 2018; Mabhaudhi et al. 2019). Finger millet grows successfully on marginal lands with poor soil fertility (Thilakarathna and Raizada 2015) and exhibits a higher degree of salt tolerance in comparison to other cereals (Bray et al. 2000; Shailaja and Thirumeni 2007; Rahman et al. 2014). Common glasswort (Salicornia europaea L.), an orphan annual dicot with diverse uses (Loconsole et al. 2019), is one of the most salt-tolerant species worldwide (Patel 2016). Other orphan crops have been used in phytoremediation (Mkumbo et al. 2012), the most sustainable way of rehabilitating polluted lands through the use of plants to extract heavy metals from soil (Raskin et al. 1997). Plants of the Amaranthaceae family, including Salicornia brachiata (Sharma et al. 2010), Amaranthus spinosus (Chinmayee et al. 2012), Amaranthus retroflexus L. var. retroflexus and Amaranthus hybridus L., have been reported to be tolerant to various heavy metals (Mohsenzade et al. 2009; Zhang et al. 2010). African yam bean (AYB) (Sphenostylis stenocarpa Harms) and Jatropha curcas have been reported as excellent phytoremediators for heavy metal (i.e., Al, Fe, Cr, Mn, Ar, Zn, Cd and Pb) contaminated soil (Jamil et al. 2009; Ochekwu and Eneh 2012; Chandra et al. 2016a). These examples present opportunities for the promotion of these crops to a higher level of production.
Medicinal/pharmaceutical/cosmetic value
Many people in the developing countries have depended on orphan crops for medicine, pharmaceuticals and cosmetics for centuries. The African eggplant has been used traditionally in the management of a range of ailments from weight reduction and hypertension (Miller et al. 1999; Odetola et al. 2004; Ogunka-Nnoka et al. 2018) to treatment of several conditions such as diabetes (Ezugwu et al. 2005), anticonvulsant (Gbile and Adesina 1988), skin infections (Oliver-Bever 1986), rheumatic disease, swollen joint pains (Anosike et al. 2012), colic, ulcers, gastro-esophageal reflux disease and constipation (Gbile and Adesina 1988; Ezugwu et al. 2005). In addition to the high levels of vitamin C and β-carotene, the African eggplant has significant levels of alkaloids, saponins, flavonoids, tannins, ascorbic acid and steroids (Chinedu et al. 2011a, b; Neugart et al. 2017; Sekulya et al. 2018) making it a potential source of precursors for pharmaceutical drugs. There are several reports on the medicinal properties of Solanum anguivi (Ripperger and Himmelreich 1994; Elekofehinti et al. 2012, 2013, 2015), confirming its traditional use as medicine in certain parts of Africa. Breadfruit {Artocarpus altilis (Parkinson) Fosberg} contains fatty acids and extracts that are used in pest management (Jones et al. 2012; Eccles et al. 2019) and has traditionally been used in Asia for the treatment of malaria, yellow fever, dengue fever (Jacob et al. 2015), liver cirrhosis, hypertension and diabetes (Wang and Wang 2010; Jones et al. 2012).
Finger millet grain, which is gluten free, has been used in the management of physiological disorders such as diabetes, hypertension, vascular fragility, hypercholesterolemia, prevention of oxidation of low-density lipoproteins (LDLs) and also to improve gastrointestinal health (Chandra et al. 2016a, b; Kumar et al. 2016; Chethan and Malleshi 2007). Tef (Eragrostis tef) has similarly received attention as a life-style crop due to its gluten-free nature (Spaenij-Dekking et al. 2005; Tadele 2019). Regular consumption of the spider plant (Cleome gynandra L.) by expectant mothers has been reported to relieve childbirth complications as well as reduce the length of the labor period (Onyango et al. 2013). Different parts of the spider plant have been used as a relief for epileptic fits, ear, eye and nostril aches, for the treatment of inflammations, headaches, scurvy, marasmus (Opole et al. 1995; Narendhirakannan et al. 2005), neuralgia, rheumatism (Chweya and Mnzava 1997) and diabetes (Shaik et al. 2013). Shea butter extracted from the shea tree (Vitellaria paradoxa or V. nilotica) is often used as a base in medicinal ointments due to its anti-inflammatory properties (Maanikuu and Peker 2017).
The cosmetic industry has benefited from the shea tree products as one of the best anti-ageing and moisturizing agents for the skin with sun-screening and collagen boosting properties (Suter et al. 2016; Montenegro and Santagati 2019). The African melon (Acanthosicyos horridus and Citrullus lanatus) has been reported to have great potential in both the food and cosmetic industry (Houdegbe et al. 2016; Cheikhyoussef et al. 2017), while the emulsifying capacity of biosurfactants from quinoa has been reported as suitable for incorporation into cosmetic emulsion formulations (Bezerraa et al. 2020). The seeds of marama bean (Tylosema esculentum) have traditionally been consumed and used as a cosmetic by the natives of the Kalahari (Cullis et al. 2019), while tiger nut (Cyperus esculentus) oil is commonly used as a cooking ingredient and in skin care (Ezeh et al. 2014).
Sources of other novel traits (nutrition, feed/fodder, biofuel)
Orphan crops play an important role in the economies of many countries, particularly in the developing world, as sources of nutrition (Jamnadass et al. 2020). It is believed that breeding for increased production of orphan crops can reduce malnutrition and stunting (Bekkering and Tian 2019; Tadele 2019). Amaranthus hypochondriacus leaves, for example, contain far more vitamin A as compared to other green leafy vegetables like spinach and cabbage (Hunter et al. 2019). Varieties of finger millet are way more nutritious than white rice in their calcium, iron, potassium, magnesium and zinc content (Tripathi and Platel 2010). The high levels of minerals, vitamins and fats in yams (Dioscorea spp.) outperform the commonly consumed potatoes (Padhan and Panda 2018, 2020). These exceptional nutritive qualities have been a major target for improving resource availability of orphan crops through whole genome sequencing (Jamnadass et al. 2020).
Several legume orphan crops species are cultivated for food, feed and fodder including winged bean (Psophocarpus tetragonolobus L.) (Hyland 1968; Hymowitz and Boyd 1977), hyacinth bean (Lablab purpureus L.) (Morris 2009a, b), lima bean (Phaseolus lunatus L.) (Andueza-noh et al. 2015), jack and sword bean (Canavalia sp.) (Akpapunam and Sefa-Dedeh 1997), mung bean (Vigna radiata L.) (Tang et al. 2014), bambara groundnut (Vigna subterranea L.) (Mayes et al. 2019), marama bean (Tylosema esculentum L.) (Cullis et al. 2019), kersting’s groundnut (Kerstingiella geocarpa Harms) (Ayenan and Ezin 2016), African yam bean (AYB) (Asare et al. 1984) and rice bean (Vigna angularis L.) (Joshi et al. 2008). All parts of the enset plant (Ensete ventricosum) are used for animal forage (Borrell et al. 2019).
The rising demand for biofuels has led to the identification of orphan crops as sources of ‘second-generation’ biofuels. The seeds of an orphan crop, Jatropha curcas (Physic nut or Jatropha), that contain 27–40% oil can be processed to produce a high-quality biodiesel fuel, usable in a standard diesel engine or further processed into jet fuel (Duraes et al. 2011; Odeh and Tan 2015). The residue (press cake) is used as feed in digesters and gasifiers to produce biogas, or as biomass feedstock to power electricity plants, or as a fertilizer due to its nitrogen, phosphorus and potassium content (Achten et al. 2007). The seeds of the African melon (Cucumeropsis mannii) have also been shown to have applications in biodiesel production (Dansi et al. 2012; Houdegbe et al. 2016).
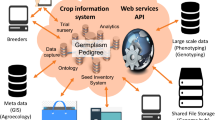
Genetics and breeding of select climate smart orphan crops
Despite their demonstrated economic importance and their beneficial contributions to agro-ecosystems, there has been a lag in the overall genetic improvement of orphan crops. The breeding methods are conventional, slow and lacking in innovation, while the breeding objectives are not well defined beyond the enhancement of domestication syndrome traits. In this section, we discuss the genetics and breeding of six climate-resilient orphan crops (Fig. 1), randomly selected from the main categories of food crops, cereals, pseudo-cereals, legumes, root and tuber crops, vegetables and fruits. These crops are seen as most promising climate resilient crops for the future and are already receiving global attention, including being incorporated as mandate crops of some of the Consultative Group of International Agricultural Research (CGIAR) centers, in some cases. For each of the crops, we briefly discuss their origin, domestication, distribution, genetic resources, economic importance and breeding status.
Finger millet
Finger millet (Eleusine coracana subsp. coracana) (2n = 4x = 36) (AABB) is an annual orphan cereal crop belonging to Poaceae family and Chloridoideae sub-family (Srinivasachary et al. 2007). There are seven other species of annual grasses in the genus Eleusine, including E. kigeziensis, E. indica, E. intermedia, E. floccifolia, E. tristachya, E. jaegeri and E. multiflora. Finger millet is believed to have been domesticated from its wild progenitor, E. coracana subsp. africana, about 5000 years ago (Dida et al. 2007). It is the only cultivated crop of the genus Eleusine and has four cultivated races, namely, elongata, plana, compacta and vulgaris (Upadhyaya et al. 2010). E. indica (AA) is the AA genome donor, while the BB genome donor remains unknown. More than 37,000 wild and cultivated finger millet germplasm has been conserved globally (Vetriventhan et al. 2016) in various gene banks, with the National Bureau of Plant Genetic Resources in India having the highest number of collections (> 10,000) followed by the International Crops research Institute for the Semi-Arid Tropics (ICRISAT) (7519) (Odeny et al. 2020). East Africa and India are considered the primary and secondary centers of diversity, respectively (Bisht and Mukai 2002) for finger millet, and accessions from the two regions appear to be genetically and morphologically distinct (Arya et al. 2013; Babu et al. 2014a; Ramakrishnan et al. 2015; Puranik et al. 2020).
Finger millet is largely cultivated for its nutritious gluten-free healthy grain and resilience to several biotic and abiotic stresses (Rodriguez et al. 2020). Traditionally, genetic improvement in finger millet was limited to pedigree-based selection for larger seed size, higher yield and less shattering, with a focus on enhancing its domestication. The inclusion of finger millet as a mandate crop of ICRISAT led to a relatively more structured breeding with the main objectives as enhancing resistance to blast disease (Magnaporthe grisea teleomorph: Pyricularia grisea), S. hermonthica (parasitic witchweed), lodging, tolerance to stressful soil and moisture conditions, and grain that can be more easily dehulled and ground (National Research Council 1996). Hybridization in finger millet has been undertaken manually, or using the plastic bag technique, hot water treatment, or the use of chemicals (Kunguni et al. 2015) due to the lack of cytoplasmic male sterile (CMS) lines. Its self-pollinating nature (Hilu and de Wet 1980) and the tiny floral architecture have hindered bulk hybridizations, especially in Africa, where most improved varieties released are from selections. Most of the hybridization-based breeding has been done in Asia and, in some cases, included African germplasm leading to the release of ‘Indaf’ varieties (Deba et al. 2008) with improved yields. There are now a few programs in E. Africa employing hybridization-based breeding through hand emasculation and pollination.
Genomics-assisted breeding has been limited in finger millet due to lack of a robust set of molecular markers until recently. The first partial finger millet genetic map was constructed by Dida et al. (2007) using Restriction Fragment Length polymorphism (RFLP), Amplified Fragment Length Polymorphism (AFLP) and Single Strand Conformation Polymorphic (SSCP) expressed sequenced tags. The map was constructed using an interspecific F2 mapping population between Eleusine coracana subsp. coracana (Okhale 1) and Eleusine coracana subsp. africana (MD-20), and contained 327 loci that were mapped to either A or B genomes. More recently, a more robust single nucleotide polymorphism (SNP) linkage map was developed using F2:3 families of the same interspecific cross between Okhale 1 and MD-20 (Qi et al. 2018). This recent map used 4453 SNP markers in 18 linkage groups that were designated the same as in Dida et al. (2007) and incorporated a subset of markers that had been mapped in the first linkage map. There is currently no linkage map generated exclusively from a cross involving the cultivated subspecies only. Trait mapping has been limited in finger millet, and the few studies undertaken so far used association mapping, albeit with less than optimal numbers of genotypes. Most of the traits mapped are agronomic (Babu et al. 2014a; Lule et al. 2018; Sharma et al. 2018), although there are also reports of association mapping for blast disease resistance (Babu et al. 2014b) as well as for nutrition-related traits (Babu et al. 2014c; Puranik et al. 2020).
Amaranthus hypochondriacus
Amaranthus hypochondriacus (2n = 2x = 32) is one of the more than 70 species of the genus Amaranthus, mostly annuals, distributed across the world’s tropical and temperate regions. It is also one out of the three Amaranth grain species considered to have desirable agronomic characteristics besides A. cruentus L. and A. caudatum L (Grubben and Denton 2004) that form part of the hybridus complex together with the two potential wild ancestors, A. hybridus and A. quitensis. A. hypochondriacus crosses easily with the four species within the hybridus complex making it difficult to fully understand the taxonomic relationships within the complex. There are several hypotheses on the origin of the three-grain amaranths (Sauer 1967,1976; Kirkpatrick 1995; Xu and Sun 2001; Mallory et al. 2008) although more recent molecular evidence supports a monophyletic origin (Kietlinski et al. 2014; Viljoen et al. 2018), perhaps from two or three different, geographically separated lineages of A. hybridus (Stetter and Schmid 2017; Stetter et al. 2020). The first record of A. hypochondriacus domestication was by the Aztec civilization in central Mexico (Brenner et al. 2010) but most of its cultivation today occurs in India. Approximately 61 diverse collections of amaranth genetic resources are being maintained in at least 11 countries of the world (Das 2016; Joshi et al. 2018), majority of which reside in the USDA germplasm collection with a total of 3338 accessions from 40 countries (Trucco and Tranel 2011; Brenner 2015).
Amaranthus hypochondriacus is cultivated mainly as a pseudo-cereal but also for fodder (Abbasi et al. 2012) and as an ornamental (Pandey and Singh 2011). The grains are highly nutritious (Ssepuuya et al. 2018) with medicinal properties (Aditya and Bhattacharjee 2018), and the plants have incredible agronomic versatility (Rodriguez et al. 2020). The plants are considered autogamous but there are varying amounts of interspecific and inter-varietal hybridization (Suresh et al. 2014) that have further resulted in significant morphological and genetic diversity (Stetter et al. 2017) and a wider adaptability to different eco-geographical environments (Lee et al. 2008). Domestication syndrome remains indistinct in amaranth with strong photoperiod sensitivity and very small shattering seeds (Sauer 1967; Brenner et al. 2010). The frequent outcrossing with the wild-weedy relatives has not only complicated further the proper analysis of amaranth domestication (Stetter et al. 2016), but also led to a constraint for pure seed production (Brenner et al. 2013). The tiny intricate flowers make hand emasculation and pollination in amaranth difficult (Stetter et al. 2016) on a large scale. Alternative methods of hybridization include hot water treatment (Stetter et al. 2016) and the use of male sterility (Peters and Jain 1987; Brenner 2019). Although the application of hybrid breeding in amaranth is very promising (Lehmann et al. 1991), genetic improvement in the past has been achieved mainly through pedigree-based selection of suitable genotypes from landraces (Stetter et al. 2016). The major breeding objectives in amaranth include reduced seed shattering, reduced plant height (1.0–1.5 m), resistance to lodging, flowering above the leaf canopy for mechanical harvest, high grain yield, synchronized maturity, high grain quality, reduced leafiness in the green head area, and resistance to diseases and pests (Joshi et al. 2018).
The genetics of most of the agronomic traits have been determined (Joshi et al. 2018) including flowering time (Kulakow and Jain 1985), plant height, leaf length and width, panicle length and width (Kulakow and Jain 1987), harvest index, 1000 seed weight, grain yield (Pandey 1984), grain protein percentage (Pandey and Pal 1985), starch content of grain (Okuno and Sakoguchi 1982), seed coat color, inflorescence color and purple leaf mark (Gupta and Gudu 1990). Despite the availability of a 16-group SNP linkage map that has been constructed using an interspecific F2 mapping population between A. hypochondriacus x A. caudatus (Maughan et al. 2011), trait mapping has been limited due to the lack of robust mapping populations. The map comprises 411 SNP markers spanning 1288 cM with an average marker density of 3.1 cM per marker. The availability of a reference genome (Lightfoot et al. 2017) has made it possible to use SNP markers generated from genotyping-by-sequencing (GBS) for diversity analysis (Wu and Blair 2017) but is yet to be used for bi-parental or association mapping of traits.
Grass pea
Grass pea (Lathyrus sativus L.) (2n = 2x = 14) is a member of the Fabaceae family. The genus consists of more than 150 species, which are further divided into 13 sections based on morphological traits (Kupicha 1983). Grass pea belongs to the section Lathyrus along with 33 other species and is the only cultivated pulse crop (Allkin 1986) in the genus. The primary gene pool consists of the highly variable L. sativus accessions (Yunus and Jackson 1991), while the secondary gene pool includes L. amphicarpos, L. cicero, L. chrysanthus, L. gorgoni, L. marmoratus and L. pseudocicera, L. blepharicarpus, L. hierosolymitanus and L. hirsutus. Grass pea cultivation originated around 6000 BC in the Balkan peninsula (Kislev 1989) and is believed to have been the first crop to be domesticated in Europe (Kislev 1989). Its production has now spread to other parts of the world, both temperate and tropical, including North and South America, the Canary Islands, the Mediterranean region, East Africa and Asia. Almost 20,000 accessions of Lathyrus spp. are maintained in different gene banks across 27 countries (Shehadeh 2011; Vaz Patto and Rubiales 2014), with a safe duplication of 3239 accessions in the global collection at the International Centre for Agricultural Research in Dryland Areas (ICARDA). The CROP TRUST (https://www.croptrust.org/crop/grass-pea/) is currently supporting 4,451 unique grass pea germplasm, of which 3595 are backed up at the Svalbard Global Seed Vault. France, India, Bangladesh and Chile hold the largest collections of L. sativus (Hillocks and Maruthi 2012) globally, while Ethiopia has the largest collection of grass pea in Africa (Girma and Korbu 2012).
Grass pea is cultivated for its seeds as a healthy food (Lambein et al. 2019) and feed (Smulikowska et al. 2008), as well as for fodder (Singh and Roy 2013). It is a hardy and resilient crop (Campbell 1997) that is rarely affected by pests and diseases (Rahman et al. 1995) and is one of the most climate and environmental change tolerant legumes (Kislev 1989; Yunus and Jackson 1991; Chowdhury and Slinkard 2000; Vaz Patto et al. 2006).
Grass pea is predominantly autogamous, although some level of cross-pollination has been reported (Rahman et al. 1995; Hanson and Street 2008; Ghorbel et al. 2014). Crop improvement has been achieved through conventional selection from landraces (Yunus and Jackson 1991; Vaz Patto et al. 2006), with the initial objective of improving domestication syndrome traits. Grass pea has also been included as a mandate crop of ICARDA, thereby enhancing its breeding structure. Finding high yielding cultivars with low ODAP content (< 0.2%) has been the main breeding objective (Campbell 1997; Girma and Korbu 2012) due to the high risk of lathyrism (Barone and Tulumello 2020) that would result from the consumption of high ODAP genotypes. High genetic variation for ODAP content, ranging from 0.02 to 2.59%, has been reported within and among populations of grass pea (Campbell 1997; Tay et al. 2000; Granati et al. 2003; Tadesse and Bekele 2003; Vaz Patto et al. 2006; Girma and Korbu 2012) that have led to the release of a number of cultivars with low ODAP (Campbell 1997; Granati et al. 2003; Tadesse and Bekele 2003; Vaz Patto et al. 2006; Girma and Korbu 2012; Hillocks and Maruthi 2012; Vaz Patto and Rubiales 2014) through conventional breeding. Hybridity is largely achieved through hand emasculation and pollination.
The first linkage map in grass pea was constructed using molecular and isozyme markers on an F2 population of 100 individuals derived from a cross between a blue-flowered and a white-flowered parent (Chowdhury and Slinkard 1999). The map comprised 71 RAPD, three isozymes and one morphological marker spread across 14 linkage groups and spanning 898 cM with an average distance between markers of 17.2 cM. About 12% of the markers used showed distorted segregation. The second linkage map was constructed using a backcross population of 92 individuals segregating for resistance to ascochyta blight (Mycosphaerella pinodes) (Skiba et al. 2004). The same study mapped two QTLs responsible for 12 and 9% of trait variation in linkage groups 1 and 2 using 47 RAPDs, 7 STMS and 13 STS/CAPS markers that spanned 803.1 cM across nine linkage groups. There are no records of any additional trait mapping studies either using bi-parental or diverse populations. Both simple sequence repeat (SSR) and SNP markers have been developed in grass pea (Yang et al. 2014; Hao et al. 2017), and a draft genome sequence is now available (Emmrich et al. 2020) to enable routine molecular analysis for trait mapping and characterization. More robust populations will also need to be developed to enable precise analysis of complex traits.
Water yam
Water yam (Dioscorea alata L.) (2n = 2x = 40), also known as the greater yam, is one of the oldest cultivated yam species (Lebot 2009) and the most widely cultivated yam species worldwide (Abraham and Nair 1990; Obidiegwu et al. 2009). It belongs to the genus Dioscorea which comprises over 600 species distributed primarily in the tropics and subtropics (Rao et al. 1973). The genus Dioscorea was historically assembled into 32–59 sections (Knuth 1924; Ayensu 1972). The section Enantiophyllum Uline is the most important section as it contains the three economically important species, D. alata, D. cayenensis and D. rotundata, all of which are cultivated worldwide. The other important species in the genus include D. bulbifera, D. esculenta, D. opposita, D. japonica, D. nummularia, D. pentaphylla, D. transversa, D. trifida and D. dumetorum (Dahiya et al. 2015; Efraín González Ramírez and García 2019). The species belonging to the Enantiophyllum section that includes water yam are considered unique to Southeast Asia (Malapa et al. 2005) suggesting they likely originated from this part of the world. D. alata is believed to have been domesticated about 6000 years ago (Lebot 2009) in Melanesia, where the greatest phenotypic variability has been observed (Lebot et al. 1998). Although previous studies reported that D. alata was close to D. nummularia and D. transversa (Malapa et al. 2005; Wilkin et al. 2005), this school of thought has been recently challenged (Caetano et al. 2016) and the true ancestry of D. alata remains unknown. Ex site germplasm collections have been assembled at the Central Tuber Crops Research Institute, Kerala, India (431 accessions), Centre de Ressources Biologiques Plantes Tropicales INRA-CIRAD, Guadeloupe, France (181) and at the International Institute of Tropical Agriculture (IITA) in Ibadan, Nigeria (772 accessions) (Arnau et al. 2017).
Water yam has a wide geographical distribution and is especially desirable for production due to high yield potential, ease of propagation, early growth vigor for weed suppression, long storability of tubers (Sartie and Asiedu 2014) and high nutritional content of tubers (Fauziah et al. 2020). It is dioecious (produces separate male and female plants) (Egesi et al. 2002; Obidiegwu et al. 2009; Ajayi and Oyetayo 2009; Baboli and Safe Kordi 2010) with ploidy levels ranging from diploids (2n = 2x = 40), triploids (2n = 3x = 60) and tetraploids (2n = 4x = 80) (Abraham and Gopinathan Nair 1991; Egesi et al. 2002; Obidiegwu et al. 2009; Arnau et al. 2009; Baboli and Safe Kordi 2010). Water yam is almost exclusively clonally propagated using small tubers or small pieces of tubers (Arnau et al. 2017), which provides agronomical advantages but has the disadvantage of enhancing the spread of diseases (Arnau et al. 2017). The major breeding objectives in water yam include resistance to anthracnose disease [Colletotrichum gloeosporioides (Penz.)] (Bhartiya et al. 2017), high tuber quality and yield potential (Arnau et al. 2016). Conventional hybridization through ploidy induction and manipulation (Arnau et al. 2010; Baboli and Safe Kordi 2010) has been successfully used to improve resistance to biotic and abiotic stresses (Darkwa et al. 2020), while higher vigor and tuber yield advantage were reported with tetraploid (2n = 4x = 80) and triploid (2n = 3x = 60) water yam compared to its diploid (2n = 2x = 40) counterpart (Arnau et al. 2007; Lebot 2009; Lebot et al. 2019). Artificially induced polyploidy has positive effects on chlorophyll content, leaf shape, stomata density, plant width, vine size and length and fruit (Kenji et al. 2005; Ajayi et al. 2010; Baboli and Safe Kordi 2010; Abraham et al. 2013). Successful interspecific hybridization that has been reported between D. alata and D. nummularia under artificial hand pollination (Lebot et al. 2017) provides further opportunities to introgress superior traits from D. nummularia such as resistance to anthracnose disease, high dry matter content of the tubers, high vigor and robustness, resistance to cyclones and tolerance to acid rain (Lebot et al. 2017). There are no reports of successful hybridization between D. alata and either D. rotundata and D. cayenensis (Rao et al. 1973; Arnau et al. 2007; Lopez-Montes et al. 2012).
Wide morphological and genetic variation has been reported in water yam (Arnau et al. 2017; Agre et al. 2019), which has been exploited in a few cases using molecular tools to identify genomic regions responsible for traits of interest. The first intraspecific genetic linkage map of D. alata was constructed using 523 polymorphic AFLP markers that were mapped onto 20 linkage groups spanning 1233 cM with a mean marker spacing of 2.31 cM (Mignouna et al. 2002). This linkage map also led to the identification of an AFLP marker linked to anthracnose resistance although only 10% of phenotypic variance was explained (Mignouna et al. 2002). Petro et al. (2011) later developed a more saturated AFLP linkage map and identified nine QTLs linked to anthracnose resistance that explained a range of 7–32.9% of phenotypic variance. More recently, Bhartiya et al. (2017) used EST-SSRs to map resistance to anthracnose and identified a consistent QTL on LG14 that explained 68.5% of the total phenotypic variation. The most recent linkage map of water yam was developed using SNP markers generated from genotyping-by-sequencing (GBS) and led to the identification of a major sex determination QTL on LG6 (Carrillo-Perdomo et al. 2019). Besides their use for linkage mapping, molecular markers have also been used in water yam for hybridity testing (Sartie and Asiedu 2011).
African eggplant
The African eggplant (Solanum aethiopicum) (2n = 2x = 24) belongs to the Solanaceae family and genus Solanum. It is one of the only three cultivated eggplants together with the Gboma eggplant (S. macrocarpon) and brinjal eggplant (S. melongena), all of which belong to the Leptostemonum clade, and to a species-rich sub-clade native to the Old World; Africa, Australia, and Asia (Lester and Daunay 2003; Acquadro et al. 2017). Studies based on seed protein and amplified fragment length polymorphism (AFLP) markers confirmed that S. aethiopicum is more related to S. macrocarpon than to S. melongena (Daunay et al. 2001; Sękara et al. 2007). The African eggplant is believed to have been domesticated in Africa from its wild progenitor Solanum anguivi (Sakata and Lester 1997; Lester 1998), which forms part of its primary genepool, and hybrids between S. aethiopicum and S. anguivi are fully fertile (Lester and Niakan 1986; Plazas et al. 2014; Taher et al. 2017). Successful crosses are possible between the African eggplant and both Gboma eggplant and brinjal eggplant, as well as with their respective ancestors S. dasyphyllum and S. insanum with intermediate fertility (Daunay et al. 1991; Prohens et al. 2012; Plazas et al. 2014). The three eggplants are also related to a large number of wild species (Syfert et al. 2016), which are well adapted to a wide range of conditions, from desert to swampy areas and environments with wide ranges of temperatures. GENESYS (2017) records 798 accessions of S. aethiopicum with possible additional collections in India and the Institute of Vegetables and Flowers, China (Taher et al. 2017). Although other unreported collections of S. aethiopicum may exist in different countries, more global collections need to be urgently done to avoid genetic erosion in this nutritious climate resilient vegetable crop. The African eggplant cultivation is mostly restricted to Africa, but is also cultivated in the Caribbean and Brazil (Schippers 2000) as well as in some areas of the southern part of Italy (Bukenya 1994; Sunseri et al. 2010).
The African eggplant is a hypervariate species (Lester et al. 1986; Plazas et al. 2014) with four recognized cultivar groups; Shum, Gilo, Kumba and Aculeatum (Lester 1986; Lester et al. 1986; Lester and Daunay 2003) that are completely inter-fertile (Lester and Niakan 1986). The Shum is used for its leaves; Kumba for both fruits and leaves; and Gilo for its fruits, and Aculeatum is used as an ornamental (Lester 1986; Schippers 2000; Lester and Daunay 2003). The crop is predominantly self-pollinating with up to 30% cross-pollination (Adeniji et al. 2012). It has been used as a source of resistance genes that have been introduced into other Solanaceae crops (Collonnier et al. 2001; Toppino et al. 2008; Rizza et al. 2002). Crop improvement has been achieved through the selection of landraces to enhance the domestication syndrome traits (non-shattering, reduced dormancy, increased seed size etc.), as well as improve resilience to biotic and abiotic stresses (Sseremba et al. 2018a, 2018b). There is now an established drought screening protocol (Nakanwagi et al. 2020) that can be used to identify drought resilient genotypes for use in generating relevant populations for future genetic studies. Its wild progenitor, S. anguivi, is a good source of novel alleles for disease resistance (Schippers 2000) and high number of fruits per inflorescence (Bukenya-Ziraba 2004; Osei et al. 2010; Afful et al. 2018).
The use of molecular markers within the African eggplant has been mainly for germplasm or genome characterization (Sakata et al. 1991; Sakata and Lester 1994; Gramazio et al. 2016; Song et al. 2019). There is currently no association or linkage mapping studies reported in the literature despite the recent availability of a reference genome (Song et al. 2019).
Breadfruit
Breadfruit belongs to the genus Artocarpus (Moraceae), which consists of approximately 60 species native to the Oceania region (Kochummen 2000; Zerega et al. 2004). It is believed to have been domesticated from its wild ancestor, Artilis camansi Blanco (breadnut), in western Pacific about 3000 years ago (Ragone 2006; Zerega et al. 2006) from where it was spread by humans throughout the tropics (Roberts-Nkrumah 2007; Omubuwajo 2007; Ragone 1997). The cultivated breadfruit (Artocarpus altilis, (Parkinson) Fosberg, Moraceae), together with its wild relatives, A. camansi, A. mariannensis Trécul and natural hybrids (A. altilis x A. mariannensis) make up the breadfruit complex (Ragone 2007; Zerega et al. 2015). The National Tropical Botanical Garden (NTBG) in Hawaii is the main breadfruit conservation center and manages a field genebank of 220 accessions from 18 Pacific Island groups, the Philippines, the Seychelles, Indonesia and Honduras (Ragone 2007; Breadfruit Conservation Strategy 2007). Additional 33 accessions, including 24 duplicates from the NTBG collection, are maintained in the USDA/ARS National Plant Germplasm System at the Pacific Basin Tropical Plant Genetic Resources Management Unit in Hawaii and the National Germplasm Repository in Puerto Rico (Breadfruit Conservation Strategy 2007). There are other collections in Vanuatu (36 accessions), Samoa (200 collections from 14 countries), the University of the West Indies (33 accessions) (Ragone 2007) and several minor collections spread across the Pacific, Caribbean and West Africa (Breadfruit Conservation Strategy 2007). Morphological and molecular characterization of breadfruit collections and cultivars reveal a complex origin (Zerega et al. 2004) and high diversity (Sreekumar et al. 2007; Jones et al. 2012; Zerega et al. 2015).
Seedless cultivars of breadfruit, which are either triploids (2n = 3x = ~ 84) or sterile diploids (2n = 2x = 56), are an important source of starch (Zerega et al. 2004) throughout Oceania, the Caribbean islands, and some parts of Africa and Asia. Breadfruit is grown mainly for its starchy fruit, which is a rich source of carbohydrates, fiber, vitamins, minerals flavonoids and complete protein (Rincon and Padilla 2004; Ijarotimi and Aroge 2005; Ragone and Cavaletto 2006; Jones et al. 2011, 2013; Liu et al. 2015). Different parts of the plant have pharmacological (Nwokocha et al. 2012; Jalal et al. 2015; Weng et al. 2018) and insect-repelling properties (Jones et al. 2012). Although seeded breadfruit cultivars can be propagated using seeds, vegetative propagation is the preferred method. Plants raised from seeds are not always true to type and lack uniformity (Ragone 2006; Deivanai and Bhore 2010). Vegetative propagation is done using rooted shootlets or root cuttings, air layering, budding and grafting onto seedling rootstocks (Deivanai and Bhore 2010). In vitro propagation using tissue culture has been optimized and is the preferred method of germplasm exchange besides its use for mass propagation (Murch et al. 2008). Breadfruit breeding objectives include improved resistance to lodging through wind damage during typhoons and cyclones (Daley et al. 2012; Zhou and Underhill 2019), resistance/tolerance to prolonged drought stress, resistance to mealybugs and breadfruit flies (Bactrocera frauenfeldi and B. umbrosa), fruit and root rots (Phellinus noxius and Phytophthora palmivora) (Ragone 2006; Zhou et al. 2014). Crop improvement has been achieved through traditional selection from landraces resulting in unique high yielding cultivars that can be distinguished morphologically (Lincoln and Ladefoged 2014). Yields of up to 50 tons/ha have been recorded (Roberts-Nkrumah 1998) despite the lack of agronomic and breeding research (Lincoln et al. 2018, 2019; Zhou et al. 2014).
Molecular studies in breadfruit have focused on understanding its evolution, domestication and overall genetic characterization using AFLPs (Sreekumar et al. 2007), RAPDs (Ifah et al. 2018), SSRs (De Bellis et al. 2016) and SNPs (Laricchia et al. 2018). Although a draft genome sequence (Sahu et al. 2019) was recently published, we found no record of association or linkage mapping studies. The availability of the draft genome provides a great opportunity for gene discovery, trait mapping and comparative genomics.
Development of genetic and genomic resources in climate resilient orphan crops
Advances made in biotechnology and genomics, especially in next-generation sequencing (NGS), have significantly improved our understanding of orphan crops over the last two decades. Funded initiatives and web resources related to orphan crop genetics and genomics have become available (Padulosi 2017; Chiurugwi et al. 2019; Gregory et al. 2019; Jamnadass et al. 2020) leading to rapid genome characterization and candidate gene identification. There are now publicly available genome analysis tools enabling the utilization of resources from major crops for the exploitation of minor/orphan crops. There are also great opportunities to transfer the benefits of advanced breeding resources such as whole genome and transcriptome sequencing, genomic selection, genome editing and speed breeding from major crops to closely related climate resilient orphan crops. We discuss the processes and lessons to be learnt during the development of such resources in orphan crops under the following five main topics.
Genomes and transcriptomes
Over the last five years alone, 30 orphan crops representing 13 families have had their genomes sequenced (Table 1). The selection criteria for genome sequencing included importance to local food security and nutritional value (Chang et al. 2019; Hendre et al. 2019; Jamnadass et al. 2020), and tolerance to environmental stresses (Song et al. 2019; Emmrich et al. 2020). The sizes of genomes sequenced ranged from 0.217 Gb (Moringa oleifera) to about 1.5 Gb (Eleusine coracana) (Table 1), which is relatively small compared to the full range of plant genome size (Liu et al. 2019). Only 8 out of the 30 genomes sequenced were polyploids (Table 1) highlighting a possible bias towards simple genomes, especially because the sequencing was mostly done using second-generation platforms, resulting in the assembly of draft genomes. Although some of these draft genomes will be more than adequate for utilization in molecular breeding, a third-generation (PacBio; Hi-C reads) sequencing tool will be needed to improve complex genomes as has been done for tef (VanBuren et al. 2020).
Most whole genome sequencing projects are often coupled with the generation of the respective transcriptomes, which enable the full annotation of the genome generated. While the preferred transcriptome for annotation would be from the same species as was done for wild mustard (Brassica juncea) (Yang et al. 2016; Pati et al. 2019), the African eggplant (Song et al. 2019) and tef (VanBuren et al. 2020), there are also cases where the existing transcriptome of a close relative or a well-annotated transcriptome of a model crop has been used due to resource limitations. An earlier reference genome of finger millet, for example, was annotated using data from maize (Hittalmani et al. 2017). Other transcriptomes of orphan crops have been generated in response to specific biological questions, and the method of choice has been RNA sequencing (RNA-seq) (Ozsolak and Milos 2011). For example, Ranasinghe et al. (2019) identified 2416 differentially expressed genes while profiling for response to salt stress in quinoa (Chenopodium quinoa). In Jute-mallow, a transcription analysis was done to identify drought stress-related genes (Yang et al. 2017). Microarrays were the methods of choice for transcriptome analysis before the advent of NGS and were also applied in several orphan crops such as white lupin (Zhu et al. 2010), tef (Degu 2019), African nightshade (Solanum nigrum) (Schmidt and Baldwin 2009), wild mustard (Srivastava et al. 2015) and buckwheat (Fagopyrum esculentum) (Golisz et al. 2008) to detect expression profiles relevant to abiotic stress resilience.
Molecular markers and genomic selection
A robust set of molecular markers is an important breeding resource in all crops but is often lacking in many orphan crops. Diversity Arrays Technology (DArT) (Jaccoud et al. 2001) has been one of the most relevant methods for molecular marker development in orphan crops as it is hybridization-based and therefore does not require prior sequence information. This technology transformed the genetic characterization and linkage mapping of a number of crops that were considered orphan two decades ago including pigeonpea (Yang et al. 2006; Yang et al. 2011) and cassava (Manihot esculenta Crantz) (Hurtado et al. 2008). More recently, DArT has been combined with NGS in a procedure called DArT-sequencing (DArT-seq) (Sansaloni et al. 2011) that enables high throughput genotyping for rapid SNP discovery in many orphan crops. DArT sequencing is now being used in the characterization of many climate-resilient orphan crops including Bambara groundnut (Redjeki et al. 2020), finger millet (Dida et al. 2020), Kersting’s groundnut (Kerstingiella geocarpa) (Kafoutchoni et al. 2020), lupin (Lupinus albus) (Raman et al. 2014) and grass pea (Almeida et al. 2016).
Aside from DArT-sequencing, other restriction-associated DNA sequencing (RADseq) (Davey et al. 2011) genotyping methods including genotyping-by-sequencing (GBS) (Elshire et al. 2011) have also been exploited in the characterization and linkage mapping of orphan crops, especially where a reference genome is available as was done in white Guinea yam (Dioscorea rotundata) (Tamiru et al. 2017). These sequence-based genotyping platforms are the future of genotyping in all crops including orphan crops with no reference genomes. With the dropping costs of NGS, most of the climate resilient orphan crops will most likely have their genomes sequenced in the next decade. Where whole genome sequences will not be available, comparative genomics (Feltus et al. 2006) could be exploited alongside other tools that would enable more precise SNP calling from NGS data in the absence of reference genomes (Lu et al. 2013; Russell et al. 2014; Melo et al. 2016).
Availability of a robust set of molecular markers would pave the way for genomic selection (GS), a form of marker-assisted selection that uses dense markers covering the whole genome to estimate the breeding value of selection candidates for a quantitative trait (Goddard 2009). Genomic selection promises to increase genetic gain in crops (Voss-Fels et al. 2019) and would therefore provide an opportunity for the much-needed progress in the crop improvement of orphan crops. A lot of successes have been reported in the implementation of GS in several crops including relatively underutilized crop species such as sorghum (Fernandes et al. 2018), cassava (Ozimati et al. 2018; Torres et al. 2019) and Kersting’s groundnut (Akohoue et al. 2020). For GS to work optimally in most of the climate resilient orphan crops, there will be need to develop data analysis tools that would enable the parallel analysis of NGS genotyping data alongside high-quality phenotypic data. Luckily, several digital tools and programs exist that support orphan crops including the Breeding Management System (BMS) (Shrestha et al. 2012), which is a product of the Integrated Breeding Platform (IBP; https://www.integratedbreeding.net). There are also training programs such as the African Plant Breeding Academy (http://pba.ucdavis.edu/PBA_in_Africa/) with the goal of training orphan crop breeders in the most advanced theory and technologies for plant breeding in support of critical decisions for crop improvement.
Identification of climate smart genes in orphan crops for use in major crops
Several genes involved in the response to extreme stress conditions have been identified in orphan crops, and in some cases, used to improve major crops, or functionally validated in model crops such as Arabidopsis thaliana or Nicotiana tabacum (Table 2). Majority of the genes reported for drought and/or salt stress are transcription factors, which are known to play important roles in regulating response of plants to abiotic stresses (Joshi et al. 2016; van Zelm et al. 2020). BjDREB1B, a DREB gene cloned from Brassica juncea, led to the accumulation of higher levels of free proline in tobacco confirming its role in response to drought and salinity (Cong et al. 2008). Another DREB transcription factor, VrDREB2A, cloned from mung bean (Vigna radiata), significantly increased the tolerance of transgenic Arabidopsis plants to drought and salt stresses (Chen et al. 2016).
Management of pests and diseases has also benefitted from genes and other resources identified from orphan crops. Within the Solanaceae family, resistance to Fusarium oxysporium f. sp. melongenae (Rizza et al. 2002; Toppino et al. 2008) in brinjal eggplant was introduced from the African eggplant, while resistance to late blight (Phytophthora infestans) in potato (Solanum tuberosum) has been traditionally managed through the introgression of major genes from underutilized relatives (Ross 1986; Gebhardt and Valkonen 2001; Van Der Vossen et al. 2003; Ghislain et al. 2019). A recent whole genome analysis confirmed the abundance of disease resistance genes in the African eggplant (Song et al. 2019), making it a great resource for future introgression and R gene cloning. The African rice (Oryza glaberrima Steud.), an underutilized rice species cultivated in West Africa, is a major source of resistance to Rice yellow mottle virus (RYMV) (Pidon et al. 2017), bacterial blight (Xanthomonas oryzae pv. Oryzae) (Neelam et al. 2020), blast disease (Magnaporthe oryzae) (Dong et al. 2020), green rice leafhopper (Nephotettix nigropictus) (Fujita et al. 2010), as well as rice gall midge (Orseolia oryzae) (Ukwungwu et al. 1998) to the Asian rice.
An Ascorbate peroxidase (APX) gene cloned from yam (Dioscorea alata) was shown to enhance tolerance to flood and chilling stresses in transgenic Arabidopsis (Rosa et al. 2010; Bonifacio et al. 2011; Chen et al. 2019). A high-quality reference genome of mung bean enabled fast identification of synteny blocks associated with seed weight and nematode resistance through comparative analysis with soybean (Glycine max) and led to the development of functional markers for mung bean (Kang et al. 2014).
Genome editing
Genome editing is a conventional method that is applied to alter the genotype and phenotype of organisms (Zhang et al. 2017) and involves the exploitation of both natural and induced mutations in crop improvement. Several genome editing tools are now available (Please see Hassanin et al. 2019) although the clustered regularly interspaced short palindromic repeats (CRISPR)–CRISPR-associated protein-9 nuclease (Cas9) (CRISPR–Cas9) (Doudna and Charpentier 2014) is the most common. Genome editing might be used to rapidly modify undesirable traits in orphan plants and accelerate the process of domestication. This can be through the reduction of the plant content of secondary metabolites, which are often toxic (Jørgensen et al. 2005; Østerberg et al. 2017). While genome editing using CRISPR-Cas9 has been suggested as a promising method for improving domestication syndrome traits in both diploids (Lemmon et al. 2018) and polyploids (Tripathi et al. 2019; Zaman et al. 2019), there are some limiting factors in orphan crops, including lack of a well-annotated genome, sub-optimal tissue culture regeneration protocols and lack of genetic transformation methods. For closely related crops, some of these techniques could be transferred from model and/or well-studied crops and replicated to achieve similar results in promising orphan crops in a fraction of the time that it took for the major crops (Chiurugwi et al. 2019; Pareek et al. 2020). Lemmon et al. (2009) successfully applied genome editing using CRISPR-Cas9 in a tomato wild relative, groundcherry (Physalis pruinosa) and improved domestication and productivity traits. An efficient CRISPR/Cas9‐based genome editing has also been established for banana (Kaur et al. 2017), a polyploid that is relatively under-researched, making it a good example for other orphan crops with complex genomes.
Speed breeding
A recent review highlights speed breeding as one of the key technologies that would revolutionize the breeding of orphan crops (Chiurugwi et al. 2019). Proven methods of shortening the growth cycle of crops include the combination of at least two of the following; extending the duration of exposure to light (Ghosh et al. 2018), improved hand pollination and emasculation techniques (Stetter et al. 2016), growing the crops in a growth chamber (Ghosh et al. 2018), doubled haploidy (Chaudhary et al. 2019), optimal temperatures and humidity (Connor et al. 2013) and early seed harvest (Ghosh et al. 2018). Speed breeding protocols have been optimized for cereals such as wheat (Triticum aestivum L.) (Alahmad et al. 2018) and rice (Oryza sativa L.) (Ohnishi et al. 2011), and legumes including groundnut (Arachis hypogaea L.) (O'Connor et al. 2013) and chickpea (Cicer arietinum L.) (Samineni et al. 2020). These existing protocols could be tested in closely related orphan crops, perhaps with minor modifications. There is already a rapid production protocol for grain amaranths, which was developed through a combination of controlled growth conditions and efficient crossing methods (Stetter et al. 2016). Other protocols have been developed for grass pea and quinoa, which could soon lead to new varieties (Ghosh et al. 2018). Speed breeding, therefore, looks very promising and could be implemented right away to enhance orphan crops.
Challenges in breeding climate smart orphan crops
Lack of research investments
Despite the demonstrated importance of breeding orphan crops for climate change resilience and the progress that is being made, orphan crops still face several investment challenges. Most national, private sector and international agricultural research funding is skewed toward major crops (Naluwairo 2011). Rice, maize and wheat remain the highest priority crops in most countries (Shiferaw et al. 2013; Shrestha et al. 2019). The methodologies that are currently being used for priority setting for agricultural research investment rely on areas of production and numbers of beneficiaries, which often leave out climate smart orphan crops that may be the only source of livelihoods for some of the most vulnerable populations. A different framework for prioritizing agricultural research investment needs to be considered. There is increasing evidence of reductions in future productivity of major crops due to climate change (Lizumi and Ramankutty 2016; Zhao et al. 2017) that should be used to justify more investments in climate resilient orphan crops.
A better investment plan would enhance the development of genetic and genomic resources, improve crop breeding and enable the exploitation of the demonstrated benefits under different climate change scenarios. The first priority would be to train breeders in the use of advanced breeding tools, as is currently being done by the African Plant Breeding Academy. Functional networks of breeders working on same crops or addressing the same challenges would need to be formed to provide learning platforms that would reduce duplication of activities and enhance utilization of funds invested. The implementation of digital tools that would enhance the proper utilization and interpretation of both genotypic and phenotypic data will also need to be done before advanced breeding methods such as GS and speed breeding are implemented. The use of additional modern tools such as bioinformatics (Armstead et al. 2009), GS, mutational R gene enrichment sequencing (MutRenSeq; Steuernagel et al. 2016); genome editing (Lemmon et al. 2018), high throughput phenotyping (Mir et al. 2019) and nanotechnology (Jan et al. 2020) would make rapid improvement of orphan crops possible.
Collection, documentation and characterization of germplasm
The rich diversity that exists in the majority of these orphan crops is threatened with extinction unless the germplasm is conserved and fully characterized (Bhattacharjee 2009). There have been some national and international efforts (Sogbohossou et al. 2018; Daley et al. 2020; http://www.ntbg.org/breadfruit/) to conserve a few orphan crops, but in most cases, the germplasm collections are not optimum and lacking full genetic characterization. An evaluation of the world's largest breadfruit germplasm collection found that approximately 50% of the typical East Polynesian seedless triploid cultivars were represented by a single genotype (Zerega et al. 2015). The genetic gains made from the Green Revolution (Evenson and Gollin 2003) have been attributed to the conservation, characterization and exchange of germplasm (Pingali 2012). Both in situ and ex situ germplasm collections will be required followed by full characterization to enable successful crop improvement.
Underdeveloped extension services and seed systems
Agricultural extension services in most countries have been built around a few major crops and may be minimal or non-existent for orphan crops. Yet these services have been described as the main conduit for disseminating information on farm technologies, support rural adult learning and assist farmers in developing their farm technical and managerial skills (Danso-Abbeam et al. 2018). Extension agents also serve as feedback channels between farmers and the global research community with respect to proven best practices (Kabunga et al. 2011). In some cases, these services have been digitized and therefore capable of reaching farmers using mobile phones in some of the remotest of villages (Fu and Akter 2016), as long as the verified information on the target crop is available. There is need to structure extension services by region and target crops in order to provide the relevant information for orphan crops as well, especially those that are climate resilient and form a major part of livelihoods in specific regions. For example, teff is the most important cereal crop in Ethiopia (VanBuren et al. 2020), but still lacking adequate extension services (Teshome and Tegegne 2020) beyond the delivery of a package with improved variety and fertilizer (Abraham 2015).
Related to extension services are the seed systems. Orphan crops generally have underdeveloped seed systems (Mabhaudhi et al. 2019) that result in the recycling of poor quality seeds and subsequently, extremely low yields. For vegetatively propagated orphan crops, development of rapid regeneration protocols under sterile laboratory conditions may be necessary as has been done for breadfruit (Murch et al. 2008) to ensure the distribution of disease-free quality seedlings to farmers. This takes time and resources and would need the establishment of special laboratories and trained personnel. But even for sexually propagated orphan crops, different forms of quality seed supply should be tested and regulated to suit the needs of the crop and the targeted agro-ecologies (Ahmed et al. 2009).
Marketing
The value chains for orphan crops are not well developed resulting in poor quality products that may be unattractive to the end-user and fetching way below the true value. Finger millet in East Africa, for example, can be processed and marketed as a high value malt drink or in the baking and breakfast cereals industry but is instead mainly marketed for household porridge preparation. Teff value chain is often described as untraceable (Amentae et al. 2016) and lacking in value addition (Lee 2018). The increasing demand for healthy products in the west and among the growing middle and upper class in developing nations has the potential to drive the demand for healthy orphan crops. However, value addition, better presentation and packaging (Opole 2019) will be needed for these products to appeal to consumers.
Future perspectives
In order to reduce the impact of climate change, there is a need to shift away from global dependence on a limited number of crop species (Mayes et al. 2012). Several orphan crops continue to make a difference in the livelihoods of many households, especially with the increasing effects of climate change. Some crops such as cassava (ICGMC 2015), a perennial with extensive root systems, and chickpea (Garg et al. 2011; Jain et al. 2013), that were considered orphan crops two decades ago, are poised to become the new major crops under the future low inputs climate smart agriculture (CSA) regime. The next-generation crop plants need to be water and nutrient use efficient and have sustainable yields over a wider range of environmental conditions (Pareek et al. 2020). The potential that has been observed in several of these orphan crops will need to be translated for regular profitable production by an average farmer by improving their genetics and agronomy to meet the global demands. The wide range of tools and techniques to enhance sustainable crop production and resilience to climate change that have been developed for major crops will need to be tested and validated for use in orphan crops to fast track their performance. Conventional breeding alongside advanced tools such as GS, speed breeding and genome editing will play a big role in accelerating the process of domestication, through the reduction of toxic plant content (Jørgensen et al. 2005; Østerberg et al. 2017) and enhancing of phenotypes for better yields under climate smart agriculture (Chandrasekaran et al. 2016; Li et al. 2017; Lu and Jian-Kang 2017; Pareek et al. 2020). Value addition, better presentation and packaging of these crops and their products will go a long way in enhancing their adoption, especially with the increasing interest in healthy foods and the need to protect environments through the production of climate-smart crops. The success of future climate resilient crops will require a multidisciplinary research effort and multi-stakeholder funding prioritization.
Change history
22 July 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00122-021-03904-0
References
Abbasi D, Rouzbehan Y, Rezaei J (2012) Effect of harvest date and nitrogen fertilization rate on the nutritive value of amaranth forage (Amaranthus hypochondriacus). Anim Feed Sci Technol 171:6–13
Abraham R (2015) Achieving food security in ethiopia by promoting productivity of future world food tef: a review. Adv Plants Agric Res 2:00045
Abraham K, Nair GP (1990) Floral biology and artificial pollination in Dioscorea alata L. Euphytica 48:45–51. https://doi.org/10.1007/BF00028959
Abrouk M, Ahmed HI, Cubry P, Simonikova D et al (2020) Fonio millet genome unlocks African orphan crop diversity for agriculture in a changing climate. BioRxiv. https://doi.org/10.1101/2020.04.11.037671
Achten WM, Mathijs E, Verchot L, Singh VP et al (2007) Jatropha biodiesel fueling sustainability? Biofuels Bioprod Biorefining 1:283–291. https://doi.org/10.1002/bbb.39
Acquadro A, Barchi L, Gramazio P, Portis E et al (2017) Coding SNPs analysis highlights genetic relationships and evolution pattern in eggplant complexes. PLoS ONE 12:1–20. https://doi.org/10.1371/journal.pone.0180774
Adeniji OT, Kusolwa PM, Reuben SOWM (2012) Genetic diversity among accessions of Solanum aethiopicum L. groups based on morpho-agronomic traits. Plant Genet Resour Charact Util 10:177–185. https://doi.org/10.1017/S1479262112000226
Aditya M, Bhattacharjee S (2018) Foliar anti-diabetic and antioxidant potential of a promising accession of Amaranthus hypochondriacus L.: GC-MS based evidences. J Phytopharmacol 7:121–126
Afful NT, Nyadanu D, Akromah R, Amoatey HM, Annor C, Diawouh RG (2018) Evaluation of crossability studies between selected eggplant accessions with wild relatives S. torvum, S. anguivi and S. aethopicum (Shum group). J Plant Breed Crop Sci 10:1–2
Ahmed HMI, Gregg BR, Louwaars NP (2009) Seed systems for underutilized crops. Acta Hortic 806:459–464. https://doi.org/10.17660/ActaHortic.2009.806.
Ajayi OB, Oyetayo FL (2009) Potentials of Kerstingiella geocarpa as a health food. J Med Food 12:184–187. https://doi.org/10.1089/jmf.2008.0100
Akohoue F, Achigan-Dako EG, Sneller C, Van Deynze A, Sibiya J (2020) Genetic diversity, SNP-trait associations and genomic selection accuracy in a west African collection of Kersting’s groundnut [Macrotyloma geocarpum(Harms) Maréchal and Baudet]. PLoS ONE 15(6):e0234769. https://doi.org/10.1371/journal.pone.0234769
Akpapunam MA, Sefa-Dedeh S (1997) Jack bean (Canavalia ensiformis): nutrition related aspects and needed nutrition research. Plant Foods Hum Nutr 50:93–99. https://doi.org/10.1007/BF02436029
Alahmad S, Dinglasan E, Leung KM, Riaz A, Derbal N, Voss-Fels KP, Able JA, Bassi FM, Christopher J, Hickey LT (2018) Speed breeding for multiple quantitative traits in durum wheat. Plant methods 14(1):36
Allkin R (1986) Names and synonyms of species and subspecies in the Vicieae: issue 3 Vicieae database project. University of Southampton Biology Department, Southampton
Almeida NF, Gonçalves L, Lourenço M, Julião N, Aznar-Fernández T, Rubiales D, Vaz Patto MC (2016) The pursuit of resistance sources to biotic stresses in Lathyrus sativus. In: 2nd International Legume Society Conference. Tróia, Portugal, 11–14 Oct 2016.
Amentae TK, Tura EG, Gebresenbet G, Ljungberg D (2016) Exploring value chain and post-harvest losses of teff in Bacho and Dawo districts of central Ethiopia. J Stored Prod Postharvest Res 7:11–28
Andueza-Noh RH, Martínez-Castillo J, Chacón-Sánchez MI (2015) Domestication of small-seeded lima bean (Phaseolus lunatus L.) landraces in Mesoamerica: evidence from microsatellite markers. Genetica 143:657–669. https://doi.org/10.1007/s10709-015-9863-0
Aneja B, Yadav NR, Kumar N, Yadav RC (2015) Hsp transcript induction is correlated with physiological changes under drought stress in Indian mustard. Physiol Mol Biol Plants 21:305–316. https://doi.org/10.1007/s12298-015-0305-3
Anosike CA, Obidoa O, Ezeanyika LU (2012) The anti-inflammatory activity of garden egg (Solanum aethiopicum) on egg albumin-induced oedema and granuloma tissue formation in rats. Asian Pac J Tropical Med 5:62–66
Armstead I, Huang L, Ravagnani A, Robson P, Ougham H (2009) Bioinformatics in the orphan crops. Brief Bioinform 10:645–653. https://doi.org/10.1093/bib/bbp036
Arya L, Verma M, Gupta VK, Seetharam A (2013) Use of genomic and genic SSR markers for assessing genetic diversity and population structure in Indian and African finger millet (Eleusine coracana (L.) Gaertn.) germplasm. Plant Syst Evol 299:1395–1401
Asare EO, Shehu Y, Agishi EA (1984) Prelimininary studies on indigenous species for dry season grazing in the northern savanna zone of Nigeria. Trop Grassl 18:148–152
Ayanlade A, Radeny M, Morton JF, Muchaba T (2018) Rainfall variability and drought characteristics in two agro-climatic zones: an assessment of climate change challenges in Africa. Sci Tot Env 630:728–737. https://doi.org/10.1016/j.scitotenv.2018.02.196
Ayenan MAT, Ezin VA (2016) Potential of Kersting’s groundnut (Macrotyloma geocarpum Harms) Maréchal and Baudet and prospects for its promotion. Agric Food Secur 5:2–9. https://doi.org/10.1186/s40066-016-0058-4
Babitha KC, Vemanna RS, Nataraja KN, Udayakumar M (2015a) Overexpression of EcbHLH57 transcription factor from Eleusine coracana L. in tobacco confers tolerance to salt, oxidative and drought stress. PLoS ONE 10:1–21. https://doi.org/10.1371/journal.pone.0137098
Babitha KC, Ramu SV, Nataraja KN, Sheshshayee MS, Udayakumar M (2015b) EcbZIP60, a basic leucine zipper transcription factor from Eleusine coracana L. improves abiotic stress tolerance in tobacco by activating unfolded protein response pathway. Mol Breed 35:1–17. https://doi.org/10.1007/s11032-015-0374-6
Baboli ZM, Safe Kordi AA (2010) Characteristics and composition of watermelon seed oil and solvent extraction parameters effects. J Am Oil Chem Soc 87:667–671. https://doi.org/10.1007/s11746-010-1546-5
Babu BK, Dinesh P, Agrawal PK, Sood S et al (2014a) Comparative genomics and association mapping approaches for blast resistant genes in finger millet using SSRs. PLoS ONE 9(6):e99182. https://doi.org/10.1371/journal.pone.0099182
Babu BK, Agrawal PK, Pandey D, Jaiswal JP, Kumar A (2014b) Association mapping of agro-morphological characters among the global collection of finger millet genotypes using genomic SSR markers. Mol Biol Rep 41:5287–5297. https://doi.org/10.1007/s11033-014-3400-6
Babu BK, Agrawal PK, Pandey D, Kumar A (2014c) Comparative genomics and association mapping approaches for opaque2 modifier genes in finger millet accessions using genic, genomic and candidate gene-based simple sequence repeat markers. Mol Breed 34:1261–1279. https://doi.org/10.1007/s11032-014-0115-2
Bae MJ, Kim YS, Kim IS, Choe YH, Lee EJ, Kim YH, Park HM, Yoon HS (2013) Transgenic rice overexpressing the Brassica juncea gamma-glutamylcysteine synthetase gene enhances tolerance to abiotic stress and improves grain yield under paddy field conditions. Mol Breed 31:931–945. https://doi.org/10.1007/s11032-013-9846-8
Bálint M, Domisch S, Engelhardt CHM, Haase P, Lehrian S, Sauer J, Theissinger K, Pauls S, Nowak C (2011) Cryptic biodiversity loss linked to global climate change. Nat Clim Chang 1:313–318. https://doi.org/10.1038/NCLIMATE1191
Barone M, Tulumello R (2020) Grass pea the β-ODAP toxin and neurolathyrism health and safety considerations. Lathyrus sativus and Nutrition 2020. Springer, Cham, pp 45–53
Bekkering CS, Tian L (2019) Thinking outside of the cereal box: breeding underutilized (pseudo) cereals for improved human nutrition. Front Genet 10:1–7. https://doi.org/10.3389/fgene.2019.01289
Bezerraa KG, Durvala IJ, Silvab IA, Fabiola CG (2020) Emulsifying capacity of biosurfactants from Chenopodium quinoa and Pseudomonas aeruginosa UCP 0992 with focus of application in the cosmetic Industry. Chem Eng 79:211–216
Bhartiya A, Aditya J, Pal R, Bajeli J (2017) Agromorphological, nutritional and antioxidant properties in horsegram [Macrotyloma Uniflorum (Lam.) Verdc.] Germplasm collection from diverse altitudinal range of North Western Himalayan hills of India. Vegetos 30:1–7. https://doi.org/10.4172/2229-4473.1000215
Bhatt ID, Dauthal P, Rawat S, Gaira KS et al (2012) Characterization of essential oil composition, phenolic content, and antioxidant properties in wild and planted individuals of Valeriana jatamansi Jones. Sci Hortic (Amsterdam) 136:61–68. https://doi.org/10.1016/j.scienta.2011.12.032
Bhattacharjee R (2009) Harnessing biotechnology for conservation and increased utilization of orphan crops. Atdf J 6:24–33
Bonifacio A, Martins MO, Ribeiro CW, Fontenele AV, Carvalho FEL, Margis-Pinheiro M, Silveira JAG (2011) Role of peroxidases in the compensation of cytosolic ascorbate peroxidase knockdown in rice plants under abiotic stress. Plant Cell Environ 34:1705–1722. https://doi.org/10.1111/j.1365-3040.2011.02366.x
Borelli T, Hunter D, Padulosi S, Amaya N, Meldrum G, de Oliveira Beltrame DM, Samarasinghe G, Wasike VW, Güner B, Tan A, Dembélé YK, Lochetti G, Sidibé A, Tartanac F (2020) Local solutions for sustainable food systems: the contribution of orphan crops and wild edible species. Agronomy 10:1–25. https://doi.org/10.3390/agronomy10020231
Borrell JS, Biswas MK, Goodwin M, Blomme G (2019) Enset in Ethiopia: a poorly characterized but resilient starch staple. Ann Bot 123:747–766. https://doi.org/10.1093/aob/mcy214
Bray EA, Bailey-Serres J, Weretilnyk E (2000) Responses to abiotic stresses. In: Gruissem W, Buchannan B, Jones R (eds) Biochemistry and molecular biology of plants. American Society of Plant Biologists, Rockville, MD, pp 158–1249
Breadfruit Conservation Strategy (2007) https://cdn.croptrust.org/wp/wp-content/uploads/2019/05/Breadfruit_Strategy_FINAL_14Sept07.pdf. Last accessed 4 Dec 2020
Brenner DM (2015) Status of the Amaranthus seed collection. http://www.amaranthinstitute.org/sites/default/files/Ama%20Inst%202015%2007%20Brenner%20small.pdf. Last accessed 26 Nov 2020.
Brenner DM (2019) Registration of DB 199313, cytoplasmic male sterile grain Amaranth genetic stock. J Plant Regist 13:251–253. https://doi.org/10.3198/jpr2018.06.0042crgs
Brenner DM, Baltensperger DD, Kulakow PA, Lehmann JW, Myers RL, Slabbert MM, Sleugh BB (2010) Genetic resources and breeding in Amaranthus. In: Janick J (ed) Plant breeding reviews 19. Wiley, New York, USA, pp 227–285
Brenner DM, Johnson WG, Sprague CL et al (2013) Crop–weed hybrids are more frequent for the grain amaranth ‘Plainsman’ than for ‘D136-1.’ Genet Resour Crop Evol 60:2201–2205. https://doi.org/10.1007/s10722-013-0043-8
Bukenya Z (1994) Solanum macrocarpon: an Underutilised but potential vegetable in Uganda. In: Malawi J, Seyani H, Chikuni A (eds.) AETFAT 8th Plenary Meeting. pp 17–24
Bukenya-Ziraba R (2004) ‘Solanum anguivi Lam’. Record from Grubben GJH, Denton OA (ed) Protabase. PROTA (Plant Resources of Tropical Africa), Wageningen, The Netherlands. http://database.prota.org
Caetano BFR, de Moura NA, Almeida APS, Dias MC et al (2016) Yacon (Smallanthus sonchifolius) as a food supplement: health-promoting benefits of fructooligosaccharides. Nutrients 8:1–13. https://doi.org/10.3390/nu8070436
Campbell CG (1997) Grass pea. Lathyrus sativus L. Promoting the conservation and use of underutilized and neglected crops. 18. Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute, Rome, Italy
Carrillo-Perdomo E, Raffiot B, Ollivier D, Deulvot C et al (2019) Identification of novel sources of resistance to seed weevils (Bruchus spp.) in a faba bean germplasm collection. Front Plant Sci 9:1–12. https://doi.org/10.3389/fpls.2018.01914
Chakraborty S, Chakraborty N, Datta A (2000) Increased nutritive value of transgenic potato by expressing a nonallergenic seed albumin gene from Amaranthus hypochondriacus. Proc Natl Acad Sci U S A 97:3724–3729. https://doi.org/10.1073/pnas.97.7.3724
Chandra V, Bajpai O, Singh N (2016a) Energy crops in sustainable phytoremediation. Renew Sustain Energy Rev 54:58–73. https://doi.org/10.1016/j.rser.2015.09.078
Chandra D, Chandra S, Pallavi SAK (2016b) Review of finger millet (Eleusine coracana (L.) Gaertn): A power house of health benefiting nutrients. Food Sci Hum Wellness 5:149–155. https://doi.org/10.1016/j.fshw.2016.05.004
Chandrasekaran J, Brumin M, Wolf D, Leibman D et al (2016) Development of broad virus resistance in non-transgenic cucumber using CRISPR / Cas9 technology. Mol Plant Pathol 17:1140–1153. https://doi.org/10.1111/mpp.123757:1140-1153
Chang Y, Liu H, Liu M, Liao X et al (2018) The draft genomes of five agriculturally important African orphan crops. Gigascience 8:1–16. https://doi.org/10.1093/gigascience/giy152
Chaudhary HK, Sharma P, Manoj NV, Singh K (2019) New frontiers in chromosome elimination-mediated doubled haploidy breeding: Focus on speed breeding in bread and durum wheat. Indian J Genet 79(1 Suppl 254):263
Cheikhyoussef N, Kandawa-Schulz M, Böck R (2017) Characterization of Acanthosicyos horridus and Citrullus lanatus seed oils: two melon seed oils from Namibia used in food and cosmetics applications. 3 Biotech 7:297. https://doi.org/10.1007/s13205-017-0922-3
Chen Y, Guo H, Jiang Y, Li C, Zhao W, Tian Y (2003) Disease-tolerance of transgenic tobacco plants expressing Ah-AMP gene of Amaranthus hypochondriacus *. Prog Nat Sci 13:362–366. https://doi.org/10.1080/10020070312331343680
Chen HJ, Su CT, Lin CH, Huang GJ, Lin YH (2010) Expression of sweet potato cysteine protease SPCP2 altered developmental characteristics and stress responses in transgenic Arabidopsis plants. J Plant Physiol 167:838–847. https://doi.org/10.1016/j.jplph.2010.01.005
Chen H, Liu L, Wang L, Wang S, Cheng X (2016) VrDREB2A, a DREB-binding transcription factor from Vigna radiata, increased drought and high-salt tolerance in transgenic Arabidopsis thaliana. J Plant Res 129:263–273. https://doi.org/10.1007/s10265-015-0773-0
Chen Z, Lu HH, Hua S, Lin KH, Chen N, Zhang Y, You Z, Kuo YW, Chen SP (2019) Cloning and overexpression of the ascorbate peroxidase gene from the yam (Dioscorea alata) enhances chilling and flood tolerance in transgenic Arabidopsis. J Plant Res 132:857–866. https://doi.org/10.1007/s10265-019-01136-4
Chethan S, Malleshi NG (2007) Finger Millet Polyphenols: Characterization and their Nutraceutical Potential. Am J Food Technol 2:582–592
Chiang CM, Chen CC, Chen SP, Lin KH, Chen LR, Su YH, Yen HC (2017) Overexpression of the ascorbate peroxidase gene from eggplant and sponge gourd enhances flood tolerance in transgenic Arabidopsis. J Plant Res 130:373–386. https://doi.org/10.1007/s10265-016-0902-4
Chinedu SN, Olasumbo AC, Eboji OK, Emiloju OC, Olajumoke K, Dania DI (2011a) Proximate and phytochemical analyses of Solanum aethiopicum L. and Solanum macrocarpon L. Fruits Res J Chem Sci 1:63–71
Chinedu SN, Olasumbo AC, Eboji OK, Emiloju OC et al (2011b) Proximate and phytochemical analyses of Solanum aethiopicum L. and Solanum macrocarpon L. Fruits Res J Chem Sci 1:63–71
Chinmayee MD, Mahesh B, Pradesh S, Mini I, Swapna TS (2012) The assessment of phytoremediation potential of invasive weed Amaranthus spinosus L. Appl Biochem Biotechnol 167:1550–1559. https://doi.org/10.1007/s12010-012-9657-0
Chiurugwi T, Kemp S, Powell W, Hickey LT (2019) Speed breeding orphan crops. Theor Appl Genet 132:607–616. https://doi.org/10.1007/s00122-018-3202-7
Chowdhury MA, Slinkard AE (2000) Genetic diversity in grass pea (Lathyrus sativus L.). Genet Resour Crop Evol 47:163–169
Chowdhury S, Basu A, Kundu S (2017) Overexpression of a new osmotin-like protein gene (sindOLP) confers tolerance against biotic and abiotic stresses in sesame. Front Plant Sci 8:1–16. https://doi.org/10.3389/fpls.2017.00410
Chweya JA, Mnzava NA (1997) Cat’s Whiskers. Cleome Gynandra L, Bioversity International Vol, p 11
Collonnier C, Mulya K, Fock I, Mariska I, Servaes A, Vedel F, Siljak-Yakovlev S, Souvannavong V, Ducreux G, Sihachakr D (2001) Source of resistance against Ralstonia solanacearum in fertile somatic hybrids of eggplant (Solanum melongena L.) with Solanum aethiopicum L. Plant Sci 160:301–313
Cong L, Chai TY, Zhang YX (2008) Characterization of the novel gene BjDREB1B encoding a DRE-binding transcription factor from Brassica juncea L. Biochem Biophys Res Commun 371:702–706. https://doi.org/10.1016/j.bbrc.2008.04.126
Cui XH, Hao FS, Chen H, Chen J, Wang XC (2008) Expression of the Vicia faba VfPIP1 gene in Arabidopsis thaliana plants improves their drought resistance. J Plant Res 121:207–214. https://doi.org/10.1007/s10265-007-0130-z
Cullis C, Kunert KJ (2017) Unlocking the potential of orphan legumes. J Exp Bot 68:1895–1903. https://doi.org/10.1093/jxb/erw437
Cullis C, Chimwamurombe P, Barker N, Kunert K, Vorster J (2018) Orphan legumes growing in dry environments: marama bean as a case study. Front Plant Sci 9:1–7. https://doi.org/10.3389/fpls.2018.01199
Cullis C, Lawlor DW, Chimwamurombe P, Bbebe N, Kunert K, Vorster J (2019) Development of marama bean, an orphan legume, as a crop. Food Energy Secur 8:e00164. https://doi.org/10.1002/fes3.164
Dahiya PK, Linnemann AR, Van Boekel MAJS, Khetarpaul N et al (2015) Mung bean: Technological and Nutritional Potential. Crit Rev Food Sci Nutr 55:670–688. https://doi.org/10.1080/10408398.2012.671202
Daley O, Gloster M, Roberts-Nkrumah LB (2012) Assessment and characterization of damage by hurricane Tomas to major tree crops with special emphasis on breadfruit (Artocarpus altilis) and breadnut (Artocarpus camansi) in St. Lucia and St. Vincent and the Grenadines. Proc Caribb Food Crop Soc 48:124–131
Daley OO, Roberts-Nkrumah LB, Alleyne AT (2020) Morphological diversity of breadfruit [Artocarpus altilis (Parkinson) Fosberg] in the Caribbean. Sci Hortic 266:109278. https://doi.org/10.1016/j.scienta.2020.109278
Dansi A, Vodouhè R, Azokpota P, Yedomonhan H, Assogba P, Adjatin A, Loko YL, Dossou-Aminon I, Akpagana K (2012) Diversity of the neglected and underutilized crop species of importance in Benin. Sci World J 2012:932947. https://doi.org/10.1100/2012/932947
Danso-Abbeam G, Ehiakpor DS, Aidoo R (2018) Agricultural extension and its effects on farm productivity and income: insight from Northern Ghana. Agric & Food Secur 7:74. https://doi.org/10.1186/s40066-018-0225-x
Das S (2016) Infrageneric classification of amaranths. In: Das S (ed) Amaranthus: a promising crop of future. Singapore, Springer Nature, pp 49–56
Dauber J, Miyake S (2016) To integrate or to segregate food crop and energy crop cultivation at the landscape scale? Perspectives on biodiversity conservation in agriculture in Europe. Energ Sustain Soc 6:25. https://doi.org/10.1186/s13705-016-0089-5
Daunay MC, Lester RN, Laterrot H (1991) The use of wild species for the genetic improvement of Brinjal eggplant (Solanum melongena) and tomato (Lycopersicon esculentum). In: Hawkes JG, Lester RN, Estrada N (eds) Solanaceae III: Taxonomy-Chemistry-Evolution, vol 3. The Linnean Society of London. Royal Botanical Gardens Kew, London, UK, pp 389–412
Daunay MC, Lester R, Gebhardt C, Hennart J, Jahn M (2001) Genetic Resources of eggplant (Solanum melongena L) and allied species: a new challenge for molecular geneticists and eggplant breeders. In: Van Den Berg R, Barendse G, Mariani C (eds) Solanaceae V. Nijmegan University Press, Nijmegen The Netherlands
Davey JW, Hohenlohe PA, Etter PD, Boone JQ, Catchen JM, Blaxter ML (2011) Genome-wide genetic marker discovery and genotyping using next-generation sequencing. Nat Rev Genet 12:499–510
Davis AR, Perkins-Veazie P, Sakata Y, López-Galarza S, Maroto JV, Lee SG, Huh YC, Sun Z, Miguel A, King SR, Cohen R, Lee JM (2008) Cucurbit grafting. CRC Crit Rev Plant Sci 27:50–74. https://doi.org/10.1080/07352680802053940
Dawson I, Jaenicke H (2006) Underutilised plant species: the role of biotechnology. International Centre for Underutilized Crops, Colombo
Dawson IK, McMullin S, Kindt R, Muchugi A, Hendre P, Lillesø J-PB, Jamnadass R (2019) Delivering perennial new and orphan crops for resilient and nutritious farming systems. In: Rosenstock TS, Girvetz E, Nowak A (eds) The climate-smart agriculture papers. Springer, Cham, pp 113–125
De Bellis F, Malapa R, Kagy V, Lebegin S, Billot C, Labouisse J-P (2016) New development and validation of 50 SSR markers in breadfruit (Artocarpus altilis, Moraceae) by next-generation sequencing. Appl Plant Sci 4:apps. 1600021
Deba F, Xuan TD, Yasuda M, Tawata S (2008) Chemical composition and antioxidant, antibacterial and antifungal activities of the essential oils from Bidens pilosa Linn. var. Radiata Food Control 19:346–352. https://doi.org/10.1016/j.foodcont.2007.04.011
Degu HD (2019) Analysis of Differentially Expressed Genes. Niger J Biotechnol 36:167–187. https://doi.org/10.1017/cbo9780511615535.010
Deivanai S, Bhore SJ (2010) Breadfruit (Artocarpus altilis Fosb.) an underultilized and neglected fruit plant species. Middle-east J Sci Res 6:1–13. Available from: www.idosi.org/pdf Accessed 21 Aug 2014
Dida MM, Srinivasachary RS, Bennetzen JL et al (2007) The genetic map of finger millet, Eleusine coracana. Theor Appl Genet 114:321
Dida MM, Oduori CA, Manthi SJ, Avosa MO, Mikwa EO, Ojulong HF, Odeny DA (2020) Novel sources of resistance to blast disease in finger millet. Crop Sci. https://doi.org/10.1002/csc2.20378
Dong L, Liu S, Kyaing MS, Peng Xu et al (2020) Identification and fine mapping of Pi69(t), a new gene conferring broad-spectrum resistance against Magnaporthe oryzae from Oryza glaberrima Steud. Front Plant Sci 11:1190. https://doi.org/10.3389/fpls.2020.01190
Doudna JA, Charpentier E (2014) The new frontier of genome engineering with CRISPR-Cas9. Science 28:346
Durães FOMH, Laviola BG, Alves AA (2011) Potential and challenges in making physic nut (Jatropha curcas L.) a viable biofuel crop: the Brazilian perspective. CAB Rev Perspect Agric Vet Sci Nutr Nat Resour 6:1–8. https://doi.org/10.1079/PAVSNNR20116043
Elekofehinti OO, Adanlawo IG, Saliu JA, Sodehinde SA (2012) Saponins from Solanum anguivi fruits exhibit hypolipidemic potential in Rattus novergicus. Der Pharmacia Lett 4:811–814
Elekofehinti OO, Kamdem JP, Boligon AA, Athayde ML, Lopes SR, Waczuk EP et al (2013) African eggplant (Solanum anguivi Lam) fruits with bioactive polyphenolic compounds exert in vitro antioxidant properties and inhibit Ca2+ induced mitochondrial swelling. Asian Pac J Trop Biomed 3:757–766
Elekofehinti OO, Kamdem JP, Meinerz DF et al (2015) Saponin from the fruit of Solanum anguivi protects against oxidative damage mediated by Fe2+ and sodium nitroprusside in rat brain synaptosome P2 fraction. Arch Pharm Res. https://doi.org/10.1007/s12272-014-0536-9
Elshire RJ, Glaubitz JC, Sun Q, Poland JA, Kawamoto K, Buckler ES et al (2011) A robust, simple Genotyping-by-Sequencing (GBS) approach for high diversity species. PLoS ONE 6(5):e19379. https://doi.org/10.1371/journal.pone.0019379
Emmrich PMF, Sarkar A, Njaci I, Kaithakottil GG et al (2020) A draft genome of grass pea (Lathyrus sativus) a resilient diploid legume. BioRxiv. https://doi.org/10.1101/2020.04.24.058164
Evenson RE, Gollin D (2003) Assessing the impact of the Green Revolution, 1960 to 2000. Science 300:758–762. https://doi.org/10.1126/science.1078710
Ezeh O, Gordon MH, Niranjan K (2014) Tiger nut oil (Cyperus esculentus L.): a review of its composition and physico-chemical properties. Eur J Lipid Sci Technol 116:783–794
Ezugwu C, Okonta J, Nwodo N (2005) Antidiabetic properties of ethanolic fruit extract of Solanium aethiopicum L. J Pharm Allied Sci 2:251–254. https://doi.org/10.4314/jophas.v2i2.34986
Fan W, Lou HQ, Gong YL, Liu MY et al (2015) Characterization of an inducible C2H2-type zinc finger transcription factor VuSTOP1 in rice bean (Vigna umbellata) reveals differential regulation between low pH and aluminum tolerance mechanisms. New Phytol 208:456–468. https://doi.org/10.1111/nph.13456
Fang ZW, Xu XY, Gao JF, Wang PK, Liu ZX, Feng BL (2015) Characterization of FeDREB1 promoter involved in cold- and drought-inducible expression from common buckwheat (Fagopyrum esculentum). Genet Mol Res 14:7990–8000. https://doi.org/10.4238/2015.July.17.7
FAO (2015) Coping with climate change—the roles of genetic resources for food and agriculture. Rome
Feltus FA, Singh HP, Lohithaswa HC, Schulze SR, Silva TD, Paterson AH (2006) A comparative genomics strategy for targeted discovery of single-nucleotide polymorphisms and conserved-noncoding sequences in orphan crops. Plant Physiol 140(4):1183–1191
Fernandes SB, Dias KOG, Ferreira DF, Brown PJ (2018) Efficiency of multi-trait, indirect, and trait-assisted genomic selection for improvement of biomass sorghum. Theor Appl Genet 131(3):747–755. https://doi.org/10.1007/s00122-017-3033-y
Feulner G (2017) Global challenges: climate change. Glob Chall 1:5–6. https://doi.org/10.1002/gch2.1003
Fu X, Akter S (2016) The Impact of mobile phone technology on agricultural extension services delivery: Evidence from India. J Dev Studies 52:1561–1576. https://doi.org/10.1080/00220388.2016.1146700
Fujita D, Doi K, Yoshimura A, Yasui H (2010) A major QTL for resistance to green rice leafhopper (Nephotettix cincticeps Uhler) derived from African rice (Oryza glaberrima Steud.). Breed Sci 60:336–341. https://doi.org/10.1270/jsbbs.60.336
Gao F, Zhou J, Deng RY, Zhao HX et al (2017) Overexpression of a tartary buckwheat R2R3-MYB transcription factor gene, FtMYB9, enhances tolerance to drought and salt stresses in transgenic Arabidopsis. J Plant Physiol 214:1–28. https://doi.org/10.1016/j.jplph.2017.04.007
Garg R, Patel RK, Tyagi AK, Jain M (2011) De novo assembly of chickpea transcriptome using short reads for gene discovery and marker identification. DNA Res 18:53–63. https://doi.org/10.1093/dnares/dsq028
Gbile Z, Adesina S (1988) Nigerian Solanum species of economic importance. Ann Missouri Bot Gard 75:862–865
Gebhardt C, Valkonen JPT (2001) Organization of genes controlling disease resistance in the potato genome. Annu Rev Phytopathol 39:79–102
GENESYS (2017) The Global Gateway to Genetic Resources. Available online at: https://www.genesys-pgr.org (Accessed December 6th, 2020).
Ghislain M, Byarugaba AA, Magembe E, Njoroge A et al (2019) Stacking three late blight resistance genes from wild species directly into African highland potato varieties confers complete field resistance to local blight races. Plant Biotechnol J 17:1119–1129. https://doi.org/10.1111/pbi.13042
Ghorbel M, Marghali S, Trifi-Farah N, Chtourou-Ghorbel N (2014) Phylogeny of Mediterranean Lathyrus species using Inter Simple Sequence Repeats markers. Acta Bot Gall 161:91–98. https://doi.org/10.1080/12538078.2013.878854
Ghosh S, Watson A, Gonzalez-Navarro OE et al (2018) Speed breeding in growth chambers and glasshouses for crop breeding and model plant research. Nat Protoc 13:2944–2963. https://doi.org/10.1038/s41596-018-0072-z
Girma D, Korbu L (2012) Review of genetic improvement of grass pea (Lathyrus sativus) in Ethiopia : an unfulfilled promise. Plant Breed 131:231–236. https://doi.org/10.1111/j.1439-0523.2011.01935.x
Gnanaraj M, Udhayakumar N, Rajiv Gandhi R, Manoharan K (2015) Isolation and gene expression analysis of phospholipase C in response to abiotic stresses from Vigna radiata (L.) Wilczek. Indian J Exp Biol 53:335–341
Goddard M (2009) Genomic selection: prediction of accuracy and maximisation of long term response. Genetica 136:245–257. https://doi.org/10.1007/s10709-008-9308-0
Golisz A, Sugano M, Fujii Y (2008) Microarray expression profiling of Arabidopsis thaliana L. in response to allelochemicals identified in buckwheat. J Exp Bot 59:3099–3109. https://doi.org/10.1093/jxb/ern168
Gramazio P, Blanca J, Ziarsolo P et al (2016c) Transcriptome analysis and molecular marker discovery in Solanum incanum and S. aethiopicum two close relatives of the common eggplant (Solanum melongena) with interest for breeding. BMC Genom 17:300. https://doi.org/10.1186/s12864-016-2631-4
Granati E, Bisignano V, Chiaretti D, Crino P et al (2003) Characterization of Italian and exotic Lathyrus germplasm for quality traits. Genet Resour Crop Evol 50:273–280
Gregory PJ, Mayes S, Hui CH, Jahanshiri E, Julkifle A, Kuppusamy G, Kuan HW, Lin TX, Massawe F, Suhairi TASTM, Azam-Ali SN (2019) Crops For the Future (CFF): an overview of research efforts in the adoption of underutilised species. Planta 250:979–988. https://doi.org/10.1007/s00425-019-03179-2
Grubben GJH, Denton OA (2004) Plant resources of tropical Africa 2. Vegetables, PROTA Foundation, Netherlands, p 668
Gupta VK, Gudu S (1990) Inheritance of some morphological traits in grain amaranthus. Euphytica 46:79–84. https://doi.org/10.1007/BF00057621
Habiyaremye C, Matanguihan JB, D’Alpoim Guedes J, Ganjyal GM et al (2017) Proso millet (Panicum miliaceum L.) and its potential for cultivation in the Pacific Northwest, U.S.: A review. Front Plant Sci 7:1–17. https://doi.org/10.3389/fpls.2016.01961
Hanbury CD, White CL, Mullan BP, Siddique KHM (2000) A review of the potential of Lathyrus sativus L. and L. cicera L. grain for use as animal feed. Anim Feed Sci Technol 87:1–27. https://doi.org/10.1016/S0377-8401(00)00186-3
Hanson J, Street K (2008) Regeneration Guidelines: grass pea. In: Dulloo M, Thormann I, Jorge M, Hanson J (eds) Crop Specific regeneration guidelines [CD-ROM]. CGIAR System-wide Genetic Resource Programme, Rome, Italy, p 9
Hao X, Yang T, Liu R, Hu J et al (2017) An RNA sequencing transcriptome analysis of grass pea (Lathyrus sativus L) and development of SSR and KASP Markers. Front Plant Sci 8:1873. https://doi.org/10.3389/fpls.2017.01873
Hassanin HAM, Koko M, Abdalla M, Mu W, Jiang B (2019) Detarium microcarpum: A novel source of nutrition and medicine: A review. Food Chem 274:900–906. https://doi.org/10.1016/j.foodchem.2018.09.070
Hendre PS, Muthemba S, Kariba R, Muchugi A, Fu Y, Chang Y, Song B, Liu H, Liu M, Liao X, Sahu SK, Wang S, Li L, Lu H, Peng S, Cheng S, Xu X, Yang H, Wang J, Liu X, Simons A, Shapiro HY, Mumm RH, Van Deynze A, Jamnadass R (2019) African Orphan crops consortium (AOCC): status of developing genomic resources for African orphan crops. Planta 250:989–1003. https://doi.org/10.1007/s00425-019-03156-9
Hillocks R, Maruthi MN (2012) Grass pea (Lathyrus sativus): Is there a case for further crop improvement? Euphytica. https://doi.org/10.1007/s10681-012-0702-4
Hinojosa L, González JA, Barrios-Masias FH, Fuentes F, Murphy KM (2018) Quinoa abiotic stress responses: a review. Plants 7:1–32. https://doi.org/10.3390/plants7040106
Hittalmani S, Mahesh HB, Shirke MD, Biradar H et al (2017) Genome and transcriptome sequence of Finger millet (Eleusine coracana (L.) Gaertn.) provides insights into drought tolerance and nutraceutical properties. BMC Genom 18:465
Hooper AM, Tsanuo MK, Chamberlain K, Tittcomb K, Scholes J, Hassanali A, Khan ZR, Pickett JA (2010) Isoschaftoside, a C-glycosylflavonoid from Desmodium uncinatum root exudate, is an allelochemical against the development of Striga. Phytochemistry 71:904–908. https://doi.org/10.1016/j.phytochem.2010.02.015
Houdegbe CA, Sogbohossou OED, Achigan-Dako EG (2016) Utilization and breeding perspective in the egusi gourd Melothria sphaerocarpa (Cogn.) H. Schaef. et S.S. Renner (syn: Cucumeropsis mannii Naudin). Genet Resour Crop Evol 63:545–559. https://doi.org/10.1007/s10722-015-0361-0
Huang Y, Zhao H, Gao F, Yao P, Deng R, Li C, Chen H, Wu Q (2018) A R2R3-MYB transcription factor gene, FtMYB13, from tartary buckwheat improves salt/drought tolerance in Arabidopsis. Plant Physiol Biochem 132:238–248. https://doi.org/10.1016/j.plaphy.2018.09.012
Hunter D, Borelli T, Beltrame DMO, Oliveira CNS et al (2019) The potential of neglected and underutilized species for improving diets and nutrition. Planta 250:709–729. https://doi.org/10.1007/s00425-019-03169-4
Hurtado P, Olsen KM, Buitrago C, Ospina C, Marin J, Duque M, de Vicente C, Wongtiem P, Killian A, Adeleke M, Fregene M (2008) Comparison of simple sequence repeat (SSR) and diversity array technology (DArT) markers for assessing genetic diversity in cassava (Manihot esculenta Crantz). Plant Genet Resources 6(3):208–214
Hyland HL (1968) Plant Inventory No 171. USDA, Washington, D.C.
Hymowitz T, Boyd J (1977) Origin, Ethnobotany and Agricultural Potential of the Winged Bean: Psophocarpus tetragonolobus. Published by : Springer on behalf of New York Botanical Garden Press Origin, Ethnobotany and Agricultural Potential of the Winged Bean- Psophocarpus tetragon. Econ Bot 31:180–188
ICGMC (2015) High-Resolution Linkage Map and Chromosome-Scale Genome Assembly for Cassava (Manihot esculenta Crantz) from 10 Populations. Genes Genome Genet 5:133–144. https://doi.org/10.1534/g3.114.015008
Ifah AA, Yuniastuti E, Parjanto S (2018) Analysis of breadfruit plant diversity (Artocarpus altilis P.) by random amplified polymorphic DNA (RAPD) in DIY. AIP Conf Proc. https://doi.org/10.1063/1.5062802
Ijarotimi SO, Aroge F (2005) Evaluation of the nutritional composition, sensory, and physical properties of a potential weaning food from locally available food materials-breadfruit (Artocarpus altilis) and soybean (Glycine max). Pol J Food Nutr Sci 14:411–415
Jaccoud D, Peng K, Feinstein D, Kilian A (2001) Diversity arrays: a solid state technology for sequence information independent genotyping. Nucleic Acids Res 29:e25
Jacob A, Etong I, Tijjani A (2015) Proximate, mineral and anti-nutritional compositions of Melon (Citrullus lanatus) Seeds. Br J Res 2:142–151
Jain M, Misra G, Patel RK, Priya P, Jhanwar S, Khan AW, Shah N, Singh VK, Garg R, Jeena G, Yadav M, Kant C, Sharma P, Yadav G, Bhatia S, Tyagi AK, Chattopadhyay D (2013) A draft genome sequence of the pulse crop chickpea (Cicer arietinum L.). Plant J 74:715–729. https://doi.org/10.1111/tpj.12173
Jalal TK, Ahmed IA, Mikail M et al (2015) Evaluation of antioxidant, total phenol and flavonoid content and antimicrobial activities of Artocarpus altilis (Breadfruit) of underutilized tropical fruit extracts. Appl Biochem Biotechnol 175:3231–3243. https://doi.org/10.1007/s12010-015-1499-0
Jami SK, Clark GB, Turlapati SA, Handley C, Roux SJ, Kirti PB (2008) Ectopic expression of an annexin from Brassica juncea confers tolerance to abiotic and biotic stress treatments in transgenic tobacco. Plant Physiol Biochem 46:1019–1030. https://doi.org/10.1016/j.plaphy.2008.07.006
Jamil S, Abhilash PC, Singh N, Sharma PN (2009) Jatropha curcas: A potential crop for phytoremediation of coal fly ash. J harzadous Mater 172:269–275
Jamnadass R, Mumm RH, Hale I, Hendre P, Muchugi A, Dawson IK, Powell W, Graudal L, Yana-Shapiro H, Simons AJ, Van Deynze A (2020) Enhancing African orphan crops with genomics. Nat Genet 52:356–360. https://doi.org/10.1038/s41588-020-0601-x
Jan A, Pirzadah TB, Malik B (2020) Nanotechnology: an innovative tool to enhance crop production. In: Hakeem K, Pirzadah T (eds) Nanobiotechnology in agriculture. Nanotechnol life Sci. Springer, Cham
Jarvis A, Upadhyaya H, Gowda C, Aggarwal P, Fujisaka S, Anderson B (2009) Climate change and its effect on conservation and use of plant genetic resources for food and agriculture and associated biodiversity for food security. http://www.fao.org/docrep/013/i1500e/i1500e16.pdf. Accessed
Jiang J, Su M, Chen Y, Gao N, Jiao C, Sun Z, Li F, Wang C (2013) Correlation of drought resistance in grass pea (Lathyrus sativus) with reactive oxygen species scavenging and osmotic adjustment. Biol 68:231–240. https://doi.org/10.2478/s11756-013-0003-y
Jones AMP, Ragone D, Aiona K, Lane WA, Murch SJ (2011) Nutritional and morphological diversity of breadfruit (Artocarpus, Moraceae): Identification of elite cultivars for food security. J Food Comp Anal 24:1091–1102
Jones AMP, Klun JA, Cantrell CL, Ragone D, Chauhan KR, Brown PN, Murch SJ (2012) Isolation and identification of mosquito (Aedes aegypti) biting deterrent fatty acids from male inflorescences of breadfruit (Artocarpus altilis (Parkinson) Fosberg). J Agric Food Chem 60:3867–3873. https://doi.org/10.1021/jf300101w
Jones AMP, Murch SJ, Wiseman J et al (2013) Morphological diversity in breadfruit (Artocarpus, Moraceae): insights into domestication, conservation, and cultivar identification. Genet Resour Crop Evol 60:175–192. https://doi.org/10.1007/s10722-012-9824-8
Jørgensen K, Bak S, Busk PK, Sørensen C et al (2005) Cassava plants with a depleted cyanogenic glucoside content in leaves and tubers. Distribution of cyanogenic glucosides, their site of synthesis and transport, and blockage of the biosynthesis by RNA interference technology. Plant Physiol 139:363–374. https://doi.org/10.1104/pp.105.065904
Joseph SK, Bolla S, Joshi K, Bhat M et al (2017) Determination of chemical composition and nutritive value with fatty acid compositions of African Mangosteen (Garcinia Livingstonei). Erwerbs-Obstbau 59:195–202. https://doi.org/10.1007/s10341-016-0311-9
Joshi KD, Bhandari B, Gautam R, Bajracharya J, Hollington PA (2008) Ricebean: a multipurpose underutilised legume. Southampton, UK
Joshi R, Wani SH, Singh B, Bohra A, Dar ZA, Lone AA, Pareek A, Singla-Pareek SL (2016) Transcription factors and plants response to drought stress: Current understanding and future directions. Front Plant Sci 7:1–15. https://doi.org/10.3389/fpls.2016.01029
Joshi DC, Sood S, Hosahatti R et al (2018) From zero to hero: the past, present and future of grain amaranth breeding. Theor Appl Genet 131:1807–1823. https://doi.org/10.1007/s00122-018-3138-y
Kabunga NS, Dubois T, Qaim M (2011) Information asymmetries and technology adoption: the case of tissue culture bananas in Kenya. Discussion paper No. 74, Georg-August. Goettingen: Universität Göttingen
Kafoutchoni KM, Agoyi EE, Agbahoungba S, Assogbadjo AE, Agbangla C (2020) Genetic diversity and population structure in a regional collection of Kersting’s groundnut (Macrotyloma geocarpum (Harms) Maréchal and Baudet). Preprints 2020:2020090097. https://doi.org/10.20944/preprints202009.0097.v1
Kang YJ, Kim SK, Kim MY, Lestari P et al (2014) Genome sequence of mungbean and insights into evolution within Vigna species. Nat Commun 5:1–9. https://doi.org/10.1038/ncomms6443
Kaur N, Alok A, Shivani Kaur N, Pandey P, Awasthi P, Tewari S (2017) CRISPR/Cas9-mediated efficient editing in phytoene desaturase (PDS) demonstrates precise manipulation in banana cv. Rasthali genome Functional and Integrative Genomics 18:89–99
Kietlinski KD, Jimenez F, Jellen EN, Maughan PJ, Smith SM, Pratt DB (2014) Relationships between the weedy Amaranthus hybridus (Amaranthaceae) and the grain amaranths. Crop Sci 54:220–228. https://doi.org/10.2135/cropsci2013.03.0173
King AJ, Montes LR, Clarke JG, Itzep J (2015) Identification of QTL markers contributing to plant growth, oil yield and fatty acid composition in the oilseed crop Jatropha curcas L. Biotechnol Biofuels 8:1–17. https://doi.org/10.1186/s13068-015-0326-8
Kiranmai K, Lokanadha Rao G, Pandurangaiah M, Nareshkumar A et al (2018) A novel WRKY transcription factor, MuWRKY3 (Macrotyloma uniflorum Lam. Verdc.) enhances drought stress tolerance in transgenic groundnut (Arachis hypogaea L.) plants. Front Plant Sci 9:1–12. https://doi.org/10.3389/fpls.2018.00346
Kirkpatrick BA (1995) Interspecific relationships within the genus Amaranthus (Amaranthaceae). Texas A and M Univ, USA, PhD Thesis
Kislev M (1989) Origins of the cultivation of Lathyrus sativus and L. cicera (Fabaceae). Economic Bot 43:262–270
Kochummen KM (2000a) Artocarpus JR and G Forster, nom. conserv. In: Soepadmo E, Saw LG (eds) Tree flora of Sabah and Sarawak. Sabah Forestry Department, Sarawak Forestry Department, Forest Research Institute Malaysia, Kuala Lumpur, Malaysia, pp 187–212
Krishnamurthy L, Upadhyaya HD, Kashiwagi J, Purushothaman R, Dwivedi SL, Vadez V (2016) Variation in drought-tolerance components and their interrelationships in the minicore collection of finger millet germplasm. Crop Sci 56:1914–1926. https://doi.org/10.2135/cropsci2016.03.0191
Kulakow PA, Jain SK (1985) The inheritance of flowering in Amaranthus species. J Genet 64:85–100
Kulakow P, Jain S (1987) Genetics of grain amaranths. Theoret Appl Genetics 74:113–120. https://doi.org/10.1007/BF00290093
Kumar A, Metwal M, Kaur S, Gupta AK, Puranik S, Singh S, Singh M, Gupta S, Babu BK, Sood S, Yadav R (2016) Nutraceutical value of finger millet [Eleusine coracana (L.) Gaertn.], and their improvement using omics approaches. Front Plant Sci 7:1–14. https://doi.org/10.3389/fpls.2016.00934
Kunguni JS, Odeny DA, Dangasuk OG, Matasyoh LG, Oduori COA (2015) Response of elite Kenyan finger millet (Eleusine coracana, L. Gaertn) genotypes to Ethrel application. International Letters of Natural Sciences 48:43–52
Kupicha F (1983) The infrageneric structure of Lathyrus. Notes Roy Bot Gard Edinb 41:209–244
Lambein F, Travella S, Kuo YH, Van Montagu M, Heijde M (2019) Grass pea (Lathyrus sativus L.): orphan crop, nutraceutical or just plain food? Planta. https://doi.org/10.1007/s00425-018-03084-0
Laricchia KM, Johnson MG, Ragone D, Williams EW, Zerega NJC, Wickett NJ (2018c) A transcriptome screen for positive selection in domesticated breadfruit and its wild relatives (Artocarpus spp.). Am J Bot 105(5):915–926
Lebot V (2009) Tropical root and tuber crops: cassava, sweet potato, yams and aroids. CABI, Wallingford, p 413
Lebot V, Trilles B, Noyer JL, Modesto J (1998) Genetic relationships between Dioscorea alata L. cultivars. Genet Res and Crop Evol 45:499–508
Lee JR, Hong GY, Dixit A, Chung JW, Ma KH, Lee JH, Kang HK, Cho YH, Gwag JG, Park YL (2008) Characterization of microsatellite loci developed for Amaranthus hypocondiacus and their cross amplification in wild species. Conserv Genet 9:243–246
Lehman JW, Clark RL, Frey KJ (1991) Biomass heterosis and combining ability in interspecific and intraspecific matings of grain amaranths. Crop Sci 31:1111–1116
Lemmon ZH, Reem NT, Dalrymple J et al (2018) Rapid improvement of domestication traits in an orphan crop by genome editing. Nature Plants 4:766–770. https://doi.org/10.1038/s41477-018-0259-x
Lester RN (1986) Taxonomy of Scarlet Eggpants, Solanum aethiopicum L. Acta Hortic 182:123–132
Lester RN (1998) Genetic resources of capsicum and eggplant. Xth EUCARPIA meeting on genetics and breeding of capsicum and eggplant. Avignon, France, pp 25–30
Lester RN, Daunay MC (2003) Diversity of African vegetable Solanum species and its implications for a better understanding of plant domestication. In: Knüpffer H, Ochsmann J (eds) Schriften zu genetischen ressourcen. Springer, Berlin, pp 137–152
Lester RN, Niakan L (1986) Origin and domestication of the scarlet eggplant Solanum aetbiopicum from, S. anguivi in Africa. Biology and systematics. Columbia University Press, New York, pp 431–456
Lester RN, Hakiza JJH, Stavropoulos N, Teixiera MM (1986) Variation patterns in the African Scarlet eggplant, Solanum aethiopicum L. In: Styles BT (ed) Infraspecific classification of wild and cultivated plants. Oxford : Published for the Systematics Association by Clarendon Press, 1986.
Levizou E, Drilias P, Kyparissis A (2004) Exceptional photosynthetic performance of Capparis spinosa L. under adverse conditions of Mediterranean summer. Photosynthetica 42:229–235. https://doi.org/10.1023/B:PHOT.0000040594.85407.f4
Li P, Pei Y, Sang X, Ling Y, Yang Z, He G (2009) Transgenic indica rice expressing a bitter melon (Momordica charantia) class I chitinase gene (McCHIT1) confers enhanced resistance to Magnaporthe grisea and Rhizoctonia solani. Eur J Plant Pathol 125:533–543. https://doi.org/10.1007/s10658-009-9501-8
Li C, Yue J, Wu X, Xu C, Yu J (2014) An ABA-responsive DRE-binding protein gene from Setaria italica, SiARDP, the target gene of SiAREB, plays a critical role under drought stress. J Exp Bot 65:5415–5427. https://doi.org/10.1093/jxb/eru302
Li J, Sun Y, Du J, Zhao Y, Xia L (2017) Generation of targeted point mutations in rice by a modified CRISPR/Cas9 System. Mol Plant 10:526–529. https://doi.org/10.1016/j.molp.2016.12.001
Lightfoot DJ, Jarvis DE, Ramaraj T, Lee R et al (2017) Single-molecule sequencing and Hi-C-based proximity-guided assembly of amaranth (Amaranthus hypochondriacus) chromosomes provide insights into genome evolution. BMC Biol 15:1–15. https://doi.org/10.1186/s12915-017-0412-4
Lincoln NK, Ladefoged TN (2014) Agroecology of pre-contact Hawaiian dryland farming: The spatial extent, yield and social impact of Hawaiian breadfruit groves in Kona, Hawai’i. J Archeological Sci 49:192–202
Lincoln NK, Radovich T, Acosta K, Isele E, Cho A (2019b) Toward standardized leaf sampling for foliar nutrient analysis in breadfruit. Hort Technol 29:443–449
Liu Y, Ragone D, Murch S (2015) Breadfruit (Artocarpus altilis): a source of high-quality protein for food security and novel food products. Amino Acids 47:847–856
Liu H, Wei J, Yang T, Mu W, Song B et al (2019) Molecular digitization of a botanical garden: High-depth whole-genome sequencing of 689 vascular plant species from the Ruili Botanical Garden. Gigascience 8:1–9. https://doi.org/10.1093/gigascience/giz007
Lizumi T, Ramankutty N (2016) Changes in yield variability of major crops for 1981–2010 explained by climate change. Environ Res Lett 26:034003
Loconsole D, Cristiano G, De Lucia B (2019) Glassworts: From wild salt marsh species to sustainable edible crops. Agric 9:1–12. https://doi.org/10.3390/agriculture9010014
Lu Y, Jian-Kang Z (2017) Precise editing of a target base in the rice genome using a modified CRISPR/Cas9 system. Mol Plant 10:523–525. https://doi.org/10.1016/j.molp.2016.11.013
Lu F, Lipka AE, Glaubitz J, Elshire R, Cherney JH, Cherney JH et al (2013) (2013) Switchgrass genomic diversity, ploidy, and evolution: novel insights from a network-based SNP discovery protocol. PLoS Genet 9(1):e1003215. https://doi.org/10.1371/journal.pgen.1003215
Lv X, Lan S, Guy KM, Yang J, Zhang M, Hu Z (2016) Global Expressions Landscape of NAC Transcription Factor Family and Their Responses to Abiotic Stresses in Citrullus lanatus. Sci Rep 6:1–14. https://doi.org/10.1038/srep30574
Mabhaudhi T, Chimonyo VGP, Hlahla S, Massawe F, Mayes S, Nhamo L, Modi AT (2019) Prospects of orphan crops in climate change. Planta 250:695–708. https://doi.org/10.1007/s00425-019-03129-y
Mallory MA, Hall RV, McNabb AP, Pratt DB et al (2008) Development and characterization of microsatellite markers for the grain amaranths. Crop Sci 48:1098–1106
Masand S, Yadav SK (2016) Overexpression of MuHSP70 gene from Macrotyloma uniflorum confers multiple abiotic stress tolerance in transgenic Arabidopsis thaliana. Mol Biol Rep 43:53–64. https://doi.org/10.1007/s11033-015-3938-y
Massange-Sánchez JA, Palmeros-Suárez PA, Espitia-Rangel E, Rodríguez-Arévalo I et al (2016) Overexpression of grain amaranth (Amaranthus hypochondriacus) AhERF or AhDOF transcription factors in Arabidopsis thaliana increases water deficit-and salt-stress tolerance, respectively, via contrasting stress-amelioration mechanisms. PLoS ONE 11:1–43. https://doi.org/10.1371/journal.pone.0164280
Maughan PJ, Turner TB, Coleman CE, Elzinga DB et al (2009) Characterization of Salt Overly Sensitive 1 (SOS1) gene homoeologs in quinoa (Chenopodium quinoa Willd.). Genome 52:647–657. https://doi.org/10.1139/G09-041
Maughan P, Smith S, Fairbanks D, Jellen E (2011) Development, characterization, and linkage mapping of Single Nucleotide Polymorphisms in the grain amaranths (Amaranthus sp.). The Plant Genome 4:92–101. https://doi.org/10.3835/plantgenome2010.12.0027
Mayes S, Massawe F, Alderson PG, Roberts JA, Azam-Ali SN (2012) The potential for underutilized crops to improve security of food production. Oxford Journals 63:1075–1079
Mayes S, Ho WK, Chai HH et al (2019) Bambara groundnut: an exemplar underutilised legume for resilience under climate change. Planta 250:803–820. https://doi.org/10.1007/s00425-019-03191-6
Mayes S, Redjeki ES, Ho WK, Shah N, Ardiarini NR (2020) Understanding the genetic relationships between Indonesian bambara groundnut landraces and investigating their origins. Genome 2020.
Melo ATO, Bartaula R, Hale I (2016) GBS-SNP-CROP: a reference-optional pipeline for SNP discovery and plant germplasm characterization using variable length, paired-end genotyping-by-sequencing data. BMC Bioinformatics 17:29. https://doi.org/10.1186/s12859-016-0879-y
Midega C, Khan Z, Amudavi D, Pittchar J, Pickett J (2010) Integrated management of Striga hermonthica and cereal stemborers in finger millet (Eleusine coracana (L.) Gaertn.) through intercropping with Desmodium intortum. Int J Pest Manag 56:145–151. https://doi.org/10.1080/09670870903248843
Midega CAO, Wasonga CJ, Hooper AM, Pickett JA, Khan ZR (2017) Drought-tolerant Desmodium species effectively suppress parasitic striga weed and improve cereal grain yields in western Kenya. Crop Prot 98:94–101. https://doi.org/10.1016/j.cropro.2017.03.018
Miller JD, Arteca RN, Pell EJ (1999) Senescence-associated gene expression during ozone-induced leaf senescence in Arabidopsis. Plant Physiol 120:1015–1024. https://doi.org/10.1104/pp.120.4.1015
Min JH, Ju HW, Yang KY, Chung JS, Cho BH, Kim CS (2014) Heterologous expression of the gourd E3 ubiquitin ligase gene LsRZF1 compromises the drought stress tolerance in arabidopsis thaliana. Plant Physiol Biochem 77:7–14. https://doi.org/10.1016/j.plaphy.2014.01.010
Mir RR, Reynolds M, Pinto F, Khan MA, Bhat MA (2019) High-throughput phenotyping for crop improvement in the genomics era. Plant Sci 282:60–72
Mishra S, Behura R, Awasthi JP, Dey M et al (2014) Ectopic overexpression of a mungbean vacuolar Na+/H+ antiporter gene (VrNHX1) leads to increased salinity stress tolerance in transgenic Vigna unguiculata L. Walp Mol Breed 34:1345–1359. https://doi.org/10.1007/s11032-014-0120-5
Mkumbo S, Mwegoha W, Renman G (2012) Assessment of the phytoremediation potential for Pb. Zn and Cu of indigenous plants growing in a gold mining area in Tanzania 2:2425–2434. https://doi.org/10.6088/ijes.00202030123
Mohsenzade F, Nasseri S, Mesdaghinia A, Nabizadeh R, Zafari D, Chehregani A (2009) Phytoremediation of petroleum-contaminated soils: Pre-screening for suitable plants and rhizospheral fungi. Toxicol Environ Chem 91:1443–1453. https://doi.org/10.1080/02772240902744451
Montenegro L, Santagati LM (2019) Use of Vegetable Oils to Improve the Sun Protection Factor of Sunscreen Formulations. Cosmetics 6:25
Morris JB (2009a) Morphological and reproductive characterization in hyacinth bean, Lablab purpureus (L.) sweet germplasm with clinically proven nutraceutical and pharmaceutical traits for use as a medicinal food. J Diet Suppl 6:263–279. https://doi.org/10.1080/19390210903070830
Morris JB (2009b) Morphological and reproductive characterization in Hyacinth Bean, Lablab purpureus (L.) sweet germplasm with clinically proven nutraceutical and pharmaceutical traits for use as a medicinal Food. Journal Diet Suppl 6:263–279. https://doi.org/10.1080/19390210903070830
Murch SJ, Ragone D, Lei Shi W, Ala AR, Saxena PK (2008) In vitro conservation and sustained production of breadfruit (Artocarpus altilis, Moraceae): modern technologies for a traditional tropical crop. Naturwissenschaften 95:99–107
Nakanwagi MJ, Sseremba G, Kabod NP, Masanza M, Kizito EB (2020) Identification of growth stage-specific watering thresholds for drought screening in Solanum aethiopicum Shum. Sci Rep 21:862. https://doi.org/10.1038/s41598-020-58035-1
Naluwairo R (2011) Investing in orphan crops to improve food and livelihood security of uganda’s rural poor: policy gaps opportunities and recommendations. ACODE Policy Research Series, Kampala
Narendhirakannan RT, Kandaswamy M, Subramanian S (2005) Anti-inflammatory activity of Cleome gynandra L. on hematological and cellular constituents in adjuvantinduced arthritic rats. Indian J Med Food 8:93–99
National Research Council (1996) Lost crops of Africa. The National Academies Press, Washington
Neelam K, Mahajan R, Gupta V et al (2020) (2020) High-resolution genetic mapping of a novel bacterial blight resistance gene xa-45(t) identified from Oryza glaberrima and transferred to Oryza sativa. Theor Appl Genet 133:689–705. https://doi.org/10.1007/s00122-019-03501-2
Neshamba SM (2010) Variability for drought tolerance in finger millet (Eleusine coracana (L.) Gaertn.] accessions from Zambia. The University of Zambia
Neugart S, Baldermann S, Ngwene B, Wesonga J, Schreiner M (2017) Indigenous leafy vegetables of Eastern Africa—a source of extraordinary secondary plant metabolites. Food Res Int. https://doi.org/10.1016/j.foodres.2017.02.014
Nkansah GO, Ahwireng AK, Amoatey C, Ayarna AW (2013) Grafting onto African eggplant enhances growth, yield and fruit quality of tomatoes in tropical Forest ecozones. J Appl Hortic 15:16–20. https://doi.org/10.37855/jah.2013.v15i01.03
Nwokocha CR, Owu DU, McLaren M, Murray J et al (2012) Possible mechanisms of action of the aqueous extract of Artocarpus altilis (breadfruit) leaves in producing hypotension in normotensive Sprague-Dawley rats. Pharmaceut Biol 50:1096–1102. https://doi.org/10.3109/13880209.2012.658113
Obidiegwu JE, Asiedu R, Ene-Obong E, Muoneke C, Kolesnikova-Allen M (2009) Genetic characterization of some water yam (Dioscorea alata L.) accessions in West Africa with simple sequence repeats. J Food Agric Environ 7:634–638
Ochekwu EB, Eneh CO (2012) Phytoremediation potential of sorghum (Sorghum bicolor (L) Moench) and African yam bean (Sphenostylis sternocarpa) Hoechest ex A. Rich) harms on crude oil polluted soil. NISEB J 1:12
O’Connor DJ, Wright GC, Dieters MJ, George DL, Hunter MN, Tatnell JR, Fleischfresser DB (2013) Development and application of speed breeding technologies in a commercial peanut breeding program. Peanut Sci 40(2):107–114
Odeh I, Tan D (2015) Expanding biofuel production in Australia: opportunities beyond. Farm Policy J 4:29–39
Odeny DA, Niazi A, Tesfaye K, Lule D, Wanyonyi S, Kunguni J (2020) Genomic designing for climate smart finger millet. In: Kole C (ed) Genomic designing of climate-smart cereal crops. Springer, Berlin, Heidelberg, pp 287–307
Odetola AA, Iranloye YO, Akinloye O (2004) Hypolipidaemic potentials of Solanum melongena and Solanum gilo on Hypercholesterolemic Rabbits. Pakistan J Nutr 3:180–187
Ogunka-Nnoka C, Bm O-O, Omeje H (2018) Effects of ethanol extracts of S. aethiopicum stalks on lipid profile and haematological parameters of Wistar Albino rats. Int J Sci Res Methodol 10:215–229
Ohnishi T, Yoshino M, Yamakawa H, Kinoshita T (2011) The biotron breeding system: a rapid and reliable procedure for genetic studies and breeding in rice. Plant Cell Physiol 52:1249–1257
Oibiokpa FI, Godwin IA, Abubakar NS, Kudirat OS (2014) Nutritional composition of Detarium microcarpum fruit. Afr J Food Sci 8:342–350. https://doi.org/10.5897/ajfs2014.1161
Okuno K, Sakoguchi S (1982) Inheritance of starch characteristics in perisperm of Amaranthus hypochondriacus. J Hered 73:467
Oliver-Bever BE (1986) Medicinal plants in tropical West Africa. Cambridge University Press, Cambridge
Omubuwajo TO (2007) Breadfruit as a key component of sustainable livelihoods in Nigeria: prospects, opportunities and challenges. Acta Hortic 757:121–124
Onyango CM, Kunyanga CN, Ontita EG, Narla RD, Kimenju JW (2013) Current status on production and utilization of spider plant (Cleome gynandra L.) an underutilized leafy vegetable in Kenya. Genet Resour Crop Evol 60:2183–2189
Opole R (2019) Opportunities for enhancing production, utilization and marketing of finger millet in Africa. Afr J Food Agric Nutri Dev. 19:13863–13882. https://doi.org/10.18697/ajfand.84.BLFB1004
Opole M, Chweya JA, Imungi JK (1995). Indigenous Vegetables of Kenya: indigenous knowledge, agronomy and nutritive value. Field and Laboratory Experience Report
Osei MK, Oluoch MO, Osei CK, Banful B (2010) Morphological characterisafion of African Eggplant (Solanum spp.) Germplasm in some African countries. Agric Innov Sust Dev 163:63–71
Østerberg JT, Xiang W, Olsen LI, Edenbrandt AK et al (2017) Accelerating the domestication of new crops: feasibility and Approaches. Trends Plant Sci 22:373–384. https://doi.org/10.1016/j.tplants.2017.01.004
Ozimati A, Kawuki R, Esuma W, Kayondo IS, Wolfe M, Lozano R, Rabbi I, Kulakow P, Jannink JL (2018) Training population optimization for prediction of cassava brown streak disease resistance in west african clones. G3 8:3903–3913. https://doi.org/10.1534/g3.118.200710
Ozsolak F, Milos PM (2011) RNA sequencing: advances, challenges and opportunities. Nat Rev Genet 12(2):87–98
Padhan B, Panda D (2018) Variation of photosynthetic characteristics and yield in wild and cultivated species of yams (Dioscorea spp.) from Koraput. India Photosynthetica 56:1010–1018
Padhan B, Panda D (2020) Potential of neglected and underutilized yams (Dioscorea spp.) for improving nutritional security and health benefits. Front Pharmacol 11:1–13. https://doi.org/10.3389/fphar.2020.00496
Padulosi S (2017) Bring NUS back to the table! GREAT Insights Mag 64:21–22
Palmeros-Suárez PA, Massange-Sánchez JA, Martínez-Gallardo NA, Montero-Vargas JM et al (2015) The overexpression of an Amaranthus hypochondriacus NF-YC gene modifies growth and confers water deficit stress resistance in Arabidopsis. Plant Sci 240:25–40. https://doi.org/10.1016/j.plantsci.2015.08.010
Pandey RM (1984) Genetic studies of yield contributing traits in Amaranthus. Theoret Appl Genet 68:121–125. https://doi.org/10.1007/BF00252326
Pandey RM, Pal M (1985) Genetics of grain protein in Amaranthus. Crop Improv 12:55–58
Pandey RM, Singh R (2011) Genetic divergence in grain amaranth (Amaranthus hypochondriacus L.). Genetika 43:41–49
Pandurangaiah M, Lokanadha Rao G, Sudhakarbabu O, Nareshkumar A, Kiranmai K, Lokesh U, Thapa G, Sudhakar C (2014) Overexpression of horsegram (Macrotyloma uniflorum Lam. Verdc.) NAC transcriptional factor (MuNAC4) in groundnut confers enhanced drought tolerance. Mol Biotechnol 56:758–769. https://doi.org/10.1007/s12033-014-9754-0
Pareek A, Dhankher OP, Foyer CH (2020) Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J Exp Bot 71:451–456. https://doi.org/10.1093/jxb/erz518
Patel S (2016) Salicornia: evaluating the halophytic extremophile as a food and a pharmaceutical candidate. 3 Biotech 6:1–10. https://doi.org/10.1007/s13205-016-0418-6
Patel MK, Joshi M, Mishra A, Jha B (2015) Ectopic expression of SbNHX1 gene in transgenic castor (Ricinus communis L.) enhances salt stress by modulating physiological process. Plant Cell Tissue Organ Cult 122:477–490. https://doi.org/10.1007/s11240-015-0785-4
Pati K, Zhang F, Batley J (2019) First report of genome size and ploidy of the underutilized leguminous tuber crop Yam Bean (Pachyrhizus erosus and P. tuberosus) by flow cytometry. Plant Genet Resour Charact Util 17:456–459. https://doi.org/10.1017/S1479262119000170
Peng Y, Zhang J, Cao G, Xie Y, Liu X, Lu M, Wang G (2010) Overexpression of a PLDα1 gene from Setaria italica enhances the sensitivity of Arabidopsis to abscisic acid and improves its drought tolerance. Plant Cell Rep 29:793–802. https://doi.org/10.1007/s00299-010-0865-1
Peters I, Jain S (1987) Genetics of grain amaranths III. 3. Gene-Cytoplasmic Male-Sterility J Hered 78:251–256
Pidon H, Ghesquiere A, Cheron S, Issaka S, Hebrard E, Sabot F, Kolade O, Silue D, Albar L (2017) Fine mapping of RYMV3: a new resistance gene to Rice yellow mottle virus from Oryza glaberrima. Theor Appl Genet 130:807–818
Pingali PL (2012) Green revolution: impacts, limits, and the path ahead. Proc Natl Acad Sci USA 109:12302–12308. https://doi.org/10.1073/pnas.0912953109
Plazas M, Prohens J, Cuñat A, Vilanova S (2014) Reducing capacity, chlorogenic acid content and biological activity in a collection of scarlet (Solanum aethiopicum) and Gboma (S. macrocarpon) eggplants. Int J Mol Sci 15:17221–17241. https://doi.org/10.3390/ijms151017221
Poola J, Ojuederie O, Omonhinmin C, Adegbite A (2019) Neglected and underutilized legume crops: improvement and Future prospects. In: Shah F (ed) Recent advances in grain crops research. IntechOpen, London, pp 1–22
Prohens J, Plazas M, Raigón MD, Seguí-Simarro JM, Stommel JR, Vilanova S (2012) Characterization of interspecific hybrids and first backcross generations from crosses between two cultivated eggplants (Solanum melongena and S. aethiopicum Kumba group) and implications for eggplant breeding. Euphytica 186:517–538. https://doi.org/10.1007/s10681-012-0652-x
Puranik S, Bahadur RP, Srivastava PS, Prasad M (2011) Molecular cloning and characterization of a membrane associated NAC family gene, SiNAC from foxtail millet [Setaria italica (L.) P. Beauv.]. Mol Biotechnol 49:138–150. https://doi.org/10.1007/s12033-011-9385-7
Puranik S, Sahu PP, Beynon S, Srivastava RK, Sehgal D, Ojulong H, Yadav R (2020) Genome-wide association mapping and comparative genomics identifies genomic regions governing grain nutritional traits in finger millet [Eleusine coracana (L.) Gaertn.]. Plants People Planet 00:1–14. https://doi.org/10.1002/ppp3.10120
Ragone D (1997) Breadfruit. Artocarpus altilis (Parkinson) Fosb, promoting the conservation and use of underutilized and neglected crops. 10. Institute of Plant Genetics and Crop Plant Research, Gatersleben, Germany and International. Plant Genetic Resources Institute, Rome, Italy
Ragone D (2007) Breadfruit: diversity, conservation and potential. Acta Hortic 757:19–30. https://doi.org/10.17660/ActaHortic.2007.757.1
Ragone D, Cavaletto C (2006) Sensory evaluation of fruit quality and nutritional composition of 20 breadfruit (Artocarpus, Moraceae) cultivars. Econ Bot 60:335–346
Rahman M, Kumar J, Rahman M, Afzal M (1995) Natural outcrossing in lathyrus satlvus L. Indian J Genet Plant Breed 55:204–207
Rahman H, Jagadeeshselvam N, Valarmathi R, Sachin B, Sasikala R, Senthil N, Sudhakar D, Robin S, Raveendran M (2014) Transcriptome analysis of salinity responsiveness in contrasting genotypes of finger millet (Eleusine coracana L.) through RNA-sequencing. Plant Mol Biol 85:485–503. https://doi.org/10.1007/s11103-014-0199-4
Rahman H, Ramanathan V, Nallathambi J, Duraialagaraja S, Raveendran M (2016) Over-expression of a NAC 67 transcription factor from finger millet (Eleusine coracana L.) confers tolerance against salinity and drought stress in rice. BMC Biotechnol 16:7–20. https://doi.org/10.1186/s12896-016-0261-1
Ramakrishnan M, Ceasar SA, Duraipandiyan V, Al-Dhabi NA, Ignacimuthu S (2015) Using molecular markers to assess the genetic diversity and population structure of finger millet (Eleusine coracana (L.) Gaertn) from various geographical regions. Genet Resour Crop Evol. https://doi.org/10.1007/s10722-015-0255-1
Ramegowda V, Gill US, Sivalingam PN, Gupta A, Gupta C, Govind G, Nataraja KN, Pereira A, Udayakumar M, Mysore KS, Senthil-Kumar M (2017) GBF3 transcription factor imparts drought tolerance in Arabidopsis thaliana. Sci Rep 7:1–13. https://doi.org/10.1038/s41598-017-09542-1
Ramesh R, Achari G, Asolkar T, Dsouza M, Singh NP (2016) Management of bacterial wilt of brinjal using wild brinjal (Solanum torvum) as root stock. Indian Phytopathol 69:260–265
Ramírez-Ortega FA, Herrera-Pola PS, Toscano-Morales R, Xoconostle-Cazares B, Ruiz-Medrano R (2014) Overexpression of the pumpkin (cucurbita maxima) 16 kda phloem protein CmPP16 increases tolerance to water deficit. Plant Signal Behav 9:11. https://doi.org/10.4161/15592324.2014.973823
Ranasinghe RASN, Maduwanthi SDT, Marapana RAUJ (2019) Nutritional and health benefits of Jackfruit (Artocarpus heterophyllus Lam.): a review. Int J Food Sci 2019:1–12. https://doi.org/10.1155/2019/4327183
Raskin I, Smith RD, Salt DE (1997) Phytoremediation of metals: using plants to remove pollutants from the environment. Curr Opin Biotechnol 8:221–226
Redjeki ES, Ho WK, Shah N, Molosiwa OO, Ardiarini N, Kuswanto Mayes S (2020) Understanding the genetic relationships between Indonesian bambara groundnut landraces and investigating their origins. Genome 999:1–9
Rincon AM, Padilla FC (2004) Physicochemical properties of Venezuelan breadfruit (Artocarpus altilis) starch. Arch Latinoam Nutr 54:449–456
Ripperger H, Himmelreich U (1994) Anguivine and isoanguivine, steroid alkaloid glycosides from Solanum anguivi. Phytochem 37:1725–1727. https://doi.org/10.1016/s0031-9422(00)89600-4
Rizza F, Mennella G, Collonnier C, Sihachakr D, Kashyap V, Rajam M, Presterà M, Rotino G (2002) Androgenic dihaploids from somatic hybrids between Solanum melongena and S. aethiopicum group gilo as a source of resistance to Fusarium oxysporum f. sp. melongenae. Plant Cell Rep 20:1022–1032. https://doi.org/10.1007/s00299-001-0429-5
Roberts-Nkrumah LB (2007) An overview of breadfruit (Artocarpus altilis) in the Caribbean. Acta Hortic 757:51–60
Rodríguez JP, Rahman H, Thushar S, Singh RK (2020) Healthy and resilient cereals and pseudo-cereals for marginal agriculture: molecular advances for improving nutrient bioavailability. Front Genet 11:49. https://doi.org/10.3389/fgene.2020.00049
Rosa SB, Caverzan A, Teixeira FK, Lazzarotto F, Silveira JAG, Ferreira-Silva SL, Abreu-Neto J, Margis R, Margis-Pinheiro M (2010) Cytosolic APx knockdown indicates an ambiguous redox responses in rice. Phytochemistry 71:548–558. https://doi.org/10.1016/j.phytochem.2010.01.003
Ross H (1986) Potato breeding. Problems perspectives. Adv Plant Breed Suppl 13:82–86
Ruiz-Carrasco K, Antognoni F, Coulibaly AK, Lizardi S, Covarrubias A, Martínez EA, Molina-Montenegro MA, Biondi S, Zurita-Silva A (2011) Variation in salinity tolerance of four lowland genotypes of quinoa (Chenopodium quinoa Willd.) as assessed by growth, physiological traits, and sodium transporter gene expression. Plant Physiol Biochem 49:1333–1341. https://doi.org/10.1016/j.plaphy.2011.08.005
Russell J, Hackett C, Hedley P et al (2014) The use of genotyping by sequencing in blackcurrant (Ribes nigrum): developing high-resolution linkage maps in species without reference genome sequences. Mol Breeding 33:835–849. https://doi.org/10.1007/s11032-013-9996-8
Sadumpati V, Kalambur M, Vudem DR, Kirti PB, Khareedu VR (2013) Transgenic indica rice lines, expressing Brassica juncea Nonexpressor of pathogenesis-related genes 1 (BjNPR1), exhibit enhanced resistance. J Biotechnol 166:114–121. https://doi.org/10.1016/j.jbiotec.2013.04.016
Sahu SK, Liu M, Yssel A, Kariba R et al (2020) Draft genomes of two artocarpus plants, jackfruit (A. heterophyllus) and breadfruit (A. altilis). Genes (Basel) 11:1–17. https://doi.org/10.3390/genes11010027
Saikia JP, Konwar BK (2012) Physicochemical properties of starch from aroids of north east India. Int J Food Prop 15:1247–1261. https://doi.org/10.1080/10942912.2010.491929
Sakata Y, Lester RN (1994) Chloroplast DNA diversity in eggplant (Solanum melongena) and its related species S. incanum and S. marginatum. Euphytica 80:1–4. https://doi.org/10.1007/BF00039291
Sakata Y, Lester RN (1997) Chloroplast DNA diversity in brinjal eggplant (Solanum melongena L.) and related species. Euphytica 97:295–301. https://doi.org/10.1023/A:1003000612441
Sakata Y, Nishio T, Monma S (1989) Resistance of Solanum species to Verticillium wilt and bacterial wilt. In: Proceeding of the VIIth EUCARPIA meeting on Genetics and Breeding of Capsicum and Eggplant, Kragujevac, Yugoslavia pp 27–30
Sakata Y, Nishio T, Matthews PJ (1991) Chloroplast DNA analysis of eggplant (Solanum melongena) and related species for their taxonomic affinity. Euphytica 55:21–26. https://doi.org/10.1007/BF00022555
Samarajeewa KBDP, Horiuchi T, Oba S (2006) Finger millet (Eleusine corocana L. Gaertn.) as a cover crop on weed control, growth and yield of soybean under different tillage systems. Soil Tillage Res 90:93–99. https://doi.org/10.1016/j.still.2005.08.018
Samardžić JT, Nikolić DB, Timotijević GS, Jovanović ŽS et al (2010) Tissue expression analysis of FeMT3, a drought and oxidative stress related metallothionein gene from buckwheat (Fagopyrum esculentum). J Plant Physiol 167:1407–1411. https://doi.org/10.1016/j.jplph.2010.05.016
Samineni S, Sen M, Sajja SB, Gaur PM (2020) Rapid generation advance (RGA) in chickpea to produce up to seven generations per year and enable speed breeding. The Crop J 8(1):164–169
Sansaloni C, Petroli C, Jaccoud D et al (2011) (2011) Diversity arrays technology (DArT) and next-generation sequencing combined: genome-wide, high throughput, highly informative genotyping for molecular breeding of Eucalyptus. BMC Proc 5:P54. https://doi.org/10.1186/1753-6561-5-S7-P54
Sauer JD (1967) The grain amaranths and their relatives: A revised taxonomic and geographic survey. Ann Missouri Bot Gard 54:103–137
Sauer JD (1976) Grain amaranths. In: Simmonds NW (ed) Evolution of crop plants. Longman, London, pp 4–7
Savary S, Willocquet L, Pethybridge SJ, Esker P, McRoberts N, Nelson A (2019) The global burden of pathogens and pests on major food crops. Nat Ecol Evol 3:430–439. https://doi.org/10.1038/s41559-018-0793-y
Schippers RR (2000) African indigenous vegetables : an overview of the cultivated species. University of Greenwich, Natural Resources Institute, London, UK
Schmidt S, Baldwin IT (2009) Down-regulation of systemin after herbivory is associated with increased root allocation and competitive ability in Solanum nigrum. Oecologia 159:473–482. https://doi.org/10.1007/s00442-008-1230-8
Sękara A, Cebula S, Kunicki E (2007) Cultivated eggplants-origin, breeding objectives and genetic resources, a review. FOLIA Hortic 19:97–114
Sekulya S, Nandutu A, Namutebi A, Ssozi J, Masanza M, Kabod B (2018) Antioxidant potential of the farmer preferred selections of Solanum aethiopicum vegetable consumed in central Uganda. Eur J Biol Res 8:26–33
Shaik K, Shaik A, Kumar D, Kadirvel D (2013) Evaluation of preliminary phytochemical properties and hypoglycemic activity of Cleome gynandra L. Int J Pharm Pharm Sci 5:824–828
Shailaja HB, Thirumeni S (2007) Evaluation of salt-tolerance in finger millet (Eleusine coracana) genotypes at seedling stage. Indian J Agric Sci 77:672–674
Sharma A, Gontia I, Agarwal PK, Jha B (2010) Accumulation of heavy metals and its biochemical responses in Salicornia brachiata, an extreme halophyte. Mar Biol Res 6:511–518. https://doi.org/10.1080/17451000903434064
Shehadeh AA (2011) Ecogeographic, genetic and genomic studies of the genus Lathyrus L. Thesis submitted to University of Birmingham, UK
Shiferaw B, Smale M, Braun H et al (2013) Crops that feed the world 10. Past successes and future challenges to the role played by wheat in global food security. Food Sec 5:291–317. https://doi.org/10.1007/s12571-013-0263-y
Shinozaki K, Uemura M, Bailey-Serres J, Bray EA, Weretilnyk E (2015) Responses to abiotic stress. In: Buchanan BB, Gruissem W, Jones RL (eds) Biochemistry and molecular biology. Wiley, Chichester, pp 1051–1100
Shortridge J (2019) Observed trends in daily rainfall variability result in more severe climate change impacts to agriculture. Clim Chang 157:429–444. https://doi.org/10.1007/s10584-019-02555-x
Shrestha R, Matteis L, Skofic M, Portugal A, McLaren G, Hyman G, Arnaud E (2012) Bridging the phenotypic and genetic data useful for integrated breeding through a data annotation using the crop ontology developed by the crop communities of practice. Front Physiol 3:326. https://doi.org/10.3389/fphys.2012.00326
Shrestha R, Joshi B, Shrestha J, Timilsina A (2019) Crop groups based on research priority: priority crops, neglected and underutilized (NUS), future smart food (FSF). In: Proceedings of a national workshop on working crop groups, 21–22 June 2018, Kathmandu, Nepal
Siadjeu C, Pucker B, Viehöver P, Albach DC, Weisshaar B (2020) High contiguity de novo genome sequence assembly of trifoliate yam (Dioscorea dumetorum) using long read sequencing. Genes 11:1–11. https://doi.org/10.3390/genes11030274
Singh A, Roy AK (2013) Variability for forage yielding traits in exotic grass pea (Lathyrus sativa L.). Forage Res 38:230–233
Skiba B, Ford R, Pang ECK (2004) Construction of a linkage map based on a Lathyrus sativus backcross population and preliminary investigation of QTLs associated with resistance to ascochyta blight. Theor Appl Genet 109:1726–1735. https://doi.org/10.1007/s00122-004-1812-8
Smulikowska S, Rybinski W, Czerwinski J, Taciak M, Mieukowska A (2008) Evaluation of selected mutants of grass pea (Lathyrus sativus L.) var. Krab as an ingredient in broiler chicken diet. J Anim Feed Sci 17:75–87
Sogbohossou EOD, Achigan-Dako EG, Maundu P, Solberg S et al (2018) A roadmap for breeding orphan leafy vegetable species: a case study of Gynandropsis gynandra (Cleomaceae). Hortic Res 5:1–15. https://doi.org/10.1038/s41438-017-0001-2
Song B, Song Y, Fu Y, Kizito EB, Kamenya SN et al (2019) Draft genome sequence of Solanum aethiopicum provides insights into disease resistance, drought tolerance, and the evolution of the genome. Gigascience 8:1–16. https://doi.org/10.1093/gigascience/giz115
Spaenij-Dekking L, Kooy-Winkelaar Y, Koning F (2005) The Ethiopian cereal tef in celiac disease [6]. N Engl J Med 353:1748–1749. https://doi.org/10.1056/NEJMc051492
Sreekumar VB, Binoy AM, George ST (2007) Genetic and morphological variation in breadfruit (Artocarpus altilis (Park.) Fosberg) in the Western Ghats of India using AFLP markers. Genet Resour Crop Evol 54:1659–1665. https://doi.org/10.1007/s10722-007-9282-x
Srinivasachary DMM, Gale MD, Devos KM (2007) Comparative analyses reveal high levels of conserved colinearity between the finger millet and rice genomes. Theor Appl Genet 115:489–499
Srivastava S, Srivastava AK, Sablok G, Deshpande TU, Suprasanna P (2015) Transcriptomics profiling of Indian mustard (Brassica juncea) under arsenate stress identifies key candidate genes and regulatory pathways. Front Plant Sci 6:1–14. https://doi.org/10.3389/fpls.2015.00646
Ssepuuya G, Katongole J, Tumuhimbise G (2018) Contribution of instant amaranth (Amaranthus hypochondriacus L.)-based vegetable soup to nourishment of boarding school adolescents. Food Sci Nutr. https://doi.org/10.1002/fsn3.664
Sseremba G, Tongoona P, Eleblu J, Danquah E, Kaweesi T, Baguma Y, Masanza M, Kizito E (2018a) Stability of Solanum aethiopicum Shum accessions under varied water deficit stress levels and identification of pertinent breeding traits for resistance to water shortage. Euphytica 214:0014–2336. https://doi.org/10.1007/s10681-017-2097-8
Sseremba G, Tongoona P, Eleblu J, Danquah EY, Kizito EB (2018b) Heritability of drought resistance in Solanum aethiopicum Shum group and combining ability of genotypes for drought tolerance and recovery. Sci Hortic (Amsterdam) 240:213–220. https://doi.org/10.1016/j.scienta.2018.06.028
Stetter MG, Schmid KJ (2017) Analysis of phylogenetic relationships and genome size evolution of the Amaranthus genus using GBS indicates the ancestors of an ancient crop. Mol Phylogenet Evol 109:80–92
Stetter MG, Zeitler L, Steinhaus A, Kroener K, Biljecki M, Schmid KJ (2016) Crossing Methods and Cultivation Conditions for Rapid Production of Segregating Populations in Three Grain Amaranth Species. Front Plant Sci 7:816. https://doi.org/10.3389/fpls.2016.00816
Stetter MG, Müller T, Schmid KJ (2017) Genomic and phenotypic evidence for an incomplete domestication of South American grain amaranth (Amaranthus caudatus). Mol Ecol 26:871–886. https://doi.org/10.1111/mec.13974
Stetter MG, Vidal-Villarejo M, Schmid KJ (2020) Parallel seed color adaptation during multiple domestication attempts of an ancient new world grain. Mol Biol Evol 37:1407–1419. https://doi.org/10.1093/molbev/msz304
Steuernagel B, Periyannan SK, Hernández-Pinzón I, Witek K et al (2016) Rapid cloning of disease-resistance genes in plants using mutagenesis and sequence capture. Nat Biotechnol 34:652–655
Sunseri F, Polignano GB, Alba V, Lotti C et al (2010) Genetic diversity and characterization of African eggplant germplasm collection. African J Plant Sci 4:231–241
Suresh S, Chung J-W, Cho G-T, Sung J-S, Park J-H, Gwag J-G, Baek H-J (2014) Analysisof molecular genetic diversity and population structure in Amaranthus germplasm using SSR markers. Plant Biosyst 148:635–644. https://doi.org/10.1080/11263504.2013.788095
Syfert MM, Castañeda-Álvarez NP, Khoury CK, Särkinen T et al (2016) Crop wild relatives of the brinjal eggplant (Solanum melongena): poorly represented in genebanks and many species at risk of extinction. Am J Bot 103:635–651. https://doi.org/10.3732/ajb.1500539
Tadele Z (2019) Orphan crops: their importance and the urgency of improvement. Planta 250:677–694. https://doi.org/10.1007/s00425-019-03210-6
Tadele Z, Bartels D (2019) Promoting orphan crops research and development. Planta 250:675–676. https://doi.org/10.1007/s00425-019-03235-x
Tadesse W, Bekele E (2003) Phenotypic diversity of Ethiopian grass pea (Lathyrus sativus L.) in relation to geographical regions and altitudinal range. Genet Res Crop Evol 50:497–505
Taher D, Solberg SØ, Prohens J, Chou Y, Rakha M, Wu T (2017) World Vegetable Center eggplant collection: origin, composition, seed dissemination and utilization in breeding. Front Plant Sci 8:1484. https://doi.org/10.3389/fpls.2017.01484
Tamiru M, Natsume S, Takagi H, White B et al (2017) Genome sequencing of the staple food crop white Guinea yam enables the development of a molecular marker for sex determination. BMC Biol 15:1–20. https://doi.org/10.1186/s12915-017-0419-x
Tang M, Liu X, Deng H, Shen S (2011) Over-expression of JcDREB, a putative AP2/EREBP domain-containing transcription factor gene in woody biodiesel plant Jatropha curcas, enhances salt and freezing tolerance in transgenic Arabidopsis thaliana. Plant Sci 181:623–631. https://doi.org/10.1016/j.plantsci.2011.06.014
Tang D, Dong Y, Ren H, Li L, He C (2014) A review of phytochemistry, metabolite changes, and medicinal uses of the common food mung bean and its sprouts (Vigna radiata). Chem Cent J 8:1–9. https://doi.org/10.1186/1752-153X-8-4
Tay J, Valenzuela A, Venegas F (2000) Collecting and evaluating Chilean germplasm of grass pea (Lathyrus sativus L.). Lathyrus Lathyrism Newsl 1:21
Teshome S, Tegegne B (2020) Determinants of adoption of improved teff varieties by smallholder farmers: the case of Kobo District, North Wollo Zone, Amhara Region, Ethiopia. Int J Agric Econ 5:114–122. https://doi.org/10.11648/j.ijae.20200504.14
Thilakarathna MS, Raizada MN (2015) A review of nutrient management studies involving finger millet in the semi-arid tropics of Asia and Africa. Agronomy 5:262–290. https://doi.org/10.3390/agronomy5030262
Tlili N, Elfalleh W, Saadaoui E, Khaldi A, Triki S, Nasri N (2011) The caper (Capparis L.): ethnopharmacology, phytochemical and pharmacological properties. Fitoterapia 82:93–101. https://doi.org/10.1016/j.fitote.2010,09.006
Tolera A, Sundstøl F (2000) Supplementation of graded levels of Desmodium intortum hay to sheep feeding on maize stover harvested at three stages of maturity. 2. Rumen fermentation and nitrogen metabolism. Anim Feed Sci Technol 87:215–229. https://doi.org/10.1016/S0377-8401(00)00205-4
Toppino L, Valè G, Rotino GL (2008) Inheritance of Fusarium wilt resistance introgressed from Solanum aethiopicum Gilo and Aculeatum groups into cultivated eggplant (S. melongena) and development of associated PCR-based markers. Mol Breed 22:237–250. https://doi.org/10.1007/s11032-008-9170-x
Torres LG, Vilelade Resende MD, Azevedo CF, Fonsecae Silva F, de Oliveira EJ (2019) Genomic selection for productive traits in biparental cassava breeding populations. PLoS ONE 14(7):e0220245. https://doi.org/10.1371/journal.pone.0220245
Tripathi B, Platel K (2010) Finger millet (Eleusine coracana) flour as a vehicle for fortification with zinc. J Trace Elem Med Biol 24:46–51. https://doi.org/10.1016/j.jtemb.2009.09.001
Tripathi L, Ntui VO, Tripathi JN (2019) Application of genetic modification and genome editing for developing climate-smart banana. Food Energy Sec 8(4):e00168
Trucco F, Tranel PJ (2011) Amaranthus. In: Kole C (ed) Wild crop relatives: genomic and breeding resources. Springer, Berlin, Heidelberg
Tyagi J, Varma A, Pudake RN (2017) Evaluation of comparative effects of arbuscular mycorrhiza (Rhizophagus intraradices) and endophyte (Piriformospora indica) association with finger millet (Eleusine coracana) under drought stress. Eur J Soil Biol 81:1–10. https://doi.org/10.1016/j.ejsobi.2017.05.007
Ukwungwu MN, Williams CT, Okhidievbie O (1998) Screening of African rice Oryza glaberrima Steud, for resistance to the African rice gall midge Orseolia oryzivora Harris and Gagné. Insect Sci Appl 18:167–170. https://doi.org/10.1017/S1742758400007827
Umesh MR, Angadi S, Gowda P, Ghimire R, Begna S (2019) Climate-resilient minor crops for food security. In: Hasanuzzaman M (ed) Agronomic crops. Springer, Singapore, pp 19–32
UNCCD (2017) Global land outlook. 1st ed. Bonn: UN Convention to Combat Desertification
Upadhyaya HD, Sarma NDRK, Ravishankar CR, Albrecht T, Narasimhudu Y et al (2010) Developing a mini-core collection in finger millet using multi-location data. Crop Sci 50:1924–1931
Van Der Vossen E, Sikkema A, BtL H, Gros J, Stevens P, Muskens M, Wouters D, Pereira A, Stiekema W, Allefs S (2003) An ancient R gene from the wild potato species Solanum bulbocastanum confers broad-spectrum resistance to Phytophthora infestans in cultivated potato and tomato. Plant J 36:867–882. https://doi.org/10.1046/j.1365-313X.2003.01934.x
van Zelm E, Zhang Y, Testerink C (2020) Salt Tolerance Mechanisms of Plants. Annu Rev Plant Biol 71:403–433. https://doi.org/10.1146/annurev-arplant-050718-100005
VanBuren R, Man Wai C, Wang X, Pardo J, Yocca AE, Wang H, Chaluvadi SR, Han G, Bryant D, Edger PP, Messing J, Sorrells ME, Mockler TC, Bennetzen JL, Michael TP (2020) Exceptional subgenome stability and functional divergence in the allotetraploid Ethiopian cereal teff. Nat Commun 11:1–11. https://doi.org/10.1038/s41467-020-14724-z
Vaz Patto MC, Rubiales D (2014) Lathyrus diversity: available resources with relevance to crop improvement—L. sativus and L. cicera as case studies. Ann Bot 113:895–908
Vaz Patto MC, Skiba B, Pang ECK, Ochatt SJ, Lambein F, Rubiales D (2006) Lathyrus improvement for resistance against biotic and abiotic stresses: from classical breeding to marker assisted selection. Euphytica 147:133–147
Vetriventhan M, Upadhyaya HD, Dwivedi SL, Pattanashetti SK, Kumar Singh S (2016) Finger and foxtail millets. In: Singh M, Upadhyaya HD (eds) Genetic and genomic resources for grain cereals improvement. Academic Press, Cambridge, Massachusetts, USA, pp 291–319
Vietnameyer ND, Borlaugh NE, Axtell J, Burton GW et al (1996) Fonio. Lost crops of Africa. National Academy Press, Washington D.C., pp 39–58
Viljoen E, Odeny DA, Coetzee MPA et al (2018) Application of chloroplast phylogenomics to resolve species relationships within the plant genus Amaranthus. J Mol Evol 86:216–239. https://doi.org/10.1007/s00239-018-9837-9
Voss-Fels KP, Cooper M, Hayes BJ (2019) Accelerating crop genetic gains with genomic selection. Theor Appl Genet 132:669–686. https://doi.org/10.1007/s00122-018-3270-8
Waiganjo MM, Muriuki J, Mbugua GW (2007) Potential of indigenous leafy vegetables as companion crops for pest management of high-value legumes: a case study of gynandropsis gynandra in Kenya. Acta Hortic 752:319–321. https://doi.org/10.17660/ActaHortic.2007.752.54
Wang Z, Wang S (2010) Patent Application Publication (10) Pub. No.: US 2010/0035098 A1 Patent Application Publication. 1:1–5
Wang JW, Li Y, Zhang YX, Chai TY (2013) Molecular cloning and characterization of a Brassica juncea yellow stripe-like gene, BjYSL7, whose overexpression increases heavy metal tolerance of tobacco. Plant Cell Rep 32:651–662. https://doi.org/10.1007/s00299-013-1398-1
Wang F, Ren G, Li F, Qi S et al (2018) A chalcone synthase gene AeCHS from Abelmoschus esculentus regulates flavonoid accumulation and abiotic stress tolerance in transgenic Arabidopsis. Acta Physiol Plant 40:1–13. https://doi.org/10.1007/s11738-018-2680-1
Weng JR, Bai LY, Ko HH, Tsai YT (2018) Cyclocommunol induces apoptosis in human oral squamous cell carcinoma partially through a Mcl-1-dependent mechanism. Phytomed 39:25–32
Wu X, Blair MW (2017) Diversity in grain amaranths and relatives distinguished by genotyping by sequencing (GBS). Front Plant Sci 8:1960. https://doi.org/10.3389/fpls.2017.01960
Xu F, Sun M (2001) Comparative analysis of phylogenetic relationships of grain amaranths and their wild relatives (Amaranthus; Amaranthaceae) using internal transcribed spacer, amplified fragment length polymorphism and double primer fluorescent inter simple sequence repeat markers. Mol Phylogenet Evol 21:372–387
Xue Y, Yin N, Chen B, Liao F, Win AN, Jiang J, Wang R, Jin X, Lin NA, Chai Y (2017) Molecular cloning and expression analysis of two FAD2 genes from chia (Salvia hispanica). Acta Physiol Plant 39:1–12. https://doi.org/10.1007/s11738-017-2390-0
Yang SY, Saxena RK, Kulwal PA, Ash GJ, Dubey A, Harper DI et al (2011) The first genetic map of pigeonpea based on diversity arrays technology (DArT) markers. J Genet 90:103–109
Yang T, Jiang J, Burlyaeva M, Hu J, Coyne CJ, Kumar S et al (2014) Large-scale microsatellite development in grass pea (Lathyrus sativus L.) an orphan legume of the arid areas. BMC Plant Biol 14:65. https://doi.org/10.1186/1471-2229-14-65
Yang S, Pang W, Ash G, Harper J, Carling J, Wenzl P et al (2016) Low level of genetic diversity in cultivated pigeonpea compared to its wild relatives is revealed by diversity arrays technology. Theor Appl Genet 113:585–595
Yang Z, Dai Z, Lu R, Wu B, Tang Q, Xu Y, Cheng C, Su J (2017) Transcriptome analysis of two species of jute in response to polyethylene glycol (PEG)—induced drought stress. Sci Rep 7:1–11. https://doi.org/10.1038/s41598-017-16812-5
Yao L, Jiang Y, Lu X, Wang B, Zhou P, Wu T (2016) A R2R3-MYB transcription factor from Lablab purpureus induced by drought increases tolerance to abiotic stress in Arabidopsis. Mol Biol Rep 43:1089–1100. https://doi.org/10.1007/s11033-016-4042-7
Yao PF, Li CL, Zhao XR, Li MF, Zhao HX, Guo JY, Cai Y, Chen H, Wu Q (2017) Overexpression of a tartary buckwheat gene, FtbHLH3, enhances drought/oxidative stress tolerance in transgenic Arabidopsis. Front Plant Sci 8:1–17. https://doi.org/10.3389/fpls.2017.00625
Yue J, Li C, Liu Y, Yu J (2014) A remorin gene SiREM6, the target gene of SiARDP, from foxtail millet (Setaria italica) promotes high salt tolerance in transgenic Arabidopsis. PLoS ONE 9:1–10. https://doi.org/10.1371/journal.pone.0100772
Yunus AG, Jackson MT (1991) The gene pools of the grass pea (Lathyrus sativus L.). Plant Breed 106:319–328. https://doi.org/10.1111/j.1439-0523.1991.tb00517.x
Zaman QU, Li C, Cheng H, Hu Q (2019) Genome editing opens a new era of genetic improvement in polyploid crops. Crop J 7(2):141–150
Zerega NJC, Ragone D, Motley TJ (2004) Complex origins of breadfruit (Artocarpus altilis Moraceae): implications for human migrations in Oceania. Am J Bot 91:760–766. https://doi.org/10.3732/ajb.91.5.760
Zerega NJC, Ragone D, Motley TJ (2006) Breadfruit origins, diversity, and human-facilitated distribution. In: Motley TJ, Zerega NJC, Cross H (eds) Darwin’s harvest: new approaches to the origins, evolution, and conservation of crops. Columbia University Press, New York, pp 213–238
Zerega N, Wiesner-Hanks T, Ragone D et al (2015) Diversity in the breadfruit complex (Artocarpus, Moraceae): genetic characterization of critical germplasm. Tree Genet Genom. https://doi.org/10.1007/s11295-014-0824-z
Zhang FL, Niu B, Wang YC, Chen F, Wang SH, Xu Y, Jiang LD, Gao S, Wu J, Tang L, Jia YJ (2008) A novel betaine aldehyde dehydrogenase gene from Jatropha curcas, encoding an enzyme implicated in adaptation to environmental stress. Plant Sci 174:510–518. https://doi.org/10.1016/j.plantsci.2008.01.018
Zhang X, Zhang S, Xu X, Li T, Gong G, Jia Y, Li Y, Deng L (2010) Tolerance and accumulation characteristics of cadmium in Amaranthus hybridus L. J Hazard Mater 180:303–308. https://doi.org/10.1016/j.jhazmat.2010.04.031
Zhang L, Li X, Ma B, Gao Q et al (2017) The tartary buckwheat genome provides insights into Rutin biosynthesis and abiotic stress tolerance. Mol Plant 10:1224–1237. https://doi.org/10.1016/j.molp.2017.08.013
Zhao C, Liu B, Piao S, Wang X et al (2017) Temperature increase reduces global yields of major crops in four independent estimates. Proc Natl Acad Sci 114:9326–9331
Zhou Y, Underhill SJ (2017) Breadfruit (Artocarpus altilis) DELLA genes: gibberellin-regulated stem elongation and response to high salinity and drought. Plant Growth Regul 83:375–383
Zhou Y, Underhill SJ (2019) A dwarf phenotype identified in breadfruit (Artocarpus altilis) plants growing on marang (A. odoratissimus) rootstocks. Horticulturae 5:40
Zhou Y, Taylor M, Underhill S (2014) Dwarfing of breadfruit (Artocarpus altilis) trees: opportunities and challenges. J Exp Agric Int 4:1743–1763. https://doi.org/10.9734/AJEA/2014/12012
Zhu YY, Zeng HQ, Dong CX, Yin XM, Shen QR, Yang ZM (2010) microRNA expression profiles associated with phosphorus deficiency in white lupin (Lupinus albus L.). Plant Sci 178:23–29. https://doi.org/10.1016/j.plantsci.2009.09.011
Acknowledgement
This work was supported by the CGIAR Research Program (CRP) on Grain Legumes and Dryland Cereals and the National key research and development project of China (2020YFE0202300) and the Agricultural Science and Technology Innovation Program.
Funding
None.
Author information
Authors and Affiliations
Contributions
DAO and BS planned the review, generated an outline, oversaw the general structure and write-up and edited the manuscript. EOM and SNK searched the literature, assembled data on orphan crops, generated review tables and drafted sections of the review.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interests.
Additional information
Communicated by Brent Hulke.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kamenya, S.N., Mikwa, E.O., Song, B. et al. Genetics and breeding for climate change in Orphan crops. Theor Appl Genet 134, 1787–1815 (2021). https://doi.org/10.1007/s00122-020-03755-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00122-020-03755-1