Abstract
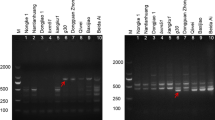
Fusarium wilt caused by the fungus Fusarium oxysporum f. sp. cubense race 4 (FOC4) results in vascular tissue damage and ultimately death of banana (Musa spp.) plants. Somaclonal variants of in vitro micropropagated banana can hamper success in propagation of genotypes resistant to FOC4. Early identification of FOC4 resistance in micropropagated banana plantlets is difficult, however. In this study, we identified sequence-characterized amplified region (SCAR) markers of banana associated with resistance to FOC4. Using pooled DNA from resistant or susceptible genotypes and 500 arbitrary 10-mer oligonucleotide primers, 24 random amplified polymorphic DNA (RAPD) products were identified. Two of these RAPD markers were successfully converted to SCAR markers, called ScaU1001 (GenBank accession number HQ613949) and ScaS0901 (GenBank accession number HQ613950). ScaS0901 and ScaU1001 could be amplified in FOC4-resistant banana genotypes (“Williams 8818-1” and Goldfinger), but not in five tested banana cultivars susceptible to FOC4. The two SCAR markers were then used to identify a somaclonal variant of the genotype “Williams 8818-1”, which lost resistance to FOC4. Hence, the identified SCAR markers can be applied for a rapid quality control of FOC4-resistant banana plantlets immediately after the in vitro micropropagation stage. Furthermore, ScaU1001 and ScaS0901 will facilitate marker-assisted selection of new banana cultivars resistant to FOC4.






Similar content being viewed by others
Abbreviations
- FOC:
-
Fusarium oxysporum f. sp. cubense
- FOC4:
-
Fusarium oxysporum f. sp. cubense race 4
- RAPD:
-
Random amplified polymorphic DNA
- SCAR:
-
Sequence-characterized amplified region
- p.i.:
-
Post inoculation
References
Food and Agricultural Organization (FAO, Geneva) (2009) www.fao.org/production/faostat
Risterucci AM, Hippolyte I, Perrier X, Xia L, Caig V, Evers M, Huttner E, Kilian A, Glaszmann JC (2009) Development and assessment of diversity arrays technology for high-throughput DNA analyses in Musa. Theor Appl Genet 119:1093–1103. doi:10.1007/s00122-009-1111-5
Ploetz RC, Herbert J, Sebasigari K, Hernandez JH, Pegg KG, Ventura JA, Mayato LS (1990) Importance of Fusarium wilt in different banana-growing regions. In: Ploetz RC (ed) Fusarium wilt of banana. American Phytopathological Society, St. Paul, pp 9–16
Getha1 K, Vikineswary S (2002) Antagonistic effects of Streptomyces violaceusniger strain G10 on Fusarium oxysporum f. sp. cubense race 4: indirect evidence for the role of antibiosis in the antagonistic process. J Ind Microbiol Biotechnol 28:303–310. doi:10.1038/sj.jim.7000247
Moore NY, Pegg KG, Bentley S, Smith LJ (2001) Fusarium wilt of banana: global problems and perspectives. In: Molina AB, Nikmasdek NH, Liew KW (eds) Banana fusarium wilt management: towards sustainable cultivation. INIBAP-ASPNET, Los Banos, pp 11–30
Saravanan T, Muthusamy M, Marimuthu T (2003) Development of integrated approach to manage the fusarial wilt of banana. Crop Prot 22:1117–1123. doi:10.1016/S0261-2194(03)00146-7
Pegg KG, Moore NY, Bentley S (1966) Fusarium wilt of banana in Australia: a review. Aust J Agric Res 47:637–650
Bentley S, Pegg KG, Moore NY, Davis RD, Buddenhagen IW (1998) Genetic variation among vegetative compatibility groups of Fusarium oxysporum f. sp. cubense analyzed by DNA fingerprinting. Phytopathology 88:1283–1293
Ploetz RC (2005) Panama disease, an old nemesis rears its ugly head: part 1, the beginnings of the banana export trades. Plant Health Prog. doi:10.1094/PHP-2005-1221-01-RV
Ploetz R, Pegg K (2000) Fungal disease of the root, corm and pseudosteam. In: Jones DR (ed) Diseases of banana abaca and enset. CABI, Wallingford, pp 143–171
Lemanceau P, Bakker PAHM, Dekogel WJ, Alabouvette C, Schippers B (1992) Effect of pseudobactin 358 production of Pseudomonas putida wcs 358 on suppression of Fusarium wilt of carnations by non pathogenic Fusarium oxysporum FO47. Appl Environ Microbiol 58:2978–2982
Nel B, Steinberg C, Labuschagne N, Viljoen A (2006) The potential of nonpathogenic Fusarium oxysporum and other biological control organisms for suppressing Fusarium wilt of banana. Plant Pathol 55:217–223. doi:10.1111/j.1365-3059.2006.01344.x
Leeman M, Vanpelt JA, Den Ouden FM, Heinsbroek M, Bakker PAHM, Schippers B (1996) Iron availability affects induction of systematic resistance to Fusarium wilt to radish by Pseudomonas fluorescens. Phytopathology 86:149–155
Pocasangre L (2000) Biological enhancement of banana tissue culture plantlets with endophytic fungi for the control of the burrowing nematode Radopholus similes and the Panama disease (Fusarium oxysporum f. sp. cubense). PhD Thesis, University of Bonn, Bonn
Vu TT, Hauschild R, Sikora RA (2006) Fusarium oxysporum endophytes induced systemic resistance against Radopholus similis on banana. Nematology 8:847–852. doi:10.1163/156854106779799259
Mendoza AR, Sikora RA (2009) Biological control of Radopholus similis in banana by combined application of the mutualistic endophyte Fusarium oxysporum strain 162, the egg pathogen Paecilomyces lilacinus strain 251 and the antagonistic bacteria Bacillus firmus. Biocontrol 54:263–272. doi:10.1007/s10526-008-9181-x
Weber OB, Muniz CR, Vitor AO, Freire FCO, Oliveira VM (2007) Interaction of endophytic diazotrophic bacteria and Fusarium oxysporum f. sp. cubense on plantlets of banana ‘Maça’. Plant Soil 298:47–56. doi:10.1007/s11104-007-9335-0
Paparu P, Dubois T, Gold CS, Niere B, Adipala E, Coyne D (2008) Screenhouse and field persistence of nonpathogenic endophytic Fusarium oxysporum in Musa tissue culture plants. Microb Ecol 55:561–568. doi:10.1007/s00248-007-9301-7
Forsyth LM, Smith LJ, Aitken EAB (2006) Identification and characterization of non-pathogenic Fusarium oxysporum capable of increasing and decreasing Fusarium wilt severity. Mycol Res 110:929–935. doi:10.1016/j.mycres.2006.03.008
Diener AC, Ausubel FM (2005) Resistance to Fusarium oxysporum 1, a dominant Arabidopsis disease-resistance gene, is not race specific. Genetics 171:305–321. doi:10.1534/genetics.105.042218
Brotman Y, Kovalski I, Dogimont C, Pitrat M, Portnoy V, Perl-Treves NKR (2005) Molecular markers linked to papaya ring spot virus resistance and Fusarium race 2 resistance in melon. Theor Appl Genet 110:337–345. doi:10.1007/s00122-004-1845-z
Wang PZ, Su L, Qin L, Hu B, Guo WZ, Zhang TZ (2009) Identification and molecular mapping of a Fusarium wilt resistant gene in upland cotton. Theor Appl Genet 119:733–739. doi:10.1007/s00122-009-1084-4
Swetha PR, Subramanian RB (2008) Isolation and molecular analysis of R-gene in resistant Zingiber officinale (ginger) varieties against Fusarium oxysporum f. sp. zingiberi. Bioresour Technol 99:4540–4543
Sharma KD, Winter P, Kahl G, Muehlbauer FJ (2004) Molecular mapping of Fusarium oxysporum f. sp. ciceris race 3 resistance gene in chickpea. Theor Appl Genet 108:1243–1248. doi:10.1007/s00122-003-1561-0
Simons G, Groenendijk J, Wijbrandi J, Reijans M, Groenen J, Diergaarde P, Lee TVD, Bleeker M, Onstenk J, Both DM, Haring M, Mes J, Cornelissen B, Zabeau M, Vos P (1998) Dissection of the Fusarium I2 gene cluster in tomato reveals six homologs and one active gene copy. Plant Cell 10:1055–1068
Mutlu N, Boyaci FH, Göçmen M, Abak K (2008) Development of SRAP, SRAP-RGA, RAPD and SCAR markers linked with a Fusarium wilt resistance gene in eggplant. Theor Appl Genet 117:1303–1312. doi:10.1007/s00122-008-0864-6
Dita MA, Waalwijk C, Buddenhagen IW, Souza MT Jr, Kema GHJ (2010) A molecular diagnostic for tropical race 4 of the banana Fusarium wilt pathogen. Plant Pathol 59:348–357. doi:10.1111/j.1365-3059.2009.02221.x
Lin YH, Chang JY, Liu ET, Chao CP, Huang JW, Chang PFL (2009) Development of a molecular marker for specific detection of Fusarium oxysporum f. sp. cubense race 4. Eur J Plant Pathol 123:353–365. doi:10.1007/s10658-008-9372-4
Xiao W, Huang XL, Huang X, Chen YP, Dai XM, Zhao JT (2007) Plant regeneration from protoplasts of Musa acuminate cv. Mas (AA) via somatic embryogenesis. Plant Cell Tiss Organ Cult 90:191–200. doi:10.1007/s11240-007-9241-4
Murashige CT, Skoog FA (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Plant Physiol 15:473–497
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight DNA. Nucleic Acids Res 8:4321–4325
Paran I, Michelmore R (1993) Development of reliable PCR-based markers linked to downy mildew resistance genes in lettuce. Theor Appl Genet 85:985–993. doi:10.1007/BF00215038
Nash SM, Snyder WC (1962) Quantitative estimations by plate counts of propagules of the bean root rot Fusarium in field soil. Phytopathology 52:567–572
Muralidharan K, Wakeland EK (1993) Concentration of primer and template qualitatively affects products in random amplified polymorphic DNA PCR. Biotechniques 14:362–364
Agarwal M, Shrivastava N, Padh H (2008) Advances in molecular marker techniques and their applications in plant sciences. Plant Cell Rep 27:617–631. doi:10.1007/s00299-008-0507-z
Arens P, Mansilla C, Deinum D et al (2010) Development and evaluation of robust molecular markers linked to disease resistance in tomato for distinctness, uniformity and stability testing. Theor Appl Genet 120:655–664. doi:10.1007/s00122-009-1183-2
Cao W, Hughes GR, Ma H, Dong Z (2001) Identification of molecular markers for resistance to Seportia nodorum blotch in durum wheat. Theor Appl Genet 102:551–554. doi:10.1007/s001220051681
Singh M, Chaudhary K, Singal HR, Magill CW, Boora KS (2006) Identification and characterization of RAPD and SCAR markers linked to anthracnose resistance gene in sorghum [Sorghum bicolor (L.) Moench]. Euphytica 149:179–187. doi:10.1007/s10681-005-9065-4
Larsen HH, Masur H, Kovacs JA, Gill VJ, Silcott VA, Kogulan P, Maenza J, Smith M, Lucey DR, Fischer SH (2002) Development and evaluation of a quantitative, touch-down, real-time PCR assay for diagnosing Pneumocystis carinii pneumonia. J Clin Microbiol 40:490–494. doi:10.1128/JCM.40.2.490-494.2002
Ramage CM, Borda AM, Hamill SD, Smith MK (2004) A simplified PCR test for early detection of dwarf off-types in micropropagated Cavendish banana (Musa spp. AAA). Sci Hort 103:145–151. doi:10.1016/j.scienta.2004.04.015
Li J, Chen S, Evans DH (2001) Typing and subtyping influenza virus using DNA microarrays and multiplex reverse transcriptase PCR. J Clin Microbiol 39:696–704. doi:10.1128/JCM.39.2.696-704.2001
Wu YL, Yi GJ, Peng XX (2010) Rapid screening of Musa species for resistance to Fusarium wilt in an in vitro bioassay. Eur J Plant Pathol 128:409–415
Martin GB, Brommonschenkel SH, Chunwongse J, Frary A, Ganal MW, Spivey R, Wu T, Earle ED, Tanksley SD (1993) Map-based cloning of a protein kinase gene conferring disease resistance in tomato. Science 262:1432–1436. doi:10.1126/science.7902614
Acknowledgments
This research was financed by funds from the National Key Basic Research Special Foundation of China (2005DKA21000-5-05) and supported by the Programs for the National Public Welfare Scientific Research Institution Foundation (NYS200703) and the Hainan Provincial Natural Science Foundation (No. 807039).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, W., Hu, Y., Sun, D. et al. Identification and evaluation of two diagnostic markers linked to Fusarium wilt resistance (race 4) in banana (Musa spp.). Mol Biol Rep 39, 451–459 (2012). https://doi.org/10.1007/s11033-011-0758-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-011-0758-6