Abstract
The ecosystem services approach to conservation is becoming central to environmental policy decision making. While many negative biological invasion-driven impacts on ecosystem structure and functioning have been identified, much less was done to evaluate their ecosystem services. In this paper, we focus on the often-overlooked ecosystem services provided by three notable exotic ecosystem engineering bivalves, the zebra mussel, the quagga mussel, and the golden mussel. One of the most significant benefits of invasive bivalves is water filtration, which results in water purification and changes rates of nutrient cycling, thus mitigating the effects of eutrophication. Mussels are widely used as sentinel organisms for the assessment and biomonitoring of contaminants and pathogens and are consumed by many fishes and birds. Benefits of invasive bivalves are particularly relevant in human-modified ecosystems. We summarize the multiple ecosystem services provided by invasive bivalves and recommend including the economically quantifiable services in the assessments of their economic impacts. We also highlight important ecosystem disservices by exotic bivalves, identify data limitations, and future research directions. This assessment should not be interpreted as a rejection of the fact that invasive mussels have negative impacts, but as an attempt to provide additional information for scientists, managers, and policymakers.
Similar content being viewed by others
Introduction
Ecosystem services consist of flows of materials, energy, and information from natural capital stocks that are of fundamental importance to humankind (Costanza et al., 1997, 2014; Millennium Ecosystem Assessment, 2005). Interest in the evaluation of ecosystem services has grown rapidly in the last decades, partly fostered by estimates suggesting that their economic assets (~ 33 trillion US$ per year) exceed the global gross domestic product (Costanza et al., 1997, 2014). The concept of ecosystem services gained further attention in 2005, when the United Nations published its millennium ecosystem assessment (MEA) (Millennium Ecosystem Assessment, 2005). The objective of the MEA was to assess the consequences of ecosystem changes for human well-being and to establish the scientific bases for the actions needed to ensure the conservation and sustainable use of ecosystems and their contributions to humankind. Ecosystem services are defined by the MEA as “the benefits that people can obtain from ecosystems”. The Common International Classification of Ecosystem Services recognized three broad categories of services: Provisioning Services (e.g., provision of food, water, fuel), Regulating and Maintenance Services (e.g., water purification, climate regulation, pollination), and Cultural Services (e.g., education, recreation, tourism, aesthetic, and spiritual values) (Millennium Ecosystem Assessment, 2005; Haines-Young & Potschin, 2011).
The ecosystem services approach to conservation is becoming central to many areas of environmental policy decision making, and supporting information, both economic and non-economic, is increasingly needed. In this context, many negative biological invasion-driven effects on the structure and functioning of ecosystems have been identified, but much less has been done evaluating and/or monetizing the ecosystem services provided by invasive species. The economic impacts of invasive species on these services are often neither quantified nor incorporated into economic impact assessments (e.g., Diagne et al., 2021), and many of their ecosystem services that are difficult to monetize are regularly ignored (Pejchar & Mooney, 2009; Thompson, 2014; Jernelöv, 2017; Boltovskoy et al., 2022). A vivid example are the three notable exotic ecosystem engineering bivalves, Dreissena polymorpha (Pallas, 1771) (the zebra mussel), D. rostriformis bugensis (Andrusov, 1897) (the quagga mussel), and Limnoperna fortunei (Dunker, 1857) (the golden mussel).
Until around the seventeenth century, the geographic range of D. polymorpha was limited to the Ponto-Caspian basin, but in late 1800s—early 1900s the species started spreading rapidly across eastern and western Europe using canals built to connect shipping routes between the Black Sea and the Baltic Sea basins, and in the 1980s it reached North America, most probably with ship ballast water (Mordukhai-Boltovskoi, 1960; Kerney & Morton, 1970; Kinzelbach, 1992; Starobogatov & Andreeva, 1994; Karatayev et al. 2003, 2007b, 2008; Pollux et al., 2010; bij de Vaate, 2010; bij de Vaate et al., 2014). In contrast to zebra mussels, D. r. bugensis started spreading beyond its native range only in the middle of the twentieth century. Its geographic expansion was initially slow but has increased dramatically since the 1980s in both Europe and North America (Zhulidov et al., 2004, 2010; Karatayev et al., 2007b, 2011a, 2015a; van der Velde et al., 2010; Benson, 2014; Matthews et al., 2014; Orlova, 2014), fostering intensive research on the species (reviewed in Karatayev et al., 2015a).
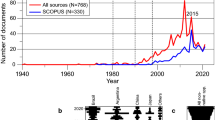
Although L. fortunei is native to southern China, it colonized Cambodia, Vietnam, Laos, Thailand in historical times, and northern China, Taiwan and Korea around the 1940s to 1980s, its main expansion beyond continental Asia started in the early 1990s (Japan, South America; Darrigran & Pastorino, 2004; Boltovskoy, 2015b). Thus, not only did dreissenids started spreading earlier, but they also colonized countries with much higher scientific outputs (Europe, North America) than the golden mussel (several Asian and South American countries). As of December 2021, ~ 5000 documents on Dreissena had been published since 1771 (13% of them on D. r. bugensis alone or together with D. polymorpha) (Limanova, 1964, 1978; reviewed in Karatayev et al., 2015a; Karatayev and Burlakova, accepted; SCOPUS), and ~ 330 on Limnoperna. Therefore, information on D. polymorpha is much more abundant than that on D. r. bugensis, and especially on L. fortunei. Inevitably, this imbalance is reflected in this research work, which is largely based on surveys on dreissenids, chiefly D. polymorpha, and on both dreissenid species, as they have often been treated jointly (Karatayev et al., 2007b; Ward & Ricciardi, 2007; Higgins & Vander Zanden, 2010; Kelley et al., 2010; Kissman et al., 2010), although they differ substantially in their spread dynamics, environmental tolerance, and within-waterbody distribution patterns, often resulting in different ecosystem impacts (Karatayev et al., 1997, 1998, 2002, 2011b, 2014a, 2015a, 2021a; Nalepa, 2010; Benson, 2014).
Dreissena spp. and L. fortunei have similar life histories and share many ecological and functional traits. As opposed to almost all other freshwater bivalves that lack a free-living planktonic stage and whose adults burrow in the sediments, Dreissena spp. and L. fortunei have planktonic larvae and their adults are epifaunal, attaching to the substrate by a byssus (Zhadin, 1946; Darrigran & Damborenea, 2005). Thus, they occupy a novel ecological niche in the invaded freshwaters of the northern and southern hemispheres (Johnson & Carlton, 1996; Karatayev et al., 1997, 2007a; Darrigran, 2002). Due to their high rates of spread and densities, the large numbers of waterbodies colonized, and the extent of their ecological and economic impacts, both species of Dreissena and L. fortunei are considered among the most aggressive freshwater invaders (Karatayev et al., 2007b, 2011a, 2015a; Higgins & Vander Zanden, 2010; Kelley et al., 2010; Boltovskoy & Correa, 2015; Ludwig et al., 2021). The biomass of dreissenids often exceeds the combined biomass of all native benthos by at least one order of magnitude and can represent over 90% of the combined biomass of all pelagic and bottom invertebrates combined (Vanderploeg et al., 2002). These exotic bivalves are very effective ecosystem engineers (i.e., species that “directly or indirectly control the availability of resources to other organisms by causing physical state changes in biotic or abiotic materials”; Jones et al., 1994, 1997), altering both ecosystem structure and function, and can have dramatic impacts on the waterbodies invaded (reviewed in Karatayev et al., 2002, 2007a; Vanderploeg et al., 2002; Gutierrez et al., 2003; Zhu et al., 2006; Sousa et al., 2009; Darrigran & Damborenea, 2011; Boltovskoy, 2015a). These services may be particularly significant in “novel ecosystems” (i.e., systems with significant novel elements such as invasive species that are the result of deliberate or inadvertent human action, Hobbs et al., 2006) defined by Hobbs et al. (2013) as “a system of abiotic, biotic, and social components (and their interactions) that, by virtue of human influence, differ from those that prevailed historically, having a tendency to self-organize and manifest novel qualities without intensive human management”.
Even though these mussels (especially D. polymorpha) have been studied intensively, assessments of their effects in the areas invaded vary widely, from almost entirely negative to largely mixed and often clearly positive. While in North America the effects of dreissenids are generally considered as predominantly negative (Nalepa, 2010; Ward & Ricciardi, 2013), many positive influences have been described, especially in Europe (e.g., Binimelis et al., 2007; Karatayev et al., 1994a, b; Smit et al., 1993; Ram & Palazzolo, 2008; Dionisio Pires et al., 2010; Gomes et al., 2018; Wang et al., 2021). Mixed effects have also been reported for L. fortunei (Boltovskoy & Correa, 2015; Boltovskoy, 2017).
These mussels are also notorious for producing major negative economic impacts by biofouling of human-made facilities, including power generating, drinking water and other industrial plants, water conveyance structures, and watercraft, often requiring costly maintenance operations conceivably in the range of hundreds to thousands of millions of US$ per year worldwide (see “Disservices, caveats, and unresolved issues” section). Ecological impacts include the overgrowth and/or competition for food with native benthic and pelagic species, promotion, in certain conditions, of the growth of bottom filamentous algae and blooms of blue-green algae, transfer of contaminants up the food web, etc. (see “Disservices, caveats, and unresolved issues” section).
As with all organisms, both native and introduced, the effects of Dreissena spp. and L. fortunei are often mixed, context- and stakeholder-dependent. However, most policy and management actions concerning invasive species, as well as much of the scientific literature, rely on the assumption that these species have overwhelmingly negative impacts (Perrings et al., 2001; Pimentel, 2011; Simberloff & Vitule, 2013; Diagne et al., 2021).
Taking into account the large body of literature describing the negative impacts of these invasive mussels, in this paper we focus on their positive effects and review their ecosystem and economic services. Our rationale is based on the assumption that if a particular effect of a native species is considered as a service (e.g., water clarity increase by native unionid mussels; Vaughn, 2017; Vaughn & Hoellein, 2018), the same effect is also a service when provided by exotic bivalves. Further, if a baneful process (e.g., water pollution, eutrophication) driven by other mechanisms or organisms is mitigated by these exotic bivalves, we also consider this mitigation as a service. For example, water filtration by native mussels (Unionidae) can offset the negative impact of declines in water transparency, which is considered a service (Vaughn & Hoellein, 2018). However, because they commonly occur in higher densities, invasive mussels (Dreissena spp., L. fortunei) are much more efficient than native mussels in the clarification of water (Diggins, 2001; Leuven et al., 2014; Collas et al., 2020), but when only their negative impacts are considered (which, admittedly, are sizeable, especially for the industry; see “Disservices, caveats, and unresolved issues” section), they get no credit for these benefits (Boltovskoy et al., 2022).
In the sections below we have made efforts to follow the framework of the MEA (Table 1), but the assignment of the effects discussed to either of these categories is debatable. Thus, “Provisioning” and “Regulating” services are often tightly intertwined, and the regulation of a feature or process can often involve the provisioning of resources. For example, habitat modification by invasive mussels involves the regulation of environmental variables on the substrates colonized, but also the provisioning of food for invertebrates that co-inhabit them (see below). In addition, the role invasive mussels play in the ecosystem (ecosystem functions) are much wider than the ecosystem services defined by the MEA as those that directly or indirectly contribute to human well-being. Ecosystem functions are “dynamic processes that determine the amount, forms, distribution, fluxes, import and export of energy and various materials” (Strayer, 2012), and while many contribute to ecosystem services, others are not directly linked to human benefits. The maintenance of ecological functions, and thus ecosystem services, is key for conservation in the race to cope with global environmental change, and exotic species that perform ecological functions similar to those of natives, should be valued and deserve recognition (Vizentin-Bugoni et al., 2019; Leuzinger & Rewald, 2021).
Analysis of published studies
A search of SCOPUS performed in December 2021 using “Dreissena” or “zebra mussel” or “quagga mussel” in the abstract, title or keywords yielded 2853 publications, whereas 333 documents were retrieved for “Limnoperna” or “golden mussel” (after eliminating duplicates and non-applicable titles). All the publications were separated into eight major categories: (1) ecosystem services (including positive impacts on ecosystems/communities, such as water purification, reduction in phytoplankton, nutrient cycles (e.g., phosphorus reduction), food for other animals, positive effects on the native benthos consumed by fish and birds, macrophytes etc., biomonitoring, bioremediation, various uses, mussel farming, etc.); (2) general biology (including anatomy and morphology, physiology, life history traits, symbionts, parasites, and ecotoxicology); (3) ecology (ecological traits and habitat requirements); (4) management (risk analysis, early detection, prevention, control, management, eradication, etc.); (5) impacts (studies that assess negative ecological and/or economic impacts on ecosystems or native communities/species, including competition, transfer of contaminants up the food chain, and others, see below); (6) dispersal (past and future dispersal, vectors and pathways); (7) taxonomy, systematics and evolution (phylogeny, phylogeography, genetic diversity, taxonomy, systematics), and (8) other studies that did not fit into the above categories. Publications were separated based on our interpretation of the results provided by authors. Documents often covered more than one subject but were tallied only once and assigned to the most relevant category only. As mentioned above, ecosystem functions (defined by the MEA as those that directly or indirectly contribute to human well-being) are much wider than the ecosystem services provided by natural capital assets. Therefore, papers dealing with ecosystem functions resulting in predominantly negative ecological impact were included in the impact category, while publications dealing with predominantly positive ecological impacts were included in the ecosystem services category.
Considering the three species jointly, the largest number of publications was centered on ecosystem services (33%), followed by general biology (19%) and ecology (17%) (Fig. 1). Publications on impact and management constituted 10% each, 8% of the papers were on dispersal, and 2% on taxonomy, systematics and evolution. This analysis indicates that despite the large economic and ecological consequences of freshwater mussel invasions, there is ample recognition of the ecosystem functions and services they provide. For dreissenids, in the ecosystem services group, the largest number of publications (28%) were on the use of dreissenids as environmental bioindicators, 16% reported fish and bird consumption of dreissenids, 14% on reductions in phytoplankton, 9% described their positive effect on the benthos, 6 and 7%, respectively, were on improvement in water clarity and their effects on nutrient cycles. The largest number of papers in the impact category was on the effect of dreissenids on unionids (32%), impacts on water utilities (10%), their negative effect on the deep-water amphipod Diporeia (11%) and fishes (8%), and on the transfer of contaminants (10%).
Regulating and supporting/maintenance
One of the most significant impacts of invasive bivalves is their water filtration for feeding and respiration, which involves water purification, enhancement of water clarity, the rates of nutrient recycling and their sequestration, and decreases in phytoplanktonic primary production. These services are particularly relevant in already highly impacted ecosystems.
Biofiltration (water purification)
Mussels filter water for both feeding and respiration. As water is moved across their gills, particulates are removed from the water column. Both dreissenids and the golden mussel feed on organic seston in general, including detritus, bacterioplankton, phytoplankton, and zooplankton (reviewed in Karatayev et al., 2007a), retaining particles from < 1 to > 750 μm in size (Ten Winkel & Davids, 1982; Mikheev et al., 1994; Roditi et al., 1996; Boltovskoy et al., 2015; Rojas Molina et al., 2015; Xia et al., 2020). Filtered particles are either ingested (producing feces), or rejected (pseudofeces), but in both cases they are bound in mucus and deposited on the bottom (Baker et al., 2000; Morton, 2015). Filtration rates vary depending on temperature, mussel species and size, seston composition and concentration, water velocity, etc. (Kryger & Riisgard, 1988; Karatayev et al., 1997; Baldwin et al., 2002; Elliott et al., 2008; Boltovskoy et al., 2015; Tokumon et al., 2015; Xia et al., 2020). Karatayev et al. (1997) estimated zebra mussel filtration rates at 35–110 mL per g (total wet weight) per hour, which is within the range of values reported for L. fortunei (Boltovskoy et al., 2015). For quagga mussels, similar or higher rates have been reported (Ackerman, 1999; Diggins, 2001; Baldwin et al., 2002; Naddafi & Rudstam, 2013; Mei et al., 2016).
Consumed particulate organic matter is thus metabolized, transformed into mussel tissue and shell, becoming available for a wide range of animals that cannot feed on small, suspended particles (see below). Shell materials (e.g., C, Ca, Na, Cl, K, Mg, Ba, Sr, U; Immel et al., 2016) are removed from the pelagic system and are recycled or buried after mussel death (reviewed in Karatayev et al., 1997, 2002; Strayer & Malcom, 2007b; Ozersky et al., 2015). However, in South America preservation of dead L. fortunei (and native mollusc) shells is restricted by the low concentrations of Ca in the water (Boltovskoy et al., 2009b; Correa et al., 2015).
In the summer, mussels can potentially filter volumes of water equivalent to those of the entire waterbody in a few days to a few months (Karatayev et al., 1997, 2007a; Boltovskoy et al., 2009a). Mussel filtration has dramatic impacts on lakes and rivers yielding particulate suspended matter reductions of up to over 60%, Secchi disc depth increases up to 200%, and 50–250% increases in light penetration (Karatayev et al., 1997, 2018a; Effler & Siegfried, 1998; Strayer et al., 1999; Boltovskoy et al., 2009a; Higgins & Vander Zanden, 2010; Kelly et al., 2010; Higgins, 2014; Mayer et al., 2014; Barbiero et al., 2018; Tokumon et al., 2018). In Embalse de Río Tercero (Argentina), L. fortunei was estimated to potentially be able to filter the entire volume of the waterbody (0.48 km3) in 1–2 days (Boltovskoy et al., 2009a). Sediment accumulation rates can double after the invasion (Lvova, 1977, 1979; Tokumon et al., 2018), and the percent of organic matter in the sediments associated with the mussels increase up to threefold (Howell et al., 1996; Roditi et al., 1997; Sardiña et al., 2008; Tokumon et al., 2018).
In economic terms, the services conveyed by the mussels’ filtration have been assessed in several surveys providing information on the potential gains and losses involved. One measure is based on the changes in waterfront property values in association with changes in the water clarity of the waterbodies involved (Limburg et al., 2010; Walsh et al., 2016), illustrating that economic impacts can be captured by the market economy and used in economic cost–benefit analyses of invasive species (see details under “Cultural Services” below).
Economic benefits of mussel filtration are not restricted to changes in property values, but can also alleviate the costs of drinking water treatment. Although clean raw surface water (in this context, mussel pre-filtered) is significantly cheaper to process than turbid water (Price & Heberling, 2018), to the best of our knowledge only one survey attempted to evaluate this difference due to filtration by invasive mussels in economic terms. Wang et al. (2021) estimated that biofiltration by D. polymorpha in several Dutch rivers saves potabilization plants from 110 to 12,000 € per million m3 of water processed, and under a scenario of reduced metal pollution (and, therefore, enhanced filtration by Dreissena), these savings were estimated to increase by 89 € per million of m3. A few studies analyzed the feasibility of using L. fortunei beds or their shells for the removal of contaminants from water, wastewater, or the mussels’ tissues (Rombaldi et al., 2015; Zhang et al., 2015; Gomes et al., 2018; Cerqueira et al., 2019; Mantovani et al., 2020), but none has yet been applied beyond experimental settings.
Reduction of phytoplanktonic primary production
Invasive mussels can improve water quality by consuming a large fraction of the phytoplanktonic production. The last seven decades of changes in the North American Laurentian Great Lakes are a prime example of this process. In the 1950s and 1960s, excessive algal growth due to anthropogenic eutrophication, caused by increasing human settlement and phosphorus loadings, was identified as a major threat to the water quality of the Great Lakes (Beeton, 1961, 1965; Ayers, 1962). In attempts to reverse this trend, in 1972 the USA and Canada signed the Great Lakes Water Quality Agreement (GLWQA; International Joint Commission—IJC, 1972), which enforced improvements in the treatment of sewage and reductions of point sources of phosphorus. The target of the GLWQA was to restore the open waters of the upper Great Lakes (Superior, Huron, and Michigan) to oligotrophic conditions, and lakes Erie and Ontario to mesotrophic/oligo-mesotrophic conditions (IJC, 1978). In the early 1970s, none of the lakes, except Lake Superior, met the target trophic status, but currently the open waters of all these lakes (with the exception of the western and central basins of Lake Erie) have over accomplished it (Dove & Chapra, 2015). This oligotrophication was associated with a dramatic decrease in anthropogenic nutrient inputs into the Great Lakes, suggesting that the concerted binational management actions were a major success. However, the most dramatic system-wide changes in the Great Lakes occurred only after the proliferation of large populations of quagga mussels had caused pronounced and long-lasting impacts, including increases in Secchi depths, declines in total phosphorus, chlorophyll, phytoplanktonic primary production, and phytoplankton and zooplankton biomass, resulting in the oligotrophication of lakes Michigan, Huron, and the eastern basin of Lake Erie (Barbiero & Tuchman, 2004; Dobiesz & Negel, 2009; Dove, 2009; Mida et al., 2010; Barbiero et al., 2011, 2012, 2018; Evans et al., 2011; Bunnell et al., 2014; Pothoven & Fahnenstiel, 2014; Reavie et al., 2014; Dove & Chapra, 2015; Pothoven & Vanderploeg, 2020; reviewed in Karatayev & Burlakova, accepted). Significant reductions in phytoplankton and/or chlorophyll a concentrations due to L. fortunei were also noticed in South America, both in enclosure experiments (Cataldo et al., 2012a), and in field observations comparing before versus after L. fortunei introduction conditions (Boltovskoy et al., 2009a).
The increased water clarity is largely the result of mussel grazing of suspended particulate matter and its deposition on the bottom bound in mucus (see above). In southern Lake Michigan, dreissenids were estimated to consume over 50% of the annual net primary production (Tyner et al., 2015), and 26–77% in the western basin of Lake Erie (Madenjian, 1995; Boegman et al., 2008). For organic carbon, the grazing and subsequent quagga mussel-mediated deposition rates of offshore Lake Michigan are 1.4–4.1 times higher than passive sedimentation. These populations can graze 100% of the offshore organic material reaching the lake bottom, thus consuming all water-borne offshore carbon in 18–42 days, depending on the season (Tyner et al., 2015).
The ability of zebra mussels to reduce phytoplankton biomass and increase water clarity has long been recognized (reviewed in Karatayev et al., 1997, 2002, 2015a; Higgins & Vander Zanden, 2010), and mussels are used in several European waterbodies for biomanipulation purposes to decrease the effects of anthropogenic eutrophication (see “Bioremediation” below).
Nutrient cycling and sequestration
Benthic filter feeders can exert strong bottom-up effects on aquatic systems by altering the stoichiometry and the rates of nutrient recycling, as well as the spatial distribution of nutrients (Stanczykowska & Lewandowski, 1993; Mellina et al., 1995; Arnott & Vanni, 1996; Naddafi et al., 2009). The nearshore shunt hypothesis posits that dreissenids have modified the physical environment, altered nutrient recycling pathways, and increased nutrient retention in the nearshore (Hecky et al., 2004). In the Great Lakes, in recent years the colonization of the mid-depths and the profundal zones by quagga mussels have expanded these impacts to deeper regions as well (Vanderploeg et al., 2010; Karatayev et al., 2021a).
In lakes and reservoirs, phosphorus (P) concentrations are regulated by the balance of P sources and sinks, including inputs from the watershed, removal with outflow, and net burial in the sediments (Katsev, 2017; Li et al., 2018a ). In the sediments, a large fraction of the deposited P can be recycled and released back to the water column, but the proportions of recycled P vary widely. Filter-feeding dreissenids remove particulate P from water, and their P deposition rates are tenfold greater than passive P settling rates, which involves a major increase in the rates of transfer of P to the benthos, and shorter P residence times in the water column (Mosley & Bootsma, 2015). This may explain the long-term declines in total P concentrations in lakes colonized by quagga mussels (Mayer et al., 2014; Dove & Chapra, 2015). The Laurentian Great Lakes are a dramatic example of large scale reorganization of biogeochemical cycles due to the impacts of a single benthic species, the quagga mussel (Li et al., 2021). Before dreissenids invaded the Great Lakes, the recycled fraction of sedimented P varied from 10% (Lake Michigan) to 60% (Lake Erie), contributing 15–48% of all (internal and external) P inputs to the water column (Katsev, 2017). On a yearly basis, profundal mussels recycle approximately 10 times more P than the amount they sequester in their biomass (Mosley & Bootsma, 2015). Most of the P is re-mobilized (Stoeckmann & Garton, 1997), being either excreted into the water in dissolved form, or deposited on the sediment surface as feces and pseudofeces (Arnott & Vanni, 1996; Mosley & Bootsma, 2015), where it is eventually re-mineralized to dissolved P via microbial decomposition (Giles & Pilditch, 2006). However, given their huge densities in the Great Lakes colonized by dreissenids, the tissues and shells of quagga mussels now contain nearly as much phosphorus as the entire water column (Li et al., 2021).
Analysis of seven North American and European lakes with long-term data (> 15 years) spanning both pre- and post-Dreissena periods, and pre- and post-P input reductions, showed significantly lower total phosphorus in the water column after the introduction of Dreissena (Mayer et al., 2014). A meta-analysis of data from 57 lakes, 11 rivers, and 18 enclosure experiments concluded that in lakes the presence of Dreissena is associated with particulate phosphorus declines of ~ 21%, total phosphorus declines of ~ 18%, but no significant changes in soluble reactive phosphorus (Higgins, 2014). The declines were found to be persistent for up to ~ 10–20 years after the mussel’s invasion.
Johengen et al. (1995) estimated that after dreissenid populations reached a stable density in Saginaw Bay (in the early 1990s), between 52 and 682 t of P became locked up in the mussels’ tissues. Pennuto et al. (2014) found that mussels accounted for up to 95% of the benthic biomass (dry weight) in southern Lake Erie and sequestered in their tissues approximately 500 t of P (6.2 mg per g of dry weight), and 6426 t of N. In nearshore areas of Lake Ontario, the amount of P sequestered in mussel tissue was around 160 t (Pennuto et al., 2012). Similar values were reported for European waterbodies. Goedkoop et al. (2011) quantified the biomass and accumulation of P and N in zebra mussels in eutrophic Lake Ekoln (Sweden), with a total standing stock of dreissenid biomass (dry weight) of 362 t, representing around 3.4 t of P and 36.6 t of N. Considering an average dreissenid life span of 2–3 years, the annual retention by mussels was estimated at 1.2–1.8 t of P, corresponding to 50–77% of the annual P influx from the Uppsala sewage treatment plant to the lake. The annual retention of N by zebra mussels was 13–20 t, largely equaling the annual input of N from atmospheric sources on the lake’s surface. The authors stressed that while these retention rates correspond to only a fraction of the annual P load from agricultural sources, the role of zebra mussels in nutrient budgets would be much larger if these budgets were adjusted for the bias introduced by including the input of a large fraction of refractory P (and N) (Goedkoop et al., 2011) (see also “Bioremediation" section).
The long-term P (as well as C and N) sink represented by spent Dreissena shells is also likely an overlooked ecosystem service provided by the mussels, as ~ 15–20% of whole mussel P is associated with their shells (Goedkoop et al., 2021). Dreissenids may produce up to 10 kg dry mass of shells per m2 annually and, in standing and/or hard waters, shell production rates far exceed their rates of decay, further enhancing P withdrawal (Strayer & Malcom, 2007b; Ozersky et al., 2015). Due to the high resistance to erosion of their aragonitic shells (Pathy & Mackie, 1993; Meng et al., 2018), the accumulating deposits of spent shells are likely a long-term sink for nutrients (Ozersky et al., 2015). In contrast, in South America, mollusc shells (including Limnoperna) dissolve rapidly after death due to the low Ca concentrations in the water, and very rarely preserve in the sediments (Boltovskoy et al., 2009b; Correa et al., 2015; Karatayev et al., 2015b).
The effects of these invasive mussels on nitrogen compounds are also complex and highly context-dependent. In their meta-analysis of lakes (littoral and profundal zones), rivers and enclosure experiments with and without zebra mussels, Higgins and Vander Zanden (2010) estimated differences in the concentrations of N in its various forms. Although in absolute terms differences with and without Dreissena were often high (up to 73%), due to their large variability none of 17 comparisons yielded statistically significant figures. After the introduction of L. fortunei in Río Tercero Reservoir (Argentina), total N increased by ~ 270%, chiefly due to ammonia, whereas the concentrations of nitrites and nitrates did not change significantly. In experimental conditions, N was observed to increase in the sediments (Tokumon et al., 2018) and in the water column (Cataldo et al., 2012a) in response to the presence of L. fortunei, but experimental settings may not reflect system-wide responses adequately (Higgins & Vander Zanden, 2010; Higgins, 2014; Tokumon et al., 2018).
While P and N are of particular importance due to their relevance for primary producers, Dreissena plays a major role in the recycling and sequestration of many other elements as well, including As, Ba, C, Ca, Cr, Cu, Fe, Ni, Ti, Mg, Mn, Pb, Si, Zn, V, and Al (Walz, 1978; Karatayev et al., 1994a; Wojtal-Frankiewicz & Frankiewicz, 2010; Schaller & Planer-Friedrich, 2017; Balogh et al., 2022). Dreissenids can uptake and depurate metals through their interaction with the environment depending on the seasonal change in metabolic activity, in a regulated way in the case of essential metals and passively in the case of non-essential ones (Balogh et al., 2022). Fish feeding on dreissenids accumulate < 2% of the microelements, and although 37% is released back to the water, the rest is buried in the sediments and excluded from the cycle for decadal periods (Karatayev et al., 1994a). Significant reductions in magnesium and calcium ion concentrations in the presence of zebra mussels, especially during periods of higher and more stable temperatures, were found in field experiments (Wojtal-Frankiewicz & Frankiewicz, 2010). The large lake-wide declines in calcium concentrations in Lake Erie and Ontario in the 1990s were likely due to calcium uptake by dreissenid mussels. In Lake Ontario, these declines in calcium may have further reduced the frequency and/or intensity of summer whiting events, resulting in dramatic increases in summer epilimnetic water clarity (Barbiero et al., 2006; Chapra et al., 2012). In the Finger Lakes (New York state, USA) the introduction of zebra mussels decreased calcite precipitation that began to rise in early 1800s due to chemical weathering induced by naturally acidic rains falling on freshly deforested and tilled landscapes and was further accelerated in the 1940s following industrialization and acidic rainfall associated with World War II (Lajewski et al., 2003).
Habitat modification
Engineering native and invasive organisms can cause physical changes of the environment, modifying existing and creating new habitats (Crooks, 2002). Bivalves have hard, calcium carbonate shells that increase the substratum available for other sessile species that need hard substrata for survival. Dreissena spp. and L. fortunei attach to hard substrata, conspecifics, and other organisms with byssal proteinaceous threads, creating complex two- and three-dimensional reef-like habitats for a wide range of sessile and mobile organisms that would otherwise be absent or scarce. Although in marine systems a variety of animals have a similar function (e.g., mussel beds, barnacles, coral reefs), in freshwaters only dreissenids and mytilids play this role (reviewed in Karatayev et al., 2002, 2007a; Vanderploeg et al., 2002; Gutierrez et al., 2003; Zhu et al., 2006; Sousa et al., 2009; Darrigran & Damborenea, 2011; Burlakova et al., 2012; Sylvester & Sardiña, 2015). These reefs made of live mussels and spent shells are used by many invertebrates as refuge from predation, and from physical (waves, currents) and physiological (temperature, desiccation) stress (reviewed in Karatayev et al., 1997, 2002; Stewart et al., 1998, 1999; Gutierrez et al., 2003; Burlakova et al., 2012). The effect of increased habitat complexity in mussel aggregations is reinforced by the trophic subsidy of the mussels due to their organic matter-rich feces and pseudofeces, partly retained within the colonies, and partly dispersed around them on the sediment surface. Field studies, sediment trap, mesocosm, and enclosure experiments indicate that organic matter, carbon, and total mass fluxes to the benthos are strongly increased in the presence of these mussels (Gergs et al., 2009; Cataldo et al., 2012b; Ozersky et al., 2015; Tokumon et al., 2018), enhancing the food subsidy for benthic deposit feeders (Karatayev et al., 1994a, 2002, 2007a, b; Karatayev & Burlakova, 1992, 1995; Botts & Patterson, 1996; Stewart et al., 1998; Burlakova et al., 2005, 2012; Sylvester & Sardiña, 2015).
Another source of food for benthic invertebrates inhabiting the mussels’ beds are the algal and bacterial communities that grow on the shells and in their aggregations. Over 150 algal species were identified in periphyton samples collected from zebra and golden mussel shells (Makarevich et al., 2008; Carvalho Torgan et al., 2009), and the total area of additional hard substrate represented by dreissenid shells can exceed 11% of the lake surface (Makarevich et al., 2008). Significant increases in periphyton biomass, in association with zebra and golden mussels, were reported in field assessments and enclosure experiments (Cataldo et al., 2012b; Higgins, 2014). Compared to bare sediments, dreissenids also increase heterotrophic bacterial density dramatically (up to ~ 2000%, Higgins & Vander Zanden, 2010), and enhance bacterial activity and metabolic diversity (Lohner et al., 2007).
Increased water clarity caused by the filtration of invasive bivalves favors the growth of submerged aquatic vegetation (also ecosystem engineers), which in turn affects water flow and provides resources such as food and habitat for many animals (Reeders & bij de Vaate, 1990; Lyakhnovich et al., 1988; Skubinna et al., 1995; Lowe & Pillsbury, 1995; Strayer et al., 1999), as well as many other ecosystem services (Thomaz, 2021). Macrophyte biomass and coverage increase as improved light penetration allows the plants to colonize deeper layers. One of their salient effects is the competition with phytoplankton for nutrients (Karatayev et al., 1997, 2002; Vanderploeg et al., 2002; Zhu et al., 2006; Ibelings et al., 2007; Higgins & Vander Zanden, 2010; Mayer et al., 2014; Noordhuis et al., 2016; Wegner et al., 2019), which in lakes, ponds and lagoons often triggers shifts from turbid to clear water states (Ibelings et al., 2007; Karatayev et al., 2014b; Mayer et al., 2014; Noordhuis et al., 2016). A meta-analysis of waterbodies invaded by dreissenids concluded that the areal coverage of macrophytes increased by approximately 180% between pre- and post-invasion periods, and the depth of the littoral zone increased by ~ 0.6 m in rivers, and 1.1 m in lakes (Higgins, 2014). In some waterbodies, however, increased light penetration can stimulate the growth of filamentous algae, which can be a nuisance for navigation and recreational activities (see “Disservices, caveats, and unresolved issues” section). Submerged macrophytes associated with clearer waters may provide refuge for zooplankton (Mayer et al., 2001; Schriver et al., 1995, but see also Meerhoff et al., 2006), enhance the abundance and diversity of benthic invertebrates, birds, and fish larvae, providing them with additional food, shelter, and substrate (e.g., MacIsaac, 1996; Mayer et al., 2001; Luukkonen et al., 2014; Musin et al., 2015).
Wastewater treatment
The ability of dreissenids to filter and clean water from organic pollution and toxic substances, including heavy metals, is attracting increasing attention (Selegean & Heidtke, 1994; Elliot et al., 2008; Binelli et al., 2014; Gomes et al., 2018). Early results of experimental trials with zebra mussels exposed to diluted activated sewage sludge for 96 h showed that the animals removed and biodeposited nearly all seston and P, significantly improving the clarity and decreasing the biochemical oxygen demand of the sludge (Mackie & Wright, 1994). Biofilters consisting of zebra mussel-overgrown artificial cage-like modules were successfully tested for wastewater treatment in Germany (Kusserov et al., 2010). Mussels can also eliminate pathogenic organisms (e.g., Escherichia coli, enteric viruses, Toxoplasma gondii, Giardia duodenalis) from wastewaters (Mezzanotte et al., 2016; Géba et al., 2020).
Mussel shells are composed primarily (> 80%) of calcium carbonate and can be used as a P-binding agent for the removal of P from wastewater effluents, helping to combat eutrophication. In test trials, Dreissena shell fragments had the highest phosphorus adsorption capacity compared to other media (Van Weelden & Anderson, 2003). McCorquodale-Bauer & Cicek (2020) suggested the use the zebra mussel shells as an alternative source to mined calcium carbonate for the production of lime to remove phosphorus in wastewater. Experimental trials with zebra mussel shells removed over 99% of the P, suggesting that they may be an efficient alternative for the precipitation of P in wastewaters.
Bioremediation
The use of zebra mussels for culling the effects of eutrophication were the object of many studies dating back to the 1980s, mostly in Europe (Piesik, 1983), and particularly in The Netherlands (Smit et al., 1993; Waajen et al., 2016), where many shallow freshwater lakes suffer from severe algal blooms (Noordhuis et al., 1992; Reeders et al., 1993). Successful field experiments using ponds with and without zebra mussels were carried out in the early 1990s (Reeders & bij de Vaate, 1990; Noordhuis et al., 1992). With respect to the control (without mussels), the treatment pond (with mussels) experienced a steady increase in Secchi disc depths (with a ~ 1 m difference remaining stable throughout the first year of the experiment), declines in both organic and inorganic suspended matter, increases in light penetration, and did not develop cyanobacterial blooms. Even filamentous Cyanobacteria, such as Aphanizomenon flos-aquae and Oscillatoria spp., too large to be consumed by zebra mussels, disappeared from the treated pond, likely because water clarification driven by the zebra mussels weakened their competitive advantage (Noordhuis et al., 1992). Using an in situ enclosure experiment, Waajen et al. (2016) concluded that quagga mussels can reduce the phytoplankton (including Cyanobacteria) biomass of a hypertrophic urban pond, and induce a clear water state. In a review of studies on lake restoration in north-western Europe, the use of zebra mussels was suggested as a promising approach to curtail phytoplankton, including toxic Cyanobacteria, growth (Gulati et al., 2008). It should be noticed, however, that the impacts of both dreissenids and of L. fortunei on Cyanobacteria are still controversial (see “Disservices, caveats, and unresolved issues” section).
Dreissenid mussel farming can be a realistic option and a potentially profitable industry in coastal waters (Stybel et al., 2009; Schernewski et al., 2012; Friedland et al., 2019). Schernewski et al. (2019) concluded that mussel farming is the most promising option for tackling eutrophication and facilitating macrophyte restoration to improve the water quality of the heavily eutrophicated large Oder (Szczecin) Lagoon (southern Baltic Sea). Further, dreissenids were found to be an adequate and potentially profitable source of food for zoo and farm animals and fish aquaculture plants (see “Harvest of mussels for farm animal and cultivated fish fodder” section). Goedkoop et al. (2021) estimated that a single 0.5 ha mussel farm could compensate for the total annual input of P from 23 ha of the watershed, or the biologically available P from 49 ha of agricultural land. While in their experimental conditions mussel farming and harvesting was found economically unsustainable, the development of ad hoc industrial methods and economic incentives for nutrient reductions in the lake could make this approach feasible.
Deterioration of environmental conditions in the coastal zone of the Baltic Sea along the Latvian, Lithuanian and Kaliningrad coasts during the 1980–1990s resulted in declines of major macrobenthic communities and keystone species (Mytilus edulis, Furcellaria lumbricalis, and Dreissena polymorpha in estuaries) and affected the spawning grounds of the Baltic herring. Artificial reefs and substrates were constructed for the cultivation of Furcellaria, Mytilus and Dreissena to restore these spawning grounds. The spawning success and survival of herring eggs depended on local hydrodynamics, specific materials, and types of substrates (Korolevs & Kondratjeva, 2006).
Dreissenids are very efficient at concentrating some noxious polluting substances in their shells, with very high shell:ambient water concentration ratios (e.g., U: 35,897, Ba: 1290, Zn: 800; Immel et al., 2016). Thus, by incorporating these elements from the medium and retaining them on the bottom, they effectively purify the water-column, but can also transfer these toxicants up the food web when predated upon (see “Disservices, caveats, and unresolved issues” section). Bacterial biofilms on L. fortunei shells were reported to significantly enhance the degradation of glyphosate, the most widely used herbicide in agriculture worldwide and a major contaminant of freshwaters (although this process can also favor the release of nutrients and, therefore, eutrophication; Flórez Vargas et al., 2019).
Environmental monitors and indicators
Invasive mussels are used extensively as model organisms for quality assessment and biomonitoring of freshwaters, and some of the major benefits they provide are often associated with waterbodies impacted by human activities, including hazardous chemicals, pathogens, and hypoxia.
Hazardous chemicals
The surveillance of chemical contamination of surface waters involves two main objectives: to determine whether contamination levels are compliant with the regulatory environmental standards, and to evaluate the temporal trends of contamination in different environmental compartments of aquatic ecosystems (Besse et al., 2012). Selecting a proper biological model is crucial for both objectives. The drawback of biomonitoring lies in the fact that different organisms behave differently, and therefore a contaminant that is noxious to one organism may be innocuous to many others (Faria et al., 2010).
Mussels are widely used as sentinel organisms to monitor chemical pollution in aquatic environments because they are filter feeding, sessile bottom dwellers that bioaccumulate many contaminants with little metabolic transformation and provide time-integrated information of chemical contamination in the environment (Roesijadi et al., 1984). In marine ecosystems, Mytilus spp. have historically been used as sentinel organisms worldwide to monitor the contamination by some persistent organic pollutants, heavy metals, organotin compounds, radionuclides, and pharmaceuticals. In freshwaters, dreissenids and L. fortunei have all the characteristics required for a good model: they have a remarkable filtering capacity which ensures active interaction with the medium; are widespread in lentic, lotic and estuarine environments and are available throughout the year; their size makes them easy to collect and manipulate, they are sessile and relatively long living (Karatayev et al., 2006); and they survive well in laboratory conditions (Slooff et al., 1983; Jenner et al., 1989; reviewed in Borcherding, 1992; Binelli et al., 2015).
In Europe, zebra mussels have been used extensively as model organisms for the quality assessment and biomonitoring of freshwaters since the late 1970s. They can accumulate large amounts of a wide range of pollutants in their soft tissues and shells, making them suitable for biomonitoring of heavy metals (Cd, Co, Cr, Cu, Hg, Ni, Pb, Zn, Hg, Se), organic compounds such as methylmercury, dichlorodiphenyltrichloroethane and several related pesticides (DDTs), polychlorinated biphenyls (PCBs), polycyclic aromatic hydrocarbons (PAHs), hexachlorobenzene (HCB), hexachlorocyclohexanes (HCHs), organophosphate insecticides, and even radioactive contamination (Neumann & Jenner, 1992; reviewed in Binelli et al., 2015). Moreover, even transplanted zebra mussels exposed for a period of six weeks can accumulate micropollutants up to levels comparable to those measured in resident mussels (Binelli et al., 2015). Zebra mussels were found to be good indicators of endocrine disrupting chemicals (Quinn et al., 2004). Bashnin et al. (2019) found that bioaccumulated pesticides and metals in transplanted zebra mussels can give an insight not only into their bioavailability in the environment, but also into the ecological responses of the benthic communities affected by these toxicants.
Dreissenids were selected as target organisms in the North American Great Lakes by a country-wide program using bivalves for water quality monitoring (the National Status and Trends Mussel Watch Project, National Oceanic and Atmospheric Administration—NOAA, USA) that examines contaminants at nearshore sites on a biennial basis providing crucial information for the identification of the levels and distribution of toxicants (Apeti & Lauenstein, 2006). Monitoring of contaminant concentrations in dreissenids over time can also be used to assess the efficiency of remediation-oriented initiatives (Kimbrough et al., 2014). In addition to the assessment of bioaccumulated contaminants in their bodies, invasive mussels are also intensively used as both in-vivo and in-vitro biomarkers, and in transcriptomics and proteomics studies to provide information on the potential impact of pollutants on the health of other organisms (reviewed in Binelli et al., 2015).
Monitoring of pollution with the aid of dreissenids is not restricted to the assessment of contaminants in their tissues and shells. As most bivalves, when exposed to stressful conditions, including toxic substances in the surrounding water, dreissenids shut their valves more often and for longer periods than normal. This behavior can be monitored automatically in ad hoc flow-through devices with mussels at the intake end of water treatment plants, triggering an alarm when the proportion of closed shells are above predefined threshold values. As of 2006, 13 such devices (“Dreissena-Monitors”) were used by German drinking water treatment plants and successfully functioning as an early warning system for the intake of polluted water (Borcherding, 2006).
Although the golden mussel is more resilient to adverse conditions than dreissenids, it also has been used to monitor the presence of environmental pollutants using chemical and genotoxic assessments (do Amaral et al., 2019; El Haj et al., 2019; Balsamo Crespo et al., 2020; Gattás et al., 2020; Nunes et al., 2020; Pazos et al., 2020; Besen & Marengoni, 2021; Girardello et al., 2021; Mendes Sene et al., 2021; Miranda et al., 2021; Oliveira et al., 2021).
Pathogens
Aquatic pollution by pathogenic organisms, including viruses such as the ones responsible for the COVID-19 pandemic (Le Guernic et al., 2022), can be monitored using dreissenids. Fecal coliforms and Escherichia coli are used as bioindicators to evaluate water quality and wastewater treatment efficiency, and the use of mussels as indicators of contamination by fecal bacteria provide advantages compared with traditional monitoring methods (Selegean et al., 2001; reviewed in Gomes et al., 2018). Zebra mussels were found to host 132 times more E. coli and other intestinal enterococci than ambient water for up to two days after pulse exposures to the bacteria, thus providing a time-integrating and much more sensitive indicator of bacterial contamination than water samples (Bighiu et al., 2019). Bacteria can also be a food source for the bivalves (Mikheev et al., 1994), and D. polymorpha and Corbicula fluminea (also invasive) were found to clear E. coli more rapidly that native unionids (Silverman et al., 1997).
Analyses of pathogenic protists (Cryptosporidium parvum, Giardia duodenalis, Giardia lamblia, Toxoplasma gondii, Cyclospora sp., Enterocytozoon intestinalis, E. hellem, E. bieneusi) in the water requires filtration of large volumes because their densities are often low. Mussel filtration concentrates their numbers, often proportionally to their values in the water, allowing usage of the bivalve’s tissues as indicators and a good integrative matrix for biomonitoring of these pathogens (Lucy et al., 2008, 2010; Lucy, 2009; Conn et al., 2014; Ladeiro et al., 2014; Gomes et al., 2018; Géba et al., 2020). In addition, due to the digestion of C. parvum and T. gondii oocysts, mussels can be used as a bioremediation tool to mitigate contamination by pathogenic protists (Géba et al., 2021a, b). Zebra mussels were found useful in assessing viral contamination by measuring the accumulation of indicators of viral pollution F-specific RNA bacteriophages in their tissue (Capizzi-Banas et al., 2021). They can also accumulate a low pathogenic form of the avian influenza virus H5N1 (Stumpf et al., 2010; reviewed in Gomes et al., 2018). In spiked treated municipal wastewater, Mezzanotte et al. (2016) found a significant reduction in rotavirus that can cause gastroenteritis, and although the viruses remained in the soft zebra mussel tissues or in the liquid phase, they were not transmissible to other species. The ability to bioaccumulate microcystins by zebra and golden mussels can potentially be used for biomonitoring of toxic cyanobacteria blooms (Paldavičienė et al., 2015; Minillo et al., 2016).
Hypoxia
Hypoxia is a key global stressor in freshwater, estuarine and marine benthic ecosystems (Diaz & Rosenberg, 2008; Tellier et al., 2022) that is predicted to increase worldwide due to ongoing human-induced eutrophication and global warming (Villnäs et al., 2012). Both zebra and quagga mussels are intolerant of even moderate hypoxia; thus, monitoring the occurrence and length-frequency distribution of Dreissena spp. can be an effective tool for mapping the extent and frequency of hypoxia in freshwaters (Karatayev et al., 2018b, 2021b). In contrast to pelagic organisms and some motile benthic species, dreissenids cannot migrate to escape hypoxia, and their planktonic larvae and extremely high fecundity allow them to disperse rapidly and recolonize substrates even after large-scale die-offs. Due to their long lifespan, their populations usually consist of multiyear cohorts allowing the detection of even rare hypoxic events. Further, due to their large body size and high densities, in clear waters they can be surveyed using remote sensing techniques (e.g., underwater video), allowing swift collection of information on their distribution over large areas, and both conventional (bottom grabs) and video surveys proved to be efficient tools in mapping hypoxic areas (Karatayev et al., 2018c, 2021b; Burlakova et al., 2022), providing a record of the recent history, rather than instantaneous snapshots, of hypoxic events.
In contrast to dreissenids, the golden mussel is tolerant of very low oxygen concentrations (~ 0.5 mg L−1; Karatayev et al., 2007b; Perepelizin & Boltovskoy, 2011). In urbanized stretches of the Río de la Plata Estuary (Argentina-Uruguay) the golden mussel thrives in areas polluted with raw sewage and runoff from storm water outlets and in areas where dissolved oxygen levels are extremely low (Boltovskoy et al., 2006). Although massive die-offs associated with system-wide dissolved oxygen drops have also been reported (Oliveira et al., 2010), its ability to survive at low dissolved oxygen levels limit the use of this mussel as an indicator of hypoxia.
Provisioning services
Food for other species
Food for fishes
Both dreissenid species and the golden mussel provide an abundant food resource for fishes (Vorobiev, 1949; Zhadin, 1952; Karatayev et al., 1994b, 2015b; Molloy et al., 1997; Bartsch et al., 2005; Cataldo, 2015; Paolucci & Thuesen, 2015). At least 58 species of benthivorous fishes in Europe and in North America feed on adult dreissenids, and > 50 in South America on L. fortunei.
Dreissenids are particularly important in fish diets in their native range, where fishes are evolutionarily adapted to consume mussels (reviewed in Karatayev et al., 1994b). In the North Caspian Sea about 90% of the annual production of mussels (13,000 t, wet weight) are consumed by fishes (Yablonskaya, 1985). In the Azov Sea, fishes consume annually 13,800,000 t of benthos, roughly half of which is represented by the bivalves (Vorobiev, 1949). Since many European fishes are adapted to feed on mussels, the introduction of zebra mussels into new European waterbodies is often associated with increases in fish productivity and commercial catches (Lvova, 1977; Lyakhnovich et al., 1988; Karatayev & Burlakova, 1995; Karatayev et al., 1997, 2002, 2010a). A vivid example is the roach (Rutilus rutilus), the most prominent consumer of dreissenids (reviewed in Karatayev et al., 1994b, 1997; Molloy et al., 1997), which in invaded lakes is characterized by much higher growth rates, larger size, and higher lipid content compared to pre-invasion periods (Lyagina & Spanowskaya, 1963; Poddubny, 1966).
In North America, predation on dreissenid mussels in the Great Lakes has been documented for many commercially important native fishes, including whitefish (Coregonus clupeaformis) (Pothoven & Madenjian, 2008; Madenjian et al., 2010), lake sturgeon (Acipenser fulvescens) (Jackson et al., 2002; Bruestle et al., 2018), blue catfish (Ictalurus furcatus) and channel catfish (I. punctatus) (Thorp et al., 1998), freshwater drum (Aplodinotus grunniens), round whitefish (Prosopium cylindraceum) (Turschak & Bootsma, 2015), and yellow perch (Perca flavescens) (Morrison et al., 1997; Watzin et al., 2008; Shields & Beckman, 2015). In Oneida Lake (New York state), dreissenid mussels are a substantial component of lake sturgeon diets, especially for the larger individuals (Jackson et al., 2002). Dreissena spp. comprises a major part of the diet of the endangered silver chub (Macrhybopsis storeriana), having largely replaced other bivalves (Sphaeriidae) and Gastropoda (Kocovsky, 2019). A similar shift from a pre-invasion diet of other benthic littoral invertebrates to zebra mussels was recorded for several species of Lepomis (Molloy et al., 1997; Mercer et al., 1999; Magoulick & Lewis, 2002; Colborne et al., 2015). Non-dreissenid nearshore invertebrates, which have benefited from dreissenid-mediated benthification, became the primary forage of nearly all nearshore fish species (Turschak & Bootsma, 2015). After Lake Erie was invaded by dreissenids, benthic resources were estimated to support 75–95% of the potential fish production (Johannsson et al., 2000). In Lake Ida (USA), 10 (out of 11) fish species increased the use of littoral carbon after the establishment of the zebra mussel, with the mean use of littoral carbon increasing from 43 to 67% (Morrison et al., 2021). In the Hudson River (USA), littoral macrophyte primary production doubled after zebra mussel colonized the river (Caraco et al., 2000), and many fishes associated with vegetated shallows where they feed chiefly on benthic invertebrates increased after the invasion, although pelagic fishes declined (Strayer et al., 2004). In contrast, dreissenid-induced loss of primary production and oligotrophication of the Great Lakes resulted in large declines in pelagic fish (see “Disservices, caveats, and unresolved issues” section), suggesting the need for actions oriented at managing benthic-oriented native fishes, such as coregonids and lake trout, better suited to ongoing ecosystem changes (Dettmers et al., 2012; Kao et al., 2018). Consequences of dreissenid introductions to fisheries in the Great Lakes, however, have been much more significant than those in other inland lakes (Nienhuis et al., 2014).
In addition to native fish species, several introduced fishes feed on both zebra and quagga mussels, as well as on another Ponto-Caspian invader, the round goby (Neogobius melanostomus) (Jude et al., 1992; Charlebois et al., 1997; Molloy et al., 1997; Watzin et al., 2008). The round goby, which feeds intensively on the mussels, provided a very important trophic link between dreissenids and commercially and recreationally valuable fish species, including lake trout (Salvelinus namaycush) (Dietrich et al., 2006), burbot (Lota lota) (Madenjian et al., 2011), yellow perch (Perca flavescens) (Weber et al., 2011), smallmouth bass (Micropterus dolomieu) (Crane & Einhouse, 2016), walleye (Sander vitreus) (Pothoven et al., 2017), and lake sturgeon (Acipenser fulvescens) (Jacobs et al., 2017). Lake sturgeon is a species of conservation concern in many U.S. states and Canadian provinces (Peterson et al., 2007). In the lower Niagara River, which hosts one of the few remnant lake sturgeon populations in New York state, three non-native species became dominant in its diet: the round goby, the amphipod Echinogammarus ischnus, and dreissenids (Bruestle et al., 2018). Due to their high consumption rates of round gobies, adult lake sturgeon in the lower Niagara River are now primarily piscivorous, and their recovery is supported by the high availability of energetically rich non-native food resources (Bruestle et al., 2018).
Of the > 50 fish species that feed on L. fortunei in South America (reviewed in Cataldo, 2015), some became dependent on this resource almost exclusively (e.g., the boga, Megaleporidens obtusidens; Penchaszadeh et al., 2000), whereas others, formerly omnivorous, iliophagous, and ichthyophagous species, shifted from plants, detritus and other items to adult mussels after mussel introduction (Ferriz et al., 2000). In Japan and in South America, predator inclusion/exclusion experiments indicate that up to > 90% of the mussel’s production is consumed, presumably mostly by fishes (Sylvester et al., 2007a; Nakano et al., 2010; Duchini et al., 2018). In the Uruguay River (Argentina-Uruguay), stable isotope mixing models (δ13C and δ15N) show that L. fortunei is responsible for up to 66% of the biomass of 8 dominant fish species (González-Bergonzoni et al., 2020).
Many midsized fishes that feed on golden mussels are in turn consumed by larger, piscivorous fishes with high commercial and recreational value, suggesting that mussels are likely to have a positive impact on these large species as well (reviewed in Boltovskoy & Correa, 2015; Karatayev et al., 2015b). In addition, similar to dreissenids, golden mussels transfer large amounts of organic matter from the pelagic to the benthic domains through their feeding and filtering activities and the formation of feces and pseudofeces, which likely boosts the biomass of many iliophagous species, including the sábalo (Prochilodus lineatus), an intensively exploited and strictly iliophagous species which represents up to > 60% of the overall fish catches and biomass in the Río de la Plata basin, and ~ 80–90% of Argentine freshwater fish exports (Boltovskoy et al., 2006; Scarabotti et al., 2021).
In Europe and North America, at least 17 species of fish (primarily fry) have been reported to consume planktonic larvae of zebra mussels (reviewed in Molloy et al., 1997; Chrisafi et al., 2007; Watzin et al., 2008; Turschak & Bootsma, 2015) as veligers represent an abundant and, because of their limited dodging capabilities, easily available prey for early feeding fish larvae, and may partially offset the apparent low consumption of other prey sources (Marin Jarrin et al., 2015; Withers et al., 2015). During the summer, Dreissena veligers often comprise up to 73% of total zooplankton density, and up to 40% of the zooplankton biomass and production (Wiktor, 1958; Kornobis, 1977; Lvova et al., 1994; David et al., 2009; Karatayev et al., 2010a; Withers et al., 2015; Lazareva et al., 2016; Bowen et al., 2018). In Salto Grande Reservoir (Argentina–Uruguay), in 2006–2019, golden mussel larvae comprised ~ 80% of the combined densities of larvae, Cladocera and Copepoda (Boltovskoy et al., 2021a). Consumption of golden mussel veligers by fish larvae could be even more significant than the consumption of adult mussels (Boltovskoy & Correa, 2015). Golden mussel veligers are not only more abundant and easier to capture than crustacean zooplankton, but they also represent an energetically more profitable food resource yielding significantly higher growth rates than crustaceans (Paolucci et al., 2010). Golden mussels have been suggested to increase Argentine freshwater fish landings and exports significantly (Boltovskoy et al., 2006), but the multiple variables potentially involved in this rise are difficult to tease apart.
In addition to the facilitating effects of the consumption of mussel larvae and adults, fishes also benefit from the enhancement of mussel-associated invertebrates (Lyakhnovich et al., 1983, 1988; Karatayev & Burlakova 1992, 1995; Stewart & Haynes, 1994; Sylvester et al., 2007b; Sardiña et al., 2008, 2011) (see “Habitat modification” section).
A major concern associated with mussels consumed by other aquatic animals is whether they facilitate the transfer of bioaccumulated contaminants up the food web, and if these exotic mussels are effectively more deleterious in transferring the contaminants than other native or introduced prey species (see “Disservices, caveats, and unresolved issues” section).
Food for birds
Consumption of dreissenids has been recorded for at least 36 bird species, including 21 in Europe and 20 in North America (ducks, pochards, scaups, coots, rails, etc.; reviewed in Karatayev et al., 1994b; Molloy et al., 1997). Zebra mussels are often very abundant, requiring low search and handling times (Leuzinger & Schuster, 1970; Kornobis, 1977; Draulans, 1982; Suter, 1982b; Wormington & Leach, 1992). In terms of biomass, zebra mussels are the most abundant macroinvertebrate prey for ducks in the Rhine River and in lakes Ijsselmeer and Markermeer (The Netherlands), where large numbers of mussel-consuming ducks are present from October to April and consume up to ~ 30% of the annual zebra mussel production (Smit et al., 1993). Since zebra mussels invaded Lake Constance (Germany) in the 1960s, the number of overwintering waterbirds increased four-fold, decreasing mussel biomass in shallow areas by > 90%. The birds remove ~ 750 t of mussels per km2, or 1390 g (whole wet weight with shell) of mussels per bird per day (Werner et al., 2005). Migrating waterfowl can quickly locate areas with dense mussel populations (reviewed in Molloy et al., 1997) and forage most commonly on mussels between autumn and spring, when flocks are either temporarily present during their migrations (Mitchell & Carlson, 1993; Hamilton et al., 1994), or overwintering on site (bij de Vaate, 1991; Cleven & Frenzel, 1993). A 92-fold increase in scaups (Aythya spp.), and a noticeable increase in other waterfowl were recorded in Long Point Bay on Lake Erie, one of the most important waterfowl staging areas on the Great Lakes, after dreissenids colonized the bay (Petrie & Knapton, 1999). The use of Lake St. Clair (USA-Canada) by scaups, canvasback (Aythya valisineria), and redhead ducks (Aythya americana) in U.S. waters during fall migrations increased from 1.1 million use-days before dreissenid establishment, to 2.1 million after it. While scaups prey on dreissenids directly, canvasbacks likely responded to increased submerged aquatic macrophyte food associated with greater water clarity due to mussel colonization (Luukkonen et al., 2014). The declining European population of greater scaup A. marila strongly depends on the non-native zebra mussels that constitute > 90% of their food by biomass. In the brackish lagoons of the Odra River Estuary (south-western Baltic Sea), an important area for the species during the non-breeding season in Europe, greater scaups consume an average of 5400 t of zebra mussels annually (Marchowski et al., 2015).
The introduction of zebra mussels was associated not only with dramatic increases in waterfowl numbers, but also affected their migration patterns (reviewed in Molloy et al., 1997). In Swiss lakes, prior to the introduction of zebra mussels, waterfowl fed on aquatic macrophytes and migrated to the south in the fall after plant die-back, whereas presently, large numbers overwinter locally (Leuzinger & Schuster, 1970). In winter, however, ice formation precludes foraging, except in waterbodies where open waters are available year-round as in cooling water reservoirs for power plants where large flocks of mallards (Anas platyrhynchos) regularly overwinter and consume large quantities of zebra mussels in shallow open waters (Karatayev et al., 1994b; Kozulin, 1995). In England, the geographical range of the tufted duck (Aythya fuligula) expanded due in part to the spread of zebra mussels (Olney, 1963). Food abundance and availability, particularly Dreissena, were suggested as the main factors governing lake choice by overwintering diving ducks in Switzerland (Pedroli, 1981; Suter, 1994). Soon after the arrival of zebra mussels in western Lake Constance in the late 1960s, 10–50-fold increases in overwintering tufted ducks, pochards (Aythya spp.), and coots (Fulica spp.) were observed, and goldeneye (Bucephala clangula) began to arrive earlier (Suter, 1982b). Conversely, in areas where zebra mussel populations declined, diving birds showed a tendency to leave overwintering areas earlier, likely due to lower food availability (Suter, 1982a).
In addition to the direct consumption of dreissenids, waterfowl also prey on the invertebrates associated with the mussels. The abundance of macroinvertebrates (mostly Oligochaeta, Chironomidae, and Ephemeroptera) associated with Dreissena colonies were significantly reduced in unprotected enclosures in shallow areas of Lake Constance, presumably due to waterfowl predation (Mörtl et al., 2010). In addition, birds also benefit from increased macrophyte coverage (see “Habitat modification” section). For example, the bay of Lucerne (Switzerland) has become an internationally important wintering site for red-crested pochard (Netta rufinadue) due to recolonization of the lake by stoneworts (Characeae) after zebra mussels were introduced in the 1980s (Schwab et al., 2001). Both charophytes and zebra mussels are considered keystone species defining ecosystem resilience in some lakes, and careful management of these species is as important as the control of nutrients (Ibelings et al., 2007).
In South America, areas invaded by the golden mussel host hundreds of aquatic bird species, many of which feed on submerged organisms. However, their consumption of L. fortunei has never been studied. Circumstantial observations in Embalse de Río Tercero reservoir (Argentina) suggest that, after the introduction of the golden mussel, the densities of some coot species (Fulica leucoptera, F. armillata) increased significantly, probably in response to the growth of aquatic macrophytes, on which the birds feed. Birds have also been observed to dive and emerge with clusters of L. fortunei in their beaks (M. Hechem, pers. comm.), suggesting that they may also feed on the mussel, as do other coot species on Dreissena in North America (see above).
Food for other animals
In addition to fishes and birds, several other animals have been reported to feed on dreissenids. In Europe and North America, several species of crayfish (Cambarus affinis, C. robustus, Orconectes limosus, O. virilis, O. propinquus) and muskrats (Ondatra zibethicus) have been observed eating zebra mussels (reviewed in Karatayev et al., 1994b; Molloy et al., 1997). According to Piesik (1974), during the summer adult stages of the crayfish O. limosus can consume 6000 young dreissenids per crayfish potentially limiting their population growth. Blue crabs (Callinectes sapidus) were recorded actively feeding on dreissenids in the Hudson River, causing mussels populations to crash near Catskill (New York, USA) in 1992 (Molloy et al., 1994). Dreissena was found in the guts of the mudpuppy salamander (Necturus maculosus), a declining Laurentian Great Lakes native species (Beattie et al., 2017). Experiments in The Zoological Garden of Osnabrück (Germany) showed that mongooses (Mungos mungo) and the oriental small-clawed otters (Aonyx cinerea) can feed on zebra mussels; raccoons (Procyon lotor) prefer zebra mussels over other food items, while Arctic foxes (Vulpes lagopus) feed on mussels reluctantly (Schernewski et al., 2019). In Lake Opinicon (Canada), zebra mussels constitute up to 36% of the diet of the northern map turtle (Graptemys geographica) which can consume over 3000 kg of zebra mussels per year (Bulté & Blouin-Demers, 2008). In addition to G. geographica, the stinkpot turtle Sternotherus odoratus was found to prey heavily on invasive mussels in the Laurentian Great Lakes (Lindeman, 2006; Patterson & Lindeman, 2009). In trials involving multi-prey assemblages, two native mysid species from the St. Lawrence middle estuary, Neomysis americana and Mysis stenolepis, exhibited high predation rates on zebra mussel veligers (Winkler et al., 2007).
Comparisons of L. fortunei densities on unprotected artificial substrates with those on substrates protected with 5–40 mm meshes indicate that up to over 90% of their yearly production is lost to predators, presumably fishes and invertebrates (Sylvester et al., 2007a; Nakano et al., 2010; Duchini et al., 2018). In South America, two crab species and one turtle have been observed to feed on L. fortunei (Bujes et al., 2007; Torres et al., 2012; Carvalho et al., 2013), but the importance of this item in their diets is unknown.
Harvest of mussels for farm animal and cultivated fish fodder
The possibility of taking advantage of the large biomass of invasive bivalves has been envisioned since the 1950s. Zhadin (1952) and Karatayev et al. (1994b) reviewed the Russian literature on the use of dreissenids as food for livestock and poultry in dry food blends, and to extract vitamins. In terms of fresh weight, zebra mussels contain 8.4% of protein, 0.8% of fat, 0.14% of P, 1.3% of raw ash and 89.3% of water (Schernewski et al., 2019), which makes them a potential substitute or additive for protein-rich fodder for farm animals. In Tsimlyanskoe Reservoir (Russia), the annual production of zebra mussels was estimated at 1,000,000 t, or 242,000 t in tissue biomass. An annual harvest of only 2% of this biomass (to prevent overharvesting and the ensuing eutrophication) can produce 5,000 t of dreissenid tissue containing ~ 500 t of protein (Miroshnichenko, 1990). From 6 to 60 t of molluscs can be harvested from one hectare of the reservoir’s bottom. In Pyalovsk Reservoir (Russia), 20–40 kg of mussels can be collected from 15 to 20 running meters of the bottom; harvesting by hand can yield 100–200 kg h−1, and up to 500 kg h−1 with the aid of motorboats (reviewed in Karatayev et al., 1994b). In waterbodies with high mussel densities, artificial ad hoc substrates can produce up to 20–30 kg of molluscs per square meter annually (reviewed in Karatayev et al., 1994b).
Zebra mussel shells washed ashore were used as a food supplement for chicken (reviewed in Karatayev et al., 1994b), as well as for ducks and pigs (Gasunas, 1959, 1965). Ducks fed with zebra mussels grew better than those fed with the traditional food, and the farms became more profitable. In 1964 alone, 7000 t of dreissenids were harvested for duck farms in the Curonian Lagoon (Baltic Sea). Harvested zebra mussels have also been used to produce food for cultivated fish. Grinded mussels (raw or boiled) were used as a food supplement for several species including carp, sterlet, sturgeon, bester, and salmon (Karatayev et al., 1994b). Zebra mussels were found to be a palatable food supplement for chickens: their high levels of calcium were essential for egg shell formation, and mussel-supplemented diets did not show any adverse effects (McLaughlan et al., 2014).
Although L. fortunei reaches very high densities too, harvesting mussels for commercial purposes has never been reported, and would probably be economically unviable given their very patchy distribution. On the other hand, cleaning of industrial facilities and fish farms often produces huge amounts of dead mussels, which have occasionally been evaluated for the production of farm animal or cultivated fish fodder (Almeida et al., 2006; Bayerle et al., 2017; Wachholz et al., 2017), and for the neutralization of soil acidity and the supply of nutrients for plants (Barbosa, 2009; Silva, 2016). Although some results are encouraging, none has yet been applied on industrial scales, and the presence of contaminants in the mussels’ residues were often considered a major obstacle (see “Disservices, caveats, and unresolved issues” section).
Materials
Mussel shells are composed primarily (> 80%) of calcium carbonate (Immel et al., 2016), and thus can be used as a source of this salt. Most calcium carbonate is sourced from mined limestone. Calcium carbonate is widely used in medical and nutritional applications (Feldman, 1996; Chang et al., 2007), and in agriculture as a soil pH neutralizer and buffer (Oates, 2008). In construction it has a variety of uses, including crushed limestone as a road aggregate, and especially in the production of lime (calcium oxide), widely used in the manufacturing of cement, several adhesives, and steel slag (Oates, 2008). The use of zebra mussel shells washed ashore or derived from cleaning industrial facilities was suggested as an alternative source for calcium carbonate (McCorquodale-Bauer & Cicek, 2020).
Dreissenids and L. fortunei can be also used as a fertilizer, soil amendment, or mulch in agriculture (Barbosa, 2009; Mackie & Claudi, 2010; Silva, 2016). Mussel shells have occasionally been used to raise the pH of soils. Ontario Hydro (Toronto, Canada) and the Monroe Power Plant (Michigan, USA) used the zebra mussel remains removed from their biofouled pipes for composting to cover a landfill on the property. At Detroit’s Edison Monroe power plant (USA), zebra mussels remains and associated debris were mixed, piled into windrows, and spread onto grounds where coal used to be piled to enhance grass growth (McDonnell, 1996). Cornell University researchers tested recipes for composting zebra mussel shells and found that a mixture of 1:14:17:18 parts by weight of peat, sawdust, poultry litter and water, mixed 1:1 with zebra mussel remains helped to maintain a good pore structure and enhanced air flow. After three months of maturing, the compost was mixed with topsoil in various proportions, and tomatoes and radishes were grown in the mixtures. Most seedlings grew better than on topsoil alone (McDonnell, 1996).
Cultural services
Aesthetics, leisure, and property values
The effects of invasive species on cultural services, defined as non-consumptive attributes of an ecosystem (i.e., recreation, tourism, history, education, science, heritage, inspiration, spirituality and aesthetics), are difficult to assess because they are based on personal and local value systems (Pejchar & Mooney, 2009; Cassini, 2020). Invasive species can alter these cultural services, either negatively or positively, and sometimes in opposition to their impacts on other services.
As discussed above, suspension-feeding bivalves significantly enhance water transparency, particularly in already highly impacted ecosystems. Improvements in water clarity increase its aesthetical perception, reduce some negative impacts such as odor and toxic cyanobacterial blooms (but see “Disservices, caveats, and unresolved issues” section), and facilitate most water-related recreational activities. Divers enjoy the increased visibility in the Great Lakes, and the scuba diving industry has boomed around the lakes as a result (https://www.shipwreckexplorers.com/invasive-species/). Improved water clarity has had a strong impact on visitors of the Thunder Bay National Marine Sanctuary, an area of 4,300 square miles of Lake Huron off the coast of Alpena, Michigan, where nearly 100 shipwrecks have been identified (Williams, 2020), and the wrecks and the clear waters made Thunder Bay an important attraction for scuba divers from around the world. Steve Kroll, a 69 year-old retired math teacher from Alpena, remembers what it was like to dive the wrecks before the mussels, when underwater visibility was often poor: “A good day of diving was when you could see 5–7 feet. Now if you have 5–7 feet of visibility there are probably some people thinking we shouldn’t be diving…Now I think if we went back, we’d lose a lot of divers.” (Williams, 2020).
By clarifying the water, invasive bivalves can increase the property value of neighboring real estate since waterfront, water-view and neighboring property owners benefit more than the general public from increases in surface water quality (Jakus et al., 2013). With respect to changes in property values, the services conveyed by the mussels’ filtration have been assessed in a few surveys. In the vacation region of North Central Wisconsin (USA), increased lake water clarity associated with the presence of dreissenids was suggested to increase property values by about 10% (Johnson & Meder, 2013). In Otter Tail and Becker Counties (Minnesota, USA), the presence of zebra mussels in the lakes per se did not affect lakeshore property values, suggesting that buyers and sellers in the lakeshore housing market were not being swayed by the presence or absence of zebra mussels in their purchasing decisions (Mcgrew, 2013).
Walsh et al. (2016) estimated that the decrease of the water clarity of Lake Mendota (USA) by ~ 1 m (due to the invasion of the zooplanktivorous spiny water flea—Bythotrephes longimanus—which decimated algae-grazing zooplankton), produced an overall loss in neighboring properties of US$ 140 million. Using the method and assumptions employed by Walsh et al. (2016), we performed a rough estimate of the benefits conferred to properties around some of the Great Lakes by the increased water clarity from dreissenid filtration. In lakes Michigan and Huron, mussels increased Secchi depths from < 10 m in the 1980–1990s, to ~ 20 m in 2015–2019 (Barbiero et al., 2018; Bunnell et al., 2021; U.S. Environmental Protection Agency’ Great Lakes National Program Office 1983–2019 long-term monitoring water quality data, https://cdx.epa.gov/). A 9 m in Secchi depth increase and the same increase (i.e., willingness to pay) in neighboring property value per meter of extra water clarity as the one used by Walsh et al. (2016) (in decreased value due to water turbidity) yields US$ 767.52 per house (adjusted to present values). Lake Michigan's shoreline hosts ~ 12 million people (Lake Michigan Facts, https://www.livescience.com/32011-lake-michigan.html), and 3 million Americans and Canadians (ca. 50% each) live on Lake Huron (Lake Huron Basin Statistics, https://www.glerl.noaa.gov/education/ourlakes/lakes.html). The average U.S. household hosts 2.5 people (https://www.statista.com/statistics/183648/average-size-of-households-in-the-us/), and 2.9 in Canada (https://www.statista.com/statistics/478954/average-family-size-in-canada-by-province/). Thus, the combined potential economic gain for properties on both lakes would have been over 37 billion US$. While admittedly very crude, these estimates suggest economically large benefits and call for more precise calculations of benefits to be included in overall cost–benefit analyses.
Several studies analyzed the relationships between property values and water clarity based on surveys of owners of waterfront property. The underlying assumption is that the market value of lakefront properties is tightly associated with the lake’s water quality, which in turn is chiefly perceived through the lake’s water clarity (Steinnes, 1992; Michael et al., 1996, 2000; Leggett & Bockstael, 2000; Gibbs et al., 2002; Boyle & Bouchard, 2003; Krysel et al., 2003; Ara et al., 2006; reviewed in Jakus et al., 2013). Indeed, water clarity is the second most important lake characteristic for property purchasers (the overall scenic beauty of the lake being the first), affecting market prices around 5–23%, depending on the market group (Michael et al., 2000). A one-meter improvement in lake water clarity increases property prices from $11 to $382 per foot of water frontage (Steinnes, 1992; Michael et al., 1996; Jakus et al., 2013), and an overall property increase of 0.9–6.6% (Gibbs et al., 2002; Boyle & Bouchard, 2003).
The increased water clarity due to dreissenid filtration can in turn induce the growth of macrophytes and bottom algae (see “Habitat modifications” above, and “Disservices, caveats, and unresolved issues” section). While increased water clarity can be classified as an ecosystem service (in terms of human enjoyment), the nuisance due to filamentous algae can represent a disservice. To quantify the human perceptions of the effects of these beneficial and baneful impacts, and examine how they translate into economic terms (i.e., changes in property values), Limburg et al. (2010) surveyed business owners and homeowners along the shores of Lake Ontario to the western end of the St. Lawrence River (New York state, USA) to calculate the effects of dreissenids on property values due to both increased water quality and nuisance filamentous algae. The authors scaled up their results by multiplying the number of coastal households by the average increase in property value (approximately US$ 3500 due to increased water clarity) and subtracted the losses due to filamentous algae (approximately US$ 750 per household), concluding that the net benefit would be in the multiple millions of dollars. Thus, the benefits were ranked significantly higher than the losses by both business owners and homeowners, indicating that mussels produced mixed, but dominantly beneficial, impacts.
In these examples water clarity was used as a proxy for a variety of ecosystem functions (such as bioturbation, nutrient cycling, and phytoplanktonic primary productivity), but using a proxy response variable for ecosystem functions may overlook other key ecosystem processes, and does not provide information on the magnitude, direction, or rate of change to the underlying ecosystem functions (reviewed in Flood et al., 2020). These simple calculations also do not take into account changes in global markets, regional differences in housing values, geographic differences in the degree of impact, and many other factors, but despite all the shortcomings, they illustrate the point that positive economic impacts can be captured by the market economy.
In addition to recreation and aesthetics, mussels can be valued or reviled for their role in symbolic (inspiration, spirituality, religion, ceremony and tradition) services. The impacts of invasive mussels on these important cultural components are obviously less relevant than those of many much more charismatic organisms, such as trees, birds, reptiles or mammals, but conceivably not negligible, although their significance is difficult to quantify.
Information and knowledge
The value of exotic mussels as a tool in the area of intellectual and experiential (scientific and educational) services has received limited recognition. Our analysis of the literature, however, revealed that almost a third of the publications in the Ecosystem function and services category are centered on the application of mussels as biomonitors and bioindicatiors (see “Wastewater treatment”, “Bioremediation” and “Environmental monitors and indicators” sections).
The biochemical processes involved in the production and adhesion of byssus threads are investigated to prevent biofouling and to gain insights into the challenging task of engineering adhesive bonds underwater, anticorrosives, metal-sequestering reagents, and novel biomimetic polymers (Rzepecki & Waite, 1995; Brazee & Carrington, 2006; Luo et al., 2006; Andrade et al., 2015; Ohkawa & Nomura, 2015; Li et al., 2018b, c). The stable isotope C and O composition of zebra mussel shells has been used as a proxy of paleoclimatological and palaeolimnological variations (Wurster & Patterson, 2001; Apolinarska, 2013).
The accumulated knowledge on exotic mussel ecology, spread, distribution and competition, as well as mussels themselves as easy obtainable test organism, are used widely in K-12 and higher education (e.g., https://nsgl.gso.uri.edu/ilin/ilinf96001/ilinf96001.htm; https://www.hws.edu/fli/lessons_teach_quagga.aspx; Kean & Enochs, 2001). A variety of lesson plans using zebra mussels are offered by Teachers Pay Teachers Program to a community of more than 7 million educators (https://www.teacherspayteachers.com/Browse/Search:zebra%20mussels).
Disservices, caveats, and unresolved issues
A major economic impact of these byssate exotic bivalves is the fouling of human-made facilities, including industrial plants, water conveyance structures, fish culture cages, and watercraft. Mussels clog water intakes and conduits, sieves and filters, heat exchangers, and many other components, requiring costly maintenance operations. Several assessments of the expenditures involved have been produced at various spatial and temporal scales (Roberts, 1990; Office of Technology Assessment, 1993; Khalanski, 1997; O'Neill, 1997; Pimentel, 2005; Connelly et al., 2007; Rebelo et al., 2018), and although the values reported have been questioned (Lovell et al., 2006; Connelly et al., 2007; Ram & Palazzolo, 2008), they most probably range in the hundreds to thousands of millions US$ per year worldwide.
Exotic mussels are known to have negative effects on some species due to their overgrowth and/or competition for food (e.g., other filter-feeding bivalves such as Unionidae, Sphaeriidae, and the deep-water amphipod Diporeia). Probably the most notorious and best studied negative effect of dreissenids is that on native bivalves (Mackie, 1991; Haag et al., 1993; Gillis & Mackie, 1994; Schloesser & Nalepa 1994; Ricciardi et al., 1996; Schloesser et al., 1996; Karatayev et al., 1997, Burlakova et al., 2000, 2014; Aldridge et al., 2004; Strayer & Malcom, 2007a; Lucy et al., 2014). Although in some cases unionid populations have been almost or completely extirpated (Ricciardi et al., 1998), the largest impacts are typically found in lentic habitats, where dreissenids attain the highest densities, while native mussels are most abundant and diverse in streams and rivers (Karatayev et al., 2018d; Vaughn & Hoellein, 2018). Further, in some cases the impact on native bivalves seems to be a transient event (Strayer & Malcom, 2007a; Burlakova et al., 2014). Similar negative effects were reported for L. fortunei (Darrigran, 2002; Mansur et al., 2003; Ezcurra de Drago, 2004; Lopes et al., 2009; Karatayev et al., 2010b; Rojas Molina & Williner, 2013), but the impacts on the hosts have not been quantified.
Except for these rather clear-cut negative economic and ecological effects, many others are largely mixed, being positive for some species and processes, and, simultaneously, negative for others (Karatayev & Burlakova, accepted; Dermott and Kerec, 1997; reviewed in Karatayev et al., 1997, 2010b; Higgins & Vander Zanden, 2010). These effects result in direct and indirect changes, both positive and negative, in species diversity, and in promoting biogenic homogenization (Sardiña et al., 2011; Burlakova et al., 2012). A vivid example are the mixed effects of dreissenids on benthic communities, where collector and scraper species (e.g., isopods, amphipods, gastropods, oligochaetes, and chironomids) typically increase in association with mussel beds due to direct and indirect positive interactions (Karatayev et al., 1997; Burlakova et al., 2005, 2012), but native deep-water amphipods and suspension feeders including sphaeriids, unionids, and some chironomids can be outcompeted (Lvova-Kachanova & Izvekova, 1978; Karatayev et al., 1997, 2002; Burlakova et al., 2005, 2012, 2018; Ward & Ricciardi, 2007; Barbiero et al., 2011). Mixed effects also involve planktonic communities, where mussels may selectively consume some species at the expense of others (Cotner et al., 1995; Fahnenstiel et al., 2010; Barbiero et al., 2012; Rojas-Molina et al., 2015).
Among the impacts of both dreissenids and of L. fortunei on the phytoplankton, the most controversial is their effect on Cyanobacteria (reviewed in Kelly et al., 2010; Karatayev et al., 2015a, b; Boltovskoy et al., 2015; Reynolds & Aldridge, 2021). While most European, South American and some North American studies found that these mussels feed on Cyanobacteria, including toxic Microcystis strains (Reeders & bij de Vaate, 1990; Strayer et al., 1999; Baker & Levinton, 2003; Dionisio Pires et al., 2005; McLaughlan & Aldridge, 2013; Boltovskoy et al., 2015; Noordhuis et al., 2016; Reynolds & Aldridge, 2021), a number of reports suggested that they may facilitate toxic blooms of Microcystis spp. by selective grazing and promotion of colony formation, rejection of toxic strains, and/or enhancement of nutrient loadings (Makarewicz et al., 1999; Vanderploeg et al., 2001, 2009; Conroy et al., 2005a, b; Cataldo et al., 2012b). Opposite impacts seem to be very species- and context-dependent, and even depend on variations in median Microcystis colony size (White & Sarnelle, 2014). In North America, the positive effect of dreissenids on Microcystis spp. seems to be restricted to lakes with low to moderate total phosphorus concentrations (< 25 µg total P L−1), whereas those with high nutrient loadings are not affected (Vanderploeg et al., 2001; Nicholls et al., 2002; Sarnelle et al., 2005; Knoll et al., 2008). On the other hand, in South America L. fortunei can promote cyanobacterial blooms at total phosphorus > 50–100 µg L−1 (Cataldo et al., 2012b). Clearly, the effects of exotic bivalves on Cyanobacteria require further investigation in different systems.
Another important effect with mixed consequences recorded in many lakes is the proliferation of bottom filamentous algae and macrophytes, usually as a result of enhanced water clarity and the concomitant increase in colonizable depth, increasing substrate for attachment, and higher bioavailability of nutrients due to less competition with phytoplankton (Arnott & Vanni, 1996; Conroy et al., 2005a, b). In the lower North American Great Lakes (Erie, Michigan, Ontario) Cladophora blooms, common from the 1950s through the early 1980s, were largely eradicated through the implementation of the Great Lakes Water Quality Agreement, but returned in the mid-1990s due to the expansion of dreissenids (reviewed in Higgins et al., 2008). It should be noticed that while these plants are generally perceived as a nuisance for recreational activities (Limburg et al., 2010), they have many positive impacts on the fauna (see above).
Although clarification of the water due to the mussels’ filtration activity is usually recognized as an environmental and economic asset, and an important ecosystem service, mussel beds along the coast, as well as their fouling of recreational watercraft, can be a nuisance for leisure activities, including potential injuries to bathers and, in the case of massive die-offs, the organic matter pollution and odor of dead, decomposing animals. Further, changes from turbid to clear waters are not free from ecosystem disservices, as they can involve higher CO2 effluxes (Jeppesen et al., 2015). The oligotrophication of the Laurentian Great Lakes due to dreissenids over the benchmarks intended by the Great Lakes Water Quality Agreement was suggested to be “excessive”, and negative for zooplankton phenology and abundance (Pothoven & Vanderploeg, 2020, 2022), pelagic fish prey and predatory fishes (Pothoven et al., 2001; Hoyle et al., 2008; Nalepa et al., 2009a, b; Nalepa, 2010). The extent of light penetration modifies the depth of the euphotic layer, and the ensuing growth of vegetation may affect fish habitats. Higher water clarity can also increase the penetration of ultraviolet radiation affecting the survival or distribution of some organisms, induce changes in fish schooling, reproductive behavior, territoriality (Bunnell et al., 2021), and even modify temperature and dissolved oxygen vertical stratification patterns (Yu & Culver, 2000). While nutrient sequestration from the water column by invasive bivalves, especially in lakes, has been well documented, studies of changes in their concentrations (in particular P and N) in the water yielded quite dissimilar results, with both increases and decreases having been reported, as well as differences in the changes of their various compounds (see “Nutrient cycling and sequestration” section; Lindim, 2015), suggesting that these impacts are context-dependent.
Another major concern associated with exotic filter feeding bivalves consumed by a large number of fishes, birds and other aquatic animals, is whether they facilitate the transfer of contaminants up the food web by providing a novel route for the less accessible sediment-deposited toxicants (Bruner et al., 1994; Roper et al., 1996; Johns & Timmerman, 1998; Robertson & Lauenstein, 1998; Kwon et al., 2006; Schummer et al., 2010). As described above (“Environmental monitors and indicators” section) mussels bioaccumulate many toxicants, which upon consumption can be transferred to other animals, and eventually to humans. Although bioaccumulation and trophic transfer vary among species (Matthews et al., 2015) and pollutant types (Perez-Fuentetaja et al., 2015), the key issue is whether invasive mussels are effectively more noxious in this respect than other prey. We are not aware of large-scale, comprehensive studies on this problem, but although some contaminants were reported to bioaccumulate very significantly in the mussels’ tissue or shells (see “Environmental monitors and indicators” section), for others (e.g., PBDEs—polybrominated diphenyl ethers) the bioaccumulation rates have been found to be much lower than for zooplankton and amphipods (Perez-Fuentetaja et al., 2015), which may decrease the levels of these compounds in mussel-feeding fishes (Hahm et al., 2009). Thus, while the bioaccumulation of contaminants by exotic mussels may indeed be a major problem, most aquatic organisms bioaccumulate pollutants, and further research is needed in order to assess whether these mussels are effectively more harmful in transferring contaminants than other native or introduced prey species.
Reverse ecosystem changes due to mussel population crashes
The ecosystem-wide changes caused by invasive mussels may not be permanent, as their populations can decline or be extirpated due to pollution, oxygen declines or other factors (Noordhuis et al., 2016; Karatayev et al., 2018a). After Lake Lukomlskoe (Belarus) was colonized by zebra mussels in the late 1960s, summer Secchi depths increased from 1.8 to > 4 m, seston and phytoplankton densities declined, macrophyte coverage increased, and the lake switched from eutrophic to meso-oligotrophic (Lyakhnovich et al., 1988; Karatayev & Burlakova, 1992; Karatayev et al., 1997). However, a tenfold decline in zebra mussel biomass occurred 30 years after mussel numbers peaked, most likely driven by an increase in nutrient loads and oxygen depletion caused by the fish hatchery launched in the lake in 1989 (Mitrakhovich et al., 2008). This decline was associated with a relapse of almost all environmental parameters to pre-invasion values, returning the lake to a turbid water state (Mitrakhovich et al., 2008; Karatayev et al., 2021c).
Effects of domestic and industrial wastewater discharges into the Rhine River watershed became evident in the second part of the nineteenth and first part of the twentieth century, resulting in strong population reductions and large scale extinctions of many riverine organisms, including the extinction of zebra mussels in the late 1960s. After measures were undertaken to improve the water quality, signs of recovery of macroinvertebrate and fish communities became apparent in the second half of the 1970s (bij de Vaate et al., 2006). In Lake Veluwe (The Netherlands) the increased nutrient load of the late 1960s to early 1970s caused a shift from a clear to a turbid water state, along with sharp declines in macrophytes and Secchi depths, increases in chlorophyll concentrations, and extirpation of zebra mussels (Ibelings et al., 2007). After reductions in phosphorous loads and manipulation of fish (reduction in bream density), clear water was re-established allowing the return of zebra mussels, whose high filtration capacity helped in maintaining clear waters (Ibelings et al., 2007; Noordhuis et al., 2016). Similar changes, including shifts from clear to turbid water states and extirpation of zebra mussels, were recorded in several other lakes, including Lake Eem (The Netherlands), due to an increase in external nutrient loads (Noordhuis et al., 2016). Again, reduction in nutrients loads and increased catches of benthivorous bream triggered a change to a clear water state, that was facilitated by the recolonization by zebra mussels in 1996, and especially by quagga mussels in 2010, leading to a strong increase in transparency and macrophyte abundance, and a decrease of cyanobacterial blooms (Noordhuis et al., 2016).
Reverse changes in environmental parameters were also observed in the central basin of Lake Erie, associated with a decline in the biomass of quagga mussels due to the return of hypoxic conditions around 2000. The decline in dreissenid densities in the basin was concomitant with a decrease in spring dissolved silica concentrations and an increase in total phosphorus and near-bottom turbidity, neither of which were recorded in the western or eastern basins. This sharp decline of Dreissena coincided with a shift from clear to turbid waters, indicating that dreissenid-related shifts in the water quality of the waterbodies invaded are reversible (Karatayev et al., 2018a). Further, although in many Northern Hemisphere lakes and rivers quagga mussels tend to replace earlier invasions by zebra mussels (Karatayev et al., 2011a, 2015a; bij de Vaate et al., 2014; Strayer et al., 2019), a reversal to zebra mussel dominance has also been noticed (Rudstam & Gandino, 2020; Karatayev et al., 2021b), which may likely involve important ecological changes.
Coastal wetlands associated with the Great Lakes are critically important to a diverse array of wetland-dependent organisms, but only half of the wetlands remain intact due to both historic and contemporary wetland losses. In the Inner Long Point Bay of eastern Lake Erie submerged aquatic vegetation (SAV) provides food and habitat for a diversity of fish and wildlife, and its abundance and community structure serve as indicators of ecosystem health. Colonization by zebra mussels in the early 1990s increased water clarity and has been associated with changes in SAV abundance, distribution, and community composition (Petrie & Knapton, 1999). Between 1992 and 2009, dreissenid populations declined over 90%, likely due to increased eutrophication, sediment loads and predation by both fish and waterfowl. Declines in the abundance of filter feeding mussels and the associated increase in phytoplankton reduced light availability and induced declines of the five most abundant SAV species, lowering the bay’s carrying capacity for waterfowl, fish and other wildlife (Churchill et al., 2016).
The loss in filtration services provided by invasive dreissenid mussels following a mass mortality event was estimated in the river Meuse in the Netherlands in 2016–2017. During the summer, the filtration capacity of dreissenids was sufficient to filter up to over 12% of the discharge in a 25 km impounded stretch of the river. This service was completely lost for 17 months following a mass mortality, until recolonization restored the filtration capacity (Collas et al., 2020).
In the Great Lakes, the impacts of dreissenids on nutrients suggest that, while monitoring of external phosphorus loads remains important, the monitoring of dreissenid populations is now more important than ever. Even if P concentrations in the water column remain low, accumulation of P in the mussels’ biomass involves a high risk of large and unpredictable changes in the system if mussel densities decline releasing P into the water column (Li et al., 2021).
Records of long-term changes of L. fortunei densities are very few. In the Lower Delta of the Paraná River, occasional visual observations of mussel bed densities on coastal revetments suggest periodic variations, but no quantitative data are available. In the reservoir Embalse de Río Tercero (Argentina), adult densities (~ 900–1000 ind./m2 in 1996; Boltovskoy et al., 2009a) started collapsing dramatically around 2014, allegedly due to the blooms of another invasive species, the dinoflagellate Ceratium spp. (C. furcoides, C. hirundinella; Mariñelarena et al., 2016), but the timings of Ceratium blooms and the decline of L. fortunei do not match, and the dinoflagellate has never been reported to be harmful to the mussel. Presently these populations have recovered, but the reasons for the decline are unknown. In Brazil’s Pantanal wetland, total population wipeouts are a recurring event due to seasonal hypoxic events, but recolonization is swift (Oliveira et al., 2010). Mass mortalities of L. fortunei have also been observed in Japan (Uchida et al., 2007), but the reasons involved are unclear.
Concluding remarks
In this paper we summarized the most salient ecosystem and economic services provided by three widespread freshwater exotic bivalves (Fig. 2), and highlighted the major caveats and exceptions associated with them. One important conclusion of this work is that, with few exceptions (e.g., biofouling of industrial facilities), the effects of the invasive mussels reviewed are mixed; facilitation of a given process, community or species almost invariably occurs at the expense of a different process, community, or species. Our appreciation of the net outcome is largely anthropocentric, insofar as we confer a higher value to some organisms and ecosystem characteristics than to others. Higher values are often associated with a more pristine state, the one that characterized the system decades or centuries ago and did not host these introduced species. But when these species help to reverse other natural or human-induced undesirable effects (e.g., eutrophication vs. water clarification), or yield resources for exploitable or otherwise valued organisms (fishes, birds), our perception changes. We advocate for a broader and more holistic outlook of biological invasions in general, which should provide a more objective perspective of their impacts on the environment and on human well-being (Tassin & Kull, 2015; Boltovskoy et al., 2022). In this respect, novel ecosystems (Hobbs et al., 2013) deserve particular interest. This notion involves viewing the role of alien species more pragmatically, and even considering some “new” species as desirable elements (Hui & Richardson, 2017), as shown in the examples above. At the same time, we should make it abundantly clear that we do not argue in favor of biological introductions, and do not deny the need to eradicate some clearly baneful invasives, or to pursue successful policies aimed at culling new introductions. We agree with the notion that the invasion risks involved are too high, and the results are unpredictable and occasionally disastrous, but also with the fact that while invaded systems differ from those that prevailed historically, they are not necessarily less desirable than the latter (Hobbs et al., 2006) and, as shown above, there are many examples where these invaders have been responsible for the amelioration of major ecosystems impacted by human activities and helped the recovery of declining native species.
This review includes three freshwater invasive mussels. As noticed above, the volume of information on the zebra mussel is much larger than those on the quagga mussel, and especially on the golden mussel. Because the three species share many important traits (see “Introduction” section), comparisons between them are inevitable, but they also display major biological differences, and the areas invaded by dreissenids are ecologically very dissimilar from those invaded by the golden mussel (Karatayev et al., 2007a, 2015b). Thus, extrapolations of the impacts of the zebra mussel to the quagga mussel, and especially the golden mussel, can often be misguiding. Further, because impacts are strongly context-dependent, even the same invader can have very different effects in different environments (Karatayev et al., 2021a) and at different times, and the effects measured often depend on the methodological approach employed (Boltovskoy et al., 2021b). Because differences in coverage between the three species are very large, vacant areas of knowledge are also dissimilar. However, in all cases the long-term behavior of their populations still poses major questions.
In closing, we would like to stress the need to recognize the benefits conferred by invasive mussels, and particularly to include their economically quantifiable services in the assessments of their economic impacts. Damage estimates by invasive species attract more attention than their benefits (Jernelöv, 2017; Guerin et al., 2018), and assessments of damage only are dominant in the literature (Perrings et al., 2001; Pimentel, 2011; Diagne et al., 2021). However, as shown above, these invasive species do have sizable benefits, many of which involve major economic gains, but their ecosystemic and economic benefits are normally ignored, minimized, or deemed “non monetizable” (Boltovskoy et al., 2022). Given the widespread distribution of invasive mussels and the long-term focus of scientists and managers on the negative aspects of their dispersal, it is important to assess quantitatively their positive ecological effects and economic benefits. This assessment should not be interpreted as a rejection of the fact that invasive mussels have negative impacts on some communities and, in particular, on productive activities, but “rather as an opportunity to provide an additional piece of information for scientists, managers and policymakers” (Vimercati et al., 2020).
Data availability
Data sharing not applicable to this review article as only publicly available datasets were analyzed during the current study.
References
Ackerman, J. D., 1999. Effect of velocity on the filter feeding of dreissenid mussels (Dreissena polymorpha and Dreissena bugensis): implications for trophic dynamics. Canadian Journal of Fisheries and Aquatic Sciences 56: 1551–1561.
Aldridge, D. C., P. Elliott & G. D. Moggridge, 2004. The recent and rapid spread of the zebra mussel (Dreissena polymorpha) in Great Britain. Biological Conservation 119: 253–261.
Almeida, H. C., A. P. C. Suszek, S. N. T. G. C. Mendonça & R. S. C. Flauzino, 2006. Estudo do Limnoperna fortunei (mexilhão dourado) como ingrediente na ração animal, através das características físico-químicas, microbiológicas e presença de mercúrio. Higiene Alimentar 20: 61–65.
Andrade, R. G., J. L. Ferrerira de Araújo, A. Nakamura Filho, A. C. Paganini Guañabens, M. D. de Carvalho & A. V. Cardoso, 2015. Functional surface of the molluscan foot of Limnoperna fortunei (Dunker, 1857): morphology, organic structures and the role of cilia on underwater adhesion. Materials Science and Engineering C 54: 32–42.
Apeti, D. A. & G. G. Lauenstein, 2006. An assessment of mirex concentrations along the southern shorelines of the Great Lakes, USA. American Journal of Environmental Sciences 2: 95–103.
Apolinarska, K., 2013. Stable isotope compositions of recent Dreissena polymorpha (Pallas) shells: paleoenvironmental implications. Journal of Paleolimnology 50: 353–364.
Ara, S., E. Irwin & T. Haab, 2006. The influence of water quality on the housing price around Lake Erie. Proceedings of American Agricultural Economics Association Annual Meeting, Long Beach, CA, July, 2006 [available on https://ideas.repec.org/p/ags/aaea06/21275.html].
Arnott, D. L. & M. J. Vanni, 1996. Nitrogen and phosphorus recycling by the zebra mussel (Dreissena polymorpha) in the western basin of Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 53: 646–659.
Ayers, J. C., 1962. Great Lakes waters, their circulation and physical and chemical characteristics. American Association for the Advancement of Science 71: 71–89.
Baker, S. M. & J. S. Levinton, 2003. Selective feeding by three native North American freshwater mussels implies food competition with zebra mussels. Hydrobiologia 505: 97–105.
Baker, S. M., J. S. Levinton & W. J. Evan, 2000. Particle transport in the zebra mussel, Dreissena polymorpha (Pallas). Biological Bulletin (Woods Hole) 199: 116–125.
Baldwin, B. S., M. S. Mayer, J. Dayton, N. Pau, J. Mendilla, M. Sullivan, A. Moore, A. Ma & E. L. Mills, 2002. Comparative growth and feeding in zebra and quagga mussels (Dreissena polymorpha and Dreissena bugensis): implications for North American lakes. Canadian Journal of Fisheries and Aquatic Sciences 59: 680–694.
Balogh, C., J. Kobak, Z. Kovács, J. Serfőző, N. Faragó & Z. Serfőző, 2022. Contribution of invasive bivalves (Dreissena spp.) to element distribution: phase interaction, regional and seasonal comparison in a large shallow lake. Biogeochemistry 158: 91–111.
Balsamo Crespo, E., P. J. Pereyra, A. Silvestro, K. Hidalgo & G. Bulus Rossini, 2020. Acute toxicity of Cd2+, Cr6+, and Ni2+ to the golden mussel Limnoperna fortunei (Dunker 1857). Bulletin of Environmental Contamination and Toxicology 104: 748–754.
Barbiero, R. P. & M. L. Tuchman, 2004. Long-term dreissenid impacts on water clarity in Lake Erie. Journal of Great Lakes Research 30: 557–565.
Barbiero, R. P., M. L. Tuchman & E. S. Millard, 2006. Post-dreissenid increases in transparency during summer stratification in the offshore waters of Lake Ontario: is a reduction in whiting events the cause? Journal of Great Lakes Research 32: 131–141.
Barbiero, R. P., B. M. Lesht & G. J. Warren, 2011. Evidence for bottom–up control of recent shifts in the pelagic food web of Lake Huron. Journal of Great Lakes Research 37: 78–85.
Barbiero, R. P., B. M. Lesht & G. J. Warren, 2012. Convergence of trophic state and the lower food web in Lakes Huron, Michigan and Superior. Journal of Great Lakes Research 38: 368–380.
Barbiero, R. P., B. M. Lesht, G. J. Warren, L. G. Rudstam, J. M. Watkins, E. D. Reavie, K. E. Kovalenko & A. Y. Karatayev, 2018. A comparative examination of recent changes in nutrients and lower food web structure in Lake Michigan and Lake Huron. Journal of Great Lakes Research 44: 573–589.
Barbosa, D. B. P., 2009. Utilização do resíduo moído de mexilhão dourado (Limnoperna fortunei Dunker, 1857) como corretivo da acidez do solo e fonte de nutrientes para as plantas. PhD Thesis, Universidade Federal do Rio Grande do Sul (Brazil).
Bartsch, M. R., L. A. Bartsch & S. Gutreuter, 2005. Strong effects of predation by fishes on an invasive macroinvertebrate in a large floodplain river. Journal of the North American Benthological Society 24: 168–177.
Bashnin, T., V. Verhaert, M. De Jonge, L. Vanhaecke, J. Teuchies & L. Bervoets, 2019. Relationship between pesticide accumulation in transplanted zebra mussel (Dreissena polymorpha) and community structure of aquatic macroinvertebrates. Environmental Pollution 252: 591–598.
Bayerle, D. F., R. V. Nunes, C. A. Gonçalves Jr., L. Wachholz, C. Scherer, I. M. da Silva, T. M. Moraes de Oliveira & J. G. de Vargas Jr, 2017. Golden mussel (Limnoperna fortunei) in feed for broiler chicks using tannin as a sequestrant of toxic metals. Ciências Agrárias 38: 834–854.
Beattie, A. M., M. R. Whiles & P. W. Willink, 2017. Diets, population structure, and seasonal activity patterns of mudpuppies (Necturus maculosus) in an urban, Great Lakes coastal habitat. Journal of Great Lakes Research 43: 132–143.
Beeton, A. M., 1961. Environmental changes in Lake Erie. Transactions of the American Fisheries Society 90: 153–159.
Beeton, A. M., 1965. Eutrophication of the St. Lawrence Great Lakes. Limnology & Oceanography 10: 240–254.
Benson, A. J., 2014. Chronological history of zebra and quagga mussels (Dreissenidae) in North America, 1988–2010. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control 2nd ed. CRC Press, Boca Raton: 9–31.
Besen, M. A. & N. G. Marengoni, 2021. Bioaccumulation of metals and evaluation of golden mussels encrusted on different screens of net cages. Boletim Do Instituto De Pesca 47: e624.
Besse, J. P., O. Jeffard & M. Coquery, 2012. Relevance and applicability of active biomonitoring in continental waters under the water framework. Trends in Analytical Chemistry 36: 113–127.
Bighiu, M. A., A. Norman Haldén, W. Goedkoop & J. Ottoson, 2019. Assessing microbial contamination and antibiotic resistant bacteria using zebra mussels (Dreissena polymorpha). Science of the Total Environment 650: 2141–2149.
Binelli, A., S. Magni, C. Soave, F. Marazzi, E. Zuccato, S. Castiglion, M. Parolini & V. Mezzanotte, 2014. The biofiltration process by the bivalve D. polymorpha for the removal of some pharmaceuticals and drugs of abuse from civil wastewaters. Ecological Engineering 71: 710–721.
Binelli, A., C. Della Torre, S. Magni & M. Parolini, 2015. Does zebra mussel (Dreissena polymorpha) represent the freshwater counterpart of Mytilus in ecotoxicological studies? A critical review. Environmental Pollution 196: 386–403.
Binimelis, R., W. Born, I. Monterroso & B. Rodrıguez-Labajos, 2007. Biological invasions. In Nentwig, D. (ed), Ecological studies 193 Springer-Verlag, Berlin: 331–347.
Boegman, L., M. R. Loewen, P. F. Hamblin & D. A. Culver, 2008. Vertical mixing and weak stratification over zebra mussel colonies in western Lake Erie. Limnology & Oceanography 53: 1093–1110.
Boltovskoy, D., 2015. Limnoperna fortunei: The ecology, distribution and control of a swiftly spreading invasive fouling mussel, Springer, Cham: 1–476.
Boltovskoy, D., 2015. Distribution and colonization of Limnoperna fortunei: special traits of an odd mussel. In Boltovskoy, D. (ed), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Springer International Publishing, Cham: 301–311.
Boltovskoy, D., 2017. Traits and impacts of invasive species: myths and evidences from the perspective of introduced freshwater mussels. Aquatic Ecosystem Health and Management 20: 334–343.
Boltovskoy, D. & N. Correa, 2015. Ecosystem impacts of the invasive bivalve Limnoperna fortunei (golden mussel) in South America. Hydrobiologia 746: 81–95.
Boltovskoy, D., N. Correa, D. Cataldo & F. Sylvester, 2006. Dispersion and ecological impact of the invasive freshwater bivalve Limnoperna fortunei in the Río de la Plata watershed and beyond. Biological Invasions 8: 947–963.
Boltovskoy, D., A. Karatayev, L. Burlakova, D. Cataldo, V. Karatayev, F. Sylvester & A. Mariñelarena, 2009a. Significant ecosystem-wide effects of the swiftly spreading invasive freshwater bivalve Limnoperna fortunei. Hydrobiologia 636: 271–284.
Boltovskoy, D., F. Sylvester, A. Otaegui, V. Leytes & D. Cataldo, 2009b. Environmental modulation of the reproductive activity of the invasive mussel Limnoperna fortunei in South America. Austral Ecology 34: 719–730.
Boltovskoy, D., N. Correa, F. Sylvester & D. Cataldo, 2015. Nutrient recycling, phytoplankton grazing, and associated impacts of Limnoperna fortunei. In Boltovskoy, D. (ed), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Springer International Publishing, Cham: 153–176.
Boltovskoy, D., F. Bordet, V. Leites & D. Cataldo, 2021a. Multiannual trends (2004–2019) in the abundance of larvae of the invasive mussel Limnoperna fortunei and crustacean zooplankton in a large South American reservoir. Austral Ecology 46: 1221–1235.
Boltovskoy, D., N. Correa, L. E. Burlakova, A. Y. Karatayev, E. V. Thuesen, F. Sylvester & E. M. Paolucci, 2021b. Traits and impacts of introduced species: a quantitative review of meta-analyses. Hydrobiologia 848: 2225–2258.
Boltovskoy, D., R. Guiaşu, L. Burlakova, A. Karatayev, M. Schlaepfer & N. Correa, 2022. Misleading estimates of economic impacts of biological invasions: including the costs but not the benefits. Ambio. https://doi.org/10.1007/s13280-022-01707-1.
Borcherding, J., 1992. Another early warning system for the detection of toxic discharges in the aquatic environment based on valve movements of the freshwater mussel Dreissena polymorpha. Limnologie Aktuell 4: 127–146.
Borcherding, J., 2006. Ten years of practical experience with the Dreissena Monitor, a biological early warning system for continuous water quality monitoring. Hydrobiologia 556: 417–426.
Botts, P. S. & B. A. Patterson, 1996. Zebra mussel effects on benthic invertebrates: physical or biotic? Journal of the North American Benthological Society 15: 179–184.
Bowen, K. L., A. J. Conway & W. J. S. Currie, 2018. Could dreissenid veligers be the lost biomass of invaded lakes? Freshwater Science 37: 315–329.
Boyle, K. & R. Bouchard, 2003. Water quality effects on property prices in Northern New England. LakeLine 23: 24–27.
Brazee, S. L. & E. Carrington, 2006. Interspecific comparison of the mechanical properties of mussel byssus. Biological Bulletin 211: 263–274.
Bruestle, E., C. Karboski, A. Hussey, A. Fisk, K. Mehler, C. Pennuto & D. Gorsky, 2018. Novel trophic interaction between lake sturgeon (Acipenser fulvescens) and non-native species in an altered food web. Canadian Journal of Fisheries and Aquatic Sciences 76: 6–14.
Bruner, K. A., S. W. Fisher & P. F. Landrum, 1994. The role of the zebra mussel, Dreissena polymorpha, in contaminant cycling: II. Zebra mussel contaminant accumulation from algae and suspended particles, and transfer to the benthic invertebrate, Gammarus fasciatus. Journal of Great Lakes Research 20: 735–750.
Bujes, C. S., I. Ely & L. Verrastro, 2007. Trachemys dorbigni (Brazilian Slider) diet. Herpetological Review 38: 335.
Bulté, G. & G. Blouin-Demers, 2008. Northern map turtles (Graptemys geographica) derive energy from the pelagic pathway through predation on zebra mussels (Dreissena polymorpha). Freshwater Biology 53: 497–508.
Bunnell, D. B., R. P. Barbiero, S. A. Ludsin, C. P. Madenjian, G. J. Warren, D. M. Dolan, T. O. Brenden, R. Briland, O. T. Gorman, Ji. X. He, T. H. Johengen, B. F. Lantry, B. M. Lesht, T. F. Nalepa, S. C. Riley, C. M. Riseng, T. J. Treska, I. Tsehaye, M. G. Walsh, D. M. Warner & B. C. Weidel, 2014. Changing ecosystem dynamics in the Laurentian Great Lakes: bottom-up and top-down regulation. BioScience 64: 26–39.
Bunnell, D. B., S. A. Ludsin, R. L. Knight, L. G. Rudstam, C. E. Williamson, T. O. Höök, P. D. Collingsworth, B. M. Lesht, R. P. Barbiero, A. E. Scofield, E. S. Rutherford, L. Gaynor, H. A. Vanderploeg & M. A. Koops, 2021. Consequences of changing water clarity on the fish and fisheries of the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 78: 1524–1542.
Burlakova, L. E., A. Y. Karatayev & D. K. Padilla, 2000. The impact of Dreissena polymorpha (Pallas) invasion on unionid bivalves. International Review of Hydrobiology 85: 529–541.
Burlakova, L. E., A. Y. Karatayev & D. K. Padilla, 2005. Functional changes in benthic freshwater communities after Dreissena polymorpha (Pallas) invasion and consequences for filtration. In Dame, R. & S. Olenin (eds), The Comparative Roles of Suspension Feeders in Ecosystems. NATO Science Series: IV – Earth and Environmental Sciences Springer, Cham: 263–275.
Burlakova, L. E., A. Y. Karatayev & V. A. Karatayev, 2012. Invasive mussels induce community changes by increasing habitat complexity. Hydrobiologia 685: 121–134.
Burlakova, L. E., B. L. Tulumello, Y. Karatayev, A. Krebs, D. W. Schloesser, D. T. Zanatta, W. L. Patterson, T. A. Griffith, M. W. Scott & T. Crail, 2014. Competitive replacement of invasive congeners may relax impact on native species: interactions among zebra, quagga, and native unionid mussels. PLoS ONE 9(12): E114926. https://doi.org/10.1371/journal.pone.0114926.
Burlakova, L. E., R. P. Barbiero, A. Y. Karatayev, S. E. Daniel, E. K. Hinchey & G. Warren, 2018. The benthic community of the Laurentian Great Lakes: analysis of spatial gradients and temporal trends from 1998–2014. Journal of the Great Lakes Research 44: 600–617.
Burlakova, L. E., A. Y. Karatayev, K. Mehler & E. K. Hinchey, 2022. Exploring Great Lakes benthoscapes: can we visually delineate hypoxic habitats? Hydrobiologia. https://doi.org/10.1007/s10750-022-04821-z.
Capizzi-Banas, S., M. P. Ladeiro, F. Bastien, I. Bonnard, N. Boudaud, C. Gantzer & A. Geffard, 2021. The utility of Dreissena polymorpha for assessing the viral contamination of rivers by measuring the accumulation of F-Specific RNA bacteriophages. Water (Switzerland) 13: 904.
Caraco, N. F., J. J. Cole, S. E. G. Findlay, D. T. Fischer, G. G. Lampman, M. L. Pace & D. L. Strayer, 2000. Dissolved oxygen declines in the Hudson River associated with the invasion of the zebra mussel (Dreissena polymorpha). Environmental Science and Technology 34: 1204–1210.
Carvalho, D. A., P. A. Collins & C. J. De Bonis, 2013. Predation ability of freshwater crabs: age and prey-specific differences in Trichodactylus borellianus (Brachyura: Trichodactylidae). Journal of Freshwater Ecology 28: 573–584.
Carvalho Torgan, L., S. E. Salomoni & A. Brugalli Bicca, 2009. Diatomáceas sobre Limnoperna fortunei (Dunker), molusco introduzido no Lago Guaíba, Sul do Brasil. Revista Brasileira De Botanica 32: 23–31.
Cassini, M. H., 2020. A review of the critics of invasion biology. Biological Reviews 95: 1467–1478.
Cataldo, D., 2015. Trophic relationships of Limnoperna fortunei with adult fishes. In Boltovskoy, D. (ed), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Springer International Publishing, Cham: 231–248.
Cataldo, D., A. Vinocur, I. O’Farrell, E. Paolucci, V. Leites & D. Boltovskoy, 2012. The introduced bivalve Limnoperna fortunei boosts Microcystis growth in Salto Grande Reservoir (Argentina): evidence from mesocosm experiments. Hydrobiologia 680: 25–38.
Cataldo, D., I. O’Farrell, E. Paolucci, F. Sylvester & D. Boltovskoy, 2012. Impact of the invasive golden mussel (Limnoperna fortunei) on phytoplankton and nutrient cycling. Aquatic Invasions 7: 91–100.
Cerqueira, M. B. R., L. Kupski, S. S. Caldas & E. G. Primel, 2019. Golden mussel shell and water in matrix solid phase dispersion: a suitable combination for the extraction of acetylsalicylic and salicylic acids from sewage sludge. Microchemical Journal 148: 102–107.
Chang, F., G. Li, M. Haws & T. Niu, 2007. Element concentrations in shell of Pintada margaritifera from French Polynesia and evaluation for using as a food supplement. Food Chemistry 104: 1171–1176.
Chapra, S. C., A. Dove & G. J. Warren, 2012. Long-term trends of Great Lakes major ion chemistry. Journal of Great Lakes Research 38: 550–560.
Charlebois, P. M., J. E. Marsden, R. G. Goettel, R. K. Wolfe, D. J. Jude & S. Rudnicka, 1997. The round goby, Neogobius melanostomus (Pallas), a review of European and North American literature. Illinois Natural History Survey Special Publications. No. 20.
Chrisafi, E., P. Kaspiris & G. Katselis, 2007. Feeding habits of sand smelt (Atherina boyeri, Risso 1810) in Trichonis Lake (Western Greece). Journal of Applied Ichthyology 23: 209–214.
Churchill, R. L. T., M. L. Schummer, S. A. Petrie & H. A. L. Henry, 2016. Long-term changes in distribution and abundance of submerged aquatic vegetation and dreissenid mussels in Long Point Bay, Lake Erie. Journal of Great Lakes Research 42: 1060–1069.
Cleven, E. J. & P. Frenzel, 1993. Population dynamics and production of Dreissena polymorpha (Pallas) in River Seerhein, the outlet of Lake Constance (Obersee). Archiv Für Hydrobiologie 127: 395–407.
Colborne, S. F., A. D. M. Clapp, F. J. Longstaffe & B. D. Neff, 2015. Foraging ecology of native pumpkinseed (Lepomis gibbosus) following the invasion of zebra mussels (Dreissena polymorpha). Canadian Journal of Fisheries and Aquatic Sciences 72: 983–990.
Collas, F. P. L., K. R. Koopman, G. van der Velde & R. Leuven, 2020. Quantifying the loss of filtration services following mass mortality of invasive dreissenid mussels. Ecological Engineering 149: 105781.
Conn, D. B., F. E. Lucy & T. K. Graczyk, 2014. Dreissenid mussels as sentinel biomonitors for human and zoonotic pathogens. Chapter 17. In Nalepa, T. & D. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control CRC Press/Taylor and Francis, Boca Raton: 265–272.
Connelly, N. A., C. R. O’Neill, B. A. Knuth & T. L. Brown, 2007. Economic impacts of zebra mussels on drinking water treatment and electric power generation facilities. Environmental Management 40: 105–112.
Conroy, J. D., D. D. Kane, D. M. Dolan, W. J. Edwards, M. N. Charlton & D. A. Culver, 2005a. Temporal trends in Lake Erie plankton biomass: roles of external phosphorus loading and dreissenid mussels. Journal of Great Lakes Research 31(Suppl. 2): 89–110.
Conroy, J. D., W. J. Edwards, R. A. Pontius, D. D. Kane, H. Zhang, J. F. Shea, J. N. Richey & D. A. Culver, 2005b. Soluble nitrogen and phosphorus excretion of exotic freshwater mussels (Dreissena spp.): potential impacts for nutrient remineralization in western Lake Erie. Freshwater Biology 50: 1146–1162.
Correa, N., P. Sardiña, P. V. Perepelizin & D. Boltovskoy, 2015. Limnoperna fortunei colonies: structure, distribution and dynamics. In Boltovskoy, D. (ed), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Springer, Cham: 119–143.
Costanza, R., R. d’Arge, R. de Groot, S. Farber, M. Grasso, B. Hannon, K. Limburg, S. Naeem, R. V. O’Neill, J. Paruelo, R. G. Raskin, P. Sutton & M. van den Belt, 1997. The value of the world’s ecosystem services and natural capital. Nature 387: 253–260.
Costanza, R., R. de Groot, P. Sutton, S. van der Ploeg, S. J. Anderson, I. Kubiszewski, S. Farber & R. K. Turner, 2014. Changes in the global value of ecosystem services. Global Environmental Change 26: 152–158.
Cotner, J. B., W. S. Gardner, J. R. Johnson, R. H. Sada, J. F. Cavaletto & R. T. Heath, 1995. Effects of zebra mussels (Dreissena polymorpha) on bacterioplankton: evidence for both size-selective consumption and growth stimulation. Journal of Great Lakes Research 21: 517–528.
Crane, D. P. & D. W. Einhouse, 2016. Changes in growth and diet of smallmouth bass following invasion of Lake Erie by the round goby. Journal of Great Lakes Research 42: 405–412.
Crooks, J. A., 2002. Characterizing ecosystem-level consequences of biological invasions: the role of ecosystem engineers. Oikos 97: 153–166.
Darrigran, G., 2002. Potential impact of filter feeding invaders on temperate inland freshwater environments. Biological Invasions 4: 145–156.
Darrigran, G. & C. Damborenea, 2005. A South American bioinvasion case history: Limnoperna fortunei (Dunker, 1857), the golden mussel. American Malacological Bulletin 20: 105–112.
Darrigran, G. & C. Damborenea, 2011. Ecosystem engineering impact of Limnoperna fortunei in South America. Zoological Science 28: 1–7.
Darrigran, G. & G. Pastorino, 2004. Distribution of the golden mussel Limnoperna fortunei (Dunker, 1857) (Bivalvia: Mytilidae) after 10 years invading America. Journal of Conchology 3: 95–101.
David, K. A., B. M. Davis & R. D. Hunter, 2009. Lake St Clair zooplankton: evidence for post-Dreissena changes. Journal of Freshwater Ecology 24: 199–209.
bij de Vaate, A., 1991. Distribution and aspects of population dynamics of the zebra mussel, Dreissena polymorpha Pallas 1771 in the Lake IJsselmeer area (The Netherlands). Oecologia (Heidelb.) 86: 40–50.
bij de Vaate, A., 2010. Some evidence for ballast water transport being the vector of the quagga mussel (Dreissena rostriformis bugensis Andrusov 1897) introduction into Western Europe and subsequent upstream dispersal in the River Rhine. Aquatic Invasions 5: 207–209.
bij de Vaate, A., R. Breukel & G. van der Velde, 2006. Long-term developments in ecological rehabilitation of the main distributaries in the Rhine delta: fish and macroinvertebrates. Hydrobiologia 565: 229–242.
bij de Vaate, A., G. Van der Velde, R. S. E. W. Leuven & K. C. M. Heiler, 2014. Spread of the quagga mussel, Dreissena rostriformis bugensis, in Western Europe. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control 2nd ed. CRC Press, Boca Raton: 83–92.
Dermott, R. & D. Kerec, 1997. Changes to the deep water benthos of eastern Lake Erie since the invasion of Dreissena: 1979-1993. Canadian Journal of Fisheries and Aquatic Sciences 54: 922–930.
Dettmers, J. M., C. I. Goddard & K. D. Smith, 2012. Management of alewife using pacific salmon in the Great Lakes: whether to manage for economics or the ecosystem? Fisheries 37(11): 495–501.
Diagne, C., B. Leroy, A.-C. Vaissière, R. E. Gozlan, D. Roiz, I. Jarić, J.-M. Salles, C. J. A. Bradshaw & F. Courchamp, 2021. High and rising economic costs of biological invasions worldwide. Nature 592: 571–576.
Diaz, R. J. & R. Rosenberg, 2008. Spreading dead zones and consequences for marine ecosystems. Science 321: 926–929.
Dietrich, J. P., B. J. Morrison & J. A. Hoyle, 2006. Alternative ecological pathways in the eastern Lake Ontario food web—round goby in the diet of lake trout. Journal of Great Lakes Research 32: 395–400.
Diggins, T. P., 2001. A seasonal comparison of suspended sediment filtration by quagga (Dreissena bugensis) and zebra (D. polymorpha) mussels. Journal of Great Lakes Research 27: 457–466.
Dionisio Pires, L. M., B. M. Bontes, E. Van Donk & B. W. Ibelings, 2005. Grazing on colonial and filamentous, toxic and non-toxic cyanobacteria by the zebra mussel Dreissena polymorpha. Journal of Plankton Research 27: 331–339.
Dionisio Pires, L. M., B. W. Ibelings & E. Van Donk, 2010. Zebra mussels as a potential tool in the restoration of eutrophic shallow lakes dominated by toxic cyanobacteria. In van der Velde, G., S. Rajagopal & A. bij de Vaate (eds), The Zebra Mussel in Europe Backhuys Publishers, Leiden: 361–372.
do Amaral, Q. D. F., E. Da Rosa, J. G. Wronski, L. Zuravski, M. V. M. Querol, B. dos Anjos, C. F. F. de Andrade, M. M. Machado & L. F. S. de Oliveira, 2019. Golden mussel (Limnoperna fortunei) as a bioindicator in aquatic environments contaminated with mercury: cytotoxic and genotoxic aspects. Science of the Total Environment 675: 343–353.
Dobiesz, N. E. & P. L. Negel, 2009. Changes in mid-summer water temperature and clarity across the Great Lakes between 1968 and 2002. Journal of Great Lakes Research 35: 371–384.
Dove, A., 2009. Long-term trends in major ions and nutrients in Lake Ontario. Aquatic Ecosystem Health & Management 12: 281–295.
Dove, A. & S. C. Chapra, 2015. Long-term trends of nutrients and trophic response variables for the Great Lakes. Limnology & Oceanography 60: 696–721.
Draulans, D., 1982. Foraging and size selection of mussels by the tufted duck, Aythya fuligula. Journal of Animal Ecology 51: 943–956.
Duchini, D., D. Boltovskoy & F. Sylvester, 2018. The invasive freshwater bivalve Limnoperna fortunei in South America: multiannual changes in its predation and effects on associated benthic invertebrates. Hydrobiologia 817: 431–446.
Effler, S. W. & C. Siegfried, 1998. Tributary water quality feedback from the spread of zebra mussels: Oswego River, New York. Journal of Great Lakes Research 24: 453–463.
El Haj, Y., S. Bohn & M. M. Souza, 2019. Tolerance of native and invasive bivalves under herbicide and metal contamination: an ex vivo approach. Environmental Science and Pollution Research International 26: 31198–31206.
Elliott, P., D. C. Aldridge & G. D. Moggridge, 2008. Zebra mussel filtration and its potential uses in industrial water treatment. Water Research 42: 1664–1674.
Evans, M. A., G. Fahnenstiel & D. Scavia, 2011. Incidental oligotrophication of North American Great Lakes. Environmental Science and Technology 45: 3297–3303.
Ezcurra de Drago, I., 2004. Biodiversidad de Porifera en el Litoral Argentino. Grado de competencia con el bivalvo invasor Limnoperna fortunei (Dunker, 1857) (Bivalvia, Mytilidae). Instituto Superior de Correlación Geológica (INSUGEO). Miscelanea 12: 195–204.
Fahnenstiel, G., S. Pothoven, H. Vanderploeg, D. Klarer, T. Nalepa & D. Scavia, 2010. Recent changes in primary production and phytoplankton in the offshore region of southeastern Lake Michigan. Journal of Great Lakes Research 36(Suppl.3): 20–29.
Faria, M., M. A. López, M. Fernández-Sanjuan, S. Lacorte & C. Barata, 2010. Comparative toxicity of single and combined mixtures of selected pollutants among larval stages of the native freshwater mussels (Unio elongatulus) and the invasive zebra mussel (Dreissena polymorpha). Science of the Total Environment 408: 2452–2458.
Feldman, M., 1996. Comparison of the effects of over-the-counter famotidine and calcium carbonate antacid on posprandial gastric acid. Journal of American Medical Association 225: 1428–1431.
Ferriz, R. A., C. A. Villar, D. Colautti & C. Bonetto, 2000. Alimentacion de Pterodoras granulosus (Valenciennes) (Pisces, Doradidae) en la baja Cuenca del Plata. Revista Del Museo Argentino De Ciencias Naturales 2: 151–156.
Flood, P. J., A. Duran, M. Barton, A. E. Mercado-Molina & J. C. Trexler, 2020. Invasion impacts on functions and services of aquatic ecosystems. Hydrobiologia 847: 1571–1586.
FlórezVargas, R. P., J. F. Saad, M. Graziano, M. dos Santos Afonso, I. Izaguirre & D. Cataldo, 2019. Bacterial composition of the biofilm on valves of Limnoperna fortunei and its role in glyphosate degradation in water. Aquatic Microbial Ecology 83: 83–94.
Friedland, R., A.-L. Buer, S. Dahlke & G. Schernewski, 2019. Spatial effects of different zebra mussel farming strategies in an eutrophic Baltic Lagoon. Frontiers in Environmental Science 6: 158. https://doi.org/10.3389/fenvs.2018.00158.
Gasunas, I. I., 1959. Forage benthos of Coronian Lagoon. In Yankevichus, K. (ed.) Kurshu Mares, Vilnus (Lithuania): 139–280.
Gasunas, I. I., 1965. Distribution and economic importance of mollusc Dreissena polymorpha (Pallas) in Lithuanian waterbodies. In: Molluscs: Issues of Theoretical and Applied Malacology, Abstracts of 2nd Meeting on the Investigation of Molluscs, Nauka Press, Leningrad: 66.
Gattás, F., M. Espinosa, P. Babay, H. Pizarro & D. Cataldo, 2020. Invasive species versus pollutants: potential of Limnoperna fortunei to degrade glyphosate-based commercial formulations. Ecotoxicology and Environmental Safety 201: 110794.
Géba, E., D. Aubert, L. Durand, S. Escotte, S. La Carbona, C. Cazeaux, I. Bonnard, F. Bastien, M. Palos Ladeiro, J. P. Dubey, I. Villena, A. Geffard & A. Bigot-Clivot, 2020. Use of the bivalve Dreissena polymorpha as a biomonitoring tool to reflect the protozoan load in freshwater bodies. Water Research 2020(170): 115297.
Géba, E., D. Rioult, O. Palluel, O. Dedourge-Geffard, S. Betoulle, D. Aubert & A. Bigot-Clivot, 2021a. Resilience of Dreissena polymorpha in wastewater effluent: use as a bioremediation tool? Journal of Environmental Management 278: 111513.
Géba, E., A. Rousseau, A. Le Guernic, S. Escotte-Binet, L. Favennec, S. La Carbona, G. Gargala, J. P. Dubey, I. Villena, S. Betoulle, D. Aubert & A. Bigot-Clivot, 2021b. Survival and infectivity of Toxoplasma gondii and Cryptosporidium parvum oocysts bioaccumulated by Dreissena polymorpha. Journal of Applied Microbiology 130: 504–515.
Gergs, R., K. Rinke & K.-O. Rothhaupt, 2009. Zebra mussels mediate benthic-pelagic coupling by biodeposition and changing detrital stoichiometry. Freshwater Biology 54: 1379–1391.
Gibbs, J. P., J. M. Halstead, K. J. Boyle & J.-C. Huang, 2002. An hedonic analysis of the effects of lake water clarity on New Hampshire lakefront properties. Agricultural and Resource Economics Review 31: 39–46.
Giles, H. & C. A. Pilditch, 2006. Effects of mussel (Perna canaliculus) biodeposit decomposition on benthic respiration and nutrient fluxes. Marine Biology 150: 261–271.
Gillis, P. L. & G. L. Mackie, 1994. Impact of the zebra mussel, Dreissena polymorpha, on populations of Unionidae (Bivalvia) in Lake St. Clair. Canadian Journal of Zoology 72: 1260–1271.
Girardello, F., C. C. Leite, L. B. Touguinha, M. Roesch-Ely, A. N. Fernandes, R. M. D. Oliveira, D. L. G. Borges, C. K. H. D. Silva, M. Salvador, I. V. Villela & J. A. P. Henriques, 2021. ZnO nanoparticles alter redox metabolism of Limnoperna fortunei. Research Square. https://doi.org/10.21203/rs.3.rs-210424/v1.
Goedkoop, W., R. Naddafi & U. Grandin, 2011. Retention of N and P by zebra mussels (Dreissena polymorpha Pallas) and its quantitative role in the nutrient budget of eutrophic Lake Ekoln, Sweden. Biological Invasions 13: 1077–1086.
Goedkoop, W., M. I. Choudhury, D. C. P. Lau & U. Grandin, 2021. Inverting nutrient fluxes across the land-water interface – Exploring the potential of zebra mussel (Dreissena polymorpha) farming. Journal of Environmental Management 281: 111889.
Gomes, J., A. Matos, R. M. Quinta-Ferreira & R. C. Martins, 2018. Environmentally applications of invasive bivalves for water and wastewater decontamination. Science of the Total Environment 630: 1016–1027.
González-Bergonzoni, I., I. Silva, F. Teixeira de Mello, A. D’Anatro, L. Bocardi, S. Stebniki, E. Brugnoli, G. Tesitore, N. Vidal & D. E. Naya, 2020. Evaluating the role of predatory fish on the invasion of the Asian golden mussel (Limnoperna fortunei) in a subtropical river. Journal of Applied Ecology 57: 717–728.
Guerin, G. R., I. Martín-Forés, B. Sparrow & A. J. Lowe, 2018. The biodiversity impacts of non-native species should not be extrapolated from biased single-species studies. Biodiversity and Conservation 27: 785–790.
Gulati, R. D., L. M. Dionisio Pires & E. Van Donk, 2008. Lake restoration studies: failures, bottlenecks and prospects of new ecotechnological measures. Limnologica 38: 233–247.
Gutierrez, J. L., C. G. Jones, D. L. Strayer & O. O. Iribarne, 2003. Mollusks as ecosystem engineers: the role of shell production in aquatic habitats. Oikos 101: 79–90.
Haag, W. R., D. R. Berg, D. W. Garton & J. L. Farris, 1993. Reduced survival and fitness in native bivalves in response to fouling by the introduced zebra mussel (Dreissena polymorpha) in western Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 50: 13–19.
Hahm, J., J. B. Manchester-Neesvig, D. DeBord & W. C. Sonzogni, 2009. Polybrominated diphenyl ethers (PBDEs) in Lake Michigan forage fish. Journal of Great Lakes Research 35: 154–158.
Haines-Young, R. & M. Potschin-Young, 2011. Common international classification of ecosystem services (CICES): 2011 update. Expert Meet Ecosystem Accounts 33: 1–17.
Hamilton, D. J., C. D. Ankney & R. C. Bailey, 1994. Predation of zebra mussels by diving ducks: an exclosure study. Ecology 75: 521–531.
Hecky, R. E., R. E. H. Smith, D. R. Barton, S. J. Guildford, W. D. Taylor, M. N. Charlton & E. T. Howell, 2004. The near shore phosphorus shunt: a consequence of ecosystem engineering by dreissenids in the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 61: 1285–1293.
Higgins, S. N., 2014. Meta-analysis of dreissenid effects on freshwater ecosystems. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control 2nd ed. CRC Press, Boca Raton: 487–494.
Higgins, S. N. & M. J. Vander Zanden, 2010. What a difference a species makes: a meta-analysis of dreissenid mussel impacts on freshwater ecosystems. Ecological Monographs 80: 179–196.
Higgins, S. N., S. Y. Malkin, E. Todd Howell, S. J. Guildford, L. Campbell, V. Hiriart-Baer & R. E. Hecky, 2008. An ecological review of Cladophora glomerata (Chlorophyta) in the Laurentian Great Lakes. Journal of Phycology 44: 839–854.
Hobbs, R. J., S. Arico, J. Aronson, J. S. Baron, P. Bridgewater, V. A. Cramer, P. R. Epstein, J. J. Ewel, C. A. Klink, A. E. Lugo, D. Norton, D. Ojima, D. M. Richardson, E. W. Sanderson, F. Valladares, M. Vilà, R. Zamora & M. Zobel, 2006. Novel ecosystems: theoretical and management aspects of the new ecological world order. Global Ecology and Biogeography 15: 1–7.
Hobbs, R. J., E. S. Higgs & C. M. Hall, 2013. Defining novel ecosystems. In Hobbs, R. J., E. S. Higgs & C. M. Hall (eds), Novel Ecosystems: Intervening in the New Ecological World Order Wiley, Chichester: 58–60.
Howell, E. T., C. H. Marvin, R. W. Bilyea, P. B. Kauss & K. Somers, 1996. Changes in environmental conditions during Dreissena colonization of a monitoring station in eastern Lake Erie. Journal of Great Lakes Research 22: 744–756.
Hoyle, J. A., J. N. Bowlby & B. J. Morrison, 2008. Lake whitefish and walleye population responses to dreissenid mussel invasion in eastern Lake Ontario. Aquatic Ecosystem Health and Management 11: 403–411.
Hui, C. & D. M. Richardson, 2017. Invasion Dynamics, Oxford University Press, Oxford: 1–322.
Ibelings, B. W., R. Portielje, E. H. R. R. Lammens, R. Noordhuis, M. S. van den Berg, W. Joosse & M. L. Meijer, 2007. Resilience of alternative stable states during the recovery of shallow lakes from eutrophication: Lake Veluwe as a case study. Ecosystems 10: 4–16.
IJC, 1972. Great Lakes Water Quality Agreement with annexes and texts and terms of reference, between the United States and Canada, Signed at Ottawa, April 15, 1972, International Joint Commission, Windsor. Retrieved from https://treaties.un.org/doc/Publication/UNTS/Volume%20837/volume-837-I-11982-English.pdf.
IJC, 1978. Revised Great Lakes Water Quality Agreement of 1978, with Annexes and Terms of Reference, Between the United States and Canada, Signed at Ottawa, November 22, 1978, International Joint Commission, Windsor: https://www.ijc.org/sites/default/files/GLWQA_e.pdf
Immel, F., C. Broussard, B. Catherinet, L. Plasseraud, G. Alcaraz, I. Bundeleva & F. Marin, 2016. The shell of the invasive bivalve species Dreissena polymorpha: biochemical, elemental and textural investigations. PLOS ONE. https://doi.org/10.1371/journal.pone.0154264.
Jackson, J., A. VanDeValk, T. Brooking, O. VanKeeken & L. Rudstam, 2002. Growth and feeding dynamics of lake sturgeon, Acipenser fulvescens, in Oneida Lake, New York: results from the first five years of a restoration program. Journal of Applied Ichthyology 18: 439–443.
Jacobs, G. R., E. L. Bruestle, A. Hussey, D. Gorsky & A. T. Fisk, 2017. Invasive species alter ontogenetic shifts in the trophic ecology of Lake Sturgeon (Acipenser fulvescens) in the Niagara River and Lake Ontario. Biological Invasions 19: 1533–1546.
Jakus, P., M. J. Kealy, J. Loomis, N. Nelson, J. Ostermiller, C. Stanger & N. von Stackelberg, 2013. Economic Benefits of Nutrient Reductions in Utah’s Waters. Report Prepared for the State of Utah Utah Division of Water Quality, CH2M Hill, Salt Lake City [available on https://documents.deq.utah.gov/water-quality/standards-technical-services/DWQ-2020-000676.pdf].
Jenner, H. A., F. Noppert & T. Sikking, 1989. A now system for the detection of valve-movement response or bivalves. KCMA Scientific & Technical Reports 7: 91–98.
Jeppesen, E., D. Trolle, T. A. Davidson, R. Bjerring, M. Søndergaard, L. S. Johansson, T. L. Lauridsen, A. Nielsen, S. E. Larsen & M. Meerhoff, 2015. Major changes in CO2 efflux when shallow lakes shift from a turbid to a clear water state. Hydrobiologia 778: 33–44.
Jernelöv, A., 2017. The Long-Term Fate of Invasive Species. Aliens Forever or Integrated Immigrants with Time?, Springer, Cham: 1–296.
Johannsson, O. E., R. Dermott, D. M. Graham, J. A. Dahl, E. Scott Millard, D. D. Myles & J. LeBlanc, 2000. Benthic and pelagic secondary production in lake erie after the invasion of Dreissena spp. with implications for fish production. Journal of Great Lakes Research 26: 31–54.
Johengen, T. H., T. F. Nalepa, G. L. Fahnenstiel & G. Goudy, 1995. Nutrient changes in Saginaw Bay, Lake Huron, after establishment of the zebra mussel (Dreissena polymorpha). Journal of Great Lakes Research 21: 449–464.
Johns, C. & B. E. Timmerman, 1998. Total cadmium, copper, and zinc in two dreissenid mussels, Dreissena polymorpha and Dreissena bugensis, at the outflow of Lake Ontario. Journal of Great Lakes Research 24: 55–64.
Johnson, M. F. & M. E. Meder, 2013. Effects of aquatic invasive species on home prices: evidence from wisconsin. Human Settlement & Movement eJournal [available on https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2316911].
Johnson, L. E. & J. T. Carlton, 1996. Post-establishment spread in large-scale invasions: dispersal mechanisms of the zebra mussel Dreissena polymorpha. Ecology 77: 1686–1690.
Jones, C. G., J. H. Lawton & M. Shachak, 1994. Organisms as ecosystem engineers. Oikos 69: 373–386.
Jones, C. G., J. H. Lawton & M. Shachak, 1997. Positive and negative effects of organisms as physical ecosystem engineers. Ecology 78: 1946–1957.
Jude, D. J., R. H. Reider & G. R. Smith, 1992. Establishment of Gobiidae in the Great Lakes basin. Great Lakes Research Review 3: 27–34.
Kao, Y.-C., M. W. Rogers & D. B. Bunnell, 2018. Evaluating stocking efficacy in an ecosystem undergoing oligotrophication. Ecosystems 21(4): 600–618.
Karatayev, A. Y. & L. E. Burlakova, 1992. Changes in tropic structure of macrozoobenthos of an eutrophic lake, after invasion of Dreissena polymorpha. Biologiya Vnutrennikh Vod. Informatsionnyi Bulletin 93: 67–71 (in Russian).
Karatayev, A. Y. & L. E. Burlakova, 1995. The role of Dreissena in lake ecosystems. Russian Journal of Ecology 26(3): 207–211.
Karatayev, A. Y., V. P. Lyakhnovich, S. A. Afanasiev, L. E. Burlakova, V. P. Zakutsky, S. M. Lyakhov, M. P. Miroshnichenko, T. G. Moroz, M. Y. Nekrasova, I. A. Skalskaya, T. G. Kharchenko & A. A. Protasov, 1994a. The place of species in ecosystem. In Starobogatov, J. I. (ed), Freshwater Zebra Mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, Ecology, Practical Meaning Nauka Press, Moscow: 180–195 (in Russian).
Karatayev, A. Y., V. P. Mikheev, S. A. Afanasiev, M. Y. Kirpichenko, A. A. Protasov, L. V. Shevtsova & T. G. Kharchenko, 1994b. Practical uses and control on artificial structures. In Starobogatov, J. I. (ed), Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae). Systematics, ecology, practical meaning Nauka Press, Moscow: 206–221 (in Russian).
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 1997. The effects of Dreissena polymorpha (Pallas) invasion on aquatic communities in eastern Europe. Journal of Shellfish Research 16: 187–203.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 1998. Physical factors that limit the distribution and abundance of Dreissena polymorpha (Pall.). Journal of Shellfish Research 17: 1219–1235.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2002. Impacts of zebra mussels on aquatic communities and their role as ecosystem engineers. In Leppakoski, E., S. Gollach & S. Olenin (eds), Invasive Aquatic Species of Europe: Distribution, Impacts and Management Kluwer Academic Publishers, Dordrecht: 433–446.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2003. Patterns of spread of the zebra mussel (Dreissena polymorpha (Pallas)): the continuing invasion of Belarussian lakes. Biological Invasions 5: 213–221.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2006. Growth rate and longevity of Dreissena polymorpha (Pallas): a review and recommendations for future study. Journal of Shellfish Research 25: 23–32.
Karatayev, A. Y., D. Boltovskoy, D. K. Padilla & L. E. Burlakova, 2007a. The invasive bivalves Dreissena polymorpha and Limnoperna fortunei: parallels, contrasts, potential spread and invasion impacts. Journal of Shellfish Research 26: 205–213.
Karatayev, A. Y., D. K. Padilla, D. Minchin, D. Boltovskoy & L. E. Burlakova, 2007b. Changes in global economies and trade: the potential spread of exotic freshwater bivalves. Biological Invasions 9: 161–180.
Karatayev, A. Y., L. E. Burlakova, S. E. Mastitsky & S. Olenin, 2008. Past, current, and future of the Central European Corridor for aquatic invasions in Belarus. Biological Invasions 10: 215–232.
Karatayev, A. Y., L. E. Burlakova, V. A. Karatayev & D. Boltovskoy, 2010. Limnoperna fortunei versus Dreissena polymorpha: Population densities and benthic community impacts of two invasive freshwater bivalves. Journal of Shellfish Research 29: 975–984.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2010. Dreissena polymorpha in Belarus: history of spread, population biology, and ecosystem impacts. In van der Velde, G., S. Rajagopal & A. bij de Vaate (eds), The Zebra Mussel in Europe Backhuys Publishers, Leiden: 101–112.
Karatayev, A. Y., L. E. Burlakova, S. E. Mastitsky, D. K. Padilla & E. L. Mills, 2011a. Contrasting rates of spread of two congeners, Dreissena polymorpha and Dreissena rostriformis bugensis, at different spatial scales. Journal of Shellfish Research 30: 923–931.
Karatayev, A. Y., S. E. Mastitsky, D. K. Padilla, L. E. Burlakova & M. M. Hajduk, 2011b. Differences in growth and survivorship of zebra and quagga mussels: size matters. Hydrobiologia 668: 183–194.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2014a. General overview of zebra and quagga mussels what we do and do not know. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control 2nd ed. CRC Press, Boca Raton: 695–703.
Karatayev, V. A., A. Y. Karatayev, L. E. Burlakova & L. D. Rudstam, 2014b. Eutrophication and Dreissena invasion as drivers of biodiversity: a century of change in the mollusc community of Oneida Lake. PLoS ONE 9(7): e101388.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2015a. Zebra versus quagga mussels: a review of their spread, population dynamics, and ecosystem impacts. Hydrobiologia 746: 97–112.
Karatayev, A., D. Boltovskoy, L. Burlakova & D. Padilla, 2015b. Parallels and contrasts between Limnoperna fortunei and Dreissena species. In Boltovskoy, D. (ed), Limnoperna fortunei, Invading Nature – Springer Series in Invasion Ecology Springer, Cham: 261–297.
Karatayev, A. Y., L. E. Burlakova, K. Mehler, R. P. Barbiero, E. K. Hinchey, P. D. Collingsworth, K. E. Kovalenko & G. Warren, 2018a. Life after Dreissena: the decline of exotic suspension feeder may have significant impacts on lake ecosystems. Journal of Great Lakes Research 44: 650–659.
Karatayev, A. Y., L. E. Burlakova, K. Mehler, S. A. Bocaniov, P. D. Collingsworth, G. Warren, R. T. Kraus & E. K. Hinchey, 2018b. Biomonitoring using invasive species in a large lake: Dreissena distribution maps hypoxic zones. Journal of Great Lakes Research 44: 639–649.
Karatayev, A. Y., L. E. Burlakova, K. Mehler, E. K. Hinchey & G. Warren, 2018c. Benthic video image analysis facilitates monitoring of Dreissena populations across spatial scales. Journal of the Great Lakes Research 44: 629–638.
Karatayev, A. Y., L. E. Burlakova & D. K. Padilla, 2018d. Can introduced species replace lost biodiversity? A test with freshwater molluscs. Hydrobiologia 810: 45–56.
Karatayev, A. Y., V. A. Karatayev, L. E. Burlakova, K. Mehler, M. D. Rowe, A. K. Elgin & T. F. Nalepa, 2021a. Lake morphometry determines Dreissena invasion dynamics. Biological Invasions 23: 2489–2514.
Karatayev, A. Y., L. E. Burlakova, K. Mehler, E. K. Hinchey, M. Wick, M. Bakowska & N. Mrozinska, 2021b. Rapid assessment of Dreissena population in Lake Erie using underwater videography. Hydrobiologia 848: 2421–2436.
Karatayev, V. A., L. G. Rudstam, A. Y. Karatayev, L. E. Burlakova, B. V. Adamovich, H. A. Zhukava, K. T. Holeck, A. L. Hetherington, J. R. Jackson, C. W. Hotaling, T. V. Zhukova, T. M. Mikheyeva, R. Z. Kovalevskaya, O. A. Makarevich & D. V. Kruk, 2021c. Serial invasions can disrupt the time course of ecosystem recovery. BioRxiv. https://doi.org/10.1101/2021.10.29.466526.
Karatayev, A. Y. & L. E. Burlakova, accepted. What we know and don’t know about the invasive zebra (Dreissena polymorpha) and quagga (Dreissena rostriformis bugensis) mussels. Hydrobiologia.
Katsev, S., 2017. When large lakes respond fast: A parsimonious model for phosphorus dynamics. Journal of Great Lakes Research 43: 199–204.
Kean, W. F. & L. G. Enochs, 2001. Urban field geology for K-8 teachers. Journal of Geoscience Education 49: 358–363.
Kelly, D. W., L.-M. Herborg & H. J. MacIsaak, 2010. Ecosystem changes associated with Dreissena invasions: recent developments and emerging issues. In van der Velde, G., S. Rajagopal & A. bij de Vaate (eds), The Zebra Mussel in Europe Backhuys Publishers, Leiden: 199–209.
Kerney, M. P. & B. S. Morton, 1970. The distribution of Dreissena polymorpha (Pallas) in Britain. Journal of Conchology 27: 97–100.
Khalanski, M., 1997. Industrial and ecological consequences of the introduction of new species in continental aquatic ecosystems: the zebra mussel and other invasive species (Dreissena polymorpha, Corbicula fluminea, Corophium curvispinum). Bulletin Français De La Peche Et De La Pisciculture 344(345): 385–404.
Kimbrough, K. L., W. E. Johnson, A. P. Jacob & G. G. Lauenstein, 2014. Contaminant concentrations in dreissenid mussels from the Laurentian Great Lakes: a summary of trends from the mussel watch program. Chapter 18. In Nalepa, T. & D. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control CRC Press/Taylor and Francis, Boca Raton: 273–284.
Kinzelbach, R., 1992. The main features of the phylogeny and dispersal of the zebra mussel Dreissena polymorpha. In Neumann, D. & H. A. Jenner (eds), The Zebra Mussel Dreissena polymorpha: Ecology, Biological Monitoring and First Applications in the Water Quality Management Gustav Fisher, Stuttgart: 5–17.
Kissman, C. E. H., L. B. Knoll & O. Sarnelleb, 2010. Dreissenid mussels (Dreissena polymorpha and Dreissena bugensis) reduce microzooplankton and macrozooplankton biomass in thermally stratified lakes. Limnology and Oceanography 55: 1851–1859.
Knoll, L. B., O. Sarnelle, S. K. Hamilton, C. E. H. Kissman, A. E. Wilson, J. B. Rose & M. R. Morgan, 2008. Invasive zebra mussels (Dreissena polymorpha) increase cyanobacterial toxin concentrations in low-nutrient lakes. Canadian Journal of Fisheries and Aquatic Sciences 65: 448–455.
Kocovsky, P. M., 2019. Diets of endangered silver chub (Macrhybopsis storeriana, Kirtland, 1844) in Lake Erie and implications for recovery. Ecology of Freshwater Fish 28: 33–40.
Kornobis, S., 1977. Ecology of Dreissena polymorpha (Pall.) (Dreissenidae, Bivalvia) in lakes receiving heated water discharges. Polish Archives of Hydrobiology 24: 531–545.
Korolevs A. & N. Kondratjeva, 2006. Artificial reefs and substrata in the coastal zone of the Baltic – The problems and decisions. 2006 IEEE US/EU Baltic International Symposium, pp. 1–14, doi: https://doi.org/10.1109/BALTIC.2006.7266190.
Kozulin, A., 1995. Ecology of Mallards Anas platyrhynchos wintering in low temperature conditions in Belarus. Acta Ornithologica (Warsaw) 30: 125–134.
Kryger, J. & H. U. Riisgard, 1988. Filtration rate capacities in 6 species of European freshwater bivalves. Oecologia 77: 34–38.
Krysel, C., E. M. Boyer, C. Parson & P. Welle, 2003. Lakeshore property values and water quality: evidence from property sales in the Mississippi Headwaters Region. Submitted to the Legislative Commission on Minnesota Resources by the Mississippi Headwaters Board and Bemidji State University [available on https://www.mississippiheadwaters.org/files/bsu_study.pdf].
Kusserov, R., M. Mörtl, J. Mählmann, D. Uhlmann & I. Röske, 2010. The design of a Zebra-Mussel-Biofilter. In van der Velde, G., S. Rajagopal & A. bij de Vaate (eds), The Zebra Mussel in Europe Backhuys Publishers, Leiden: 323–330.
Kwon, T.-D., S. W. Fisher, G. W. Kim, H. Hwang & J.-E. Kim, 2006. Trophic transfer and biotransformation of polychlorinated biphenyls in zebra mussel, round goby, and smallmouth bass in Lake Erie, USA. Environmental Toxicology and Chemistry 25: 1068–1078.
Ladeiro, M. P., D. Aubert, I. Villena, A. Geffard & A. Bigot, 2014. Bioaccumulation of human waterborne protozoa by zebra mussel (Dreissena polymorpha): Interest for water biomonitoring. Water Research 48: 148–155.
Lajewski, C. K., H. T. Mullins, W. P. Patterson & C. W. Callinan, 2003. Historic calcite record from the Finger Lakes, New York: impact of acid rain on a buffered terrane. Bulletin of the Geological Society of America 115: 373–384.
Lazareva, V. I., A. I. Kopylov, E. A. Sokolova & E. G. Pryanichnikova, 2016. Veliger larvae of dreissenids (Bivalvia, Dreissenidae) in the plankton foodweb of Rybinsk Reservoir. Biology Bulletin 43: 1313–1321.
Le Guernic, A., M. Palos Ladeiro, N. Boudaud, J. Do Nascimento, C. Gantzer, J.-C. Inglard, J.-M. Mouchel, C. Pochet, L. Moulin, V. Rocher, P. Waldman, S. Wurtzer & A. Geffard, 2022. First evidence of SARS-CoV-2 genome detection in zebra mussel (Dreissena polymorpha). Journal of Environmental Management 301: 113866.
Leggett, C. G. & N. E. Bockstael, 2000. Evidence of the effects of water quality on residential land prices. Journal of Environmental Economics and Management 39: 121–144.
Leuven, R. S. E. W., F. P. L. Collas, K. R. Koopman, J. Matthews & G. Van der Velde, 2014. Mass mortality of invasive zebra and quagga mussels by desiccation during severe winter conditions. Aquatic Invasions 9: 243–252.
Leuzinger, S. & B. Rewald, 2021. The who or the how? Species vs. ecosystem function priorities in conservation ecology. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2021.758413.
Leuzinger, H. & S. Schuster, 1970. Auswirkungen der massenvermehrung de wandermuschel Dreissena polymorpha auf die wasservögel des bodensees. Der Ornithologische Beobachter 67: 269–274.
Li, J., Y. Zhang & S. Katsev, 2018a. Phosphorus recycling in deeply oxygenated sediments in Lake Superior controlled by organic matter mineralization. Limnology & Oceanography 63: 1372–1385.
Li, S., Y. Chen, Y. Gao, Z. Xia & A. Zhan, 2018b. Chemical oxidants affect byssus adhesion in the highly invasive fouling mussel Limnoperna fortunei. Science of the Total Environment 646: 1367–1375.
Li, S., Z. Xia, Y. Chen, Y. Gao & A. Zhan, 2018c. Byssus structure and protein composition in the highly invasive fouling mussel Limnoperna fortunei. Frontiers Physiology 9: 418.
Li, J., V. Ianaiev, A. Huff, J. Zalusky, T. Ozersky & S. Katsev, 2021. Benthic invaders control the phosphorus cycle in the world’s largest freshwater ecosystem. Proceedings of the National Academy of Sciences of the United States of America 118: e2008223118.
Limanova, N. A., 1964. Dreissena: bibliography. Biology of Dreissena and its control. Proceedings of Institute of Biology of Inland Waters of Academy of Science of the USA 7: 83–136 (in Russian).
Limanova, N. A., 1978. Dreissena: Bibliographic Guide. Moscow: pp. 115 (in Russian).
Limburg, K. E., V. A. Luzadis, M. Ramsey, K. L. Schulz & C. M. Mayer, 2010. The good, the bad, and the algae: Perceiving ecosystem services and disservices generated by zebra and quagga mussels. Journal of Great Lakes Research 36: 86–92.
Lindeman, P. V., 2006. Zebra and Quagga Mussels (Dreissena spp.) and other prey of a Lake Erie population of common map turtles (Emydidae: Graptemys geographica). Copeia 2: 268–273.
Lindim, C., 2015. Modeling the impact of zebra mussels (Dreissena polymorpha) on phytoplankton and nutrients in a lowland river. Ecological Modelling 301: 17–26.
Lohner, R., V. Sigler, C. Mayer & C. Balogh, 2007. A comparison of the benthic bacterial communities within and surrounding Dreissena clusters in lakes. Microbial Ecology 54: 469–477.
Lopes, M. N., J. P. Vieira & M. D. M. Burns, 2009. Biofouling of the golden mussel Limnoperna fortunei (Dunker, 1857) over the Anomura crab Aegla platensis Schmitt, 1942. Pan-American Journal of Aquatic Sciences 4: 222–225.
Lovell, S. J., S. F. Stone & L. Fernandez, 2006. The economic impacts of aquatic invasive species: a review of the literature. Agricultural and Resource Economics Review 35: 195–208.
Lowe, R. L. & R. W. Pillsbury, 1995. Shifts in benthic algal community structure and function following the appearance of zebra mussel (Dreissena polymorpha) in Saginaw Bay, Lake Huron. Journal of Great Lakes Research 21: 558–566.
Lucy, F. E., 2009. Zebra mussels: review of ecology and impacts since invasion in Ireland. In Mackie, G. & R. Claudi (eds), Monitoring and Control of Macrofouling Molluscs in Freshwater Systems CRC Press, Boca Raton: 386–389.
Lucy, F. E., T. K. Graczyk, L. Tamang, A. Miraflor & D. Minchin, 2008. Biomonitoring of surface and coastal water for Cryptosporidium, Giardia, and human-virulent microsporidia using molluscan shellfish. Parasitology Research 103: 1369.
Lucy, F. E., M. Connolly, T. K. Graczyk, L. Tamang, M. Sullivan & S. E. Mastitsky, 2010. Zebra mussels (Dreissena polymorpha) are effective sentinels of water quality irrespective of their size. Aquatic Invasions 5: 49–57.
Lucy, F. E., L. E. Burlakova, A. Y. Karatayev, S. E. Mastitsky & D. T. Zanatta, 2014. Zebra mussel impacts on unionids: a synthesis of trends in North America and Europe. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control, 2nd ed. CRC Press, Boca Raton: 623–646.
Ludwig, S., E. H. R. Sari, H. Paixão, L. C. Montresor, J. Araújo, C. F. A. Brito, G. Darrigran, A. R. Pepato, T. H. D. A. Vidigal & C. B. Martinez, 2021. High connectivity and migration potentiate the invasion of Limnoperna fortunei (Mollusca: Mytilidae) in South America. Hydrobiologia 848: 499–513.
Luo, F., L. Liu, Z. You, W. Huang, H. Deng & S. Ouyang, 2006. Study on solvability of Limnoperna fortunei byssus cohering in raw water pipe. Water and Wastewater Engineering 3: 29–32.
Luukkonen, D. R., E. N. Kafcas, B. T. Shirkey & S. R. Winterstein, 2014. Impacts of dreissenid mussels on the distribution and abundance of diving ducks on Lake St. Clair. Chapter 41. In Nalepa, T. & D. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control CRC Press/Taylor and Francis, Boca Raton: 647–659.
Lvova, A. A., 1979. The role of Dreissena polymorpha Pallas in the self-cleaning of Uchinskoe reservoir. In Likharev, I. M. (ed), Molluscs: Main Results of Their Study Abstracts of the 6th Meeting on the Investigation of Molluscs Nauka Press, Leningrad: 219–220 (in Russian).
Lvova, A. A., A. Y. Karatayev & G. E. Makarova, 1994. Planktonic larva. In Starobogatov, J. I. (ed), Freshwater Zebra Mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae) Systematics, Ecology, Practical Meaning Nauka Press, Moscow: 149–155 (in Russian).
Lvova, A. A., 1977. The ecology of Dreissena polymorpha (Pall.) in Uchinskoe Reservoir. Candidate Dissertation, Moscow State University, Moscow, USSR (in Russian).
Lvova-Kachanova, A. A. & E. I. Izvekova, 1978. Dreissena and chironomids from Uchinskoe reservoir. In Dunaeva, T. N., et al., (eds), Plant and Animal Life in Moscow and its Environments Moscow University Press, Moscow: 119–121 (in Russian).
Lyagina, T. N. & V. D. Spanowskaya, 1963. Morphological distinctions of some fish in Uchinskoe reservoir. In Sokolova, N. Y. (ed), Uchinskoe and Mozhaiskoe Reservoirs Moskovskii Universitet Press, Moscow: 269–310 (in Russian).
Lyakhnovich, V. P., A. Y. Karatayev & P. A. Mitrakhovich, 1983. Effect of Dreissena polymorpha Pallas on ecosystem of a eutrophic lake. Biologiya Vnutrennikh Vod. Informacionnyi Bulleten 60: 25–28 (in Russian).
Lyakhnovich, V. P., A. Y. Karatayev, P. A. Mitrakhovich, L. V. Guryanova & G. G. Vezhnovets, 1988. Productivity and prospects for utilizing the ecosystem of Lake Lukoml, thermoelectric station cooling reservoir. Soviet Journal of Ecology 18: 255–259.
MacIsaac, H. J., 1996. Potential abiotic and biotic impacts of zebra mussels on the inland waters of North America. American Zoologist 36: 287–299.
Mackie, G. L., 1991. Biology of the exotic zebra mussel, Dreissena polymorpha, in relation to native bivalves and its potential impact in Lake St. Clair. Hydrobiologia 219: 251–268.
Mackie, G. L. & R. Claudi (eds), 2010. Monitoring and Control of Macrofouling Mollusks in Fresh Water Systems. CRC Press, Boca Raton.
Mackie, G. L. & C. A. Wright, 1994. Ability of the zebra mussel, Dreissena polymorpha to biodeposit and remove phosphorus and bod from diluted activated sewage sludge. Water Research 28: 1123–1130.
Madenijan, C. P., S. A. Pothoven, P. J. Schneeberger, M. P. Ebener, L. C. Mohr, T. F. Nalepa & J. R. Bence, 2010. Dreissenid mussels are not a “dead end” in Great Lakes food webs. Journal of Great Lakes Research 36: 73–77.
Madenjian, C. P., 1995. Removal of algae by the zebra mussel Driessena polymorpha population in western Lake Erie—a bioenergetics approach. Canadian Journal of Fisheries and Aquatic Sciences 52: 381–390.
Madenjian, C. P., M. A. Stapanian, L. D. Witzel, S. A. Pothoven, D. W. Einhouse & H. L. Whitford, 2011. Evidence for predatory control of the invasive round goby. Biological Invasions 13: 987–1002.
Magoulick, D. D. & L. C. Lewis, 2002. Predation on exotic zebra mussels by native fishes: effects on predator and prey. Freshwater Biology 47: 1908–1918.
Makarevich, T. A., S. E. Mastitsky & Y. V. Savich, 2008. Phytoperiphyton on the shells of Dreissena polymorpha (Pallas) in Lake Naroch. Aquatic Invasions 3: 283–295.
Makarewicz, J. C., T. W. Lewis & P. Bertram, 1999. Phytoplankton composition and biomass in the offshore waters of Lake Erie: Pre- and post-Dreissena introduction (1983–1993). Journal of Great Lakes Research 25: 135–148.
Mansur, M. C. D., C. P. Santos, G. Darrigran, I. Heydrich, C. T. Callil & F. R. Cardoso, 2003. Primeiros dados quali-quantitativos do mexilhão-dourado, Limnoperna fortunei (Dunker), no Delta do Jacuí, no Lago Guaíba e na Laguna dos Patos, Rio Grande do Sul, Brasil e alguns aspectos de sua invasão no novo ambiente. Revista Brasileira De Zoologia 20: 75–84.
Mantovani, D., H. B. Quesada, R. A. de Souza, L. F. Cusioli, L. Nishi, A. Diório, P. F. Soares, R. Bergamasco & M. F. Vieira, 2020. Adsorption of methylene blue from effluent using golden mussel (Limnoperna fortunei) shell as a low-cost material. Desalination and Water Treatment 188: 232–238.
Marchowski, D., G. Neubauer, Ł Ławicki, A. Woźniczka, D. Wysocki, S. Guentzel & M. Jarzemski, 2015. The importance of non-native prey, the zebra mussel Dreissena polymorpha, for the declining greater scaup Aythya marila: a case study at a key European staging and wintering site. PLoS ONE 10: e0145496.
Marin Jarrin, J. R., K. L. Pangle, J. M. Reichert, T. B. Johnson, J. Tyson & S. A. Ludsin, 2015. Influence of habitat heterogeneity on the foraging ecology of first feeding yellow perch larvae, Perca flavescens, in western Lake Erie. Journal of Great Lakes Research 41: 208–214.
Mariñelarena, A., M. E. MacDonagh, J. Donadelli & M. A. Casco, 2016. Un caso inusual de eutrofización en el Embalse Río Tercero: el posible rol de dos bioinvasores. Biologia Acuática 31: 10–18.
Matthews, J., G. Van der Velde, A. bij de Vaate, F. Collas, K. Koopman & R. S. E. W. Leuven, 2014. Rapid range expansion of the invasive quagga mussel in relation to zebra mussel presence in The Netherlands and Western Europe. Biological Invasions 16: 23–42.
Matthews, J., A. M. Schipper, A. J. Hendriks, T. T. Le Yen, A. bij de Vaate, G. van der Velde & R. S. E. W. Leuven, 2015. A dominance shift from the zebra mussel to the invasive quagga mussel may alter the trophic transfer of metals. Environmental Pollution 203: 183–190.
Mayer, C. M., L. G. Rudstam, E. L. Mills, S. G. Cardiff & C. A. Bloom, 2001. Zebra mussels (Dreissena polymorpha), habitat alteration, and yellow perch (Perca flavescens) foraging: system-wide effects and behavioural mechanisms. Canadian Journal of Fisheries and Aquatic Sciences 58: 2459–2467.
Mayer, C. M., L. E. Burlakova, P. Eklöv, D. Fitzgerald, A. Y. Karatayev, S. A. Ludsin, S. Millard, E. L. Mills, A. P. Ostapenya, L. G. Rudstam, B. Zhu & T. V. Zhukova, 2014. The benthification of freshwater lakes: exotic mussels turning ecosystems upside down. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control, 2nd ed. CRC Press, Boca Raton: 575–586.
McCorquodale-Bauer, K. & N. Cicek, 2020. Zebra mussel shells as an alternative mineral resource for lime production as a phosphorus precipitant. Environmental Technology 22: 1–12.
McDonnell, E., 1996. Composting Zebra Mussels. Cornell Composting. Cornell Waste Management Institute, Department of Crop and Soil Sciences, Cornell University [available on http://compost.css.cornell.edu/zebra.html].
Mcgrew, S. C., 2013. Exploring the impact of zebra mussels (Dreissena polymorpha) on residential lakeshore property values in Otter Tail and Becker counties, Minnesota. M. A. Thesis, University of North Dakota. Theses and Dissertations. 1453 [available on https://commons.und.edu/theses/1453].
McLaughlan, C. & D. C. Aldridge, 2013. Cultivation of zebra mussels (Dreissena polymorpha) within their invaded range to improve water quality in reservoirs. Water Research 47: 4357–4369.
McLaughlan, C., P. Rose & D. C. Aldridge, 2014. Making the best of a pest: the potential for using invasive Zebra mussel (Dreissena polymorpha) biomass as a supplement to commercial chicken feed. Environmental Management 54(5): 1102–1109.
Meerhoff, M., C. Fosalba, C. Bruzzone, N. Mazzeo, W. Noordoven & E. Jeppesen, 2006. An experimental study of habitat choice by Daphnia: plants signal danger more than refuge in subtropical lakes. Freshwater Biology 51: 1320–1330.
Mei, X., X. Zhang, S.-S. Kassam & L. G. Rudstam, 2016. Will the displacement of zebra mussels by quagga mussels increase water clarity in shallow lakes during summer? Results from a mesocosm experiment. PLoS ONE 11(12): e0168494.
Mellina, E., J. B. Rasmussen & E. L. Mills, 1995. Impact of zebra mussel (Dreissena polymorpha) on phosphorus cycling and chlorophyll in lakes. Canadian Journal of Fisheries and Aquatic Sciences 52: 2553–2573.
Mendes Sene, A., D. Melo Rosa, S. M. Millan Gutierre & P. Santos Pompeu, 2021. Freshwater mollusks as proxies for assessing agrochemicals hazards in Volta Grande Reservoir, Brazil. Revista Ambiente e Agua. https://doi.org/10.4136/ambi-agua.2681.
Meng, Y., Z. Guo, S. C. Fitzer, A. Upadhyay, V. B. S. Chan, C. Li, M. Cusack, H. Yao, K. W. K. Yeung & V. Thiyagarajan, 2018. Ocean acidification reduces hardness and stiffness of the Portuguese oyster shell with impaired microstructure: a hierarchical analysis. Biogeosciences 15: 6833–6846.
Mercer, J. L., M. G. Fox & C. D. Metcalfe, 1999. Changes in benthos and three littoral zone fishes in a shallow, eutrophic ontario lake following the invasion of the zebra mussel (Dreissena polymorpha). Lake and Reservoir Management 15: 310–323.
Mezzanotte, V., F. Marazzi, M. Bissa, S. Pacchioni, A. Binelli, M. Parolini, S. Magni, F. M. Ruggeri, C. D. C. Morghen, C. Zanotto & A. Radaelli, 2016. Removal of enteric viruses and Escherichia coli from municipal treated effluent by zebra mussels. Science of the Total Environment 539: 395–400.
Michael, H. J., K. J. Boyle & R. Bouchard, 2000. Does the measurement of environmental quality affect implicit prices estimated from hedonic models? Land Economics 76: 283–298.
Michael, H. J., K. J. Boyle & R. Bouchard, 1996. Water quality affects property prices: a case study of selected Maine lakes. Maine Agricultural and Forest Experiment Station Miscellaneous Report 398 [available on https://digitalcommons.library.umaine.edu/cgi/viewcontent.cgi?article=1003&context=aes_miscreports].
Mida, J. L., D. Scavia, G. L. Fahnenstiel, S. A. Pothoven, H. A. Vanderploeg & D. M. Dolan, 2010. Long-term and recent changes in southern Lake Michigan water quality with implications for present trophic status. Journal of Great Lakes Research 36: 42–49.
Mikheev, V. P., A. Y. Karatayev & L. E. Burlakova, 1994. Feeding. In Starobogatov, J. I. (ed), Freshwater Zebra Mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae) Systematics, Ecology, Practical Meaning Nauka, Moscow: 120–137 (in Russian).
Millennium Ecosystem Assessment, 2005. Ecosystems and Human Well-being, Island Press, Washington, DC [available on https://www.millenniumassessment.org/en/index.html.]
Minillo, A., M. Casali, W. Isique, M. Leite & O. Rocha, 2016. Acumulação de microcistinas no mexilhão dourado Limnoperna fortunei e riscos para a biota aquática. Revista Brasileira De Ciências Ambientais 41: 42–57.
Miranda, C. E., C. D. Clauser, V. L. Lozano, D. H. Cataldo & H. N. Pizarro, 2021. An invasive mussel is in trouble: How do glyphosate, 2,4-D and its mixture affect Limnoperna fortuneiʹs survival? Aquatic Toxicology 239: 105957.
Miroshnichenko, M. P., 1990. Growth, production and practical use of Dreissena polymorpha (Pallas) in foow web of Tsimlyanskoe reservoir. In Khmeleva, N. N., A. P. Golubev, N. Y. Sokolova & V. E. Roschin (eds), Species Within their Range: Biology, Ecology, and Productivity of Aquatic Invertebrates Navuka i Tekhnika Press, Minsk: 141–146. (in Russian).
Mitchell, C. A. & J. Carlson, 1993. Lesser scaup forage on zebra mussels at Cook Nuclear Plant, Michigan. Journal of Field Ornithology 64: 219–222.
Mitrakhovich, P. A., V. M. Samoilenko, Z. K. Kartashevich, A. A. Svirid, E. A. Kozlov, G. N. Koolev & N. A. Papko, 2008. Ecosystem of Lukoml thermoelectric station cooling reservoir, Pravo and Economica Press, Minsk (Belarus).
Molloy, D. P., J. Powell & P. Ambrose, 1994. Short-term reduction of adult zebra mussels (Dreissena polymorpha) in the Hudson River near Catskill, New York: An effect of juvenile blue crab (Callinectes sapidus) predation? Journal of Shellfish Research 13: 367–371.
Molloy, D. P., A. Y. Karatayev, L. E. Burlakova, D. P. Kurandina & F. Laruelle, 1997. Natural enemies of zebra mussels: predators, parasites and ecological competitors. Reviews in Fisheries Science 5: 27–97.
Mordukhai-Boltovskoi, F. D., 1960. Caspian Fauna in the Azov and Black Sea Basins, Academia Nauk Press, Moscow: (in Russian).
Morrison, T. W., W. E. Lynch Jr. & K. Dabrowski, 1997. Predation on zebra mussels by freshwater drum and yellow perch in western Lake Erie. Journal of Great Lakes Research 23: 177–189.
Morrison, A. L., M. A. Thelen, S. E. Howe, K. D. Zimmer, B. R. Herwig, D. F. Staples & M. C. McEachran, 2021. Impacts of zebra mussels (Dreissena polymorpha) on isotopic niche size and niche overlap among fish species in a mesotrophic lake. Biological Invasions 23: 2985–3002.
Mörtl, M., S. Werner & K. O. Rothhaupt, 2010. Effects of predation by wintering water birds on zebra mussels and on associated macroinvertebrates. In van der Velde, G., S. Rajagopal & A. bij de Vaate (eds), The Zebra Mussel in Europe Backhuys Publishers, Leiden: 239–249.
Morton, B., 2015. The biology and anatomy of Limnoperna fortunei, a significant freshwater bioinvader: blueprints for success. In Boltovskoy, D. (ed), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Springer, Cham: 3–41.
Mosley, C. & H. Bootsma, 2015. Phosphorus recycling by profunda quagga mussels (Dreissena rostriformis bugensis) in Lake Michigan. Journal of Great Lakes Research 41: 38–48.
Musin, G. E., F. Rojas Molina, F. Giri & V. Williner, 2015. Structure and density population of the invasive mollusc Limnoperna fortunei associated with Eichhornia crassipes in lakes of the Middle Paraná floodplain. Journal of Limnology 74: 537–548.
Naddafi, R. & L. G. Rudstam, 2013. Predator induced behavioral defenses in two competitive invasive species. Animal Behavior 86: 1275–1284.
Naddafi, R., P. Eklöv & K. Pettersson, 2009. Stoichiometric constraints do not limit successful invaders: zebra mussels in Swedish lakes. PLoS ONE 4(4): e5345.
Nakano, D., T. Kobayashi & I. Sakaguchi, 2010. Predation and depth effects on abundance and size distribution of an invasive bivalve, the golden mussel Limnoperna fortunei, in a dam reservoir. Limnology 11: 259–266.
Nalepa, T. F., D. L. Fanslow & G. A. Lang, 2009a. Transformation of the offshore benthic community in Lake Michigan: recent shift from the native amphipod Diporeia spp. to invasive mussel Dreissena rostriformis bugensis. Freshwater Biology 54: 466–479.
Nalepa, T. F., S. A. Pothoven & D. L. Fanslow, 2009b. Recent changes in benthic macroinvertebrate populations in Lake Huron and impact on the diet of lake whitefish (Coregonus clupeaformus). Aquatic Ecosystem Health and Management 12: 2–10.
Nalepa, T. F., 2010. An overview of the spread, distribution, and ecological impacts of the quagga mussel, Dreissena rostriformis bugensis, with possible implications to the Colorado River system. Proceedings, Colorado River Basin Science and Resource Management Symposium. Coming Together, Coordination of Science and Restoration Activities for the Colorado River Ecosystem, Scottsdale, AZ, November 18–20, 2008. U.S. Geological Survey Scientific Investigations Report 2010–5135.
Neumann, D. & H. A. Jenner, 1992. Studies on the ecology and ecotoxicology of the zebra mussel Dreissena polymorpha. In Neumann, D. & H. A. Jenner (eds), The Zebra Mussel Dreissena polymorpha Ecology, Biology, Monitoring and First Applications in the Water Quality Management Gustav Fischer, New York: 1–4.
Nicholls, K. H., L. Heintsch & E. Carney, 2002. Univariate step-trend and multivariate assessments of the apparent effects of P loading reductions and zebra mussels on the phytoplankton of the Bay of Quinte, Lake Ontario. Journal of Great Lakes Research 28: 15–31.
Nienhuis, S., T. J. Haxton & T. C. Dunkley, 2014. An empirical analysis of the consequences of zebra mussel invasions on fisheries in inland, freshwater lakes in Southern Ontario. Management of Biological Invasions 5: 287–302.
Noordhuis, R. H., H. Reeders & A. bij de Vaate, 1992. Filtration rate and pseudofeces production in zebra mussels and their application in water quality management. In Neumann, D. & H. A. Jenner (eds), The Zebra Mussel Dreissena polymorpha: Ecology, Biological Monitoring and First Applications in the Water Quality Management Gustav Fisher, Stuttgart: 101–114.
Noordhuis, R., B. G. van Zuidam, E. T. H. M. Peeters & G. J. van Geestet, 2016. Further improvements in water quality of the Dutch Border lakes: two types of clear states at different nutrient levels. Aquatic Ecology 50: 521–539.
Nunes, S. M., L. Müller, C. Simioni, L. C. Ouriques, M. A. Gelesky, D. Fattorini, F. Regoli, J. M. Monserrat & J. Ventura-Lima, 2020. Impact of different crystalline forms of nTiO2 on metabolism and arsenic toxicity in Limnoperna fortunei. Science of the Total Environment 728: 138318.
Oates, J. A. H., 2008. Lime and Limestone: Chemistry and Technology, Production and Uses. John Wiley and Sons. Toronto, Weinheim, Germany.
Office of Technology Assessment, 1993. Harmful Non-indigenous Species in the United States (Publication OTA-F-565), United States Congress, Washington [available on http://www.princeton.edu/~ota/disk1/1993/9325/9325.pdf].
Ohkawa, K. & T. Nomura, 2015. Control of Limnoperna fortunei fouling: antifouling materials and coatings. In Boltovskoy, D. (ed), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Springer International Publishing, Cham: 395–415.
Oliveira, M. D., S. K. Hamilton, D. F. Calheiros & C. M. Jacobi, 2010. Oxygen depletion events control the invasive golden mussel (Limnoperna fortunei) in a tropical floodplain. Wetlands 30: 705–716.
Oliveira, M. M., E. S. Silva, S. H. Calazans, F. C. Fernandes, M. H. C. Baeta Neves, A. S. Ferrão Filho, R. A. Hauser Davis, R. M. Lopes, F. F. Bastos, V. L. F. Cunha Bastos & J. Cunha Bastos, 2021. Microcystin bioaccumulation in Limnoperna fortunei following Microcystis aeruginosa exposure, analysis of in vivo enzymatic phosphatase, acetylcholinesterase and carboxylesterase effects and in vitro experiments. Ecotoxicology and Environmental Contamination 16: 35–43.
Olney, P., 1963. The food and feeding habits of tufted duck Aythya fuligula. Ibis 105: 55–62.
O’Neill, C., 1997. Economic impact of Zebra Mussels: Results of the 1995 Zebra Mussel Information Clearinghouse Study. Great Lakes Research Review 3: 35–42.
Orlova, M. I., 2014. Origin and spread of quagga mussels (Dreissena rostriformis bugensis) in eastern Europe with notes on size structure of populations. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control 2nd ed. CRC Press, Boca Raton: 93–102.
Ozersky, T., D. O. Evans & B. K. Ginn, 2015. Invasive mussels modify the cycling, storage and distribution of nutrients and carbon in a large lake. Freshwater Biology 60: 827–843.
Paldavičiene, A., A. Zaiko, H. Mazur-Marzec & A. Razinkovas-Baziukas, 2015. Bioaccumulation of microcystins in invasive bivalves: a case study from the boreal lagoon ecosystem. Oceanologia 57: 93–101.
Paolucci, E. M. & E. V. Thuesen, 2015. Trophic relationships of Limnoperna fortunei with larval fishes. In Boltovskoy, D. (ed), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Springer International Publishing, Cham: 211–229.
Paolucci, E., E. Thuesen, D. Cataldo & D. Boltovskoy, 2010. Veligers of an introduced bivalve (Limnoperna fortunei) are a new food resource that enhances growth of larval fish in the Paraná River (South America). Freshwater Biology 55: 1831–1844.
Pathy, D. A. & G. L. Mackie, 1993. Comparative shell morphology of Dreissena polymorpha, Mytilopsis leucophaeata, and the “quagga” mussel (Bivalvia: Dreissenidae) in North America. Canadian Journal of Zoology 71: 1012–1023.
Patterson, J. C. & P. V. Lindeman, 2009. Effects of zebra and quagga mussel (Dreissena spp.) invasion on the feeding habits of Sternotherus odoratus (Stinkpot) on Presque Isle. Northwestern Pennsylvania. Northeastern Naturalist 16: 365–374.
Pazos, R. S., F. Spaccesi & N. Gómez, 2020. First record of microplastics in the mussel Limnoperna fortunei. Regional Studies in Marine Science 38: 101360.
Pedroli, J.-C., 1981. La phénologie des Fuligules hivernants sur le lac de Neuchâtel. Nos Oiseaux 36: 157–163.
Pejchar, L. & H. A. Mooney, 2009. Invasive species, ecosystem services and human well-being. Trends in Ecology & Evolution 24: 497–504.
Penchaszadeh, P. E., G. A. Darrigran, C. Angulo, A. Averbuj, M. Brögger, A. Dogliotti & N. Pírez, 2000. Predation of the invasive freshwater mussel Limnoperna fortunei (Dunker, 1857) (Mytilidae) by the fish Leporinus obtusidens Valenciennes, 1846 (Anostomidae) in the Rio de la Plata, Argentina. Journal of Shellfish Research 19: 229–231.
Pennuto, C. M., E. T. Howell, T. W. Lewis & J. C. Makarewicz, 2012. Dreissena population status in nearshore Lake Ontario. Journal of Great Lakes Research 38(Suppl. 4): 161–170.
Pennuto, C. M., L. E. Burlakova, A. Y. Karatayev, J. Kramer, A. Fischer & C. Mayer, 2014. Spatiotemporal characteristics of nitrogen and phosphorus in the benthos of nearshore Lake Erie. Journal of Great Lakes Research 40: 541–549.
Perepelizin, P. V. & D. Boltovskoy, 2011. Resistance of the invasive pest mussel Limnoperna fortunei to anoxia. Journal of the American Water Works Association 103: 79–85.
Perez-Fuentetaja, A., S. A. Mackintosh, L. R. Zimmerman, M. D. Clapsadl, M. Alaee & D. S. Aga, 2015. Trophic transfer of flame retardants (PBDEs) in the food web of Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 72: 1886–1896.
Perrings, C., M. Williamson & S. Dalmazzone (eds), 2001. The Economics of Biological Invasions. Edward Elgar, Northampton.
Peterson, D. L., P. Vecsei & C. A. Jennings, 2007. Ecology and biology of the lake sturgeon: a synthesis of current knowledge of a threatened North American Acipenseridae. Reviews in Fish Biology and Fisheries 17: 59–76.
Petrie, S. & R. Knapton, 1999. Rapid increase and subsequent decline of zebra and quagga mussels in Long Point Bay, Lake Erie: possible influence of waterfowl predation. Journal of Great Lakes Research 25: 772–782.
Piesik, Z., 1974. The role of the crayfish Orconectes limosus (Raf.) in extinction of Dreissena polymorpha (Pall.) subsisting on steelon net. Polish Archives of Hydrobiology 21: 401–410.
Piesik, Z., 1983. Biology of Dreissena polymorpha (Pall.) settling on stylon nets and the role of this mollusc in eliminating the seston and the nutrients from the watercourse. Polish Archives of Hydrobiology 30: 353–361.
Pimentel, D., 2005. Aquatic nuisance species in the New York State Canal and Hudson River systems and the Great Lakes Basin: an economic and environmental assessment. Environmental Management 35: 692–701.
Pimentel, D., 2011. Biological invasions, Economic and Environmental Costs of Alien Plant Animal and Microbe Species 2nd ed. CRC Press, Boca Raton: 1–449.
Poddubnyi, A. G., 1966. Adaptive response of Rutilus rutilus to variable environmental conditions. Proceedings of Institute of Biology of Inland Waters of the Academy of Science of the USSR 10: 131–138 (in Russian).
Pollux, B. J. A., G. van der Velde & A. bij de Vaate, 2010. A perspective on global spread of Dreissena polymorpha: a review on possibilities and limitations. In van der Velde, G., S. Rajagopal & A. bij de Vaate (eds), The Zebra Mussel in Europe Backhuys Publishers, Leiden: 45–58.
Pothoven, S. A. & G. L. Fahnenstiel, 2014. Lake Michigan after dreissenid mussel invasion. In Nalepa, T. F. & D. W. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control 2nd ed. CRC Press, Boca Raton: 545–553.
Pothoven, S. A. & C. P. Madenjian, 2008. Changes in consumption by alewives and lake whitefish after dreissenid mussel invasions in Lake Michigan and Huron. North American Journal of Fisheries Management 28: 308–320.
Pothoven, S. A. & H. A. Vanderploeg, 2020. Seasonal patterns for Secchi depth, chlorophyll a, total phosphorus, and nutrient limitation differ between nearshore and offshore in Lake Michigan. Journal of Great Lakes Research 46: 519–527.
Pothoven, S. A. & H. A. Vanderploeg, 2022. Variable changes in zooplankton phenology associated with the disappearance of the spring phytoplankton bloom in Lake Michigan. Freshwater Biology 67: 365–377.
Pothoven, S. A., T. Nalepa, P. Schneeberger & S. Brandt, 2001. Changes in diet and body condition of lake whitefish in southern Lake Michigan associated with changes in benthos. North American Journal of Fisheries Management 21: 876–883.
Pothoven, S. A., C. P. Madenjian & T. O. Hook, 2017. Feeding ecology of the walleye (Percidae, Sander vitreus), a resurgent piscivore in Lake Huron (Laurentian Great Lakes) after shifts in the prey community. Ecology of Freshwater Fish 26: 676–685.
Price, J. I. & M. T. Heberling, 2018. The effects of source water quality on drinking water treatment costs: a review and synthesis of empirical literature. Ecological Economics 151: 195–209.
Quinn, B., F. Gagné, M. Costello, C. McKenzie, J. Wilson & C. Mothersill, 2004. The endocrine disrupting effect of municipal effluent on the zebra mussel (Dreissena polymorpha). Aquatic Toxicology 66: 279–292.
Ram, J. L. & S. M. Palazzolo, 2008. Globalization of an aquatic pest: economic costs, ecological outcomes, and positive applications of zebra mussel invasions and expansions. Geography Compass 2(6): 1755–1776.
Reavie, E. D., R. P. Barbiero, L. E. Allinger & G. J. Warren, 2014. Phytoplankton trends in the Laurentian Great Lakes: 2001–2011. Journal of Great Lakes Research 40: 618–639.
Rebelo, M. F., L. F. Afonso, J. A. Americo, L. da Silva, J. L. B. Neto, F. Dondero, A. Crisanti & Q. Zhang, 2018. A sustainable synthetic biology approach for the control of the invasive golden mussel (Limnoperna fortunei). PeerJ. https://doi.org/10.7287/peerj.preprints.27164v1.
Reeders, H. H. & A. bij de Vaate, 1990. Zebra mussels (Dreissena polymorpha): a new perspective for water quality management. Hydrobiologia 200(201): 437–450.
Reeders, H. H., A. bij de Vaate & R. Noordhuis, 1993. Potential of the zebra mussel (Dreissena polymorpha) for water quality management. In Nalepa, T. F. & D. W. Schloesser (eds), Zebra Mussels. Biology, Impacts, and Control, Lewis Publishers, Ann Arbor: 439–451.
Reynolds, S. A. & D. C. Aldridge, 2021. Impacts of invasive quagga mussels (Dreissena rostriformis bugensis) on reservoir water quality, as revealed by progressive-change BACIPS analysis. Water Research 197: 117105.
Ricciardi, A., F. G. Whoriskey & J. B. Rasmussen, 1996. Impact of the Dreissena invasion on native unionid bivalves in the upper St. Lawrence River. Canadian Journal of Fisheries and Aquatic Sciences 53: 1434–1444.
Ricciardi, A., R. J. Neves & J. B. Rasmussen, 1998. Impending extinctions of North American freshwater mussels (Unionoida) following the zebra mussel (Dreissena polymorpha) invasion. Journal of Animal Ecology 67: 613–619.
Roberts, L., 1990. Zebra mussel invasion threatens U.S. waters. Science 249: 1370–1372.
Robertson, A. & G. G. Lauenstein, 1998. Distribution of chlorinated organic contaminants in dreissenid mussels along the southern shores of the Great Lakes. Journal of Great Lakes Research 24: 608–619.
Roditi, H. A., N. F. Caraco, J. J. Cole & D. L. Strayer, 1996. Filtration of Hudson River water by the zebra mussel (Dreissena polymorpha). Estuaries 19: 824–832.
Roditi, H. A., D. L. Strayer & S. E. G. Findlay, 1997. Characteristics of zebra mussel Dreissena polymorpha biodeposits in a tidal freshwater estuary. Archiv Für Hydrobiologie 140: 207–219.
Roesijadi, G., J. S. Young, A. S. Drum & J. M. Gurtisen, 1984. Behavior of trace metals in Mytilus edulis during a reciprocal transplant field experiment. Marine Ecology Progress Series 18: 155–170.
Rojas Molina, F. & V. Williner, 2013. First record of the non-indigenous mussel Limnoperna fortunei (Bivalvia, Mytilidae) as an epibiont of the crab Trichodactylus borellianus (Decapoda, Trichodactylidae). Crustaceana 86: 682–692.
Rojas Molina, F., S. José de Paggi & J. C. Paggi, 2015. Impacts of Limnoperna fortunei on zooplankton. In Boltovskoy, D. (ed), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Springer, Cham: 177–190.
Rombaldi, C., J. L. de Oliveira Arias, G. I. Hertzog, S. S. Caldas, J. P. Vieira & E. G. Primel, 2015. New environmentally friendly MSPD solid support based on golden mussel shell: characterization and application for extraction of organic contaminants from mussel tissue. Analytical and Bioanalytical Chemistry 407: 4805–4814.
Roper, J. M., D. S. Cherry, J. W. Simmers & H. E. Tatem, 1996. Bioaccumulation of toxicants in the zebra mussel, Dreissena polymorpha, at the times beach confined disposal facility, Buffalo, New York. Environmental Pollution 94: 117–129.
Rudstam, L. G. & C. J. Gandino, 2020. Zebra or quagga mussel dominance depends on trade-offs between growth and defense - Field support from Onondaga Lake, NY. PLoS ONE 15: e0235387.
Rzepecki, L. M. & J. H. Waite, 1995. Wresting the muscle from mussel beards: research and applications. Molecular Marine Biology and Biotechnology 4: 313–322.
Sardiña, P., D. Cataldo & D. Boltovskoy, 2008. The effects of the invasive mussel, Limnoperna fortunei, on associated fauna in South American freshwaters: importance of physical structure and food supply. Fundamental and Applied Limnology/archiv Für Hydrobiologie 173: 135–144.
Sardiña, P., E. Chaves & M. Marchese, 2011. Benthic community responses to invasion by the golden mussel, Limnoperna fortunei Dunker: biotic homogenization vs. environmental driving forces. Journal of the North American Benthological Society 30: 1009–1023.
Sarnelle, O., A. E. Wilson, S. K. Hamilton, L. B. Knoll & D. F. Raikow, 2005. Complex interactions between the zebra mussel, Dreissena polymorpha, and the harmful phytoplankter, Microcystis aeruginosa. Limnology & Oceanography 50: 896–904.
Scarabotti, P. A., L. O. Lucifora, L. A. Espínola, A. P. Rabuffetti, J. Liotta, J. E. Mantinian, J. P. Roux, N. Silva, L. Balboni, F. Vargas, L. D. Demonte & S. Sánchez, 2021. Long-term trends of fishery landings and target fish populations in the lower La Plata basin. Neotropical Ichthyology 19: e210013.
Schaller, J. & B. Planer-Friedrich, 2017. The filter feeder Dreissena polymorpha affects nutrient, silicon, and metal(loid) mobilization from freshwater sediments. Chemosphere 174: 531–537.
Schernewski, G., N. Stybel & T. Neumann, 2012. Zebra mussel farming in the Szczecin (Oder) Lagoon: water-quality objectives and cost-effectiveness. Ecology and Society 17: 4.
Schernewski, G., R. Friedland, A.-L. Buer, S. Dahlke, B. Drews, S. Höft, T. Klumpe, M. Schadach, J. Schumacher & A. Zaiko, 2019. Ecological-social-economic assessment of zebra-mussel cultivation scenarios for the Oder (Szczecin) Lagoon. Journal of Coastal Conservation 23: 913–929.
Schloesser, D. W. & T. F. Nalepa, 1994. Dramatic decline of unionid bivalves in offshore waters of western Lake Erie after infestation of the zebra mussel, Dreissena polymorpha. Canadian Journal of Fisheries and Aquatic Sciences 51: 2234–2242.
Schloesser, D. W., T. F. Nalepa & G. L. Mackie, 1996. Zebra mussel infestation of unionid bivalves (Unionidae) in North America. American Zoologist 36: 300–310.
Schriver, P., J. Bogestrand, E. Jeppesen & M. Sondergaard, 1995. Impact of submerged macrophytes on fish-zooplankton phytoplankton interactions: large-scale enclosure experiments in a shallow eutrophic lake. Freshwater Biology 33: 255–270.
Schummer, M. L., S. S. Badzinski, S. A. Petrie, Y. W. Chen & N. Belzile, 2010. Selenium accumulation in sea ducks wintering at Lake Ontario. Archives of Environmental Contamination and Toxicology 58: 854–862.
Schwab, A., U. Bornhauser-Sieber & V. Keller, 2001. Wintering waterbirds in the Lucerne part of Vierwaldstättersee (Switzerland) 1954/55-2000/01. Ornithologische Beobachter 98: 179–208.
Selegean, J. P. & T. M. Heidtke, 1994. Helpful zebra mussels? Water Environment and Technology 6: 31–32.
Selegean, J. P. W., R. Kusserow, R. Patel, T. M. Heidtke & J. L. Ram, 2001. Using zebra mussels to monitor Escherichia coli in environmental waters. Journal of Environmental Quality 30: 171–179.
Shields, R. C. & D. W. Beckman, 2015. Assessment of variation in age, growth, and prey of freshwater drum (Aplodinotus grunniens) in the Lower Missouri River. Southwestern Naturalist 60: 360–365.
Silva, A. C. S., 2016. Quantificação de metais potencialmente tóxicos em mexilhão dourado (Limnoperna fortunei) por espectrometria de absorção atômica com fonte contínua e alta resolução empregando amostragem direta de sólidos. Ms. Sc. Thesis, Universidade Estadual Paulista “Júlio de Mesquita Filho”, Brazil.
Silverman, H., J. S. Cherry, J. W. Lynn, T. H. Dietz, S. J. Nichols & E. Achberger, 1997. Clearance of laboratory-cultured bacteria by freshwater bivalves: differences between lentic and lotic unionids. Canadian Journal of Zoology 75: 1857–1866.
Simberloff, D. & J. R. S. Vitule, 2013. A call for an end to calls for the end of invasion biology. Oikos 123: 408–413.
Skubinna, J. P., T. G. Coon & T. R. Batters, 1995. Increased abundance and depth of submersed macrophytes in response to decreased turbidity in Saginaw Bay, Lake Huron. Journal of Great Lakes Research 21: 476–478.
Slooff, W., D. de Zwart & J. M. Marquenie, 1983. Detection limits of a biological monitoring system for chemical water pollution based on mussel activity. Bulletin of Environmental Contamination and Toxicology 30: 400–405.
Smit, H., A. bij de Vaate, H. H. Reeders, E. H. van Nes & R. Noordhuis, 1993. Colonization, ecology, and positive aspects of zebra mussels (Dreissena polymorpha) in The Netherlands. In Nalepa, T. F. & D. Schloesser (eds), Zebra Mussels: Biology, Impacts, and Control Lewis Publishers, Boca Raton: 55–77.
Sousa, R., J. L. Gutierrez & D. C. Aldridge, 2009. Nonindigenous invasive bivalves as ecosystem engineers. Biological Invasions 11: 2367–2385.
Stanczykowska, A. & K. Lewandowski, 1993. Thirty years of studies of Dreissena polymorpha ecology in Mazurian Lakes of Northeastern Poland. In Nalepa, T. F. & D. W. Schloesser (eds), Zebra Mussels. Biology Impacts and Control Lewis Publishers, Boca Raton: 3–33.
Starobogatov, J. I. & S. I. Andreeva, 1994. Distribution and history. In Starobogatov, J. I. (ed), Freshwater zebra mussel Dreissena polymorpha (Pall.) (Bivalvia, Dreissenidae) Systematics, Ecology, Practical Meaning Nauka Press, Moscow: 47–55 (in Russian).
Steinnes, D. N., 1992. Measuring the economic value of water quality: the case of lakeshore land. Annals of Regional Science 26: 171–176.
Stewart, T. W. & J. M. Haynes, 1994. Benthic macroinvertebrate communities of southwestern Lake Ontario following invasion of Dreissena. Journal of Great Lakes Research 20: 479–493.
Stewart, T. W., J. G. Miner & R. L. Lowe, 1998. Quantifying mechanisms for zebra mussel effects on benthic macroinvertebrates: organic matter production and shell-generated habitat. Journal of the North American Benthological Society 17: 81–94.
Stewart, T. W., J. C. Gafford, J. G. Miner & R. L. Lowe, 1999. Dreissena-shell habitat and antipredator behavior: combined effects on survivorship of snails co-occurring with molluscivorous fish. Journal of the North American Benthological Society 18: 274–283.
Stoeckmann, A. M. & D. W. Garton, 1997. A seasonal energy budget for zebra mussels (Dreissena polymorpha) in western Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 54: 2743–2751.
Strayer, D. L., 2012. Eight questions about invasions and ecosystem functioning. Ecology Letters 15: 1199–1210.
Strayer, D. L. & H. M. Malcolm, 2007. Shell decay rates of native and alien freshwater bivalves and implications for habitat engineering. Freshwater Biology 52: 1611–1617.
Strayer, D. L. & H. M. Malcom, 2007. Effects of zebra mussels (Dreissena polymorpha) on native bivalves: the beginning of the end or the end of the beginning? Journal of the North American Benthological Society 26: 111–122.
Strayer, D. L., N. F. Caraco, J. J. Cole, S. Findlay & M. L. Pace, 1999. Transformation of freshwater ecosystems by bivalves - a case study of zebra mussels in the Hudson River. BioScience 49: 19–28.
Strayer, D., K. Hattala & A. Kahnle, 2004. Effects of an invasive bivalve (Dreissena polymorpha) on fish in the Hudson River estuary. Canadian Journal of Fisheries and Aquatic Sciences 61: 924–941.
Strayer, D. L., B. V. Adamovich, R. Adrian, D. C. Aldridge, C. Balogh, L. E. Burlakova, H. Fried-Petersen, L. G. Tóth, A. L. Hetherington, T. S. Jones, A. Y. Karatayev, J. B. Madill, O. A. Makarevich, J. E. Marsden, A. L. Martel, D. Minchin, T. F. Nalepa, R. Noordhuis, T. J. Robinson, L. G. Rudstam, A. N. Schwalb, D. R. Smith, A. D. Steinman & J. M. Jeschke, 2019. Long-term population dynamics of dreissenid mussels (Dreissena polymorpha and D. rostriformis): a cross-system analysis. Ecosphere 10(4): e02701.
Stumpf, P., K. Failing, T. Papp, J. Nazir, R. Böhm & R. E. Marschang, 2010. Accumulation of a low pathogenic avian influenza virus in zebra mussels (Dreissena polymorpha). Avian Diseases 54: 1183–1190.
Stybel, N., C. Fenske & G. Schernewski, 2009. Mussel cultivation to improve water quality in the Szczecin Lagoon. Journal of Coastal Research 56: 1459–1463.
Suter, W., 1982a. Die Bedeutung von Untersee-Ende/Hochrhein (Bodensee) als wichtiges Überwinterungsgewässer für Tauchenten Aythya, Bucephala und Bläßhuhn Fulica atra. Der Ornithologische Beobachter 79: 73–96.
Suter, W., 1982b. Vergleichende Nahrungsökologie von überwinternden Tauchenten Bucephala, Aythya und Bläßhuhn Fulica atra am Untersee-Ende/Hochrhein (Bodensee). Der Ornithologische Beobachter 79: 225–254.
Suter, W., 1994. Overwintering waterfowl on Swiss lakes: How are abundance and species richness influenced by trophic status and lake morphology? Hydrobiologia 279–280: 1–14.
Sylvester, F. & P. Sardiña, 2015. Relationships of Limnoperna fortunei with benthic animals. In Boltovskoy, D. (ed), Limnoperna fortunei: The Ecology, Distribution and Control of a Swiftly Spreading Invasive Fouling Mussel Springer International Publishing, Cham: 191–210.
Sylvester, F., D. Boltovskoy & D. Cataldo, 2007a. Fast response of freshwater consumers to a new trophic resource: Predation on the recently introduced Asian bivalve Limnoperna fortunei in the lower Parana River, South America. Austral Ecology 32: 403–415.
Sylvester, F., D. Boltovskoy & D. Cataldo, 2007b. The invasive bivalve Limnoperna fortunei enhances benthic invertebrate densities in South American floodplain rivers. Hydrobiologia 589: 15–27.
Tassin, J. & C. A. Kull, 2015. Facing the broader dimensions of biological invasions. Land Use Policy 42: 165–169.
Tellier, J. M., N. I. Kalejs, B. S. Leonhardt, D. Cannon, T. O. Hӧӧk & P. D. Collingsworth, 2022. Widespread prevalence of hypoxia and the classification of hypoxic conditions in the Laurentian Great Lakes. Journal of Great Lakes Research 48: 13–23.
Ten Winkel, E. N. & C. Davids, 1982. Food selection by Dreissena polymorpha Pallas (Mollusca: Bivalvia). Freshwater Biology 12: 553–558.
Thomaz, S. M., 2021. Ecosystem services provided by freshwater macrophytes. Hydrobiologia. https://doi.org/10.1007/s10750-021-04739-y.
Thompson, K., 2014. Where do Camels Belong? Why Invasive Species Aren’t All bad, Profile Books, London.
Thorp, J. H., M. D. Delong & A. F. Casper, 1998. In situ experiments on predatory regulation of a bivalve mollusc (Dreissena polymorpha) in the Mississippi and Ohio Rivers. Freshwater Biology 39: 649–661.
Tokumon, R., D. Cataldo & D. Boltovskoy, 2015. Effects of suspended inorganic matter on filtration and grazing rates of the invasive mussel Limnoperna fortunei (Bivalvia, Mytiloidea). Journal of Molluscan Studies 82: 201–204.
Tokumon, R., D. Boltovskoy & D. Cataldo, 2018. Effects of the invasive freshwater mussel Limnoperna fortunei on sediment properties and accumulation rates. Journal of Geophysical Research: Biogeosciences 123: 2002–2017.
Torres, M. V., V. Williner & F. Giri, 2012. Size selective predation on an invasive bivalve, Limnoperna fortunei (Mytilidae), by a freshwater crab, Zilchiopsis collastinensis (Trichodactylidae). Journal of Crustacean Biology 32: 698–710.
Turschak, B. A. & H. A. Bootsma, 2015. Lake Michigan trophic structure as revealed by stable C and N isotopes. Journal of Great Lakes Research 41: 185–196.
Tyner, E. H., H. A. Bootsma & B. M. Lafrancois, 2015. Dreissenid metabolism and ecosystem-scale effects as revealed by oxygen consumption. Journal of Great Lakes Research 41: 27–37.
Uchida, S., A. Shiragane, A. Uchida, Y. Tanaka, K. Doi & Y. Matsuura, 2007. A mass death after a high abundance of the invasive mussel, Limnoperna fortunei in the Yahagi River, Honshu, Japan. Report of the Yahagi River Institute 11: 35–46 (in Japanese).
van der Velde, G., S. Rajagopal & A. bij de Vaate, 2010. From zebra mussels to quagga mussels: an introduction to the Dreissenidae. In van der Velde, G., S. Rajagopal & A. bij de Vaate (eds), The Zebra Mussel in Europe Backhuys Publishers, Leiden: 1–10.
Vanderploeg, H. A., J. R. Liebig, W. W. Carmichael, M. A. Agy, T. H. Johengen, G. L. Fahnenstiel & T. F. Nalepa, 2001. Zebra mussel (Dreissena polymorpha) selective filtration promoted toxic Microcystis blooms in Saginaw Bay (Lake Huron) and Lake Erie. Canadian Journal of Fisheries and Aquatic Sciences 58: 1208–1221.
Vanderploeg, H. A., T. F. Nalepa, D. J. Jude, E. L. Mills, K. T. Holeck, J. R. Liebig, I. A. Grigorovich & H. Ojaveer, 2002. Dispersal and emerging ecological impacts of Ponto-Caspian species in the Laurentian Great Lakes. Canadian Journal of Fisheries and Aquatic Sciences 59: 1209–1228.
Vanderploeg, H. A., T. H. Johengen & J. R. Liebig, 2009. Feedback between zebra mussel selective feeding and algal composition affects mussel condition: did the regime changer pay a price for its success? Freshwater Biology 54: 47–63.
Vanderploeg, H. A., J. R. Liebig, T. F. Nalepa, G. L. Fahnenstiel & A. Pothoven, 2010. Dreissena and the disappearance of the spring phytoplankton bloom in Lake Michigan. Journal of Great Lakes Research 36: 50–59.
Vaughn, C. C., 2017. Ecosystem services provided by freshwater mussels. Hydrobiologia 810: 15–27.
Vaughn, C. C. & T. J. Hoellein, 2018. Bivalve impacts in freshwater and marine ecosystems. Annual Review of Ecology, Evolution, and Systematics 49: 183–208.
Villnäs, A., J. Norkko, K. Lukkari, J. Hewitt & A. Norkko, 2012. Consequences of increasing hypoxic disturbance on benthic communities and ecosystem functioning. PLoS ONE 7(10): e44920.
Vimercati, G., S. Kumschick, A. F. Probert, L. Volery & S. Bacher, 2020. The importance of assessing positive and beneficial impacts of alien species. NeoBiota 62: 525–545.
Vizentin-Bugoni, J., C. E. Tarwater, J. T. Foster, D. R. Drake, J. M. Gleditsch, A. M. Hruska, J. P. Kelley & J. H. Sperry, 2019. Structure, spatial dynamics, and stability of novel seed dispersal mutualistic networks in Hawai‘i. Science 364: 78–82.
Vorobiev, V. P., 1949. The Benthos of the Azov Sea, Krimizdat Press, Simpheropol (USSR) (in Russian).
Waajen, G., N. C. B. Van Bruggen, L. M. D. Pires, W. Lengkeek & M. Lurling, 2016. Biomanipulation with quagga mussels (Dreissena rostriformis bugensis) to control harmful algal blooms in eutrophic urban ponds. Ecological Engineering 90: 141–150.
Wachholz, L., R. Vianna Nunes, J. Broch & C. De Souza, 2017. Possibilidade do USO de mexilhão dourado contaminado com metais tóxicos em dietas para frangos de corte. Revista Colombiana De Ciencia Animal 9: 227–235.
Walsh, J. R., S. R. Carpenter & M. J. Vander Zanden, 2016. Invasive species triggers a massive loss of ecosystem services through a trophic cascade. Proceedings of the National Academy of Sciences USA 113: 4081–4085.
Walz, N., 1978. The production and significance of the Dreissena population in the nutrient cycle in Lake Constance. Archiv Für Hydrobiologie 82: 482–499.
Wang, J., K. R. Koopman, F. P. L. Collas, L. Posthuma, T. de Nijs, R. S. E. W. Leuven & A. J. Hendriks, 2021. Towards an ecosystem service-based method to quantify the filtration services of mussels under chemical exposure. Science of the Total Environment 763. https://doi.org/10.1016/j.scitotenv.2020.144196.
Ward, J. M. & A. Ricciardi, 2007. Impact of Dreissena invasions on benthic macroinvertebrate communities: a meta-analysis. Diversity and Distributions 13: 155–165.
Ward, J. M. & A. Ricciardi, 2013. Impacts of Dreissena on benthic macroinvertebrate communities: predictable patterns revealed by invasion history. In Nalepa, T. F. & D. Schloesser (eds), Quagga and Zebra Mussels: Biology, Impacts, and Control CRC Press, Boca Raton: 599–610.
Watzin, M. C., K. Joppe-Mercure, J. Rowder, B. Lancaster & L. Bronson, 2008. Significant fish predation on zebra mussels Dreissena polymorpha in Lake Champlain, USA. Journal of Fish Biology 73: 1585–1599.
Weber, M. J., J. M. Dettmers, D. H. Wahl & S. J. Czesnyet, 2011. Size preferences and behaviors of native yellow perch foraging on invasive round gobies. Journal of Great Lakes Research 37: 584–587.
Van Weelden T. B. & B. C. Anderson, 2003. Phosphorus adsorption capacity of zebra mussel shells as a potential filter medium in constructed wetlands. Proceedings, Annual Conference - Canadian Society for Civil Engineering: 797–805.
Wegner, B., A. L. Kronsbein, M. Gillefalk, K. van de Weyer, J. Köhler, E. Funke, M. T. Monaghan & S. Hilt, 2019. Mutual facilitation among invading nuttall’s waterweed and quagga mussels. Frontiers in Plant Science 10: 789.
Werner, S., M. Mortl, H. G. Bauer & K. O. Rothhaupt, 2005. Strong impact of wintering waterbirds on zebra mussel (Dreissena polymorpha) populations at Lake Constance, Germany. Freshwater Biology 50: 1412–1426.
White, J. D. & O. Sarnelle, 2014. Size-structured vulnerability of the colonial cyanobacterium, Microcystis aeruginosa, to grazing by zebra mussels (Dreissena polymorpha). Freshwater Biology 59: 514–525.
Wiktor, K., 1958. Larvae of Dreissena polymorpha Pall. as a food for fish spawn. Przeglad Zoologiczny 2: 182–184 (in Polish).
Williams, K., 2020. Invasive mussels clear the water and coat the wrecks at Thunder Bay National Marine Sanctuary. Great Lakes Echo [available on https://greatlakesecho.org/2020/02/27/invasive-mussels-clear-the-water-and-coat-the-wrecks-at-thunder-bay-national-marine-sanctuary/].
Winkler, G., C. Martineau, J. J. Dodson, W. F. Vincent & L. E. Johnson, 2007. Trophic dynamics of two sympatric mysid species in an estuarine transition zone. Marine Ecology Progress Series 332: 171–187.
Withers, J. L., T. M. Sesterhenn, C. J. Foley, C. D. Troy & T. O. Höök, 2015. Diets and growth potential of early stage larval yellow perch and alewife in a nearshore region of southeastern Lake Michigan. Journal of Great Lakes Research 41: 197–209.
Wojtal-Frankiewicz, A. & P. Frankiewicz, 2010. The influence of the Zebra Mussel (Dreissena polymorpha) on magnesium and calcium concentration in water. Acta Universitatis Lodziensis. Folia Biologica Et Oecologica 6: 81–101.
Wormington, A. & J. H. Leach, 1992. Concentrations of migrant diving ducks at Point Pelee National Park, Ontario, in response to invasion of zebra mussels, Dreissena polymorpha. The Canadian Field-Naturalist 106: 376–380.
Wurster, C. M. & W. P. Patterson, 2001. Seasonal variation in stable oxygen and carbon isotope values recovered from modern lacustrine freshwater molluscs: paleoclimatological implications for sub-weekly temperature records. Journal of Paleolimnology 26: 205–218.
Xia, Z., X. Cao, T. Hoxha, A. Zhan, D. G. Haffner & H. J. MacIsaac, 2020. Functional response and size-selective clearance of suspended matter by an invasive mussel. Science of the Total Environment 711: 134679.
Yablonskaya, E. A., 1985. The Caspian Sea: Fauna and Biological Productivity, Nauka, Moscow (in Russian).
Yu, N. & D. A. Culver, 2000. Can zebra mussels change stratification patterns in a small reservoir? Hydrobiologia 431: 175–184.
Zhadin, V. I., 1946. The traveling shellfish Dreissena. Priroda 5: 29–37 (in Russian).
Zhadin, V. I., 1952. Mollusks of Fresh and Brackish Waters of the USSR, Academy of Sciences of the USSR Press, Moscow (USSR) (in Russian).
Zhang, R., B. Cui & S. Huang, 2015. Degradation of forchlorfenuron by nitrification and denitrification reactions in the gut and shell biofilm of Limnoperna fortunei. Ecotoxicology 24: 381–390.
Zhu, B., D. G. Fitzgerald, C. M. Mayer, L. G. Rudstam & E. L. Mills, 2006. Alteration of ecosystem function by zebra mussels in Oneida Lake: impacts on submerged macrophytes. Ecosystems 9: 1017–1028.
Zhulidov, A. V., D. F. Pavlov, T. F. Nalepa, G. H. Scherbina, D. A. Zhulidov & TYu. Gurtovaya, 2004. Relative distributions of Dreissena bugensis and Dreissena polymorpha in the lower Don River system, Russia. International Review of Hydrobiology 89: 326–333.
Zhulidov, A. V., A. V. Kozhara, G. H. Scherbina, T. F. Nalepa, A. Protasov, S. A. Afanasiev, E. G. Pryanichnikova, D. A. Zhulidov, T. Y. Gurtovaya & D. F. Pavlov, 2010. Invasion history, distribution, and relative abundances of Dreissena bugensis in the Old World: a synthesis of data. Biological Invasions 12: 1923–1940.
Acknowledgements
LEB was supported by the Great Lakes Restoration Initiative via a cooperative agreement with Cornell University, Department of Natural Resources under Prime Agreement Award GL 00E02259-2 from the U.S. EPA “Great Lakes Long-Term Biological Monitoring Program 2017-2022” (PI L. Rudstam, Co-PIs Burlakova & Karatayev). NC was supported by Grant 273/2020 (UNDEFI, Argentina). We would like to thank Administrative Assistant S. Dickinson (GLC) for proofreading the manuscript, and the Editor and two anonymous reviewers for helpful comments. The views expressed in this publication are those of the authors and do not necessarily represent the views or policies of the U.S. EPA. Any use of trade, product or firm names is for descriptive purposes only and does not imply endorsement by the U.S. EPA.
Funding
LEB supported by a cooperative agreement with Cornell University, Department of Natural Resources under Prime Agreement Award GL 00E02259-2 from the U.S. EPA “Great Lakes Long-Term Biological Monitoring Program 2017–2022” (PI L. Rudstam, Co-PIs Burlakova & Karatayev). NC was supported by grant 273/2020 (UNDEFI, Argentina).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study. LEB, AYK and DB performed the literature search and data analysis. The first draft of the manuscript was written by LEB and all authors reviewed and commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest and no competing interests to declare regarding the publication of this article. The authors have no financial or proprietary interests in any material discussed in this article. All authors certify that they have no affiliations with or involvement in any organization or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript.
Ethical approval
Not applicable—this study is a review of published data and did not involve animals.
Consent to participate
Not applicable—this study did not involve human participants.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Guest editors: Verónica Ferreira, Luis Mauricio Bini, Katya E. Kovalenko, Andre A. Padial, Judit Padisák & María de los Ángeles González Sagrario / Aquatic Ecosystem Services
Rights and permissions
About this article
Cite this article
Burlakova, L.E., Karatayev, A.Y., Boltovskoy, D. et al. Ecosystem services provided by the exotic bivalves Dreissena polymorpha, D. rostriformis bugensis, and Limnoperna fortunei. Hydrobiologia 850, 2811–2854 (2023). https://doi.org/10.1007/s10750-022-04935-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04935-4